Introduction
Melanoma, also known as malignant melanoma, is
characterized by the abnormal proliferation of melanocytes in the
skin (1). Melanoma accounts for ~2%
of all skin cancer cases, but due to its strong invasiveness,
causes the majority of skin cancer-associated deaths (2). The incidence rate and degree of
malignancy in melanoma are high, with seriously impacts on human
health (3). Melanoma is less common
than other types of skin cancer, but more likely to invade nearby
tissues and metastasize to other regions of the body (4). Furthermore, the rate of melanoma
occurrence has increased considerably over the past few decades
(5). Currently, surgery,
chemotherapy and radiotherapy are the primary treatment options
(6), and in the early stages of
melanoma, the lesion can be successfully resected (7). Although clinical treatments are
becoming increasingly more refined, a number of disadvantages
remain, including the various side effects of radiotherapy and
chemotherapy. Due to the frequent emergence of drug resistance,
currently-available chemotherapeutics do not effectively treat
patients with advanced melanoma.
As a result of good efficacy, mild side effects, low
toxicity and its numerous targets, Traditional Chinese Medicine has
been used to treat various cancer types (8). Consequently, the identification of
effective anti-melanoma drugs from Chinese plant medicine is of
great clinical significance. Traditional Chinese Medicine comprises
complex components with low drug resistance (9), and leading antitumor components with
novel structures and significant pharmacological activity have been
confirmed (10,11). Some of these components act on
multiple targets simultaneously or cooperatively, making it
possible to screen multi-target therapeutic drugs that meet
clinical requirements (12,13). Daphnoretin (14), which is extracted from Wikstroemia
indica (15), has been proven to
exert significant antitumor effects. Nevertheless, the mechanism of
its antitumor properties in melanoma remains unclear, thus prompted
the evaluation of daphnoretin treatment in the present study.
To determine the possible intrinsic mechanism of
daphnoretin-induced melanoma cell apoptosis, the anti-
proliferative effect of daphnoretin on melanoma A375 and B16 cells
was examined using MTT and colony formation assays. Subsequently,
the apoptotic rate and the levels of apoptosis- associated proteins
were determined by Hoechst 33258 staining and western blotting. In
addition, the ROS levels in daphnoretin-treated A375 and B16 cells
were detected using DCFH-DA probes. These findings may promote
further research into novel treatments for melanoma.
Materials and methods
Cell culture and reagents
Human A375 and murine B16 malignant melanoma cells
were purchased from the cell banks of the Chinese Academy of
Sciences. Daphnoretin (cat no. X-051-191008; purity, ≥98%) was
purchased from Chengdu Herbpurify Co., Ltd. DMEM high-glucose
culture medium was procured from HyClone (Cytiva), and supplemented
with 10% FBS and 1% penicillin-streptomycin (Beijing Solarbio
Science & Technology Co., Ltd.). The cells were maintained in a
humidified incubator at 37°C (5% CO2).
MTT assay
Cells were seeded into 96-well plates at a density
of 1×105 cells/ml and cultured for 24 h, after which
different concentrations of daphnoretin (0, 10, 20, 30, 40, 50, 60,
70 and 80 µg/ml) were added for a further 24 h. Next, 20 µl MTT (5
mg/ml) was added to each well and the cells were incubated for an
additional 4 h before the media were discarded. Then, 150 µl DMSO
was added, and the plates were agitated for 10 min at room
temperature. The absorbance value was determined using a microplate
reader (Infinite 200 Pro; Tecan Group, Ltd.) at 490 nm (16).
Colony formation assay
Cells in the logarithmic phase were harvested with
0.25% trypsin and homogenized. The cells were resuspended in DMEM
medium with 10% FBS and then seeded into 6-well plates at 200
cells/well. Once adherent proliferation was established, the cells
were treated with different doses of daphnoretin (0, 20, 40 and 60
µg/ml) and incubated for 24 h. On the second day, the media were
replaced, and the cells were continuously cultured in a 37°C (5%
CO2); the media were then replaced once every three days
for 2–3 weeks. The cells were fixed with 4% paraformaldehyde
solution (Beijing Solarbio Science & Technology Co., Ltd.) for
10 min at 4°C, and then stained with 1% crystal violet for 10 min
at room temperature (both Beijing Solarbio Science & Technology
Co., Ltd.). The cells were observed under a fluorescence microscope
(DMI3000B, Leica Microsystems GmbH) and images were captured.
Hoechst 33258 staining
To observed changes in morphology, cells were seeded
into 6-well plates (2×105 cells/ml) and incubated for 24
h, after which different concentrations of daphnoretin (0, 20, 40
and 60 µg/ml) were added. After 24 h, 4% paraformaldehyde solution
was added at 4°C. After 10 min, the cells were washed twice with
PBS and stained with Hoechst 33258 for 10 min at room temperature.
Images were then captured using a fluorescence microscope (17).
Flow cytometric apoptosis
analysis
Apoptosis was detected by flow cytometry. The cells
were seeded into 6-well plates (2×105 cells/ml) and
incubated for 24 h. Then, daphnoretin was added at different
concentrations (0, 20, 40 and 60 µg/ml). The cells were collected
after 24 h, and 10 µl propidium iodide was added for 10 min,
followed by 5 µl Annexin V-FITC for a further 5 min (both at room
temperature). Flow cytometry was performed using a FACSCanto II
flow cytometer (Becton, Dickinson and Company) (18). The apoptosis rate was analyzed using
FACSDiva 6.1.3 software (Becton, Dickinson and Company).
ROS detection
Cells were seeded into 6-well plates at a density of
1.8×105 cells/ml, and then incubated for 24 h. Different
concentrations of daphnoretin were administered (0, 20, 40 and 60
µg/ml) for 24 h, after which the cells were digested with 0.25%
trypsin (Beijing Solarbio Science & Technology Co., Ltd.).
DCFH-DA (Beijing Solarbio Science & Technology Co., Ltd.) was
diluted with serum-free medium (1:1,000) to a final concentration
of 10 µM, and 1 ml was added per well prior to incubation at 37°C
for 20 min. After washing three times with serum-free medium, the
cells were analyzed by flow cytometry or photographed under a
fluorescence microscope.
Western blot analysis
Cells were seeded into 100-mm cell culture dishes
with daphnoretin administered (0, 20, 40 and 60 µg/ml) and
incubated for 24 h. The cells were then collected and washed twice
with PBS. RIPA buffer (cat. no. R0010; Beijing Solarbio Science
& Technology Co., Ltd.) was added to lyse the cells, and the
mixture was kept on ice for 30 min. The lysate was then centrifuged
at 13,000 × g for 4 min at 4°C, and the protein concentration was
measured using BCA protein assay kit, and equalized between samples
using 4X loading buffer. The mass of protein loaded per well was 80
µg. The samples were run on a 12% SDS-PAGE gel, transferred to PVDF
membranes (EMD Millipore) and then blocked with 5% milk for 1 h at
room temperature. Diluted primary antibodies against the following
targets were then added overnight at 4°C: β-actin, (cat. no. TA-09;
1:2,000; OriGene Technologies, Inc.); caspase-3 (cat. no. ab13847;
1:1,000; Abcam); cleaved caspase-3 (cat. no. 9661S; 1:1,000; Cell
Signaling Technology, Inc.); caspase-9 (cat. no. Ab202068; 1:2,000;
Abcam); cleaved caspase-9 (cat. no. 20750S; 1:1,000; Cell Signaling
Technology, Inc.); apoptotic protease-activating factor 1 (Apaf-1;
cat no. 5088S; 1:1,000; Cell Signaling Technology, Inc.);
cytochrome c (cat. no. ab110325; 1:2,000; Abcam); Bcl-2
(cat. no. ab196495; 1:1,000, Abcam); Bax (cat. no. ab182734;
1:1,000; Abcam); PI3K (cat. no. 60225-1; 1:5,000, ProteinTech
Group, Inc.); phospho (p-)PI3K (cat. no. ab182651; 1:500; Abcam);
Akt (cat. no. 9272S; 1:1,000; Cell Signaling Technology, Inc.); and
p-Akt (cat. no. 4060S; 1:2,000; Cell Signaling Technology, Inc.).
The membranes were washed four times for 5 min each (with 1X TBST
on a shaking platform), and then diluted secondary antibodies
against the following targets were added overnight at room
temperature: Peroxidase-conjugated affinipure goat anti-mouse IgG
(cat. no. ZB-2305; 1:30,000; OriGene Technologies, Inc.) and goat
anti-rabbit IgG (cat. no. ab6721; 1:20,000; Abcam). Membranes were
washed four times for 5 min with 1X TBST. Tweezers were used to
move each membrane to a culture dish, and ECL chemiluminescence
working buffer (cat. no. 34580; Thermo Fisher Scientific, Inc.) was
carefully pipetted over each membrane. Finally, the membranes were
visualized using a BioSpectrum® Imaging System (Ultra
Violet Products, Ltd.). ImageJ v1.43 software (National Institutes
of Health) was used for densitometry.
Statistical analysis
The data are presented as the mean ± standard
deviation, and each experiment was repeated at least three times.
One-way ANOVA or Students t-test followed by Bonferronis post hoc
test were performed to compare the differences between groups using
GraphPad Prism 6.0 (GraphPad Software, Inc.). P<0.05 was
considered to indicate a statistically significant difference.
Results
Daphnoretin inhibits A375 and B16 cell
proliferation and colony formation
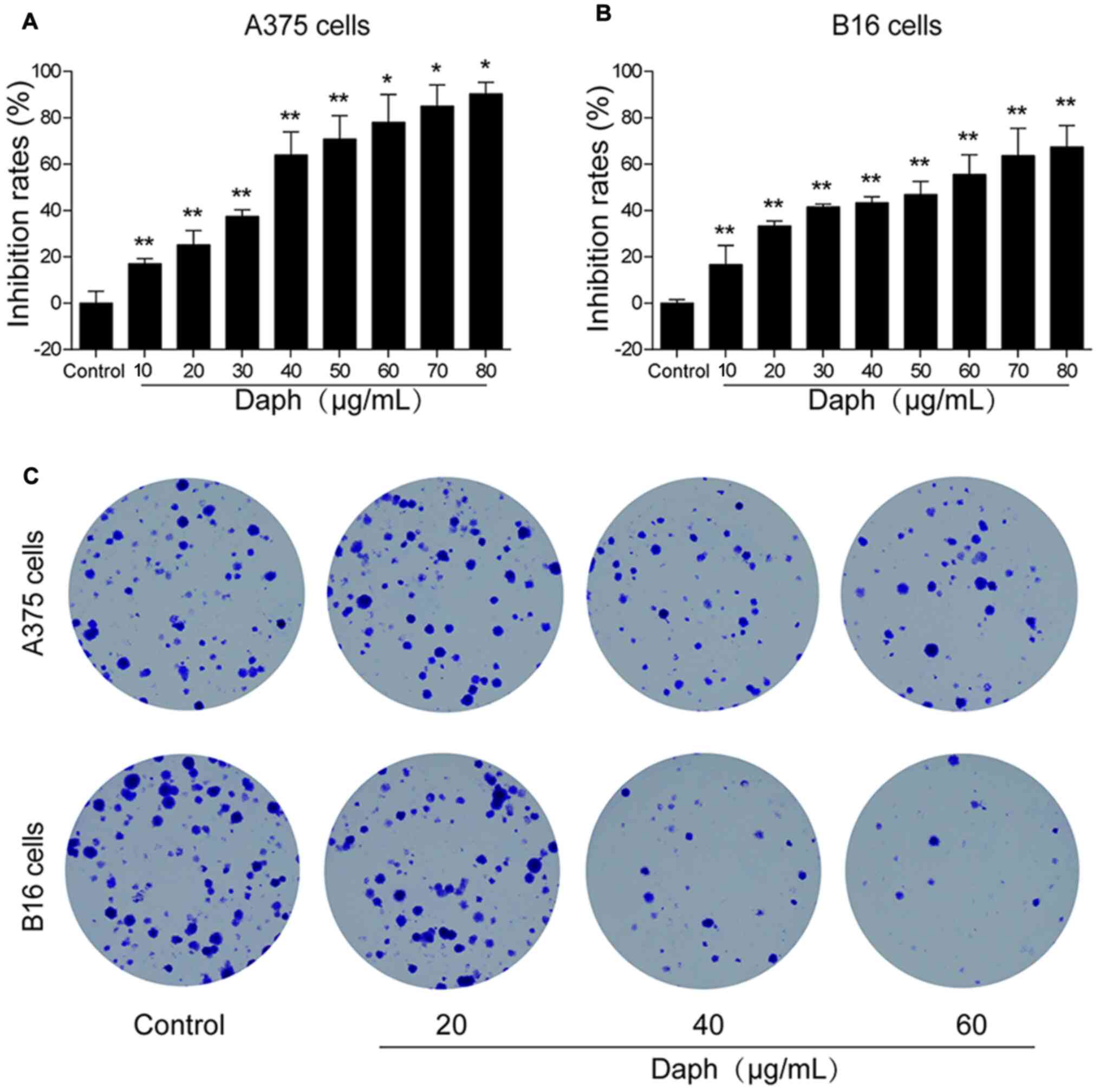
An MTT assay was performed to evaluate the antitumor
effect of daphnoretin in melanoma A375 and B16 cells. A 24-h
treatment with daphnoretin significantly suppressed cellular
proliferation, and the IC50 values for A375 and B16
cells were 37.81 and 53.46 µg/ml, respectively (Fig. 1A and B). Based on the results of the
previous experiment, a colony formation assay was conducted; the
results suggested that the number of A375 and B16 cell colonies
gradually decreased compared with that of the control group
(Fig. 1C). These results indicated
that daphnoretin inhibited cell proliferation.
Daphnoretin induces A375 and B16 cell
apoptosis
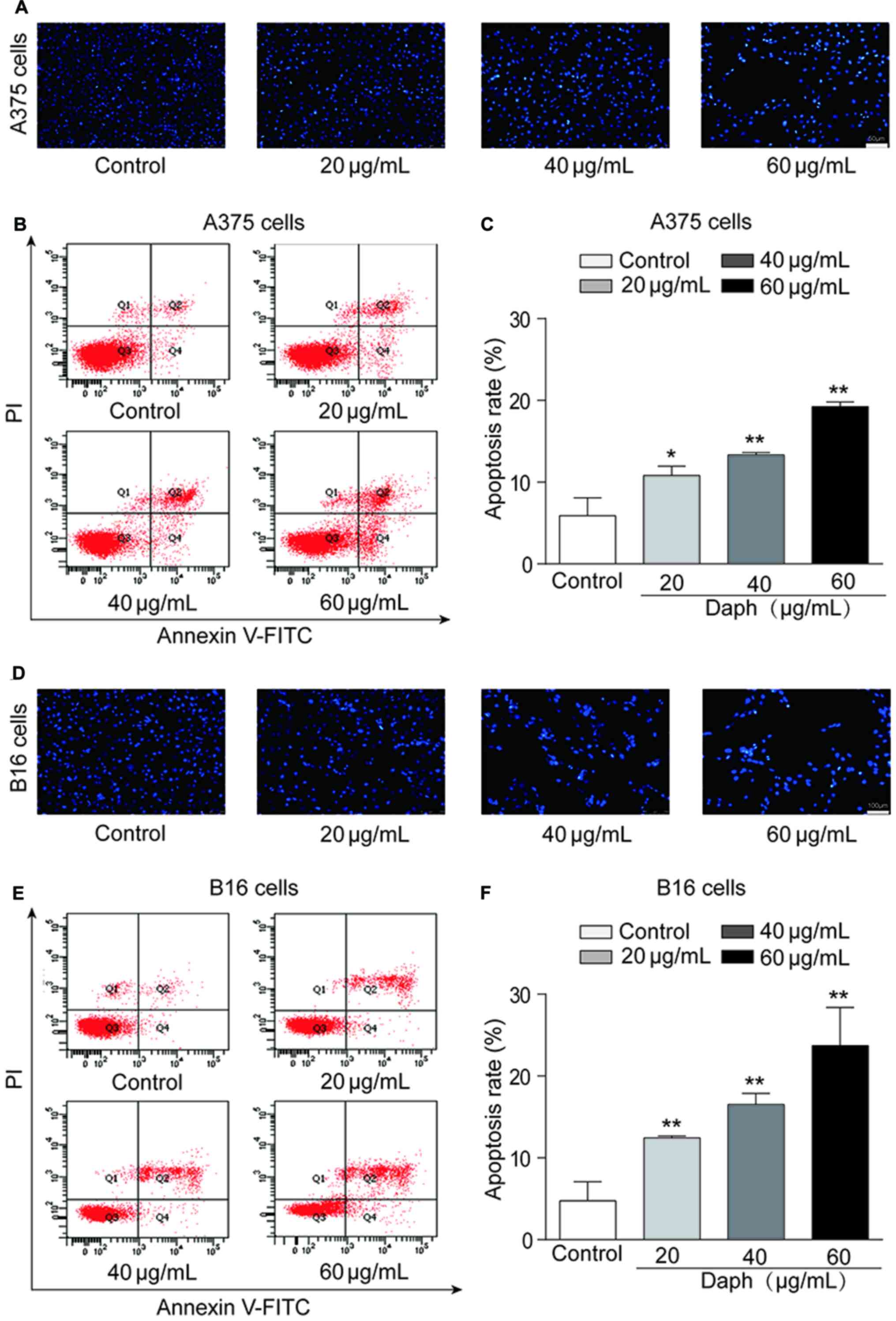
Hoechst 33258 staining was conducted to assess A375
and B16 cell apoptosis. Under a fluorescence microscope, the
formation of apoptotic bodies was observed (Fig. 2A and D). To determine the mechanism
of apoptosis in A375 and B16 cells following daphnoretin treatment,
flow cytometry was used to quantify cells positive for annexin
V-FITC and PI. Daphnoretin significantly increased the apoptotic
rate of A375 and B16 cells in a dose-dependent manner (Fig. 2B and E). Moreover, the apoptotic
rates of daphnoretin-treated A375 and B16 cells were significantly
increased compared with those of the corresponding control groups
(Fig. 2C and F). These findings
suggested that daphnoretin induced apoptosis in A375 and B16
melanoma cells.
Daphnoretin induces melanoma cell
apoptosis in a caspase-dependent manner
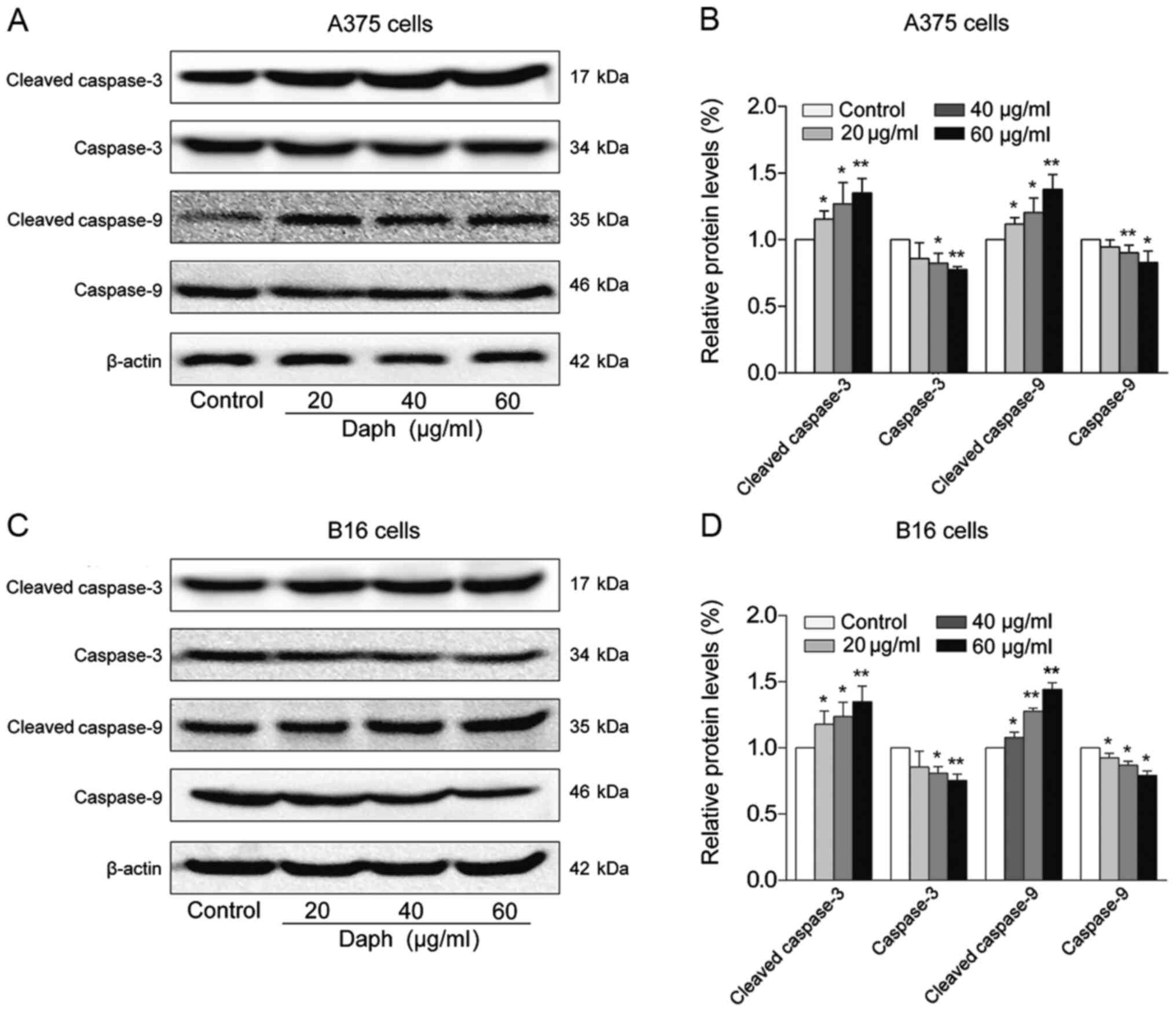
The effects of daphnoretin on the levels of
apoptosis-related proteins were evaluated using A375 and B16 cells.
Western blotting revealed that the protein levels of caspase-3 and
−9 were both significantly decreased, while those of cleaved
caspase-3 and −9 were dose-dependently increased in
daphnoretin-treated A375 cells (Fig. 3A
and B). Similarly, the expression levels of caspase-3 and −9
were upregulated, and those of cleaved caspase-3 and −9 were
downregulated in daphnoretin-treated B16 cells, compared with the
control group (Fig. 3C and D). These
results suggested that daphnoretin induced apoptosis in A375 and
B16 cells by a caspase-dependent mechanism.
Daphnoretin induces melanoma cell
apoptosis via the Bax/Bcl-2 pathway
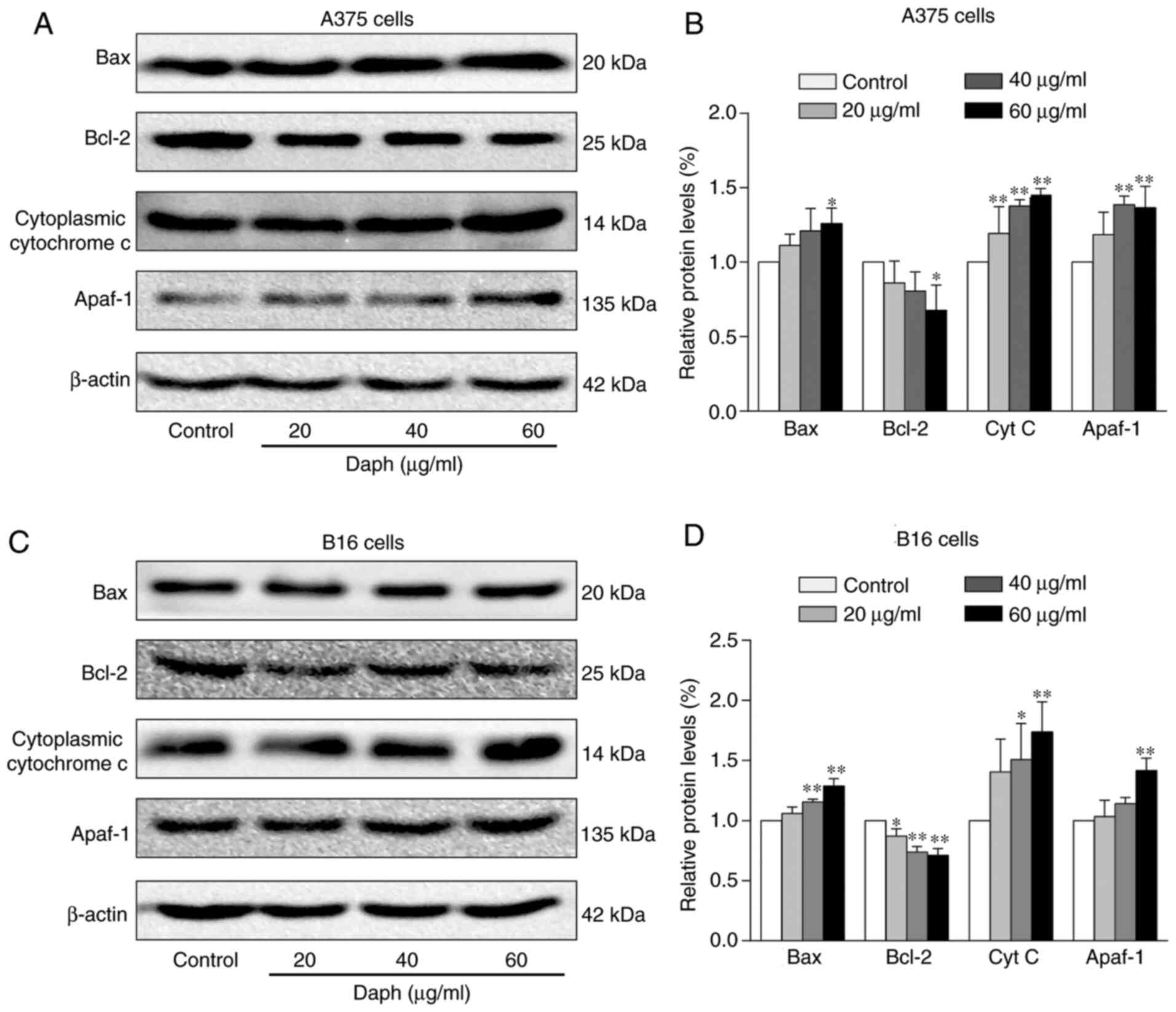
The expression levels of another set of
apoptosis-related proteins were measured by western blotting. The
data showed that the levels of Bcl-2 were upregulated, whereas Bax,
cytochrome c and Apaf-1 were downregulated in
daphnoretin-treated A375 cells (Fig. 4A
and B). Similarly, the levels of Bcl-2 increased, while those
of Bax, cytochrome c and Apaf-1 decreased in B16 cells
following treatment with daphnoretin (Fig. 4C and D). These results suggested that
daphnoretin induced apoptosis in A375 and B16 cells by a
Bax/Bcl-2-dependent pathway.
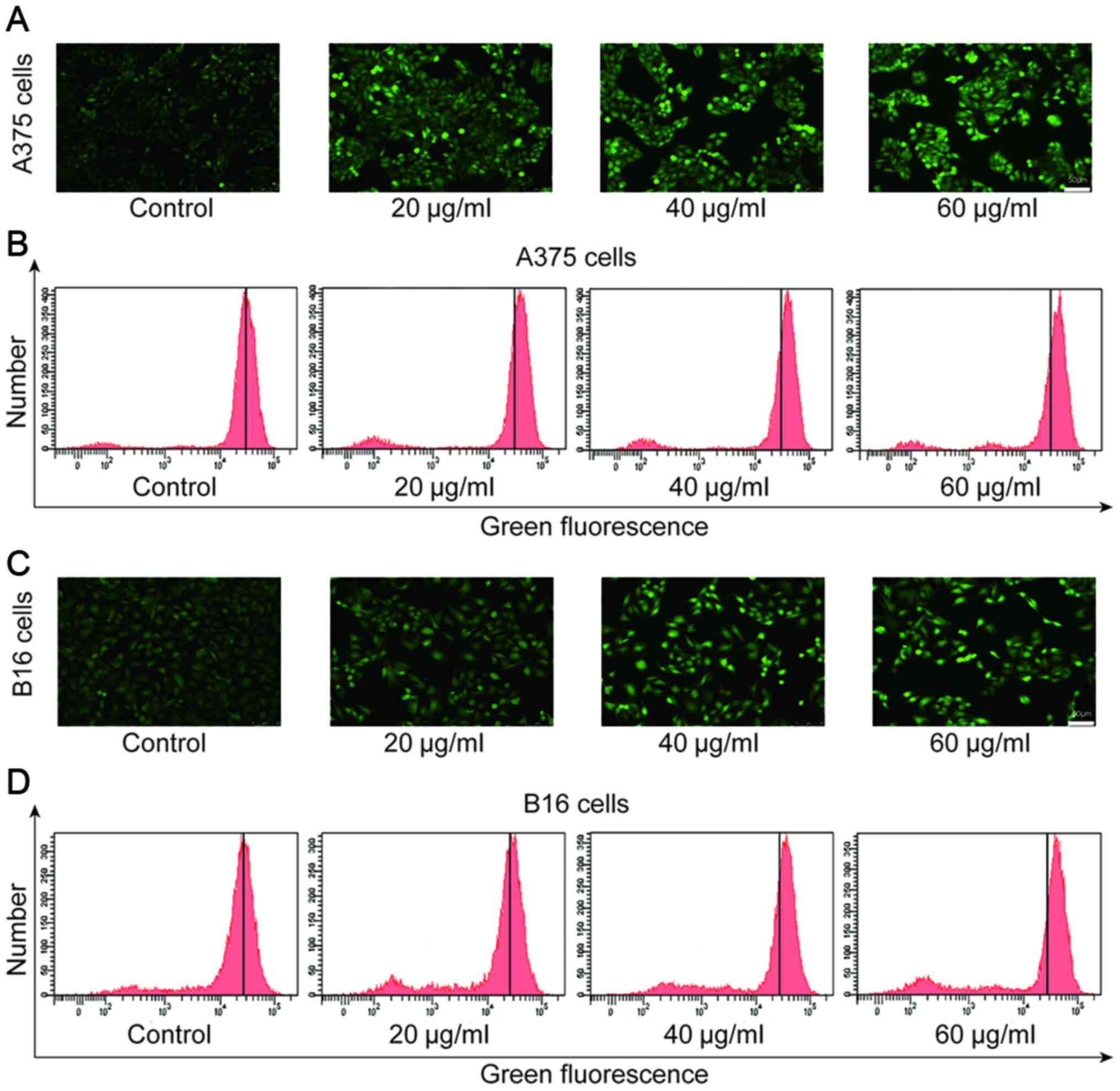
Daphnoretin increases the level of ROS
production in melanoma cells
ROS plays a regulatory role in the process of
apoptosis induced by various antitumor drugs (19). It has been confirmed that the
increase in ROS may induce apoptosis (20). In the present study, the level
DCF-associated green fluorescence was gradually increased in A375
and B16 cells exposed to daphnoretin (Fig. 5A and C). In addition, the flow
cytometry results showed that 24 h after daphnoretin treatment, the
level of ROS in A375 and B16 cells dose-dependently increased
compared with the corresponding control group (Fig. 5B and D). The data indicated that
daphnoretin increased the level of ROS in melanoma cells.
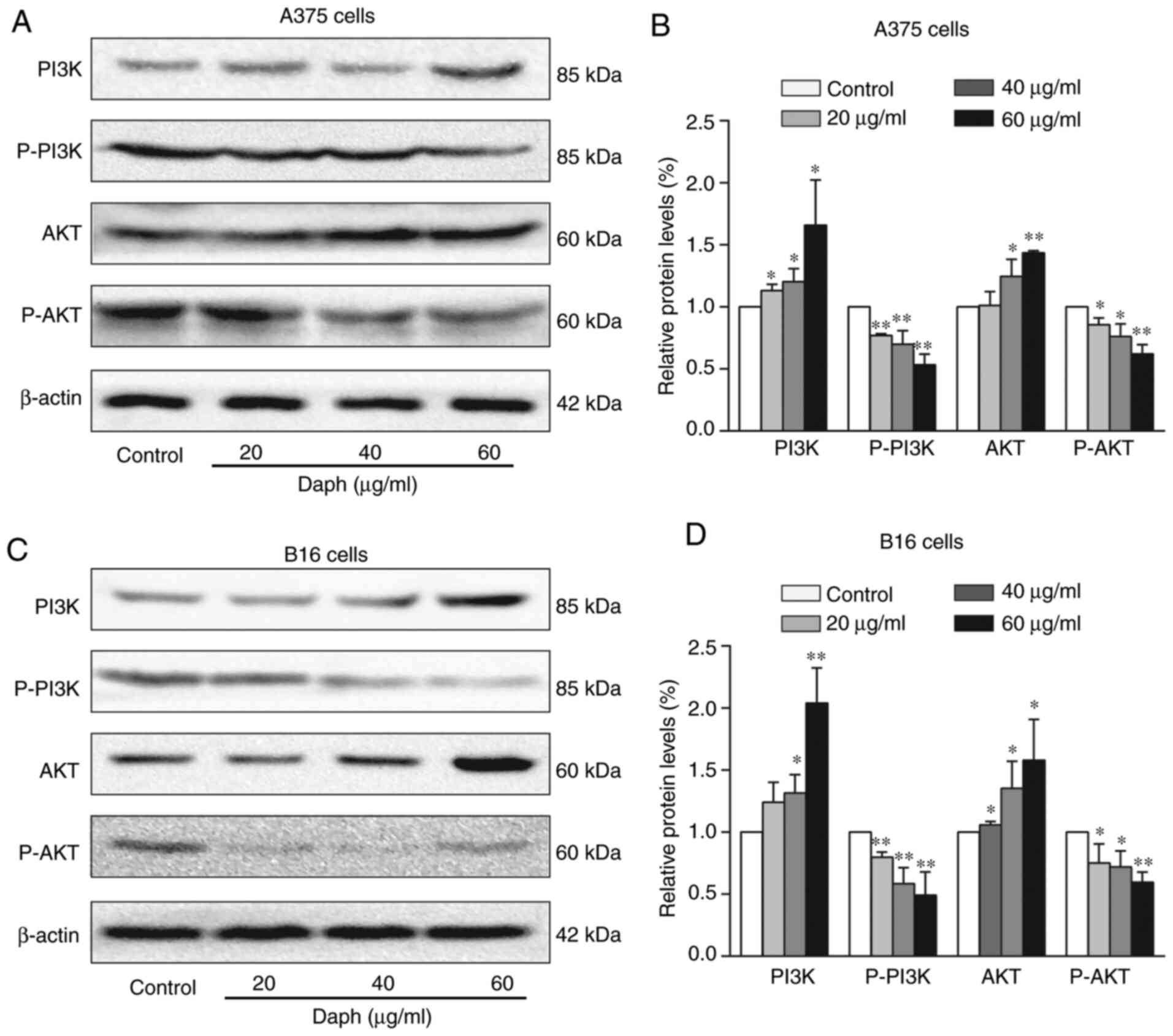
Daphnoretin regulates the PI3K/Akt
signaling pathway in A375 and B16 cells
The PI3K/Akt signaling pathway is an important
pathway closely related to tumor genesis and development, thus the
protein levels of PI3K/Akt were assessed by western blotting. The
results showed that the protein expression levels of PI3K and Akt
were increased, whereas p-PI3K and p-Akt were decreased in
daphnoretin-treated A375 and B16 cells (Fig. 6A-D). These results indicated that
daphnoretin regulated the PI3K/Akt signaling pathway.
Discussion
Daphnoretin exerts antitumor effects in specific
tumor types. A study demonstrated that daphnoretin induced
apoptosis in human osteosarcoma cells by triggering G2/M
cell cycle arrest and activating the caspase-3 pathway (21). In another study, daphnoretin was
shown to induce the mitochondria-mediated apoptosis of HeLa cells
(22). Daphnoretin has also been
reported to influence the proliferation and apoptosis of A549 lung
cancer cells in vitro (23).
Moreover, daphnoretin was found to inhibit the proliferation,
invasiveness and migration of colon cancer HCT116 cells, and to
promote apoptosis by regulating Akt signaling pathway activity
(24). The results of the present
study demonstrated that daphnoretin inhibited proliferation and
promoted apoptosis via the ROS-mediated mitochondrial apoptosis and
the PI3K/Akt signaling pathways.
In the present study, daphnoretin was first found to
significantly inhibit the proliferation of A375 and B16 melanoma
cells in a concentration-dependent manner. To further confirm the
mechanisms associated with the daphnoretin treatment, the formation
of apoptotic bodies was observed in A375 and B16 cells following
Hoechst staining, and the cellular apoptotic rate was significantly
increased compared with the control group. These findings suggest
that daphnoretin induced apoptosis in melanoma A375 and B16
cells.
Apoptosis is a complex process in which various
protein signals are transmitted through several different pathways
(25). Caspases, which are the
convergence points of multiple apoptotic pathways, regulate the
final execution of apoptosis (26,27).
Thus, caspase activation is an essential link in apoptotic signal
transduction (28). Under the
stimulation of apoptotic signals, cytochrome c is released
into the cytoplasm and combines with Apaf-1 to activate the
caspase-9 precursor; activated caspase-9 then cleaves the caspase-3
proenzyme, which activates caspase-3, thus promoting its cleavage.
Finally, a caspase cascade reaction is triggered, and apoptosis is
induced (29,30). The present findings indicate that the
levels of caspase-3 and −9 were significantly decreased, whereas
the levels of cleaved caspase-3 and −9 were markedly increased
compared with the control group.
Several studies have suggested that the Bcl-2 family
plays a vital role in the apoptotic process (31,32). The
expression and regulation of Bcl-2 family proteins are essential in
signal transduction pathways and apoptosis. The pro-apoptotic
protein Bax, and the anti-apoptotic protein Bcl-2, are essential
members of the Bcl-2 family, and their coordinated action plays an
important role in apoptosis (33).
Bcl-2 and Bax usually exist as heterodimers, which jointly regulate
apoptosis (34,35). When the expression of Bcl-2 is high,
a large number of heterodimers with Bax are formed, inhibiting the
activity of Bax and preventing apoptosis; when the expression of
Bax exceeds a certain point, Bax homodimers predominate, which
induces apoptosis (35,36). The present results showed that the
expression levels of Bax increased, while those of Bcl-2 decreased,
suggesting that the Bcl-2 family regulates daphnoretin-induced
apoptosis. Previous studies have shown that the Bcl-2 family
members release cytochrome c and activated various caspases
(37,38). Therefore, the results of the present
study indicate that daphnoretin induces the activity of the Bcl-2
family proteins, and promote the release of cytochrome c and
Apaf-1, thereby activating caspase-9 and −3, and ultimately
inducing apoptosis.
An impairment of the ROS balance is associated with
cancer cell proliferation inhibition. Compared with normal
untransformed cells, ROS levels are increased in cancer cells
(39). As signaling molecules in
cellular regulation, ROS play an important role in apoptosis by
regulating apoptotic factors (40).
Deoxynivalenol induces the cytotoxicity of IPEC-J2 cells in a
dose-dependent manner, in which apoptosis is potentially the result
of increased ROS production (41).
After cells receive pro-apoptotic signals, ROS generation
increases, which promotes Ca2+ influx, upregulates the
expression of Bax, initiates mitochondrial permeability transition
pore formation, and activates caspases, which ultimately leads to
apoptosis. A previous study revealed that ROS-induced apoptosis may
be related to the ROS-dependent upregulation of Bax, and a
reduction in Bcl-2 expression (42,43).
Another study indicated that the activation of caspase-3 was
regulated by ROS (44). To
investigate these mechanisms in A375 and B16 cells treated with
daphnoretin, ROS generation was measured by flow cytometry, which
revealed increased levels of ROS in A375 and B16 cells. These
results indicated that daphnoretin induced ROS-mediated apoptosis
in melanoma A375 and B16 cells.
ROS have also been shown to play important roles in
various signaling pathways, including the PI3K/Akt, Wnt/β-catenin
and MAPK pathways (45). It has been
reported that genistein induces G2/M cell cycle arrest
and apoptosis in human bladder transitional cell carcinoma T24
cells through the ROS-mediated PI3K/Akt signaling pathway (46), which is also important for regulating
ROS generation in HCT116 cells (47). Therefore, the potential association
between PI3K/Akt and the daphnoretin-induced inhibition of
proliferation and increased apoptosis were investigated using A375
and B16 cells. The findings suggested that the levels of PI3K and
Akt increased, and that those of p-PI3K and p-Akt decreased, in
daphnoretin-treated A375 and B16 cells, indicating that the
PI3K/Akt signaling pathway is involved in the antitumor effect of
daphnoretin in melanoma cells.
In conclusion, daphnoretin markedly inhibited
cellular proliferation and induced apoptosis through the
ROS-mediated mitochondrial apoptosis and PI3K/Akt signaling
pathways in melanoma A375 and B16 cells. These findings demonstrate
that daphnoretin has the potential to be used as an anti-melanoma
drug. However, the future clinical application of daphnoretin
requires further investigation.
Acknowledgements
Not applicable.
Funding
The present study was funded by the National Natural
Science Foundation of China (grant no. 31870338), the Key Research
and Development Program of Shandong Province of China (grant no.
2019GSF108214), Taishan Scholars Construction Engineering of
Shandong Province (grant no. tsqn201812099), the Dominant
Disciplines Talent Team Development Scheme of Higher Education of
Shandong Province (grant no. 2016052410) and the Introduction and
Cultivation Project for Young Creative Talents of Higher Education
of Shandong Province (grant no. 20191008198).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors contributions
HW, XH, DL and QZ designed the experiments. HW and
XH obtained the experimental data and wrote the manuscript. HW, ML
and ZP performed Hoechst 33258 staining, flow cytometric apoptosis
analysis, western blot analysis and ROS detection. HW and DL
interpreted and analyzed the data. HW and QZ assessed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
George Liddell H and Scott R: Drugs in
clinical development for melanoma. Pharmaceut Med. 26:171–183.
2012.
|
|
2
|
Saavedra-Alonso S, Zapata-Benavides P,
Chavez-Escamilla AK, Manilla-Muñoz E, Zamora-Avila DE,
Franco-Molina MA and Rodriguez-Padilla C: WT1 shRNA delivery using
transferrin-conjugated PEG liposomes in an in vivo model of
melanoma. Exp Ther Med. 12:3778–3784. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pippa C, Mirela H, Kate F and Christine P:
Management of melanoma. Br Med Bull. 111:149–162. 2014. View Article : Google Scholar
|
|
4
|
Deiana M, Dalle Carbonare L, Serena M,
Cheri S, Parolini F, Gandini A, Marchetto G, Innamorati G, Manfredi
M, Marengo E, et al: New insights into the runt domain of RUNX2 in
melanoma cell proliferation and migration. Cells. 7:2202018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen L and Jin S: Trends in mortality
rates of cutaneous melanoma in East Asian populations. PeerJ.
4:e28092016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wada-Ohno M, Ito T and Furue M: Adjuvant
therapy for melanoma. Curr Treat Options Oncol. 20:632019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tchernev G: One step melanoma surgery for
patient with thick primary melanomas: ‘To break the rules, you must
first master them!’. Open Access Maced J Med Sci. 6:367–371. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hsu SC and Chung JG: Anticancer potential
of emodin. Biomedicine. 2:108–116. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun X, Yan H, Zhang Y, Wang X, Qin D and
Yu J: Preparative separation of diterpene lactones and flavones
from Andrographis paniculate using off-line two-dimensional
high-speed counter-current chromatography. Molecules. 24:6202019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mao W and Xia Q: Anti-tumor effects of
traditional Chinese medicine give a promising perspective. J Cancer
Res Ther. 10 (Suppl 1):S1–S2. 2014. View Article : Google Scholar
|
|
11
|
Luo F, Gu J, Chen L and Xu X: Systems
pharmacology strategies for anticancer drug discovery based on
natural products. Mol Biosyst. 10:1912–1917. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhaokun Y, Zijun L and Jiumao L:
Anticancer properties of traditional Chinese medicine. Comb Chem
High Throughput Screen. 20:423–429. 2017.
|
|
13
|
Xiang Y, Guo Z, Zhu P, Chen J and Huang Y:
Traditional Chinese medicine as a cancer treatment: Modern
perspectives of ancient but advanced science. Cancer Med.
8:1958–1975. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen CA, Liu CK, Hsu ML, Chi CW, Ko CC,
Chen JS, Lai CT, Chang HH, Lee TY, Lai YL and Chen YJ: Daphnoretin
modulates differentiation and maturation of human dendritic cells
through down-regulation of c-Jun N-terminal kinase. Int
Immunopharmacol. 51:25–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lu CL, Li YM, Fu GQ, Yang L, Jiang JG, Zhu
L, Lin FL, Chen J and Lin QS: Extraction optimisation of
daphnoretin from root bark of Wikstroemia indica (L.) C.A. and its
anti-tumour activity tests. Food Chem. 124:1500–1506. 2011.
View Article : Google Scholar
|
|
16
|
Van MJ, Kaspers GJ and Cloos J: Cell
sensitivity Assays: The MTT assay. Methods Mol Biol. 88:237–245.
2011.
|
|
17
|
Wu S, Zhu G, Ni Y, Zhang T and Jiang W:
Cucurbitacin I (JSI-124)-induced apoptosis of HepG2 cells via p53
signaling pathway. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 33:33–38.
2017.(In Chinese). PubMed/NCBI
|
|
18
|
Yurinskaya V, Aksenov N, Moshkov A, Model
M, Goryachaya T and Vereninov A: A comparative study of U937 cell
size changes during apoptosis initiation by flow cytometry, light
scattering, water assay and electronic sizing. Apoptosis.
22:1287–1295. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luu HN, Wen W, Li H, Dai Q, Yang G, Cai Q,
Xiang YB, Gao YT, Zheng W and Shu XO: Are dietary antioxidant
intake indices correlated to oxidative stress and inflammatory
marker levels? Antioxid Redox Signal. 22:951–959. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang B, Peng X, Li G, Xu Y, Xia X and
Wang Q: Oxidative stress is involved in Patulin induced apoptosis
in HEK293 cells. Toxicon. 94:1–7. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gu S and He J: Daphnoretin induces cell
cycle arrest and apoptosis in human osteosarcoma (HOS) cells.
Molecules. 17:598–612. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang ZY, Kan JT, Cheng ZY, Wang XL, Zhu YZ
and Guo W: Daphnoretin-induced apoptosis in HeLa cells: A possible
mitochondria-dependent pathway. Cytotechnology. 66:512014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang HF, Wu Z, Bai X, Zhang Y and He P:
Effect of daphnoretin on the proliferation and apoptosis of A549
lung cancer cells in vitro. Oncol Lett. 8:1139–1142. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu S, Guo H, Gao X, Li M and Bian H:
Daphnoretin: An invasion inhibitor and apoptosis accelerator for
colon cancer cells by regulating the Akt signal pathway. Biomed
Pharmacother. 111:1013–1021. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Olechowska-Jarząb A, Ptak-Belowska A and
Brzozowski T: Terapeutic importance of apoptosis pathways in
pancreatic cancer. Folia Med Cracov. 56:61–70. 2016.
|
|
26
|
Li D, Hu X, Han T, Liao J, Xiao W, Xu S,
Li Z, Wang Z, Hua H and Xu J: NO-releasing enmein-type diterpenoid
derivatives with selective antiproliferative activity and effects
on apoptosis-related proteins. Molecules. 21:11932016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lkhagvasuren K and Kim JK: Ziyuglycoside
II induces caspases-dependent and caspases-independent apoptosis in
human colon cancer cells. Toxicol In Vitro. 59:255–262. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Palai TK and Mishra SR: Caspases: An
apoptosis mediator. J Adv Veterinary Animal Res. 2:18–22. 2015.
View Article : Google Scholar
|
|
29
|
Frejlich E, Rudno-Rudzińska J, Janiszewski
K, Salomon L and Kielan W: Caspases and their role in gastric
cancer. Adv Clin Exp Med. 22:593–602. 2013.PubMed/NCBI
|
|
30
|
Zhao Y, Jing Z, Lv J, Zhang Z, Lin J, Cao
X, Zhao Z, Liu P and Mao W: Berberine activates
caspase-9/cytochrome c- mediated apoptosis to suppress
triple-negative breast cancer cells in vitro and in vivo. Biomed
Pharmacother. 95:18–24. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sheng M, Zhou Y, Yu W, Weng Y, Xu R and Du
H: Protective effect of Berberine pretreatment in hepatic ischemia/
reperfusion injury of rat. Transplant Proc. 47:275–282. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kvansakul M and Hinds MG: The Bcl-2
family: Structures, interactions and targets for drug discovery.
Apoptosis. 20:136–150. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Galluzzi L, Kepp O, Trojel-Hansen C and
Kroemer G: Mitochondrial control of cellular life, stress, and
death. Circ Res. 111:11982012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sawa A and Sedlak TW: Oxidative stress and
inflammation in schizophrenia. Schizophr Res. 176:1–2. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Jin S and Dai CL: Attenuation of
reperfusion-induced hepatocyte apoptosis is associated with
reversed bcl-2/bax ratio in hemi-hepatic artery-preserved portal
occlusion. J Surg Res. 174:298–304. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ding H, Wang J, Jia FP, Yi J and Zhang M:
Research on the A549 cell apoptosis mechanism of the nude mouse
model using MenSC-sTRAIL. Eur Rev Med Pharmacol Sci. 21:3218–3222.
2017.PubMed/NCBI
|
|
37
|
Burrer CM, Foight GW, Keating AE and Chan
GC: Selective peptide inhibitors of antiapoptotic cellular and
viral Bcl-2 proteins lead to cytochrome c release during
latent Kaposis sarcoma-associated herpesvirus infection. Virus Res.
211:86–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gupta R and Ghosh S: Putative roles of
mitochondrial Voltage-dependent anion channel, Bcl-2 family
proteins and c-Jun N-terminal Kinases in ischemic stroke associated
apoptosis. Biochimie Open. 4:47–55. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jutooru I, Guthrie AS, Chadalapaka G,
Pathi S, Kim K, Burghardt R, Jin UH and Safe S: Mechanism of action
of phenethylisothiocyanate and other reactive oxygen
species-inducing anticancer agents. Mol Cell Biol. 34:2382–2395.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hong M, Li J, Li S and Almutairi MM:
Acetylshikonin sensitizes hepatocellular carcinoma cells to
apoptosis through ROS-mediated caspase activation. Cells.
8:14662019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kang R, Li R, Dai P, Li Z, Li Y and Li C:
Deoxynivalenol induced apoptosis and inflammation of IPEC-J2 cells
by promoting ROS production. Environ Pollut. 251:689–698. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Choi YH, Kang YJ, Kim SH, Sung B and Kim
ND: Abstract 1773: MHY-449 induces apoptotic cell death through
ROS- and caspase-dependent pathways in AGS human gastric cancer
cells. Cancer Res. 75:17732015.
|
|
43
|
AlBasher G, AlKahtane AA, Alarifi S, Ali
D, Alessia MS, Almeer RS, Abdel-Daim MM, Al-Sultan NK, Al-Qahtani
AA, Ali H and Alkahtani S: Methotrexate-induced apoptosis in human
ovarian adenocarcinoma SKOV-3 cells via ROS-mediated
bax/bcl-2-cyt-c release cascading. Onco Targets Ther. 12:21–30.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen L, Gong MW, Peng ZF, Zhou T, Ying MG,
Zheng QH, Liu QY and Zhang QQ: The marine fungal metabolite,
dicitrinone B, induces A375 cell apoptosis through the ROS-related
caspase pathway. Mar Drugs. 12:1939–1958. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lee SY, Jeong EK, Ju MK, Jeon HM, Kim MY,
Kim CH, Park HG, Han SI and Kang HS: Induction of metastasis,
cancer stem cell phenotype, and oncogenic metabolism in cancer
cells by ionizing radiation. Mol Cancer. 16:102017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Park C, Cha HJ, Lee H, Hwang-Bo H, Ji SY,
Kim MY, Hong SH, Jeong JW, Han MH, Choi SH, et al: Induction of
G2/M cell cycle arrest and apoptosis by genistein in human bladder
cancer T24 cells through inhibition of the ROS-dependent PI3k/Akt
signal transduction pathway. Antioxidants (Basel). 8:3272019.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gao X, Li X, Ho CT, Lin X and Chen Z:
Cocoa tea (Camellia ptilophylla) induces mitochondria-dependent
apoptosis in HCT116 cells via ROS generation and PI3K/Akt signaling
pathway. Food Res Int. 129:1088542019. View Article : Google Scholar : PubMed/NCBI
|