Introduction
According to the 2018 global cancer statistics, the
incidence rate of breast cancer in women accounts for 11.6% of the
total cancer cases (1). Breast
cancer is the most common cancer among women, and is also the
leading cause of cancer-associated deaths (1). In 2014, the incidence rate of breast
cancer in China was 18.65%, and it is growing at a rate of 3% per
year (2), which has become one of
the main threats to the health of women; it is a great burden on
society and requires further research. There are numerous
treatments for breast cancer, including targeted molecular therapy,
chemotherapy, radiotherapy and surgery (3). Although considerable progress has been
achieved in the medical treatment of breast cancer, there is no
radically effective treatment for patients with breast cancer, and
the prognosis of clinical treatment remains extremely poor.
Additionally, breast cancer, especially triple-negative breast
cancer, is prone to recurrence and metastasis (4). Therefore, in-depth study of breast
cancer and the identification of novel biomarkers or active
therapeutic targets are particularly important research
directions.
MicroRNA (miRNA/miR)-223-3p is located in the q12
locus of the X chromosome (5).
miR-223-3p is highly conserved and has potential roles in major
physiological changes. For example, miR-223-3p acts as an oncogene
in certain cancer types, such as T-cell acute lymphoblastic
leukemia (6), gastric cancer
(7) and prostate cancer (8), whereas in acute myeloid leukemia
(9), cervical cancer (10) and non-small cell lung cancer
(11) it functions as a tumor
suppressor. Currently, the role of miR-223-3p in tumorigenesis has
not been fully determined. Therefore, it is important to understand
the role of miR-223-3p in tumorigenesis and the development of
disease. It has been revealed that miR-223-3p has the potential to
promote the proliferation and invasion of cancer cells. For
instance, the proliferation and invasion of breast cancer cells are
enhanced following transfer of miR-223-3p into cells (12). However, to the best of our knowledge,
the mechanism of miR-223-3p in breast cancer has not been
previously described.
Epithelial-mesenchymal transition (EMT) is a
reversible cellular process that temporarily puts epithelial cells
in a quasi-mesenchymal state (13).
EMT is associated with wound healing, tissue regeneration and organ
fibrosis, and is involved in metastasis and cancer progression
(14). In addition, EMT activation
in tumor cells affects the development of various types of
malignancies, such as breast and pancreatic cancer (15,16).
During tumor development, this pleiotropic process induces, in a
single cancer cell, multiple features associated with advanced
malignancies (17,18). Metastatic breast cancer is largely
incurable (19). Therefore,
increased understanding of EMT regulation in breast cancer may
enable the development of novel targeted treatment strategies.
However, to the best of our knowledge, the regulatory mechanisms of
miR-223-3p and EMT in breast cancer have not been studied.
The Hippo signaling pathway was first discovered in
Drosophila. It can inhibit cell proliferation and regulate
apoptosis to limit the size of tissue morphological growth
(20). In addition to regulating
stem/progenitor cell expansion, inhibiting cell proliferation,
stimulating apoptosis and controlling organ size, the Hippo
signaling pathway also serves a crucial role in the proliferation
of tumor cell proliferation (21).
Yes-related protein (Yap) is a key effector in the Hippo signaling
pathway. It has been reported that the Hippo signaling pathway is
inactivated by inhibition of Yap activity, and increased Yap
activity can inhibit the expression of tumor suppressor genes in
tumor cells (22). EMT and the Hippo
signaling pathway serve a regulatory role in the proliferation and
growth of tumor cells (23).
However, to the best of our knowledge, the role of the Hippo
signaling pathway in breast cancer has not been previously
determined. Therefore, the roles of EMT and the Hippo signaling
pathway are novel research targets in breast cancer.
The present study aimed to investigate the effects
of miR-223-3p on the proliferation, migration and invasion of
breast cancer cells via the Hippo/Yap signaling pathway, and to
further evaluate the underlying mechanism of miR-223-3p in breast
cancer cells to provide a research basis for the clinical treatment
of breast cancer.
Materials and methods
Cell culture
Human breast cancer MDA-MB-231 and MCF-7 cells (both
purchased from Peking Union Medical College) were cultured in DMEM
(Gibco; Thermo Fisher Scientific, Inc.) containing 10% FBS
(Invitrogen; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin
and 100 µg/ml streptomycin. Human normal mammary epithelial cells
(MCF-10A, purchased from Peking Union Medical College) cells were
cultured in growth medium consisting of DMEM/F-12 (1:1; Gibco;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS, 100 U/ml
penicillin and 100 U/ml streptomycin. Cells were incubated at 37°C
in a 5% CO2 atmosphere. Cells were cultured at saturated
humidity and 37°C, changing culture medium every 2 days. When cells
grew to 90%, they were trypsinized (25%) and then centrifuged for
10 min at 4°C and 18,750 × g before resuspension. The suspension
was added to a new petri dish.
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from cells using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) or the Ultrapure RNA kit (CoWin Biosciences) according to the
manufacturer's protocols. RNA (0.5 µg) was subjected to reverse
transcription using ReverTra Ace qPCR RT Master mix with gDNA
Remover (Toyobo Life Science). After adding oligo(dT) into RNA, it
was heated at 70°C for 10 min and put in an ice bath for 1 min;
m-mlv, dNTP and rnasin were then added, mixed well and centrifuged,
then stored at 42°C for 40–90 min, 95°C for 10 min and finally at
4°C. qPCR was performed using a Bio-Rad IQ5 instrument (Bio-Rad
Laboratories, Inc.) with TransStart® Top Green qPCR
SuperMix (Beijing Transgen Biotech Co., Ltd.). The reactions were
performed under the following conditions as suggested by the
manufacturer: 94°C for 30 sec, followed by 40 cycles of 94°C for 5
sec and 60°C for 30 sec, followed by a dissociation protocol. The
following primer sequences were used: miR-223-3p forward,
5′-TAAAGCAACCGAGCACTGAGA-3′ and reverse,
5′-ACGGTAGAGGTCCTTTCCTTTG-3′; and 18S rRNA forward,
5′-AGGCGCGCAAATTACCCAATCC-3′ and reverse,
5′-GCCCTCCAATTGTTCCTCGTTAAG-3′. The relative expression was
normalized to 18S rRNA expression (24), used as a loading control. Relative
gene expression was analyzed using the 2−∆∆Cq method
(25).
Cell transfection assay
MCF-7 cells were cultured in a 6-well plate at 37°C
for 24 h. Transfection was performed when the cell density grew to
~70%. Subsequently, cells were transfected with miRVana miRNA
inhibitors of miR-223-3p (miR-223-3p inhibitor; 50 nM; cat. no.
4464084; Thermo Fisher Scientific, Inc.) and negative control (NC;
50 nM; cat. no. R10034; Guangzhou RiboBio Co., Ltd.) using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol, for 6 h at 37°C.
Subsequently, the medium was changed to DMEM containing 10% serum
and incubated at 37°C for 12 h before subsequent experiments
(26).
Cell proliferation assay
A Cell Counting Kit 8 (CCK8; Beyotime Institute of
Biotechnology) assay was used to detect the proliferation of
mammary cancer cells. Briefly, at a cell growth density of ~70%,
control (untransfected cells), NC- and miR-223-3p
inhibitor-transfected MCF-7 cells were cultured in 96-well cell
culture plates. After incubation at 37°C for 12, 24, 48 and 72 h,
10 µl CCK8 reagent was added to each well. The cells were placed in
a cell incubator at 37°C for 0.5–1.5 h. Subsequently, the optical
density value of each well was determined on a microplate reader at
450 nm.
Apoptosis assay
The cells were cultured in 6-well plates and divided
into three groups: Control group (untransfected MCF-7 cells), NC
group and miR-223-3p inhibitor MCF-7 group. After transfection,
1×106 cells were collected, washed in 1 ml 1X binding
buffer and centrifuged at 4°C and 18,750 × g for 10 min.
Subsequently, 1 ml 1X binding buffer was added to the collected
cells, and then 10 µl Annexin V-FITC (Sungene Biotech Co., Ltd.)
reagent was added to each group of collected cells. After
incubation in the dark at 37°C for 15 min, 5 µl PI (Sungene Biotech
Co., Ltd.) solution was added for 10 min at room temperature and
apoptosis was analyzed by flow cytometry (CytoNova; CapitalBio
Technology, Inc.). Results were analyzed using the Kaluza software
(v2.1.1; Beckman Coulter, Inc.). The apoptotic rate was calculated
as the percentage of early and late apoptotic cells.
Cell migration assay
Cell migration was measured using a wound healing
assay. A total of 5×104 cells were cultured in each well
of a 24-well plate and cultured overnight in the incubator at 37°C
to enable the cells to adhere to the bottom of the plate. Once the
cells grew to ~80% confluence, a straight-line scratch was made
with a 200-µl pipette tip, followed by washing with PBS and the
medium was replaced with FBS-free medium. Subsequently, the scratch
area was imaged using an optical light microscope (Olympus CKX53;
Olympus Corporation; magnification, ×100) to record the results at
0 h. The cells were then placed in a cell incubator. After 24 h at
37°C, cell migration area size was analyzed with ImageJ software
(v1.8.0; National Institutes of Health), comparing the findings
with the previous time point. The experiments were repeated three
times.
Cell invasion assay
The invasive ability of cells was examined using a
Transwell (Corning, Inc.) assay. The upper surface of the bottom
membrane of the Transwell chamber was coated with 50 mg/l Matrigel
(1:8) for 30 min at 37°C and air dried at 4°C. The residual liquid
in the culture plate was aspirated and 50 µl serum-free culture
medium containing 10 g/l BSA (Thermo Fisher Scientific, Inc.) was
added into each well at 37°C for 30 min. The chamber was placed in
the culture plate, and 300 µl pre-warmed serum-free medium was
added to the upper chamber, which was left at room temperature for
15–30 min to rehydrate the matrix gel and absorb the remaining
culture medium. Cells were starved in serum-free medium for 12–24 h
before preparing the cell suspension. Cells were added to 24-well
culture plates and Transwell chambers were placed in them.
Moreover, 500 µl medium containing FBS was added to the lower
chamber of the orifice plate. The cells were cultured at 37°C for
24 h. The cells invading the chamber were stained with 0.1% crystal
violet for 1 h at room temperature, and 3–5 fields of view were
randomly selected for imaging. Stained cells were counted under an
optical light microscope (Olympus CKX53; Olympus Corporation;
magnification, ×100).
Western blotting
Proteins were extracted from treated cells using
lysis buffer (Beyotime Institute of Biotechnology) and quantified
using a bicinchoninic acid protein assay kit (Beyotime Institute of
Biotechnology). A total of 10 µg protein/lane was separated by 10%
SDS-PAGE and then transferred to PVDF membranes. The membranes were
blocked with 5% skimmed milk at room temperature for 60 min and
incubated with primary antibodies at 4°C overnight. Rabbit
monoclonal primary antibodies against GAPDH (cat. no. ab181602),
macrophage stimulating 1 (MST1; cat. no. ab79199), large tumor
suppressor kinase 1 (LATS1; cat. no. ab70561), phosphorylated-Yap1
(cat. no. ab76252), Yap1 (cat. no. ab52771) and MMP14 (cat. no.
ab51074), and the anti-rabbit immunoglobulin G secondary antibody
(cat. no. ab7090) were obtained from Abcam. MMP9 (cat. no.
10375-2-AP), MMP2 (cat. no. 10373-2-AP), CDK2 (cat. no.
10122-1-AP), Cyclin E1 (cat. no. 11554-1-AP), cyclin-dependent
kinase inhibitor 1 (p21; cat. no. 10355-1-AP), E-cadherin (cat. no.
20874-1-AP), N-cadherin (cat. no. 22018-1-AP) and vimentin (cat.
no. 10366-1-AP) antibodies were obtained from ProteinTech Group,
Inc. All antibodies were diluted as recommended in the
specifications (dilution ratio, 1:1,000). The blots were then
incubated with HRP-conjugated secondary antibody (1:2,000; cat. no.
7074; Cell Signaling Technology, Inc.) at room temperature for 1 h.
After extensive washing in TBST, protein bands were revealed with
Super Signal West Femto Maximum Sensitivity Substrate (Thermo
Fisher Scientific, Inc.) and visualized with Image Quant LAS 500
(Cytiva).
Statistical analysis
All results were obtained from ≥3 independent
experiments and data are presented as the mean ± SD. Data were
analyzed using SPSS software (version 20.0; IBM Corp.). GraphPad
Prism software (version 6.0; GraphPad Software, Inc.) was used to
generate figures. One-way ANOVA was used to analyze the
significance of differences among different groups. Comparisons
between multiple groups were performed using the Scheffe post-hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
miR-223-3p expression levels in
different breast cancer cells
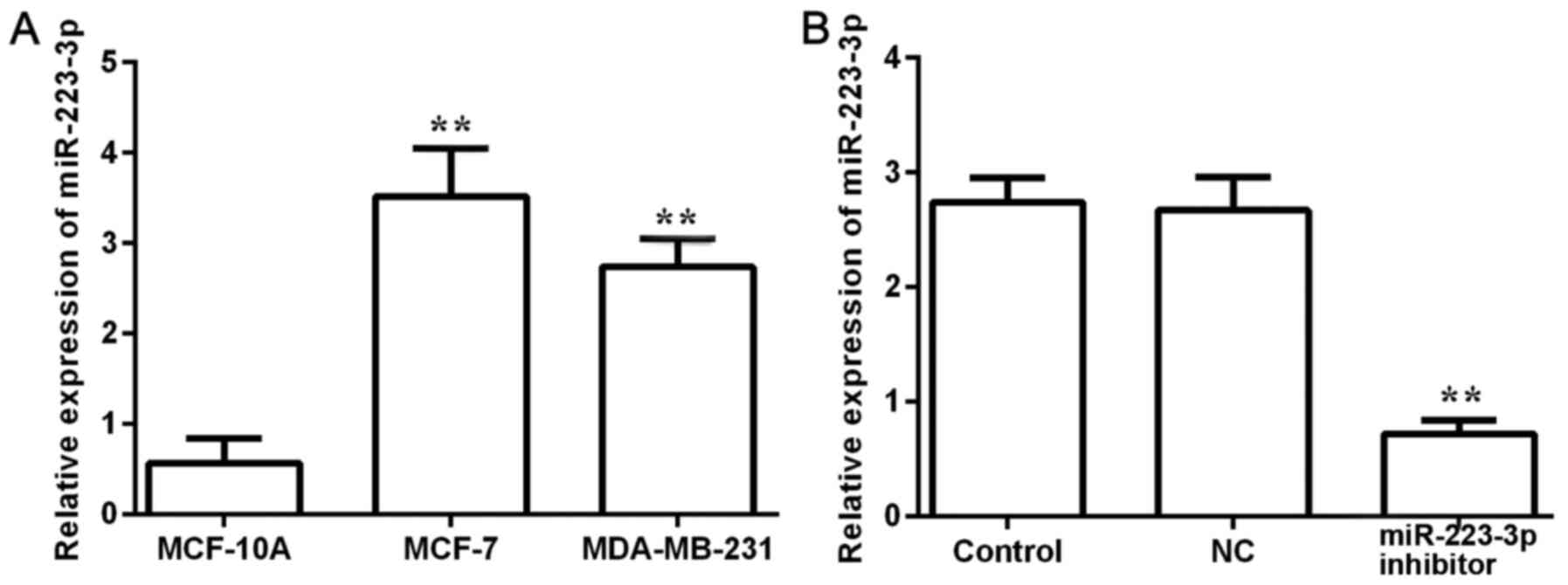
miR-223-3p expression levels were detected in
MCF-10A normal breast cells and in two different breast cancer cell
lines, MCF-7 and MDA-MB-231 (Fig.
1A). The expression levels of miR-223-3p in breast cancer cells
were significantly higher compared with expression in normal cells.
As the highest miR-223-3p expression level was observed in MCF-7
cells, these cells were selected for subsequent experiments. After
miR-223-3p inhibitor was transfected into MCF-7 cells, miR-223-3p
expression was detected (Fig. 1B).
The results demonstrated that miR-223-3p inhibitor could inhibit
miR-223-3p expression.
Downregulation of miR-223-3p inhibits
the proliferation of breast cancer cells in vitro
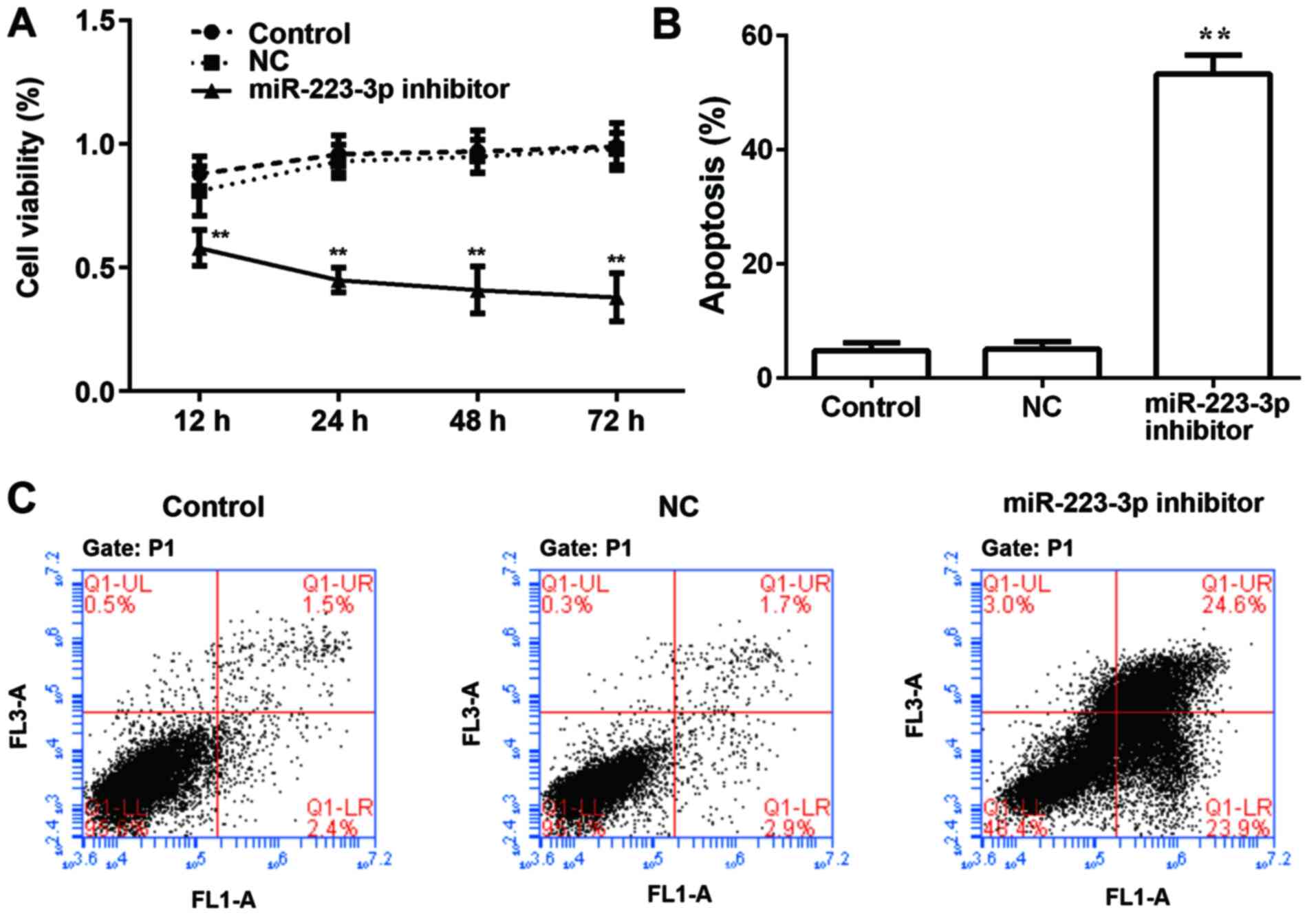
To examine the role of miR-223-3p on the
proliferation of MCF-7 cells, miR-223-3p inhibitor was transfected
into MCF-7 cells and results were compared with the control group.
Subsequently, cell viability was detected using a CCK8 assay at
different time points, and a growth curve was generated based on
the results. As presented in Fig.
2A, there was no significant difference in the proliferation
rate between the MCF-7 cell control group and the NC group, whereas
the cell proliferation rate of the miR-223-3p inhibitor group was
significantly lower compared with that of the control group. By
detecting the apoptosis rate, it was found that inhibiting
miR-223-3p expression significantly increased apoptosis (Fig. 2B and C).
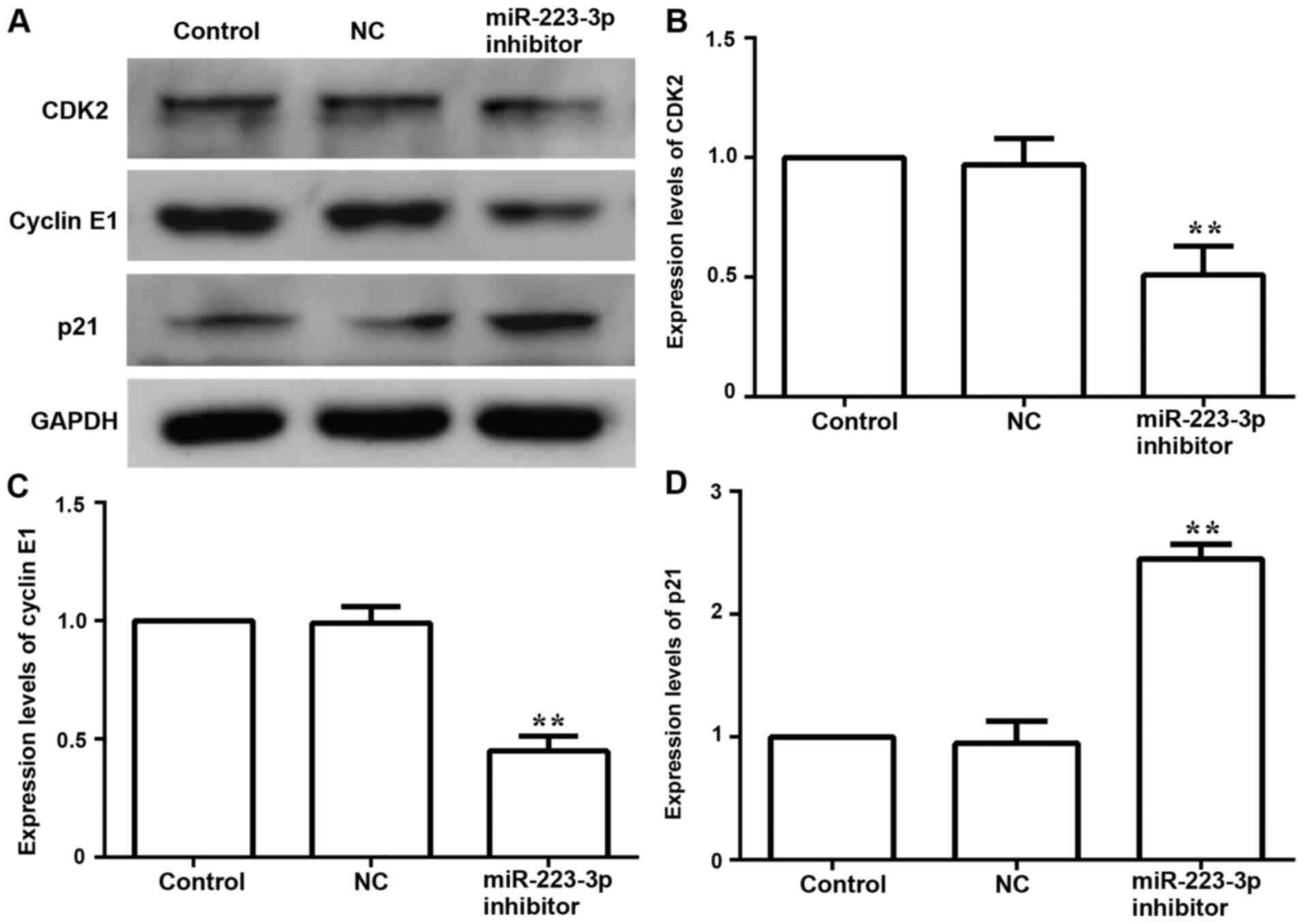
Subsequently, the expression levels of cell
cycle-related proteins CDK-2, cyclin-related proteins Cyclin E1 and
p21 were examined to further evaluate the effect of miR-223-3p on
cell proliferation. Compared with the control group, the protein
expression levels of CDK-2 and Cyclin E1 were downregulated,
whereas the expression of p21 was upregulated in the miR-223-3p
inhibitor group (Fig. 3). These
results suggested that inhibiting miR-223-3p expression could
inhibit the proliferation of breast cancer cells.
miR-223-3p inhibition suppresses the
migration and invasion of breast cancer cells
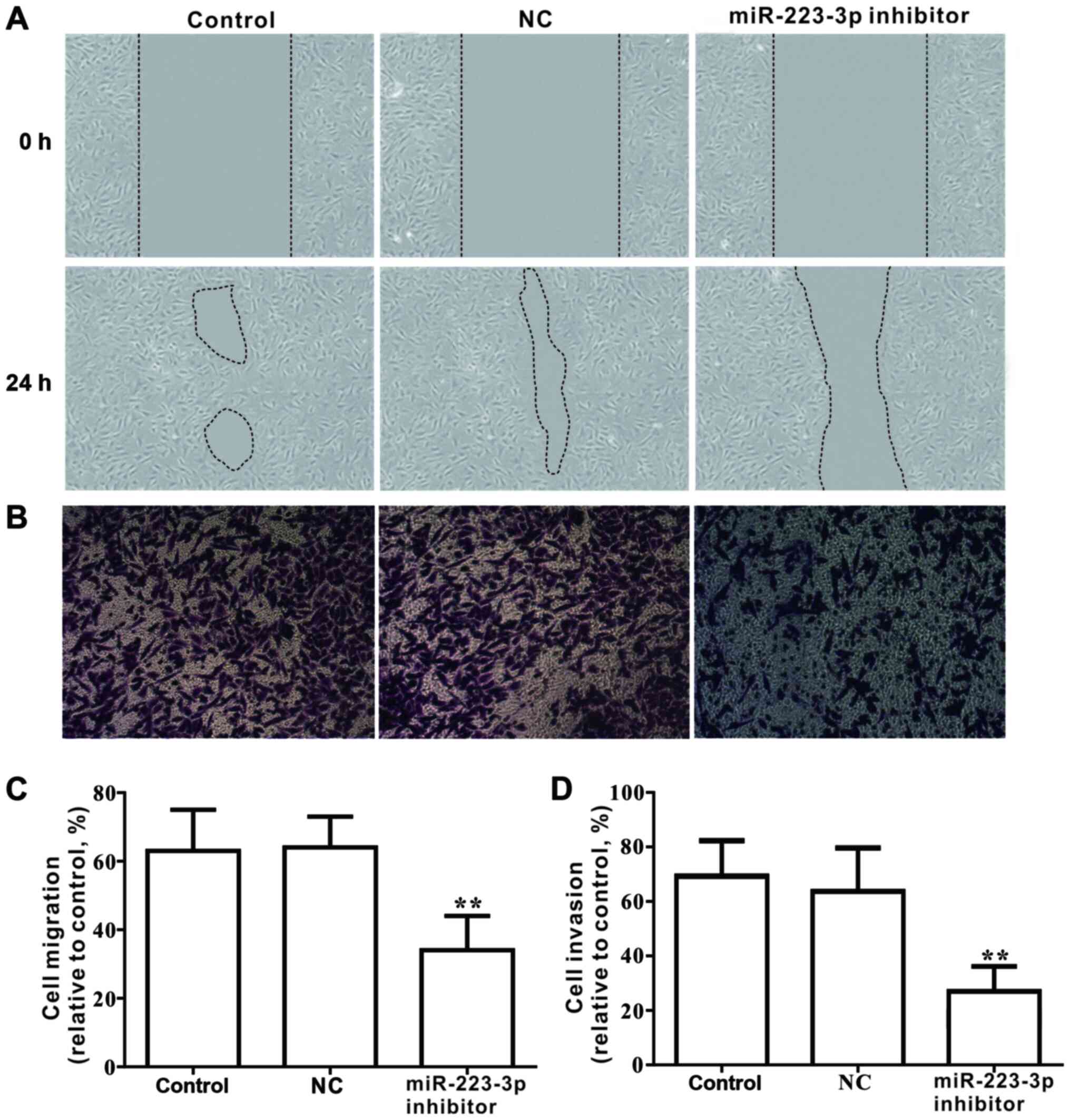
The effects of miR-223-3p on the migration and
invasion of MCF-7 cells were investigated. Results from the wound
healing assay demonstrated that loss of miR-223-3p expression
significantly inhibited the migratory ability of MCF-7 cells
(Fig. 4A and C). Similarly,
Transwell analysis indicated that the invasive capacity of cells
transfected with miR-223-3p inhibitor was significantly declined
compared with that of the control group (Fig. 4B and D). Furthermore, in cells
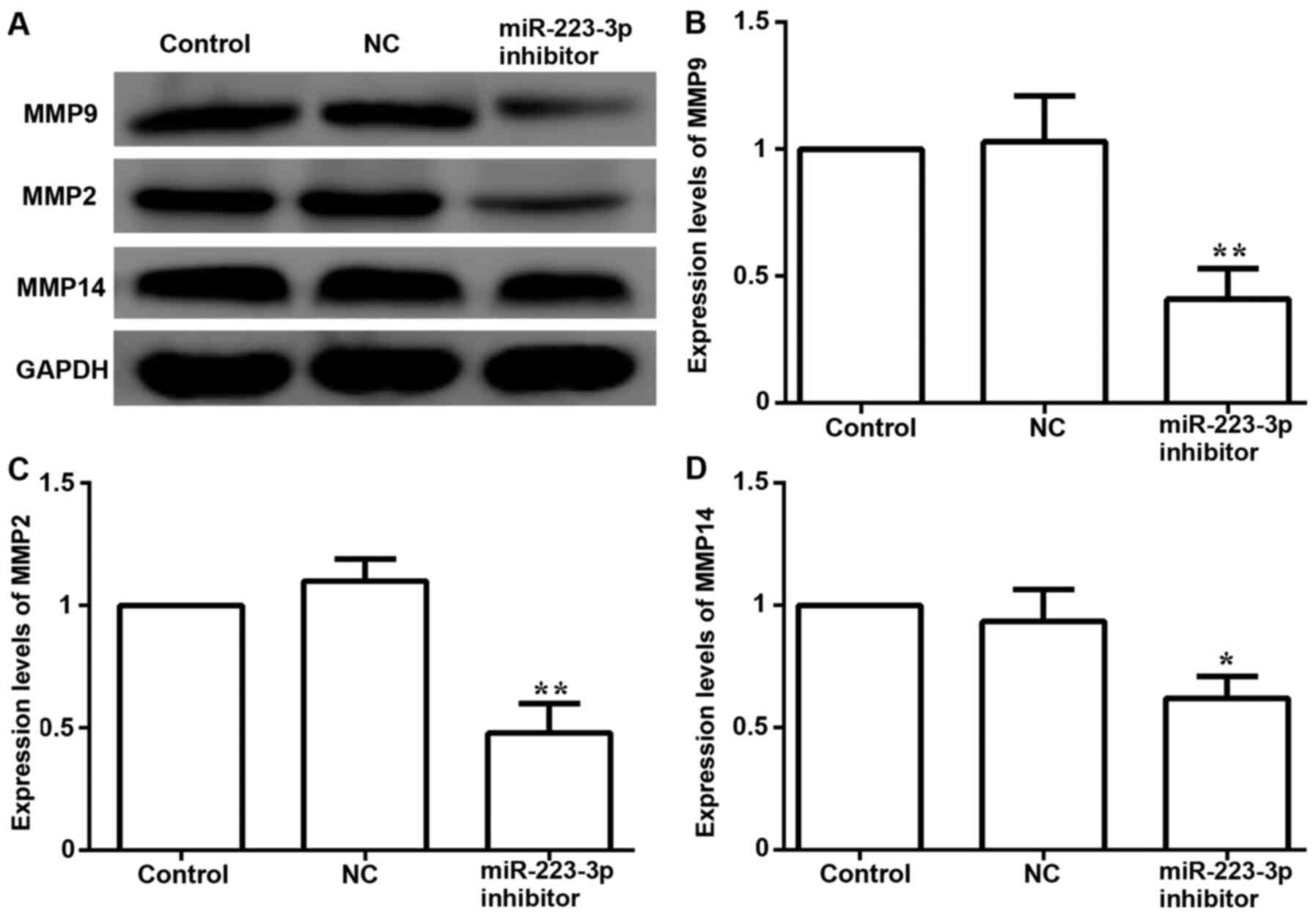
transfected with miR-223-3p inhibitor, the protein expression
levels of metastasis-associated proteins, including MMP9, MMP2 and
MMP14, were significantly decreased compared with the control group
expression levels (Fig. 5). These
data suggested that inhibiting miR-223-3p expression may decrease
migration and invasion in MCF-7 cells.
Inhibition of mir-223-3p decreases EMT
in breast cancer cells
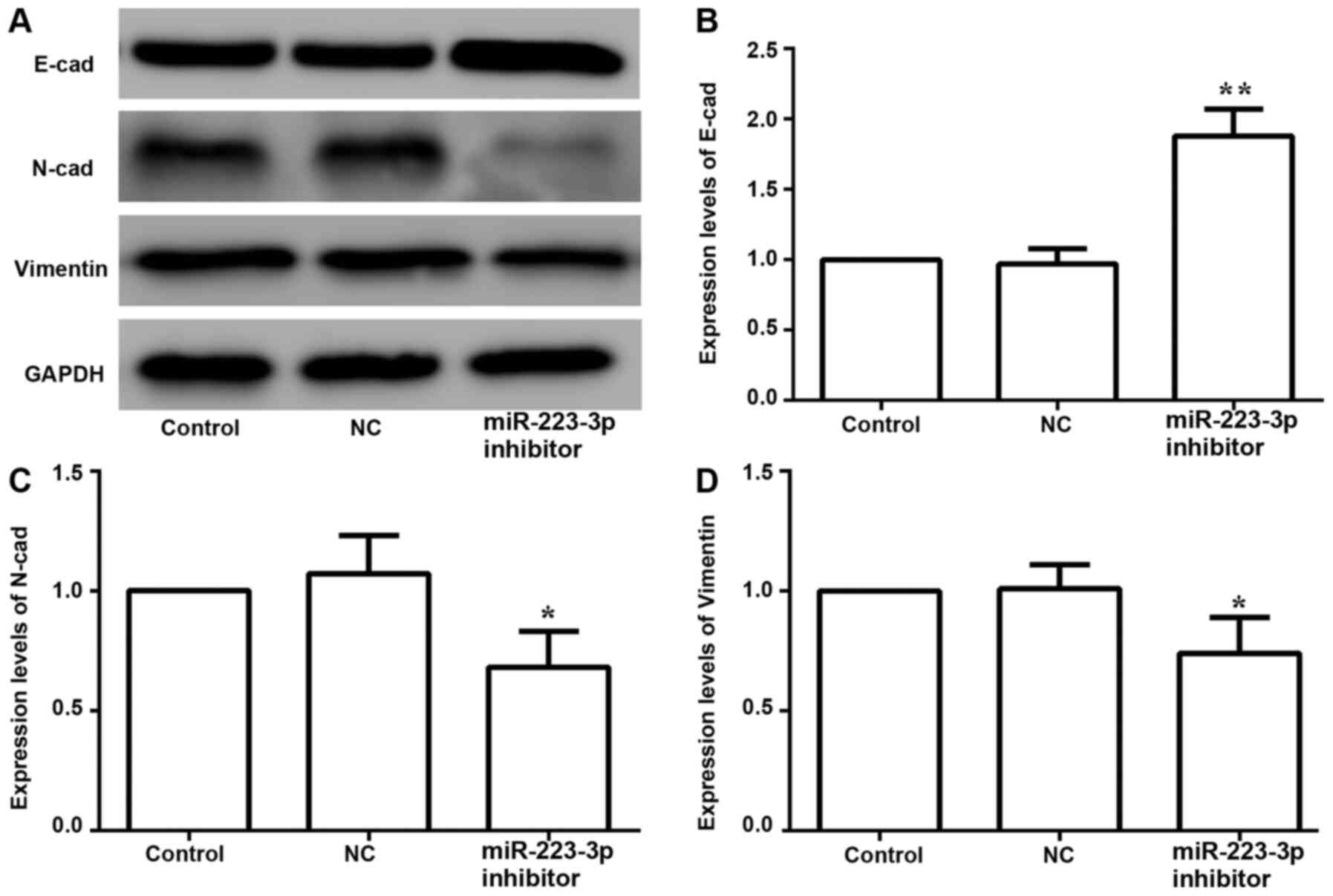
The expression levels of EMT-associated proteins
were examined to determine the effects of miR-223-3p on EMT in
breast cancer cells. The results suggested that inhibiting the
expression of miR-223-3p increased the protein expression of
E-cadherin, and decreased N-cadherin and vimentin expression levels
(Fig. 6). Thus, these data indicated
that inhibition of miR-223-3p may reverse EMT in breast cancer
cells.
miR-223-3p and the Hippo/Yap signaling
pathway
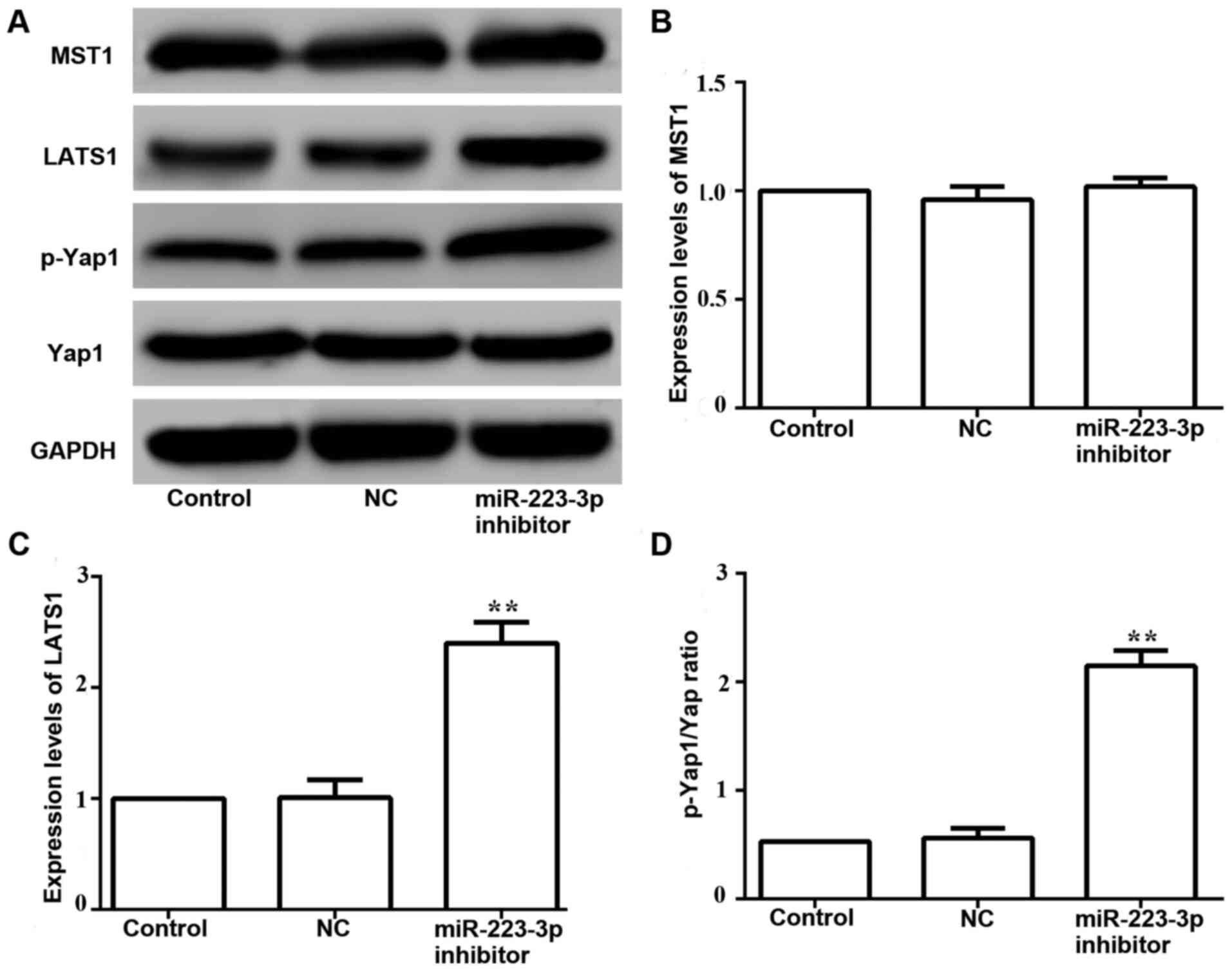
The western blotting results demonstrated that the
expression levels of phosphorylated Yap1 were increased in MCF-7
cells transfected with miR-223-3p inhibitor compared with those in
control cells (Fig. 7A and D).
Moreover, no significant difference was found between the protein
expression levels of MST1 in cells transfected with miR-223-3p
inhibitor and control cells (Fig. 7A and
B). It was identified that LATS1 expression was significantly
upregulated in miR-223-3p inhibitor-transfected cells (Fig. 7A and C). These results suggested that
miR-223-3p may activate the Hippo/Yap signaling pathway.
Discussion
The role of miR-223-3p in tumor growth has recently
been described. For example, miR-223-3p overexpression promotes the
proliferation, migration and invasion of ovarian cancer cells
(27). Conversely, ectopic
expression of miR-223-3p could inhibit the invasion, migration,
growth and proliferation of breast cancer cells (28). It has also been shown that the MMP
protein family, including MMP2, MMP9 and MMP14, is involved in cell
development and migration (29). In
the present study, the expression levels of miR-223-3p were
compared between normal and breast cancer cells. It was observed
that the transcription levels of miR-223-3p were markedly increased
in breast cancer cells. It was also found that following
transfection of miR-223-3p inhibitor into cells, miR-223-3p
expression could be significantly inhibited. Furthermore, in
association with inhibition of miR-223-3p, the activity of tumor
cells was markedly decreased, and the apoptosis rate was increased.
Thus, the present study demonstrated that miR-223-3p may serve an
important role in the proliferation, metastasis and invasion of
breast cancer cells.
CDK and the regulatory subunit cyclin can directly
affect the progression of the cell cycle (30). The present study demonstrated that
miR-223-3p inhibitor markedly inhibited the proliferation of MCF-7
cells by downregulating the expression levels of CDK2 and Cyclin
E1, and upregulating p21 expression. In addition, miR-223-3p
inhibition significantly decreased the migration and invasion of
MCF-7 cells, and the results demonstrated that the expression
levels of MMP family proteins were inhibited. These characteristics
may contribute to the understanding of miR-223-3p expression and
the invasive biological behavior of breast cancer cells.
EMT serves a key role in both normal cell
development and tumor development (31). EMT is the process of epithelial cells
losing epithelial proteins, including E-cadherin, which is
responsible for tight junctions (32), and members of the miR-200 family,
which help maintain an epithelial phenotype (33). The cell moves towards a more
mesenchymal phenotype as it gains mesenchymal markers, such as
N-cadherin, which provides the cells with migratory potential, and
vimentin and fibronectin, which are proteins excreted to help form
the extracellular matrix (34).
However, when proteins involved in EMT are dysregulated, epithelial
cell polarity is lost, which increases the invasive ability of
tumor cells and accelerates the occurrence and development of
tumors (35). EMT serves an
important role in cancer progression and can stimulate cells to
migrate and invade, increasing their metastatic potential (36,37).
Therefore, it was hypothesized that miR-223-3p may affect EMT in
breast cancer cells. The present results demonstrated that
miR-223-3p inhibition significantly increased the expression levels
of the epithelial marker E-cadherin and significantly decreased the
expression levels of mesenchymal markers, such as N-cadherin and
vimentin, suggesting that miR-223-3p may affect the migration and
invasion of MCF-7 cells and aggravate the occurrence of breast
cancer. However, compared with control cells, inhibition of
miR-223-3p could increase the expression levels of E-cadherin and
decrease the expression levels of N-cadherin and vimentin,
suggesting that decreasing miR-223-3p expression could inhibit the
progression of EMT, and thus inhibit the invasiveness of tumors,
which was consistent with the effect of inhibiting the
transcription of miR-223-3p on the proliferative, migratory and
invasive abilities of MCF-7 cells. However, the mechanism through
which miR-223-3p induces EMT requires further clarification.
Studies in Drosophila and vertebrates have
revealed that the Hippo signaling pathway has important regulatory
effects on organ growth (38). As
research progresses, increasing evidence suggests that the Hippo
signaling pathway is dysregulated in the development of human
tumors (21). Yap functions as a key
downstream effector of the Hippo signaling pathway, mainly through
phosphorylation, to inhibit tumor progression (39). Previous studies have reported that
the abnormal activation of the Hippo signaling pathway is
associated with tumor progression, and abnormal Yap expression is
associated with the occurrence of various tumors (38), such as liver cancer (40), breast cancer (40) and pancreatic cancer (41). Studies have also revealed that
abnormal activation of Yap serves a key regulatory role in the
process of tumorigenesis and development (39,42,43). The
present results demonstrated that miR-223-3p inhibition increased
the phosphorylation of Yap.
MST1, LATS1 and LATS2 are key kinases in the core
kinase cassette of the mammalian Hippo signaling pathway (44,45).
Once the Hippo signaling pathway is activated, the MST kinase
phosphorylates LATS and the transcriptional co-activator Yap,
thereby inactivating Yap (46).
Overexpression of Yap can reverse the inhibition of the Hippo
signaling pathway by blocking the activity of LATS (47). The present study demonstrated that
miR-223-3p inhibition increased the phosphorylation of Yap and
upregulated the expression level of the Hippo signaling pathway
kinase LATS1. Abnormal Yap overexpression is associated with basic
cellular processes, such as cell proliferation, migration, invasion
and EMT (48). Therefore, inhibition
of miR-223-3p expression may regulate Yap expression via the Hippo
signaling pathway, thus inhibiting the proliferation, migration,
invasion and EMT of breast cancer cells.
It has been reported that miR-223-3p has the
potential to promote the proliferation and invasion of cancer cells
(6–11), and the proliferation and invasion of
breast cancer cells are significantly enhanced after miR-223-3p
transfection into breast cancer cells (12,49,50). The
present report, as an initial study of miR-223-3p in breast cancer,
aimed to preliminarily investigate whether this miRNA has a
regulatory effect on breast cancer cells and to analyze its pathway
of action. There are certain limitations, such as the requirement
for in vivo experiments to support the present hypothesis.
However, relevant animal experiments to verify the regulatory role
of miR-223-3p in tumors have been conducted by our group, but
relevant experimental data are being submitted, and thus they are
not shown in the current manuscript. Future studies will continue
to examine the role of miR-223-3p in the occurrence, metastasis and
upstream regulators of breast cancer, and investigate whether it
affects other tumor regulatory pathways.
In conclusion, the present study demonstrated that
miR-223-3p may regulate breast cancer cell proliferation,
migration, invasion and EMT via the Hippo/Yap signaling pathway.
This phenomenon may be ameliorated after miR-223-3p inhibition. The
present results provide a basis for the study of breast cancer and
novel ideas for treatment.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from Jilin
Provincial Science and Technology Development Project (grant nos.
20200201577JC and 20180520229JH).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TD performed gene and protein expression, cell
viability, apoptosis, cell migration and cell invasion assays. DW
performed E-cadherin, N-cadherin and vimentin protein detection. XW
performed Mst1, LATS1, Yap1 and phosphorylated Yap1 protein
detection. BL designed and supervised the study, and collaborated
to discuss the results. JX and QX analyzed the data. TD wrote and
modified the manuscript and summarized the experiment based on the
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen W, Sun K, Zheng R, Zeng H, Zhang S,
Xia C, Yang Z, Li H, Zou X and He J: Cancer incidence and mortality
in China, 2014. Chin J Cancer Res. 30:1–12. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rong C, Meinert ÉFRC and Hess J: Estrogen
receptor signaling in radiotherapy: From molecular mechanisms to
clinical studies. Int J Mol Sci. 19:7132018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Mahmood S, Sapiezynski J, Garbuzenko OB
and Minko T: Metastatic and triple-negative breast cancer:
Challenges and treatment options. Drug Deliv Transl Res.
8:1483–1507. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jang HJ, Lee HS, Burt BM, Lee GK, Yoon KA,
Park YY, Sohn BH, Kim SB, Kim MS, Lee JM, et al: Integrated genomic
analysis of recurrence-associated small non-coding RNAs in
oesophageal cancer. Gut. 66:215–225. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mavrakis KJ, Van Der Meulen J, Wolfe AL,
Liu X, Mets E, Taghon T, Khan AA, Setty M, Rondou P, Vandenberghe
P, et al: A cooperative microRNA-tumor suppressor gene network in
acute T-cell lymphoblastic leukemia (T-ALL). Nat Genet. 43:673–678.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li J, Guo Y, Liang X, Sun M, Wang G, De W
and Wu W: MicroRNA-223 functions as an oncogene in human gastric
cancer by targeting FBXW7/hCdc4. J Cancer Res Clin Oncol.
138:763–774. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wei Y, Yang J, Yi L, Wang Y, Dong Z, Liu
Z, Ou-Yang S, Wu H, Zhong Z, Yin Z, et al: MiR-223-3p targeting
SEPT6 promotes the biological behavior of prostate cancer. Sci Rep.
4:75462014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fazi F, Racanicchi S, Zardo G, Starnes LM,
Mancini M, Travaglini L, Diverio D, Ammatuna E, Cimino G, Lo-Coco
F, et al: Epigenetic silencing of the myelopoiesis regulator
microRNA-223 by the AML1/ETO oncoprotein. Cancer Cell. 12:457–466.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tang Y, Wang Y, Chen Q, Qiu N, Zhao Y and
You X: MiR-223 inhibited cell metastasis of human cervical cancer
by modulating epithelial-mesenchymal transition. Int J Clin Exp
Pathol. 8:11224–11229. 2015.PubMed/NCBI
|
|
11
|
Zhao FY, Han J, Chen XW, Wang J, Wang XD,
Sun JG and Chen ZT: miR-223 enhances the sensitivity of non-small
cell lung cancer cells to erlotinib by targeting the insulin-like
growth factor-1 receptor. Int J Mol Med. 38:183–191. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yoshikawa M, Iinuma H, Umemoto Y,
Yanagisawa T, Matsumoto A and Jinno H: Exosome-encapsulated
microRNA-223-3p as a minimally invasive biomarker for the early
detection of invasive breast cancer. Oncol Lett. 15:9584–9592.
2018.PubMed/NCBI
|
|
13
|
Nieto MA, Huang RYJ, Jackson RA and Thiery
JP: EMT: 2016. Cell. 166:21–45. 2106. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y and Zhou BP: Epithelial-mesenchymal
transition-a hallmark of breast cancer metastasis. Cancer Hallm.
1:38–49. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye X, Tam WL, Shibue T, Kaygusuz Y,
Reinhardt F, Ng Eaton E and Weinberg RA: Distinct EMT programs
control normal mammary stem cells and tumour-initiating cells.
Nature. 525:256–260. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Krebs AM, Mitschke J, Lasierra Losada M,
Schmalhofer O, Boerries M, Busch H, Boettcher M, Mougiakakos D,
Reichardt W, Bronsert P, et al: The EMT-activator Zeb1 is a key
factor for cell plasticity and promotes metastasis in pancreatic
cancer. Nat Cell Biol. 19:518–529. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan
A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al: The
epithelial-mesenchymal transition generates cells with properties
of stem cells. Cell. 133:704–715. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Singh A and Settleman J: EMT, cancer stem
cells and drug resistance: An emerging axis of evil in the war on
cancer. Oncogene. 29:4741–4751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kar R, Jha NK, Jha SK, Sharma A, Dholpuria
S, Asthana N, Chaurasiya K, Singh VK, Burgee S and Nand P: A
‘NOTCH’ Deeper into the epithelial-to-mesenchymal transition (EMT)
program in breast cancer. Genes. 10:9612019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng Y and Pan D: The hippo signaling
pathway in development and disease. Dev Cell. 50:264–282. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan D: The hippo signaling pathway in
development and cancer. Dev Cell. 19:491–505. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao B, Lei QY and Guan KL: The Hippo-YAP
pathway: New connections between regulation of organ size and
cancer. Curr Opin Cell Biol. 20:638–646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moleirinho S, Chang N, Sims AH,
Tilston-Lünel AM, Angus L, Steele A, Boswell V, Barnett SC, Ormandy
C, Faratian D, et al: KIBRA exhibits MST-independent functional
regulation of the Hippo signaling pathway in mammals. Oncogene.
32:1821–1830. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu LL, Zhao H, Ma TF, Ge F, Chen CS and
Zhang YP: Identification of valid reference genes for the
normalization of RT-qPCR expression studies in human breast cancer
cell lines treated with and without transient transfection. PLoS
One. 10:e01170582015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azimi A, Majidinia M, Shafiei-Irannejad V,
Jahanban-Esfahlan R, Ahmadi Y, Karimian A, Mir SM, Karami H and
Yousefi B: Suppression of p53R2 gene expression with specific siRNA
sensitizes HepG2 cells to doxorubicin. Gene. 642:249–255. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fang G, Liu J, Wang Q, Huang X, Yang R,
Pang Y and Yang M: MicroRNA-223-3p regulates ovarian cancer cell
proliferation and invasion by targeting SOX11 expression. Int J Mol
Sci. 18:12082017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ji Q, Xu X, Song Q, Xu Y, Tai Y, Goodman
SB, Bi W, Xu M, Jiao S, Maloney WJ and Wang Y: miR-223-3p inhibits
human osteosarcoma metastasis and progression by directly targeting
CDH6. Mol Ther. 26:1299–1312. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jabłońska-Trypuć A, Matejczyk M and
Rosochacki S: Matrix metalloproteinases (MMPs), the main
extracellular matrix (ECM) enzymes in collagen degradation, as a
target for anticancer drugs. J Enzyme Inhib Med Chem. 31:177–183.
2016. View Article : Google Scholar
|
|
30
|
Song X, Zhu M, Zhang F, Zhang F, Zhang Y,
Hu Y, Jiang L, Hao Y, Chen S, Zhu Q, et al: ZFX promotes
proliferation and metastasis of pancreatic cancer cells via the
MAPK pathway. Cell Physiol Biochem. 48:274–284. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iwatsuki M, Mimori K, Yokobori T, Ishi H,
Beppu T, Nakamori S, Baba H and Mori M: Epithelial-mesenchymal
transition in cancer development and its clinical significance.
Cancer Sci. 101:293–299. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang ZC, Yi MJ, Ran N, Wang C, Fu P, Feng
XY, Xu L and Qu ZH: Transforming growth factor-β1 induces bronchial
epithelial cells to mesenchymal transition by activating the Snail
pathway and promotes airway remodeling in asthma. Mol Med Rep.
8:1663–1668. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Korpal M, Lee ES, Hu G and Kang Y: The
miR-200 family inhibits epithelial-mesenchymal transition and
cancer cell migration by direct targeting of E-cadherin
transcriptional repressors ZEB1 and ZEB2. J Biol Chem.
283:14910–14914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rout-Pitt N, Farrow N, Parsons D and
Donnelley M: Epithelial mesenchymal transition (EMT): A universal
process in lung diseases with implications for cystic fibrosis
pathophysiology. Respir Res. 19:1362018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Royer C and Lu X: Epithelial cell
polarity: A major gatekeeper against cancer? Cell Death Differ.
18:1470–1477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mathias RA, Gopal SK and Simpson RJ:
Contribution of cells undergoing epithelial-mesenchymal transition
to the tumor microenvironment. J Proteomics. 78:545–557. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lv B, Yang X, Lv S, Wang L, Fan K, Shi R,
Wang F, Song H, Ma X, Tan X, et al: Retraction note to: CXCR4
signaling induced epithelial-mesenchymal transition by PI3K/AKT and
ERK pathways in glioblastoma. Mol Neurobiol. 54:2380. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Badouel C, Garg A and McNeill H: Herding
Hippos: Regulating growth in flies and man. Curr Opin Cell Biol.
21:837–843. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ehmer U and Sage J: Control of
proliferation and cancer growth by the Hippo signaling pathway. Mol
Cancer Res. 14:127–140. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang J, Ma L, Weng W, Qiao Y, Zhang Y, He
J, Wang H, Xiao W, Li L, Chu Q, et al: Mutual interaction between
YAP and CREB promotes tumorigenesis in liver cancer. Hepatology.
58:1011–1020. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Jiang Z, Zhou C, Cheng L, Yan B, Chen K,
Chen X, Zong L, Lei J, Duan W, Xu Q, et al: Inhibiting YAP
expression suppresses pancreatic cancer progression by disrupting
tumor-stromal interactions. J Exp Clin Cancer Res. 37:692018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu H, Du S, Lei T, Wang H, He X, Tong R
and Wang Y: Multifaceted regulation and functions of YAP/TAZ in
tumors (Review). Oncol Rep. 40:16–28. 2018.PubMed/NCBI
|
|
43
|
Zanconato F, Cordenonsi M and Piccolo S:
YAP and TAZ: A signalling hub of the tumour microenvironment. Nat
Rev Cancer. 19:454–464. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ma Y, Yang Y, Wang F, Wei Q and Qin H:
Hippo-YAP signaling pathway: A new paradigm for cancer therapy. Int
J Cancer. 137:2275–2286. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hergovich A: The roles of NDR protein
kinases in Hippo signalling. Genes. 7:212016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhao B, Lei Q and Guan KL: Mst out and HCC
in. Cancer Cell. 16:363–364. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Basu-Roy U, Bayin NS, Rattanakorn K, Han
E, Placantonakis DG, Mansukhani A and Basilico C: Sox2 antagonizes
the Hippo pathway to maintain stemness in cancer cells. Nat Commun.
6:64112015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zhang H, Liu CY, Zha ZY, Zhao B, Yao J,
Zhao S, Xiong Y, Lei QY and Guan KL: TEAD transcription factors
mediate the function of TAZ in cell growth and
epithelial-mesenchymal transition. J Biol Chem. 284:13355–13362.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang M, Chen J, Su F, Yu B, Su F, Lin L,
Liu Y, Huang JD and Song E: Microvesicles secreted by macrophages
shuttle invasion-potentiating microRNAs into breast cancer cells.
Mol Cancer. 10:1172011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cao L, Zhang X, Cao F, Wang Y, Shen Y,
Yang C, Uzan G, Peng B and Zhang D: Inhibiting inducible miR-223
further reduces viable cells in human cancer cell lines MCF-7 and
PC3 treated by celastrol. BMC Cancer. 15:8732015. View Article : Google Scholar : PubMed/NCBI
|