Introduction
The incidence of breast cancer in China and other
parts of Asia is rapidly increasing. Breast cancer is also the
second leading cause of cancer-related death in females in the
United States (1). A number of
methods are currently used to treat the breast cancer, including
chemotherapeutic agents, surgery and radiotherapy, however, these
methods do not improve the incidence of prognosis, and survival
rates. Breast cancer can be categorized according to the level of
estrogen receptor (ER) expression on the cancer cells, ER-positive
and ER-negative. The ER is expressed in ~60% of all breast cancer
cases. ER-positive breast cancer generally has a good prognosis and
patients exhibit a favorable response to antiestrogen therapy.
However ER-negative breast cancers are more aggressive and
unresponsive to antiestrogen therapy (2,3).
Therefore, an exploration of novel therapeutic agents for breast
cancer treatment and prevention is required.
Flavonoids are synthesized by plants, and are a
group of polyphenolic compounds with similar structures. The
flavonoids are categorized into subclasses, including the
anthocyanidins, flavanols, flavanones, flavanols, flavones and
isoflavones. Flavonoids carry out a number of beneficial roles in
the human body, including antioxidant, anti-inflammatory, and
anti-carcinogenic effects (4).
Silymarin that is a purified mixture of four isomeric flavonoid,
and is extracted from the seeds and fruit of the milk thistle plant
that commonly known as Silybum marianus (L.) Gaertn.
The plant contains ~65-80% silymarin flavonolignans with small
amounts of flavonoids and ~20-35% fatty acids and other
polyphenolic compounds. The major component of the silymarin
complex is silybin, which is synonymous with silibinin (5). Several clinical trials have shown that
this mixture exhibits significant hepatoprotective effects
(6). More recently, silymarin was
reported to exhibit various biological effects, including
antioxidant (7) anti-inflammatory
(8) and anti-cancer activities
(9). In addition, silymarin may
modulate the balance between pro-survival and pro-apoptotic signals
by interfering with the expression of cell cycle- and
apoptosis-regulating proteins, respectively (10).
Apoptosis and necrosis are known to intercellular
mechanisms of cell death. Apoptosis is a form of programmed cell
death, while necrosis is a form of cellular injury that induces an
inflammatory tissue response to the external leakage of
intracellular materials (11).
Apoptosis is induced by the expression of various proteins,
including the Bcl-2 family and mitogen-activated protein kinase
(MAPK) pathway proteins in response to physical or chemical
stimulation of DNA (12). The Bcl-2
family is known to inhibit the genesis and progression of cancer
via apoptosis, and is classified into the pro-apoptotic proteins
and anti-apoptotic proteins (13).
The pro-apoptotic proteins include Bax which induces apoptosis by
permeating the outer mitochondrial membrane, while the
anti-apoptotic proteins, which include Bcl-2 and Bcl-xl, inhibit
apoptosis by preserving the outer mitochondrial membrane (14). Also, the actions of caspase-9 have a
direct impact on the mitochondria as well as upstream effectors of
intrinsic apoptosis (15). The MAPKs
comprise extracellular signal-regulated protein kinase (ERK1/2),
P38 MAPK, and c-Jun N-terminal kinase/stress-activated protein
kinase (JNK/SAPK), which regulate biological activities such as
cell signaling transmission and play an important role in cell
death and proliferation (16).
These study was to determine, in MDA-MB-231 and
MCF-7 human breast cells, the inductive effects of silymarin on
apoptosis in vitro, and to explore the mechanisms of MAPK
signaling transmission. In addition, the effects of silymarin on
in vivo tumor growth were investigated.
Materials and methods
Reagents and antibodies
Silymarin was purchased from Sigma-Aldrich; Merck
KGaA (cat. no. S0292). RPMI-1640, fetal bovine serum (FBS) and
penicillin-streptomycin were purchased from Welgene, Inc.
3-(4,5-dimethylthiazol-2-yl)−2,5-diphenyltetrazolium bromide (MTT),
cell lysis buffer, 4′, 6-diamidino-2-phenylindole (DAPI) and
dimethyl sulfoxide (DMSO) were also purchased from Sigma-Aldrich;
Merck KGaA. Anti-β-actin, anti-Bax, anti-Bcl-2, anti-caspase-9,
anti-poly [ADP-ribose] polymerase (PARP), anti-phosphorylated
(p-)P38, anti-p-JNK, anti-p-ERK1/2, anti-p38, anti-JNK, anti-ERK1/2
and goat anti-rabbit IgG antibodies were purchased from Cell
signaling Technology, Inc.
Cell line and culture
The human MDA-MB-231 and MCF-7 breast cancer cell
lines were obtained from the Korean Cell Line Bank (Seoul, Korea).
And cultured in RPMI-1640 medium, supplemented with 10% FBS and 1%
penicillin-streptomycin. The cells were maintained at 37°C (5%
CO2) in a humidified atmosphere. And the culture medium
was replaced every two to three days.
Cell viability assay
Silymarin-induced changes in breast cancer cell
viability were assessed using a MTT assay. MDA-MB-231 and MCF-7
cells were seeded into 96-well plate at a density of
2×104 cells/ml (200 µl/well). Following incubation for
24 h at 37°C (5% CO2), the cells were treated with
silymarin 0, 25, 50, 75, 100, 150 and 200 µg/ml for 24 h. We
checked the concentrations that based on the previous experiment in
the reference (9,10). Silymarin was dissolved in alcohol and
diluted in the media. After treatment, the medium was discarded,
and 40 µl MTT solution (5 mg/ml) was added prior to incubation for
a further 2 h. Subsequently, 100 µl of DMSO was added to each well
to dissolve the formazan crystals and the absorbance was recorded
at 595 nm using a microplate reader (Bio-Rad, Laboratories, Inc).
Untreated control cells were used as the comparative control.
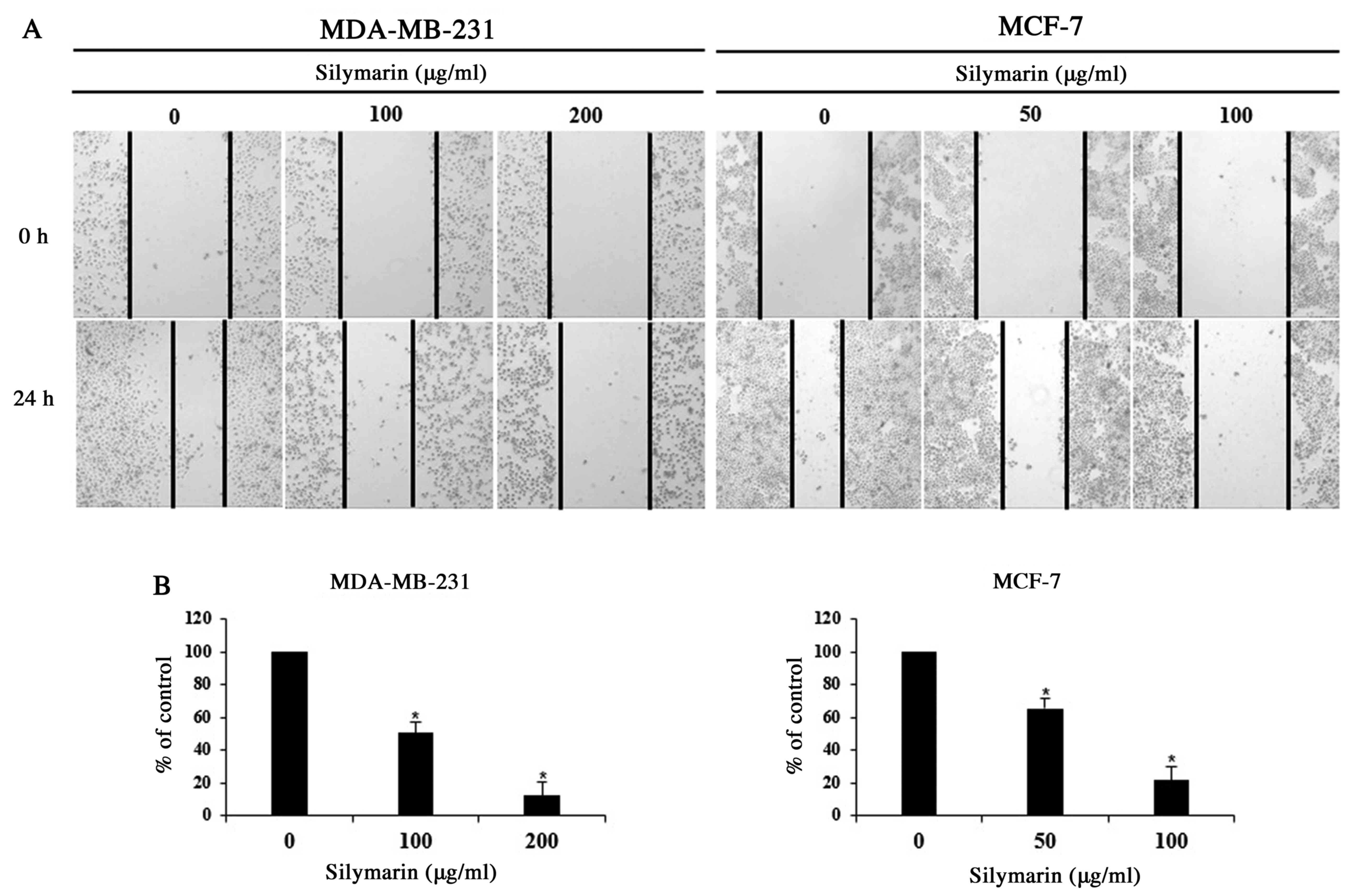
Wound healing assay
MDA-MB-231 and MCF-7 cells were seeded in growth
medium into culture diches and incubated for 24 h. After confirming
the formation of a complete monolayer, a uniform scratch was
created in each dish using a sterile 1-ml pipette tip, and the
cells were washed three times with PBS. The medium was replaced
with 5% serum medium in the presence of absence of silymarin, and
the cells were incubated for 24 h. The wound closure rate was
assessed at 0 and 24 h using images captured with a phase-contrast
microscope (magnification, ×200).
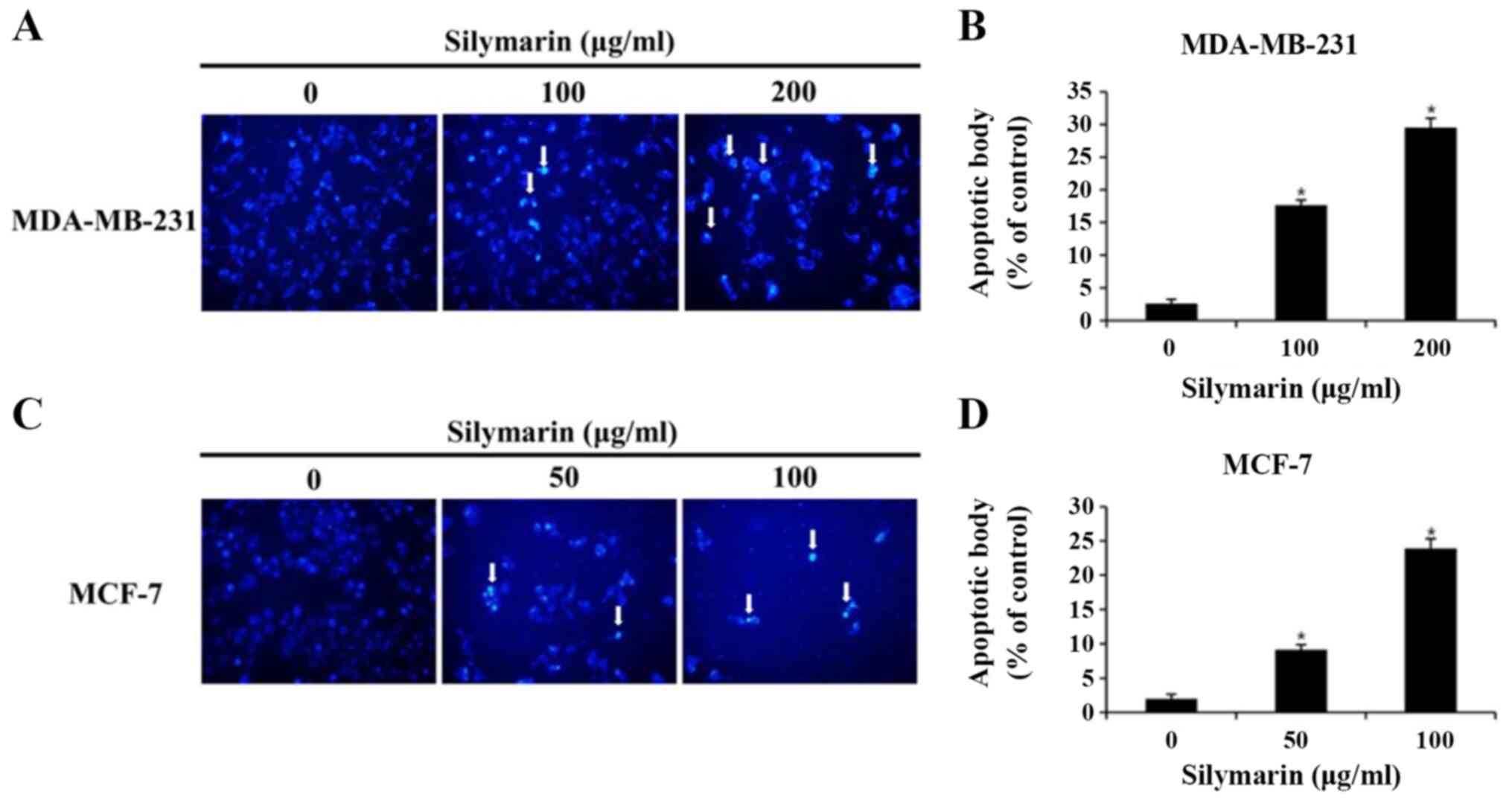
DAPI staining
DAPI staining was performed to detect the chromatin
condensation and nuclear fragmentation, known characteristics of
the apoptotic cells. MDA-MB-321 and MCF-7 cells were seeded into
60-mm culture dishes and allowed to attach for 24 h. The cells were
then treated PBS or various concentrations of silymarin for 24 h.
After discarding the media, the cells were washed with PBS and
incubated with 4% formalin and methanol for 15 min each at room
temperature. DAPI solution was added and the cells were incubated
at room temperature for 10 min under light protection. Chromatin
condensation was observed under a fluorescence microscope
(magnification, ×200) and apoptotic bodies were counted three times
in 100 randomly selected cells.
Western blot analysis
MDA-MB-231 and MCF-7 cells were treated with various
concentrations of silymarin for 24 h, and then the cells were
harvested and lysed for 30 min on ice. A Bradford protein assay
(Bio-Rad Laboratories, Inc.) was used to determine the
concentration of each protein. The proteins were separated using
6–14% SDS PAGE gels, and subsequently transferred onto
nitrocellulose membranes. The membranes were blocked with blocking
buffer 5% non-fat dry milk in Tris-buffered saline with Tween-20
(TBS-T) for 1 h at room temperature, and then were further
incubated with primary antibodies (diluted in blocking solution)
overnight at 4°C. The following primary antibodies were purchased
from Cell Signaling Technology, Inc.: Anti-β-actin (1:1,000; cat.
no. 4967), anti-Bax (1:1,000; cat. no. 2772), anti-Bcl-2 (1:1,000;
cat. no. 2876), anti-caspase-9 (1:1,000; cat. no. 9502), anti-PARP
(1:1,000; cat. no. 9542), anti-p-P38 (1:1,000; cat. no. 4631),
anti-p38 (1:1,000; cat. no. 9212), anti-p-JNK (1:1,000; cat. no.
4668), anti-JNK (1:1,000; cat. no. 9252), anti-p-ERK1/2 (1:1,000;
cat. no. 9101), anti-ERK1/2 (1:1000; cat. no. 9102; Cell Signaling
Technology, Inc.). After washing with TBS-T, the membranes were
incubated with horseradish peroxidase (HRP)-conjugated, goat
anti-rabbit IgG secondary antibodies (1:1,000; cat. no. 7074; Cell
Signaling Technology, Inc.) for 1 h at room temperature. After
further washing with TBS-T, the protein bands were visualized using
enhanced chemiluminescence (ECL) detection reagents (Pierce; Thermo
Fisher Scientific, Inc.) according to the manufacturer's
instructions. For each band, the concentration was quantified using
the ImageJ Launcher (version 1.48; NCBI) imaging program.
Animals and in vivo xenograft tumor
model
Male BALB/c nude mice (5-week-old, male, 20 g) were
purchased from Orient Bio Inc. (Gyeonggi-do, Korea). The mice were
maintained under a 12 h light/dark cycle and housed at a controlled
temperature (23±3°C) and humidity (40±10%) conditions. The mice
were maintained in isolated and ventilated cages (≤3 mice per cage)
and allowed access to pelleted laboratory food and water ad
libitum. MCF-7 cells were subcutaneously injected into the
right flanks of the donor nude mice. After seven days established,
MCF-7-cell tumors were detectable; when the tumors were palpable,
the mice were randomly divided into 3 groups (n=5 per group).
Silymarin was dissolved in alcohol and diluted with distilled water
(DW) and then orally administered 5 times per week at a dose of 25
or 50 mg/kg body weight, while the control group mice were
administered the vehicle (distilled water) and the same amount of
alcohol that used to dissolve the silymarin. Tumor weight and size
were measured twice a week for a total of 21 days. We confirmed the
previous experiments using silymarin to set the concentration of
silymarin that administered to mice. The duration of experiment in
21 day was established by referring in the previous silymarin
experiment of the reference. Tumor size should not exceed 1.5 cm
per IACUC guideline, so we completed the experiment in 21 day to
reduce the pain in according to the animal ethics (9). Tumor volume was measured as follows:
volume (mm3) = [0.5×(a + b)]3, where a was
the long axis, and b the short axis of the tumor. At the end of the
experimental period, the mice were sacrificed by cervical
dislocation and the tumors were excised and weighted. A portion of
the tumor was embedded in paraffin and used for TUNEL assays and
immunohistochemical analyses. The animal experiments were conducted
in accordance with the regulations of the Institutional Animal Care
and Use Committee with the approval of the Ethics Committee of
Kongju National University (Chungcheongnam-do, Korea; approval no.
KNU_2018-6).
TUNEL assay
Tumor tissues were analyzed using TUNEL in
situ apoptosis detection kit (Promega Corporation) according to
the manufacturer's protocol. Briefly, paraffin-embedded sections (5
µm) were deparaffinized and rehydrated, after which the sections
were incubated with proteinase K for 15 min at room temperature.
Equilibration buffer, biotinylated nucleotide mixture and
recombinant terminal deoxynucleotidyl transferase (rTdT) were
mixed, added to each slide, and allowed to react at 37°C for 1 h.
Then, 0.3% hydrogen peroxide in PBS was added and allowed to react
for 5 min. Streptavidin-HRP was then added to each slide, followed
by 3,3-diaminobenzidine tetrahydrochloride (DAB) solution, which
was allowed to react for 10 min. The slides were rinsed several
times in deionized water and mounted. Brown-colored apoptotic
bodies in the tumor sections from the control and silymarin-treated
mice were counted using a light microscope (magnification,
×400).
Immunohistochemistry
The paraffin-embedded sections were deparaffinized
and rehydrated by sequential immersion in xylene and alcohol
solutions. The sections were then incubated with anti-p-ERK1/2
(1:100) antibodies at 4°C overnight and subsequently incubated with
a peroxidase-conjugated goat anti-rabbit antibody for 1 h at room
temperature. The tumor sections were visualized using DAB solution.
After mounting, the sections was observed using a light microscope
(magnification, ×200).
Histological examination
The livers and kidneys of MCF-7-tumor xenograft mice
were fixed in 10% neutral formalin buffer at room temperature and
embedded in paraffin. The paraffin-embedded tissue were cut into
5-µm sections and stained with hematoxylin and eosin.
Histopathological changes were assessed using a light microscope
(magnification, ×200)
Statistical analysis
The experimental results are presented as the means
± standard deviation (SD) or standard error (SE). Statistical
analyses were conducted one-way ANOVA (SPSS Statistics 17.0; IBM
Corp.) followed by Dunnett's t-tests. When determining the
differences between the mean group values, P<0.05 was considered
to indicate a statistically significant difference.
Results
Effects of silymarin on the cell
viability of breast cancer cells
A MTT assay was performed to evaluate the effects of
silymarin on MDA-MB-231 and MCF-7 breast cancer cells. Cells were
treated with 0, 25, 50, 75, 100, 150 or 200 µg/ml silymarin for 24
h. The percentage of cell viability MDA-MB-231 cells were 114.7% at
25 µg/ml, 106.7% at 50 µg/ml, 92.2% at 75 µg/ml, 75.1% at 100
µg/ml, 63.1% at 150 µg/ml and 51.5% at 200 µg/ml silymarin
(Fig. 1A). The percentages of cell
viability MCF-7 cells were 108.0% at 25 µg/ml, 90.2% at 50 µg/ml,
69.9% at 75 µg/ml, 49.5% at 100 µg/ml, 30.5% at 150 µg/ml and 26.1%
at 200 µg/ml silymarin (Fig. 1B).
These results suggest that silymarin has significantly effects the
viability of MDA-MB-231 and MCF-7 human breast cancer cells. Also,
MCF-7 cells were more sensitive to silymarin than MDA-MB-231
cells.
Effects of silymarin on the
proliferation of breast cancer cells
Cancer cells can generate new tumors by infiltrating
into distant tissues via the lymphatic ducts and blood, or by
proliferating into the surrounding tissues (17). As proliferation is one of the primary
characteristics of cancer cells, MDA-MB-231 and MCF-7 cells were
treated with concentrations of silymarin that significantly
impacted the cell viability (as determined by MTT assay), and
proliferation was assessed using a wound-healing assay. We checked
the survival rate through the screening that used the MTT assay.
Based on these results, we set the concentrations at MDA-MB-231 and
MCF-7. Following treatment with silymarin (MDA-MB-231 cells, 100
and 200 µg/ml and MCF-7 cells 50 and 100 µg/ml), the proliferation
of both breast cancer cells was inhibited in a
concentration-dependent manner (Fig.
2A). In addition, the cells percentages at the 24 h time point
were 100% (untreated), 50.6% (100 µg/ml)), and 12.1% (200 µg/ml)
for MDA-MB-231 and 100% (untreated), 65.3% (50 µg/ml), and 21.6%
(100 µg/ml) for MCF-7 cells, compared with those of the control
group, indicating concentration-dependent inhibition of cellular
proliferation (Fig. 2B).
Accordingly, these findings indicate that silymarin exerts
inhibitory effects on the proliferation of MDA-MB-231 and MCF-7
breast cancer cells.
Silymarin-induced morphological
changes in breast cancer cells
To investigated whether the silymarin-induced
effects on breast cancer cells viability resulted from the
induction of apoptosis, chromatin condensation and morphological
changes to the nucleus were observed using DAPI staining, which
specifically interacts with DNA (11). DAPI-positive cells were considerably
more likely to be found in silymarin-treated MDA-MB-231 and MCF-7
breast cancer cells than in the untreated control cells (Fig. 3A and B). To assess the degree of
apoptosis, images of 100 cells from five random fields were
captured using a fluorescence microscope (magnification, ×200). The
percentage increases in apoptotic cells were 2.6, 17.6 and 29.5% in
MDA-MB-231 cells, and 2.0, 9.1 and 23.9% in MCF-7 cells at the
respective silymarin concentrations (Fig. 3C and D). These results suggest that
the silymarin-induced decrease in MDA-MB-231 and MCF-7 cell
viability was closely associated with apoptosis.
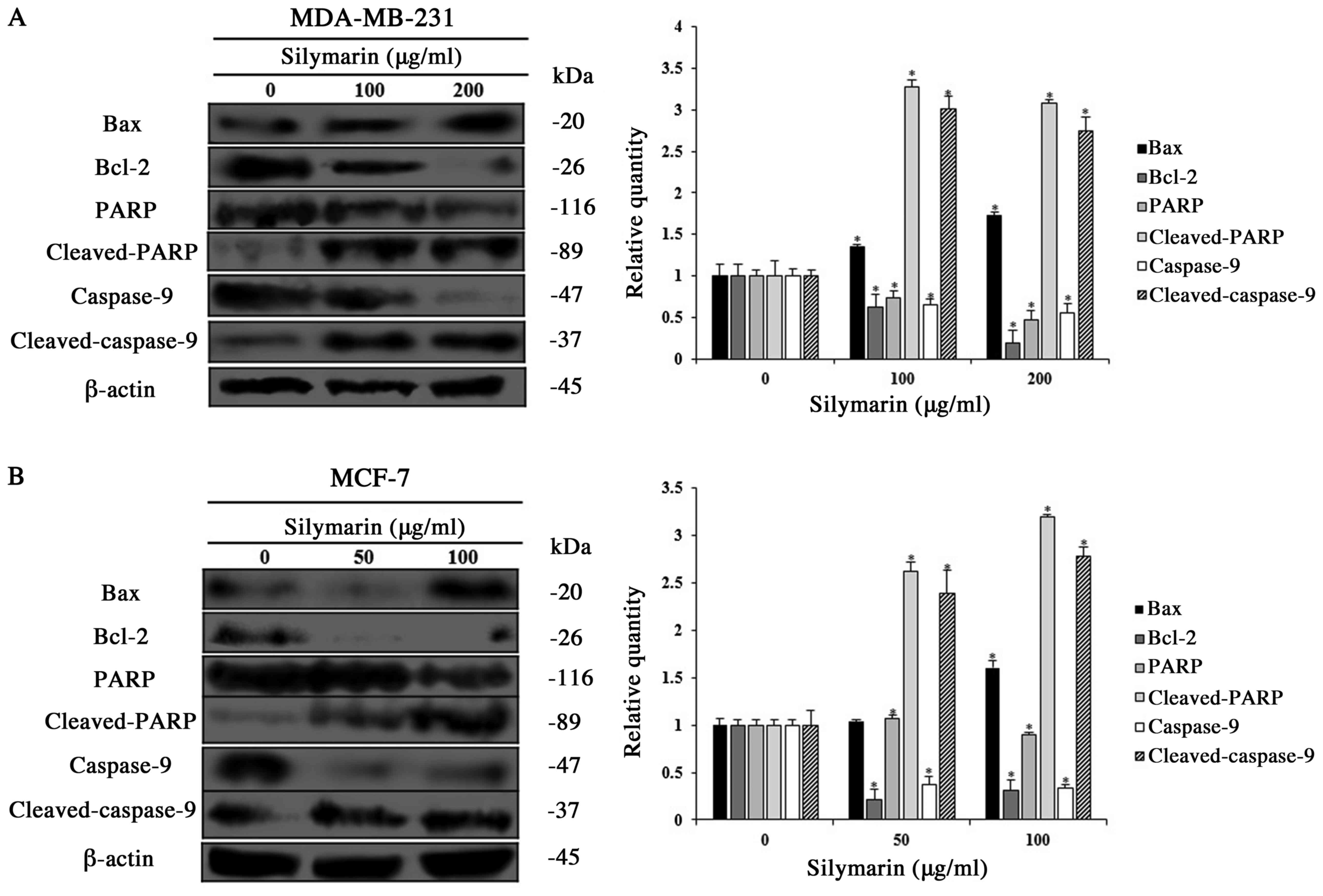
Effects of silymarin on
apoptosis-related proteins in breast cancer cells
Apoptosis occurs via various protein interactions.
Among the proteins involved, the Bcl-2 family regulates the balance
between pro-apoptotic and anti-apoptotic proteins (12). PARP, which resides in the nucleus is
decomposed by activation of the caspase cascade, which induces
apoptosis (13). Therefore, western
blotting was conducted to investigate the changes in the
apoptosis-related protein expression in silymarin-treated
MDA-MB-231 and MCF-7 cells treated with silymarin. The expression
levels of Bax, cleaved PARP, and cleaved caspase-9 (pro-apoptotic
proteins) were increased in MDA-MB-231 and MCF-7 cells treated with
silymarin, while the expression of Bcl-2 (anti-apoptotic protein)
expression was decreased (Fig. 4A and
B). These results exhibited that the inhibitory effects of
silymarin on MDA-MB-231 and MCF-7 breast cancer cells viability
were induced by modulating the expression of apoptosis-related
proteins.
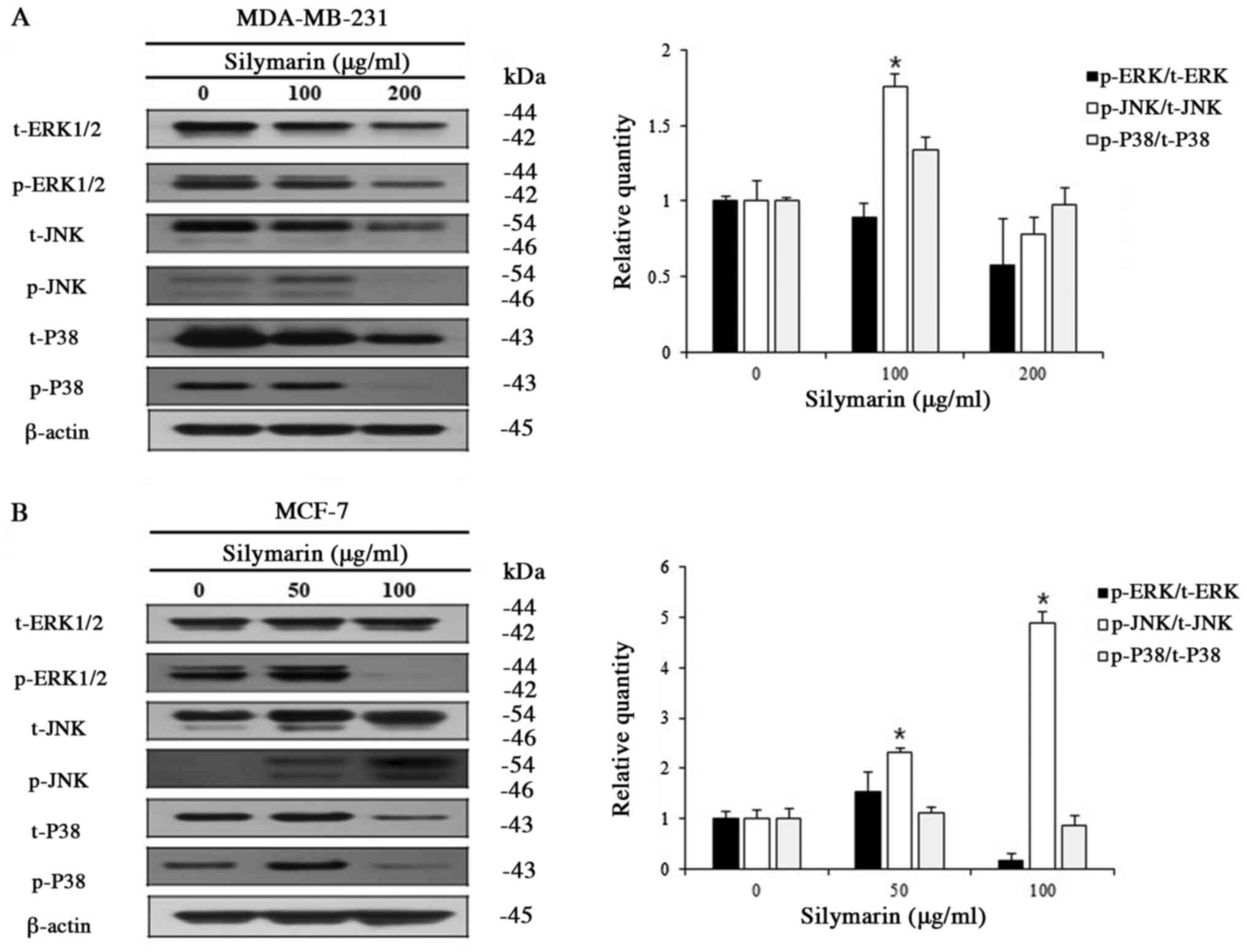
Effects of silymarin on MAPK signaling
pathways in breast cancer cells
Various kinases of the MAPK signaling pathway, which
is known as an intracellular signaling transport system, intervene
in intercellular and intracellular reactions, in response to
changes in the cellular environment (16). Generally, ERK1/2 promotes cell
proliferation, while JNK and P38 have the opposite effect.
MDA-MB-231 and MCF-7 cells were treated with different
concentrations of silymarin to examine its effects on proteins of
the MAPK signaling pathway. Silymarin treatment (at different
concentrations for 24 h) resulted in the increased expression of
p-JNK, while p-ERK1/2 and p-P38 were decreased in both MDA-MB-231
and MCF-7 cells (Fig. 5A and B).
These results confirm that silymarin-induced apoptosis in
MDA-MB-231 and MCF-7 breast cancer cells was related with the
modulation of the MAPK signaling pathway.
Effects of silymarin on breast cancer
tumor growth in an in vivo animal model
Based on the results of in vitro
experimentation, we performed a xenograft with MCF-7 breast cancer
cells. MCF-7 breast cancer cells were implanted into the hypodermis
of five-week-old male BALB/c nude mice., and the effects of
silymarin on MCF-7 tumor growth were assessed. Silymarin was
diluted in PBS and was orally administered at 25 or 50 mg/kg five
times per week for 3 weeks; the tumor size was measured twice per
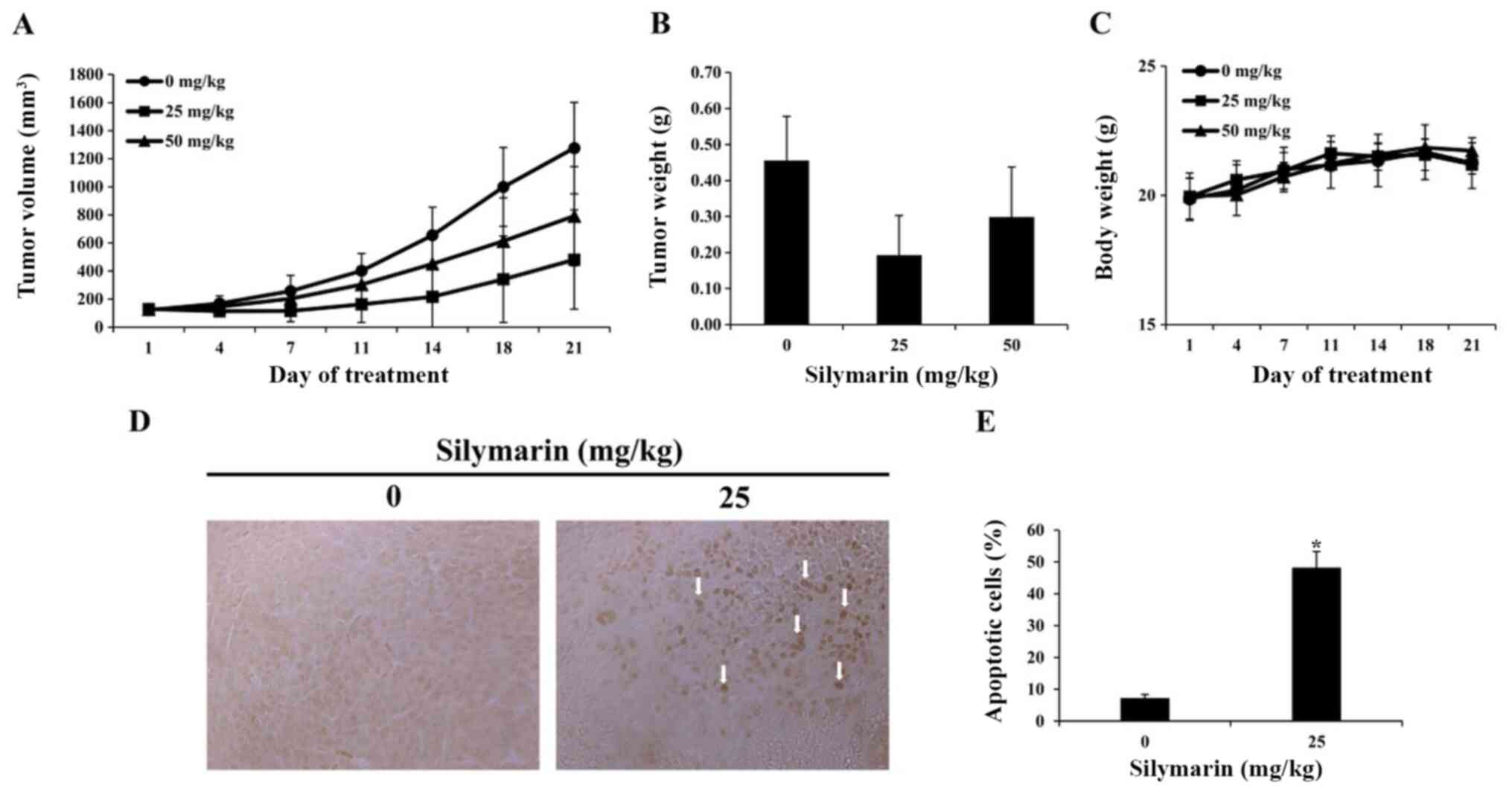
week. Compared with the control group, at 21 days post drug
administration, the tumor in administered with silymarin were
smaller than those of the control group (Fig. 6A). Compared with the control group in
21 days, tumors in the 25 and 50 mg/kg silymarin-treated groups
were 62.3 and 38.0% smaller (Table
I). The tumor weight was 0.46±0.12 g in the control group,
0.19±0.11 g in the 25 mg/kg silymarin-treated group and 0.30±0.13 g
in the 50 mg/kg silymarin-treated group (Fig. 6B). These findings suggest that
silymarin has an inhibitory effect on MCF-7 tumor growth, while the
body weights of the control and silymarin-administered groups did
not alter significantly across the 21 day period (Fig. 6C).
 | Table I.Tumor inhibition rate of xenograft
mice with MCF-7 tumors treated with silymarin. |
Table I.
Tumor inhibition rate of xenograft
mice with MCF-7 tumors treated with silymarin.
| Silymarin,
mg/kg | Pre-experiment
size, mm3 | Post-experiment
size, mm3 | Inhibition
rateb, % |
|---|
| 0a | 122.0 | 1275.4 |
|
| 25 | 129.5 | 481.3 | 62.3 |
| 50 | 126.5 | 790.5 | 38.0 |
Effects of silymarin on the apoptosis
in breast cancer tumor tissues
A TUNEL assay was performed to determine whether the
inhibitory effects of silymarin on MCF-7 tumors growth, were caused
by apoptosis. As a result, the number of TUNEL-positive cells was
increased in the group administered with 25 mg/kg silymarin
compared with that of the control group (Fig. 6D). Compared with the control group,
the group administered with 25 mg/kg silymarin witnessed a 48%
increase in TUNEL-positive cells (Fig.
6E). These findings indicate that following silymarin
treatment, DNA segmentation in MCF-7 tumors of nude mice resulted
from apoptosis, and that silymarin induced apoptosis in the MCF-7
tumors.
Effects of silymarin on MAPK signaling
pathways in breast cancer tumor tissues
In response to cellular environmental changes, the
MAPK signaling pathway, which is known as an intracellular
signaling transport system, intervene in intercellular and
intracellular reactions. ERK1/2 is an important modulator of
various biological activities that involve cell survival and
proliferation (16). In the present
study, silymarin was administered to nude mice with xenograft
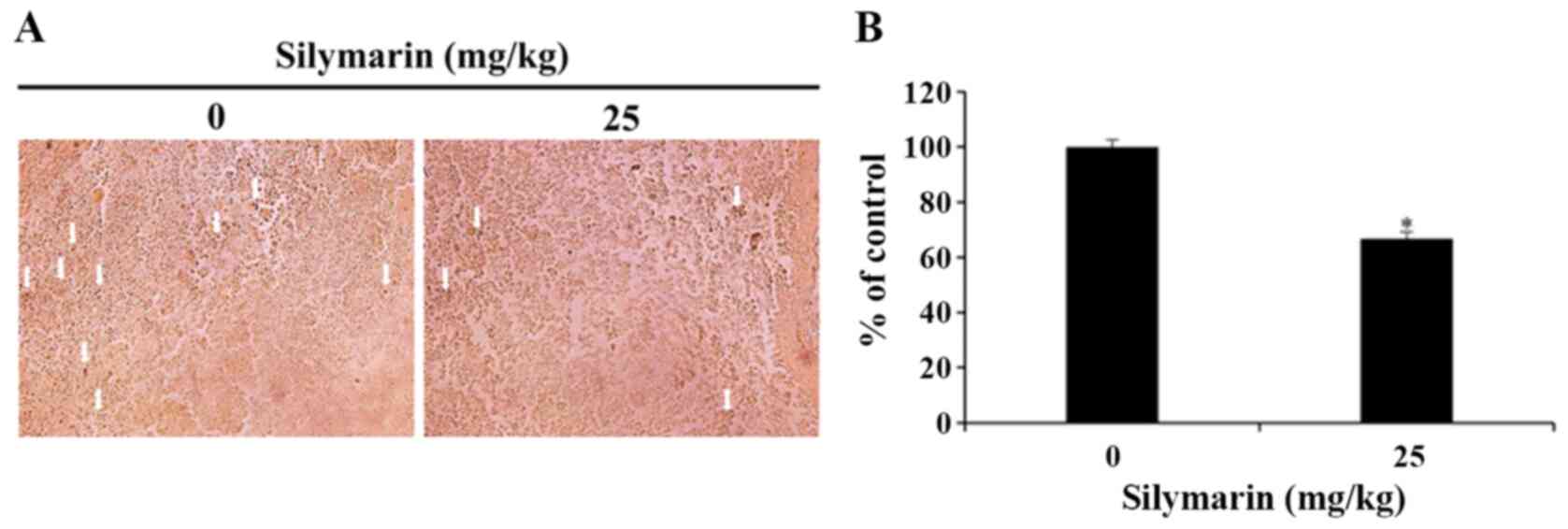
tumors, and then immunohistochemistry was used to confirm the
expression of p-ERK1/2 in the MCF-7 tumor tissues. As a result, the
25 mg/kg silymarin-treated group revealed the decrease in
expression of p-ERK1/2 in comparison with the control group
(Fig. 7A). Also, the quantitative
expression of p-ERK1/2 decreased from 100% in the control group to
66.75% in the silymarin-treated group (Fig. 7B).
Silymarin-induced histopathological
changes in liver and kidney tissues
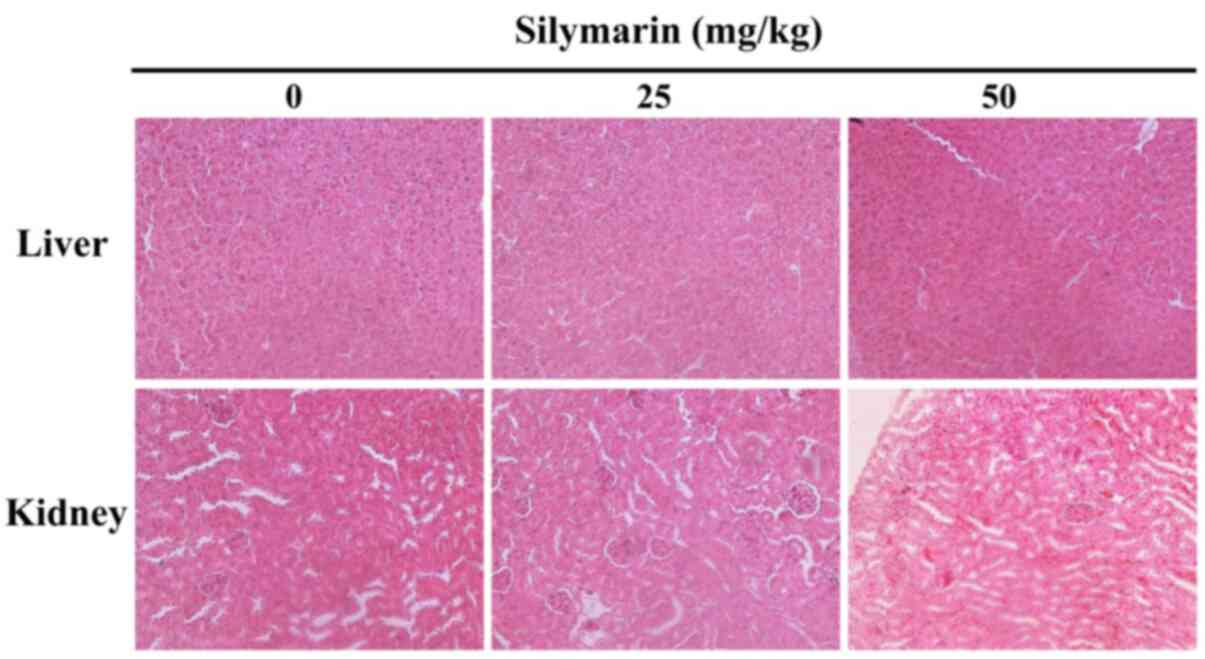
To investigate the level of silymarin-associated
organ toxicity, histological changes in the liver and kidney
tissues of MCF-7 tumor xenografted mice were confirmed by
hematoxylin and eosin staining (Fig.
8). The results confirmed that histopathological abnormalities
were not exhibited, therefore demonstrating that the apoptotic
effect of silymarin on human breast cancer cells has no
histological side-effects in a mice xenograft model.
Discussion
Owing to developments in modern medicine, the
average life expectancy has increased. However, due to changes in
diet, the rate of cancer occurrence and death rates of cancer have
also increased. Subsequently, interest in substances for cancer
prophylaxis and treatment is rising (18). Breast cancer is the most common type
of cancer in women worldwide, with continuously increasing rates of
occurrence and death. Following early detection, breast cancer
treatment can increase patient the survival rates of patients, but
is accompanied by many side-effects (19). Many studies are focused on the
anticancer and immunoregulatory effects of natural materials
without such side-effects. Among these compounds, silymarin has
been used in various developed counties, including European nations
to protect the livers of patients with chronic epilepsy. Silymarin
is a natural substance of the Composittae family obtained from the
fruit and seeds of thistles (Silybum marianus L.). Recent
studies have reported that silymarin is used as a healthy
functional food in recognition of the hepatoprotective effects and
has been reported the various effects such as inflammation (750
mg/kg/day), antioxidants (150 mg/kg−1) and anti-cancer
(25, 50 mg/kg) (6–10). In present study, the apoptosis
induction and pharmacological mechanisms of silymarin were
investigated using MDA-MB-231 and MCF-7 breast cancer cells, and
the inhibitory effects on tumor growth were verified the in
vivo.
To investigate the effects of silymarin on
MDA-MB-231 and MCF-7 breast cancer cells, the MTT assay was used to
assess the cell viability. After treatment with silymarin for 24 h,
a significant concentration-dependent decrease in the cell
viability rate, was observed, which was evident from 75 µg/ml in
MDA-MB-231 cells and 50 µg/ml in MCF-7 cells. The wound healing
assay showed that the proliferation of the MDA-MB-231 and MCF-7
breast cancer cells was also inhibited depending on the
concentration. Fan et al (20) reported a concentration-dependent
decrease in ovarian cancer cells treated with different silymarin
concentrations, where the effect was observed from 50 µg/ml. Deep
et al (10) observed a
concentration- and time-dependent decrease in the cell viability
rate of PC-3 prostate cancer cells when treated with 50 and 100
µg/ml silymarin. Additionally, Kalla et al (21) demonstrated that the viability and
proliferation of breast cancer cells were inhibited by different
concentrations of silymarin. Vaid et al (22) also observed that the viability rates
of melanoma cells were inhibited in a concentration-dependent
manner when treated with different concentrations of silymarin.
These findings support that silymarin inhibits the viability rate
and the proliferation of MDA-MB-231 and MCF-7 breast cancer cells.
MCF-7 cells were also found to be more sensitive to silymarin than
MDA-MB-231. However, the underlying reason for those differences in
sensitivity, requires further study.
When apoptosis is induced, the apoptotic bodies
appear within the cell and nuclear condensation and DNA
fragmentation occur (11). In the
present study, DAPI staining was performed to confirm whether the
effects of silymarin on breast cancer cells were caused by
apoptosis. When breast cancer cells were treated with silymarin for
24 h, MDA-MB-231 cells at 0, 100 or 200 µg/ml and MCF-7 cells at 0,
50 or 100 µg/ml, chromosomal condensation and an increase in
apoptotic bodies (hallmark characteristics of apoptosis) were
observed in both cell lines. In addition, to quantify the degree of
apoptotic induction, DAPI-positive cells were also quantified. At
the corresponding concentrations, 2.6, 17.6 and 29.5% of
MDA-MB-231, and 2.0, 9.1 and 23.9% of MCF-7 cells exhibited DAPI
staining, demonstrating a concentration-dependent increase in
DAPI-positive cells, compared with the control group. In support of
these findings, Fan et al (20) reported apoptotic bodies in ovarian
cancer cells treated with different concentrations of silymarin.
Furthermore, Katiyar et al (23) revealed that apoptotic bodies in the
skin epidermal cells were increased in a concentration-dependent
manner following silymarin treatment. To summarize, the decrease in
the viability of silymarin-treated MDA-MB-231 and MCF-7 breast
cancer cells was closely related with the induction of
apoptosis.
Various bioactivities are required to induce
apoptosis. Among these, the Bcl-2 family exists in the equilibrium
with pro-apoptotic proteins and anti-apoptotic proteins. When this
equilibrium is disrupted, apoptosis is induced (12). Therefore, western blotting was
performed to observe silymarin-induced changes in the expression
levels of apoptosis-related proteins in MDA-MB-231 and MCF-7 breast
cancer cells. As a result, expression of the pro-apoptotic protein
Bax was increased in MDA-MB-231 and MCF-7 cells, while
anti-apoptotic Bcl-2 expression was decreased. The expression
levels of cleaved caspase-9 and cleaved PARP also were increased in
both breast cancer cells. Katiyar et al (23) reported that in the skin epidermal
cells, the expression levels of Bax and cleaved PARP were
increased, while Bcl-2 expression was decreased following treatment
with different concentrations of silymarin. Fan et al
(20) also found that the expression
levels of Bax and cleaved caspase-9 in ovarian cancer cells were
increased in a concentration-dependent manner when treated with
silymarin, while Bcl-2 expression was decreased in the same manner.
These findings demonstrate that silymarin regulates the expression
levels of apoptosis proteins in MDA-MB-231 and MCF-7 breast cancer
cells and that silymarin-associated inhibitory effects on breast
cancer cell viability and proliferation were induced by
apoptosis.
The MAPK signaling pathways are known to regulators
of cell proliferation, apoptosis, proliferation, and
differentiation. Several types of MAPK signaling pathways including
those of ERK1/2, P38 MAPK, JNK/SAPK (16). In the current study, the expression
levels of ERK1/2, P38, and JNK were investigated in
silymarin-treated MDA-MB-231 and MCF-7 breast cancer cells. When
treated with different concentration of silymarin, p-ERK1/2 and
p-P38 expression levels were decreased, while p-JNK expression
levels were increased in both cell lines. Li et al (24) reported a concentration-dependent
increase in the p-P38 and p-ERK1/2 expressions in A375-S2 melanoma
cells treated with different silymarin concentrations. Furthermore,
Huang et al (25) observed
that p-P38 and p-JNK expression levels were regulated in a
concentration-dependent manner when HeLa uterine cervical cancer
cells were treated with different concentration of silymarin. Also,
Pereira et al (26) observed
that P38 MAPK inhibition results in ROS upregulation, which in turn
activates the JNK pathway via the inactivation of phosphatases,
sensitizing human tumor cells to induced apoptosis. Recent, the
natural compound in plants such as flavonoids was reported that the
effect of anti-cancer in breast cancer, including MDA-MB-231 and
MCF-7. Also, these effect of anti-cancer in flavonoids induced the
apoptosis through the various caspase, including caspase-3 and
caspase-9 that was closely related with activation of MAPK
signaling pathway such as, ERK and JNK in MDA-MB-231 (27–29) and
MCF-7 (30,31). These findings suggest that silymarin
induces apoptosis by the modulating of the MAPK signaling pathways
in MDA-MB-231 and MCF-7 breast cancer cells. Based on these
results, silymarin was used to induce apoptosis in these cells, in
order to inhibit cell growth and proliferation in vitro.
MCF-7 cells were also found to be more sensitive to silymarin
treatment than for MDA-MB-231 cells. But, to determine the relation
of between caspases and MAPK signaling pathway that was main
mechanism as the activation of MAPK signaling pathway including ERK
JNK and P38, we have to study more. Also, we should study more to
confirm the phospho-JNK mediated pathways in MDA-MB-231 and MCF-7
cells are possibly distinct and related to caspase-9 cascade in
both cases.
As a result of these findings, the MCF-7 cells were
transplanted into nude mice, and silymarin was administered to
observe the effects on MCF-7 tumors growth. Following oral
administration of silymarin at 0, 25 or 50 mg/kg for 3 weeks, the
tumor size began to decrease in comparison to in the control group
from 7 days post-administration. By the 21 day, tumor growth was
inhibited by 62.3% in the 25 mg/kg silymarin-treated group and
38.0% in the 50 mg/kg group. The tumor weights were 0.46 g in the
control group, 0.19 g in the 25 mg/kg silymarin-administered group
and 0.30 g in the 50 mg/kg silymarin-administered group, revealing
a downward trend in tumor size in the silymarin-administered
groups. Wu et al (9) found
that tumor expansion was inhibited in a concentration-dependent
manner in lung cancer tissues following the intraperitoneal
injection of 25 mg/kg or 50 mg/kg of silymarin. Also, Singh et
al (32) is reported a decrease
in A431 epidermal cancer cell tumor growth in a group injected with
silymarin for 5 weeks, compared with that in the control group.
These findings support that silymarin inhibits the growth of MCF-7
tumors.
The TUNEL assay was performed to confirm whether the
inhibition of tumor growth was caused by apoptosis induction. As a
result, TUNEL-positive apoptotic cells were increased by 48.0% in
the groups administered with 25 mg/kg of silymarin, respectively.
Singh et al (32) reported
that cells positive for apoptosis bodies were increased in a group
of epidermal cancer cell xenografted mice with epidermal cancer
cells treated with silymarin compared with those in the control
group. These results demonstrate that DNA fragmentation in the
nucleus was due to apoptosis, and that silymarin inhibits the MCF-7
tumor growth via apoptotic induction.
ERK is as an important regulator of apoptosis, with
known roles in cell proliferation and differentiation (16). After the administration of silymarin
to xenograft mice, immunohistochemistry was performed to observe
ERK1/2 activities in MCF-7 tumor tissues. As a result, the level of
p-ERK1/2 expression was decreased in the 25 mg/kg silymarin-treated
group compared with those in the control group, reflecting the same
results obtained from the western blotting in vitro.
Histopathological studies confirmed that silymarin
treatment does not result in obvious lesions of the liver and
kidney. In addition, body weight observations indicated no signs of
systemic toxicity. To the best of our knowledge, the in vivo
results of the present study demonstrate the apoptotic effects of
silymarin in human breast cancer are not associated with
side-effects, as has been previously indicated (33,34).
However, we have to study more for the lack of assessment of
apoptosis and toxicity in healthy tissues following silymarin
treatment as a potential limitation to the experiments.
To summarize, the findings of the present study
indicate that breast tumor growth was inhibited by silymarin as the
result of apoptosis induction was the cause of such inhibition,
suggesting that silymarin inhibits the growth of breast cancer
cells both in vitro and in vivo. Taken together,
these results illustrate a novel effect of silymarin and
demonstrate its potentially chemotherapeutic use in patient with
breast cancer.
Acknowledgements
Not applicable.
Funding
This research was supported by the Basic Science
Research Program through the National Research Foundation of Korea
(NRF) funded by the Ministry of Education, Science and Technology
(NRF 2017R1A2B4005516).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SHK, GSC, HJK and JYJ designed the experiments to
perform, and then wrote the manuscript. SHK, GSC, ESY, JSW, JHL,
SHH and SHJ performed the experiments. SHK, GSC and ESY analyzed
and interpreted the results. GSC performed the statistical
analysis. ESY and JSW assembled the data. JYJ contributed to every
process as a supervisor. SHK, GSC, ESY, JSW, JHL, SHH and JYJ
assessed and confirmed the authenticity of all the raw data. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The animal experiments were conducted in accordance
with the regulations of the Institutional Animal Care and Use
Committee with the approval of the Ethics Committee of Kongju
National University (Yesan, South Korea).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu MM, Huang Y and Wang J: Developing
phytoestrogens for breast cancer prevention. Anticancer Agents Med
Chem. 12:1306–1313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Z, Qin B, Qi X, Mao J and Wu D:
Isoalantolactone induces apoptosis in human breast cancer cells via
ROS-mediated mitochondrial pathway and downregulation of SIRT1.
Arch Pharm Res. 39:1441–1453. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nizamutdinova IT, Lee GW, Son KH, Jeon SJ,
Kang SS, Kim YS, Lee JH, Seo HG, Chang KC and Kim HJ: Tanshinone I
effectively induces apoptosis in estrogen receptor-positive (MCF-7)
and estrogen receptor-negative (MDA-MB-231) breast cancer cells.
Int J Oncol. 33:485–491. 2008.PubMed/NCBI
|
|
4
|
Panche AN, Diwan AD and Chandra SR:
Flavonoids: An overview. J Nutr Sci. 5:e472016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Comelli MC, Mengs U, Schneider C and
Prosdocimi M: Toward the definition of the mechanism of action of
silymarin: Activities related to cellular protection from toxic
damage induced by chemotherapy. Integr Cancer Ther. 6:120–129.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen IS, Chen YC, Chou CH, Chuang RF,
Sheen LY and Chiu CH: Hepatoprotection of silymarin against
thioacetamide-induced chronic liver fibrosis. J Sci Food Agric.
92:1441–1447. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sherif IO and Al-Gayyar MM: Antioxidant,
anti-inflammatory and hepatoprotective effects of silymarin on
hepatic dysfunction induced by sodium nitrite. Eur Cytokine Netw.
24:114–121. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Lakkany NM, Hammam OA, El-Maadawy WH,
Badawy AA, Ain-Shoka AA, Ebeid FA and EI-Lakkany NM:
Anti-inflammatory/anti-fibrotic effects of the hepatoprotective
silymarin and the schistosomicide praziquantel against Schistosoma
mansoni-induced liver fibrosis. Parasit Vectors. 5:92012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu T, Liu W, Guo W and Zhu X: Silymarin
suppressed lung cancer growth in mice via inhibiting
myeloid-derived suppressor cells. Biomed Pharmacother. 81:460–467.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Deep G, Singh RP, Agarwal C, Kroll DJ and
Agarwal R: Silymarin and silibinin cause G1 and G2-M cell cycle
arrest via distinct circuitries in human prostate cancer PC3 cells:
A comparison of flavanone silibinin with flavanolignan mixture
silymarin. Oncogene. 25:1053–1069. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Han SI, Kim YS and Kim TH: Role of
apoptotic and necrotic cell death under physiologic conditions. BMB
Rep. 41:1–10. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Oda E, Ohki R, Murasawa H, Nemoto J,
Shibue T, Yamashita T, Tokino T, Taniguchi T and Tanaka N: Noxa, a
BH3-only member of the Bcl-2 family and candidate mediator of
p53-induced apoptosis. Science. 288:1053–1058. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Donovan M and Cotter TG: Control of
mitochondrial integrity by Bcl-2 family members and
caspase-independent cell death. Biochim Biophys Acta. 1644:133–147.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase-3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang Y, Zhu X, Chen Y, Wang X and Chen R:
p38 and JNK MAPK, but not ERK1/2 MAPK, play important role in
colchicine-induced cortical neurons apoptosis. Eur J Pharmacol.
576:26–33. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Joneson T, White MA, Wigler MH and
Bar-Sagi D: Stimulation of membrane ruffling and MAP kinase
activation by distinct effectors of RAS. Science. 271:810–812.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doll R: The lessons of life: Keynote
address to the nutrition and cancer conference. Cancer Res. 52
(Suppl 7):2024s–2029s. 1992.PubMed/NCBI
|
|
19
|
Porter PL: Global trends in breast cancer
incidence and mortality. Salud Publica Mex. 51 (Suppl 2):s141–s146.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fan L, Ma Y, Liu Y, Zheng D and Huang G:
Silymarin induces cell cycle arrest and apoptosis in ovarian cancer
cells. Eur J Pharmacol. 743:79–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kalla PK, Chitti S, Aghamirzaei ST,
Senthilkumar R and Arjunan S: Anti-cancer activity of silymarin on
MCF-7 and NCIH-23 cell lines. Adv Biol Res (Faisalabad). 8:57–61.
2014.
|
|
22
|
Vaid M, Singh T, Prasad R and Katiyar SK:
Silymarin inhibits melanoma cell growth both in vitro and in vivo
by targeting cell cycle regulators, angiogenic biomarkers and
induction of apoptosis. Mol Carcinog. 54:1328–1339. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Katiyar SK, Roy AM and Baliga MS:
Silymarin induces apoptosis primarily through a p53-dependent
pathway involving Bcl-2/Bax, cytochrome c release, and caspase
activation. Mol Cancer Ther. 4:207–216. 2005.PubMed/NCBI
|
|
24
|
Li LH, Wu LJ, Tashiro SI, Onodera S,
Uchiumi F and Ikejima T: The roles of Akt and MAPK family members
in silymarin's protection against UV-induced A375-S2 cell
apoptosis. Int Immunopharmacol. 6:190–197. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang Q, Wu LJ, Tashiro S, Onodera S, Li
LH and Ikejima T: Silymarin augments human cervical cancer HeLa
cell apoptosis via P38/JNK MAPK pathways in serum-free medium. J
Asian Nat Prod Res. 7:701–709. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pereira L, Igea A, Canovas B, Dolado I and
Nebreda AR: Inhibition of p38 MAPK sensitizes tumour cells to
cisplatin-induced apoptosis mediated by reactive oxygen species and
JNK. EMBO Mol Med. 5:1759–1774. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui L, Bu W, Song J, Feng L, Xu T, Liu D,
Ding W, Wang J, Li C, Ma B, et al: Apoptosis induction by
alantolactone in breast cancer MDA-MB-231 cells through reactive
oxygen species-mediated mitochondrion-dependent pathway. Arch Pharm
Res. 41:299–313. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin KL, Su JC, Chien CM, Tseng CH, Chen
YL, Chang LS and Lin SR: Naphtho[1,2-b]furan-4,5-dione induces
apoptosis and S-phase arrest of MDA-MB-231 cells through JNK and
ERK signaling activation. Toxicol In Vitro. 24:61–70. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu P, Zhang C, Gao CY, Ma T, Zhang H, Zhou
MM, Yang YW, Yang L and Kong LY: Anti-proliferation of
triple-negative breast cancer cells with physagulide P: ROS/JNK
signaling pathway induces apoptosis and autophagic cell death.
Oncotarget. 8:64032–64049. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Palit S, Kar S, Sharma G and Das PK:
Hesperetin induces apoptosis in breast carcinoma by triggering
accumulation of ROS and activation of ASK1/JNK pathway. J Cell
Physiol. 230:1729–1739. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rabi T and Banerjee S: Novel synthetic
triterpenoid methyl 25-hydroxy-3-oxoolean-12-en-28-oate induces
apoptosis through JNK and p38 MAPK pathways in human breast
adenocarcinoma MCF-7 cells. Mol Carcinog. 47:415–423. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Singh RP, Tyagi AK, Zhao J and Agarwal R:
Silymarin inhibits growth and causes regression of established skin
tumors in SENCAR mice via modulation of mitogen-activated protein
kinases and induction of apoptosis. Carcinogenesis. 23:499–510.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kim LH, Khadka S, Shin JA, Jung JY, Ryu
MH, Yu HJ, Lee HN, Jang B, Yang IH, Won DH, et al: Nitidine
chloride acts as an apoptosis inducer in human oral cancer cells
and a nude mouse xenograft model via inhibition of STAT3.
Oncotarget. 8:91306–91315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Shivakumar P, Rani MU, Reddy AG and
Anjaneyulu Y: A study on the toxic effects of Doxorubicin on the
histology of certain organs. Toxicol Int. 19:241–244. 2012.
View Article : Google Scholar : PubMed/NCBI
|