Introduction
Cell fusion is a phenomenon that exists widely in
the physiological and pathological conditions of organisms
(1). Cell fusion involves two cells
merging together through their plasma membranes, causing their
cytoplasm to mix to form hybrids, obtaining new biological
characteristics, functions and phenotypes (1). Some types of cells, such as gametes,
myoblasts, macrophages and syncytiotrophoblasts, have the ability
to form fused cells or polyploids, which are important for species
passage, development and the maintenance of normal physiological
functions (2). Recent studies have
demonstrated that cell fusion can also occur in the occurrence and
progression of some diseases, such as viral infections and tumors
(3–5). The present review discusses the effects
of cell fusion in malignant tumors and provides support and
reference for tumor research and treatment. The present study
discusses the phenomenon and mechanism of cell fusion in humans,
and cell fusion events in the tumor microenvironment and their
roles in tumor progression. In addition, potential cancer treatment
options targeting cell fusion are considered.
Pattern of cell fusion
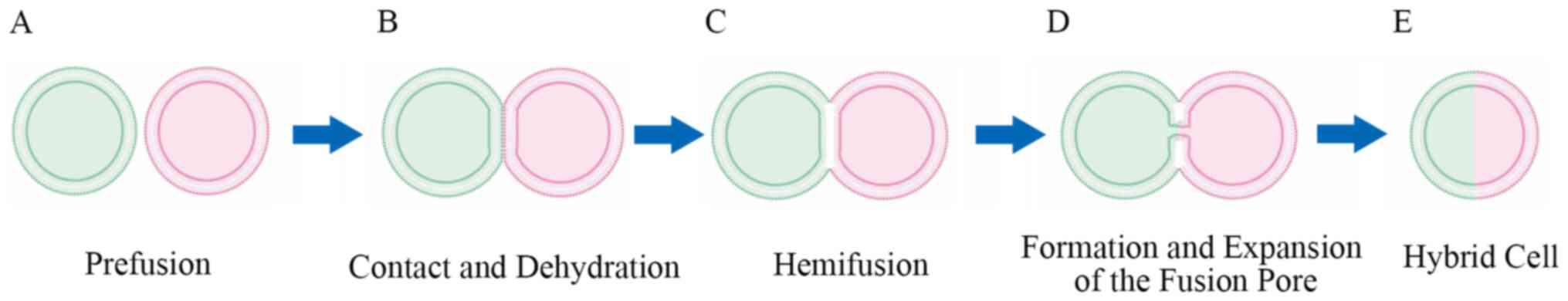
The pattern of cell fusion can be divided into three
phases: i) Contact and dehydration, ii) hemifusion and iii) the
formation and expansion of a fusion pore (3), all of which are energy consuming
(6). Prefusion preparation is a
prerequisite for cell fusion to accurately fuse specific cells
(Fig. 1A) (7). Hernández and Podbilewicz (8) further divided the prefusion preparation
process into three steps, differentiation, recognition and
adhesion. The expression of specific recognition or
adhesion-associated proteins during preparation is sufficient and
necessary for cell fusion; however, this does not mean that they
are directly involved in the fusion process itself; they just help
specific cells maintain proximity (8,9). In this
process, the cells are close enough (<10 nm), and the distance
gradually becomes <1 nm under the activation of some proteins
accompanied by the removal of water molecules between cells during
dehydration (Fig. 1B) (10,11). At
such a close distance, the plasma membrane begins to bend, and the
outer layer of the phospholipid bilayer merges, which is also known
as hemifusion (Fig. 1C) (12,13).
Consequently, the inner layer further merges and forms a fusion
pore between the cells (6) (Fig. 1D). As the fusion pores expand, the
cytoplasm is completely mixed to form a hybrid containing the
genomes and several organelles, such as mitochondria of the two
parental cells (Fig. 1E) (14). The proteins that are activated during
cytoplasmic membrane fusion that directly mediate and induce cell
fusion are referred to as fusogens (15,16).
Fusogens assemble into unilateral or bilateral complexes, which
determine the site of cell fusion and overcome the energy barriers
that are required to prevent the anti-fusion mechanism (17). There are four families of fusogens
that are explicitly involved in cell-cell fusion, of which only one
is expressed in human cells, syncytins, which play a key role in
the development of human placental syncytiotrophoblasts (8). Given that differentiated cells do not
share the same molecular mechanism, there are studies on fusogens
in different types of human cells (18–20).
Notably, a recent study demonstrated that different fusogens share
similar structural folds, which may provide insight for the
discovery of novel fusogens (21).
Cell fusion in physiological processes
Cell fusion is a widespread physiological phenomenon
in several living organisms, from fungi to mammals. Cell fusion
participates in various processes, including reproduction, growth
and development, and involves complex genetic and molecular
mechanisms that remain unclear (2).
Previous studies have reported that different differentiated cells
may not share the same mechanism in cell fusion, such as having
different adhesion or recognition molecules and fusogens (8,22).
Molecules involved in some cell fusion phenomena that occur under
physiological conditions in mammals are summarized in Table I.
 | Table I.Cell fusion related molecules under
mammalian physiological conditions. |
Table I.
Cell fusion related molecules under
mammalian physiological conditions.
| Molecule | Expression | Essential for
fusion | Type | Function |
|---|
| CD9 | Oocyte
microvilli | Yes | Heterotypic | Recognition |
| IZUMO1 | Sperm | Yes | Heterotypic | Recognition |
| Juno | Oocyte | Yes | Heterotypic | Recognition |
| Syncytin-1 | Placenta, myoblast
and brain | Yes | Homotypic | Fusogen |
| Syncytin-2 | Placenta | Yes | Homotypic | Fusogen |
| GCM1 | Placenta | Unclear | Homotypic | Regulates
syncytins |
| MRF | Macrophage | Yes | Homotypic | Recognition,
combine with CD46 |
| CD-STAMP | Macrophage | Yes | Homotypic | Unclear |
| CD44 | Macrophage | No | Homotypic | Recognition |
| CCL2 | Macrophage | Unclear | Homotypic | Regulator |
| ADAM12 | Myoblast | Yes | Homotypic | Adhesion |
| Myomaker | Myoblast | Yes | Homotypic | Unclear |
| FGFRL1 | Myoblast | No | Homotypic | Unclear |
| GRAF1 | Myoblast | Unclear | Homotypic | Regulator |
Sperm-oocyte fusion in fertilization is the earliest
and most common understanding of cell fusion (23). CD9, expressed on the microvilli of
oocytes, and IZUMO1, expressed on sperm, have been demonstrated to
play important roles in sperm-oocyte fusion (24). CD9 knockout mice exhibited an
abnormal morphology of microvilli in oocytes (25), and CD9 may be associated with cell
membrane curvature via interaction with IgSF (24). IZUMO1 forms an adhesion complex by
binding to the receptor Juno and mediates the specific recognition
of sperm and oocytes during fertilization (26). The IZUMO1-JUNO complex is an
essential molecule in cell contact but is not directly involved in
plasma membrane merger (27). The
fusogens involved in mammalian sperm-egg fusion remain unclear.
The only human fusogen, syncytins, which depend on
cell fusion, are present in placental formation (28). Following implantation of the embryo,
trophoblast cells differentiate into the inner layer of
cytotrophoblasts (CTBs) and the outer layer of syncytiotrophoblasts
(STBs) (29). Syncytin-1 is
predominantly expressed in STBs (28) and is also present in some tumors
(30), myoblasts (31), osteoclasts (32) and oligodendrocytes (33). Syncytin-2 is predominantly expressed
in CTBs, and its receptor, major facilitator superfamily domain
containing 2, is present in STBs (34). The function and receptor of
syncytin-3 remain unknown.
Macrophages exert physiological functions by forming
syncytia under certain conditions, such as osteoclasts that
regulate skeletal stability and multinucleated giant cells, which
participate in immune responses during infection (35). For macrophages, at least three
receptors are essential for cell fusion, including macrophage
fusion receptor (MFR), dendritic cell-specific transmembrane
protein (DC-STAMP) and CD44 (36).
The receptor for MFR is CD47, both of which belong to the
immunoglobulin superfamily and are expressed on the macrophage
membrane (37). Hyaluronan is
considered a ligand for CD44, and CD44 antibodies can inhibit the
process of osteoclastogenesis (38).
DC-STAMP is an important component of the formation of osteoclasts
and multinucleated giant cells (39). The differentiation of myoblasts is a
prerequisite for cell fusion, including the expression of
adhesion-, migration-, and cytoskeletal rearrangement-associated
molecules (40). Recently, in
mammals, a new fusogen candidate in myoblasts was discovered,
myomaker, which controls the formation of muscle fibers and induces
non-fusogenic cells to form multinucleated cells (41,42).
Cell fusion in cancer hallmarks
Almost 120 years ago, the zoologist, Theodor Boveri,
speculated that cancer may originate from the abnormal formation of
aneuploidy (43). Cell fusion is an
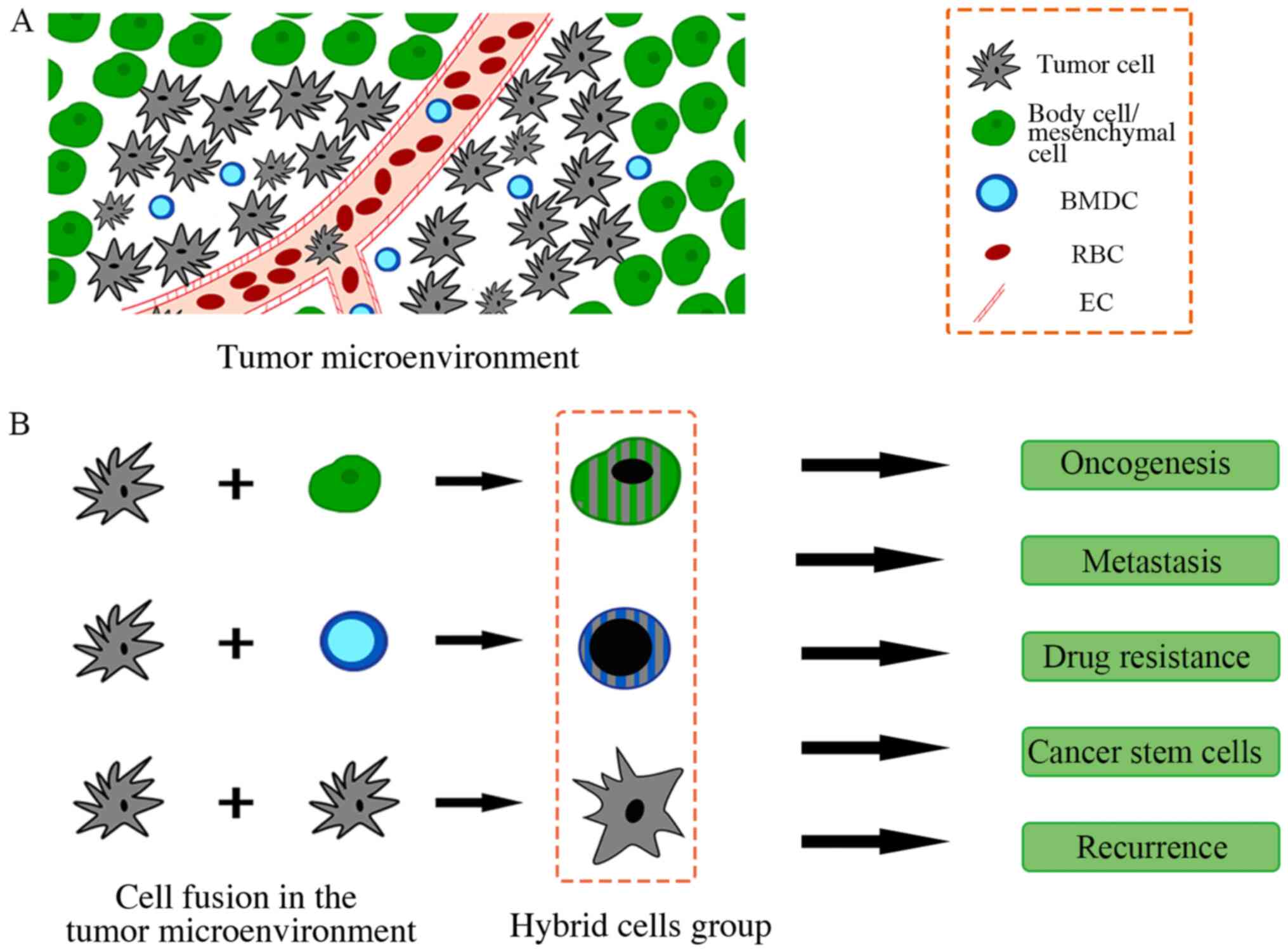
important pathway for aneuploidy formation (3). Currently, the phenomenon of cell fusion
in tumors has been gradually recognized, and several fusion cases
have been observed in the tumor microenvironment, such as cancer
cells fusing with mesenchymal stem cells (MSCs) (44–46),
macrophages (47,48), fibroblasts (49) or endothelial cells (50,51). In
addition, cumulative reports have demonstrated that cancer cells
can obtain hallmarks from cell fusions within the microenvironment
(52–54). The reported functions of cell fusions
in tumors are summarized in Fig.
2.
Heterogeneity
In addition to genetic or epigenetic alterations in
oncogenes or tumor suppressor genes, tumorigenesis is closely
associated with chromosomal instability (55). However, previous studies have
reported that there are some diploid tumor cells with no obvious
mutations at the genetic level, challenging the traditional somatic
mutation theory (SMT) (56,57). Aneuploidy is observed in several
malignancies, revealing the genetic instability of cancer cells
(58). A hypothesis called the
heterokaryon-to-synkaryon transition provides an explanation for
the heterogeneity of tumors (59),
which suggests that the tumor forms a heterokaryon (containing the
respective nuclei) and further forms a synkaryon (containing only
one nuclei) by rearrangement of the chromosome (60). When homotypic or heterotypic cells
fuse, genomic instability caused by chromosomal rearrangement is
likely to be fatal (55). Zhou et
al (61) also detected DNA
double-strand damage and translocation in hybrid cells.
Furthermore, Delespaul et al (55) confirmed that hybrids of partly
transformed fibroblasts can detect genomic instability and induce
hybrid cell tumor formation in mice. Dittmar et al (62) demonstrated that cell fusion in breast
cancer, as a mechanism of gene transfer, is involved in the
emergence of tumor heterogeneity in evolution. Hybrid progenies
overexpress or lose specific genes via chromosome rearrangement,
which not only increases tumor heterogeneity but also enhances the
ability of cancer cells to adapt to diverse tumor microenvironments
(63). Delespaul et al
(55) also demonstrated that tumors
formed by fused cells can rapidly promote tumor progression if they
have the appropriate genome. However, Su et al reported that
cell fusion (such as in breast cancer) can also regulate tumor
heterogeneity through epigenetics rather than genetics (64). These phenomena provide a new
understanding of the role of cell fusion in heterogeneity, and
there are other complex regulatory mechanisms for the formation of
tumor heterogeneity.
Oncogenesis
In some cases, genetic instability in aneuploid
hybrid cells is likely to trigger the malignant transformation of
cells and induce malignant cell behaviors (3). As early as 1992, Munzarova et al
(65) observed that advanced
melanoma gradually exhibits the biological characteristics of
lymphocytes and macrophages, and hypothesized that melanoma may
derive from the fusion of host melanocytes and macrophages. Zhou
et al (61) reported the
fusion of small intestinal epithelial cells (IEC-6 cells) through
PEG-informed mice and detected aneuploidy in 40% of the clones.
Some fused cells exhibited transformed phenotypes, such as
resistance to apoptosis, enhanced proliferation capacity and
chromosomal rearrangement (61). He
et al (66) also confirmed
that 84.1% of progeny cells fused with gastric epithelial cells and
MSCs were aneuploid and malignant transformation occurred, in the
laboratory. However, the association between tumor formation and
cell fusion remains unclear. In some studies, hybrid cells have
played key roles in suppressing malignant behaviors following cell
fusion. For example, in the liver of mice, the fusion of liver
tumor cells and stem cells has been demonstrated to suppress
tumorigenesis (67). Furthermore,
Israel and Schaeffer (68) performed
cell fusion between the original cloned normal and transformed
liver epithelial cells, and the survival time of hybrid cell
transplanted mice was significantly longer. Taken together, these
findings confirm the tumor suppressive effect of normal cytoplasm,
making the role of cell fusion in tumors more complicated.
Recently, it has been speculated that the SMT cannot
explain various tumorigenesis phenomena. Theories that abnormal
mitochondria mediate tumorigenesis have been proposed (69,70).
Given that the fusion of cytoplasm is involved in the process of
cell fusion, the role of mitochondria in cell fusion cannot be
ignored (70). According to Seyfried
and Shelton (71), the offspring of
normal cell nuclei transplanted into the enucleated cytoplasm of
tumor cells can still have the characteristics of malignant
behavior. This means that metabolic abnormalities caused by
cytoplasmic fusion, such as abnormal mitochondrial function, may be
the cause of tumors rather than nuclear gene changes (70). However, malignant transformation of
normal cells via cell fusion in vivo, and cell fusion have
not been observed in all tumors (72). Similarly, Duelli and Lazebnik have
reported that the appearance of fused cells in solid tumors is a
rare phenomenon (~1%) (73). A
hypothesis called the ‘dark matter hypothesis’ states that because
hybrid cells currently identified in tumors mostly rely on the
expression of cell surface biomarkers and parental DNA, the
instability of progeny cell genes may lead to the inability to
continuously express relevant biomarkers (74). In addition, the fusion between tumor
cells may be more difficult to detect, resulting in a lower
incidence of cell fusion events detected in tumors (74).
Metastasis
Tumor metastasis is a multistep and multistage
complex process. Among these multistage processes,
epithelial-to-mesenchymal transition (EMT) is a key step (75). EMT is an important adaptive process
for tumors to move away from the primary site to distant tissues
during tumor metastasis (76).
During EMT, the number of adhesion molecules on the surface of
tumor cells decreases to express the interstitial phenotype and
gain migration capacity (77).
Several aggressive cancer cells exhibit metastasis, secretion and
phagocytosis, similar to bone marrow-derived cells (BMDCs)
(78). One theory is that tumor
cells acquire a mesenchymal phenotype derived from the fusion of
tumors and BMDCs, such as macrophages (76). Spontaneous fusion of BMDCs with tumor
cells in vivo has been observed in both mice (79) and humans (80), and hybrid cells express several genes
associated with tumor invasion and metastasis, such as SPARC, MCR1
and MET (76). Recently, Gast et
al (48) demonstrated that BMDCs
can increase their heterogeneity by fusing with tumors, allowing
tumors to acquire a migration phenotype. In addition,
macrophage-tumor fusion cells are detected in the peripheral blood
of patients with cancer, an observation that is closely associated
with the tumor stage and prognosis (48). Furthermore, the tumor-BMDC fusion
hypothesis gives tumor metastasis an explanation for the preference
of different organs (76). The
liver, lungs and bone are usually the preferred metastatic sites
for several tumors, and these sites usually have large numbers of
BMDCs (81). The migration induced
by BMDC-tumor fusion may be more suitable for a new
microenvironment (78). In addition
to BMDCs, some studies have also demonstrated that MSCs,
endothelial cells and fibroblasts can also induce tumor metastasis
by spontaneous fusion with cancer cells in the tumor
microenvironment (82). Noubissi
et al (83) demonstrated that
the migratory ability of the nonmetastatic breast cancer cell
lines, T47Ds and MCF7s, is significantly enhanced following
induction of fusion with MSCs. Similar findings were observed in
vascular epithelial cells (84) and
tumor-associated fibroblasts (85).
In a coculture model of mesenchymal cells and prostate cancer cells
by Wang et al (86),
spontaneously fused hybrid cells were formed that had the ability
to sustain growth, genotype changes and increase malignancy.
Conversely, it has been reported that the fusion of mesenchymal
cells and tumor cells plays a role in tumor suppression (45). For example, Wei et al
(45) demonstrated that FOXF1 can
decrease the malignancy of tumors by regulating the fusion of lung
cancer cells and MSCs. Thus, the role of cell fusion in tumor
progression requires further research and discussion.
Notably, Clawson et al (87) demonstrated that macrophage-tumor cell
fusions (MTFs) extracted from the peripheral blood of patients with
pancreatic ductal adenocarcinoma (PDAC) have the phenotypes of
macrophages, stem cells and PDACs. However, in the orthotopic
xenograft tumor model in nude mice, only well-differentiated cell
islands were observed in the pancreas, and many disseminated cell
populations, such as lungs and liver, were present, but no obvious
tumor formation was observed (87).
A similar phenomenon has been demonstrated in melanoma (47). For instance, the extracted MTFs did
not form transplanted tumors in the subcutaneous area of nude mice
but produced metastatic lesions in other organs (47). Collectively, these findings suggest
that the fused cells do not directly form tumor metastases, but
they form a niche that facilitates tumor metastasis in the tissues
they disseminate (88). These
seemingly contradictory studies make the theory of tumor fusion
cell metastasis controversial.
Drug resistance
The formation of tumor resistance involves several
mechanisms, including changes in receptor activity, drug
transporters and enzymes that produce inactivated drugs (89). Intercellular gene exchange via cell
fusion may potentially cause rapid changes in cancer cell
resistance and form subpopulations that are dominant in the
microenvironment. Subpopulations of cells with different drug
resistance capacities can acquire multidrug resistance through cell
fusion (90). Miller et al
(91) demonstrated that the
5-fluorouracil-resistant 44FTO cell line spontaneously fuses with
the methotrexate-resistant 168FAR cell line to form a
double-resistant hybrid cell. Nonresistant tumor cells can also
acquire resistance through cell fusion, such as drug-resistant
cells formed by tumors and BMDCs (92). Uygur et al (93) recently discovered that in prostate
cancer, the fusion of cancer cells with surrounding muscle cells
enhances the resistance of tumors. Song et al (94) reported that in hybridization
experiments of the oral cancer cell lines, SCC9 and HUVECs, hybrid
cells exhibited parental phenotypic characteristics and
significantly improved resistance to chemotherapy drugs. Following
fusion of melanoma cells with fibroblasts and macrophages, Searles
et al (95) observed that
functional gene exchange between parental cells produced enhanced
resistance in progeny cells. Tumor cells can increase drug
resistance by forming polyploid giant cancer cells under the
induction of chemotherapy drugs (96). Taken together, these findings suggest
that cell fusion can be used as a mechanism to allow cell
subpopulations to acquire new or enhanced drug resistance in a
complex tumor microenvironment.
Based on the theory that mitochondrial abnormalities
cause tumors, the role of cytoplasmic fusion in drug resistance
cannot be ignored (70). Due to the
hypoxia of the tumor microenvironment and the impaired
mitochondrial function of tumor cells, ATP synthesis in several
tumor cells occurs mainly through mitochondrial substrate level
phosphorylation and glycolysis (97). The switch of metabolic modes will
lead to the enhancement of drug resistance. Xu et al
(98) demonstrated that cells with
mitochondrial defects or hypoxia have an increase in glycolytic
activity and drug resistance compared with normal cells. By
inhibiting glycolysis, the resistance of tumor cells to the
original chemotherapeutic drugs can be overcome (98). Thus, cytoplasmic fusion can provide
novel insights into drug resistance from the perspective of
metabolism.
Cancer stem cells (CSCs)
CSCs are a special subpopulation of tumor cells that
play important roles in tumorigenicity, drug resistance and
recurrence (99). CSCs possess
several characteristics, such as a low proliferation rate,
anti-apoptosis, downregulation of anti-proliferative pathways, drug
resistance and a more efficient DNA damage repair capacity, which
often make them the source of tumor drug resistance and recurrence
(100). There are several
hypotheses about the origin of CSCs, one of which is that CSCs are
derived from the fusion of stem cells and differentiated cells, as
recurrent tumors often exhibit different characteristics and
phenotypes compared with the original tumors (101). Wei et al (45) reported that spontaneous fusion can
occur in lung cancer and MSCs, and that progenies exhibit a
decrease in the proliferation rate and stem cell-like status.
Dittmar et al (62) also
observed stem cell-like features in hybrid cells fused to breast
epithelial cells and breast cancer cells. Similarly, Bartosh et
al observed cancer cell cannibalizing MSCs in a 3D coculture
model of breast cancer and MSCs. Cancer cells appeared dormant to
protect against the hypoxic and undernourished microenvironment
(102), which may provide an
explanation for tumor recurrence and drug resistance. Under this
condition, the hybrid cells enter a state similar to hibernation by
decreasing the metabolic level, which cannot be damaged by
chemotherapy drugs for an extensive period, and plays a role in the
process of tumor recurrence (102).
Similarly, Uygur et al (93)
demonstrated that under the action of syncytins and AnxA5, the
fusion of prostate cancer cells and muscle cells significantly
increases the expression of CD133, indicating an increase in tumor
stemness. Given the important role of CSCs in tumor recurrence and
drug resistance, the specific mechanism of CSCs generated through
cell fusion is still worth further investigation.
Targeting cell fusion for tumor
treatment
Cell fusion plays an important role in tumor
progression; thus, targeting cell fusion for therapeutic approaches
to cancer is also within the scope of this discussion. Currently,
research on targeted tumor therapy for the cell fusion process is
very scarce; however, there has been some progress in using cell
fusion as a tumor therapeutic strategy.
Block cell fusion
Due to the various negative effects of cell fusion
in tumors, scientists are naturally driven towards inhibiting
cancer heterogeneity, drug resistance, stemness and EMT by blocking
cell fusion. Li et al (103)
successfully blocked the occurrence and progression of
rhabdomyoblastoma in vivo by inhibiting IL-4 receptors
(mediating myoblast fusion). The inhibition of cell fusion in some
colon cancer models has also yielded positive results (61). However, not all cell fusions in the
body are pathological, and scholars have also noted that in some
cases of tumor and somatic cell fusion, hybrid cells exhibit more
benign phenotypes rather than promoting tumor progression (104). Further understanding of the role of
cell fusion in tumors is required, and specific agents that inhibit
the cell fusion process of specific tumors are lacking. Reliable
inhibitors for cell fusion require further investigation.
Fusogens are an important part of cell fusion, and
understanding their function is key to the development of specific
cell fusion inhibitors. Fusogens are very complex in composition
and function (21). Some loss of
functions for fusogens indicate that the lack of fusogens is
associated with diseases, such as infertility and muscle
dystrophies (18). Defects in SNAREs
can cause neurocutaneous CEDNIK syndrome and centronuclear myopathy
(18). In addition, the structure
and function of several fusogens remain unclear, and further
research is required.
Immunomodulatory functions
The fusion of BMDCs with tumor cells may be an
important mechanism for tumor metastasis and tumor stem cell
formation. Due to the immunoregulatory function of BMDCs, some
scientists have tried to use hybrids of BMDCs and tumor cells to
activate tumor immunity and suppress the progression of tumors
(76,105). In the study of Koido et al,
the progeny cells fused with tumor cells and dendritic cells (DCs)
were used to make cell fusion vaccines to induce anti-tumor
specific immunity. This vaccine utilizes DCs to expose entire
tumor-associated antigens, and present antigens to activate CD8+
and CD4+ T cells (106,107). Previous studies have reported that
newly fused hybrid cells are prone to necrosis, and release a large
number of proteins locally (108),
which may also be presented by DCs as tumor antigens to activate
the immune system (72).
Some new biomaterial technologies have also been
incorporated into the idea of cell fusion-targeted tumor therapy.
Recently, Liu et al (109)
tried to construct immunotherapeutic nanoplatforms from hybrid cell
membranes derived from cancer cells and DCs to achieve more
efficient and precise photodynamic therapy (PDT). Utilizing
DC-tumor hybrid cell membranes for tumor tropism successfully
enriches PDT nanomaterials to tumor entities (109). This tumor-specific immunotherapy
method expands the method of cell fusion for tumor treatment.
Cell fusion in radiotherapy
Radiotherapy is a common method used to treat
malignancies, and radiation is also an important inducer of cell
fusion (110). Thus, the phenomenon
of cell fusion during radiotherapy is worthy of discussion. Rizvi
et al (111) reported that
gamma-ray radiation can induce the fusion of small intestinal stem
cells and BMDCs, and this effect is significantly increased in
small intestine tumors. Further research in the BMDC-transplanted
mouse model demonstrated that the proliferation of epithelial cells
increased significantly following radiation, which was associated
with the increase in fusion of BMDCs and the small intestinal
epithelium (111). And as the
radiation dose increases, the number of fused cells also increases
(112). Garvin et al
(113) reported that CD163
(macrophage phenotype)-positive tumor cells were detected in some
patients with breast cancer undergoing breast-conserving surgery
and radiotherapy. The increase in CD163-positive cancer cells is
associated with the infiltration of macrophages in the tumor
stroma, which may be due to radiation-induced macrophage-tumor
fusion. These CD163-positive cells have strong resistance to
radiotherapy, and indicate a poor prognosis (113). The spontaneous fusion hybrid of
MCF-7 cells and macrophages in vitro and in vivo
confirmed its radioresistance and DNA repair abilities, which makes
the treatment of tumors more difficult (114). In addition, Yeh et al
(115) demonstrated that the fusion
of macrophages and small intestinal stromal cells caused by
radiation can increase chronic fibrosis of the intestinal stroma.
Collectively, these findings suggest that it is important to
consider the influence of radiation on tumor cell fusion when
undergoing radiotherapy.
Diagnosis and prognosis
The increase in tumor heterogeneity caused by cell
fusion is closely associated with the grade and prognosis of the
tumor. The degree of tumor malignancy and prognosis can be
determined by detecting the frequency of tumor cell fusion
(48). Gast et al (48) successfully constructed hybrid cells
of macrophages and tumor cells in vitro and detected tumor
hybrid cells in circulating blood in mice. The number of hybrid
cells in peripheral blood was significantly associated with the
tumor stage and survival of mice (48). This suggests that hybrid cells may
also be detected in human peripheral blood and used as a diagnostic
tool to determine the cancer stage and patient prognosis. However,
several details about the mechanism of cell fusion in tumors are
yet to be investigated, and thus, there is no effective way to
prevent tumor cell fusion. Furthermore, the direct application of
hybrid cells to treat tumors requires full verification of the
safety of the progeny (116).
Conclusions
Cell fusion is essential for the normal growth and
development of organisms, but the consequences of unexpected cell
fusion may also be catastrophic, such as initiating cancer.
Although the theory that cancer originates from cell fusion has
been proposed for a century, in recent decades, the existence of
spontaneous cell fusion in human tumors has been confirmed.
Currently, the effects on cell fusion in tumors are focused on the
following aspects: i) Whether hybrid cells can cause tumor
formation; ii) which cells can hybridize with tumor cells; iii) how
to detect hybrid cells in tumors; iv) the association between cell
fusion and tumor progression; v) the role of cytoplasm in tumor
fusion and vi) the clinical value of hybrid cells in tumors
(3,8,61,72,74).
According to the SMT, offspring genome changes via cell fusion
cause cells to acquire new phenotypes and biological
characteristics. This directly triggers further cell invasion,
metastasis, drug resistance and recurrence (57). In addition, given that cell genetic
abnormalities do not exist in all tumor cells, changes via
cytoplasmic fusion, particularly mitochondrial abnormalities, can
also induce malignant characteristics (69). Metabolic disorders caused by cell
fusion are also an important driving force for the progression of
cancer cells (97). Recently, some
targeting cell fusion treatment methods have gradually been
proposed. However, due to the large heterogeneity of cell fusion in
different tumors, these treatment options are unstable and cannot
be applied for short-term use (106,107,109,116).
Cell fusion is widespread in the tumor microenvironment. Based on
the limited understanding of tumor cell fusion, its scientific
value is worthy of further investigation.
Acknowledgements
Not applicable.
Funding
The present review was partly supported by a grant
from the National Natural Science Foundation of China (grant no.
81572488, to WX).
Availability of data and materials
Not applicable.
Authors' contributions
HFW and WX collected most of the data and drafted
the initial manuscript. BZX, YHW, and DYY interpreted the data. HYZ
and XBJ made critical revisions to the article. PF supervised all
of the research work and gave the final approval for the
publication of this article. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Brukman NG, Uygur B, Podbilewicz B and
Chernomordik LV: How cells fuse. J Cell Biol. 218:1436–1451. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Oren-Suissa M and Podbilewicz B: Cell
fusion during development. Trends Cell Biol. 17:537–546. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bastida-Ruiz D, Van Hoesen K and Cohen M:
The dark side of cell fusion. Int J Mol Sci. 17:6382016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ku JWK, Chen Y, Lim BJW, Gasser S, Crasta
KC and Gan YH: Bacterial-induced cell fusion is a danger signal
triggering cGAS-STING pathway via micronuclei formation. Proc Natl
Acad Sci USA. 117:15923–15934. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laberge GS, Duvall E, Haedicke K and
Pawelek J: Leukocyte-cancer cell fusion-genesis of a deadly
journey. Cells. 8:1702019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Willkomm L and Bloch W: State of the art
in cell-cell fusion. Methods Mol Biol. 1313:1–19. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zito F, Lampiasi N, Kireev I and Russo R:
United we stand: Adhesion and molecular mechanisms driving cell
fusion across species. Eur J Cell Biol. 95:552–562. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hernández JM and Podbilewicz B: The
hallmarks of cell-cell fusion. Development. 144:4481–4495. 2017.
View Article : Google Scholar
|
|
9
|
Raj I, Sadat Al Hosseini H, Dioguardi E,
Nishimura K, Han L, Villa A, de Sanctis D and Jovine L: Structural
basis of egg coat-sperm recognition at fertilization. Cell.
169:1315–1326.e17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Augustine GJ and Weninger K:
Kinetics of complexin binding to the SNARE complex: Correcting
single molecule FRET measurements for hidden events. Biophys J.
93:2178–2187. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Donaldson SH Jr, Lee CT Jr, Chmelka BF and
Israelachvili JN: General hydrophobic interaction potential for
surfactant/lipid bilayers from direct force measurements between
light-modulated bilayers. Proc Natl Acad Sci USA. 108:15699–15704.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chernomordik LV, Kozlov MM, Leĭkin SL,
Markin VS and Chizmadzhaev IuA: Membrane fusion: Local interactions
and structural rearrangements. Dokl Akad Nauk SSSR. 288:1009–1013.
1986.(In Russian). PubMed/NCBI
|
|
13
|
Chernomordik LV and Kozlov MM: Membrane
hemifusion: Crossing a chasm in two leaps. Cell. 123:375–382. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Skehel JJ and Wiley DC: Receptor binding
and membrane fusion in virus entry: The influenza hemagglutinin.
Annu Rev Biochem. 69:531–569. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eckert DM and Kim PS: Mechanisms of viral
membrane fusion and its inhibition. Annu Rev Biochem. 70:777–810.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Weber T, Zemelman BV, McNew JA, Westermann
B, Gmachl M, Parlati F, Söllner TH and Rothman JE: SNAREpins:
Minimal machinery for membrane fusion. Cell. 92:759–772. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calder LJ and Rosenthal PB: Cryomicroscopy
provides structural snapshots of influenza virus membrane fusion.
Nat Struct Mol Biol. 23:853–858. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Segev N, Avinoam O and Podbilewicz B:
Fusogens. Curr Biol. 28:R378–R380. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Mercapide J, Rappa G and Lorico A: The
intrinsic fusogenicity of glioma cells as a factor of
transformation and progression in the tumor microenvironment. Int J
Cancer. 131:334–343. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Esnault C, Priet S, Ribet D, Vernochet C,
Bruls T, Lavialle C, Weissenbach J and Heidmann T: A
placenta-specific receptor for the fusogenic, endogenous
retrovirus-derived, human syncytin-2. Proc Natl Acad Sci USA.
105:17532–17537. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fédry J, Liu Y, Péhau-Arnaudet G, Pei J,
Li W, Tortorici MA, Traincard F, Meola A, Bricogne G, Grishin NV,
et al: The ancient gamete fusogen HAP2 is a eukaryotic class II
fusion protein. Cell. 168:904–915.e10. 2017. View Article : Google Scholar
|
|
22
|
Aguilar PS, Baylies MK, Fleissner A,
Helming L, Inoue N, Podbilewicz B, Wang H and Wong M: Genetic basis
of cell-cell fusion mechanisms. Trends Genet. 29:427–437. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Okabe M: Sperm-egg interaction and
fertilization: Past, present, and future. Biol Reprod. 99:134–146.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Primakoff P and Myles DG: Cell-cell
membrane fusion during mammalian fertilization. FEBS Lett.
581:2174–2180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Runge KE, Evans JE, He ZY, Gupta S,
McDonald KL, Stahlberg H, Primakoff P and Myles DG: Oocyte CD9 is
enriched on the microvillar membrane and required for normal
microvillar shape and distribution. Dev Biol. 304:317–325. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Aydin H, Sultana A, Li S, Thavalingam A
and Lee JE: Molecular architecture of the human sperm IZUMO1 and
egg JUNO fertilization complex. Nature. 534:562–565. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ohto U, Ishida H, Krayukhina E, Uchiyama
S, Inoue N and Shimizu T: Structure of IZUMO1-JUNO reveals
sperm-oocyte recognition during mammalian fertilization. Nature.
534:566–569. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mi S, Lee X, Li X, Veldman GM, Finnerty H,
Racie L, LaVallie E, Tang XY, Edouard P, Howes S, et al: Syncytin
is a captive retroviral envelope protein involved in human
placental morphogenesis. Nature. 403:785–789. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gude NM, Roberts CT, Kalionis B and King
RG: Growth and function of the normal human placenta. Thromb Res.
114:397–407. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bjerregaard B, Holck S, Christensen IJ and
Larsson LI: Syncytin is involved in breast cancer-endothelial cell
fusions. Cell Mol Life Sci. 63:1906–1911. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bjerregard B, Ziomkiewicz I, Schulz A and
Larsson LI: Syncytin-1 in differentiating human myoblasts:
Relationship to caveolin-3 and myogenin. Cell Tissue Res.
357:355–362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Søe K, Andersen TL, Hobolt-Pedersen AS,
Bjerregaard B, Larsson LI and Delaisse JM: Involvement of human
endogenous retroviral syncytin-1 in human osteoclast fusion. Bone.
48:837–846. 2011. View Article : Google Scholar
|
|
33
|
Antony JM, van Marle G, Opii W,
Butterfield DA, Mallet F, Yong VW, Wallace JL, Deacon RM, Warren K
and Power C: Human endogenous retrovirus glycoprotein-mediated
induction of redox reactants causes oligodendrocyte death and
demyelination. Nat Neurosci. 7:1088–1095. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dupressoir A, Vernochet C, Bawa O, Harper
F, Pierron G, Opolon P and Heidmann T: Syncytin-A knockout mice
demonstrate the critical role in placentation of a fusogenic,
endogenous retrovirus-derived, envelope gene. Proc Natl Acad Sci
USA. 106:12127–12132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vignery A: Macrophage fusion: The making
of osteoclasts and giant cells. J Exp Med. 202:337–340. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Helming L and Gordon S: Molecular
mediators of macrophage fusion. Trends Cell Biol. 19:514–522. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Saginario C, Sterling H, Beckers C,
Kobayashi R, Solimena M, Ullu E and Vignery A: MFR, a putative
receptor mediating the fusion of macrophages. Mol Cell Biol.
18:6213–6223. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kania JR, KehatStadler T and Kupfer SR:
CD44 antibodies inhibit osteoclast formation. J Bone Miner Res.
12:1155–1164. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yagi M, Miyamoto T, Toyama Y and Suda T:
Role of DC-STAMP in cellular fusion of osteoclasts and macrophage
giant cells. J Bone Miner Metab. 24:355–358. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Horsley V and Pavlath GK: Forming a
multinucleated cell: Molecules that regulate myoblast fusion. Cells
Tissues Organs. 176:67–78. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Quinn ME, Goh Q, Kurosaka M, Gamage DG,
Petrany MJ, Prasad V and Millay DP: Myomerger induces fusion of
non-fusogenic cells and is required for skeletal muscle
development. Nat Commun. 8:156652017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Mitani Y, Vagnozzi RJ and Millay DP: In
vivo myomaker-mediated heterologous fusion and nuclear
reprogramming. FASEB J. 31:400–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Boveri T: Concerning the origin of
malignant tumours by Theodor Boveri. Translated and annotated by
Henry Harris. J Cell Sci. 121 (Suppl 1):S1–S84. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun C, Zhao D, Dai X, Chen J, Rong X, Wang
H, Wang A, Li M, Dong J, Huang Q and Lan Q: Fusion of cancer stem
cells and mesenchymal stem cells contributes to glioma
neovascularization. Oncol Rep. 34:2022–2030. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wei HJ, Nickoloff JA, Chen WH, Liu HY, Lo
WC, Chang YT, Yang PC, Wu CW, Williams DF, Gelovani JG and Deng WP:
FOXF1 mediates mesenchymal stem cell fusion-induced reprogramming
of lung cancer cells. Oncotarget. 5:9514–9529. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Melzer C, von der Ohe J and Hass R: In
vitro fusion of normal and neoplastic breast epithelial cells with
human mesenchymal stroma/stem cells partially involves tumor
necrosis factor receptor signaling. Stem Cells. 36:977–989. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Clawson GA, Matters GL, Xin P,
Imamura-Kawasawa Y, Du Z, Thiboutot DM, Helm KF, Neves RI and
Abraham T: Macrophage-tumor cell fusions from peripheral blood of
melanoma patients. PLoS One. 10:e01343202015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gast CE, Silk AD, Zarour L, Riegler L,
Burkhart JG, Gustafson KT, Parappilly MS, Roh-Johnson M, Goodman
JR, Olson B, et al: Cell fusion potentiates tumor heterogeneity and
reveals circulating hybrid cells that correlate with stage and
survival. Sci Adv. 4:eaat78282018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yu L, Guo W, Zhao S, Wang F and Xu Y:
Fusion between cancer cells and myofibroblasts is involved in
osteosarcoma. Oncol Lett. 2:1083–1087. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Powell AE, Anderson EC, Davies PS, Silk
AD, Pelz C, Impey S and Wong MH: Fusion between intestinal
epithelial cells and macrophages in a cancer context results in
nuclear reprogramming. Cancer Res. 71:1497–1505. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Huang CM, Yan TL, Xu Z, Wang M, Zhou XC,
Jiang EH, Liu K, Shao Z and Shang ZJ: Hypoxia enhances fusion of
oral squamous carcinoma cells and epithelial cells partly via the
epithelial-mesenchymal transition of epithelial cells. Biomed Res
Int. 2018:50152032018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Lu X and Kang Y: Cell fusion as a hidden
force in tumor progression. Cancer Res. 69:8536–8539. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yin L, Hu P, Shi X, Qian W, Zhau HE,
Pandol SJ, Lewis MS, Chung LWK and Wang R: Cancer cell's
neuroendocrine feature can be acquired through cell-cell fusion
during cancer-neural stem cell interaction. Sci Rep. 10:12162020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Dörnen J, Myklebost O and Dittmar T: Cell
fusion of mesenchymal stem/stromal cells and breast cancer cells
leads to the formation of hybrid cells exhibiting diverse and
individual (stem cell) characteristics. Int J Mol Sci. 21:96362020.
View Article : Google Scholar
|
|
55
|
Delespaul L, Merle C, Lesluyes T, Lagarde
P, Le Guellec S, Pérot G, Baud J, Carlotti M, Danet C, Fèvre M, et
al: Fusion-mediated chromosomal instability promotes aneuploidy
patterns that resemble human tumors. Oncogene. 38:6083–6094. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hedley DW, Leary JA and Kirsten F:
Metastatic adenocarcinoma of unknown primary site: Abnormalities of
cellular DNA content and survival. Eur J Cancer Clin Oncol.
21:185–189. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Baker SG: A cancer theory kerfuffle can
lead to new lines of research. J Natl Cancer Inst. 107:dju4052014.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mertens F, Johansson B, Höglund M and
Mitelman F: Chromosomal imbalance maps of malignant solid tumors: A
cytogenetic survey of 3185 neoplasms. Cancer Res. 57:2765–2780.
1997.PubMed/NCBI
|
|
59
|
Bjerkvig R, Tysnes BB, Aboody KS, Najbauer
J and Terzis AJ: Opinion: The origin of the cancer stem cell:
Current controversies and new insights. Nat Rev Cancer. 5:899–904.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Mohr M, Zaenker KS and Dittmar T: Fusion
in cancer: An explanatory model for aneuploidy, metastasis
formation, and drug resistance. Methods Mol Biol. 1313:21–40. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Zhou X, Merchak K, Lee W, Grande JP,
Cascalho M and Platt JL: Cell fusion connects oncogenesis with
tumor evolution. Am J Pathol. 185:2049–2060. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Dittmar T, Schwitalla S, Seidel J,
Haverkampf S, Reith G, Meyer-Staeckling S, Brandt BH, Niggemann B
and Zänker KS: Characterization of hybrid cells derived from
spontaneous fusion events between breast epithelial cells
exhibiting stem-like characteristics and breast cancer cells. Clin
Exp Metastas. 28:75–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Goldenberg DM, Rooney RJ, Loo M, Liu D and
Chang CH: In-vivo fusion of human cancer and hamster stromal cells
permanently transduces and transcribes human DNA. PLoS One.
9:e1079272014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Su Y, Subedee A, Bloushtain-Qimron N,
Savova V, Krzystanek M, Li L, Marusyk A, Tabassum DP, Zak A,
Flacker MJ, et al: Somatic cell fusions reveal extensive
heterogeneity in basal-like breast cancer. Cell Rep. 11:1549–1563.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Munzarova M, Lauerova L and Capkova J: Are
advanced malignant melanoma cells hybrids between melanocytes and
macrophages? Melanoma Res. 2:127–129. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
He X, Li B, Shao Y, Zhao N, Hsu Y, Zhang Z
and Zhu L: Cell fusion between gastric epithelial cells and
mesenchymal stem cells results in epithelial-to-mesenchymal
transition and malignant transformation. BMC Cancer. 15:242015.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Faggioli F, Sacco MG, Susani L, Montagna C
and Vezzoni P: Cell fusion is a physiological process in mouse
liver. Hepatology. 48:1655–1664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Israel BA and Schaeffer WI: Cytoplasmic
suppression of malignancy. In Vitro Cell Dev Biol. 23:627–632.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Seyfried TN: Cancer as a mitochondrial
metabolic disease. Front Cell Dev Biol. 3:432015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hsu CC, Tseng LM and Lee HC: Role of
mitochondrial dysfunction in cancer progression. Exp Biol Med
(Maywood). 241:1281–1295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Seyfried TN and Shelton LM: Cancer as a
metabolic disease. Nutr Metab (Lond). 7:72010. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Platt JL, Zhou X, Lefferts AR and Cascalho
M: Cell fusion in the war on cancer: A perspective on the inception
of malignancy. Int J Mol Sci. 17:11182016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Duelli D and Lazebnik Y: Cell fusion: A
hidden enemy? Cancer Cell. 3:445–448. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Weiler J and Dittmar T: Cell fusion in
human cancer: The dark matter hypothesis. Cells. 8:1322019.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mittal V: Epithelial mesenchymal
transition in tumor metastasis. Annu Rev Pathol. 13:395–412. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Pawelek JM and Chakraborty AK: Fusion of
tumour cells with bone marrow-derived cells: A unifying explanation
for metastasis. Nat Rev Cancer. 8:377–386. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kalluri R and Neilson EG:
Epithelial-mesenchymal transition and its implications for
fibrosis. J Clin Invest. 112:1776–1784. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Seyfried TN and Huysentruyt LC: On the
origin of cancer metastasis. Crit Rev Oncog. 18:43–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chakraborty AK, Sodi S, Rachkovsky M,
Kolesnikova N, Platt JT, Bolognia JL and Pawelek JM: A spontaneous
murine melanoma lung metastasis comprised of host × tumor hybrids.
Cancer Res. 60:2512–2519. 2000.PubMed/NCBI
|
|
80
|
Yilmaz Y, Lazova R, Qumsiyeh M, Cooper D
and Pawelek J: Donor Y chromosome in renal carcinoma cells of a
female BMT recipient: Visualization of putative BMT-tumor hybrids
by FISH. Bone Marrow Transplant. 35:1021–1024. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Fidler IJ: Timeline: The pathogenesis of
cancer metastasis: The ‘seed and soil’ hypothesis revisited. Nat
Rev Cancer. 3:453–458. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jiang E, Yan T, Xu Z and Shang Z: Tumor
microenvironment and cell fusion. Biomed Res Int. 2019:50135922019.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Noubissi FK, Harkness T, Alexander CM and
Ogle BM: Apoptosis-induced cancer cell fusion: A mechanism of
breast cancer metastasis. FASEB J. 29:4036–4045. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Choi H and Moon A: Crosstalk between
cancer cells and endothelial cells: Implications for tumor
progression and intervention. Arch Pharm Res. 41:711–724. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Kalluri R: The biology and function of
fibroblasts in cancer. Nat Rev Cancer. 16:582–598. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang R, Sun X, Wang CY, Hu P, Chu CY, Liu
S, Zhau HE and Chung LW: Spontaneous cancer-stromal cell fusion as
a mechanism of prostate cancer androgen-independent progression.
PLoS One. 7:e426532012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Clawson GA, Matters GL, Xin P, McGovern C,
Wafula E, dePamphilis C, Meckley M, Wong J, Stewart L, D'Jamoos C,
et al: ‘Stealth dissemination’ of macrophage-tumor cell fusions
cultured from blood of patients with pancreatic ductal
adenocarcinoma. PLoS One. 12:e01844512017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Clawson G: The fate of fusions. Cells.
8:132018. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Kachalaki S, Ebrahimi M, Mohamed
Khosroshahi L, Mohammadinejad S and Baradaran B: Cancer
chemoresistance; biochemical and molecular aspects: A brief
overview. Eur J Pharm Sci. 89:20–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Vasan N, Baselga J and Hyman DM: A view on
drug resistance in cancer. Nature. 575:299–309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Miller FR, Mohamed AN and McEachern D:
Production of a more aggressive tumor cell variant by spontaneous
fusion of two mouse tumor subpopulations. Cancer Res. 49:4316–4321.
1989.PubMed/NCBI
|
|
92
|
Nagler C, Hardt C, Zanker KS and Dittmar
T: Co-cultivation of murine BMDCs with 67NR mouse mammary carcinoma
cells give rise to highly drug resistant cells. Cancer Cell Int.
11:212011. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Uygur B, Leikina E, Melikov K, Villasmil
R, Verma SK, Vary CPH and Chernomordik LV: Interactions with muscle
cells boost fusion, stemness, and drug resistance of prostate
cancer cells. Mol Cancer Res. 17:806–820. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Song K, Song Y, Zhao XP, Shen H, Wang M,
Yan TL, Liu K and Shang ZJ: Oral cancer/endothelial cell fusion
experiences nuclear fusion and acquisition of enhanced survival
potential. Exp Cell Res. 328:156–163. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Searles SC, Santosa EK and Bui JD:
Cell-cell fusion as a mechanism of DNA exchange in cancer.
Oncotarget. 9:6156–6173. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Mirzayans R and Murray D: Intratumor
heterogeneity and therapy resistance: Contributions of dormancy,
apoptosis reversal (Anastasis) and cell fusion to disease
recurrence. Int J Mol Sci. 21:13082020. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Seyfried TN, Arismendi-Morillo G,
Mukherjee P and Chinopoulos C: On the origin of ATP synthesis in
cancer. iScience. 23:1017612020. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Xu RH, Pelicano H, Zhou Y, Carew JS, Feng
L, Bhalla KN, Keating MJ and Huang P: Inhibition of glycolysis in
cancer cells: A novel strategy to overcome drug resistance
associated with mitochondrial respiratory defect and hypoxia.
Cancer Res. 65:613–621. 2005.PubMed/NCBI
|
|
99
|
Beck B and Blanpain C: Unravelling cancer
stem cell potential. Nat Rev Cancer. 13:727–738. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Dittmar T, Nagler C, Schwitalla S, Reith
G, Niggemann B and Zänker KS: Recurrence cancer stem cells-made by
cell fusion? Med Hypotheses. 73:542–547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bartosh TJ, Ullah M, Zeitouni S, Beaver J
and Prockop DJ: Cancer cells enter dormancy after cannibalizing
mesenchymal stem/stromal cells (MSCs). Proc Natl Acad Sci USA.
113:E6447–E6456. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Li G, Kikuchi K, Radka M, Abraham J, Rubin
BP and Keller C: IL-4 receptor blockade abrogates satellite cell:
Rhabdomyosarcoma fusion and prevents tumor establishment. Stem
Cells. 31:2304–2312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Platt JL and Cascalho M: Cell fusion in
malignancy: A cause or consequence? a provocateur or cure? Cells.
8:5872019.PubMed/NCBI
|
|
105
|
Fais S and Overholtzer M: Cell-in-cell
phenomena in cancer. Nat Rev Cancer. 18:758–766. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Koido S, Homma S, Okamoto M, Namiki Y,
Takakura K, Uchiyama K, Kajihara M, Arihiro S, Imazu H, Arakawa H,
et al: Fusions between dendritic cells and whole tumor cells as
anticancer vaccines. Oncoimmunology. 2:e244372013. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Koido S: Dendritic-tumor fusion cell-based
cancer vaccines. Int J Mol Sci. 17:8282016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Platt JL and Cascalho M: IgM in the
kidney: A multiple personality disorder. Kidney Int. 88:439–441.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Liu WL, Zou MZ, Liu T, Zeng JY, Li X, Yu
WY, Li CX, Ye JJ, Song W, Feng J and Zhang XZ: Expandable
immunotherapeutic nanoplatforms engineered from cytomembranes of
hybrid cells derived from cancer and dendritic cells. Adv Mater.
31:e19004992019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Hass R, von der Ohe J and Ungefroren H:
Potential role of MSC/cancer cell fusion and EMT for breast cancer
stem cell formation. Cancers (Basel). 11:14322019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Rizvi AZ, Swain JR, Davies PS, Bailey AS,
Decker AD, Willenbring H, Grompe M, Fleming WH and Wong MH: Bone
marrow-derived cells fuse with normal and transformed intestinal
stem cells. Proc Natl Acad Sci USA. 103:6321–6325. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Davies PS, Powell AE, Swain JR and Wong
MH: Inflammation and proliferation act together to mediate
intestinal cell fusion. PLoS One. 4:e65302009. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Garvin S, Oda H, Arnesson LG, Lindström A
and Shabo I: Tumor cell expression of CD163 is associated to
postoperative radiotherapy and poor prognosis in patients with
breast cancer treated with breast-conserving surgery. J Cancer Res
Clin Oncol. 144:1253–1263. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Lindström A, Midtbö K, Arnesson LG, Garvin
S and Shabo I: Fusion between M2-macrophages and cancer cells
results in a subpopulation of radioresistant cells with enhanced
DNA-repair capacity. Oncotarget. 8:51370–51386. 2017. View Article : Google Scholar
|
|
115
|
Yeh MH, Chang YH, Tsai YC, Chen SL, Huang
TS, Chiu JF and Ch'ang HJ: Bone marrow derived macrophages fuse
with intestine stromal cells and contribute to chronic fibrosis
after radiation. Radiother Oncol. 119:250–258. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Willenbring H: Therapeutic cell fusion. Br
J Surg. 92:923–924. 2005. View Article : Google Scholar : PubMed/NCBI
|