Introduction
Cervical cancer is considered as the 4th most common
type of cancer in global clinical practice (1). Globally, cervical cancer caused 569,847
new cases, accounting for 3.2% of all new cancer cases, and 311,365
deaths, accounting for 3.3% of all cancer-associated deaths, in
2018 alone (2). Human papillomavirus
(HPV) infection is a major risk factor for cervical cancer
(3). With the increased
understanding of the molecular mechanisms of HPV infection
(4,5), as well as the popularization of HPV
screening and vaccination (6,7), the
incidence and mortality rates of cervical cancer have significantly
dropped over the past decades (6,7).
However, the HPV screening rate in developing countries, such as
China, remains low, and most patients with cervical cancer are
diagnosed at advanced stages and have a poor survival (8).
A considerable number of molecular pathways are
involved in the pathogenesis of cervical cancer (9). Understanding the roles of these
molecular signaling pathways provides novel insights into the
development of targeted therapies (10). The development and progression of
cancer, such as lung cancer, liver cancer and CSCC, involve the
regulation of non-coding RNAs (ncRNAs), which do not encode
proteins but regulate the expression of cancer-associated genes at
different levels (11).
ncRNA-targeted therapies have provided valuable insights into
cancer treatment (11). However, the
functions of most ncRNAs remain unknown. ncRNAs, such as long
(>200 nucleotides) ncRNAs (lncRNAs), have no protein-coding
information, but they participate in human diseases by affecting
protein synthesis (11). PSMG3-AS1
has been characterized as an oncogenic lncRNA in breast cancer
(12), while its role in other types
of cancer is unknown. A previous bioinformatics study revealed that
PSMG3-AS1 may be a potential target of microRNA (miRNA/miR)-4417, a
critical player in cancer biology (13). Therefore, the present study aimed to
investigate the interaction between miR-4417 and PSMG3-AS1 in
cervical squamous cell carcinoma (CSCC), which is a major subtype
of cervical cancer.
Materials and methods
Sample collection
The present study was approved by the Ethics
Committee of Weifang People's Hospital (Weifang, China; approval
no. WPH011). A total of 64 patients with CSCC (all females; age
range, 42–64 years; mean age, 51.7±6.7 years) admitted at the
aforementioned hospital between December 2012 and December 2014
were enrolled in the present study. All patients were newly
diagnosed cases, and no other severe clinical disorders, such as
chronic diseases, severe infections, heart diseases or other types
of cancer, were observed among these patients. No therapy was
initiated before the admission of patients. All patients provided
written informed consent. Fine-needle aspiration was performed to
collect CSCC and paired adjacent (within 3 cm around tumors)
non-tumor tissues from all patients before therapy.
Histopathological examinations were performed to confirm all tissue
samples. Tissue samples were stored in liquid nitrogen before
subsequent experiments. The clinical data of all 64 patients are
listed in Table I.
 | Table I.Clinical data of the 64 patients with
cervical squamous cell carcinoma. |
Table I.
Clinical data of the 64 patients with
cervical squamous cell carcinoma.
| Characteristic | Value |
|---|
| Clinical stage,
n |
|
| I or
II | 29 |
| III or
IV | 35 |
| Mean age ± SD,
years | 51.7±6.7 |
| Tumor multiplicity,
n |
|
|
Single | 40 |
|
Multiple | 24 |
| Differentiation,
n |
|
| G1 | 22 |
| G2 | 24 |
| G3 | 18 |
| Body mass index,
n |
|
| ≥24 | 20 |
|
<24 | 44 |
| Smoking, n |
|
| Yes | 18 |
| No | 46 |
Treatment and follow-up
The 64 patients with CSCC included 12, 17, 20 and 15
cases at clinical stage I, II, III and IV, respectively, based on
the American Joint Committee on Cancer (AJCC) staging (14). Based on the health conditions and
AJCC stage of the patients, anticancer therapies, such as surgical
resection, chemotherapy, radiotherapy or combined therapy, were
performed. From the day of admission, all patients were followed up
for 5 years (through phone call and/or outpatient visit) in a
monthly manner to record survival, and patients who died of causes
other than CSCC were excluded from the present study (77 patients
with CSCC were enrolled at the beginning of the study, and 13
patients who died of causes unrelated to CSCC were excluded).
Cell culture
The CSCC SiHa and C33A cell lines were obtained from
the American Type Culture Collection. Cells were cultured in
Eagle's Minimum Essential Medium with 10% FBS (both Gibco; Thermo
Fisher Scientific, Inc.) at 37°C with 5% CO2 at 95%
humidity. Cells were harvested at ~85% confluence and used in
subsequent transient transfection experiments.
Cell transfection
The PSMG3-AS1 expression vector was constructed
using pcDNA3.1 vector (Invitrogen; Thermo Fisher Scientific, Inc.)
as the backbone. miR-4417 mimic (5′-GGUGGGCUUCCCGGAGGG-3′) and its
non-targeting negative control (NC) miRNA
(5′-UGCCACGUGGCAUGCAGUG-3′) were purchased from Sigma-Aldrich
(Merck KGaA). SiHa and C33A cells were transfected with 10 nM
PSMG3-AS1 expression vector or 45 nM miR-4417 mimic using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Incubation with transfection mixture was
performed at 37°C for 6 h. Empty vector- or NC miRNA-transfected
cells were used as NC cells. Untransfected cells were used as the
control (C) cells. Subsequent experiments were performed 48 h
post-transfection.
Luciferase reporter assay
The pGL3-PSMG3-AS1 luciferase reporter vector
(Promega Corporation) was established. Cells were transfected with
miR-4417 mimic + pGL3-PSMG3-AS1 (miR-4417 group) or NC miRNA +
pGL3-PSMG3-AS1 (NC miRNA group) using the aforementioned
transfection method. Luciferase activity was measured after 48 h
using the Firefly Luciferase Assay kit 2.0 (Biotium, Inc.). Firefly
luciferase activity was normalized to Renilla luciferase
activity.
RNA extraction
Total RNA was extracted from tissue samples and
cultivated cells using RNAzol (Sigma-Aldrich; Merck KGaA). To
harvest miRNAs, 85% ethanol was used to precipitate and wash RNA
samples. RNA concentrations were measured using a NanoDrop 2000
spectrophotometer (Thermo Fisher Scientific, Inc.). RNA samples
were digested with DNase I to remove genomic DNA.
Reverse transcription-quantitative
(RT-q)PCR
Preparation of cDNA samples was performed via RT
using the QuantiTect Reverse Transcription kit (Qiagen China Co.,
Ltd.) in accordance with the manufacturer's instructions. The
templates were RNA samples from both tissue and cultivated cells.
The expression levels of PSMG3-AS1 were measured using BlazeTaq™
One-Step SYBR-Green RT-qPCR kit (GeneCopoeia, Inc.) following the
manufacturer's instructions, with β-actin as the internal control.
The expression levels of mature miR-4417 were measured using
All-in-One™ miRNA qRT-PCR Detection kit (GeneCopoeia, Inc.) with U6
as the internal control. All PCR reactions were performed in
triplicate, and the 2−ΔΔCq method (15) was used to normalize Ct values: ΔCT =
Ct (target gene) - Ct (internal control). The sample with the
biggest ΔCt value was set to 1, and all other samples were
normalized to this sample. The primer sequences were as follows:
PSMG3-AS1 forward, 5′-GAAGCAGAACCAACGCACAG-3′ and reverse,
5′-GCATAATCCAATCCCTCAAGAA-3′; β-actin forward,
5′-TGACGGGGTCACCCACACTGTGCCCATCTA-3′ and reverse,
5′-CTAGAAGCATTTGCGGTGGACGATGGAGGG-3′; miR-4417 forward,
5′-GGTGGGCTTCCCGGA-3′. Universal reverse primer and U6 primers were
included in the aforementioned All-in-One™ miRNA qRT-PCR Detection
kit. The qPCR conditions consisted of 95°C for 1 min, followed by
40 cycles of 95°C for 10 sec and 58°C for 40 sec.
RNA interaction prediction
The potential interaction between miR-4417 and
PSMG3-AS1 was predicted using IntaRNA 2.0 (16). In this program, miR-4417 was used as
short sequence and PSMG3-AS1 was used as long sequence.
Transwell assay
SiHa and C33A cells were harvested 48 h
post-transfection and subjected to Transwell assay using
Transwell® Cell Culture Plate Inserts (8.0-µm pore;
Corning, Inc.). Briefly, 600 cells in 0.1 ml serum-free EMEM were
seeded in the upper chamber. The lower chamber was filled with EMEM
with 20% FBS. Matrigel (EMD Millipore)-coated membranes (6 h at
37°C) were used in invasion assays, while uncoated membranes were
used in migration assays. Cells were cultivated for 12 h at 37°C,
followed by staining of the lower surface of membranes with 1%
crystal violet (Sigma-Aldrich; Merck KGaA) for 20 min at room
temperature in the dark. Cells were counted under a light
microscope (magnification, ×20) and images were obtained.
Statistical analysis
GraphPad Prism 6 (GraphPad Software, Inc.) was used
to process and analyze data. Data were expressed as the mean ± SEM
of three biological replicates. Paired Student's t-test was used to
compare gene expression levels between CSCC and non-tumor tissues.
One-way ANOVA and Tukey's post-hoc test were used to compare
multiple groups. The 64 patients with CSCC were divided into high
and low PSMG3-AS1 expression groups (n=32) based on the median
relative PSMG3-AS1 expression level (4.27) in CSCC tissues.
Survival curves were plotted based on the 5-year follow-up data
using GraphPad Prism 6 with the ‘Survival’ panel and ‘Comparing two
groups’ function. Survival curves were compared using the log-rank
test. Correlations were analyzed using Pearson's correlation
coefficient. χ2 test was performed to analyze the
association between the expression levels of miR-4417, PSMG3-AS1
and the patients' clinical data. P<0.05 was considered to
indicate a statistically significant difference.
Results
Upregulation of PSMG3-AS1 expression
in CSCC tissues predicts a poor survival
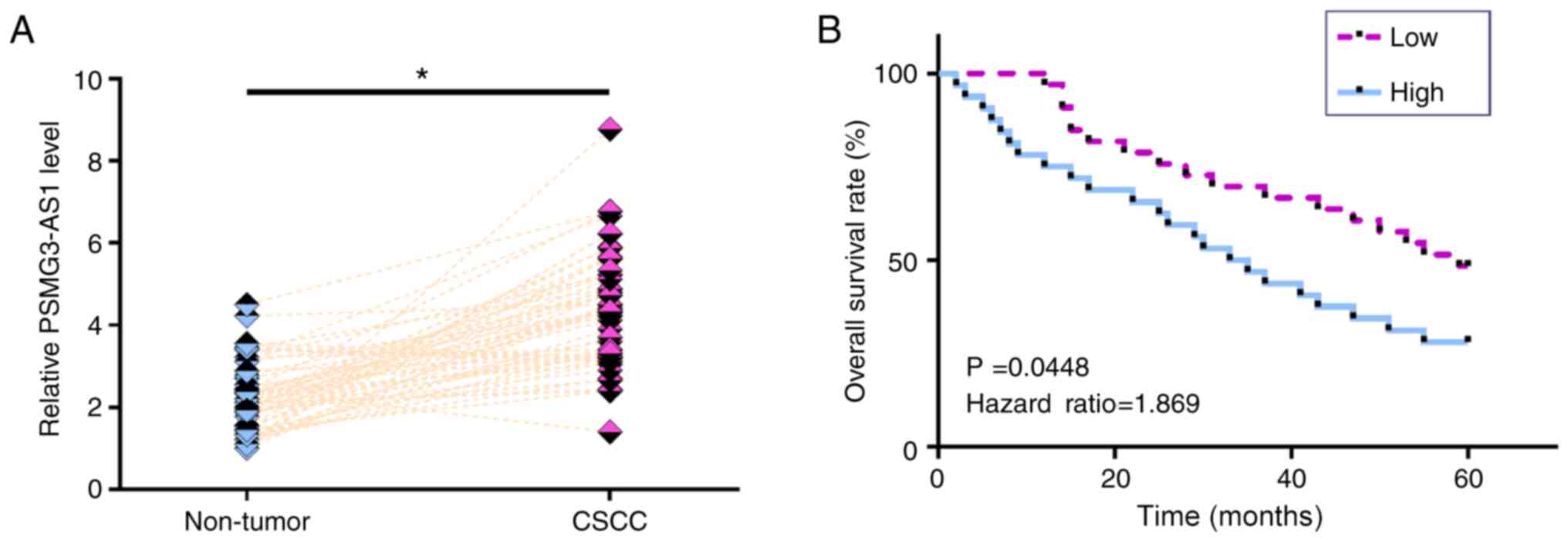
The expression levels of PSMG3-AS1 in both CSCC and
non-tumor tissues from the 64 patients with CSCC were measured via
RT-qPCR. Compared with non-tumor tissues, the expression levels of
PSMG3-AS1 were significantly increased in CSCC tissues (Fig. 1A; P<0.05). Survival curve analysis
revealed that the overall survival rate of patients in the high
PSMG3-AS1 expression group was significantly lower than that in the
low PSMG3-AS1 expression group (Fig.
1B; P=0.0448). χ2 test revealed that the expression
levels of PSMG3-AS1 were not significantly associated with age,
smoking habit, HPV infection and clinical stage (data not
shown).
miR-4417 expression is downregulated
in CSCC tissues and inversely correlated with PSMG3-AS1
expression
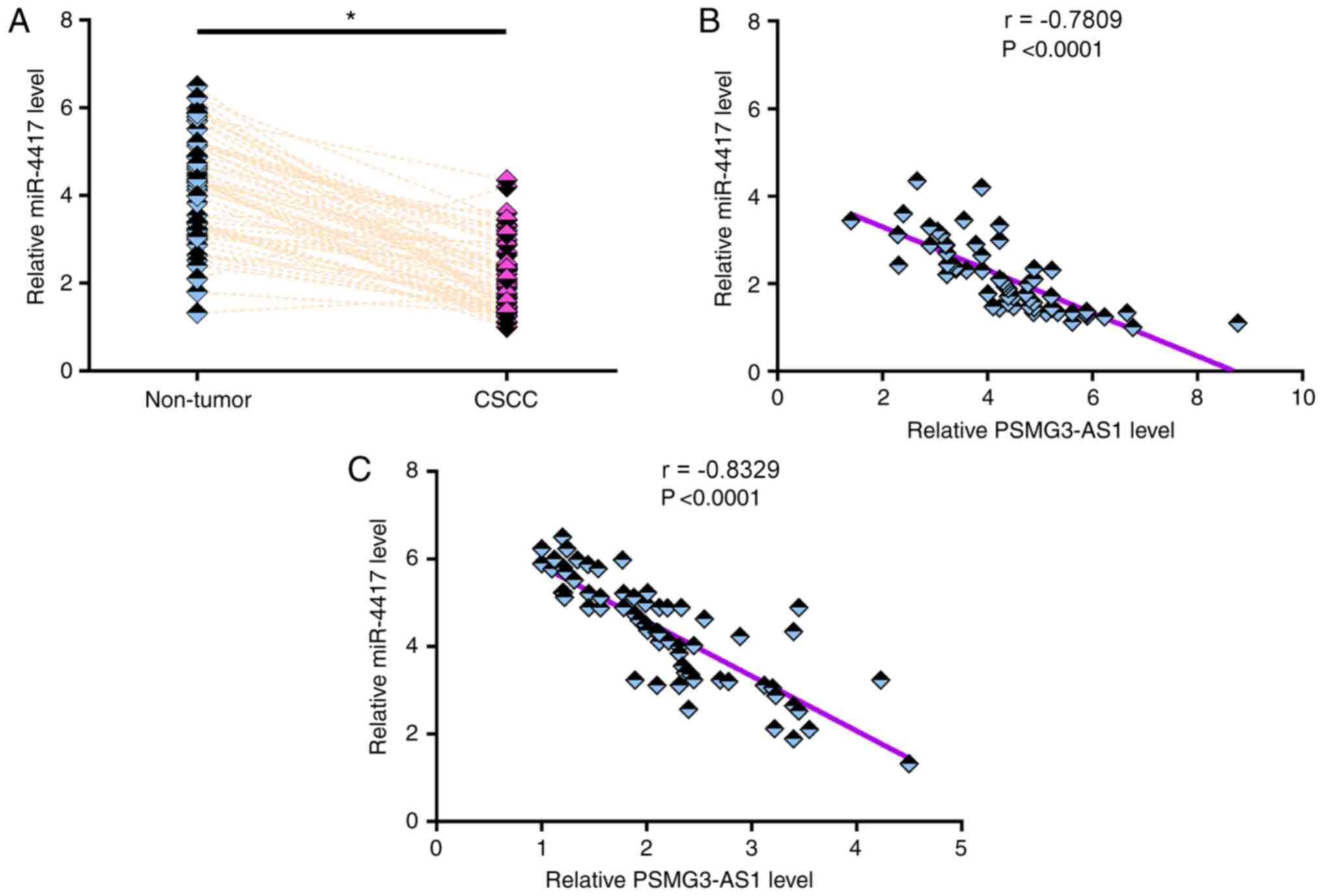
The expression levels of miR-4417 in both CSCC and
non-tumor tissues from the 64 patients with CSCC were measured via
RT-qPCR. Compared with non-tumor tissues, the expression levels of
miR-4417 were significantly lower in CSCC tissues (Fig. 2A; P<0.05). Pearson's correlation
coefficient analysis revealed that the expression levels of
miR-4417 were inversely and significantly correlated with the
expression levels of PSMG3-AS1 in both CSCC tissues (Fig. 2B) and non-tumor tissues (Fig. 2C). χ2 test revealed that
the expression levels of miR-4417 were not significantly associated
with age, smoking habit, HPV infection and clinical stage (data not
shown).
miR-4417 targets PSMG3-AS1 in CSCC
cells
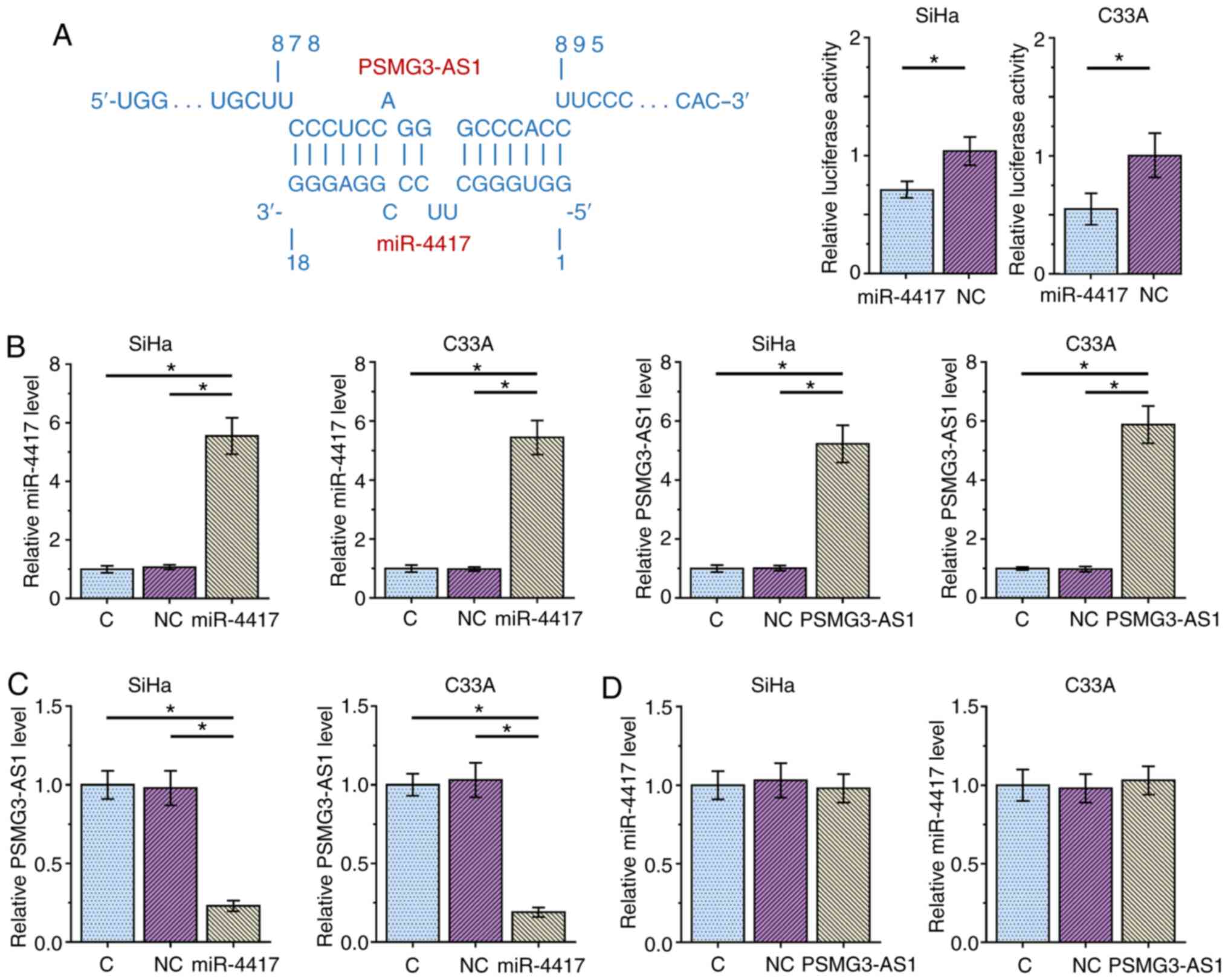
The potential interaction between miR-4417 and
PSMG3-AS1 was predicted using IntaRNA 2.0 (14). It was observed that miR-4417 and
PSMG3-AS1 may form multiple base pairing (Fig. 3A). Luciferase reporter assay revealed
that, compared with the NC miRNA group, the miR-4417 group
exhibited significantly decreased luciferase activity, indicating a
direct interaction between miR-4417 and PSMG3-AS1 (Fig. 3A). SiHa and C33A cells were
transfected with either miR-4417 mimic or PSMG3-AS1 expression
vector, and the overexpression of miR-4417 and PSMG3-AS1 were
confirmed 48 h post-transfection via RT-qPCR (Fig. 3B; P<0.05). Compared with the C and
NC groups, overexpression of miR-4417 significantly decreased the
expression levels of PSMG3-AS1 (Fig.
3C; P<0.05), while overexpression of PSMG3-AS1 did not
affect miR-4417 expression (Fig. 3D)
in both cell lines. These results suggested that miR-4417 may
target PSMG3-AS1 to downregulate its expression, while PSMG3-AS1
did not regulate miR-4417 expression.
miR-4417 downregulates PSMG3-AS1 to
suppress cancer cell invasion and migration
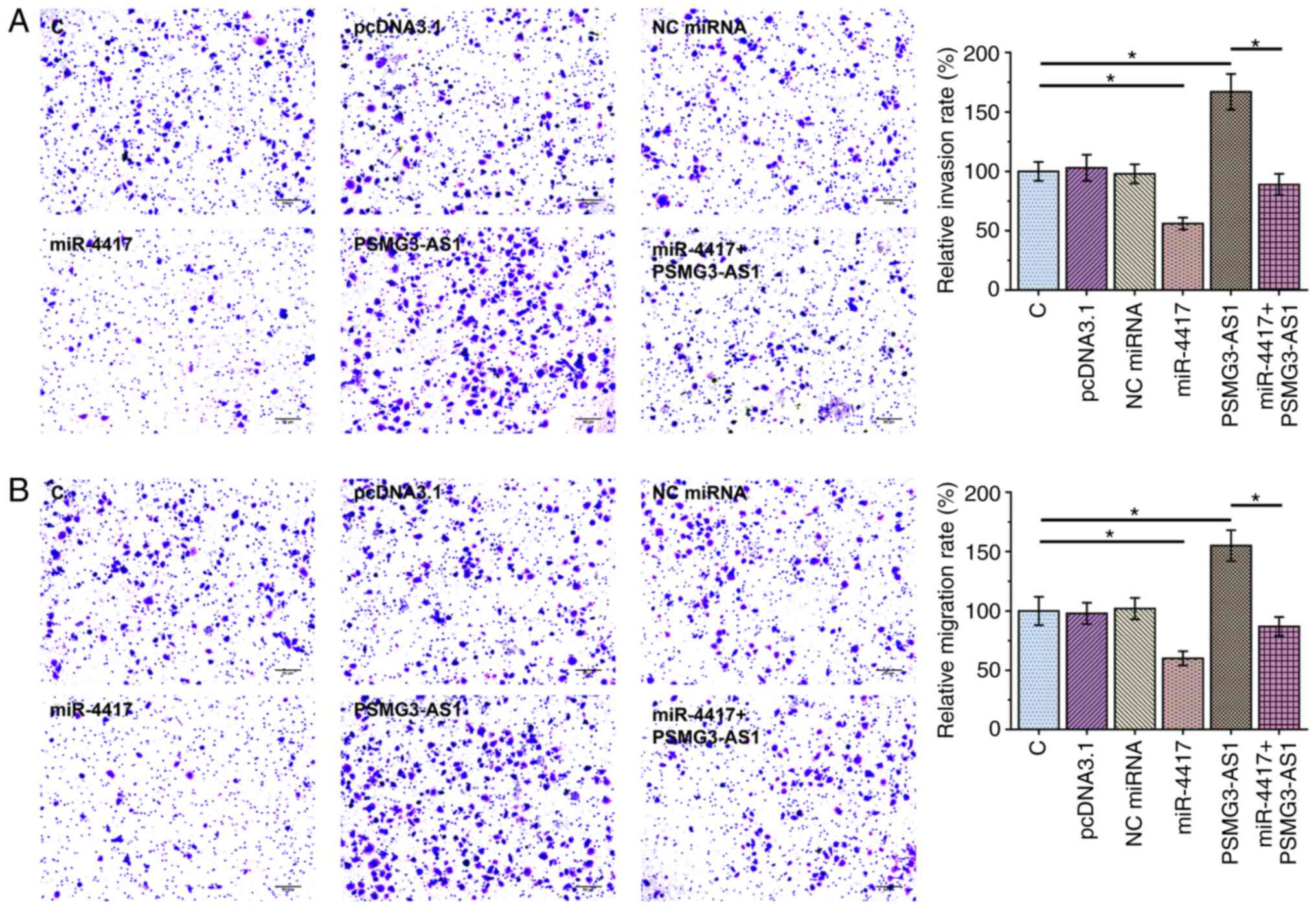
Transwell assay was performed to assess the effect
of overexpression of PSMG3-AS1 and miR-4417 on invasion (Fig. 4A) and migration (Fig. 4B) of SiHa and C33A cells.
Overexpression of PSMG3-AS1 significantly increased invasion and
migration rates of cancer cells compared with control cells, while
overexpression of miR-4417 significantly inhibited invasion and
migration of cancer cells and attenuated the effect of PSMG3-AS1
overexpression (Fig. 4A and B;
P<0.05). The expression levels of miR-4417 (Fig. 5A; P<0.05) and PSMG3-AS1 (Fig. 5B; P<0.05) in all groups included
in Transwell assays were also measured via RT-qPCR. It was observed
that the cell invasion and migration patterns were consistent with
the expression pattern of PSMG3-AS1, with the highest expression
level of PSMG3-AS1 being observed in the PSMG3-AS1 group and the
lowest PSMG3-AS1 level in the miR-4417 group (Fig. 5B).
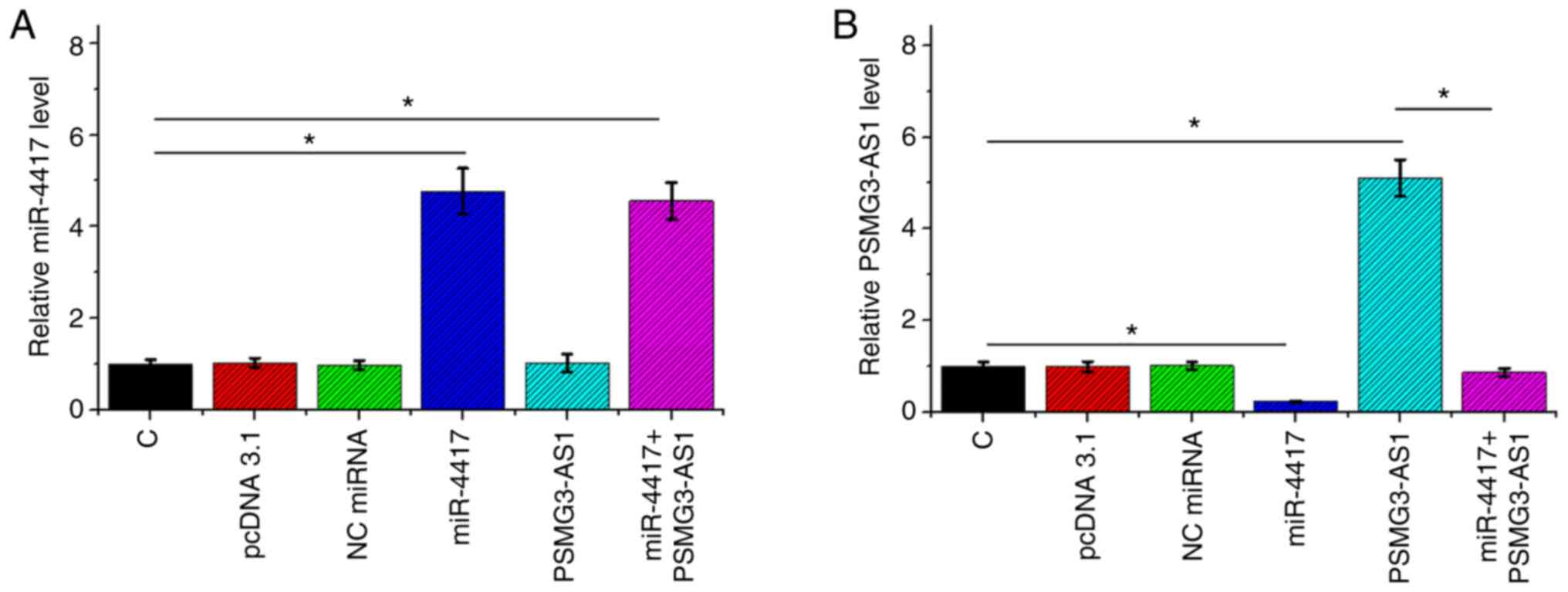 | Figure 5.miR-4417 and PSMG3-AS1 expression in
C, pcDNA 3.1, NC miRNA, miR-4417, PSMG3-AS1 and miR-4417+PSMG3-AS1
groups included in Transwell assay. Expression levels of (A)
miR-4417 and (B) PSMG3-AS1 in C, pcDNA 3.1, NC miRNA, miR-4417,
PSMG3-AS1 and miR-4417+PSMG3-AS1 groups involved in Transwell
assays were also measured by reverse transcription-quantitative
PCR. All experiments were performed in three biological replicates,
and the data are presented as the mean ± SEM. *P<0.05.
miR/miRNA, microRNA; C, control; NC, negative control. |
Discussion
The present study investigated the interaction
between miR-4417 and PSMG3-AS1 in CSCC. It was observed that the
expression levels of miR-4417 and PSMG3-AS1 were altered in CSCC,
and that miR-4417 may target PSMG3-AS1 to suppress the invasion and
migration of CSCC cells.
It has been previously demonstrated that PSMG3-AS1
expression is upregulated in breast cancer and may sponge
miR-143-3p to promote migration and proliferation of breast cancer
cells (12). To the best of our
knowledge, the present study was the first to report the
upregulation of PSMG3-AS1 expression in CSCC. In addition,
increased cell invasion and migration rates of CSCC cells were
observed after the overexpression of PSMG3-AS1. Therefore,
PSMG3-AS1 may serve an oncogenic role in CSCC.
HPV infection screening has significantly increased
the early diagnostic rate of CSCC (6,7).
However, in China, the HPV screening rate remains low and HPV
vaccination is not beneficial for patients who have been already
infected (8). Consequently, a
considerable number of patients with CSCC are diagnosed at advanced
stages and their survival is generally poor (6–8). In the
present study, it was demonstrated that high expression levels of
PSMG3-AS1 in cancer tissues of patients with CSCC may predict a
poor survival. Therefore, measuring the expression levels of
PSMG3-AS1 in cancer tissues before therapy may guide the
determination of treatment strategies, thereby improving the
survival of patients. However, the viability of this approach needs
to be further studied.
miR-4417 serves different roles in different types
of cancer (13,17). It has been reported that miR-4417
expression is downregulated in triple-negative breast cancer, and
overexpression of miR-4417 suppresses cancer cell migration and
invasion, indicating its tumor suppressive role in this disease
(13). By contrast, miR-4417
expression is upregulated in hepatocellular carcinoma and regulates
the phosphorylation of pyruvate kinase muscle 2 to suppress
apoptosis and promote proliferation of cancer cells, suggesting its
oncogenic role (14). In the present
study, it was observed that miR-4417 expression was downregulated
in CSCC tissues and miR-4417 overexpression had inhibitory effects
on cancer cell invasion and migration. Therefore, miR-4417 may be a
tumor suppressor in CSCC. In addition, it was revealed that
miR-4417 may target PSMG3-AS1 to exert its tumor suppressive role
and that miR-4417 expression was inversely correlated with
PSMG3-AS1 expression in both CSCC and non-tumor tissues. However,
the present study is limited by the small number of patients.
Future studies with more patients are required to further confirm
the current findings.
In conclusion, miR-4417 expression was downregulated
and PSMG3-AS1 expression was upregulated in CSCC. miR-4417 may
target PSMG3-AS1 to suppress cell invasion and migration in
CSCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WZ designed the experiments and drafted the
manuscript. SM, XL and WZ performed the experiments. SM and WZ
analyzed the data. All authors confirmed the authenticity of the
data, and have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Weifang People's Hospital (Weifang, China; approval
no. WPH011). The research was performed in accordance with the
World Medical Association Declaration of Helsinki. All patients
provided written informed consent prior to their inclusion in the
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CSCC
|
cervical squamous cell carcinoma
|
|
HPV
|
human papillomavirus
|
|
lncRNA
|
long non-coding RNA
|
References
|
1
|
Small W Jr, Bacon MA, Bajaj A, Chuang LT,
Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR,
Viswanathan AN, et al: Cervical cancer: A global health crisis.
Cancer. 123:2404–2412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wentzensen N, Schiffman M, Palmer T and
Arbyn M: Triage of HPV positive women in cervical cancer screening.
J Clin Virol. 76 (Suppl 1):S49–S55. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Drews CM, Case S and Vande Pol SB: E6
proteins from high-risk HPV, low-risk HPV, and animal
papillomaviruses activate the Wnt/β-catenin pathway through
E6AP-dependent degradation of NHERF1. PLoS Pathog. 15:e10075752019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Basukala O, Mittal S, Massimi P, Bestagno
M and Banks L: The HPV-18 E7 CKII phospho acceptor site is required
for maintaining the transformed phenotype of cervical
tumour-derived cells. PLoS Pathog. 15:e10077692019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koh WJ, Greer BE, Abu-Rustum NR, Apte SM,
Campos SM, Cho KR, Chu C, Cohn D, Crispens MA, Dorigo O, et al:
Cervical cancer, version 2.2015. J Natl Compr Canc Netw.
13:395–404; quiz 404. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang B, He M, Chao A, Engelgau MM, Saraiya
M and Wang L and Wang L: Cervical cancer screening among adult
women in China, 2010. Oncologist. 20:627–634. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ibeanu OA: Molecular pathogenesis of
cervical cancer. Cancer Biol Ther. 11:295–306. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Crafton SM and Salani R: Beyond
chemotherapy: An overview and review of targeted therapy in
cervical cancer. Clin Ther. 38:449–458. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chandra Gupta S and Nandan Tripathi Y:
Potential of long non-coding RNAs in cancer patients: From
biomarkers to therapeutic targets. Int J Cancer. 140:1955–1967.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cui Y, Fan Y, Zhao G, Zhang Q, Bao Y, Cui
Y, Ye Z, Chen G, Piao X, Guo F, et al: Novel lncRNA PSMG3 AS1
functions as a miR 143 3p sponge to increase the proliferation and
migration of breast cancer cells. Oncol Rep. 43:229–239.
2020.PubMed/NCBI
|
|
13
|
Wong CK, Gromisch C, Ozturk S, Papageorgis
P, Abdolmaleky HM, Reinhard BM, Thiagalingam A and Thiagalingam S:
MicroRNA-4417 is a tumor suppressor and prognostic biomarker for
triple-negative breast cancer. Cancer Biol Ther. 20:1113–1120.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu CS, Spirtos A, Khulpateea BR and Lea
JS: AJCC versus FIGO staging of cervical cancer to predict disease
severity. Gynecol Oncol. 149:1552018. View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−ΔΔC(T)) μethod. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mann M, Wright PR and Backofen R: IntaRNA
2.0: Enhanced and customizable prediction of RNA-RNA interactions.
Nucleic Acids Res. 45:W435–W439. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song L, Zhang W, Chang Z, Pan Y, Zong H,
Fan Q and Wang L: miR-4417 targets tripartite motif-containing 35
(TRIM35) and regulates pyruvate kinase muscle 2 (PKM2)
phosphorylation to promote proliferation and suppress apoptosis in
hepatocellular carcinoma cells. Med Sci Monit. 23:1741–1750. 2017.
View Article : Google Scholar : PubMed/NCBI
|