Introduction
Gastric cancer is one of the most common malignant
tumor types in the digestive system, with a steadily increasing
incidence in numerous countries. There are ~1 million new cases
with gastric cancer reported each year (1). Early clinical manifestations of gastric
cancer are mild and difficult to be diagnosed. Once the patients
are diagnosed with relevant clinical manifestations, the majority
have reached a stage with metastasis, thereby losing the best time
for treatment (2). At present,
neoadjuvant chemotherapy (NACT) has been found to have significant
clinical efficacy in the treatment of solid tumors, including liver
cancer, gastric cancer and breast cancer. In this case, NACT may
decrease the risk of postoperative recurrence and metastasis, while
improving the survival rate and quality of life of patients
(3–5). However, not all patients respond to
NACT due to the presence of a tumor resistance mechanism affecting
the change in drug efficacy during treatment. Therefore, it is
vital to select a reasonable treatment regimen and optimize the
prognosis of patients to identify a reliable predictive index of
chemotherapy sensitivity and early diagnosis of gastric cancer.
Autophagy is a process of cellular self-digestion
under stress. In recent years, an increasing number of studies have
demonstrated that autophagy serves an important role in the growth
and differentiation of tumor cells (6,7). The
formation of autophagosomes requires the participation of various
protein complexes and small molecules, including the hallmark
protein LC3. LC3-mediated autophagy has been revealed to be
critical in a variety of gastric diseases (8,9).
Mitochondrial gene beta-lactamases (LACTB) encoding a mitochondrial
membrane protein may either inhibit cell proliferation by
regulating the mitochondrial lipid metabolism and tumor cell state,
or suppress tumor growth by directly binding to the tumor
suppressor gene, P53 (10). It has
been demonstrated that abnormal expression of LACTB was associated
with obesity and atherosclerosis (11). Furthermore, the occurrence of gastric
cancer has been proven to be associated with intracellular
mechanisms underlying lipid accumulation in gastric epithelial
neoplasms (12). These findings
suggested that the association between LACTB and diseases
associated with abnormal lipid metabolism has potential research
value. We hypothesized that the expression of LACTB and LC3 may be
associated with the occurrence and development of gastric
cancer.
In the present study, the clinical data of 51
patients with advanced gastric cancer treated with NACT were
analyzed, and the effect of NACT on the expression of LACTB and LC3
in gastric cancer tissues was investigated. The present study
investigated the relevant efficacy and prognosis, thereby laying an
evidence-based basis for the screening of chemotherapy regimens for
gastric cancer.
Materials and methods
Patients and samples
A total of 51 patients with histologically-confirmed
advanced gastric cancer were enrolled between June 2015 and June
2017 in the Department of Gastrointestinal Surgery of Guizhou
Medical University (Guizhou, China). All patients underwent
pretreatment clinical evaluation, including a complete medical
history review, physical examination, gastroscopy, whole abdomen
enhanced CT and tumor marker examination. These patients were aged
between 34 and 80 years with a mean age of 61.67±12.18 years and
consisted of 35 males and 16 females. The following
clinicopathological factors were recorded: Age, sex, tumor
location, Borrmman classification (13), Tumor-Node-Metastasis (TNM),
histological differentiation, response to NACT and LC3, and the
expression level of LACTB. The TNM staging was defined according to
the rules of the American Joint Commission on Cancer system manual,
7th edition (14). The Human Ethics
Review Committee of the Affiliated Hospital of Guizhou Medical
University approved the present study, and written informed consent
was obtained from the patients for the use of their biopsy
materials.
Eligibility criteria
Patients with histologically-confirmed locally
advanced or metastatic gastric cancer were considered eligible for
the present study. Furthermore, the patients met the following
criteria: i) No other uncontrolled or severe primary malignant
tumors or underlying disease; ii) aged between 20 and 80 years; and
iii) fulfilling the selection criteria for preoperative NACT, as
well as agreeing to undergo NACT.
Neoadjuvant chemotherapy
All patients in the present study underwent NACT
treatment with the oxaliplatin plus S-1 (SOX) (cat. no. H20040817;
Jiangsu Hengrui Pharmaceutical Co., Ltd.); oxaliplatin (130
mg/m2) was intravenously injected on day 1, while S-1
(50 mg bid) was administered orally from day 1 to day 14. The
aforementioned procedure was repeated every 3 weeks for 2–4
cycles.
Evaluation of efficacy
To determine whether patients could continue
chemotherapy, they underwent routine blood and biochemical tests
prior to the start of each chemotherapy cycle, including
granulocyte, hemoglobin, platelet, liver function and kidney
function tests. Following 2 cycles of chemotherapy, the patients
were assessed by ultrasonic gastroscopy, whole abdomen enhanced CT
examination and tumor marker, such as carcinoembryonic antigen,
carbohydrate antigen 125, carbohydrate antigen 199 monitoring. The
following treatment options were applied based on the evaluation:
Patients directly underwent surgery if the lesions had progressed;
those with smaller lesions continued one cycle of NACT; those
without significant improvement in the lesions communicated with
their family members and voluntarily selected direct surgery or
continued 1–2 cycles of chemotherapy.
NACT was conducted as mentioned earlier, and the
tumor response to neoadjuvant treatment was reviewed using the
Response Evaluation Criteria in Solid Tumors (15). A complete response (CR) and a partial
response (PR) were defined as complete disappearance of the lesion
and >30% decrease in the maximum transverse diameter of the
primary lesion, respectively. Additionally, cases with new lesions
or >20% increase in the maximum transverse diameter of primary
lesions were evaluated as progressive disease (PD), while those who
failed to meet these criteria were classified as having stable
disease (SD).
Immunohistochemistry (IHC)
The cancer tissue specimens were fixed in 10%
formaldehyde at 4°C for 3–24 h, embedded in paraffin, and
continuously sectioned at a thickness of 4 µm. The specimens were
attached to the slides treated with poly-L-lysine and baked at 80°C
for 50 min. Sections were incubated with 3% hydrogen peroxide for
10 min at room temperature to block the endogenous peroxidase
activity. The tissue sections were then incubated with rabbit
polyclonal antibodies against LACTB (dilution, 1:200; cat. no.
ab244454; Abcam) and LC3 (dilution, 1:500; cat. no. ab63817; Abcam)
overnight at 4°C, washed 3 times with phosphate buffered saline
(PBS) containing 0.1% Tween-20, and exposed to the horseradish
peroxidase-labeled goat anti-rabbit secondary antibody IgG
(1:2,000; cat. no. ab6112; Abcam) for 30 min at 20°C.
Immunoreactions were detected by the DAKO REAL EnVision Detection
System-HRP (Dako; Agilent Technologies, Inc.). The color reaction
was performed using 3,3′-diaminobenzidine after incubation for 5
min at room temperature, and the sections were stained with
hematoxylin and eosin (H&E) for 5 and 3 min at room
temperature, respectively. The images of IHC and H&E staining
were obtained using the OLYMPUS BX53 light microscope
(magnification, ×400) (Olympus Corporation), and the digital slides
were analyzed by the software Image J v.1.8.0 (National Institutes
of Health). The expression and subcellular distribution of LACTB
and LC3 in the slices were observed under a light microscope, and
five high-power fields (magnification, ×400) were selected for each
slice. Staining intensity was categorized as follows: No staining,
0 points; light yellow, 1 point; dark yellow, 2 points; and brown
or tan, 3 points. Additionally, the rate of positive staining cells
was scored as: 0 points for l<10%, 1 point for 10–25%, 2 points
for 26–50%, 3 points for 51–75%, and 4 points for >76% (16,17). The
final score was then calculated by multiplying the staining
intensity by the percentage of positive cells in staining areas.
With the cut-off value of LACTB and LC3 as 5 and 3, respectively, a
final score of greater than the value was defined as high
expression, whereas that of less than the value was determined as
low expression (18,19). The staining was scored independently
by two senior pathologists in a double-blinded manner.
Immunofluorescence
The tissues were rinsed with PBS 3 times for 5 min
each, blocked with 5% normal goat serum in a wet box for 30 min at
room temperature, and then incubated with mouse monoclonal primary
antibodies against LC3 (dilution, 1:100; cat. no. ab243506; Abcam)
or LACTB (dilution, 1:100; cat. no. ab244455; Abcam) at 4°C for 24
h. Following being rinsed 3 times with PBS, the tissues were
incubated with FITC-labeled goat anti-mouse secondary antibody IgG
(1:200; cat. no. ab150113; Abcam) at room temperature for 1 h in
the dark, and then rinsed 3 times with PBS. No primary antibody was
added to the negative control. The tissues were counterstained with
DAPI for 5 min at room temperature, and images were captured under
a fluorescence microscope (magnification, ×400) (Leica AF6000 cell
station). Images of the nucleus (DAPI staining, blue fluorescence)
and cell body (LC3 or LACTB staining, red fluorescence) were
captured separately, and the two images were merged for an
overlapping image for counting of cells positively stained for LC3
or LACTB.
Western blotting
Tissues were lysed in RIPA protein lysis solution
(Biouniquer Technology Co., Ltd.), and extracted protein was
quantified using the BCA protein quantification method. A total of
45 g of protein mixed with SDS protein sampling buffer was
separated by 8–15% SDS-PAGE and electro-transferred onto a PVDF
membrane. Following being blocked with TBST blocking solution
containing 5% skimmed milk powder for 100 min at room temperature,
the membrane was incubated with diluted anti-LACTB antibody
(dilution, 1:1,000; Abcam) or anti-LC3 antibody (dilution, 1:1,000;
MBL International Co.) overnight at 4°C. On the following day, the
membrane was washed with TBST and subjected to an incubation with
HRP-labeled secondary antibody (dilution, 1:2,000; Beijing Kangwei
Biological Technology Co., Ltd.) at room temperature for 1.5 h.
GAPDH was used as an internal control. Finally, target proteins in
the membrane were exposed using enhanced chemiluminescence working
fluid (Merck Millipore). The shaded value was calculated using
Image Lab software v.4.0 (Bio-Rad Laboratories Inc.), and the ratio
of the shaded value of LACTB and LC3 expression to that of GADPH
expression was determined as the relative expression amount of the
protein.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from tissue using the
TRIzol® reagent (Thermo Fisher Scientific, Inc.).
Following identification of quality and purity, 2 µg RNA was
reverse transcribed into cDNA using SuperScript® III
Reverse Transcriptase (Thermo Fisher Scientific, Inc.) at 42°C for
15 min. Next, RT-qPCR was performed using SYBR Green I (Roche
Diagnostics GmbH). GAPDH was used as the internal control. The
primer sequences were as follows: GAPDH forward,
5′-CCTCGTCTCATAGACAAGATGGT-3′ and reverse,
5′-GGGTAGAGTCATACTGGAACATG-3′; LACTB forward,
5′-CTGCTGCACAGGATCAAGGA-3′ and reverse, 5′-ATCCAGTTTCCCTGCTTCCC-3′.
The following thermocycling conditions were applied: 95°C for 30
sec, followed by 50 cycles of denaturation at 95°C for 5 sec, and
60°C for 30 sec. At the end of the experiment, the cycle threshold
(Ct) value of each sample was obtained. Data were analyzed by using
the comparative 2−ΔΔCq method (20).
Statistical analysis
Statistical analysis was performed using SPSS 21.0
(IBM Corp.). The significant associations between the expression of
LACTB or LC3 and various clinicopathological parameters were
determined by the Pearson's chi-square test or Fisher's exact test.
Based on the normality of the distribution, the results are
expressed as either the mean ± standard deviation or the median and
interquartile range. Differences between two groups were compared
by the Student's t-test. The Mann-Whitney U test was used to
investigate the difference between the mRNA expression of LACTB
prior to and following NACT. The correlation between the expression
of LACTB and LC3 prior to and following NACT was analyzed using the
Spearman correlation coefficient. P<0.05 was used to indicate a
statistically significant difference.
Results
Clinical factors and their association
with the expression of LACTB and LC3 proteins in gastric cancer
prior to SOX regimen NACT
The clinicopathological data of 51 patients with
gastric cancer were obtained, and their associations with the
expression levels of LACTB and LC3 were analyzed. As summarized in
Table I, there were no statistically
significant differences in the expression levels of LACTB and LC3
among different clinicopathological subgroups in terms of sex, age,
histological differentiation, tumor location, Borrmman type and TNM
stage (P>0.05).
 | Table I.Clinicopathological factors and their
association with the expression of LACTB and LC3 proteins in
gastric cancer prior to neoadjuvant chemotherapy. |
Table I.
Clinicopathological factors and their
association with the expression of LACTB and LC3 proteins in
gastric cancer prior to neoadjuvant chemotherapy.
|
|
| LACTB | LC3 |
|---|
|
|
|
|
|
|---|
| Clinical
parameter | Number of cases,
n | Low | High | Low | High |
|---|
| Sex |
|
|
|
|
|
|
Male | 35 | 20 | 15 | 25 | 8 |
|
Female | 16 | 8 | 8 | 14 | 2 |
| χ2 |
|
| 2.519 |
|
|
| P-value |
|
| 0.112 |
| 0.464 |
| Age |
|
|
|
|
|
| <65
years | 24 | 14 | 10 | 20 | 4 |
| ≥65
years | 27 | 14 | 13 | 21 | 6 |
| χ2 |
|
| 0.216 |
|
|
| P-value |
|
| 0.642 |
| 0.731 |
| Histological
differentiation |
|
|
|
|
|
|
Moderately-differentiated
gastric adenocarcinoma | 16 | 13 | 3 | 12 | 4 |
|
Poorly-differentiated gastric
adenocarcinoma | 35 | 28 | 7 | 29 | 6 |
| P-value |
|
| 1.000 |
| 0.705 |
| Tumor location |
|
|
|
|
|
|
Fundus | 6 | 3 | 2 | 5 | 1 |
|
Body | 9 | 5 | 4 | 7 | 2 |
|
Antral | 36 | 25 | 11 | 28 | 7 |
| P-value |
|
| 0.316 |
| 0.578 |
| Borrmman type |
|
|
|
|
|
|
I–II | 16 | 10 | 6 | 12 | 4 |
|
III–IV | 35 | 20 | 15 | 29 | 6 |
| χ2 |
|
| 0.165 |
|
|
| P-value |
|
| 0.685 |
| 0.705 |
| TNM stage |
|
|
|
|
|
|
I–II | 7 | 2 | 5 | 5 | 2 |
|
III–IV | 44 | 26 | 18 | 36 | 8 |
| P-value |
|
| 0.221 |
| 0.612 |
Effect of SOX regimen NACT on the
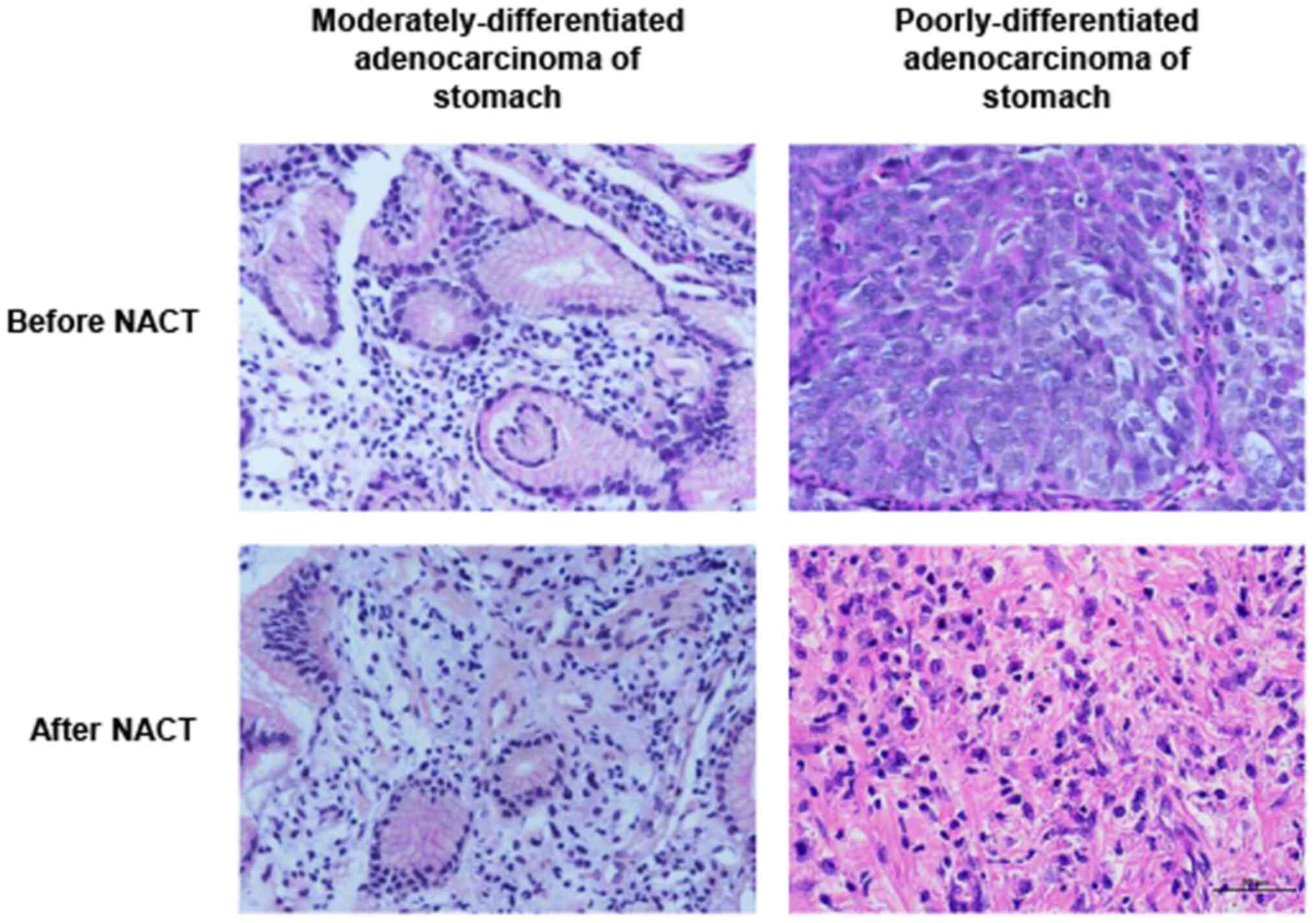
pathomorphology of gastric cancer
Prior to NACT, moderately-differentiated
adenocarcinoma with glandular duct structure in the stomach can be
observed, and the cancer cells were columnar or cubic in shape and
arrangement. Poorly-differentiated gastric adenocarcinomas only
displayed a tendency to form glandular duct structures. Cancer
cells were short columnar, cubic or amorphous, forming strange
nuclear cells, tumor giant cells or cancer cells with strip or
scattered distribution, unclear nuclear structure and irregular
shape, while pathological mitotic phases may be easily identified.
By contrast, few cancer cells were identified in the interstitium
in a strip-like or nest-like arrangement following NACT with SOX.
As shown in Fig. 1, only a single
cancer cell remained in well-differentiated gastric
adenocarcinoma.
Effect of SOX regimen NACT on the
expression distribution of LACTB and LC3 in gastric cancer
tissues
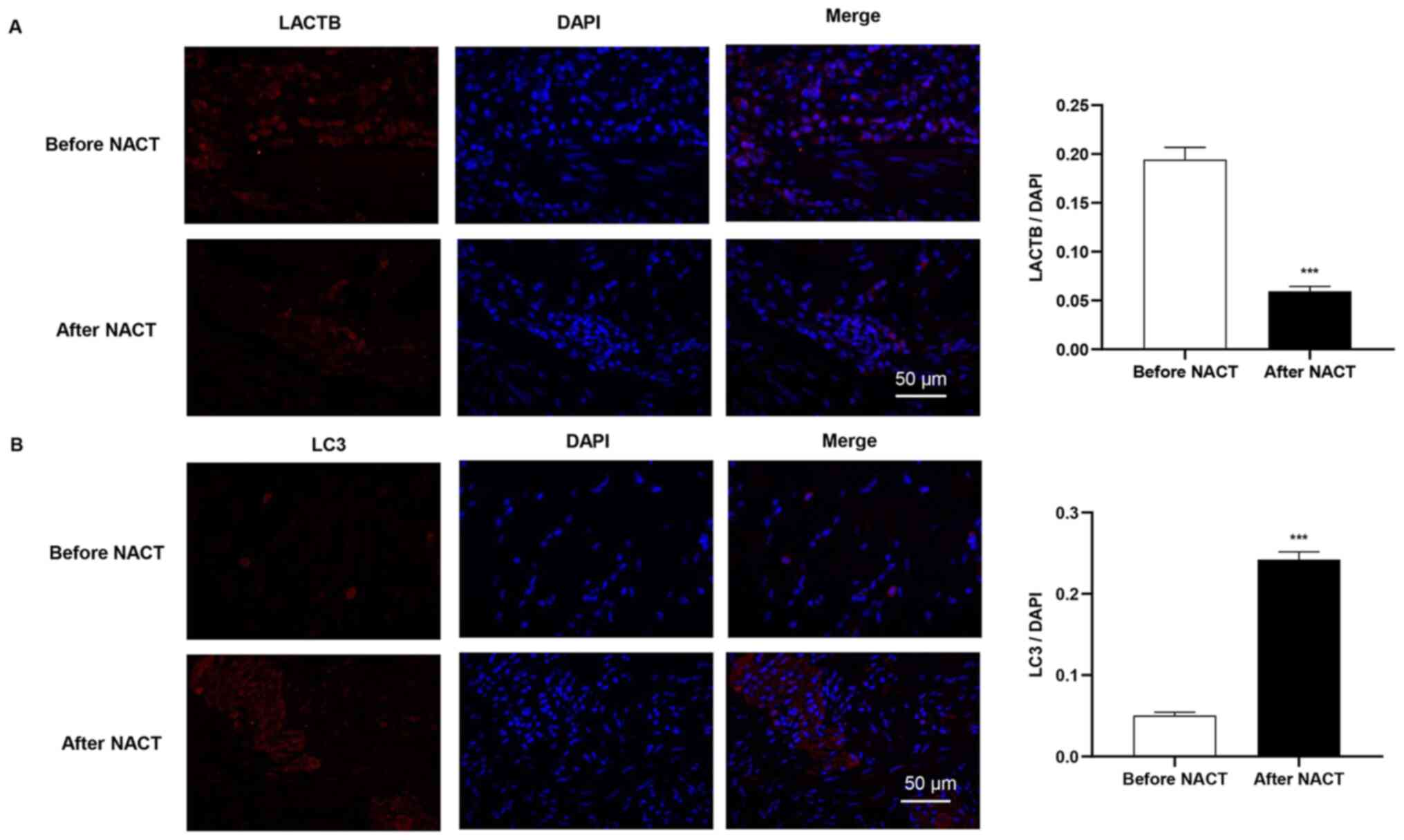
The present study further analyzed the subcellular
distribution of LACTB and LC3 protein in the gastric cancer cells
by using immunofluorescence. As depicted in Fig. 2, LACTB and LC3 were mostly localized
to the cytoplasm or cell membrane, exhibiting needle-tip-like
signals. Following NACT, a significant decrease in LACTB expression
was detected in the gastric cancer tissues; negative staining for
LACTB was present in the majority of the gastric cancer tissues,
while very little cytoplasmic LACTB expression was observed. By
contrast, LC3 was highly expressed in the cytoplasm of gastric
cancer cells following NACT.
Effect of SOX regimen NACT on the
expression levels of LACTB and LC3 in gastric cancer
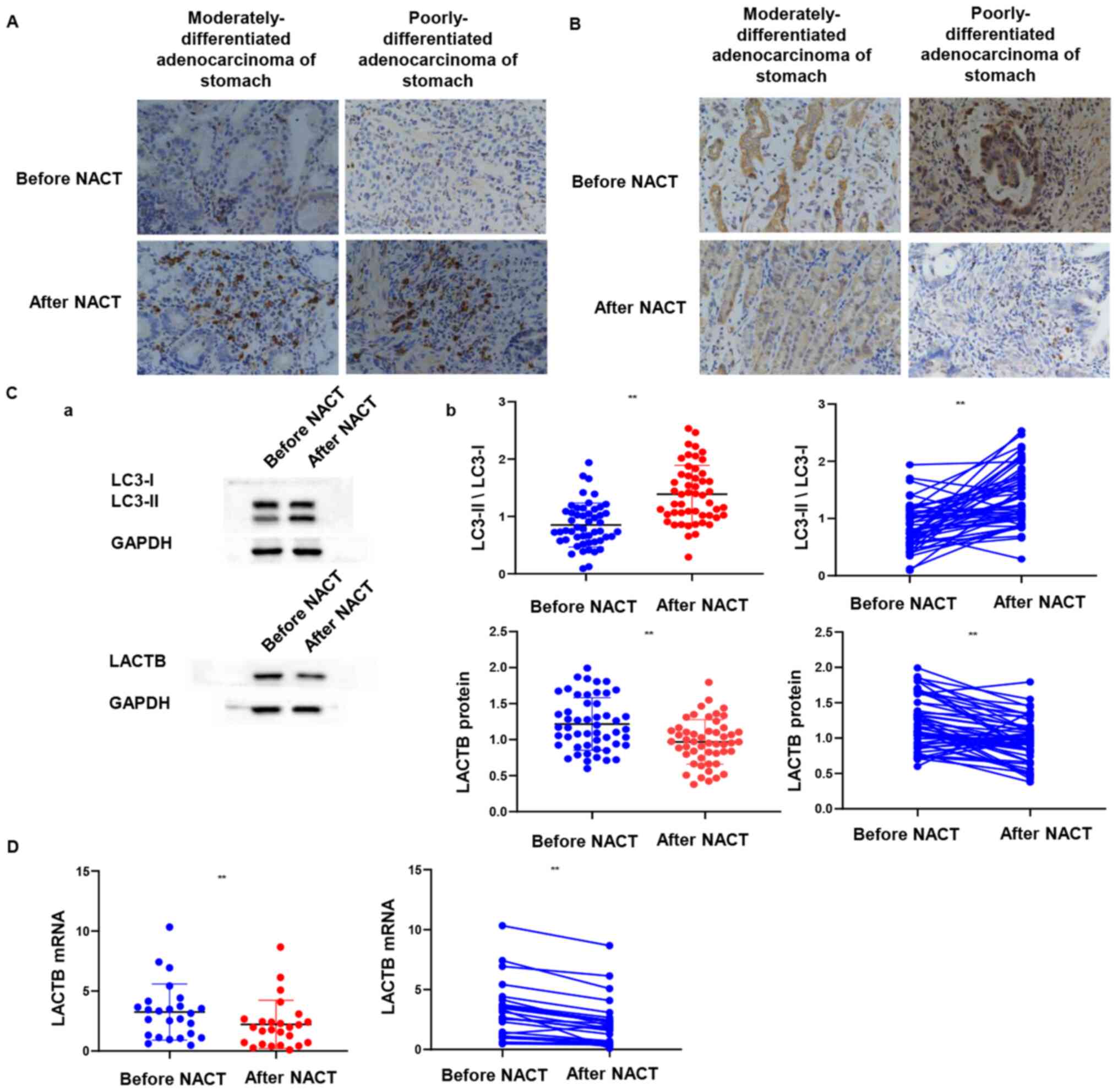
IHC revealed that while LACTB staining was
visualized as brown-yellow granules localized to the cell membrane
and cytoplasm (Fig. 3A), LC3 was
stained positively as brown granules in the cytoplasm and
occasionally in the nuclei of the cancer cells (Fig. 3B). Among 51 gastroscopic biopsy
specimens of gastric cancer prior to SOX NACT, 29 (56.9%) and 11
(21.6%) were positive for LACTB and LC3 expression, respectively.
Notably, following NACT, LACTB and LC3 were expressed in 14 (27.5%)
and 31 (60.78%) gastric cancer specimens, respectively.
Furthermore, Western blotting demonstrated that the expression of
LACTB in gastric cancer tissues prior to NACT was significantly
higher than that following the treatment. By contrast, LC3 protein
expression was significantly increased during this period
(P<0.01; Fig. 3C). To verify this
observation, the mRNA expression level of LACTB was analyzed using
RT-qPCR and it was revealed that LACTB expression levels in gastric
cancer tissues following NACT were significantly lower than that
prior to the treatment (P<0.01; Fig.
3D).
The expression of LACTB and LC3
predicts response of gastric cancer to NACT with SOX
Among 51 patients undergoing NACT, 3 (5.88%), 27
(52.94%), 13 (25.49%) and 8 (15.69%) displayed CR, PR, SD and PD,
respectively. While the rate of decreased LACTB expression was
68.6%, the rate of increased LC3 expression was 60.8%. As shown in
Table II, there was a significant
negative correlation between the expression of LACTB and LC3 prior
to and following NACT (P<0.001). Furthermore, it was observed
that the levels of LACTB and LC3 following NACT were significantly
correlated with the curative effect of NACT (P<0.01; P<0.01;
Table III).
 | Table II.Correlation between the expression of
LACTB and LC3 prior to and following neoadjuvant chemotherapy. |
Table II.
Correlation between the expression of
LACTB and LC3 prior to and following neoadjuvant chemotherapy.
|
|
| LC3 |
|
|
|---|
|
|
|
|
|
|
|---|
| LACTB | n | Decreased | Invariant | Increased | rs | P-value |
|---|
| Decreased | 32 | 6 | 1 | 25 |
|
|
| Invariant | 5 | 1 | 0 | 4 | −0.785 | 0.000 |
| Increased | 14 | 10 | 2 | 2 |
|
|
 | Table III.Difference between the expression of
LACTB or LC3 in gastric cancer prior to and following neoadjuvant
chemotherapy. |
Table III.
Difference between the expression of
LACTB or LC3 in gastric cancer prior to and following neoadjuvant
chemotherapy.
|
|
| Clinical efficacy
of chemotherapy |
|
|
|---|
|
|
|
|
|
|
|---|
| Protein | Number of cases
(n) | CR | PR | SD | PD | χ2 | P-value |
|---|
| LACTB |
|
|
|
|
|
|
|
|
Decreased | 32 | 0 | 13 | 12 | 7 | 17.648 | 0.007 |
|
Invariant | 5 | 1 | 2 | 1 | 1 |
|
|
|
Increased | 14 | 2 | 12 | 0 | 0 |
|
|
| LC3 |
|
|
|
|
|
|
|
|
Decreased | 17 | 3 | 13 | 1 | 0 |
| 0.000 |
|
Invariant | 3 | 0 | 2 | 1 | 0 |
|
|
|
Increased | 31 | 0 | 12 | 11 | 8 |
|
|
Discussion
Gastric cancer is the second leading cause of
cancer-related mortality worldwide, and is the third leading cause
of cancer-related mortality in males and the second leading cause
of cancer-related mortality in females in China (21). With the development of chemotherapy
technology for gastric cancer, particularly the discovery of a
series of novel therapeutic targets, a growing number of NACT
regimens have been applied in the clinical treatment of gastric
cancer. NACT with SOX is currently one of the commonly used
chemotherapy regimens for the treatment of advanced gastric cancer
(22). The present retrospective
study demonstrated that, as a neoadjuvant regimen for advanced
gastric cancer, SOX chemotherapy had a total effective rate (CR+PR)
of 58.8%, which was similar to that reported previously (23). However, in clinical practice, a
considerable proportion of patients have tumor progression during
chemotherapy and a delay in treatment, thereby affecting the
overall efficacy. Therefore, molecular biomarkers are required for
guiding neoadjuvant treatment. The present study reported that the
expression of LACTB and LC3 in gastric cancer was significantly
associated with the curative effect of NACT. To the best of our
knowledge, the present study was the first to identify the role of
LACTB and LC3 in gastric cancer with neoadjuvant chemotherapy.
The present study demonstrated that there was no
correlation between the expression level of LACTB or LC3 proteins
and clinicopathological indices of gastric cancer, including sex,
age, histological differentiation, tumor location, Borrmman type
and TNM stage. Based on the aforementioned findings, the expression
of LC3 and LACTB was further investigated in the biopsies of 51
patients with advanced gastric cancer undergoing preoperative
chemotherapy by immunohistochemistry and Western blotting. The
staining revealed that LC3 and LACTB are mainly localized in the
cytoplasm. The aforementioned observations are consistent with the
subcellular localization of LC3 in resected gastrointestinal cancer
tissues reported in a previous study (24). Following NACT, LC3 was significantly
expressed in 31 cases (60.78%), while LACTB was only significantly
expressed in 14 cases of gastric cancer (27.5%). Previous studies
have reported that the autophagy activity of numerous malignant
tumor types is lower than that of normal tissues, while radiation
or antitumor drugs may increase autophagy activity and induce
autophagic death of tumor cells (25,26).
Tumor resistance to anticancer therapies, including chemotherapy
and radiation therapy, may be enhanced through upregulation of
transcription of autophagy-inducible factors (27). LC3 is divided into two subtypes, type
I LC3 (LC3-I) and type II LC3 (LC3-II). In the physiological state,
LC3 synthesized in cells is routinely processed into cytoplasmic
soluble LC3-I. Upon autophagy induction, LC3-I undergoes a
ubiquitin-like modification to bind with phosphatidylethanolamine
on the surface of the autophagy membrane to form LC3-II. Therefore,
the ratio of LC3-II/LC3-I is an important index to evaluate the
occurrence and level of autophagy. Clinicopathological studies of
liver tumors have demonstrated that LC3 expression may serve as an
important prognostic factor for hepatocellular carcinoma,
particularly for those patients undergoing surgical resection
(28). The association between
increased LC3 expression and a poor prognosis in gastric cancer has
been extensively studied (29–31). All
these studies have indicated that LC3 may be used as an independent
prognostic indicator for patients with gastric cancer. As a tumor
suppressor, LACTB is highly expressed in numerous solid tumor
types. Zhang et al (32)
reported that downregulation of LACTB was significantly associated
with a poor clinical prognosis in breast cancer. Furthermore, Li
et al (33) demonstrated that
LACTB-overexpression in glioma cells may inhibit cell
proliferation, invasion and angiogenesis, while low expression of
LACTB was associated with a poor prognosis in patients with glioma.
Furthermore, it has been demonstrated that patients with colorectal
cancer with a low expression of LACTB have a poor overall prognosis
(34).
Previous studies have demonstrated that the main
obstacle to the development of PD during chemotherapy is multidrug
resistance (35,36). The development of multidrug
resistance will lead to an increase in cancer-associated mortality.
The resistance to NACT in triple-negative breast cancer, ovarian
cancer and bladder cancer has been reported in previous studies
(37–39). In the present study, among 51
patients with advanced gastric cancer undergoing NACT, 36 displayed
an increase in the expression level of LC3; of the 36 patients, 8
developed PD. Meanwhile, 29 out of 51 patients receiving the
treatment had a decrease in the expression level of LACTB; 6 of the
29 patients had PD. Notably, 2 of the 6 patients with unchanged
expression level of LACTB following NACT developed PD. Numerous
factors are associated with chemoresistance. However, the
expression of resistance genes is obtained by reprogramming
following chemotherapy. In addition, the development of drug
resistance may be associated with the dose of chemotherapeutic
drugs, course of treatment and treatment regimen.
Taken together, these data suggested that certain
patients with gastric cancer undergoing NACT may develop multidrug
resistance, and abnormal expression of LACTB and LC3 may indicate a
poor prognosis, leading to resistance to NACT. Further studies are
required to investigate the association between the expression of
LACTB or LC3 and the drug resistance of gastric cancer cells, as
well as the molecular mechanism underlying the involvement of LACTB
in the occurrence and development of gastric cancer. Furthermore,
given the small number of patients, the absence of a retrospective
design and survival analysis and the presence of confounding
factors, including environmental factors, in the present study,
further validation of the findings is required.
In conclusion, the present study revealed a
correlation between the expression level of LACTB and LC3 following
NACT in gastric cancer and the efficacy of clinical chemotherapy,
suggesting that the expression of LACTB and LC3 may potentially
serve as a prognostic factor for patients with gastric cancer.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Guizhou
Provincial Department of Education Project [grant no. YJSCXJH
(2019) 069] and the Hospital-Level Project of Guizhou Cancer
Hospital (grant no. YT2019019).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
FY was responsible for the conception and design of
the study. ZY was responsible for the collection and entry of data.
WN, ZL, ZC and WW were responsible for the collection and assembly
of data. CS organized the data. GF was responsible for the
experimental operations. YY was responsible for funding and project
design. FY and ZY confirm the authenticity of all the raw data. All
authors read and approved the final manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Ethical approval for the present study was provided
by the Ethics Committee of the Affiliated Hospital of Guizhou
medical university (Guizhou, China). All patients provided written
informed consent for participations.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lott PC and Carvajal-Carmona LG: Resolving
gastric cancer aetiology: An update in genetic predisposition.
Lancet Gastroenterol Hepatol. 3:874–883. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tan Z: Recent advances in the surgical
treatment of advanced gastric cancer: A review. Med Sci Monit.
25:3537–3541. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Derks MGM and van de Velde CJH:
Neoadjuvant chemotherapy in breast cancer: More than just
downsizing. Lancet Oncol. 19:2–3. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Das M: Neoadjuvant chemotherapy: Survival
benefit in gastric cancer. Lancet Oncol. 18:e3072017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akateh C, Black SM, Conteh L, Miller ED,
Noonan A, Elliott E, Pawlik TM, Tsung A and Cloyd JM: Neoadjuvant
and adjuvant treatment strategies for hepatocellular carcinoma.
World J Gastroenterol. 25:3704–3721. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wei X, Duan W, Li Y, Zhang S, Xin X, Sun
L, Gao M, Li Q and Wang D: AT101 exerts a synergetic efficacy in
gastric cancer patients with 5-FU based treatment through promoting
apoptosis and autophagy. Oncotarget. 7:34430–34441. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu M, Li CM, Chen ZF, Ji R, Guo QH, Li Q,
Zhang HL and Zhou YN: Celecoxib regulates apoptosis and autophagy
via the PI3K/Akt signaling pathway in SGC-7901 gastric cancer
cells. Int J Mol Med. 33:1451–1458. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Masuda GO, Yashiro M, Kitayama K, Miki Y,
Kasashima H, Kinoshita H, Morisaki T, Fukuoka T, Hasegawa T,
Sakurai K, et al: Clinicopathological correlations of
autophagy-related proteins LC3, beclin 1 and p62 in gastric cancer.
Anticancer Res. 36:129–136. 2016.PubMed/NCBI
|
|
9
|
Shida M, Kitajima Y, Nakamura J,
Yanagihara K, Baba K, Wakiyama K and Noshiro H: Impaired mitophagy
activates mtROS/HIF-1α interplay and increases cancer
aggressiveness in gastric cancer cells under hypoxia. Int J Oncol.
48:1379–1390. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pascual G, Avgustinova A, Mejetta S,
Martín M, Castellanos A, Attolini CS, Berenguer A, Prats N, Toll A,
Hueto JA, et al: Targeting metastasis-initiating cells through the
fatty acid receptor CD36. Nature. 541:41–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lu JB, Yao XX, Xiu JC and Hu YW:
MicroRNA-125b-5p attenuates lipopolysaccharide-induced monocyte
chemoattractant protein-1 production by targeting inhibiting LACTB
in THP-1 macrophages. Arch Biochem Biophys. 590:64–71. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Enjoji M, Kohjima M, Ohtsu K, Matsunaga K,
Murata Y, Nakamuta M, Imamura K, Tanabe H, Iwashita A, Nagahama T
and Yao K: Intracellular mechanisms underlying lipid accumulation
(white opaque substance) in gastric epithelial neoplasms: A pilot
study of expression profiles of lipid-metabolism-associated genes.
J Gastroenterol Hepatol. 31:776–781. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim JH, Lee HH, Seo HS, Jung YJ and Park
CH: Borrmann type 1 cancer is associated with a high recurrence
rate in locally advanced gastric cancer. Ann Surg Oncol.
25:2044–2052. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Edge SB BD, Compton CC, Fritz AG, Greene
FL and Trotti A: AJCC cancer staging manual. 7th edition. Springer;
New York, NY: 2010
|
|
15
|
Armato SG III and Nowak AK: Revised
modified response evaluation criteria in solid tumors for
assessment of response in malignant pleural mesothelioma (version
1.1). J Thorac Oncol. 13:1012–1021. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jaffer S, Orta L, Sunkara S, Sabo E and
Burstein DE: Immunohistochemical detection of antiapoptotic protein
X-linked inhibitor of apoptosis in mammary carcinoma. Hum Pathol.
38:864–870. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Park JM, Huang S, Wu TT, Foster NR and
Sinicrope FA: Prognostic impact of Beclin 1, p62/sequestosome 1 and
LC3 protein expression in colon carcinomas from patients receiving
5-fluorouracil as adjuvant chemotherapy. Cancer Biol Ther.
14:100–107. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Xue C, He Y, Zhu W, Chen X, Yu Y, Hu Q,
Chen J, Liu L, Ren F, Ren Z, et al: Low expression of LACTB
promotes tumor progression and predicts poor prognosis in
hepatocellular carcinoma. Am J Transl Res. 10:4152–4162.
2018.PubMed/NCBI
|
|
19
|
Wang J, Pan XL, Ding LJ, Liu DY, Da-Peng L
and Jin T: Aberrant expression of beclin-1 and LC3 correlates with
poor prognosis of human hypopharyngeal squamous cell carcinoma.
PLoS One. 8:e690382013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Papachristopoulou G, Tsapralis N,
Michaelidou K, Ardavanis-Loukeris G, Griniatsos I, Scorilas A and
Talieri M: Human kallikrein-related peptidase 12 (KLK12) splice
variants discriminate benign from cancerous breast tumors. Clin
Biochem. 58:78–85. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ferlay J, Shin HR, Bray F and Mathers C:
GLOBOCAN 2008, cancer incidence and mortality worldwide: IARC
Cancer Base no. 10. International Agency for Research on Cancer;
Lyon: 2010
|
|
22
|
Yamada Y, Higuchi K, Nishikawa K, Gotoh M,
Fuse N, Sugimoto N, Nishina T, Amagai K, Chin K, Niwa Y, et al:
Phase III study comparing oxaliplatin plus S-1 with cisplatin plus
S-1 in chemotherapy-naive patients with advanced gastric cancer.
Ann Oncol. 26:141–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li T and Chen L: Efficacy and safety of
SOX regimen as neoadjuvant chemotherapy for advanced gastric
cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 14:104–106. 2011.(In
Chinese). PubMed/NCBI
|
|
24
|
Yoshioka A, Miyata H, Doki Y, Yamasaki M,
Sohma I, Gotoh K, Takiguchi S, Fujiwara Y, Uchiyama Y and Monden M:
LC3, an autophagosome marker, is highly expressed in
gastrointestinal cancers. Int J Oncol. 33:461–468. 2008.PubMed/NCBI
|
|
25
|
Kimmelman AC and White E: Autophagy and
tumor metabolism. Cell Metab. 25:1037–1043. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amaravadi R, Kimmelman AC and White E:
Recent insights into the function of autophagy in cancer. Genes
Dev. 30:1913–1930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu YL, Jahangiri A, Delay M and Aghi MK:
Tumor cell autophagy as an adaptive response mediating resistance
to treatments such as antiangiogenic therapy. Cancer Res.
72:4294–4299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee YJ, Hah YJ, Kang YN, Kang KJ, Hwang
JS, Chung WJ, Cho KB, Park KS, Kim ES, Seo HY, et al: The
autophagy-related marker LC3 can predict prognosis in human
hepatocellular carcinoma. PLoS One. 8:e815402013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang X, Wu WKK, Gao J, Li Z, Dong B, Lin
X, Li Y, Li Y, Gong J, Qi C, et al: Autophagy inhibition enhances
PD-L1 expression in gastric cancer. J Exp Clin Cancer Res.
38:1402019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cao Y, Luo Y, Zou J, Ouyang J, Cai Z, Zeng
X, Ling H and Zeng T: Autophagy and its role in gastric cancer.
Clin Chim Acta. 489:10–20. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim JS, Bae GE, Kim KH, Lee SI, Chung C,
Lee D, Lee TH, Kwon IS and Yeo MK: Prognostic significance of LC3B
and p62/SQSTM1 expression in gastric adenocarcinoma. Anticancer
Res. 39:6711–6722. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang J, He Y, Yu Y, Chen X, Cui G, Wang
W, Zhang X, Luo Y, Li J, Ren F, et al: Upregulation of miR-374a
promotes tumor metastasis and progression by downregulating LACTB
and predicts unfavorable prognosis in breast cancer. Cancer Med.
7:3351–3362. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li HT, Dong DY, Liu Q, Xu YQ and Chen L:
Overexpression of LACTB, a mitochondrial protein that inhibits
proliferation and invasion in glioma cells. Oncol Res. 27:423–429.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zeng K, Chen X, Hu X, Liu X, Xu T, Sun H,
Pan Y, He B and Wang S: LACTB, a novel epigenetic silenced tumor
suppressor, inhibits colorectal cancer progression by attenuating
MDM2-mediated p53 ubiquitination and degradation. Oncogene.
37:5534–5551. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yan C and Li TS: Dual role of mitophagy in
cancer drug resistance. Anticancer Res. 38:617–621. 2018.PubMed/NCBI
|
|
36
|
Cichocka-Radwan A and Lelonek M: Annual
prognostic factors in chronic heart failure in patients over 80
years old. Kardiol Pol. 75:164–173. 2017.PubMed/NCBI
|
|
37
|
Echeverria GV, Ge Z, Seth S, Zhang X,
Jeter-Jones S, Zhou X, Cai S, Tu Y, McCoy A, Peoples M, et al:
Resistance to neoadjuvant chemotherapy in triple-negative breast
cancer mediated by a reversible drug-tolerant state. Sci Transl
Med. 11:eaav09362019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Glasgow MA, Argenta P, Abrahante JE,
Shetty M, Talukdar S, Croonquist PA, Khalifa MA and Starr TK:
Biological insights into chemotherapy resistance in ovarian cancer.
Int J Mol Sci. 20:21312019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Buttigliero C, Tucci M, Vignani F,
Scagliotti GV and Di Maio M: Molecular biomarkers to predict
response to neoadjuvant chemotherapy for bladder cancer. Cancer
Treat Rev. 54:1–9. 2017. View Article : Google Scholar : PubMed/NCBI
|