Introduction
Since 1966, cancer of the nasopharynx has been
featured among the top 5 cancers in Malaysia. Between 1981 and
1982, cancer of the nasopharynx was the second most common cancer
among males in Sarawak (1).
Specifically, from January 1981 to December 1982, the Land Dayak
(the name then for Bidayuh, a native ethnic group of Sarawak), had
a crude rate of cancer of the nasopharynx of 9.0 per 100,000
population per year, followed by Sarawak Chinese with 5.8 per
100,000 (1).
While Bidayuh was formerly known as Land Dayak, Iban
was once referred to as Sea Dayak (2). With regards to history, Dayak comprises
the inland or interior groups of people in Borneo (2) and Bidayuh means ‘people of the land’
(3). Today, Dayak refers to the
Iban, Bidayuh and Orang Ulu; Iban forms the largest indigenous
category (4). The Bidayuh community
is categorised into 4 main groups: i) Biatah; ii) Bau-Jagoi; iii)
Bukar-Sadung; and iv) Salako-Rara (5). The estimated Bidayuh population size in
2020 was 220,000 (6).
The island of Borneo comprises the East Malaysian
states of Sabah and Sarawak, the Malaysian federal territory of
Labuan, the country Brunei and the Indonesian territory known as
Kalimantan. The initial settlement of the Bidayuh in Sarawak,
~1,000 years ago, was Bung Bratak, among others. The ancestors of
Bung Bratak inhabitants were originally from Gunong Sunkong,
Kalimantan (2). The Bidayuh migrated
from the western part of Borneo island (3) or West Kalimantan, while the Iban
generally migrated from Kalimantan (7).
Between the years 2012–2016, cancer of the
nasopharynx was the fifth most common cancer among Malaysian
residents. Also, cancer of the nasopharynx was the fifth most
common cancer in Malaysian male residents. It was the fourth most
common cancer in Malaysian Chinese males. Notably, nasopharynx
cancer is the top common cancer among males in Sarawak (8). The uniqueness of this situation in
Sarawak contrasts with the pattern of cancer incidence in other
states in Malaysia, where colorectal cancer and lung cancer exceed
nasopharyngeal cancer (8).
Additionally, in 2007–2011, the nasopharynx was the most common
cancer site among Sarawak males of Bidayuh or Iban ethnicity
(9). Nevertheless, other ethnic
groups of Sarawak, such as Chinese, Malay, and Melanau were also at
increased risk (9). From 2007–2011,
the age-standardised rate (ASR) in Sarawak was 11.6 per 100,000
population and 4.8 per 100,000 in males and females, respectively;
with a male to female ASR ratio of 2.4:1 (9). Specifically, the ASR for Bidayuh males
and females, respectively, was 24.6 and 9.3 per 100,000 (9). The Iban people had an ASR of 12.0 per
100,000 for males and 4.7 per 100,000 females (9). Among the Chinese, the ASR was 11.2 per
100,000 for males and 4.1 per 100,000 for females (9). The ASR for Malay males was 8.1 per
100,000, while for Malay females, the ASR was 4.5 per 100,000
(9). Finally, amongst Melanau, the
ASR was 4.7 per 100,000 and 1.9 per 100,000 in males and females,
respectively (9). Notwithstanding
ethnicity, the incidence ratio was 2–3 folds higher in males
compared with females. Worldwide, between 2008–2012, Zhongshan
City, China, had the highest ASR for males (25 per 100,000) while
Zhuhai, China, had the highest ASR for females (9 per 100,000)
(10). The ASR among Bidayuh people
was very similar to the highest ASR internationally. Meanwhile,
GLOBOCAN 2020 estimates the world ASR at 2.2 per 100,000 for males
and 0.8 per 100,000 for females (11). The reasons males are more frequently
affected compared with females may be due to genetic susceptibility
affected by the X-chromosome (12),
or oestrogen sex hormone-associated (13), lifestyle, behaviour, environment or a
combination of these factors (14).
Several types of tumours may develop at the
nasopharynx, the most common being nasopharyngeal carcinoma (NPC)
(15,16). The Fossa of Rosenmüller is usually
the site of origin (17). NPC has
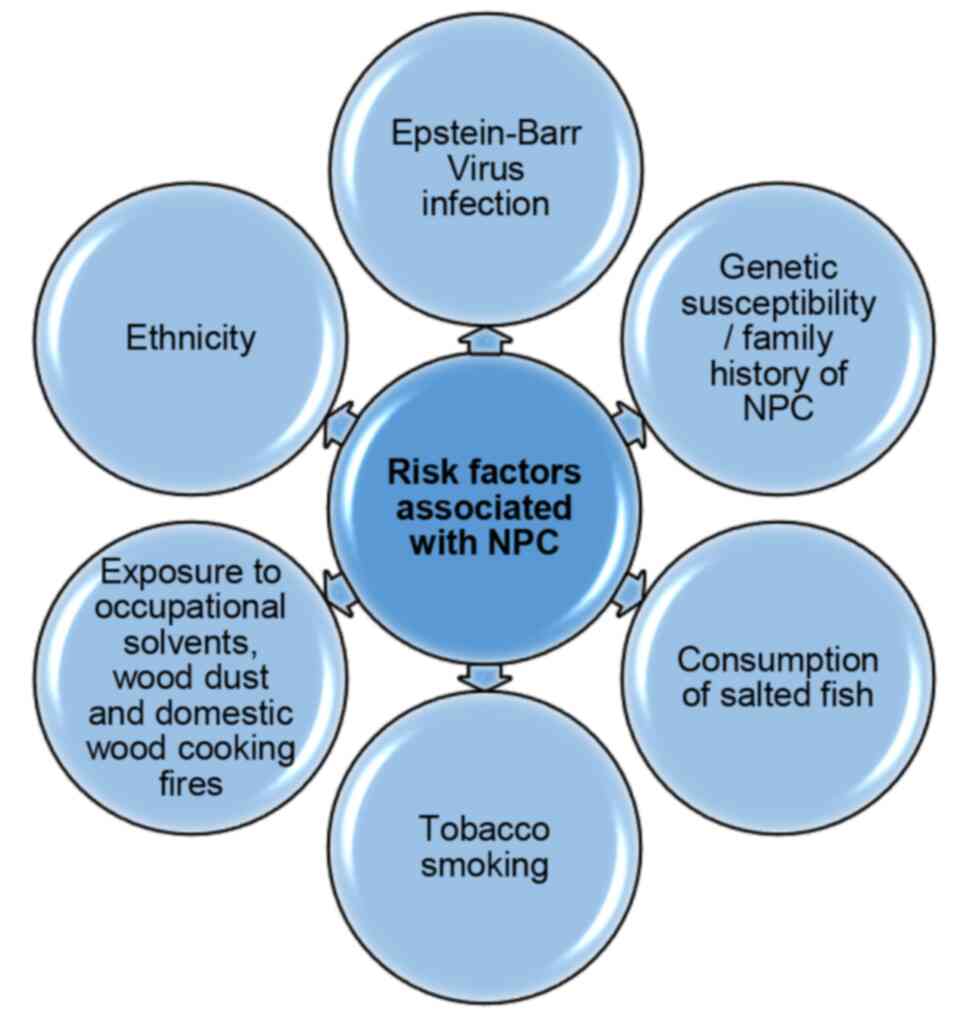
been associated with various risk factors (Fig. 1) (18,19),
suggesting multifactorial causation. The present review discusses
several of these risk factors focusing on the Malaysian scenario,
particularly among the Bidayuh of Sarawak.
Epstein-Barr virus infection
The risk for certain types of cancers increases with
persistent infection of bacteria, parasites, or viruses (20). Among the cancer-causing viruses are
Epstein-Barr Virus (EBV), which may cause Hodgkin and certain
non-Hodgkin lymphomas, stomach cancer and NPC (20). EBV belongs to the herpesvirus family,
and it is ubiquitous (21). It is
present in >90% of the world's population (21). In places where NPC is endemic,
>95% of cases are associated with EBV (22). EBV infection in NPC is predominantly
latent (23,24). Recently, specific EBV variants such
as non-synonymous EBV variants within BALF2 (SNP 162476_C and SNP
163364_T), were suggested to be associated with NPC (25–29).
Inspite of multiple in vitro and in vivo experiments,
studies have demonstrated that EBV infection by itself is
insufficient for the tumorigenic transformation of epithelial cells
(23,30). The role of EBV in the pathogenesis of
NPC has been investigated widely and comprehensive reports are
available (23,31–37). The
present review will not cover the topic of EBV-encoded latent genes
such as, EBV-encoded small RNAs, EBV nuclear antigen 1, latent
membrane protein 1, latent membrane protein 2 and Bam H1 A
rightward open reading frame 1 and lytic genes, such as the
immediate early transactivator BZLF1.
Genetic predisposition
Inheritance of cancer-predisposing genetic mutations
is linked to certain cancers, including leukaemias and lymphomas,
breast, ovarian, pancreatic, prostate, skin cancer and NPC
(20). A family history of any
cancer, especially NPC, contributes independently or mutually to
NPC risk factors (38,39). Individuals with a first-degree
relative with NPC have an elevated risk of developing NPC (38). In endemic regions, this familial risk
is substantial due to shared genetic susceptibility among relatives
or environmental risk factors (38).
In the endemic area of Guangdong, China, the combination of family
history of cancer or history of NPC and consumption of
salt-preserved fish was significantly associated with risk of NPC;
higher than the individual risk of family history alone or
consumption of salt-preserved fish alone (38). A recent report suggests that familial
risk of NPC may be associated with host response to EBV (40).
A genetic hereditary study of the Chinese population
of Southern China and the Bidayuh population found that the Bidayuh
genetic material shared similarity with ancestor lineage that could
have originated from South China or Indochina 10,000-30,000 years
ago, preceding the Austronesian expansion (41). From a linguistic perspective, the
language of the Bidayuh belongs to the Austronesian family
(42). Based on genetic and
anthropological epidemiology, cultural and linguistic similarity,
it was hypothetically proposed that a common ancestral origin
exists between the Bidayuh and the Bai-Yue tribe
(proto-Austronesian people), the reference population postulated
for NPC origin of Southern China (43). In addition, it was found that
mitochondrial DNA (mtDNA) of the Bidayuh was 60% similar to the
Bai-Yue (41,44). As ancestral populations migrated out
from Taiwan, one of the proposed dispersal centres of Austronesian
speakers (45), the inheritance of
NPC susceptibility gene(s) from Austronesian ancestors may explain
why the Bidayuh and Bai-Yue have a genetic predisposition to NPC
(16). A 1998–2003 study involving
patients with NPC in Guangzhou, China, detected a novel 4981 bp
deletion in mtDNA in 93% of cases, in contrast to the most abundant
4977 bp common deletion (CD) (46).
The aforementioned report indicated that CD-mtDNA mutation may be
implicated in NPC development and progression (46). In 1997–1998, a study in patients with
NPC in Guangzhou, China, demonstrated somatic mutations and
polymorphisms in mtDNA, such as A→G substitution at np15769 and C→T
substitution at np15970 (47).
Polymorphisms found included np 292T→C, np16242C→A, np16038A→G and
np15787T→C (47). Sequence variants
in mtDNA had been reported to be associated with genetic
susceptibility to familial NPC in the Zhejiang area, China
(48). A 2001–2004 study of patients
with NPC within the Chaoshan population of China on mtDNA sequence
variation identified variants significantly associated with NPC
risk (49). In addition, mtDNA
haplogroup distribution has demonstrated that the haplogroup R9 and
its sub-haplogroup F1 are positively associated with NPC risk
(49). However, the manner in which
susceptibility genes cause NPC remains unknown, and the biological
impacts of mtDNA mutations are still unclear (46,47). In
addition, to the best of our knowledge to date there has been no
study on the mtDNA of the Bidayuh that may contribute to NPC.
Studies of HLA allele frequencies are essential for
anthropology and understanding human migration patterns (50). In 2009, a study encompassing the
Bidayuh pinpointed the frequent detection of alleles A*3401 and
B*1521 (51). These alleles were
also common in Australian aborigines, but rare in most other
Southeast Asians (51). Furthermore,
the Bidayuh also have high frequencies of DRB1*1602, similar to the
Papua New Guinea Lowlanders who linguistically also have
Austronesian origins (51).
Comparisons of HLA allele frequencies showing genetic similarities
further strengthen the school of thought that the Bidayuh and
ancient Bai-Yue Chinese tribe share common ancestry of
Austronesians but were geographically separated via migration
waves.
HLA alleles are not known oncogenes (52). Hence, HLA by itself, is not
responsible for NPC development (53). Instead, suppression of immune
response presented by HLA alleles may enable immune evasion of
EBV-infected NPC cells (23), hence
permitting NPC cell survival. According to an HLA polymorphism
study, the genetic distance between Bidayuh and Iban was relatively
short, with a value of 0.00986, explaining the Iban's genetic
predisposition to NPC being similar to the Bidayuh (50). Studies of the genetic linkage of the
Bidayuh and Iban especially in regard to immune responses warrants
further attention.
Diet
Sarawak's people take pride in their ethnic native
food, which has become the state's identity (54). Sarawak's geographical terrain,
particularly during pre-independence and the lifestyle then,
resulted in the forests becoming the source of traditional foods
(54). Given that the exclusive
preparation methods of ethnic cuisine are passed down from one
generation to another, traditional food is continually consumed
(54).
A high salt diet posed an increased risk of
developing various types of cancers, such as gastric cancer
(55,56), whereas salted fish consumption showed
a discernible positive association with NPC (57). Notably, the ethnic group with the
highest incidence rates for both nasopharyngeal carcinoma and
gastric cancers amongst the men in Sarawak was reported to be the
Bidayuh, followed by the Chinese and Ibans (9). The Bidayuh population consumes
preserved pork and fish (58) that
could have increased their risk of developing NPC, a hypothetical
observation given the diet of the Chinese, another high-risk NPC
population, which consists of a high amount of salted food
(59). In accordance, in Guangdong
province, Southern China, a significant relationship exists between
monthly regular salted fish consumption and increased NPC risk
(60), especially during childhood
(61). Salted vegetables and
preserved meat are other risk factors for NPC (61). Among Malaysian Chinese in the state
of Selangor, a history of any kind of salted fish intake in
childhood which may continue into adolescence was a significant
risk factor for NPC (62,63).
Salt is used to preserve food, such as raw or cooked
meat and vegetables to inhibit microorganism growth during extended
storage (64). Unless the food
undergoes a quality check to monitor the amount and usage of
preservative compounds, which is possible if produced in factories,
there is no limit set for homemade preserved food (64). Salted fish contains N-nitrosamines.
On top of that, salt used in preserving food is crude sea salt
(62). Unlike pure sodium chloride,
sea salt contains nitrates and nitrites which could produce
nitrosamine, a carcinogenic compound (65) formed from various stomach reactions
between nitrates and nitrites with secondary amines (62,66).
Nitrosamines induce methylation of DNA that may activate
proto-oncogenes, such as ras or myc or inactivate suppressor genes
such as, p53 or cyclin-dependent kinase inhibitor 2A (67,68). In
addition, N-nitroso compounds and high salt consumption was found
to reactivate EBV from the latent stage (69,70),
which could further increase the risk of NPC (71).
Environmental exposure
Exposure to hazardous environmental pollutants,
occupational agents and inhaled carcinogens could increase an
individual's risk of developing NPC (63,72).
This relationship could be attributed to the nasopharynx's ability
to trap dust and smoke particles from inhaled air, which eventually
leads to inflammation (63,72). Given that dust and smoke are of the
correct particulate matter size and weights able to be deposited in
the nasopharynx, inhaled dust and smoke present as reasonable
biological risk factors for NPC (63,72).
In Sarawak, the timber industry is an important
economic industry which contributes to numerous wood-based products
for export (73). Sawmills that
process timber for wood planks use various hazardous chemicals,
such as phenol-formaldehyde resin and urea (74,75). The
usage of phenol-formaldehyde increases the chance of getting NPC
1.83-fold (76). A study conducted
in the United States found that occupational exposure to
formaldehyde increases NPC risk (77). The Guangdong province of Southern
China, with high NPC incidence, is also well-known for wood-based
industries (78). Wood-based
productions emit significant amounts of formaldehyde and other
total volatile organic compounds, such as terpene (78). Wood dust was associated with a
considerable risk of developing upper respiratory cancers in a
study conducted in New York, United States (79). In a 1990–1992 study of NPC (all
squamous cell carcinoma) among the Malaysian Chinese of Selangor
and Federal Territory of Kuala Lumpur, wood dust was associated
with NPC (72). The significant
association between all kinds of dust and exposure to wood smoke
with NPC was also reported earlier among the Chinese of Selangor
(63). In Nigeria, exposure to
cooking wood fumes was the commonest and a significant risk factor
associated with malignancies in the nasopharynx among females
(80). Some communities in Sarawak
still prefer food cooked using firewood (81). The smoke produced by burning charcoal
contains various hazardous chemicals that are carcinogenic, such as
dimethylbenzathracene and benzanthracene (82). Incomplete burning of wood fires used
in cooking produces smoke particles that could be deposited in the
nasopharynx (63,72).
Rubber processing factories use chemicals during
vulcanisation to change raw rubber properties to more durable
products, such as tyres, window seals, tubes and shoe soles
yielding nitrosamines, such as N-nitrosodimethylamine,
N-nitrosodiethylamine, N-nitrosodibutylamine, N-nitrosopiperidine,
N-nitrosopyrrolidine and N-nitrosomorpholine (83,84).
Exposure to a high concentration of nitrosamines in German rubber
factory workers increased their risk of developing respiratory
cancers (85). Rubber is a main
contributor from the agricultural sector to Malaysia's economy
(86). Sarawak's administration and
farmers favour the rubber industry compared to rice because of its
price and market demand (87,88).
Malaysian rubber tappers are exposed to formic acid, wood smoke and
talc powder while producing rubber sheets (63). Rubber processing was significantly
related to NPC development in Selangor Chinese (63). Formaldehyde is another principal
chemical used in the production of natural rubber (78). It is currently speculated that
formaldehyde is a significant risk factor for NPC, especially among
rubber industrial workers exposed to various chemicals without
proper precautions or personal protection equipment (78). Rubber industrial workers have a
higher chance of developing NPC compared with individuals not
exposed to formaldehyde (78).
As of 2010, almost half of the Bidayuh population
are working in the service sector (6). However, back in 1947, close to 99% of
Bidayuh participated in agriculture (6). Given the high dependency of Sarawak's
people on agricultural produce, open burning is still practised
during land clearing before planting, primarily in rural areas
(89,90). The smoke produced may adversely
affect the inhabitants’ health. Burning incense and mosquito coils
were also found to increase the risk of NPC by 5.9-folds in users
compared with non-users (75).
To the best of our knowledge, no studies have been
performed to date associating environmental exposure to
occupational solvents, wood dust, and domestic wood cooking fires
to the high incidences of NPC among the general Sarawak population
and the Bidayuh in particular. Hence, future epidemiological
studies addressing these risk factors within the Bidayuh of Sarawak
need to be conducted.
Tobacco smoking
The risk of developing cancer increases with smoking
tobacco, which exposes an individual to toxins that may cause
genetic mutations, such as nicotine and tar (20). Smoking tobacco causes cancers of the
lung, nasal cavity, nasopharynx, oral cavity, digestive system and
urogenital system (20). There is no
level of exposure to tobacco smoke, including exposure to
second-hand smoke, which is considered safe (20). Notwithstanding the specific
indigenous category, natives of Sarawak had a 37.9% prevalence of
second-hand smoke exposure at home (91). In comparison, exposure at work was
25.7% (91). Tobacco smoking is one
of the critical risk factors for NPC, supported by multiple
studies, including those conducted in Malaysia (72) and Thailand (92).
Cigarette smoking is another factor that contributes
to NPC (18). Cigarettes contain
compounds that are carcinogenic for humans, including nitrosamine,
polycyclic aromatic hydrocarbons and free radicals (68,93).
Cigarette smoke may damage DNA in mucosal cells that line the
airway (93), which may initiate
cancer formation. A meta-analysis revealed a modest, but
significant association between current cigarette smokers and NPC
vs. those who have never smoked (94). Smokers, especially among older people
(95,96) and early age of exposure to smoking
(94,97), have a higher risk of developing NPC
compared with non-smokers. The risk of developing NPC increases
with the intensity of cigarettes smoked per day and smoking
duration (92,94,98).
Among Sarawak natives, the prevalence of any tobacco product use
was 26.9%; 24.5% for tobacco smoking and 23.2% for cigarette
smoking (91). Irrespective of sex,
the prevalence of current cigarette smokers in Malaysia aged 15–19
years is 11.6% (91). A study
conducted in Sarawak showed that the habit of smoking could start
as early as 12 years old, with ~30% prevalence among students
(99).
None of the aforementioned studies investigated the
association between tobacco smoking and NPC development in the
Sarawak population in general or the Bidayuh in particular.
Nevertheless, it is plausible that such prevalence of smoking may
largely contribute to the high risk of developing NPC, especially
when smoking begins at a young age.
Other challenges
In 1947, ~99% of the Bidayuh were rural dwellers
(6). As of 2010, ~70% of Bidayuh
lived in rural areas (6). Patients
with NPC may live in rural areas, where geographical terrain and
accessibility pose a challenge (100). Hospitals may be very far and there
may be no efficient transport facilities other than logging roads
or rivers (100). Such challenges
could deter patients from seeking treatment (100). Hence, NPC may impact populations
with less access to healthcare.
Lack of awareness about NPC among the public was
identified as a challenge for the early detection of NPC (101). A Malaysian study to determine the
proportions of health literacy levels found that 41.5% of rural
respondents had limited health literacy (91). Meanwhile, between ethnicity, the
prevalence of possessing limited health literacy was 41.9% for
natives of Sarawak (91). Limited
health literacy in the Disease Prevention domain had a 37.6%
prevalence among Sarawak respondents who were natives (91). The Disease Prevention domain refers
to the capability to access, evaluate, interpret and understand
information about risk factors and to make informed decisions on
the basis of this information (91).
Cancer patients tend to seek some form of
complementary and alternative medicine. Complementary medicine was
defined by the National Cancer Institute (NCI) as treatments used
together with standard medical treatments (20). Alternative medicine, on the other
hand, refers to treatments used in place of standard medical
treatments (20). However, a number
of these complementary and alternative medicines have not been well
studied and it remains unknown if these are safe or effective
(20). Malaysia is a multicultural
and multifaith society that has practised traditional medicine for
generations (102). Although by
2010, slightly >80% of the Bidayuh have embraced Christianity,
looking back to 1947, about 91% of the Bidayuh were firm believers
of tribal religion (2,6). Numerous Malaysians believe traditional
medicine is more effective and less detrimental to the quality of
life compared with modern medicine (102–104).
Influence may come from relatives or friends who persuade patients
to opt for alternative medicine, believing that it would affect the
quality of life less negatively and may be more effective compared
with modern medicine (102–104). Patients may resort to alternative
medicine halfway through modern medicine without finishing the
scheduled prescribed treatment. Incomplete treatment may contribute
to ineffective outcomes (102–104).
Seeking additional or different treatment from traditional healers
may interrupt or delay a patient's scheduled hospital treatment
(102–104). Some patients may blindly trust
traditional healers, who usually attract interest due to their
lower fees compared with doctors (102–104).
Regrettably, traditional healers lack knowledge of cancer and
cannot treat patients with cancer appropriately (105). Consulting traditional healers has
partially contributed to delayed cancer diagnosis and treatment,
causing late presentation, advanced stage diagnosis and higher
cancer mortality rates (105).
Sometimes patients do not disclose their use of alternative
medicine (106). Non-disclosure
could be due to fear that their scheduled treatment may be put on
hold if discovered by doctors (106). Such a habit may be dangerous as
alternative medicines may counteract with prescription drugs.
Limited health literacy may cause patients to choose alternative
medicine while abandoning modern medicine (105,107).
Lack of awareness about NPC, topography limitations and usage of
different treatments are 3 challenges conceivably present in the
Bidayuh community that may deter early detection of NPC and the
pursuit of practical and modern treatment.
Limitations of this review are the absence of
studies associating dietary habits, tobacco smoking and
environmental exposure to NPC development in the Sarawak population
in general or the Bidayuh in particular. Investigations addressing
these risk factors within this population are warranted. The
novelty of this review is that there has not been an article
focusing on the trend and high incidence rate of NPC among the
Bidayuh of Sarawak, Malaysia. The relevance from an anthropological
perspective was also presented in the present review, linking the
Bidayuh to the Bai-Yue population of Southern China postulated for
NPC origin.
Conclusion
The aetiology of NPC is probably multifactorial,
with all the factors acting together (18,19).
Studies investigating the cause of the high incidence of NPC
amongst the Bidayuh population would be useful to help us
understand this cancer. The possible roles of viral, environmental,
dietary factors, host genetics and immune factors make the study of
NPC a unique opportunity to investigate multifactorial interaction
in cancer's aetiopathogenesis.
Acknowledgements
We thank Tan Sri Dr Noor Hisham Abdullah, the
Director General of Health, Ministry of Health, Malaysia, for his
permission to publish this article.
Funding
No funding was received.
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
REL and MD were equal and major contributors in
writing the manuscript and confirm the authenticity of all the raw
data. ASBK critically revised the manuscript for important
intellectual content. DCYC and MV were involved in drafting the
manuscript. PMN contributed to the manuscript conception and design
and reviewed the final version to be published. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dawie Usop AK: Epidemiology of cancer in
Sarawak, East Malaysia. Southeast Asian J Trop Med Public Health.
16:584–590. 1985.PubMed/NCBI
|
|
2
|
Tan CB: Communal associations of the
indigenous communities of Sarawak: A study of ethnicity and
national integration. Institute of Advanced Studies; University of
Malaya, Kuala Lumpur: 1994
|
|
3
|
Johari S, Shuib A, Ramachandran S, Herman
S and Kunasekaran P: Bidayuh Community of Malaysia: Presenting
culture and nature as a package for sustainable development of
indigenous tourism. Balancing Development and Sustainability in
Tourism Destinations: Proceedings of the Tourism Outlook Conference
2015. Saufi A, Andilolo IR, Othman N and Lew AA: Springer
Singapore; Singapore: pp. 89–96. 2016
|
|
4
|
Tan CB: Indigenous people, the state and
ethnogenesis: A study of the communal associations of the ‘Dayak’
communities in Sarawak, Malaysia. J Southeast Asian Stud.
28:263–284. 1997. View Article : Google Scholar
|
|
5
|
Bongarrá M, Kayad FG and Campbell YM: The
Bidayuh-languages or dialects? In: Selected Papers of the Bidayuh
Language Development and Preservation Project (2003–2017). Bongarrá
M, Arritt M and Kayad FG: Dayak Bidayuh National Association;
Kuching, Sarawak: pp. 207–219. 2017
|
|
6
|
Kheung LC and Adruce SA: The demographic
profile and sustainability growth of the Bidayuh population of
Sarawak. Int J Acad Res Bus Soc Sci. 8:69–68. 2018.
|
|
7
|
Chang YM, Swaran Y, Phoon YK, Sothirasan
K, Sim HT, Lim KB and Kuehn D: Haplotype diversity of 17
Y-chromosomal STRs in three native Sarawak populations (Iban,
Bidayuh and Melanau) in East Malaysia. Forensic Sci Int Genet.
3:e77–e80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Azizah AM, Hashimah B, Nirmal K, Siti
Zubaidah AR, Puteri NA, Nabihah A, Sukumaran R, Balqis B, Nadia SM,
Sharifah SS, et al: Malaysia national cancer registry report
2012–2016. National Cancer Institute Malaysia. 1–116. 2019.
|
|
9
|
Epidemiology of cancer in Sarawak
2007–2011. Sarawak Cancer Registry Malaysia: 2017
|
|
10
|
Bray F, Colombet M, Mery L, Piñeros M,
Znaor A, Zanetti R and Ferlay J: Cancer Incidence in Five
Continents. Vol 11 (electronic version). International Agency for
Research on Cancer; Lyon: 2017
|
|
11
|
Ferlay J, Ervik M, Lam F, Colombet M, Mery
L, Piñeros M, Znaor A, Soerjomataram I and Bray F: Global Cancer
Observatory: Cancer Today. International Agency for Research on
Cancer; Lyon: 2020
|
|
12
|
Zuo XY, Feng QS, Sun J, Wei PP, Chin YM,
Guo YM, Xia YF, Li B, Xia XJ, Jia WH, et al: X-chromosome
association study reveals genetic susceptibility loci of
nasopharyngeal carcinoma. Biol Sex Differ. 10:132019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xie SH, Yu IT, Tse LA, Mang OW and Yue L:
Sex difference in the incidence of nasopharyngeal carcinoma in Hong
Kong 1983–2008: Suggestion of a potential protective role of
oestrogen. Eur J Cancer. 49:150–155. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang LL, Chen WQ, Xue WQ, He YQ, Zheng RS,
Zeng YX and Jia WH: Global trends in incidence and mortality of
nasopharyngeal carcinoma. Cancer Lett. 374:22–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan JJC, Pilch BZ, Kuo TT, Wenig BM and
Lee AW: Tumours of the Nasopharynx. World Health Organization
Classification of Tumours: Pathology and Genetics of Head and Neck
Tumours. Barnes L, Eveson JW, Reichart P and Sidransky D: IARC
Press; Lyon: 2005
|
|
16
|
Poh SS, Chua ML and Wee JT: Carcinogenesis
of nasopharyngeal carcinoma: An alternate hypothetical mechanism.
Chin J Cancer. 35:92016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khoo AS and Pua KC: Diagnosis and clinical
evaluation of nasopharyngeal carcinoma. Nasopharyngeal Carcinoma.
Busson P: Advances in Experimental Medicine and Biology. 778.
Springer, Landes Bioscience; New York, NY: pp. 1–9. 2013,
View Article : Google Scholar
|
|
18
|
Chang ET and Adami HO: The enigmatic
epidemiology of nasopharyngeal carcinoma. Cancer Epidemiol
Biomarkers Prev. 15:1765–1777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tao Q and Chan AT: Nasopharyngeal
carcinoma: Molecular pathogenesis and therapeutic developments.
Expert Rev Mol Med. 9:1–24. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
AACR Cancer Progress Report 2016, . Saving
Lives Through Research. Clinical cancer research. American
Association for Cancer Research; pp. S1–S137. 2016
|
|
21
|
Soong YL and Tham IW: Epidemiology of
nasopharyngeal carcinoma. Nasopharyngeal Carcinoma: Management
Strategies. Yom SS and Wee J: Future Medicine Ltd.; London: pp.
5–19. 2014, View Article : Google Scholar
|
|
22
|
Stelow EB and Wenig BM: Update from the
4th edition of the world health organization classification of head
and neck tumours: Nasopharynx. Head Neck Pathol. 11:16–22. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsao SW, Tsang CM, Pang PS, Zhang G, Chen
H and Lo KW: The biology of EBV infection in human epithelial
cells. Semin Cancer Biol. 22:137–143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tsao SW, Tramoutanis G, Dawson CW, Lo AK
and Huang DP: The significance of LMP1 expression in nasopharyngeal
carcinoma. Semin Cancer Biol. 12:473–487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsai MH, Raykova A, Klinke O, Bernhardt K,
Gärtner K, Leung CS, Geletneky K, Sertel S, Münz C, Feederle R and
Delecluse HJ: Spontaneous lytic replication and epitheliotropism
define an Epstein-Barr virus strain found in carcinomas. Cell Rep.
5:458–470. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bristol JA, Djavadian R, Albright ER,
Coleman CB, Ohashi M, Hayes M, Romero-Masters JC, Barlow EA,
Farrell PJ, Rochford R, et al: A cancer-associated Epstein-Barr
virus BZLF1 promoter variant enhances lytic infection. PLoS Pathog.
14:e10071792018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Correia S, Bridges R, Wegner F, Venturini
C, Palser A, Middeldorp JM, Cohen JI, Lorenzetti MA, Bassano I,
White RE, et al: Sequence variation of Epstein-Barr virus: Viral
types, geography, codon usage, and diseases. J Virol.
92:e01132–01118. 2018. View Article : Google Scholar
|
|
28
|
Hui KF, Chan TF, Yang W, Shen JJ, Lam KP,
Kwok H, Sham PC, Tsao SW, Kwong DL, Lung ML and Chiang AK: High
risk Epstein-Barr virus variants characterized by distinct
polymorphisms in the EBER locus are strongly associated with
nasopharyngeal carcinoma. Int J Cancer. 144:3031–3042. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu M, Yao Y, Chen H, Zhang S, Cao SM,
Zhang Z, Luo B, Liu Z, Li Z, Xiang T, et al: Genome sequencing
analysis identifies Epstein-Barr virus subtypes associated with
high risk of nasopharyngeal carcinoma. Nat Genet. 51:1131–1136.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tsao SW, Wang X, Liu Y, Cheung YC, Feng H,
Zheng Z, Wong N, Yuen PW, Lo AK, Wong YC and Huang DP:
Establishment of two immortalized nasopharyngeal epithelial cell
lines using SV40 large T and HPV16E6/E7 viral oncogenes. Biochim
Biophys Acta. 1590:150–158. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gourzones C, Busson P and Raab-Traub N:
Epstein-Barr virus and the pathogenesis of nasopharyngeal
carcinomas. Nasopharyngeal Carcinoma. Busson P: Advances in
Experimental Medicine and Biology. 778. Springer, Landes
Bioscience; New York, NY: pp. 42–60. 2013, View Article : Google Scholar
|
|
32
|
Tsang CM, Deng W, Yip YL, Zeng M-S, Lo KW
and Tsao SW: Epstein-Barr virus infection and persistence in
nasopharyngeal epithelial cells. Chin J Cancer. 33:549–555.
2014.PubMed/NCBI
|
|
33
|
Tsang CM and Tsao SW: The role of
Epstein-Barr virus infection in the pathogenesis of nasopharyngeal
carcinoma. Virol Sin. 30:107–121. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tsao SW, Tsang CM and Lo KW: Epstein-Barr
virus infection and nasopharyngeal carcinoma. Philos Trans R Soc
Lond B Biol Sci. 372:201602702017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Young LS and Dawson CW: Epstein-Barr virus
and nasopharyngeal carcinoma. Chin J Cancer. 33:581–590.
2014.PubMed/NCBI
|
|
36
|
Tsao SW, Yip YL, Tsang CM, Pang PS, Lau
VM, Zhang G and Lo KW: Etiological factors of nasopharyngeal
carcinoma. Oral Oncol. 50:330–338. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsao SW, Tsang CM, To KF and Lo KW: The
role of Epstein-Barr virus in epithelial malignancies. J Pathol.
235:323–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ren ZF, Liu WS, Qin HD, Xu YF, Yu DD, Feng
QS, Chen LZ, Shu XO, Zeng YX and Jia WH: Effect of family history
of cancers and environmental factors on risk of nasopharyngeal
carcinoma in Guangdong, China. Cancer Epidemiol. 34:419–424. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jia WH and Qin HD: Non-viral environmental
risk factors for nasopharyngeal carcinoma: A systematic review.
Semin Cancer Biol. 22:117–126. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu G, Hsu WL, Coghill AE, Yu KJ, Wang CP,
Lou PJ, Liu Z, Jones K, Vogt A, Wang M, et al: Whole-exome
sequencing of nasopharyngeal carcinoma families reveals novel
variants potentially involved in nasopharyngeal carcinoma. Sci Rep.
9:99162019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jinam TA, Hong LC, Phipps ME, Stoneking M,
Ameen M, Edo J; HUGO Pan-Asian SNP Consortium, ; Saitou N:
Evolutionary history of continental southeast Asians: ‘early train’
hypothesis based on genetic analysis of mitochondrial and autosomal
DNA data. Mol Biol Evol. 29:3513–3527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Noeb J and Ridu RS: Language development
in bidayuh: Past, present and future. Selected papers of the
Bidayuh language development and preservation project (2003–2017).
Bongarrá M, Arritt M and Kayad FG: Dayak Bidayuh National
Association; Sarawak: pp. 3–46. 2017
|
|
43
|
Wee JT, Ha TC, Loong SL and Qian CN: Is
nasopharyngeal cancer really a ‘Cantonese cancer’? Chin J Cancer.
29:517–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wee J, Ha TC, Loong S and Qian CN: High
incidence of nasopharyngeal cancer: Similarity for 60% of
mitochondrial DNA signatures between the Bidayuhs of Borneo and the
Bai-yue of southern China. Chin J Cancer. 31:455–456. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Trejaut J, Lee CL, Yen JC, Loo JH and Lin
M: Ancient migration routes of Austronesian-speaking populations in
oceanic Southeast Asia and Melanesia might mimic the spread of
nasopharyngeal carcinoma. Chin J Cancer. 30:96–105. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shao JY, Li Y, Gao HY, Mai HQ, Zhang Y,
Guo X and Zeng YX: High frequency of common deletion (4981 bp) in
mitochondrial DNA in nasopharyngeal carcinoma and its correlation
with patient age and clinical stages. Cancer Biol Ther.
3:1270–1274. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pang LJ, Shao JY, Liang XM, Xia YF and
Zeng YX: Mitochondrial DNA somatic mutations are frequent in
nasopharyngeal carcinoma. Cancer Biol Ther. 7:98–207. 2008.
View Article : Google Scholar
|
|
48
|
Peng Z, Xie C, Wan Q, Zhang L, Li W and Wu
S: Sequence variations of mitochondrial DNA D-loop region are
associated with familial nasopharyngeal carcinoma. Mitochondrion.
11:327–333. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hu SP, Du JP, Li DR and Yao YG:
Mitochondrial DNA haplogroup confers genetic susceptibility to
nasopharyngeal carcinoma in Chaoshanese from Guangdong, China. PLoS
One. 9:e877952014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dhaliwal JS, Shahnaz M, Azrena A, Irda YA,
Salawati M, Too CL and Lee YY: HLA polymorphism in three indigenous
populations of Sabah and Sarawak. Tissue Antigens. 75:166–169.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jinam TA, Saitou N, Edo J, Mahmood A and
Phipps ME: Molecular analysis of HLA class I and II genes in four
indigenous Malaysian populations. Tissue Antigens. 75:151–158.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Goldsmith DB, West TM and Morton R: HLA
associations with nasopharyngeal carcinoma in southern Chinese: A
meta-analysis. Clin Otolaryngol Allied Sci. 27:61–67. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Lu CC, Chen JC, Jin YT, Yang HB, Chan SH
and Tsai ST: Genetic susceptibility to nasopharyngeal carcinoma
within the HLA-A locus in Taiwanese. Int J Cancer. 103:745–751.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ting H, Tan SR and John AN: Consumption
intention toward ethnic food: Determinants of Dayak food choice by
Malaysians. J Ethn Foods. 4:21–27. 2017. View Article : Google Scholar
|
|
55
|
Tsugane S: Salt, salted food intake, and
risk of gastric cancer: Epidemiologic evidence. Cancer Sci. 96:1–6.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Song P, Wu L and Guan W: Dietary nitrates,
nitrites, and nitrosamines intake and the risk of gastric cancer: A
meta-analysis. Nutrients. 7:9872–9895. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Armstrong RW, Imrey PB, Lye MS, Armstrong
MJ, Yu MC and Sani S: Nasopharyngeal carcinoma in Malaysian
Chinese: Salted fish and other dietary exposures. Int J Cancer.
77:228–235. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Dealwis C: Exogamous marriages between
migrant indians with local dayaks and the identity of their
offspring. International Symposium on Dynamics of Marriage/Divorce
Migration Flow in Asia Research Institute for Languages and
Cultures of Asia and Africa (ILCAA). Tokyo University of Foreign
Studies. 53–74. 2011.
|
|
59
|
Poirier S, Ohshima H, de-Thé G, Hubert A,
Bourgade MC and Bartsch H: Volatile nitrosamine levels in common
foods from Tunisia, south China and Greenland, high-risk areas for
nasopharyngeal carcinoma (NPC). Int J Cancer. 39:293–296. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Putera I, Ramadhan MG, Anindya S, Sutanto
NR, Kurniawan A, Hosea FN and Safitri ED: Relationship between
salted fish consumption and nasopharyngeal carcinoma: An
evidence-based case report. Acta Med Indones. 47:72–77.
2015.PubMed/NCBI
|
|
61
|
Jia WH, Luo XY, Feng BJ, Ruan HL, Bei JX,
Liu WS, Qin HD, Feng QS, Chen LZ, Yao SY and Zeng YX: Traditional
cantonese diet and nasopharyngeal carcinoma risk: A large-scale
case-control study in Guangdong, China. BMC Cancer. 10:4462010.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Armstrong RW and Eng AC: Salted fish and
nasopharyngeal carcinoma in Malaysia. Soc Sci Med. 17:1559–1567.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Armstrong RW, Armstrong MJ, Yu MC and
Henderson BE: Salted fish and inhalants as risk factors for
nasopharyngeal carcinoma in Malaysian Chinese. Cancer Res.
43:2967–2970. 1983.PubMed/NCBI
|
|
64
|
Babji AS, Aishah S and Aminah A: Nitrite
content of some foods in Malaysia. Pertanika. 7:39–41. 1984.
|
|
65
|
Tricker AR and Preussmann R: Carcinogenic
N-nitrosamines in the diet: Occurrence, formation, mechanisms and
carcinogenic potential. Mutat Res. 259:277–289. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Lin JK: Nitrosamines as potential
environmental carcinogens in man. Clin Biochem. 23:67–71. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Belinsky SA, Devereux TR and Anderson MW:
Role of DNA methylation in the activation of proto-oncogenes and
the induction of pulmonary neoplasia by nitrosamines. Mutat Res.
233:105–116. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Nasution II, Lutan R, Munir D and Wahyuni
AS: Relationship between cigarette smoking and nasopharyngeal
carcinoma. Int J Nasopharyngeal Carcinoma. 1:17–23. 2019.
View Article : Google Scholar
|
|
69
|
Shao YM, Poirier S, Ohshima H, Malaveille
C, Zeng Y, de Thé G and Bartsch H: Epstein-Barr virus activation in
Raji cells by extracts of preserved food from high risk areas for
nasopharyngeal carcinoma. Carcinogenesis. 9:1455–1457. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Huang SY, Fang CY, Tsai CH, Chang Y,
Takada K, Hsu TY and Chen JY: N-methyl-N’-nitro-N-nitrosoguanidine
induces and cooperates with
12-O-tetradecanoylphorbol-1,3-acetate/sodium butyrate to enhance
Epstein-Barr virus reactivation and genome instability in
nasopharyngeal carcinoma cells. Chem Biol Interact. 188:623–634.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Zheng X, Yan L, Nilsson B, Eklund G and
Drettner B: Epstein-Barr virus infection, salted fish and
nasopharyngeal carcinoma. A case-control study in southern China.
Acta Oncol. 33:867–872. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Armstrong RW, Imrey PB, Lye MS, Armstrong
MJ, Yu MC and Sani S: Nasopharyngeal carcinoma in Malaysian
Chinese: Occupational exposures to particles, formaldehyde and
heat. Int J Epidemiol. 29:991–998. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kaur A: A history of forestry in Sarawak.
Mod Asian Stud. 32:117–147. 1998. View Article : Google Scholar
|
|
74
|
Niemelä R and Vainio H: Formaldehyde
exposure in work and the general environment. Occurrence and
possibilities for prevention. Scand J Work Environ Health.
7:95–100. 1981. View Article : Google Scholar
|
|
75
|
West S, Hildesheim A and Dosemeci M:
Non-viral risk factors for nasopharyngeal carcinoma in the
Philippines: Results from a case-control study. Int J Cancer.
55:722–727. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
IARC Working Group on the Evaluation of
Carcinogenic Risks to Humans, . Formaldehyde, 2-butoxyethanol and
1-tert-butoxypropan-2-ol. International Agency for Research on
Cancer; Lyon: 2006
|
|
77
|
Vaughan TL, Stewart PA, Teschke K, Lynch
CF, Swanson GM, Lyon JL and Berwick M: Occupational exposure to
formaldehyde and wood dust and nasopharyngeal carcinoma. Occup
Environ Med. 57:376–384. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Menicagli R, Bolla G, Menicagli L and
Esseiridou A: Industrial pollutants and nasopharyngeal cancer: An
open question. Gulf J Oncolog. 1:70–74. 2017.PubMed/NCBI
|
|
79
|
Jayaprakash V, Natarajan KK, Moysich KB,
Rigual NR, Ramnath N, Natarajan N and Reid ME: Wood dust exposure
and the risk of upper aero-digestive and respiratory cancers in
males. Occup Environ Med. 65:647–654. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Adoga AA, Kokong DD, Nimkur TL and Ma'an
ND: Environmental and life-style related risk factors for sinonasal
and nasopharyngeal malignancies among a prospective cohort in Jos,
Nigeria. Int J Otolaryngol. 2018:85248612018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Cravioto J, Ohgaki H, Che HS, Tan C,
Kobayashi S, Toe H, Long B, Oudaya E, Rahim NB and Farzeneh H: The
effects of rural electrification on quality of life: A southeast
Asian perspective. Energies. 13:24102020. View Article : Google Scholar
|
|
82
|
Kodama AM and Dollar AM: Indoor fires as a
possible cause of cancers of the upper respiratory and digestive
systems in certain underdeveloped countries. J Environ Health.
46:88–90. 1983.
|
|
83
|
Spiegelhalder B and Preussmann R:
Occupational nitrosamine exposure. 1. Rubber and tyre industry.
Carcinogenesis. 4:1147–1152. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Incavo JA and Schafer MA: Simplified
method for the determination of N-nitrosamines in rubber
vulcanizates. Anal Chim Acta. 557:256–261. 2006. View Article : Google Scholar
|
|
85
|
Straif K, Weiland SK, Bungers M,
Holthenrich D, Taeger D, Yi S and Keil U: Exposure to high
concentrations of nitrosamines and cancer mortality among a cohort
of rubber workers. Occup Environ Med. 57:180–187. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ratnasingam J, Ramasamy G, Wai LT, Senin
AL and Muttiah N: The prospects of rubberwood biomass energy
production in Malaysia. BioRes. 10:2526–2548. 2015. View Article : Google Scholar
|
|
87
|
Mertz O, Egay K, Bruun TB and Colding TS:
The last Swiddens of Sarawak, Malaysia. Hum Ecol. 41:109–118. 2012.
View Article : Google Scholar
|
|
88
|
Warren-Thomas E, Dolman PM and Edwards DP:
Increasing demand for natural rubber necessitates a robust
sustainability initiative to mitigate impacts on tropical
biodiversity. Conserv Lett. 8:230–241. 2015. View Article : Google Scholar
|
|
89
|
Hatch T: Shifting cultivation in Sarawak:
Past, present and future. In: Tropical Ecology and Development:
Proceedings of the Vth International Symposium of Tropical Ecology.
Furtado JI: International Society for Tropical Ecology; Kuala
Lumpur: pp. 483–496. 1979
|
|
90
|
Kendawang JJ, Tanaka S, Shibata K, Yoshida
N, Sabang J, Ninomiya I and Sakurai K: Effects of shifting
cultivation on soil ecosystems in Sarawak, Malaysia. III. Results
of burning practice and Changes in soil organic matter at niah and
bakam experimental sites. Soil Sci Plant Nutr. 51:515–523. 2005.
View Article : Google Scholar
|
|
91
|
National Health and Morbidity Survey
(NHMS) 2019. Technical Report - Volume 1, . NCDs-non-communicable
diseases: Risk Factors and Other Health Problems. Institute for
Public Health (IPH). National Institutes of Health, Ministry of
Health; Malaysia: 2020
|
|
92
|
Fachiroh J, Sangrajrang S, Johansson M,
Renard H, Gaborieau V, Chabrier A, Chindavijak S, Brennan P and
McKay JD: Tobacco consumption and genetic susceptibility to
nasopharyngeal carcinoma (NPC) in Thailand. Cancer Causes Control.
23:1995–2002. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Randerath E, Danna TF and Randerath K: DNA
damage induced by cigarette smoke condensate in vitro as assayed by
32P-postlabeling. Comparison with cigarette smoke-associated DNA
adduct profiles in vivo. Mutat Res. 268:139–153. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Long M, Fu Z, Li P and Nie Z: Cigarette
smoking and the risk of nasopharyngeal carcinoma: A meta-analysis
of epidemiological studies. BMJ Open. 7:e0165822017. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Chen CJ, Liang KY, Chang YS, Wang YF,
Hsieh T, Hsu MM, Chen JY and Liu MY: Multiple risk factors of
nasopharyngeal carcinoma: Epstein-Barr virus, malarial infection,
cigarette smoking and familial tendency. Anticancer Res.
10:547–553. 1990.PubMed/NCBI
|
|
96
|
Vaughan TL, Shapiro JA, Burt RD, Swanson
GM, Berwick M, Lynch CF and Lyon JL: Nasopharyngeal cancer in a
low-risk population: Defining risk factors by histological type.
Cancer Epidemiol Biomarkers Prev. 5:587–593. 1996.PubMed/NCBI
|
|
97
|
Hsu WL, Chen JY, Chien YC, Liu MY, You SL,
Hsu MM, Yang CS and Chen CJ: Independent effect of EBV and
cigarette smoking on nasopharyngeal carcinoma: A 20-year follow-up
study on 9,622 males without family history in Taiwan. Cancer
Epidemiol Biomarkers Prev. 18:1218–1226. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chow WH, McLaughlin JK, Hrubec Z, Nam JM
and Blot WJ: Tobacco use and nasopharyngeal carcinoma in a cohort
of US veterans. Int J Cancer. 55:538–540. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Juslina O, Leelavathi M, Khairani O and
Iryani T: Prevalence of smoking among secondary school students in
Sarawak. Malays Fam Physician. 6:66–67. 2011.PubMed/NCBI
|
|
100
|
Devi BCR, Tang TS and Corbex M: Setting up
home-based palliative care in countries with limited resources: A
model from Sarawak, Malaysia. Ann Oncol. 19:2061–2066. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
101
|
World Cancer Day 2019: Factsheet for
Healthcare Providers. Disease Control Division. Ministry of Health
Malaysia. 2019.
|
|
102
|
Ariff KM and Beng KS: Cultural health
beliefs in a rural family practice: A Malaysian perspective. Aust J
Rural Health. 14:2–8. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Alshagga MA, Al-Dubai SA, Muhamad Faiq SS
and Yusuf AA: Use of complementary and alternative medicine among
asthmatic patients in primary care clinics in Malaysia. Ann Thorac
Med. 6:115–119. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Lee PY, Abang Taha AB, Lin K, Ghazali SR
and Syed Ahmad Al-Mashoor SH: Usage of complementary and
alternative medicine among primary care clinic attendees, Kuching,
Sarawak, Malaysia, January-April 2004. Asia Pac Fam Med.
6:2007.
|
|
105
|
Merriam S and Muhamad M: Roles traditional
healers play in cancer treatment in Malaysia: Implications for
health promotion and education. Asian Pac J Cancer Prev.
14:3593–3601. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Farooqui M, Hassali MA, Abdul Shatar AK,
Shafie AA, Seang TB and Farooqui MA: Complementary and alternative
medicine (CAM) use by Malaysian oncology patients. Complement Ther
Clin Pract. 18:114–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Muhamad M, Merriam S and Suhami N: Why
breast cancer patients seek traditional healers. Int J Breast
Cancer. 2012:6891682012. View Article : Google Scholar : PubMed/NCBI
|