Introduction
Pancreatic cancer is a highly malignant type of
tumor of the digestive system, with a 5-year survival rate of 3%
(1). Most patients present with
advanced stage disease at diagnosis, upon which surgery is no
longer an option (2). Even after
surgical resection, the estimated overall survival rate remains low
(20%) and most patients will develop metastatic disease within 5
years (3). The lethality of
pancreatic cancer is attributed to several factors, including the
lack of effective screening, delayed presentation and complex tumor
biology and genetics (4). At
present, there is no effective treatment for pancreatic cancer,
especially in advanced stages, and there has been no successful
breakthrough in the development of new targeted therapies (5). Therefore, there is an urgent need to
identify effective therapeutics for patients with pancreatic cancer
(6).
As a programmed death mechanism, apoptosis serves an
important role in maintaining homeostasis (7). A number of oncogenic pathways inhibit
apoptosis, and the imbalance of apoptosis has an important role in
tumor occurrence and development (8). TNF-related apoptosis-inducing ligand
(TRAIL) can induce apoptosis of various cancer cells while sparing
most normal cells, and has been shown to be a relatively promising
and effective anticancer agent (9,10).
TRAIL-induced apoptosis is mediated by an extrinsic pathway via
death receptor (DR)4 and/or DR5. The interaction between the DR
Fas-associated protein with death domain (FADD) and pro-caspase-8
promotes the formation of death-inducible signaling complex (DISC)
(11). With the formation of DISC,
pro-caspase-8 is activated, which then activates caspase-3
(10). Caspase-8 may also
proteolytically process Bid to initialize the intrinsic pathway
(12). However, a subsequent study
found that a multitude of cancer cells avoid TRAIL-induced
apoptosis in different ways, such as via loss of cell surface
expression of TRAIL receptors and imbalance of stoichiometric
ratios of pro- and anti-apoptotic proteins (12). Additionally, another study has
revealed that the function of lipid rafts is associated with TRAIL
resistance in NSCLC cells (13).
Lipid rafts are membrane microdomains within the lipid bilayer
structure containing special lipids and proteins, which provide a
membrane aggregation platform for DRs and serve an important role
in initiating death signaling transmission (14).
Most tumor cell membranes contain acid phospholipids
(3–9%), making the tumor cell surface negatively charged (15,16). In
a previous study (17), the soluble
TRAIL 114–121 amino acid coding sequence (VRERGPQR) was selected
and constructed into the ‘RRRRRRRR’ sequence to obtain a novel
TRAIL mutant, TRAIL-Mu3. The antitumor effects of TRAIL-Mu3 on
colorectal cancer in vitro and in vivo were significantly improved
(18). The present study
investigated the antitumor effects of TRAIL-Mu3 on pancreatic
cancer cells and its possible mechanism.
Materials and methods
Cell culture and treatment
MIAPaca-2 cells (Shanghai Institutes for Biological
Sciences; Chinese Academy of Sciences) were maintained in RPMI-1640
medium (Thermo Fisher Scientific, Inc.) supplemented with 1 mM
sodium pyruvate and 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.). PANC-1 and PA-TU8988S cells (Shanghai Institutes
for Biological Sciences; Chinese Academy of Sciences) were
maintained in low-glucose DMEM (Thermo Fisher Scientific, Inc.)
supplemented with 10% fetal bovine serum. All cells were incubated
in 5% CO2 at 37°C. Cytotoxicity was evaluated using
medium containing 10% Cell Counting Kit-8 reagent (Dojindo
Molecular Technologies, Inc.) for 1.5 h at 37°C. Pancreatic cancer
cells were treated with TRAIL-Mu3 (Initial concentration 0.1/1/100
µg/ml) or TRAIL (Initial concentration 100/100/300 µg/ml) (Chengdu
Huachuang Biotechnology Co., Ltd.) for 48 h at 37°C. A microplate
reader was used to measure the raw values at a wavelength of 490 nm
(Infinite F50; Tecan Group, Ltd.). The criterium for sensitivity or
resistance was the half maximal inhibitory concentration
(IC50 <10 µg/ml, the cell was sensitive to the
protein sample; IC50 ≥10 µg/ml, the cell was resistant
to the protein sample). Cytotoxicity assays were performed in
triplicate and repeated at least three times.
Assessment of apoptosis
PANC-1 cells (2×106/ml) were seeded in
6-well plates and incubated with TRAIL-Mu3 or TRAIL (0.0025 µg/ml)
for 24 h at 37°C. Subsequently, the cells were collected and washed
twice with cold phosphate-buffered saline (PBS). The cells were
resuspended in 500 µl 1X Annexin V binding buffer containing 5 µl
Annexin V-FITC and 5 µl PI solution (Dojindo Molecular
Technologies, Inc.). The percentage of early and late apoptotic
cells was evaluated with a FACSCalibur instrument (Cytoflex;
Beckman Coulter, Inc.) and analyzed using CytExpert 2.0 (Beckman
Coulter, Inc.).
PANC-1 cells (5×103/ml) were seeded in
96-well plates, pretreated with or without the broad-spectrum
caspase inhibitor Z-VAD-FMK (10 µM; Selleck Chemicals) and
incubated with TRAIL-Mu3 (initial concentration 0.1 µg/ml) or TRAIL
(initial concentration 0.1 µg/ml) for 48h at 37°C. Cytotoxicity was
evaluated using the Cell Counting Kit-8, as aforementioned.
Analysis of affinity by
immunofluorescence
The Pierce FITC Antibody Labeling kit (Thermo Fisher
Scientific, Inc.) was used to label TRAIL-Mu3 or TRAIL according to
the manufacturers instructions. PANC-1 cells (5×104/ml)
were seeded in 24-well plates that contained slides and then
incubated with labeled TRAIL-Mu3 or TRAIL. After 1 h at 37°C, cells
were washed with PBS and fixed with 4% paraformaldehyde for 20 min
at room temperature. Cells were then stained with DiI (Beyotime
Institute of Biotechnology) for 10 min and Hoechst 33342 (Beyotime
Institute of Biotechnology) for 5 min at room temperature. Antifade
mounting medium (Beyotime Institute of Biotechnology) was used to
mount the cells, and the samples were analyzed by fluorescence
microscopy (magnification, ×400; AX10 Imager A2; Carl Zeiss
AG).
Analysis of the distribution of DRs in
lipid rafts by immunofluorescence
PANC-1 cells (5×104/ml) were seeded and
incubated with TRAIL-Mu3 or TRAIL (0.0025 µg/ml) in 24-well plates
with slides and fixed in 4% paraformaldehyde at 4°C. After 20 min,
cells were blocked with 3% bovine serum albumin (BSA; Thermo Fisher
Scientific, Inc.) for 30 min at room temperature. For double
staining, anti-DR4 or anti-DR5 mouse monoclonal primary antibodies
(1:100; cat. nos. sc-8411 and sc-166624, respectively; Santa Cruz
Biotechnology, Inc.) were used to label the cells at 4°C. After 1
h, the cells were incubated with rhodamine-conjugated goat
anti-mouse IgG secondary antibody (1:62.5; cat. no. sc-358922;
Santa Cruz Biotechnology, Inc.) for 45 min at 4°C. FITC-conjugated
rabbit anti-choleratoxin B (12.5 µg/ml; cat. no. C1655;
Sigma-Aldrich; Merck KGaA) was simultaneously added with the
secondary antibody to stain the membrane rafts. Fluorescence
microscopy (magnification, ×400; AX10 Imager A2/AX10 Cam HRC; Carl
Zeiss AG) was used to analyze the cells.
Western blot analysis
PANC-1 cells were seeded in a 60-mm culture dish and
incubated with TRAIL-Mu3 or TRAIL (0.0025 µg/ml) at 37°C. After 2,
4 and 6 h, cells were collected and lysed at 4°C with 200 µl buffer
containing 1% Triton X-100 and protease and phosphatase inhibitors.
Protein concentration was determined using a bicinchoninic acid
assay. Proteins (50 µg/lane) from lysed cells were separated via
15% SDS-PAGE and transferred to polyvinylidene difluoride
membranes, which were blocked with TBS buffer containing 3% BSA
(Thermo Fisher Scientific, Inc.) for 2 h at room temperature and
subsequently probed with primary antibodies overnight at 4°C,
followed by secondary antibodies for 2 h at 4°C. Protein bands were
visualized by electrochemiluminescence (Thermo Fisher Scientific,
Inc.). Primary antibodies for caspase-3 (cat. no. 9665; 1:1,000),
caspase-8 (cat. no. 9746; 1:1,000), β-actin (cat. no. 4970;
1:1,000) and poly (ADP-ribose) polymerase (PARP; cat. no. 9542;
1:1,000) were purchased from Cell Signaling Technology, Inc.
HRP-conjugated anti-mouse IgG (cat. no. 7076; 1:1,000) and
anti-rabbit IgG (cat. no. 7074; 1:1,000) secondary antibodies were
purchased from Cell Signaling Technology, Inc. ImageJ.JS (powered
by ImJoy) was used for densitometry.
In parallel, PANC-1 cells (5×103/ml) were
seeded in 96-well plates, incubated with TRAIL-Mu3 or TRAIL (0.0025
µg/ml) for 2, 4 and 6 h at 37°C. Cytotoxicity was evaluated using
the Cell Counting Kit-8, as aforementioned.
Flow cytometric analysis of DRs
PANC-1 cells (2×106/ml) were seeded in 6-well plates
and incubated with TRAIL-Mu3 or TRAIL (0.0025 µg/ml) for 24 h at
37°C. Subsequently, cells were collected and resuspended in PBS
containing 1% BSA. Next, FITC-labeled mouse anti-human DR4 (cat.
no. A15746) or DR5 monoclonal antibodies (cat. no. A15750) (both
1:20; both from Thermo Fisher Scientific, Inc.) were added for 30
min at 4°C. FITC-labeled control IgG isotypes (1:20; cat. no.
MA5-18096; Thermo Fisher Scientific, Inc.) were used to assess
non-specific staining at 4°C. After 30 min, a FACSCalibur
instrument (CytoFlex; Beckman Coulter, Inc.) and CytExpert 2.0
(Beckman Coulter, Inc.) were used to analyze the cells.
Statistical analysis
SPSS software (version 19.0; IBM Corp.) or OriginPro
software (version 9.0; OriginLab) were used to perform
computer-based statistical analysis. Results were presented as the
mean ± SD of at least three different experiments. Statistical
significance was evaluated using unpaired Students t-test for
comparisons between 2 groups and one-way ANOVA followed by Tukeys
post-hoc test for comparisons among >2 groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
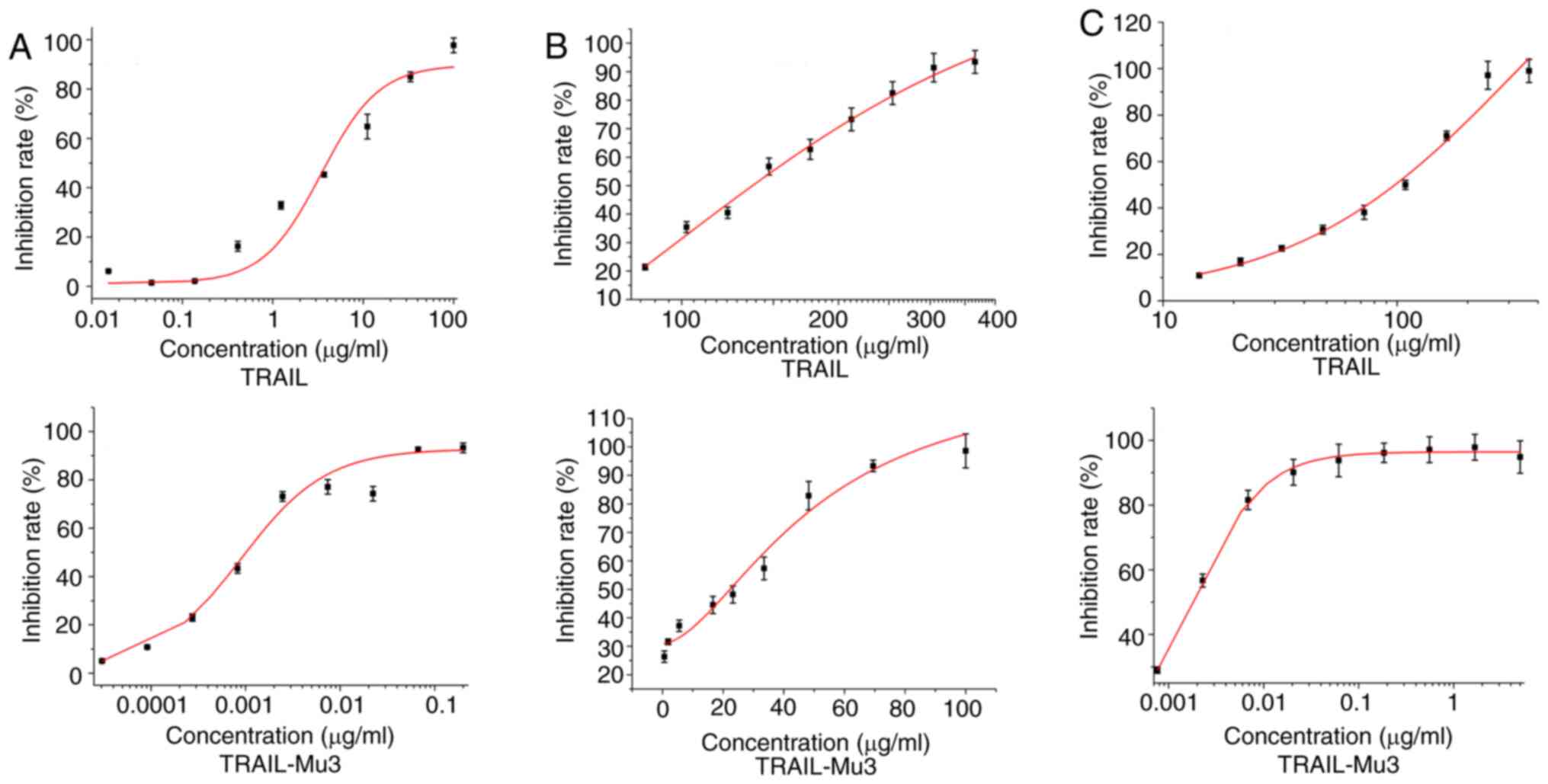
Cytotoxic ability of TRAIL-Mu3
The present study assessed the cytotoxic ability of
TRAIL-Mu3 in MIAPaca-2, PA-TU8988S and PANC-1 cells. TRAIL-Mu3
exhibited markedly higher cytotoxicity on pancreatic cancer cell
lines compared with TRAIL (Fig.
1A-C). A significant decrease in the IC50 of
TRAIL-Mu3 was observed in the three cancer cell lines compared with
TRAIL (Table I).
 | Table I.Comparison of IC50 values
between pancreatic cancer cell lines. |
Table I.
Comparison of IC50 values
between pancreatic cancer cell lines.
|
| IC50,
µg/ml |
|---|
|
|
|
|---|
| Cell line | TRAIL | TRAIL-Mu3 |
|---|
| MIAPaca-2 | 5.7252±0.9613 |
0.0008±0.0002a |
| PA-TU8988S |
128.4667±2.2460 |
30.4600±2.7700b |
| PANC-1 |
145.1920±5.5636 |
0.0028±0.0017c |
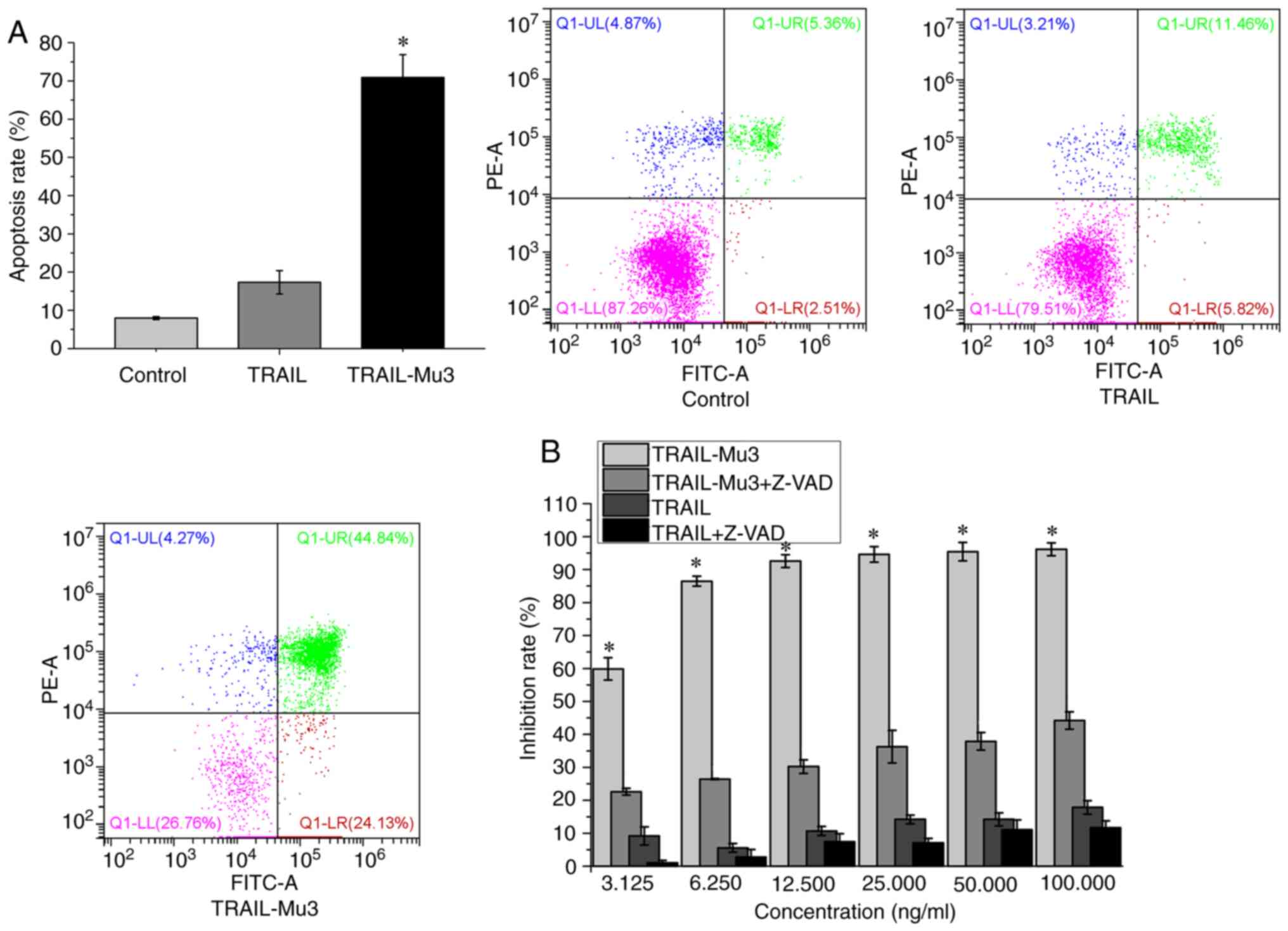
Pro-apoptotic effects of TRAIL-Mu3 are
significantly improved and TRAIL-Mu3-induced PANC-1 cell apoptosis
is dependent on the caspase cascade
The present study selected the PANC-1 cell line,
which is TRAIL-resistant and TRAIL-Mu3-sensitive, for subsequent
experiments. The apoptosis rate was significantly higher in the
TRAIL-Mu3 group compared with in the TRAIL group (Fig. 2A). TRAIL-Mu3 exhibited significantly
higher antitumor activity compared with TRAIL at each tested dose
(Fig. 2B). However, when PANC-1
cells were pretreated with the broad-spectrum caspase inhibitor
Z-VAD-FMK, the antitumor activity of TRAIL and TRAIL-Mu3 was
inhibited (Fig. 2B).
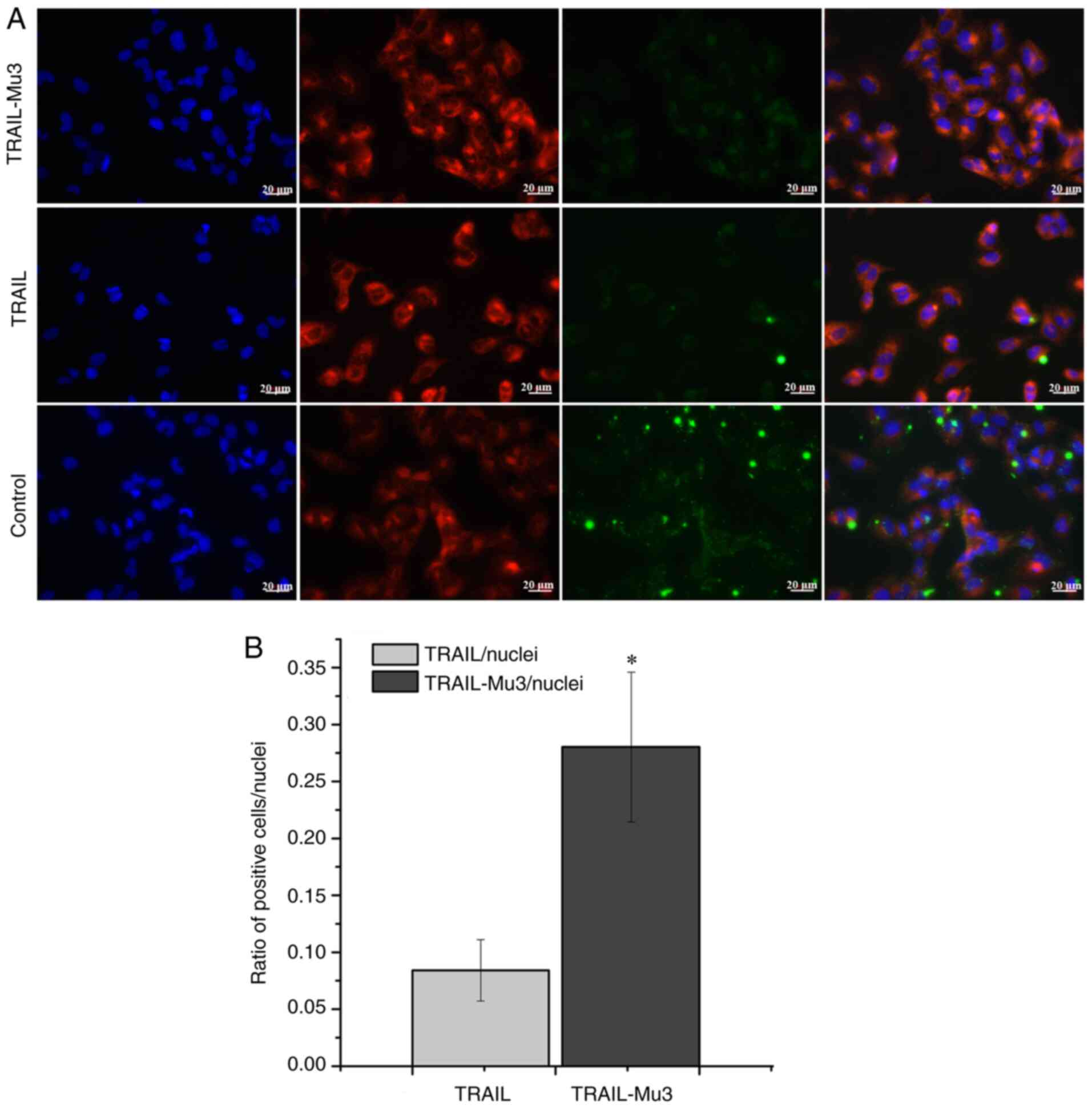
Affinity of TRAIL-Mu3 to PANC-1 cell
membranes is significantly enhanced
Immunofluorescence assays were performed to assess
the difference in affinity between TRAIL-Mu3 and TRAIL in PANC-1
cells. Following exposure to labeled TRAIL-Mu3 and TRAIL for 1 h,
PANC-1 cells were found to combine with a large amount of Green
fluorescence in the TRAIL-Mu3 group, but this was not observed for
the TRAIL group (Fig. 3A). The
quantification of the signal expressed as a ratio of TRAIL or
TRAIL-Mu3-positive cells to the number of nuclei is shown in
Fig. 3B, indicating that the ratio
of TRAIL-Mu3-positive cells/nuclei was significantly increased
compared with the ratio of TRAIL-positive cells/nuclei.
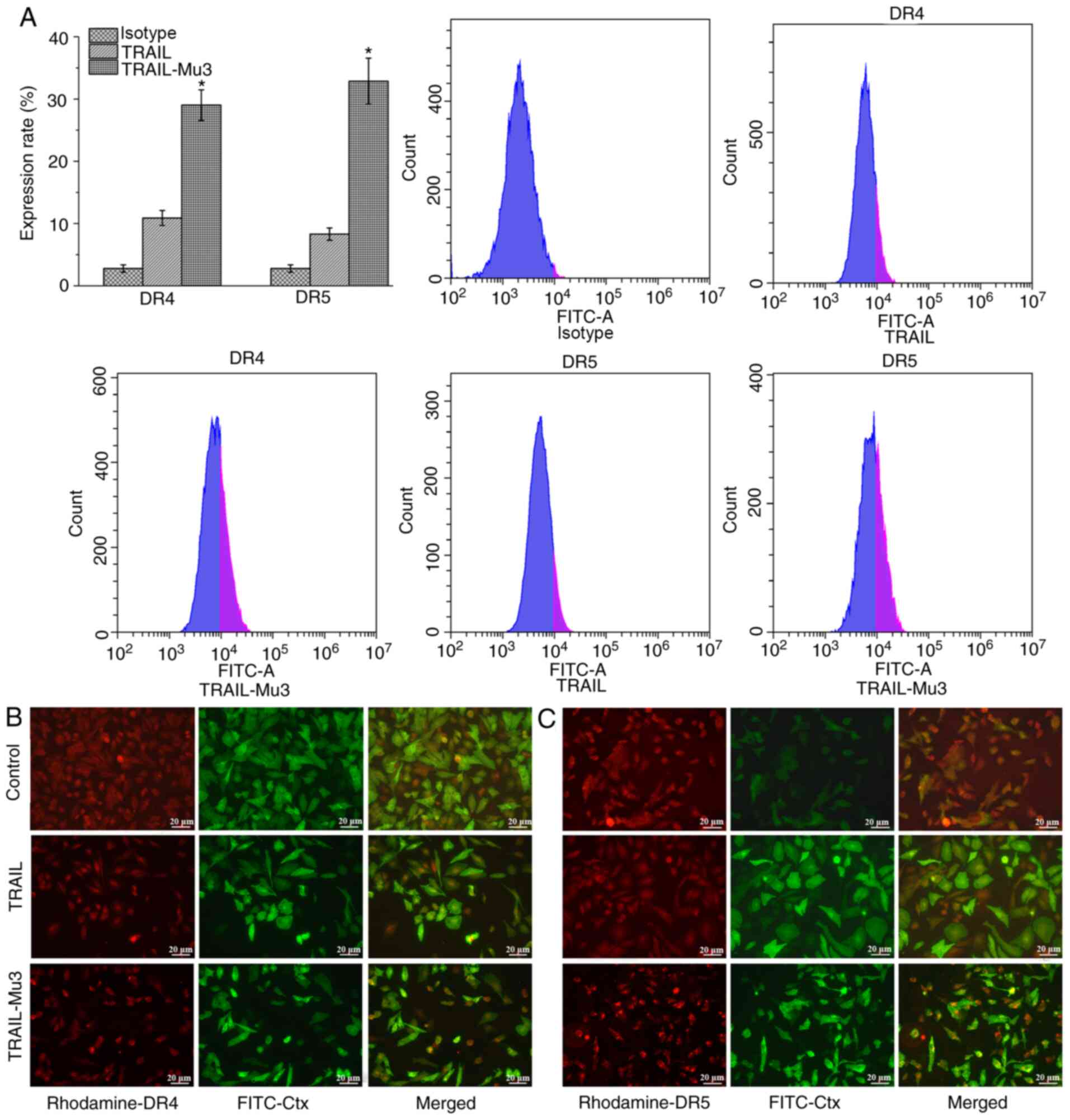
TRAIL-Mu3 upregulates DR expression
and promotes DR5 clustering into lipid rafts in PANC-1 cells
TRAIL-induced apoptosis is mediated by an extrinsic
pathway via DR4 and DR5 (11).
Therefore, the cell surface expression levels of DR4 and DR5 were
evaluated by flow cytometry. Compared with the TRAIL group, DR4 and
DR5 expression was increased in the TRAIL-Mu3 group (Fig. 4A). Cells treated with or without
TRAIL or TRAIL-Mu3 for 24 h were assessed by immunofluorescence.
The samples were stained using antibodies against DR4, DR5 and
FITC-coupled choleratoxin B (FITC-Ctx), which forms a complex with
the raft GM1-ganglioside component. In the TRAIL-Mu3 group, DR5
colocalized with FITC-Ctx, but this was not observed in the TRAIL
group; no DR4 colocalization with FITC-Ctx was observed in both
TRAIL and TRAIL-Mu3 groups (Fig. 4B and
C).
TRAIL-Mu3 activates the caspase
cascade in a faster and more efficient manner compared with TRAIL
in PANC-1 cells
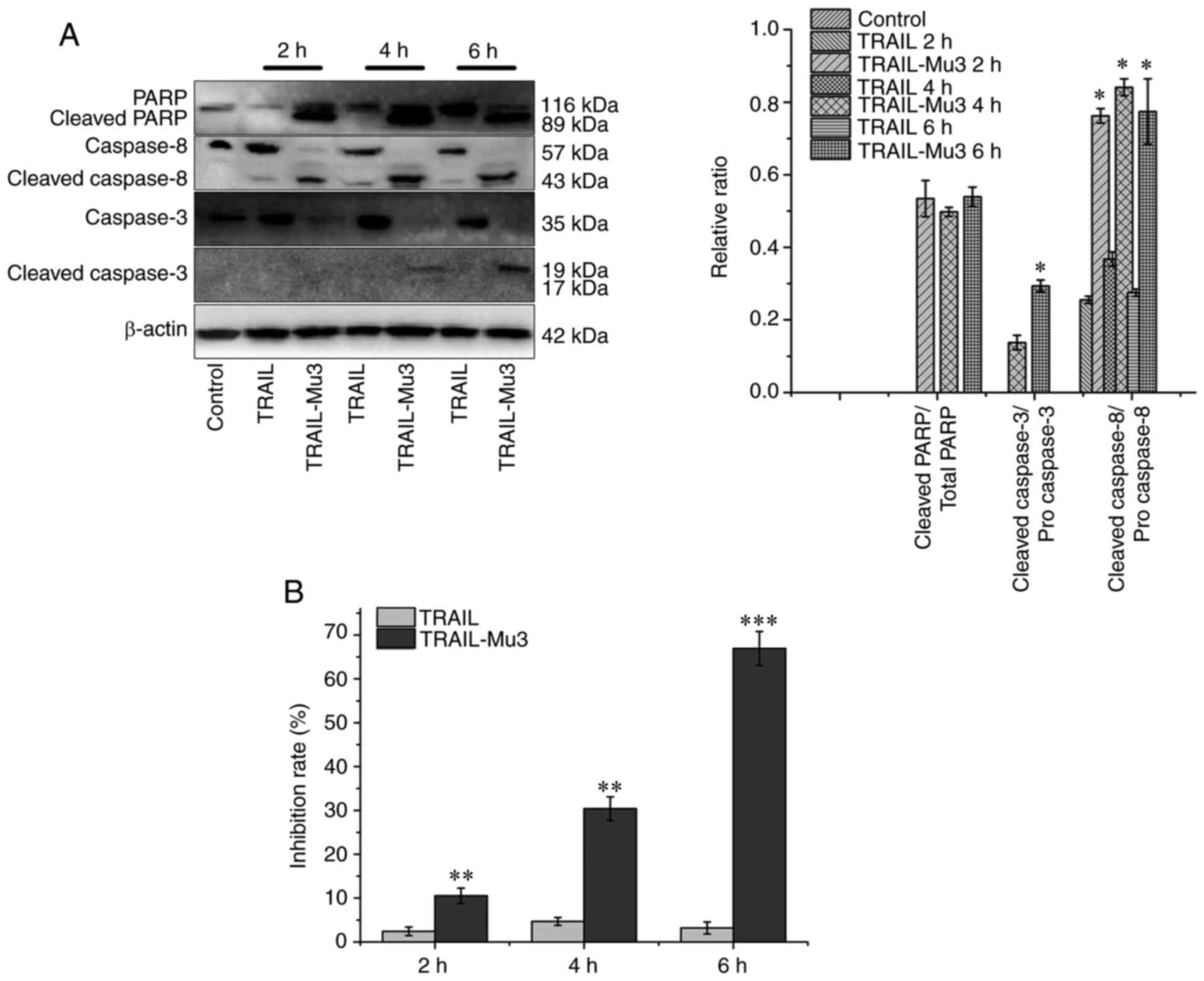
Based on the western blot analysis, treatment with
TRAIL did not result in caspase cleavage (mainly of caspase-3),
while TRAIL-Mu3 induced cleavage of both caspases-8 and −3 as early
as 2 h (Fig. 5A). In a parallel
experiment, the inhibition rate increased gradually after 2, 4 and
6 h in the TRAIL-Mu3 group (Fig.
5B). Cleavage of caspase-3 in PANC-1 cells may be essential in
the TRAIL-Mu3 group, since its substrate PARP was also cleaved in
the TRAIL-Mu3 group and the inhibition rate increased (Fig. 5A and B).
Discussion
Pancreatic cancer is one of the most lethal types of
malignant tumor, with rising death rates (19). Due to late detection of pancreatic
cancer in advanced stages, minimal efficacy of currently available
therapies and its extremely aggressive nature, it remains a
challenging problem in the clinical field (20). TRAIL can induce apoptosis of cancer
cells without causing toxicity in mice, which has led to the
in-depth study of pro-apoptotic TRAIL receptor (TRAIL-R) signaling
(9). To date, numerous
biotherapeutic drug candidates that activate TRAIL-Rs have been
developed (10,21). However, the results of clinical
trials with TRAIL-R agonists have been disappointing, due to
inadequate delivery methods, poor agonistic activity of these
agents and TRAIL resistance (22,23). To
translate these promising agents into clinical application, studies
have aimed to develop TRAIL derivatives with higher therapeutic
effects (24,25).
The novel recombinant protein TRAIL-Mu3 used in the
present study exhibited significantly higher cytotoxicity compared
with TRAIL. TRAIL-Mu3 considerably decreased the IC50 in
all cell lines tested, and was able to overcome TRAIL resistance of
several pancreatic cancer cell lines. In addition, experimental
results revealed that TRAIL-Mu3-induced apoptosis in PANC-1 cells
was greater compared with that induced by TRAIL. Our previous study
demonstrated that TRAIL-Mu3 repressed the growth of PANC-1
×enografts in nude mice and investigated the possible mechanism of
this effect (17). The present study
then assessed the underlying mechanism of action of TRAIL-Mu3 to
further understand the improvement in the biological activity of
TRAIL-Mu3.
Based on immunofluorescence results, it was revealed
that the affinity of TRAIL-Mu3 to PANC-1 cell membranes was
markedly enhanced compared with that of TRAIL. Aside from the
N-terminal amino acid coding sequence, TRAIL-Mu3 has a similar
structure to soluble TRAIL. The N-terminal of the TRAIL-Mu3
sequence (amino acids 114–121) was constructed into the ‘RRRRRRRR’
sequence, thereby increasing the positive charge of TRAIL-Mu3
(17). It has been found that most
tumor cell membranes contain acid phospholipids (3–9%), making the
tumor cell surface negatively charged (15,16).
Electrostatic interactions may enhance the affinity of TRAIL-Mu3 to
cell membranes. A previous study has confirmed that one class of
cell-penetrating peptides, including Arg-rich peptides, may
penetrate cells through electrostatic interactions and hydrogen
bonding, and seem to be energy-independent (26). TRAIL-Mu3 is Arg-rich with an
increased positive charge, which may contribute to the enhanced
affinity of TRAIL-Mu3 to cell membranes. With the increased
affinity, the chance of TRAIL-Mu3 binding to the receptor is also
increased, which may activate the extrinsic pathway inducing tumor
cell apoptosis (27).
TRAIL-induced apoptosis is mediated by an extrinsic
pathway via DR4 and DR5 (22). The
flow cytometry assay results revealed that treatment with TRAIL-Mu3
increased DR4 and DR5 cell surface expression. Previous studies
revealed that TRAIL toxicity in tumors can be regulated by TRAIL-R
expression. Hu et al (28)
reported that chaetospirolactone reversed the apoptotic resistance
towards TRAIL in pancreatic cancer by upregulating DR4 expression.
In addition, Yang et al (29)
found that high HOX transcript antisense RNA (HOTAIR) expression
increased the resistance of pancreatic cancer cells to
TRAIL-induced apoptosis, and short hairpin RNA-mediated
HOTAIR-knockdown in TRAIL-resistant PANC-1 cells sensitized them to
TRAIL-induced apoptosis via upregulation of DR5 expression.
Notably, in the present study, TRAIL-Mu3 promoted DR5 clustering
into lipid rafts. A previous study has confirmed that various
compounds that promote the clustering of DRs into lipid rafts can
account for TRAIL sensitization (13). Xu et al (30) reported that β-elemene increased the
sensitivity of gastric cancer cells to TRAIL by promoting DR5
clustering and translocation of caspase-8, DR5 and FADD into lipid
rafts.
A time-course assay was performed in PANC-1 cells in
the present study. Cells were incubated with TRAIL and TRAIL-Mu3,
followed by western blot analysis of activated caspase-8, caspase-3
and the corresponding substrate PARP. Compared with TRAIL,
TRAIL-Mu3 activated the caspase cascade in a faster and more
efficient manner. Caspase-8 activation following ligation of
DR4/DR5 by TRAIL is the apical caspase in the extrinsic pathway;
subsequently, caspase-3 is cleaved by activated caspase-8, followed
by the cleavage of death substrates and cell death (31). PARP inactivated by caspase cleavage
serves an important role in DNA repair (32). PARP cleavage by caspase-3 is an
important signal of apoptosis (33).
In the present study, TRAIL-Mu3 enhanced the cleavage of caspase-3
and its substrate PARP. A previous study has suggested that
combined use of PARP inhibitors may sensitize pancreatic cancer
cells to TRAIL (34).
In conclusion, the TRAIL-Mu3-enhanced pro-apoptotic
potential in pancreatic cancer cells may be associated with the
strengthening of the apoptotic signaling pathway by increased
affinity to the cell membrane, upregulation of DR4 and DR5
expression, clustering of DR5 in lipid rafts and efficient caspase
activation. However, there were several limitations in the present
study. The molecular mechanisms by which TRAIL-Mu3 induced the
redistribution of DR5 in lipid rafts were not very clear.
Mechanistic studies should be performed in the future. Overall, the
present study provided insight into the clinical application of
TRAIL-Mu3 in the treatment of pancreatic cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the
National Natural Scientific Foundation of China (grant no.
81372444) and the Natural Science Foundation of Chengdu Medical
College (grant no. CYZ18-15).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
MH performed the experiments and drafted the
manuscript. MH and YH were responsible for confirming the
authenticity of the data. CY and WJT performed the apoptosis and
affinity analyses. JY and XZH performed the cytotoxicity assays. MH
and LJW performed the analysis of the distribution of DRs in lipid
rafts, western blot analysis and flow cytometric analysis of DRs.
SCC and YH conceived and designed the experiments. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wolpin BM: Pancreatic cancer. Hematol
Oncol Clin North Am. 29:xiii–xiv. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chu LC, Goggins MG and Fishman EK:
Diagnosis and detection of pancreatic cancer. Cancer J. 23:333–342.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chatzizacharias NA, Tsai S, Griffin M,
Tolat P, Ritch P, George B, Barnes C, Aldakkak M, Khan AH, Hall W,
et al: Locally advanced pancreas cancer: Staging and goals of
therapy. Surgery. 163:1053–1062. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Draper A: Updates in pancreatic cancer:
Modest gains and hopeful targets. J Oncol Pharm Pract. 25:101–109.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sadeghi S, Davoodvandi A, Pourhanifeh MH,
Sharifi N, ArefNezhad R, Sahebnasagh R, Moghadam SA, Sahebkar A and
Mirzaei H: Anti-cancer effects of cinnamon: Insights into its
apoptosis effects. Eur J Med Chem. 178:131–140. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and DOrazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging (Albany NY). 8:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
von Karstedt S, Montinaro A and Walczak H:
Exploring the TRAILs less travelled: TRAIL in cancer biology and
therapy. Nat Rev Cancer. 17:352–366. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhong HH, Wang HY, Li J and Huang YZ:
TRAIL-based gene delivery and therapeutic strategies. Acta
Pharmacol Sin. 40:1373–1385. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan X, Gajan A, Chu Q, Xiong H, Wu K and
Wu GS: Developing TRAIL/TRAIL death receptor-based cancer
therapies. Cancer Metastasis Rev. 37:733–748. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shahwar D, Iqbal MJ, Nisa MU, Todorovska
M, Attar R, Sabitaliyevich UY, Farooqi AA, Ahmad A and Xu B:
Natural product mediated regulation of death receptors and
intracellular machinery: Fresh from the pipeline about
TRAIL-mediated signaling and natural TRAIL sensitizers. Int J Mol
Sci. 20:202019. View Article : Google Scholar
|
|
13
|
Ouyang W, Yang C, Liu Y, Xiong J, Zhang J,
Zhong Y, Zhang G, Zhou F, Zhou Y and Xie C: Redistribution of DR4
and DR5 in lipid rafts accounts for the sensitivity to TRAIL in
NSCLC cells. Int J Oncol. 39:1577–1586. 2011.PubMed/NCBI
|
|
14
|
Aroui S, Brahim S, Hamelin J, De Waard M,
Breard J and Kenani A: Conjugation of doxorubicin to cell
penetrating peptides sensitizes human breast MDA-MB 231 cancer
cells to endogenous TRAIL-induced apoptosis. Apoptosis.
14:1352–1365. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Papo N, Seger D, Makovitzki A, Kalchenko
V, Eshhar Z, Degani H and Shai Y: Inhibition of tumor growth and
elimination of multiple metastases in human prostate and breast
xenografts by systemic inoculation of a host defense-like lytic
peptide. Cancer Res. 66:5371–5378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kirson ED, Dbalý V, Tovarys F, Vymazal J,
Soustiel JF, Itzhaki A, Mordechovich D, Steinberg-Shapira S,
Gurvich Z, Schneiderman R, et al: Alternating electric fields
arrest cell proliferation in animal tumor models and human brain
tumors. Proc Natl Acad Sci USA. 104:10152–10157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang M, Zhu H, Yi C, Yan J, Wei L, Yang
X, Chen S and Huang Y: A novel TRAIL mutant-TRAIL-Mu3 enhances the
antitumor effects by the increased affinity and the up-expression
of DR5 in pancreatic cancer. Cancer Chemother Pharmacol.
82:829–838. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu H, Yan J, Xu Q, Wei L, Huang X, Chen S
and Yi C: TRAIL mutant membrane penetrating peptide alike (TMPPA)
TRAIL-Mu3 enhances the antitumor effects of TRAIL in vitro and in
vivo. Mol Med Rep. 16:9607–9612. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Neoptolemos JP, Kleeff J, Michl P,
Costello E, Greenhalf W and Palmer DH: Therapeutic developments in
pancreatic cancer: Current and future perspectives. Nat Rev
Gastroenterol Hepatol. 15:333–348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spano C, Grisendi G, Golinelli G,
Rossignoli F, Prapa M, Bestagno M, Candini O, Petrachi T, Recchia
A, Miselli F, et al: Soluble TRAIL armed human MSC as gene therapy
for pancreatic cancer. Sci Rep. 9:17882019.https://doi.org/10.1038/s41598-018-37433-6 View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Miguel D, Lemke J, Anel A, Walczak H
and Martinez-Lostao L: Onto better TRAILs for cancer treatment.
Cell Death Differ. 23:733–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bellail AC, Qi L, Mulligan P, Chhabra V
and Hao C: TRAIL agonists on clinical trials for cancer therapy:
The promises and the challenges. Rev Recent Clin Trials. 4:34–41.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lemke J, von Karstedt S, Zinngrebe J and
Walczak H: Getting TRAIL back on track for cancer therapy. Cell
Death Differ. 21:1350–1364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Legler K, Hauser C, Egberts JH, Willms A,
Heneweer C, Boretius S, Röcken C, Glüer CC, Becker T, Kluge M, et
al: The novel TRAIL-receptor agonist APG350 exerts superior
therapeutic activity in pancreatic cancer cells. Cell Death Dis.
9:4452018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gallego-Lleyda A, De Miguel D, Anel A and
Martinez-Lostao L: lipid nanoparticles decorated with TNF-related
aptosis-inducing ligand (TRAIL) are more cytotoxic than soluble
recombinant TRAIL in sarcoma. Int J Mol Sci. 19:192018. View Article : Google Scholar
|
|
26
|
Gupta B, Levchenko TS and Torchilin VP:
Intracellular delivery of large molecules and small particles by
cell-penetrating proteins and peptides. Adv Drug Deliv Rev.
57:637–651. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li R, Yang H, Jia D, Nie Q, Cai H, Fan Q,
Wan L, Li L and Lu X: Fusion to an albumin-binding domain with a
high affinity for albumin extends the circulatory half-life and
enhances the in vivo antitumor effects of human TRAIL. J Control
Release. 228:96–106. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hu W, Jia X, Gao Y and Zhang Q:
Chaetospirolactone reverses the apoptotic resistance towards TRAIL
in pancreatic cancer. Biochem Biophys Res Commun. 495:621–628.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yang SZ, Xu F, Zhou T, Zhao X, McDonald JM
and Chen Y: The long non-coding RNA HOTAIR enhances pancreatic
cancer resistance to TNF-related apoptosis-inducing ligand. J Biol
Chem. 292:10390–10397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu L, Guo T, Qu X, Hu X, Zhang Y, Che X,
Song H, Gong J, Ma R, Li C, et al: β-elemene increases the
sensitivity of gastric cancer cells to TRAIL by promoting the
formation of DISC in lipid rafts. Cell Biol Int. 42:1377–1385.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Beyer K, Baukloh AK, Stoyanova A, Kamphues
C, Sattler A and Kotsch K: Interactions of tumor necrosis
factor-related apoptosis-inducing ligand (TRAIL) with the immune
system: Implications for inflammation and cancer. Cancers (Basel).
11:112019. View Article : Google Scholar
|
|
32
|
Pilié PG, Tang C, Mills GB and Yap TA:
State-of-the-art strategies for targeting the DNA damage response
in cancer. Nat Rev Clin Oncol. 16:81–104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hsu HY, Lin TY, Hu CH, Shu DTF and Lu MK:
Fucoidan upregulates TLR4/CHOP-mediated caspase-3 and PARP
activation to enhance cisplatin-induced cytotoxicity in human lung
cancer cells. Cancer Lett. 432:112–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu F, Sun Y, Yang SZ, Zhou T, Jhala N,
McDonald J and Chen Y: Cytoplasmic PARP-1 promotes pancreatic
cancer tumorigenesis and resistance. Int J Cancer. 145:474–483.
2019. View Article : Google Scholar : PubMed/NCBI
|