Introduction
Lung cancer is a common malignancy, ranking first
among all causes of cancer-related mortality worldwide, with a
5-year survival rate of <20% (1).
There has been significant progress in lung cancer molecular
targeted therapies, such as small-molecule inhibitors targeting
molecular aberrations, such as epidermal growth factor and
anaplastic lymphoma kinase, during recent years (2). However, the prognosis of patients with
advanced lung cancer remains poor (2,3). Viral
therapy, using oncolytic viruses, eliminating neoplasms through
cell lysis and death mechanisms, is urgently required as one of the
novel treatment strategies. The avian paramyxovirus Newcastle
disease virus (NDV) possesses antitumor activity and is safe for
the host (4,5), as demonstrated in animal models and
several phase II clinical trials (6–8).
Numerous investigations have been conducted into the antitumor
mechanisms of NDV, including its ability to stimulate
immunoreactive cells and to induce tumor cell death (9–13). The
latter has been called the oncolytic effect of NDV, and involves
multiple tumor cell death mechanisms (14). However, how NDV exerts its oncolytic
effect on lung cancer remains to be investigated.
MicroRNAs (miRNAs/miRs) are non-coding small RNA
molecules that regulate gene expression after transcription, and
thus affect numerous biological processes, including cell
proliferation, differentiation, development and apoptosis. In
recent years, the involvement of miRNA in the regulation of tumor
cell apoptosis through post-transcriptional regulation of cascade
signaling pathways has been revealed (15). Therefore, combined with NDV-induced
tumor cell death, we hypothesized that miRNAs play a regulatory
role as tumor suppressors in the NDV-induced oncolysis of tumor
cells. miR-204 as a tumor suppressor plays an important role in
various types of cancer cells, including lung cancer cells
(16). Additionally, miR-204
expression is downregulated in non-small cell lung cancer (17). In the present study, the role of
miR-204 in the promotion of NDV-induced oncolysis of lung cancer
A549 cells is described. The study assessed whether overexpression
or inhibition of miR-204 was significantly associated with
NDV-induced oncolysis in A549 cells, and also investigated whether
major regulators of the apoptosis pathway were regulated by
miR-204. The study evaluated whether miR-204 plays a role as a
tumor suppressor in NDV-induced oncolysis in lung cancer cells.
Materials and methods
Cell lines and virus
The human lung cancer A549 cell line were maintained
in Ham's F-12 medium (Kaign's modification) supplemented with 10%
FBS, 100 U/ml penicillin and 100 µg/ml streptomycin (all Gibco;
Thermo Fisher Scientific, Inc.). The cells were cultured at 37°C in
a 5% CO2 incubator.
NDV strain 7793 was obtained from the laboratory in
the Department of Microbiology of Guangxi Medical University
(Nanning, China). A stock of infectious virus was propagated in
embryonated chicken eggs. The allantoic fluid was collected from
the eggs and centrifuged (300-400 × g for 30 min at 4°C), and then
subjected to ultracentrifugation (50,000 × g for 60 min at 4°C).
The pellet was resuspended in phosphate-buffered saline (PBS) and
purified twice using a 35% sucrose gradient and ultracentrifugation
(97,000 × g for 60 min at 4°C). Purified virus was resuspended in
PBS containing 0.1% EDTA. NDV titers were determined using
hemagglutination tests, with a single hemagglutination unit (HU)
defined as the lowest virus concentration leading to visible
agglutination of chicken erythrocytes. The ultraviolet (UV)-NDV
7793 was produced by inactivating NDV 7793 with UV light for 30 min
(254 nm; 2 mW/cm2; 30 cm).
A549 cell culture with NDV or UV-NDV
in vitro
A549 cells (1×105) were seeded in 6-well
plates until reaching 80–90% confluency in triplicate. Prior to
culture with virus, the cells were washed twice with PBS. The A549
cells were cultured with NDV 7793 (25 HU/105 cells) or
UV-NDV 7793 (25 HU/105 cells) at 37°C with 5% CO2 and
were collected at 24, 48 and 72 h. As the positive control, A549
cells were cultured with 20 mg/l cisplatin (Sigma-Aldrich; Merck
KGaA). As the negative control (NC), A549 cells were mock-cultured
in parallel and processed in the same way. Cells were collected by
centrifugation (300-400 × g for 10 min at 4°C), washed twice in PBS
and used in western blotting, reverse transcription- quantitative
PCR, apoptosis and cytotoxicity assays.
All experiments using living virus and cell culture
were conducted in biosafety level-2 containment of the Department
of Microbiology of Guangxi Medical University and were approved by
Guangxi Nanning Health Committee (protocol no. 00003).
Cytotoxicity assays
Cell Counting Kit-8 (CCK-8) (Dojindo Molecular
Technologies, Inc.) was used according to the manufacturer's
instructions to measure the viability and proliferation of cells.
A549 cells (1×105) were treated with various titers of
NDV (64, 128, 256, 512, 1024 and 2048 HU) and incubated for 24, 48
and 72 h at 37°C. Subsequently, 10 µl CCK-8 solution was added to
each well and incubated for 2 h at 37°C. The absorbance was
measured at 450 nm using a microplate reader. Cell viability was
calculated using the following formula: Cell viability = (OD of
control - OD of treatment)/(OD of control - OD of blank) × 100. The
assay was repeated 3 times.
NDV-triggered oncolysis in tumor cells was analyzed
using an Annexin V-fluorescein isothiocyanate (FITC) Apoptosis
Detection kit containing PI (Becton-Dickinson and Company) in
accordance with the manufacturer's instructions. Briefly,
1×106 cells were washed with cold PBS twice, and
re-suspended in 300 µl binding buffer. After incubation with 3 µl
Annexin V-FITC at room temperature for 10 min in the dark, the
cells were mixed with 200 µl binding buffer prior to flow cytometry
using a FACSVerse flow cytometer (BD Pharmingen; BD Biosciences).
The data were analyzed using ModFit LT (version 2.0; Verity
Software House, Inc.) to determine cell distribution. Results are
presented as means from triplicate cultures.
One Step TUNEL Apoptosis assay kit (Beyotime
Institute of Biotechnology) was used according to the
manufacturer's instructions to measure the apoptosis of cells.
Briefly, 1×105 A549 cells were immobilized with 4%
paraformaldehyde for 30 min at room temperature and permeabilized
using 0.2% Triton X-100. The TdT reaction mixture (100 µl) was
prepared according to the manufacturer's instructions and applied
to cells for 1 h at 37°C in the dark. After sealing with
anti-fluorescence quenching sealing solution, the films were
observed under a fluorescence microscope (EVOS FL; Thermo Fisher
Scientific, Inc.). The cells in five random fields (magnification,
×100) were counted.
Cytopathic assays
In brief, for cell cytopathic assays,
1×105 A549 cells were seeded in 6-well plates until
reaching 80–90% confluency in triplicate. Prior to culture with
virus, the cells were washed twice with PBS. The A549 cells were
cultured with NDV 7793 (25 HU/105 cells) at 37°C with 5%
CO2 for 24, 48 and 72 h. For the negative control (NC), A549 cells
were treated with PBS in parallel and processed in the same way.
Finally, cells in each group were captured using a light microscope
with a green filter (magnification, ×100) and six areas were
randomly selected to be captured.
Transfection and RT-quantitative PCR
analysis
A549 cells were seeded in 6-well plates at
1×105 cells/ml/well 1 day before the transfection. On
the following day, transfection was performed when the cells had
reached ~70% confluence. miR-204 mimics and inhibitors were
purchased from GenePharma (Shanghai, China) and the sequences for
these are shown in Table I. The NCs
used for the mimic and inhibitor were non-targeting sequences. The
final concentration of miRNA used was 50 nM. Transfections were
conducted with Lipofectamine™ 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
transfection medium was replaced 4–6 h after transfection. Cells
were cultured at 37°C and collected 48 h post-transfection for
subsequent experimentations.
 | Table I.Sequences of miR-204 mimics and
inhibitors. |
Table I.
Sequences of miR-204 mimics and
inhibitors.
| miRNA | Sense | Antisense |
|---|
| miR-204 mimics |
5′-UUCCCUUUGUCAUCCUAUGCCU-3′ |
5′-AAGGGAAACAGUAGGAUUCGGA-3′ |
| Mimic-NC |
5′-UUUGUACUACACAAAAGUACUG-3′ |
5′-AAACAUGAUGUGUUUUCAUGAC-3′ |
| miR-204
inhibitor |
5′-AGGCUUAGGAUGACAAAGGGAA-3′ |
|
| Inhibitor-NC |
5′-CAGUACUUUUGUGUAGUACAAA-3′ |
|
Total RNA was extracted using a MiniBEST Universal
RNA Isolation kit (Takara Biotechnology Co., Ltd.) and quantified
by the NanoDrop ND-2000 (Thermo Scientific, USA). cDNA was obtained
using PrimeScript™ RT reagent kit (Takara Biotechnology Co., Ltd.)
according to the manufacturer's instructions. The cDNA samples were
used for quantitative PCR analysis in triplicate to determine the
expression levels of miR-204, caspase-3 and Bax using a QuantiFast
SYBR-Green PCR kit (Qiagen, Inc.) and an ABI 7500 Real-Time PCR
instrument. Primers were purchased from Sangon Biotech, Co., Ltd.,
and sequences are shown in Table
II. The thermocycling conditions were as follows: 95°C for 10
min, followed by 40 cycles at 95°C for 10 sec, 60°C for 30 sec and
72°C for 30 sec. miRNA expression levels were normalized to U6
expression levels and the mRNA levels of caspase-3 and Bax were
normalized to β-actin levels using the 2−∆∆Cq method
(18).
 | Table II.Primers used to detect miRNA,
caspase-3 and BAX expression levels with reverse
transcription-quantitative PCR. |
Table II.
Primers used to detect miRNA,
caspase-3 and BAX expression levels with reverse
transcription-quantitative PCR.
| Gene | Forward primer | Reverse primer |
|---|
| miR-204-5p |
5′-TCCCTTTGTCATCCTATGCCTAA-3′ |
5′-AAGGGAAACAGUAGGAUUCGGA-3′ |
| U6 |
5′-GGAACGATACAGAGAAGATTAGC-3′ |
5′-TGGAACGCTTCACGAATTTGCG-3′ |
| Caspase-3 |
5′-AAGGCAGAGCCATGGACCAC-3′ |
5′-CTGGCAGCATCATCCACACATAC-3′ |
| BAX |
5′-AGATGTGGTCTATAATGCGTTTTCC-3′ |
5′-CAGAAGGCACTAATCAAGTCAAGGT-3′ |
| β-actin |
5′-TGGCACCCAGCACAATGAA −3′ |
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ |
Western blotting analysis
The cells were treated with 10 µl/mg RIPA lysis
buffer (cat. no. P0013B; Beyotime Institute of Biotechnology) and
centrifuged at 4°C at 25,000 × g for 10 min. The protein
concentration was determined using a BCA Protein Assay kit (cat.
no. P0009; Beyotime Institute of Biotechnology). The protein
samples (50 µg/lane) were separated by 10% SDS-PAGE (Bio-Rad
Laboratories, Inc.) and transferred to a PVDF membrane (EMD
Millipore). The membrane was blocked with a 5% non-fat milk
solution at room temperature for 1 h and subsequently incubated
with the following primary antibodies at 4°C overnight: Mouse
antibodies against full-length caspase-3 (cat. no. 9668; 1:1,000;
Cell Signaling Technology, Inc.), mouse anti-Bax (cat. no. 89477S;
1:1,000; Cell Signaling Technology, Inc.) and mouse anti-β-actin
(cat. no. 3700T; 1:2,000; Cell Signaling Technology, Inc.; used as
an internal control). The next day, the blots were incubated at
room temperature with HRP-conjugated anti-mouse IgG secondary
antibody (cat. no. 7076; 1:2,500; Cell Signaling Technology, Inc.).
Immunoblots were developed using an enhanced chemiluminescence
detection system (Wuhan Boster Biological Technology, Ltd.) and
quantified using Quantity One V4.62 software (Bio-Rad Laboratories,
Inc.). β-actin was used as the loading control.
Statistical analysis
The data are presented as the mean ± standard
deviation of three independent experiments, unless otherwise shown.
Data were analyzed using SPSS 22.0 (IBM, Corp.). One-way analysis
of variance followed by Tukey's post hoc test was used to identify
significant differences among multiple groups. An unpaired
Student's t-test was used to compare two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
NDV-induced oncolysis of human lung
cancer A549 cells
To evaluate the oncolytic effects of exposure of
A549 cells to NDV-7793, the present study investigated whether
stimulation with NDV decreased A549 cell viability in a
dose-dependent manner. The apoptotic effect of A549 cells after
treatment with NDV was observed. There was a marked and rapid
decrease in the viability of A549 cells 48 h after treatment with
NDV in comparison with 24 or 72 h of treatment, and the viability
of the A549 cells after treatment with NDV at 1:2048 for 48 h was
the lowest (Fig. 1A). The effects of
NDV-induced cytopathy in A549 cells were also observed (Fig. 1B), including the appearance of A549
cell lesions after stimulation with NDV for 24 h, a clear
cytopathic effect at 48 h and a large number of dead cells at 72 h.
Observation under TUNEL fluorescence microscopy showed apoptotic
A549 cells after exposure to NDV-7793 (Fig. 1C). After treatment with NDV, the
oncolysis rate was increased compared with that in the PBS group
(P<0.05) (Fig. 1D). Therefore,
NDV induced the oncolysis of A549 cells. When NDV was inactivated
by UV treatment, oncolysis was significantly but not completely
decreased (P<0.05), which may be due to the various mechanisms
of NDV-induced oncolysis. Hemagglutinin neuraminidase, as a viral
envelope protein of NDV, has the ability alone to induce oncolysis
(19).
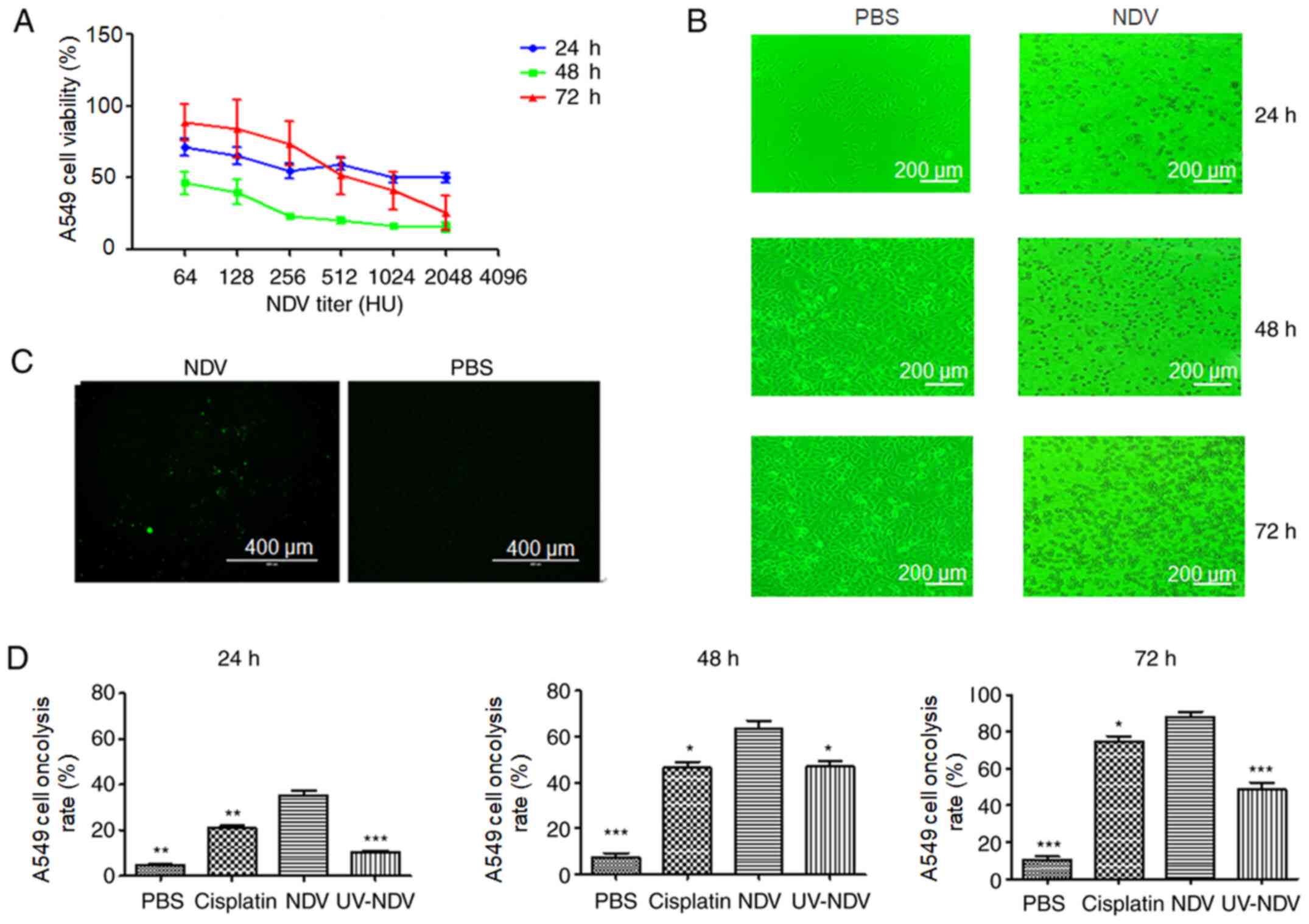 | Figure 1.Oncolysis induction in NDV-stimulated
human lung cancer A549 cells in vitro. (A) A549 cell
viability after NDV stimulation at 24, 48 and 72 h, respectively,
was detected by Cell Counting Kit-8 test. (B) NDV-induced
cytopathic effect was observed through light microscopy with a
green filter (magnification, ×100; scale bar, 200 µm). (C)
Observation under TUNEL fluorescence microscopy showed that
apoptotic cells appeared when A549 cells were exposed to NDV-7793
(magnification, ×100; scale bar, 400 µm). (D) Oncolysis induction
in NDV-stimulated human lung cancer A549 cells in vitro was
analyzed using Annexin V-fluorescein isothiocyanate. *P<0.05,
**P<0.01 and ***P<0.001 vs. NDV. Results are representative
of three independent experiments. NDV, Newcastle disease virus; UV,
ultraviolet; PBS, phosphate-buffered saline. |
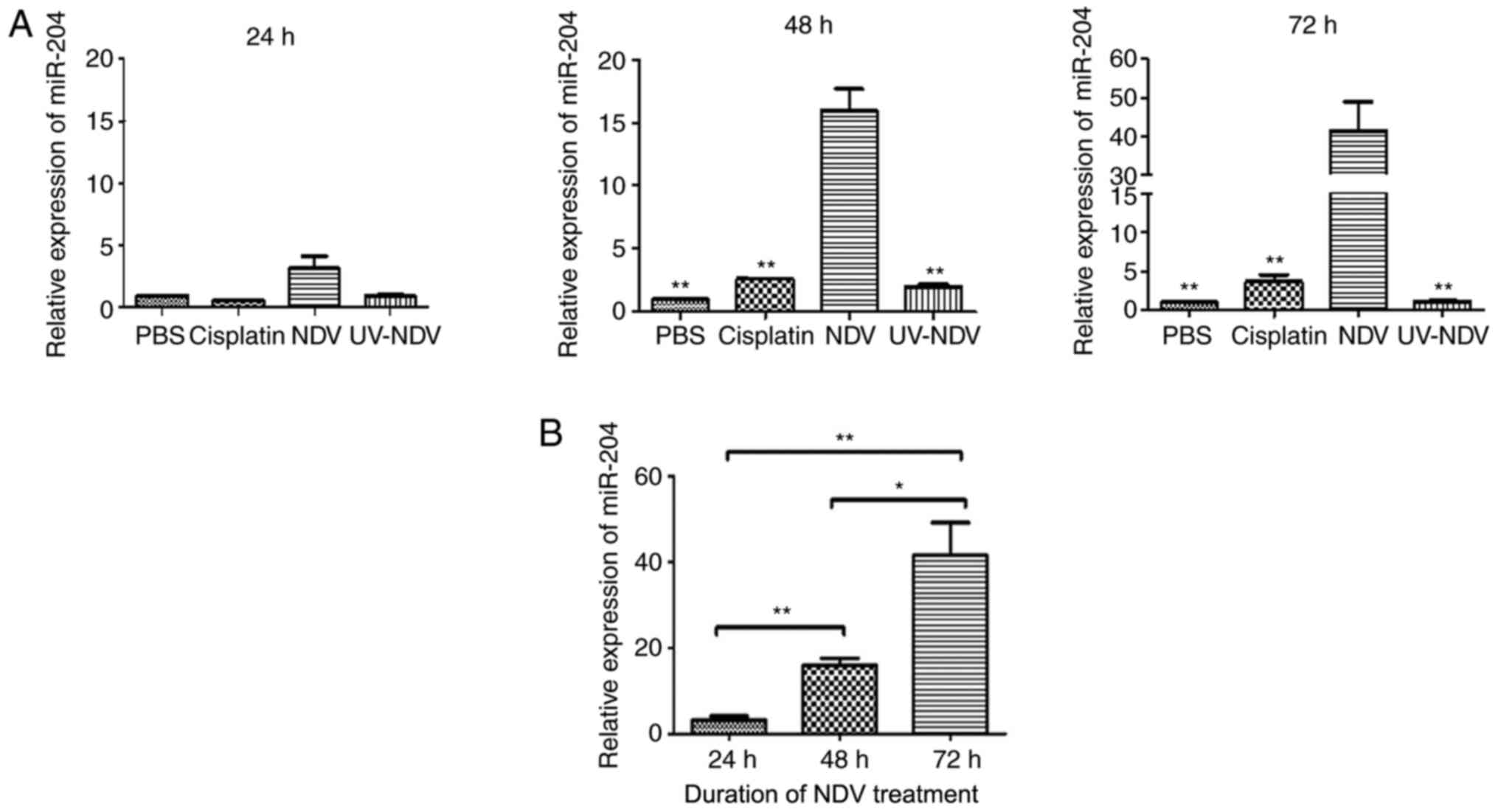
Expression of miR-204 in NDV-treated
A549 cells
It has been shown that miR-204 regulates apoptosis
and that miR-204 is significantly downregulated in lung cancer cell
lines, including A549 (20). To
determine whether treatment with NDV affected the expression of
miR-204, q-PCR assays were conducted to assess miR-204 expression
levels in the present study. miR-204 was significantly upregulated
in NDV-treated A549 cells (P<0.05) compared with that in control
cells at 48 and 72 h (Fig. 2A).
There was an increase in miR-204 levels 24 h and 48 h after
treatment with NDV, with the peak after 72 h (Fig. 2B).
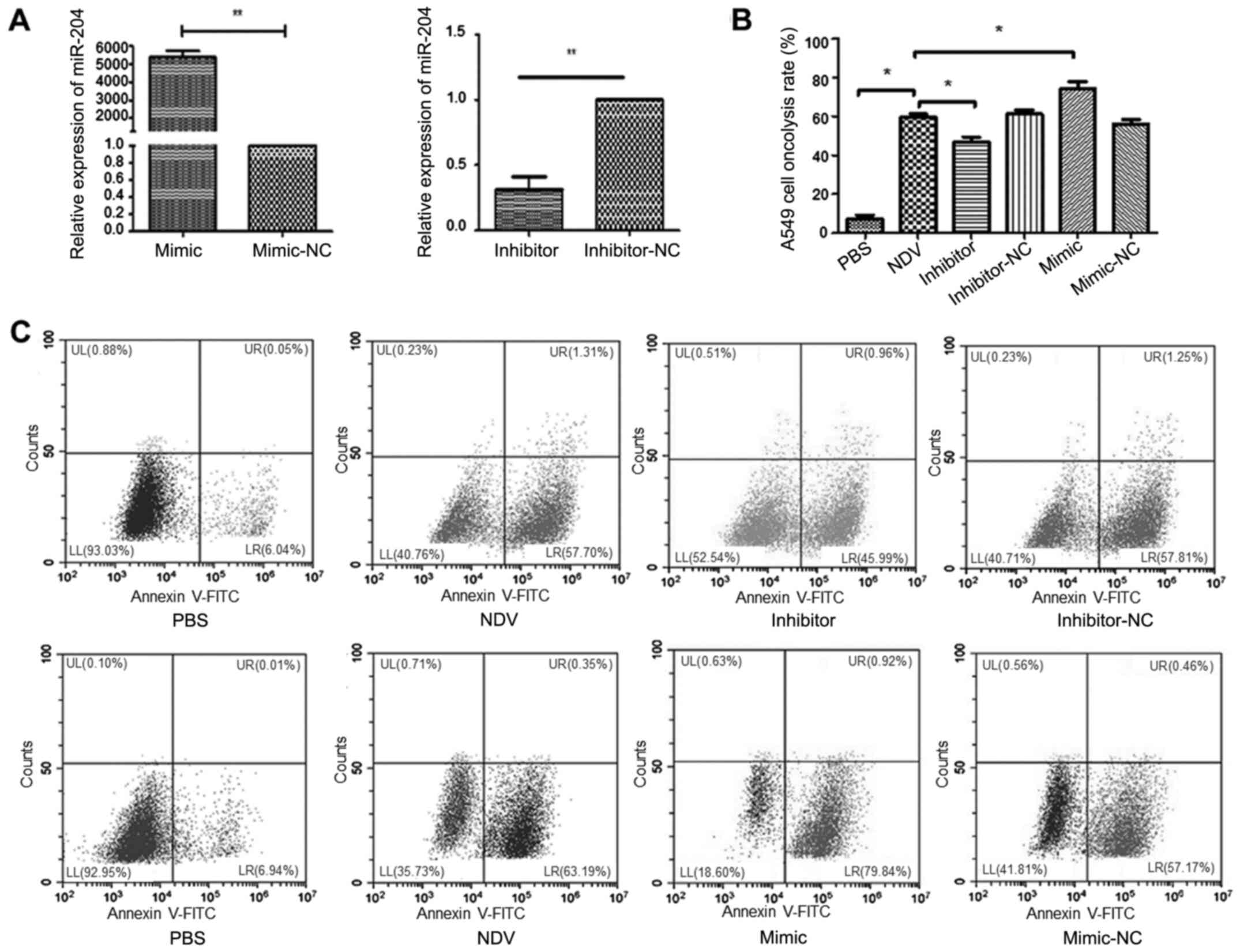
Next, the study investigated whether expression of
miR-204 was significantly associated with oncolytic capacity in
A549 cells treated with NDV, using miR-204 inhibitors or mimics.
The miR-204 expression level was significantly increased and
decreased after transfection of the miR-204 inhibitor and mimic,
respectively, in the A549 cells (both P<0.01; Fig. 3A). Compared with that in the PBS
group, the proportion of oncolysis was significantly increased in
NDV-induced A549 cells (P<0.05; Fig.
3B and C). Compared with that in the NDV group, the proportion
of oncolysis was significantly decreased or increased after
transfection with miR-204 inhibitors or mimics, respectively
(P<0.05; Fig. 3B and C).
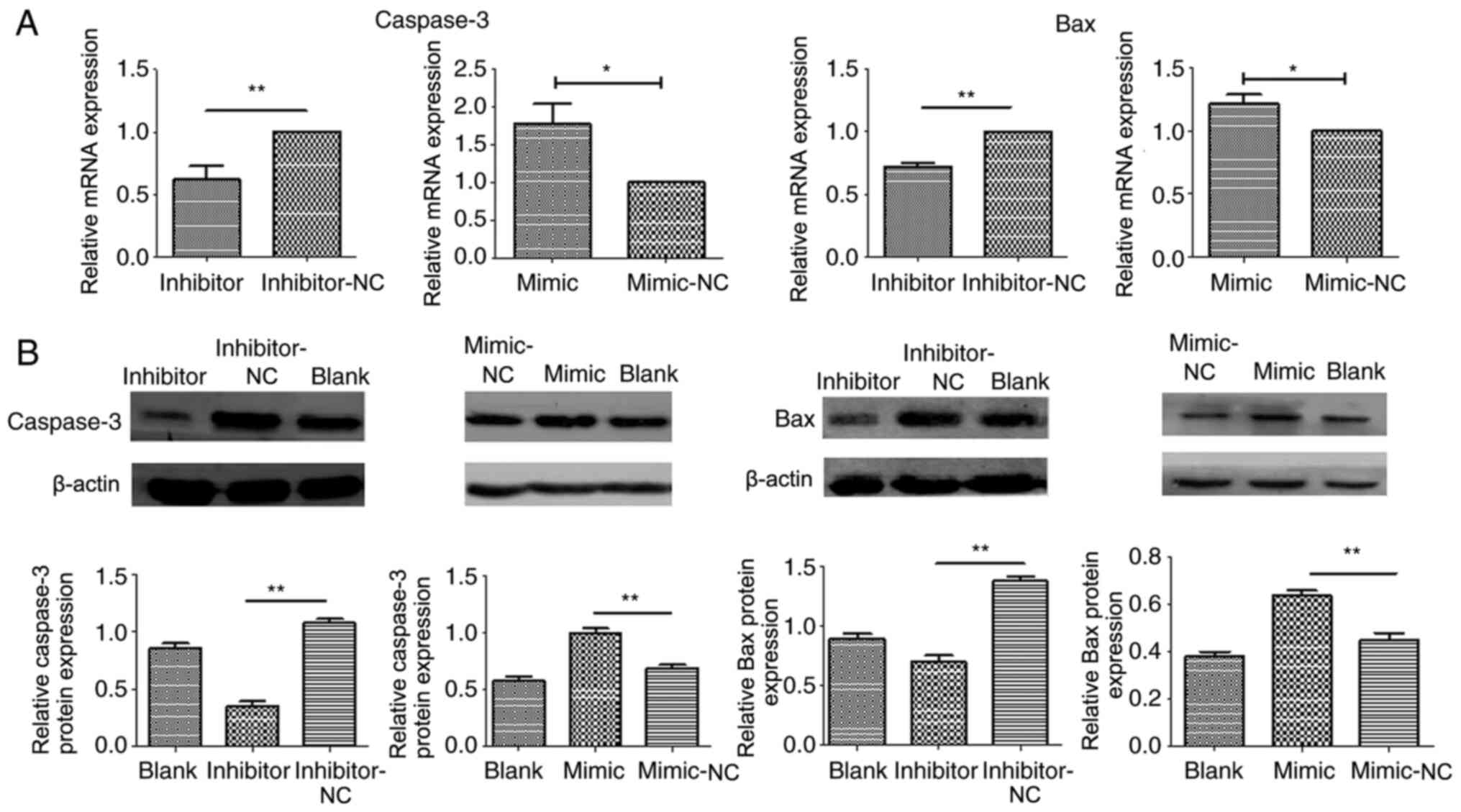
Association between apoptosis-related
proteins and miR-204 in NDV-inducted oncolysis
To investigate whether the oncolysis pathway
regulated by miR-204 was dependent on caspase-3 activity, A549
cells were incubated with miR-204 inhibitors or mimics following
stimulation with NDV. A decrease in caspase-3 and Bax mRNA levels
was found after treatment with the miR-204 inhibitor compared with
the NC (both P<0.01), while an increase in caspase-3 and Bax was
found after treatment with the mir-204 mimic (both P<0.05)
(Fig. 4A). Similar to the mRNA
results, a clear decrease in the levels of Bax and caspase-3
protein levels was also observed for NDV-stimulated A549 cells
pretreated with miR-204 inhibitors compared with the NC, while an
increase in levels was found following treatment with the mimic
(Fig. 4B).
Discussion
The avian paramyxovirus NDV is a single-stranded
negative-sense RNA virus that causes Newcastle disease in poultry,
but almost certainly does not infect mammals. NDV is almost safe
for a human host and possesses tumor-selective oncolytic activity.
Impairment of antiviral type I IFN signaling in tumor cells is
believed to be the reason for effective tumor-selective death. The
virus-selective oncolytic effect is apoptosis dependent, and
related to higher levels of viral transcription, translation and
progeny virus formation (21). In
the present study, the effect of NDV-7793 was observed in human
lung adenocarcinoma A549 cells in vitro and its therapeutic
potential in lung cancer was explored. Cisplatin, the standard
first-line choice of chemotherapy drugs in the clinical treatment
of various types of solid cancer, such as lung and cervical cancer,
and melanoma, was used as positive control in this study (22). Apoptotic A549 cells began to appear
at 24 h after virus treatment, indicating that NDV-7793 infected
the A549 cells and induced apoptosis. However, a marked and rapid
decrease in the viability of A549 cells was found after 48 h of
treatment with NDV in comparison with that at 24 or 72 h of
treatment. We hypothesize that these unexpected results may be due
to the complicated interactions between NDV and cancer cells.
Depending on the severity of the clinical manifestations observed
in chickens and the tropism of the virus, NDV has been classified
into the following five pathotypes: Asymptomatic enteric,
lentogenic, mesogenic, viscerotropic velogenic and neurotropic
velogenic (23). NDV7793 is a
lentogenic strain that has low virulence or is non-virulent. The
release of NDV after budding from the plasma membrane is not
efficient. Therefore, the generation and release of offspring
viruses is limited. From the aspect of the tumor cells in the
present study, some A549 cells died after NDV treatment, and the
remaining living cells continued to grow and reproduce. Therefore,
this may be one of the reasons that in in vivo studies, it
is necessary to infuse NDV several times to achieve a significant
and sustained antitumor effect (24). In future studies, the recombinant NDV
strains can be considered to make up for this deficiency. UV
radiation could not completely destroy the cytotoxicity of NDV,
which may be due to various mechanisms of NDV-induced cytotoxicity.
Hemagglutinin neuraminidase, as a viral envelope protein of NDV,
has the ability alone to induce cytotoxicity (18).
A number of NDV strains have been studied for
oncolytic activity in various cancer cells (25–28). For
example, the effects of NDV strain D90, which was isolated in
China, against oral squamous cell carcinoma cells have been
identified, and the cell death induced by D90 was apoptotic
(28). The results of the present
study are in agreement with those of several previous studies
(25–28). However, the mechanisms that lead to
this activity are unclear. In the current study, the role of
miR-204 in NDV-induced oncolytic activity was described. miR-204 is
closely associated with the regulation of apoptosis, proliferation
and metastasis in tumor cells. For example, miR-204 inhibits the
proliferation and migration of non-small cell carcinoma cells by
targeting Atf2 (29). The present
study examined the expression of miR-204 and explored its role in
oncolytic NDV-induced apoptosis. The results suggested the
existence of miR-204-dependent, NDV-induced tumor oncolytic
activity. Previous studies have shown that the expression of
miR-204 is significantly downregulated in a number of lung cancer
cell lines, including A549. Furthermore, miR-204 overexpression
induces apoptosis in lung cancer cells (16). In the present study, it was found
that the expression of miR-204 was significantly upregulated when
A549 cells were cultured with NDV in vitro. To examine the
role of miR-204 in NDV-triggered oncolytic activity, miR-204 was
overexpressed or inhibited in lung cancer A549 cells. The
overexpression of miR-204 increased NDV-triggered oncolysis while
inhibition via an miR-204 inhibitor decreased oncolysis. Therefore,
miR-204 levels showed significant associations with the NDV
oncolytic effect. A similar phenomenon has been demonstrated in
other oncolytic virus infections. Coxsackievirus-B3-induced
apoptosis of cardiomyocytes is regulated by miR-34a (30). miR-31 and miR-128 modulate oncolytic
measles virus infection and oncolysis through PVRL4 (31). Another study has shown that miR-99b
and miR-485 function as enhancers of adenoviral oncolysis in
pancreatic cancer (32). These
results indicate that virus infection can change the miRNA
expression profile in tumor cells and some miRNAs may participate
in effective virus-triggered oncolysis. Previous studies have shown
that potential mRNAs that were targeted by miR-204 were selected
and identified to reveal various tumor-selective cell death
pathways significantly associated with miRNA expression (33–35). At
present, our group is performing transcriptome analysis of A549
cells infected by NDV. Data from further prediction algorithms and
luciferase reporter constructs should be obtained to select
potential target mRNAs. Additionally, the present results were
obtained from in vitro experimental systems, so the miRNA
regulation activity of NDV upon oncolytic activity should be
demonstrated further in animal models.
Although the level of miR-204 was significantly
associated with the NDV oncolytic effect, it was also found that
miR-204 inhibitors or mimics had a limited effect on oncolysis.
Cells have very strong compensatory mechanisms (36). Each gene may be targeted by hundreds
of miRNAs, and each miRNA may regulate hundreds of genes (37). Therefore, this limited effect might
be due to the existence of a large number of cellular regulatory
factors, which can offset or mask the effect of inhibiting
apoptosis.
Apoptosis consists of death receptor
ligand/extrinsic and mitochondrial/intrinsic pathways. It has been
confirmed that NDV induces tumor cell apoptosis through both of
these pathways (38). The intrinsic
and extrinsic pathways converge downstream at regulatory factor
caspase-3, which is required to participate in tumor cell apoptosis
induced by NDV (39). In the present
study, miR-204 overexpression resulted in upregulation of
full-length caspase-3, while inhibition via an miR-204 inhibitor
decreased the level of full-length caspase-3. Therefore, miR-204
may regulate NDV oncolysis in A549 cells through the expression of
full-length caspase-3. Activated initiator caspases subsequently
free full-length caspase-3 of their short inhibitory prodomain,
allowing them to cleave a large set of cellular substrates
(40). Therefore, not only
full-length caspase-3, but also the levels of cleaved caspase-3
should be considered in future studies in order to demonstrate the
role of caspase-3 in the process of apoptosis. Bax, a member of the
Bcl-2 family, is another signaling protein involved in apoptosis
regulation by binding to the permeability transition pore complex,
which is responsible for the regulation of mitochondrial membrane
permeability. The present study also demonstrated that
pharmacological inhibitors and mimics of miR-204 decreased and
promoted the expression of Bax, respectively, in A549 cells,
indicating that miR-204 regulates NDV oncolysis in A549 cells.
In summary, the present study provides preliminary
evidence that NDV induces apoptosis in tumor cells via an
miR-204-dependent pathway. miR-204 plays a role as a tumor
suppressor in NDV-induced apoptosis in tumor cells. These findings
indicate that the regulation of apoptosis signaling by miR-204
influences tumor survival in NDV-treated lung cancer. These results
highlight the miRNA-regulatory activity of NDV upon oncolytic
activity.
Acknowledgements
Not applicable.
Funding
This study was supported by a grant from the Natural
Science Foundation of Guangxi, China (no. 2018GXNSFDA281043), the
National Natural Science Foundation of China (no. 81960511) and the
Guangxi First-class Discipline Project for Basic and Medicine
Sciences (no. GXFCDP-BMS-2018).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL contributed significantly to the statistical
analysis and writing the manuscript. JJH performed the PCR and
transfection experiments. WYT performed the western blotting and
the apoptosis analysis. LXG performed the virus purification and
cell cultures. XHF contributed to the study design and provided
crucial experiment materials. YL, WYT, JJH, LXG and XHF confirm the
authenticity of the raw data. All authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hirsch FR, Scagliotti GV, Mulshine JL,
Kwon R, Curran WJ Jr, Wu YL and Paz-Ares L: Lung cancer: Current
therapies and new targeted treatments. Lancet. 389:299–311. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adam V, Dooms C and Vansteenkiste J: Lung
cancer at the intensive care unit: The era of targeted therapy.
Lung Cancer. 89:218–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fournier P and Schirrmacher V: Oncolytic
Newcastle disease virus as cutting edge between tumor and host.
Biology (Basel). 2:936–975. 2013.PubMed/NCBI
|
|
5
|
Lam HY, Yeap SK, Pirozyan MR, Omar AR,
Yusoff K, Abd-Aziz S and Alitheen NB: Corrigendum to ‘Safety and
clinical usage of Newcastle disease virus in cancer therapy’.
BioMed Res Int. 2017:45294372017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Batliwalla FM, Bateman BA, Serrano D,
Murray D, Macphail S, Maino VC, Ansel JC, Gregersen PK and
Armstrong CA: A 15-year follow-up of AJCC stage III malignant
melanoma patients treated postsurgically with Newcastle disease
virus (NDV) oncolysate and determination of alterations in the CD8
T cell repertoire. Mol Med. 4:783–794. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cassel WA and Murray DR: A ten-year
follow-up on stage II malignant melanoma patients treated
postsurgically with Newcastle disease virus oncolysate. Med Oncol
Tumor Pharmacother. 9:169–171. 1992.PubMed/NCBI
|
|
8
|
Schirrmacher V, van Gool S and Stuecker W:
Breaking therapy resistance: An update on oncolytic Newcastle
disease virus for improvements of cancer therapy. Biomedicines.
7:662019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cuadrado-Castano S, Sanchez-Aparicio MT,
García-Sastre A and Villar E: The therapeutic effect of death:
Newcastle disease virus and its antitumor potential. Virus Res.
209:56–66. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Keshavarz M, Nejad ASM, Esghaei M,
Bokharaei-Salim F, Dianat-Moghadam H, Keyvani H and Ghaemi A:
Oncolytic Newcastle disease virus reduces growth of cervical cancer
cell by inducing apoptosis. Saudi J Biol Sci. 27:47–52. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu Q, Rangaswamy US, Wang W, Robbins SH,
Harper J, Jin H and Cheng X: Evaluation of Newcastle disease virus
mediated dendritic cell activation and cross-priming tumor-specific
immune responses ex vivo. Int J Cancer. 146:531–541. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jarahian M, Watzl C, Fournier P, Arnold A,
Djandji D, Zahedi S, Cerwenka A, Paschen A, Schirrmacher V and
Momburg F: Activation of natural killer cells by Newcastle disease
virus hemagglutinin-neuraminidase. J Virol. 83:8108–8121. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Washburn B, Weigand MA, Grosse-Wilde A,
Janke M, Stahl H, Rieser E, Sprick MR, Schirrmacher V and Walczak
H: TNF-related apoptosis-inducing ligand mediates tumoricidal
activity of human monocytes stimulated by Newcastle disease virus.
J Immunol. 170:1814–1821. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koks CA, Garg AD, Ehrhardt M, Riva M,
Vandenberk L, Boon L, De Vleeschouwer S, Agostinis P, Graf N and
Van Gool SW: Newcastle disease virotherapy induces long-term
survival and tumor-specific immune memory in orthotopic glioma
through the induction of immunogenic cell death. Int J Cancer.
136:E313–E325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ors-Kumoglu G, Gulce-Iz S and Biray-Avci
C: Therapeutic microRNAs in human cancer. Cytotechnology.
71:411–425. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li P, Wang Q and Wang H: MicroRNA-204
inhibits the proliferation, migration and invasion of human lung
cancer cells by targeting PCNA-1 and inhibits tumor growth in vivo.
Int J Mol Med. 43:1149–1156. 2019.PubMed/NCBI
|
|
17
|
Guo W, Zhang Y, Zhang Y, Shi Y, Xi J, Fan
H and Xu S: Decreased expression of miR-204 in plasma is associated
with a poor prognosis in patients with non-small cell lung cancer.
Int J Mol Med. 36:1720–1726. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghrici M, El Zowalaty M, Omar AR and
Ideris A: Induction of apoptosis in MCF-7 cells by the
hemagglutinin-neuraminidase glycoprotein of Newcastle disease virus
Malaysian strain AF2240. Oncol Rep. 30:1035–1044. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li N, Guo X, Liu L, Wang L and Cheng R:
Molecular mechanism of miR-204 regulates proliferation, apoptosis
and autophagy of cervical cancer cells by targeting ATF2. Artif
Cells Nanomed Biotechnol. 47:2529–2535. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Raihan J, Ahmad U, Yong YK, Eshak Z,
Othman F and Ideris A: Regression of solid breast tumours in mice
by Newcastle disease virus is associated with production of
apoptosis related-cytokines. BMC Cancer. 19:3152019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ghosh S: Cisplatin: The first metal based
anticancer drug. Bioorg Chem. 88:1029252019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dimitrov KM, Afonso CL, Yu Q and Miller
PJ: Newcastle disease vaccines - A solved problem or a continuous
challenge? Vet Microbiol. 206:126–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yurchenko KS, Zhou P, Kovner AV, Zavjalov
EL, Shestopalova LV and Shestopalov AM: Oncolytic effect of
wild-type Newcastle disease virus isolates in cancer cell lines in
vitro and in vivo on xenograft model. PLoS One. 13:e01954252018.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lazar I, Yaacov B, Shiloach T, Eliahoo E,
Kadouri L, Lotem M, Perlman R, Zakay-Rones Z, Panet A and
Ben-Yehuda D: The oncolytic activity of Newcastle disease virus
NDV-HUJ on chemoresistant primary melanoma cells is dependent on
the proapoptotic activity of the inhibitor of apoptosis protein
Livin. J Virol. 84:639–646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Al-Shammari AM, Salman MI, Saihood YD,
Yaseen NY, Raed K, Shaker HK, Ahmed A, Khalid A and Duiach A: In
vitro synergistic enhancement of Newcastle disease virus to
5-fluorouracil cytotoxicity against tumor cells. Biomedicines.
4:32016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kalyanasundram J, Hamid A, Yusoff K and
Chia SL: Newcastle disease virus strain AF2240 as an oncolytic
virus: A review. Acta Trop. 183:126–133. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang CX, Ye LW, Liu Y, Xu XY, Li DR, Yang
YQ, Sun LL and Yuan J: Antineoplastic activity of Newcastle disease
virus strain D90 in oral squamous cell carcinoma. Tumour Biol.
36:7121–7131. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang S, Gao L, Thakur A, Shi P, Liu F,
Feng J, Wang T, Liang Y, Liu JJ, Chen M, et al: miRNA-204
suppresses human non-small cell lung cancer by targeting ATF2.
Tumour Biol. 37:11177–11186. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang D, Li M, Yu Y, Shi H and Chen R:
microRNA-34a aggravates coxsackievirus B3-induced apoptosis of
cardiomyocytes through the SIRT1-p53 pathway. J Med Virol.
91:1643–1651. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Geekiyanage H and Galanis E: MiR-31 and
miR-128 regulates poliovirus receptor-related 4 mediated measles
virus infectivity in tumors. Mol Oncol. 10:1387–1403. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Rovira-Rigau M, Raimondi G, Marín MA,
Gironella M, Alemany R and Fillat C: Bioselection reveals miR-99b
and miR-485 as enhancers of adenoviral oncolysis in pancreatic
cancer. Mol Ther. 27:230–243. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li T, Pan H and Li R: The dual regulatory
role of miR-204 in cancer. Tumour Biol. 37:11667–11677. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Santoni G, Morelli MB, Santoni M, Nabissi
M, Marinelli O and Amantini C: Targeting transient receptor
potential channels by MicroRNAs drives tumor development and
progression. Adv Exp Med Biol. 1131:605–623. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tajbakhsh A, Mokhtari-Zaer A, Rezaee M,
Afzaljavan F, Rivandi M, Hassanian SM, Ferns GA, Pasdar A and Avan
A: Therapeutic potentials of BDNF/TrkB in breast cancer; current
status and perspectives. J Cell Biochem. 118:2502–2515. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang X, Jia Y, Wang X, Wang C, Lv C, Li X,
Chu Z, Han Q, Xiao S, Zhang S, et al: MiR-375 has contrasting
effects on Newcastle disease virus growth depending on the target
gene. Int J Biol Sci. 15:44–57. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Trobaugh DW and Klimstra WB: MicroRNA
regulation of RNA virus replication and pathogenesis. Trends Mol
Med. 23:80–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bian J, Wang K, Kong X, Liu H, Chen F, Hu
M, Zhang X, Jiao X, Ge B, Wu Y, et al: Caspase- and
p38-MAPK-dependent induction of apoptosis in A549 lung cancer cells
by Newcastle disease virus. Arch Virol. 156:1335–1344. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yan Y, Liang B, Zhang J, Liu Y and Bu X:
Apoptotic induction of lung adenocarcinoma A549 cells infected by
recombinant RVG Newcastle disease virus (rL-RVG) in vitro. Mol Med
Rep. 11:317–326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lamkanfi M and Kanneganti TD: Caspase-7: A
protease involved in apoptosis and inflammation. Int J Biochem Cell
Biol. 42:21–24. 2010. View Article : Google Scholar : PubMed/NCBI
|