Introduction
Lung cancer is the most common malignancy and the
leading cause of cancer-associated mortality, worldwide (1). More than ninety million people
worldwide are at risk of developing lung cancer, which has been
recognized as a major health problem for numerous years (2). According to the cancer statistics by
the National Central Cancer Registry of China in China in 2015,
non-small lung cancer (NSCLC) accounts for ~80% of all lung cancer
(3). More than 75% of lung cancer
cases are advanced at diagnosis, as there is no practical way to
screen a large number of people at risk (4). Despite recent advances in the
understanding of the molecular biology of the lungs and the
treatment of lung cancer, the prognosis of this malignancy remains
poor (5). Currently, the 5-year
overall survival rate of lung cancer remains unsatisfactory
(6). Based on these TNM groupings,
current 5-year survival estimates in NSLCC ranged from 73% in stage
IA disease to 13% in stage IV disease in 2016 (7). Therefore, accurate diagnosis, improved
prognosis, and effective treatment are urgently required to improve
the treatment of NSCLC.
MicroRNAs (miRNAs/miR) are a class of short
non-coding ribonucleic acid molecules, ~23 nucleotides in length,
that play important regulatory roles in gene expression at the
post-transcriptional level (8).
Accumulating evidence indicates that altered expression levels of
miRNA is crucial for cancer initiation and progression and
functions as tumor suppressive or oncogenic miRNA (9,10).
miRNAs play important roles in regulating cell proliferation,
migration, invasion, differentiation and apoptosis (11). In addition, miRNAs have been found to
be a potential non-invasive biomarker (12). Recently, miR-891a-5p was found to be
increased in lung adenocarcinoma cells compared with that in normal
lung cells, and miR-891a-5p could enhance tumor cell proliferation
(13). In addition, a study by Lee
et al (14) also reported
that the mRNA expression level of miR-891a-5p was significantly
higher in patients with NSCLC. However, the clinical value of
miR-891a-5p in NSCLC remains unclear and its regulatory role on
tumor cell invasion and migration.
In the present study, the expression level of
miR-891a-5p in serum and tissue samples from patients with NCSLC
and in NSCLC cell lines was investigated, and the diagnostic and
prognostic value of miR-891a-5p was also determined. Furthermore,
the effects of miR-891a-5p on NSCLC cell proliferation, migration
and invasion were investigated using in vitro experiments,
and the potential mechanisms of miR-891a-5p in the development of
NSCLC was initially determined by analyzing the potential target
genes.
Materials and methods
Patients and clinical sample
collection
A total of 120 patients with NSCLC were included in
the present study, who were pathologically diagnosed with NSCLC and
underwent resection surgery between April 2011 and March 2014 at
Yueqing People's Hospital (Yueqing, China). None of the patients
received any anti-tumor therapy and the electronic medical record
data of all the patients was complete. In addition, 68 age- and
sex-matched healthy volunteers were recruited during the same time
period as controls. The controls had routine physical examinations
at Yueqing People's Hospital (Yueqing, China) and no history of
malignancy. Before the patients underwent surgery, venous blood
samples were collected and were immediately centrifuged for serum
extraction. Blood samples were collected from all participants and
immediately centrifuged at 1,500 × g for 10 min at 4°C for serum
extraction. A total of 120 paired NSCLC and adjacent normal
tissues, which were at least 3 cm away from the edge of tumors,
were collected at the time of surgery and were frozen using liquid
nitrogen and stored at −80°C. Demographic and clinicopathological
characteristics, as well as survival information, from a 5-year
follow-up survey (range, 0–60 months), were recorded for the
subsequent analyses. Cases who died of other events, such as
diseases other than NSCLC or an accident were excluded from the
study. The experimental procedures were approved by the Ethics
Committee of Yueqing People's Hospital (Yueqing, China; approval
no. 0112983).
Cell culture and transfection
The NSCLC cell lines (H1299, HCC827, H460 and A549)
and the normal lung cell line (NHBE) were purchased from The Cell
Bank of Chinese Academy of Sciences. All the cells were incubated
in Dulbecco's modified Eagles medium, supplemented with 10% fetal
bovine serum (FBS) (all from Invitrogen; Thermo Fisher Scientific,
Inc.) at 37°C in a humidified atmosphere with 5%
CO2.
Cell transfection was used to increase or decrease
the expression level of miR-891a-5p, using miR-891a-5p mimics and
miR-891a-5p inhibitor. These and their respective negative controls
(mimic NC and inhibitor NC, respectively) were synthesized by
Shanghai GenePharma Co., Ltd. and transfected into the NSCLC cells
using Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The miR concentration and sequences were as
follows: 50 nM miR-891a-5p mimic, 5′-UGCAACGAACCUGAGCCACUGA-3′; 100
nM miR-891a-5p inhibitor, 5′-UCAGUGGCUCAGGUUCGUUGCA-3′; 50 nM mimic
NC, 5′-UUCUCCGAACGUGUCACGU-3′; and 100 nM inhibitor NC
5′-CAGUACUUUUGUGUAGUACAA-3′. The cells transfected with the
transfection reagents only, were used as the control group. Cell
transfection was performed for 6 h at 37°C, then culture medium was
replaced and the cells were used for subsequent analyses 48 h after
transfection.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the fresh tissue, serum
samples and cells using TRIzol® (Invitrogen; Thermo
Fisher Scientific, Inc.), then the RNA was reverse transcribed into
single-stranded cDNA using the PrimeScript reverse transcriptase
kit (Takara Bio, Inc.) according to the manufacturer's guidelines.
The mRNA expression level of miR-891a-5p and HOXA5 was detected
using qPCR, and a SYBR-Green I Master Mix kit (Invitrogen; Thermo
Fisher Scientific, Inc.) on a 7500 Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). U6 was used as an
endogenous control for miR-891a-5p and GAPDH was used as an
endogenous control for HOXA5. The thermocycling conditions were as
follows: initial denaturation at 95°C for 10 min, then 40 cycles of
denaturation at 95°C for 30 sec, annealing at 60°C for 20 sec and
extension at 72°C for 15 sec. The oligonucleotide primer sequences
were as follows: miR-891a-5p forward, 5′-GCCGAGTCAGUGGCTCAGGT-3′
and reverse, 5′-CTCAACTGGTGTCGTGGA-3′; HOXA5 forward,
5′-AGCCACAAATCAAGGACACA-3′ and reverse, 5′-GCTCGCTCACGGAACTATG-3′;
U6 forward, 5′-GCTTCGGCAGCACATATACTAAAAT-3′ and reverse:
5′-CGCTTCACGAATTTGCGTGTCAT-3′; and GAPDH, forward: 5′-
TGCACCACCAACTGCTTAGC-3′ and reverse, 5′-GGCATGCACTGTGGTCATGAG-3′.
The final expression value was calculated using the
2−ΔΔCq method (15).
Cell proliferation analysis
A Cell Counting Kit-8 (CCK-8) assay was used to
detect the proliferation of the cells. The cells were seeded into
96-well plate (2×103 cells/well) and incubated for 0,
24, 48 and 72 h. Then, 10 µl CCK-8 reagent (Beyotime Institute of
Biotechnology) was added to each well and further incubated for 2
h. The optical density was measured at 450 nm using a microplate
reader.
Transwell assay
Transwell chambers (Corning Inc.) were used to the
measure the migration and invasion of the NSCLC cells. The
Transwell chambers precoated with Matrigel (Corning Inc.) at 37°C
for 1 h were used for the invasion assays, while the chambers
without Matrigel coating were used for the migration assays. The
transfected cells, at a density of 2×105 cells/chamber,
were seeded into the upper chambers with serum-free medium, while
culture medium, supplemented with 10% FBS, was added to the lower
chambers, as a chemoattractant. After incubation at 37°C for 48 h,
the cells in the lower chambers were stained using 0.1% crystal
violet at room temperature for 20 min and counted under an inverted
light microscope (Olympus Corporation; magnification, ×200).
Dual-luciferase reporter assay
Based on bioinformatics analysis, potential target
genes can be predicted using the TargetScan online tool (http://www.targetscan.org/vert_72) (16). The wild-type (WT) and mutant type
(MUT) of the HOXA5 3′-untranslated region (UTR) sequences, that
contained the binding site of miR-891a-5p, were incorporated into
the luciferase reporter vector, pGL3-luciferase (Promega
Corporation). The recombinant vectors were co-transfected into the
tumor cells with miR-891a-5p mimics, miR-891a-5p inhibitor or their
respective NCs, which sequences are the same as the ones used for
transfection, using Lipofectamine® 2000. The miR
concentration and sequences were as follows: 50 nM miR-891a-5p
mimic, 5′-UGCAACGAACCUGAGCCACUGA-3′; 100 nM miR-891a-5p inhibitor,
5′-UCAGUGGCUCAGGUUCGUUGCA-3′; 50 nM mimic NC,
5′-UUCUCCGAACGUGUCACGU-3′; and 100 nM inhibitor NC
5′-CAGUACUUUUGUGUAGUACAA-3′. After incubation for 48 h, the
relative luciferase activity was analyzed using a Dual-Luciferase
Reporter Assay System (Promega Corporation) and normalized to
Renilla luciferase activity.
Statistical analysis
Statistical analyses were either performed using
SPSS v21.0 (IBM Corp.) or GraphPad v7.0 (GraphPad Software, Inc.).
Data are presented as the mean ± SD. The differences in the
miR-891a-5p expression level in serum samples between patients with
NSCLC and the healthy controls were analyzed using an unpaired
t-test, while the differences in the expression level of
miR-891a-5p and HOXA5 between tumor and adjacent normal tissues
were analyzed using a paired t-test. The differences between
multiple groups were compared using one-way analysis of variance
and a Tukey's post hoc test. The patients were divided into the
miR-891a-5p high expression group (n=62) and miR-891a-5p low
expression group (n=58) according to the mean value of miR-891a-5p
expression levels in NSCLC tissues (2.091). A χ2 test
was used to evaluate the association between miR-891a-5p expression
level and the clinicopathological characteristics of the patients
with NSCLC. According to the serum expression levels of
miR-891a-5p, receiver operating characteristic (ROC) curve was
plotted to evaluate its diagnostic value, the optimal cut-off value
was calculated according to the Youden index, and the corresponding
cut-off value when the Youden index was maximal was the optimal
diagnostic cut-off value (Youden index = Sensitivity + Specificity-
1). The Kaplan-Meier survival method and multivariate Cox
regression analyses were used to examine the prognostic value of
miR-891a-5p. Pearsons correlation analysis was used to analyze the
correlation between the expression of miR-891a-5p and HOXA5.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression level of miR-891a-5p in
NSCLC
To further understand the role of miR-891a-5p in
NSCLC, the mRNA expression levels of miR-891a-5p in the NSCLC serum
and tissue samples, and the NSCLC cells lines was determined using
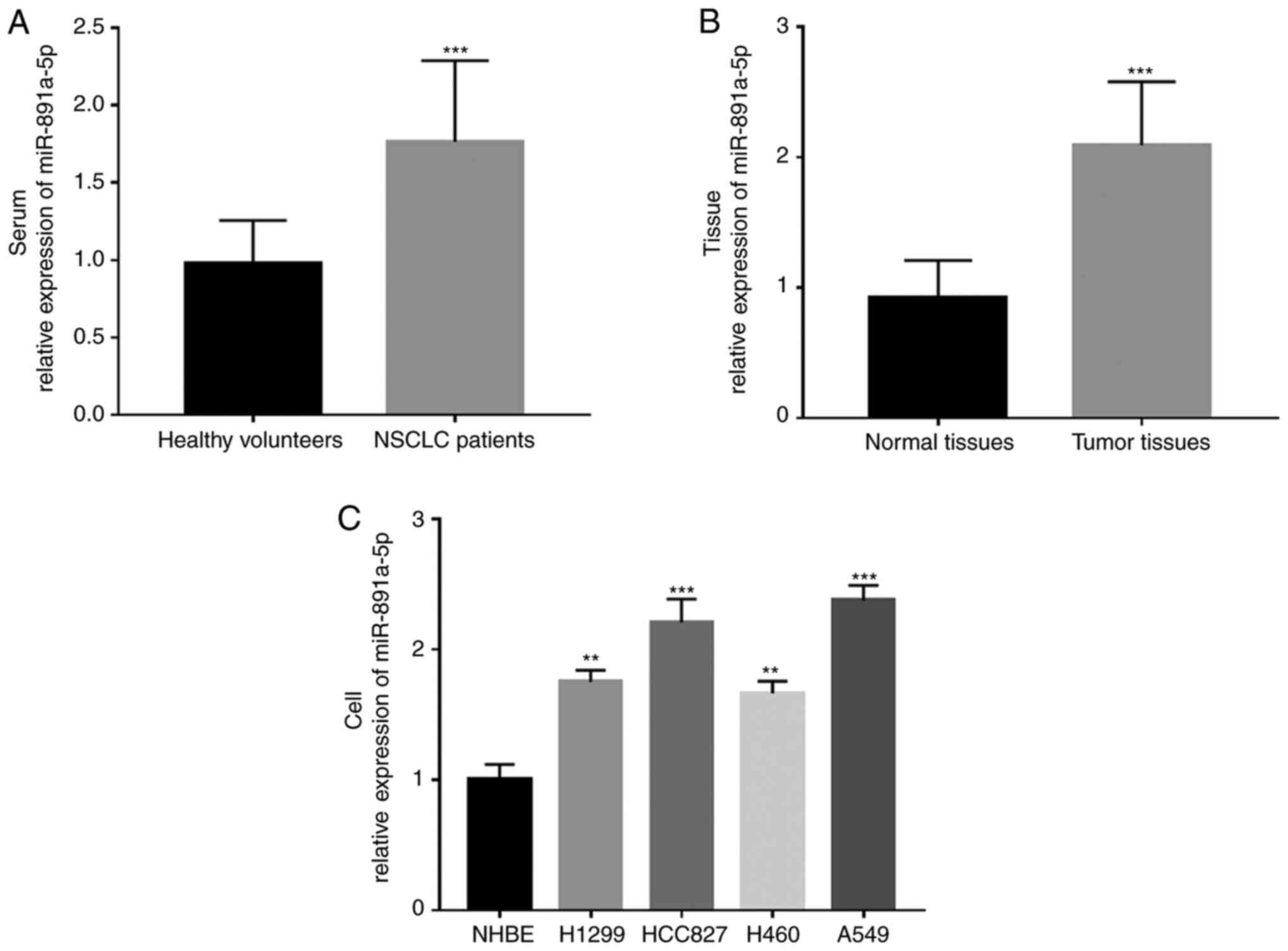
RT-qPCR. It was found that the mRNA expression levels of
miR-891a-5p were significantly increased in the serum and tissue
samples from the patients with NSCLC compared with that in the
healthy or adjacent normal controls, respectively (both P<0.001)
(Fig. 1A and B). Meanwhile, the mRNA
expression levels of miR-891a-5p in the NSCLC cell lines were also
investigated and it was found that the expression level of
miR-891a-5p was significantly higher in the NSCLC cell lines
(H1299, HCC827, H460 and A549) compared with that in the normal
NHBE cells (all P<0.01) (Fig.
1C).
Association between the expression
level of miR-891a-5p and the clinicopathological features of
patients with NSCLC
Data analysis was performed to determine the
association between the expression level of miR-891a-5p in the
NSCLC tissues and the clinicopathological data of the patients, and
to investigate the role of miR-891a-5p in the development of NSCLC.
Firstly, the mean expression value of miR-891a-5p (2.091) in NSCLC
tissues was used as the cutoff value to classify the patients into
low and high miR-891a-5p expression groups. It was found that the
miR-891a-5p expression level was associated with lymph node
metastasis (P=0.029), differentiation (P=0.041) and TNM stage
(P=0.019; Table I). In contrast,
there was no association between the expression level of
miR-891a-5p and the other parameters, such as tumor size, sex, age
and smoking history (all P>0.05).
 | Table I.Association between miR-891a-5p and
the clinical characteristics in patients with non-small cell lung
cancer. |
Table I.
Association between miR-891a-5p and
the clinical characteristics in patients with non-small cell lung
cancer.
|
|
| miR-891a-5p
expression level |
|
|---|
|
|
|
|
|
|---|
| Characteristics | Total number
(n=120) | Low (n=58) | High (n=62) | P-value |
|---|
| Age, years, n |
|
|
| 0.765 |
|
≤60 | 48 | 24 | 24 |
|
|
>60 | 72 | 34 | 38 |
|
| Sex, n |
|
|
| 0.916 |
|
Female | 47 | 23 | 24 |
|
|
Male | 73 | 35 | 38 |
|
| Smoking status,
n |
|
|
| 0.389 |
|
Non-smoker | 49 | 26 | 23 |
|
|
Smoker | 71 | 32 | 39 |
|
| Tumor size, cm,
n |
|
|
| 0.140 |
| ≤3 | 62 | 34 | 28 |
|
|
>3 | 58 | 24 | 34 |
|
| Differentiation,
n |
|
|
| 0.041 |
|
Well/moderate | 65 | 37 | 28 |
|
|
Poor | 55 | 21 | 34 |
|
| Lymph node
metastasis, n |
|
|
| 0.029 |
|
Negative | 58 | 34 | 24 |
|
|
Positive | 62 | 24 | 38 |
|
| TNM stage, n |
|
|
| 0.019 |
|
I/II | 55 | 33 | 22 |
|
|
III/IV | 65 | 25 | 40 |
|
Clinical significance of miR-891a-5p
in the diagnosis and prognosis of NSCLC
The expression levels of miR-891a-5p were increased
in the NSCLC tissue and serum samples, and its diagnostic and
prognostic significance in patients with NSCLC was further
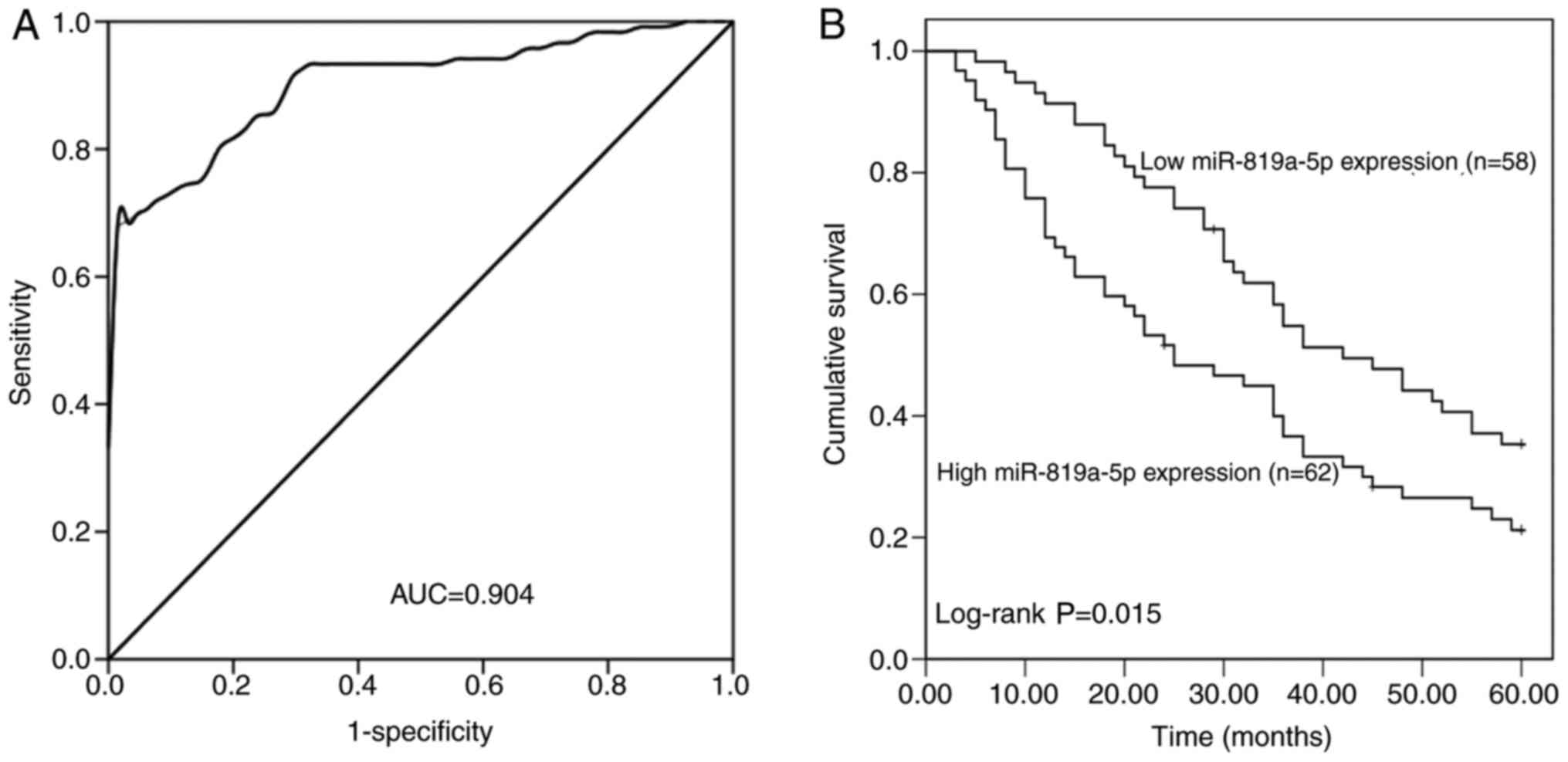
investigated. By analyzing the mRNA expression level of miR-891a-5p
in the serum, a ROC curve was constructed (Fig. 2A), and the area under the cure (AUC)
was found to be 0.904, which demonstrated that miR-891a-5p had high
diagnostic value. With a cut-off value of 1.236, the sensitivity
was 82.5% and the specificity was 80.9%, which was the optimal
relative expression value to distinguish the patients with NSCLC
from the healthy controls.
Kaplan-Meier survival curves (Fig. 2B) were plotted to evaluate the
association between the mRNA expression level of miR-891a-5p and
the overall survival rate of the patients with NSCLC, which
indicated that the patients with low miR-891a-5p expression levels
had improved overall survival compared with those with high
miR-891a-5p expression levels (log-rank; P=0.015). Furthermore, Cox
analysis indicated that miR-891a-5p [hazard ratio (HR), 1.808; 95%
CI, 1.129–2.895; P=0.014] and TNM stage (HR, 1.625; 95% CI,
1.026–2.573; P=0.039) were two independent prognostic factors for
the survival of patients with NSCLC (Table II).
 | Table II.Multivariate Cox regression analysis
in patients with non-small cell lung cancer. |
Table II.
Multivariate Cox regression analysis
in patients with non-small cell lung cancer.
|
| Multivariate
analysis |
|---|
|
|
|
|---|
|
Characteristics | HR | 95% CI | P-value |
|---|
| miR-819a-5p (high
vs. low) | 1.808 | 1.129–2.895 | 0.014 |
| Age, years (>60
vs. ≤60) | 1.215 | 0.731–2.020 | 0.452 |
| Sex (male vs.
female) | 1.080 | 0.684–1.706 | 0.741 |
| Smoking status (yes
vs. no) | 1.278 | 0.825–1.981 | 0.272 |
| Tumor size, cm
(>3 vs. ≤3) | 1.094 | 0.691–1.731 | 0.701 |
| Differentiation
(yes vs. no) | 1.473 | 0.918–2.362 | 0.108 |
| Lymph node
metastasis (yes vs. no) | 1.253 | 0.789–1.989 | 0.339 |
| TNM stage (III/IV
vs. I/II) | 1.625 | 1.026–2.573 | 0.039 |
Regulatory effects of miR-891a-5p on
NSCLC cell proliferation
To further investigate the function of miR-891a-5p
in NSCLC tumor progression, in vitro experiments were
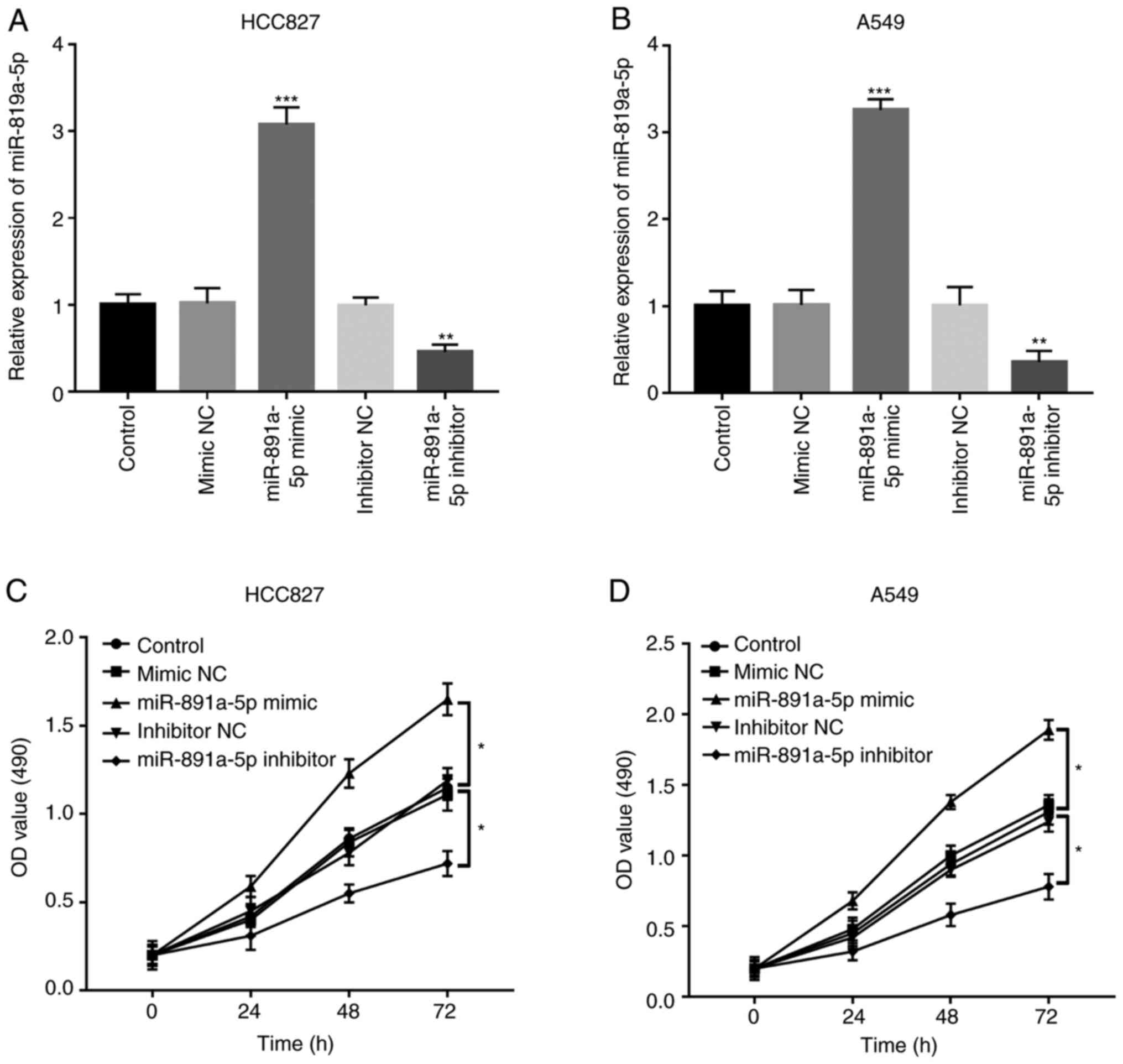
performed using the HCC827 and A549 cell lines. Following
transfection with miR-891a-5p mimics and inhibitors, the mRNA
expression level of miR-891a-5p was successfully upregulated and
downregulated, respectively, in both the HCC827 and A549 cell lines
(all P<0.001) (Fig. 3A and B).
Following which, using a CCK-8 assay, cell proliferation was
significantly increased in cells transfected with miR-891a-5p
mimics, but was downregulation in cells transfected with the
miR-891a-5p inhibitor compared with that in the control (both
P<0.05) (Fig. 3C and D).
Regulatory effects of miR-891a-5p on
NSCLC cell migration and invasion
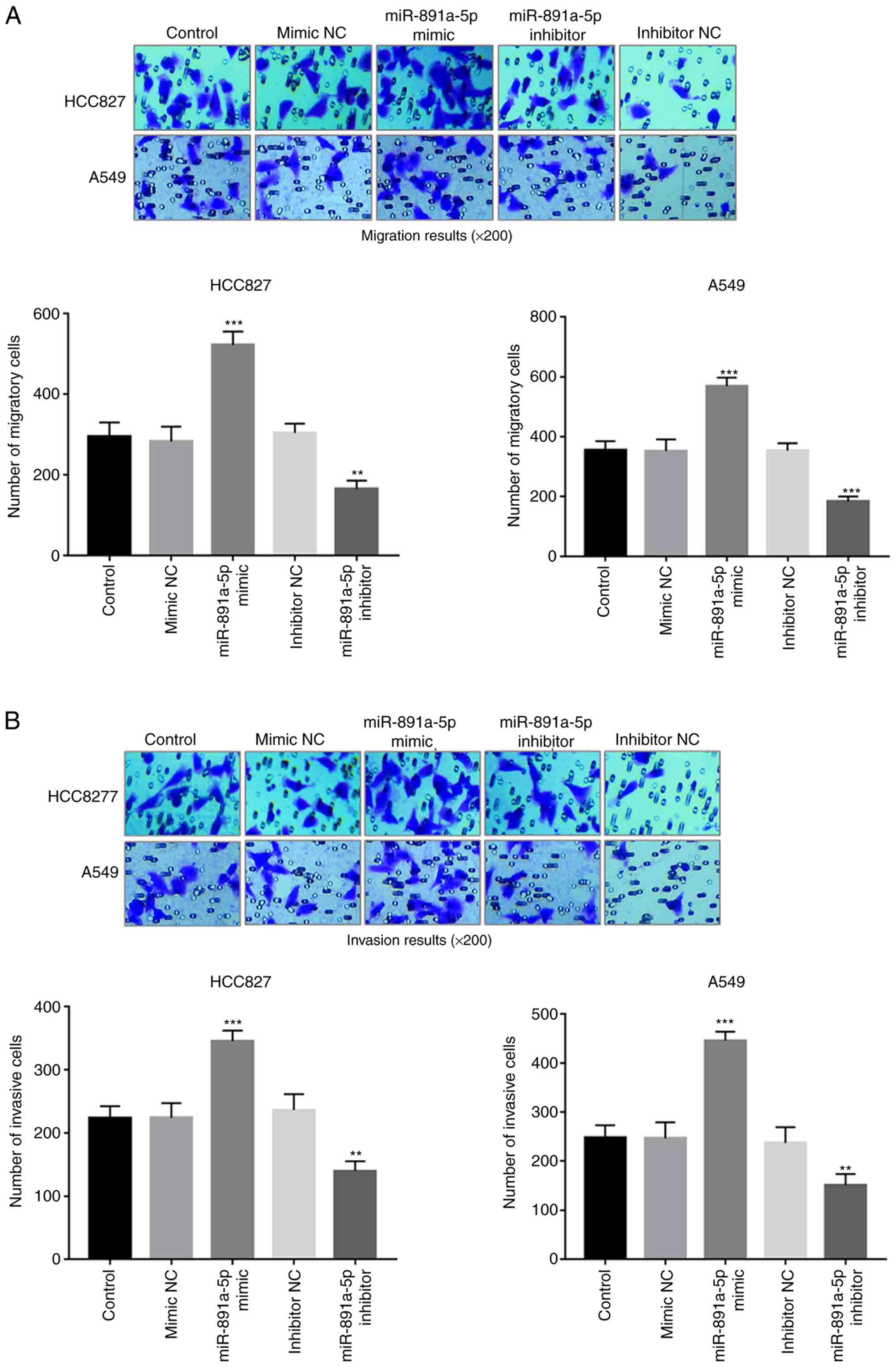
The effects of miR-891a-5p on cell migration and
invasion in the HCC827 and A549 cells were also investigated using
a Transwell system. Upregulation of miR-891a-5p resulted in
enhanced cell migration, whereas downregulation of miR-891a-5p
resulted in decreased cell migration, in both cell lines (all
P<0.01) (Fig. 4A). Similarly, the
overexpression of miR-891a-5p also promoted the invasive ability of
the NSCLC cells, but knockdown of miR-891a-5p inhibited the
invasive ability (all P<0.01) (Fig.
4B).
HOXA5 is a direct target of
miR-891a-5p in the NSCLC cells
According to the results of the TargetScan online
analysis, HOXA5 was predicted to be a target of miR-891a-5p, with a
complementary sequence at its 3′-UTR (Fig. 5A). The binding site for miR-891a-5p
was found in the HOXA5 gene, and luciferase reporter assays were
used to detect the interaction between miR-891a-5p and HOXA5. A
dual-luciferase reporter assay result indicated that the luciferase
activity in the HOXA5-WT group was decreased by the upregulated
expression level of miR-891a-5p, but was increased by the decreased
expression of miR-891a-5p in both the HCC827 and A549 cells (all
P<0.05) (Fig. 5B and C). However,
there was no significant change in luciferase activity in the MUT
group (all P>0.05). Detection of HOXA5 mRNA expression levels in
the HCC827 and A549 cells revealed that miR-891a-5p mimics
inhibited HOXA5 expression levels, whereas silencing of miR-891a-5p
enhanced the expression of HOXA5 (all P<0.01) (Fig. 5D and E). In addition, the expression
level of HOXA5 in the tumor tissues was significantly lower
compared with that in the adjacent normal lung tissues in patients
with NSCLC (P<0.001; Fig. 5F),
which was found to be negatively correlated with the expression
level of miR-891a-5p (r, −0.760; P<0.001; Fig. 5G). The aforementioned results
indicated that HOXA5 may be a direct target of miR-891a-5p in
NSCLC.
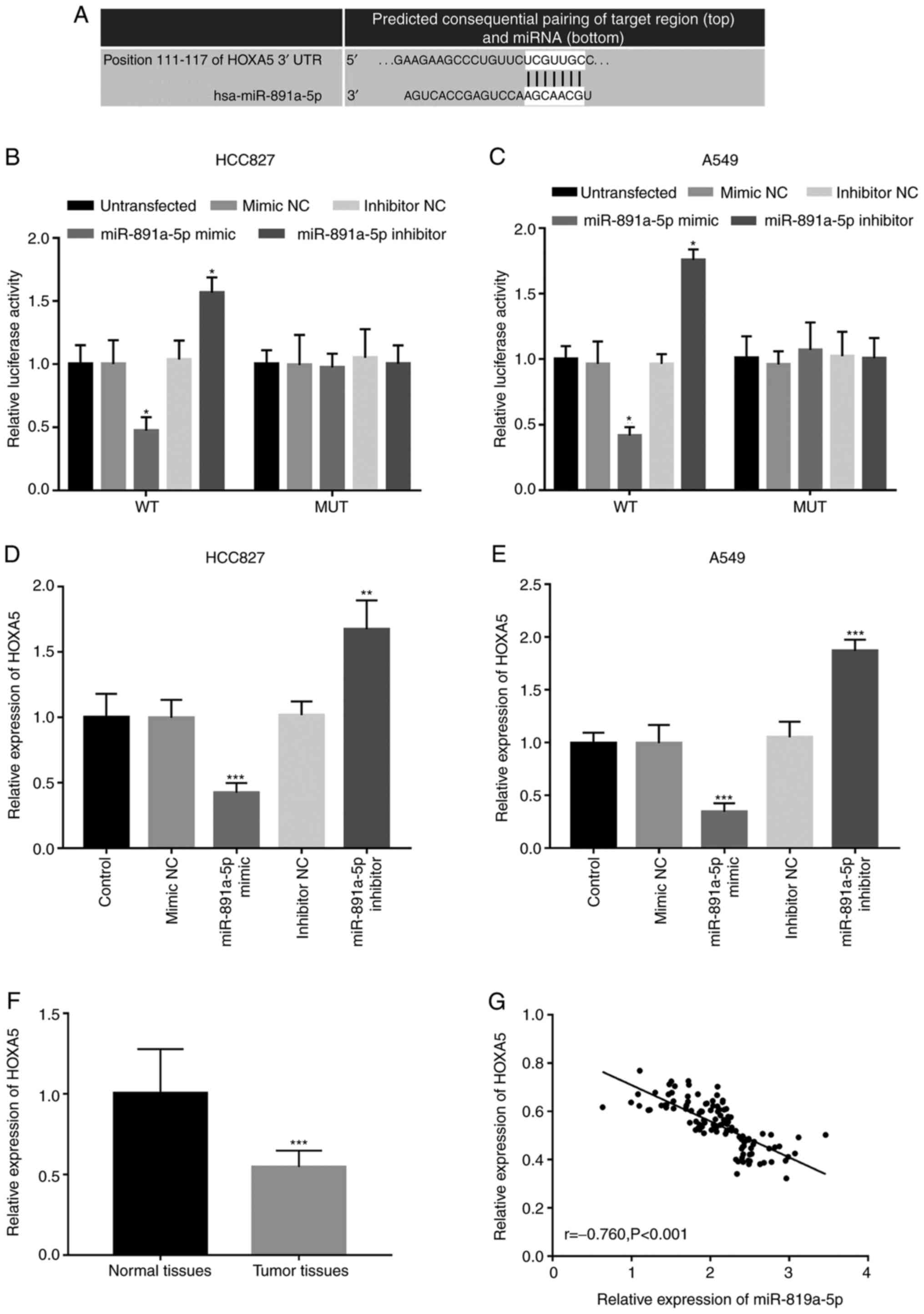 | Figure 5.HOXA5 is a direct target of
miR-891a-5p in NSCLC cells. (A) The predicted target sequence
between HOXA5 and miR-891a-5p. In the (B) HCC827 and (C) A549 NSCLC
cell lines, the luciferase activity of the HOXA5-WT was decreased
following the overexpression of miR-891a-5p, but was increased by
the knockdown of miR-891a-5p expression. *P<0.05 vs.
untransfected. The expression level of HOXA5 level in the (D)
HCC827 and (E) A549 cells revealed that the miR-891a-5p mimics
inhibited the expression level of HOXA5, while knockdown of
miR-891a-5p increased the expression level. **P<0.01,
***P<0.001 vs. control. (F) The expression level of HOX5 in
adjacent normal tissue was significantly increased compared with
that in the NSCLC tissue. ***P<0.001 vs. normal tissues. (G) The
expression level of HOXA5 was negatively correlated with the
expression level of miR-891a-5p. miR, microRNA; NSCLC, non-small
cell lung cancer; NC, negative control; WT, wild-type; MUT, mutant;
UTR, untranslated region. |
Discussion
NSCLC is the most common type of lung cancer,
worldwide and has numerous pathological features (17). Its estimated 5-year survival rate is
only 15.9%, a figure that has only slightly improved over the past
few decades (18). The occurrence
and development of NSCLC can result in changes to a wide range of
pathological processes, including complex changes in the mRNA
expression level of various oncogenes and tumor suppressor genes,
such as miR-182-5p, miRNA-148a, miRNA-200a-3p (19–21),
that play a key role in cell proliferation and apoptosis (5). Therefore, a deeper understanding of the
genes and related mechanisms involved in the progression of NSCLC
is urgently required to improve the current treatments and the
prognosis of this disease.
miRNA has been shown to be involved in the
pathogenesis of several types of cancer, and some miRNA families
show functions similar to oncogenes or tumor-suppressor genes
(22). For example, it was
demonstrated that the expression level of miR-519a was decreased in
gastric cancer cell lines compared with that in normal gastric
cells, and the proliferation, migration and invasion of tumor cells
could be inhibited by the overexpression of miR-519a (23). In osteosarcoma tissues, the
expression levels of miR-99b were downregulated and this was
associated with the clinical stage and distant metastasis of the
patients with osteosarcoma, suggesting it could be used as a
biomarker for this type of malignancy (24). Liang et al (25) found that the expression level of
miR-146a-5p was increased in triple-negative breast cancer. The
aforementioned study showed that patients with high levels of
miR-146a-5p in tumor tissues had poor survival, therefore,
increased expression of miR-146a-5p in triple-negative breast
cancer cells was associated with poor prognosis (25). All the aforementioned studies
suggested that functional miRNAs may serve as a new direction into
the research for cancer targeted therapy. The role of some miRNA
has been investigated in NSCLC, and their clinical significance and
functional role have also been determined in previous studies. For
example, miR-940 inhibited the proliferation of cancer cells by
targeting FAM83F and further inhibited the progression of NSCLC
(26). Feng et al (27) found that miR-16-1-3p was
significantly downregulated in NSCLC cells compared with that in
normal lung cells. In addition, decreased cell proliferation,
inhibited cell migration and invasion following transfection with
miR-16-1-3p mimics, compared with that in the negative control
group.
As a functional miRNA, miR-891a-5p has become a hot
topic, particularly when investigating tumors, cell proliferation
and cell differentiation (28).
Zhang et al found that low expression levels of miR-891a-5p
in breast cancer tissue were significantly associated with low
distant metastasis-free survival in patients with breast cancer,
suggesting that the expression level of miR-891a-5p could be a
potential prognostic marker for metastatic human breast cancer
(29). However, the biological
function and clinical significance of miR-891a-5p have rarely been
reported in NSCLC. Therefore, the role of miR-891a-5p in the
progression of NSCLC is warranted. In the present study, RT-qPCR
was used to determine the mRNA expression levels of miR-891a-5p in
NSCLC tissue and serum samples and it was found that the mRNA
expression of miR-891a-5p was significantly increased in NSCLC
tissue and serum samples compared with that in the corresponding
normal controls. Due to the increase in miR-891a-5p mRNA expression
level in NSCLC, the clinical significance of miR-891a-5p in the
diagnosis and prognosis of NSCLC was also investigated (30). Therefore, ROC analysis was performed
based on the serum expression level of miR-891a-5p. The results
indicated that the AUC, from the ROC curve, was 0.904, and that an
AUC above 0.9 indicated high diagnostic accuracy (31). Thus, miR-891a-5p may have high
sensitivity and specificity and could be a potential diagnostic
biomarker. According to the Kaplan-Meier survival curves and Cox
regression analysis, it was also found that patients with NSCLC and
high expression levels of miR-891a-5p had poor overall survival
rates and miR-891a-5p was an independent prognostic factor for
NSCLC. Furthermore, the increased miR891a-5p expression level was
found to be associated with differentiation, lymph node metastasis
and TNM stage in patients with NSCLC, indicating that the increased
expression level of miR-891a-5p might be associated with the
development of NSCLC. However, no significant relationship was
found between miR-891a-5p and tumor size, although the regulatory
effects of miR-891a-5p on tumor progression were observed in NSCLC
cells. In addition, cell proliferation migration and invasion of
the tumor cells are not manifested solely by the size of the tumor,
but are also associated with the differentiation and TNM stage of
the tumor (32). Taken together, it
is hypothesized that miR-891a-5p may serve as a candidate
diagnostic and prognostic biomarker in patients with NSCLC.
To understand the biological function of miR-891a-5p
in the progression of NSCLC, further cellular experiments were
performed. The results indicated that the downregulation of
miR-891a-5p resulted in suppressed tumor cell migration,
proliferation and invasion, but the upregulation of miR-891a-5p led
to enhanced tumor cell biological behaviors. The aforementioned
results confirmed that miR-891a-5p may play a role in promoting
tumorigenesis in NSCLC progression. In addition, the mechanisms of
miR-891a-5p in NSCLC was further analyzed. The results of the
dual-luciferase reporter assay revealed that miR-891a-5p could
directly bind to HOXA5 in NSCLC cell lines. In addition, the
results of the cell experiments showed that changing the expression
level of cellular miR-891a-5p could affect the expression level of
HOXA5 in the cells. Altered HOXA5 methylation levels were found to
affect disease development, for example in type II diabetes
(33) and colorectal cancer
(34). In addition, it was
previously shown that HOXA5 was regulated by miRNAs to play a role
in numerous types of cancer and affect the biological activity of
cancer cells. For example, miR-196a was found to act as a tumor
suppressor in gastric cancer by inhibiting cell viability, colony
formation, proliferation, cell cycle progression and promoting cell
apoptosis by targeting HOXA5 (35).
In colon cancer, HOXA5 decreased expression could induce the
phenotypic loss of tumor stem cells, thereby preventing tumor
progression and metastasis (36).
HOXA5 expression was altered in patients with different diseases,
such as lung cancer, primary pulmonary hypertension and chronic
obstructive pulmonary disease (COPD) (37). A previous study by Chang et al
(38) showed that overexpression of
HOXA5 could reduce the invasive ability of several lung cancer cell
lines via a p53-independent pathway. Therefore, it is hypothesized
that miR-891a-5p may be involved in NSCLC tumorigenesis by
targeting HOXA5.
The present study, has several limitations, such as
the limited sample size, the lack of paracancerous tissue results
and the absence of animal experiments. In addition, the effects of
other factors, such as methylation, on the expression level of
HOXA5 were not investigated, which may interfere with the
regulation of HOXA5 by miR-891a-5p. Therefore, in future studies,
the results from the present study should be confirmed using cancer
tissues, paracancerous tissues and adjacent normal tissues from a
larger study cohort. The functional role and mechanisms of
miR-891a-5p in the tumorigenesis of NSCLC should also be further
investigated using animal cancer models, and the specific
regulatory mechanism of the HOXA5/miRNA 891a-5p axis.
In conclusion, the results from the present study
indicated that miR-891a-5p expression was increased in NSCLC
tissues and serum samples, and may be used as a diagnostic and
prognostic biomarker. The overexpression of miR-891a-5p was shown
to promote NSCLC cell proliferation, migration and invasion, which
indicated that miR-891a-5p could be an oncogene in NSCLC
progression, and has the potential to be used to improve targeted
therapy for NSCLC. HOXA5 was also identified as a target gene of
miR-891a-5p, which may mediate the biological function of
miR-891a-5p in NSCLC progression.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
NW and JZ designed the study, performed clinical
studies, analyzed data and wrote the manuscript. NW performed the
cell experiments. NW and JZ confirm the authenticity of all raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was provided by each
patient and the experimental procedures were all in accordance with
the guidelines of the Ethics Committee of Yueqing People's Hospital
(Yueqing, China; approval no. 0112983).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nasim F, Sabath BF and Eapen GA: Lung
Cancer. Med Clin North Am. 103:463–473. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Võsa U, Vooder T, Kolde R, Vilo J,
Metspalu A and Annilo T: Meta-analysis of microRNA expression in
lung cancer. Int J Cancer. 132:2884–2893. 2013. View Article : Google Scholar
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu J, Bian T, Feng J, Qian L, Zhang J,
Jiang D, Zhang Q, Li X, Liu Y and Shi J: miR-335 inhibited cell
proliferation of lung cancer cells by target Tra2β. Cancer Sci.
109:289–296. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu YL, Sun Y, Zhou CC, Zhang L, Yu SY, Ma
SL, Han LL, Zhang XQ and Orlando M: Survival without common
toxicity criteria grade 3/4 toxicity following second-line
treatment with pemetrexed for nonsquamous non-small cell lung
cancer in Chinese patients. Chin Med J (Engl). 126:4624–4628.
2013.PubMed/NCBI
|
|
7
|
Woodard GA, Jones KD and Jablons DM: Lung
Cancer Staging and Prognosis. Cancer Treat Res. 170:47–75. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Turchinovich A, Weiz L and Burwinkel B:
Extracellular miRNAs: The mystery of their origin and function.
Trends Biochem Sci. 37:460–465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Barbato S, Solaini G and Fabbri M:
MicroRNAs in oncogenesis and tumor suppression. Int Rev Cell Mol
Biol. 333:229–268. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Xu X, Ge G, Zang X, Shao M, Zou
S, Zhang Y, Mao Z, Zhang J, Mao F, et al: miR 498 inhibits the
growth and metastasis of liver cancer by targeting ZEB2. Oncol Rep.
41:1638–1648. 2019.PubMed/NCBI
|
|
11
|
Yuan J, Su Z, Gu W, Shen X, Zhao Q, Shi L,
Jin C, Wang X, Cong H and Ju S: MiR-19b and miR-20a suppress
apoptosis, promote proliferation and induce tumorigenicity of
multiple myeloma cells by targeting PTEN. Cancer Biomark.
24:279–289. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ganju A, Khan S, Hafeez BB, Behrman SW,
Yallapu MM, Chauhan SC and Jaggi M: miRNA nanotherapeutics for
cancer. Drug Discov Today. 22:424–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin K, Xu T, He BS, Pan YQ, Sun HL, Peng
HX, Hu XX and Wang SK: MicroRNA expression profiles predict
progression and clinical outcome in lung adenocarcinoma.
OncoTargets Ther. 9:5679–5692. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee HY, Han SS, Rhee H, Park JH, Lee JS,
Oh YM, Choi SS, Shin SH and Kim WJ: Differential expression of
microRNAs and their target genes in non-small-cell lung cancer. Mol
Med Rep. 11:2034–2040. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin Z, Zhao J, Wang X, Zhu X and Gong L:
Overexpression of microRNA-497 suppresses cell proliferation and
induces apoptosis through targeting paired box 2 in human ovarian
cancer. Oncol Rep. 36:2101–2107. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen Z, Fillmore CM, Hammerman PS, Kim CF
and Wong KK: Non-small-cell lung cancers: A heterogeneous set of
diseases. Nat Rev Cancer. 14:535–546. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Akhurst T: Staging of non-small-cell lung
cancer. PET Clin. 13:1–10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao L, Yan SB, Yang J, Kong JL, Shi K, Ma
FC, Huang LZ, Luo J, Yin SY, He RQ, et al: MiR-182-5p and its
target HOXA9 in non-small cell lung cancer: A clinical and
in-silico exploration with the combination of RT-qPCR, miRNA-seq
and miRNA-chip. BMC Med Genomics. 13:32020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen Y, Min L, Ren C, Xu X, Yang J, Sun X,
Wang T, Wang F, Sun C and Zhang X: miRNA-148a serves as a
prognostic factor and suppresses migration and invasion through
Wnt1 in non-small cell lung cancer. PLoS One. 12:e01717512017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tan T, Xu XH, Lu XH and Wang XW:
MiRNA-200a-3p suppresses the proliferation, migration and invasion
of non-small cell lung cancer through targeting IRS2. Eur Rev Med
Pharmacol Sci. 24:712–720. 2020.PubMed/NCBI
|
|
22
|
Zhang L, Deng T, Li X, Liu H, Zhou H, Ma
J, Wu M, Zhou M, Shen S, Li X, et al: microRNA-141 is involved in a
nasopharyngeal carcinoma-related genes network. Carcinogenesis.
31:559–566. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cai H, Lin H, Cao W, Sun J, Huang Y and
Fang Y: The downregulation of miR-519a predicts poor prognosis and
contributes to tumor progression in gastric cancer. Int J Clin Exp
Pathol. 12:2496–2505. 2019.PubMed/NCBI
|
|
24
|
Shi X and Guan X: MicroRNA-99b predicts
clinical outcome of osteosarcoma and suppresses tumor cell
proliferation, migration and invasion. Diagn Pathol. 14:1172019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang H, Huang W, Wang Y, Ding L and Zeng
L: Overexpression of MiR-146a-5p Upregulates lncRNA HOTAIR in
triple-negative breast cancer cells and predicts poor prognosis.
Technol Cancer Res Treat. 18:15330338198829492019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gu GM, Zhan YY, Abuduwaili K, Wang XL, Li
XQ, Zhu HG and Liu CL: MiR-940 inhibits the progression of NSCLC by
targeting FAM83F. Eur Rev Med Pharmacol Sci. 22:5964–5971.
2018.PubMed/NCBI
|
|
27
|
Feng QQ, Dong ZQ, Zhou Y, Zhang H and Long
C: miR-16-1-3p targets TWIST1 to inhibit cell proliferation and
invasion in NSCLC. Bratisl Lek Listy. 119:60–65. 2018.PubMed/NCBI
|
|
28
|
Yao S, Hu M, Hao T, Li W, Xue X, Xue M,
Zhu X, Zhou F, Qin D, Yan Q, et al: MiRNA-891a-5p mediates HIV-1
Tat and KSHV Orf-K1 synergistic induction of angiogenesis by
activating NF-κB signaling. Nucleic Acids Res. 47:27002019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang Z, Xu L, He L, Wang J, Shi X, Li Z,
Shi S, Hou K, Teng Y and Qu X: MiR-891a-5p as a prognostic marker
and therapeutic target for hormone receptor-positive breast cancer.
J Cancer. 11:3771–3782. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu X and Lu J: The significance of
detection of serum miR-423-5p and miR-484 for diagnosis of
colorectal cancer. Clin Lab. 61:187–190. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kamarudin AN, Cox T and Kolamunnage-Dona
R: Time-dependent ROC curve analysis in medical research: Current
methods and applications. BMC Med Res Methodol. 17:532017.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Enane FO, Saunthararajah Y and Korc M:
Differentiation therapy and the mechanisms that terminate cancer
cell proliferation without harming normal cells. Cell Death Dis.
9:9122018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao W, Xu Y, Luo D, Saeed M and Sun C:
Hoxa5 promotes adipose differentiation via increasing DNA
methylation level and inhibiting PKA/HSL signal pathway in Mice.
Cell Physiol Biochem. 45:1023–1033. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li D, Bai Y, Feng Z, Li W, Yang C, Guo Y,
Lin C, Zhang Y, He Q, Hu G, et al: Study of promoter methylation
patterns of HOXA2, HOXA5, and HOXA6 and its clinicopathological
characteristics in colorectal cancer. Front Oncol. 9:3942019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu Y, Zhou T, Tang Q and Xiao J: HOXA5
inhibits tumor growth of gastric cancer under the regulation of
microRNA-196a. Gene. 681:62–68. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ordóñez-Morán P, Dafflon C, Imajo M,
Nishida E and Huelsken J: HOXA5 counteracts stem cell traits by
inhibiting Wnt signaling in colorectal cancer. Cancer Cell.
28:815–829. 2015. View Article : Google Scholar
|
|
37
|
Liu XH, Lu KH, Wang KM, Sun M, Zhang EB,
Yang JS, Yin DD, Liu ZL, Zhou J, Liu ZJ, et al: MicroRNA-196a
promotes non-small cell lung cancer cell proliferation and invasion
through targeting HOXA5. BMC Cancer. 12:3482012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chang CJ, Chen YL, Hsieh CH, Liu YJ, Yu
SL, Chen JJ and Wang CC: HOXA5 and p53 cooperate to suppress lung
cancer cell invasion and serve as good prognostic factors in
non-small cell lung cancer. J Cancer. 8:1071–1081. 2017. View Article : Google Scholar : PubMed/NCBI
|