Introduction
Adrenocortical carcinoma (ACC) is an endocrine
tumour with high malignancy, invasiveness and recurrence rate, and
has a poor prognosis (1). The
majority of patients are diagnosed at an advanced stage with
invasion of adjacent organs or metastatic disease (2). Currently, complete resection is the
only feasible method to treat ACC. However, the prognosis of
patients with relapsed or metastatic disease is unfavourable as
patients with metastatic ACC have an overall 5-year survival rate
of <20% (3). Chemotherapy remains
an effective method of adjuvant therapy in patients with a high
risk of relapse and metastasis, and mitotane is the only drug
approved for the systemic treatment of ACC (4). However, mitotane has a narrow
therapeutic range, poor bioavailability and side effects (5). Long-term treatment causes obvious
adverse toxic effects, such as adrenal insufficiency, sexual
dysfunction, hypothyroidism and neurotoxicity (6–8).
Moreover, mitotane cannot improve long-term survival rates for
patients with metastatic ACC or inoperable ACC, although it may
delay tumour progression (9).
Therefore, safe treatment strategies to overcome drug tolerance and
minimize side effects are needed to improve ACC treatment.
Curcumin is a plant polyphenolic compound and a
major component in turmeric (Curcuma longa) (10). Curcumin has been reported to have
good efficacy and biological safety for treating cancer, with
targeted inhibitory effects on gastric, colorectal, breast and
cervical cancer (11–14). Curcumin induces the apoptosis of
colon cancer cells with wild-type p53 and mutant p53 in a dose- and
time-dependent manner (15). In
addition, curcumin inhibits the nuclear translocation of NF-κB
through the inhibition of IκB kinase, leading to apoptosis of
prostate carcinoma cells (16) and
inducing apoptosis associated with endoplasmic reticulum (ER)
stress in lung cancer cells (17).
Moreover, curcumin induces cytotoxic effects in papillary thyroid
cancer and cancer stem-like cells by targeting the JAK/STAT3
signalling pathway (18). In
addition, clinical trials have indicated that curcumin is safe and
exhibits therapeutic efficacy in patients with progressive,
advanced types of cancer. A study by Sharma et al (19) indicated that curcumin extract may be
administered safely for several months at daily doses of up to 2.2
g (equivalent to 180 mg of curcumin), resulting in a clinical
benefit in patients with advanced colorectal cancer. Curcumin also
has anticancer activity and is tolerated and safe in patients with
breast and prostate cancer (20,21).
However, whether curcumin can induce apoptosis of ACC cells or
inhibit tumour growth is unclear, and the possible mechanisms
remain undefined.
The current study evaluated the effect of curcumin
on ACC and analysed the underlying mechanisms by high-throughput
sequencing. The systemic analysis of curcumin-induced
whole-transcriptome alterations provided precise molecular targets
and signalling pathways, contributing to the improved application
of curcumin for ACC treatment.
Materials and methods
Cell culture and drugs
The human SW-13 cell line was purchased from the
National Collection of Authenticated Cell Cultures, Chinese Academy
of Sciences (cat. no. TCHu221). The human NCI-H295R cell line was
purchased from American Type Culture Collection (cat. no.
ATCC® CRL-2128™). The cells were cultured in high
glucose DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin solution (Beijing Solarbio Science &
Technology Co., Ltd.). Mycoplasma testing was routinely carried out
to ensure that the cell lines were mycoplasma-free. Cells were
cultured in an incubator containing 5% CO2 at 37°C.
Curcumin was purchased from Sigma-Aldrich; Merck KGaA, dissolved in
DMSO and then diluted in DMEM to the desired concentrations.
Cell viability assay
SW-13 and NCI-H295R cells were plated in 96-well
plates at a density of 5×103 cells/well. Cells were
allowed to attach overnight. The following day, cells were treated
with 0, 10, 20, 30, 40, 50, 60, 80 or 100 µM curcumin for 24 or 48
h at 37°C. Cell viability was then measured with a Cell Counting
Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.).
Briefly, CCK-8 solution (10 µl) was added to each well and
incubated for an additional 1 h at 37°C, optical density values at
450 nm were assessed using a microplate reader (Fluoroskan Ascent™;
Thermo Fisher Scientific, Inc.). The half-maximal inhibitory
concentration (IC50) values were calculated by SPSS
v20.0 software (IBM Corp.)
Transwell migration and invasion
assays
SW-13 and NCI-H295R cells were treated with 0, 20,
30 or 40 µM curcumin for 24 h at 37°C. For the migration assay,
2×105 cells suspended in serum-free medium were seeded
in the upper compartment of Transwell chambers (Corning, Inc.).
Subsequently, 500 µl medium containing 10% FBS was added to the
lower compartment. For the invasion assay, the upper chambers were
coated with Matrigel for 30 min at 37°C (Corning, Inc.). After
incubation for 24 h at 37°C in 5% CO2, the cells on the
upper chambers were removed using a cotton swab, and the filters
were washed with PBS. Cells that migrated to the bottom side of the
membrane were fixed with 4% paraformaldehyde for 15 min at room
temperature and stained with 0.1% crystal violet for 15 min at room
temperature. Images were captured under a light microscope at a
magnification of ×100 (Nikon Corporation) and counted in five
randomly selected visual fields using ImageJ software version 1.8.0
(National Institutes of Health).
Flow cytometry analyses of
apoptosis
SW-13 and NCI-H295R cells were treated with 0, 20,
30 or 40 µM curcumin for 24 h at 37°C. Cells were harvested and
resuspended in binding buffer at a concentration of
1×106 cells/ml. Subsequently, 100 µl cell suspension, 5
µl Annexin V-APC and 5 µl 7-AAD (BD Biosciences) solution were
added to a culture tube and incubated for 15 min in the dark at
room temperature. After 400 µl binding buffer was added to each
tube, apoptosis was analysed with a FACSCalibur™ flow cytometer (BD
Biosciences). Data were analysed with FlowJo v10.5.3 software
(FlowJo LLC).
RNA library preparation,
high-throughput sequencing and RNA sequencing (RNA-seq) data
analysis
Total RNA was extracted from control- and
curcumin-treated groups of ACC SW-13 cells using TRIzol®
(Takara Biotechnology Co., Ltd.). PCR amplification, product
purification and quantification, and sequencing were carried out on
the Illumina HiSeq platform at Sangon Biotech Co., Ltd. The RNA
concentration was measured using a Qubit® RNA Assay kit
(Life Technologies, Inc.) on a Qubit® 2.0 fluorometer
(Thermo Fisher Scientific, Inc.), and the RNA integrity was
assessed using an RNA Nano 6000 Assay kit on the Bioanalyser 2100
system (both from Agilent Technologies, Inc.). A total of 2 µg of
RNA per sample was used as the input material, and mRNA was
purified from total RNA using poly-T oligo-attached magnetic beads.
Sequencing libraries were generated using the Hieff NGS™ MaxUp
Dual-mode mRNA Library Prep Kit for Illumina® (Yeasen
Biotech, Inc.) according to the manufacturer's protocol. PCR was
performed with Phusion High-Fidelity DNA polymerase, Universal PCR
primers and Index (X) Primer. Finally, the products were purified
(AMPure XP system), and the library quality was assessed on the
Agilent Bioanalyser 2100 system. Paired-end sequencing of the
library was performed on HiSeq XTen sequencers (Illumina, Inc.),
and the quality of the sequencing data was evaluated with FastQC
(version 0.11.2). The significant differentially expressed genes
(DEGs) were determined using a of false discovery rate (FDR)
corrected P-value <0.05 and a fold change >2 as the
threshold. Gene Ontology (GO) enrichment was performed with TopGO
(version 2.24.0). Kyoto Encyclopedia of Genes and Genomes (KEGG)
enrichment analysis was performed with ClusterProfiler (version
3.0.5), and a protein-protein interaction (PPI) network was
constructed with the R igraph package and the STRING database
(http://string-db.org).
Reverse transcription-quantitative
(RT-q)PCR
After total RNA was extracted from ACC SW-13 cells,
NCI-H295R cells and xenograft tumour tissues using
TRIzol®, and cDNA was synthesised using the Takara RT
kit (Takara Biotechnology Co., Ltd.) at 37°C for 15 min, 85°C for 5
sec, and stored at 4°C until subsequent experimentation. Next, mRNA
expression levels were measured via qPCR using
SYBR®-Green PCR Master Mix (Takara Biotechnology Co.,
Ltd.) in an Applied Biosystems 7500 Real-Time PCR system (Thermo
Fisher Scientific, Inc.). The thermocycling conditions were 95°C
for 30 sec, followed by 40 cycles at 95°C for 5 sec and 60°C for 34
sec. GAPDH was selected as the reference gene and expression levels
were calculated according to the 2−ΔΔCq method (22). The primer sequences used are listed
in Tables I and II.
 | Table I.Human primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Human primer sequences for reverse
transcription-quantitative PCR.
| Gene name | Forward primer,
5–3 | Reverse primer,
5–3 |
|---|
| GAPDH |
CAGGAGGCATTGCTGATGAT |
GAAGGCTGGGGCTCATTT |
| HSP70 |
AAGAACGCCCTGGAGTCCTACG |
CTTGTCCGCCTCGCTGATCTTG |
| JNK |
ACACCACAGAAATCCCTAGAAG |
CACAGCATCTGATAGAGAAGGT |
| p38 |
ATTTCAGTCCATCATTCATGCG |
GTAAAAACGTCCAACAGACCAA |
| c-Jun |
AAGATGGAAACGACCTTCTACG |
CTTAGGGTTACTGTAGCCGTAG |
| c-Fos |
CTTCCCAGAAGAGATGTCTGTG |
TGGGAACAGGAAGTCATCAAAG |
| ATF4 |
ATGGATTTGAAGGAGTTCGACT |
AGAGATCACAAGTGTCATCCAA |
| CHOP |
GAGAATGAAAGGAAAGTGGCAC |
ATTCACCATTCGGTCAATCAGA |
| Bax |
CGAACTGGACAGTAACATGGAG |
CAGTTTGCTGGCAAAGTAGAAA |
| Bcl-2 |
TGCATCCCAAACAAGCTCCC |
TGCCCTTGGTCTTCTGTGGA |
 | Table II.Mouse primer sequences for reverse
transcription-quantitative PCR. |
Table II.
Mouse primer sequences for reverse
transcription-quantitative PCR.
| Gene name | Forward primer,
5-3 | Reverse primer,
5-3 |
|---|
| GAPDH |
GGTTGTCTCCTGCGACTTCA |
TGGTCCAGGGTTTCTTACTCC |
| HSP70 |
CAACAAGATCACCATCACCAAC |
TTCATGTTGAAGGCATAGGACT |
| JNK |
TTGAAAACAGGCCTAAATACGC |
GTTTGTTATGCTCTGAGTCAGC |
| p38 |
AGGAATTCAATGACGTGTACCT |
AGGTCCCTGTGAATTATGTCAG |
| c-Jun |
GAAAGCTGTGTCCCCTGTCTG |
CACACCATCTTCTGGTGTACAGT |
| c-Fos |
TCTCTAGTGCCAACTTTATCCC |
GAGATAGCTGCTCTACTTTGCC |
| ATF4 |
AGTTTAGAGCTAGGCAGTGAAG |
CATACAGATGCCACTGTCATTG |
| CHOP |
CTCCAGATTCCAGTCAGAGTTC |
ACTCTGTTTCCGTTTCCTAGTT |
| Bax |
TTGCCCTCTTCTACTTTGCTAG |
CCATGATGGTTCTGATCAGCTC |
| Bcl-2 |
GATGACTTCTCTCGTCGCTAC |
GAACTCAAAGAAGGCCACAATC |
Western blotting
Cells or tumour tissues were lysed with RIPA lysis
buffer containing 1 mM PMSF (Beijing Solarbio Science and
Technology Co., Ltd.). Protein concentrations were determined with
a BCA protein assay kit (Beyotime Institute of Biotechnology). A
total of 50 µg of total protein was separated using 10 or 12%
SDS-PAGE and transferred to PVDF membranes (MilliporeSigma).
Membranes were blocked with 5% non-fat milk for 1 h at room
temperature. Subsequently, primary antibodies specific for JNK
(cat. no. 9252), phosphorylated (p)-JNK (cat. no. 4668), p38 (cat.
no. 9212), p-p38 (cat. no. 4511), c-Jun (cat. no. 9165), Bax (cat.
no. 2772), Bcl-2 (cat. no. 3498) (all 1:1,000; Cell Signaling
Technology, Inc.), heat shock protein 70 (HSP70; cat. no. 48597),
c-Fos (cat. no. 49310), activating transcription factor 4 (ATF4;
cat. no. 49174) and C/EBP homologous protein (CHOP; cat. no. 49418)
(all 1:1,000; Signalway Antibody LLC) were added, and membranes
were incubated overnight at 4°C. After washing with 0.1%
TBS-Tween-20, horseradish peroxidase-conjugated secondary
antibodies (1:10,000; cat. no. L3012; Signalway Antibody LLC) were
added for 1 h at room temperature. Protein bands were visualised
using an Odyssey infrared laser scanner (LI-COR Biosciences) and
analysed with ImageJ software v1.8.0 (National Institutes of
Health). Membranes were also probed with an antibody specific for
GAPDH (cat. no. 21612; 1:10,000; Signalway Antibody LLC), which was
used as the loading control.
Lentiviral transfection
CHOP knockdown and negative control (GV493,
scrambled sequence) lentiviruses were designed and synthesized by
Shanghai GeneChem Co., Ltd. The selected targeting sequence of CHOP
(CHOP shRNA) was CGAATGGTGAATCTGCACCAA, and the sequence of the
negative control vector (NC) was TTCTCCGAACGTGTCACGT. SW-13 cells
were plated into 12-well plates at a density of 1×105
cells/well and incubated overnight. Subsequently, the medium was
replaced with medium containing lentivirus (CHOP shRNA or NC,
MOI=50). After 12 h, the medium was replaced with DMEM supplemented
with 10% FBS, and the expression of green fluorescent protein was
observed using fluorescence microscopy at a magnification of ×200
(Nikon Corporation) after 72 h. The cells were selected with 2
µg/ml puromycin (Beijing Solarbio Science & Technology Co.,
Ltd.) for 24 h at 37°C to kill the non-transfected cells. Finally,
the medium was replaced with complete medium. RT-qPCR and western
blot analyses were performed to determine the efficiency of CHOP
knockdown.
Xenograft transplantation and
therapy
BALB/c nude male mice (4-week-old; 18–20 g; n=8)
were obtained from the Experimental Animal Center of Guangxi
Medical University (Nanning, China) and raised under standard
conditions (20-26°C temperature; 40–60% humidity; 12 h light/12 h
dark cycle; food, drinking water and litter changed every 2 days)
in the Experimental Animal Center of Guangxi Medical University
(Nanning, China). All animal experiments were approved by the
Animal Ethics and Welfare Committee of Guangxi Medical University.
A total of 2×106 SW-13 cells in 100 µl PBS per mouse
were injected subcutaneously into the right flank. The tumours were
measured every other day, and the tumour volumes were calculated
with the following formula: Volume = length × width2 ×
1/2. When the tumour volumes were 100 mm3, the mice were
randomly divided into three groups of eight mice per group.
Curcumin dissolved in olive oil (50 or 100 mg/kg) was administered
via intraperitoneal injection to mice once daily for 2 weeks, while
mice in the control group received injections of olive oil. At the
end of treatment, the mice were anaesthetised with 1% sodium
pentobarbital and then sacrificed using cervical dislocation. The
tumours were removed and weighed for subsequent studies.
Electron microscopy
Tumour tissues were fixed with phosphate buffer (pH
7.4) containing 2.5% glutaraldehyde overnight at 4°C. Tissues were
then post-fixed with 1% OsO4 for 1 h at room
temperature, stained with 1% uranyl acetate for 1 h at room
temperature, dehydrated through graded acetone solutions and
embedded in Epon overnight at room temperature. Areas containing
tissues were mounted in blocks and sliced into 70-nm-thick
sections. Sections were examined under a transmission electron
microscope (HT 7800; Hitachi Ltd.) at a magnification of ×2,500 or
×7,000.
Statistical analysis
Data are presented as the mean ± SD and were
analysed using SPSS v20.0 software (IBM Corp.). One-way ANOVA
combined with Bonferroni's post-hoc test was employed to analyse
the differences between sets of data. P<0.05 was considered to
indicate a statistically significant difference.
Results
Curcumin inhibits the viability,
migration and invasion of ACC cells
The CCK-8 assay was used to evaluate the viability
of SW-13 and NCI-H295R cells. SW-13 and NCI-H295R cells were
exposed to various concentrations of curcumin (0, 10, 20, 30, 40,
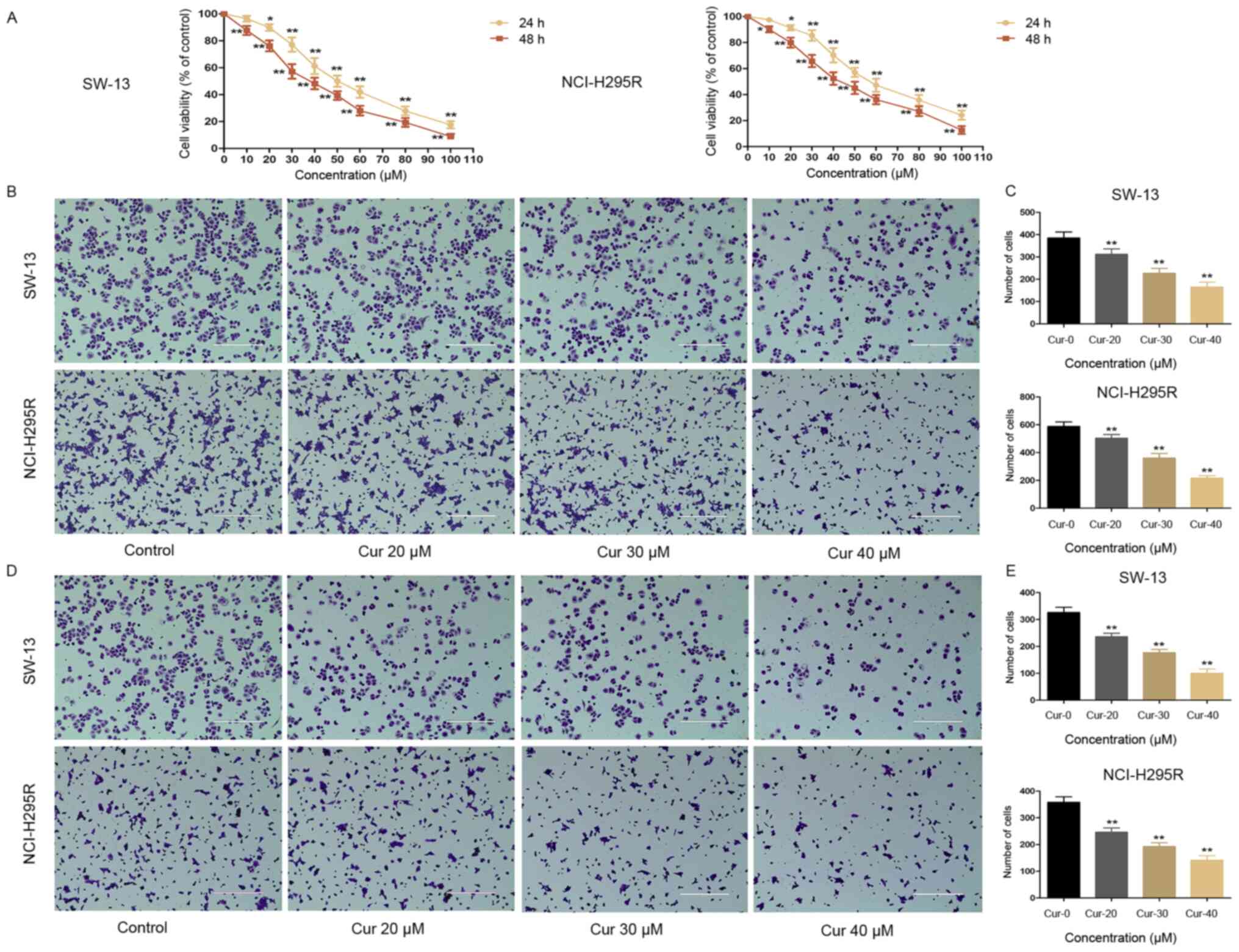
50, 60, 80 or 100 µM) for 24 or 48 h. As shown in Fig. 1A, cell viability decreased
significantly in a concentration-dependent manner. The
IC50 values for curcumin in SW-13 and NCI-H295R cells
were 50.809±1.706 and 59.271±1.773 at 24 h and 35.917±1.555 and
42.317±1.627 at 48 h, respectively (Table III). In the cell motility assay,
both the migration and invasion of SW-13 and NCI-H295R cells were
significantly decreased by exposure to curcumin for 24 h (Fig. 1B-E).
 | Table III.IC50 values for curcumin
in adrenocortical carcinoma cell lines. |
Table III.
IC50 values for curcumin
in adrenocortical carcinoma cell lines.
| Cell lines | IC50 at
24 h ± SD, µM | IC50 at
48 h ± SD, µM |
|---|
| SW-13 | 50.809±1.706 | 35.917±1.555 |
| NCI-H295R | 59.271±1.773 | 42.317±1.627 |
Curcumin induces apoptosis of ACC
cells
To investigate the effects of curcumin on the
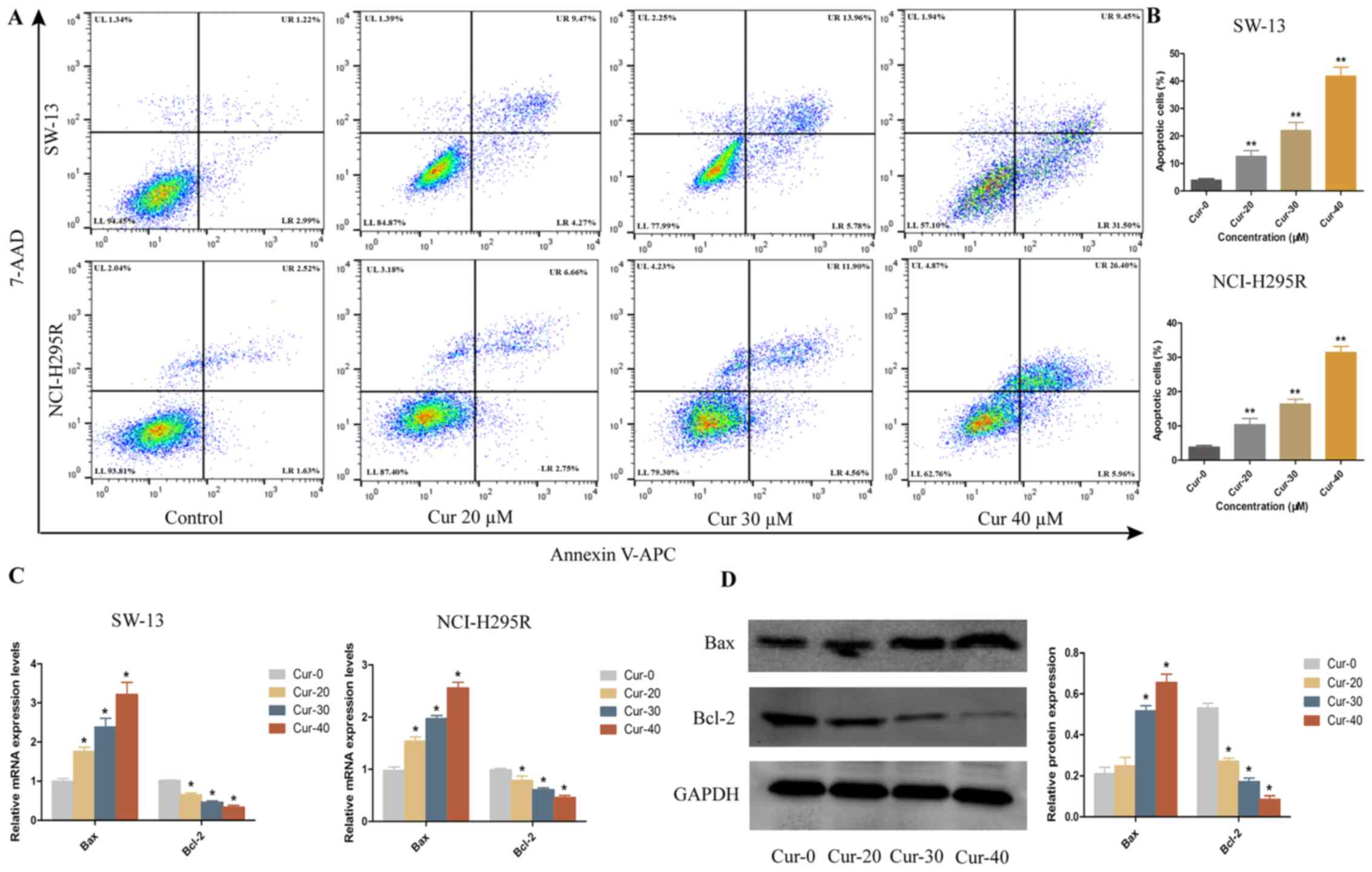
apoptosis of SW-13 and NCI-H295R cells, flow cytometric analysis
was performed using Annexin V-APC/7-AAD, revealing that curcumin
induced apoptosis in a dose-dependent manner (Fig. 2A and B). Next, the expression levels
of the apoptotic factor Bax and the anti-apoptotic factor Bcl-2
were examined by RT-qPCR and western blotting. As the concentration
of curcumin increased, the mRNA and protein expression levels of
the apoptotic factor Bax significantly increased, while those of
the anti-apoptotic factor Bcl-2 significantly decreased (Fig. 2C and D). These results indicated that
curcumin induced apoptosis in ACC cells.
RNA-seq and bioinformatic analyses of
the mechanism of curcumin in ACC
The experimental conditions for transcriptome
analysis were determined based on the aforementioned cell viability
assays. The CCK-8 assay results showed that 50 µM curcumin, which
resulted in a cell viability of 49.9%, was the closest to the
IC50 of curcumin in SW-13 cells after incubation for 24
h (Fig. 1A). Therefore,
transcriptome analysis was performed on SW-13 cells after treatment
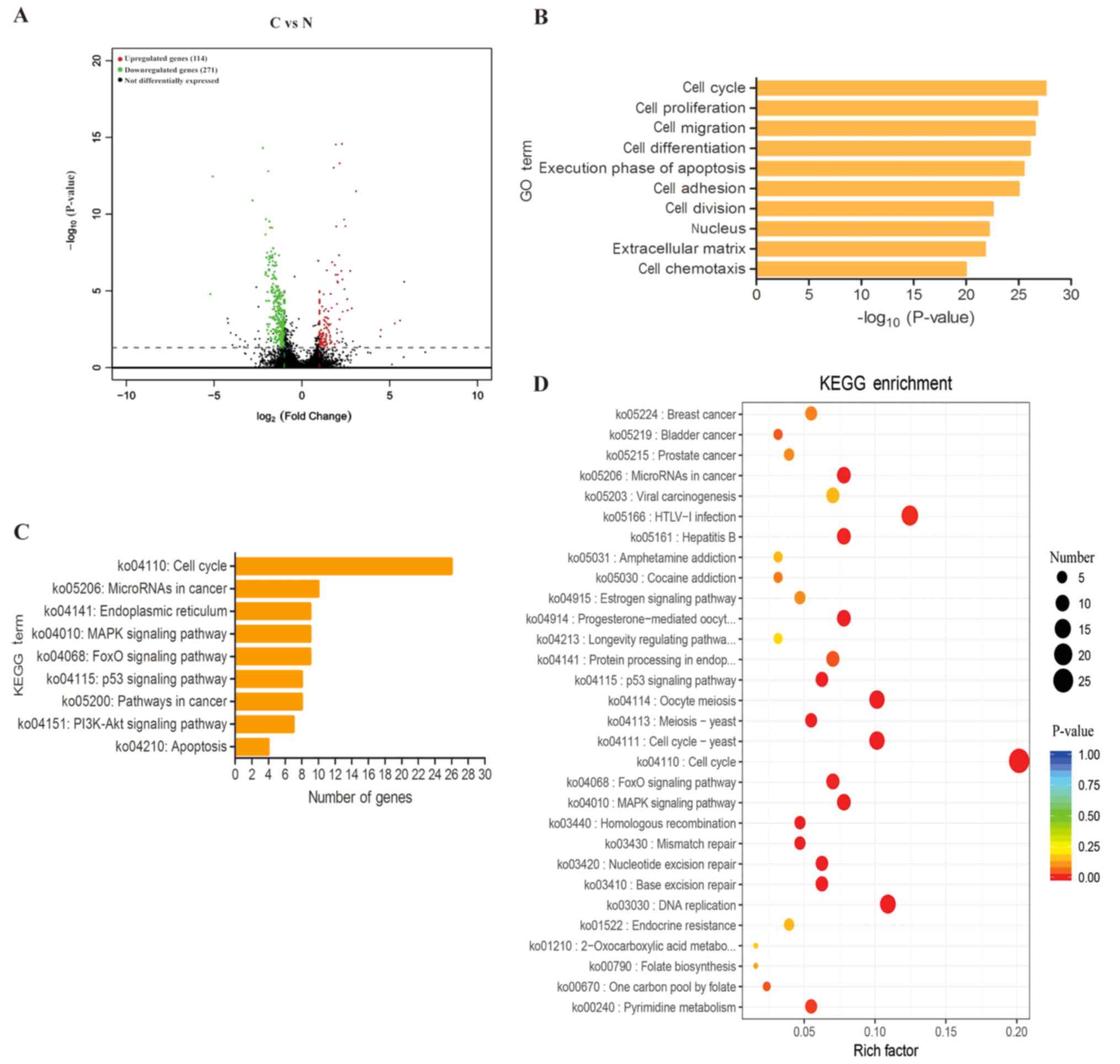
with 50 µM curcumin for 24 h. In total, 385 DEGs were identified in
the curcumin-treated group compared with the control group; 114
genes were upregulated and 271 genes were downregulated (Fig. 3A). GO enrichment analysis was used to
examine the processes in which the DEGs were involved, and the top
ten enriched GO terms were associated with in ‘cell cycle’, ‘cell
proliferation’, ‘cell migration’, ‘cell differentiation’ and
‘execution phase of apoptosis’ (Fig.
3B). KEGG enrichment analysis demonstrated that
curcumin-induced apoptosis of ACC cells was mediated primarily
through the ‘cell cycle’, ‘microRNAs in cancer’, ‘endoplasmic
reticulum’ and the ‘MAPK signalling pathway’ (Fig. 3C and D). These altered genes and
pathways may improve the understanding of the mechanisms underlying
curcumin-induced apoptosis of ACC cells.
Confirmation of DEGs by RT-qPCR and
western blotting
The GO and KEGG analysis results showed that the
‘MAPK signalling pathway’ and ‘endoplasmic reticulum’ pathway may
play notable roles in the curcumin-induced apoptosis of ACC cells.
These results were validated using RT-qPCR and western blotting
(Fig. 4A-C). HSP70 is a molecular
chaperone that inhibits apoptosis (23). After curcumin treatment, HSP70
expression was downregulated, while the expression levels of
p-JNK/JNK, p-p38/p38, c-Jun, c-Fos, ATF4 and CHOP increased. These
results indicated that the JNK, p38 MAPK and ER stress pathways
were activated by curcumin treatment.
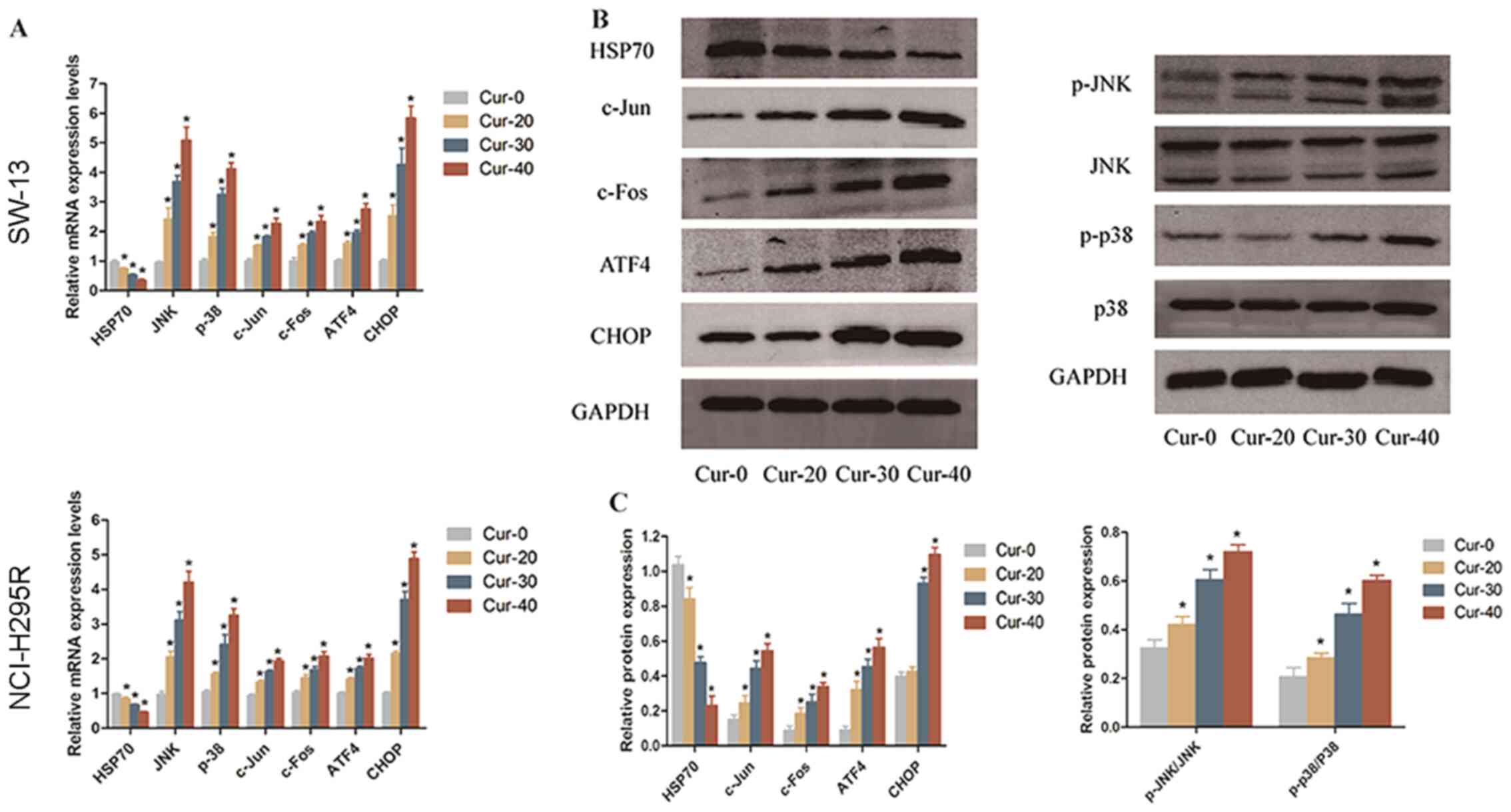 | Figure 4.Curcumin induces apoptosis of
adrenocortical carcinoma cells through the JNK, p38 MAPK and
endoplasmic reticulum stress pathways. (A) mRNA expression levels
of HSP70, JNK, p38, c-Jun, c-Fos, ATF4 and CHOP in SW-13 cells and
NCI-H295R cells treated with 0, 20, 30 or 40 µM curcumin. (B)
Protein expression levels in SW-13 cells were analysed by western
blotting. (C) Quantification of the western blotting results.
*P<0.05 vs. Cur-0. HSP70, heat shock protein 70; ATF4,
activating transcription factor 4; CHOP, C/EBP homologous protein;
p, phosphorylated. |
CHOP-knockdown inhibits
curcumin-induced apoptosis of SW-13 cells
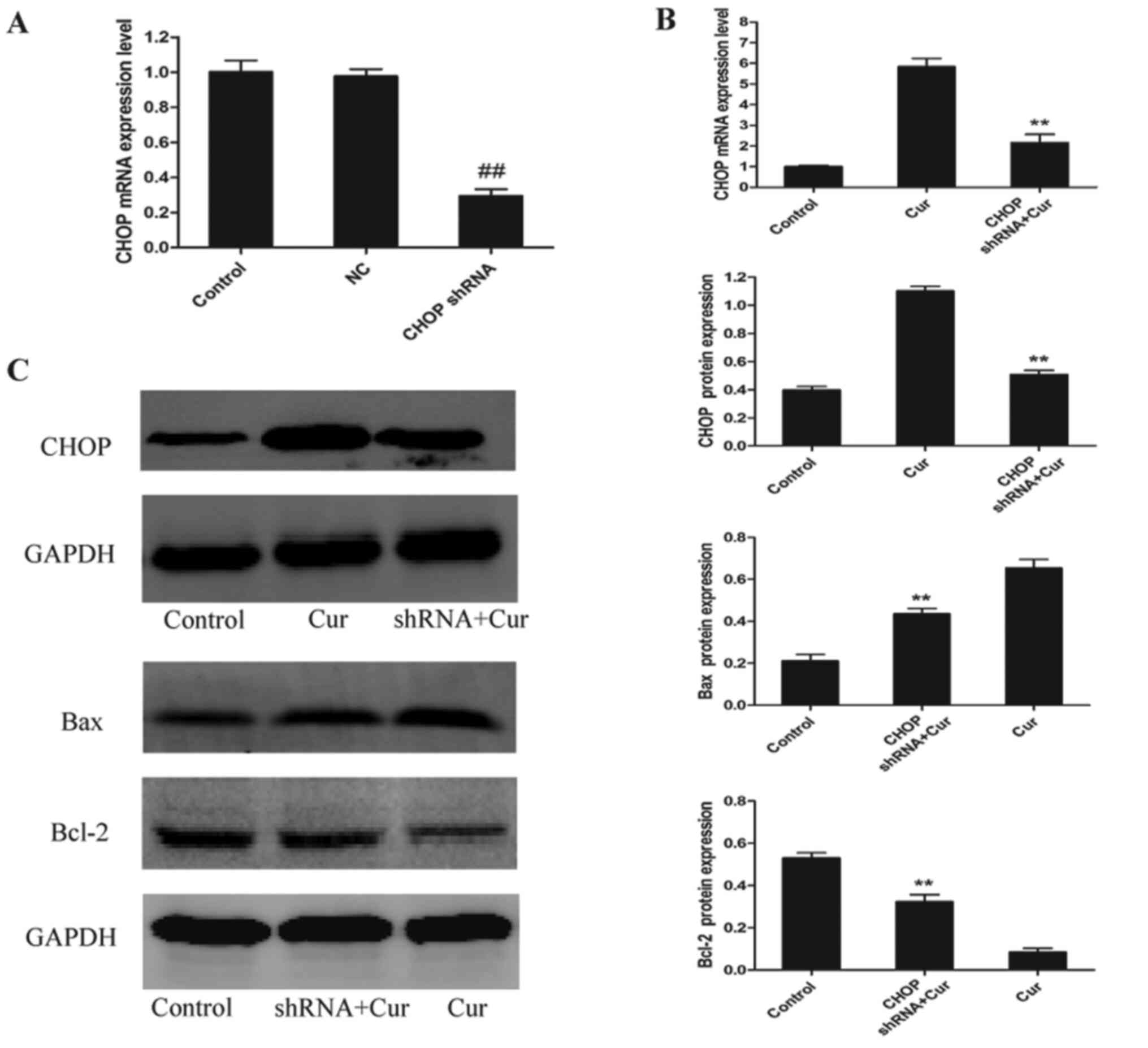
To further determine whether CHOP plays a notable
role in curcumin-induced apoptosis, a shRNA was used to knock down
CHOP expression. As shown in Fig.
5A, CHOP mRNA expression was significantly decreased by ~70%
compared with that in cells transfected with the NC. Following
treatment with 40 µM curcumin, there was a significant decrease in
CHOP mRNA and protein expression compared with the curcumin-treated
group (Fig. 5B and C). Moreover, the
protein expression levels of the apoptotic factor Bax were
decreased, while the protein expression levels of the
anti-apoptotic factor Bcl-2 were increased compared with the
curcumin-treated group (Fig.
5C).
Curcumin inhibits SW-13 ×enograft
tumour growth
Based on the inhibitory effects of curcumin on ACC
cell proliferation in vitro, a SW-13 ×enograft tumour model
was established in nude mice. After intraperitoneal injection of 50
or 100 mg/kg curcumin or vehicle alone for 2 weeks, a significant
decrease in the tumour weight and volume was observed in the groups
treated with 50 and 100 mg/kg curcumin (Fig. 6A-C). As shown in Fig. 6D, the body weights of the mice in the
different treatment groups did not change significantly. Curcumin
at a dose of 100 mg/kg exhibited a greater inhibitory effect on
tumour growth compared with the other groups, and expansion of the
ER was observed under an electron microscope (Fig. 6E). The expression levels of genes in
the JNK, p38 MAPK and ER stress pathways were measured in xenograft
tumours by RT-qPCR and western blotting, as shown in Fig. 7. After curcumin treatment, HSP70 and
Bcl-2 expression was significantly decreased, while Bax expression
was significantly increased (Fig. 7A, B
and D). The JNK, p38 MAPK and ER stress pathways were
activated, and the levels of p-JNK/JNK, p-p38/p38, c-Jun, c-Fos,
ATF4 and CHOP were significantly increased in the 50 and 100 mg/kg
curcumin-treated groups (Fig. 7A-C).
These data indicated that curcumin exhibited antitumour activity,
was safe to use in vivo and inhibited xenograft tumour
growth through the JNK, p38 MAPK and ER stress pathways.
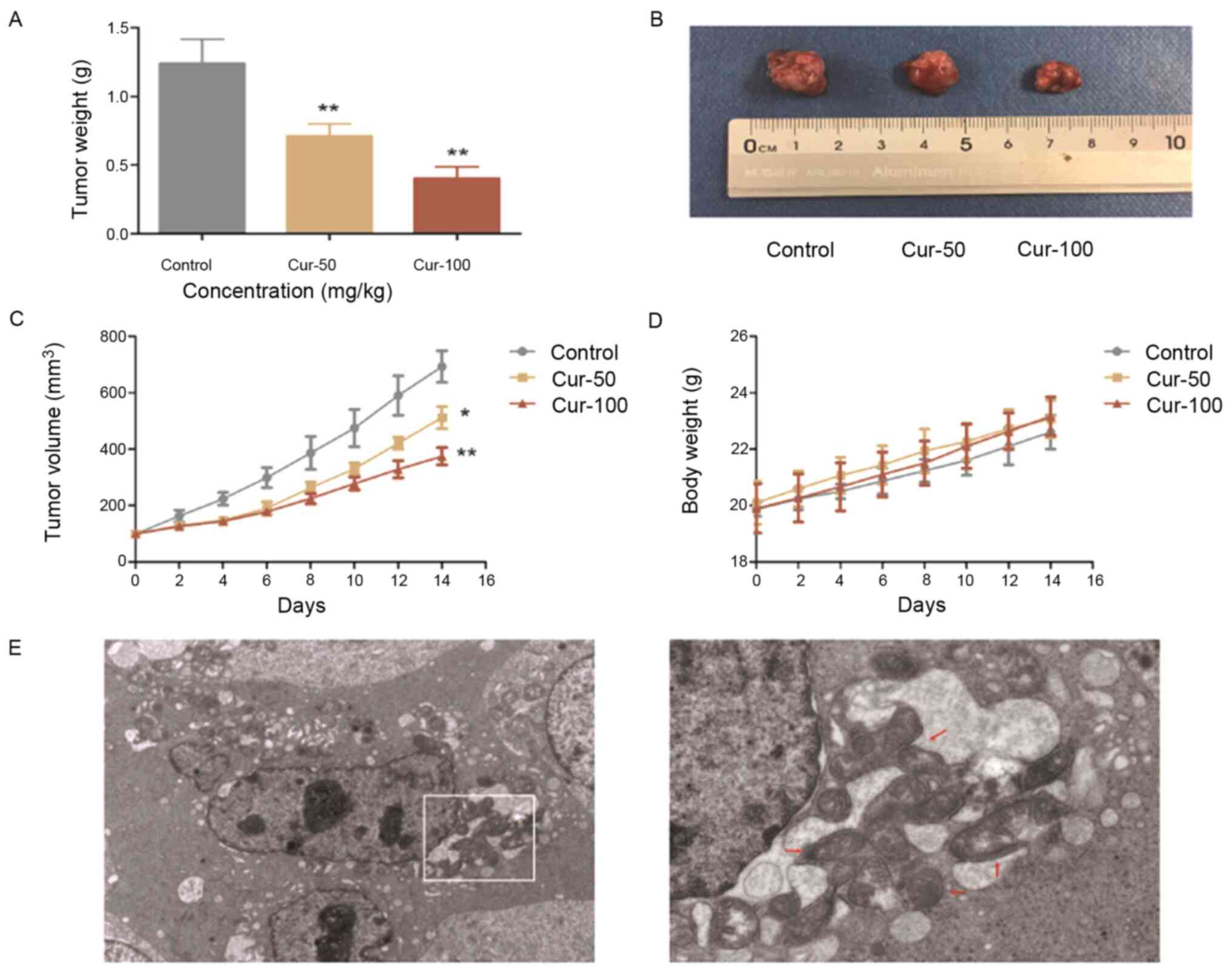 | Figure 6.Curcumin inhibits SW-13 ×enograft
tumour growth in vivo. (A) Tumour weights of mice in the
experimental groups. (B) SW-13 ×enograft tumours. (C) Tumour
volumes at 0, 2, 4, 6, 8, 10, 12 and 14 days. (D) Changes in the
body weight of mice in the different groups. (E) ER morphology was
examined with an electron microscope and revealed ER expansion (red
arrows) in xenograft tumours treated with curcumin. Original
magnification, ×2,500 and ×7,000. *P<0.05 and **P<0.01 vs.
control. ER, endoplasmic reticulum; Cur, curcumin. |
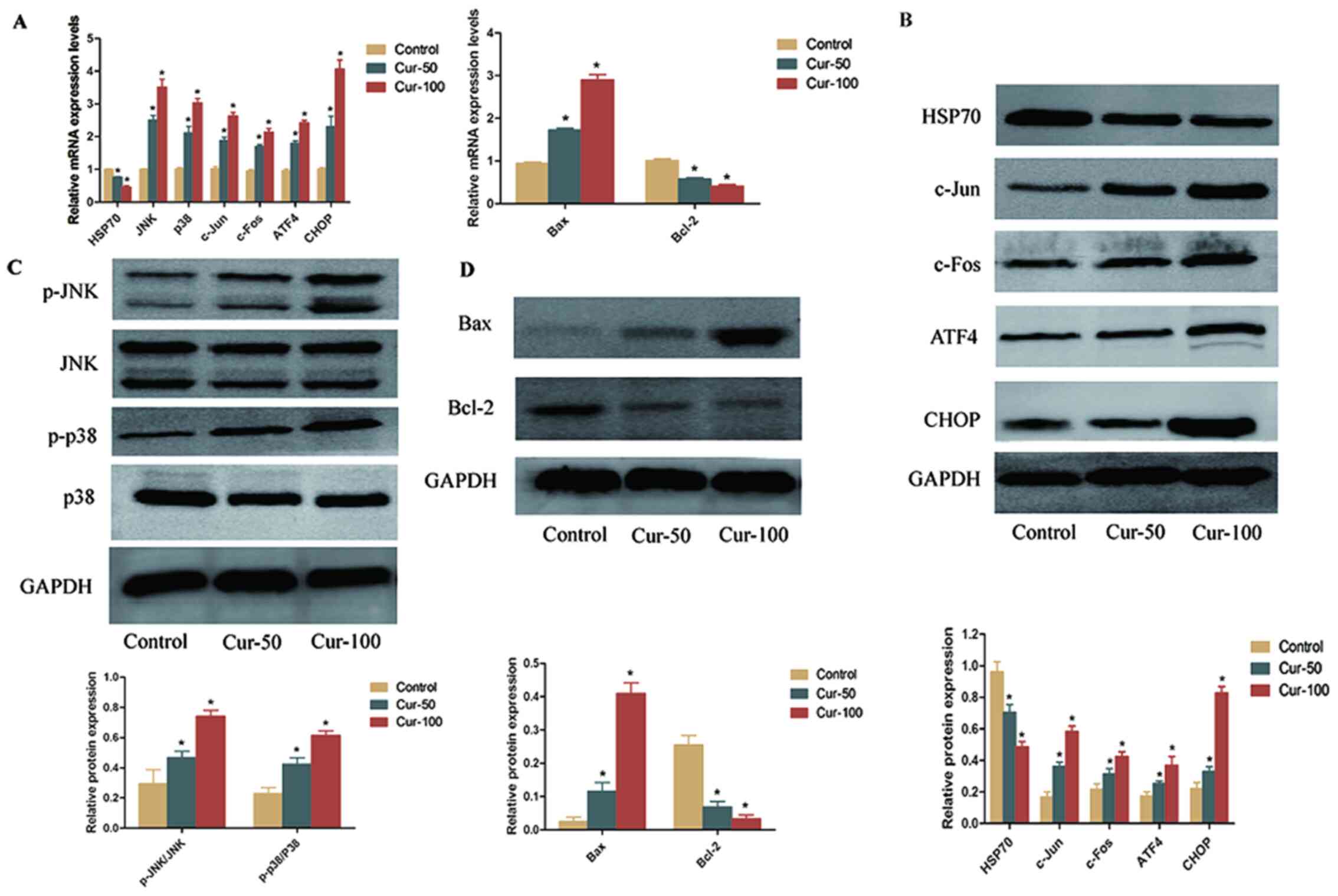 | Figure 7.Expression levels of differentially
expressed genes in the JNK, p38 MAPK and endoplasmic reticulum
stress pathways in xenograft tumours treated with 50 or 100 mg/kg
curcumin. (A) mRNA expression levels of HSP70, JNK, p38, c-Jun,
c-Fos, ATF4, CHOP, Bax and Bcl-2 in xenograft tumours. (B) Protein
expression levels of HSP70, c-Jun, c-Fos, ATF4, and CHOP in
xenograft tumours. (C) Protein expression levels of p-JNK/JNK and
p-p38/p38 in xenograft tumours. (D) Protein expression levels of
Bax and Bcl-2 in xenograft tumours. *P<0.05 vs. control. HSP70,
heat shock protein 70; ATF4, activating transcription factor 4;
CHOP, C/EBP homologous protein; p, phosphorylated; Cur,
curcumin. |
Discussion
Curcumin is a natural potential anticancer agent
with few side effects (24).
Curcumin potently inhibits tumour cell apoptosis and has been
reported to inhibit the proliferation of human non-small cell lung
carcinoma cells (25). In addition,
animal studies have shown that curcumin inhibits tumorigenesis and
tumour metastasis. Zhu et al (26) reported that curcumin inhibits the
proliferation and invasion of monocytic leukaemia cells in
vivo. Curcumin was also shown to inhibit the growth and
angiogenesis of colorectal cancer CT26 ×enografts in nude mice
(27) and to decrease tumour growth
and augment the cisplatin antitumour effects in Sprague-Dawley rats
with 7,12-dimethyl benz[a]anthracene-induced breast cancer
(28). ACC is malignant and
aggressive; therefore, the majority of patients who relapse or who
have metastatic disease do not survive (29). The present study used two ACC cell
lines to demonstrate that curcumin inhibits the viability,
migration and invasion, and induces apoptosis in ACC cells in
vitro. Moreover, curcumin was well tolerated and effectively
inhibited tumour growth in vivo in a nude mouse xenograft
model. However, since it has low bioavailability, its derivative
curcumin liposomes should be used to enhance solubility in future
studies.
Curcumin can inhibit cancer by exerting several
biological effects, including decreasing proliferation, inducing
apoptosis, inhibiting invasion and regulating the cell cycle
(30–32). Curcumin increases glioblastoma cell
death by inhibiting the PI3K/Akt/mTOR signaling pathway (33). In addition, curcumin inhibits bladder
cancer cell proliferation and bladder tumour growth by decreasing
Sp1, Sp3 and Sp4 protein levels (34). However, curcumin exerts an antitumour
effect by inducing apoptosis in gastric and lung cancer cells by
activation of Bax, caspases and pro-apoptotic ER stress (35,36). In
the present study, transcriptomic analysis was performed in ACC
cells to explore potential target genes and signalling mechanisms
active after curcumin treatment. Hub genes and signalling pathways
that may serve important roles in the curcumin-induced apoptosis of
ACC cells were identified. A total of 385 DEGs, 114 upregulated and
271 downregulated, were identified. GO and KEGG pathway enrichment
analyses showed that the ‘cell cycle’, ‘microRNAs in cancer’, ‘MAPK
signaling pathway’ and ‘endoplasmic reticulum’ were the predominant
pathways associated with curcumin-induced apoptosis of ACC
cells.
HSP70 is a molecular chaperone required for the
regulation of cellular homeostasis via the control of protein
folding, translocation, biogenesis and degradation (37). HSP70, a member of the HSP family, has
been reported to be highly expressed in numerous types of cancer
and is associated with higher tumour grade, metastasis,
chemotherapeutic resistance and poor prognosis (38,39).
Nuclear translocation and expression of the HSP70 protein is
correlated with the stage and the prognosis of patients with
papillary thyroid cancer (40). The
overall survival rates of patients with nasopharyngeal carcinoma
with positive expression of HSP70 were significantly lower than
those with negative expression (41). Sheng et al (42) showed that inhibition of HSP70
expression enhances the sensitivity of gastric cancer cells to
cisplatin via the MAPK signalling pathway and that HSP70 may be a
therapeutic target in gastric cancer. The present study suggested
that the MAPK signalling pathway was involved in the
curcumin-induced apoptosis of ACC cells. JNK and p38 are essential
components of the MAPK signal transduction pathway, leading to
programmed cell death in response to certain stimuli (43,44). The
present results indicated that as curcumin concentration increased,
HSP70 expression decreased, and JNK and p38 were activated and
phosphorylated. JNK and p38 are typically described as
stress-activated kinases that mediate apoptotic signals (45). HSP70 senses the accumulation of
abnormal proteins after heat shock and other stresses to regulate
JNK and p38 (46). Furthermore, JNK
and p38 orchestrate cellular responses by mediating their
downstream transcription factor activator protein-1, a c-Jun/c-Fos
heterodimer. JNK and p38 are activated to modify the expression of
CHOP, and participate in apoptosis induced by the ER stress pathway
(47,48).
Curcumin has been reported to increase the
expression of ATF4 and CHOP, which are considered the hallmarks of
ER stress-induced apoptosis (49,50).
During severe or prolonged ER stress, ATF4 is activated and binds
to AARE1 on the CHOP promoter, further promoting the expression of
the ER stress-associated apoptotic factor CHOP (51–53).
After curcumin treatment, the expression levels of ATF4, and CHOP
increased in ACC cells in vitro and in vivo. The
current study investigated the role of CHOP in curcumin-induced
apoptosis using lentivirus transfection. The shRNA-mediated
knockdown of CHOP expression inhibited curcumin-induced apoptosis
in ACC SW-13 cells. The current results are consistent with those
of previous studies (54–56). Therefore, activation of JNK and p38
to stimulate ER stress appears to be a major contributor to the
curcumin-induced apoptosis of ACC cells.
In conclusion, curcumin inhibited ACC growth and
induced apoptosis. Curcumin activated JNK and p38 MAPK, and
stimulated ER stress, which may serve a notable role in apoptosis.
Thus, the present results may facilitate the search for novel
treatments of ACC and for understanding the molecular mechanisms
underlying the effects of curcumin. Curcumin may be a promising
candidate for ACC therapy. Nonetheless, future studies should
investigate the mechanism of the cell cycle and microRNA pathways
in curcumin-induced apoptosis of ACC cells in curcumin liposome
experiments.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81060220, 81860146
and 81660138), The Ministry of Science and Technology (grant nos.
2016YFC0901200 and 2016YFC0901205), The Nanning Scientific Research
and Technology Development Plan Project (grant no. zc20203009) and
The Guangxi Medical and Health Self-financing Project (grant no.
Z20200122).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available in the [SRA] repository, [http://www.ncbi.nlm.nih.gov/bioproject/699600].
Authors' contributions
XH, DL, YM and ZL conceived and designed the study.
XH, CL, HY, XL, XD, XHL, LL, ZH, DL and ZL conducted the study and
analysed the data. XH and ZL confirmed the authenticity of all the
raw data. XH drafted the initial manuscript. DL, YM and ZL guided
the writing. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The in vivo animal experiments were approved
by The Animal Ethics and Welfare Committee of Guangxi Medical
University (Nanning, China; approval no. 201903022).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ACC
|
adrenocortical carcinoma
|
|
DEGs
|
differentially expressed genes
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
PPI
|
protein-protein interaction
|
|
shRNA
|
short hairpin RNA
|
|
ER
|
endoplasmic reticulum
|
|
HSP70
|
heat shock protein 70
|
References
|
1
|
Roca E, Berruti A, Sbiera S, Rapa I, Oneda
E, Sperone P, Ronchi CL, Ferrari L, Grisanti S, Germano A, et al:
Topoisomerase 2α and thymidylate synthase expression in
adrenocortical cancer. Endocr Relat Cancer. 24:319–327. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simon G, Pattou F, Mirallié E, Lifante JC,
Nominé C, Arnault V, de Calan L, Caillard C, Carnaille B, Brunaud
L, et al: Surgery for recurrent adrenocortical carcinoma: A
multicenter retrospective study. Surgery. 161:249–256. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Assié G, Antoni G, Tissier F, Caillou B,
Abiven G, Gicquel C, Leboulleux S, Travagli JP, Dromain C, Bertagna
X, et al: Prognostic parameters of metastatic adrenocortical
carcinoma. J Clin Endocrinol Metab. 92:148–154. 2007. View Article : Google Scholar
|
|
4
|
Ruggiero C, Doghman-Bouguerra M, Ronco C,
Benhida R, Rocchi S and Lalli E: The GRP78/BiP inhibitor HA15
synergizes with mitotane action against adrenocortical carcinoma
cells through convergent activation of ER stress pathways. Mol Cell
Endocrinol. 474:57–64. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hermsen IG, Haak HR, de Krijger RR,
Kerkhofs TM, Feelders RA, de Herder WW, Wilmink H, Smit JW,
Gelderblom H, de Miranda NF, et al: Mutational analyses of
epidermal growth factor receptor and downstream pathways in
adrenocortical carcinoma. Eur J Endocrinol. 169:51–58. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Baudin E, Pellegriti G, Bonnay M,
Penfornis A, Laplanche A, Vassal G and Schlumberger M: Impact of
monitoring plasma 1,1-dichlorodiphenildichloroethane (o,p'DDD)
levels on the treatment of patients with adrenocortical carcinoma.
Cancer. 92:1385–1392. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Muratori L, Pia A, Reimondo G, Pisano C,
La Salvia A, Puglisi S, Scagliotti GV and Sperone P: Prolonged
adrenal insufficiency after the discontinuation of mitotane
therapy. Endocr Metab Immune Disord Drug Targets. 20:485–487. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Else T, Kim AC, Sabolch A, Raymond VM,
Kandathil A, Caoili EM, Jolly S, Miller BS, Giordano TJ and Hammer
GD: Adrenocortical carcinoma. Endocr Rev. 35:282–326. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seidel E, Walenda G, Messerschmidt C,
Obermayer B, Peitzsch M, Wallace P, Bahethi R, Yoo T, Choi M,
Schrade P, et al: Generation and characterization of a
mitotane-resistant adrenocortical cell line. Endocr Connect.
9:122–134. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schaffer M, Schaffer PM and Bar-Sela G: An
update on Curcuma as a functional food in the control of cancer and
inflammation. Curr Opin Clin Nutr Metab Care. 18:605–611. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun C, Zhang S, Liu C and Liu X: Curcumin
Promoted miR-34a expression and suppressed proliferation of gastric
cancer cells. Cancer Biother Radiopharm. 34:634–641. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Milacic V, Banerjee S, Landis-Piwowar KR,
Sarkar FH, Majumdar AP and Dou QP: Curcumin inhibits the proteasome
activity in human colon cancer cells in vitro and in vivo. Cancer
Res. 68:7283–7292. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Somasundaram S, Edmund NA, Moore DT, Small
GW, Shi YY and Orlowski RZ: Dietary curcumin inhibits
chemotherapy-induced apoptosis in models of human breast cancer.
Cancer Res. 62:3868–3875. 2002.PubMed/NCBI
|
|
14
|
Sreekanth CN, Bava SV, Sreekumar E and
Anto RJ: Molecular evidences for the chemosensitizing efficacy of
liposomal curcumin in paclitaxel chemotherapy in mouse models of
cervical cancer. Oncogene. 30:3139–3152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Watson JL, Hill R, Yaffe PB, Greenshields
A, Walsh M, Lee PW, Giacomantonio CA and Hoskin DW: Curcumin causes
superoxide anion production and p53-independent apoptosis in human
colon cancer cells. Cancer Lett. 297:1–8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Killian PH, Kronski E, Michalik KM,
Barbieri O, Astigiano S, Sommerhoff CP, Pfeffer U, Nerlich AG and
Bachmeier BE: Curcumin inhibits prostate cancer metastasis in vivo
by targeting the inflammatory cytokines CXCL1 and −2.
Carcinogenesis. 33:2507–2519. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Xiao J, Zhou H, Yang S, Wu X,
Jiang C, Zhao Y, Liang D, Li X and Liang G: A novel monocarbonyl
analogue of curcumin,
(1E,4E)-1,5-bis(2,3-dimethoxyphenyl)penta-1,4-dien-3-one, induced
cancer cell H460 apoptosis via activation of endoplasmic reticulum
stress signaling pathway. J Med Chem. 54:3768–3778. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khan AQ, Ahmed EI, Elareer N, Fathima H,
Prabhu KS, Siveen KS, Kulinski M, Azizi F, Dermime S, et al:
Curcumin-mediated apoptotic cell death in papillary thyroid cancer
and cancer stem-like cells through targeting of the JAK/STAT3
signaling pathway. International journal of molecular sciences.
212020.
|
|
19
|
Sharma RA, McLelland HR, Hill KA, Ireson
CR, Euden SA, Manson MM, Pirmohamed M, Marnett LJ, Gescher AJ, et
al: Pharmacodynamic and pharmacokinetic study of oral Curcuma
extract in patients with colorectal cancer. Clin Cancer Res.
7:1894–1900. 2001.PubMed/NCBI
|
|
20
|
Saghatelyan T, Tananyan A, Janoyan N,
Tadevosyan A, Petrosyan H, Hovhannisyan A, Hayrapetyan L,
Arustamyan M, Arnhold J, et al: Efficacy and safety of curcumin in
combination with paclitaxel in patients with advanced, metastatic
breast cancer: A comparative, randomized, double-blind,
placebo-controlled clinical trial. Phytomedicine. 70:1532182020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi YH, Han DH, Kim SW, Kim MJ, Sung HH,
Jeon HG, Jeong BC, Seo SI, Jeon SS, Lee HM, et al: A randomized,
double-blind, placebo-controlled trial to evaluate the role of
curcumin in prostate cancer patients with intermittent androgen
deprivation. Prostate. 79:614–621. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Boudesco C, Cause S, Jego G and Garrido C:
Hsp70: A cancer target inside and outside the cell. Methods Mol
Biol. 1709:371–396. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee WH, Loo CY, Young PM, Traini D, Mason
RS and Rohanizadeh R: Recent advances in curcumin nanoformulation
for cancer therapy. Expert Opin Drug Deliv. 11:1183–1201. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shishodia S, Potdar P, Gairola CG and
Aggarwal BB: Curcumin (diferuloylmethane) down-regulates cigarette
smoke-induced NF-kappaB activation through inhibition of
IkappaBalpha kinase in human lung epithelial cells: Correlation
with suppression of COX-2, MMP-9 and cyclin D1. Carcinogenesis.
24:1269–1279. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu G, Shen Q, Jiang H, Ji O, Zhu L and
Zhang L: Curcumin inhibited the growth and invasion of human
monocytic leukaemia SHI-1 cells in vivo by altering MAPK and MMP
signalling. Pharm Biol. 58:25–34. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moradi-Marjaneh R, Hassanian SM, Rahmani
F, Aghaee-Bakhtiari SH, Avan A and Khazaei M: Phytosomal curcumin
elicits anti-tumor properties through suppression of angiogenesis,
cell proliferation and induction of oxidative stress in colorectal
cancer. Curr Pharm Des. 24:4626–4638. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kumar P, Barua CC, Sulakhiya K and Sharma
RK: Curcumin ameliorates cisplatin-induced nephrotoxicity and
potentiates its anticancer activity in sd rats: Potential role of
curcumin in breast cancer chemotherapy. Front Pharmacol. 8:1322017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kerkhofs TM, Ettaieb MH, Hermsen IG and
Haak HR: Developing treatment for adrenocortical carcinoma. Endocr
Relat Cancer. 22:R325–R338. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen L, Zhan CZ, Wang T, You H and Yao R:
Curcumin inhibits the proliferation, migration, invasion, and
apoptosis of diffuse large B-cell lymphoma cell line by regulating
MiR-21/VHL axis. Yonsei Med J. 61:20–29. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hassanalilou T, Ghavamzadeh S and Khalili
L: Curcumin and gastric cancer: A review on mechanisms of action. J
Gastrointest Cancer. 50:185–192. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He YC, He L, Khoshaba R, Lu FG, Cai C,
Zhou FL, Liao DF and Cao D: Curcumin nicotinate selectively induces
cancer cell apoptosis and cycle arrest through a P53-mediated
mechanism. Molecules. 24:41792019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maiti P, Plemmons A and Dunbar GL:
Combination treatment of berberine and solid lipid curcumin
particles increased cell death and inhibited PI3K/Akt/mTOR pathway
of human cultured glioblastoma cells more effectively than did
individual treatments. PLoS One. 14:e02256602019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chadalapaka G, Jutooru I, Chintharlapalli
S, Papineni S, Smith R III, Li X and Safe S: Curcumin decreases
specificity protein expression in bladder cancer cells. Cancer Res.
68:5345–5354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Firouzi Amoodizaj F, Baghaeifar S, Taheri
E, Farhoudi Sefidan Jadid M, Safi M, Seyyed Sani N, Hajazimian S,
Isazadeh A and Shanehbandi D: Enhanced anticancer potency of
doxorubicin in combination with curcumin in gastric adenocarcinoma.
J Biochem Mol Toxicol. 34:e224862020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lin SS, Huang HP, Yang JS, Wu JY, Hsia TC,
Lin CC, Lin CW, Kuo CL, Gibson Wood W and Chung JG: DNA damage and
endoplasmic reticulum stress mediated curcumin-induced cell cycle
arrest and apoptosis in human lung carcinoma A-549 cells through
the activation caspases cascade- and mitochondrial-dependent
pathway. Cancer Lett. 272:77–90. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Das JK, Xiong X, Ren X, Yang JM and Song
J: Heat Shock Proteins in Cancer Immunotherapy. J Oncol.
2019:32672072019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kumar S, Stokes J III, Singh UP, Scissum
Gunn K, Acharya A, Manne U and Mishra M: Targeting Hsp70: A
possible therapy for cancer. Cancer Lett. 374:156–166. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yun CW, Kim HJ, Lim JH and Lee SH: Heat
shock proteins: agents of cancer development and therapeutic
targets in anti-cancer therapy. Cells. 9:602019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Avdalyan AM, Ivanov AA, Lushnikova EL,
Molodykh OP and Vikhlyanov IV: The relationship of immunoexpression
of Ki-67 and Hsp70 with clinical and norphological parameters and
prognosis of papillary thyroid cancer. Bull Exp Biol Med.
168:688–693. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Feng J, Zhan Y, Zhang Y, Zheng H, Wang W
and Fan S: Increased expression of heat shock protein (HSP) 10 and
HSP70 correlates with poor prognosis of nasopharyngeal carcinoma.
Cancer Manag Res. 11:8219–8227. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sheng L, Tang T, Liu Y, Ma Y, Wang Z, Tao
H, Zhang Y and Qi Z: Inducible HSP70 antagonizes cisplatin induced
cell apoptosis through inhibition of the MAPK signaling pathway in
HGC 27 cells. Int J Mol Med. 42:2089–2097. 2018.PubMed/NCBI
|
|
43
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yue J and Lopez JM: Understanding MAPK
signaling pathways in apoptosis. Int J Mol Sci. 21:2346212020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Sui X, Kong N, Ye L, Han W, Zhou J, Zhang
Q, He C and Pan H: p38 and JNK MAPK pathways control the balance of
apoptosis and autophagy in response to chemotherapeutic agents.
Cancer Lett. 344:174–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gabai VL, Meriin AB, Mosser DD, Caron AW,
Rits S, Shifrin VI and Sherman MY: Hsp70 prevents activation of
stress kinases. A novel pathway of cellular thermotolerance. J Biol
Chem. 272:18033–18037. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zheng QY, Li PP, Jin FS, Yao C, Zhang GH,
Zang T and Ai X: Ursolic acid induces ER stress response to
activate ASK1-JNK signaling and induce apoptosis in human bladder
cancer T24 cells. Cell Signal. 25:206–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Choi JH, Jeong YJ, Yu AR, Yoon KS, Choe W,
Ha J, Kim SS, Yeo EJ and Kang I: Fluoxetine induces apoptosis
through endoplasmic reticulum stress via mitogen-activated protein
kinase activation and histone hyperacetylation in SK-N-BE(2)-M17
human neuroblastoma cells. Apoptosis. 22:1079–1097. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Mathur A, Abd Elmageed ZY, Liu X,
Kostochka ML, Zhang H, Abdel-Mageed AB and Mondal D: Subverting
ER-stress towards apoptosis by nelfinavir and curcumin coexposure
augments docetaxel efficacy in castration resistant prostate cancer
cells. PLoS One. 9:e1031092014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zinszner H, Kuroda M, Wang X, Batchvarova
N, Lightfoot RT, Remotti H, Stevens JL and Ron D: CHOP is
implicated in programmed cell death in response to impaired
function of the endoplasmic reticulum. Genes Dev. 12:982–995. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu TH, Tu WQ, Tao WC, Liang QE, Xiao Y
and Chen LG: Verification of resveratrol inhibits intestinal aging
by downregulating ATF4/Chop/Bcl-2/Bax signaling pathway: Based on
network pharmacology and animal experiment. Front Pharmacol.
11:10642020. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang X, Zhuang Y, Fang Y, Cao H, Zhang C,
Xing C, Guo X, Li G, Liu P, Hu G, et al: Endoplasmic reticulum
stress aggravates copper-induced apoptosis via the PERK/ATF4/CHOP
signaling pathway in duck renal tubular epithelial cells. Environ
Pollut. 272:1159812021. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Khatun H, Wada Y, Konno T, Tatemoto H and
Yamanaka KI: Endoplasmic reticulum stress attenuation promotes
bovine oocyte maturation in vitro. Reproduction. 159:361–370. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Liang T, Zhang X, Xue W, Zhao S, Zhang X
and Pei J: Curcumin induced human gastric cancer BGC-823 cells
apoptosis by ROS-mediated ASK1-MKK4-JNK stress signaling pathway.
Int J Mol Sci. 15:15754–15765. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yu X, Zhong J, Yan L, Li J, Wang H, Wen Y
and Zhao Y: Curcumin exerts antitumor effects in retinoblastoma
cells by regulating the JNK and p38 MAPK pathways. Int J Mol Med.
38:861–868. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhang W, Chen L, Shen Y and Xu J:
Rifampicin-induced injury in L02 cells is alleviated by 4-PBA via
inhibition of the PERK-ATF4-CHOP pathway. Toxicol In Vitro.
36:186–196. 2016. View Article : Google Scholar : PubMed/NCBI
|