Introduction
Prostate cancer is one of the most common malignant
tumors in men. Historically, the incidence rate of prostate cancer
has been lower in China compared with in Western countries
(1). However, with lifestyle changes
due to economic growth, the incidence rate of prostate cancer has
been rapidly increasing in recent years. A high-fat diet and lack
of exercise are among the factors that contribute to prostate
cancer (2). In addition, patients
with local infiltration and distant metastasis often have a poor
prognosis (3). Therefore, inhibiting
metastasis in prostate cancer has become a major challenge for
clinicians.
Circular RNAs (circRNAs) are natural endogenous RNAs
that regulate transcriptional and post-transcriptional steps
(4). circRNAs are evolutionarily
conserved and stable. Although they are considered a novel type of
non-coding RNA, numerous studies have shown that some circRNAs can
encode proteins (5). circRNAs have
tissue- and cell-specific expression patterns (6). circRNA is involved in the occurrence
and development of tumors. Its biological functions include acting
as a scaffold for protein complexes, regulating gene expression,
alternative splicing and participating in the interaction between
RNA and protein (7). Cerebellar
degeneration-related protein 1, anti-sense (circCDR1as;
hsa_circ_0001946) is derived from the antisense transcript of CDR1,
which is located on chromosome Xq27.1 (8). circCDR1as is highly expressed in
prostate cancer, but its role in prostate cancer is unclear
(9).
microRNAs (miRNAs/miRs) are a class of endogenous
single-chain non-coding RNAs consisting of 19–25 nucleotides.
miRNAs are widely found in eukaryotic cells and play an important
role in gene regulation. They bind to the 3′-untranslated regions
(3′-UTRs) of target mRNAs to regulate gene expression, and cause
either the translation or degradation of the target mRNAs (10,11).
miRNA-mediated inhibition of gene expression helps to regulate cell
development, proliferation and adhesion (12).
Recent studies have shown that most circRNAs exist
in the cytoplasm and their mechanisms of action are mediated by
miRNA sponge interactions through binding to miRNAs (4,13).
X-linked inhibitor of apoptosis protein (XIAP) is a major member of
a newly identified IAP family of proteins. It is the strongest
inhibitor of apoptosis in IAP family. It can directly inhibit
caspases and regulate apoptosis in multiple ways. XIAP is
overexpressed in prostate cancer cell lines, and its expression is
closely associated with tumor progression, recurrence, prognosis
and chemoresistance (14). Combined
with the results of bioinformatics analysis, the present study used
approaches to overexpress and suppress circCDR1as to elucidate its
molecular role in prostate cancer invasion at the cellular and
molecular levels. The current findings provide the scientific basis
for the design of strategies to inhibit invasion with circCDR1as as
the target.
Materials and methods
Bioinformatics analysis
The relative expression of circCDR1as was analyzed
using MiOncoCirc (https://mioncocirc.github.io/), the relative
expression of circCDR1as was analyzed in cancer tissues(ACC,
adrenocortical carcinoma; BLCA, bladder urothelial carcinoama;
BRCA, breast invasive carcinoma; chol, cholangiocarcinoma; colo,
colorectal cancer; esca, esophageal carcinoma; gbm, glioblastoma
multiforme; hcc, hepatocellular carcinoma; hnsc, head and neck
squamous cell carcinoma; kdny, kidney cancer; lung, lung cancer;
maly, malignant lymphoma; nbl, neuroblastoma; ov, ovarian serous
cystadenocarcinoma; paad, pancreatic adenocarcinoma; prad, prostate
adenocarcinoma; sarc, sarcoma; secr, cecretory cancer; skcm, skin
cutaneous melanoma. and the location and regulatory miRNAs of
circCDR1as were identified using circBase (http://www.circbase.org/). The target genes regulated
by miRNA were analyzed using TargetScan (http://www.targetscan.org/cgi-bin/targetscan/vert_72/view_gene.cgi?rs=ENST00000371199.3&taxid=9606&members=miR-641/3617-5p&showcnc=1&shownc=1&shownc_nc=1&showncf1=1&subset=1).
Cell culture and transfection
LNCaP, 22Rv1, PC-3, RWPE-1 and 293T cell lines were
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences. The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM)/F12 containing 10% newborn bovine
serum (Thermo Fisher Scientific, Inc.) at 37°C in 5%
CO2. The cells were digested and subcultured with 0.25%
trypsin every 2–3 days. Cells in the logarithmic growth period and
with a trypan blue rejection rate >95% were used in all
experiments.
The construction of a lentivirus vector expressing
siRNA targeting circCDR1as was carried out by Shanghai GenePharma
Co., Ltd. 293T cells in logarithmic growth phase were inoculated at
a density of 5×106/ml and cultured at 37°C with 5%
CO2. The recombinant si-circCDT1as vector was packaged
with 10 ug plasmid and incubated with Lipofectamine®
3000 to form a complex. The cells were treated with DMEM containing
1% FBS 24 h after transfection. After 48 h of transfection, the
culture medium was collected. The transfection sequences were as
follows: miR-641 Mature sequence (MI0003656),
5′-UGGGUGAAAGGAAGGAAAGACAUAGGAUAGAGUCACCUCUGUCCUCUGUCCUCUACCUAUAGAGGUGACUGUCCUAUGUCUUUCCUUCCUCUUACCCCU-3′;
miRNA mimic negative control (scrambled sequences, mimic-NC,
Guangzhou Ruibo Biotechnology Co., Ltd.), 5′-GUUACCAUGGAUGUAAU-3′;
small interfering (si)-circCDR1as, 5′-GCACCTGTGTCAAGGTCTTTT-3′ and
siRNA-NC (scrambled sequence), 5′-CGGGATTGGGATAAGCC-3′. siRNA-NC
was used for the transfection of si-XIAP (Guangzhou Ruibo
Biotechnology Co., Ltd.). si-XIAP sequences are presented in
Table SI and the most effective
sequence was used for subsequent studies. In total, 20 pmol of
siRNAs, miR-641 mimic and respective controls (cell group,
untransfected control cells) were transfected to PC-3 cell line
using Lipofectamine® 3000 (Thermo Fisher Scientific,
Inc.) according to the manufacturer's instructions. Transfections
were performed at room temperature for 6 h.
Reverse transcription-quantitative
(RT-q)PCR
Primer 6.0 (Canada primer, inc. www.premierbiosoft.com) was used to design the
primers (Table I), which were
synthesized by Thermo Fisher Scientific, Inc. LNCaP, 22Rv1, PC-3,
and RWPE-1 cells were cultured and treated with trypsin to make a
cell suspension. After counting the cells with a cell counting
plate, a cell suspension containing ~1.0×106 cells was
transferred to an RNase-free centrifuge tube to collect the cells.
TRIzol® reagent was used to extract RNA (Invitrogen;
Thermo Fisher Scientific, Inc.), and M-MLV reverse transcriptase
(Thermo Fisher Scientific, Inc.) was used to reverse transcribed
RNA into cDNA. Reverse transcription was performed at 16°C for 30
min, 42°C for 40 min and 85°C for 5 min. cDNA was used as a
template for PCR reaction using SYBR Green qPCR supermax ((Takara
Biotechnology Co., Ltd.). Under the following conditions: 50°C For
2 min, 95°C for 2 min, 95°C for 15 sec and 60°C for 32 sec for 40
cycles. GAPDH and U6 were used as internal references, and the
2−ΔΔCq method was used quantify the results (15).
 | Table I.Primers for genes. |
Table I.
Primers for genes.
| Gene | Forward, 5′-3′ | Reverse, 5′-3′ |
|---|
| GAPDH |
GTTGGTGGTGCAGGAGGCA |
CTCGCTTCGGCAGCACA |
| circCDR1 |
GGTTTCCGATGGCACCTG |
GGTTTCCGATGGCACCTG |
| miR-641 |
AAAGACATAGGATAGAGTCACCT C |
GTGCAGGGTCCGAGGT |
| XIAP |
CATCCATGGCAGATTATGAAGCA |
CTTCACTGGGCTTCCAATCAGTTAG |
| RT-miRNA |
TCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGA |
AACGCTTCACGAATTTGCGT |
| U6 |
GTTCCCATCTCGCTTCGGCAGCACA |
AACGCTTCACGAATTTGCGT |
MTT assay
There were six experimental groups: Cell group
(untransfected cells), siRNA-NC group (negative control sequence +
Lipofectamine 3000), miR-641 (miR-641 mimic + Lipofectamine 3000),
mimic-NC group (negative control miRNA mimic + Lipofectamine 3000),
si-circCDR1as (siRNA circCDR1as + Lipofectamine 3000), si-XIAP
(siRNA XIAP + Lipofectamine 3000). PC-3 cells in the logarithmic
growth phase were collected in serum-free DMEM/F12 and the cell
concentration was adjusted to 1.0×105 cells/ml with
dilution with serum-free DMEM/F12. Next, 96-well plates were
inoculated with 100 µl/well. After 24, 48 and 72 h, 20 µl MTT
solution [5 mg/ml in phosphate-buffered saline (PBS)] was added to
each well. The cells were incubated for 4 h at 37°C and the culture
supernatant was carefully removed from each well and discarded.
Suspended cells were centrifuged (1,000 × g at 37°C for 3 mins)
prior to removing the culture medium. Then, 150 µl dimethyl
sulfoxide was added to each well, and the formazan crystals were
dissolved by shaking for 10 min. The optical absorption value of
each well at a wavelength of 490 nm was measured using a plate
reader.
Transwell assay
The six aforementioned experimental groups were
analyzed. Precooled serum-free medium was used to dilute the
membrane matrix (Matrigel; Corning Inc.) at a volume ratio of 1:3,
40 µl of which was added to the precooled Transwell cells and
incubated at 37°C for 2 h to coagulate the membrane matrix. Excess
liquid was removed from the chamber, and 100 and 600 µl serum-free
medium was added to the upper and lower chambers, respectively. The
cells were incubated overnight at 37°C. On the second day of cell
transfection, 1.0×105 cells were counted use a cell
counter (Thermo Fisher Scientific, Inc.), and resuspended in 100 µl
serum-free DMEM/F12. The cells were added to the upper chamber of
the Transwell chamber and 600 µl DMEM/F12 containing 10% newborn
bovine serum was added to the lower chamber. After incubation at
37°C and 5% CO2 for 24 and 48 h, the cells in the upper
chamber were removed and wiped with a cotton swab (15). The cells in the lower chamber were
observed under an inverted light microscope and images were
captured. The cells were stained with crystal violet (37°C for 10
min), which was eluted in 33% acetic acid, and absorbance at a
wavelength of 570 nm was measured using a plate reader.
Scratch assay
The aforementioned six experimental groups were
analyzed. When the cell fusion rate reached 100%, cells in the
logarithmic growth stage were washed three times with PBS after
being scratched vertically with a pipette tip. The cells debris
were removed and added to the serum-free medium for further
culture. At 0 and 48 h after scratching, images were captured under
a light microscope and the mean distance between cells was
calculated using ImageJ version 3.0 (National Institutes of Health)
according the following formula: Healing rate (%)=(1-measured
width/0 h mean measured width) ×100 (16).
Western blotting
The cells grouped were the same as aforementioned.
The PC3 cells of the different experimental groups were collected,
and protein was extracted using a nuclear extraction kit (Thermo
Fisher Scientific, Inc.). Protein concentration was measured using
the BCA method. Samples of 30 µg protein were loaded per lane and
separated using 10% gels and SDS-PAGE and transferred to a PVDF
membrane. The PVDF membrane was incubated in a PBS solution
containing 5% skimmed milk powder first for 2 h at room temperature
and then overnight at 4°C to block non-specific binding sites. The
membrane was incubated with the following antibodies: Anti-XIAP
(1;500; cat. no. ab229050; Abcam, Inc.), anti-GAPDH (1:500; cat.
no. ab9485; Abcam) at room temperature for 3 h, washed three times
with 0.1% Tween 20, and incubated at room temperature for 1 h with
a horseradish peroxidase-labeled mouse anti-XIAP, anti-GAPDH IgG
antibody (1:1,000; cat. no. ab205719; Abcam). The protein bands
were analyzed by AlphaEaseFC 4.0 software (Protein Simple) and
semi-quantitative quantification was performed.
Luciferase reporter assay
There were five experimental groups: Cell group
(untreated cells), NC group [NC sequence +
psiCHECK-2-circCDR1as-wild-type (wt), psiCHECK-2-circCDR1as-mutant
(mut), psiCHECK-2-XIAP-3′-untranslated region (UTR)-wt or
psiCHECK-2-XIAP-3′-UTR-mut], inhibitor-NC group (inhibitor-NC +
psiCHECK-2-circCDR1as-wt/psiCHECK-2-circCDR1as-mut or
psiCHECK-2-XIAP-3′-UTR-WT/psiCHECK-2-XIAP-3′-UTR-mut), miR-641
group (miR-641 mimic +
psiCHECK-2-circCDR1as-wt/psiCHECK-2-circCDR1as-mut or
psiCHECK-2-XIAP-3′-UTR-WT/psiCHECK-2-XIAP-3′-UTR-mut) and miR-641
inhibitor group (miR-641 inhibitor +
psiCHECK-2-circCDR1as-wt/psiCHECK-2-circCDR1as-mut or
psiCHECK-2-XIAP-3′-UTR-wt/psiCHECK-2-XIAP-3′-UTR-mut). psiCHECK-2
vector purchased from Promega Corporation. The genomic DNA of PC-3
cells was extracted as a PCR amplification template, this was
performed as aforementioned, and the 3′-UTR sequences of linear
circCDR1as and XIAP were amplified with genomic DNA as a template.
The PCR amplification products were double digested with
restriction enzymes Xho and NotIR, and the
amplification fragments were ligated to the psiCHECK-2 vector. DH5a
Escherichia coli were transformed with the ligated products.
Positive clones were screened using blue and white spot analysis
and enzyme digestion identification, and were sent for sequencing
and double fluorescence detection (17). Luciferase activity was calculated
using the formula: (Firefly Luciferase/Rellina Luciferase) and was
measured using a Dual Luciferase Reporter Assay System kit (Promega
Corporation) on a Tecan M200 Pro luminescence reader according to
the manufacturer's protocol (Beijing KeyuXingye Science and
Technology Development Co., Ltd.).
Anti-Ago2 RNA-binding protein
immunoprecipitation (RIP) assay
There were three experimental groups: Input group
(input), IgG group (IgG antibody) and Ago2 group (anti-Ago2
antibody). Biotin-coupled miRNA and circRNA pull-down assays were
performed as previously described (11). Briefly, PC-3 cells were transfected
with 20 nM of 5′-end biotinylated circCDR1as
(digoxin-5′-CTCAGGATTATCTGGAAGAC-3′) (digoxin; Guangzhou RiboBio
Co., Ltd.) for 1 day. Biotin-coupled RNA complexes were pulled down
by incubating the cell lysates with MyOne Streptavidin C1 Dynabeads
(Invitrogen; Thermo Fisher Scientific, Inc.). The abundance of
circCDR1as or miRNA in the bound fractions was evaluated by RT-qPCR
analysis (18).
Statistical analysis
Data are shown as the mean ± standard deviation. The
experiment repeated three times. SPSS 20.0 (IBM, Corp.) was used to
process the data, and one-way ANOVA followed by Bonferroni's
correction test was used to analyze the significance of differences
between different groups. Unpaired t-tests were used for
comparisons between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
circCDR1as is highly expressed in
prostate cancer and there are interactions between circCDR1as and
miR-641 and between miR-641 and XIAP
Bioinformatics analysis indicated that the relative
expression of circCDR1as was significantly higher in breast cancer,
prostate cancer and sarcoma samples compared with other cancer
tissues. Binding sites were identified between circCDR1as and
miR-641 and between miR-641 and XIAP. These results indicated that
there is a regulatory relationship between circCDR1as, miR-641 and
XIAP (Fig. 1).
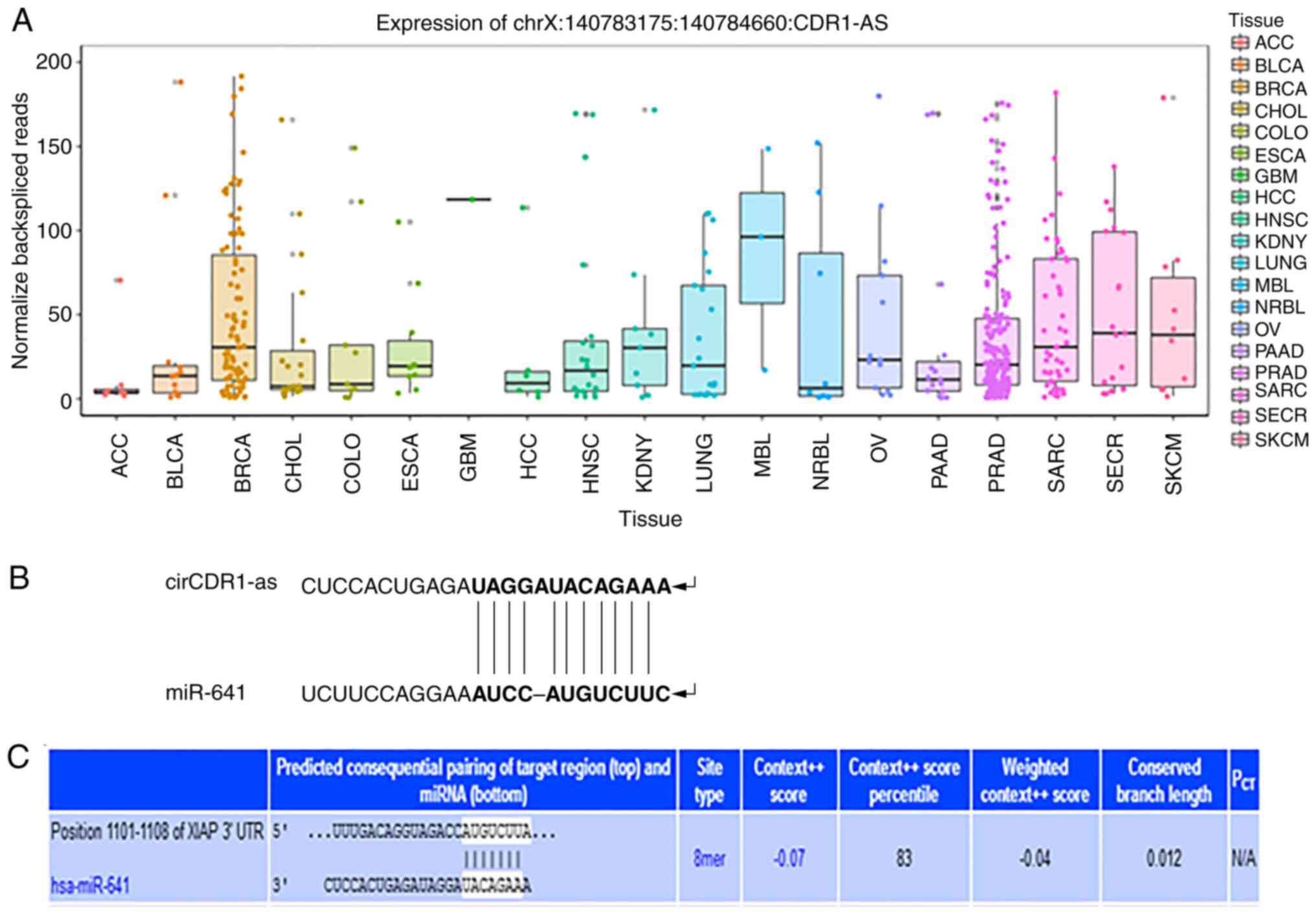 | Figure 1.Bioinformatics software prediction
results. (A) Relative expression levels of circCDR1as in cancer
tissue. (B) Schematic diagram of the binding site between
circCDR1as and miR-641. (C) Schematic diagram of the binding site
between miR-641 and XIAP. circCDR1as, circular RNA cerebellar
degeneration-related antigen 1, anti-sense; miR, microRNA; XIAP,
X-linked inhibitor of apoptosis protein; hsa, homo sapien; ACC,
adrenocortical carcinoma; BLCA, bladder urothelial carcinoama;
BRCA, breast invasive carcinoma; chol, cholangiocarcinoma; colo,
colorectal cancer; esca, esophageal carcinoma; gbm, glioblastoma
multiforme; hcc, hepatocellular carcinoma; hnsc, head and neck
squamous cell carcinoma; kdny, kidney cancer; lung, lung cancer;
maly, malignant lymphoma; nbl, neuroblastoma; ov, ovarian serous
cystadenocarcinoma; paad, pancreatic adenocarcinoma; prad, prostate
adenocarcinoma; sarc, sarcoma; secr, cecretory cancer; skcm, skin
cutaneous melanoma. |
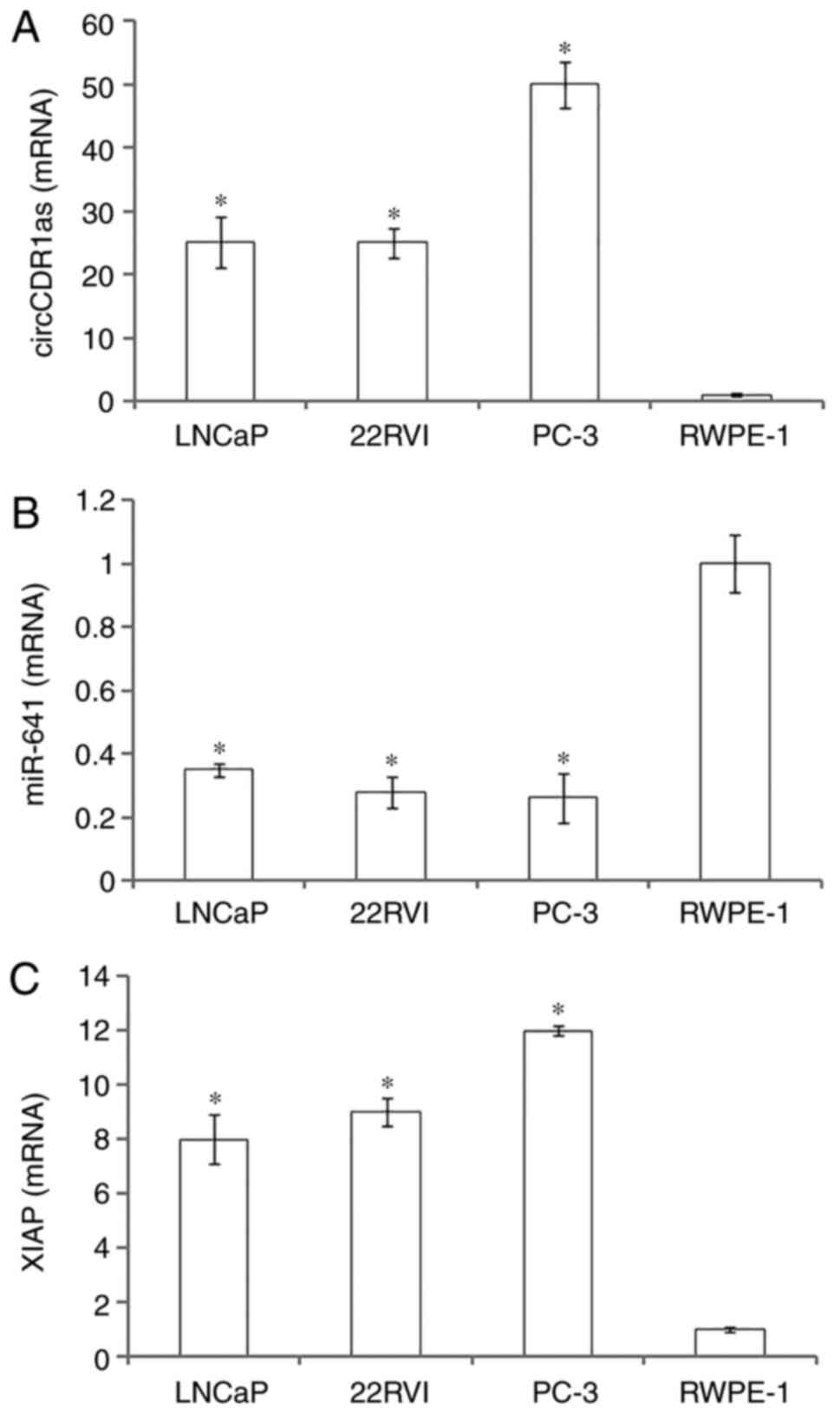
circCDR1as and XIAP expression is high
in prostate cancer cell lines and miR-641 expression is low in
prostate cancer cell lines
RT-qPCR showed that circCDR1as and XIAP mRNA were
highly expressed in prostate cancer cell lines (LNCaP, 22Rv1 and
PC-3) compared with normal prostate epithelial cells (RWPE-1), with
the highest expression in PC-3 cells. In contrast, miR-641 was
expressed at a low level in prostate cancer cells compared with
normal prostate epithelial cells. The results suggested that
circCDR1as and XIAP may promote the occurrence and development of
prostate cancer, and that miR-641 may inhibit the occurrence and
development of prostate cancer (Fig.
2).
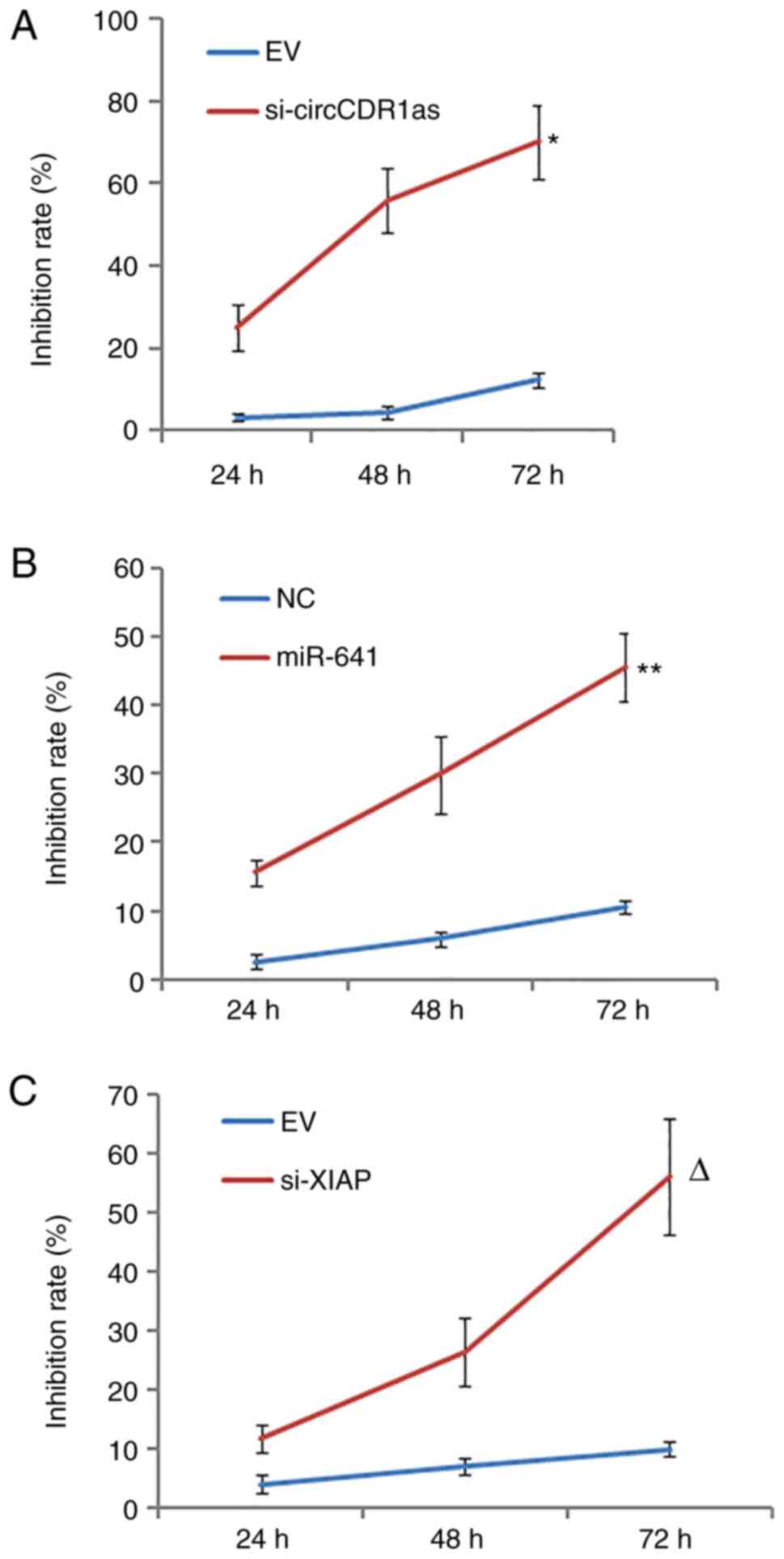
Reduction of circCDR1as and XIAP
expression and overexpression of miR-641 inhibit the proliferation
of PC-3 cells
After the expression levels of circCDR1as and XIAP
were suppressed and miR-641 was overexpressed (Fig. S1). MTT assay results showed that
si-circCDR1as, si-XIAP and miR-641 mimic inhibited the
proliferation of PC-3 cells in a time-dependent manner compared
with respective controls (Fig.
3).
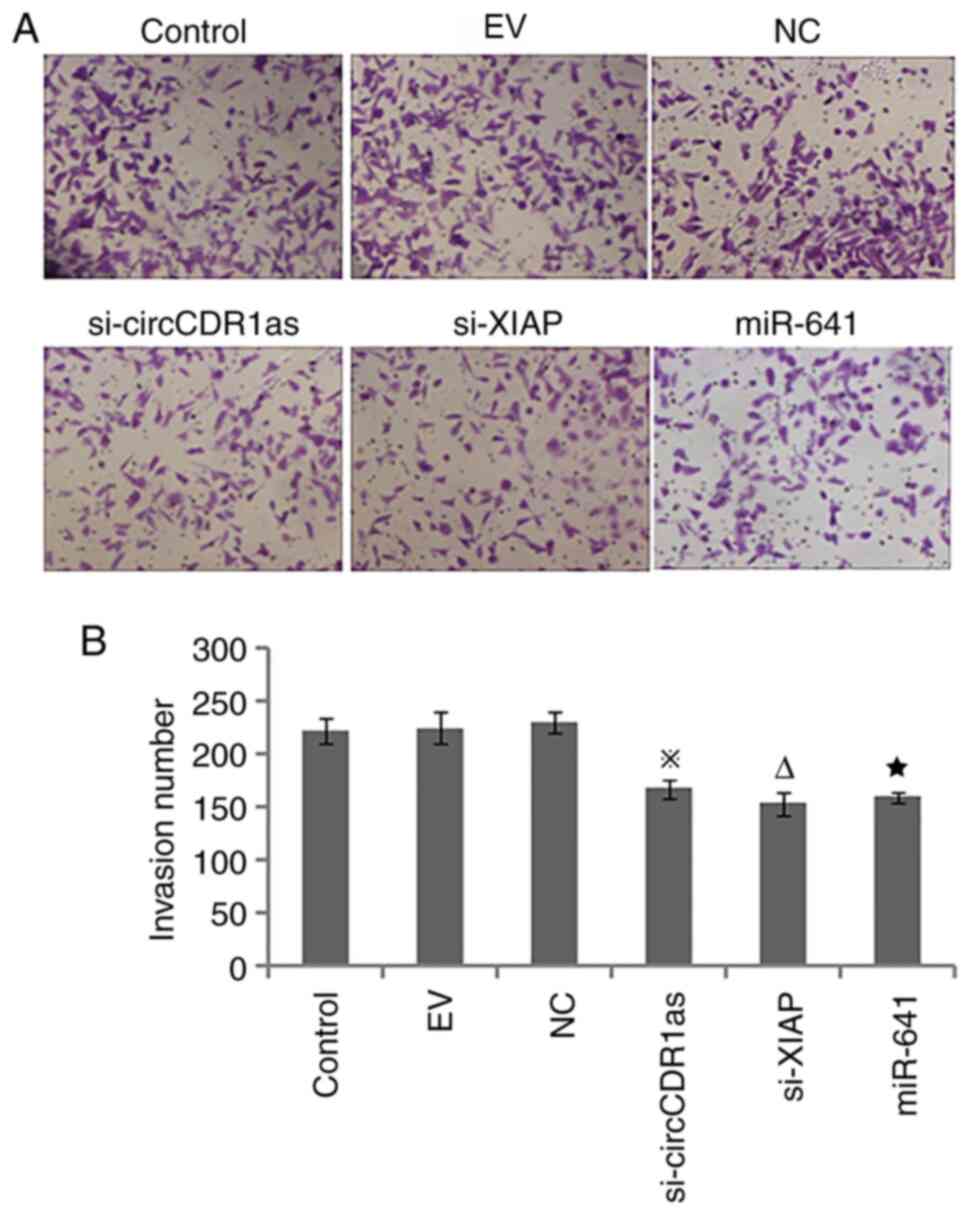
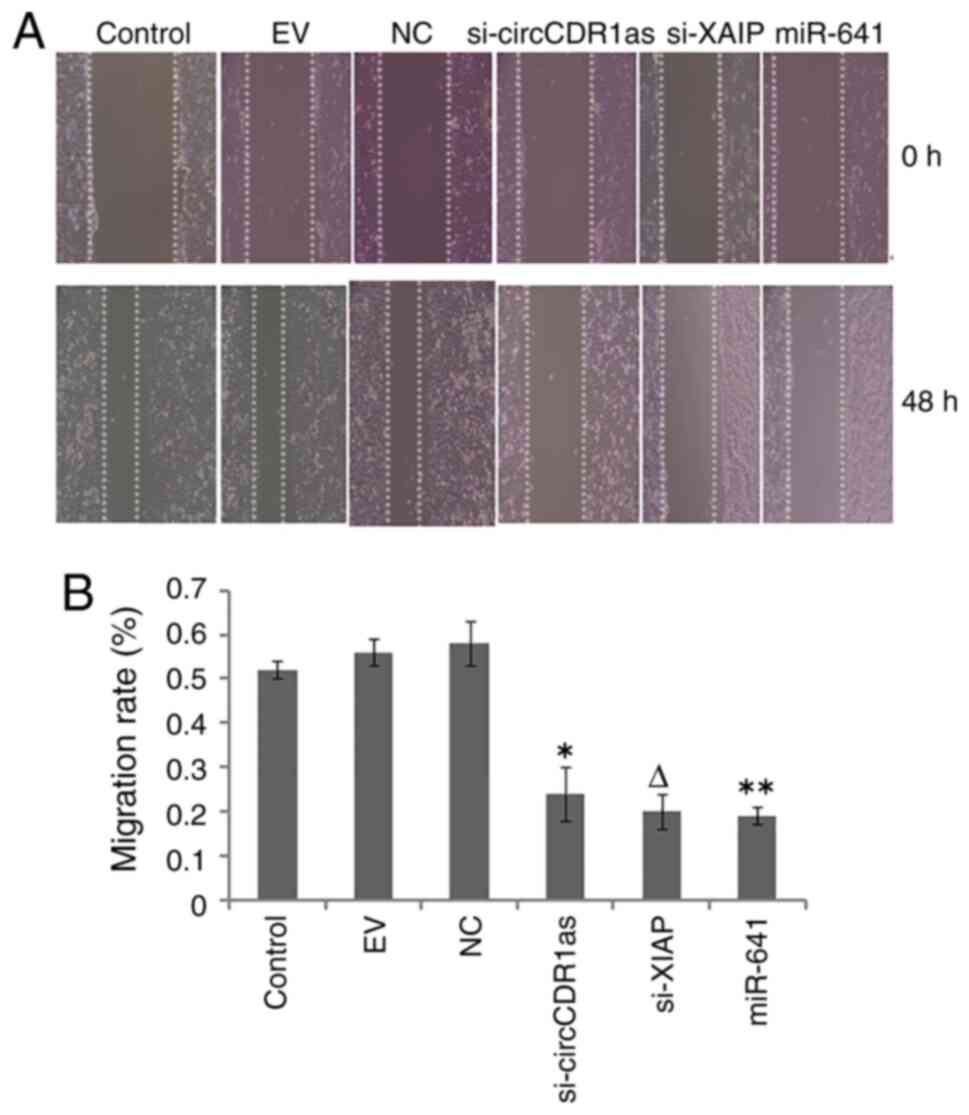
Reduction of circCDR1as and XIAP
expression and overexpression of miR-641 inhibit the invasion and
migration of PC-3 cells
After the expression of circCDR1as and XIAP were
suppressed and miR-641 was overexpressed, a Transwell assay showed
that si-circCDR1as, si-XIAP and miR-641 mimic inhibited the
invasion of PC-3 cells (Fig. 4). A
scratch assay showed that the migration of PC-3 cells could be
inhibited by si-circCDR1as, si-XIAP and miR-641 mimic (Fig. 5). These findings showed that the
invasion and migration of PC-3 cells could be inhibited by
decreasing the expression of circCDR1as and XIAP and increasing the
expression of miR-641.
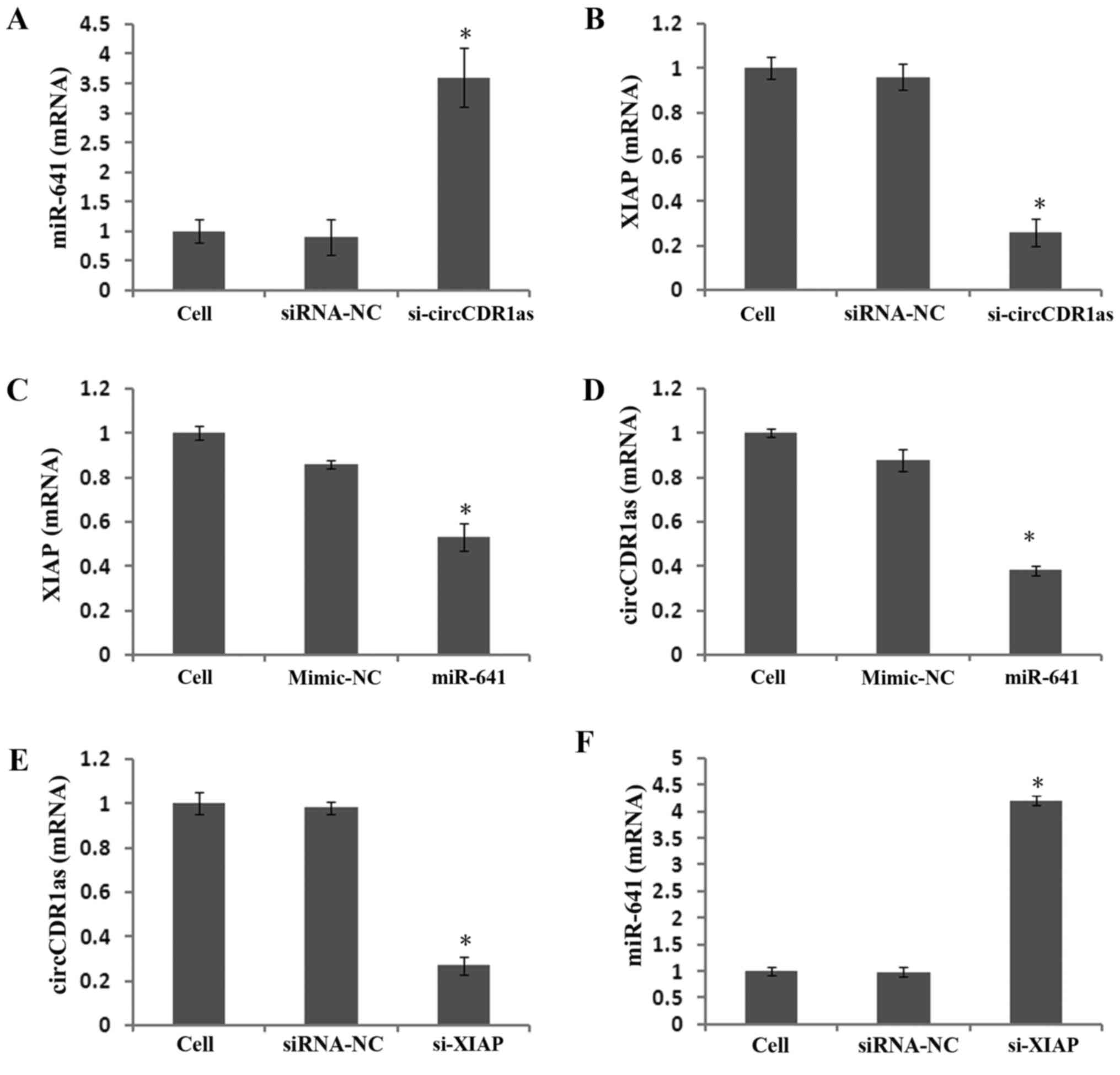
Reducing circCDR1as expression
promotes miR-641 expression and inhibits XIAP expression
To study the expression and regulatory mechanism of
circCDR1as, an anti-Ago2 RIP assay was used to detect binding
between circCDR1as and miR-641. The results showed that the
relative expression of miR-641 was 11 times higher compared with
that of the negative control group in the Ago2 antibody-binding RNA
group, indicating binding between circCDR1as and miR-641 (Fig. 6A and B). A double fluorescent
reporter gene vector was constructed to confirm the binding site
between miR-641 and the 3′-UTR of XIAP. The results showed that the
fluorescence activity of 293T cells co-transfected with miR-641
mimic and the 3′-UTR of XIAP was clearly reduced (Fig. 6C), but the fluorescence activity of
293T cells co-transfected with miR-641 mimic and the mut-3′-UTR of
XIAP showed no obvious change (Fig.
6D), indicating that miR-641 could bind to the 3′-UTR of XIAP.
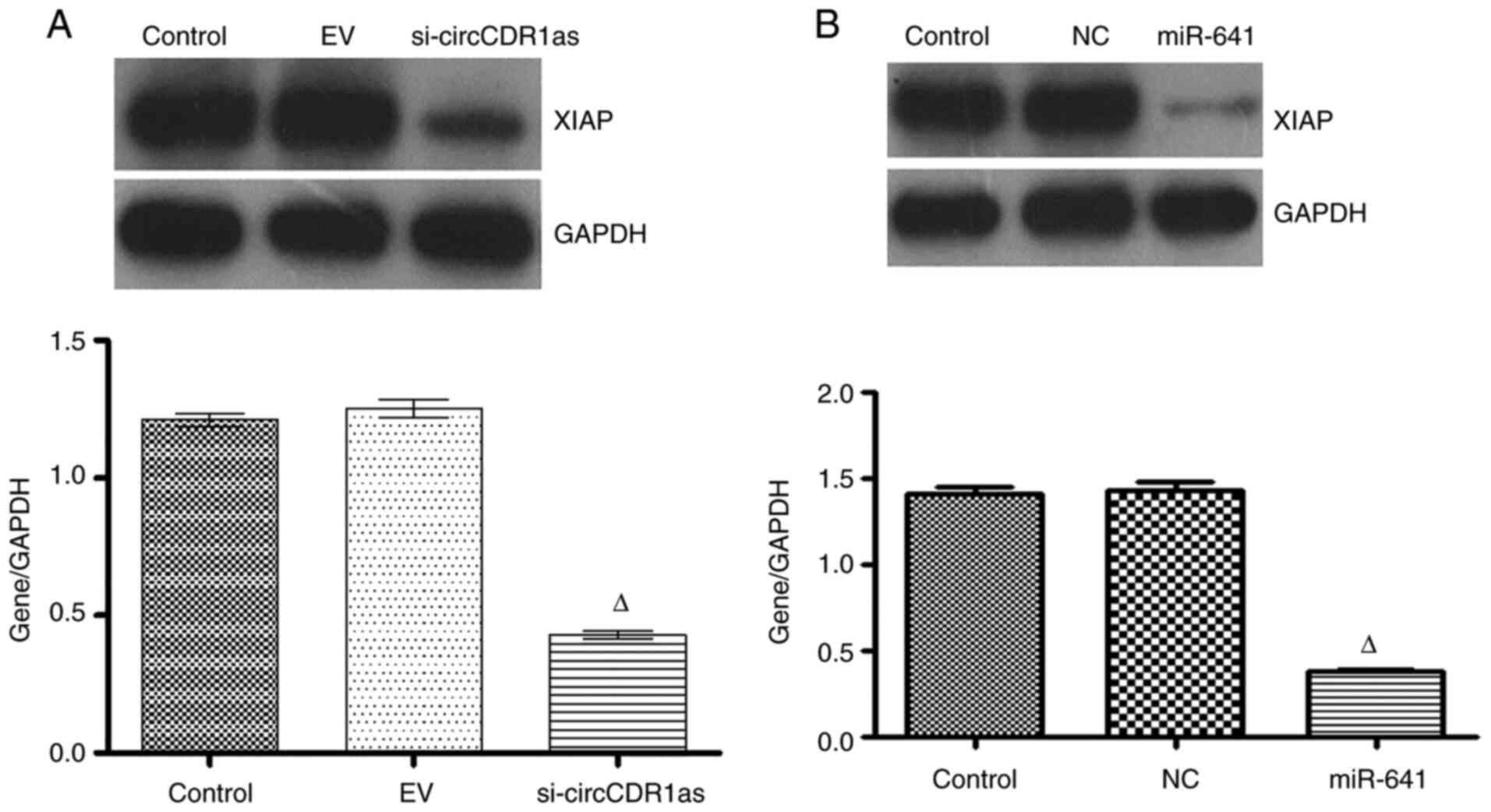
At 48 h after transfecting PC-3 cells with si-circCDR1as, miR-641
expression was significantly increased (Fig. 7A), whereas XIAP expression was
downregulated (Figs. 7B and 8A). At 48 h after transfecting PC-3 cells
with miR-641 mimic, the expression of circCDR1as (Fig. 7D) and XIAP was also downregulated
(Figs. 7C and 8B). After PC-3 cells were transfected with
si-XIAP, circCDR1as expression was downregulated and miR-641
expression was upregulated (Fig. 7E and
F, respectively). These results showed that decreasing
circCDR1as expression promotes the expression of miR-641 and
inhibits the expression of XIAP.
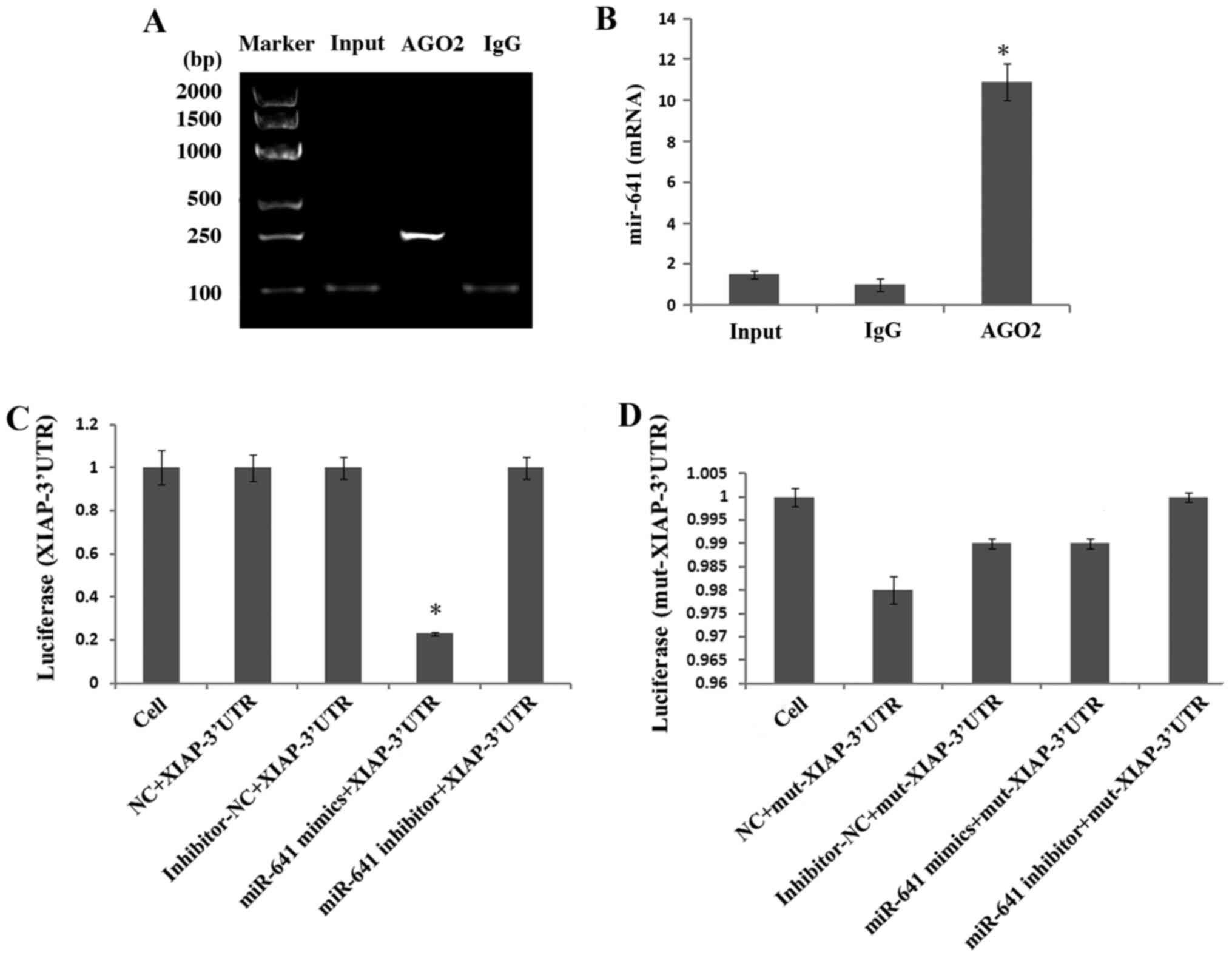 | Figure 6.Detection of interactions between
genes. (A) Anti-ago2 RIP electrophoretogram. (B) Anti-Ago2 RIP
assay was used to detect binding between circCDR1as and miR-641.
(C) Comparison of the fluorescence activity of 293T cells
co-transfected with miR-641 mimic and wild-type XIAP-3′-UTR.
*P<0.05 compared with the cell, NC, inhibitor-NC and miR-641
inhibitor groups (D) Comparison of the fluorescence activity of
293T cells co-transfected with miR-641 mimic and mut XIAP-3′-UTR.
circCDR1as, circular RNA cerebellar degeneration-related antigen 1,
anti-sense; miR, microRNA; XIAP, X-linked inhibitor of apoptosis
protein; EV, empty vector; NC, negative control; UTR, untranslated
region; mut, mutant. |
Discussion
circRNAs are generated by reverse splicing of
pre-mRNAs, which differs from the production of traditional linear
RNAs. In the process of reverse splicing, the upstream 5′ splicing
receptor is added to the downstream 3′ splicing donor in the
opposite direction, and the binding of 3′ and 5′ phosphodiester
bonds at the splicing site results in circRNA molecules forming
rings (4,18). circRNA expression is related to a
variety of diseases and tumor progression. Due to their wide
expression, unique stable structure, and tissue-specific
expression, circRNAs play important physiological functions in
different biological processes (19). In the present study, circCDR1as was
highly expressed in prostate cancer cell lines (LNCaP, 22Rv1, and
PC-3), and its expression was reduced in order to inhibit the
proliferation and invasion of PC-3 cells. Taken together with the
results of bioinformatics analysis, these results suggest that
circCDR1as may be a potential diagnostic marker for prostate
cancer.
circRNAs exist in the cytoplasm and that their
mechanisms of action are mediated mostly by miRNA sponge
interactions via binding to miRNAs (8–10).
miR-641 exerts an antitumor effect, and overexpression of miR-641
can inhibit the proliferation and migration of lung cancer cells
(20). XIAP expression elevated in
prostate cancer tissues (21). XIAP
is a therapeutic target to induce apoptosis in prostate cancer
cells because XIAP plays an important role in human prostate
carcinogenesis (22,23).
In the present study, the relative expression of
circCDR1as and XIAP was higher in prostate cancer cell lines
compared with in normal prostate epithelial cells (RWPE-1), whereas
miR-641 expression was higher in prostate epithelial cells compared
with in prostate cancer cell lines. After effectively reducing the
expression of circCDR1as and XIAP and increasing the expression of
miR-641, the proliferation, invasion and migration of PC-3 cells
were effectively inhibited. circCDR1as could bind to miR-641, which
targets the 3′-UTR of XIAP. The current results showed that the
regulatory axis of circCDR1as/miR-641/XIAP affected the
proliferation and invasion of PC-3 cells.
The role of circCDR1as has been investigated in
previous studies. After knocking down the expression of circCDR1as
in hepatocellular carcinoma cells with high circCDR1as expression,
the expression of miR-7 was upregulated, the expression of
G1/S-specific cyclin E1 and
phosphatidylinositol-4,-5-bisphosphate 3-kinase catalytic subunit δ
genes are downregulated, and tumor differentiation and invasion are
inhibited (24). circCDR1as also
upregulates N-cadherin and inhibits E-cadherin to promote the
epithelial-mesenchymal transition via miR-7 for osteosarcoma
migration (25). Silencing
circCDR1as may inhibit the expression of proteasome activating
factor γ by removing the competitive inhibitory effect on miR-7,
thereby enhancing the sensitivity of drug-resistant breast cancer
cells (26). Silencing circCDR1as
increases the expression of miR-135b-5p and decreases the
expression of hypoxia-inducible factor 1-α inhibitor, thereby
increasing the proliferative capacity of ovarian cancer cells
(27). An in-depth study on the
expression and regulatory mechanism of circCDR1as in tumors may
indicate the direction for the targeted therapy of tumors.
In summary, the present study is the first to
demonstrate that the circCDR1as/miR-641/XIAP regulatory axis
affects the invasion and migration of the prostate cancer PC-3 cell
line. These findings may provide a new direction for targeted gene
therapy of prostate cancer and lay a theoretical foundation for
elucidating the molecular mechanism of prostate cancer. The present
study also has some limitations. For example, the expression of
circCDR1as in blood was not detected. In future it should be
determined whether the circCDR1as/miR-641/XIAP regulatory axis
affects the proliferation and metastasis of tumors in animals.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This work was supported by grants from the Medical
and Health Science and Technology Project of Panyu District,
Guangzhou (grant nos. 2017-Z04-18, 2018-Z04-59 and 2019-Z4-02), and
the Guangzhou Health and Family Planning Commission Program (grant
nos. 20181A011118, 20192A011027 and 20191A011119).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YLN and YLZ contributed to the design of the present
study, developed the methodology, analyzed the results and wrote
the manuscript. YLZ collected the bioinformatics data. JHH
performed the experiments. YLZ and KL confirmed the authenticity of
all raw data. CGX contributed to the design of the study and
critically revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The study was conducted with the written informed
consent of all patients and was approved by The Ethics Committee of
the Affiliated Hospital of Guizhou Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
circCDR1as
|
circular RNA cerebellar
degeneration-related antigen 1, anti-sense
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
miRNA
|
microRNA
|
|
NC
|
negative control
|
|
RIP
|
RNA-binding protein
immunoprecipitation
|
|
RT
|
reverse transcription
|
|
siRNA
|
small interfering RNA
|
|
UTR
|
untranslated region
|
|
XIAP
|
X-linked inhibitor of apoptosis
protein
|
References
|
1
|
Minimally invasive group of urology male
genitourinary cancer professional committee of China anti cancer
association, . Consensus of Chinese prostate cancer surgical
treatment experts. Zhejiang Med. 40:217–220. 2018.
|
|
2
|
Welch HG and Albertsen PC: Reconsidering
prostate cancer mortality-the Future of PSA screening. N Engl J
Med. 382:1557–1563. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
He JH, Zhang JZ, Han ZP, Wang L, Lv YB and
Li YG: Reciprocal regulation of PCGEM1 and miR-145 promote
proliferation of LNCaP prostate cancer cells. J Exp Clin Canc Res.
33:722014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang S, Li X, Zheng H, Si X, Li B, Wei G,
Li C, Chen Y, Chen Y, Liao W, et al: Loss of
super-enhancer-regulated circRNA Nfix induces cardiac regeneration
after myocardial infarction in adult mice. Circulation.
139:2857–2876. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang R, Xing L, Zheng X, Sun Y, Wang X and
Chen J: The circRNA circAGFG1 acts as a sponge of miR-195-5p to
promote triple-negative breast cancer progression through
regulating CCNE1 expression. Mol Cancer. 18:42019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang K, Shi H, Xi H, Wu X, Cui J, Gao Y,
Liang W, Hu C, Liu Y, Li J, et al: Genome-wide lncRNA microarray
profiling identifies novel circulating lncRNAs for detection of
gastric cancer. Theranostics. 7:213–227. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang M, Ma L, Liu Y, He Y, Li G, An X and
Cao B: CircRNA-006258 sponge-adsorbs miR-574-5p to regulate cell
growth and milk synthesis via EVI5L in goat mammary epithelial
cells. Genes (Basel). 11:7182020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu L, Zhang M, Zheng X, Yi P, Lan C and Xu
M: The circular RNA ciRS-7 (Cdr1as) acts as a risk factor of
hepatic microvascular invasion in hepatocellular carcinoma. J
Cancer Res Clin Oncol. 143:17–27. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Vo JN, Cieslik M, Zhang Y, Shukla S, Xiao
L, Zhang Y, Wu YM, Dhanasekaran SM, Engelke CG, Cao X, et al: The
landscape of circular RNA in cancer. Cell. 176:869–881.e13. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Do DN, Dudemaine PL, Fomenky BE and
Ibeagha-Awemu EM: Integration of miRNA weighted gene co-expression
network and miRNA-mRNA co-expression analyses reveals potential
regulatory functions of miRNAs in calf rumen development. Genomics.
111:849–859. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang C, Zhou B, Liu M, Liu Y and Gao R:
miR-126-5p restoration promotes cell apoptosis in cervical cancer
by targeting Bcl2l2. Oncol Res. 25:463–470. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sheng Y, Hu R, Zhang Y and Luo W:
MicroRNA-4317 predicts the prognosis of breast cancer and inhibits
tumor cell proliferation, migration, and invasion. Clin Exp Med.
20:417–425. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu T, Ye P, Ye Y, Lu S and Han B:
Circular RNA hsa_circRNA_002178 silencing retards breast cancer
progression via microRNA-328-3p-mediated inhibition of COL1A1. J
Cell Mol Med. 24:21892020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barrera-Vázquez OS, Cancio-Lonches C,
Hernández-González O, Chávez-Munguia B, Villegas-Sepúlveda N and
Gutiérrez-Escolano AL: The feline calicivirus leader of the capsid
protein causes survivin and XIAP downregulation and apoptosis.
Virology. 527:146–158. 2019. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
He JH, Han ZP, Zhou JB, Chen WM, Lv YB, He
ML and Li YG: MiR-145 affected the circular RNA expression in
prostate cancer LNCaP cells. J Cell Biochem. 119:9168–9177. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
He JH, Han ZP, Zou MX, He ML, Li YG and
Zheng L: CDX2/mir-145-5p/SENP1 pathways affect LNCaP cells invasion
and migration. Front Oncol. 9:4772019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang XM, Li ZL, Li JL, Xu Y, Leng KM, Cui
YF and Sun DJ: A novel prognostic biomarker for cholangiocarcinoma:
circRNA Cdr1as. Eur Rev Med Pharmacol Sci. 22:365–371.
2018.PubMed/NCBI
|
|
19
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kong Q, Shu N, Li J and Xu N: miR-641
functions as a tumor suppressor by targeting MDM2 in human lung
cancer. Oncol Res. 26:735–741. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Devi GR: XIAP as target for therapeutic
apoptosis in prostate cancer. Drug News Perspect. 17:127–134. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu J, Li M and Xia S: Expression and
clinical significance of antiapoptosis gene XIAP in prostate
cancer. Zhonghua Nan Ke Xue. 10:832–835. 2004.(In Chinese).
PubMed/NCBI
|
|
23
|
Berezovskaya O, Schimmer AD, Glinskii AB,
Pinilla C, Hoffman RM, Reed JC and Glinsky GV: Increased expression
of apoptosis inhibitor protein XIAP contributes to anoikis
resistance of circulating human prostate cancer metastasis
precursor cells. Cancer Res. 65:2378–2386. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yu L, Gong X, Sun L, Zhou Q, Lu B and Zhu
L: The circular RNA Cdr1as act as an oncogene in hepatocellular
carcinoma through targeting miR-7 expression. PLoS One.
11:e01583472016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu B, Yang T, Wang Z, Zhang Y, Liu S and
Shen M: CircRNA CDR1as/miR-7 signals promote tumor growth of
osteosarcoma with a potential therapeutic and diagnostic value.
Cancer Manag Res. 10:4871–4880. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang W, Yang X, Wang X, Gu J, Zhou D, Wang
Y, Yin B, Guo J and Zhou M: Silencing CDR1as enhances the
sensitivity of breast cancer cells to drug resistance by acting as
a miR-7 sponge to down-regulate REGγ. J Cell Mol Med. 23:4921–4932.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen H, Mao M, Jiang J, Zhu D and Li P:
Circular RNA CDR1as acts as a sponge of miR-135b-5p to suppress
ovarian cancer progression. Onco Targets Ther. 12:3869–3879. 2019.
View Article : Google Scholar : PubMed/NCBI
|