Introduction
Immunotherapy has become the mainstay treatment for
cancer. One of the most successful, and still rapidly progressing
areas in immunotherapy is the blockade of inhibitory immune
checkpoint molecules expressed on T cells, such as programmed cell
death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated
protein 4 (CTLA-4). T cells are the primary regulators of immune
cells that participate in immune surveillance and cancer immunity.
Due to the potency of T cells and the cascade amplification effect
in immune responses initiated by T cells, the activation state of T
cells has to be intricately regulated. Inhibitory immune
checkpoints, such as PD-1 and CTLA-4, negatively regulate the
function and dampen the antitumor immunity of activated T cells.
Therefore, unleashing a pre-existing immune response against tumors
through neutralization antibodies targeting inhibitory immune
checkpoints can enhance the antitumor activity of T cells.
Currently, anti-PD-1 antibodies have been approved by the U.S. Food
and Drugs Administration and are used in the treatment of malignant
melanoma (1), lung cancer (2), renal cancer (3) and lymphoma (4).
T cell immunoglobulin and mucin domain 3 (TIM3)
represents a new target beyond the first generation of immune
checkpoints, CTLA-4 and PD-1. TIM3 is one of the major negative
regulators on the surface of activated T cells. The TIM family has
eight members in mice, but only three members in humans (TIM1, TIM3
and TIM4) located on chromosome 5 (5). TIM3 was identified as a molecule
expressed by interferon γ-producing CD4+ and
CD8+ T cells (6) and
numerous other cell types, including regulatory T cells, myeloid
cells (7), natural killer cells
(8) and mast cells (9). Previous data have revealed that TIM3
served a critical role in regulating the activities of different
innate immune cells, and it was associated with autoimmune
diseases, chronic viral infections and cancer (10). Several ligands of TIM3 have been
identified, including galectin-9, phosphatidylserine (PtdSer),
carcinoembryonic antigen-related cell adhesion molecule 1
(CEACAM1), and high-mobility group box 1 protein (11,12).
TIM3 and its ligand galectin-9 may constitute one of the negative
regulatory pathways that can inhibit T cell immunity (6). Therefore, the blockade of TIM3 is
currently being investigated in clinical trials for the treatment
of cancer (13).
Single-domain antibodies (sdAbs) are antibody
fragments, typically derived from the heavy chain variable fragment
(VH) domain, with a molecular weight of ~12 kD. The best studied
sdAbs include camelid VHH, cartilaginous fish
VNAR and human VH. Due to the small size, excellent
thermal stability, reversible refolding capacity and other
outstanding properties, sdAbs have demonstrated significant
potential in medical applications or as research tools (14,15).
Numerous sdAbs are being actively evaluated in clinical trials, and
one sdAb drug, caplacizumab (a humanized camelid VHH),
has been recently approved for the treatment of acquired thrombotic
thrombocytopenic purpura and thrombosis (16,17).
Rabbits have been identified as a convenient model to generate VH
domain antibodies via immunization and phage display (18). In the present study, a similar
strategy was adopted to generate TIM3 inhibitory sdAbs that could
be combined with chimeric antigen receptor (CAR) T cell therapy to
boost CAR T cell efficacy in cancer treatment.
Materials and methods
Cell lines
The human mesothelioma cell line H226
(ATCC® CRL-5826™; Genetimes ExCell Technology, Inc.) was
maintained as an adherent monolayer culture in RPMI-1640 medium
(Invitrogen; Thermo Fisher Scientific, Inc.) supplemented with 10%
FBS (HyClone; Cytiva), 2 mM L-glutamine and 100 IU/ml
penicillin-streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). H9 (Feng Lab, Huazhong Agricultural University, Wuhan,
China) cells were also used, which were an engineered human
epithelial carcinoma A431 (ATCC® CRL-1555; Genetimes
ExCell Technology, Inc.) cell line (mesothelin negative) that
overexpressed cell surface mesothelin. A431 and all A431-derived
cells were maintained in DMEM (Invitrogen; Thermo Fisher
Scientific, Inc.), and incubated in 5% CO2 at 37°C.
These cells were harvested, and the media were refreshed twice a
week. Human 293F cells (cat. no. 11625019, Invitrogen; Thermo
Fisher Scientific, Inc.) were maintained in SMM 293-TII Expression
medium (cat. no. M293TII; SinoBiological, Inc.) in 5%
CO2 at 37°C. Cells were passaged every 4 days at the
seeding density of 3×105 cells/ml.
Preparation of recombinant
proteins
The extracellular domain (ECD) of TIM3 (NP_033665.1,
a.a. 22–202) was fused with human IgG1-Fc and cloned into mammalian
expression vector pFUSE-hIgG1-Fc2 (pfuse-hg1fc2; InvivoGen) to
generate TIM3-hFc. Galectin-9 (NP_033665.1, a.a. 2-355) was fused
with rabbit IgG-Fc and cloned into mammalian expression vector
pFUSE-rIgG-Fc2 (pfuse-rfc2; InvivoGen) to generate Gal9-rFc. The
mesothelin-coding sequence (NP_005814.2, a.a. 296-580) was fused
with either human IgG1-Fc or rabbit IgG-Fc and cloned into the same
expression vector. The expression vectors were then introduced into
293F cells using polyethylenimine (PEI) (cat. no. 23966-1,
Polysciences, Inc.) transfection. The PEI was dissolved at 1 µg/µl
in endotoxin-free deionized water that had been heated to ~65°C.
For transfection, 300 µg expression plasmid and 750 µl PEI was
pre-diluted in 5 ml Opti-MEM medium (Invitrogen; Thermo Fisher
Scientific, Inc.) separately and then mixed for 20 min at room
temperature before being added to 3×108 293F cells that
were passaged in 100 ml of SMM 293-TII Expression medium. Following
24 h of transfection, 200 ml fresh cell culture medium was added
and cells were cultured at 37°C and 5% CO2, with gently
shaking at 110–175 rotation per minute. The cell density was
maintained at 6×106 cells/ml by daily supplementation
with fresh medium. The cell culture medium was collected 1 week
after transfection, centrifuged at 4°C and 10,000 × g for 1 h and
then filtered through 0.2-µm Nalgene Bottle Top filters (cat. no.
291-4520; Thermo Fisher Scientific, Inc.) and subsequently loaded
onto the Protein A affinity chromatography column (cat. no.
C600952-0501; Sangon Biotech Co., Ltd.). After being washed with
PBS buffer, the recombinant protein was eluted with pH 2.0 buffer
and immediately neutralized with pH 8.0 Tris-HCl buffer. Protein
concentration was measured by bicinchoninic acid (BCA) method. The
purity of the preparations was analyzed by 10% SDS-PAGE, and 5 µg
protein was loaded per lane. The protein band was visualized with
Coomassie Blue staining solution at room temperature for 4 h.
Construction of phage-displayed sdAb
library
In total, 2 rabbits (3–4 months old, 1.5–2.0 kg,
provided by the Animal Facility of Huazhong Agricultural
University, Wuhan, China) were immunized with the recombinant
TIM3-hFc. Each rabbit was primed with a subcutaneous injection of
100 µg protein mixed with 100 µl Complete Freund's adjuvant
(Sigma-Aldrich; Merck KGaA). Then, 14 days later, rabbits were
boosted with half the amount of the protein mixed with Incomplete
Freund's adjuvant. Seven days later after boosting, rabbits were
euthanized by ear vein injection of sodium pentobarbital at 100
mg/kg according to the previous protocol (19), and the immunized spleens were
harvested. The total splenic RNA was isolated using
TRIzol® (cat. no. 15596018; Invitrogen; Thermo Fisher
Scientific, Inc.) and reverse transcribed into cDNA at 37°C for 15
min using a PrimeScript™ kit (Takara Bio, Inc.). PCR amplification
of the VH domains was performed as previously described (18). The VH fragments were digested with
the restriction enzyme SfiI and cloned into the phage
display vector pComb3× (cat. no. 3498425, Biovector NTCC, Inc.).
Then, 10 µg ligation product was introduced into 0.5 ml competent
E.coli TG1 cells (cat. no. DE1055; Shanghai Weidi Biotechnology
Co., Ltd.) by electroporation using the following parameters: 25
µF, 200 Ω and 1.8 kV. The resultant library contained
1×108 original transformants.
Isolation of TIM3 sdAbs
Briefly, TIM3-hFc protein was immobilized on a
96-well Nunc MaxiSorp™ plate (Thermo Fisher Scientific, Inc.) at a
concentration of 100 µg/ml in PBS for 30 min. After being washed
with PBS-0.1% Tween-20 (PBST) buffer, 50 µl phage solution was
added to the plate and incubated for 1 h at room temperature. After
being washed with PBST four times, the bound phage was eluted with
pH 2.0 buffer (0.2 M glycine-hydrochloric acid) and re-amplified in
TG1 cells for the next round of panning. After three rounds of
panning, single colonies were randomly selected and monoclonal
phage enzyme-linked immunosorbent assays (ELISAs) were performed to
identify the TIM3-specific binders. The detailed phage panning and
phage ELISA methods have been previously described (20).
Production of TIM3 sdAb
Rabbit VH domain antibodies were fused with His-FLAG
tag, inserted into the expression vector pFUSE-hIgG1-Fc2
(pfuse-hg1fc2; InvivoGen), and expressed as a VH-His-FLAG-hFc
format in 293F cells according to the aforementioned method. The 6×
His tag was used for Nickel column affinity purification and the
hFc tag was used in the cell binding assay by flow cytometry that
used goat anti-human polyclonal antibody (cat. no. 109-135-170,
Jackson ImmunoResearch Laboratories, Inc.) as secondary
antibody.
ELISAs
A 96-well MaxiSorp plate was coated with 5 µg/ml
TIM3-hFc protein and incubated at 37°C for 30 min. After being
washed twice with PBST, the plate was blocked for 30 min with
blocking buffer (2% BSA in PBS buffer). After removing the blocking
buffer, biotinylated TIM3 sdAbs were incubated with the plate at
variable concentrations starting from 200 µg/ml and followed by 1:2
serial dilutions. TIM3 sdAbs binding was detected using
HRP-conjugated streptavidin (Invitrogen; Thermo Fisher Scientific,
Inc.; 1:4,000).
Neutralization assay of TIM3
sdAbs
The neutralization assay was performed using a
competitive ELISA to determine the ability of the TIM3 sdAbs to
block TIM3 from binding to its ligand galectin-9. Briefly, a
96-well MaxiSorp plate was coated with 5 µg/ml TIM3-hFc at 37°C for
30 min. After blocking, variable amounts of TIM3 sdAbs were added
to the plate and incubated at 37°C for 30 min to allow the sdAbs to
bind to TIM3-hFc. Following the incubation, 5 µg/ml biotinylated
galectin-9-rFc was directly added to the wells without removing the
TIM3 sdAbs solution and galetin-9-rFc binding was performed at 37°C
for 30 min. After the plate was washed twice with PBST,
galectin-9-rFc binding was detected by HRP-conjugated streptavidin
(1:4,000; cat. no. D111054-0001, Sangon Biotech Co. Ltd.).
Subsequently, the plate was washed four times and the binding was
visualized using the HRP substrate 3,3′,5,5′-tetramethylbenzidine
(Beyotime Institute of Biotechnology), and quantified using a
spectrometer at 450 nm.
Packaging of lentiviral virus
Lentivirus was generated from 293T cells
(ATCC® CRL-3216; Genetimes ExCell Technology, Inc.,
China) that were co-transfected with a 3rd generation lentiviral
expression plasmid pLenti-LG or pLenti-MesoCAR-IRES-mScarletI (both
were constructed in Feng Lab, Huazhong Agricultural University,
Wuhan, China) carrying the gene of interest, together with
packaging plasmid psPAX2 and enveloping plasmid pMD2.G. A total of
10 µg of the plasmids were mixed at 1:3:1 ratio in 0.5 ml Opti-MEM
medium, associated with 25 µg PEI that was diluted in 0.5 ml
Opti-MEM medium. The complex of DNA-PEI was formed by incubating at
room temperature for 20 min, and then transferred to
1×107 293T cells seeded on T-75 flask. The lentivirus
was collected at 72 h post transfection, then filtered with 0.45-µm
filter unit (EMD Millipore), immediately used or stored at −80°C
before the transduction experiment. For the transductions, the
lentiviral particles were directly added to the target cell culture
at MOI of 10, followed by 24 h of co-incubation. After changing the
medium, transduced cells were cultured for 3 days before subsequent
experimentation.
Production of CAR T cells
The CAR expression cassette was based on the second
generation format, and was constructed by de novo gene
synthesis, which consisted of the CD8a leader sequence
(NP_001139345, a.a. 1–21), followed by anti-mesothelin scFv YP158
(21), a CD8α hinge (NP_001139345,
a.a. 138–182), a CD8α transmembrane region (NP_001139345, a.a.
183–206), a 4-1BB intracellular domain (NP_001552.2, a.a. 214–255),
a CD3ζ intracellular domain (NP_932170, a.a. 52–164), an internal
ribosome entry site (from encephalomyocarditis virus) and a red
fluorescent protein mScarlet-I. The amino acid sequence of the
CAR-YP158 cassette was as follows: MAL PVT ALL LPL ALL LHA ARP
DIQSLEESGGDLVKPGASLTLTCTASGFSFSGDYYMCWVRQAPGKGLEWIACIGGGSNTATYYATWAKGRFTISKTSSTTVTLQMTSLTAADTATYFCARDLGFVDYALELWGPGTLVTVSSGGGGSGGGGSGGGGSDVVMTQTPASVEVAVGGTVTIKCQASENMYNSLAWYQQKPGQPPKLLIYRASTLESGVPSRFKGSGSGTEYTLTISDLECADAATYYCQCTFYSHNNNYGGAFGGGTEVVVKSGT
TTP APR PPT PAP TIA SQP LSL RPE ACR PAA GGA VHTRGL DFA CDI YIW APL
AGT CGV LLL SLV ITL YCK RGRKKL LYI FKQ PFM RPV QTT QEE DGC SCR FPE
EEE GGCELR VKF SRS ADA PAY QQG QNQ LYN ELN LGR REE YDV LDK RRG RDP
EMG GKP QRR KNP QEGL YNE LQK DKM AEA YSE IGM KG ERR RGK GHD GLY QGL
STA TKD TYD ALH MQA LPP R (underlined sections correspond to the
cloning enzyme sites EcoRV and BspEI).
The whole blood samples were collected with informed
consent from healthy donors using a protocol approved by the Wuhan
Blood Center. Ethics of using human blood samples was reviewed and
approved by Biomedical Research Ethical Committee, Huazhong
Agriculture University and ethics committee-approved oral consent
was obtained from donors. Human peripheral blood mononuclear cells
(PBMCs) were isolated by Ficoll gradient centrifugation (Stemcell
Technologies, Inc.). The PBMCs were activated with Dynabeads™
CD3/CD28 Human T-Activator (cat. no. 11131D; Thermo Fisher
Scientific, Inc.) and cultured in RPMI-1640 medium supplemented
with 200 IU/ml human recombinant interleukin (IL)-2 (cat. no.
GMP-TL104, T&L Biological Technology) for 3 days, according to
the manufacturer's protocol. The activated T cells were
subsequently transduced with the lentivirus carrying the CAR
cassette by co-incubation for 24 h. After transduction, fresh
medium containing 200 IU/ml IL-2 was supplemented to maintain the
growth of CAR T cells for an additional 3 days before the
functional studies. The expression of CAR genes was indicated by
the expression of mScarlet-I protein, which can be detected by flow
cytometry.
In vitro cytotoxicity assay of CAR T
cells
The cancer cell lines, A431, H9 and H226, were
stably transduced with a 3rd generation lentiviral vector pLenti-LG
(Feng Lab, Huazhong Agricultural University, Wuhan, China; details
mentioned previously) to express firefly luciferase (ffLuc2)-green
fluorescent protein (GFP) fusion protein. The ffLuc2-GFP gene was
synthesized by GenScript and the pLenti-LG plasmid was constructed
by the Feng Lab, Huazhong Agricultural University, Wuhan, China.
Subsequently, 200 µl cancer cells were plated into a 96-well plate
(1×104 cells/well), followed by the addition of CAR T
cells at a 5:1 ratio (CAR T: Cancer cells). Following an 18 h
co-incubation, the surviving cells were collected by centrifuging
the plate at 400 × g at 4°C for 10 min. After removing the medium,
the cell pellet was resuspended in 100 µl PBS buffer and lysed by
two rounds of freezing-thawing. The activity of the released
luciferase was measured with 10 µl cell lysate mixed with 100 µl
substrate D-Luciferin buffer (Pierce Firefly Luc One-Step Glow
Assay Kit, cat. no. 16196, Thermo Fisher Scientific Inc.) according
to the manufacturer's instruction. The enzymatic activity was used
to quantify the cell viability. The interval between CAR T cell
transduction and activity measurement was 3 days. The viability
percentage (%) was, therefore, equal to the experimental
signal/maximal signal.
Flow cytometry
Unstimulated PBMCs and CAR T cells were co-incubated
with 10 µg/ml TIM3 sdAbs that had the fused hFc tag for 1 h on ice.
Cell binding of TIM3 sdAbs was detected with APC-conjugated goat
anti-human polyclonal antibody (1:1,000; cat. no. 109-135-170,
Jackson ImmunoResearch Laboratories, Inc.). The fluorescence
intensity was measured on a FACS Calibur flow cytometer (BD
Biosciences) and used to analyze sdAbs binding. The expression
level of CAR in CAR T cells was quantified by measuring the
fluorescence intensity of the co-expressed mScarlet-I.
In vivo animal studies
The therapeutic potential of sdAb TIM3-R53 was
tested using H9 ×enograft model mice. Four to six-week old female
athymic nude mice (average ~22 g, provided by Animal Facility of
Huazhong Agricultural University, Wuhan, China) were assigned to 4
groups, 5 mice per group. Mice were housed at 25°C, 45% humidity,
12 h light/dark cycle and had free access to food and water.
Briefly, nude mice were injected subcutaneously on the right flank
with 2×106 H9 cells. After the tumor had formed and
reached a size of 100 mm3, treatment was started by
intraperitoneal injection of 5×106 mesothelin-targeted
CAR T cells or control PBMCs. TIM3 sdAb was intravenously delivered
every two days at a concentration of 10 mg/kg. Tumor growth was
measured every two days with a caliper; the tumor volume
(mm3) was calculated using the following formula:
(a × b2) ×0.5, where a is the tumor
length and b is the tumor width in mm. Five mice per group
were assigned and mice were considered at the end point when tumor
size exceeded 1,000 mm3. Mice were euthanized with
inhaled CO2 when the tumors grew to >1,000
mm3. The flow rate for CO2 was 10% as the
volume displacement per min, and death was verified by lack of
cardiac pulse and fixed and dilated pupils.
Statistical analysis
For the quantitative experiments, ≥3 experimental
repeats were performed. All statistical analyses were conducted
using GraphPad Prism 5 software (GraphPad Software, Inc.). The data
are expressed as the mean ± SEM. Comparisons between two groups
were performed using an unpaired Student's t test (two-tailed).
Comparisons among ≥3 groups were performed using a one-way ANOVA
followed by Tukey's multiple comparison test. P<0.05 was
considered to indicate a statically significant difference.
Results
Preparation of TIM3-hFc and Gal9-rFc
proteins
The ECD of TIM3 was fused with human IgG1 Fc
(TIM3-hFc) and galectin-9 was fused with rabbit Fc (Gal9-rFc).
Recombinant mesothelin-hFc and mesothelin-rFc were used as the Fc
isotype control. Mesothelin is a
glycosylphosphatidylinositol-linked cell surface protein that has
been discovered to be highly expressed in numerous types of
malignancy, such as mesothelioma, ovarian cancer, pancreatic cancer
and non-small cell lung cancer (22). All the recombinant proteins were
expressed in 293F cells and purified via protein A chromatography.
The yield of the recombinant proteins was 1–3 mg/l of the cell
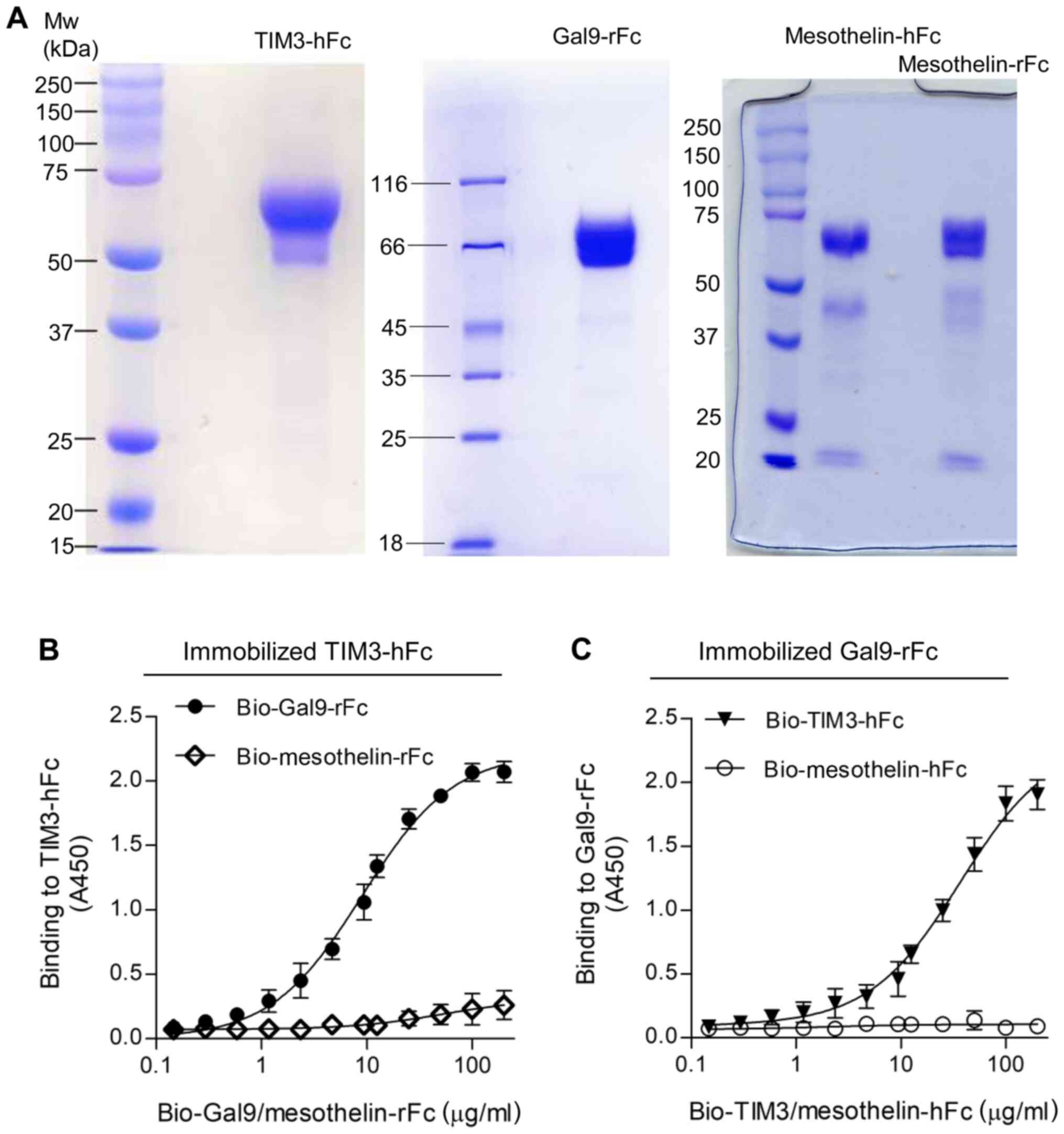
culture medium, depending on the protein. As shown in Fig. 1, SDS-PAGE analysis revealed that the
purity of the recombinant proteins was estimated to be >90%. The
reduced monomers of both TIM3-hFc and Gal9-rFc migrated at ~60 kD,
and mesothelin Fc fusions migrated at ~70 kD (Fig. 1A). The binding of Gal9-rFc to the
immobilized TIM3-hFc was confirmed by ELISA, and the half maximal
effective concentration (EC50) value was calculated to
be 138 nM (Fig. 1B). A reverse
binding assay was also performed, which tested the binding of
TIM3-hFc to immobilized Gal9-rFc (Fig.
1C). In both cases, the rFc and hFc isotype control did not
reveal any non-specific binding to TIM3 or galectin-9.
Generation of anti-TIM3 sdAbs
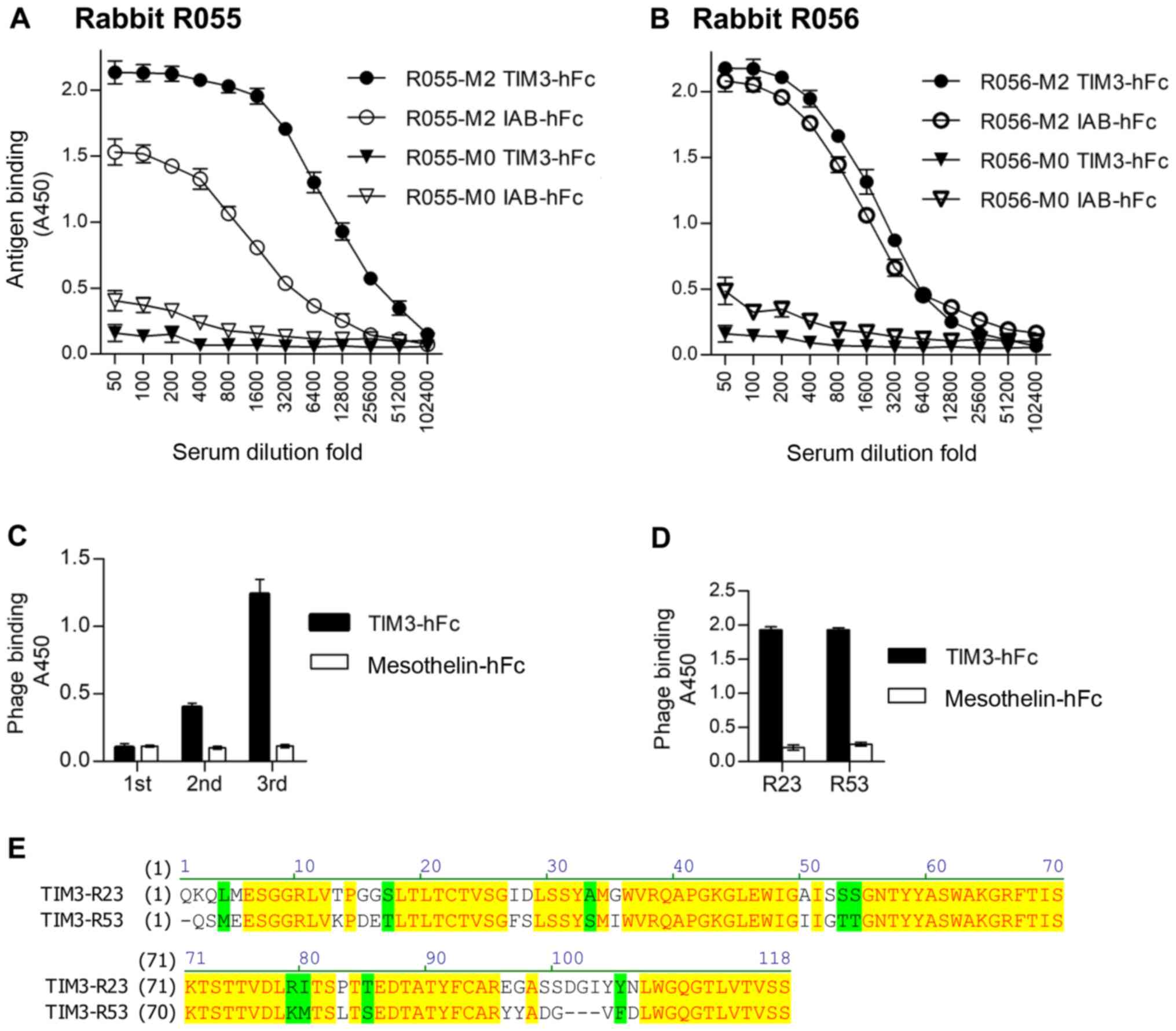
After immunization of the two rabbits with TIM3-hFc,
the titer and specificity were analyzed using an ELISA, with
IAB-hFc as the hFc control (Fig. 2A and
B). IAB was a fragment of mesothelin (Q13421, amino acid
296–359) (22). The serum of the
immunized rabbit R055 (R055-M2) showed increased binding to
TIM3-hFc compared with the IAB-hFc control. Therefore, this rabbit
was chosen to make the phage-displayed sdAb library. After three
rounds of panning, the results of polyclonal phage ELISAs from each
round of panning revealed the gradual increased binding to TIM3-hFc
(Fig. 2C). A total of 96 colonies
from the third panning were randomly picked, and a monoclonal phage
ELISA was performed to determine the TIM3-specific binders.
Sequencing of the resultant 89 ELISA-positive clones identified
that only two representative sequences, named TIM3-R23 and
TIM3-R53, were enriched. The monoclonal phage ELISA data and the
amino acid sequences of these two clones are presented in Fig. 2D and E.
Binding and neutralization activity of
TIM3 sdAbs
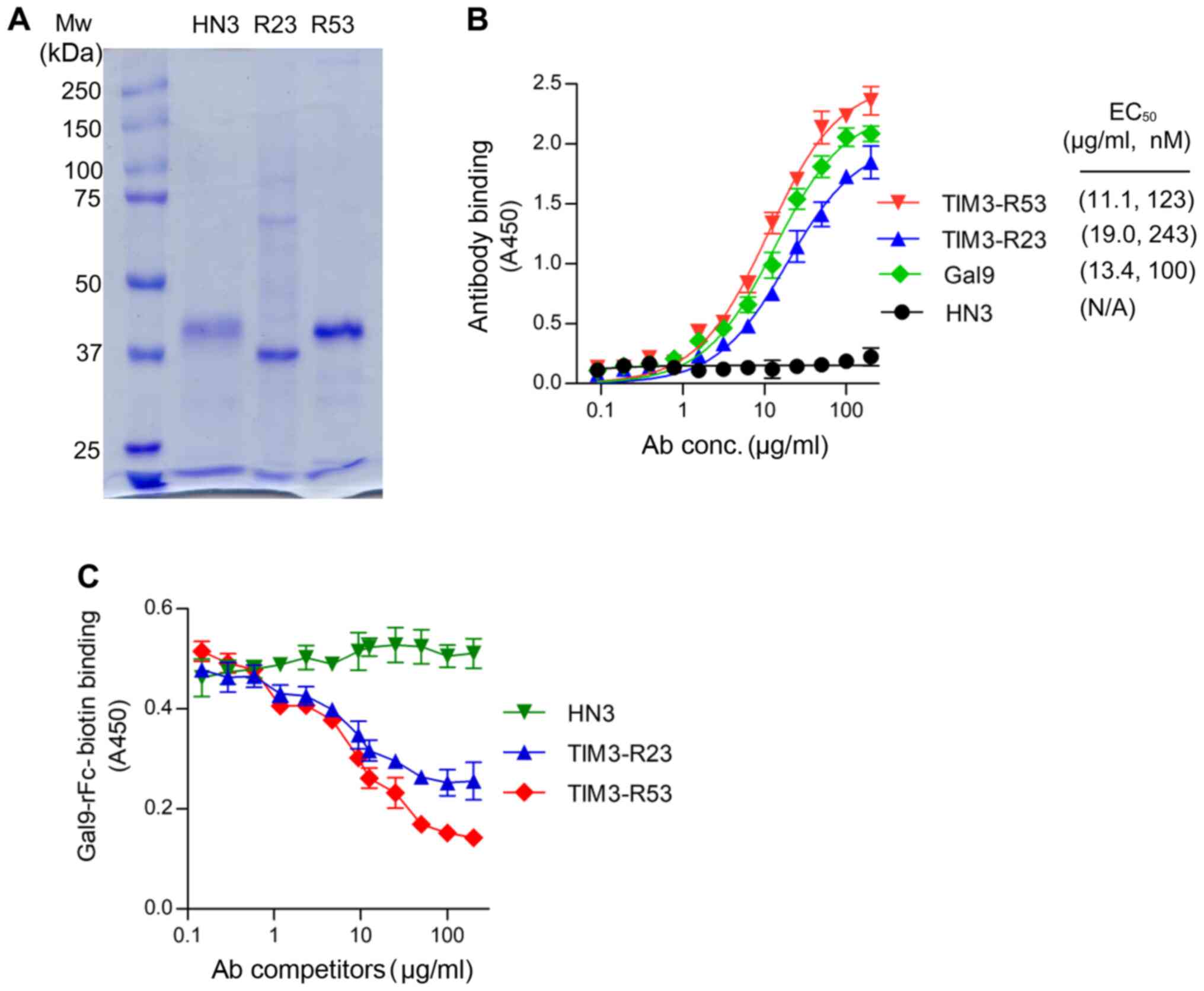
The sdAbs TIM3-R23 and TIM3-R53 were expressed in
293F cells as a VH-His-FLAG-hFc format and purified using a HiTrap
Nickel column. SDS-PAGE analysis revealed that the purified sdAbs
migrated at ~40 kD as a monomer under reducing conditions (Fig. 3A). The control sdAb HN3 is a VH
domain antibody that targets glypican-3 (20). The binding affinity of the sdAbs to
TIM3 was analyzed using ELISAs and the EC50 values for
R53 and R23 were calculated to be 123 and 243 nM, respectively
(Fig. 3B), which were similar to
that of the galectin-9 binding (100 nM). The HN3 sdAb control did
not reveal any background binding in the assay.
Subsequently, the neutralization activity of TIM3
sdAbs was analyzed by measuring its ability to inhibit TIM3 binding
to galectin-9 (Fig. 3C). The results
of the competitive ELISA illustrated that both sdAbs were able to
inhibit TIM3-galectin-9 binding (Fig.
3C); TIM3-R53 exhibited stronger blocking activity compared
with TIM3-R23, and therefore the ability of this sdAb to boost the
function of CAR T cells was further investigated in
vivo.
Cellular binding activity of TIM3
sdAbs
Cell surface TIM3 binding was analyzed using
mesothelin-targeted CAR T cells. Unstimulated PBMCs were used as
the TIM3-negative counterpart. CAR T cells were generated by
lentiviral transduction of activated PBMCs, which produced an ~15%
transduction efficiency (Fig. 4A).
Flow cytometric analysis demonstrated that the two TIM3 sdAbs
selectively bound to CAR T cells, but not to PBMCs (Fig. 4B and C). To further confirm the cell
binding and TIM3 expression kinetics on activated CAR T cells, the
CAR T cells were cultured for different days and sdAbs binding was
analyzed (Fig. 4D). The results
clearly demonstrated that the percentage of TIM3-positive CAR T
cells was increased as the culturing time was prolonged from day 5
to 9.
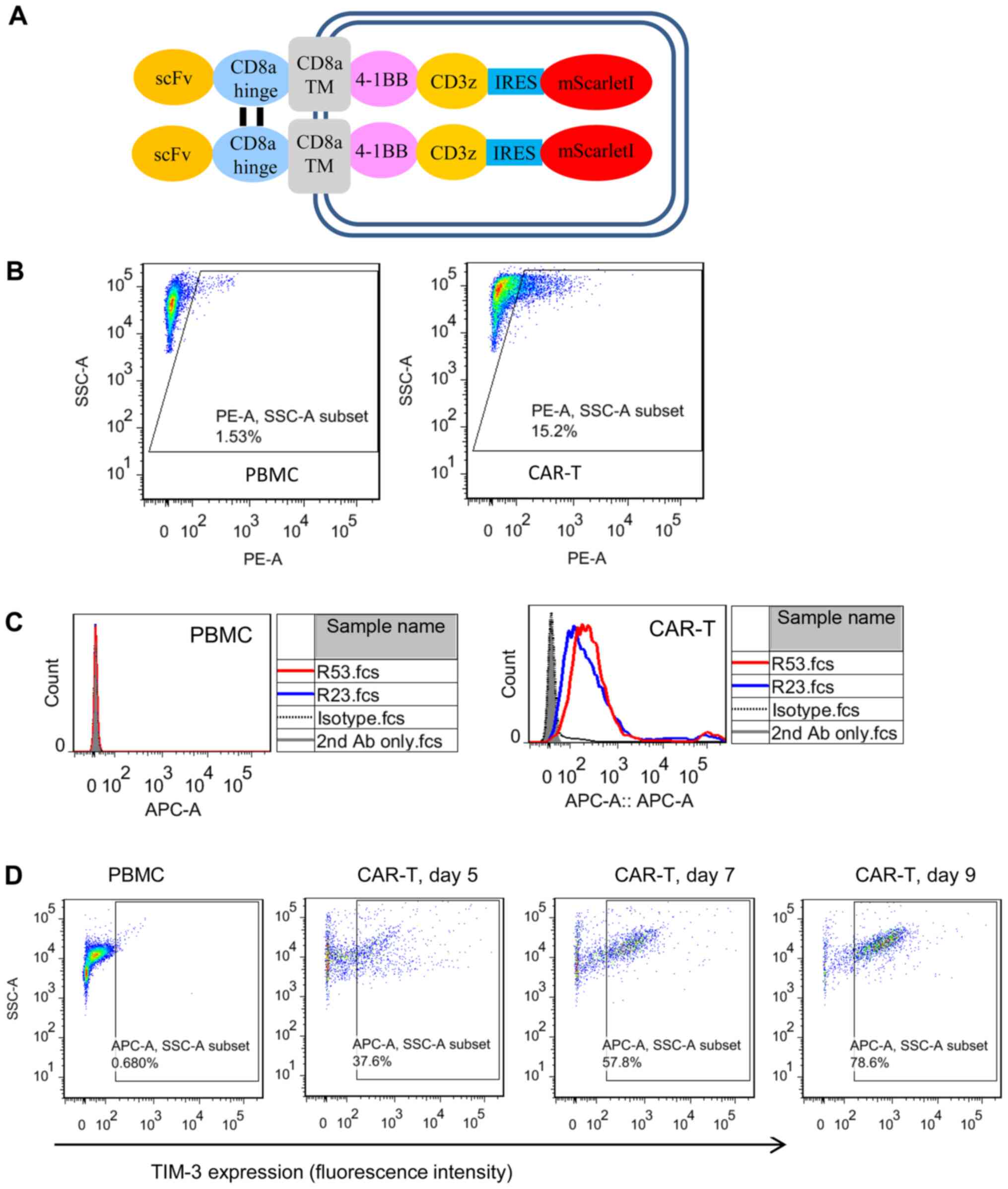 | Figure 4.Cell binding properties of TIM3
sdAbs. (A) Schematic illustration of the mesothelin-targeted CAR
structure. Anti-mesothelin scFv YP158 (21) was fused with the following domains:
CD8α hinge, CD8α transmembrane region, 4-1BB intracellular domain,
CD3ζ intracellular domain, internal ribosome entry site and a red
fluorescent protein mScarlet-I. (B) Transduction efficiency
analysis of CAR T cells. Following the transduction of PBMCs with a
CAR-bearing lentiviral vector, CAR expression was indicated by the
fluorescence of mScarletI. (C) Flow cytometric analysis of TIM3
sdAbs binding to the unstimulated PBMCs and CAR T cells. Briefly,
10 µg sdAbs were co-incubated with 1×106 cells. Antibody
binding was detected by APC-conjugated goat-anti-human IgG. HN3, a
human sdAb antibody that targeted the glypican-3, was used as the
isotype control. Shaded area, secondary antibody staining; dashed
lines, isotype control; red and blue solid line, sdAbs R53 and R23
staining, respectively. (D) Expression of TIM3 on CAR T cells
cultured for different lengths of time. TIM3, T cell immunoglobulin
and mucin domain 3; sdAbs, single-domain antibodies; CAR, chimeric
antigen receptor; PBMCs, peripheral blood mononuclear cells. |
In vivo antitumor activity of CAR T
cells is boosted by TIM3-R53
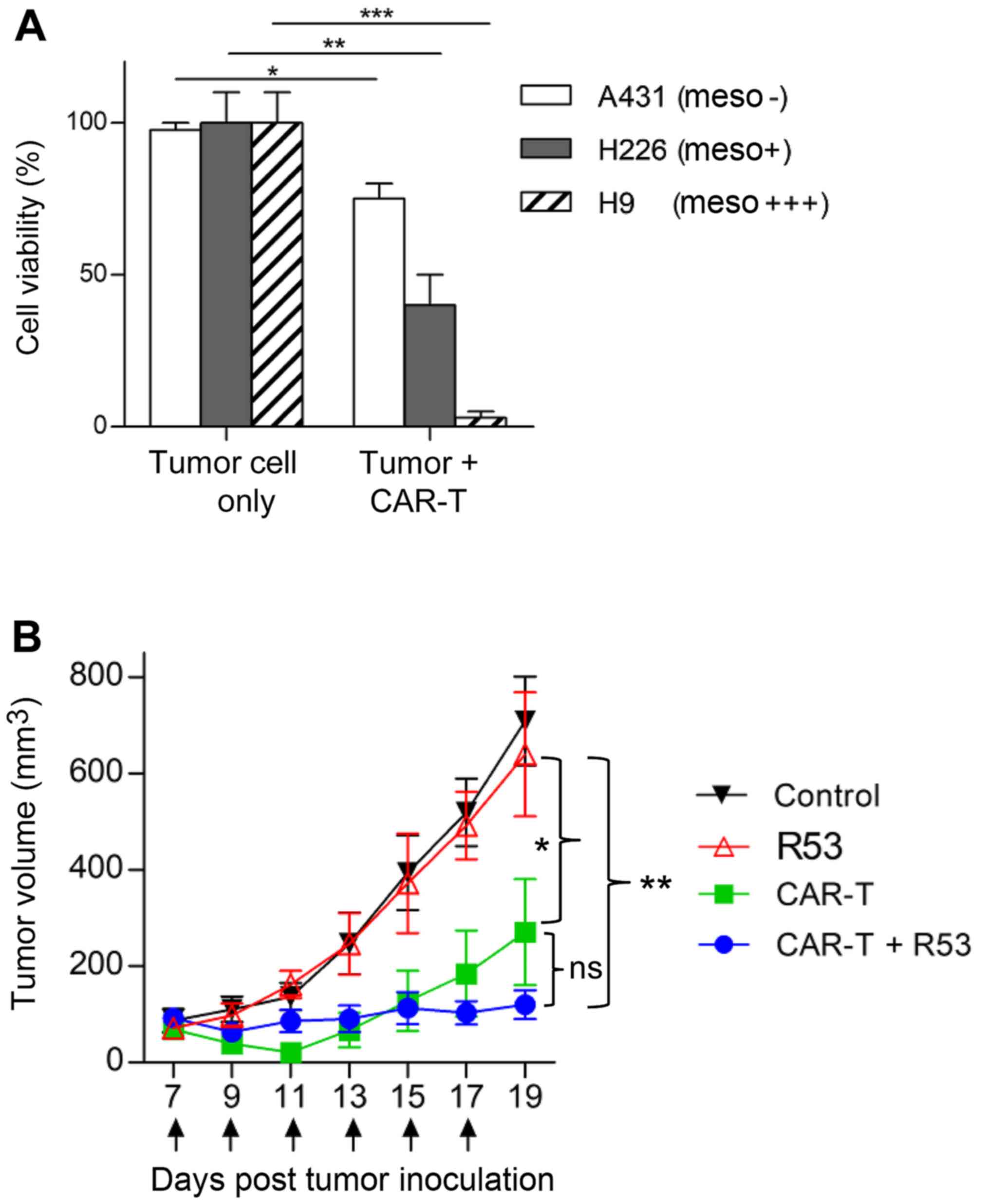
As TIM3-R53 revealed stronger in vitro
neutralization activity, it was chosen for the in vivo
study. Firstly, the in vitro cytotoxicity of the
mesothelin-targeted CAR T cells was confirmed by the co-incubation
with mesothelin-positive H226 and H9 cells and mesothelin-negative
A431 cells. The aforementioned cells were genetically labeled with
luciferase and the cell viability after CAR T cell killing was
determined by measuring the remaining luciferase activity. As shown
in Fig. 5A, the CAR T cells
efficiently killed H226 and H9 cells, with only some background
killing apparent on antigen-negative A431 cells. The in vivo
activity testing of CAR T cells was performed on H9 nude model
mice. As shown in Fig. 5B,
mesothelin-targeted CAR T cells significantly suppressed H9 tumor
growth compared with the PBS control group, and the largest tumor
size was ~14×11 mm (Table I). In
addition, the combination of TIM3-R53 and CAR T cells could
moderately boost the activity of the CAR T cells, suggesting that
the sdAb had the potential to be further explored as a TIM3
inhibitory drug candidate, but further optimization was required to
increase its potency.
 | Table I.Measurement of tumor growth. (a) ×
width (b) in mm; volume was calculated as (axb2)/2 in
mm3. |
Table I.
Measurement of tumor growth. (a) ×
width (b) in mm; volume was calculated as (axb2)/2 in
mm3.
| Group | ID# |
| Day 7 | Day 9 | Day 11 | Day 13 | Day 15 | Day 17 | Day 19 |
|---|
| Control | 790 | Measurement, axb
mm | 7.32×6.41 | 7.75×6.70 | 8.48×6.93 | 9.60×8.18 | 11.02×8.47 | 12.70×9.38 | 13.48×10.81 |
|
|
| Volume,
mm3 | 150.38 | 173.95 | 203.63 | 321.18 | 395.29 | 558.70 | 787.61 |
|
| 564 | Measurement, axb
mm | 7.38×6.18 | 7.52×6.80 | 8.19×7.02 | 9.61×9.61 | 10.92×10.88 | 11.97×10.92 | 12.57×12.06 |
|
|
| Volume,
mm3 | 140.93 | 173.95 | 201.80 | 443.75 | 646.32 | 713.69 | 913.69 |
|
| 609 | Measurement, axb
mm | 5.74×4.42 | 6.28×5.26 | 6.93×5.67 | 7.84×7.47 | 9.46×8.57 | 10.52×9.75 | 11.47×10.38 |
|
|
| Volume,
mm3 | 56.07 | 86.88 | 111.40 | 218.74 | 347.39 | 500.03 | 617.83 |
|
| 566 | Measurement, axb
mm | 5.58×4.47 | 6.14×5.04 | 6.87×5.53 | 8.74×6.47 | 12.28×8.25 | 13.27×9.06 | 14.47×10.69 |
|
|
| Volume,
mm3 | 55.75 | 77.98 | 105.05 | 182.93 | 417.90 | 544.62 | 826.90 |
|
| 565 | Measurement, axb
mm | 4.91×3.66 | 5.08×4.01 | 5.57×4.71 | 6.60×4.56 | 8.99×6.02 | 9.37×7.98 | 10.53×8.75 |
|
|
| Volume,
mm3 | 32.89 | 40.84 | 61.78 | 68.62 | 162.90 | 298.48 | 402.72 |
| R53 | 661 | Measurement, axb
mm | 7.21×5.73 | 7.8×6.45 | 8.21×6.86 | 9.53×9.21 | 11.62×10.98 | 12.01×10.88 | 13.41×12.17 |
|
|
| Volume,
mm3 | 118.51 | 162.19 | 193.22 | 404.20 | 699.93 | 711.35 | 992.81 |
|
| 611 | Measurement, axb
mm | 6.92×5.65 | 7.65×6.16 | 8.58×7.17 | 9.32×8.58 | 10.91×8.94 | 11.56×9.38 | 12.82×10.23 |
|
|
| Volume,
mm3 | 110.36 | 145.05 | 220.47 | 342.68 | 435.82 | 508.84 | 670.53 |
|
| 638 | Measurement, axb
mm | 5.67×4.65 | 6.12×5.15 | 7.89×6.94 | 8.63×7.86 | 9.33×8.09 | 10.64×9.50 | 11.14×10.02 |
|
|
| Volume,
mm3 | 61.21 | 81.23 | 189.76 | 266.63 | 305.06 | 480.37 | 559.74 |
|
| 791 | Measurement, axb
mm | 5.12×3.92 | 5.67×5.06 | 6.77×6.38 | 7.41×6.94 | 9.21×8.66 | 11.02×9.70 | 11.98×11.25 |
|
|
| Volume,
mm3 | 39.24 | 72.49 | 137.91 | 178.35 | 345.64 | 518.30 | 757.56 |
|
| 792 | Measurement, axb
mm | 4.24×3.86 | 4.98×3.67 | 5.83×4.58 | 5.59×3.89 | 6.71×4.92 | 9.21×7.78 | 8.87×7.34 |
|
|
| Volume,
mm3 | 31.60 | 33.52 | 61.24 | 42.38 | 81.13 | 278.94 | 239.25 |
| CAR-T | 947 | Measurement, axb
mm | 7.32×5.64 | 6.20×4.58 | 4.90×3.38 | 7.63×5.75 | 9.35×6.83 | 9.62×8.12 | 11.51×8.86 |
|
|
| Volume,
mm3 | 116.42 | 65.03 | 27.99 | 126.05 | 218.08 | 317.40 | 451.46 |
|
| 568 | Measurement, axb
mm | 7.17×4.59 | 6.70×3.98 | 6.58×3.43 | 8.34×6.56 | 9.96×8.02 | 10.28×9.43 | 10.95×10.23 |
|
|
| Volume,
mm3 | 75.53 | 52.94 | 38.71 | 179.62 | 320.33 | 456.99 | 612.79 |
|
| 610 | Measurement, axb
mm | 6.23×4.63 | 5.31×3.98 | 3.74×3.31 | 3.87×3.67 | 5.89×5.13 | 6.35×5.45 | 7.03×6.32 |
|
|
| Volume,
mm3 | 66.65 | 41.99 | 20.44 | 26.00 | 77.43 | 94.24 | 140.36 |
|
| 563 | Measurement, axb
mm | 5.45×4.59 | 4.13×3.84 | 3.66×3.30 | 4.23×1.66 | 4.89×2.95 | 5.20×4.00 | 6.79×5.30 |
|
|
| Volume,
mm3 | 57.42 | 30.45 | 19.97 | 5.82 | 21.30 | 41.56 | 95.22 |
|
| 578 | Measurement, axb
mm | 4.07×3.66 | 2.57×2.07 | 1.00×1.00 | 0.00×0.00 | 0.00×0.00 | 2.91×2.75 | 4.96×4.92 |
|
|
| Volume,
mm3 | 27.26 | 5.51 | 0.50 | 0.00 | 0.00 | 11.00 | 60.00 |
| CAR-T+R53 | 960 | Measurement, axb
mm | 7.02×5.50 | 6.42×5.10 | 7.55×6.14 | 7.63×6.60 | 7.88×7.32 | 7.56×7.08 | 8.56×7.16 |
|
|
| Volume,
mm3 | 106.18 | 83.49 | 142.24 | 165.94 | 211.11 | 189.65 | 219.65 |
|
| 794 | Measurement, axb
mm | 6.78×5.46 | 5.98×5.02 | 6.53×5.49 | 6.87×5.80 | 7.38×6.28 | 6.72×5.73 | 7.58×6.19 |
|
|
| Volume,
mm3 | 101.00 | 75.30 | 98.50 | 115.40 | 145.70 | 110.30 | 145.20 |
|
| 585 | Measurement, axb
mm | 6.55×5.20 | 5.43×4.77 | 7.08×5.78 | 7.47×5.69 | 7.24×5.79 | 6.82×5.20 | 7.28×5.34 |
|
|
| Volume,
mm3 | 88.56 | 61.77 | 118.41 | 120.81 | 121.51 | 92.21 | 103.76 |
|
| 665 | Measurement, axb
mm | 6.21×5.18 | 5.34×4.39 | 6.10×4.02 | 6.53×3.55 | 6.98×4.49 | 6.54×4.79 | 6.74×5.03 |
|
|
| Volume,
mm3 | 83.20 | 51.50 | 49.30 | 41.20 | 70.30 | 75.10 | 85.20 |
|
| 636 | Measurement, axb
mm | 6.50×5.01 | 6.23×3.80 | 5.03×3.07 | 4.18×2.28 | 4.42×2.74 | 6.19×3.88 | 6.36×3.86 |
|
|
| Volume,
mm3 | 81.58 | 44.98 | 23.64 | 10.89 | 16.61 | 46.49 | 47.44 |
Discussion
TIM3 is generally considered as an inhibitory immune
checkpoint that is preferentially expressed on activated T cells
and other immune cells. TIM3 interacts with multiple ligands
including galectin-9, CEACAM1, PtdSer, HMGB1, and causes exhaustion
and apoptosis of antigen-specific Th1 cells and CTLs, which
correlates with impaired antitumor immune response (23). The blockade of TIM3 has exhibited
beneficial effects in T cell-based immunotherapies in some
preclinical studies (24,25). However, more studies showed that
antibodies to TIM3 could enhance T cell function, but the efficacy
looks moderate or may work best in synergy with PD1/PDL1 blockade
(26–28). A recently completed phase Ia/b study
on non-small cell lung cancer indicated that TIM3 blocking antibody
LY3321367 had acceptable safety profile with favorable
pharmacokinetics/pharmacodynamics but only modest antitumor
activity (27). In such a
complicating and disappointing scenario, it was worthwhile to
develop and test more antibodies with different epitopes and
binding properties more rigorously in the preclinical studies.
To date, most TIM3-blockade antibodies are
conventional IgG types. Plenty of preclinical studies heavily
relied on one rat monoclonal antibody, RMT3-23, as a research tool
to block TIM3 function (11,29,30).
RMT3-23 is commercially available as research reagent and
recognizes mouse TIM3 but not human TIM3. However, detailed
characterization showed that RMT3-23 weakly blocks CEACMA1 and
PtdSer from binding to TIM3, but not galectin-9 (11). Very recently, a fully human
anti-human TIM3 monoclonal antibody M6903 was developed from human
immunoglobulin (Ig) transgenic rats (OmniRat®) immunized
with His-tagged recombinant human TIM3 extracellular domain
(31). M6903 binds human TIM3 with
strong affinity (Kd, 2.3 nM), but moderately blocks the
engagement of three TIM3 ligands, PtdSer (IC90, ~1
µg/ml), CEACAM1 (IC90, ~0.5 µg/ml), and galectin-9
(IC90, ~1.5 µg/ml). However the in vivo antitumor
activity by M6903 monotherapy was still moderate (31), probably due to the insufficient
blocking activity. Apart from the aforementioned IgGs, one camel
sdAb to human TIM3 was previously generated (32), however this sdAb has not been
rigorously tested for the binding and blocking activity in
vitro and in vivo. Taken together, good monoclonal
antibodies that could potently block human TIM3 from binding to any
of its ligands are still lacking. The present study aimed to
develop new sdAbs that could block the binding of galectin-9 to
human TIM3.
By using protein immunization and phage display, the
current study successfully isolated two rabbit TIM3 sdAbs, R53 and
R23. The binding affinity of these two sdAbs to TIM3 was similar
and close to that of the ligand galectin-9, all of which were
within the 100 nM range. As lead molecules, these sdAbs
demonstrated weak but acceptable affinity and it is still possible
to further improve their affinity through antibody engineering. The
neutralization assay revealed that sdAb R53 inhibited
galectin-9-TIM3 binding to a greater extent compared with R23.
Thus, the present study focused on R53 for the following functional
studies.
It was reported that CAR T cells could overexpress
TIM3 for ~10 days after transduction (33). The present study confirmed the cell
binding specificity of the sdAbs through their ability to
selectively bind to mesothelin-targeted CAR T cells, but not to
PBMCs. In addition, the expression of TIM3 in the CAR T cells was
discovered to gradually increase with the extended culture time. As
a proof-of-concept study on the in vivo antitumor activity
of the sdAb, mesothelin-targeted CAR T cells were constructed and
the efficacy of CAR T cells in combination with TIM3 sdAbs were
analyzed. The combination of R53 could boost the CAR T cell
activity moderately (Fig. 5B),
probably due to its low affinity. The affinity of R53 was about
50-fold lower than M6903 (31),
however their in vivo efficacy seems quite similar, if
disregarding the difference of the experimental conditions, such as
tumor cell lines, immune effector cells, and the immune competency
of the mice. To the best of our knowledge, R53 and M6903 are the
only reported monoclonal antibodies against human TIM3 that have
been well characterized and functionally tested in vitro and
in vivo; further antibody engineering may improve their
efficacy.
It has been reported that PD-1 blockade therapy
could produce adaptive resistance in numerous patients, and that
the combined therapy of anti-PD-1 and anti-TIM3 significantly
inhibited the growth of tumors in preclinical studies (34,35).
Therefore, it is worthwhile to further evaluate the efficacy of the
sdAb isolated in the present study in more diverse and
translational settings.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant. no. 31670943), the
Fundamental Research Funds for the Central Universities (grant nos.
2662016PY113, 2662017PY111 and 2662019YJ013) and the Applied Basic
Research Program of Wuhan Science and Technology Bureau (grant nos.
2017060201010195 and 2019020701011438).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY performed the experiments, analyzed the data,
prepared the figures and drafted the manuscript. XC, QW and XM
performed the flow cytometry experiments. CW, DZ and YZ measured
the CAR T cell killing ability both in vitro and in
vivo. HH, LH, JL and CX provided assistance with the
experiments and data analysis. SH analyzed the data and revised the
manuscript. XY and MF designed the study and finalized the
manuscript. LY and XC confirmed the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All the procedures used in the animal studies were
approved by the Animal Care and Use Committee of Huazhong
Agricultural University (approval no. HZAUMO-2017-051). Peripheral
blood materials used in this study were obtained from healthy
donors with informed consent. The use of human blood samples was
reviewed and approved by Biomedical Research Ethical Committee,
Huazhong Agriculture University (approval no. HZAUHU-2017-002).
Patient consent for publication
Not applicable.
Competing interests
A patent (patent no. CN110938146A, submitted on Nov.
20th, 2019) has been filed based on the domain antibodies and their
applications. The authors have declared no other competing
interest.
References
|
1
|
Hamid O, Robert C, Ribas A, Hodi FS,
Walpole E, Daud A, Arance AS, Brown E, Hoeller C, Mortier L, et al:
Antitumour activity of pembrolizumab in advanced mucosal melanoma:
A post-hoc analysis of KEYNOTE-001, 002, 006. Br J Cancer.
119:670–674. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Garon EB, Rizvi NA, Hui R, Leighl N,
Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L,
et al: Pembrolizumab for the treatment of non-small-cell lung
cancer. N Engl J Med. 372:2018–2028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Motzer RJ, Escudier B, McDermott DF,
George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G,
Plimack ER, et al: Nivolumab versus everolimus in advanced
renal-cell carcinoma. N Engl J Med. 373:1803–1813. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Langer CJ, Gadgeel SM, Borghaei H,
Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins
RG, Stevenson JP, Jalal SI, et al: Carboplatin and pemetrexed with
or without pembrolizumab for advanced, non-squamous non-small-cell
lung cancer: A randomised, phase 2 cohort of the open-label
KEYNOTE-021 study. Lancet Oncol. 17:1497–1508. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mcintire JJ, Umetsu SE, Akbari O, Potter
M, Kuchroo VK, Barsh GS, Freeman GJ, Umetsu DT and Dekruyff RH:
Identification of Tapr (an airway hyperreactivity regulatory locus)
and the linked Tim gene family. Nat Immunol. 2:1109–1116. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhu C, Anderson AC, Schubart A, Xiong H,
Imitola J, Khoury SJ, Zheng XX, Strom TB and Kuchroo VK: The Tim-3
ligand galectin-9 negatively regulates T helper type 1 immunity.
Nat Immunol. 6:1245–1252. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Anderson AC, Anderson DE, Bregoli L,
Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW,
Hirashima M, et al: Promotion of tissue inflammation by the immune
receptor Tim-3 expressed on innate immune cells. Science.
318:1141–1143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ndhlovu LC, Lopez-Vergès S, Barbour JD,
Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF and
Lanier LL: Tim-3 marks human natural killer cell maturation and
suppresses cell-mediated cytotoxicity. Blood. 119:3734–3743. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Phong BL, Avery L, Sumpter TL, Gorman JV,
Watkins SC, Colgan JD and Kane LP: Tim-3 enhances FcεRI-proximal
signaling to modulate mast cell activation. J Exp Med.
212:2289–2304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han G, Chen G, Shen B and Li Y: Tim-3: An
activation marker and activation limiter of innate immune cells.
Front Immunol. 4:4492013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sabatos-Peyton CA, Nevin J, Brock A,
Venable JD, Tan DJ, Kassam N, Xu F, Taraszka J, Wesemann L, Pertel
T, et al: Blockade of Tim-3 binding to phosphatidylserine and
CEACAM1 is a shared feature of anti-Tim-3 antibodies that have
functional efficacy. Oncoimmunology. 7:e13856902017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dolina JS, Braciale TJ and Hahn YS:
Liver-primed CD8+ T cells suppress antiviral adaptive immunity
through galectin-9-independent T-cell immunoglobulin and mucin 3
engagement of high-mobility group box 1 in mice. Hepatology.
59:1351–1365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Markwick LJ, Riva A, Ryan JM, Cooksley H,
Palma E, Tranah TH, Manakkat Vijay GK, Vergis N, Thursz M, Evans A,
et al: Blockade of PD1 and TIM3 restores innate and adaptive
immunity in patients with acute alcoholic hepatitis.
Gastroenterology. 148:590–602.e10. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Khodabakhsh F, Behdani M, Rami A and
Kazemi-Lomedasht F: Single-domain antibodies or nanobodies: A class
of next-generation antibodies. Int Rev Immunol. 37:316–322. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wesolowski J, Alzogaray V, Reyelt J, Unger
M, Juarez K, Urrutia M, Cauerhff A, Danquah W, Rissiek B,
Scheuplein F, et al: Single domain antibodies: Promising
experimental and therapeutic tools in infection and immunity. Med
Microbiol Immunol. 198:157–174. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elverdi T and Eskazan AE: Caplacizumab as
an emerging treatment option for acquired thrombotic
thrombocytopenic purpura. Drug Des Devel Ther. 13:1251–1258. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Scully M, Cataland SR, Peyvandi F, Coppo
P, Knöbl P, Kremer Hovinga JA, Metjian A, de la Rubia J, Pavenski
K, Callewaert F, et al: Caplacizumab treatment for acquired
thrombotic thrombocytopenic purpura. N Engl J Med. 380:335–346.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shinozaki N, Hashimoto R, Fukui K and
Uchiyama S: Efficient generation of single domain antibodies with
high affinities and enhanced thermal stabilities. Sci Rep.
7:57942017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
AVMA Panel on Euthanasia. American
Veterinary Medical Association, . 2000 Report of the AVMA panel on
euthanasia. J Am Vet Med Assoc. 218:669–696. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Feng M, Gao W, Wang R, Chen W, Man YG,
Figg WD, Wang XW, Dimitrov DS and Ho M: Therapeutically targeting
glypican-3 via a conformation-specific single-domain antibody in
hepatocellular carcinoma. Proc Natl Acad Sci USA. 110:E1083–E1091.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang YF and Ho M: Humanization of rabbit
monoclonal antibodies via grafting combined Kabat/IMGT/Paratome
complementarity-determining regions: Rationale and examples. MAbs.
9:419–429. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaneko O, Gong L, Zhang J, Hansen JK,
Hassan R, Lee B and Ho M: A binding domain on mesothelin for
CA125/MUC16. J Biol Chem. 284:3739–3749. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Baghdadi M, Takeuchi S, Wada H and Seino
K: Blocking monoclonal antibodies of TIM proteins as orchestrators
of anti-tumor immune response. MAbs. 6:1124–1132. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herrera-Camacho I, Anaya-Ruiz M,
Perez-Santos M, Millán- Pérez Peña L, Bandala C and Landeta G:
Cancer immunotherapy using anti-TIM3/PD-1 bispecific antibody: A
patent evaluation of EP3356411A1. Expert Opin Ther Pat. 29:587–593.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu JF, Wu L, Yang LL, Deng WW, Mao L, Wu
H, Zhang WF and Sun ZJ: Blockade of TIM3 relieves immunosuppression
through reducing regulatory T cells in head and neck cancer. J Exp
Clin Cancer Res. 37:442018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou G, Sprengers D, Boor PPC, Doukas M,
Schutz H, Mancham S, Pedroza-Gonzalez A, Polak WG, de Jonge J,
Gaspersz M, et al: Antibodies against immune checkpoint molecules
restore functions of tumor-infiltrating T cells in hepatocellular
carcinomas. Gastroenterology. 153:1107–1119.e10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Harding JJ, Moreno V, Bang YJ, Hong MH,
Patnaik A, Trigo J, Szpurka AM, Yamamoto N, Doi T, Fu S, et al:
Blocking TIM-3 in treatment-refractory advanced solid tumors: A
phase Ia/b study of LY3321367 with or without an anti-PD-L1
antibody. Clin Cancer Res. 27:2168–2178. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim JE, Patel MA, Mangraviti A, Kim ES,
Theodros D, Velarde E, Liu A, Sankey EW, Tam A, Xu H, et al:
Combination therapy with Anti-PD-1, anti-TIM-3, and focal radiation
results in regression of murine gliomas. Clin Cancer Res.
23:124–136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ngiow SF, von Scheidt B, Akiba H, Yagita
H, Teng MW and Smyth MJ: Anti-TIM3 antibody promotes T cell
IFN-γ-mediated antitumor immunity and suppresses established
tumors. Cancer Res. 71:3540–3551. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kurtulus S, Sakuishi K, Ngiow SF, Joller
N, Tan DJ, Teng MW, Smyth MJ, Kuchroo VK and Anderson AC: TIGIT
predominantly regulates the immune response via regulatory T cells.
J Clin Invest. 125:4053–4062. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang D, Jiang F, Zaynagetdinov R, Huang
H, Sood VD, Wang H, Zhao X, Jenkins MH, Ji Q, Wang Y, et al:
Identification and characterization of M6903, an antagonistic
anti-TIM-3 monoclonal antibody. Oncoimmunology. 9:17449212020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Homayouni V, Ganjalikhani-Hakemi M, Rezaei
A, Khanahmad H, Behdani M and Lomedasht FK: Preparation and
characterization of a novel nanobody against T-cell immunoglobulin
and mucin-3 (TIM-3). Iran J Basic Med Sci. 19:1201–1208.
2016.PubMed/NCBI
|
|
33
|
Jiang Z, Jiang X, Chen S, Lai Y, Wei X, Li
B, Lin S, Wang S, Wu Q, Liang Q, et al: Anti-GPC3-CAR T cells
suppress the growth of tumor cells in patient-derived xenografts of
hepatocellular carcinoma. Front Immunol. 7:6902017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sakuishi K, Apetoh L, Sullivan JM, Blazar
BR, Kuchroo VK and Anderson AC: Targeting Tim-3 and PD-1 pathways
to reverse T cell exhaustion and restore anti-tumor immunity. J Exp
Med. 207:2187–2194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Koyama S, Akbay EA, Li YY, Herter-Sprie
GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ,
Asahina H, et al: Adaptive resistance to therapeutic PD-1 blockade
is associated with upregulation of alternative immune checkpoints.
Nat Commun. 7:105012016. View Article : Google Scholar : PubMed/NCBI
|