Introduction
Prostate cancer (PCa) is one of the most common
types of malignant cancer of the prostatic epithelium (1,2). Age and
heredity are the main risk factors associated with PCa development
(3). The age of onset ranges from 65
to 74 years, with a median age of 66 years (4). It is estimated that >1,600,000 new
cases are diagnosed annually (5).
The incidence of PCa is significantly higher in the Western
population than in the Asian one (6). In the United States, ~1 out of 5 men
will be diagnosed with PCa (4),
while in China, the incidence of PCa is 7.1/100,000 individuals
(7). Although PCa has a 5-year
survival rate of 98.6% (4,8), an in-depth understanding of its
pathogenesis and new therapeutic strategies are still urgently
required.
Circular RNAs (circRNAs), a large class of
non-coding RNAs, have attracted increasing attention over the last
decade (9). Preliminary studies have
revealed that circRNAs serve an important role in
post-transcriptional regulation by acting as microRNA (miRNA/miR)
sponges or by encoding peptides (10–12).
CircRNAs have been reported to have unique expression profiles in
different cancer subtypes and they regulate multiple biological
processes, such as development, differentiation, apoptosis and cell
proliferation (13). Some circRNAs
have already been considered as tumor-associated circRNAs, such as
CDR1as, circUBAP2 and circPTV1 (14). Among these, circUBAP2 has been
reported as a critical regulator involved in the progression of
several types of cancer, such as ovarian cancer, esophageal
squamous cell carcinoma and pancreatic adenocarcinoma (15–17).
Recently, Chen et al (18) revealed the circRNA transcriptional
landscape with functional annotation in localized PCa. In their
circRNA profile of PCa, circUBAP2 was significantly abundantly
expressed in PCa (18). However, the
role of circUBAP2 in the progression of PCa remains unclear. Hence,
the present study aimed to clarify the regulatory mechanism of
circUBAP2 in the progression of PCa.
Materials and methods
Cell culture
Four human PCa cell lines (LNCaP, V16A, DU145 and
PC-3) were purchased from the American Type Culture Collection
(ATCC) and cultured in RPMI 1640 medium (Gibco; Thermo Fisher
Scientific Inc.) with 10% fetal bovine serum (FBS; Thermo Fisher
Scientific Inc.) and 1% penicillin-streptomycin (18,19).
Human prostate epithelial cell line (RWPE-1) was purchased from
ATCC and cultured in Keratinocyte serum-free medium (KSFM; Thermo
Fisher Scientific Inc.) (20). All
cells were maintained in a 37°C humidified atmosphere with 5%
CO2.
Clinical tissues
A total of 20 male patients who were diagnosed as
prostate cancer and underwent prostatectomy at The First Affiliated
Hospital of Xinjiang Medical University (Urumqi, China) from
January 2020 to March 2020 were included in the present study.
Patients with immunological diseases, metabolic diseases,
hypertension diabetes or any other type of tumor diseases were
excluded. The mean age of enrolled patients was 74.38±3.32 years
(age range, 68–81 years). PCa and paired adjacent normal tissues (2
cm distance from tumor tissue) were obtained after prostatectomy
and stored in liquid nitrogen. Written informed consent was
obtained from each participant. All study protocols were approved
by the Ethics Committee of The First Affiliated Hospital of
Xinjiang Medical University (approval no. XJ20200025; Urumqi,
China).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA from PCa cell lines and tissues was
extracted using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific Inc.). Nuclear and cytoplasmic RNA were separated
using a Cytoplasmic and Nuclear RNA Purification kit (Norgen Biotek
Corp.). The miRNAs were reverse transcribed to cDNA using the
All-in-one™ miRNA First-Strand cDNA Synthesis kit (GeneCopoeia
Inc.) and the mRNAs were reverse transcribed to cDNA using the
Oligo(dT) priming method (GeneCopoeia Inc.) according to the
manufacturer's protocol. SYBR Green qPCR Master Mix (Thermo Fisher
Scientific Inc.) was used to detect the expression levels of miRNAs
and mRNAs via qPCR. The qPCR thermocycling conditions were as
follows: Enzyme activation at 95°C for 30 sec, followed by 40
two-step cycles (95°C for 5 sec; 60°C for 30 sec). The related
expression levels of miRNAs and mRNAs were normalized to U6 and
GAPDH, respectively, according to the 2−∆∆Cq method
(21).
The primers were as follows: circUBAP2 forward,
5′-AGCCTCAGAAGCCAACTCCTTTG-3′ and reverse,
5′-TCAGGTTGAGATTTGAAGTCAAGAT-3′; MAP3K2 forward,
5′-CCCCAGGTTACATTCCAGATGA-3′ and reverse,
5′-GCATTCGTGATTTTGGATAGCTC-3′; ERK1 forward,
5′-TACACCAACCTCTCGTACATCG-3′ and reverse,
5′-CATGTCTGAAGCGCAGTAAGATT-3′; JNK forward,
5′-TGTGTGGAATCAAGCACCTTC-3′ and reverse,
5′-AGGCGTCATCATAAAACTCGTTC-3′; p53 forward,
5′-CAGCACATGACGGAGGTTGT-3′ and reverse, 5′-TCATCCAAATACTCCACACGC3′;
GAPDH forward, 5′-ACCACAGTCCATGCCATCAC-3′ and reverse,
5′-TCCACCACCCTGTTGCTGTA-3′; miR-1244 forward,
5′-ACACTCCAGCTGGGAAGTAGTTGGTTTGTATGAG-3′ and reverse,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGAACCATCT-3′; and U6 forward,
5′-AAAGCAAATCATCGGACGACC-3′ and reverse,
5′-GTACAACACATTGTTTCCTCGGA-3′.
miRNA and mRNA expression profiles
analysis
The expression profiles of miRNA (GSE76260) and mRNA
(GSE30994) were downloaded from the Gene Expression Omnibus (GEO)
database (22,23). The differentially expressed miRNAs
and mRNAs were detected using the GEO2R tool (https://www.ncbi.nlm.nih.gov/geo/geo2r),
which allowed users to compare two or more groups of samples in a
GEO Series. After eliminating duplicates, |fold-change (FC)| ≥2 and
adjusted P<0.05 were set as the criteria for differentially
expressed miRNAs.
Competitive endogenous RNA (ceRNA)
network
The circUBAP2-targeted miRNAs were predicted using
circBank (circbank.cn) and circular RNA interactome
(circinteractome.nia.nih.gov) (24).
The miRNAs predicted by both of these websites were considered as
potential target miRNAs of circUBAP2. The expression levels of
these potential target miRNAs in PCa were further confirmed based
on the GSE76260 miRNA expression profile. The potential target
mRNAs were screened out using miRWalk (mirwalk.umm.uni), TargetScan
(targetscan.org), miRDB (mirdb.org) and TargetMiner (isical.ac.in)
(25). Their expression levels in
PCa were also validated based on the GSE30994 mRNA expression
profile. The circUBAP2-associated ceRNA network was constructed
using Cytoscape (https://cytoscape.org/).
Inhibition of miR-1244 and
circUBAP2
The miR-1244 inhibitor (the sequences were not
provided) and scrambled negative control (the sequences were not
provided) was designed and synthesized by Suzhou GenePharma Co.,
Ltd. The sequence of circUBAP2 was obtained using circBase
(http://www.circbase.org/). The small
interfering (si)RNA targeting circUBAP2 (si-circUBAP2; siRNA-1,
5′-GCTTCTAAGCTTTCTGAAACA-3′; siRNA-2, 5′-CAGCTTCTAAGCTTTCTGAAA-3′;
and siRNA-3, 5′-CCCAGCTTCTAAGCTTTCTGA-3′) and scrambled negative
control (si-control; the sequences were not provided) were also
designed and synthesized by Suzhou GenePharma Co., Ltd. (26).
Cell transfection
The miR-1244 inhibitor or si-circUBAP2 (both 100 nM)
were transfected into PCa cells using Lipofectamine®
3000 (Thermo Fisher Scientific Inc.) and then the cells were
maintained in a 37°C humidified atmosphere with 5% CO2
for 6 h, following which culture medium was replaced. After 48 h,
RT-qPCR was performed to detect the mRNA expression levels.
Luciferase reporter gene assay
The luciferase reporter gene plasmid pGL6-miR
(Beyotime Institute of Biotechnology) containing the putative
binding site of miR-1244 was constructed and validated by Sangon
Biotech Co., Ltd. Human embryonic kidnFS (293T) cells were
purchased from the American Type Culture Collection (ATCC) and
cultured in Dulbecco's modified Eagle's medium (DMEM; Thermo Fisher
Scientific, Inc.) with 10% fetal bovine serum (FBS; Gibco; Thermo
Fisher Scientific, Inc.) and 1% penicillin-streptomycin.
Subsequently, the 293T cells were seeded into a 24-well plate at a
density of 2×105 cells/well and then cultured at 37°C
with 5% CO2 and 95% humidity. 293T cells were
transfected with pGL6-miR with or without miR-1244 mimics or NC
(Suzhou GenePharma Co., Ltd.) using Lipofectamine 3000. Luciferase
protein was stained via immunofluorescence according to the
manufacturer's instructions. The luciferase activity was measured
48 h after transfection with a Dual-Luciferase Reporter Assay
System (Promega Corporation) using a fluorescence spectrophotometer
(Infinite M200; Tecan Group, Ltd.), which was then normalized to
Renilla luciferase activity.
Western blotting
Total protein from PCa cells were extracted by using
the Total Protein Extraction kit (cat. no. PE001; Signalway
Antibody LLC) and quantified using the bicinchoninic acid (BCA)
protein quantitative method. The proteins were subjected to 10%
SDS-PAGE with equal amounts (50 µg/lane) and then transferred onto
PVDF membranes. After blocking with 5% skimmed milk for 1 h at room
temperature, membranes were washed by TBST with 0.5% Tween 20 5
times and incubated on a plate shaker with primary antibodies
overnight at 4°C. Subsequently, membranes were washed again and
probed by secondary antibodies for 1 h at room temperature.
Finally, the protein bands were visualized using an ECL
luminescence reagent (Sangon Biotech Co., Ltd.). Primary antibodies
against MAP3K2 (1:10,000; cat. no. ab240926), ERK (1:1,000; cat.
no. ab32081), JNK (1:1,000; cat. no. ab110724), p38 (1:1,000; cat.
no. ab170099), β-actin (1:5,000; cat. no. ab179467) and secondary
horse-radish peroxidase conjugated antibodies (goat anti-rabbit
IgG; 1:5,000, cat. no. ab7090) used in the present study were all
purchased from Abcam. β-actin was used as the loading control.
Cell counting kit-8 (CCK-8) assay
CCK-8 assay was performed to evaluate the effects of
circUBAP2-knockdown on the proliferation of PCa LNCaP, V16A, DU145
and PC-3 cell lines. The PCa cells were seeded into 96-well plates
and cultured at 37°C. Subsequently, the CCK-8 solution (10 µl/well)
(Beyotime Institute of Biotechnology) was added for 2 h at each
time point (48, 96 and 144 h post-transfection). The cell
proliferative activity was reflected by measuring the optical
density at a wavelength of 450 nm using Multiskan Spectrum (Thermo
Fisher Scientific Inc.).
Statistical analysis
The | FC | ≥2 and adjusted P<0.05 indicated that
miRNA and mRNA were significantly differentially expressed. The
data of RT-qPCR and CCK-8 assays were expressed as the mean ± SD of
3 repeats. The paired Student's t-test was used to compare the
differences between two groups. One-way ANOVA (parametric) was used
to compare the differences among multiple groups, with Tukey's test
as the post-hoc test. The correlation analysis was determined using
Pearson's correlation coefficient. Statistical analysis was
performed using GraphPad Prism 7.0 (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Result
circUBAP2 is highly expressed in four
PCa cell lines
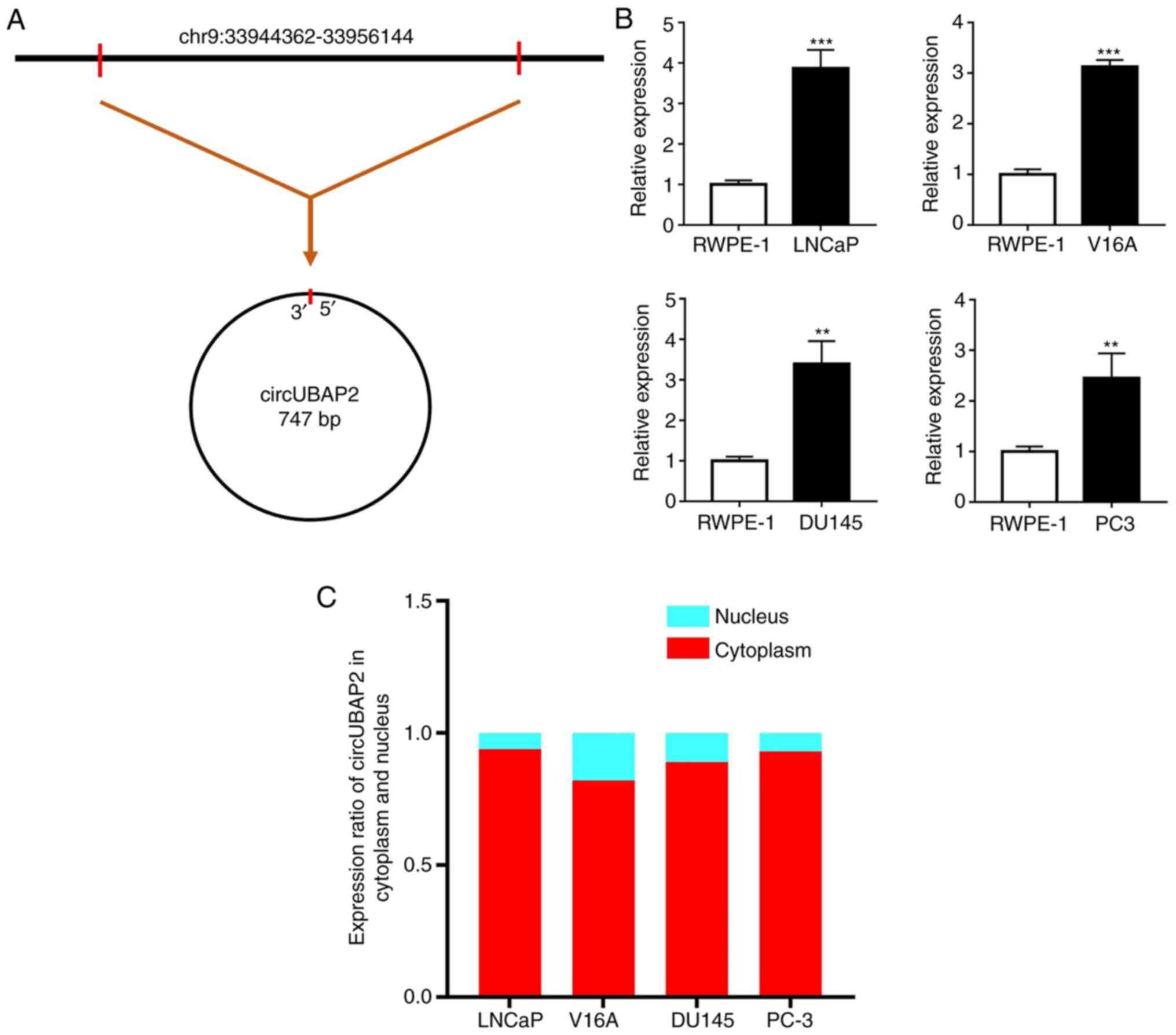
According to circBase, circUBAP2 is generated from
the UBAP2 gene and its genomic position, as shown in Fig. 1A. The expression levels of circUBAP2
were detected in four human PCa cell lines (LNCaP, V16A, DU145 and
PC-3) and in a human normal prostate cell line (RWPE-1). The
results revealed that circUBAP2 expression was significantly
upregulated in all four PCa cell lines compared with in RWPE-1
cells (Fig. 1B). Among these PCa
cell lines, LNCaP exhibited the highest expression levels of
circUBAP2. Additionally, the expression levels of circUBAP2 were
further validated in the nucleus and cytoplasm of PCa cell lines
separately to confirm its location in PCa cells. The results
indicated that circUBAP2 was mainly expressed in the cytoplasm,
which was important for its function prediction (Fig. 1C).
Potential targets of circUBAP2
To further explore the regulatory effect of
circUBAP2 in PCa cells, its target miRNAs were predicted using
circular RNA interactome and circBank. Venn diagram analysis was
further used to identify the common miRNAs in both websites
(Fig. 2A). The results indicated
that miR-1244, miR-665, miR-1200, miR-582-3p, miR-637, miR-892b,
miR-571 and miR-1305 were the potential target miRNAs of circUBAP2
(Fig. 2B). Their expression levels
in PCa cells were then validated based on the miRNA expression
dataset GSE76260, which included 172 dysregulated miRNAs; however,
only miR-1244 was found to be differentially expressed in PCa
(Fig. 2C). Subsequently, the target
mRNAs of miR-1244 were predicted using miRDB, TargetScan, miRWalk
and TargetMiner. A total of 22 mRNAs were co-predicted as potential
target mRNAs of circUBAP2/miR-1244 (Fig.
2D). Among these mRNAs, adducin 3 (ADD3), dynein cytoplasmic 1
intermediate chain 1 (DYNC1I1), MAP3K2 and Rho GTPase activating
protein 20 (ARHGAP20) were found to be differentially expressed
(upregulated) in the mRNA expression profile GSE30994 (Fig. 2E). Finally, a ceRNA network was
constructed to assess the association of circUBAP2, miR-1244, ADD3,
DYNC1I1, MAP3K2 and ARHGAP20 (Fig.
2F).
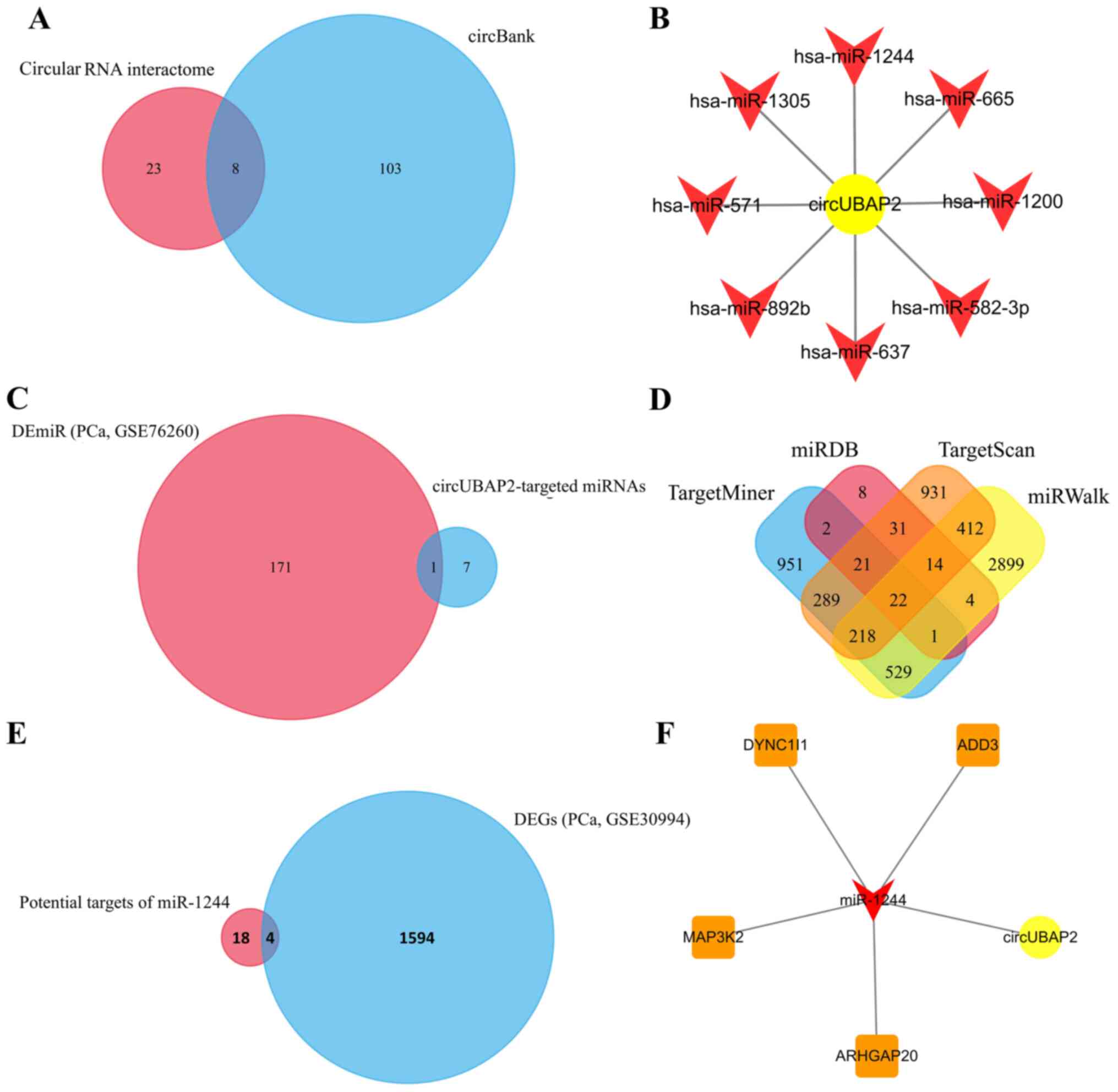 | Figure 2.Prediction of the
circUBAP2-associated axis. (A) A total of 31 and 111 miRNAs were
predicted as targets of circUBAP2 using circular RNA interactome
and circBank, respectively, and 8 of these miRNAs were
co-predicted. (B) The potential regulatory network of circUBAP2 and
its targets miR-1244, miR-665, miR-1200, miR-582-3p, miR-637,
miR-892b, miR-571 and miR-1305. (C) miR-1244 was the only DEmiR in
the miRNA profile GSE76260. (D) A total of 22 mRNAs were predicted
as the potential target mRNAs of miR-1244, which were co-predicted
using miRDB, TargetScan, miRWalk and TargetMiner. (E) Four
co-predicted DEGs (ADD3, DYNC1I1, MAP3K2 and ARHGAP20) in the mRNA
expression profile GSE30994. (F) circUBAP2-associated competitive
endogenous RNA network. circUBAP2, circular RNA UBAP2; DE,
differentially expressed; DEGs, DE genes; miR/miRNA, microRNA;
ADD3, adducin 3; DYNC1I1, dynein cytoplasmic 1 intermediate chain
1; ARHGAP20, Rho GTPase activating protein 20; PCa, prostate
cancer. |
Validation of PCa-associated
circUBAP2/miR-1244/MAP3K2 axis
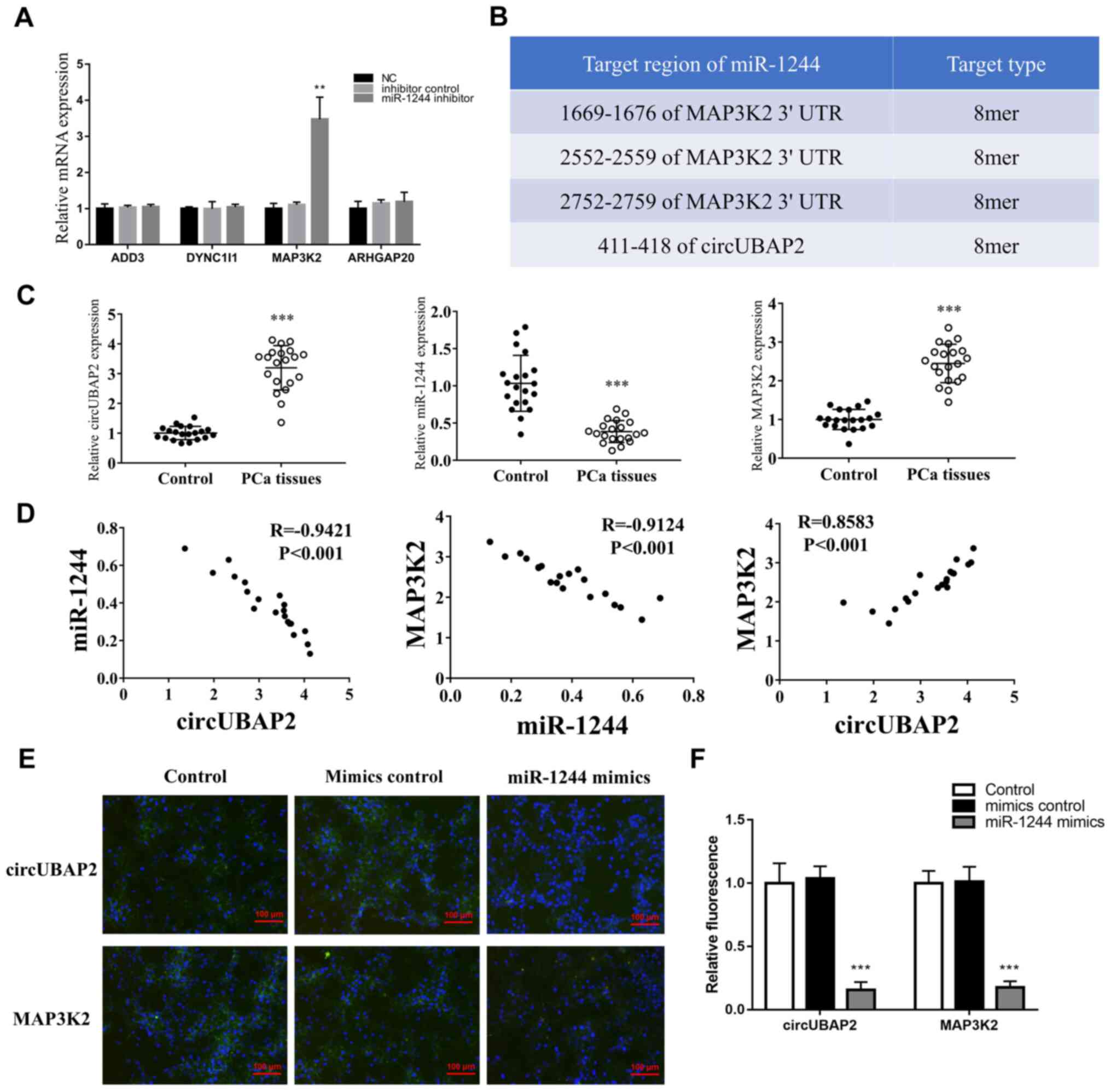
In the present study, miR-1244 expression was
knocked down using a miR-1244 inhibitor to validate its target
mRNAs in PCa cells. Only MAP3K2 expression was significantly
upregulated following miR-1244-knockdown, indicating that MAP3K2
was the final target mRNA of the circUBAP2/miR-1244 axis (Fig. 3A). Additionally, their potential
binding sites were validated using TargetScan (Fig. 3B). Subsequently, the expression
levels of circUBAP2, miR-1244 and MAP3K2 were detected in tissues
of patients with PCa. The results indicated that circUBAP2 and
MAP3K2 expression was significantly upregulated, while miR-1244
expression was significantly downregulated in PCa tissues compared
with in normal tissues (Fig. 3C).
Furthermore, circUBAP2 expression was negatively correlated with
miR-1244 expression (Fig. 3D;
R=−0.9421; P<0.001), but positively correlated with MAP3K2
expression (Fig. 3D; R=0.8583;
P<0.001). Additionally, a negative correlation was observed
between miR-1244 and MAP3K2 expression (Fig. 3D; R=−0.9124; P<0.001). In
addition, the expression of circUBAP2 was partly inhibited by
overexpression of miR-1244, but promoted by inhibition of miR-1244
(Fig. S1). The luciferase reporter
gene assay was further performed to validate the binding sites of
miR-1244 on circUBAP2 and MAP3K2. The results indicated that the
luciferase activity was significantly decreased in 293T cells
co-transfected with the circUBAP2/MAP3K2 reporter plasmid (pGL6-miR
plasmid) and miR-1244 mimics (Fig.
S2A) compared with the other groups (Fig. 3E and F), while there was no
difference between the circUBAP2 and MAP3K2 groups.
Functional verification of the
circUBAP2/miR-1244/MAP3K2 axis in LNCaP cells
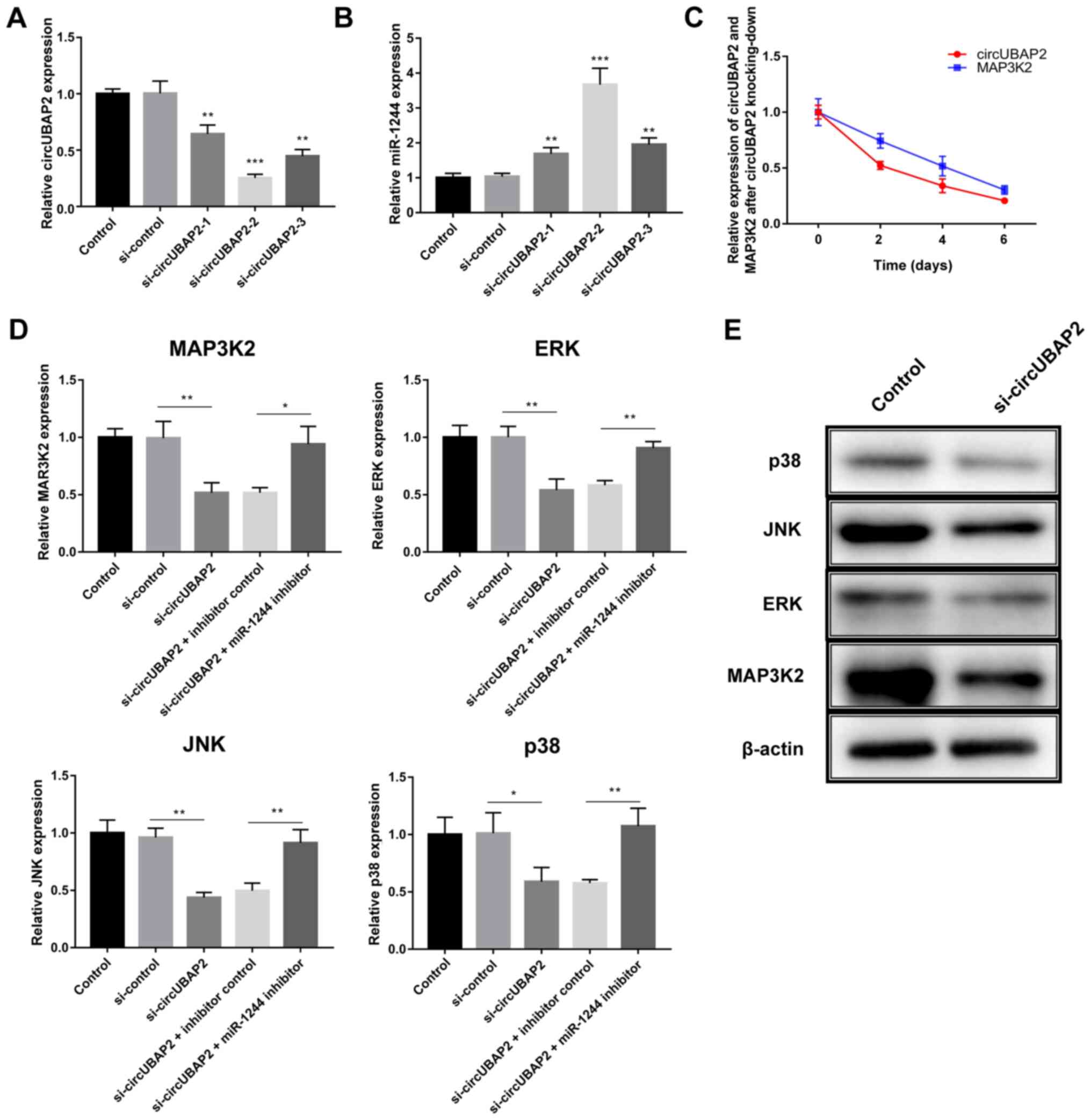
As the LNCaP cell line presented the highest
circUBAP2 expression, the regulating role of circUBAP2 was further
explored in the LNCaP cell line. First, the effect of three
si-circUBAP2 was examined on knocking down circUBAP2 expression,
indicating that si-circUBAP2-2 had the most significant effect on
knocking down circUBAP2 expression and increasing miR-1244
expression (Fig. 4A and B).
Additionally, circUBAP2 expression was decreased in tandem with
MAP3K2 expression (Fig. 4C).
Subsequently, the miR-1244 inhibitor was used for rescue assays
(Fig. S2B). The mRNA expression
levels of MAP3K2, ERK, JNK and p38 were all significantly decreased
48 h after circUBAP2-knockdown (Fig.
4D). However, their expression levels were significantly
reversed by co-transfection with the miR-1244 inhibitor (Fig. 4D). Since the mRNA expression of
MAP3K2, ERK, JNK and p38 were significantly reversed by rescue
experiments, which indicated that knocking down circUBAP2 could
suppress the expression of these genes via targeting miR-1244.
Hence, the inhibitory effects of knocking-down circUBAP2 on protein
expression of MAP3K2, ERK, JNK and p38 were further validated. The
western blotting results also confirmed that the protein expression
levels of MAP3K2, ERK, JNK and p38 were decreased at day 6 (the
most effective time point) after si-circUBAP2 transfection compared
with the untransfected group (Fig.
4E).
Effect of circUBAP2 on cell
proliferation of four PCa cell lines
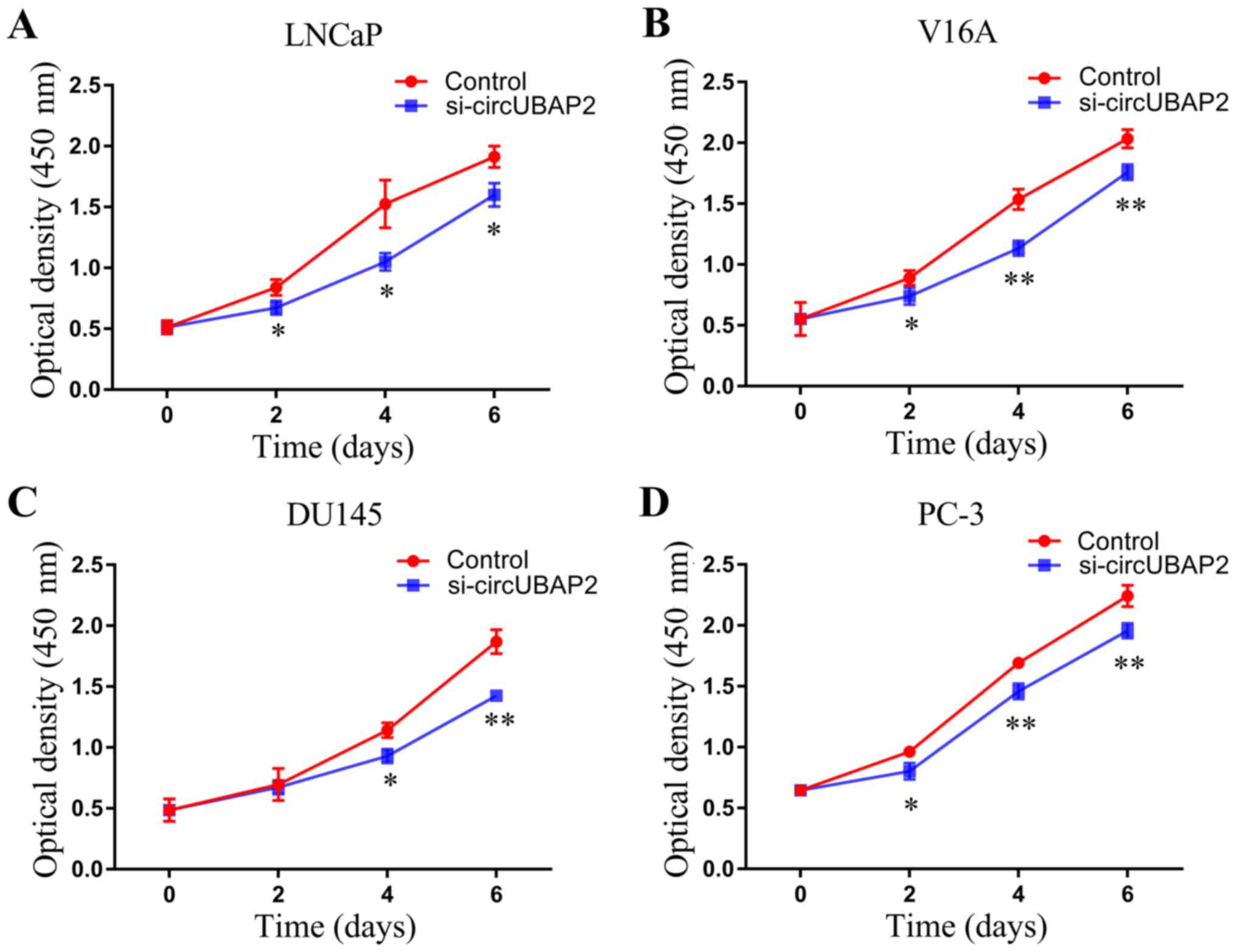
The effect of circUBAP2 on the proliferation of
LNCaP cells was measured using the CCK-8 assay. The proliferation
of LNCaP, V16A, DU145 and PC-3 cells was tested every 2 days after
circUBAP2-knockdown. As shown in Fig.
5, the proliferative activity of all PCa cell lines was
significantly decreased following circUBAP2-knockdown.
Discussion
circRNAs are critical regulators that serve an
imperative role in gene expression (14,27).
circRNA biomarkers and circRNA-targeted therapy have attracted
increasing attention in recent years (28,29).
Previous studies have revealed the differential expression profile
of circRNAs in PCa, indicating that circRNAs may act as important
factors in regulating the progression of PCa (30,31).
circUBAP2 is a well-known tumor-associated circRNA. Wu et al
(16) indicated that circ-UBAP2
promoted the malignant behaviors of esophageal squamous cell
carcinoma via the miR-422a/Rab10 axis. Zhao et al (17) found that circUBAP2 modulated
pancreatic adenocarcinoma by regulating the infiltration and
function of immune cells. Additionally, Sheng et al
(15) identified circUBAP2 as a
critical circRNA that promoted the progression of ovarian cancer by
targeting miR-144. A preliminary study screened 171 circRNAs that
were differentially expressed in PCa tissues, including circUBAP2
(upregulated) (18). However, the
regulatory effect of circUBAP2 in the progression of PCa remains
unclear.
Thus, the present study aimed to further explore the
regulatory effect and mechanism of circUBAP2 in PCa. First, the
expression levels of circUBAP2 were detected in four PCa cell
lines, indicating that circUBAP2 expression was upregulated in PCa
cells and that circUBAP2 was mainly located in the cytoplasm.
Subsequently, site prediction websites were used to predict the
targets of circUBAP2 in regulating the progression of PCa. Based on
the miRNA expression profile GSE76260 and the mRNA expression
profile GSE30994, it was determined that the
circUBAP2/miR-1244/MAP3K2 axis may be critical for PCa progression.
miR-1244 has been reported to act as a suppressor in various types
of cancer, such as ovarian cancer and lung carcinoma by inhibiting
its target mRNAs, such as pyruvate kinase M1/2 and myocyte enhancer
factor 2D (14,32,33).
Moreover, the miRNA expression profile GSE76260 revealed that
miR-1244 was downregulated in PCa. MAP3K2 is a stress-activated
protein kinase in the MAPK signaling pathway, which is essential in
promoting cancer cell proliferation, including in PCa (23,34).
However, the regulatory association between miR-1244 and MAP3K2 has
not been fully defined. Notably, MAP3K2 expression in the present
study was found to be upregulated in PCa tissues, which
corresponded to the expression trend of circUBAP2, while it was
negatively correlated with miR-1244 expression. Hence, it was
hypothesized that the upregulation of circUBAP2 in PCa cells may
suppress miR-1244 expression and promote MAP3K2 expression. High
MAP3K2 expression may thereby promote cell proliferation of PCa
cells.
To further prove this hypothesis, several lines of
evidence were provided. First, the binding sites of miR-1244 on
circUBAP2 and MAP3K2 were further confirmed by a luciferase
reporter gene assay. Functional assays were then performed to
verify the effect of circUBAP2 on the expression levels of MAP3K2
and MAPK key factors, including ERK, JNK and p38.
circUBAP2-knockdown using si-circUBAP2 significantly decreased the
expression levels of MAP3K2, ERK, JNK and p38 in LNCaP cells; their
expression levels were then rescued by co-transfection with the
miR-1244 inhibitor. Additionally, a CCK-8 assay was performed to
verify the effect of circUBAP2 on the proliferation of PCa cells.
The results revealed that circUBAP2-knockdown significantly
decreased cell proliferation, indicating that circUBAP2 may have a
role in promoting cell proliferation.. Overall, the current data
suggested that circUBAP2 may promote the proliferation of PCa cells
via the miR-1244/ MAP3K2 axis.
In summary, the present study indicated that the
circUBAP2/miR-1244 MAP3K2 axis may promote the proliferation of PCa
cells, since circUBAP2-knockdown suppressed cell proliferation and
the progression of PCa. These findings may be essential in
providing new therapeutic strategies for PCa.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Department of
Science and Technology of Xinjiang Uygur Autonomous Region, China
(grant no. 2017DOIC294).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request. The GSE76260 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE76260)
and GSE30994 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi)
datasets are available in the Gene Expression Omnibus
repository.
Authors' contributions
XL and YW designed and supervised the study, as well
as organized and wrote the manuscript. XL, BA and WW performed the
data analysis. XL, MR, and CX contributed to acquisition of data.
XL, BA, WW, MR, CX and YW confirm the authenticity of the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
participants. All study protocols were approved by the Ethics
Committee of The First Affiliated Hospital of Xinjiang Medical
University (approval no. XJ20200025).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Szentirmai E and Giannico GA: Intraductal
carcinoma of the prostate. Pathologica. 112:17–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xin L: Cells of origin for prostate
cancer. Adv Exp Med Biol. 1210:67–86. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sartor O and de Bono JS: Metastatic
prostate cancer. N Engl J Med. 378:645–657. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Abrams DI: An integrative approach to
prostate cancer. J Altern Complement Med. 24:872–880. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merriel SWD, Funston G and Hamilton W:
Prostate cancer in primary care. Adv Ther. 35:1285–1294. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou CK, Check DP, Lortet-Tieulent J,
Laversanne M, Jemal A, Ferlay J, Bray F, Cook MB and Devesa SS:
Prostate cancer incidence in 43 populations worldwide: An analysis
of time trends overall and by age group. Int J Cancer.
138:1388–1400. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pang C, Guan Y, Li H, Chen W and Zhu G:
Urologic cancer in China. Jpn J Clin Oncol. 46:497–501. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shan X, Danet-Desnoyers G, Aird F, Kandela
I, Tsui R, Perfito N and Iorns E: Replication study: Androgen
receptor splice variants determine taxane sensitivity in prostate
cancer. PeerJ. 6:e46612018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kristensen LS, Andersen MS, Stagsted L,
Ebbesen KK, Hansen TB and Kjems J: The biogenesis, biology and
characterization of circular RNAs. Nat Rev Genet. 20:675–691. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen G, Wang Q, Li Z, Yang Q, Liu Y, Du Z,
Zhang G and Song Y: Circular RNA CDR1as promotes adipogenic and
suppresses osteogenic differentiation of BMSCs in steroid-induced
osteonecrosis of the femoral head. Bone. 133:1152582020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu P, Mo Y, Peng M, Tang T, Zhong Y, Deng
X, Xiong F, Guo C, Wu X, Li Y, et al: Emerging role of
tumor-related functional peptides encoded by lncRNA and circRNA.
Mol Cancer. 19:222020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Q, Wang W, Zhou Q, Chen C, Yuan W,
Liu J, Li X and Sun Z: Roles of circRNAs in the tumour
microenvironment. Mol Cancer. 19:142020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tang Q and Hann SS: Biological roles and
mechanisms of circular RNA in human cancers. Onco Targets Ther.
13:2067–2092. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lei M, Zheng G, Ning Q, Zheng J and Dong
D: Translation and functional roles of circular RNAs in human
cancer. Mol Cancer. 19:302020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sheng M, Wei N, Yang HY, Yan M, Zhao QX
and Jing LJ: CircRNA UBAP2 promotes the progression of ovarian
cancer by sponging microRNA-144. Eur Rev Med Pharmacol Sci.
23:7283–7294. 2019.PubMed/NCBI
|
|
16
|
Wu Y, Zhi L, Zhao Y, Yang L and Cai F:
Knockdown of circular RNA UBAP2 inhibits the malignant behaviours
of esophageal squamous cell carcinoma by microRNA-422a/Rab10 axis.
Clin Exp Pharmacol Physiol. 47:1283–1290. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhao R, Ni J, Lu S, Jiang S, You L, Liu H,
Shou J, Zhai C, Zhang W, Shao S, et al: CircUBAP2-mediated
competing endogenous RNA network modulates tumorigenesis in
pancreatic adenocarcinoma. Aging (Albany NY). 11:8484–8501. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen S, Huang V, Xu X, Livingstone J,
Soares F, Jeon J, Zeng Y, Hua JT, Petricca J, Guo H, et al:
Widespread and functional RNA circularization in localized prostate
cancer. Cell. 176:831–843. e22. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dizeyi N, Hedlund P, Bjartell A, Tinzl M,
Austild-Taskén K and Abrahamsson PA: Serotonin activates MAP kinase
and PI3K/Akt signaling pathways in prostate cancer cell lines. Urol
Oncol. 29:436–445. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan C, Zhang L, Meng X, Qin H, Xiang Z,
Gong W, Luo W, Li D and Han X: Chronic exposure to microcystin-LR
increases the risk of prostate cancer and induces malignant
transformation of human prostate epithelial cells. Chemosphere.
263:1282952021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim J, Mizokami A, Shin M, Izumi K, Konaka
H, Kadono Y, Kitagawa Y, Keller ET, Zhang J and Namiki M: SOD3 acts
as a tumor suppressor in PC-3 prostate cancer cells via hydrogen
peroxide accumulation. Anticancer Res. 34:2821–2831.
2014.PubMed/NCBI
|
|
23
|
Wang LL and Zhang M: miR-582-5p is a
potential prognostic marker in human non-small cell lung cancer and
functions as a tumor suppressor by targeting MAP3K2. Eur Rev Med
Pharmacol Sci. 22:7760–7767. 2018.PubMed/NCBI
|
|
24
|
Chen G, Wang Q, Yang Q, Li Z, Du Z, Ren M,
Zhao H, Song Y and Zhang G: Circular RNAs hsa_circ_0032462,
hsa_circ_0028173, hsa_circ_0005909 are predicted to promote CADM1
expression by functioning as miRNAs sponge in human osteosarcoma.
PLoS One. 13:e02028962018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Q, Yang Q, Chen G, Du Z, Ren M, Wang
A, Zhao H, Li Z, Zhang G and Song Y: LncRNA expression profiling of
BMSCs in osteonecrosis of the femoral head associated with
increased adipogenic and decreased osteogenic differentiation. Sci
Rep. 8:91272018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai J, Zhuang Y, Tang M, Qian Q and Chen
JP: CircRNA UBAP2 facilitates the progression of colorectal cancer
by regulating miR-199a/VEGFA pathway. Eur Rev Med Pharmacol Sci.
24:7963–7971. 2020.PubMed/NCBI
|
|
27
|
Sulaiman SA, Abdul Murad NA, Mohamad Hanif
EA, Abu N and Jamal R: Prospective advances in circular RNA
investigation. Adv Exp Med Biol. 1087:357–370. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arnaiz E, Sole C, Manterola L,
Iparraguirre L, Otaegui D and Lawrie CH: CircRNAs and cancer:
Biomarkers and master regulators. Semin Cancer Biol. 58:90–99.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Holdt LM, Kohlmaier A and Teupser D:
Circular RNAs as therapeutic agents and targets. Front Physiol.
9:12622018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hua JT, Chen S and He HH: Landscape of
noncoding RNA in prostate cancer. Trends Genet. 35:840–851. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan Z, Xiao Y, Chen Y and Luo G: Screening
and identification of epithelial-to-mesenchymal transition-related
circRNA and miRNA in prostate cancer. Pathol Res Pract.
216:1527842020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, He X, Chen Y and Cao D: Long
non-coding RNA LINC00504 regulates the Warburg effect in ovarian
cancer through inhibition of miR-1244. Mol Cell Biochem. 464:39–50.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang R, Zhang Y and Li H:
miR-1244/myocyte enhancer factor 2D regulatory loop contributes to
the growth of lung carcinoma. DNA Cell Biol. 34:692–700. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lv X, Wang M, Qiang J and Guo S: Circular
RNA circ-PITX1 promotes the progression of glioblastoma by acting
as a competing endogenous RNA to regulate miR-379-5p/MAP3K2 axis.
Eur J Pharmacol. 863:1726432019. View Article : Google Scholar : PubMed/NCBI
|