Introduction
Hepatocellular carcinoma (HCC) is one of the most
common malignancies, and the third most common cause of
cancer-associated mortality worldwide (1). In 2018, the estimated global incidence
rate of liver cancer per 100,000 person-years was 9.3, and the
corresponding mortality rate was 8.5; HCC accounts for 75–80% of
liver cancer cases (2). In addition,
chronic hepatitis B virus (HBV) infections account for 75–80% of
HCC cases worldwide (3); in China,
HBV infections account for >80% of all HCC cases (4). Radical resection of liver cancer and
liver transplantation are the current potential curative treatments
for selected patients with HCC based on the Milan Criteria
(5,6). However, long-term surgical outcomes
remain unsatisfactory due to high tumor recurrence rates (7,8).
Previous studies have reported that liver- and tumor-related
characteristics such as cirrhosis, tumor size, number and vascular
invasion are definitive risk factors associated with long-term
survival after curative resection of HCC (9,10).
Cancer antigen 125 (CA125) is a
high-molecular-weight glycoprotein identified with a murine
monoclonal antibody against epithelial ovarian cancer (11), and serum CA125 levels have been
proposed as a marker for monitoring the course of disease in
patients with epithelial ovarian cancer (12). In addition, CA125 is used for the
diagnosis and prognosis of various types of tumor, such as ovarian
cancer, cholangiocarcinoma and lung cancer (13–16).
Previous studies have demonstrated that CA125 levels are high in
patients with acute and chronic liver disease, particularly in
those with cirrhotic ascites (17,18). The
expression levels of serum CA125 are elevated in HCC compared with
health subject and benign liver diseases, and CA125 exhibits 92%
sensitivity for the diagnosis of HCC; however, the specificity is
only 48.5%, which is significantly lower compared with that of
α-fetoprotein (AFP) (19). Despite
the numerous biomarkers proposed for HCC, such as AFP-L3,
des-γ-carboxy prothrombin and osteopontin (20), the first one that was identified,
AFP, remains the most utilized. Previous studies have demonstrated
that CA125 provides a reference value for the prognostic evaluation
of patients with various types of tumor, such as pancreatic,
epithelial ovarian and lung cancer (21–23).
Zhou et al (24) have
reported that high preoperative serum CA125 levels predict a large
tumor diameter and poor prognosis following liver resection in
patients with HCC with AFP levels ≤200 ng/ml. To date, the
association between CA125 levels and prognosis of patients with
HBV-related HCC has not been reported in detail; although certain
retrospective reports have identified an association between them
(13,24), further studies are needed to confirm
this. Based on the worldwide predominance of HBV-related HCC, it is
essential to investigate the association between CA125 and
prognosis in HBV-related HCC.
To address this question, the present study
retrospectively analyzed the records of 306 patients with
HBV-related HCC treated by curative resection and assessed the
potential associations between CA125 levels prior to tumor
resection and post-operative disease-free survival (DFS) and
overall survival (OS).
Materials and methods
Patients
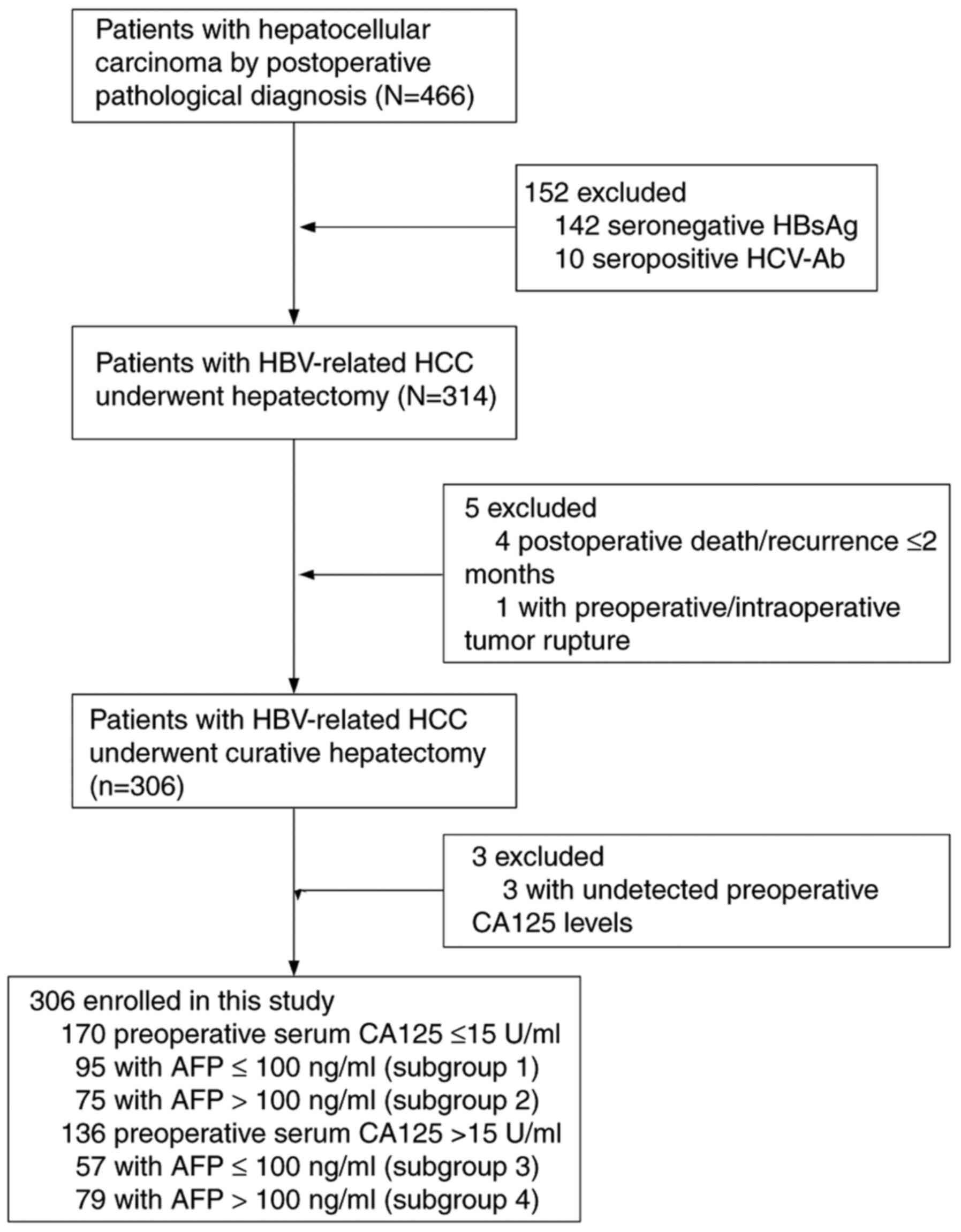
Between April 2013 and October 2018, a retrospective
study was conducted using the data from 466 consecutive patients
(392 men, 74 women; median age, 54.4 years) with HBV-related HCC
who underwent hepatic resection at Liuzhou People's Hospital
Affiliated to Guangxi Medical University (Liuzhou, China). The
inclusion criteria were as follows: i) Patients ≥18 years; ii) HCC
was confirmed by postoperative pathologic examination; iii)
hepatitis B surface antigen was positive; iv) patient agreed to
liver resection; v) curative treatment (pathological confirmation
of negative resection margin); and vi) no local recurrence within 2
months after surgery. The exclusion criteria were as follows: i)
Metastatic or recurrent liver cancer was confirmed by postoperative
pathological examination; ii) hepatitis C virus or human
immunodeficiency virus; iii) significant lesions of the heart, lung
or kidney; iv) patient received transarterial chemoembolization or
other antitumor therapy before surgery; and v) severe complications
or adverse events (including postoperative mortality) within 2
months following surgery. Based on these criteria, 160 patients
were excluded, and the remaining 306 patients were enrolled. The
patient selection flow diagram is presented in Fig. 1. The Clinical Research Ethics
Committee of Liuzhou People's Hospital Affiliated to Guangxi
Medical University approved the study, and written informed consent
was obtained from each patient before surgery.
All patients received conventional perioperative
prophylactic antibiotics, drugs inhibiting gastric acid secretion,
liver protection therapy and nutritional support. The primary
outcome of the study was DFS and OS after hepatectomy. Secondary
outcomes included tumor characteristics, intraoperative blood loss
and intraoperative transfusion.
Based on a previous study, an AFP level of 100 ng/ml
was selected as the stratification cutoff point (25). According to the stratification of
preoperative serum CA125 and AFP levels, the patients were
classified into four subgroups: i) Subgroup 1, CA125 ≤15 U/ml and
AFP ≤100 ng/ml (n=95); ii) subgroup 2, CA125 ≤15 U/ml and AFP
>100 ng/ml (n=75); iii) subgroup 3, CA125 >15 U/ml and AFP
≤100 ng/ml (n=57); and iv) subgroup 4, CA125 >15 U/ml and AFP
>100 ng/ml (n=79). Patients received antiviral therapy
conforming to the Chronic Hepatitis B Practice Guidelines of the
Asian Pacific Association for the Study of the Liver (26). Antiviral therapy was administered
before or after surgery (oral 100 mg lamivudine, 10 mg adefovir
dipivoxil or 0.5 mg entecavir daily).
Clinical diagnosis and
definitions
Diagnosis of HCC was confirmed by pathology. OS was
calculated from the day of surgery until the day of mortality or
last contact. The DFS was defined as the time from the day of
surgery to the day of confirmed tumor recurrence, or from the day
of surgery to the day of mortality or last contact for patients who
did not experience recurrent disease.
Biochemical tests and follow-up
Patient blood tests were performed in the morning of
the second day after hospital admission. Baseline examinations
(within 7 days prior to surgery) included regular routine blood,
liver function, blood coagulation function, hepatitis B surface
antigen (HBsAg), HBsAg antibody (anti-HBs), hepatitis B e antigen
(HBeAg), HBeAg antibody (anti-HBe), antibody to hepatitis core
antigen (anti-HBc), AFP and CA125 concentration, and HBV DNA level
tests. Serum HBV viral loads were measured using a PCR HBV
monitoring kit (Roche Diagnostics K.K) with a lower detection limit
of 200 IU/ml. The concentration of serum CA125 and AFP was detected
by ELISA (cat. nos. Q8WX17 and E-EL-H0070c; Wuhan Huamei Biotech
Co., Ltd) according to the manufacturer's instructions.
Patients were followed up at Liuzhou People's
Hospital every 3 months during the first postoperative year and
every 3–6 months thereafter. The follow-up period ended on August
12, 2019. Blood tests and liver ultrasonography were performed
during each visit by independent doctors. A computed tomography
(CT) scan of the abdomen was performed every 6 months. If
recurrence was suspected, CT or magnetic resonance imaging was
immediately performed to confirm the diagnosis. Patients with
confirmed recurrence were subjected to further treatment. If the
recurrent tumor was localized, a second liver resection,
radiofrequency ablation or percutaneous ethanol injection was
suggested. If the recurrent tumor was multiple or diffuse,
transcatheter arterial chemoembolization (TACE) was performed.
Treatment decisions were based on the pattern of recurrence and
liver function reserve.
Statistical analysis
For the demographic data, normally distributed
continuous data are presented as the mean ± SD, and non-normally
distributed continuous data are presented as the median and
interquartile range. Categorical variables are presented as
percentages. Statistical analyses were conducted using the
independent samples Student's t-test (or Mann-Whitney U test
for non-normally distributed data), one-way analysis of variance
(or Kruskal-Wallis test for non-normally distributed data) and the
χ2 test, as appropriate. DFS and OS were assessed using
Kaplan-Meier analysis, and log-rank tests were used to evaluate
differences between groups. The Bonferroni post hoc test was used
for multiple comparisons. For the laboratory parameters, the cutoff
values were the upper limit of the normal values. Univariate and
multivariate analyses were performed by the Cox proportional
hazards regression model. Following the univariate analysis,
multivariate analysis of survival was performed. All analyses were
conducted with SPSS 23.0 software for windows (IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Basis patient characteristics
The median follow-up time of the patients included
in the present study was 35.0 months. Table I summarizes the characteristics of
the patients in the high (>15 U/ml) and low (≤15 U/ml)
preoperative serum CA125 groups. The majority of the assessed
variables were similar between the two groups. However, significant
differences were noted in the platelet count, bilirubin, AFP and
serum total bilirubin levels, which may be due to the patients with
high AFP levels or severe liver damage having a tendency for
long-term chronic liver disease and high serum CA125 levels. As
presented in Table II, patients
with high preoperative serum AFP levels were younger compared with
those with low preoperative serum AFP levels (subgroup 1 vs. 2,
P=0.001; subgroup 3 vs. 4, P<0.001). Patients with the same
baseline AFP levels but with high serum CA125 levels presented with
poorer liver function compared with that in patients with low serum
CA125 levels. There were no significant differences in the
preoperative liver function between the preoperative high and low
serum AFP level groups when the baseline CA125 levels were the
same.
 | Table I.Comparison of clinicopathological
characteristics between two groups of patients stratified by the
CA125 levels. |
Table I.
Comparison of clinicopathological
characteristics between two groups of patients stratified by the
CA125 levels.
| Variable | Total, n=306 | CA125 >15 U/ml,
n=136 | CA125 ≤15 U/ml,
n=170 | P-value |
|---|
| Age, years, mean ±
SD | 51.9±11.7 | 50.7±12.1 | 52.8±11.4 | 0.116a |
| Sex, male, n
(%) | 261 (85.3%) | 114 (83.8%) | 147 (86.5%) | 0.516 |
| Diabetes, n
(%) | 68 (22.2%) | 37 (27.2%) | 31 (18.2%) | 0.061 |
| Hypertension, n
(%) | 45 (14.7%) | 22 (16.2%) | 23 (13.5%) | 0.516 |
| Platelet count,
×109/l | 178 (135–235) | 199 (149–257) | 171 (129–216) | 0.004b |
| TBIL, µmol/l | 12.7
(9.8–18.0) | 13.7
(10.6–20.9) | 11.8
(9.2–16.0) | 0.001b |
| ALT, IU/l | 54 (30–95) | 56 (33–94) | 45 (27–96) | 0.440b |
| AST, IU/l | 80 (37–130) | 82 (42–132) | 72 (32–125) | 0.503b |
| ALB, g/l, mean ±
SD | 37.9±4.47 | 36.8±4.38 | 38.8±4.33 |
<0.001a |
| PT, sec, mean ±
SD | 14.9±1.67 | 15.0±1.69 | 14.9±1.66 | 0.507a |
| HBV DNA, IU/ml
(%) |
|
|
| 0.085 |
|
≤2,000 | 193 (63.1%) | 93 (68.4%) | 100 (58.8%) |
|
|
>2,000 | 113 (36.9%) | 43 (31.6%) | 70 (41.2%) |
|
| AFP, ng/ml |
|
|
| 0.015 |
|
≤100 | 152 (49.7%) | 57 (41.9%) | 95 (55.9%) |
|
|
>100 | 154 (50.3%) | 79 (58.1%) | 75 (44.1%) |
|
| ASA |
|
|
| 0.286 |
| I | 189 (61.8%) | 82 (60.3%) | 107 (62.9%) |
|
| II | 105 (34.3%) | 46 (33.8%) | 59 (56.2%) |
|
|
III | 12 (3.9%) | 8 (5.9%) | 4 (2.4%) |
|
| Child-Pugh
grade |
|
|
| 0.088 |
| A | 243 (79.4%) | 114 (83.8%) | 129 (75.9%) |
|
| B | 63 (20.6%) | 22 (16.2%) | 41 (24.1%) |
|
| Cirrhosis, n
(%) | 259 (84.6%) | 113 (83.1%) | 146 (85.9%) | 0.501 |
| Ascites, n (%) | 80 (26.1%) | 41 (31.1%) | 39 (22.9%) | 0.154 |
| Tumor diameter,
cm | 5.5 (3.7–9.0) | 7.0 (4.4–11.1) | 5.0 (3.5–7.5) | <0.001 |
| Tumor number, n
(%) |
|
|
| 0.186 |
|
Solitary | 238 (77.8%) | 101 (74.3%) | 137 (80.6%) |
|
|
Multiple | 68 (22.2%) | 35 (25.7%) | 33 (19.4%) |
|
| TNM stage, n
(%) |
|
|
| <0.001 |
| I | 152 (49.7%) | 55 (40.4%) | 97 (57.1%) |
|
| II | 50 (16.3%) | 18 (13.2%) | 32 (18.8%) |
|
|
III | 104 (34.0%) | 63 (46.3%) | 41 (24.1%) |
|
| Venous
invasion (n,%) | 114 (36.3%) | 80 (37.6%) | 34 (33.7%) | 0.503 |
| Tumor
differentiation (n,%) |
|
|
| 0.352 |
| Well
differentiated | 31 (10.1%) | 10 (7.4%) | 21 (12.4%) |
|
|
Moderately differentiated | 258 (84.3%) | 118 (86.8%) | 140 (82.4%) |
|
| Poorly
differentiated | 17 (5.6%) | 8 (5.9%) | 9 (5.3%) |
|
|
Intraoperative blood loss,
ml | 500 (200–825) | 500
(200–1,000) | 450 (200–800) | 0.058b |
|
Intraoperative transfusion
(n,%) | 118 (38.6%) | 65 (47.8%) | 53 (31.2%) | 0.003 |
| Surgery
time, min | 180 (144–210) | 180 (150–235) | 180 (140–206) | 0.162b |
| TACE, n (%) |
|
|
| 0.175 |
| ≤3 | 268 (87.6%) | 123 (90.4%) | 145 (85.3%) |
|
|
>3 | 38 (12.4%) | 13 (9.6%) | 25 (14.7%) |
|
 | Table II.Comparison of clinicopathologic
characteristics among the patient subgroups stratified by CA125 and
AFP levels. |
Table II.
Comparison of clinicopathologic
characteristics among the patient subgroups stratified by CA125 and
AFP levels.
| Variables | Subgroup 1,
n=95 | Subgroup 2,
n=75 | Subgroup 3,
n=57 | Subgroup 4,
n=79 | P1 | P2 | P3 | P4 |
|---|
| Age, years, mean ±
SD | 55.3±10.2 | 49.7±12.0 | 56.4±11.7 | 46.6±10.8 | 0.001a | 0.577a | 0.104a |
<0.001a |
| Sex, male, n
(%) | 79 (83.2%) | 68 (90.7%) | 51 (89.5%) | 63 (79.7%) | 0.155 | 0.284 | 0.057 | 0.129 |
| Hypertension, n
(%) | 16 (16.8%) | 4 (5.3%) | 7 (12.3%) | 5 (6.3%) | 0.021 | 0.447 | – | 0.227 |
| Diabetes, n
(%) | 19 (10.0%) | 12 (16.0%) | 16 (28.1%) | 21 (26.6%) | 0.502 | 0.253 | 0.110 | 0.847 |
| Platelet count,
×109/l | 177.6±62.2 | 178.7±66.8 | 178.0±74.2 | 221.0±93.8 | 0.912a | 0.970a | 0.002a | 0.005a |
| TBIL, µmol/l | 11.6
(9.4–15.4) | 11.9
(8.8–16.6) | 16.1
(11.6–21.8) | 12.8
(9.9–20.8) | 0.787b | 0.001b | 0.097b | 0.083b |
| ALT, IU/l | 40 (25–84) | 66 (36–114) | 49 (29–94) | 58 (37–85) | 0.008b | 0.245b | 0.548b | 0.457b |
| AST, IU/l | 71 (31–145) | 87 (43–126) | 80 (41–119) | 84 (48–132) | 0.350b | 0.812b | 0.622b | 0.260b |
| PT, sec | 14.8±1.6 | 15±1.6 | 15.3±1.7 | 14.8±1.6 | 0.520a | 0.086a | 0.510a | 0.088a |
| HBV DNA IU/ml , n
(%) |
|
|
|
| 0.971 | 0.085 | 0.078 | 0.266 |
|
≤2,000 | 39 (41.1%) | 31 (41.3%) | 21 (36.8%) | 22 (27.8%) |
|
|
|
|
|
>2,000 | 56 (58.9%) | 44 (58.7%) | 36 (63.2%) | 57 (72.2%) |
|
|
|
|
| Albumin, g/l | 39.3
(36.6–40.7) | 38.8
(36.5–42.0) | 36.4
(33.5–39.3) | 37.5
(33.3–40.4) | 0.651b |
<0.001b | 0.006b | 0.225b |
| ASA, n (%) |
|
|
|
| 0.202 | 0.326 | 0.213 | 0.231 |
| I | 60 (63.2%) | 47 (62.7%) | 30 (52.6%) | 52 (65.8%) |
|
|
|
|
| II | 31 (32.6%) | 28 (37.3%) | 22 (38.6%) | 24 (30.4%) |
|
|
|
|
|
III | 4 (4.2%) | 0 | 5 (8.8%) | 3 (3.8%) |
|
|
|
|
| Child-Pugh grade, n
(%) |
|
|
|
| 0.293 | 0.424 | 0.084 | 0.917 |
| A | 75 (78.9%) | 54 (72.0%) | 48 (84.2%) | 66 (83.5%) |
|
|
|
|
| B | 20 (21.1%) | 21 (28.0%) | 9 (15.8%) | 13 (16.5%) |
|
|
|
|
| Cirrhosis | 78 (82.1%) | 68 (90.7%) | 48 (84.2%) | 65 (82.3%) | 0.111 | 0.739 | 0.129 | 0.767 |
| Ascites | 23 (24.2%) | 16 (21.3%) | 17 (29.8%) | 24 (30.4%) | 0.658 | 0.447 | 0.201 | 0.944 |
| TNM stage, n
(%) |
|
|
|
| 0.027 | 0.162 | 0.007 | 0.005 |
| I | 57 (60.0%) | 40 (53.3%) | 30 (52.7%) | 25 (31.6%) |
|
|
|
|
| II | 22 (23.2%) | 10 (13.3%) | 10 (17.5%) | 8 (10.2%) |
|
|
|
|
|
III | 16 (16.8%) | 25 (33.3%) | 17 (29.8%) | 46 (58.2%) |
|
|
|
|
| Tumor diameter,
cm | 5.5±3.4 | 6.2±3.8 | 6.2±4.0 | 8.9±4.4 | 0.247 | 0.302 | <0.001 | <0.001 |
| Tumor number, n
(%) |
|
|
|
| 0.010 | 0.464 | 0.004 | 0.289 |
|
Solitary | 70 (73.7%) | 67 (89.3%) | 45 (78.9%) | 56 (70.9%) |
|
|
|
|
|
Multiple | 25 (26.3%) | 8 (10.7%) | 12 (21.1%) | 23 (29.1%) |
|
|
|
|
| Venous invasion, n
(%) | 27 (28.4%) | 28 (37.3%) | 18 (31.6%) | 39 (49.4%) | 0.217 | 0.680 | 0.132 | 0.038 |
| Tumor
differentiation, n (%) |
|
|
|
| 0.083 | 0.945 | 0.185 | 0.004 |
| Well
differentiated | 16 (16.8%) | 5 (6.7%) | 9 (15.8%) | 1 (1.3%) |
|
|
|
|
|
Moderately differentiated | 73 (76.8%) | 67 (89.3%) | 45 (78.9%) | 73 (92.4%) |
|
|
|
|
| Poorly
differentiated | 6 (6.3) | 3 (4.0%) | 3 (5.3%) | 5 (6.3) |
|
|
|
|
| Intraoperative
blood loss | 400 (200–700) | 400 (200–900) | 400
(200–1,000) | 600
(300–1,200) | 0.308b | 0.906b | 0.045b | 0.020b |
| Intraoperative | 24 (25.3%) | 29 (38.7%) | 22 (38.6%) | 43 (54.4%) | 0.061 | 0.083 | 0.050 | 0.068 |
| Surgery time
transfusion, n (%) | 180 (135–210) | 180 (140–202) | 180 (125–194) | 180 (150–240) | 0.786b | 0.825b | 0.069b | 0.034b |
| TACE, n (%) |
|
|
|
| 0.009 | 0.009 | 0.210 | 0.148 |
| ≤3 | 75 (78.9%) | 70 (93.3%) | 54 (94.7%) | 69 (87.3%) |
|
|
|
|
|
>3 | 20 (21.1%) | 5 (6.7%) | 3 (5.3%) | 10 (12.7%) |
|
|
|
|
DFS and OS analysis
DFS
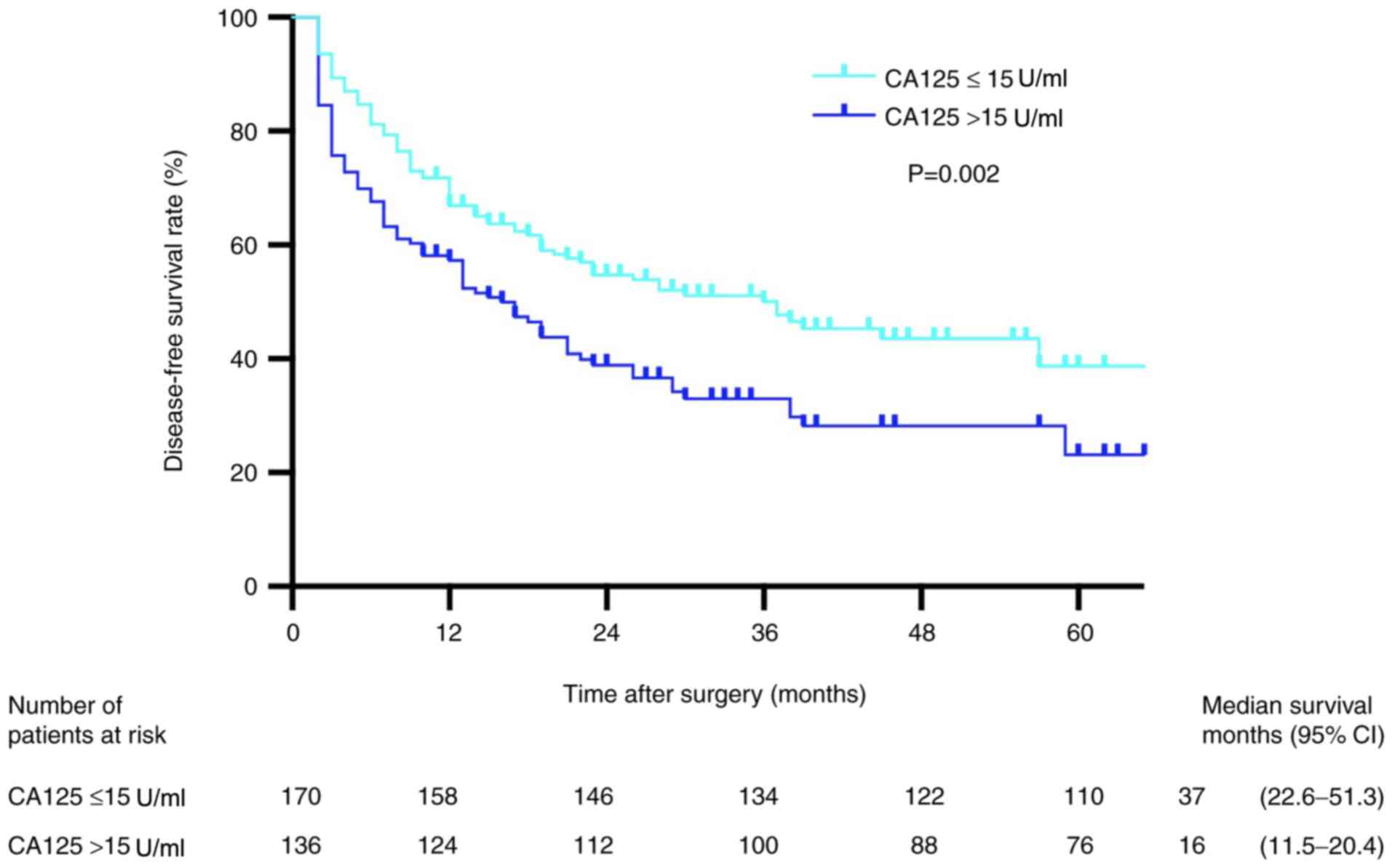
Patients in the low preoperative CA125 group
presented with higher DFS rates compared with those in the high
preoperative CA125 group (P=0.002; Fig.
2). The 1-, 2- and 3-year DFS rates in the low preoperative
CA125 group were 93.5, 87.0 and 79.3%, respectively, whereas the
corresponding rates in the high preoperative CA125 group were
100.0, 84.6 and 75.7%, respectively. Univariate analysis results
demonstrated that male sex, platelet count
<100×109/l, TNM stage III, tumor diameter >5 cm,
multiple tumors, venous invasion, tumor differentiation,
intraoperative blood loss >1,000 ml, intraoperative transfusion
and high serum CA125 levels were independent risk factors
associated with DFS (Table III).
Multivariate analysis revealed that tumor diameter >5 cm,
multiple tumors and vascular invasion at the time of resection were
independent risk factors associated with short DFS (Table III).
 | Table III.Univariate and multivariate analyses
of DFS and OS in all patients (n=306). |
Table III.
Univariate and multivariate analyses
of DFS and OS in all patients (n=306).
|
| DFS | OS |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Index | HR | P-value | HR (95% CI) | P-value | HR | P-value | HR (95% CI) | P-value |
|---|
| Age | 0.763 | 0.267 |
|
| 0.873 | 0.623 |
|
|
| Sex | 0.599 | 0.035 |
|
| 0.539 | 0.036 |
|
|
| Hypertension | 1.325 | 0.284 |
|
| 1.082 | 0.795 |
|
|
| Diabetes | 1.356 | 0.077 |
|
| 1.455 | 0.064 |
|
|
| ALT | 1.274 | 0.135 |
|
| 0.999 | 0.439 |
|
|
| AST | 1.076 | 0.673 |
|
| 1.000 | 0.822 |
|
|
| Platelet count
(≥100 vs. <100×109/l) | 0.489 | 0.022 |
|
| 0.665 | 0.240 |
|
|
| PT (≤14 vs. >14
sec) | 1.139 | 0.430 |
|
| 1.047 | 0.815 |
|
|
| TBIL (≤17 vs.
>17 µmol/l) | 1.118 | 0.504 |
|
| 1.193 | 0.373 |
|
|
| HBV DNA (≤2,000 vs.
>2,000 IU/ml) | 1.325 | 0.284 |
|
| 1.196 | 0.356 |
|
|
| AFP (≤100 vs.
>100 ng/ml) | 1.310 | 0.077 |
|
| 1.052 | 0.026 |
|
|
| TNM stage |
|
|
|
|
|
|
|
|
| I | (Ref.) | (Ref.) |
|
| (Ref.) | (Ref.) |
|
|
| II | 1.144 | 0.555 |
|
| 1.073 | 0.809 |
|
|
|
III | 1.781 | <0.001 |
|
| 2.220 | <0.001 |
|
|
| ASA |
|
|
|
|
|
|
|
|
| I | (Ref.) | (Ref.) |
|
| (Ref.) | (Ref.) |
|
|
| II | 1.111 | 0.515 |
|
| 1.115 | 0.571 |
|
|
|
III | 1.785 | 0.097 |
|
| 0.955 | 0.929 |
|
|
| Child-Pugh grade (A
vs. B) | 0.919 | 0.661 |
|
| 0.708 | 0.151 |
|
|
| Cirrhosis | 0.959 | 0.839 |
|
| 1.370 | 0.254 |
|
|
| Ascites | 1.370 | 0.058 |
|
| 1.192 | 0.387 |
|
|
| Tumor diameter (≤5
vs. >5 cm) | 2.069 | <0.001 | 1.725 | 0.002 | 2.377 | <0.001 | 1.746 | 0.010 |
|
|
|
| (1.229–2.419) |
|
|
| (1.142–2.668) |
|
| Tumor number
(Multiple vs. solitary) | 1.985 | <0.001 | 1.751 | 0.001 | 2.122 | <0.001 | 1.739 | 0.006 |
|
|
|
| (1.253–2.446) |
|
|
| (1.175–2.573) |
|
| Venous invasion
(Yes vs. no) | 1.960 | <0.001 | 1.668 | 0.001 | 2.280 | <0.001 | 1.819 | 0.002 |
|
|
|
| (1.223–2.277) |
|
|
| (1.254–2.638) |
|
| Tumor
differentiation |
|
|
|
|
|
|
|
|
| Well
differentiated | (Ref.) | (Ref.) |
|
| (Ref.) | (Ref.) |
|
|
|
Moderately differentiated | 2.283 | 0.008 |
|
| 3.541 | 0.006 |
|
|
| Poorly
differentiated | 2.339 | 0.052 |
|
| 2.029 | 0.068 |
|
|
| Intraoperative
blood loss (≤1,000 vs. >1,000 ml) | 1.561 | 0.008 |
|
| 1.649 | 0.010 |
|
|
| Intraoperative
transfusion (Yes vs. no) | 1.453 | 0.015 |
|
| 1.640 | 0.007 |
|
|
| TACE (≤3 vs.
>3) | 1.102 | 0.653 |
|
| 0.861 | 0.578 |
|
|
| CA125 (≤15 vs.
>15 U/ml) | 1.597 | 0.002 |
|
| 2.112 | <0.001 | 1.709 | 0.005 |
|
|
|
|
|
|
|
| (1.177–2.482) |
|
OS
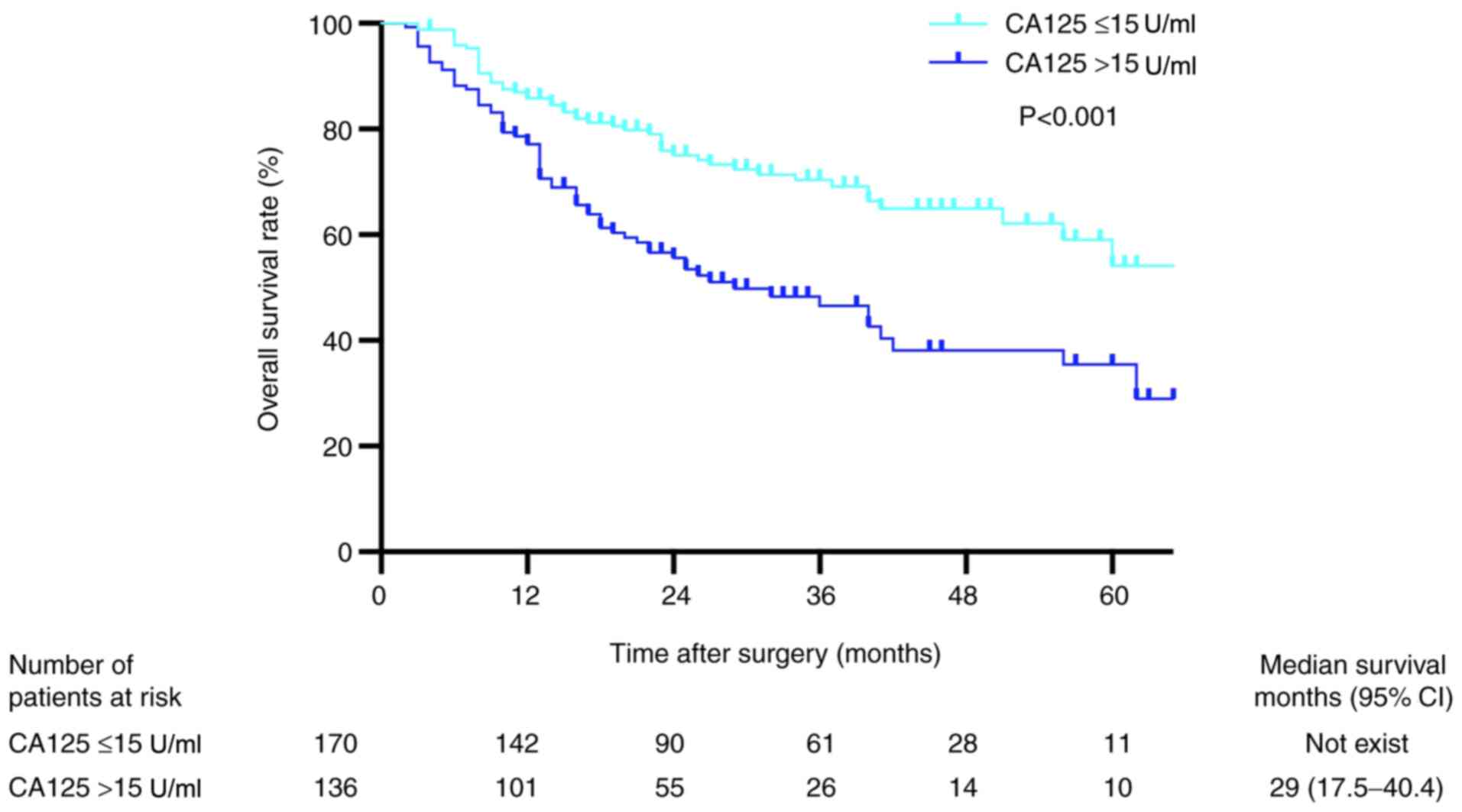
Kaplan-Meier analysis revealed that the OS rates in
patients with preoperative serum CA125 levels ≤15 U/ml were
significantly higher compared with those in patients with
preoperative serum CA125 levels >15 U/ml (P<0.001; Fig. 3). The 1-, 2- and 3-year OS rates in
the low preoperative serum CA125 group were 95.2, 86.9 and 84.4%,
respectively, whereas the corresponding rates in the high
preoperative serum CA125 group were 91.2, 83.1 and 77.1%,
respectively. Univariate analysis revealed that sex, AFP >100
ng/ml, TNM stage III, tumor diameter >5 cm, multiple tumors,
venous invasion, tumor differentiation, intraoperative blood loss
>1,000 ml, intraoperative transfusion and serum CA125 levels
were independent risk factors associated with OS (Table III). The results of the
multivariate analysis demonstrated that tumor diameter >5 cm,
multiple tumors, venous invasion and preoperative serum CA125
levels were independent risk factors associated with short OS
(Table III).
Analysis based on the stratification
of preoperative serum CA125 and AFP levels
All patients were stratified by the baseline AFP and
preoperative serum CA125 levels, and the association of CA125
levels with long-term prognosis was evaluated in each stratum.
Table II summarizes the
characteristics of these four subgroups.
Impact of preoperative serum CA125
levels on the prognosis of patients with high baseline AFP
In the 154 patients in the high preoperative serum
AFP level group (subgroups 2 and 4), Kaplan-Meier analysis
demonstrated that high preoperative serum CA125 levels were
associated with lower DFS and OS rates compared with those in the
low CA125 group (both P<0.001; Fig.
4A and B). Univariate and multivariate Cox models revealed that
high preoperative serum CA125 levels along with certain tumor
characteristics (TNM stage III, tumor diameter >5 cm, multiple
tumors and the presence of venous invasion) were risk factors for
short DFS and OS rates in the high preoperative serum AFP group
(Table IV).
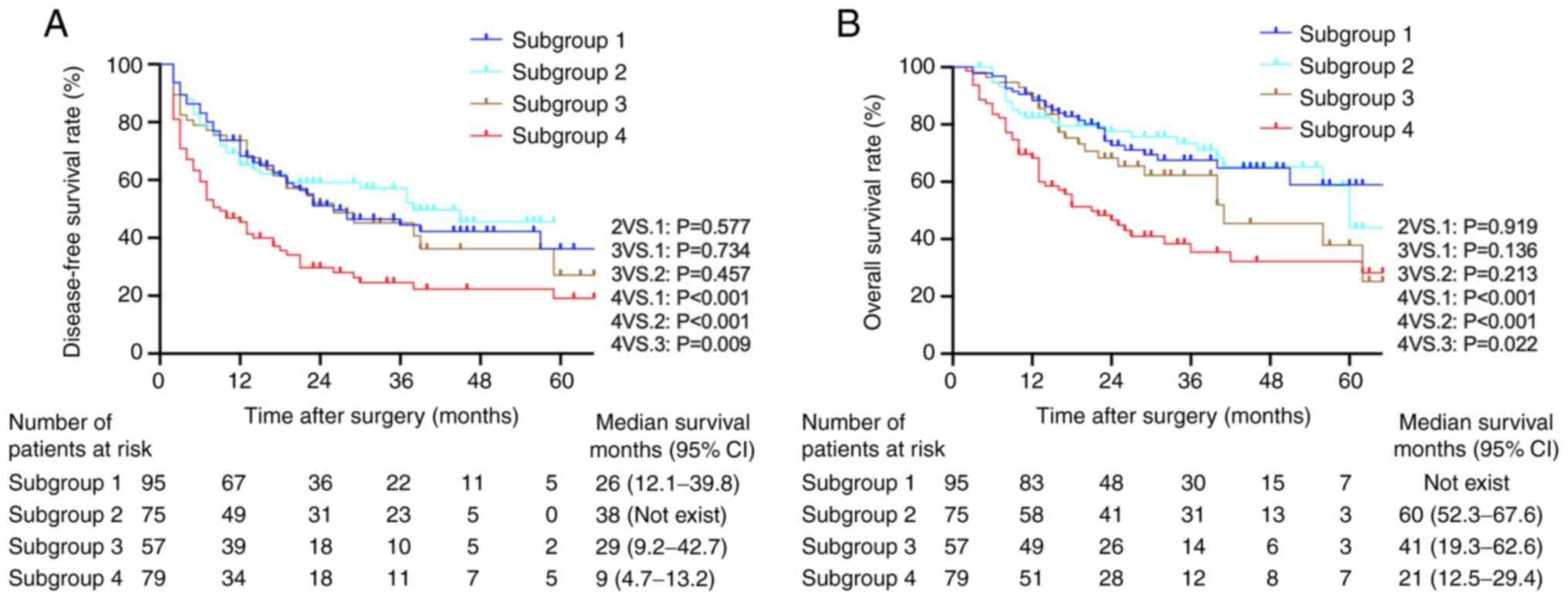 | Figure 4.Kaplan-Meier survival curves of
patients in high and low preoperative serum CA125 subgroups
following stratification by baseline AFP levels. (A) Disease-free
survival rates among the four subgroups of patients. (B) Overall
survival rates among the four subgroups of patients (log-rank test:
Subgroup 4 vs. 1, P<0.001; subgroup 4 vs. 2, P<0.001;
subgroup 4 vs. 3, P=0.022; subgroup 1 vs. 2, P=0.919; subgroup 1
vs. 3, P=0.136; subgroup 2 vs. 3, P=0.213). Subgroup 1,
preoperative serum CA125 ≤15 U/ml and preoperative serum AFP ≤100
ng/ml (n=95); subgroup 2, preoperative serum CA125 ≤15 U/ml and
preoperative serum AFP >100 ng/ml (n=75); subgroup 3,
preoperative serum CA125 >15 U/ml and preoperative serum AFP
≤100 ng/ml (n=57); subgroup 4, preoperative serum CA125 >15 U/ml
and preoperative serum AFP ≥100 ng/ml (n=79). CA125, cancer antigen
125; AFP, α-fetoprotein; CI, confidence interval. |
 | Table IV.Univariate and multivariate analyses
of DFS and OS in patients with high preoperative serum AFP >100
ng/ml (n=154). |
Table IV.
Univariate and multivariate analyses
of DFS and OS in patients with high preoperative serum AFP >100
ng/ml (n=154).
|
| DFS | OS |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Index | HR | P-value | HR (95% CI) | P-value | HR | P-value | HR (95% CI) | P-value |
|---|
| Age | 0.407 | 0.126 |
|
| 0.419 | 0.226 |
|
|
| Sex | 0.742 | 0.337 |
|
| 0.651 | 0.232 |
|
|
| Hypertension | 1.337 | 0.528 |
|
| 1.447 | 0.532 |
|
|
| Diabetes | 1.220 | 0.408 |
|
| 1.207 | 0.501 |
|
|
| ALT | 1.164 | 0.511 |
|
| 1.516 | 0.145 |
|
|
| AST | 1.051 | 0.838 |
|
| 1.351 | 0.318 |
|
|
| Platelet count
(≥100 vs. <100×109/l) | 0.472 | 0.056 |
|
| 0.621 | 0.264 |
|
|
| PT (≤14 vs. >14
sec) | 1.075 | 0.745 |
|
| 1.157 | 0.573 |
|
|
| TBIL (≤17 vs.
>17 µmol/l) | 1.137 | 0.578 |
|
| 1.148 | 0.258 |
|
|
| HBV DNA (≤2,000 vs.
>2,000 IU/ml) | 1.055 | 0.806 |
|
| 1.290 | 0.331 |
|
|
| TNM stage |
|
|
|
|
|
|
|
|
| I | (Ref.) | (Ref.) |
|
| (Ref.) | (Ref.) |
|
|
| II | 0.683 | 0.361 |
|
| 0.779 | 0.614 |
|
|
|
III | 1.961 | 0.002 |
|
| 1.386 | 0.001 |
|
|
| ASA |
|
|
|
|
|
|
|
|
| I | (Ref.) | (Ref.) |
|
| (Ref.) | (Ref.) |
|
|
| II | 1.109 | 0.637 |
|
| 0.983 | 0.946 |
|
|
|
III | 1.249 | 0.758 |
|
| 0.826 | 0.851 |
|
|
| Child-Pugh grade (A
vs. B) | 0.823 | 0.450 |
|
| 0.609 | 0.119 |
|
|
| Cirrhosis | 1.006 | 0.984 |
|
| 1.397 | 0.374 |
|
|
| Ascites | 1.391 | 0.145 |
|
| 1.042 | 0.882 |
|
|
| Tumor diameter (≤5
vs. >5 cm) | 1.779 | 0.014 |
|
| 2.105 | 0.008 |
|
|
| Tumor number
(Multiple vs. solitary) | 1.864 | 0.009 |
|
| 2.488 | 0.001 | 1.862 | 0.021 |
|
|
|
|
|
|
|
| (1.096–3.162) |
|
| Venous invasion
(Yes vs. no) | 2.101 | <0.001 | 1.943 | 0.002 | 2.104 | 0.002 | 1.822 | 0.015 |
|
|
|
| (1.282–2.943) |
|
|
| (1.121–2.960) |
|
| Tumor
differentiation |
|
|
|
|
|
|
|
|
| Well
differentiated | (Ref.) | (Ref.) |
|
| (Ref.) | (Ref.) |
|
|
|
Moderately differentiated | 2.418 | 0.217 |
|
| 1.983 | 0.341 |
|
|
| Poorly
differentiated | 3.049 | 0.173 |
|
| 1.649 | 0.584 |
|
|
| Intraoperative
blood loss (≤1,000 vs. >1,000 ml) | 1.484 | 0.071 |
| 1.427 | 0.155 |
|
|
|
| Intraoperative
transfusion (Yes vs. no) | 1.078 | 0.717 |
|
| 1.218 | 0.411 |
|
|
| TACE (≤3 vs.
>3) | 0.893 | 0.735 |
|
| 0.840 | 0.644 |
|
|
| CA125 (≤15 vs.
>15 U/ml) | 2.120 | <0.001 | 1.965 | 0.002 | 2.496 | <0.001 | 2.170 | 0.003 |
|
|
|
| (1.286–3.004) |
|
|
| (1.299–3.624) |
|
Impact of preoperative serum CA125
levels on the prognosis of patients with low baseline AFP
Kaplan-Meier analysis identified no significant
differences in the OS rates between the high and low preoperative
serum CA125 level groups in patients with low baseline AFP levels
(subgroup 1 vs. 3, P=0.136; Fig.
4B). Univariate analysis identified the following factors as
significantly associated with OS: Diabetes, tumor size, tumor
number, venous invasion, tumor differentiation and intraoperation
transfusion. The results of the multivariate analysis demonstrated
that tumor size, venous invasion and intraoperative transfusion
were independent risk factors for OS (Table V). In the DFS analysis, no
significant differences were observed in the DFS rates between the
high and low preoperative serum CA125 groups in patients with low
baseline AFP levels (subgroup 1 vs. 3, P=0.743; Fig. 4A). Both univariate and multivariate
analyses demonstrated that intraoperative transfusion and tumor
size were prognostic factors associated with DFS and OS in these
subgroups (Table V).
 | Table V.Univariate and multivariate analyses
of DFS and OS in patients with high preoperative serum AFP ≤100
ng/ml (n=152). |
Table V.
Univariate and multivariate analyses
of DFS and OS in patients with high preoperative serum AFP ≤100
ng/ml (n=152).
|
| DFS | OS |
|---|
|
|
|
|
|---|
|
| Univariate
analysis | Multivariate
analysis | Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Index | HR | P-value | HR (95% CI) | P-value | HR | P-value | HR (95% CI) | P-value |
|---|
| Age (years) | 1.020 | 0.943 |
|
| 1.284 | 0.439 |
|
|
| Sex (M; n,%) | 0.458 | 0.049 |
|
| 0.390 | 0.071 |
|
|
| Hypertension | 1.214 | 0.551 |
|
| 0.798 | 0.540 |
|
|
| Diabetes | 1.512 | 0.095 |
|
| 1.855 | 0.039 |
|
|
| ALT | 1.273 | 0.297 |
|
| 0.945 | 0.841 |
|
|
| AST | 1.005 | 0.984 |
|
| 0.725 | 0.277 |
|
|
| Platelet count
(≥100 vs. <100×109/l) | 0.491 | 0.166 |
|
| 0.698 | 0.547 |
|
|
| PT (≤14 vs. >14
sec) | 1.214 | 0.431 |
|
| 0.911 | 0.756 |
|
|
| TBIL (≤17 vs.
>17 µmol/l) | 1.126 | 0.624 |
|
| 1.049 | 0.878 |
|
|
| HBV DNA (≤2,000 vs.
>2,000 IU/ml) | 1.106 | 0.668 |
|
| 1.110 | 0.724 |
|
|
| TNM stage |
|
|
|
|
|
|
|
|
| I | (Ref.) | (Ref.) |
|
| (Ref.) | (Ref.) |
|
|
| II | 1.576 | 0.103 |
|
| 1.331 | 0.433 |
|
|
|
III | 1.280 | 0.373 |
|
| 1.700 | 0.107 |
|
|
| ASA |
|
|
|
|
|
|
|
|
| I | (Ref.) | (Ref.) |
|
| (Ref.) | (Ref.) |
|
|
| II | 1.106 | 0.676 |
|
| 0.809 | 0.729 |
|
|
|
III | 2.210 | 0.053 |
|
| 1.080 | 0.902 |
|
|
| Child-Pugh grade (A
vs. B) | 1.033 | 0.909 |
|
| 0.841 | 0.638 |
|
|
| Cirrhosis | 0.911 | 0.753 |
|
| 1.313 | 0.505 |
|
|
| Ascites | 1.336 | 0.237 |
|
| 1.402 | 0.258 |
|
|
| Tumor diameter (≤5
vs. >5 cm) | 2.305 | <0.001 | 1.945 | 0.006 | 2.527 | 0.003 | 2.043 | 0.026 |
|
|
|
| (1.206–3.137) |
|
|
| (1.091–3.825) |
|
| Tumor number
(Multiple vs. solitary) | 2.270 | 0.001 | 2.099 | 0.003 | 1.924 | 0.029 |
|
|
| Venous invasion
(Yes vs. no) | 1.673 | 0.029 | (1.298–3.396) |
| 2.261 | 0.004 | 2.016 | 0.016 |
|
|
|
|
|
|
|
| (1.142–3.559) |
|
| Tumor
differentiation |
|
|
|
|
|
|
|
|
| Well
differentiated | (Ref.) | (Ref.) |
|
| (Ref.) | (Ref.) |
|
|
|
Moderately differentiated | 2.181 | 0.029 |
|
| 4.309 | 0.015 |
|
|
| Poorly
differentiated | 1.770 | 0.344 |
|
| 3.989 | 0.093 |
|
|
| Intraoperative
blood loss (≤1,000 vs. >1,000 ml) | 1.547 | 0.101 |
|
| 1.851 | 0.056 |
|
|
| Intraoperative
transfusion (Yes vs. no) | 2.047 | 0.002 | 1.916 | 0.006 | 2.184 | 0.006 | 1.932 | 0.024 |
|
|
|
| (1.208–3.040) |
|
|
| (1.091–3.419) |
|
| TACE (≤3 vs.
>3) | 1.387 | 0.254 |
|
| 0.935 | 0.862 |
|
|
| CA125 (≤15 vs.
>15 U/ml) | 1.093 | 0.703 |
|
| 1.557 | 0.119 |
|
|
Patients with high preoperative serum
CA125 and AFP levels have a poor prognosis
Kaplan-Meier analysis demonstrated that subgroup 4
had a significantly lower DFS rate compared with that in the other
subgroups (subgroup 4 vs. 1, P<0.001; subgroup 4 vs. 2,
P<0.001; and subgroup 4 vs. 3, P=0.009). Among subgroups 1, 2
and 3, the differences were not significant (Fig. 4). In addition, patients in subgroup 4
had a significantly lower OS rate compared with that in the other
three subgroups (subgroup 4 vs. 1, P<0.001; subgroup 4 vs. 2,
P<0.001; and subgroup 4 vs. 3, P=0.022). Comparisons between
subgroups 2 and 3 and between subgroups 1 and 2 revealed no
significant differences (Fig.
4).
Discussion
The key result of the present study was the
identification of high preoperative serum CA125 levels as an
independent risk factor for the prognosis of patients with
HBV-related HCC. In addition, patients with both high preoperative
serum CA125 and AFP levels presented with a poorer prognosis
compared with that of patients with only high preoperative serum
CA125 or AFP levels. However, no significant differences were
observed in the DFS or OS rates between the high and low
preoperative serum CA125 level groups in patients with baseline
preoperative serum AFP levels ≤100 ng/ml. Thus, the present study
identified a new approach to predict the prognosis of patients with
HBV-related HCC patients using preoperative serum AFP and CA125
concentrations. This may provide a novel potential strategy to
predict the prognosis and assess the course of treatment in
patients with HBV-related HCC.
CA125 has been extensively used as a biomarker for
ovarian cancer, and its upregulation has been observed in several
types of human malignancy, such as lung, breast and pancreatic
cancer (27–29). The normal reference value of CA125 is
0.1–35 U/ml (12); however, the 35
U/ml cutoff is not absolute. In one study, the serum CA125
concentration in healthy subjects was 7.9±8.0 (mean ± SD) in women
and 8.0±9.4 U/ml in men (12).
Another study reported that the concentration was 13.2±6.8 and
9.7±3.2 U/ml in women and men, respectively (30). With the benchmark cutoff of 35 U/ml,
28% of patients with non-gynecological cancers had elevated antigen
levels; by contrast, antigen levels were elevated in >80% of
women with non-mucinous ovarian cancer (12). Lopez et al (19) demonstrated that the CA125 cutoff
values for patients with HCC were 55 U/ml in women and 12 U/ml in
men. To date, the major focus of studies on CA125 levels and HCC
was on the diagnosis, and only a limited number of studies have
focused on the prognosis of HCC (13,15,24). In
addition, the association between CA125 and prognosis in patients
with HBV-related HCC has not been widely reported. The present
study used a CA125 cutoff value of 15 U/ml to investigate the
association between preoperative serum CA125 levels and the
prognosis of patients with HBV-related HCC.
In the present study, patients with high
preoperative serum CA125 levels had significantly higher DFS and OS
rates compared with those with low serum CA125 levels in all study
populations. Zhou et al (24)
have reported that high preoperative serum CA125 levels predict a
poor prognosis and large tumor sizes following liver resection in
patients with HCC and AFP ≤200 ng/ml. However, the aforementioned
study did not identify high preoperative serum CA125 levels as an
independent risk factor for the prognosis of HCC. Although Huang
et al (13) have demonstrated
that high preoperative serum CA125 levels served as an independent
prognostic factor of OS and DFS in patients with HCC, the cutoff
values for CA125 and AFP levels in their study were 35 U/ml and 20
ng/ml, respectively. On the other hand, the 3-year overall survival
rates in the normal and high CA125 HCC groups in their study were
53.6 and 36.4%, respectively (13),
which were slightly lower compared with those observed in the
present study. The main reason for these differences may be the
inclusion of patients with non-HBV-related HCC in the previous
study. In the present study, the results of the multivariate
analysis demonstrated that tumor diameter, multiple tumors and
venous invasion were independent risk factors for DFS and OS, and a
high preoperative serum CA125 level was an independent risk factor
associated with a short OS time in patients with HBV-related
HCC.
In the stratification analyses in the present study,
a low preoperative serum CA125 level was associated with a
favorable prognosis (both OS and DFS rates) in patients with high
baseline AFP levels; the same was observed in patients with low
preoperative serum AFP and high baseline CA125 levels. However, in
patients with low baseline AFP levels, no significant differences
were observed in the DFS or OS rates between the preoperative high
and low serum CA125 level subgroups. A notable outcome of the
present study was that patients with high preoperative serum CA125
and AFP levels presented with a poorer prognosis compared with that
in the other three groups. Certain tumor characteristics of
subgroup 4 (TNM stage III, tumor diameter, multiple tumors,
presence of venous invasion and tumor differentiation) were
associated with higher malignancy compared with those in the other
groups, which may be due to the patients with both high
preoperative serum CA125 and AFP levels exhibiting a tendency for a
poor prognosis. A previous study has demonstrated that high serum
AFP levels tend to indicate highly malignant tumors with
histological features of aggressiveness such as poor
differentiation, vascular invasion, satellitosis and a fast growth
rate (31). High serum CA125 levels
predict a large tumor diameter (24), which was observed in the present
study in patients with the same baseline AFP levels. In the
stratification analyses, multivariate analysis demonstrated that
tumor diameter, multiple tumors and the presence of venous invasion
were independent risk factors for DFS and OS. Based on low
preoperative serum AFP and CA125 levels, multivariate analysis
demonstrated that intraoperative transfusion was an independent
risk factor for DFS and OS. One previous meta-analysis has
demonstrated that perioperative blood transfusion is associated
with adverse clinical outcomes in patients following resection of
HCC (32). The explanation proposed
by the authors of the aforementioned study is that allogenic blood
transfusion may induce immunosuppression and decrease natural
killer (NK) cell and/or T helper cell activities. Thus, the
prognosis of patients with HBV-related HCC may be associated not
only with the biological characteristics of the tumor, but also
with the perioperative management of patients.
However, further studies are required to determine
the underlying mechanism of the effects of CA125 in tumor
development. Previous studies have demonstrated that upregulation
of CA125 leads to tumor growth and metastasis (33–35),
which may explain the poor prognosis of patients with HBV-related
HCC. The MUC16 gene, which is also termed CA125, has been
identified to be one of the top three frequently mutated genes
(36), and its upregulation has been
associated with a poor prognosis in multiple types of malignancy,
such as pancreatic cancer, cholangiocarcinoma and bladder cancer
(21,37–39).
CA125 has been demonstrated to modulate the innate immune response
against ovarian cancer cells by directly inhibiting the function of
NK cells, thus aiding cancer cells to escape the host immune
response (40,41). In addition, MUC16 has been
implicated in cancer cell signaling; knockdown of MUC16
expression inhibits the proliferation of ovarian and breast cancer
cell lines by inducing caspase-dependent or independent apoptosis
(42). Furthermore, the
aforementioned study has also reported that MUC16 knockdown
suppresses the colony-forming, adhesive, migratory and invasive
abilities of ovarian and breast cancer cells. Thus, CA125 may
become a novel target for the treatment of HBV-related HCC.
The present study identified that patients with high
preoperative serum AFP levels were younger compared with those with
low preoperative serum AFP levels, which was consistent with the
results of a previous study (43).
In addition, in the present study, patients with the same baseline
AFP levels but with high serum CA125 levels presented with poorer
liver function compared with that in patients with low serum CA125
levels, whereas no significant differences were observed in the
preoperative liver function between the preoperative high and low
serum AFP level groups when the baseline CA125 levels were the
same. These results suggested that patients with high CA125 levels
may have a longer liver disease history. Previous studies have
reported that high levels of CA 125 were associated with the
severity of liver disease, particularly in patients with cirrhosis
(17,44,45),
which is a late stage of liver disease; however, this should be
validated in a prospective randomized controlled study by
monitoring the dynamic levels of CA125. Therefore, the results of
the current study should be interpreted carefully.
The present study had various limitations. First,
the study utilized a retrospective design, and the patient sample
size was relatively small following stratification. Second, a
subset of patients who underwent surgery in 2018 was included;
therefore, the follow-up was not sufficiently long, which
potentially affected the survival analysis outcome. Third, the
effect of abdominal inflammation before surgery could not be ruled
out, and an increase in CA125 levels may be associated with
peritoneal inflammation (17).
Fourth, the dynamic changes in CA125 levels after surgery were not
monitored, and whether high postoperative serum CA125 level is a
risk factor for poor prognosis in patients with HBV-related HCC
following resection is unknown. In addition, in the OS curves, a
small gap was observed between subgroups 1 and 3, although no
significant difference existed; thus, a longer follow-up period and
a larger number of cases is required to address this issue. The
results of the present study need to be validated further in
prospective randomized large-sample multicenter studies.
In conclusion, the present study used a 15 U/ml
cutoff value of CA125 and demonstrated that preoperative serum
CA125 levels may be a prognostic factor for survival in patients
with HBV-related HCC. However, in patients with low AFP levels, the
association between preoperative serum CA125 levels and prognosis
in HBV-related HCC remains unclear and requires further
investigation.
Acknowledgements
The authors would like to thank Mr. Guangdou Yuan
(The First Affiliated Hospital of Guangxi Medical University,
Nanning, China) for his technical support.
Funding
This study was supported by The Liuzhou Medical
Basic Research Foundation (grant no. 1ry201705) and the Self-funded
Project of Guangxi Health Commission (grant no. Z20170685).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CQ and SH conceived and designed the current study.
CQ wrote and revised the manuscript. SH performed the majority of
the experiments. YG participated in the research design, the
collection, sorting and statistical analysis of research data, and
helped to edit the manuscript. SH and CH confirmed the authenticity
of all the raw data and analyzed statistical data. CH and JL
interpreted the data and revised the manuscript. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The study complied with the standards of the
declaration of Helsinki and was approved by The Clinical Research
Ethics Committee of Liuzhou People's Hospital Affiliated to Guangxi
Medical University (approval no. 2020-KY-E-13-01). The need for
informed consent was waived owing to the retrospective nature of
the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HBV
|
hepatitis B virus
|
|
HCC
|
hepatocellular carcinoma
|
|
AST
|
aspartate aminotransferase
|
|
ALT
|
alanine aminotransferase
|
|
AFP
|
α-fetoprotein
|
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zamor PJ, deLemos AS and Russo MW: Viral
hepatitis and hepatocellular carcinoma: Etiology and management. J
Gastrointest Oncol. 8:229–242. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Nguyen VT, Law MG and Dore GJ: Hepatitis
B-related hepatocellular carcinoma: Epidemiological characteristics
and disease burden. J Viral Hepat. 16:453–463. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bruix J, Gores GJ and Mazzaferro V:
Hepatocellular carcinoma: Clinical frontiers and perspectives. Gut.
63:844–855. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Miura JT, Johnston FM, Tsai S, Eastwood D,
Banerjee A, Christians KK, Turaga KK and Gamblin TC: Surgical
resection versus ablation for hepatocellular carcinoma</=3 cm: A
population-based analysis. HPB (Oxford). 17:896–901. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rahbari NN, Mehrabi A, Mollberg NM, Müller
SA, Koch M, Büchler MW and Weitz J: Hepatocellular carcinoma:
Current management and perspectives for the future. Ann Surg.
253:453–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akamatsu N and Kokudo N: Liver
transplantation for hepatocellular carcinoma from living-donor vs.
Deceased donor. Hepatobiliary Surg Nutr. 5:422–428. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hyun MH, Lee YS, Kim JH, Lee CU, Jung YK,
Seo YS, Yim HJ, Yeon JE and Byun KS: Hepatic resection compared to
chemoembolization in intermediate- to advanced-stage hepatocellular
carcinoma: A meta-analysis of high-quality studies. Hepatology.
68:977–993. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Trevisani F, Bucci L and Garuti F: Is it
time to extend criteria for hepatic resection in the treatment of
hepatocellular carcinoma? Hepatology. 64:2257–2258. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bast RC Jr, Feeney M, Lazarus H, Nadler
LM, Colvin RB and Knapp RC: Reactivity of a monoclonal antibody
with human ovarian carcinoma. J Clin Invest. 68:1331–1337. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bast RC Jr, Klug TL, St John E, Jenison E,
Niloff JM, Lazarus H, Berkowitz RS, Leavitt T, Griffiths CT, Parker
L, et al: A radioimmunoassay using a monoclonal antibody to monitor
the course of epithelial ovarian cancer. N Engl J Med. 309:883–887.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang Y, Zeng J, Liu T, Lin X, Guo P, Zeng
J, Zhou W and Liu J: Prognostic significance of elevated
preoperative serum CA125 levels after curative hepatectomy for
hepatocellular carcinoma. Onco Targets Ther. 13:4559–4567. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen K, Gentry-Maharaj A, Burnell M,
Steentoft C, Marcos-Silva L, Mandel U, Jacobs I, Dawnay A, Menon U
and Blixt O: Microarray Glycoprofiling of CA125 improves
differential diagnosis of ovarian cancer. J Proteome Res.
12:1408–1418. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li Y, Li DJ, Chen J, Liu W, Li JW, Jiang
P, Zhao X, Guo F, Li XW and Wang SG: Application of Joint Detection
of AFP, CA19-9, CA125 and CEA in identification and diagnosis of
cholangiocarcinoma. Asian Pac J Cancer Prev. 16:3451–3455. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu LX, Li XF, Chen HF, Zhu YC, Wang WX, Xu
CW, Xie DF, Wan Y and Du KQ: Combined detection of CEA and CA125
for the diagnosis for lung cancer: A meta-analysis. Cell Mol Biol
(Noisy-le-grand). 64:67–70. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Devarbhavi H, Kaese D, Williams AW, Rakela
J, Klee GG and Kamath PS: Cancer antigen 125 in patients with
chronic liver disease. Mayo Clin Proc. 77:538–541. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bergmann JF, Bidart JM, George M,
Beaugrand M, Levy VG and Bohuon C: Elevation of CA 125 in patients
with benign and malignant ascites. Cancer. 59:213–217. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lopez JB, Balasegaram M and Thambyrajah V:
Serum CA 125 as a marker of hepatocellular carcinoma. Int J Biol
Markers. 11:178–182. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Piñero F, Dirchwolf M and Pessôa MG:
Biomarkers in hepatocellular carcinoma: Diagnosis, prognosis and
treatment response assessment. Cells. 9:13702020. View Article : Google Scholar
|
|
21
|
Liang C, Qin Y, Zhang B, Ji S, Shi S, Xu
W, Liu J, Xiang J, Liang D, Hu Q, et al: Oncogenic KRAS Targets
MUC16/CA125 in pancreatic ductal adenocarcinoma. Mol Cancer Res.
15:201–212. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pradjatmo H and Pradjatmo H: Impact of
preoperative serum levels of CA 125 on epithelial ovarian cancer
survival. Asian Pac J Cancer Prev. 17:1881–1886. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Isaksson S, Jönsson P, Monsef N,
Brunnström H, Bendahl PO, Jönsson M, Staaf J and Planck M: CA 19-9
and CA 125 as potential predictors of disease recurrence in
resectable lung adenocarcinoma. PLoS One. 12:e01862842017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou S, Wang Z, Li M and Wu L: Elevated
preoperative serum CA125 predicts larger tumor diameter in patients
with hepatocellular carcinoma and low AFP levels. Biomed Res Int.
2019:69596372019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chong CC, Lee KF, Ip PC, Wong JS, Cheung
SY, Wong J, Ho SC and Lai PB: Pre-operative predictors of
post-hepatectomy recurrence of hepatocellular carcinoma: Can we
predict earlier? Surgeon. 10:260–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Terrault NA, Bzowej NH, Chang KM, Hwang
JP, Jonas MM and Murad MH; American Association for the Study of
Liver Diseases, : AASLD guidelines for treatment of chronic
hepatitis B. Hepatology. 63:261–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Haridas D, Chakraborty S, Ponnusamy MP,
Lakshmanan I, Rachagani S, Cruz E, Kumar S, Das S, Lele SM,
Anderson JM, et al: Pathobiological implications of MUC16
expression in pancreatic cancer. PLoS One. 6:e268392011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lakshmanan I, Ponnusamy MP, Das S,
Chakraborty S, Haridas D, Mukhopadhyay P, Lele SM and Batra SK:
MUC16 induced rapid G2/M transition via interactions with JAK2 for
increased proliferation and anti-apoptosis in breast cancer cells.
Oncogene. 31:805–817. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lakshmanan I, Salfity S, Seshacharyulu P,
Rachagani S, Thomas A, Das S, Majhi PD, Nimmakayala RK, Vengoji R,
Lele SM, et al: MUC16 Regulates TSPYL5 for lung cancer cell growth
and chemoresistance by suppressing p53. Clin Cancer Res.
23:3906–3917. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Klug TL, Bast RC Jr, Niloff JM, Knapp RC
and Zurawski VR Jr: Monoclonal antibody immunoradiometric assay for
an antigenic determinant (CA 125) associated with human epithelial
ovarian carcinomas. Cancer Res. 44:1048–1053. 1984.PubMed/NCBI
|
|
31
|
Trevisani F, Garuti F and Neri A:
Alpha-fetoprotein for diagnosis, prognosis, and transplant
selection. Semin Liver Dis. 39:163–177. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu L, Wang Z, Jiang S, Shao B, Liu J,
Zhang S, Zhou Y, Zhou Y and Zhang Y: Perioperative allogenenic
blood transfusion is associated with worse clinical outcomes for
hepatocellular carcinoma: A meta-analysis. PLoS One. 8:e642612013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rao TD, Tian H, Ma X, Yan X, Thapi S,
Schultz N, Rosales N, Monette S, Wang A, Hyman DM, et al:
Expression of the carboxy-terminal portion of MUC16/CA125 induces
transformation and tumor invasion. PLoS One. 10:e01266332015.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Das S, Rachagani S, Torres-Gonzalez MP,
Lakshmanan I, Majhi PD, Smith LM, Wagner KU and Batra SK:
Carboxyl-terminal domain of MUC16 imparts tumorigenic and
metastatic functions through nuclear translocation of JAK2 to
pancreatic cancer cells. Oncotarget. 6:5772–5787. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Giannakouros P, Matte I, Rancourt C and
Piche A: Transformation of NIH3T3 mouse fibroblast cells by MUC16
mucin (CA125) is driven by its cytoplasmic tail. Int J Oncol.
46:91–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim N, Hong Y, Kwon D and Yoon S: Somatic
mutaome profile in human cancer tissues. Genomics Inform.
11:239–244. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen SH, Hung WC, Wang P, Paul C and
Konstantopoulos K: Mesothelin binding to CA125/MUC16 promotes
pancreatic cancer cell motility and invasion via MMP-7 activation.
Sci Rep. 3:18702013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Higashi M, Yamada N, Yokoyama S, Kitamoto
S, Tabata K, Koriyama C, Batra SK and Yonezawa S: Pathobiological
implications of MUC16/CA125 expression in intrahepatic
cholangiocarcinoma-mass forming type. Pathobiology. 79:101–116.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cotton S, Azevedo R, Gaiteiro C, Ferreira
D, Lima L, Peixoto A, Fernandes E, Neves M, Neves D, Amaro T, et
al: Targeted O-glycoproteomics explored increased sialylation and
identified MUC16 as a poor prognosis biomarker in advanced-stage
bladder tumours. Mol Oncol. 11:895–912. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Belisle JA, Horibata S, Jennifer GA,
Petrie S, Kapur A, André S, Gabius HJ, Rancourt C, Connor J,
Paulson JC and Patankar MS: Identification of Siglec-9 as the
receptor for MUC16 on human NK cells, B cells, and monocytes. Mol
Cancer. 9:1182010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gubbels JA, Felder M, Horibata S, Belisle
JA, Kapur A, Holden H, Petrie S, Migneault M, Rancourt C, Connor JP
and Patankar MS: MUC16 provides immune protection by inhibiting
synapse formation between NK and ovarian tumor cells. Mol Cancer.
9:112010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Reinartz S, Failer S, Schuell T and Wagner
U: CA125 (MUC16) gene silencing suppresses growth properties of
ovarian and breast cancer cells. Eur J Cancer. 48:1558–1569. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC
and Hsu HC: High alpha-fetoprotein level correlates with high
stage, early recurrence and poor prognosis of hepatocellular
carcinoma: Significance of hepatitis virus infection, age, p53 and
beta-catenin mutations. Int J Cancer. 112:44–50. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Edula RG, Muthukuru S, Moroianu S, Wang Y,
Lingiah V, Fung P and Pyrsopoulos NT: CA-125 significance in
cirrhosis and correlation with disease severity and portal
hypertension: A retrospective study. J Clin Transl Hepatol.
6:241–246. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Singhal A, Lander E, Karachristos A, Daly
E, Dowling P, Patel V, Maloo M and Jain A: Elevation of CA 125 and
CA 19-9 in patients with end-stage liver disease. Int J Biol
Markers. 27:e147–e151. 2012. View Article : Google Scholar : PubMed/NCBI
|