Introduction
Carbamoyl phosphate synthetase 1 (CPS1) is a
rate-limiting enzyme of the urea cycle (1). In the mitochondria, CPS1 produces
carbamoyl phosphate from ammonia, which initiates nitrogen disposal
(2). CPS1 is the antigen of the
hepatocyte paraffin 1 (Hep-Par 1) antibody, a specific marker of
hepatogenic differentiation used in clinical pathology (3). CPS1 has also been identified as a
diagnostic marker for intestinal metaplasia (IM) in Barrett's
esophagus (BE), as well as IM associated with chronic gastritis
(4). Ocak et al (5) have reported that the level of CPS1
gradually decreases in incomplete types of IM, suggesting that CPS1
may be used as a diagnostic aid in IM subtype classification. CPS1
is highly expressed in IM of the stomach (4), and is thus a sensitive indicator of
small intestine differentiation. CPS1 in expressed in normal small
intestinal, but not colonic mucosa (6). Notably, CPS1 expression is completely
lost in invasive small intestinal adenocarcinoma, but gained in
colorectal polyps with dysplasia (7). Nemolato et al (8) have demonstrated that CPS1
immunoreactivity in human colon carcinogenesis is associated with
the progression from low- to high-grade dysplasia and
adenocarcinoma. A study by Abu-Zeid and Farid (6) also supports the concept that CPS1
serves an active role in the initiation of dysplasia and the
progression of multistep colorectal carcinogenesis. However, CPS1
may also serve a suppressive role in tumor invasion and
dissemination, as its expression is inversely associated with a
number of conventional prognostic parameters, including tumor type,
grade and lymph node metastasis (6).
Similar to its role in colorectal cancer, CPS1 is highly expressed
in dysplasia and BE, with low expression levels in esophageal
adenocarcinoma (9). These
observations suggest that CPS1 expression may be down- or
upregulated at different stages of dysplasia, independent of cancer
type, although it tends to be downregulated in advanced tumors, at
least in small intestinal, colorectal and liver cancer as well as
esophageal adenocarcinoma (10).
This may be due to the poor differentiation of invasive cancer.
By contrast, a number of studies have reported that
CPS1 is upregulated during carcinogenesis. For example, in rectal
cancer, Lee et al (11) have
demonstrated that high levels of CPS1 are associated with poor
therapeutic responses and adverse outcomes in patients receiving
neoadjuvant concurrent chemo-radiotherapy. In addition, two
independent studies have revealed that the levels of CPS1 are
upregulated in lung adenocarcinoma compared with those in adjacent
non-cancerous tissues (12,13), and that CPS1 knockdown results in a
reduction in cell proliferation and the levels of metabolites
associated with the cross-compartmental pathway of pyrimidine
metabolism (14). Li et al
(15) have demonstrated that P53
inhibits the occurrence of uremia and the clearance of ammonia by
downregulating CPS1 transcription. Thus, during carcinogenesis, the
frequently lost function of p53 results in the upregulation of CPS1
and is associated with poor prognosis (15). These observations suggest a potential
role for CPS1 in the occurrence of malignancy and cell
transformation. In gastric cancer (GC), CPS1 has been reported to
be associated with a subtype of hepatoid adenocarcinomas with
diffuse positive expression (1).
Although CPS1 is highly expressed in IM-associated chronic
gastritis, its role in GC development and progression is largely
unknown. Therefore, the present study aimed to assess the levels of
CPS1 expression in Correa's cascade and to evaluate its prognostic
value in patients with GC.
Materials and methods
Patient recruitment
Pre-carcinoma gastric mucosa tissues were obtained
from patients who underwent routine gastroscopic biopsy examination
at Ruijin Hospital (Shanghai, China) between January 2018 and
January 2020. Of these patients, 10 were diagnosed with chronic
non-atrophic gastritis, 32 with chronic atrophic gastritis with IM,
30 with low-grade intraepithelial neoplasia (IN) and 32 with
high-grade IN. According to mucin expression patterns, the cases
were classified as IM type I or II. All recruited patients were
receiving gastroscopic examinations for the first time. Patients
were excluded if they had received proton pump inhibitor therapy at
the time of biopsy or had a history of other malignancies. The
tissues were routinely fixed at room temperature overnight in 10%
neutral formalin, embedded in paraffin and stored at room
temperature until use. The study was approved by the Ruijin
Hospital Ethics Committee, and patients with precancerous lesions
provided written consent to use their medical information and
biological samples.
Data from patients with GC recruited at the Nantong
Tumor Hospital (Nantong, China) between December 2000 and April
2005 were also included. The majority of data from these patients
have been reported in a previous study (16). The patients were diagnosed with GC
for the first time and had not previously received treatment.
Patients were excluded if they had a history of other malignancies
or had previously received chemotherapy or surgery. All cases were
confirmed by pathological examination after surgery, and baseline
personal information was obtained from admission records.
Clinicopathological data were extracted from medical records and
further assessed by clinicians based on laboratory tests and image
evaluation. A total of 401 patients met the full inclusion
criteria, and the latest follow-up data were available until April
2014. All patients with GC had provided written consent to use
their medical information and biological samples, and the study was
approved by the Ethics Committees of Ruijin Hospital and Nantong
Tumor Hospital.
Tissue microarray (TMA) construction
and immunohistochemical staining of CPS1
TMAs were constructed using histologically confirmed
formalin-fixed, paraffin-embedded gastric mucosa, GC and adjacent
normal gastric tissue samples by the National Engineering Center
for Biochip at Shanghai. All samples used for TMA construction were
obtained from Nantong Tumor Hospital. Immunohistochemistry was
performed using a commercially available anti-CPS1 antibody (cat.
no. ab128942; 1:200; Abcam), and CPS1 expression was evaluated
using the Leica Bond III full-automatic immunostainer (Leica
Microsystems GmbH). The sections were incubated with a
peroxidase-polymer labeled rabbit secondary antibody (cat. no.
PV-6001; 1:500; OriGene Technologies, Inc.) at room temperature for
1 h. Heat-induced epitope retrieval was performed using citrate
buffer solution, followed by visualization of the antigen using a
DAB detection kit (cat. no. ab64238; Abcam). The slides were
counterstained with hematoxylin for 20 min at room temperature.
Concurrently immunostained slides containing liver tissue known to
exhibit a positive reaction with CPS1 antibody were selected as
positive controls.
CPS1 is a mitochondrial protein that exhibits
discrete granular cytoplasmic staining (9), which was assessed based on the staining
intensity and distribution in positive cells. The staining
intensity was scored between 0 and 3 as follows: No staining, 1;
faint staining, 1; moderate staining, 2; and dark staining, 3; and
the percentage of positive cells was scored as follows: No positive
cells, 0; ≤25%, 1; 25–49%, 2; 50–75%, 3; and >75%, 4. The
immunoreactive score (IRS) was determined as the staining density
multiplied by the percentage of positively stained cells. The
samples were then divided into four categories according to these
scores: i) Strong positive, IRS, 8–12; ii) focal positive, IRS,
4–6; iii) weak positive, IRS, 2–3; and iv) negative, IRS, 0–1.
Samples with an IRS score of 2–12 were assigned to the CPS1-high
group, whereas those with an IRS score of 0 or 1 were assigned to
the CPS1-low group.
Bioinformatics analysis
The Kaplan-Meier plotter tool (https://kmplot.com/analysis/) was used to evaluate the
association between CPS1 mRNA expression levels (low vs. high) at
best cut-off point and the overall survival (OS) or
progression-free survival (PFS) of patients with GC (17). The Kaplan-Meier plotter database of
patients with gastric cancer included 1,065 histologically
confirmed samples from seven independent datasets, in which the
CPS1 mRNA levels were evaluated using a microarray platform
(17). CPS1 mRNA levels were
detected using two probes (204920_s and 217564_s_at); the OS data
were available for 881 patients, and the PFS data were available
for 503 patients. Kaplan-Meier survival plots were generated, and
the hazard ratio with 95% confidence intervals (CI) and log-rank
P-values were calculated and plotted in R using Bioconductor
packages (18). The false discovery
rate was determined to correct for multiple testing using the
‘brainwaver’ library in R (https://CRAN.R-project.org/package=brainwaver), with
the cutoff set at 5%. CPS1 status was determined using the probe
set 204920_s and 217564_s_at. MethSurv is a web tool used to
perform multivariable survival analysis using DNA methylation data
(https://biit.cs.ut.ee/methsurv/)
(19) that utilizes methylome data
from 395 histologically confirmed gastric adenocarcinoma patients
recruited by The Cancer Genome Atlas (TCGA) consortium, and uses
the Cox proportional hazards model to develop an interactive web
interface for survival analysis. In the present study, CPS1
methylation status was evaluated using the CpG site cg21967368.
Statistical analysis
Categorical data are presented as the count number
and percentage. Associations between the CPS1 expression levels in
GC tissues and patient clinicopathological characteristics were
assessed using the χ2 or Fisher's exact test as
indicated. The OS of patients with GC was defined as the time from
surgical treatment to the time of death from any cause, and the
data were censored as the last follow-up point. The Kaplan-Meier
plot along with the log-rank and Gehan-Breslow-Wilcoxon (weighted
for early time points) test was used to compare the OS of patients
with high or low CPS1 expression. Univariate and multivariate Cox
proportional hazards regression models were used to assess the
association between CPS1 expression and the OS of patients with GC.
The distribution of the methylation level of CpG site cg21967368 at
different clinical stages is presented as combined violin and box
plots. All statistical analyses were performed using GraphPad prism
version 8.4.0 (GraphPad Software, Inc.) and/or R software (version
4.0.2) (18). A two-sided P<0.05
was considered to indicate a statistically significant
difference.
Results
CPS1 is a specific marker of IM in the
gastric mucosa
In the present study, CPS1 was not expressed in the
epithelial cells of patients with non-atrophic gastritis, but
diffuse strong expression was observed in epithelial cells of
patients with IM in 32 cases (100%) of chronic atrophic gastritis.
Both IM type I and II exhibited strong positive CPS1 staining
(Fig. 1A and B). CPS1 was also
strongly expressed in all 102 (100%) cases of IM from IN or GC,
including 30 cases of low-grade IN with IM, 28 cases of high-grade
IN with IM, and 44 cases of GC with IM.
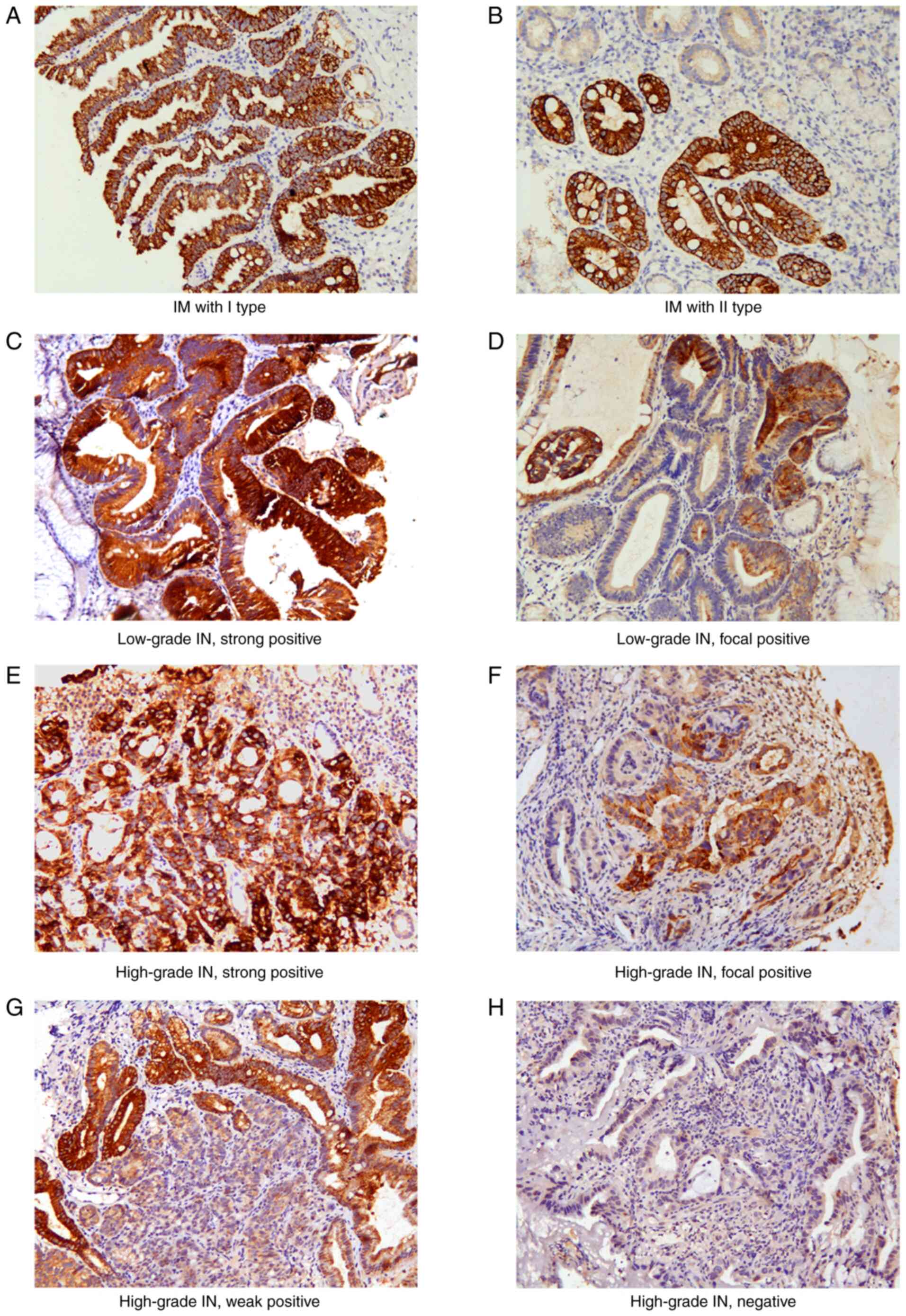 | Figure 1.Representative images of CPS1
staining in different types of IM and gastric tumor tissues. (A) IM
type I, strong positive; (B) IM type II, strong positive; (C)
low-grade IN, strong positive; (D) low-grade IN, focal positive;
(E) high-grade IN, strong positive; (F) high-grade IN, focal
positive; (G) high-grade IN, weak positive; (H) high-grade IN,
negative CPS1 expression. Original magnification, ×200. CPS1,
carbamoyl phosphate synthetase 1; IM, intestinal metaplasia; IN,
intraepithelial neoplasia. |
CPS1 expression is gradually
downregulated in Correa's cascade
In low-grade IN, 21 (70%) and 9 (30%) cases
exhibited strong and focal positive expression, respectively. The
expression of CPS1 was significantly lower in high-grade IN and
intestinal-type GC compared with that in low-grade IN. In
high-grade IN, only 4 cases (12.5%) exhibited strong expression, 7
(21.8%) presented with focal positive staining, 7 (21.8%) had weak
expression, and no expression was observed in 14 (43.8%) cases
(Fig. 1C-H). In 172 cases of
intestinal-type GC, only 14 (8%) retained strong-positive staining,
14 (8%) presented with focal positive staining, 37 (22%) exhibited
weak expression, and 107 (62%) had no expression. These results
suggested that CPS1 expression was downregulated as Correa's
cascade progressed, and the differences in expression were
statistically significant (P<0.05; Table I; Fig.
S1).
 | Table I.Expression of CPS1 in different types
of intestinal metaplasia, IN and GC. |
Table I.
Expression of CPS1 in different types
of intestinal metaplasia, IN and GC.
| Immunoreactive
score | Gastric mucosa
(n=10) | Intestinal
metaplasia (n=32) | Low-grade IN
(n=40) | High-grade IN
(n=60) | Intestinal-type GC
(n=172) | Diffuse-type GC
(n=144) | Mixed-type GC
(n=172) |
|---|
| Strong (8–12) | 0 | 32 (100) | 21 (70) | 4
(12.5) | 14 (8) | 37
(26) | 11 (13) |
| Focal (4–6) | 0 | 0 | 9
(30) | 7
(21.8) | 14 (8) | 10 (7) | 4 (5) |
| Weak (2–3) | 0 | 0 | 0 | 7
(21.8) | 37
(22) | 22
(15) | 19 (22) |
| Negative (0–1) | 10 (100) | 0 | 0 | 14 (43.8) | 107 (62) | 75
(52) | 51 (60) |
| P-value |
|
<0.001a |
<0.001b |
<0.001c | 0.047d | 0.005e | 0.652f |
Expression patterns of CPS1 differ
according to gastric tumor type
In cases of diffuse-type GC, 37 (26%) exhibited
strong positive CPS1 expression, 10 (7%) were focal positive, 22
(15%) presented with weak positive expression, and 75 (52%) had no
CPS1 expression. For mixed-type GC, 11 cases (13%) were strong
positive, 4 (5%) were focal positive, 19 (22%) exhibited weak
positive expression, and 51 (60%) were CPS1-negative. Among the
three types of tumor, a relatively high proportion of patients with
diffuse-type GC exhibited strong positive CPS1 expression (26%;
P<0.001; Table I; Fig. 2).
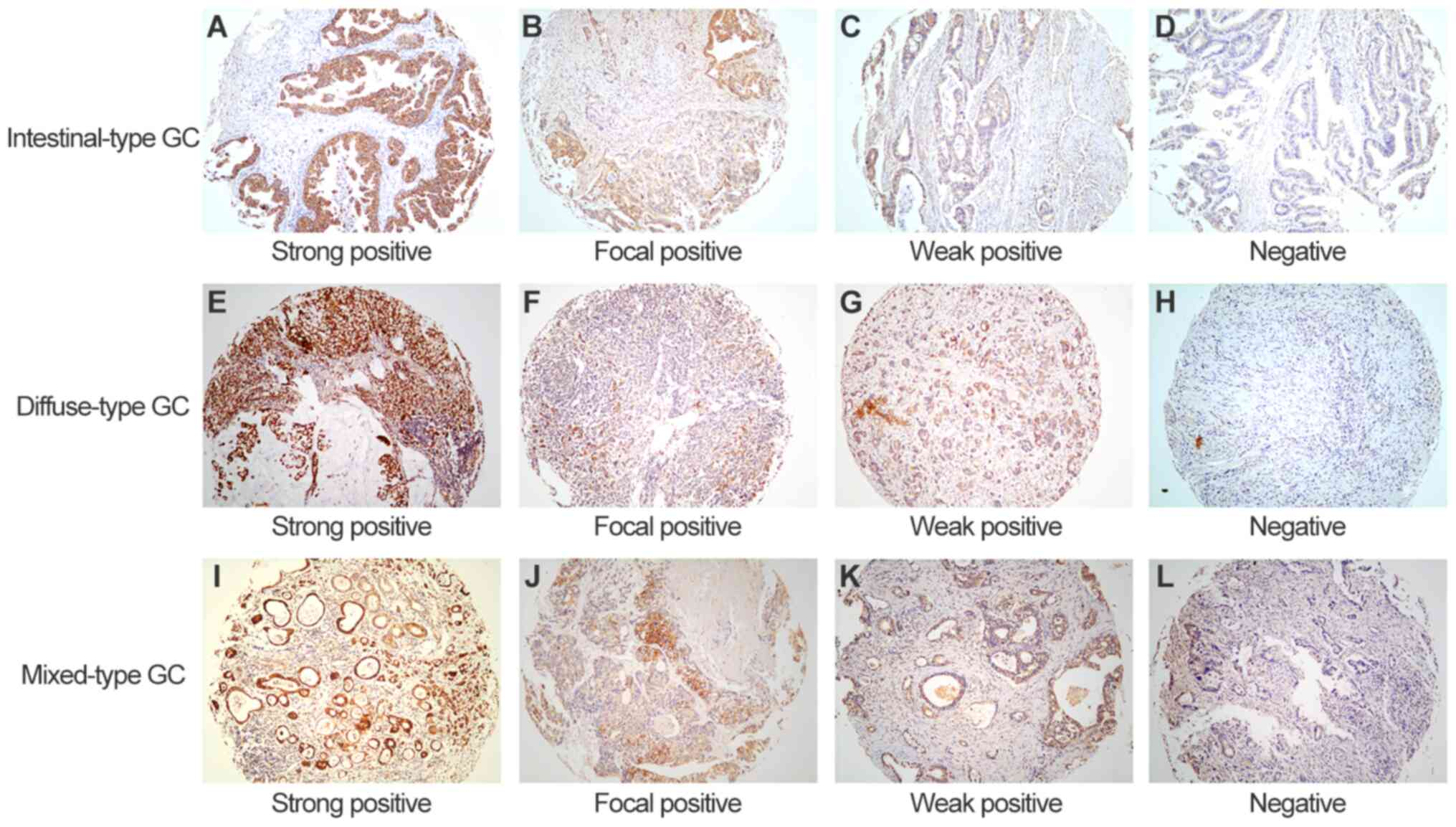 | Figure 2.Representative images of CPS1
staining in intestinal-, diffuse- and mixed-type GC tissues. (A-D)
Intestinal-type GC samples with (A) strong positive, (B) focal
positive, (C) weak positive and (D) negative CPS1 expression. (E-H)
Diffuse-type GC samples with (E) strong positive, (F) focal
positive, (G) weak positive and (H) negative CPS1 expression. (I-L)
Mixed-type GC samples with (I) strong positive, (J) focal positive,
(K) weak positive and (L) negative CPS1 expression. Original
magnification, ×100. CPS1, carbamoyl phosphate synthetase 1; GC,
gastric cancer; IHC, immunohistochemistry. |
Association between CPS1 expression
and the clinicopathological characteristics of patients with
GC
Low CPS1 expression levels were significantly
associated with an advanced TNM stage (P=0.031) and the depth of
invasion (P=0.037) in intestinal-type GC, but was not associated
with age, sex, lymph node metastasis or distant metastasis.
Furthermore, CPS1 expression was not significantly associated with
any clinical parameters in patients with diffuse- or mixed-type GC
(Table II).
 | Table II.Association of CPS1 expression and
patient clinical characteristics in various types of GC according
to Lauren's classification. |
Table II.
Association of CPS1 expression and
patient clinical characteristics in various types of GC according
to Lauren's classification.
|
| Intestinal-type GC,
n=172 | Diffuse-type GC,
n=144 | Mixed-type GC,
n=85 |
|---|
|
|
|
|
|
|---|
| Variable | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-value |
|---|
| All patients | 107 (62.2) | 65 (37.8) |
| 75 (52.1) | 69 (47.9) |
| 51 (60.0) | 34 (40.0) |
|
| Age, years |
|
| 0.9134 |
|
| 0.287 |
|
| 0.064 |
|
<65 | 60 (56.1) | 37 (56.9) |
| 52 (69.3) | 42 (60.9) |
| 37 (72.5) | 18 (52.9) |
|
|
≥65 | 47 (43.9) | 28 (43.1) |
| 23 (30.7) | 27 (39.1) |
| 14 (27.5) | 16 (47.1) |
|
| Sex |
|
| 0.8267 |
|
| 0.211 |
|
| 0.852 |
|
Male | 79 (73.8) | 47 (72.3) |
| 14 (20.3) | 14 (20.3) |
| 34 (66.7) | 22 (64.7) |
|
|
Female | 28 (26.2) | 18 (27.7) |
| 55 (79.7) | 55 (79.7) |
| 17 (33.3) | 12 (35.3) |
|
| Depth of
invasion |
|
| 0.037a |
|
| 0.586 |
|
| 0.544 |
|
T1-2 | 17 (15.9) | 19 (29.2) |
| 11 (14.7) | 8
(11.6) |
| 4 (7.8) | 4
(11.8) |
|
|
T3-4 | 90 (84.1) | 46 (70.8) |
| 64 (85.3) | 61 (88.4) |
| 47 (92.2) | 30 (88.2) |
|
| Lymph node
metastasis |
|
| 0.091 |
|
| 0.656 |
|
| 0.708b |
| N0 | 31 (29.0) | 27 (41.5) |
| 11 (14.7) | 12 (17.4) |
| 9
(17.6) | 10 (29.4) |
|
|
N1/N2/N3 | 76 (71.0) | 38 (58.5) |
| 64 (85.3) | 57 (82.6) |
| 42 (82.4) | 24 (70.6) |
|
| Distant
metastasis |
|
| 0.506b |
|
| 0.104 |
|
| 0.271b |
| M0 | 102 (95.3) | 60 (92.3) |
| 63 (84.0) | 64 (92.8) |
| 48 (94.1) | 34 (100) |
|
| M1 | 5
(4.7) | 5 (7.7) |
| 12 (16.0) | 5 (7.2) |
| 3 (5.9) | 0 (0) |
|
| TNM stage |
|
| 0.031a |
|
| 0.965 |
|
| 0.182 |
|
I–II | 32 (29.9) | 30 (46.2) |
| 15 (20.0) | 14 (20.3) |
| 10 (19.6) | 11 (32.4) |
|
|
III–IV | 75 (70.1) | 35 (53.8) |
| 60 (80.0) | 55 (79.7) |
| 41 (80.4) | 23 (67.6) |
|
Low CPS1 expression levels are
associated with a poor prognosis in the three GC types
The follow-up times of the 401 patients in the
Nantong cohort ranged between 0.3 and 113 months, with a median
time of 45.3 months; 125 of these patients succumbed to the
disease, and 276 patients were alive at the last follow-up. The
results of univariate analysis demonstrated that the depth of
invasion, lymph node metastasis and TNM stage were associated with
the OS of patients with intestinal-type GC. Lymph node metastasis
and TNM stage were also associated with the OS of patients with
diffuse-type GC, whereas lymph node metastasis and distant
metastasis were associated with the OS of patients with mixed-type
GC (Table III). Low CPS1
expression levels were associated with a short OS time in patients
with intestinal-type (log-rank and Gehan-Breslow-Wilcoxon test,
P<0.001), diffuse-type (log-rank and Gehan-Breslow-Wilcoxon
test, P<0.001) and mixed-type GC (log-rank test, P=0.010;
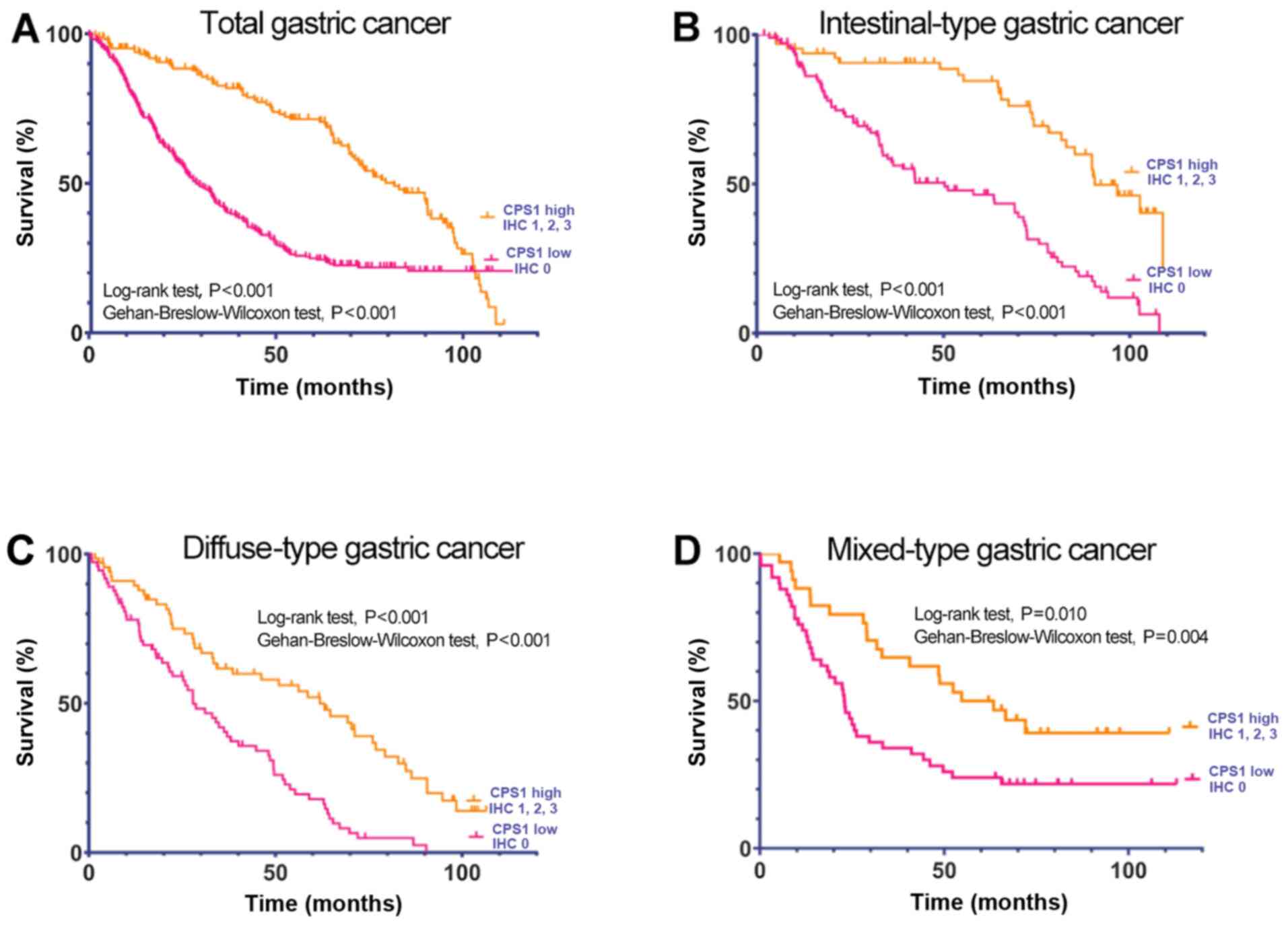
Gehan-Breslow-Wilcoxon test, P=0.004) (Fig. 3). Univariate Cox regression analysis
revealed hazard ratio (HR) values of 2.49 (95% CI, 1.59–3.88;
P<0.001) for intestinal-type, 1.89 (95% CI, 1.28–2.78; P=0.001)
for diffuse-type and 2.04 (95% CI, 1.19–3.49; P=0.011) for
mixed-type GC. Furthermore, the results of the multivariate Cox
regression analysis demonstrated that CPS1 expression was
independently associated with the OS of patients in the Nantong
cohort (intestinal-type GC, HR=2.38; 95% CI, 1.53–3.69; P<0.001;
diffuse-type GC, HR=1.81; 95% CI, 1.22–2.71; P=0.003; and
mixed-type GC, HR=1.92; 95% CI, 1.09–3.37; P=0.018; Table IV).
 | Table III.Univariate analysis of the overall
survival and clinical characteristics of patients with GC in the
Nantong Cohort. |
Table III.
Univariate analysis of the overall
survival and clinical characteristics of patients with GC in the
Nantong Cohort.
|
| Intestinal-type GC
(n=172) | Diffuse-type GC
(n=144) | Mixed-type GC
(n=85) |
|---|
|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≥65 vs. <65
years) | 1.01
(0.68–1.49) | 0.968 | 0.87
(0.59–1.3) | 0.508 | 1.22
(0.54–2.74) | 0.633 |
| Sex (Female vs.
male) | 1.05
(0.68–1.62) | 0.816 | 0.72
(0.49–1.07) | 0.102 | 0.81
(0.47–1.42) | 0.464 |
| Depth of
invasion | 1.73
(1.01–2.96) | 0.045a | 1.58
(0.86–2.88) | 0.14 | 1.80
(0.65–4.98) | 0.257 |
| Lymph node
metastasis | 2.27
(1.43–3.59) |
<0.001a | 2.55
(1.36–4.78) | 0.003a | 2.13
(1.07–4.22) | 0.031a |
| Distant
metastasis | 1.54
(0.75–3.18) | 0.240 | 1.22
(0.69–2.13) | 0.49 | 3.39
(1.05–10.94) | 0.041a |
| TNM stage | 2.18
(1.39–3.40) |
<0.001a | 1.76
(1.05–2.95) | 0.033a | 1.63
(0.88–3.02) | 0.122 |
| CPS1 (High vs.
low) | 2.49
(1.59–3.88) |
<0.001a | 1.89
(1.28–2.78) | 0.001a | 2.04
(1.19–3.49) | 0.011a |
 | Table IV.Multivariate analysis of the overall
survival and clinical characteristics of patients with GC in the
Nantong Cohort. |
Table IV.
Multivariate analysis of the overall
survival and clinical characteristics of patients with GC in the
Nantong Cohort.
|
| Intestinal-type GC
(n=172) | Diffuse-type GC
(n=144) | Mixed-type GC
(n=85) |
|---|
|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age (≥65 vs. <65
years) | 0.95
(0.64–1.42) | 0.812 | 0.91
(0.61–1.37) | 0.659 | 1.19
(0.69–2.07) | 0.516 |
| Sex (Female vs.
male) | 1.23
(0.79–1.91) | 0.357 | 0.81
(0.54–1.21) | 0.301 | 0.86
(0.49–1.51) | 0.603 |
| TNM stage | 2.06
(1.30–3.23) | 0.001a | 1.68
(0.99–2.84) | 0.053 | 1.49
(0.78–2.84) | 0.222 |
| CPS1 (High vs.
low) | 2.38
(1.53–3.69) | 0.000a | 1.81
(1.22–2.71) | 0.003a | 1.92
(1.09–3.37) | 0.018a |
Low CPS1 mRNA expression levels are
associated with a short OS time in patients with GC
Survival analysis of the association between the
survival outcome and CPS1 expression levels in GC was performed
using the Kaplan-Meier plotter database data from seven independent
patient cohorts. In this pooled patient cohort, low CPS1 mRNA
expression levels were associated with a poor prognosis in patients
with GC. The results demonstrated that low CPS1 mRNA levels were
associated with a short OS time, as determined using both probes
(Figs. 4A-C and S2). In addition, low mRNA expression
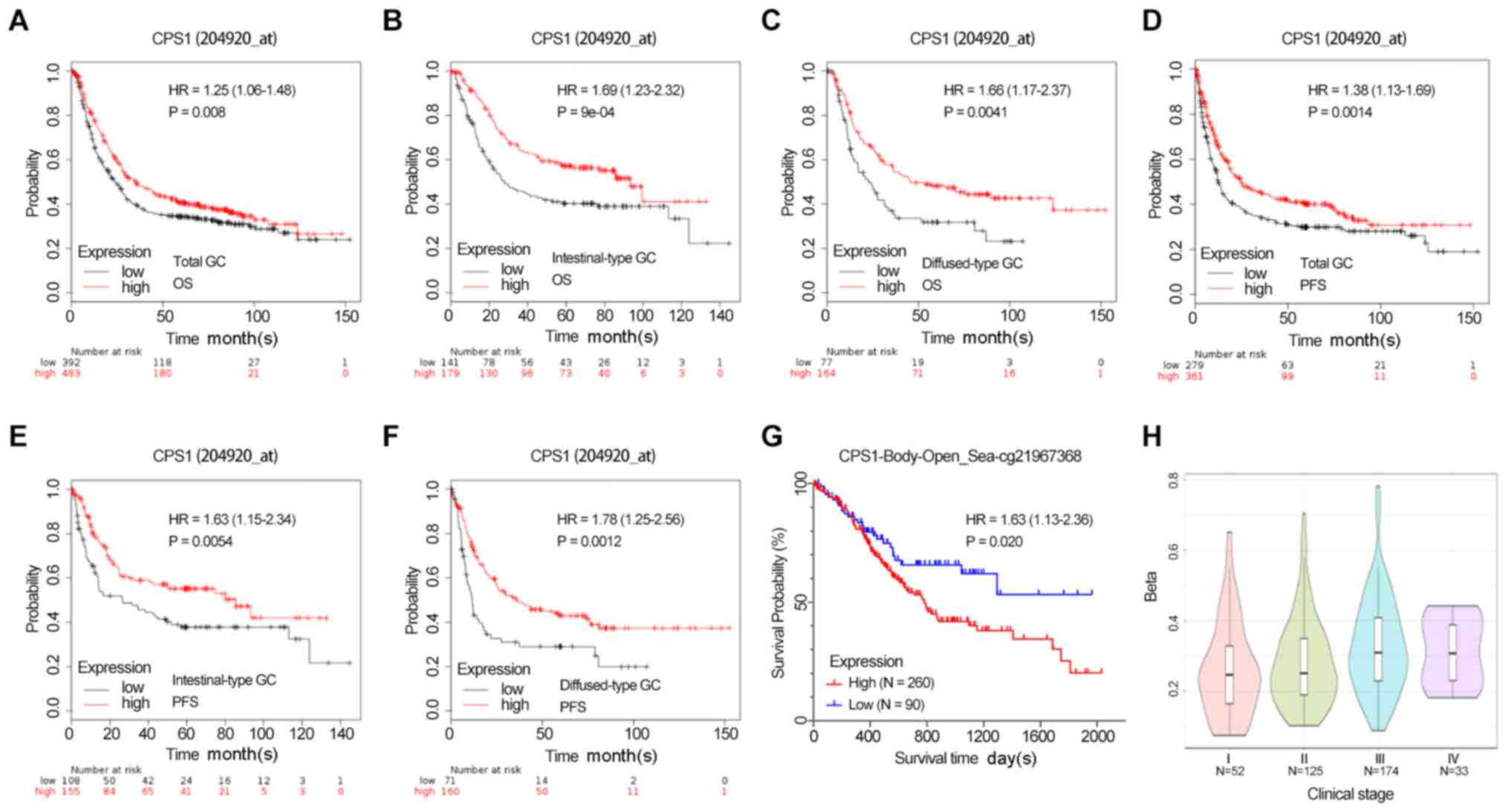
levels of CPS1 were also associated with a short PFS time in data
from both gene detection probes (probe 204920_s: Total GC, HR=1.38;
95% CI, 1.13–1.69; P=0.001; intestinal-type GC, HR=1.63; 95% CI,
1.15–2.34; P=0.005; and diffuse-type GC, HR=1.78; 95% CI,
1.25–2.56; P=0.001; Fig. 4D-F). A
similar trend was observed using probe 217564_s_at (total GC,
HR=1.41; 95% CI, 1.15–1.73; P=0.007; intestinal-type GC, HR=1.61;
95% CI, 1.14–2.28; P=0.007; and diffuse-type GC, HR=1.96; 95% CI,
1.34–2.87; P=0.004; Fig. S2).
The MethSurv database was used to assess the
association between the survival outcome and CpG methylation status
of the CPS1 promoter region in patients with GC. Among 395 patients
from the TCGA stomach adenocarcinoma datasets released in March
2017, a high methylation level of CPS1 at cg21967368 was associated
with a short OS time (HR=1.63; 95% CI, 1.13–2.36; P=0.027), and the
methylation level of cg21967368 was increased in clinical stages
III and IV compared with that in stages I and II (Fig. 4G-H).
Discussion
In the present study, the expression of CPS1 was not
observed in the epithelial cells of chronic non-atrophic gastritis
lesions; however, strongly positive expression was observed in the
epithelia of the gastric mucosa of patients with IM. These results
suggested that diffusely and strongly positive expression of CPS1
may be one of the primary characteristics of IM, which may be a
useful diagnostic marker in the clinic. Notably, the expression
levels of CPS1 were progressively downregulated during gastric
carcinogenesis from IM to low-grade IN, high-grade IN and
intestinal GC. These results suggested that the loss of CPS1
expression in dysplasia may be a malignant transformation marker of
IM, which was consistent with the results of previous studies that
have reported downregulation of CPS1 during esophageal
adenocarcinoma (9) and colorectal
cancer (6) progression.
Whether CPS1 is the initiating factor of IM or is
simply an associated phenomenon remains to be elucidated. Molecular
markers of IM can be divided into two categories according to the
differentiation direction; the first category comprises gastric
differentiation-related proteins, such as transcription factor SOX2
(20), mucin-5AC and mucin-6
(21), whereas the second category
includes intestinal differentiation-related proteins, such as
intestinal specific transcription factor homeobox protein CDX2
(22), mucin-2 and VILLIN (23). By contrast, CPS1 is typically a
hepatogenic differentiation marker (3). Although CPS1 is primarily expressed in
the mucosa of the small intestine, its expression and enzyme
activity is 10–20-fold higher in the liver (24). The stomach, intestine, liver and
pancreas all originate from endodermal progenitors, and previous
studies have reported that human gastric epithelial cells or
hepatocytes differentiate into multipotent endodermal progenitors
using defined small molecules, such as A83-01 and hepatocyte growth
factor (25,26). Thus, we hypothesize that gastric
epithelial cells may also differentiate into multipotent endodermal
progenitor cells under the stimulation of Helicobacter
pylori infection or bile acid, two of the most common
environmental factors for IM induction. Although only the
intestinal GC phenotype can be morphologically observed, the
potential to identify other phenotypes at the molecular level
cannot be excluded. Fatty acid binding protein-1 (FABP-1), a type
of FABP present in the liver, is highly expressed in BE (9). A previous study has revealed that
FABP-1 is a more reliable diagnostic marker of BE compared with
CDX2, and that its expression levels are downregulated at the
dysplasia stage of adenocarcinoma (9). Hepatocyte nuclear factor 4α (HNF4α), a
liver-enriched transcription factor, is one of the major regulators
of hepatocyte differentiation and proliferation (27). At present, HNF4α is considered to be
a key early component in the development of BE (28). The ectopic expression of HNF4α in the
esophagus generates columnar metaplasia (28). HNF4α has recently been identified as
a crucial regulator of intestinal regeneration in an organoid model
of intestinal damage and repair (29). Therefore, partial hepatocyte
differentiation may occur during the process of IM at the early
stage of carcinogenesis, but the expression of hepatocyte
differentiation genes may be lost at the stage of malignant
transformation or development into invasive cancer. However,
further investigation is required to confirm this hypothesis.
In the present study, CPS1 expression levels were
associated with the tumor invasion depth and clinical stage in
intestinal-type GC, and the loss of CPS1 expression was associated
with a short OS time following surgical treatment, regardless of
tumor type. These results suggested that CPS1 downregulation may be
involved in the pathogenesis and progression of GC, and that CPS1
may serve as a potential prognostic biomarker or therapeutic target
for patients with GC. The results of the prognostic analysis in the
present study were consistent with those from a study of
hepatocellular carcinoma, which suggested that low levels of CPS1
were associated with a poor OS rate (30). Similar findings in GC have been
reported by Fan et al (31),
indicating that CPS1 expression levels are negatively associated
with the depth of invasion and may be a potential independent
prognostic indicator. By contrast, we hypothesize that CPS1
expression is likely to be lost rather than upregulated in
intestinal-type GC, as this subtype originates from the IM mucosa,
and diffuse strong CPS1 expression was observed at the IM stage in
the present study. In addition, the results of the present study
suggested that diffuse-type GC with signet ring cells presented
with a higher rate of strong positive CPS1 expression compared with
that in intestinal-type GC, which was in contrast to the results of
a previous study by Fan et al (31). However, similar to the present study,
Maitra et al (32)
demonstrated that strong positive CPS1 expression was observed in
3/10 gastric tumors, all of which were poorly differentiated
signet-ring or mixed intestinal-signet-ring cell carcinomas. Since
intestinal-type and diffuse-type tumors have distinct origins, the
role of CPS1 may differ between them.
Previous studies have reported that CPS1 is
regulated by liver-enriched transcription factors. For example,
Chen et al (33) have
demonstrated that HNF3β serves an essential role in CPS1 expression
and promotes the metabolism of ammonia. The transcription of CPS1
has also been reported to be associated with the glucocorticoid and
glucagon signaling pathways (34,35). DNA
methylation is one of the critical mechanisms regulating CPS1
expression. Liu et al (36)
have reported that DNA methylation suppresses CPS1 expression in
primary human hepatocellular carcinoma by regulating its promoter
activity. Comprehensive and integrative genomic characterization of
hepatocellular carcinoma data from TCGA database has also revealed
that CPS1 is downregulated by hypermethylation, which results in
metabolic reprogramming (37). In
the present study, data acquired from the MethSurv database also
demonstrated that in GC, low levels of CPS1 expression may be
associated with altered DNA methylation patterns.
In conclusion, the results of the present study
demonstrated that CPS1 was a highly specific marker of IM in the
gastric mucosa, and CPS1 expression was gradually lost during the
progression of intestinal-type GC. This loss of CPS1 expression was
significantly associated with tumor invasion and the clinical stage
of intestinal-type GC, and low CPS1 levels were associated with a
short OS time in the clinic. Downregulation of CPS1 may occur due
to hypermethylation in its promoter region. Further studies are
warranted to elucidate the underlying mechanisms of the effects of
CPS1 in IM, the dynamic changes in the progression of
intestinal-type cancer and the role of high CPS1 expression levels
in diffuse-type GC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was supported by the National Key R&D
Program of China (grant no. 2017YFC0907001), the Natural Science
Foundation of Shanghai (grant no. 20ZR1434100), Shanghai Municipal
Commission of Health and Family Planning (grant nos. 20164Y0250 and
201840160) and the Science and Technology Commission of Jiading
Distinct (grant nos. JDKW-2017-W09 and JDKW-2018-W08).
Availability of data and materials
All datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XF, XW, EX, FY and PC performed the data analyses
and wrote the manuscript. XF, XW, EX, FY and PC revised the
manuscript and provided constructive criticism for the final
version. EX and FL contributed materials and performed statistical
analysis, as well as interpreted the data. QL and QM analyzed the
data and performed the immunohistochemistry staining. PC and FY
contributed to the conception and design of the study. XF, FY and
PC confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
The use of human tissues in the present study was
approved by the Ethics Committees of Nantong Tumor Hospital and
Ruijin Hospital, and patients provided written consent to use their
medical information and biological samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Butler SL, Dong H, Cardona D, Jia M, Zheng
R, Zhu H, Crawford JM and Liu C: The antigen for Hep Par 1 antibody
is the urea cycle enzyme carbamoyl phosphate synthetase 1. Lab
Invest. 88:78–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ali EZ, Khalid MK, Yunus ZM, Yakob Y, Chin
CB, Abd Latif K and Hock NL: Carbamoylphosphate synthetase 1 (CPS1)
deficiency: Clinical, biochemical, and molecular characterization
in Malaysian patients. Eur J Pediatr. 175:339–346. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leong AS, Sormunen RT, Tsui WM and Liew
CT: Hep Par 1 and selected antibodies in the immunohistological
distinction of hepatocellular carcinoma from cholangiocarcinoma,
combined tumours and metastatic carcinoma. Histopathology.
33:318–324. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chu PG, Jiang Z and Weiss LM: Hepatocyte
antigen as a marker of intestinal metaplasia. Am J Surg Pathol.
27:952–959. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ocak GA, Yildirim G and Elpek GO:
Hepatocyte antigen expression in subtypes of intestinal metaplasia
of the stomach. West Indian Med J. 61:659–664. 2012.PubMed/NCBI
|
|
6
|
Abu-Zeid RM and Farid RM: Role of
Hepatocyte Paraffin 1 antigen in the course of colorectal
carcinogenesis. Int J Physiol Pathophysiol Pharmacol. 5:177–183.
2013.PubMed/NCBI
|
|
7
|
Cardona DM, Zhang X and Liu C: Loss of
carbamoyl phosphate synthetase I in small-intestinal
adenocarcinoma. Am J Clin Pathol. 132:877–882. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nemolato S, Ravarino A, Fanni D, Coni P,
Di Felice E, Senes G and Faa G: Hepatocyte paraffin 1
immunoreactivity in early colon carcinogenesis. Gastroenterology
Res. 2:277–281. 2009.PubMed/NCBI
|
|
9
|
Srivastava S, Kern F, Sharma N, McKeon F,
Xian W, Yeoh KG, Ho KY and The M: FABP1 and Hepar expression levels
in Barrett's esophagus and associated neoplasia in an Asian
population. Dig Liver Dis. 49:1104–1109. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kinoshita M and Miyata M: Underexpression
of mRNA in human hepatocellular carcinoma focusing on eight loci.
Hepatology. 36:433–438. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee YY, Li CF, Lin CY, Lee SW, Sheu MJ,
Lin LC, Chen TJ, Wu TF and Hsing CH: Overexpression of CPS1 is an
independent negative prognosticator in rectal cancers receiving
concurrent chemoradiotherapy. Tumour Biol. 35:11097–11105. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Celiktas M, Tanaka I, Tripathi SC,
Fahrmann JF, Aguilar-Bonavides C, Villalobos P, Delgado O, Dhillon
D, Dennison JB, Ostrin EJ, et al: Role of CPS1 in cell growth,
metabolism and prognosis in LKB1-inactivated lung adenocarcinoma. J
Natl Cancer Inst. 109:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu G, Zhao Z, Yan Y, Zhou Y, Wei J, Chen
X, Lin W, Ou C, Li J, Wang X, et al: CPS1 expression and its
prognostic significance in lung adenocarcinoma. Ann Transl Med.
8:3412020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim J, Hu Z, Cai L, Li K, Choi E, Faubert
B, Bezwada D, Rodriguez-Canales J, Villalobos P, Lin YF, et al:
CPS1 maintains pyrimidine pools and DNA synthesis in
KRAS/LKB1-mutant lung cancer cells. Nature. 546:168–172. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Mao Y, Zhao L, Li L, Wu J, Zhao M,
Du W, Yu L and Jiang P: p53 regulation of ammonia metabolism
through urea cycle controls polyamine biosynthesis. Nature.
567:253–256. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen P, Guo H, Wu X, Li J, Duan X, Ba Q
and Wang H: Epigenetic silencing of microRNA-204 by Helicobacter
pylori augments the NF-κB signaling pathway in gastric cancer
development and progression. Carcinogenesis. 41:430–441. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Szasz AM, Lanczky A, Nagy A, Förster S,
Hark K, Green JE, Boussioutas A, Busuttil R, Szabó A and Győrffy B:
Cross-validation of survival associated biomarkers in gastric
cancer using transcriptomic data of 1,065 patients. Oncotarget.
7:49322–49333. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
R Core Team, . R: A language and
environment for statistical computing. R Foundation for Statistical
Computing; Vienna, Austria: 2014
|
|
19
|
Modhukur V, Iljasenko T, Metsalu T, Lokk
K, Laisk-Podar T and Vilo J: MethSurv: A web tool to perform
multivariable survival analysis using DNA methylation data.
Epigenomics. 10:277–288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan T, Ni Z, Han C, Min Y, Sun N, Liu C,
Shi M, Lu W, Wang N, Du F, et al: SOX2 interferes with the function
of CDX2 in bile acid-induced gastric intestinal metaplasia. Cancer
Cell Int. 19:242019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Camilo V, Garrido M, Valente P, Ricardo S,
Amaral AL, Barros R, Chaves P, Carneiro F, David L and Almeida R:
Differentiation reprogramming in gastric intestinal metaplasia and
dysplasia: Role of SOX2 and CDX2. Histopathology. 66:343–350. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mutoh H, Hakamata Y, Sato K, Eda A, Yanaka
I, Honda S, Osawa H, Kaneko Y and Sugano K: Conversion of gastric
mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice.
Biochem Biophys Res Commun. 294:470–479. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi XY, Bhagwandeen B and Leong AS: CDX2
and villin are useful markers of intestinal metaplasia in the
diagnosis of Barrett esophagus. Am J Clin Pathol. 129:571–577.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ryall J, Nguyen M, Bendayan M and Shore
GC: Expression of nuclear genes encoding the urea cycle enzymes,
carbamoyl-phosphate synthetase I and ornithine carbamoyl
transferase, in rat liver and intestinal mucosa. Eur J Biochem.
152:287–292. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Qin J, Wang S, Zhang W, Duan J,
Zhang J, Wang X, Yan F, Chang M, Liu X, et al: Conversion of Human
gastric epithelial cells to multipotent endodermal progenitors
using defined small molecules. Cell Stem Cell. 19:449–461. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim Y, Kang K, Lee SB, Seo D, Yoon S, Kim
SJ, Jang K, Jung YK, Lee KG, Factor VM, et al: Small
molecule-mediated reprogramming of human hepatocytes into bipotent
progenitor cells. J Hepatol. 70:97–107. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Watt AJ, Garrison WD and Duncan SA: HNF4:
A central regulator of hepatocyte differentiation and function.
Hepatology. 37:1249–1253. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Colleypriest BJ, Burke ZD, Griffiths LP,
Chen Y, Yu WY, Jover R, Bock M, Biddlestone L, Quinlan JM, Ward SG,
et al: Hnf4α is a key gene that can generate columnar metaplasia in
oesophageal epithelium. Differentiation. 93:39–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Montenegro-Miranda PS, van der Meer JHM,
Jones C, Meisner S, Vermeulen JLM, Koster J, Wildenberg ME,
Heijmans J, Boudreau F, Ribeiro A, et al: A novel organoid model of
damage and repair identifies HNF4α as a critical regulator of
intestinal epithelial regeneration. Cell Mol Gastroenterol Hepatol.
10:209–223. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin Y, Liang ZY, Zhou WX and Zhou L:
Combination with CK19 Might increase the prognostic power of hep
par 1 in hepatocellular carcinoma after curative resection. J
Invest Surg. 31:412–419. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan Z, Li J, Dong B and Huang X:
Expression of Cdx2 and hepatocyte antigen in gastric carcinoma:
Correlation with histologic type and implications for prognosis.
Clin Cancer Res. 11:6162–6170. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Maitra A, Murakata LA and Albores-Saavedra
J: Immunoreactivity for hepatocyte paraffin 1 antibody in hepatoid
adenocarcinomas of the gastrointestinal tract. Am J Clin Pathol.
115:689–694. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Z, Tang N, Wang X and Chen Y: The
activity of the carbamoyl phosphate synthase 1 promoter in human
liver-derived cells is dependent on hepatocyte nuclear factor
3-beta. J Cell Mol Med. 21:2036–2045. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takiguchi M and Mori M: Transcriptional
regulation of genes for ornithine cycle enzymes. Biochem J.
312:649–659. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guei TR, Liu MC, Yang CP and Su TS:
Identification of a liver-specific cAMP response element in the
human argininosuccinate synthetase gene. Biochem Biophys Res
Commun. 377:257–261. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu H, Dong H, Robertson K and Liu C: DNA
methylation suppresses expression of the urea cycle enzyme
carbamoyl phosphate synthetase 1 (CPS1) in human hepatocellular
carcinoma. Am J Pathol. 178:652–661. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cancer Genome Atlas Research Network.
Electronic address, . simplewheeler@bcm.edu; Cancer
Genome Atlas Research Network: Comprehensive and integrative
genomic characterization of hepatocellular carcinoma. Cell.
169:1327–1341 e23. 2017. View Article : Google Scholar : PubMed/NCBI
|