Introduction
Head and neck squamous cell carcinoma (HNSCC) is the
sixth leading cancer by incidence worldwide (1). Treatment modalities for HNSCC have
advanced, but there is still a high incidence of recurrence after
initial therapy with rates of 40–50% reported for hypopharyngeal
carcinoma (2,3). The cancer stem cell hypothesis
(4,5)
allows us to explain the heterogeneity and resistance to anticancer
treatment of a malignant tumor, including head and neck cancers.
Numerous studies about the detection and control of this cell have
been reported, but there are no clinically approved treatments to
date (6).
Cyclooxygenase (COX) is an enzyme catalyzing the
conversion of arachidonic acid to prostaglandins (PGs). COX-1 is
constitutively expressed in various tissues throughout the body,
whereas COX-2 expression is induced in sites of inflammation,
including cancer and premalignant lesions. COX-2 expression is
elevated in HNSCCs (7–9), and seems to have a negative correlation
with survival (10–13). This is explained by multiple reasons,
including promotion in tumor progression (12), proliferation (14), angiogenesis (10), and lymph node metastasis (15). We have previously reported that COX-2
expression is related to lymph node metastasis in oropharyngeal
carcinomas (16) and that COX-2
inhibition can have an anti-metastatic effect through the
suppression of epithelial to mesenchymal transition (EMT) in
pharyngeal carcinoma (17).
Furthermore, of the four downstream receptors of PG E2 (PGE2),
which are EP1-4, we have recently reported that PG E receptor 2
(EP2) plays an efficient role in EMT in hypopharyngeal carcinoma
(18). COX-2 expression is also
related to resistance to anticancer therapies, such as chemotherapy
(13,19–21) and
radiotherapy (22) in other cancer
sites. Recently, the interaction of the COX2/PGE2/EP axis and
cancer stemness (23–26) has been reported, but little has been
studied in HNSCCs. Moreover, no studies have reported the
association of COX-2 expression and cancer stemness, especially
chemo-sensitivity in HNSCCs.
Here, we aimed to investigate the effect of COX-2 on
cancer stem cell (CSC) property and to reveal its effect on
chemo-resistance by in vitro and clinicopathological assays in
HNSCCs.
Materials and methods
Cell lines
Human pharyngeal carcinoma cell lines (FaDu and
Detroit 562) were purchased from American Type Culture Collection
(ATCC).
Cell culture
Cell lines were cultured in Eagle's Minimum
Essential Medium (Sigma-Aldrich; Merck KGaA) supplemented with 10%
fetal bovine serum (FBS, US origin) and 1% penicillin-streptomycin
(solution stabilized, Sigma-Aldrich; Merck KGaA), and incubated in
a humidified incubator (37°C, 5% carbon dioxide). Cells were
subcultured continuously according to the ATCC protocol.
Drugs and reagents
The selective COX-2 inhibitor (celecoxib), selective
EP2 antagonist (PF-04418948), and docetaxel (DTX) were purchased
from Toronto Research Chemicals, Cayman Chemical, and Sigma-Aldrich
(Merck KGaA), respectively. Dimethyl sulfoxide (DMSO) was used as a
solvent and vehicle control.
Reverse transcription-quantitative
PCR
The RNeasy mini kit (Qiagen) was used for RNA
extraction, and the SuperScript™ III First-Strand Synthesis System
(Invitrogen; Thermo Fisher Scientific, Inc.) for complementary DNA
synthesis. Quantitative real-time polymerase chain reaction (PCR)
was performed using the 7500 Fast Real-Time PCR system instrument
and software (Applied Biosystems; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. Primers and probes were
purchased from Applied Biosystems (TaqMan® Gene
Expression Assays) with the following IDs: β-actin (actin beta,
Hs01060665_g1), OCT3/4 (POU class 5 homeobox 1, Hs04260367_gH),
NANOG (nanog homeobox, Hs04399610_g1), SOX-2 (SRY-box 2,
Hs01053049_s1), ALDH1A1 (aldehyde dehydrogenase 1 family member A1,
Hs00946916_m1), CD44 (CD44 molecule, Hs01075861_m1), COX-2
(prostaglandin-endoperoxide synthase 2, Hs00153133_m1), EP1
(prostaglandin E receptor 1, Hs00168752_m1), EP2 (prostaglandin E
receptor 2, Hs00168754_m1), EP3 (prostaglandin E receptor 3,
Hs00168755_m1), and EP4 (prostaglandin E receptor 4,
Hs00168761_m1). The PCR amplification conditions were as follows:
20 sec at 95°C followed by 40 cycles of 3-sec denaturation at 95°C
and 30 sec annealing at 60°C. We quantified the relative gene
expression levels using the standard curve method, and compared the
levels to β-actin, which was used as an endogenous control.
COX-2 inhibition and EP2 inhibition
for messenger RNA extraction
Cells were seeded at a density of 200/µl into a
six-well dish and incubated in a medium containing 10% FBS.
Twenty-four hours later, the cells were treated with celecoxib (5
µM) or PF-04418948 (10 µM). These concentrations of the reagents
were found to be optimal with no toxic effect on cell viability up
to at least 48 h in our preliminary experiments. Treatment with
DMSO was used as controls. Cells were collected 12 h later and used
for total RNA extraction. The experiment in each condition was
performed at least three times to assess consistency.
COX-2 knockdown
Cells were seeded at a density of 10,000/ml into a
six-well dish in a serum-reduced medium (Opti-MEM, Thermo Fisher
Scientific, Inc.). Twenty-four hours later, the medium was changed
and siRNA for the COX-2 gene PTGS2 (Silencer®
Pre-designed siRNA, Life Technologies) and negative control siRNA
(Silencer® Select Negative Control siRNA, Life
Technologies) were added at a density of 20 pmol with lipofectamine
(Thermo Fisher Scientific, Inc.). Twenty-four hours later, the
cells were scraped and collected for analysis.
Cell proliferation assay
Cells were seeded to a 96-well dish at a density of
1,000 cells/200 µl/well, and incubated in a medium containing 10%
FBS overnight. The medium was changed the next day and treated with
the following drugs: i) multiple density of DTX between 0.005 nM
and 50 µM+DMSO; ii) multiple density of DTX between 0.005 nM and 50
µM+celecoxib (5 µM); and iii) multiple density of DTX between 0.005
nM and 50 µM+PF-04418948 (10 µM). Cell viability was checked 72 h
later with the CellTiter 96® AQueous One Solution Cell
Proliferation Assay (Promega), as per the manufacturer's
instruction. Briefly, 20 µl of the reagent containing the
tetrazolium compound and phenazine ethosulfate were added to each
well, and the plate was incubated for 4 h at 37°C. Viable cells
were quantified by measuring the optical density values of
absorbance at 490 nm using a microplate reader. The experiment was
performed three times and run in triplicate each time.
Immunofluorescence staining
For immunofluorescence staining of Ki-67, FaDu and
Detroit 562 cells were seeded in slide chambers (Thermo Fisher
Scientific, Inc.) and treated with DMSO alone, 10 µM of celecoxib,
50 nM of DTx, and 10 µM of celecoxib + 50 nM of DTX for 24 h. After
washing the cells extensively with phosphate-buffered saline (PBS),
the cells were fixed with 4% paraformaldehyde fixative for 15 min.
After washing with PBS, the cells were incubated with anti-Ki-67
mouse antibody (ab245113, Abcam) at 1:100 overnight. Goat
anti-Mouse IgG Alexa Fluor (Thermo Fisher Scientific, Inc.) was
used for secondary antibody, and Hoechst 33258 was used for nuclear
staining. Ki-67 positive cells were counted from four randomly
chosen areas at 10× magnification.
Sphere formation assay
Cells were seeded with a serum-free medium into an
ultra-low attachment dish (Corning) at a density of 500 cells/ml.
The medium was supplemented with 20 ng/ml of the human basic
fibroblast growth factor (Sigma-Aldrich, catalog no. F0291) and 20
ng/ml of the human epidermal growth factor (Sigma-Aldrich, catalog
no. E5036). Celecoxib was added at two different densities; 1 and
10 nM, and DMSO was used for control. Cells were cultured for 7
days, and the number of spheres per well was counted manually on
day 7.
Patients and tissue specimens
In order to assess the pathological effect of
chemotherapy, patients who were diagnosed as hypopharyngeal
carcinoma after biopsy and received surgical resection of the tumor
after induction chemotherapy at Keio University Hospital between
April 1, 2010 and March 31, 2015 were analyzed. Tissue samples from
the hospital tissue bank and their medical records were obtained
retrospectively. The protocols for the use of the clinical
materials were approved by the Institutional Ethics Review Board of
the Ethics Committee of Keio University School of Medicine
(reference nos. 2010-013 and 2010-013-2). Informed consent was
obtained in the form of opt-out on the web-site and by information
in the hospital. All procedures for clinical tissues were performed
in accordance with the principles of the 1964 Helsinki Declaration
and its later amendments.
Pathological judgement was used because it is
difficult to accurately measure the size of hypopharyngeal lesions
under radiographic evaluations and pathological assessment is more
direct. Pretreatment biopsy specimens and surgically resected tumor
specimens from 12 pathologically diagnosed hypopharyngeal carcinoma
patients who received induction chemotherapy (DTX 60
mg/m2, cisplatin 60 mg/m2, fluorouracil 700
mg/m2) after biopsy were analyzed. All patients had no
history of other head and neck carcinoma and had not received prior
treatment, including chemotherapy and radiotherapy. All specimens
were fixed with 10% formalin, embedded with paraffin, and sliced at
5 µm each. The pathological effect of induction chemotherapy was
evaluated by one trained head and neck pathologist, who was blinded
to the data concerning COX-2 expression. The effects were graded
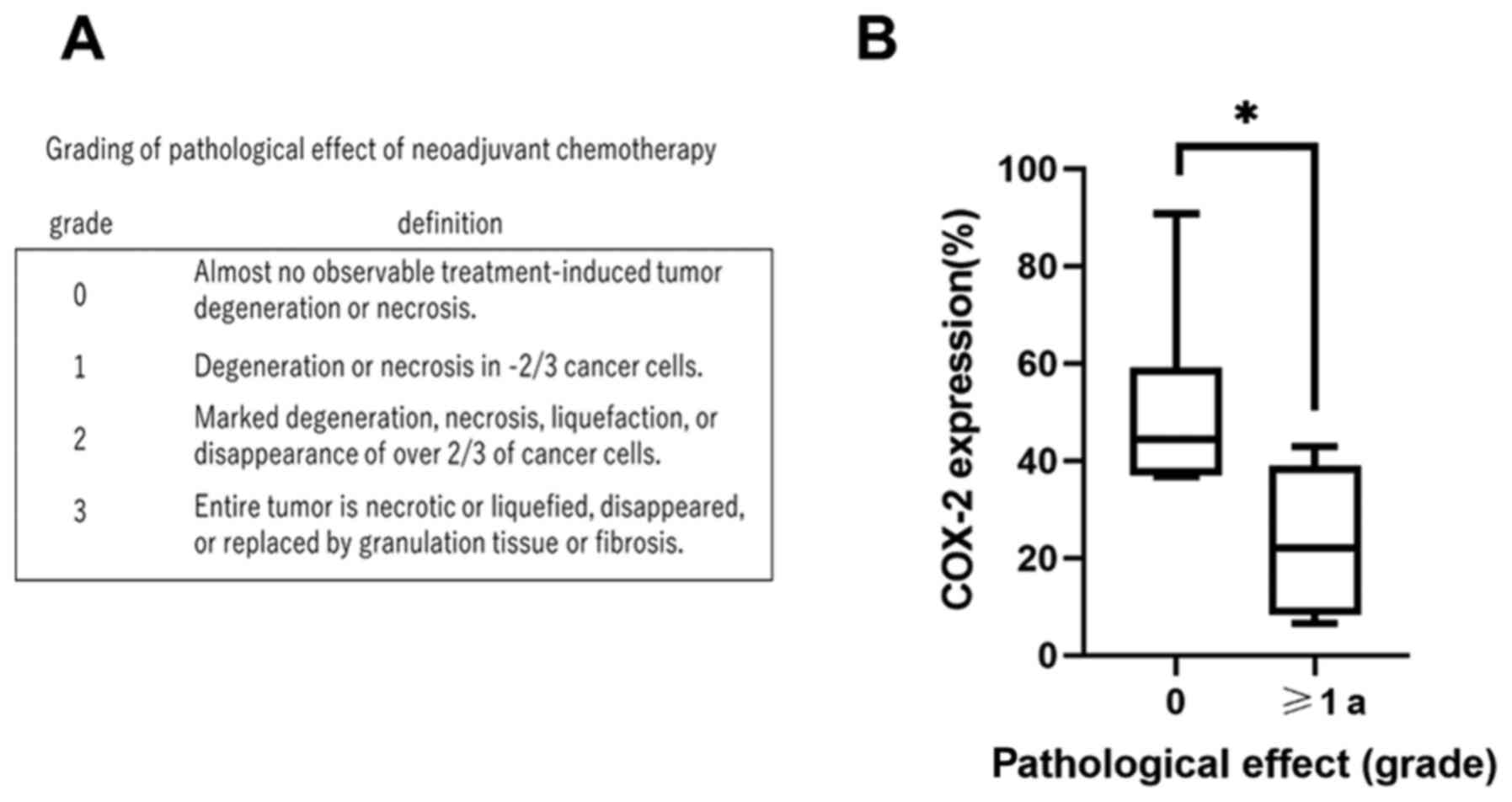
according to the following grading system: Grade 0, no effect;
grade 1, slight effect; grade 2, moderate effect; and grade 3,
significant effect (Fig. 1A)
(19). Univariate analyses of the
pathological effect of chemotherapy and age, the T stage, N stage,
clinical stage, and COX-2 expression were performed.
Immunohistochemistry
Immunostainings were performed with the automated
immunostaining machine Ventana Discovery XT (Roche
Diagnostics/Ventana Medical Systems), as per the manufacturer's
instructions and using the ultraView Universal DAB Detection Kit
(Roche/Ventana). The COX-2 primary antibody (catalog number
760–4254, product code 518101862) was purchased from Roche
Diagnostics K.K. The ratio of COX-2 positive tumor cells was
calculated by using the computational software Tissue
Studio® (Definiens, Inc.). For each slide, the region of
interest (ROI) was set for the whole tumor or for biopsy specimens
for the tumorous area. A hematoxylin threshold of 0.1, typical
nucleus size of 60 µm2, maximum cell growth of 10, and
classification of 0.1 were set, and the expression of COX-2 was
automatically calculated by the number of COX-2-positive tumor
cells divided by the number of total tumor cells.
Statistical analysis
The data repeatedly obtained in the in vitro
assays are presented as the mean ± standard deviation of three or
more independent experiments. GraphPad Prism 8.3.0 (GraphPad
Software, Inc.) was used to perform the statistical analysis.
Fisher's exact test was used to analyze the association between
patient clinicopathological characteristics and COX-2 expression.
The difference in COX-2 expression by pathological response was
analyzed using the Wilcoxon rank-sum test. Results of the cell
proliferation assay were analyzed using non-linear regression
analysis. Student's t-test was used for mRNA expression comparison.
Sphere formation assay was analyzed using one-way ANOVA followed by
Dunnett's multiple comparison test. P<0.05 was considered to
indicate a statistically significant difference.
Results
COX-2 expression is significantly
associated with the pathological effect of induction
chemotherapy
Pretreatment biopsy specimens and surgical specimens
after induction chemotherapy were obtained from 12 patients with
hypopharyngeal carcinoma. Patients' characteristics are summarized
in Table I. The COX-2 expression
varied from 6.6 to 91%, with a mean of 36%. In this study, in order
to classify COX-2 expression, we used this mean as a cutoff, and
divided the group into two; above mean, and below mean. COX-2
expression was classified into two groups, as its positive cutoff
rate was 35%. There was a negative correlation between COX-2
expression and the pathological effect of induction chemotherapy
(Table II and Fig. 1B), showing that tumors with high
pretreatment COX-2 expression tended to be resistant to induction
chemotherapy. According to univariate analysis, the relationship
between the pathological effect of chemotherapy and COX-2
expression was statistically significant (P=0.015) (Table II). Median pretreatment COX-2
expression in patients with no pathological response to
chemotherapy was 44%, and that in patients who showed a response
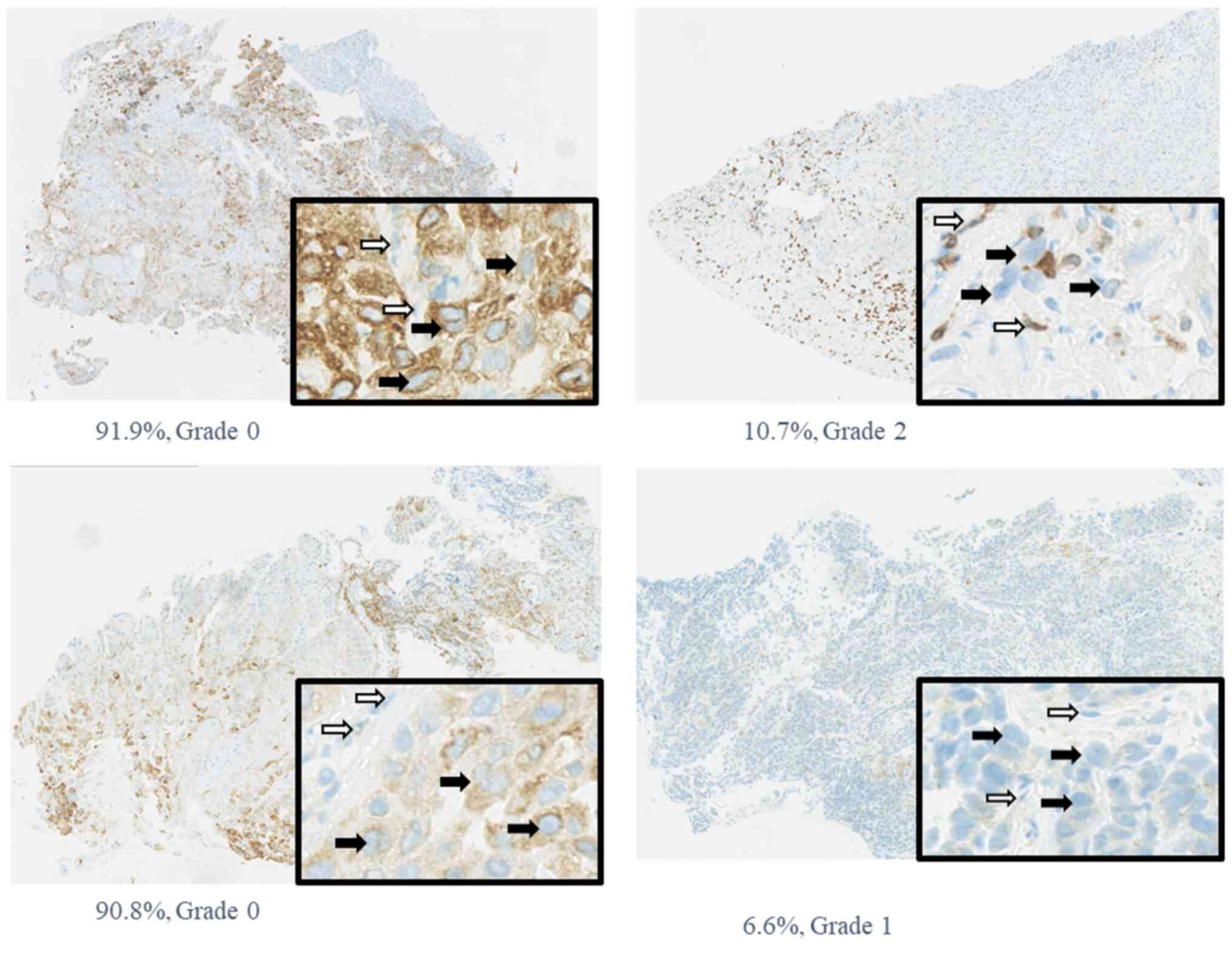
was 22%; this difference was statistically significant (P=0.03).
Representative cases are shown in Fig.
2.
 | Table I.Clinicopathological characteristics
of 12 patients with hypopharyngeal carcinoma. |
Table I.
Clinicopathological characteristics
of 12 patients with hypopharyngeal carcinoma.
|
Characteristics | Value |
|---|
| Sex, male/female,
n | 12/0 |
| Mean age (range),
years | 63 (49–80) |
| Subsite, n |
|
|
Piriform sinus | 11 |
|
Posterior wall | 1 |
|
Post-cricoid | 0 |
| T stage 1/2/3/4,
n | 2/7/2/1 |
| N stage 0/1/2/3,
n | 4/2/5/1 |
| Stage I/II/III/IV,
n | 1/2/3/6 |
 | Table II.Association between pathological
effect of chemotherapy and clinicopathological characteristics. |
Table II.
Association between pathological
effect of chemotherapy and clinicopathological characteristics.
|
| Pathological
effect |
|
|---|
|
|
|
|
|---|
| Characteristic | 0 | ≥1a | P-value |
|---|
| Age, years |
|
| 0.54 |
|
≤65 | 3 | 5 |
|
|
>65 | 3 | 1 |
|
| T stage |
|
| 0.18 |
|
1+2 | 6 | 3 |
|
|
3+4 | 0 | 3 |
|
| N stage |
|
| 0.08 |
|
0+1 | 5 | 1 |
|
|
2+3 | 1 | 5 |
|
| Stage |
|
| 0.18 |
|
1+2 | 3 | 0 |
|
|
3+4 | 3 | 6 |
|
| COX-2 expression,
% |
|
| 0.015a |
|
≤36 | 0 | 5 |
|
|
>36 | 6 | 1 |
|
COX-2 inhibitor improves
chemo-sensitivity to docetaxel in head and neck squamous cell
carcinoma cell lines
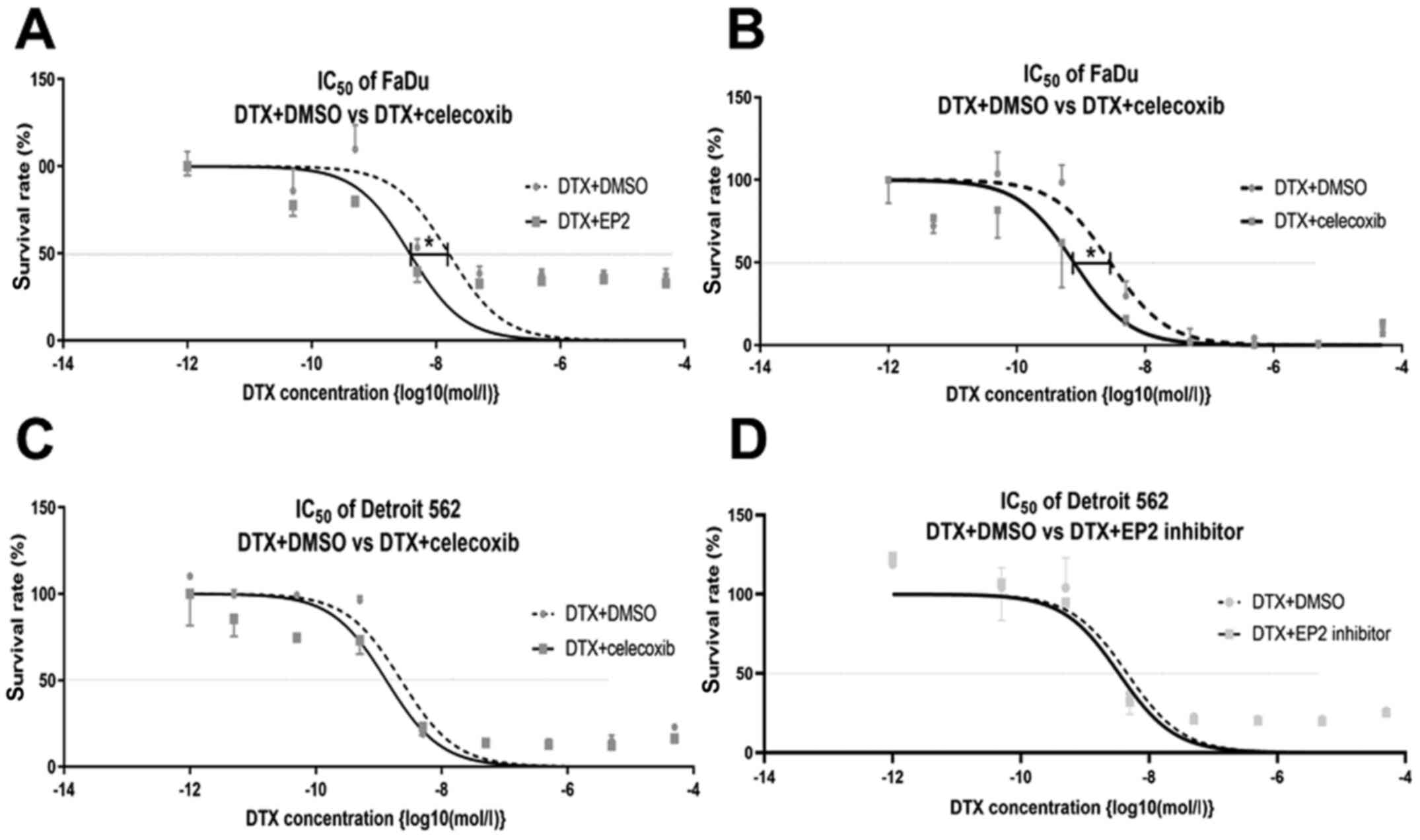
The result of the cell viability assay with the
addition of celecoxib to multiple densities of DTX is shown in
Fig. 3. The IC50
decreased significantly with the addition of celecoxib in FaDu
(LogIC50 DTX versus [vs.] DTX+celecoxib: −8.542 vs.
09.111; 95% CI of LogIC50 DTX vs. DTX+celecoxib: −8.804
to −8.291 vs. −9.480 to −8.817). The addition of celecoxib also
decreased the IC50 of Detroit 562, but this was not
statistically significant (LogIC50 DTX and
DTX+celecoxib: −8.644 and −8.881, respectively; 95% CI of
LogIC50 DTX and DTX+celecoxib: −9.077 to −8.448 and
−9.309 to −8.847, respectively) (Fig. 3A
and B).
EP2 inhibitor tends to improve
chemo-sensitivity to docetaxel in head and neck squamous cell
carcinoma cell lines
The addition of the selective EP2 antagonist
(PF-04418948) tended to improve chemo-sensitivity; however, it was
not statistically significant in FaDu (LogIC50 DTX and
DTX+PF-04418948: −7.793 and −8.422, respectively; 95% CI of
LogIC50 DTX and DTX+PF-04418948: −8.475 to −6.925 and
−9.017 to −7.772, respectively) nor Detroit 562 (LogIC50
DTX and DTX+PF-04418948: −8.320 and −8.470, respectively; 95% CI of
LogIC50 DTX and DTX+PF-04418948: −8.688 to −7.959 and
−8.829 to −8.098, respectively) (Fig. 3C
and D).
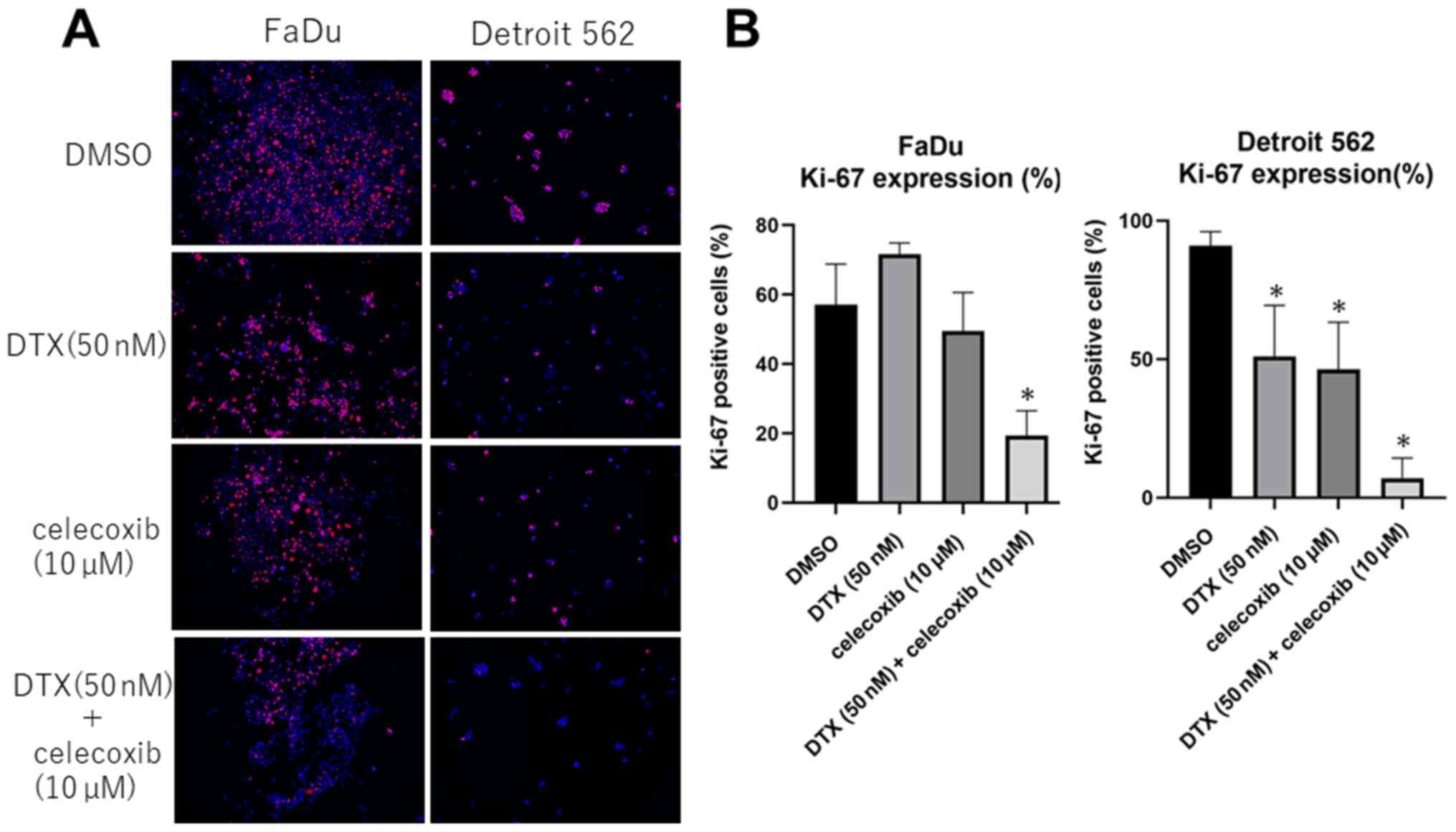
COX-2 inhibitor suppresses Ki-67
expression in head and neck squamous cell carcinoma cell lines
From immunofluorescence staining, Ki-67 expression
significantly decreased in Detroit 562 after celecoxib treatment
and combined treatment of DTX and celecoxib. In FaDu, celecoxib
alone did not show significant difference, whereas combination
treatment showed significant suppression of Ki-67 expression.
(Fig. 4)
Baseline messenger RNA expression of
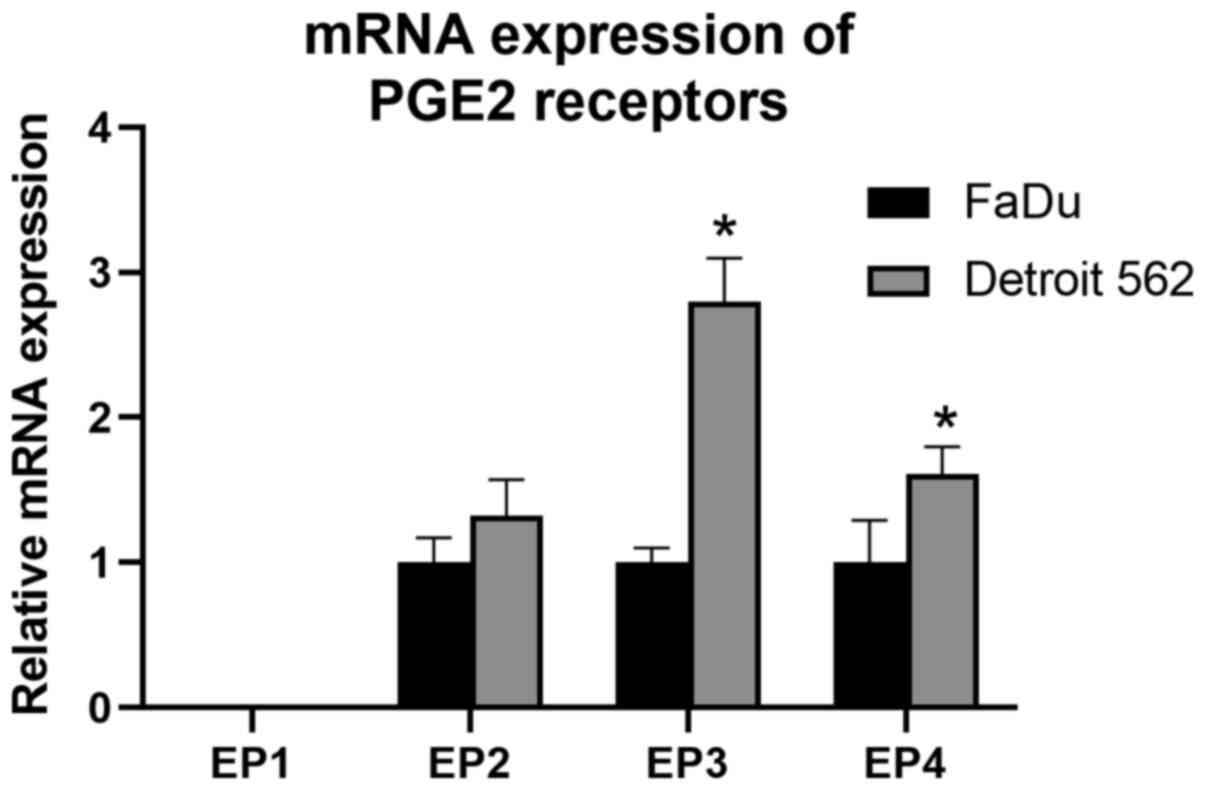
prostaglandin E2 receptor genes vary between cell lines
Baseline messenger RNA (mRNA) expression of PGE2
receptors is shown in Fig. 5.
Expression of EP1 was not detected in either cell lines. Relative
quantification of PGE2 receptors against β-actin varied between the
two cell lines. Detroit 562 showed a higher degree of expression in
all receptor genes compared to FaDu, significantly in EP3 and
EP4.
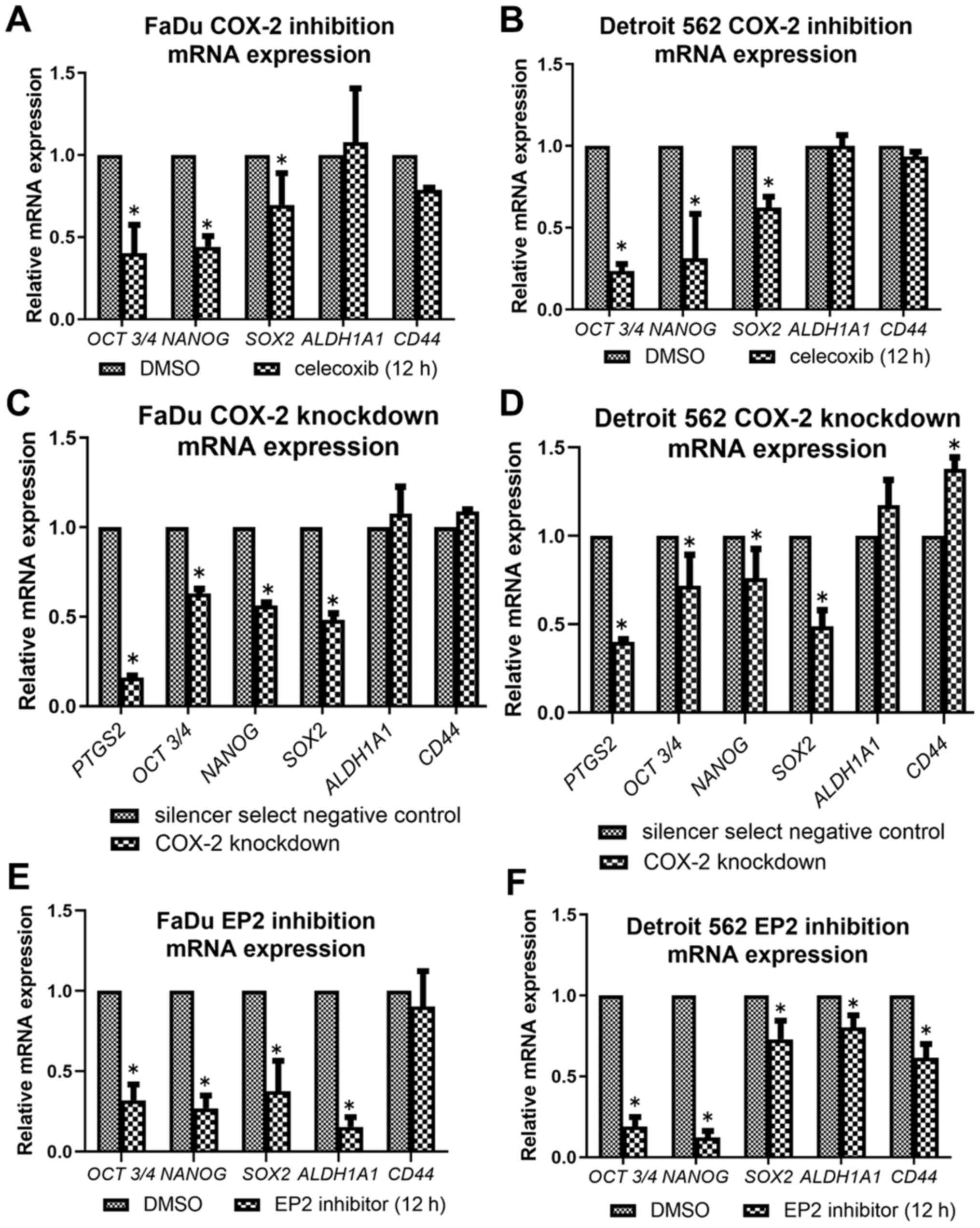
COX-2 inhibitors reduce messenger RNA
expression of stemness-related genes
COX-2 inhibition by celecoxib led to downregulation
of expressions in OCT3/4, NANOG, and SOX-2 in both cell lines
(Fig. 6A and B). There was no
significant change in ALDH1A1 expression throughout the inhibition
assay. COX-2 knockdown cells also showed a similar alteration
compared to celecoxib treatment, decreasing the expressions of
OCT3/4, NANOG, and SOX-2 (Fig. 6C and
D). PTGS2 was significantly decreased in both cell lines
compared to negative control, confirming that transfection
successfully knocked down COX-2. EP2 inhibition showed a similar
but slightly different alteration, decreasing the expressions of
OCT3/4, NANOG, SOX-2 and ALDH1A1 in both cell lines, and CD44 in
Detroit 562 (Fig. 6E and F).
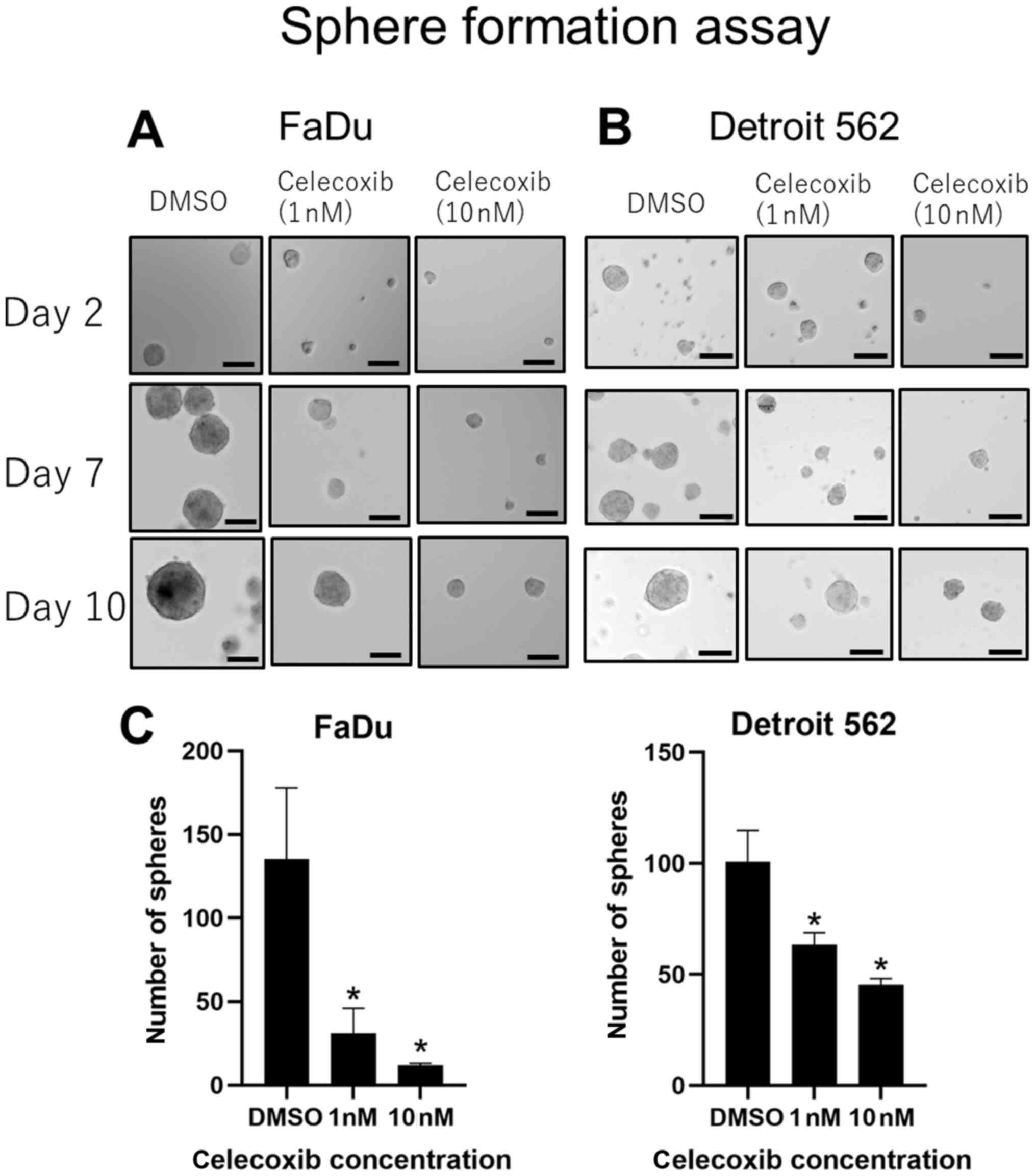
COX-2 inhibitors suppress sphere
formation in head and neck squamous cell carcinoma cell lines
Cells cultured with celecoxib established smaller
spheres, whereas cells cultured in DMSO developed larger spheres in
both cell lines (Fig. 7A and B).
Celecoxib of 1 and 10 nM showed a significant suppression in the
number of spheres established compared with DMSO (Fig. 7C), and this effect was observed in a
concentration dependent manner. EP2 inhibitors did not show any
positive effect (data not shown).
Discussion
From our clinicopathological assays, patients with
hypopharyngeal carcinoma who had high COX-2 expression showed
tolerance to the following induction chemotherapy, indicating that
COX-2 expression is related to chemotherapeutic resistance. Similar
studies using pretreatment biopsy specimens to predict
chemo-sensitivity have been reported in esophageal carcinoma
(27) and nasopharyngeal carcinoma
(19), but there are no reports of
hypopharyngeal squamous cell carcinomas. Our result was compatible
with findings of these previous reports showing that tumors with
high COX-2 expression pretreatment were resistant to the following
chemotherapy.
Furthermore, we found that COX-2 inhibition improves
chemo-sensitivity in HNSCCs in vitro. Using pharyngeal carcinoma
cell lines, the chemo-sensitivity to DTX improved with the addition
of celecoxib. Immunofluorescence analysis revealed that combination
treatment of DTX and celecoxib suppresses Ki-67 in a significant
manner. Celecoxib itself showed effect on Ki-67 expression in
Detroit 562, but considering the low dose of celecoxib we used and
the fact that addition of celecoxib to DTX significantly decreased
Ki-67 in both cell lines regardless of the effect of single
celecoxib treatment, celecoxib seems to have anti-cancer effects
other than proliferation suppression. Previous studies have shown
that celecoxib enhances anti-tumor activity by promoting apoptosis
(28–30) and inhibiting DNA repair (22,31).
Besides these pathways, controlling cancer stemness can also be one
reason for improvement of chemo-resistance (32–34).
COX-2 and its metabolic product PGE2 play an
important role in maintaining cancer stemness and activating
repopulation (35). In this study,
celecoxib downregulated cancer stem cell-related genes such as
OCT3/4, NANOG, and SOX-2 in pharyngeal carcinoma cell lines, and
led to the inhibition of sphere formation, one of the
characteristics of cancer stem cells. Similarly, knockdown of PTGS2
led to downregulation of OCT3/4, NANOG, and SOX-2. Previous reports
also demonstrated that COX-2 was co-expressed with CSC markers
including SOX-2, OCT3/4, and ALDH (36), and upregulation of COX-2 was
associated with increased chemo-resistance in CSC-like side
population cells (37). The
mechanism regulating cancer stemness by COX-2 has not been fully
elucidated, but interaction between the COX2/PGE2/EP axis and
cancer stemness by signaling molecules, such as Wnt (23–25) and
STAT3 (26), are assumed to control
this effect. All of these documented data suggest that COX-2
expression is related to CSC, and can play a role in
chemo-resistance. Therefore, COX-2 inhibition can be an attractive
target for HNSCC, especially for combination use with
chemotherapy.
Despite the multiple promising results of COX-2
inhibition in vivo and in vitro, clinical trials have
failed to prove an absolute positive effect of celecoxib. A
meta-analysis including malignancies such as lung cancers, prostate
cancers, breast cancers, ovarian cancers, and colorectal cancers,
concluded that the addition of celecoxib increased the overall
response rate in non-small-cell lung cancer (NSCLC), but had no
effect on other malignancies (38).
However, a phase III randomized trial of advanced NSCLC showed no
benefit of celecoxib on survival (39). As for head and neck carcinomas, a
phase 2–3 study for advanced nasopharyngeal carcinoma showed
improvement in 2-year local control but none in survival with the
addition of low-dose celecoxib to chemotherapy (40).
These inconsistent results and the fact that COX-2
expression in tumor or stromal cells has no impact on the effect of
celecoxib (39,41) may be explained by the various
expression patterns of the downstream PGE2 receptors, which vary
between organs and cell lines (42).
Multiple HNSCC cell lines have been reported with wide variation of
EP expression patterns (14,43). In our study, the mRNA expression
level of EP1-4 genes varied between the two cell lines (Fig. 4). EP1 was absent in both cell lines,
and FaDu showed a lower expression in the other receptors (EP2, 3,
and 4) than Detroit 562, which may be the reason for the different
response to celecoxib and the EP2 inhibitor.
The four types of PGE2 receptors, EP1-4, induce
various signals, and each play different roles in malignancy
(42,44). The expression patterns, therefore,
can affect the molecular functions of COX-2 inhibitors. EP1 shows a
tumor-promoting role by activating pathways related to cell
migration and invasion in various organs (45,46), but
may have an anti-tumoral effect in breast cancer (47). The role of EP3 in malignancy is
unclear but seems to promote cancers (48) including HNSCCs (14,43). EP2
and EP4 receptors have similar responses, and are both linked to Gs
proteins and activating adenylate cyclase, leading to increased
cAMP levels. EP2 receptors induce angiogenesis (49) and suppress anti-tumor immune response
(50). We have also previously
reported that activation of EP2 receptors can promote EMT in HNSCCs
(18).
Based on our present study, EP2 pathway activation
may be related with cancer stemness, and targeting it can be useful
in effectively improving chemo-sensitivity to DTX. Although EP2
inhibition improved chemo-sensitivity and downregulated cancer
stemness-related genes, we could not show suppression in the sphere
formation assay as celecoxib did. This may be explained by the
relatively short half-life time of PF-04418948 compared to
celecoxib (51). Furthermore, we
have performed same assays using an EP4 antagonist, and could not
determine any positive effect concerning control of cancer stemness
(data not shown). As celecoxib inhibits all PGE2 receptors,
combination blocking of specific receptors (such as simultaneous
inhibition of EP2 and EP4) may be effective and needs to be further
elucidated. Although celecoxib failed to show an absolute positive
effect in clinical trials and long-term use of COX-2 inhibitors can
lead to elevated cardiovascular risk (52), further detailed analysis of PGE2
receptor expression and downstream signaling may provide a possible
therapeutic target.
There are limitations to our study. First of all,
the clinical sample size was relatively small. This was due to the
limited number of hypopharyngeal carcinoma patients who received
surgery after induction chemotherapy. Second, the alteration of
cancer stemness related genes were analyzed by PCR, and whether
proteins of stemness markers were affected, needs further analysis.
Last, although we were able to show that COX-2 inhibition improves
chemosensitivity, and that COX-2 inhibition leads to suppression of
cancer stemness, the precise mechanism underlying these two
phenomenon needs further investigation. Whether COX-2 inhibition
removed chemoresistance by directly blocking cancer stemness or by
a different pathway remains unknown.
In conclusion, COX-2 inhibition can improve
chemo-resistance to DTX in hypopharyngeal carcinomas through the
inhibition of cancer stemness. Downstream PGE2 receptor expression
seems to be a key factor to assess the effect of celecoxib and
further study is awaited.
Acknowledgements
The authors would like to thank Dr Shintarou
Nakamura and Dr Makoto Hosoya of Keio University School of Medicine
(Tokyo, Japan) for technical assistance. The abstract was presented
at the 2018 Annual Meeting of the American Association for Cancer
Research April 14–18 in Chicago (IL, USA) and published as abstract
no. 914 in Cancer Res 2018;78 (13 Suppl).
Funding
The present study was funded by a grant from the
Japan Society for the Promotion of Science KAKENHI Grant-in-Aid for
Young Scientists (grant no. 16K20275).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SS collected all the data, conducted data
interpretation and analysis, and wrote the manuscript. MS, YW, FI,
YuI and NN were involved in the acquisition and analysis of the
data. KK was the pathologist who evaluated the pathological effect
of chemotherapy. HO designed the study and performed proof reading
of the article. YoI and KO were involved in designing the
experiments and troubleshooting. SS and HO confirmed the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The protocols for the use of the clinical materials
were approved by the Institutional Ethics Review Board of the
Ethics Committee of Keio University School of Medicine (reference
nos. 2010-013 and 2010-013-2). Informed consent was obtained in the
form of opt-out on the website and by information in the hospital.
All procedures for clinical tissues were performed in accordance
with the principles of the 1964 Helsinki Declaration and its later
amendments.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
American Cancer Society, . Cancer Facts
and Figures 2020. American Cancer Society; Atlanta, GA: 2020
|
|
2
|
Visini M, Giger R, Shelan M, Elicin O and
Anschuetz L: Predicting factors for oncological and functional
outcome in hypopharyngeal cancer. Laryngoscope. 131:E1543–E1549.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hall SF, Groome PA, Irish J and O'Sullivan
B: The natural history of patients with squamous cell carcinoma of
the hypopharynx. Laryngoscope. 118:1362–1371. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prince ME and Ailles LE: Cancer stem cells
in head and neck squamous cell cancer. J Clin Oncol. 26:2871–2875.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Visvader JE and Lindeman GJ: Cancer stem
cells in solid tumours: Accumulating evidence and unresolved
questions. Nat Rev Cancer. 8:755–768. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clara JA, Monge C, Yang Y and Takebe N:
Targeting signalling pathways and the immune microenvironment of
cancer stem cells-a clinical update. Nat Rev Clin Oncol.
17:204–232. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chan G, Boyle JO, Yang EK, Zhang F, Sacks
PG, Shah JP, Edelstein D, Soslow RA, Koki AT, Woerner BM, et al:
Cyclooxygenase-2 expression is up-regulated in squamous cell
carcinoma of the head and neck. Cancer Res. 59:991–994.
1999.PubMed/NCBI
|
|
8
|
Camacho M, Leon X, Fernandez-Figueras MT,
Quer M and Vila L: Prostaglandin E(2) pathway in head and neck
squamous cell carcinoma. Head Neck. 30:1175–1181. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mendes RA, Carvalho JF and Waal Iv: An
overview on the expression of cyclooxygenase-2 in tumors of the
head and neck. Oral Oncol. 45:e124–e128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gallo O, Masini E, Bianchi B, Bruschini L,
Paglierani M and Franchi A: Prognostic significance of
cyclooxygenase-2 pathway and angiogenesis in head and neck squamous
cell carcinoma. Hum Pathol. 33:708–714. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Itoh S, Matsui K, Furuta I and Takano Y:
Immunohistochemical study on overexpression of cyclooxygenase-2 in
squamous cell carcinoma of the oral cavity: Its importance as a
prognostic predictor. Oral Oncol. 39:829–835. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Saba NF, Choi M, Muller S, Shin HJ,
Tighiouart M, Papadimitrakopoulou VA, El-Naggar AK, Khuri FR, Chen
ZG and Shin DM: Role of cyclooxygenase-2 in tumor progression and
survival of head and neck squamous cell carcinoma. Cancer Prev Res
(Phila). 2:823–829. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang CC, Tu HF, Wu CH, Chang HC, Chiang
WF, Shih NC, Lee YS, Kao SY and Chang KW: Up-regulation of HB-EGF
by the COX-2/PGE2 signaling associates with the cisplatin
resistance and tumor recurrence of advanced HNSCC. Oral Oncol.
56:54–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abrahao AC, Castilho RM, Squarize CH,
Molinolo AA, dos Santos-Pinto D Jr and Gutkind JS: A role for
COX2-derived PGE2 and PGE2-receptor subtypes in head and neck
squamous carcinoma cell proliferation. Oral Oncol. 46:880–887.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang B, Jia L, Guo Q, Ren H, Hu Y and Xie
T: Clinicopathological and prognostic significance of
cyclooxygenase-2 expression in head and neck cancer: A
meta-analysis. Oncotarget. 7:47265–47277. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sekimizu M, Ozawa H, Saito S, Ikari Y,
Nakahara N, Nakamura S, Yoshihama K, Ito F, Watanabe Y, Imanishi Y,
et al: Cyclo-oxygenase-2 expression is associated with lymph node
metastasis in oropharyngeal squamous cell carcinoma under the new
TNM classification. Anticancer Res. 39:5623–5630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fujii R, Imanishi Y, Shibata K, Sakai N,
Sakamoto K, Shigetomi S, Habu N, Otsuka K, Sato Y, Watanabe Y, et
al: Restoration of E-cadherin expression by selective Cox-2
inhibition and the clinical relevance of the
epithelial-to-mesenchymal transition in head and neck squamous cell
carcinoma. J Exp Clin Cancer Res. 33:402014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Watanabe Y, Imanishi Y, Ozawa H, Sakamoto
K, Fujii R, Shigetomi S, Habu N, Otsuka K, Sato Y, Sekimizu M, et
al: Selective EP2 and Cox-2 inhibition suppresses cell migration by
reversing epithelial-to-mesenchymal transition and Cox-2
overexpression and E-cadherin downregulation are implicated in neck
metastasis of hypopharyngeal cancer. Am J Transl Res. 12:1096–1113.
2020.PubMed/NCBI
|
|
19
|
Shi C, Guan Y, Zeng L, Liu G, Zhu Y, Xu H,
Lu Y, Liu J, Guo J, Feng X, et al: High COX-2 expression
contributes to a poor prognosis through the inhibition of
chemotherapy-induced senescence in nasopharyngeal carcinoma. Int J
Oncol. 53:1138–1148. 2018.PubMed/NCBI
|
|
20
|
Saikawa Y, Sugiura T, Toriumi F, Kubota T,
Suganuma K, Isshiki S, Otani Y, Kumai K and Kitajima M:
Cyclooxygenase-2 gene induction causes CDDP resistance in colon
cancer cell line, HCT-15. Anticancer Res. 24((5A)): 2723–2728.
2004.PubMed/NCBI
|
|
21
|
Patel VA, Dunn MJ and Sorokin A:
Regulation of MDR-1 (P-glycoprotein) by cyclooxygenase-2. J Biol
Chem. 277:38915–38920. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Raju U, Ariga H, Dittmann K, Nakata E, Ang
KK and Milas L: Inhibition of DNA repair as a mechanism of enhanced
radioresponse of head and neck carcinoma cells by a selective
cyclooxygenase-2 inhibitor, celecoxib. Int J Radiat Oncol Biol
Phys. 63:520–528. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu M, Guan J, Li C, Gunter S, Nusrat L, Ng
S, Dhand K, Morshead C, Kim A and Das S: Aberrantly activated Cox-2
and Wnt signaling interact to maintain cancer stem cells in
glioblastoma. Oncotarget. 8:82217–82230. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Deng Y, Su Q, Mo J, Fu X, Zhang Y and Lin
EH: Celecoxib downregulates CD133 expression through inhibition of
the Wnt signaling pathway in colon cancer cells. Cancer Invest.
31:97–102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang C, Chen Y, Liu H, Yang J, Song X,
Zhao J, He N, Zhou CJ, Wang Y, Huang C and Dong Q: Celecoxib
targets breast cancer stem cells by inhibiting the synthesis of
prostaglandin E2 and down-regulating the Wnt pathway
activity. Oncotarget. 8:115254–115269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Q, Yuan W, Tong D, Liu G, Lan W, Zhang
D, Xiao H, Zhang Y, Huang Z, Yang J, et al: Metformin represses
bladder cancer progression by inhibiting stem cell repopulation via
COX2/PGE2/STAT3 axis. Oncotarget. 7:28235–28246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akutsu Y, Hanari N, Yusup G,
Komatsu-Akimoto A, Ikeda N, Mori M, Yoneyama Y, Endo S, Miyazawa Y
and Matsubara H: COX2 expression predicts resistance to
chemoradiotherapy in esophageal squamous cell carcinoma. Ann Surg
Oncol. 18:2946–2951. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shaik MS, Chatterjee A, Jackson T and
Singh M: Enhancement of antitumor activity of docetaxel by
celecoxib in lung tumors. Int J Cancer. 118:396–404. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Janakiraman H, House RP, Talwar S,
Courtney SM, Hazard ES, Hardiman G, Mehrotra S, Howe PH, Gangaraju
V and Palanisamy V: Repression of caspase-3 and RNA-binding protein
HuR cleavage by cyclooxygenase-2 promotes drug resistance in oral
squamous cell carcinoma. Oncogene. 36:3137–3148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choe MS, Chen Z, Klass CM, Zhang X and
Shin DM: Enhancement of docetaxel-induced cytotoxicity by blocking
epidermal growth factor receptor and cyclooxygenase-2 pathways in
squamous cell carcinoma of the head and neck. Clin Cancer Res.
13:3015–3023. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen KH, Hsu CC, Song WS, Huang CS, Tsai
CC, Kuo CD, Hsu HS, Tsai TH, Tsai CY, Woung LC, et al: Celecoxib
enhances radiosensitivity in medulloblastoma-derived CD133-positive
cells. Childs Nerv Syst. 26:1605–1612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zheng HC: The molecular mechanisms of
chemoresistance in cancers. Oncotarget. 8:59950–59964. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tong D, Liu Q, Wang LA, Xie Q, Pang J,
Huang Y, Wang L, Liu G, Zhang D, Lan W and Jiang J: The roles of
the COX2/PGE2/EP axis in therapeutic resistance. Cancer Metastasis
Rev. 37:355–368. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vinogradov S and Wei X: Cancer stem cells
and drug resistance: The potential of nanomedicine. Nanomedicine
(Lond). 7:597–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang Q, Li F, Liu X, Li W, Shi W, Liu FF,
O'Sullivan B, He Z, Peng Y, Tan AC, et al: Caspase 3-mediated
stimulation of tumor cell repopulation during cancer radiotherapy.
Nat Med. 17:860–866. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Majumder M, Xin X, Liu L, Tutunea-Fatan E,
Rodriguez-Torres M, Vincent K, Postovit LM, Hess D and Lala PK:
COX-2 induces breast cancer stem cells via EP4/PI3K/AKT/NOTCH/WNT
axis. Stem Cells. 34:2290–2305. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Guo Z, Jiang JH, Zhang J, Yang HJ, Yang
FQ, Qi YP, Zhong YP, Su J, Yang RR, Li LQ and Xiang BD: COX-2
promotes migration and invasion by the side population of cancer
stem cell-like hepatocellular carcinoma cells. Medicine
(Baltimore). 94:e18062015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen J, Shen P, Zhang XC, Zhao MD, Zhang
XG and Yang L: Efficacy and safety profile of celecoxib for
treating advanced cancers: A meta-analysis of 11 randomized
clinical trials. Clin Ther. 36:1253–1263. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Edelman MJ, Wang X, Hodgson L, Cheney RT,
Baggstrom MQ, Thomas SP, Gajra A, Bertino E, Reckamp KL, Molina J,
et al: Phase III randomized, placebo-controlled, double-blind trial
of celecoxib in addition to standard chemotherapy for advanced
non-small-cell lung cancer with cyclooxygenase-2 overexpression:
CALGB 30801 (Alliance). J Clin Oncol. 35:2184–2192. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mohammadianpanah M, Razmjou-Ghalaei S,
Shafizad A, Ashouri-Taziani Y, Khademi B, Ahmadloo N, Ansari M,
Omidvari S, Mosalaei A and Mosleh-Shirazi MA: Efficacy and safety
of concurrent chemoradiation with weekly cisplatin +/- low-dose
celecoxib in locally advanced undifferentiated nasopharyngeal
carcinoma: A phase II–III clinical trial. J Cancer Res Ther.
7:442–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Gulyas M, Mattsson JSM, Lindgren A, Ek L,
Lamberg Lundström K, Behndig A, Holmberg E, Micke P and Bergman B;
Swedish Lung Cancer Study Group, : COX-2 expression and effects of
celecoxib in addition to standard chemotherapy in advanced
non-small cell lung cancer. Acta Oncol. 57:244–250. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
O'Callaghan G and Houston A: Prostaglandin
E2 and the EP receptors in malignancy: Possible therapeutic
targets? Br J Pharmacol. 172:5239–5250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hoshikawa H, Goto R, Mori T, Mitani T and
Mori N: Expression of prostaglandin E2 receptors in oral squamous
cell carcinomas and growth inhibitory effects of an EP3 selective
antagonist, ONO-AE3-240. Int J Oncol. 34:847–852. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Markovic T, Jakopin Z, Dolenc MS and
Mlinaric-Rascan I: Structural features of subtype-selective EP
receptor modulators. Drug Discov Today. 22:57–71. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bai X, Wang J, Zhang L, Ma J, Zhang H, Xia
S, Zhang M, Ma X, Guo Y, Rong R, et al: Prostaglandin E2
receptor EP1-mediated phosphorylation of focal adhesion kinase
enhances cell adhesion and migration in hepatocellular carcinoma
cells. Int J Oncol. 42:1833–1841. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pan J, Yang Q, Shao J, Zhang L, Ma J, Wang
Y, Jiang BH, Leng J and Bai X: Cyclooxygenase-2 induced β1-integrin
expression in NSCLC and promoted cell invasion via the
EP1/MAPK/E2F-1/FoxC2 signal pathway. Sci Rep. 6:338232016.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ma X, Kundu N, Ioffe OB, Goloubeva O,
Konger R, Baquet C, Gimotty P, Reader J and Fulton AM:
Prostaglandin E receptor EP1 suppresses breast cancer metastasis
and is linked to survival differences and cancer disparities. Mol
Cancer Res. 8:1310–1318. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Amano H, Hayashi I, Endo H, Kitasato H,
Yamashina S, Maruyama T, Kobayashi M, Satoh K, Narita M, Sugimoto
Y, et al: Host prostaglandin E(2)-EP3 signaling regulates
tumor-associated angiogenesis and tumor growth. J Exp Med.
197:221–232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pan MR, Hou MF, Chang HC and Hung WC:
Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling
pathways to enhance lymphatic invasion of breast cancer cells. J
Biol Chem. 283:11155–11163. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Obermajer N and Kalinski P: Key role of
the positive feedback between PGE(2) and COX2 in the biology of
myeloid-derived suppressor cells. Oncoimmunology. 1:762–764. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
af Forselles KJ, Root J, Clarke T, Davey
D, Aughton K, Dack K and Pullen N: In vitro and in vivo
characterization of PF-04418948, a novel, potent and selective
prostaglandin EP2 receptor antagonist. Br J Pharmacol.
164:1847–1856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Solomon SD, McMurray JJ, Pfeffer MA,
Wittes J, Fowler R, Finn P, Anderson WF, Zauber A, Hawk E and
Bertagnolli M; Adenoma Prevention with Celecoxib (APC) Study
Investigators, : Cardiovascular risk associated with celecoxib in a
clinical trial for colorectal adenoma prevention. N Engl J Med.
352:1071–1080. 2005. View Article : Google Scholar : PubMed/NCBI
|