Introduction
Colorectal cancer (CRC) is a common malignancy; it
has the third highest incidence rate among all cancers and is the
second leading cause of cancer-related deaths worldwide (1). Despite advances in our understanding of
the clinicopathological features of CRC and the improvements in
diagnosis and treatment thereof, mortality from CRC is expected to
increase, with approximately 860,000 deaths reported each year
(2). Unfortunately, at the time of
diagnosis, 19.6% of patients with primary CRC have concurrent
distant metastases, with the highest frequency observed in liver
(10.9%), peritoneum (4.5%), and lung (2.4%) (3). The prognosis of patients with liver
metastasis from colorectal cancer (CRLM) has improved dramatically
with the availability of new and effective cytotoxic and targeted
agents, as well as aggressive surgical resection. Moreover, recent
improvements in innovative agents have led to an increased response
rate in unresectable CRLM. The surgical resection rate after
downstaging in initially unresectable CRLM has been reported to be
up to 40% (so called ‘conversion therapy’) (4). Despite these developments, the
oncological outcomes for patients with CRLM remains unsatisfactory;
for example, CRLM recurrence after hepatectomy is common, with a
50% rate reported in remnant liver (5–7).
Advances in molecular biology over the past decade
have facilitated a better understanding of the development of
several kinds of cancers, and a more precise use of innovative
targeted therapies. Indeed, recent studies have shown that several
cancers display intratumor genetic heterogeneity. Given the
intratumor genetic heterogeneity described in several cancers, the
metastatic process itself may result in clonal selection in the
progression from primary to metastatic disease. The proliferation,
invasion, and metastasis of cancer cells are not defined by the
nature of the cancer cell itself, but rather by the adaptation of
the microenvironment via interactions between the cancer cell and
its surrounding tissues (8,9).
It is considered that elucidating intratumor genetic
heterogeneity will lead to new diagnostic and therapeutic methods
for CRC. Herein, we performed molecular analysis on paired patients
with primary CRC and synchronous CRLM, resected at the same
institute. We investigated the molecular characteristics using
whole-exome sequencing, cancer gene panels, fusion gene panels and
microarray gene expression profiling. A notable feature of this
study is that it fully matches all of the clinicopathological data,
as the cases are from the same medical institution. Most previous
studies used metachronous or unpaired patient samples.
Consequently, the analyses presented in those studies could be
biased by intertumoral heterogeneity and the administration of
chemotherapy between the time of resection of primary CRC and
metastatic sites, which might alter the genetic characteristics of
the clones. Moreover, previous studies used a limited set of
biomarkers.
To our knowledge, no other study has analyzed such a
completely paired sample of primary CRC and synchronous CRLM using
a major complement of exhaustive genetic analyses with
next-generation sequencing.
Materials and methods
Clinical data
Surgically resected tumor specimens, normal liver
tissues and corresponding peripheral blood samples were obtained
from 22 consecutive patients who underwent both colectomy and
hepatectomy for CRC and synchronous CRLM between January 2014 and
March 2015. All patients were enrolled in Project HOPE (High-tech
Omics-based Patient Evaluation), a study launched at our institute
with the aim of evaluating the biological characteristics of cancer
by multiomics-based analyses (10).
The clinicopathological data of patients were reviewed
retrospectively. A prospective colorectal database, containing
information regarding patient characteristics, preoperative
assessment, operative characteristics, postoperative complications,
pathological characteristics, and oncological outcomes, maintained
at the hospital was used for this retrospective analysis.
Project HOPE (High-tech Omics-based
Patient Evaluation)
In the present study, we evaluated fresh frozen
tumor tissues obtained from both primary CRC and CRLM using
whole-exome sequencing (WES), cancer gene panel sequencing, fusion
gene panel sequencing and microarray-based gene expression
profiling (GEP). We also acquired peripheral blood samples from
patients and paired them with the corresponding resected tissue
samples from the HOPE study.
Ethical considerations
The research plan of Project HOPE was designed
according to the revised Ethical Guidelines for Human Genome/Gene
Analysis Research in Japan (11),
and the study protocol was approved by the Institutional Review
Board at the Shizuoka Cancer Center. Written informed consent was
obtained from all participants. This retrospective study was also
approved by the same board (Authorization no. 30-5).
Whole-exome sequencing
DNA was extracted from blood and flash-frozen
tissues using a QIAamp DNA Blood Kit (cat. no. 51185; Qiagen),
except that the tissues were treated with Proteinase (cat. no.
K19133; Qiagen). DNA was quantified using a NanoDrop
spectrophotometer (Thermo Fisher Scientific, Inc.) and a Qubit 2.0
Fluorometer (Thermo Fisher Scientific, Inc.).
DNA sample with A 260/280 ratio >1.8 was used for
DNA sequencing. The exome library used for WES was prepared using
an Ion Torrent AmpliSeq RDY Exome Kit (cat. no. A27193; Thermo
Fisher Scientific, Inc.) in accordance with the manufacturer's
instructions. A total of 100 ng of DNA was used for target
amplification under the following conditions: 99°C for 2 min,
followed by 10 cycles at 95°C for 15 sec and 60°C for 16 min, and a
final hold at 10°C. Incorporated primer sequences were partially
digested using FuPa reagent (Ion Torrent AmpliSeq RDY Exome
Kit).
Proton adapters were ligated to the amplicons at
22°C for 30 min, followed by 72°C for 10 min, and the library was
purified with Agencourt Ampure XT beads (cat. no. A63881, Thermo
Fisher Scientific, Inc.). Libraries were quantified using a
quantitative polymerase chain reaction (qPCR), and DNA (8 pM) was
sequenced using a semiconductor DNA sequencer (Ion Torrent Proton
Sequencer; Thermo Fisher Scientific, Inc.) by 200 cycles single-end
sequencing according to the manufacturer's instructions. The
average values of coverage of WES were about 100. Matched
tumor-normal pair somatic variants were identified using Ion
Reporter ver. 4.4 software (Thermo Fisher Scientific Inc.)
(12) after base calling, quality
trimming, and mapping to the hg19/GRCh37 reference genome using
Torrent Suite software ver. 4.4 (Thermo Fisher Scientific, Inc.)
(13). In this step, sequence data
derived from tumor and blood samples were analyzed separately, and
the latter were used as matched controls. In this process, only
somatic variants remain after the subtraction of variants from
blood data from those acquired from tumor data. In this
variant-call workflow, we identified somatic mutations that
satisfied the thresholds quality score ≥60 or depth of coverage
≥20. Somatic variants were inspected manually using the Integrative
Genomics Viewer (14). Annotation of
detected single nucleotide variants (SNVs) was performed using the
following databases that included germline and somatic variants:
COSMIC (15), ClinVar (16), dbSNP (17), UniProt (18) and DrugBank (19). The details are described by Nagashima
et al (10,20).
Cancer cell gene panel sequencing
The DNA library comprising 409 genes implicated in
cancer was prepared using the Ion AmpliSeq Comprehensive Cancer
Panel Kit (cat. no. 4477685; Thermo Fisher Scientific, Inc.). A
total of 10 ng of tumor DNA was used for target amplification under
the following conditions: 99°C for 2 min, followed by 12 cycles at
99°C for 15 sec and 60°C for 16 min, and a final hold at 10°C.
After adapter ligation and library purification, libraries were
quantified using a quantitative polymerase chain reaction (qPCR),
and DNA (8 pM) was sequenced using Ion Torrent Proton Sequencer
according to the manufacturer's instructions. The average values of
coverage of WES analysis were ~1,200. Data processing and
annotation were the same as described above for WES, except for
variant calls identified by subtracting 409 gene variants from WES
blood data. Ion Torrent The details are described by Shimoda et
al (21).
Comprehensive gene expression analysis
using DNA microarray
Fresh tumor and adjacent normal tissues were soaked
in RNAlater reagent (Thermo Fisher Scientific, Inc.), and total RNA
was isolated and purified using an miRNeasy Mini Kit (Qiagen)
according to the manufacturer's instructions. RNA quality was
evaluated using an RNA integrity number, which was determined using
an Agilent 2100 Bioanalyzer (Agilent Technologies). RNA samples
with an RNA integrity number ≥6.0 were used for gene expression
analysis. Gene expression analysis was performed using a SurePrint
G3 Human Gene Expression 8×60K v2 Microarray (Agilent Technologies)
kit using a One-color Low Input Quick Amp Labeling kit (Agilent
Technologies) according to the manufacturer's instructions. Data
processing to generate raw signal intensity data was performed with
GeneSpring version 13.1.1 software (Agilent Technologies). Data
analysis was performed using GeneSpring GX (Agilent Technologies)
and Microsoft Excel. Raw signal intensity values were normalized to
the 75th percentile and translated into log2 ratio
against average. Among all 50,599 probe sets, 36,022 probes which
excluded too low signal, too stable signal or abnormal data were
used for the following analysis. For the extraction of genes with
higher expression in CRLM than in CRC, the normalized signal values
for each probe were averaged in the two groups and in the group of
normal liver tissue to use further screening.
Fusion gene panel sequencing
The preparation of total RNA was described above.
Total RNAs (10 ng) were used as templates to prepare cDNAs using
the SuperScript VILO cDNA Synthesis Kit (cat. no. 11754050; Thermo
Fisher Scientific, Inc.). The Ion AmpliSeq Library Kit 2.0 (cat.
no. 4480442; Thermo Fisher Scientific, Inc.) was used to construct
an Ion Torrent adapter-ligated library in accordance with the
manufacturer's instructions, and the Ion Proton Sequencing 200 Kit
(cat. no. A26433; Thermo Fisher Scientific, Inc.) was used for
nucleotide sequencing in accordance with the manufacturer's
protocol. All data were analyzed using the Ion Reporter server.
Pre-installed software, Ion AmpliSeq RNA Fusion workflow was used
to detect fusion transcripts targeted by the fusion panel.
Fusion gene panel used in this study was
custom-made-panel, “HOPE fusion panel” which we designed to amplify
491 fusion transcripts (22). Fusion
gene data were obtained from the website of the Sanger Institute
COSMIC (15). The COSMIC database
v71 includes more than 600 fusion genes. We selected as much of the
sequence of the target fusion gene as possible and excluded
multiple fusion genes with complex structures (e.g., inversions)
with the same breakpoint, ultimately resulting in a selection of
491 fusion genes. The list of panel genes and details are described
by Urakami et al (22).
Primers for detecting fusion transcripts and their 5′ and 3′
partners were designed using the Ion Ampliseq Designer website
(https://www.ampliseq.com/; Thermo Fisher
Scientific, Inc.). The reference file for this workflow was
constructed from all fusion variants in the fusion gene panel. The
workflow used a related BED file that describes the breakpoints
between the two genes that are associated with each fusion. The
reference and BED files are included in the IonReporter
Software.
Immunohistochemical analysis
Routine pathological diagnosis was achieved using
surgically resected tumors fixed in 10% formalin and embedded in
paraffin. Paraffin sections (3 µm thickness) containing
representative histology of the tumor were used for
immunohistochemical analysis. Immunohistochemical staining was
performed using the Bond III automated stainer and BOND Polymer
Refine Detection kit (Leica Biosystems). The sections were
pretreated with Bond Epitope Retrieval Solution for 20 min at 100°C
and then reacted with the primary antibodies (Bond Epitope
Retrieval Solution 1 for osteopontin and MGP; Bond Epitope
Retrieval Solution 2 for periostin, thrombospondin 2, and GPNMB).
After reaction with diaminobenzidine chromogen, the sections were
counterstained with hematoxylin. The stained sections were
evaluated independently by two investigators (A.S. and T.S.)
blinded to patient data. Primary antibodies for osteopontin,
periostin, thrombospondin-2, and glycoprotein nonmetastatic
melanoma protein B (GPNMB) were obtained from Abcam. Primary
antibodies for matrix Gla protein (MGP) were obtained from Thermo
Fisher Scientific.
Statistical analysis
Non-parametric variables are reported as medians
(range). For comparisons between two groups, χ2 and
Wilcoxon signed rank test were used. When the expected count was
under five, Fisher's exact test was used. To compare the equality
of variances, one-way analysis of variance was performed to
calculate the F-value. The Bonferroni method was implemented to
adjust for multiple comparisons. P<0.05 was considered to be
significant. All statistical analyses were performed using IBM SPSS
Statistics software for Windows, version 24 (IBM; SPSS Inc.).
Data availability
The dataset presented in the current study has been
submitted to the National Bioscience Database Center (NBDC) under
the accession number hum0127.
Results
Patients' characteristics
The data set consisted of 22 pairs of primary CRC
and CRLM. The patients' characteristics are shown in Table I. A total of 22 paired patients were
enrolled in this study, including 13 (59.1%) colon and 9 (40.9%)
rectal cancer patients. Right-sided colon was defined as caecum,
ascending, and transverse colon. Left-sided colon was defined as
descending and sigmoid colon. All patients had synchronous liver
metastasis and did not have any other extrahepatic metastasis.
Patients with multiple cancers such as synchronous or metachronous
malignancy (within 5 years) other than carcinoma in situ, familial
adenomatous polyposis, and appendiceal cancer were excluded. The
median number of CRLM of patients was 2 (1–14). The
median size of CRLM was 30 (17–110) mm. Of the cases, 21 of 22
(95.5%) received two-stage hepatectomy. The median interval between
resection of primary CRC and CRLM was 6 (0–15) weeks. No patients
received adjuvant/neoadjuvant chemo/chemoradiotherapy before and
after the resection for primary CRC or hepatectomies for CRLM.
 | Table I.Patient characteristics (n=22). |
Table I.
Patient characteristics (n=22).
| Variable | Value |
|---|
| Sex |
|
Male | 12 |
|
Female | 10 |
| Age, years | 64.5
(27–86)a |
|
<50 | 3 |
|
≥50 | 19 |
| Primary tumor
site |
|
|
Colon | 13 |
|
Right side | 5 |
|
Left side | 8 |
|
Rectum | 9 |
| CEA (ng/ml) | 24.7
(1.8–1421)a |
|
≤5.0 | 7 |
|
>5.0 | 15 |
| Histological type
of primary site |
|
|
Differentiated | 21 |
|
Undifferentiated | 1 |
| pT stage |
|
|
pT1 | 0 |
|
pT2 | 1 |
|
pT3 | 9 |
|
pT4 | 12 |
| pN stage |
|
|
pN0 | 3 |
|
pN1 | 9 |
|
pN2 | 10 |
| Synchronous
presentation of CRLM |
|
|
Absent | 22 |
|
Present | 0 |
| Lymphatic
invasion |
|
|
Absent | 12 |
|
Present | 10 |
| Venous
invasion |
|
|
Absent | 4 |
|
Present | 18 |
| Median number of
CRLM | 2 (1–14) |
| Largest size of
CRLM, cm | 30
(17–110)a |
| Multiple CRLM |
|
|
Absent | 9 |
|
Present | 13 |
| Extrahepatic
disease |
|
|
Absent | 22 |
|
Present | 0 |
| Neoadjuvant
chemo(radio)therapy |
|
|
Absent | 22 |
|
Present | 0 |
Comparison of tumor mutation burden
between primary CRC and CRLM
The tumor mutation burden (TMB), also referred to as
‘mutation load’, represents the number of single nucleotide
variations (SNV) per mega base, and has received increasing
attention owing to its potential responses to immune checkpoint
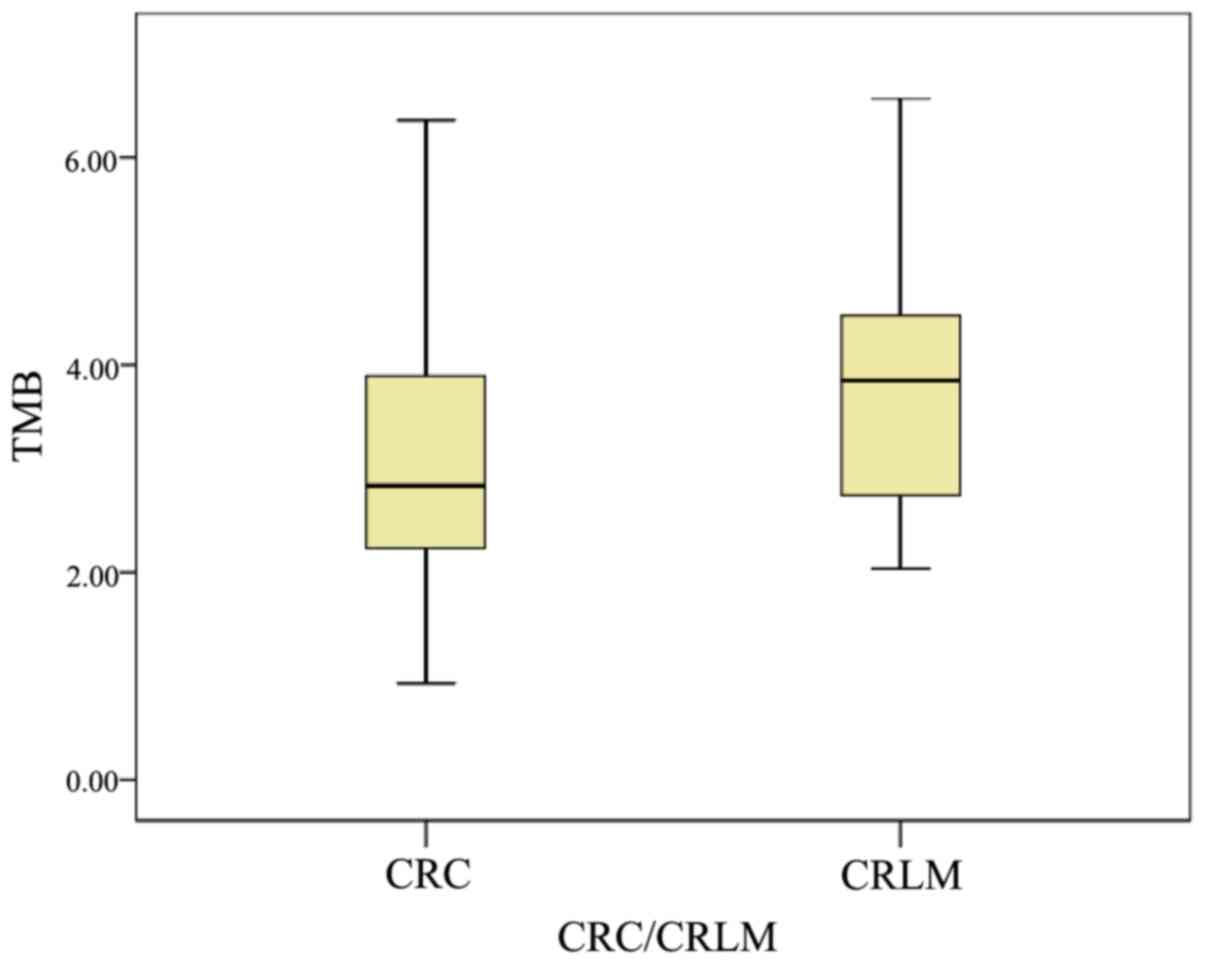
inhibitors. TMB was determined for the 22 paired samples (Fig. 1). The median TMB in primary CRC was
2.8 (0.9–6.4), whereas it was 3.8 (2.0–6.6) in CRLM. The TMB counts
in CRLM tended to be more frequent than those of primary CRC,
although there were no statistically significant differences
(P=0.07).
Gene mutation profiling
Genomic alterations contributing to tumorigenesis in
primary CRC and CRLM were analyzed using an analysis pipeline
called ‘Shizuoka Multi-omics Analysis Protocol (SMAP) (10)’. Detected genomic alterations and
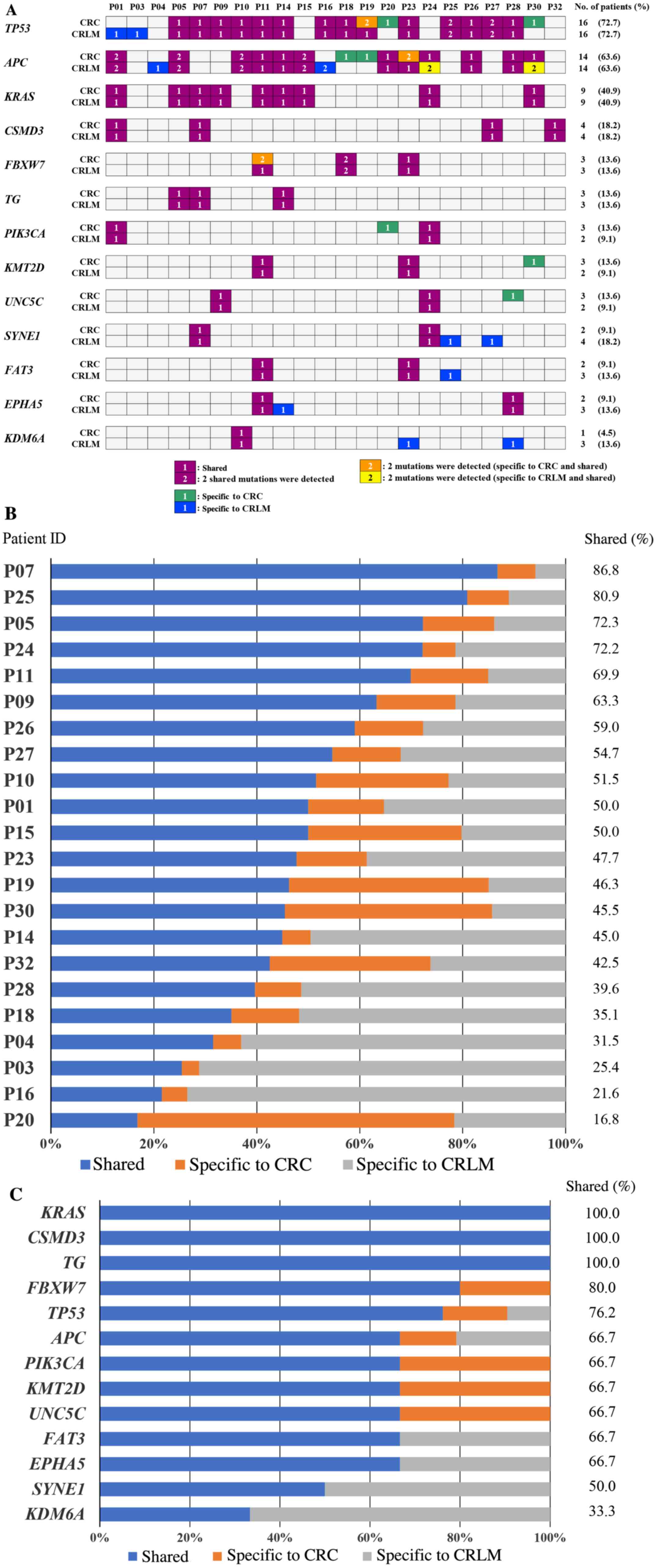
number of cases are shown in Fig.
2A. In primary CRC, the alteration of TP53, APC, and KRAS was
detected in 16 (72.7%), 14 (63.6%), and 9 (40.9%) cases,
respectively. In CRLM, the alteration of TP53, APC, and KRAS was
detected in 16 (72.7%), 14 (63.6%), 9 (40.9%) cases,
respectively.
Concordance between mutations in
matched pairs of primary CRC and CRLM
The concordance between genomic alterations in
paired primary CRC and CRLM varied from 16.8 to 86.8% in each
paired case (Fig. 2B). The
concordance between the sequence variation in KRAS, which is known
to be an important factor related to tumor metastasis was 100%. On
the other hand, the concordance in APC was only 66.7% (Fig. 2C).
Gene expression analysis using DNA
microarrays
To select candidate genes that showed significantly
higher expression in CRLM than primary CRC, gene expression was
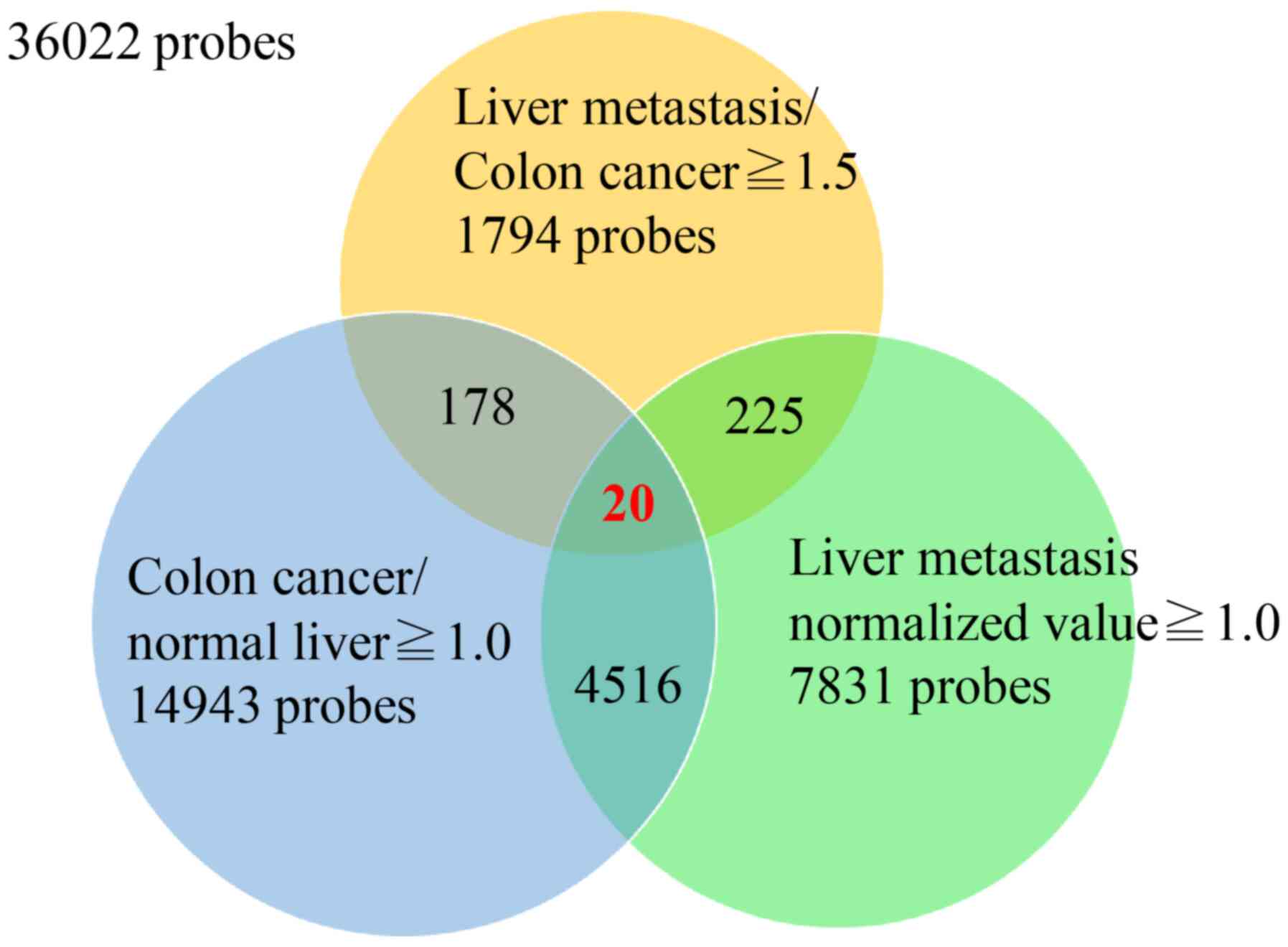
compared using the average expression value of 36,022 probes in
primary CRC, CRLM, and normal liver tissues (i.e., non-cancerous
tissues of liver metastasis cases). First, we selected 1,794 probes
whose expression was up to 1.5 times higher in CRLM than in primary
CRC. Second, to remove contamination of normal liver tissues in
liver metastasis samples, we compared expression levels in primary
CRC with those in non-cancerous liver tissues and selected 198
probes whose expression was higher in primary CRC than in normal
liver. Third, we selected 20 probes (19 genes) as highly expressed
probes in CRLM with a normalized ratio >1.0. Finally, we
examined the difference in expression level of these 20 probes in
paired samples of CRC and CRLM, and selected 11 probes (10 genes)
that showed significantly higher expression in CRLM compared to CRC
(Table II; Fig. 3). Fig.
4A shows the characteristic expression profiles of the 11
probes in the 22 paired cases. Fig.
4B shows a comparison in gene expression between primary CRC
and CRLM; all 11 probes showed significantly higher expression in
CRLM (Wilcoxon signed rank test, P<0.001).
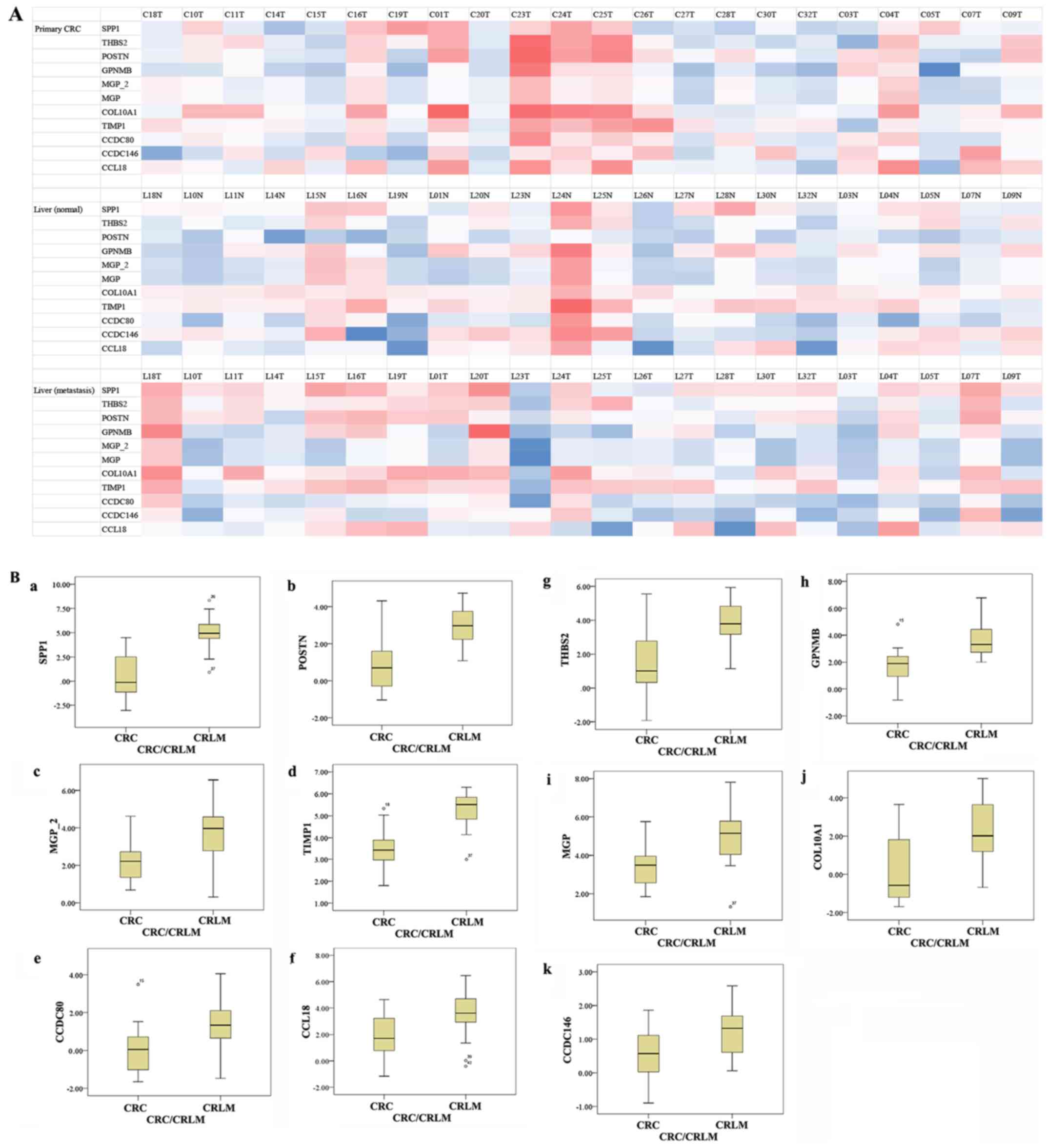 | Figure 4.(A) Heat map of the expression
profiles from the microarray analysis. Three heat maps representing
gene expression levels (z-scores) in CRC (upper), CRLM (middle) and
normal liver tissue (lower), respectively. Raw signal intensity
values of DNA microarray were log transformed and normalized to the
75th percentile. We transformed those normalized intensity value
into z-scores per probe according to the formula: Z-score=(x-α)/β.
(x: normalized intensity of the selected probe in each sample, α:
mean of the normalized values of 22 samples in the selected probe,
β: standard deviation of the normalized values of 22 samples in the
selected probe). Resultant z-score in each sample which are higher
or lower than the mean of normalized intensity in each probe are
displayed as positive value (red) or negative value (blue),
respectively. (B) Comparison of gene expression between primary CRC
and CRLM. (B-a) SPP1, (B-b) POSTN, (B-c) MGP2, (B-d) TIMP1, (B-e)
CCDC80, (B-f) CCL18, (B-g) THBS2, (B-h) GPNMB, (B-i) MGP, (B-j)
COL10A1 and (B-k) CCDC146. All 11 probes showed significantly
higher expression in CRLM than primary CRC (P<0.001). CRC,
colorectal cancer; CRLM, colorectal liver metastasis. |
 | Table II.Genes whose expression are higher in
CRLM through DNA microarray analysis. |
Table II.
Genes whose expression are higher in
CRLM through DNA microarray analysis.
|
|
|
| Variance | Bonferroni |
|---|
|
|
|
|
|
|
|---|
| Gene symbol | Gene name | Location | F-value | P-value |
<0.05/20=0.0025 |
|---|
| SPP1 | Osteopontin | ECM | 558.088 | <0.0001 | 0.0025 |
| TIMP1 | Tissue inhibitor of
metalloproteinase 1 | Secreted | 489.451 | <0.0001 | 0.0025 |
| THBS2 |
Thrombospondin-2 | ECM | 258.758 | <0.0001 | 0.0025 |
| POSTN | periostin | ECM | 248.512 | <0.0001 | 0.0025 |
| GPNMB | Glycoprotein
nonmetastatic melanoma protein B | Membrane (with ECM
domain) | 245.809 | <0.0001 | 0.0025 |
| MGP_2 | Matrix Gla
protein | ECM | 198.335 | <0.0001 | 0.0025 |
| MGP | Matrix Gla
protein | ECM | 191.511 | <0.0001 | 0.0025 |
| COL10A1 | Collagen type X α 1
chain | ECM | 130.898 | 0.0008 | 0.0025 |
| CCDC80 | Coiled-coil domain
containing 80 | Cytosol | 125.409 | 0.001 | 0.0025 |
| CCDC146 | Coiled-coil domain
containing 146 | Nucleus | 113.416 | 0.0016 | 0.0025 |
| CCL18 | Chemokine (C-C
motif) ligand 18 | Secreted | 104.601 | 0.0024 | 0.0025 |
Immunohistochemical analysis
Immunohistochemical analysis was performed to
confirm the protein expression of genes identified by DNA
microarray analysis. Among the 10 genes that were highly expressed
in CRLM, the five that were classified as encoding ‘matricellular
proteins’, i.e., osteopontin, periostin, thrombospondin-2, MGP, and
GPNMB, were selected for this analysis. GPNMB is a type I
transmembrane protein with three unique domains: an extracellular
domain, a transmembrane domain, and a cytoplasmic domain. The
extracellular domain is composed of two regions with distinct
properties: the integrin-binding motif and the polycystic kidney
disease domain. Thus, we included GPNMB as a matricellular protein
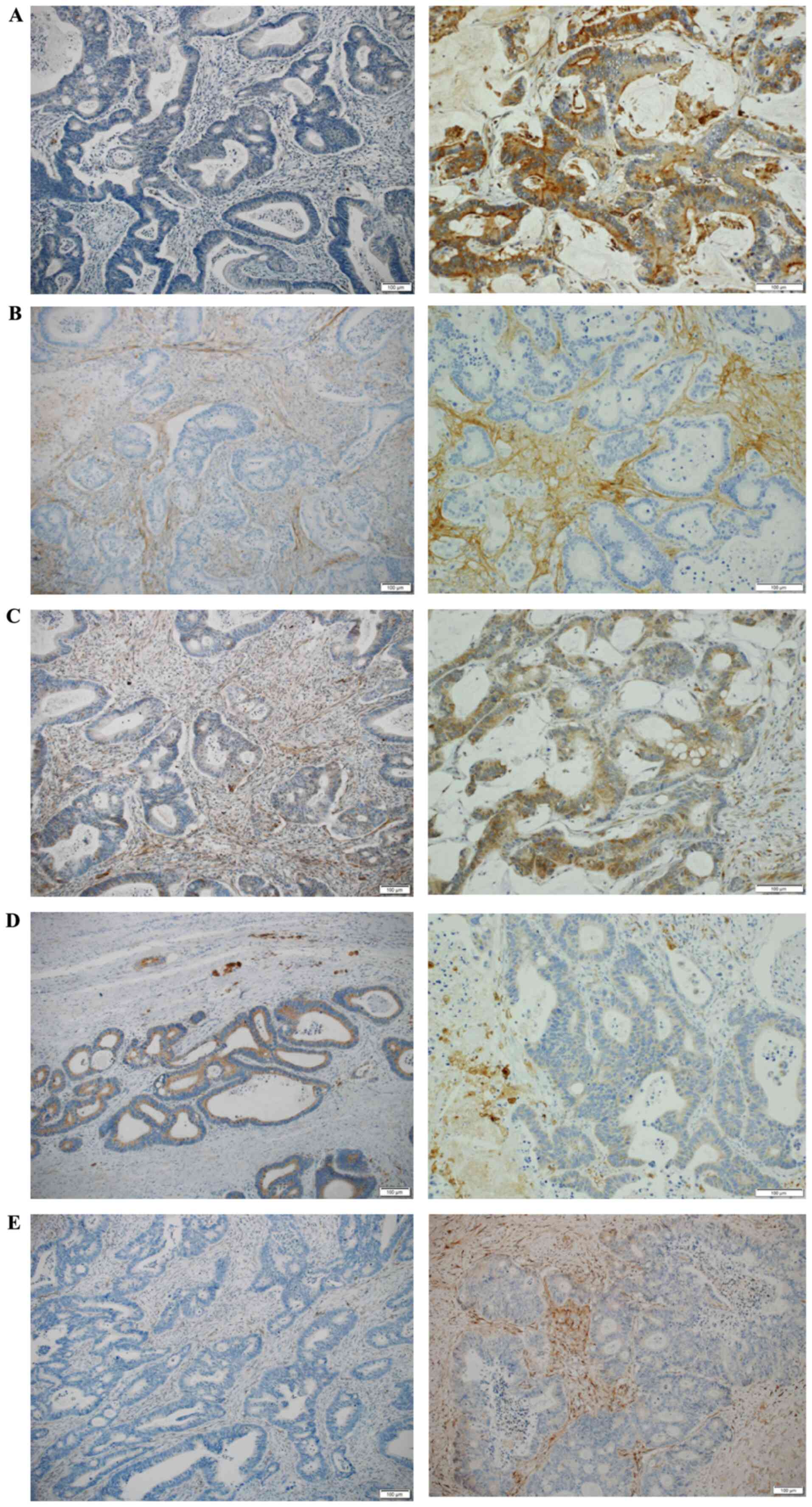
in this study. Representative images of immunohistochemical
staining are shown in Fig. 5. First,
immunoreactive scores were defined independently according to the
intensity of staining and the proportion of stained structures.
Staining intensity was scored as: 0, no staining; 1, weak staining;
and 2, strong staining. Proportions of stained tumor
cells/extracellular matrix (ECM) were classified as: 0, ≤5%
positive cells; 1, 6–25% positive cells; 2, 26–50% positive cells;
and 3, ≥51% positive cells. Total scores for intensity and
proportion were used to signify the levels of protein expression.
In this study, a score of ≤3 was considered to represent negative
expression, and a score of ≥4 was considered to represent positive
expression. Table III shows the
positive immunoreactivity scores for these five proteins.
 | Table III.Expression status of each factor by
immunostaining. |
Table III.
Expression status of each factor by
immunostaining.
|
|
| Positive | Negative |
|
|---|
|
|
|
|
|
|
|---|
| Protein | Tumor type | n | % | n | % | P-value |
|---|
| Osteopontin | Primary CRC | 0 | 0 | 22 | 100 | 0.02 |
|
| CRLM | 6 | 27.3 | 16 | 72.7 |
|
| Periostin | Primary CRC | 7 | 31.8 | 15 | 68.2 |
0.006 |
|
| CRLM | 17 | 68.2 | 5 | 31.8 |
|
|
Thrombospondin-2 | Primary CRC | 12 | 54.5 | 10 | 45.5 | 0.55 |
|
| CRLM | 10 | 45.5 | 12 | 54.5 |
|
| GPNMB | Primary CRC | 4 | 18.2 | 18 | 81.8 | 1 |
|
| CRLM | 5 | 22.7 | 17 | 77.3 |
|
| MGP | Primary CRC | 3 | 13.6 | 19 | 86.4 | 1 |
|
| CRLM | 4 | 18.2 | 18 | 81.8 |
|
Osteopontin showed strong immunoreactivity in tumor
cells and ECM of CRLM (6 of 22 cases: 27.3%) compared to no
immunoreactivity in primary CRC (0 of 22 cases: 0.0%). The
immunoreactivity score was significantly higher in CRLM than in
primary CRC (P=0.02).
Periostin also showed strong immunoreactivity in the
ECM of CRLM (17 of 22 cases: 68.2%) compared to that in primary CRC
(7 of 22 cases: 31.8%). Periostin showed no immunoreactivity in the
tumor cells of primary CRC and CRLM. Periostin was not expressed
with high frequency in primary CRC. However, when it was expressed,
it showed strong immunoreactivity in the peripheral invasive part
of primary lesions.
Thrombospondin-2 showed strong immunoreactivity in
tumor cells and ECM of both primary CRC and CRLM, and there were no
statistically significant differences (P=0.55).
GPNMB showed weak immunoreactivity in tumor cells,
but strong immunoreactivity in ECM of both primary CRC and CRLM.
These expression patterns were also observed in macrophages, but no
statistically significant differences were observed.
MGP showed strong immunoreactivity in ECM of primary
CRC (3 of 22 cases: 13.6% and CRLM (4 of 22 cases: 18.2%), but no
statistically significant differences were observed.
Comparison of number of immunoreactive
factors
To determine whether differences in the complexity
of expression patterns between primary CRC and CRLM could be
verified, and to determine genomic heterogeneity, we compared the
number of positive immunoreactive factors for 22 pairs of primary
CRC and CRLM (Table IV). Sixteen
cases (72.7%) of primary CRC showed either no or one positive
immunoreactive factor, and six cases (27.3%) were positive for two
or more immunoreactive factors. On the other hand, immunoreactivity
was more frequently observed in CRLM, with 16 cases (72.7%) sharing
two or more factors, which was significantly more than that
observed in primary CRC (P=0.007).
 | Table IV.Comparison of positive
immunoreactivity between primary CRC and CRLM. |
Table IV.
Comparison of positive
immunoreactivity between primary CRC and CRLM.
| Number of
factors | Primary CRC
(n=22) | CRLM (n=22) | P-value |
|---|
| 0 or 1 factor | 16 (72.7%) | 6 (27.3%) | 0.007 |
| 2 or more
factors | 6 (27.3%) | 16 (72.7%) |
|
Discussion
In the present study, we demonstrated the existence
of genomic heterogeneity between paired primary CRC and CRLM by
using a large complement of exhaustive genetic analyses with
next-generation sequencing. To the best our knowledge, no other
study has analyzed such a completely paired sample of primary CRC
and synchronous CRLM. Elucidation of the heterogeneity of
microenvironment-related factors on the proliferation, invasion,
and metastasis of cancer cells will lead to novel diagnostic and
therapeutic targets for CRC in the era of genome-guided
personalized cancer treatment.
Recent advances in next-generation sequencing
technologies have made it possible to analyze large numbers of
sequences, leading to international cancer genome analysis projects
such as the Cancer Genome Atlas (TCGA) and the International Cancer
Genome Consortium (ICGC). CRC can be classified into four gene
expression-based subtypes with distinguishing features, the
consensus molecular subtypes (CMSs): CMS1 (microsatellite
instability immune, 14%), CMS2 (canonical, 37%), CMS3 (metabolic,
13%), and CMS4 (mesenchymal, 23%) (23). This intertumoral heterogeneity has
led to the finding that different CRC subtypes have a different
genetic makeup, clinical behavior, pathological features, and
responses to treatment (24–26).
In addition to the intertumoral heterogeneity
mentioned above, intratumoral heterogeneity relates to the genetic
heterogeneity between cancer cells within a single tumor. During
carcinogenesis, genetic abnormalities accumulate continually,
allowing the cells an increased ability to expand and invade. As a
result of this continuous process, cancers become genetically
heterogeneous, with an indeterminate number of coexisting genomic
clones. These clones have different functional characteristics such
as the ability to form metastases or respond to chemotherapy.
Cancer cells survive and proliferate in a
microenvironment created by the cells themselves, various stromal
cells, and the stromal tissue. The stromal cells that form cancer
tissues include fibroblasts, vascular and lymphangial endothelial
cells, lymphocytes, and macrophages. Both cells interact with
cancer cells, imparting their characteristic biological features on
the cancer (27,28). Distant metastasis of cancer has also
been implicated in interactions between cancer cells and stromal
cells in the cancer microenvironment (8,9).
In this study, using the DNA microarray analysis, we
determined that many highly expressed genes were classified as
encoding ‘matricellular proteins’, which interact with the ECM.
Periostin, a secreted adhesion-related protein that is expressed in
the periosteum and periodontal ligaments, also acts as a critical
regulator in the formation and maintenance of bone and teeth, as
well as playing an important role in tumorigenesis (29). Recent studies have shown that
periostin is highly expressed in various human cancers, and it has
been suggested that periostin promotes tumor growth and metastasis
(30–32). Moreover, periostin has been reported
to enhance the metastatic growth of colon cancer by both preventing
stress-induced apoptosis in cancer cells and augmenting endothelial
cell survival to promote angiogenesis. The expression level of
periostin in metastatic tumors is reported to be noticeably higher
than that in the matched primary colon cancer (33).
Osteopontin is a multifunctional ECM phosphorylated
glycoprotein (glycol-phosphoprotein) that belongs to the Small
Integrin-Binding Ligand N-linked Glycoprotein (SIBLING) family, and
is reported to play an important role in the tumorigenesis,
progression and prognosis of various cancers by regulating
cell-matrix interactions and cell signaling through binding with
integrins and CD44 receptors (34–39). A
pooled data analysis showed that high osteopontin expression was
significantly associated with high tumor grade, invasion, lymph
node metastasis, tumor distant metastasis and poor survival in CRC
(40).
Thrombospondin-2 (THBS2) is a member of the ECM
glycoproteins that mediate ECM assembly, cell-matrix interactions,
degradation of matrix metalloproteinases (MMP)-2 and MMP-9, and
interact with multiple cell receptors and growth factors. The
implication of THBS2 expression in CRC has been controversial.
Several studies reported an inverse correlation between THBS2
expression level and malignancy grade (41,42). In
contrast, resent studies reported that THBS2 expression in CRC was
positively correlated with TNM stage and is a strong prognostic
indicator (43,44).
The GPNMB gene is reported to be overexpressed in
numerous cancers and is often associated with the metastatic
phenotype (45–49). The extracellular domain of GPNMB
interacts with integrins to facilitate the recruitment of
immune-suppressive and proangiogenic cells to the tumor
microenvironment, thereby enhancing tumor migration and invasion
(50). GPNMB expressed in immune
cells such as macrophages and dendritic cells (14,29) may
impair T-cell activation to down-modulate anti-tumor immune
responses (51–53). However, the role of GPNMB is complex;
it appears to have an inhibitory role in some cancers, but may
promote metastasis in others.
MGP is an ECM protein containing
post-translationally modified γ-carboxyglutamate residues due to
vitamin K-dependent carboxylation. MGP was initially thought to be
involved in the inhibition of calcification of arteries and
cartilage. Further investigation demonstrated that MGP had a wider
range of activities, which were dependent upon
phosphorylation-carboxylation status, protein expression and
variants. Recent studies showed that MGP plays a role in tumor
angiogenesis by increasing vascular endothelial growth factor gene
expression (54–56). Recently, MGP was reported to be
upregulated in a variety of tumors, including ovarian, breast,
urogenital and skin cancer. However, in colon and lung cancers, an
inverse correlation between MGP expression and survival was
observed (57).
In the present study, exhaustive genetic analysis
using next-generation sequencing and a comparison of immunoreactive
factors revealed the complexities of gene expression in CRLM. It is
especially notable that compared to primary CRC, CRLM has greater
genomic heterogeneity associated with the ECM. This result
corroborates our hypotheses. To proliferate in the liver, which
differs environmentally from the original colorectal tissue in
which they exist naturally, cancer cells must modify their
microenvironment to make it more amenable for survival. A suitable
microenvironment cannot be regulated by a single factor; instead,
complex factors are involved, especially in metastatic sites. The
complexity of intratumor heterogeneity, which we revealed in this
study, may be an underlying cause of the resistance to treatment
for metastatic disease.
Although differences in genomic mutational profiling
between primary and metastatic sites have been reported in some
studies (58–60), most studies have used metachronous or
unpaired patient samples. Moreover, a limited set of biomarkers was
employed to show that metastatic sites have more inherited
mutations than primary CRC. These studies have potential biases
related to both intra- and intertumoral heterogeneity.
It has been reported that the several kinds of
systemic chemotherapy could alter the genomic landscape in some
cancers (61,62). Thus, adjuvant chemotherapy has the
potential to alter the genomic mutational profiles of recurrent
cancers, changing them from those of the original primary tumors
(63). Previous analyses evaluating
the heterogeneity of metachronous tumors could be biased by the
administration of chemotherapy between the resection of primary CRC
and metastatic sites, potentially producing alterations in genomic
clones (58,64). Our study eliminates this bias since
we selected paired samples of synchronous tumors.
This study has two important limitations. First, the
study included a relatively small number of patients. We plan to
continue our analysis using an increased number of cases in the
future. Next, although the gene expression of thrombospondin-2,
GPNMB, and MGP in CRLM was more frequent than in primary CRC
according to the DNA microarray analysis, the immunohistochemical
analysis revealed no differences in expression. We consider that
the discrepancy between the tissue regions analyzed by DNA
microarray and those subjected to immunohistochemical analysis led
to this result. The relatively low immunoreactivity scores of GPNMB
and MGP may be attributed to the timing of degradation and wash-out
of these proteins from the tissue (56).
There are three future perspectives from this study.
First, we also obtained data of surgically resected tumor specimens
and corresponding peripheral blood samples not only for CRLM, but
also pulmonary metastasis, peritoneal metastasis, and ovarian
metastasis through WES, cancer gene panel sequencing, fusion gene
panel sequencing and microarray-based GEP under the framework of
‘project HOPE’. We will therefore be able to perform further
investigations using these samples. Second, studies of circulating
tumor cells (liquid biopsy) may be useful for establishing early
CRLM diagnosis. It may also lead to better predictive biomarkers to
identify patients who might benefit from adjuvant chemotherapy.
Third, the genes that were overexpressed in this study have the
potential to be new therapeutic targets. For example, the
administration of anti-periostin antibody significantly inhibited
the growth of primary tumors as well as metastatic tumors in a
murine model of breast cancer (29).
Osteopontin-inhibition is also reported to be a favorable
therapeutic approach to metastatic disease (65–68). An
antibody-drug conjugate targeting GPNMB, called glembatumumab
vedotin (CDX-011), is currently being assessed in clinical studies
for various cancers (69).
In conclusion, we examined the genomic heterogeneity
between paired primary CRC and CRLM in terms of the
microenvironment on the proliferation, invasion, and metastasis of
cancer cells. These findings will lead to new diagnostic and
therapeutic targets for CRC in the era of genome-guided
personalized cancer treatment.
Acknowledgements
The authors would like to thank Mr. Koji Muramatsu
(Division of Pathology, Shizuoka Cancer Center) for his technical
pathological assistance.
Funding
This research was funded by the prefectural
government of Shizuoka Prefecture, Japan.
Availability of data and materials
The dataset presented in the current study has been
submitted to the National Bioscience Database Center (NBDC) under
the accession number hum0127 (https://humandbs.biosciencedbc.jp/en/hum0127-v2).
Authors' contributions
AS, MK, TaS, TeS, KO, TN, KU, MS, HS and KY designed
this retrospective study. AS and TeS enrolled patients to Project
HOPE. AS, MK, TaS, MS and TN analyzed and interpreted the data. AS
and MK confirmed the authenticity of all the raw data. All authors
drafted the manuscript, revised the paper, and read and approved
the final manuscript.
Ethics approval and consent to
participate
The research plan of Project HOPE was designed
according to the revised Ethical Guidelines for Human Genome/Gene
Analysis Research in Japan (http://www.lifescience.mext.go.jp/files/pdf/n1115_01.pdf),
and the study protocol was approved by the Institutional Review
Board at the Shizuoka Cancer Center (authorization no. 30-5). This
retrospective study was also approved by the same board. Written
informed consent was obtained from all participants.
Patient consent for publication
Consent for publication was obtained from all
participants.
Competing interests
The authors declare that they have no competing
interest.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
CRLM
|
colorectal liver metastasis
|
|
Project HOPE
|
High-tech Omics-based Patient
Evaluation
|
|
WES
|
whole-exome sequencing
|
|
GEP
|
gene expression profiling
|
|
SNV
|
single nucleotide variants
|
|
GPNMB
|
glycoprotein nonmetastatic melanoma
protein B
|
|
MGP
|
matrix Gla protein
|
|
THBS2
|
thrombospondin-2
|
|
TMB
|
tumor mutation burden
|
|
SMAP
|
Shizuoka Multi-omics Analysis
Protocol
|
|
ECM
|
extracellular matrix
|
|
CMS
|
consensus molecular subtypes
|
|
TCGA
|
The Cancer Genome Atlas
|
|
ICGC
|
International Cancer Genome
Consortium
|
|
SIBLING
|
small integrin-binding ligand N-linked
glycoprotein
|
|
MMP
|
matrix metalloproteinases
|
References
|
1
|
DeSantis CE, Lin CC, Mariotto AB, Siegel
RL, Stein KD, Kramer JL, Alteri R, Robbins AS and Jemal A: Cancer
treatment and survivorship statistics, 2014. CA Cancer J Clin.
64:252–271. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hashiguchi Y, Muro K, Saito Y, Ito Y,
Ajioka Y, Hamaguchi T, Hasegawa K, Hotta K, Ishida H, Ishiguro M,
et al Japanese Society for Cancer of the Colon Rectum, : Japanese
Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019
for the treatment of colorectal cancer. Int J Clin Oncol. 25:1–42.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Folprecht G, Gruenberger T, Bechstein WO,
Raab HR, Lordick F, Hartmann JT, Lang H, Frilling A, Stoehlmacher
J, Weitz J, et al: Tumour response and secondary resectability of
colorectal liver metastases following neoadjuvant chemotherapy with
cetuximab: The CELIM randomised phase 2 trial. Lancet Oncol.
11:38–47. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Majeed AW: Surgery for colorectal liver
metastases with hepatic lymph node involvement: A systematic
review. Br J Surg. 87:17372000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Martin LW and Warren RS: Current
management of colorectal liver metastases. Surg Oncol Clin N Am.
9:853–878. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Penna C and Nordlinger B: Colorectal
metastasis (liver and lung). Surg Clin North Am. 82:1075–1090,
x-xi. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fidler IJ and Kripke ML: Metastasis
results from preexisting variant cells within a malignant tumor.
Science. 197:893–895. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Futakuchi M, Nannuru KC, Varney ML,
Sadanandam A, Nakao K, Asai K, Shirai T, Sato SY and Singh RK:
Transforming growth factor-beta signaling at the tumor-bone
interface promotes mammary tumor growth and osteoclast activation.
Cancer Sci. 100:71–81. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nagashima T, Yamaguchi K, Urakami K,
Shimoda Y, Ohnami S, Ohshima K, Tanabe T, Naruoka A, Kamada F,
Serizawa M, et al: Japanese version of The Cancer Genome Atlas,
JCGA, established using fresh frozen tumors obtained from 5143
cancer patients. Cancer Sci. 111:687–699. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Japanese ethical guidelines for human
genome/gene analysis research. https://www.mhlw.go.jp/general/seido/kousei/i-kenkyu/genome/0504sisin.htmlMarch
25–2021
|
|
12
|
Ion Reporter Software User Guide, .
Tumor-Normal pair workflow. https://tools.thermofisher.com/content/sfs/manuals/IonReporter_v50_Help.pdfMarch
25–2021
|
|
13
|
Torrent Suite v4.4.3 User and Admin Guide,
. https://assets.thermofisher.com/TFS-Assets/LSG/manuals/MAN0019144_TorrentSuite_5_14_UG.pdfMarch
25–2021
|
|
14
|
Robinson JT, Thorvaldsdóttir H, Winckler
W, Guttman M, Lander ES, Getz G and Mesirov JP: Integrative
genomics viewer. Nat Biotechnol. 29:24–26. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bamford S, Dawson E, Forbes S, Clements J,
Pettett R, Dogan A, Flanagan A, Teague J, Futreal PA, Stratton MR,
et al: The COSMIC (Catalogue of Somatic Mutations in Cancer)
database and website. Br J Cancer. 91:355–358. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Landrum MJ, Lee JM, Riley GR, Jang W,
Rubinstein WS, Church DM and Maglott DR: ClinVar: Public archive of
relationships among sequence variation and human phenotype. Nucleic
Acids Res. 42:D980–D985. 2014. View Article : Google Scholar
|
|
17
|
Sherry ST, Ward MH, Kholodov M, Baker J,
Phan L, Smigielski EM and Sirotkin K: dbSNP: The NCBI database of
genetic variation. Nucleic Acids Res. 308–311. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
UniProt Consortium: UniProt: A hub for
protein information. Nucleic Acids Res. 43:D204–D212. 2015.
View Article : Google Scholar
|
|
19
|
Wishart DS, Knox C, Guo AC, Shrivastava S,
Hassanali M, Stothard P, Chang Z and Woolsey J: DrugBank: A
comprehensive resource for in silico drug discovery and
exploration. Nucleic Acids Res. 34:D668–D672. 2006. View Article : Google Scholar
|
|
20
|
Nagashima T, Shimoda Y, Tanabe T, Naruoka
A, Saito J, Serizawa M, Ohshima K, Urakami K, Ohnami S, Ohnami S,
et al: Optimizing an ion semiconductor sequencing data analysis
method to identify somatic mutations in the genomes of cancer cells
in clinical tissue samples. Biomed Res. 37:359–366. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shimoda Y, Nagashima T, Urakami K, Tanabe
T, Saito J, Naruoka A, Serizawa M, Mochizuki T, Ohshima K, Ohnami
S, et al: Integrated next-generation sequencing analysis of whole
exome and 409 cancer-related genes. Biomed Res. 37:367–379. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Urakami K, Shimoda Y, Ohshima K, Nagashima
T, Serizawa M, Tanabe T, Saito J, Usui T, Watanabe Y, Naruoka A, et
al: Next generation sequencing approach for detecting 491 fusion
genes from human cancer. Biomed Res. 37:51–62. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guinney J, Dienstmann R, Wang X, de
Reyniès A, Schlicker A, Soneson C, Marisa L, Roepman P, Nyamundanda
G, Angelino P, et al: The consensus molecular subtypes of
colorectal cancer. Nat Med. 21:1350–1356. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sadanandam A, Lyssiotis CA, Homicsko K,
Collisson EA, Gibb WJ, Wullschleger S, Ostos LC, Lannon WA,
Grotzinger C, Del Rio M, et al: A colorectal cancer classification
system that associates cellular phenotype and responses to therapy.
Nat Med. 19:619–625. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Le DT, Durham JN, Smith KN, Wang H,
Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et
al: Mismatch repair deficiency predicts response of solid tumors to
PD-1 blockade. Science. 357:409–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Polanska UM and Orimo A:
Carcinoma-associated fibroblasts: Non-neoplastic tumour-promoting
mesenchymal cells. J Cell Physiol. 228:1651–1657. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ishii G, Ochiai A and Neri S: Phenotypic
and functional heterogeneity of cancer-associated fibroblast within
the tumor microenvironment. Adv Drug Deliv Rev. 99:186–196. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kyutoku M, Taniyama Y, Katsuragi N,
Shimizu H, Kunugiza Y, Iekushi K, Koibuchi N, Sanada F, Oshita Y
and Morishita R: Role of periostin in cancer progression and
metastasis: Inhibition of breast cancer progression and metastasis
by anti-periostin antibody in a murine model. Int J Mol Med.
28:181–186. 2011.PubMed/NCBI
|
|
30
|
Erkan M, Kleeff J, Gorbachevski A, Reiser
C, Mitkus T, Esposito I, Giese T, Büchler MW, Giese NA and Friess
H: Periostin creates a tumor-supportive microenvironment in the
pancreas by sustaining fibrogenic stellate cell activity.
Gastroenterology. 132:1447–1464. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kudo Y, Ogawa I, Kitajima S, Kitagawa M,
Kawai H, Gaffney PM, Miyauchi M and Takata T: Periostin promotes
invasion and anchorage-independent growth in the metastatic process
of head and neck cancer. Cancer Res. 66:6928–6935. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Puglisi F, Puppin C, Pegolo E, Andreetta
C, Pascoletti G, D'Aurizio F, Pandolfi M, Fasola G, Piga A, Damante
G, et al: Expression of periostin in human breast cancer. J Clin
Pathol. 61:494–498. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Moniuszko T, Wincewicz A, Koda M,
Domysławska I and Sulkowski S: Role of periostin in esophageal,
gastric and colon cancer. Oncol Lett. 12:783–787. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sodek J, Ganss B and McKee MD:
Osteopontin. Crit Rev Oral Biol Med. 11:279–303. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang J, Takahashi K, Takahashi F, Shimizu
K, Ohshita F, Kameda Y, Maeda K, Nishio K and Fukuchi Y:
Differential osteopontin expression in lung cancer. Cancer Lett.
171:215–222. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song G, Cai QF, Mao YB, Ming YL, Bao SD
and Ouyang GL: Osteopontin promotes ovarian cancer progression and
cell survival and increases HIF-1alpha expression through the
PI3-K/Akt pathway. Cancer Sci. 99:1901–1907. 2008.PubMed/NCBI
|
|
37
|
Kim JY, Bae BN, Kim KS, Shin E and Park K:
Osteopontin, CD44, and NFkappaB expression in gastric
adenocarcinoma. Cancer Res Treat. 41:29–35. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Likui W, Hong W and Shuwen Z: Clinical
significance of the upregulated osteopontin mRNA expression in
human colorectal cancer. J Gastrointest Surg. 14:74–81. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bramwell VH, Tuck AB, Chapman JA, Anborgh
PH, Postenka CO, Al-Katib W, Shepherd LE, Han L, Wilson CF,
Pritchard KI, et al: Assessment of osteopontin in early breast
cancer: Correlative study in a randomised clinical trial. Breast
Cancer Res. 16:R82014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhao M, Liang F, Zhang B, Yan W and Zhang
J: The impact of osteopontin on prognosis and clinicopathology of
colorectal cancer patients: A systematic meta-analysis. Sci Rep.
5:127132015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tokunaga T, Nakamura M, Oshika Y, Abe Y,
Ozeki Y, Fukushima Y, Hatanaka H, Sadahiro S, Kijima H, Tsuchida T,
et al: Thrombospondin 2 expression is correlated with inhibition of
angiogenesis and metastasis of colon cancer. Br J Cancer.
79:354–359. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
de Fraipont F, Nicholson AC, Feige JJ and
Van Meir EG: Thrombospondins and tumor angiogenesis. Trends Mol
Med. 7:401–407. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim H, Watkinson J, Varadan V and
Anastassiou D: Multi-cancer computational analysis reveals
invasion-associated variant of desmoplastic reaction involving
INHBA, THBS2 and COL11A1. BMC Med Genomics. 3:512010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tian Q, Liu Y, Zhang Y, Song Z, Yang J,
Zhang J, Guo T, Gao W, Dai F and He C: THBS2 is a biomarker for
AJCC stages and a strong prognostic indicator in colorectal cancer.
J BUON. 23:1331–1336. 2018.PubMed/NCBI
|
|
45
|
Rich JN, Shi Q, Hjelmeland M, Cummings TJ,
Kuan CT, Bigner DD, Counter CM and Wang XF: Bone-related genes
expressed in advanced malignancies induce invasion and metastasis
in a genetically defined human cancer model. J Biol Chem.
278:15951–15957. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kuan CT, Wakiya K, Dowell JM, Herndon JE
II, Reardon DA, Graner MW, Riggins GJ, Wikstrand CJ and Bigner DD:
Glycoprotein nonmetastatic melanoma protein B, a potential
molecular therapeutic target in patients with glioblastoma
multiforme. Clin Cancer Res. 12:1970–1982. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rose AA, Pepin F, Russo C, Abou Khalil JE,
Hallett M and Siegel PM: Osteoactivin promotes breast cancer
metastasis to bone. Mol Cancer Res. 5:1001–1014. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rose AA, Annis MG, Dong Z, Pepin F,
Hallett M, Park M and Siegel PM: ADAM10 releases a soluble form of
the GPNMB/Osteoactivin extracellular domain with angiogenic
properties. PLoS One. 5:e120932010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Rose AA, Grosset AA, Dong Z, Russo C,
Macdonald PA, Bertos NR, St-Pierre Y, Simantov R, Hallett M, Park
M, et al: Glycoprotein nonmetastatic B is an independent prognostic
indicator of recurrence and a novel therapeutic target in breast
cancer. Clin Cancer Res. 16:2147–2156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Taya M and Hammes SR: Glycoprotein
non-metastatic melanoma protein B (GPNMB) and cancer: A novel
potential therapeutic target. Steroids. 133:102–107. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhou LT, Liu FY, Li Y, Peng YM, Liu YH and
Li J: Gpnmb/osteoactivin, an attractive target in cancer
immunotherapy. Neoplasma. 59:1–5. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Selim AA: Osteoactivin bioinformatic
analysis: Prediction of novel functions, structural features, and
modes of action. Med Sci Monit. 15:MT19–MT33. 2009.
|
|
53
|
Singh M, Del Carpio-Cano F, Belcher JY,
Crawford K, Frara N, Owen TA, Popoff SN and Safadi FF: Functional
roles of osteoactivin in normal and disease processes. Crit Rev
Eukaryot Gene Expr. 20:341–357. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mertsch S, Schurgers LJ, Weber K, Paulus W
and Senner V: Matrix gla protein (MGP): An overexpressed and
migration-promoting mesenchymal component in glioblastoma. BMC
Cancer. 9:3022009. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kuzontkoski PM, Mulligan-Kehoe MJ, Harris
BT and Israel MA: Inhibitor of DNA binding-4 promotes angiogenesis
and growth of glioblastoma multiforme by elevating matrix GLA
levels. Oncogene. 29:3793–3802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gheorghe SR and Crăciun AM: Matrix Gla
protein in tumoral pathology. Clujul Med. 89:319–321.
2016.PubMed/NCBI
|
|
57
|
Caiado H, Conceição N, Tiago D, Marreiros
A, Vicente S, Enriquez JL, Vaz AM, Antunes A, Guerreiro H, Caldeira
P, et al: Evaluation of MGP gene expression in colorectal cancer.
Gene. 723:1441202020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hope NR and Murray GI: The expression
profile of RNA-binding proteins in primary and metastatic
colorectal cancer: Relationship of heterogeneous nuclear
ribonucleoproteins with prognosis. Hum Pathol. 42:393–402. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Vakiani E, Janakiraman M, Shen R, Sinha R,
Zeng Z, Shia J, Cercek A, Kemeny N, D'Angelica M, Viale A, et al:
Comparative genomic analysis of primary versus metastatic
colorectal carcinomas. J Clin Oncol. 30:2956–2962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Schrijver WAME, Selenica P, Lee JY, Ng
CKY, Burke KA, Piscuoglio S, Berman SH, Reis-Filho JS, Weigelt B,
van Diest PJ, et al: Mutation profiling of key cancer genes in
primary breast cancers and their distant metastases. Cancer Res.
78:3112–3121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Ding L, Ley TJ, Larson DE, Miller CA,
Koboldt DC, Welch JS, Ritchey JK, Young MA, Lamprecht T, McLellan
MD, et al: Clonal evolution in relapsed acute myeloid leukaemia
revealed by whole-genome sequencing. Nature. 481:506–510. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Johnson BE, Mazor T, Hong C, Barnes M,
Aihara K, McLean CY, Fouse SD, Yamamoto S, Ueda H, Tatsuno K, et
al: Mutational analysis reveals the origin and therapy-driven
evolution of recurrent glioma. Science. 343:189–193. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Harada K, Okamoto W, Mimaki S, Kawamoto Y,
Bando H, Yamashita R, Yuki S, Yoshino T, Komatsu Y, Ohtsu A, et al:
Comparative sequence analysis of patient-matched primary colorectal
cancer, metastatic, and recurrent metastatic tumors after adjuvant
FOLFOX chemotherapy. BMC Cancer. 19:2552019. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gagnière J, Dupré A, Gholami SS, Pezet D,
Boerner T, Gönen M, Kingham TP, Allen PJ, Balachandran VP, De
Matteo RP, et al: Is hepatectomy justified for BRAF mutant
colorectal liver metastases?: A multi-institutional analysis of
1497 patients. Ann Surg. 271:147–154. 2020. View Article : Google Scholar
|
|
65
|
Wu Y, Denhardt DT and Rittling SR:
Osteopontin is required for full expression of the transformed
phenotype by the ras oncogene. Br J Cancer. 83:156–163. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Nemoto H, Rittling SR, Yoshitake H, Furuya
K, Amagasa T, Tsuji K, Nifuji A, Denhardt DT and Noda M:
Osteopontin deficiency reduces experimental tumor cell metastasis
to bone and soft tissues. J Bone Miner Res. 16:652–659. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wu XL, Lin KJ, Bai AP, Wang WX, Meng XK,
Su XL, Hou MX, Dong PD, Zhang JJ, Wang ZY, et al: Osteopontin
knockdown suppresses the growth and angiogenesis of colon cancer
cells. World J Gastroenterol. 20:10440–10448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wei R, Wong JPC and Kwok HF: Osteopontin -
a promising biomarker for cancer therapy. J Cancer. 8:2173–2183.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rose AAN, Biondini M, Curiel R and Siegel
PM: Targeting GPNMB with glembatumumab vedotin: Current
developments and future opportunities for the treatment of cancer.
Pharmacol Ther. 179:127–141. 2017. View Article : Google Scholar : PubMed/NCBI
|