Introduction
According to global cancer statistics in 2018, lung
cancer is the most commonly diagnosed cancer and the leading cause
of cancer mortality, accounting for 18.4% of total cancer deaths
(1). Non-small cell lung cancer
(NSCLC) accounts for 80–85% of all cases of lung cancer (2). The optimal therapy for improving
survival rates of NSCLC patients depends on an early diagnosis at
stage I and II when surgical resection remains a feasible and
consistent option (3). However,
surgery is not an effective therapy for patients with an advanced
or metastatic NSCLC; instead, chemotherapy, radiotherapy and
targeted therapy (e.g. targeting epidermal growth factor receptor
and vascular endothelial growth factor) can be used alone or in
combination (4,5). Monoclonal antibodies blocking
immunological checkpoints, also termed immune checkpoint inhibitors
(ICIs), have become promising therapeutic options for patients
diagnosed with advanced NSCLC with or without metastasis (6). Among them, anti-programmed death-1
(PD-1) monoclonal antibodies have been widely used for treating
advanced NSCLC (7–10). Antibodies targeting PD-1 prevent
tumor cells from escaping immune-mediated destruction (11).
The PD-1 receptor is a vital immune checkpoint
molecule expressed on activated T cells that mediates
immunosuppression (12). Binding of
PD-1 to its ligands (PD-L1 and PD-L2) on cancer cells suppresses T
cells, resulting in evasion of the immune response (12). Anti-PD-1 antibodies bind to PD-1
receptors to disrupt the inhibition of T cells by tumor cells,
enhancing the antitumor effects of the immune system (12). Previous clinical trials, including
CheckMate 017 (13) and 057
(14), KEYNOTE-010(9) and KN-024
(10), have demonstrated that
compared with docetaxel, anti-PD-1 antibodies significantly
prolonged the overall survival and had a favorable safety profile
in patients with advanced NSCLC. Despite the prominent therapeutic
effects of anti-PD-1 monoclonal antibodies on patients with
advanced NSCLC, only a limited fraction of patients benefit from
this immunotherapeutic agent (15).
Therefore, developing reliable methods to predict the efficacy of
anti-PD-1 monoclonal antibody in treating patients with advanced
NSCLC may provide economic relief for non-responding patients and
save time to start other types of therapy.
At present, identification of patients with NSCLC
for anti-PD-1 therapy is mainly based on the visual assessment of
PD-1 expression levels in tumor tissue specimens by
immunohistochemistry (IHC) (16).
However, PD-1 IHC data interpretation is subjective and may be
inconsistent due to a varied response to anti-PD-1 therapy in
patients (17). Although a number
of potential indicators for predicting response to anti-PD-1
therapy have been identified, including mutational burden (18), gut microbiome (19) and tumor-infiltrating lymphocyte
abundance (20), these indicators
lack sufficient sensitivity and specificity (21). Based on next-generation sequencing,
gene expression profiling allows simultaneous assessment of a large
number of genes and has been well used to developing response
signatures for a number of types of tumor, including NSCLC
(22). The sensitivity of RNA
immune-oncology (IO) panel sequencing is >20-fold higher
compared with that of whole transcriptome sequencing; specifically,
RNA IO panel sequencing can detect lowly expressed coding genes
with high repetition and identify differential expression with
>2-fold changes (23). Thus, RNA
IO panel sequencing provides a sensitive and accurate approach for
identifying biomarkers and feature genes in clinical studies
(24).
Previous studies have reported that the expression
levels of PD-L1 are markedly different between primary tumors and
nodal metastases in patients with advanced NSCLC (25,26).
In addition, the effects of anti-PD-1 antibodies differ between
patients with primary and metastatic NSCLC (8). The present study aimed to investigate
the roles of robust biomarkers in determining whether patients with
primary or metastatic advanced NSCLC may benefit from anti-PD-1
antibody treatment.
Materials and methods
Study design
The present study was an observational study, which
was conducted in accordance with the Declaration of Helsinki and
approved by the Hunan Provincial Tumor Hospital (Changsha, China;
approval no. 2019 fast review of scientific research [08]). All
patients enrolled in the present study met the following criteria:
i) They were confirmed to be affected by advanced NSCLC; ii) they
received anti-PD-1 monoclonal antibody therapy as the second-line
treatment; iii) they were available for a 3-year follow-up period;
and iv) they signed informed consent forms. The exclusion criteria
were as follows: i) Other types of tumors; ii) history of
myocardial infarction, unstable angina, cerebral apoplexy or
uncontrollable arrhythmias; iii) pregnancy or lactation period; iv)
history of mental disorders; and v) poor compliance with the study
protocol. According to these criteria, 24 patients with NSCLC were
selected and enrolled in the study, including 13 patients with
primary and 11 with metastatic carcinoma. Primary tumor or
metastatic lymph node samples were collected from each patient by
needle biopsy prior to the start of monoclonal antibody therapy and
stored following formalin fixation and embedding in paraffin at
room temperature. All patients were evaluated by examination of the
samples and computed tomography or magnetic resonance imaging using
the Response Evaluation Criteria in Solid Tumors version 1.1
(RECIST 1.1)(27). Responses to
treatment were assessed every 6 weeks by computed tomography or
magnetic resonance imaging using RECIST 1.1 and confirmed by a
subsequent evaluation ≥4 weeks from the start of treatment. Highly
selective humanized monoclonal IgG4 antibodies against PD-1/PD-L1,
including Nivolumab, IBI308 and Duravalumab, were administered by
intravenous infusion every 2 weeks according to the treatment
regimen prescribed by each patient's primary care physician. Based
on the response to the anti-PD-1 treatment (28), the primary carcinoma group was
subdivided into the responding (n=7) and the non-responding (n=6)
groups. Similarly, the metastatic carcinoma group was classified
into the responding (n=5) and the non-responding (n=6) groups.
Overall survival was defined as the time between the start of
treatment until death. Progression-free survival defined as the
time between the start of treatment, that a patient lives with
NSCLC but it does not get worse.
RNA extraction
RNA extraction was performed using the MagMAX-96
Total RNA Isolation kit (cat. no. AM1830; Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Samples were sonicated and incubated with proteinase K for 15 min
at 56°C for protein digestion, followed by 1 h at 80°C for the
disruption of nucleic acid-protein cross-links. The digested
samples were centrifuged at 12,000 × g for 5 min at 4°C, and the
collected supernatant was treated with DNase I. Subsequently, B1
buffer and 100% ethanol were added into the supernatant to produce
the binding solution, which was transferred to a 0.45-µm cellulose
acetate microcentrifuge spin column and concentrated. Following
washing with a washing buffer, the column was eluted with an
elution buffer to collect the RNA solution. The RNA integrity
number (RIN) in the RNA solution was measured using the 2100
Bioanalyzer Instrument (Agilent Technologies, Inc.). All samples
had a RIN value >7. Finally, the RNA solution was subjected to
spectrophotometric analysis for determining the A260/A280 and
A260/A230 ratios. The A260/A280 ratio ranged between 1.9 and 2.1,
and the A260/A230 ratio was >2.0.
Reverse transcription and library
construction
The RNA was reverse transcribed into cDNA using the
High-Capacity cDNA Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions. The targeted cDNA was amplified with the multiplex
immune response primer pool (included in Oncomine IO Panel;
Genecast Biotechnology Co., Ltd.) targeting 395 genes. Following
amplification, the amplicons were partially digested using a FuPa
Reagent (10 min at 50°C, 10 min at 55°C, 20 min at 60°C and 60 min
at 10°C). Barcode adapters were subsequently ligated to the
partially digested amplicons (30 min at 22°C, 10 min at 72°C and 60
min at 10°C). The barcode-tagged amplicons were purified and
amplified. The amplified products were then dissolved in the low
EDTA TE buffer for preparing the RNA library. The library
concentration was determined using NanoDrop ND-1000
spectrophotometer (Thermo Fisher Scientific, Inc.), and the quality
of libraries was analyzed by measuring the gene length in the
library using the 2100 Bioanalyzer Instrument.
Sequencing
Qualified RNA libraries were quantified, and the
concentrations of different libraries were presented in molar
concentration according to the average fragment length. All the
libraries were pooled based on fragment lengths and the used chip,
and sequenced on the Ion Torrent S5 platform. The sequencing kits
used included Ion 540 Chef Reagents (cat. no. A27758), Ion S5 chef
solutions (cat. no. A27754), Ion S5 chef supplies (cat. no.
A27755), I Ion S5 sequencing reagents (cat. no. A27768) and Ion S5
Sequencing solutions (cat. no. A27767). The direction of sequencing
was single-end, and the loading concentration was determined by
Qubit. The nucleotide length and loading concentration of the final
library are presented in Table
I.
 | Table I.Type of sequencing and loading
concentration of the final library. |
Table I.
Type of sequencing and loading
concentration of the final library.
| Sample no. | Nucleotide length,
bp | Loading
concentration of the final library, pM |
|---|
| 201600596 | 204 | 45,177.3 |
| 201526397 | 202 | 66,084.3 |
| 201509607 | 202 | 18,998.1 |
| 201517774 | 203 | 54,449.1 |
| 201518131 | 201 | 56,903.4 |
| 201600702 | 198 | 40,450.5 |
| 201723630 | 201 | 59,812.2 |
| 201614107 | 202 | 25,179.3 |
| 201811713 | 200 | 46,904.4 |
| 201726177 | 199 | 60,266.7 |
| 201726775 | 201 | 5,9085 |
| 201619238 | 201 | 32,269.5 |
| 201613253 | 199 | 57,630.6 |
| 201729934 | 200 | 53,358.3 |
| 201729044 | 200 | 66,811.5 |
| 20180193 | 204 | 44,541.0 |
| 201817287 | 201 | 51,176.7 |
| 201814975 | 195 | 57,994.2 |
| 201811594 | 200 | 50,813.1 |
| 201800133 | 200 | 48,904.2 |
| 201801858 | 200 | 56,085.3 |
| 201703656 | 204 | 67,902.3 |
| 201715487 | 200 | 42,177.6 |
| 201816274 | 200 | 41,450.4 |
RNA IO panel sequencing and data
normalization
The RNA IO panel sequencing produced 1–2 million
reads per sample. Data normalization and processing were performed
as previously described (29).
Briefly, 10 HK genes were used as endogenous controls. The absolute
readout of each HK gene was compared against a predetermined HK
reads per million (RPM) profile. The baseline HK RPM profile was
established by measuring the average RPM of 10 replicates of
GM12878 cell line samples across various sequencing runs. The
fold-change ratio for each HK gene was calculated as follows: Ratio
of HK = absolute read count of HK/RPM profile of HK. The median
value of all HK ratios was then used as the normalization ratio for
each sample: Normalization ratio = Median of all HK ratios. The
normalized RPM (nRPM) of all genes of each sample was calculated
as: nRPM (sample S, gene G) = Absolute read count (sample S, gene
G)/Normalization ratio (sample S).
Data analysis
The sequencing data were subjected to quality
control according to the following standards: Mapped reads
≥200,000; number of detected HK genes ≥6; and valid reads
(on-target ratio) ≥67%. The sequencing quality control data of all
patients are presented in Table
II. The gene expression levels in the qualified samples were
quantified and normalized as follows: Firstly, the RPM value of HK
genes in each sample was divided by the standard HK gene value to
generate a raw value, and the values of all samples were calculated
accordingly; secondly, the median of all the values of samples was
presented as the normalized value; finally, the RPM of each sample
was divided by the normalized value to produce the corresponding
nRPM. An R/Bioconductor software package ‘limma’ (30) running on the R 3.5.3 software
(31) was used to perform
differential expression analysis according to the
log2(nRPM+1) value. The DEGs were determined using the
‘limma’ package with a false discovery rate of 0.05 and absolute
fold-change ≥2. The enrichment analysis of DEGs was performed using
the ‘ClusterProfiler’ package (32), including Gene Ontology (GO;
molecular function, biological process and cellular component;
http://geneontology.org), Kyoto Encyclopedia of
Genes and Genomes Pathway (KEGG; http://www.genome.jp/kegg) database and Reactome
Pathway Database (https://reactome.org), to determine the primary
functions of the DEGs as well as the associated metabolic and
signaling pathways. Subsequently, differential analysis of gene
sets was performed using the gene set variation analysis (GSVA)
package for R (33). A receiver
operating characteristic (ROC) curve of the DEG set was used to
calculate the cut-off values.
 | Table II.The sequencing quality control result
of all the patients. |
Table II.
The sequencing quality control result
of all the patients.
| Sample no. | Mapped reads,
n | On target reads,
n | Valid reads | Detected genes,
n | HK genes, n | QC result |
|---|
| 201600596 | 2,250,344 | 1,928,995 | 86% | 362 | 10 | Pass |
| 201526397 | 2,072,309 | 1,830,263 | 88% | 361 | 10 | Pass |
| 201509607 | 2,215,655 | 2,040,175 | 92% | 353 | 10 | Pass |
| 201517774 | 2,538,679 | 2,367,826 | 93% | 380 | 10 | Pass |
| 201518131 | 2,710,910 | 2,464,217 | 91% | 376 | 10 | Pass |
| 201600702 | 2,793,598 | 2,578,212 | 92% | 376 | 10 | Pass |
| 201723630 | 2,068,023 | 1,888,519 | 91% | 366 | 10 | Pass |
| 201614107 | 2,270,331 | 2,074,856 | 91% | 358 | 10 | Pass |
| 201811713 | 2,498,210 | 2,260,380 | 90% | 369 | 10 | Pass |
| 201726177 | 2,367,111 | 2,152,414 | 91% | 360 | 10 | Pass |
| 201726775 | 1,085,151 | 950,267 | 88% | 357 | 10 | Pass |
| 201619238 | 2,049,446 | 1,837,943 | 90% | 350 | 10 | Pass |
| 201613253 | 2,444,755 | 2,256,998 | 92% | 361 | 10 | Pass |
| 201729934 | 2,597,198 | 2,342,932 | 90% | 360 | 10 | Pass |
| 201729044 | 2,107,692 | 1,897,977 | 90% | 365 | 10 | Pass |
| 20180193 | 2,414,549 | 2,192,169 | 91% | 369 | 10 | Pass |
| 201817287 | 2,358,980 | 2,077,554 | 88% | 364 | 10 | Pass |
| 201814975 | 2,240,242 | 2,056,542 | 92% | 373 | 10 | Pass |
| 201811594 | 2,371,493 | 2,164,936 | 91% | 356 | 10 | Pass |
| 201800133 | 2,427,701 | 2,202,896 | 91% | 365 | 10 | Pass |
| 201801858 | 2,461,863 | 2,186,381 | 89% | 364 | 10 | Pass |
| 201703656 | 2,116,778 | 1,929,443 | 91% | 368 | 10 | Pass |
| 201715487 | 2,359,914 | 2,189,056 | 93% | 369 | 10 | Pass |
| 201816274 | 2,339,855 | 2,138,627 | 91% | 361 | 10 | Pass |
Statistical analysis
Data are presented as the mean ± standard deviation.
Data were analyzed by SPSS 20.0 (IBM Corp.) and R 3.5.3 software.
The expression level of the DEG set for each patient was calculated
by the mean of log2(nRPM+1) values. The mean value was
calculated by summing the four upregulated gene expression values,
subtracting the two downregulated gene expression values and by
dividing the result by 6. The survival analysis for various groups
was performed using the Kaplan-Meier survival analysis with the
log-rank test. Fisher's exact test was used for the analysis of
patient characteristics. Mann-Whitney U test was used to assess the
DEG expression levels in the responding and non-responding groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Associations between
clinicopathological features and the efficacy of anti-PD-1
monoclonal antibody treatment
The present study performed the RNA IO panel
sequencing on samples form 24 patients with NSCLC prior to
anti-PD-1 therapy. Following therapy, all 24 patients were divided
into the responding (partial response or stable disease; n=7
primary and 5 metastatic cancer cases) and non-responding
(progressive disease; n=6 primary and 6 metastatic cancer cases)
groups based on a previously reported response pattern (28). As illustrated in Table III, there were no significant
differences in the age, sex, pathological diagnosis, clinical
stage, therapeutic regimens and history of smoking between the
responding and non-responding groups (P>0.05). These results
suggested that the assessed clinicopathological features were not
associated with the efficacy of anti-PD-1 therapy in these
patients.
 | Table III.Clinicopathological features of all
patients with NSCLC. |
Table III.
Clinicopathological features of all
patients with NSCLC.
|
| Primary NSCLC
(n=13) | metastatic NSCLC
(n=11) |
|---|
|
|
|
|
|---|
| Characteristic | Responding
(n=7) | Non-responding
(n=6) | P-value | Responding
(n=5) | Non-responding
(n=6) | P-value |
|---|
| Age, years | 54.25 | 52.79 |
| 58.73 | 53.83 |
|
| Sex, n |
|
Male | 7 | 5 |
0.462 | 5 | 5 | >0.999 |
|
Female | 0 | 1 |
| 0 | 1 |
|
| Disease stage,
n |
| III
B | 1 | 0 | >0.999 | 0 | 0 | NA |
| IV | 6 | 6 |
| 5 | 6 |
|
| Pathological
diagnosis, n |
|
Adenocarcinoma | 4 | 2 |
0.592 | 3 | 4 | 0.592 |
|
Squamous cell carcinoma | 3 | 4 |
| 2 | 2 |
|
| Therapeutic
regimen, n |
|
Duravalumab | 4 | 1 |
0.266 | 3 | 1 | 0.437 |
|
IBI308 | 0 | 1 |
| 1 | 1 |
|
|
Nivolumab | 3 | 4 |
| 1 | 4 |
|
| History of smoking,
n |
| No | 6 | 2 |
0.103 | 2 | 5 | 0.242 |
|
Yes | 1 | 4 |
| 3 | 1 |
|
Identification of DEGs between the
responding and non-responding groups of patients with NSCLC
The gene expression levels in tumor tissues were
analyzed and compared between the responding and non-responding
groups of patients with NSCLC patients. As demonstrated in Fig. 1A and B, six genes were identified as
significant DEGs between the responding and non-responding groups
of patients with primary NSCLC; among them, four were upregulated
and two were downregulated in the responding group compared with
the non-responding group. Furthermore, the heatmap of DEGs revealed
the downregulation of the levels of HLA-F antisense RNA 1
(HLA-F-AS1) and neutrophil cytosolic factor 1 (NCF1), involved in
autoimmune diseases, as well as the upregulation of the levels of
transcription factor RAR-related orphan receptor C (RORC), deleted
in malignant brain tumors 1 (DMBT1), involved in the interaction
between the tumor cells and immune system, killer cell lectin-like
receptor F1 (KLRF1) and interleukin-18 (IL-18) in the responding
group compared with those in the non-responding group (Fig. 1C).
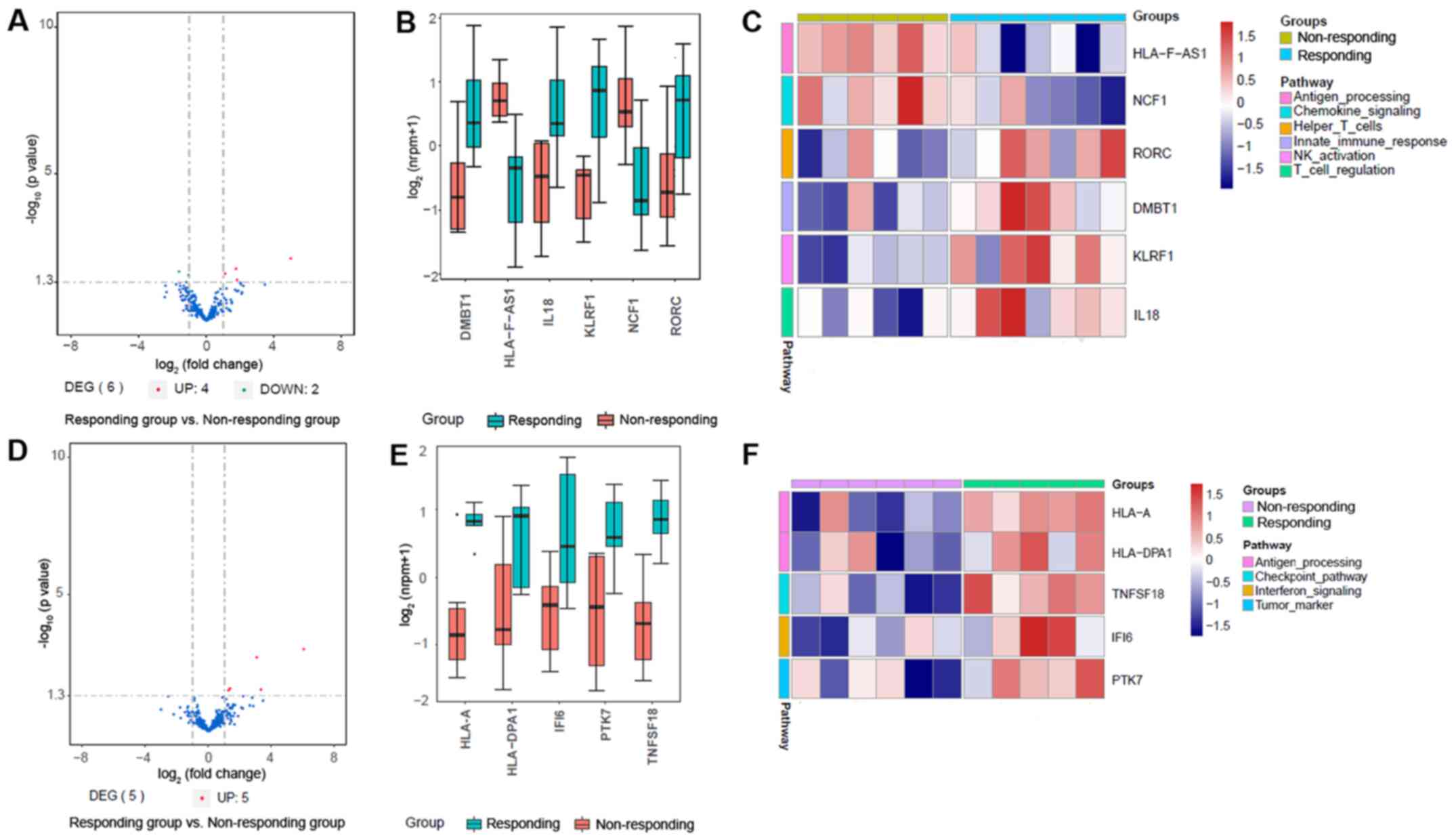 | Figure 1.Effective gene expression map of
tumor tissues from the responding and non-responding groups. (A)
Volcano plot of DEGs in patients with primary NSCLC. (B) Box plot
of DEG expression levels in patients with primary NSCLC. (C) The
expression levels of DEGs associated with various pathways in
patients with primary NSCLC. (D) volcano map in patients with
metastatic NSCLC. (E) Box plot of DEG expression levels in patients
with metastatic NSCLC. (F) The expression levels of DEGs associated
with various pathways in patients with metastatic NSCLC. NSCLC,
non-small cell lung cancer; DEG, differentially expressed gene;
HLA-F-AS1, HLA-F antisense RNA 1; NCF1, neutrophil cytosolic factor
1; RORC, RAR-related orphan receptor C; DMBT1, deleted in malignant
brain tumors 1; KLRF1, killer cell lectin-like receptor F1; IL-18,
interleukin-18; HLA-A, major histocompatibility complex class IA;
HLA-DPA1, major histocompatibility complex class II DP α1; TNFSF18,
tumor necrosis factor ligand superfamily member 18; IFI6,
interferon α-inducible protein 6; PTK7, inactive tyrosine-protein
kinase 7. |
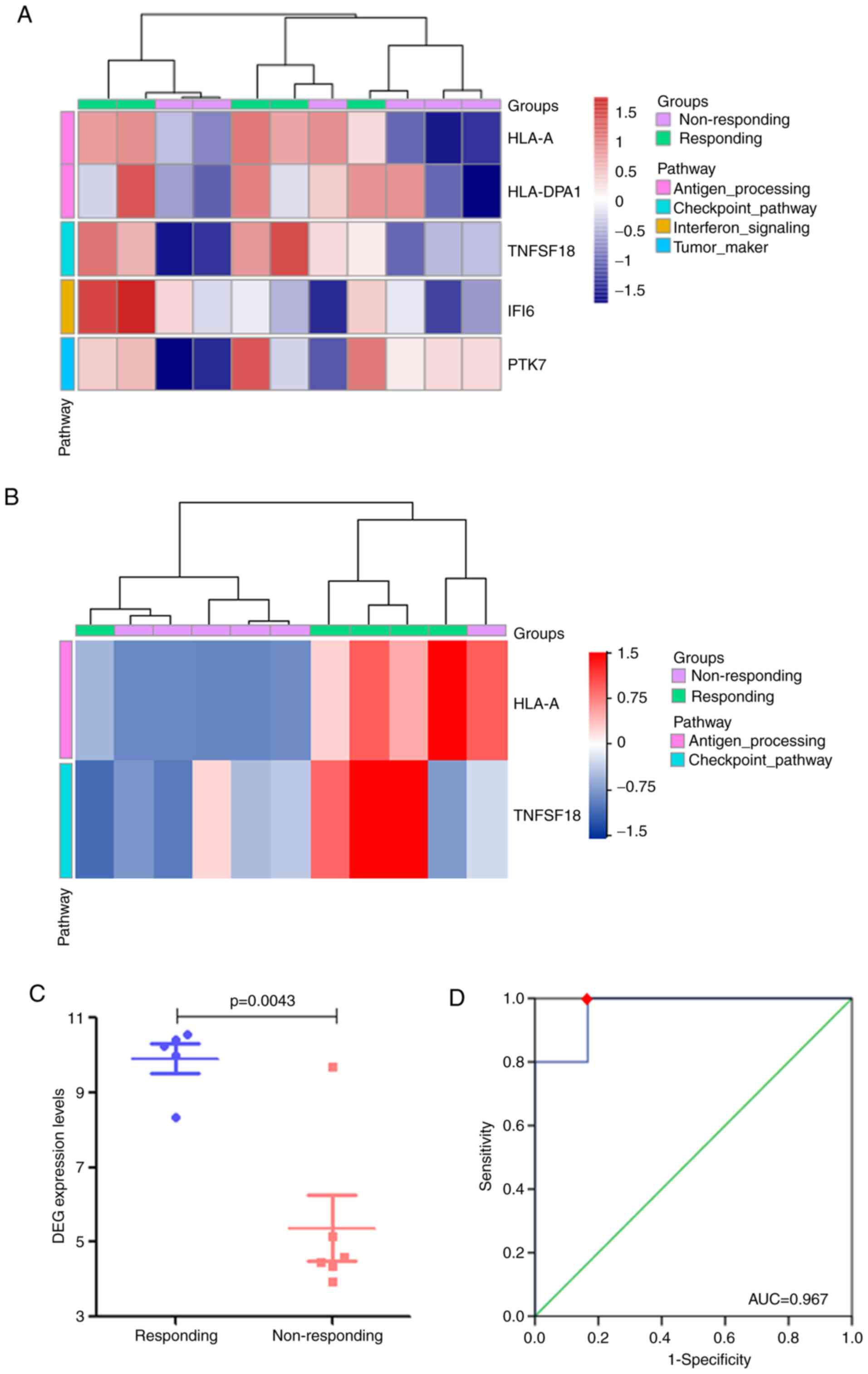
The gene expression analysis also identified major
histocompatibility complex class IA (HLA-A), major
histocompatibility complex class II DP α1 (HLA-DPA1), tumor
necrosis factor ligand superfamily member 18 (TNFSF18), interferon
α-inducible protein 6 (IFI6) and inactive tyrosine-protein kinase 7
(PTK7) as DEGs between the responding and non-responding patients
with metastatic NSCLC (Fig. 1D and
E). As demonstrated in the heatmap in Fig. 1F, the expression levels all five
DEGs were upregulated in the responding group compared with those
in the non-responding group.
Enrichment of DEGs in patients with
primary or metastatic NSCLC
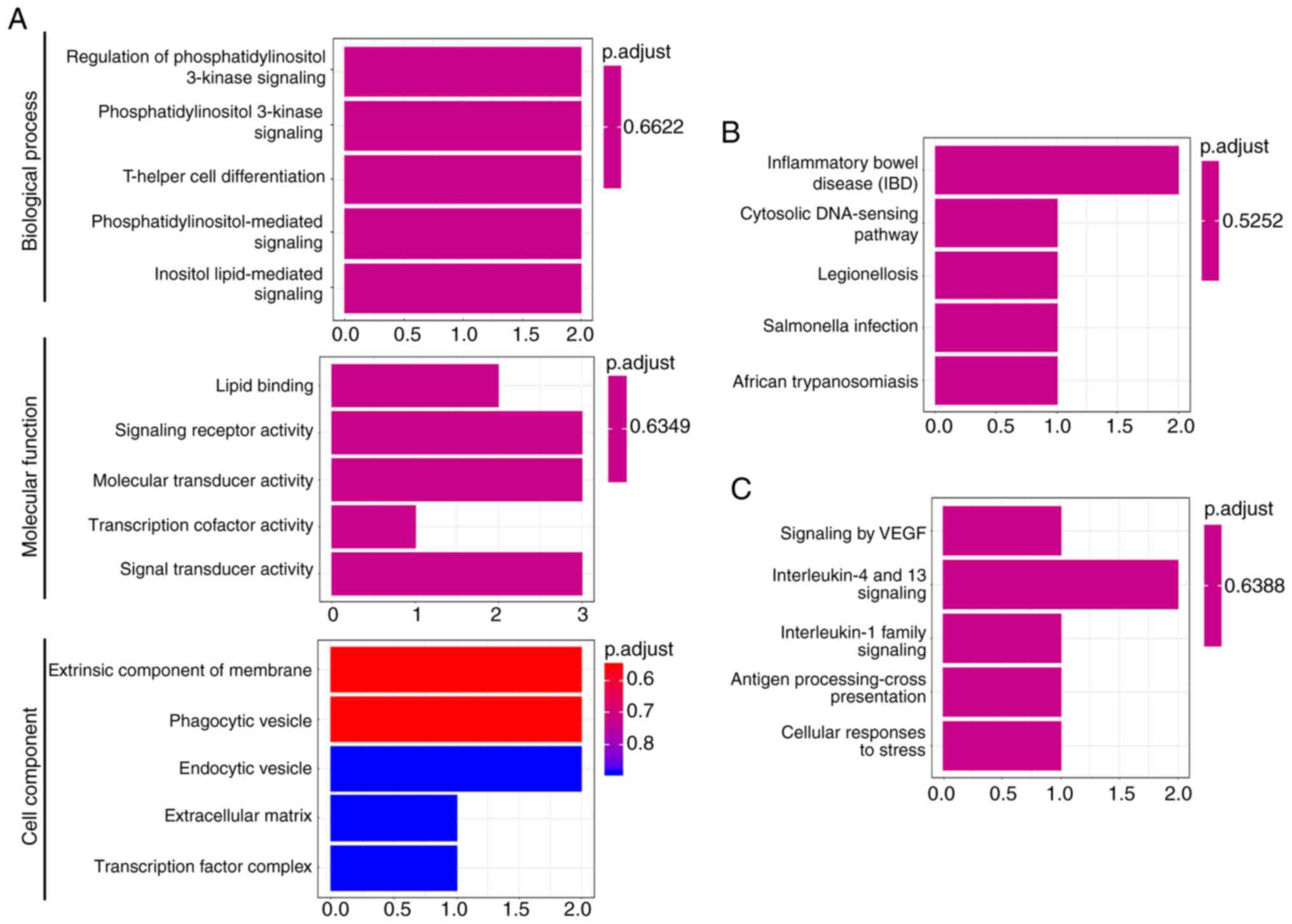
Functional enrichment analysis of the six DEGs in
patients with primary NSCLC was next performed. As presented in
Fig. 2, the six DEGs were
significantly enriched in the ‘phosphatidylinositol 3-kinase
signaling’, ‘T-helper cell differentiation’,
‘phosphatidylinositol-mediated signaling’ and ‘antigen
processing-cross presentation’ signaling pathways. Similarly, the
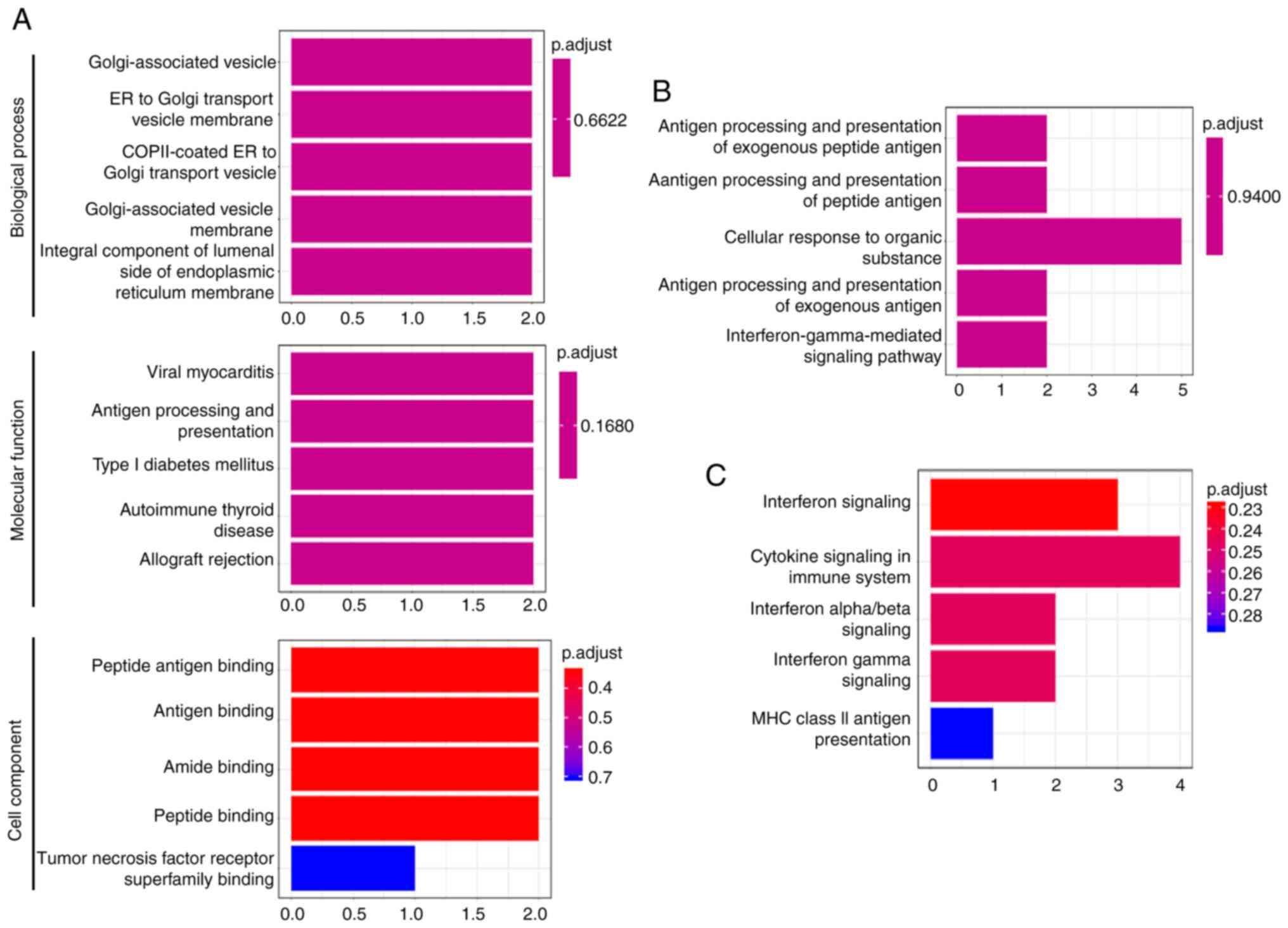
significantly enriched signaling pathways of the five DEGs in
patients with metastatic NSCLC mainly included ‘interferon
signaling’, ‘cytokine signaling in immune system’, ‘antigen
processing and presentation of exogenous peptide antigen’, ‘antigen
binding’ and ‘interferon-gamma-mediated pathway’ (Fig. 3).
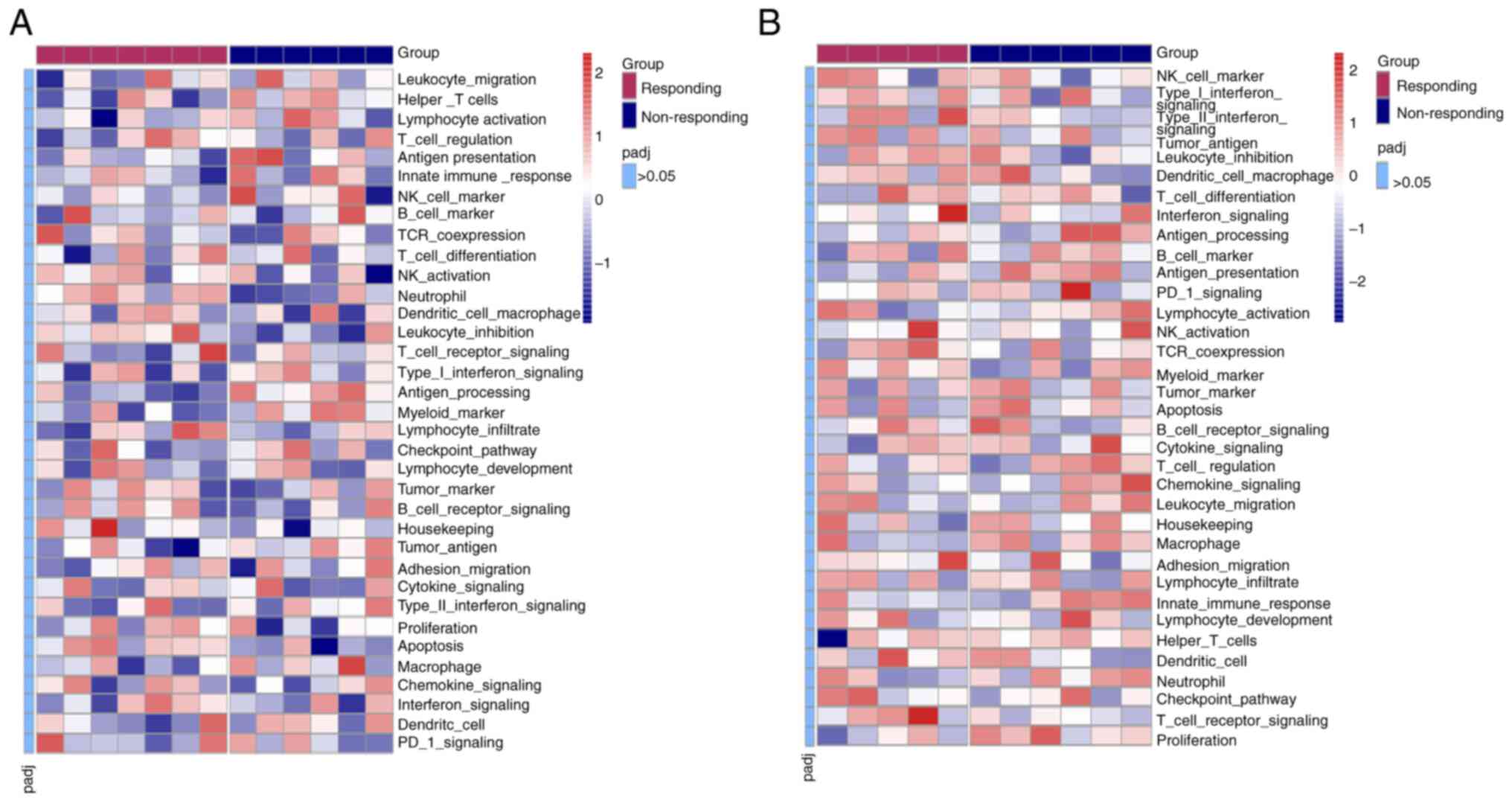
When all 395 analyzed genes in patients with primary
or metastatic NSCLC were subjected to cluster analysis of 35
immune-related signaling/functional pathways using GSVA, the
results demonstrated that there were no significant differences in
any of these pathways between the responding and non-responding
groups (adjusted P>0.05; Fig.
4).
DEG set-based prediction of the
efficacy of anti-PD-1 therapy in patients with primary NSCLC
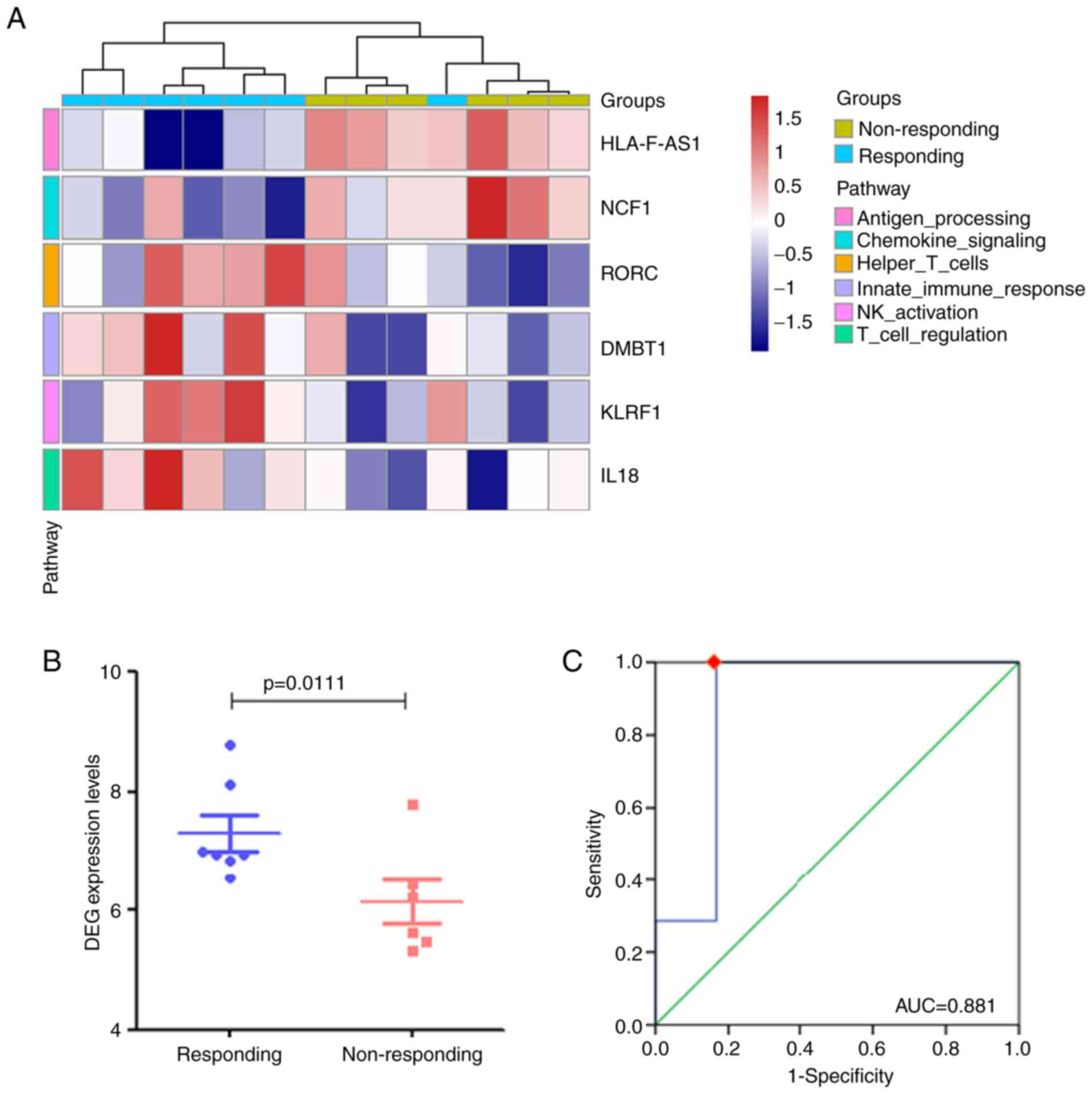
Cluster analysis of the six DEGs in 13 patients with
primary NSCLC was further performed (Fig. 5A). Although the results of one
patient deviated from the others, the remaining patients had the
correct attribution and could be distinguished, suggesting that the
gene set composed by these six DEGs could fairly distinguish the
patients between the responding and non-responding groups.
Therefore, the present study sought to determine whether the six
DEGs may form a DEG set for predicting the effect of anti-PD-1
treatment in these patients. As illustrated in Fig. 5B, the expression levels of the DEG
set in the responding group were significantly higher compared with
those in the non-responding group (P=0.011). Subsequently, the ROC
curve was used to determine the cut-off value indicating the
efficacy of anti-PD-1 treatment. The expression level of the DEG
set exhibited statistical significance in predicting the efficacy
of anti-PD-1 treatment [area under the curve (AUC) =0.881; P=0.022;
sensitivity, 100%; specificity, 83.3%; Fig. 5C). Based on the calculated cut-off
value, patients with an expression level of the DEG set >6.501
benefited from anti-PD-1 therapy.
DEG set-based prediction of the
efficacy of anti-PD-1 treatment in patients with metastatic
NSCLC
Cluster analysis of the five DEGs in patients with
metastatic NSCLC was subsequently performed (Fig. 6A). The results demonstrated that the
set of five DEGs did not fully distinguish between patients in the
responding and non-responding groups. Therefore, cluster analysis
was performed on subsets of the 5 DEGs. As presented in Fig. 6B, the DEG set comprising HLA-A and
TNFSF18 effectively distinguished the two groups of patients. The
expression level of the gene set was calculated by the mean of
log2(nRPM+1) values. Notably, the responding group
displayed a significantly higher expression levels of the DEG set
compared with those in the non-responding group (P=0.004; Fig. 6C). As indicated by the cut-off value
(Fig. 6D), the expression level of
the gene set had statistical significance in predicting the
efficacy of anti-PD-1 monoclonal antibody treatment (AUC=0.967;
P=0.011; sensitivity, 100%; specificity, 83.3%); patients with an
expression level of the DEG set >6.741 benefited from anti-PD-1
antibody therapy.
Associations between DEGs and
progression-free survival (PFS)
Lastly, the present study conducted survival
analysis on 13 patients with primary carcinoma and 10 patients with
metastatic carcinoma. As demonstrated in Fig. 7A, in the primary carcinoma group,
patients with a longer PFS exhibited higher expression levels of
DMBT1, KLRF1, RORC and the 6-gene set compared with those in
patients with a shorter PFS. Similarly, in the metastatic carcinoma
group, patients with a longer PFS displayed higher expression
levels of HLA-A, TNFSF18 and the 2-gene set compared with those in
patients with a shorter PFS (Fig.
7B).
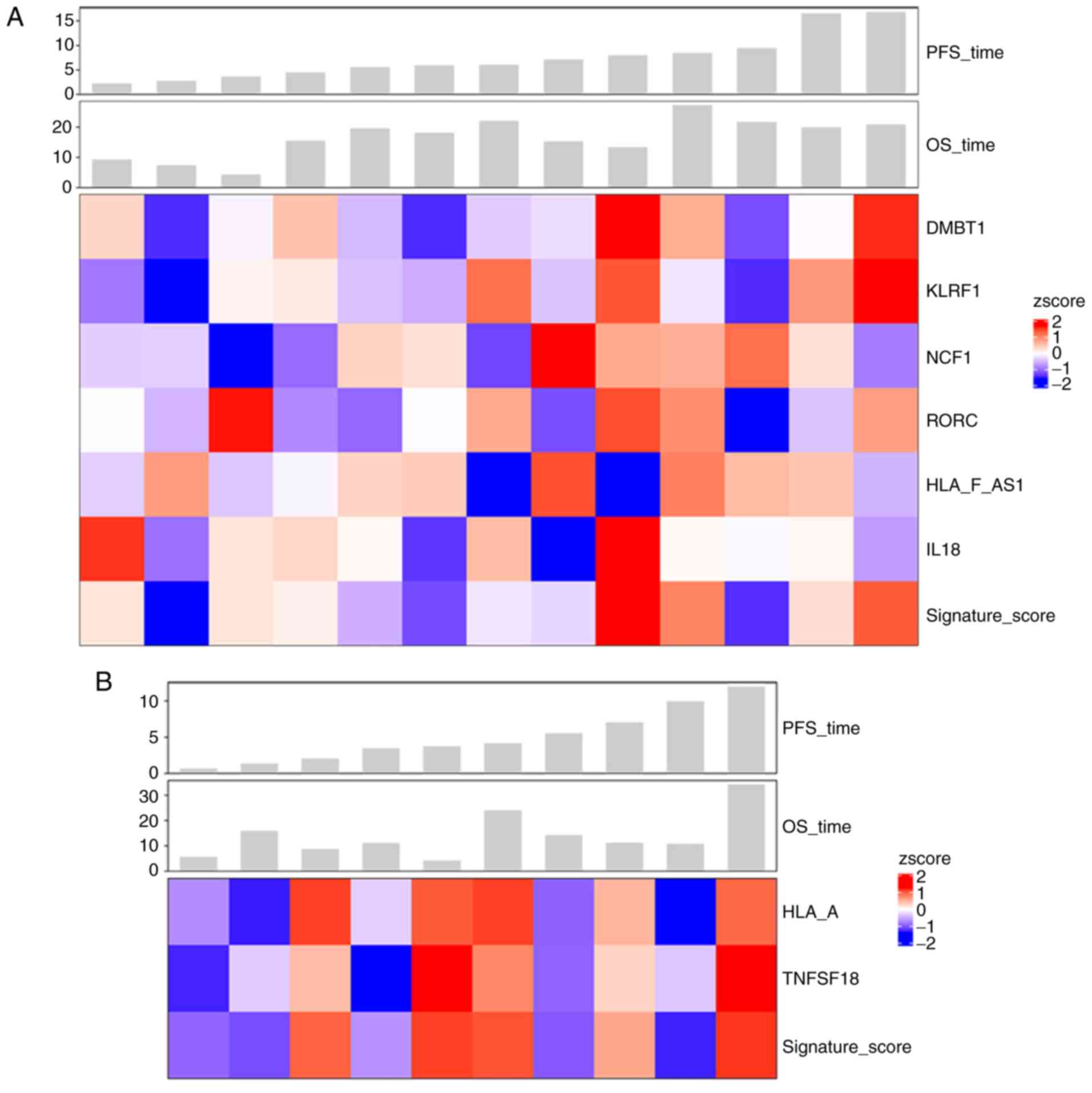 | Figure 7.Survival analysis of patients with
primary and metastatic carcinoma. (A) Among patients with primary
carcinoma, high expression levels of DMBT1, KLRF1, RORC and the
6-gene set were observed in patients with longer PFS. (B) In
metastatic carcinoma, patients with a longer PFS displayed high
expression levels of HLA-A, TNFSF18 and the 2-gene set. PFS,
progression-free survival; OS, overall survival; HLA-F-AS1, HLA-F
antisense RNA 1; NCF1, neutrophil cytosolic factor 1; RORC,
RAR-related orphan receptor C; DMBT1, deleted in malignant brain
tumors 1; KLRF1, killer cell lectin-like receptor F1; IL-18,
interleukin-18; HLA-A, major histocompatibility complex class IA;
TNFSF18, tumor necrosis factor ligand superfamily member 18. |
Discussion
Since surgery is ineffective for patients with
advanced NSCLC, chemotherapy remains the preferred treatment option
(34). Anti-PD-1 monoclonal
antibody, an immune checkpoint inhibitor, has provided a
breakthrough in the treatment of patients with advanced NSCLC
(35). The role of anti-PD-1
antibodies in the first- and second-line treatment of NSCLC or in
the local adjuvant therapy of NSCLC has been established in
previous studies (36–38). However, the effectiveness of
anti-PD-1 therapy on tumors is limited, and rapid growth of tumors
is observed in a number of patients (35). The high cost and difficulty in
predicting the efficacy have become the bottleneck for the
promotion of anti-PD-1 therapy (39). Thus, there is an urgent need to
identify efficient and precise biomarkers for screening patients
with NSCLC that may respond to anti-PD-1 antibody therapy. Analysis
of the functional mechanism of anti-PD-1 monoclonal antibody
suggests that immune-related genes and pathways serve an important
role in its antitumor effect (40).
In the present study, RNA IO panel sequencing was used to examine
the expression levels of 395 genes associated with immune pathways
in patients with primary or metastatic NSCLC prior to the standard
anti-PD-1 antibody therapy (41).
Literature review and data analysis revealed that five
immune-related genes and two gene sets may potentially be used for
predicting the therapeutic effects of PD-1 inhibitors in patients
with primary or metastatic NSCLC.
In the present study, among patients with primary
NSCLC, the responding group exhibited lower expression levels of
HLA-F-AS1 and NCF1 compared with those in the non-responding group.
HLA-F-AS1 is a long non-coding RNA that is significantly
downregulated in human lung adenocarcinoma tissues compared with
matched adjacent non-tumor tissues (42). The NCF1 protein is an essential
component of the phagocytic NADPH oxidase type 2, which is involved
in autoimmune inflammatory disorders (43). Kelkka et al (44) have reported that mice lacking NCF1
developed markedly fewer Lewis lung carcinoma tumors compared with
those in the wild-type controls. Consistently, the results of the
present study demonstrated that patients with primary NSCLC with a
longer PFS exhibited higher expression levels of HLAF-AS1 and NCF1
compared with those in patients with a shorter PFS. Thus, low
levels of HLA-F-AS1 and NCF1 may be biomarkers for predicting
response of patients with primary NSCLC to anti-PD-1 therapy. In
addition, low expression levels of HLA-F-AS1 may indicate improved
efficacy of anti-PD-1 treatment (45,46).
DMBT1 has been proposed as a candidate tumor suppressor (45,46).
DMBT1 is highly expressed in normal lung tissues, but is present at
low levels in lung cancer cell lines and primary NSCLC tissues
(45). In the present study, among
patients with primary NSCLC, the responding group exhibited higher
levels of DMBT1 compared with those in the non-responding group,
whereas increased expression levels of DMBT1 were present in
patients with a longer PFS compared with those in patients with a
shorter PFS. Although DMBT1 is lowly expressed in patients with
NSCLC, its relatively high expression levels may potentially be
used as an index for predicting the efficacy of anti-PD-1 treatment
in patients with primary NSCLC.
Among patients with metastatic NSCLC in the present
study, the responding group presented with significantly higher
levels of HLA-A and TNFSF18 compared with those in the
non-responding group. HLA-A belongs to the HLA class I antigens and
serves a crucial role in presenting tumor cell immunogenic
polypeptide to T cells as well as promoting the antitumor effects
of cytotoxic T lymphocytes (47,48).
However, HLA-A levels are markedly downregulated in the majority of
primary NSCLC tumors and all metastatic lymph nodes compared with
those in normal lung tissues (49).
TNFSF18, also termed glucocorticoid-induced TNFR-related protein
(GITRL), participates in the functioning of effector and regulatory
T cells, which is important for the development of immune responses
(50). Upregulation of GITRL has
been demonstrated to improve antitumor immunity in murine Lewis
lung carcinoma (51,52). In addition, in the present study,
patients with metastatic NSCLC with a longer PFS presented with
higher expression levels of HLA-A and TNFSF18 compared with those
in patients with a shorter PFS. Therefore, patients with metastatic
NSCLC with high expression levels of HLA-A and TNFSF18 may benefit
from anti-PD-1 treatment, suggesting that HLA-A and TNFSF18 may be
potential biomarkers for predicting the efficacy of anti-PD-1
therapy in patients with metastatic NSCLC. PTK7 is a member of the
receptor protein tyrosine kinase family (53). Studies have demonstrated that PTK7
is highly expressed in tumor tissues of patients with primary lung
adenocarcinoma, and inhibition of PTK7 reduces the number of
tumor-initiating cells and induces tumor regression (53,54).
By contrast, one study has reported that the mRNA and protein
expression levels of PTK7 are downregulated in human lung squamous
cell carcinoma compared with those in normal lung tissues, and
overexpression of PTK7 in lung cancer cells inhibits cell
proliferation, invasion and migration (55). Thus, it remains to be determined
whether PTK7 is associated with the development of NSCLC or the
response to anti-PD-1 treatment.
Single-gene predictive biomarkers are usually
considered unsatisfactory in terms of accuracy and precision. In
recent years, an increasing number of studies have demonstrated
that biomarkers consisting of gene sets (multiple DEGs) are more
accurate compared with single-gene biomarkers (56,57).
Li et al (58) have
established a 4-gene set biomarker that predicts early relapse in
advanced epithelial ovarian cancer after initial
platinum-paclitaxel chemotherapy with an accuracy ~65.5%. In
addition, a minimal driver gene set has been developed to predict
bone metastasis in breast cancer (59). Another study has proposed that an
immune gene-set based signature may serve as a promising biomarker
for estimating overall survival of patients with ovarian cancer
(60). The results of the present
study demonstrated a gene set comprising six DEGs (HLA-F-AS1, NCF1,
RORC, DMBT1, KLRF and IL-18) may be used for predicting the
efficacy of anti-PD-1 therapy in patients with primary NSCLC;
specifically, patients with a calculated expression level of the
DEG set >6.501 may benefit from anti-PD-1 therapy. In addition,
a DEG set comprising two DEGs (HLA-A and TNFSF18) may be applied to
predict the efficacy of anti-PD-1 therapy in patients with
metastatic NSCLC. Patients with an expression level of the gene set
>6.741 may benefit from anti-PD-1 monoclonal antibody
treatment.
The present study had certain limitations due to the
small sample size. In addition, there were no overlapping DEGs or
gene sets observed for both primary and metastatic NSCLC in the
present study. In two previous studies (61,62),
patients with primary and metastatic cancer also exhibited
inconsistent gene expression alterations; this problem should be
addressed in depth in future studies.
In summary, the present study conducted RNA IO panel
sequencing to identify potential biomarkers for predicting the
response to anti-PD-1 therapy in patients with primary or
metastatic NSCLC. The results of the present study demonstrated
that five immune-related DEGs and two DEG sets may be used,
respectively, as single and combination biomarkers for the
prediction of treatment efficacy. Although these results provided a
basis for identification of additional biomarkers to predict the
response to anti-PD-1 treatment, they need to be verified in
further studies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Datasets used during the present study were not
uploaded to public databases in order to protect patient privacy.
The datasets are available from the corresponding author on
reasonable request.
Authors' contributions
BC, MY and LL designed the study and performed the
experiments. YX, KL and JL collected the data. DR, JZ, LX and FX
analyzed the data. YX, DR and JZ drafted the manuscript. LL revised
the manuscript critically for important intellectual content. BC
and LL confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Cancer Hospital Affiliated to Xiangya Medical College, Central
South University (Changsha, China; approval no. 2019 fast review of
scientific research [08]). Informed consents were obtained from all
individual participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
Two of the authors (DR and JZ) are affiliated with
Genecast Biotechnology Co., Ltd., who provided the RNA
immune-oncology (IO) profiling panel for the present study. All
other authors declare that they have no competing interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Herbst RS, Morgensztern D and Boshoff C:
The biology and management of non-small cell lung cancer. Nature.
553:446–454. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pisters KM: The role of chemotherapy in
early-stage (stage I and II) resectable non-small cell lung cancer.
Semin Radiat Oncol. 10:274–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Losanno T, Rossi A, Maione P, Napolitano A
and Gridelli C: Anti-EGFR and antiangiogenic monoclonal antibodies
in metastatic non-small-cell lung cancer. Expert Opin Biol Ther.
16:747–758. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gridelli C, de Castro Carpeno J, Dingemans
AC, Griesinger F, Grossi F, Langer C, Ohe Y, Syrigos K, Thatcher N,
Das-Gupta A, et al: Safety and efficacy of bevacizumab plus
standard-of-care treatment beyond disease progression in patients
with advanced non-small cell lung cancer: The AvaALL randomized
clinical trial. JAMA Oncol. 4:e1834862018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Domagała-Kulawik J: Immune checkpoint
inhibitors in non-small cell lung cancer - towards daily practice.
Adv Respir Med. 86:142–148. 2018. View Article : Google Scholar
|
|
7
|
Horn L, Spigel DR, Vokes EE, Holgado E,
Ready N, Steins M, Poddubskaya E, Borghaei H, Felip E, Paz-Ares L,
et al: Nivolumab versus docetaxel in previously treated patients
with advanced non-small-cell lung cancer: Two-year outcomes from
two randomized, open-label, phase III trials (CheckMate 017 and
CheckMate 057). J Clin Oncol. 35:3924–3933. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vokes EE, Ready N, Felip E, Horn L, Burgio
MA, Antonia SJ, Arén Frontera O, Gettinger S, Holgado E, Spigel D,
et al: Nivolumab versus docetaxel in previously treated advanced
non-small-cell lung cancer (CheckMate 017 and CheckMate 057):
3-year update and outcomes in patients with liver metastases. Ann
Oncol. 29:959–965. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Herbst RS, Baas P, Kim DW, Felip E,
Pérez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, et al:
Pembrolizumab versus docetaxel for previously treated,
PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010):
A randomised controlled trial. Lancet. 387:1540–1550. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huang M, Pietanza MC, Samkari A,
Pellissier J, Burke T, Chandwani S, Kong F and Pickard AS: Q-TWiST
analysis to assess benefit-risk of pembrolizumab in patients with
PD-L1-positive advanced or metastatic non-small cell lung cancer.
Pharmacoeconomics. 37:105–116. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Peters S, Kerr KM and Stahel R: PD-1
blockade in advanced NSCLC: A focus on pembrolizumab. Cancer Treat
Rev. 62:39–49. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dermani FK, Samadi P, Rahmani G, Kohlan AK
and Najafi R: PD-1/PD-L1 immune checkpoint: Potential target for
cancer therapy. J Cell Physiol. 234:1313–1325. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Morgensztern D and Herbst RS: Nivolumab
and pembrolizumab for non-small cell lung cancer. Clin Cancer Res.
22:3713–3717. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shukuya T and Carbone DP: Predictive
markers for the efficacy of anti-PD-1/PD-L1 antibodies in lung
cancer. J Thorac Oncol. 11:976–988. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hellmann MD, Ciuleanu TE, Pluzanski A, Lee
JS, Otterson GA, Audigier-Valette C, Minenza E, Linardou H, Burgers
S, Salman P, et al: Nivolumab plus ipilimumab in lung cancer with a
high tumor mutational burden. N Engl J Med. 378:2093–2104. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rizvi NA, Hellmann MD, Snyder A, Kvistborg
P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, et al: Cancer
immunology. Mutational landscape determines sensitivity to PD-1
blockade in non-small cell lung cancer. Science. 348:124–128. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin Y, Dong H, Xia L, Yang Y, Zhu Y, Shen
Y, Zheng H, Yao C, Wang Y and Lu S: The diversity of gut microbiome
is associated with favorable responses to anti-programmed death-1
immunotherapy in chinese patients with NSCLC. J Thorac Oncol.
14:1378–1389. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Petrosyan F, Daw H, Haddad A, Spiro T and
Sood R: Gene expression profiling for early-stage NSCLC. Am J Clin
Oncol. 38:103–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li T, Kung HJ, Mack PC and Gandara DR:
Genotyping and genomic profiling of non-small-cell lung cancer:
Implications for current and future therapies. J Clin Oncol.
31:1039–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chim SS, Wong KK, Chung CY, Lam SK, Kwok
JS, Lai CY, Cheng YK, Hui AS, Meng M, Chan OK, et al: Systematic
selection of reference genes for the normalization of circulating
RNA transcripts in pregnant women based on RNA-Seq data. Int J Mol
Sci. 18:17092017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kamps R, Brandão RD, van den Bosch BJ,
Paulussen AD, Xanthoulea S, Blok MJ and Romano A: Next-generation
sequencing in oncology: Genetic diagnosis, risk prediction and
cancer classification. Int J Mol Sci. 18:3082017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu H, Chen X, Lin D, Zhang J, Li C, Zhang
D and Zhang X: Conformance assessment of PD-L1 expression between
primary tumour and nodal metastases in non-small-cell lung cancer.
OncoTargets Ther. 12:11541–11547. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Haragan A, Field JK, Davies MP, Escriu C,
Gruver A and Gosney JR: Heterogeneity of PD-L1 expression in
non-small cell lung cancer: Implications for specimen sampling in
predicting treatment response. Lung Cancer. 134:79–84. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Armato SG III and Nowak AK: Revised
modified response evaluation criteria in solid tumors for
assessment of response in malignant pleural mesothelioma (version
1.1). J Thorac Oncol. 13:1012–1021. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cottrell TR, Thompson ED, Forde PM, Stein
JE, Duffield AS, Anagnostou V, Rekhtman N, Anders RA, Cuda JD,
Illei PB, et al: Pathologic features of response to neoadjuvant
anti-PD-1 in resected non-small-cell lung carcinoma: A proposal for
quantitative immune-related pathologic response criteria (irPRC).
Ann Oncol. 29:1853–1860. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Paluch BE, Glenn ST, Conroy JM,
Papanicolau-Sengos A, Bshara W, Omilian AR, Brese E, Nesline M,
Burgher B, Andreas J, et al: Robust detection of immune transcripts
in FFPE samples using targeted RNA sequencing. Oncotarget.
8:3197–3205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
R Core Team: R: A language and environment
for statistical computing. R Foundation for Statistical Computing;
Vienna, Austria: https://www.R-project.org2018
|
|
32
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hänzelmann S, Castelo R and Guinney J:
GSVA: Gene set variation analysis for microarray and RNA-seq data.
BMC Bioinformatics. 14:72013. View Article : Google Scholar
|
|
34
|
Qu J, Mei Q, Liu L, Cheng T, Wang P, Chen
L and Zhou J: The progress and challenge of anti-PD-1/PD-L1
immunotherapy in treating non-small cell lung cancer. Ther Adv Med
Oncol. Feb 15–2021.(Epub ahead of print). doi:
10.1177/1758835921992968. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Valecha GK, Vennepureddy A, Ibrahim U,
Safa F, Samra B and Atallah JP: Anti-PD-1/PD-L1 antibodies in
non-small cell lung cancer: The era of immunotherapy. Expert Rev
Anticancer Ther. 17:47–59. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen R, Tao Y, Xu X, Shan L, Jiang H, Yin
Q, Pei L, Cai F, Ma L and Yu Y: The efficacy and safety of
nivolumab, pembrolizumab, and atezolizumab in treatment of advanced
non-small cell lung cancer. Discov Med. 26:155–166. 2018.PubMed/NCBI
|
|
37
|
Rizvi NA, Mazières J, Planchard D,
Stinchcombe TE, Dy GK, Antonia SJ, Horn L, Lena H, Minenza E,
Mennecier B, et al: Activity and safety of nivolumab, an anti-PD-1
immune checkpoint inhibitor, for patients with advanced, refractory
squamous non-small-cell lung cancer (CheckMate 063): A phase 2,
single-arm trial. Lancet Oncol. 16:257–265. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Rolfo C, Caglevic C, Santarpia M, Araujo
A, Giovannetti E, Gallardo CD, Pauwels P and Mahave M:
Immunotherapy in NSCLC: A promising and revolutionary weapon. Adv
Exp Med Biol. 995:97–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X
and Wu K: Biomarkers for predicting efficacy of PD-1/PD-L1
inhibitors. Mol Cancer. 17:1292018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen G, Huang AC, Zhang W, Zhang G, Wu M,
Xu W, Yu Z, Yang J, Wang B, Sun H, et al: Exosomal PD-L1
contributes to immunosuppression and is associated with anti-PD-1
response. Nature. 560:382–386. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen L and Han X: Anti-PD-1/PD-L1 therapy
of human cancer: Past, present, and future. J Clin Invest.
125:3384–3391. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yang Z, Li H, Wang Z, Yang Y, Niu J, Liu
Y, Sun Z and Yin C: Microarray expression profile of long
non-coding RNAs in human lung adenocarcinoma. Thorac Cancer.
9:1312–1322. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Holmdahl R, Sareila O, Olsson LM, Bäckdahl
L and Wing K: Ncf1 polymorphism reveals oxidative regulation of
autoimmune chronic inflammation. Immunol Rev. 269:228–247. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kelkka T, Pizzolla A, Laurila JP, Friman
T, Gustafsson R, Källberg E, Olsson O, Leanderson T, Rubin K, Salmi
M, et al: Mice lacking NCF1 exhibit reduced growth of implanted
melanoma and carcinoma tumors. PLoS One. 8:e841482013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu W, Kemp BL, Proctor ML, Gazdar AF,
Minna JD, Hong WK and Mao L: Expression of DMBT1, a candidate tumor
suppressor gene, is frequently lost in lung cancer. Cancer Res.
59:1846–1851. 1999.PubMed/NCBI
|
|
46
|
Mollenhauer J, Helmke B, Müller H,
Kollender G, Lyer S, Diedrichs L, Holmskov U, Ligtenberg T,
Herbertz S, Krebs I, et al: Sequential changes of the DMBT1
expression and location in normal lung tissue and lung carcinomas.
Genes Chromosomes Cancer. 35:164–169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Raez LE, Cassileth PA, Schlesselman JJ,
Sridhar K, Padmanabhan S, Fisher EZ, Baldie PA and Podack ER:
Allogeneic vaccination with a B7.1 HLA-A gene-modified
adenocarcinoma cell line in patients with advanced non-small-cell
lung cancer. J Clin Oncol. 22:2800–2807. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Leclerc M, Mezquita L, Guillebot De
Nerville G, Tihy I, Malenica I, Chouaib S and Mami-Chouaib F:
Recent advances in lung cancer immunotherapy: Input of T-cell
epitopes associated with impaired peptide processing. Front
Immunol. 10:15052019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xiao-Peng H, Fu-Jie S, Xiang-Yan L, Wang
Z, Li XX, Liu FY, Chen G, Jiang WP, et al: The relationship between
KRAS gene mutations and HLA class I antigen downregulation in the
metastasis of non-small cell lung cancer. J Int Med Res.
41:1473–1483. 2013. View Article : Google Scholar
|
|
50
|
Nocentini G, Ronchetti S, Petrillo MG and
Riccardi C: Pharmacological modulation of GITRL/GITR system:
Therapeutic perspectives. Br J Pharmacol. 165:2089–2099. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tian J, Ma J, Ma K, Ma B, Tang X, Baidoo
SE, Tong J, Yan J, Lu L, Xu H, et al: Up-regulation of GITRL on
dendritic cells by WGP improves anti-tumor immunity in murine Lewis
lung carcinoma. PLoS One. 7:e469362012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ma J, Wang S, Ma B, Mao C, Tong J, Yang M,
Wu C, Jiao Z, Lu L and Xu H: Dendritic cells engineered to express
GITRL enhance therapeutic immunity in murine Lewis lung carcinoma.
Cancer Lett. 301:142–150. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Chen R, Khatri P, Mazur PK, Polin M, Zheng
Y, Vaka D, Hoang CD, Shrager J, Xu Y, Vicent S, et al: A
meta-analysis of lung cancer gene expression identifies PTK7 as a
survival gene in lung adenocarcinoma. Cancer Res. 74:2892–2902.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Damelin M, Bankovich A, Bernstein J, Lucas
J, Chen L, Williams S, Park A, Aguilar J, Ernstoff E, Charati M, et
al: A PTK7-targeted antibody-drug conjugate reduces
tumor-initiating cells and induces sustained tumor regressions. Sci
Transl Med. 9:eaag26112017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kim JH, Kwon J, Lee HW, Kang MC, Yoon HJ,
Lee ST and Park JH: Protein tyrosine kinase 7 plays a tumor
suppressor role by inhibiting ERK and AKT phosphorylation in lung
cancer. Oncol Rep. 31:2708–2712. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hughey JJ: Machine learning identifies a
compact gene set for monitoring the circadian clock in human blood.
Genome Med. 9:192017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Hsu YC, Chiu YC, Chen Y, Hsiao TH and
Chuang EY: A simple gene set-based method accurately predicts the
synergy of drug pairs. BMC Syst Biol. 10 (Suppl 3):662016.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li N, Hou JL, Shi ZZ, Li XG, Li N, Sun YC,
Xu X, Cai Y, Zhang X, Zhang KT, et al: Copy number changes of
4-gene set may predict early relapse in advanced epithelial ovarian
cancer after initial platinum-paclitaxel chemotherapy. Am J Cancer
Res. 4:285–292. 2014.PubMed/NCBI
|
|
59
|
Li JN, Zhong R and Zhou XH: Prediction of
bone metastasis in breast cancer based on minimal driver gene set
in gene dependency network. Genes (Basel). 10:4662019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Shen S, Wang G, Zhang R, Zhao Y, Yu H, Wei
Y and Chen F: Development and validation of an immune gene-set
based prognostic signature in ovarian cancer. EBioMedicine.
40:318–326. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Italiano A, Vandenbos FB, Otto J, Mouroux
J, Fontaine D, Marcy PY, Cardot N, Thyss A and Pedeutour F:
Comparison of the epidermal growth factor receptor gene and protein
in primary non-small-cell-lung cancer and metastatic sites:
Implications for treatment with EGFR-inhibitors. Ann Oncol.
17:981–985. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Reinholz MM, Bruzek AK, Visscher DW,
Lingle WL, Schroeder MJ, Perez EA and Jenkins RB: Breast cancer and
aneusomy 17: Implications for carcinogenesis and therapeutic
response. Lancet Oncol. 10:267–277. 2009. View Article : Google Scholar : PubMed/NCBI
|