Introduction
Among the most common types of cancer, gastric
cancer (GC) was the second leading cause of cancer-associated
mortality worldwide until 2018, with ~1 million new cases of GC and
782,685 GC-associated mortalities occurring in 2018 (1). Radical surgery remains the most
reliable treatment for GC (2). Due
to advancements in surgical techniques, chemotherapy and
radiotherapy, the 5-year survival rate of patients with early stage
GC is >95% (2). However, early
stage GC is often asymptomatic and rarely detected, and thus,
>70% of patients with GC eventually develop advanced GC and may
be ineligible for surgical resection (2). Thus, it is important to identify novel
biomarkers associated with GC to improve the diagnosis of this
deadly disease.
Long non-coding RNAs (lncRNAs) are RNA molecules
that are >200 nucleotides in length, lack protein coding
potential, and can regulate the migration, survival and
proliferation of cancer cells (3,4). Several
studies have reported that specific lncRNAs are dysregulated in GC,
including urothelial cancer-associated 1 (5), antisense RNA in the INK4 locus
(6) and AC093818.1 (7). At the functional level, lncRNAs serve
as competing endogenous RNAs (ceRNAs) that bind to specific
microRNAs (miRNAs/miRs) in a competitive manner, thereby
upregulating the expression levels of miRNA target genes (8). Several studies have demonstrated the
interactions between lncRNAs, miRNAs and mRNAs, which influence the
development and progression of different types of cancer. For
example, lncRNA HNF1A antisense RNA 1 binds to miR-30b-3p in GC,
which promotes activation of the PI3K/AKT signaling pathway
(9). In addition, lncRNA DCST1
antisense RNA 1 regulates the survival, proliferation and invasion
of GC cells by competitively binding and sequestering miR-605-3p
(10). It has been reported that
lncRNA metallothionein 1J pseudogene can control F-box and WD
repeat domain containing 7 expression by competitively targeting
miR-92a-3p in GC cells (11). Thus,
broader analyses of lncRNA-miRNA-mRNA ceRNA networks may enable
researchers to further understand complex GC-associated gene
interactions. In addition, a ceRNA model can offer unique insights
to understand the putative roles of several uncharacterized lncRNAs
in GC development and progression.
In the present study, an integrated systematic
analysis of lncRNAs and mRNA expression patterns in datasets of
patients with GC was performed. Multiple publicly available Gene
Expression Omnibus (GEO) datasets (GSE58828, GSE72305 and GSE99416)
were normalized and integrated to identify genes that were
differentially expressed between tumor tissues and adjacent normal
tissues from patients with GC. In addition, The Cancer Genome Atlas
(TCGA) database was used to confirm the differential expression
profiles of lncRNAs in GC. A ceRNA network was constructed using
these data. Gene Ontology (GO) and protein-protein interaction
(PPI) analyses of mRNAs in the GC-related ceRNA network were
performed to further investigate the association between the
lncRNAs and GC in the ceRNA network. Receiver operating
characteristic (ROC) curve analysis was performed to determine the
diagnostic value of the central lncRNAs. Subsequently, associations
with clinicopathological characteristics were assessed, and gene
set enrichment analysis (GSEA) of the central lncRNAs was
performed.
The present study aimed to identify novel biomarkers
for the diagnosis of GC and provide insight into the mechanistic
basis of GC development and progression.
Materials and methods
Microarray datasets
Studies from the GEO database (https://www.ncbi.nlm.nih.gov/geo) were considered
eligible according to the following criteria: i) Studies with GC
tissue samples; ii) studies with information on technology and
platform used for studies. Based on these criteria, the GSE58828,
GSE72305 (12) and GSE99416
(13) GC datasets were downloaded
from the GEO database (14). Details
of each microarray study are presented in Table I. GC RNA expression profile data were
obtained from TCGA database (http://tcga-data.nci.nih.gov) (15). An overview of the bioinformatics
analysis performed to assess the GEO and TCGA datasets is presented
in Fig. S1.
 | Table I.Details of gastric cancer studies and
associated microarray datasets from the Gene Expression Omnibus
database. |
Table I.
Details of gastric cancer studies and
associated microarray datasets from the Gene Expression Omnibus
database.
|
|
| Sample size, n |
|
|
| Sex, n |
|---|
|
|
|
|
|
|
|
|
|---|
| Series accession
no. | Platform | Total | Tumor | Adjacent | DEGs, n | Upregulated, n | Downregulated,
n | Female | Male |
|---|
| GSE58828 | GPL15314 | 6 | 3 | 3 | 9,121 | 4,869 | 4,252 | 2 | 4 |
| GSE72305 | GPL15314 | 12 | 10 | 2 | 324 | 108 | 216 | NA | NA |
| GSE99416 | GPL16956 | 12 | 6 | 6 | 2,651 | 1,261 | 1,390 | 4 | 8 |
Differential expression analysis
All three datasets were initially integrated to
increase the overall sample numbers (19 tumor samples and 11 normal
samples), and introduction of unreliable results was avoided by
batch normalizing the three datasets, using the sva package
(16) (version 3.38.0; http://www.bioconductor.org/packages/release/bioc/html/sva.html)
and limma package (17) (version
3.46.0; http://bioconductor.org/packages/release/bioc/html/limma.html)
in R software (version 3.5.1; www.R-project.org). Subsequently, tumor and normal
tissue samples were compared using the limma package in R software
(17), and differential RNA
expression was detected using the following criteria: |log
fold-change (FC)|>1 and adjusted P<0.05. A volcano map was
constructed using the pheatmap package (version 1.0.12; http://cran.r-project.org/web/packages/pheatmap/index.html)
in R software.
ceRNA network construction
A ceRNA network was constructed to visualize
lncRNA-miRNA-mRNA interactions, based on the ceRNA model wherein
lncRNAs can bind and sequester miRNAs, thereby altering their
ability to influence mRNA translation (8). The miRcode database (version 11;
http://www.mircode.org) (18) was used to identify intersecting
lncRNA target miRNAs, while miRDB (version 5.0; http://mirdb.org), TargetScan 7.2 (version 7.2;
http://www.targetscan.org) (19) and miRTarBase (version 7.0; http://mirtarbase.cuhk.edu.cn/php/download.php)
(20) were used to predict
interactions between miRNAs and mRNAs.
Genes identified in at least two databases were
considered miRNA targets. The target mRNAs were further intersected
with dysregulated mRNAs in GEO GC samples. Subsequently, the ceRNA
network was constructed by combining the lncRNA-miRNA interactomes
and miRNA-mRNA interactomes, which was visualized using Cytoscape
software (version 3.6.1; http://cytoscape.org) (21).
GO and PPI analysis
To assess the functional relevance of differentially
expressed lncRNAs in the present study, GO analysis of the
molecular functions, cellular components and biological processes,
for which these genes were enriched, was performed using Webgestalt
2019 (http://www.webgestalt.org) (22). In addition, PPI network data of the
mRNAs were collected from the Search Tool for the Retrieval of
Interacting Genes (STRING) database (version 10.0; http://string-db.org) (23), and the PPI network was constructed
using Cytoscape software.
ROC curve analysis
ROC curve analysis was performed to determine the
diagnostic values of the differentially expressed lncRNAs in the
ceRNA network, using the survival ROC package (version 1.66.0;
http://bioconductor.org/packages/ROC)
in R software. An area under the curve (AUC) value of >0.50
indicated evidence for diagnosis.
Correlations between lncRNA expression
and clinicopathological characteristics
To further evaluate the clinical value of the
central lncRNAs in GC, the associations between lncRNA expression
and clinicopathological characteristics, including age, sex, grade,
stage, T classification, N classification, M classification of TCGA
data were analyzed using the χ2 test. Unpaired Student's
t-test was used to compare differences between two groups. The
tumor grading system was based on the Goseki histological grading
of GC (24).
GSEA
For GSEA, cut-off values for each lncRNA were
determined based on the median expression levels in the TCGA-STAD
database (https://portal.gdc.cancer.gov/projects/TCGA-STAD).
The ‘c2.cp.kegg.v7.0.symbols.gmt’ dataset was used for 1,000 gene
set permutations per analysis for each lncRNA, using GSEA software
(version 4.0.1; http://software.broadinstitute.org/gsea/downloads.jsp).
The present study focused on pathways with nominal P-value <0.05
and selected the most significantly enriched signaling pathways
based on their size >50. The results were generated using the
ggplot2 package (version 3.3.3; http://CRAN.R-project.org/package=ggplot2) in R
software.
Tissue sample collection
A total of 15 GC tumor tissues and paired adjacent
normal tissues (5 cm away from the tumor margin) were collected
from patients with GC who underwent radical primary tumor excision
at The First Affiliated Hospital of Chongqing Medical University
(Chongqing, China) between December 2016 and January 2019. The
inclusion criteria were as follows: i) Patients had received an
initial diagnosis of GC according to the results of gastroscopy and
pathological biopsy; ii) all patients with GC were evaluated
according to TNM stage (25) and
iii) there were no significant differences in blood routine test
indexes, liver function indexes and renal function indexes of all
patients with GC. The exclusion criteria were as follows: i)
patients with poor general condition and unable to tolerate related
examinations; ii) concurrently diagnosed with other tumors and
patients with secondary GC and iii) patients with GC who had
received radiotherapy, chemotherapy or other treatments prior to
surgery. The patients included 9 men and 6 women, with a mean age
of 64.6±4.9 years (age range, 50–75 years). Tissue samples were
stored at −80°C until subsequent experimentation. The present study
was approved by the Research Ethics Committee of the First
Affiliated Hospital of Chongqing Medical University (approval no.
2016140) and written informed consent was provided by all patients
prior to the study start.
RT-qPCR
A total of 15 pairs of tumor tissues and adjacent
normal tissues from patients with GC were used for RT-qPCR
validation. Total RNA was extracted from tissues using
TRIzol® reagent (Tiangen Biotech Co., Ltd.). The
concentration of RNA was measured using a Nanodrop 2000 instrument
(Bio-Rad Laboratories, Inc.). Total RNA (500 ng RNA) was reverse
transcribed into cDNA using the PrimeScript RT Reagent kit with
gDNA Eraser (Takara Biotechnology Co., Ltd.). qPCR was subsequently
performed using specific primers (TsingKe Biological Technology)
and TB Green® Premix ExTaq™ II (Takara Biotechnology
Co., Ltd.). The following primer sequences were used for qPCR:
LINC00501 forward, 5′-GAACAATGACCGGGGAACAG-3′ and reverse,
5′-TTCTTCCTTTGTGCTTCCGC-3′; LINC00365 forward,
5′-AGCTGCTCATCCTTCCTCAG-3′ and reverse, 5′-ACACAGGTGCCAAAATCCAC-3′;
SOX21-AS1 forward, 5′-GAGGTGCTGCAGGAGAGTTA-3′ and reverse,
5′-ACTCTCCACTCGCCTAAACC-3′; DLEU7-AS1 forward,
5′-AACAAATTTGGGGCACTGCT-3′ and reverse, 5′-CACCAAAGCACGGAAGGTAG-3′;
and GK-IT1 forward, 5′-CTGAGGTTGGGAGTTCGAGAC-3′ and reverse,
5′-GGATTACAGGCATGAGCCAC-3′; and GAPDH forward,
5′-GGTCTCCTCTGACTTCAACA-3′ and reverse, 5′-GTGAGGGTCTCTCTCTTCCT-3′.
The temperature protocol for RT was as follows: 37°C for 15 min,
85°C for 5 sec and 4°C storage. The resulting cDNA product was
stored at −20°C. The following thermocycling conditions were used
for qPCR: Initial denaturation at 95°C for 3 min, followed by 40
cycles of 95°C for 1 sec and 58°C for 30 sec. lncRNA expression
levels were calculated using the 2−∆∆Cq method (26) and normalized to the internal
reference gene GAPDH.
Statistical analysis
Statistical analysis was performed using R software
(version 3.5.1; www.R-project.org). All experiments were performed in
triplicate and data are presented as the mean ± standard deviation.
Paired Student's t-test was used to compare differences between
lncRNA expression levels in tumor tissues and adjacent normal
tissues. P<0.05 was considered to indicate a statistically
significant difference.
Results
Pooled analysis of GC-related gene
expression profiles in the GEO datasets
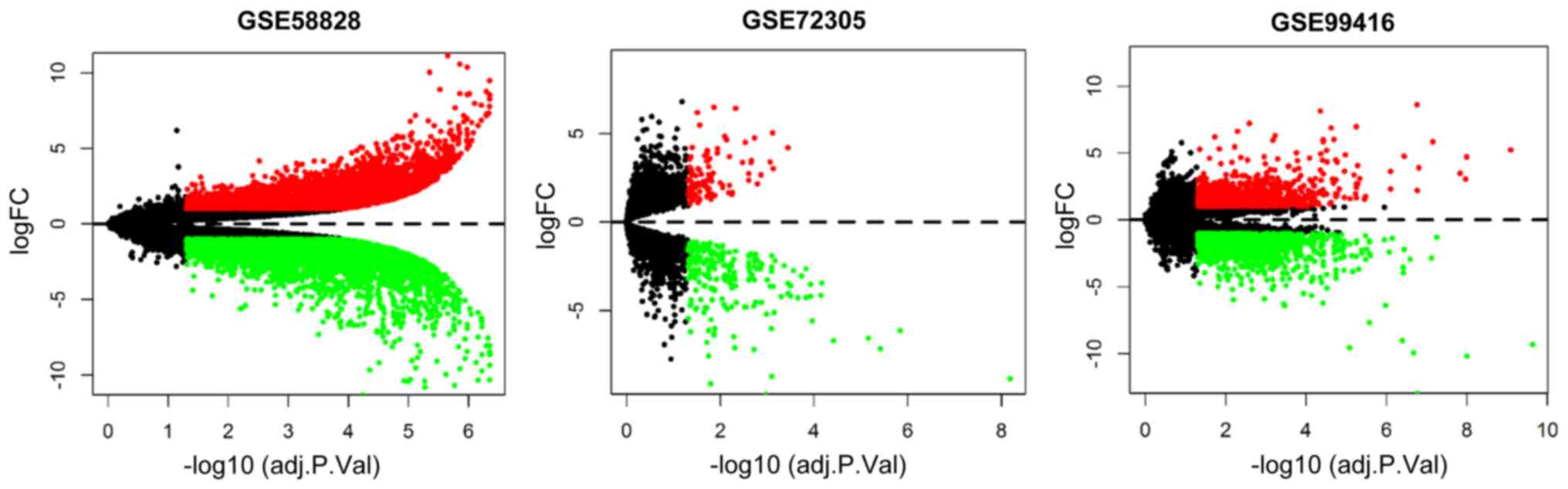
Following reannotation, 25,476, 22,053 and 32,386
genes were obtained in the GSE58828 (GPL15314), GSE72305 (GPL15314)
and GSE99416 (GPL16956) datasets, respectively. Genes that were
differentially expressed between GC tissues and normal tissues
(|log FC|>1 and false discovery rate <0.05) were identified
using the limma package. In total, 9,121 genes were differentially
expressed in the GSE58828 dataset (4,869 upregulated genes and
4,252 downregulated genes), while 324 genes were differentially
expressed in the GSE72305 dataset (108 upregulated genes and 216
downregulated genes) and 2,651 genes were differentially expressed
in the GSE99416 dataset (1,261 upregulated genes and 1,390
downregulated genes). These differentially expressed genes are
presented in volcano plots in Fig.
1. Following batch normalization, a total of 465 genes were
differentially expressed between GC tissues and normal tissues.
Overall, the present study identified 48 lncRNAs and 175 mRNAs that
were upregulated, and 45 lncRNAs and 197 mRNAs that were
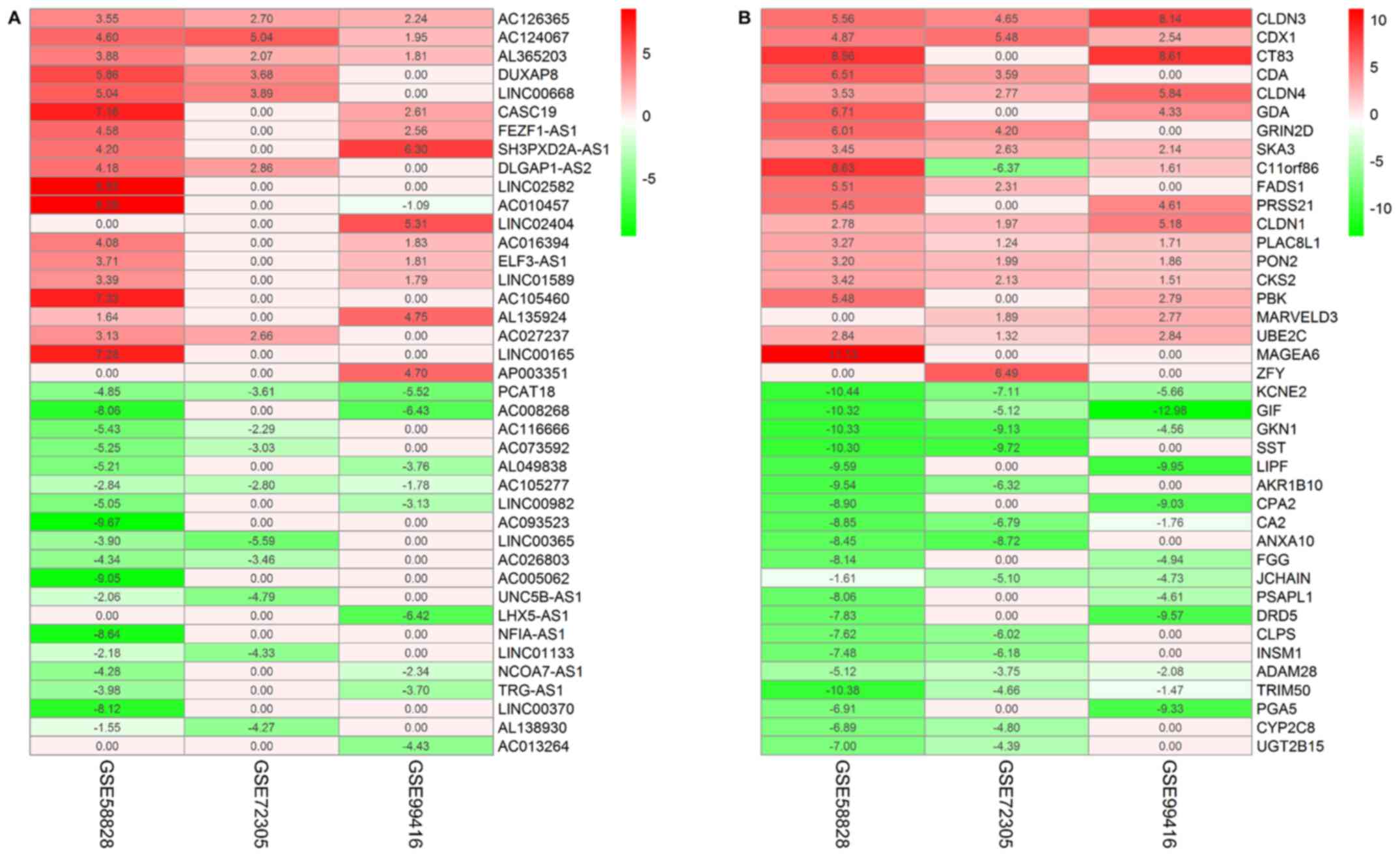
downregulated in tumor tissues. The top 20 upregulated and
downregulated lncRNAs (Fig. 2A) and
mRNAs (Fig. 2B) in these datasets
were identified, and further information regarding all the lncRNAs
and mRNAs are presented in Tables
SI and SII.
TCGA-based validation of differential
lncRNA expression
GC sample RNA expression profiles were downloaded
from TCGA database to validate the differential lncRNA expression
profiles detected in the GEO dataset analysis. Comparisons of the
GEO and TCGA datasets identified 25 differentially expressed
lncRNAs (Table II).
 | Table II.Differentially expressed long
non-coding RNAs in the Gene Expression Omnibus and The Cancer
Genome Atlas databases. |
Table II.
Differentially expressed long
non-coding RNAs in the Gene Expression Omnibus and The Cancer
Genome Atlas databases.
| lncRNA | Regulation | logFC | Adjusted
P-value |
|---|
| PGM5-AS1 | Down | −3.601 | <0.001 |
| LHX5-AS1 | Down | −4.367 | <0.001 |
| LINC02163 | Up | 5.976 | <0.001 |
| DUXAP8 | Up | 3.582 | <0.001 |
| LINC00982 | Down | −2.251 | <0.001 |
| PCAT18 | Down | −3.098 | <0.001 |
| DLEU7-AS1 | Up | 2.599 | <0.001 |
| LINC00582 | Down | −2.196 | <0.001 |
| FEZF1-AS1 | Up | 4.654 | <0.001 |
| DLGAP1-AS2 | Up | 2.174 | <0.001 |
| LINC00365 | Down | −2.210 | <0.001 |
| NCOA7-AS1 | Down | −2.018 | <0.001 |
| LINC02404 | Down | −2.802 | <0.001 |
| LINC02447 | Down | −1.205 | <0.001 |
| SOX21-AS1 | Down | −2.296 | <0.001 |
| CASC19 | Up | 2.354 | <0.001 |
| UNC5B-AS1 | Down | −1.660 | <0.001 |
| LINC00501 | Up | 2.184 | <0.001 |
| LINC01133 | Down | −1.296 | <0.001 |
| LINC00853 | Up | 1.141 | <0.001 |
| LINC01985 | Down | −1.304 | <0.001 |
| GK-IT1 | Up | 1.337 | <0.001 |
| HRAT92 | Up | 1.366 | <0.001 |
| LINC01589 | Down | −1.061 | <0.001 |
| PICSAR | Up | 1.947 | <0.001 |
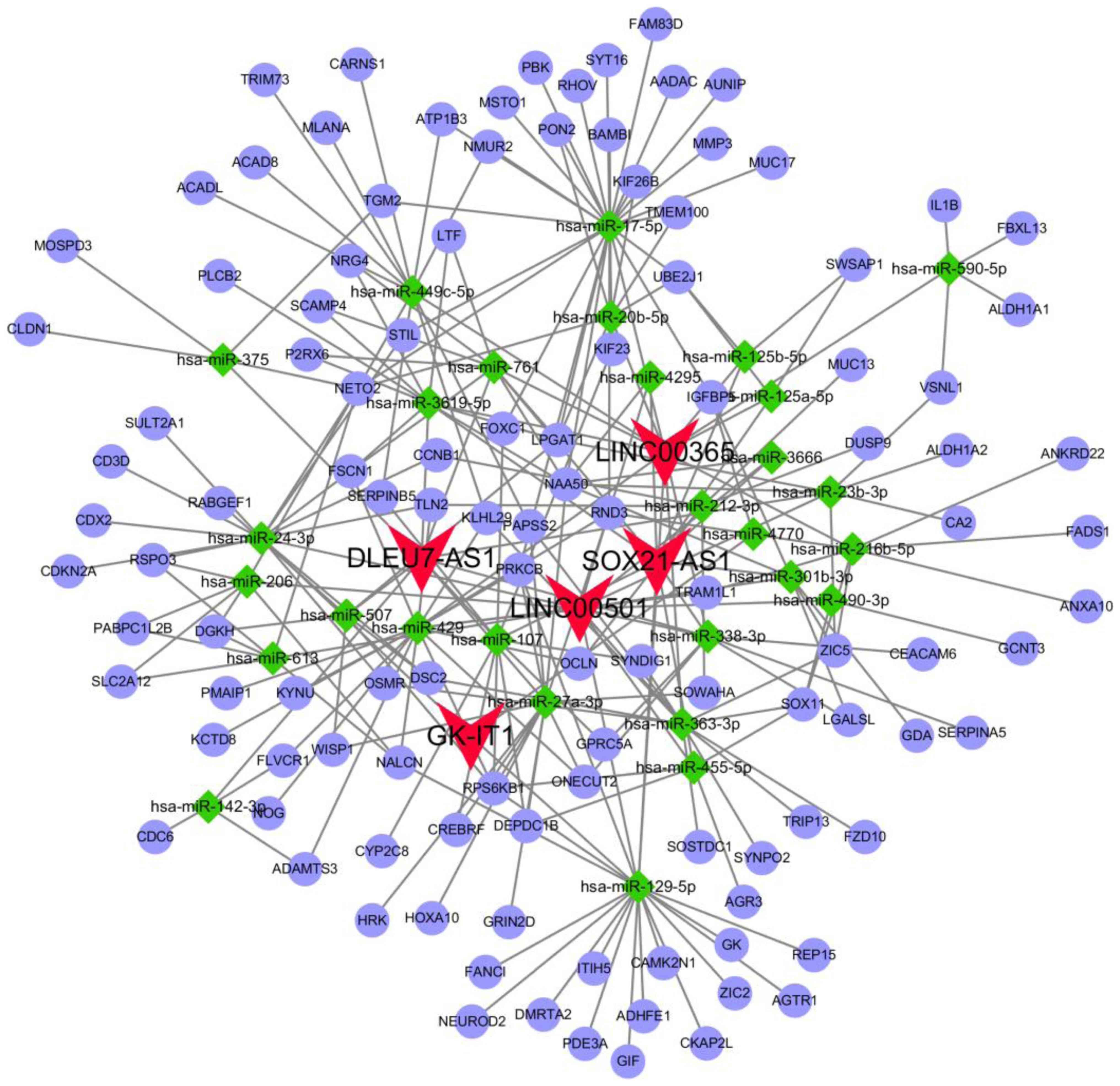
ceRNA network
Based on the lncRNA-miRNA, circRNA-miRNA and
miRNA-mRNA predicted interactions, a ceRNA network was constructed.
The network incorporated five lncRNAs (LINC00501, LINC00365,
SOX21-AS1, GK-IT1 and DLEU7-AS1), 29 miRNAs and 114 mRNAs (Fig. 3). Overall, 29 specific miRNAs were
predicted to target five specific lncRNAs (Table III), and 26 specific miRNAs
(hsa-miR-125b-5p, hsa-miR-129-5p and hsa-miR-455-5p did not target
mRNAs from the intersecting specific mRNAs) were identified to
interact with the 114 intersecting mRNAs (Table IV).
 | Table III.Specific lncRNAs that target specific
miRNAs. |
Table III.
Specific lncRNAs that target specific
miRNAs.
| lncRNAs | miRNAs |
|---|
| LINC00501 | miR-301b-3p,
miR-4295, miR-3666, miR-206, miR-613, miR-429, miR-23b-3p,
miR-24-3p, miR-363-3p, miR-338-3p, miR-455-5p, miR-129-5p,
miR-490-3p |
| LINC00365 | miR-17-5p,
miR-20b-5p, miR-429, miR-590-5p, miR-761, miR-3619-5p, miR-216b-5p,
miR-363-3p, miR-338-3p, miR-449c-5p, miR-125a-5p, miR-125b-5p,
miR-129-5p |
| SOX21-AS1 | miR-301b-3p,
miR-4295, miR-3666, miR-212-3p, miR-761, miR-3619-5p, miR-107,
miR-338-3p, miR-125a-5p, miR-125b-5p, miR-455-5p, miR-129-5p |
| GK-IT1 | miR-4770,
miR-24-3p, miR-129-5p |
| DLEU7-AS1 | miR-507,
miR-142-3p, miR-761, miR-3619-5p, miR-23b-3p, miR-27a-3p, miR-107,
miR-338-3p, miR-375 |
 | Table IV.Specific miRNAs that target specific
mRNAs. |
Table IV.
Specific miRNAs that target specific
mRNAs.
| miRNAs | mRNAs |
|---|
| miR-107 | CYP2C8, DEPDC1B,
FOXC1, CREBRF, GPRC5A, SYNDIG1, SERPINB5, KIF23, RPS6KB1, |
| miR-125a-5p | SWSAP1, UBE2J1,
SWSAP1, UBE2J1, AGTR1, DMRTA2, GK, ADHFE1, ITIH5, RPS6KB1, CAMK2N1,
REP15, NALCN, PDE3A, CKAP2L, GIF, NEUROD2, FANCI, PRKCB, ZIC2 |
| miR-142-3p | ADAMTS3, CDC6,
FLVCR1 |
| miR-17-5p | PRKCB, KIF23,
MSTO1, TMEM100, NAA50, LPGAT1, PBK, SYT16, AUNIP, ATP1B3, RHOV,
BAMBI, MUC17, MMP3, UBE2J1, AADAC, RND3, STIL, NETO2, KIF26B,
FOXC1, IGFBP5, PON2, TGM2, FAM83D, NMUR2 |
| miR-206 | RSPO3, SLC2A12,
NETO2, PABPC1L2B, NALCN, DGKH |
| miR-20b-5p | NETO2, KIF26B,
KIF23, TMEM100, LPGAT1, BAMBI, PON2, UBE2J1 |
| miR-212-3p | SOWAHA, TLN2, OCLN,
MUC13, CCNB1, DUSP9 |
| miR-216b-5p | ANKRD22, ZIC5,
FADS1, SOX11, RND3, ANXA10, TRAM1L1 |
| miR-23b-3p | VSNL1, NAA50,
LPGAT1, ALDH1A2, ZIC5, CA2 |
| miR-24-3p | CCNB1, RSPO3,
FSCN1, SULT2A1, CDX2, CD3D, STIL, NETO2, CDKN2A, DSC2, TLN2,
OSMR |
| miR-27a-3p | GRIN2D, PRKCB, HRK,
RPS6KB1, CREBRF, WISP1, RND3, HOXA10, PAPSS2, NAA50, SOWAHA,
DEPDC1B, ONECUT2 |
| miR-301b-3p | GDA, LGALSL, ZIC5,
IGFBP5, NAA50 |
| miR-338-3p | ONECUT2, GPRC5A,
LGALSL, SERPINA5, CEACAM6 |
| miR-3619-5p | NAA50, LPGAT1,
PLCB2, P2RX6, SCAMP4, LTF, NRG4, FSCN1 |
| miR-363-3p | FZD10, OSMR, DSC2,
KLHL29, SYNDIG1, PAPSS2, SOX11, TRIP13, ZIC5, SOSTDC1, SYNPO2 |
| miR-3666 | NAA50 |
| miR-375 | NETO2, CLDN1, TGM2,
MOSPD3 |
| miR-429 | ADAMTS3, NOG, RND3,
OCLN, TRAM1L1, NALCN, FLVCR1, FSCN1, KLHL29, PRKCB, ONECUT2, KCTD8,
TLN2, RPS6KB1, PMAIP1, KYNU |
| miR-4295 | NAA50 |
| miR-449c-5p | PAPSS2, ACADL,
NMUR2, TRIM73, ATP1B3, CARNS1, MLANA, ACAD8, SERPINB5, KLHL29,
NETO2, RPS6KB1, DEPDC1B, SOX11, AGR3 |
| miR-4770 | IGFBP5 |
| miR-490-3p | SOX11, GCNT3 |
| miR-507 | DGKH, DSC2,
RPS6KB1, LPGAT1, WISP1, RABGEF1 |
| miR-590-5p | FBXL13, VSNL1,
IL1B, ALDH1A1 |
| miR-613 | DGKH, RSPO3, NALCN,
NETO2, PABPC1L2B, SLC2A12 |
| miR-761 | SCAMP4, NAA50,
FSCN1, P2RX6, NRG4, LTF, LPGAT1 |
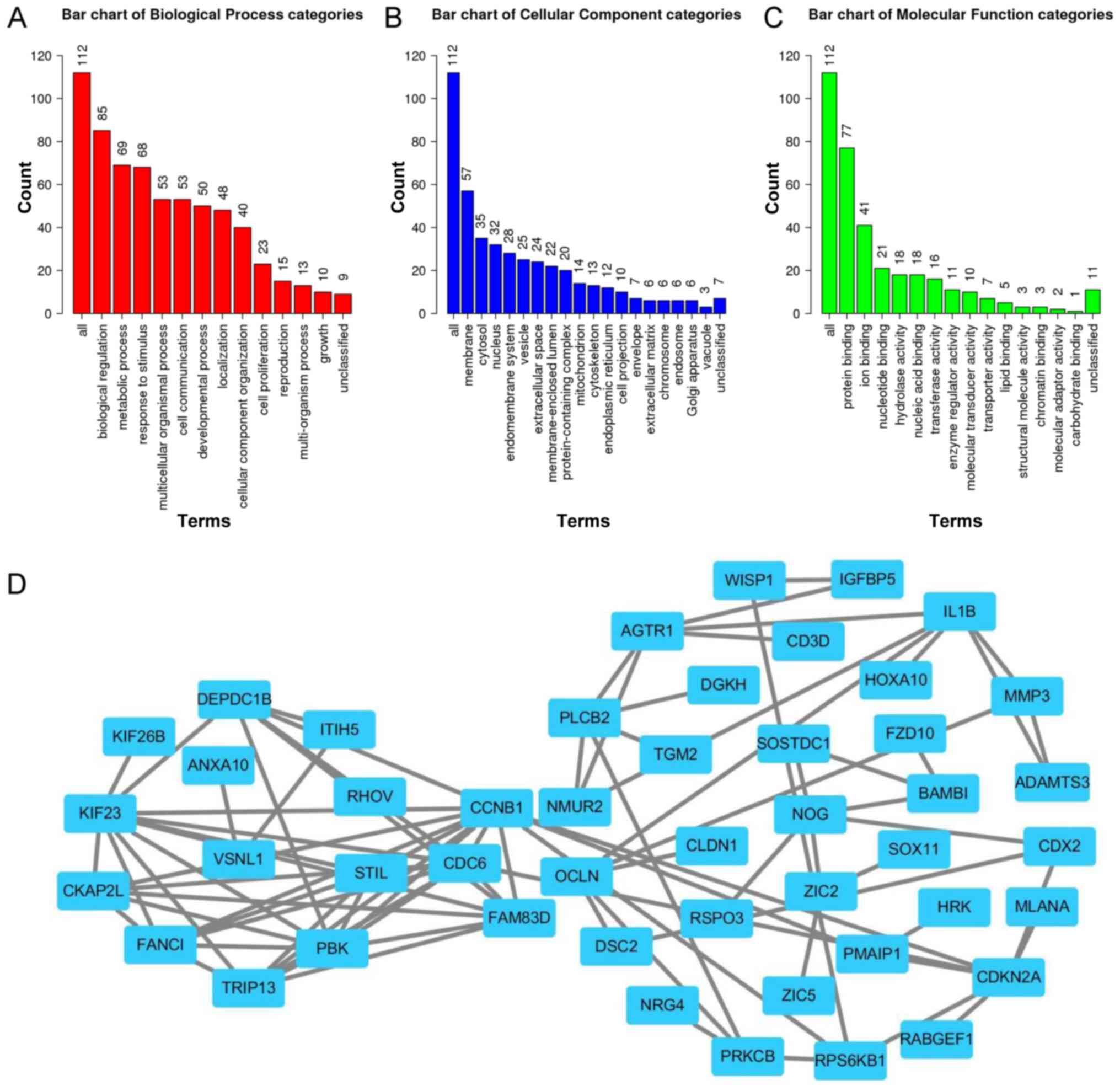
Functional enrichment and interaction
analyses
To further investigate the association between the
five lncRNAs and GC, GO and PPI network analyses of the 114 mRNAs
within the constructed ceRNA network were performed. The results
demonstrated that the mRNAs were involved in biological processes,
including ‘biological regulation’, ‘metabolic process’, ‘response
to stimulus’, ‘multicellular organismal process’, ‘cell
communication’, ‘developmental process’, ‘localization’, ‘cellular
component organization’, ‘cell proliferation’, ‘reproduction’ and
‘multi-organism process growth’ (Fig.
4A). The mRNAs were primarily enriched for cellular component
terms, including ‘membrane’, ‘cytosol’, ‘nucleus’, ‘endomembrane
system’, ‘vesicle’, ‘extracellular space’, ‘membrane-enclosed
lumen’ and ‘protein-containing complex’ (Fig. 4B). For molecular functions terms, the
mRNAs were concentrated in ‘protein binding’, ‘ion binding’,
‘nucleotide binding’, ‘hydrolase activity’, ‘nucleic acid binding’,
‘transferase activity’, ‘enzyme regulator activity’, ‘molecular
transducer activity’, ‘transporter activity’ and ‘lipid binding’
(Fig. 4C).
A PPI network incorporating the mRNAs in the ceRNA
network was constructed using the STRING database. The results
demonstrated that the lncRNAs in the ceRNA network may be involved
in regulating the cell cycle via cyclin B1 (CCNB1), family with
sequence similarity 83 member D (FAM83D) and cell division cycle 6
(CDC6) in GC (Fig. 4D).
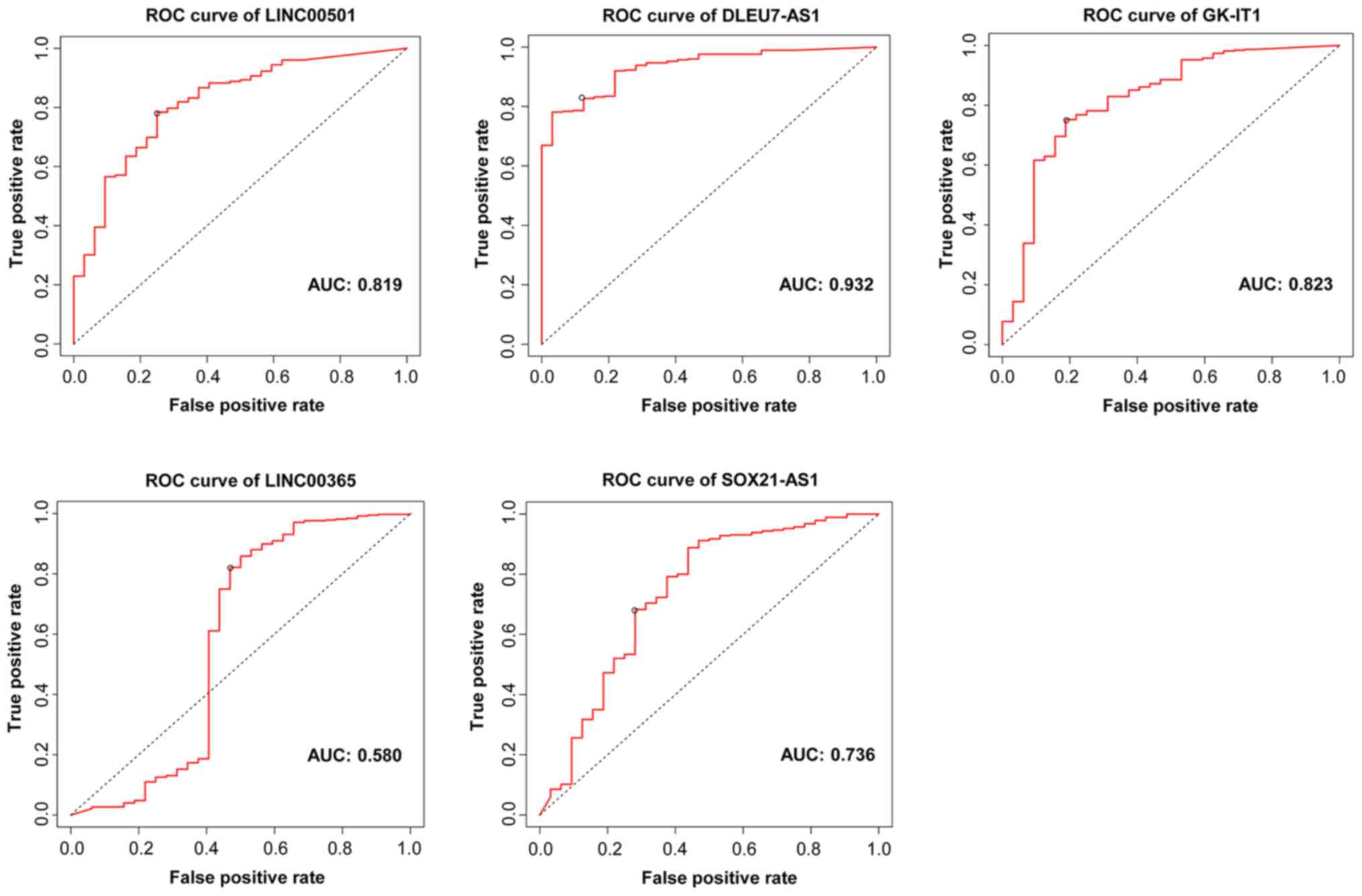
Diagnostic values of the lncRNAs
ROC curve analysis was performed to determine the
diagnostic values of the central lncRNAs in the ceRNA network. The
results generated AUC values of 0.819, 0.932, 0.823, 0.580 and
0.736 for LINC00501, DLEU7-AS1, GK-IT1, LINC00365 and SOX21-AS1,
respectively (Fig. 5), which
suggests that the five lncRNAs have diagnostic value for patients
with GC.
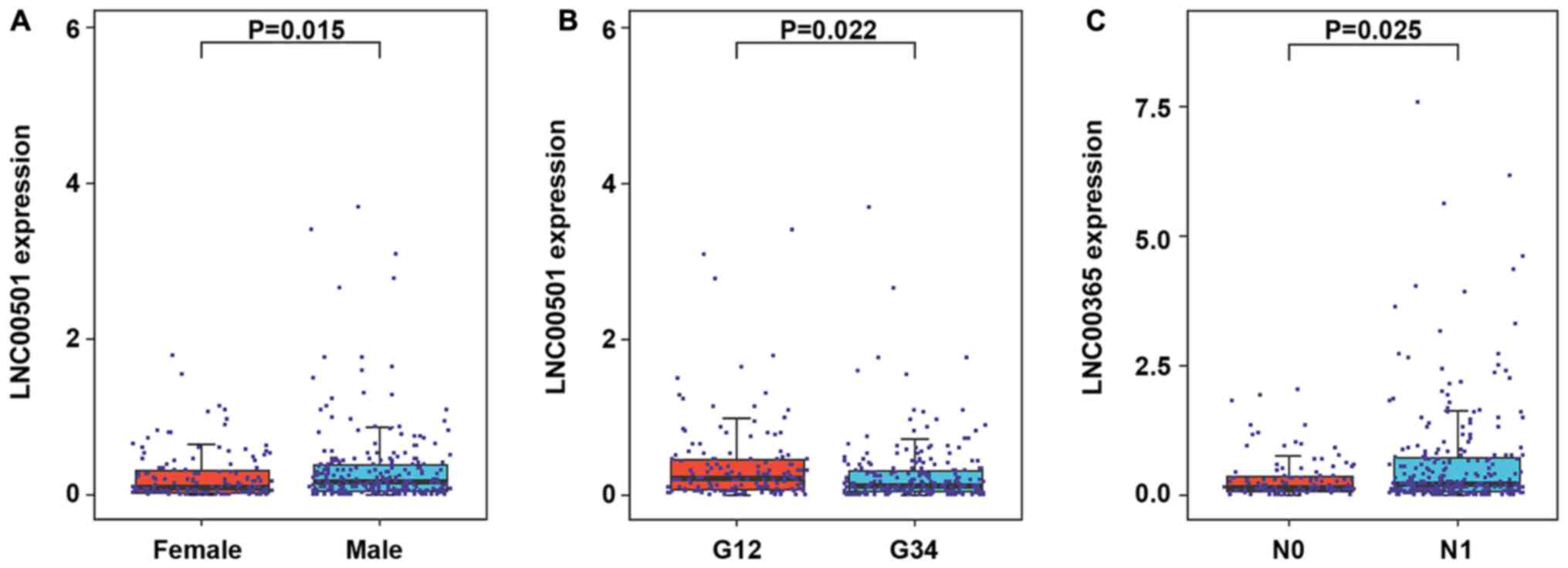
Associations between lncRNAs and
clinicopathological characteristics
The present study further assessed the clinical
value of the central lncRNAs in GC, and associations between lncRNA
expression and clinicopathological characteristics were analyzed
using TCGA database. The results demonstrated that LINC00501
expression was significantly associated with sex (P=0.015; Fig. 6A) and tumor grade (P=0.022; Fig. 6B). Furthermore, LINC00365 expression
was significantly associated with lymph node metastasis (P=0.025;
Fig. 6C). However, due to the
limited sample size, data of associations between other lncRNAs
expression and clinicopathological characteristics were
non-significant (Table SIII).
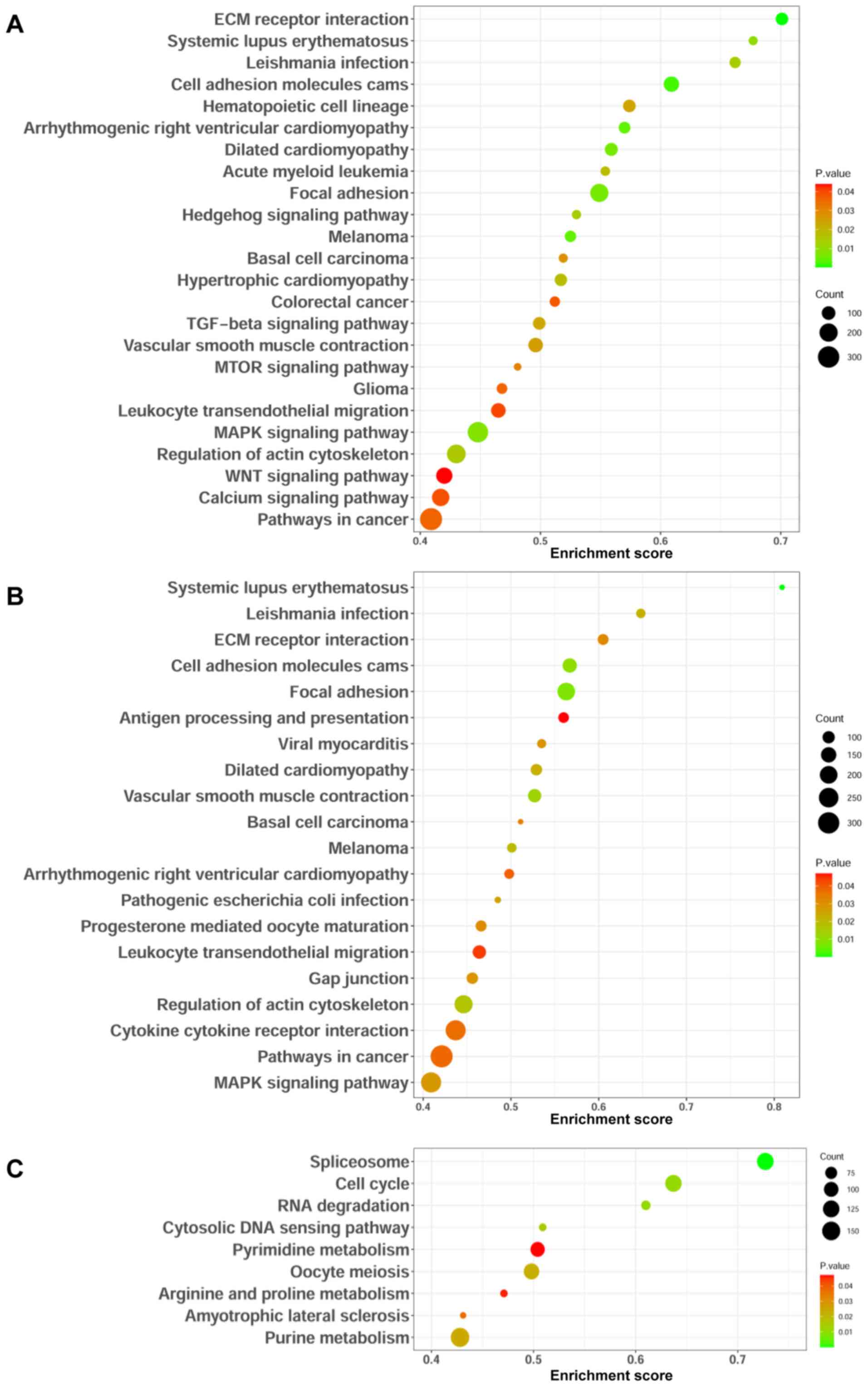
Identification of lncRNA-associated
signaling pathways
GSEA was performed to identify signaling pathways
that are differentially activated in GC. The present study focused
on signaling pathways that had nominal P<0.05 and selected the
most significantly enriched signaling pathways based on their SIZE
>50. The results demonstrated that LINC00501 was enriched in the
TGF-beta, mTOR, MAPK and WNT signaling pathways (Fig. 7A), while LINC00365 was enriched in
the MAPK signaling pathway (Fig.
7B). In addition, SOX21-AS1 was enriched in the cell cycle
(Fig. 7C). Taken together, these
results suggest that these lncRNAs may modulate these signaling
pathways, thereby regulate the development and progression of
GC.
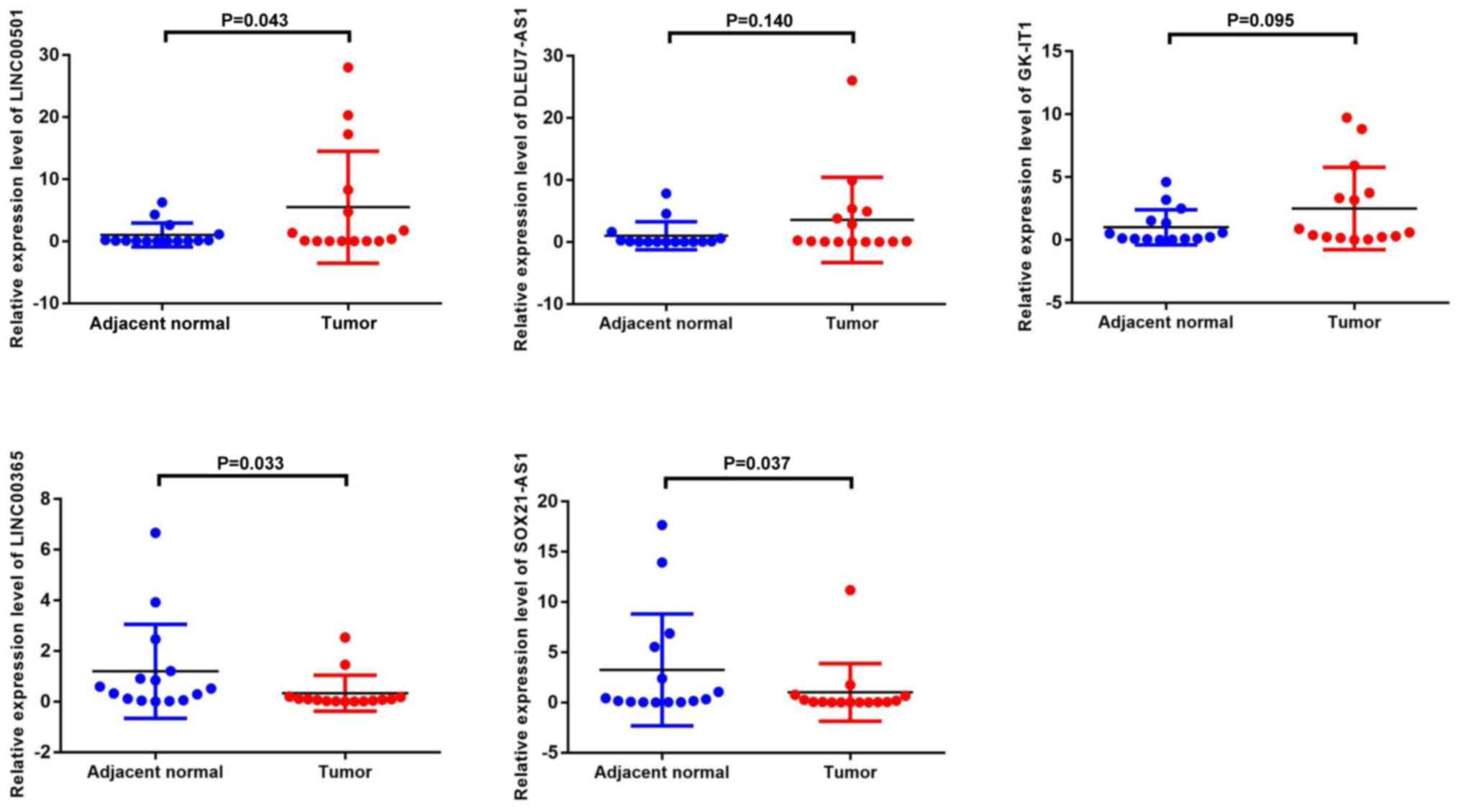
RT-qPCR verification of differential
lncRNA expression in GC tissues
To confirm that the central lncRNAs were
differentially expressed in GC tissues, RT-qPCR analysis was
performed to detect their expression levels in 15 pairs of GC
tissues and adjacent normal tissues. Consistent with the results of
the GEO and TCGA dataset analyses, the results demonstrated that
the expression levels of LINC00501, DLEU7-AS1 and GK-IT1 were
upregulated in GC tumor tissues, whereas the expression levels of
LINC00365 and SOX21-AS1 were downregulated in GC tumor tissues
(Fig. 8). Notably, LINC00501
expression was increased 5.47-fold (P=0.043), LINC00365 expression
was decreased 3.67-fold (P=0.033) and SOX21-AS1 expression was
decreased 3.24-fold (P=0.037). Although the differences in
DLEU7-AS1 and GK-IT1 expression were not significant, they
exhibited an upward trend consistent with the bioinformatics
analyses.
Discussion
Increasing evidence suggest that lncRNAs are
involved in the progression of different types of cancer (27–30).
However, the etiology of GC remains partly unknown. In addition,
the roles of lncRNAs in GC remain unclear. The present study
analyzed multiple GEO and TCGA datasets to comprehensively identify
lncRNAs associated with GC development or progression. By
constructing a ceRNA network, performing functional enrichment
analyses and ROC analysis, assessing associations with
clinicopathological characteristics, GSEA and RT-qPCR verification,
five lncRNAs (LINC00501, LINC00365, SOX21-AS1, GK-IT1 and
DLEU7-AS1) were identified that may be functionally associated with
GC development, and thus may be used as novel diagnostic biomarkers
for patients with GC.
In the present study, five lncRNAs (LINC00501,
LINC00365, SOX21-AS1, GK-IT1 and DLEU7-AS1), 29 miRNAs and 114
mRNAs were incorporated into a ceRNA network. Among the included
lncRNAs, LINC00501 and LINC00365 were associated with the most
miRNAs, suggesting that they may be key regulators of GC due to
their ceRNA functionality. Previous studies have demonstrated that
a number of the selected mRNAs and predicted miRNAs, including
miR-301b-3p (31), miR-4295
(32), miR-206 (33), miR-613 (34), miR-429 (35), forkhead box C1 (36), G protein-coupled receptor class C
group 5 member A (37) and kinesin
family member 23 (38), are
associated with GC. GO analysis revealed that the 114 mRNAs within
this network may be involved in biological processes, including
‘biological regulation’, ‘metabolic process’, ‘response to
stimulus’, ‘multicellular organismal process’, ‘cell
communication’, ‘developmental process’, ‘localization’, ‘cellular
component organization’, ‘cell proliferation’, ‘reproduction’ and
‘multi-organism process growth’. The mRNAs were primarily enriched
for cellular component terms, including ‘membrane’, ‘cytosol’,
‘nucleus’, ‘endomembrane system’, ‘vesicle’, ‘extracellular space’,
‘membrane-enclosed lumen’ and ‘protein-containing complex’. For
molecular functions terms, the mRNAs were concentrated in ‘protein
binding’, ‘ion binding’, ‘nucleotide binding’, ‘hydrolase
activity’, ‘nucleic acid binding’, ‘transferase activity’, ‘enzyme
regulator activity’, ‘molecular transducer activity’, ‘ transporter
activity’ and ‘lipid binding’. PPI analyses revealed that these
lncRNAs may promote or inhibit the development of GC by targeting a
range of cell cycle-related mRNAs, such as CCNB1 (39,40),
FAM83D (41) and CDC6 (42). The ceRNA hypothesis posits that
lncRNAs can competitively bind miRNAs, thereby altering their
ability to influence mRNA translation (8). Recent studies have demonstrated that
ceRNAs play important functional roles in GC (43,44). In
the present study, a ceRNA network was constructed, GO and PPI
analyses were performed to elucidate the mechanisms regarding how
these lncRNAs exert their functions. The results suggest LINC00501
may be involved in cell proliferation by targeting mRNA fascin
actin-bundling protein 1 (FSCN1) via miR-429. Notably, a previous
study demonstrated that miR-429 acts as a tumor suppressor by
targeting FSCN1 in GC (45), which
was consistent with the results of the present study. The results
of the present study also suggest that LINC00365 may be involved in
the cell cycle by targeting mRNA FAM83D via miR-17-5p. In addition,
SOX21-AS1 and GK-IT1 may be involved in the cell cycle by targeting
mRNA CCNB1 via miR-212-3p and miR-24-3p, respectively. Furthermore,
DLEU7-AS1 may play a role in carcinogenesis by regulating the cell
cycle through miR-142-3p/CDC6. In the present study, ROC analysis
revealed that AUC values for LINC00501, LINC00365, SOX21-AS1,
GK-IT1 and DLEU7-AS1 were all >0.50, which suggests that these
five lncRNAs have the potential to serve as valuable diagnostic
biomarkers for GC. Notably, analysis of the associations with
clinicopathological characteristics revealed that LINC00501
expression was associated with sex and tumor grade. Furthermore,
LINC00365 expression was upregulated in N1 stage samples compared
with that in N0 stage samples, suggesting that LINC00365 may be an
indicator for early stage GC diagnosis without lymph node
metastasis. GSEA of the three lncRNAs indicated that LINC00501 may
modulate GC progression via the TGF-beta, MAPK, WNT and mTOR
signaling pathways, LINC00365 via MAPK signaling and SOX21-AS1 via
the cell cycle. Several studies have highlighted the important
roles of TGF-beta signaling pathway (46), MAPK signaling pathway (47), WNT signaling pathway (48) and the cell cycle (49) in GC.
LINC00501 has been identified as a prognostic factor
associated with the overall survival of patients with
hepatocellular carcinoma (HCC) (50–52).
LINC00365 influences the Wnt/β-catenin signaling pathway, which
modulates colorectal cancer (CRC) progression (53), and breast cancer cell viability may
be regulated by the LINC00365-secretoglobin family 2A member 1
(SCGB2A1) axis, which targets NF-κB signaling (54). In GC, the expression levels of
LINC00365 and SCGB2A1 are downregulated in tumor tissues, which is
associated with a shorter survival time (55). Notably, this LINC00365/SCGB2A1 axis
and associated NF-κB suppression are associated with GC progression
(55). In the present study,
SOX21-AS1 was demonstrated to be associated with GC in the ceRNA
network. Previous studies have reported that SOX21-AS1 is
associated with different types of cancer, including cervical
cancer (56,57), HCC (58), lung adenocarcinoma (59), CRC (60) and oral cancer (61). Previous studies have demonstrated
that SOX21-AS1 can function as a ceRNA to sequester miRNAs, which
in turn modulates gene expression (57,60). For
example, SOX21-AS1 participates in cervical cancer progression by
competitively binding miR-7/voltage dependent anion channel 1
(57), while it sequesters miR-145
in CRC, which promotes tumor progression via the enhanced
expression of myosin VI (60).
SOX21-AS1 knockdown in nephroblastoma cells in vitro
disrupts the proliferation and colony formation of these cells
through a mechanism associated with p57 upregulation, and results
in cell cycle arrest (62). In
addition, SOX21-AS1 is associated with HCC progression and patient
prognosis via a mechanism associated with p21 epigenetic silencing
(58).
Although the differences in DLEU7-AS1 and GK-IT1
expression were not significant, they exhibited an upward trend
consistent with the present bioinformatics analyses. This lack of
significance may be attributed to the limited sample size in the
present study. Thus, future studies with larger sample sizes are
required to confirm the results presented here. A previous study
demonstrated that DLEU7-AS1 expression is upregulated in CRC, and
is associated with CRC stage, metastasis and poor patient prognosis
(63). From a mechanistic
perspective, DLEU7-AS1 is considered to modulate the Wnt/β-catenin
signaling pathway in CRC cells, which influences the ability of
these cells to proliferate and invade proximal or distal tissues
(63). GK-IT1 has been reported to
be positively associated with overall survival in patients with
esophageal adenocarcinoma (64).
Several studies have investigated ceRNA networks for
GC, which have aimed to screen lncRNAs involved in GC via analysis
of gene expression data obtained from TCGA database (65–67).
Different original non-coding RNA microarray data were used for the
analysis, thus different lncRNAs were identified as biomarkers for
GC. The present study aimed to screen novel lncRNAs involved in GC
via analysis of gene expression data obtained from both the GEO and
TCGA databases, which makes the results more reliable and
repeatable. However, the present study is not without limitations.
Although the expression levels of the five lncRNAs were verified in
GC tissues, the lack of validation of these biomarkers in serum is
a major limitation of the present study. Furthermore, future
studies are required to confirm the functions of the identified
lncRNAs in GC.
In conclusion, integrated analysis of TCGA and GEO
datasets and a series of analyses identified five differentially
expressed lncRNAs (LINC00501, LINC00365, SOX21-AS1, GK-IT1 and
DLEU7-AS1), which may be used as novel diagnostic biomarkers
associated with GC, and may be functionally associated with GC
development and progression. Thus, the present study provides novel
insight into the mechanistic basis and biological functions of
lncRNAs in GC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by Key Research and
Development of Social and People's Livelihood (grant no.
cstc2018jscx-mszdX0031) and the Natural Science Foundation of
Chongqing in China (grant no. cstc2019jcyj-msxmX0259).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YJ, XZ and FS conceived the present study. YJ and XZ
participated in experiment design and coordination. YJ drafted and
revised the manuscript for important intellectual content, and
performed reverse transcription-quantitative PCR analysis. LR, JS
and WZ collected the tissue samples. YH, MH, YX and YL performed
statistical analysis and revised the manuscript for important
intellectual content. YJ and FS confirmed the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Research
Ethics Committee of the First Affiliated Hospital of Chongqing
Medical University (Chongqing, China; approval no. 2016140) and
written informed consent was provided by all patients prior to the
study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Forrest ME and Khalil AM: Review:
Regulation of the cancer epigenome by long non-coding RNAs. Cancer
Lett. 407:106–112. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ponting CP, Oliver PL and Reik W:
Evolution and functions of long noncoding RNAs. Cell. 136:629–641.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cabili MN, Trapnell C, Goff L, Koziol M,
Tazon-Vega B, Regev A and Rinn JL: Integrative annotation of human
large intergenic noncoding RNAs reveals global properties and
specific subclasses. Genes Dev. 25:1915–1927. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang CJ, Zhu CC, Xu J, Wang M, Zhao WY,
Liu Q, Zhao G and Zhang ZZ: The lncRNA UCA1 promotes proliferation,
migration, immune escape and inhibits apoptosis in gastric cancer
by sponging anti-tumor miRNAs. Mol Cancer. 18:1152019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Deng W, Zhang Y, Cai J, Zhang J, Liu X,
Yin J, Bai Z, Yao H and Zhang Z: lncRNA-ANRIL promotes gastric
cancer progression by enhancing NF-kB signaling. Exp Biol Med
(Maywood). 244:953–959. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ba MC, Ba Z, Long H, Cui SZ, Gong YF, Yan
ZF, Lin KP, Wu YB and Tu YN: lncRNA AC093818.1 accelerates gastric
cancer metastasis by epigenetically promoting PDK1 expression. Cell
Death Dis. 11:642020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Russo F, Fiscon G, Conte F, Rizzo M, Paci
P and Pellegrini M: Interplay between long noncoding RNAs and
MicroRNAs in cancer. Methods Mol Biol. 1819:75–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Liu HT, Ma RR, Lv BB, Zhang H, Shi DB, Guo
XY, Zhang GH and Gao P: lncRNA-HNF1A-AS1 functions as a competing
endogenous RNA to activate PI3K/AKT signalling pathway by sponging
miR-30b-3p in gastric cancer. Br J Cancer. 122:1825–1836. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Su YZ, Cui MF, Du J and Song B: lncRNA
DCST1-AS1 regulated cell proliferation, migration, invasion and
apoptosis in gastric cancer by targeting miR-605-3p. Eur Rev Med
Pharmacol Sci. 24:1158–1167. 2020.PubMed/NCBI
|
|
11
|
Zhang G, Li S, Lu J, Ge Y, Wang Q, Ma G,
Zhao Q, Wu D, Gong W, Du M, et al: lncRNA MT1JP functions as a
ceRNA in regulating FBXW7 through competitively binding to
miR-92a-3p in gastric cancer. Mol Cancer. 17:872018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Luan M, Shi SS, Shi DB, Liu HT, Ma RR, Xu
XQ, Sun YJ and Gao P: TIPRL, a Novel tumor suppressor, suppresses
cell migration, and invasion through regulating AMPK/mTOR Signaling
pathway in gastric cancer. Front Oncol. 10:10622020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song YX, Sun JX, Zhao JH, Yang YC, Shi JX,
Wu ZH, Chen XW, Gao P, Miao ZF and Wang ZN: Non-coding RNAs
participate in the regulatory network of CLDN4 via ceRNA mediated
miRNA evasion. Nat Commun. 8:2892017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barrett T, Wilhite SE, Ledoux P,
Evangelista C, Kim IF, Tomashevsky M, Marshall KA, Phillippy KH,
Sherman PM, Holko M, et al: NCBI GEO: Archive for functional
genomics data sets-update. Nucleic Acids Res. 41((Database Issue)):
D991–D995. 2013.
|
|
15
|
Liu J, Lichtenberg T, Hoadley KA, Poisson
LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee
AV, et al: An integrated TCGA Pan-cancer clinical data resource to
drive high-quality survival outcome analytics. Cell.
173:400–416.e411. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Leek JT, Johnson WE, Parker HS, Jaffe AE
and Storey JD: The sva package for removing batch effects and other
unwanted variation in high-throughput experiments. Bioinformatics.
28:882–883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jeggari A, Marks DS and Larsson E:
miRcode: A map of putative microRNA target sites in the long
non-coding transcriptome. Bioinformatics. 28:2062–2063. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Agarwal V, Bell GW, Nam JW and Bartel DP:
Predicting effective microRNA target sites in mammalian mRNAs.
Elife. 4:e050052015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chou CH, Shrestha S, Yang CD, Chang NW,
Lin YL, Liao KW, Huang WC, Sun TH, Tu SJ, Lee WH, et al: miRTarBase
update 2018: A resource for experimentally validated
microRNA-target interactions. Nucleic Acids Res. 46:D296–D302.
2018. View Article : Google Scholar
|
|
21
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liao Y, Wang J, Jaehnig EJ, Shi Z and
Zhang B: WebGestalt 2019: Gene set analysis toolkit with revamped
UIs and APIs. Nucleic Acids Res. 47:W199–W205. 2019. View Article : Google Scholar
|
|
23
|
Szklarczyk D, Franceschini A, Wyder S,
Forslund K, Heller D, Huerta-Cepas J, Simonovic M, Roth A, Santos
A, Tsafou KP, et al: STRING v10: Protein-protein interaction
networks, integrated over the tree of life. Nucleic Acids Res.
43((Database Issue)): D447–D452. 2015. View Article : Google Scholar
|
|
24
|
Martin IG, Dixon MF, Sue-Ling H, Axon AT
and Johnston D: Goseki histological grading of gastric cancer is an
important predictor of outcome. Gut. 35:758–763. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Washington K: 7th edition of the AJCC
cancer staging manual: Stomach. Ann Surg Oncol. 17:3077–3079. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lei S, He Z, Chen T, Guo X, Zeng Z, Shen Y
and Jiang J: Long noncoding RNA 00976 promotes pancreatic cancer
progression through OTUD7B by sponging miR-137 involving EGFR/MAPK
pathway. J Exp Clin Cancer Res. 38:4702019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lu W, Zhang H, Niu Y, Wu Y, Sun W, Li H,
Kong J, Ding K, Shen HM, Wu H, et al: Long non-coding RNA linc00673
regulated non-small cell lung cancer proliferation, migration,
invasion and epithelial mesenchymal transition by sponging
miR-150-5p. Mol Cancer. 16:1182017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wei GH and Wang X: lncRNA MEG3 inhibit
proliferation and metastasis of gastric cancer via p53 signaling
pathway. Eur Rev Med Pharmacol Sci. 21:3850–3856. 2017.PubMed/NCBI
|
|
30
|
Yao N, Fu Y, Chen L, Liu Z, He J, Zhu Y,
Xia T and Wang S: Long non-coding RNA NONHSAT101069 promotes
epirubicin resistance, migration, and invasion of breast cancer
cells through NONHSAT101069/miR-129-5p/Twist1 axis. Oncogene.
38:7216–7233. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fan H, Jin X, Liao C, Qiao L and Zhao W:
MicroRNA-301b-3p accelerates the growth of gastric cancer cells by
targeting zinc finger and BTB domain containing 4. Pathol Res
Pract. 215:1526672019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yan R, Li K, Yuan DW, Wang HN, Zhang Y,
Dang CX and Zhu K: Downregulation of microRNA-4295 enhances
cisplatin-induced gastric cancer cell apoptosis through the
EGFR/PI3K/Akt signaling pathway by targeting LRIG1. Int J Oncol.
53:2566–2578. 2018.PubMed/NCBI
|
|
33
|
Deng M, Qin Y, Chen X, Wang Q and Wang J:
MiR-206 inhibits proliferation, migration, and invasion of gastric
cancer cells by targeting the MUC1 gene. Onco Targets Ther.
12:849–859. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lu Y, Tang L, Zhang Q, Zhang Z and Wei W:
MicroRNA-613 inhibits the progression of gastric cancer by
targeting CDK9. Artif Cells Nanomed Biotechnol. 46:980–984. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun T, Wang C, Xing J and Wu D: miR-429
modulates the expression of c-myc in human gastric carcinoma cells.
Eur J Cancer. 47:2552–2559. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu Y, Shao QS, Yao HB, Jin Y, Ma YY and
Jia LH: Overexpression of FOXC1 correlates with poor prognosis in
gastric cancer patients. Histopathology. 64:963–970. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu H, Zhang Y, Hao X, Kong F, Li X, Yu J
and Jia Y: GPRC5A overexpression predicted advanced biological
behaviors and poor prognosis in patients with gastric cancer.
Tumour Biol. 37:503–510. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liu Y, Chen H, Dong P, Xie G, Zhou Y, Ma
Y, Yuan X, Yang J, Han L, Chen L and Shen L: KIF23 activated
Wnt/β-catenin signaling pathway through direct interaction with
Amer1 in gastric cancer. Aging (Albany NY). 12:8372–8396. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen EB, Qin X, Peng K, Li Q, Tang C, Wei
YC, Yu S, Gan L and Liu TS: HnRNPR-CCNB1/CENPF axis contributes to
gastric cancer proliferation and metastasis. Aging (Albany NY).
11:7473–7491. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ding L, Yang L, He Y, Zhu B, Ren F, Fan X,
Wang Y, Li M, Li J, Kuang Y, et al: CREPT/RPRD1B associates with
Aurora B to regulate Cyclin B1 expression for accelerating the G2/M
transition in gastric cancer. Cell Death Dis. 9:11722018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huang M, Ma X, Shi H, Hu L, Fan Z, Pang L,
Zhu F, Yang X, Xu W, Liu B, et al: FAM83D, a microtubule-associated
protein, promotes tumor growth and progression of human gastric
cancer. Oncotarget. 8:74479–74493. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zhao B, Zhang J, Chen X, Xu H and Huang B:
Mir-26b inhibits growth and resistance to paclitaxel chemotherapy
by silencing the CDC6 gene in gastric cancer. Arch Med Sci.
15:498–503. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Cao Y, Xiong JB, Zhang GY, Liu Y, Jie ZG
and Li ZR: Long Noncoding RNA UCA1 Regulates PRL-3 expression by
sponging MicroRNA-495 to promote the progression of gastric cancer.
Mol Ther Nucleic Acids. 19:853–864. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cui Y, Pu R, Ye J, Huang H, Liao D, Yang
Y, Chen W, Yao Y and He Y: lncRNA FAM230B promotes gastric cancer
growth and metastasis by regulating the miR-27a-5p/TOP2A Axis. Dig
Dis Sci. Sep 10–2020.(Epub ahead of print). doi:
10.1007/s10620-020-06581-z. View Article : Google Scholar
|
|
45
|
Zhang M, Dong BB, Lu M, Zheng MJ, Chen H,
Ding JZ, Xu AM and Xu YH: miR-429 functions as a tumor suppressor
by targeting FSCN1 in gastric cancer cells. Onco Targets Ther.
9:1123–1133. 2016.PubMed/NCBI
|
|
46
|
Chen ZL, Qin L, Peng XB, Hu Y and Liu B:
INHBA gene silencing inhibits gastric cancer cell migration and
invasion by impeding activation of the TGF-β signaling pathway. J
Cell Physiol. 234:18065–18074. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xiang Z, Li J, Song S, Wang J, Cai W, Hu
W, Ji J, Zhu Z, Zang L, Yan R and Yu Y: A positive feedback between
IDO1 metabolite and COL12A1 via MAPK pathway to promote gastric
cancer metastasis. J Exp Clin Cancer Res. 38:3142019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gao J, Zhao C, Liu Q, Hou X, Li S, Xing X,
Yang C and Luo Y: Cyclin G2 suppresses Wnt/β-catenin signaling and
inhibits gastric cancer cell growth and migration through Dapper1.
J Exp Clin Cancer Res. 37:3172018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Zhang L, Kang W, Lu X, Ma S, Dong L and
Zou B: lncRNA CASC11 promoted gastric cancer cell proliferation,
migration and invasion in vitro by regulating cell cycle pathway.
Cell Cycle. 17:1886–1900. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hou Y, Yu Z, Tam NL, Huang S, Sun C, Wang
R, Zhang X, Wang Z, Ma Y, He X and Wu L: Exosome-related lncRNAs as
predictors of HCC patient survival: A prognostic model. Am J Transl
Res. 10:1648–1662. 2018.PubMed/NCBI
|
|
51
|
Lou X, Li J, Yu D, Wei YQ, Feng S and Sun
JJ: Comprehensive analysis of five long noncoding RNAs expression
as competing endogenous RNAs in regulating hepatoma carcinoma.
Cancer Med. 8:5735–5749. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ye J, Zhang J, Lv Y, Wei J, Shen X, Huang
J, Wu S and Luo X: Integrated analysis of a competing endogenous
RNA network reveals key long noncoding RNAs as potential prognostic
biomarkers for hepatocellular carcinoma. J Cell Biochem.
120:13810–13825. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhu Y, Bian Y, Zhang Q, Hu J, Li L, Yang
M, Qian H, Yu L, Liu B and Qian X: LINC00365 promotes colorectal
cancer cell progression through the Wnt/β-catenin signaling
pathway. J Cell Biochem. 121:1260–1272. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang L, Yan X, Yu S, Zhong X, Tian R, Xu
L, Bian X and Su J: LINC00365-SCGB2A1 axis inhibits the viability
of breast cancer through targeting NF-κB signaling. Oncol Lett.
19:753–762. 2020.PubMed/NCBI
|
|
55
|
Yan XY, Zhang JJ, Zhong XR, Yu SH, Xu L,
Tian R, Sun LK and Su J: The LINC00365/SCGB2A1 (Mammaglobin B) axis
down-regulates NF-κB signaling and is associated with the
progression of gastric cancer. Cancer Manag Res. 12:621–631. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Wang R, Li Y, Du P, Zhang X, Li X and
Cheng G: Hypomethylation of the lncRNA SOX21-AS1 has clinical
prognostic value in cervical cancer. Life Sci. 233:1167082019.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhang X, Zhao X, Li Y, Zhou Y and Zhang Z:
Long noncoding RNA SOX21-AS1 promotes cervical cancer progression
by competitively sponging miR-7/VDAC1. J Cell Physiol.
234:17494–17504. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wei C, Wang H, Xu F, Liu Z and Jiang R:
lncRNA SOX21-AS1 is associated with progression of hepatocellular
carcinoma and predicts prognosis through epigenetically silencing
p21. Biomed Pharmacother. 104:137–144. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lu X, Huang C, He X, Liu X, Ji J, Zhang E,
Wang W and Guo R: A Novel long non-coding RNA, SOX21-AS1, indicates
a poor prognosis and promotes lung adenocarcinoma proliferation.
Cell Physiol Biochem. 42:1857–1869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wei AW and Li LF: Long non-coding RNA
SOX21-AS1 sponges miR-145 to promote the tumorigenesis of
colorectal cancer by targeting MYO6. Biomed Pharmacother.
96:953–959. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang CM, Wang TH, Chen HC, Li SC, Lee MC,
Liou HH, Liu PF, Tseng YK, Shiue YL, Ger LP and Tsai KW: Aberrant
DNA hypermethylation-silenced SOX21-AS1 gene expression and its
clinical importance in oral cancer. Clin Epigenetics. 8:1292016.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang J, Hou T, Qi X, Wang J and Sun X:
SOX21-AS1 is associated with clinical stage and regulates cell
proliferation in nephroblastoma. Biosci Rep. 39:BSR201906022019.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liu XB, Han C and Sun CZ: Long non-coding
RNA DLEU7-AS1 promotes the occurrence and development of colorectal
cancer via Wnt/β-catenin pathway. Eur Rev Med Pharmacol Sci.
22:110–117. 2018.PubMed/NCBI
|
|
64
|
Yu Y, Chen X and Cang S: Cancer-related
long noncoding RNAs show aberrant expression profiles and competing
endogenous RNA potential in esophageal adenocarcinoma. Oncol Lett.
18:4798–4808. 2019.PubMed/NCBI
|
|
65
|
Pan H, Guo C, Pan J, Guo D, Song S, Zhou Y
and Xu D: Construction of a competitive endogenous RNA network and
identification of Potential regulatory axis in gastric cancer.
Front Oncol. 9:9122019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Peng J, Zhu Y, Dong X, Mao X, Lou Y, Mu Y,
Xue D and Zhou H: Construction and analysis of lncRNA-associated
ceRNA network identified potential prognostic biomarker in gastric
cancer. Transl Cancer Res. 8:1116–1128. 2019. View Article : Google Scholar
|
|
67
|
Qi M, Yu B, Yu H and Li F: Integrated
analysis of a ceRNA network reveals potential prognostic lncRNAs in
gastric cancer. Cancer Med. 9:1798–1817. 2020. View Article : Google Scholar : PubMed/NCBI
|