Introduction
Brain metastasis (BM) is a frequent complication of
systemic cancer types (1). Although
highly dependent on the primary tumour (PT) and histological
subtype (2), the prognosis of BM is
often poor (3). Epidemiological
studies have shown a BM incidence of 10–30% (4–6);
however, due to improvements in the systemic treatment of PTs,
leading to a longer survival, and the detection capabilities of
imaging modalities, this incidence is expected to increase
(3). Recent evidence has suggested
that dissemination might occur during the early stage of tumour
evolution (7–9), even before the manifestation of the PT
(10). Depending on the PT site
(3), the delay between PT diagnosis
and BM presentation can range from a few months to several years.
In fact, early undetectable disseminated tumour cells (DTCs) might
become refractory to conventional therapies after extravasation and
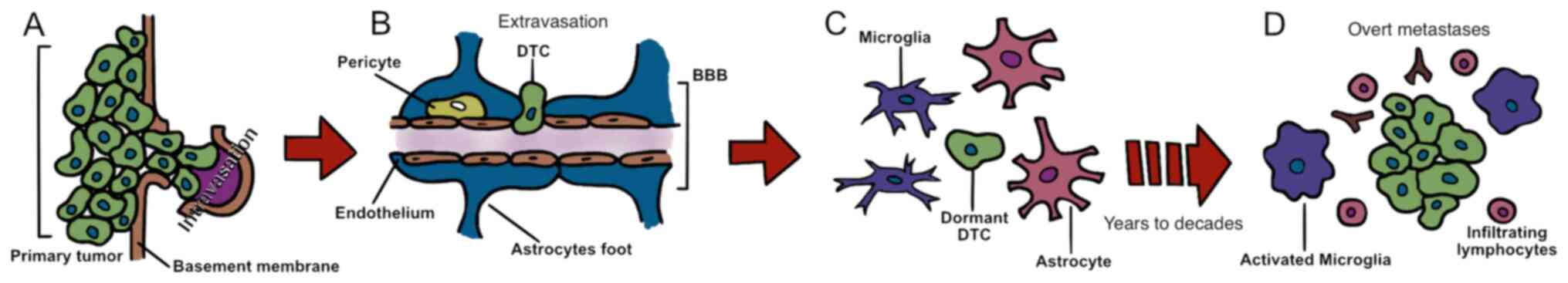
seeding in a target organ, since they enter dormancy (Fig. 1) (11–13). For
that reason, DTCs can be the source of late tumour recurrence.
Solitary tumour cell dormancy is defined as a
reversible non-proliferative cellular state characterized by
temporary mitotic and growth arrest (13), probably as the consequence of a
delayed adaptation to the microenvironment of the pre-metastatic
site (14). Further genetic and
epigenetic mutations allow dormant cells to overcome target organ
inhibitory signals, undergo mesenchymal-to-epithelial
transformation and stimulate neoangiogenesis, a required step for
proliferation (15–20). This process could result in the
delayed occurrence of metastasis. Continuous tumour growth models
have failed to explain the long interval between PT diagnosis and
distant disease recurrence, and indeed, the clinical data point to
a dormancy-based model for this delayed metastatic development
(21,22). To the best of our knowledge, no
previous study has analysed whether a selected population of
patients presenting with BM growing from dormant DTCs (i.e.,
patients with a long progression-free interval between PT and BM
diagnosis with neither PT recurrence nor other metastatic
localizations) have a prolonged survival. DTCs have been shown to
have a less aggressive phenotype than that of PTs or overt
metastases (23). Therefore, the
present study aimed at determining whether dormant BM positively
influences survival. We retrospectively collected data on patients
presenting with a BM managed at our institution between January
2004 and December 2019 to provide descriptive statistical analyses
of our cohort. We particularly focused on those patients presenting
with a long progression-free interval before the BM diagnosis. The
secondary aim was to analyse the clinical characteristics of PTs
and BMs that are linked to the phenomenon of dormancy.
Materials and methods
Patients
All patient medical records included in our
institutional (Erasmus Hospital, Free University of Bruxelles,
Belgium) neurosurgical database of BM between January 2004 and
December 2019 were reviewed. In addition, cases of cerebral
metastasis discussed in weekly multidisciplinary team meetings
during the same period were included in the medical records review,
following the exclusion of duplicates. The inclusion criterion for
further analysis was ≥1 histologically confirmed BM from a solid
extracerebral PT. Patients with a high suspicion of BM but without
a histological confirmation were, as a consequence, excluded. The
other exclusion criteria were a lack of clinical data, vertebral or
skull bone metastasis, leptomeningeal metastasis and other final
diagnoses (i.e. inflammatory process or brain primary tumour). For
each included case, the following information was collected from
the medical records: Sex, age at PT diagnosis, histological and
molecular PT type, work-up and follow-up assessment, age at BM
diagnosis and time span between BM and PT diagnoses, BM
localization and associated symptoms, presence of previous or
simultaneous distant localizations at BM diagnosis, treatment of
the BM and follow-up. Based on this information, patients were
classified into three groups. The first group included patients
with BMs from an unknown PT (uPT group), the second included
patients presenting with a BM from a known cancer with local or
systemic progression, or both (pPT group), and the last group
included patients experiencing a progression-free period following
PT treatment without local or distant recurrence at the moment of
BM diagnosis (dPT group). To exclude undetectable but already
growing BM at the time of the PT diagnosis, only patients
presenting with a minimum of 30 months of progression-free disease
were included in the dPT group (24). Only PT origins found in >2% of the
total cohort were considered independently; less frequently
observed PT origins were placed in the ‘other’ group. Since drugs
targeting specific molecular tumour profiles have a positive
influence on outcome (25), the
molecular biology phenotype was included in the survival analysis.
Since receptor expression in breast cancer allowed for specific
treatments (26),
immunohistochemistry was included in the molecular biology
group.
Statistical analysis
Statistical analysis was performed using IBM
SPSS® for Windows version 25 (IBM Corp). P<0.05 was
considered to indicate a statistically significant difference.
Categorical variables are expressed as a count (percentage),
whereas continuous data are presented as a median and interquartile
range (IQR). Kruskal-Wallis, Fisher's exact and χ2 tests
were used to analyse differences in variables between groups, as
appropriate. The Dunn's post hoc test was employed in case of
P<0.05. The Kaplan-Meier method, the log-rank test and Cox
regression were used for survival analysis. Variables with a
P<0.1 in the univariate analysis were included in the
multivariate Cox model. Furthermore, if the absolute value of the
estimated correlation between two variables was ≥0.3, one of the
variables was excluded from the multivariate analysis. A backward
stepwise method was used with variables entering the model if their
probability value was ≤5%, and leaving the model if the value was
>10%. All reported probabilities were two-sided.
Results
Included patients
After obtaining approval from the ethics committee
(approval no. P2019/319), the medical records of 346 patients
admitted to our institution (Erasmus Hospital, Free University of
Bruxelles, Belgium) for suspected BM between January 2004 and
December 2019 were reviewed. A total of 85 patients were excluded
from further analysis for the following reasons: 19 patients did
not have histological confirmation of the metastatic origin of the
brain lesion; information was missing for 12 patients; 49 patients
underwent neurosurgery for vertebral or skull bone metastasis and
not for parenchymal lesions; and for five patients, the final
histological diagnosis did not confirm the initial BM hypothesis.
Thus, 261 patients were included in the analysis. Two illustrative
case are reported herein.
Case 1
A 55-year-old woman was admitted to the neurology
department for headache and progressive cognitive decline. The only
significant previous medical issue, except for arterial
hypertension, was a serous papillary carcinoma of the left ovary
(T3cN0M0, positive for oestrogen receptors) treated by chemotherapy
(carboplatin and Taxotere) and hysterectomy with
salpingo-oophorectomy 4 years earlier; a standard follow-up
assessment showed neither local nor distant cancer recurrence.
Bradypsychia without lateralization signs was observed during the
neurological examination. Magnetic resonance imaging (MRI) of the
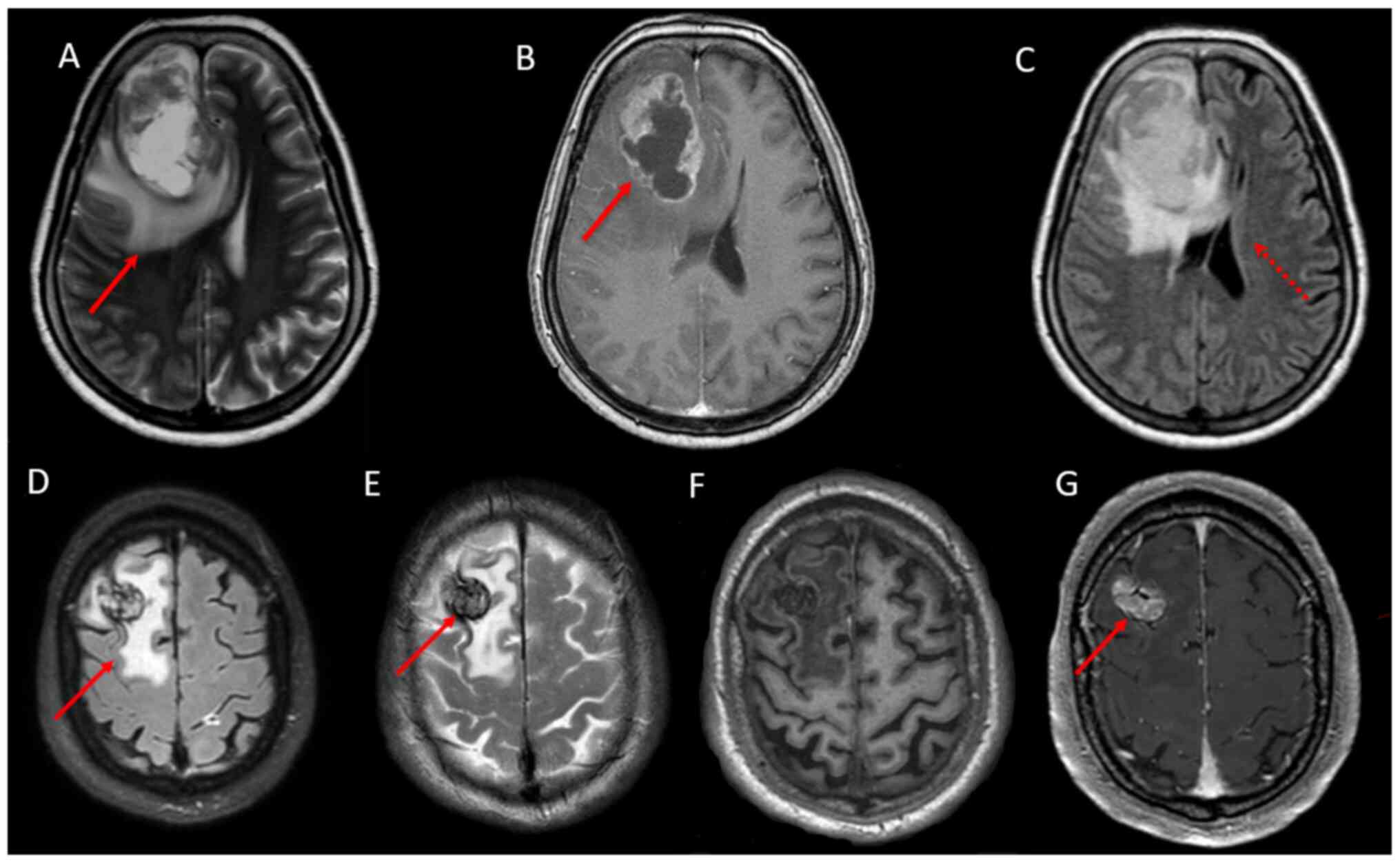
brain, performed during hospitalization (Fig. 2A-C), revealed a large ring-enhancing
right frontal lesion with extensive oedema and subfalcine
herniation. Neither local nor distant recurrences of the primary
tumour were found after a complete work-up. Resection of the brain
lesion was performed. The pathological analysis revealed oestrogen
receptor-positive ovarian cancer metastasis. Brain radiotherapy (30
Gy in 10 fractions) was delivered after neurosurgery. The BM
progressed 2 years later with the appearance of a second lesion in
the right occipital lobe that was treated by radiotherapy (30 Gy in
5 fractions). Despite treatment, the cancer progressed; a decision
for palliative care was subsequently assumed. The patient succumbed
45 months after the first BM diagnosis. This case is remarkable
compared with what has been reported in the literature (27); the patient showed a considerably
longer progression-free survival (40 vs. 24 months) and overall
survival duration after BM diagnosis (45 vs. 6.4 months).
Case 2
An 82-year-old patient was admitted to the emergency
department of our institution due to a 1-minute loss of
consciousness. The patient complained of gait instability for
several months before admission, and family members reported
progressive cognitive decline, particularly in executive functions.
The patient had a medical history of hypertension, hyperlipidaemia,
type 2 diabetes, mild cognitive impairment, obstructive sleep
apnoea, laminectomy for narrow lumbar spine canal syndrome and
carotid endarterectomy for high-grade right carotid stenosis. A
noteworthy consideration is that, 20 years earlier, he underwent
local and inguinal lymph node dissection surgery and 1 year of
interferon-α adjuvant therapy for cutaneous melanoma in the left
leg (Breslow depth 1.15 mm, Clark level 2, T2aN1aM0, Stage I);
work-up and follow-up screenings were negative for metastasis. MRI
of the brain after admission revealed a 25×5 mm right frontal
lesion surrounded by vasogenic oedema (Fig. 2D-G); a second small left temporal
lesion was also suspected. The LDH levels in blood samples were
normal (178 U/l; normal range, 135–225 U/l). A course of
dexamethasone was prescribed and the patient underwent neurosurgery
for the larger lesion a few days later. Pathological and molecular
analysis showed BRAF V600 E-mutated melanoma metastasis. A thorough
assessment revealed neither local nor distant recurrences of the
primary tumour. Gamma knife intervention was proposed for the
second lesion, but the patient declined. He was thus transferred to
a palliative care institution. Here, the case of a patient who
experienced 20 years of disease-free survival before presenting a
BM at a unique recurrence site of his primary tumour was presented;
unfortunately, due to patient refusal, despite proposed treatment
options, it was not possible to achieve long-term survival.
Patient characteristics
Patient characteristics are summarized in Table I. A slight female predominance was
present in the current cohort (n=144; 55%). The PTs most commonly
associated with BM were lung (n=138, 53%), breast (n=44; 17%),
melanoma (n=20; 8%) and colon-rectal cancer types (n=15; 6%). In
37% of cases (n=98), BM was the first manifestation of a previously
unknown PT. A total of 14 (5%) BMs were asymptomatic and were
detected during a follow-up brain imaging. A total of 178 (68%) BMs
were supratentorial, and 183 (70%) were unique. In 209 (80%) cases,
a systemic active disease was observed, which was a metastatic
localization beyond the BM and/or a PT recurrence at the first
presentation of BM. In 123 (47%) cases, molecular analysis was not
available.
 | Table I.Clinical characteristics on first
presentation with brain metastases according to primary tumour
origin compared by χ2 or Kruskal-Wallis tests. |
Table I.
Clinical characteristics on first
presentation with brain metastases according to primary tumour
origin compared by χ2 or Kruskal-Wallis tests.
| Variables | Total | Breast | Lung | Melanoma | CRC | RCC | Ovary | Uterus | Other | P-value |
|---|
| Number of patients
(%) | 261 (100) | 44 (17) | 138 (53) | 20 (8) | 15 (6) | 7 (3) | 8 (2) | 6 (2) | 23 (9) | NA |
| Median age PT,
years (IQR)a | 56 (48–62) | 45 (40–53.5) | 57
(52–63.8)c | 55 (41.8–66.5) | 58
(54.5–67)c | 60
(57.5–69)c | 59 (55–59.3) | 51 (43.5–55.3) | 56 (42.5–65.5) |
<0.001 |
| Median PT to BM,
months (IQR)a | 11 (0–33) | 34 (21.8–79.8) | 0
(0–10.8)c,e,h,j | 34 (8.75–50.3) | 23 (0–46.5) | 7 (0–25.5) | 42 (30–68.3) | 32 (18.5–34.8) | 17 (7–36) |
<0.001 |
| Median age BM,
years (IQR)a | 57 (50–65) | 49
(44.5–59.8)d,f,g | 58 (52–65) | 58 (43.8–68.3) | 60 (57–68) | 66 (60–69.5) | 62 (59.5–66) | 53 (49.5–55.5) | 59 (45.5–68.5) | 0.002 |
| Median survival,
months (IQR)a | 16 (7–33) | 27
(15.8–47.3)d,g,j | 13 (6.5–28) | 13 (6–38) | 15 (4–24) | 11 (7.5–16.5) | 20 (16–38) | 8 (7.75–12.5) | 17 (3–24) | 0.005 |
| Sex, female
(%) | 144 (55) | 44 (100) | 59 (43) | 12 (60) | 4 (26) | 3 (43) | 8 (100) | 6 (100) | 8 (34) | NA |
| BM symptoms
(%)b |
|
|
|
|
|
|
|
|
| 0.56 |
|
None | 14 (5) | 2 (4) | 9 (6) | 0 (0) | 3 (20) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
|
|
Seizure | 31 (12) | 5 (11) | 16 (12) | 3 (15) | 0 (0) | 2 (29) | 1 (13) | 0 (0) | 2 (9) |
|
|
Other | 216 (83) | 37 (84) | 114 (82) | 17 (85) | 12 (80) | 5 (71) | 7 (87) | 6 (100) | 21 (91) |
|
| BM localisation
(%)b |
|
|
|
|
|
|
|
|
| 0.46 |
|
SupraT | 178 (68) | 9 (20) | 30 (22) | 4 (20) | 2 (14) | 0 (0) | 2 (25) | 2 (33) | 5 (22) |
|
|
InfraT | 54 (20) | 29 (66) | 96 (69) | 16 (80) | 10 (66) | 6 (86) | 5 (63) | 2 (33) | 15 (65) |
|
|
Both | 29 (11) | 6 (14) | 12 (9) | 0 (0) | 3 (20) | 1 (14) | 1 (12) | 2 (34) | 3 (13) |
|
| BM number
(%)b |
|
|
|
|
|
|
|
|
| 0.48 |
|
Unique | 183 (70) | 21 (47) | 104 (75) | 14 (70) | 9 (60) | 6 (86) | 6 (75) | 2 (33) | 21 (91) |
|
| ≤3 | 246 (94) | 3 (7) | 9 (6) | 1 (5) | 1 (7) | 0 (0) | 0 (0) | 1 (17) | 1 (4) |
|
|
>3 | 15 (5) | 41 (93) | 129 (93) | 19 (95) | 14 (93) | 7 (100) | 8 (100) | 5 (83) | 22 (96) |
|
| Systemic disease
(%)b |
|
|
|
|
|
|
|
|
| 0.016 |
| No | 91 (35) | 21 (47) | 51 (37) | 6 (30) | 0 (0) | 1 (14) | 4 (50) | 1 (17) | 7 (30) |
|
|
Yes | 132 (50) | 20 (45) | 66 (48) | 11 (55) | 14 (93) | 4 (57) | 2 (25) | 4 (66) | 11 (47) |
|
| PT recurrence
(%)b |
|
|
|
|
|
|
|
|
|
<0.001 |
| No | 63 (24) | 25 (57) | 17
(12)c | 10
(50)d | 0 (0) | 2 (28) | 3 (37) | 1 (17) | 5 (22)c |
|
|
Yes | 198 (76) | 19 (43) | 121 (88) | 10 (50) | 15 (100) | 5 (71) | 5 (63) | 5 (83) | 18 (78) |
|
| PT Mol-Bio
(%)b |
|
|
|
|
|
|
|
|
|
<0.001 |
| No | 123 (47) | 1 (2)d,e,f,h,i,j | 66 (48) | 9 (45) | 7 (47) | 7 (100) | 6 (75) | 5 (83) | 22
(96)d,e |
|
|
Yes | 138 (53) | 43 (98) | 72 (52) | 11 (55) | 8 (53) | 0 (0) | 2 (25) | 1 (17) | 1 (4) |
|
Patient characteristics divided according to BM type
are summarized in Table II. A total
of 24 (10%) patients fulfilled the inclusion criteria for the dPT,
139 (53%) for the pPT and 98 (37%) for the uPT groups. Regarding BM
localization and the number of metastases, the groups did not
present any statistically significant differences. In the
univariate analysis, patients with dPT were younger [53 (IQR,
43.8–59.30 years old) vs. 57 (IQR, 52–65) in the uPT group;
P<0.033)] when the PT was diagnosed, but due to the longer time
elapsed until the BM diagnosis, the groups did not differ in terms
of age at BM diagnosis (P=0.9). The dPT patients were more
frequently female [83 vs. 59 (P=0.03) and 42% (P<0.001) in the
pPT and uPT groups, respectively]. In up to 25% of patients in the
dPT group, the clinical presentation of BM was predominantly
seizures, with a frequency 2 times higher than that in other
patients. A statistically significant difference in PT origin was
observed; breast cancer was the most common PT origin in the dPT
group (n=13; 54%), whereas lung cancer was the most common in the
uPT group (n=83; 85%). No differences were reported between the
histological and molecular subgroups for either breast or lung
cancer. The rate of PT recurrence and presence of an active
systemic disease differed between groups (P<0.01). Of note, by
definition, patients in the dPT group did not present PT recurrence
or other metastatic localizations. Thorough evaluations (e.g.
full-body CT and PET scans) did not show any metastatic
localizations, other than those in the brain, in ~1/3 of patients
from either the pPT or uPT group (n=36 and n=31, respectively). The
PT recurred in 100 (72%) patients in the pPT group. The rate of
molecular biology analysis was higher in the dPT group than in the
other groups (P<0.01).
 | Table II.Clinical characteristics on first
presentation with brain metastases according to brain metastasis
type compared by χ2, Fisher's exact or Kruskal-Wallis
tests. |
Table II.
Clinical characteristics on first
presentation with brain metastases according to brain metastasis
type compared by χ2, Fisher's exact or Kruskal-Wallis
tests.
| Parameters | dPT group | pPT group | uPT group | P-value |
|---|
| Number of patients
(%) | 24 (10) | 139 (53) | 98 (37) | NA |
| Median age PT,
years (IQR)a | 53 (43.8–59.3) | 56 (45.5–61.5) | 57
(52–65)d | 0.03 |
| Median PT to BM,
months (IQR)a | 41.5
(35.8–85.5)e,f | 22
(11–44.5)f | 0 (0) |
<0.001 |
| Median age BM,
years (IQR)a | 57 (48–64.8) | 58 (48–66) | 57 (52–65) | 0.9 |
| PT Mol-Bio
(%)c |
|
|
| <0.01 |
| No | 4 (17)e,f | 72 (51) | 47 (48) |
|
|
Yes | 20 (83) | 67 (48) | 51 (52) |
|
| Sex, female
(%)b | 20
(83)e | 82 (59) | 41
(42)d,e |
<0.001 |
| BM symptoms
(%)c |
|
|
| 0.01 |
|
None | 2 (8) | 12 (9) | 0 (0)d,e |
|
|
Seizure | 6 (25)e | 12 (9) | 13 (13) |
|
|
Other | 16 (67) | 115 (82) | 85 (86) |
|
| BM localization
(%)c |
|
|
| 0.49 |
|
InfraT | 5 (20) | 28 (20) | 21 (21) |
|
|
SupraT | 19 (79) | 94 (68) | 66 (67) |
|
|
Both | 0 (0) | 17 (12) | 11 (11) |
|
| BM number
c |
|
|
| 0.9 |
| Unique
(%) | 18 (75) | 96 (69) | 69 (70) |
|
| ≤3
(%) | 4 (16) | 34 (24) | 24 (24) |
|
| >3
(%) | 2 (8) | 9 (6) | 5 (5) |
|
| Tot
(IQR)a | 1 (1–4) | 1 (1–8) | 1 (1–13) | 0.92 |
| Systemic disease
(%)c |
|
|
|
<0.001 |
| No | 24 (100) | 36 (32) | 31 (34) |
|
|
Yes | 0 (0) | 74 (67) | 58 (66) |
|
| PT recurrence
(%)c |
|
|
|
<0.001 |
| No | 24 (100) | 39 (28) | 0 (0) |
|
|
Yes | 0 (0) | 100 (72) | 98 (100) |
|
| PT localisation
(%) |
|
|
|
|
|
Otherc | 0 (0) | 19
(14)f | 4 (4) | 0.01 |
|
Lungc | 6 (25) | 49 (35) | 83
(85)d,e |
<0.01 |
|
Mutationsc | 2 (50) | 13 (65) | 21 (43) | 0.27 |
|
Histological
subtypesc |
|
|
| 0.73 |
|
Sclc | 1 (16) | 5 (10) | 10 (12) |
|
|
Adenok | 4 (67) | 33 (67) | 60 (72) |
|
|
Squamous | 1 (16) | 7 (14) | 8 (9) |
|
|
CRCc | 0 (0) | 12 (8) | 3 (3) | 0.08 |
|
RCCc | 0 (0) | 4 (3) | 3 (3) | 0.69 |
|
Uterusc | 1 (4) | 4 (3) | 1 (1) | 0.52 |
|
Breastc | 13
(54)e | 31 (22) | 0 (0) |
<0.01 |
|
Molecular
subtypesc |
|
|
| 0.88 |
|
3neg | 5 (38) | 10 (34) | NA |
|
|
HR+/HER2+ | 2 (15) | 5 (17) | NA |
|
|
HR-/HER2+ | 2 (15) | 5 (17) | NA |
|
|
HR+/HER2- | 3 (23) | 1 (3) | NA |
|
|
Melanomac | 2 (8) | 14 (10) | 4 (4) | 0.23 |
|
Ovaryc | 2 (8) | 6 (4) | 0 (0) | 0.048 |
| BM treatment
(%)c |
|
|
| 0.92 |
|
Surgery | 0 (0) | 4 (3) | 2 (2) |
|
|
Rtp | 2 (8) | 7 (5) | 1 (1) |
|
|
Surgery+Rtp | 22 (92) | 128 (92) | 90 (92) |
|
| Median
survival, months (IQR)a | 27.5
(15.3–45.5) | 19 (7–35) | 11
(6–17)d,e |
<0.001 |
Survival analysis
Follow-up data were available for 208 patients (79%
of the entire sample); 17% of patients (n=35) were still alive at
the time of the final analysis. The results of the survival
analysis are summarized in Tables
III and SI. In the univariate
model, a longer time between PT and BM diagnoses (P=0.021), female
sex (P<0.001), breast cancer (P=0.02), presence of molecular
biology analysis (P=0.007) and treatment with neurosurgery and
radiotherapy (P=0.03) were associated with a better outcome. By
contrast, the presence of systemic disease (P=0.008), PT recurrence
(P<0.001) and older age (P=0.009) were factors that negatively
influenced prognosis.
 | Table III.Univariate and multivariate Cox
regression for survival analysis. |
Table III.
Univariate and multivariate Cox
regression for survival analysis.
|
| Overall survival
(months) | Univariate COX
regression | Multivariate Cox
regression |
|---|
|
|
|
|
|
|---|
| Parameters | median | [95% CI] | HR | [95% CI] | P-value | HR | [95% CI] | P-value |
|---|
| Sex |
|
|
|
|
|
|
|
|
| F | 21 | [18.9–27] | 0.52 | [0.38–0.72] | <0.001 | NA | NA | NA |
|
Ma | 13 | [10.9–15.1] |
|
|
|
|
|
|
| BM symptoms |
|
|
|
|
|
|
|
|
|
None | 24 |
[5.2–42.8] | 0.73 | [0.39–1.35] | 0.32 | NA | NA | NA |
|
Seizure | 18 | [12.2–23.7] | 0.84 | [0.51–1.37] | 0.49 | NA | NA | NA |
|
Othera | 16 | [12.6–19.4] |
|
|
|
|
|
|
| BM
localisation |
|
|
|
|
|
|
|
|
|
InfraT | 17 |
[3.4–30.6] | 0.58 | [0.33–1.02] | 0.23 | NA | NA | NA |
|
SupraT | 17 | [13.7–20.3] | 0.69 | [0.43–1.11] | 0.99 | NA | NA | NA |
|
Botha | 13 |
[9.5–16.5] |
|
|
|
|
|
|
| BM number |
|
|
|
|
|
|
|
|
|
Uniquea | 17 | [13.5–20.5] |
|
|
|
|
|
|
| ≤3 | 17 | [11.5–22.5] | 0.98 | [0.68–1.41] | 0.92 | NA | NA | NA |
|
>3 | 19 | [11.7–26.3] | 0.78 | [0.42–1.45] | 0.43 | NA | NA | NA |
| PT recurrence |
|
|
|
|
|
|
|
|
| No | 30 | [21.3–38.7] | 0.52 | [0.36–0.74] | <0.001 | NA | NA | NA |
|
Yesa | 14 |
[11-16.9] |
|
|
|
|
|
|
| PT Mol-Bio |
|
|
|
|
|
|
|
|
|
Noa | 13 | [10.1–15.9] |
|
|
|
|
|
|
|
Yes | 20 | [12.6–27.4] | 0.66 | [0.49–0.89] | 0.007 | NA | NA | NA |
| Systemic
disease |
|
|
|
|
|
|
|
|
| No | 20 | [11.9–28.1] | 0.62 | [0.45–0.87] | 0.008 | NA | NA | NA |
|
Yesa | 13 |
[9.9–16.1] |
|
|
|
|
|
|
| Age BM | NA | NA | 1.02 | [1.01–1.03] | 0.009 | 1.016 | [1.003–1.03] | 0.016 |
| BM treatment |
|
|
|
|
|
|
|
|
| Surgery
+ Rtp | 17 | [13.9–20.1] | 0.5 | [0.28–0.88] | 0.03 | 0.538 | [0.31–0.95] | 0.034 |
| Rtp or
surgerya | 8 |
[2.7–13.3] |
|
|
|
|
|
|
| PT
localization |
|
|
|
|
|
|
|
|
|
Breast | 34 | [24.1–43.9] | 0.64 | [0.41–0.99] | 0.02 | 0.75 | [0.50–1.13] | 0.172 |
|
Lung | 14 | [10.7–17.3] | 1.02 | [0.72–1.143] | 0.16 | NA | NA | NA |
|
Othera | 17 | [11.4–22.6] |
|
|
|
|
|
|
| dPT |
|
|
|
|
|
|
|
|
|
Yes | 28 | [17-1-38.9] | 0.58 | [0.33–0.99] | 0.048 | 0.59 | [0.34–1.02] | 0.057 |
|
Noa | 16 | [13.3–18.7] |
| [17-1-38.9] |
|
|
|
|
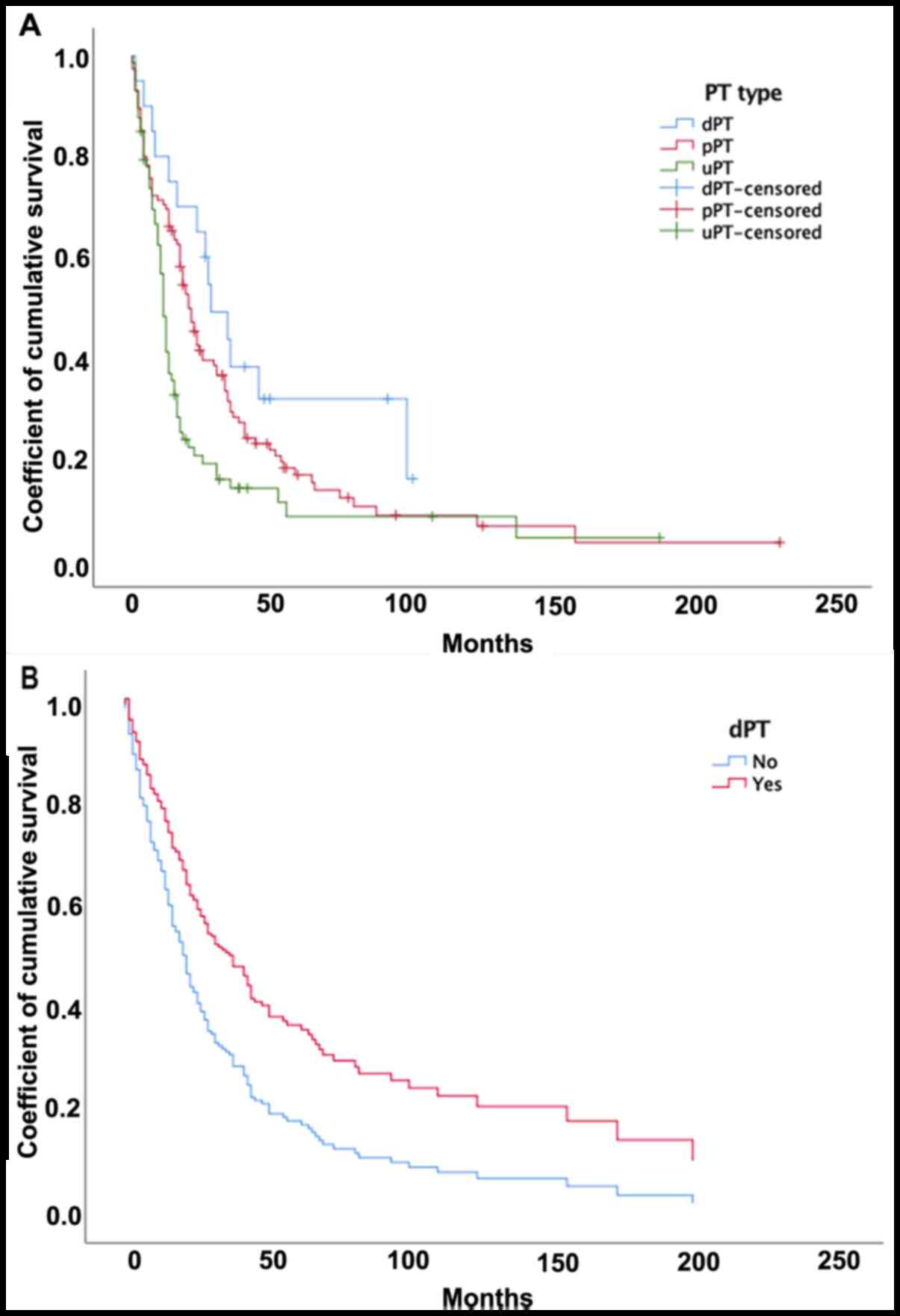
The Kaplan-Meier curves (Fig. 3) showed that patients in the dPT
group had a significantly longer survival than patients in the uPT
group [28 (IQR 15.3–45.5) vs. 11 (IQR 6–17) months; P=0.005], but
no difference was observed compared to patients in the pPT group
[19 (IQR 6–17) months; P=0.12)]. Multivariate Cox analysis
(Table III) showed that survival
was statistically correlated with a younger age at BM diagnosis,
with a 2% increase in the risk of mortality per year [hazard ratio
(HR), 1.02; P=0.016], as well as with neurosurgery and radiotherapy
treatment, which reduced the risk of mortality by ~50%, as compared
to surgery or radiotherapy alone (HR, 0.53; P=0.034). The dPT group
had a longer survival than the other groups (Fig. 3), with a 41% reduction in mortality
(HR, 0.59; P=0.057). The model failed to show improved survival for
patients with breast cancer as the PT.
Discussion
To the best of our knowledge, this was the first
study focusing specifically on the clinical characteristics and
survival of patients presenting with dormant BMs (dPT group). In
particular, in the multivariate analysis, dPT patients exhibited a
tendency, albeit not significant (P=0.057), towards an improved
outcome; dPT patients presented a median survival of 28 months and
a 5-year survival rate of 14% (vs. 16 months and 5%, respectively,
in the non-dormant group). Since there is no clinical definition of
dormant BM, the arbitrary threshold of 30 months of
progression-free disease before BM diagnosis was used, which was
derived from mathematical models developed for breast cancer
(28). Moreover, by excluding
patients with systemic disease or PT recurrence, the probability of
including BMs derived from a PT or other metastatic localizations
was reduced. Thus, even if the present study could not exclude that
BMs growing from dormant DTCs could be present in groups other than
the dPT group, the authors are confident that the current dormant
population was appropriately selected. On the other hand, this
overlap might have influenced the statistical significance of the
inter-group comparisons. Whether some specific characteristics of
dormant metastasis affect survival remains unknown.
Solitary tumour cell dormancy is defined as a
reversible non-proliferative cellular state characterized by
temporary mitotic and growth arrest (13), probably due to a delayed DTCs
adaptation to the microenvironment of the pre-metastatic site
(14). The mechanism responsible for
‘awakening’ DTC dormant cells to an actively proliferating
phenotype has not been completely elucidated, but has been found to
be dependent on the balance between intrinsic and
microenvironmental factors (29).
Following the occurrence of genetic or epigenetic mutations, such
as the activation of growth factor signalling (30) and expression of pro-angiogenetic
molecules (31), which is a required
step for proliferation (15–19), the dormant DTCs are reactivated;
however, they only grow into metastasis in the presence of a
permissive microenvironment. In addition to extracellular matrix
components or soluble ligands able to arrest proliferation
(32), brain-resident cells, such as
microglia and astrocytes, provide an environment that affects the
development of metastatic brain tumours. Early contact between DTCs
and astrocytes might lead to tumour cell death (33). However, there is accumulating
evidence that astrogliosis can provide support for tumour outgrowth
(34), and activated astrocytes can
affect microglia and T cell functions, creating an
immunosuppressive microenvironment that promotes DTC proliferation
(35). Furthermore, circulating
immune cells could put additional survival pressure on cancer cells
arrested within the cerebral capillaries prior to extravasation
(36). It could be speculated that
patients who have a more efficient immune system and exhibit a
preferential differentiation of brain-resident cells towards a
neuroprotective phenotype can more effectively control BM growth.
On the other hand, it has been demonstrated that the biology of
DTCs diverges from that of the PT or overt metastases (23); furthermore, studies have shown that,
in certain types of cancer, dissemination occurs in the early phase
or even before the PT is detectable (9). BMs growing from previously dormant DTCs
might present a milder phenotype (37) than those derived from progressing,
and thus more aggressive, PT tumours, which partially explains the
longer survival of patients with dormant metastasis. Although
certain studies have already identified the dormancy-related
genetic profiles in the PT associated with the occurrence of
delayed metastasis (15,38), the most clinically useful analyses
would focus on dormant DTC profiling (39). This approach might provide targets to
aid the development of drugs that can induce or maintain dormancy;
furthermore, follow-ups of the most frequent DTC-hosting organs,
such as a yearly evaluation of the bone marrow in patients with
breast cancer, may allow for the detection of markers of awakening
DTCs, which would necessitate therapeutic escalation (39).
With regard to BM characteristics, seizures at BM
diagnosis were more frequent in patients with dormant BM, which
could be partially due to the preponderant, albeit not significant,
supratentorial localisation of dormant BMs. Although BMs from
melanoma have been described as more likely to lead to seizures,
due to their haemorrhagic and cortical predilection (40), PT localisation did not influence BM
clinical presentation. Age negatively influenced survival in the
present sample, which was consistent with previous studies
(2,41); as a consequence, despite the lack of
statistical significance, the younger age of the dPT patients is
likely to have positively influenced their outcome. Of note, no
prognostic advantage of BMs originating from breast cancer was
observed over BMs with different PT origins (1,2,42). Since the most prevalent PT origin in
the dPT group was the breast, the fact that this location did not
influence survival reinforced the prognostic role of dormant BM. No
differences were observed in the histological subtypes of primary
cancer types (43,44), probably since the majority of the
patients included in the present study had undergone surgery,
minimizing the effect of BM from PT with a different
radiosensitivity on survival.
Molecular biology analysis of the PT was more often
available for dPT patients, and this was statistically correlated
with survival in the univariate Cox model. Despite the lack of
complete information on the medical treatment of these patients,
the use of molecular-targeted therapy, such as trastuzumab for
human epidermal growth factor receptor 2-positive breast cancers
(26), dabrafenib plus trametinib
for v-raf murine sarcoma viral oncogene homolog
B1V600-mutant melanoma (45), brigatinib for anaplastic lymphoma
kinase-positive non-small-cell lung cancer (46) or gifetinib for epidermal growth
factor receptor-positive non-small-cell lung cancer metastases
(47), which has been shown to exert
beneficial survival effects, might partially explain this finding.
As expected (48,49), the best available treatment (the
combination of neurosurgery and radiotherapy) in selected patients,
was a strong predictive factor of favourable prognosis.
The present study had several limitations. Since
only patients considered for neurosurgery or brain biopsy were
included in the study, a major source of bias in the survival
analysis could not be avoided. In fact, patients who undergo
surgery usually have a more favourable prognosis, since the
selection process for this population included criteria that
positively influence survival, such as the Karnofsky performance
status (KPS), the number of metastases and the presence of a
controlled systemic disease (50).
Although overall survival might have been overestimated, this did
not affect the statistical analysis, since all groups only included
patients who had undergone surgery. As there is no clinical
definition of dormant BM, the current study's definition of it as a
delay of 30 months between PT and BM diagnosis was questionable. In
particular, despite the fact that dormant DTCs are supposed to be
chemo-resistant (11,51), it could not be excluded that systemic
chemotherapy could have influenced progression-free-survival by
effectively limiting BM growth. Nevertheless, the same trend in
survival resulted in the multivariate survival analysis of the dPT
group including only patients presenting with a minimum of 30
months of progression-free disease under no systemic treatment (cf.
Table SII). The limited number of
patients did not allow for comparisons among all available
parameters in the multivariate models; this was the reason why no
multivariate model was built to compare differences between BM
groups. In addition, previous studies showed that the absence of
systemic disease, in the form of PT recurrence and/or metastatic
localizations in organs other than the brain, were favourable
prognostic factors for the BM population (41,52).
Since these variables were not tested in the multivariate model, it
could not be excluded that they played a role in helping the dPT
group achieve a longer survival. Due to the retrospective nature of
the present study, the KPS score was not available in all patients.
Since the KPS is a major prognostic factor (2), it could have influenced survival
analysis. Although sex was correlated with outcome in
long-surviving BM patients (53), we
preferred to include PT origin (breast) in the multivariate Cox
regression model, since these two factors were highly
correlated.
In conclusion, the results of the present study
showed that BM growing from previously dormant DTCs tends to be
associated with a better outcome than other types of BMs, possibly
due to the less aggressive phenotype of these BMs. This information
could be included in prognostication models. Moreover, the
development of therapies able to eradicate dormant DTCs could
comprise a new promising strategy to prolong survival (13).
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to express their appreciation
to Dr Dormal Giulia (Saint Luc Hospital, Catholic University of
Leuven, Belgium) and Mr. Biondi Filippo (Catholic University of
Leuven, Belgium) for their help in the statistical analysis of this
work.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FL conceived the original idea. LF, VL and LP
collected the data, performed the statistical analysis with NG, and
they all interpretated the data. LF wrote the manuscript, which was
critically revised by FL and NG. FL and LF confirm the authenticity
of all the raw data. All authors discussed the results, commented
on the manuscript and approved the final version to be published.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The ethics committee of the Erasmus Hospital (Free
University of Bruxelles, Brussels, Belgium) approved the protocol
(reference P2019/319).
Patient consent for publication
It was impossible to obtain permission for
publication from patients reported in the CASE boxes since the
patients are deceased and no further contact was possible with any
next of kin. Moreover, after verification in our institution
archives, none of the patients ever expressed any objection to
anonymous publication of personal data. For these reasons, the
present study was granted an exemption from requiring patient
consent for publication. This decision was in accordance with the
ethics committee of the Erasmus Hospital (Free University of
Bruxelles, Brussels, Belgium).
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Achrol AS, Rennert RC, Anders C, Soffietti
R, Ahluwalia MS, Nayak L, Peters S, Arvold ND, Harsh GR, Steeg PS
and Chang SD: Brain metastases. Nat Rev Dis Primer. 5:52019.
View Article : Google Scholar
|
|
2
|
Sperduto PW, Kased N, Roberge D, Xu Z,
Shanley R, Luo X, Sneed PK, Chao ST, Weil RJ, Suh J, et al: Summary
report on the graded prognostic assessment: An accurate and facile
diagnosis-specific tool to estimate survival for patients with
brain metastases. J Clin Oncol. 30:419–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lowery FJ and Yu D: Brain metastasis:
Unique challenges and open opportunities. Biochim Biophys Acta Rev
Cancer. 1867:49–57. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsukada Y, Fouad A, Pickren JW and Lane
WW: Central nervous system metastasis from breast carcinoma.
Autopsy study. Cancer. 52:2349–2354. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nayak L, Lee EQ and Wen PY: Epidemiology
of brain metastases. Curr Oncol Rep. 14:48–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tabouret E, Chinot O, Metellus P, Tallet
A, Viens P and Gonçalves A: Recent trends in epidemiology of brain
metastases: An overview. Anticancer Res. 32:4655–4662.
2012.PubMed/NCBI
|
|
7
|
Schardt JA, Meyer M, Hartmann CH, Schubert
F, Schmidt-Kittler O, Fuhrmann C, Polzer B, Petronio M, Eils R and
Klein CA: Genomic analysis of single cytokeratin-positive cells
from bone marrow reveals early mutational events in breast cancer.
Cancer Cell. 8:227–239. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hüsemann Y, Geigl JB, Schubert F, Musiani
P, Meyer M, Burghart E, Forni G, Eils R, Fehm T, Riethmüller G and
Klein CA: Systemic spread is an early step in breast cancer. Cancer
Cell. 13:58–68. 2008. View Article : Google Scholar
|
|
9
|
Harper KL, Sosa MS, Entenberg D, Hosseini
H, Cheung JF, Nobre R, Avivar-Valderas A, Nagi C, Girnius N, Davis
RJ, et al: Mechanism of early dissemination and metastasis in
Her2+ mammary cancer. Nature. 540:588–592. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rhim AD, Mirek ET, Aiello NM, Maitra A,
Bailey JM, McAllister F, Reichert M, Beatty GL, Rustgi AK,
Vonderheide RH, et al: EMT and dissemination precede pancreatic
tumor formation. Cell. 148:349–361. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Aguirre-Ghiso JA: Models, mechanisms and
clinical evidence for cancer dormancy. Nat Rev Cancer. 7:834–846.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goss PE and Chambers AF: Does tumour
dormancy offer a therapeutic target? Nat Rev Cancer. 10:871–877.
2010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sosa MS, Bragado P and Aguirre-Ghiso JA:
Mechanisms of disseminated cancer cell dormancy: An awakening
field. Nat Rev Cancer. 14:611–622. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Giancotti FG: Mechanisms governing
metastatic dormancy and reactivation. Cell. 155:750–764. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao H, Chakraborty G, Lee-Lim AP, Mo Q,
Decker M, Vonica A, Shen R, Brogi E, Brivanlou AH and Giancotti FG:
The BMP inhibitor coco reactivates breast cancer cells at lung
metastatic sites. Cell. 150:764–779. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Connell JT, Sugimoto H, Cooke VG,
MacDonald BA, Mehta AI, LeBleu VS, Dewar R, Rocha RM, Brentani RR,
Resnick MB, et al: VEGF-A and Tenascin-C produced by S100A4+
stromal cells are important for metastatic colonization. Proc Natl
Acad Sci USA. 108:16002–16007. 2011. View Article : Google Scholar
|
|
17
|
Gao D, Nolan DJ, Mellick AS, Bambino K,
McDonnell K and Mittal V: Endothelial progenitor cells control the
angiogenic switch in mouse lung metastasis. Science. 319:195–198.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aguirre-Ghiso JA, Ossowski L and Rosenbaum
SK: Green fluorescent protein tagging of extracellular
signal-regulated kinase and p38 pathways reveals novel dynamics of
pathway activation during primary and metastatic growth. Cancer
Res. 64:7336–7345. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tsai JH, Donaher JL, Murphy DA, Chau S and
Yang J: Spatiotemporal regulation of epithelial-mesenchymal
transition is essential for squamous cell carcinoma metastasis.
Cancer Cell. 22:725–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Q, Zhang H, Jiang X, Qian C, Liu Z and
Luo D: Factors involved in cancer metastasis: A better
understanding to ‘seed and soil’ hypothesis. Mol Cancer.
16:1762017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Demicheli R, Biganzoli E, Boracchi P,
Greco M and Retsky MW: Recurrence dynamics does not depend on the
recurrence site. Breast Cancer Res. 10:R832008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Retsky MW, Demicheli R, Swartzendruber DE,
Bame PD, Wardwell RH, Bonadonna G, Speer JF and Valagussa P:
Computer simulation of a breast cancer metastasis model. Breast
Cancer Res Treat. 45:193–202. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Klein CA: Selection and adaptation during
metastatic cancer progression. Nature. 501:365–372. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bilous M, Serdjebi C, Boyer A, Tomasini P,
Pouypoudat C, Barbolosi D, Barlesi F, Chomy F and Benzekry S:
Quantitative mathematical modeling of clinical brain metastasis
dynamics in non-small cell lung cancer. Sci Rep. 9:130182019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun YW, Xu J, Zhou J and Liu WJ: Targeted
drugs for systemic therapy of lung cancer with brain metastases.
Oncotarget. 9:5459–5472. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leyland-Jones B: Human epidermal growth
factor receptor 2-positive breast cancer and central nervous system
metastases. J Clin Oncol. 27:5278–5286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Piura E and Piura B: Brain Metastases from
Ovarian Carcinoma. ISRN Oncol. 5274532011.PubMed/NCBI
|
|
28
|
Demicheli R, Retsky MW, Hrushesky WJ and
Baum M: Tumor dormancy and surgery-driven interruption of dormancy
in breast cancer: Learning from failures. Nat Clin Pract Oncol.
4:699–710. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Neophytou CM, Kyriakou TC and Papageorgis
P: Mechanisms of metastatic tumor dormancy and implications for
cancer therapy. Int J Mol Sci. 20:61582019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aguirre-Ghiso JA, Liu D, Mignatti A,
Kovalski K and Ossowski L: Urokinase receptor and fibronectin
regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine
carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell.
12:863–879. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yeh AC and Ramaswamy S: Mechanisms of
cancer cell dormancy-another hallmark of cancer? Cancer Res.
75:5014–5022. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ghajar CM, Peinado H, Mori H, Matei IR,
Evason KJ, Brazier H, Almeida D, Koller A, Hajjar KA, Stainier DY,
et al: The perivascular niche regulates breast tumour dormancy. Nat
Cell Biol. 15:807–817. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Valiente M, Obenauf AC, Jin X, Chen Q,
Zhang XHF, Lee DJ, Chaft JE, Kris MG, Huse JT, Brogi E and Massagué
J: Serpins promote cancer cell survival and vascular co-option in
brain metastasis. Cell. 156:1002–1016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schulz M, Salamero-Boix A, Niesel K,
Alekseeva T and Sevenich L: Microenvironmental regulation of tumor
progression and therapeutic response in brain metastasis. Front
Immunol. 10:17132019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Priego N, Zhu L, Monteiro C, Mulders M,
Wasilewski D, Bindeman W, Doglio L, Martínez L, Martínez-Saez E,
Ramón Y, et al: STAT3 labels a subpopulation of reactive astrocytes
required for brain metastasis. Nat Med. 24:1024–1035. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lorger M and Felding-Habermann B:
Capturing changes in the brain microenvironment during initial
steps of breast cancer brain metastasis. Am J Pathol.
176:2958–2971. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schmidt-Kittler O, Ragg T, Daskalakis A,
Granzow M, Ahr A, Blankenstein TJF, Kaufmann M, Diebold J, Arnholdt
H, Muller P, et al: From latent disseminated cells to overt
metastasis: Genetic analysis of systemic breast cancer progression.
Proc Natl Acad Sci USA. 100:7737–7742. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kobayashi A, Okuda H, Xing F, Pandey PR,
Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, et al:
Bone morphogenetic protein 7 in dormancy and metastasis of prostate
cancer stem-like cells in bone. J Exp Med. 208:2641–2655. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Aguirre-Ghiso JA, Bragado P and Sosa MS:
Metastasis awakening: Targeting dormant cancer. Nat Med.
19:276–277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Noh T and Walbert T: Brain metastasis:
Clinical manifestations, symptom management, and palliative care.
Handb Clin Neurol. 149:75–88. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
D'Ambrosio AL and Agazzi S: Prognosis in
patients presenting with brain metastasis from an undiagnosed
primary tumor. Neurosurg Focus. 22:E72007. View Article : Google Scholar
|
|
42
|
Rostami R, Mittal S, Rostami P, Tavassoli
F and Jabbari B: Brain metastasis in breast cancer: A comprehensive
literature review. J Neurooncol. 127:407–414. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kuremsky JG, Urbanic JJ, Petty WJ, Lovato
JF, Bourland JD, Tatter SB, Ellis TL, McMullen KP, Shaw EG and Chan
MD: Tumor histology predicts patterns of failure and survival in
patients with brain metastases from lung cancer treated with gamma
knife radiosurgery. Neurosurgery. 73:641–647. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vern-Gross TZ, McMullen KP, Case LD,
Bourland JD, Ellis TL, Lawrence JA, Tatter SB, Shaw EG, Urbanic JJ
and Chan MD: Patterns of failure in patients receiving gamma knife
radiosurgery for breast carcinoma to the brain. Int J Radiat Oncol.
81 (Suppl):S216–S217. 2011. View Article : Google Scholar
|
|
45
|
Davies MA, Saiag P, Robert C, Grob JJ,
Flaherty KT, Arance A, Chiarion-Sileni V, Thomas L, Lesimple T,
Mortier L, et al: Dabrafenib plus trametinib in patients with
BRAFV600-mutant melanoma brain metastases (COMBI-MB): A
multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol.
18:863–873. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kim DW, Tiseo M, Ahn MJ, Reckamp KL,
Hansen KH, Kim SW, Huber RM, West HL, Groen HJM, Hochmair MJ, et
al: Brigatinib in patients with crizotinib-refractory anaplastic
lymphoma kinase-positive non-small-cell lung cancer: A randomized,
multicenter phase II trial. J Clin Oncol. 35:2490–2498. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Iuchi T, Shingyoji M, Sakaida T, Hatano K,
Nagano O, Itakura M, Kageyama H, Yokoi S, Hasegawa Y, Kawasaki K
and Iizasa T: Phase II trial of gefitinib alone without radiation
therapy for Japanese patients with brain metastases from
EGFR-mutant lung adenocarcinoma. Lung Cancer. 82:282–287. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Patchell RA, Tibbs PA, Walsh JW, Dempsey
RJ, Maruyama Y, Kryscio RJ, Markesbery WR, Macdonald JS and Young
B: A randomized trial of surgery in the treatment of single
metastases to the brain. N Engl J Med. 322:494–500. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lin X and DeAngelis LM: Treatment of brain
metastases. J Clin Oncol. 33:3475–3484. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Soffietti R, Abacioglu U, Baumert B, Combs
SE, Kinhult S, Kros JM, Marosi C, Metellus P, Radbruch A, Freixa
SSV, et al: Diagnosis and treatment of brain metastases from solid
tumors: Guidelines from the european association of neuro-oncology
(EANO). Neuro Oncol. 19:162–174. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ekici K, Temelli O, Dikilitas M, Halil
Dursun I, Bozdag Kaplan N and Kekilli E: Survival and prognostic
factors in patients with brain metastasis: Single center
experience. J BUON. 21:958–963. 2016.PubMed/NCBI
|
|
53
|
Chao ST, Barnett GH, Liu SW, Reuther AM,
Toms SA, Vogelbaum MA, Videtic GMM and Suh JH: Five-year survivors
of brain metastases: A single-institution report of 32 patients.
Int J Radiat Oncol Biol Phys. 66:801–809. 2006. View Article : Google Scholar : PubMed/NCBI
|