Introduction
Cancer development and progression is recognized as
a multi-step process involving the disruption of the
immune-mediated homeostatic balance that characterizes healthy
tissues (1). In homeostasis, the
resident immune system cells act like sentinels to safeguard tissue
and organ integrity (2). However,
the immune system and inflammation also serve a role in
tumorigenesis (3). This was first
documented in the 19th century when Dr. Rudolf Virchow observed the
presence of leukocytes within tumors, whose function has since been
elucidated (4,5). During oncogenesis, the immune system
serves a multi-faceted role in regulating cancer development from
pathogenesis to treatment. Although the immune system can suppress
factors involved in the initiation and progression of cancer,
immune cells can also promote proliferation, infiltration and
metastasis of cancer (6). Different
immune responses and cell types are involved in the formation of
the tumor microenvironment, including macrophages, neutrophils,
mast cells, myeloid derived suppressor cells, dendritic cells,
natural killer cells of the innate immune response, and the T and B
lymphocytes of the adaptive immune response (7).
A number of studies have suggested that immune cells
serve a crucial role in regulating colorectal cancer (CRC)
tumorigenesis. CRC involves multiple strategies to evade and
suppress immune system processes, including immunosurveillance,
immunoediting, antitumor immune response and conditioning of the
tumor microenvironment (8–10). Immune cells have also been identified
as prognostic indicators in CRC (9).
For example, CRC is characterized by numerous protumorigenic
inflammatory responses that selectively inhibit antitumor immune
responses and promote tumor development (11). By selectively inhibiting the
activation of antitumor cells, such as tumor infiltrating
lymphocytes (TIL), and activating suppressor T cells, such as
myeloid-derived suppressor cells and regulatory T cells, immune
cells lead to immune evasion in CRC, affecting its progression
(12–14). Given their important role in
pathogenesis and clinical outcome, immune response cells are
regarded as an independent predictor for CRC recurrence and outcome
(15). An ‘immune score’ (16) based on TIL location is, for example,
used to assess disease free and overall survival (OS), as well as
the risk of relapse and metastasis in CRC (17).
Immunotherapy involves the use of components of the
immune system to treat patients with cancer (11,14,18,19). The
main immunotherapy strategies include: Cancer vaccines and immune
stimulatory cytokines, which augment the antitumor immune response;
and the use of checkpoint inhibitors, such as the anticytotoxic T
lymphocyte-associated antigen 4 antibody, to inhibit immune
response suppression (20).
Immunotherapy has been investigated previously in CRC (21); however, further investigation is
required to elucidate the association between immune factors and
CRC.
Peripheral blood is the main component of human
physiological homeostasis. Blood connects the entire biological
system, and immune cells in the blood constitute specific immunity,
which is the third line of immune defense (22). Thus, blood cells recognize subtle
changes occurring in the body in association with injury or
disease, reflect integrated physiological responses to injury and
induce specific gene expression alterations (9,23). For
these reasons, according to the Sentinel Principle (24) peripheral blood transcriptome
profiling dynamically reflects system-wide biology (25,26).
Peripheral blood transcriptome technology has been applied in the
diagnosis of various non-hematological disorders, including various
types of cancer (27–36).
In the present study, the peripheral blood
transcriptome derived from patients with CRC was analyzed in order
to develop immune-related gene expression profiles, and to identify
CRC-specific immune genes as potential CRC diagnostic tools. The
biological functions of these genes were then characterized with
the aim of identifying new immune response-related aspects of CRC
pathogenesis, thereby investigating potential immunotherapy
techniques for CRC.
Materials and methods
Ethics
The present study was approved by The Ethics
Committees of the Affiliated Hospital of Qingdao University
(Qingdao, China) and The Seventh People's Hospital of Shanghai
University of Traditional Chinese Medicine (Shanghai, China).
Sample acquisition was performed between October 2018 and August
2019 at The Affiliated Hospital of Qingdao University and The
Seventh People's Hospital of Shanghai. A total of 121 participants
were enrolled, including 59 patients with CRC and 62 healthy
patients. Written informed consent was provided by all participants
prior to the study start.
Study population
Blood samples from 59 patients with CRC were
collected before they had undergone any form of treatment,
including radio- and chemotherapy or surgery. The patients were
selected from 74 volunteers who donated blood before routine
colonoscopy and who were subsequently diagnosed with CRC after
pathological examination. The pathologists were independent and not
involved in the present study. Healthy control samples consisted of
62 blood samples from subjects with no pathology. Tables I and II present the patient demographics and
clinical characteristics.
 | Table I.Demographics of healthy controls and
patients with colorectal cancer. |
Table I.
Demographics of healthy controls and
patients with colorectal cancer.
| Patient
demographics | Healthy
controls | Patients with
colorectal cancer | P-value |
|---|
| Sex, n |
|
|
|
|
Male | 38 | 38 |
|
|
Female | 24 | 21 |
|
| Age, years |
|
|
|
|
Min | 42 | 28 |
|
|
Max | 76 | 89 |
|
| Mean ±
standard deviation | 51.6±5.5 | 63.0±11.4 |
1.39×10−10 |
|
Year groups,
% |
|
|
|
|
21–30 | 0.0 | 1.7 |
|
|
31–40 | 0.0 | 3.4 |
|
|
41–50 | 46.8 | 10.2 |
|
|
51–60 | 48.4 | 18.6 |
|
|
61–70 | 3.2 | 37.3 |
|
|
71–80 | 1.6 | 25.4 |
|
|
81–90 | 0.0 | 3.4 |
|
| Total patients,
n | 62 | 59 |
|
 | Table II.Clinicopathological characteristics
of patients with colorectal cancer. |
Table II.
Clinicopathological characteristics
of patients with colorectal cancer.
| Characteristic | Patients, n |
|---|
| Tumor location |
|
| Left
colon | 14 |
| Right
colon | 7 |
|
Rectum | 34 |
|
Unknown | 4 |
| Tumor
differentiation |
|
|
Well | 2 |
|
Well-moderate | 1 |
|
Moderate | 42 |
|
Moderate-Poor | 4 |
|
Poor | 4 |
|
Unknown | 6 |
| Pathological
Tumor-Node-Metastasis stage |
|
| I | 7 |
| II | 22 |
|
III | 27 |
| IV | 1 |
|
Unknown | 2 |
Basic and clinicopathological
characteristics
A total of 121 blood samples were collected,
including 62 healthy controls and 59 samples from patients with
CRC. Patients with CRC were significantly older compared with the
healthy controls (P<0.01) according to analysis of variance
(F-test). The age of the controls ranged from 42–76 years, whereas
the patients with CRC ranged from 28–89 years of age. Detailed
information is presented in Table
I.
The clinicopathological characteristics of the
patients with CRC are presented in Table II, including tumor location,
differentiation and pathological Tumor-Mode-Metastasis (pTNM) stage
(37). The majority of the CRC
tissue was located in the rectum, followed by the left colon. The
main tumor differentiation type was moderate, which accounted for
~71.2% (42/59). The pTNMs were mainly stages II and III (22/59 and
27/59).
Blood collection, RNA isolation and
RNA quality control
Peripheral whole blood (2.5 ml) was collected in
PaxGene Blood RNA tubes (PreAnalytiX GmbH; Qiagen). Total RNA was
then isolated using the PaxGene Blood RNA kit (PreAnalytiX GmbH;
Qiagen) following the manufacturer's protocol. RNA quality was
assessed using a 2100 Bioanalyzer RNA 6000 Nano Chips (Agilent
Technologies, Inc.), according to the manufacturer's protocol. All
samples for microarray analysis met the following quality criteria:
RNA Integrity number ≥7.0 and 28S:18S ribosomal RNA ≥1.0. RNA
quantity was determined using a NanoDrop 1000 UV–Vis
spectrophotometer (Thermo Fisher Scientific, Inc.).
Microarray hybridization
Whole blood gene expression profiles from the 121
blood samples (59 patients with CRC and 62 controls) was analyzed
by microarray hybridization using the Gene Profiling Array cGMP
U133 P2 (cat. no. 901411), in accordance with the manufacturer's
protocol (Affymetrix; Thermo Fisher Scientific, Inc.). In brief,
200 ng of purified total RNA is transcribed into cDNA by reverse
transcription, then labeled and hybridized against the microarray,
according to the manufacturer's protocol. A total of 200 ng of each
RNA sample was used for cDNA synthesis and hybridization using the
accessory reagents of the Affymetrix microarray, according to the
manufacturer's protocol. The gene expression profiles of the RNA
samples were then processed using Affymetrix Expression Console
software (version 1.4.1; Affymetrix; Thermo Fisher Scientific,
Inc.) and normalized using the MAS5 normalization method (38) that uses a scaling factor to adjust
the global trimmed mean signal intensity value to 500 for each
array.
Microarray data and
pre-processing
To identify candidate genes for CRC, probe sets were
selected from 54,675 available sets in the Affymetrix Gene
Profiling cGMP U133 P2 microarray. The following criteria were
utilized: i) The probe sets could be detected reliably (‘present’
call) in all samples; and ii) the probe sets were present within
the microarray quality control (MAQC) list for the Affymetrix U133
P2 microarray, as reported by the MAQC Consortium (39) Immune system-related genes downloaded
from the Reactome database on December 10, 2019 (https://reactome.org/) were utilized to identify the
relevant immune-related genes (40).
All immune-related gene expression microarray data analyzed in the
present study are included in Table
SI and have been uploaded to the Gene Expression Omnibus;
accession number GSE164191. The microarray data were log
transformed to conform to a Gaussian distribution. The total data
were divided into a training set and a test set in accordance with
7:3 proportional scales.
Identification of CRC-specific immune
genes
To identify CRC-specific immune genes, feature
selection techniques were used that do not alter the original
representation of the variables but select an optimal subset from
them. To select the candidate genes efficiently and rapidly from
the vast set of gene expression signals, a one-way ANOVA F-value
was calculated to find differentially expressed genes by comparing
the expression of genes between the CRC patient group and the
healthy volunteer group following post hoc Tukey's test to
determine significant differences between the groups. Overall, 583
features were identified that were P<0.05 according to the
F-test.
This method considers each feature separately,
thereby ignoring feature dependencies that may lead to poor
classification performance (41). To
correct for this, ElasticNet regression analysis (42) was used, which takes advantage of L1
and L2 regularization to select stronger features for CRC
detection. Finally, five candidate genes with highest predictive
accuracy were selected for the classification of CRC and healthy
controls.
Model selection and performance
evaluation
A logistic regression algorithm was used to
construct a predictive model based on the five candidate genes as
described in our previous report (24) To differentiate the CRC group from the
healthy control group, area under the receiver operating
characteristic curve (ROC AUC), sensitivity, specificity and
accuracy were estimated in both the training and test groups.
Protein-protein networks and
functional enrichment analyses
The proteins that interacted with the five candidate
biomarkers were extracted from the STRING database (https://string-db.org/), with a confidence ≥0.7
(43). Gene-annotation enrichment
analysis using the clusterProfiler R package (http://bioconductor.org/packages/release/bioc/html/clusterProfiler.html;
version 3.12) was performed on signature genes and their associated
proteins (44) and the
protein-protein interaction network with the final biomarkers is
presented in Fig. 3.
Reactome pathways were identified with a strict
cut-off of P<0.05 that was corrected using the Benjamini and
Hochberg method (45) with a false
discovery rate of <0.05. Finally, protein-protein interaction
and gene networks were constructed, and the biomarkers were
identified using Cytoscape software (https://cytoscape.org/) (46).
Kaplan-Meier (KM) survival analysis
for candidate genes based on the log-rank test
A KM analysis was performed for the five candidate
genes to characterize the association between gene expression and
corresponding clinical outcome on the basis of mRNA datasets of 165
rectum adenocarcinoma included in KM plot database (http://www.kmplot.com/). With the mRNA dataset of
rectum adenocarcinoma collected in the KM database, these 165
patients were divided into high and low expression groups by using
each percentile of gene expression between the lower and upper
quartiles of expression with auto-selected best cutoff point
according to the previous reports (47,48). All
possible cutoff values between the lower and upper quartiles were
computed and the best performing threshold was used as a cutoff.
The ‘survival’ R package v2.38 (http://CRAN.R-project.org/package=survival/) was
utilized to calculate log-rank P values, hazard ratios (HR) and 95%
confidence intervals (CI).
Statistical analysis
A one-way ANOVA test (F-test) with Tukey's test was
performed to determine the age difference between the CRC patient
group and the healthy control group. An F-test following a Tukey's
test was performed to find the genes differentially expressed
between the CRC patient group and the healthy control group.
ElasticNet regression and logistic regression analysis were
performed to identify the candidate biomarkers and construct the
predictive model for CRC diagnosis by comparing expression profiles
between CRC and healthy control groups.
Results
Peripheral blood gene expression
profiling
Genome-wide gene expression profiling was applied to
the peripheral blood samples obtained from the 59 patients with CRC
and the 62 healthy controls. Genome-wide expression profiles
generated from Affymetrix GeneChip U133Plus2.0 were analyzed and
associated between the CRC and controls. The probe sets could be
detected reliably (‘present’ call) in all the samples present
within the MAQC list for the Affymetrix U133 P2 microarray, as
reported by the MAQC Consortium, and were also included in the
immune response-relevant transcriptome signatures. A final five
immune-related genes were identified to reliably distinguish CRC
from the controls, including: Protein phosphatase 3 regulatory
subunit Bα (PPP3R1), amyloid β precursor protein
(APP), cathepsin H (CTSH), proteasome activator
subunit 4 (PSME4) and DEAD-Box Helicase 3 X-Linked
(DDX3X). The corresponding gene symbols, titles of the final
five probe sets and fold changes are listed in Table III.
 | Table III.Immune-related signatures for
distinguishing colorectal cancer from controls. |
Table III.
Immune-related signatures for
distinguishing colorectal cancer from controls.
| Probe Set ID | Gene title | Gene symbol | Fold change | Regulation |
|---|
| 204506_at | Protein phosphatase
3 regulatory subunit Bα | PPP3R1 | 2.04 | Up |
| 214953_s_at | Amyloid β (A4)
precursor protein | APP | 1.84 | Up |
| 202295_s_at | Cathepsin H | CTSH | 1.23 | Up |
| 212220_at | Proteasome
activator subunit 4 | PSME4 | −1.41 | Down |
| 212514_x_at | DEAD
(Asp-Glu-Ala-Asp) box helicase 3 X-linked | DDX3X | −1.45 | Down |
Model construction and performance
evaluation
A predictive logistic regression model for
discriminating CRC from controls was constructed based on the five
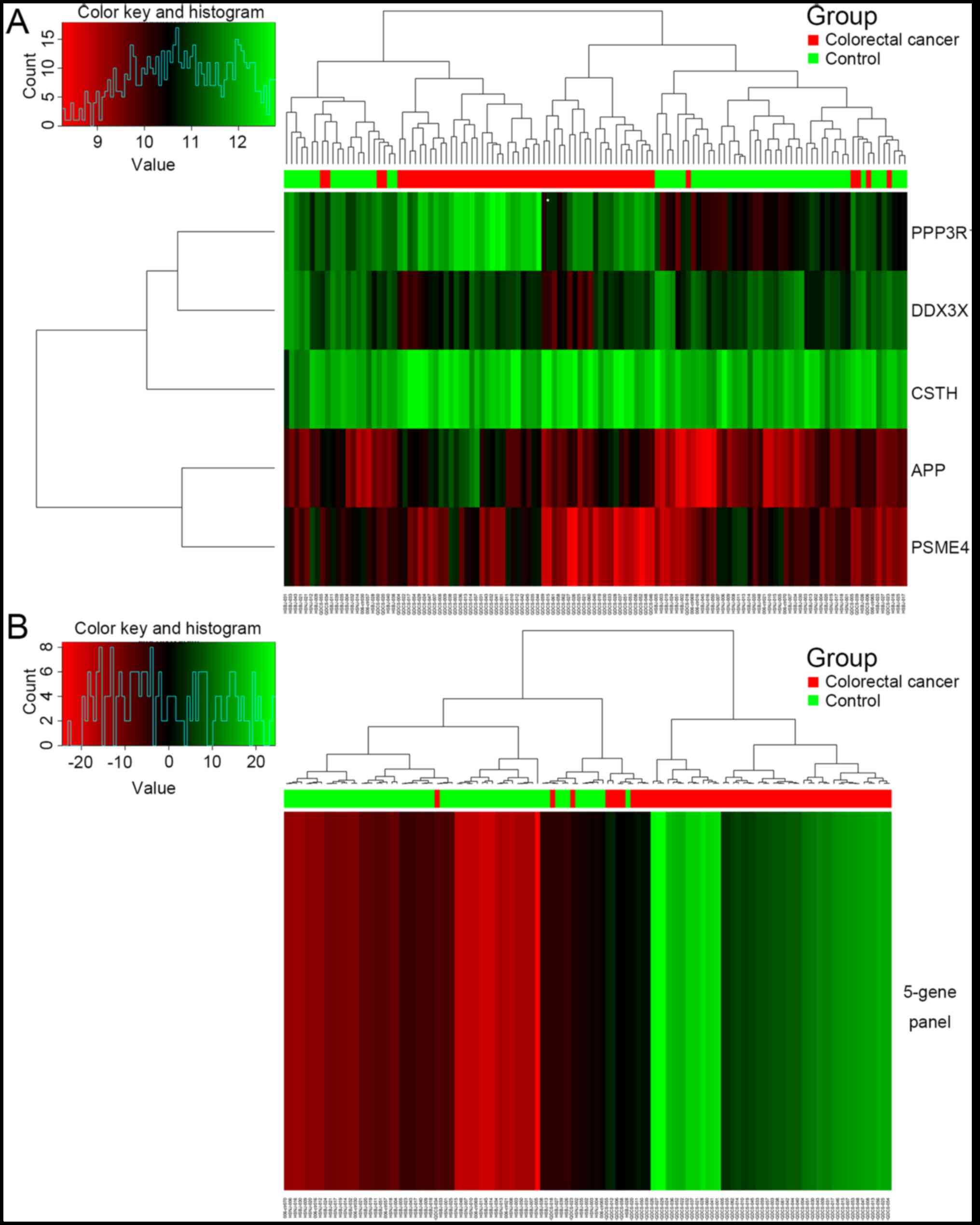
immune-related candidate genes identified. Fig. 1 presents the hierarchical cluster
diagrams that demonstrate the performance of the five genes for the
121 samples. The five-gene panel constructed by logistic regression
in Fig. 1B more clearly shows the
clustering of CRC samples compared with the control samples.
To build the predictive model, the data were divided
into training and test sets in proportions of 7:3. The training set
model contained a total of 84 samples, including 41 CRC samples and
43 control samples. The model performance was then evaluated in the
test set, which contained 37 samples, including 18 CRC samples and
19 control samples. The performances of the training set and the
test set are presented in Tables IV
and V.
 | Table IV.Predicted positive/negative
performances of the training and test set data. |
Table IV.
Predicted positive/negative
performances of the training and test set data.
| Set | Positive, n | Negative, n | Total, n |
|---|
| Training set |
|
CRC | 41 | 0 | 41 |
|
Control | 0 | 43 | 43 |
| Test set |
|
CRC | 15 | 3 | 18 |
|
Control | 1 | 18 | 19 |
 | Table V.Performance evaluation in the
training and test sets. |
Table V.
Performance evaluation in the
training and test sets.
| Set | Sensitivity, % | Specificity, % | Accuracy, % | ROC AUC |
|---|
| Training set | 100.0 | 100.0 | 100.0 | 1.00 |
| Test set | 83.3 | 94.7 | 89.2 | 0.96 |
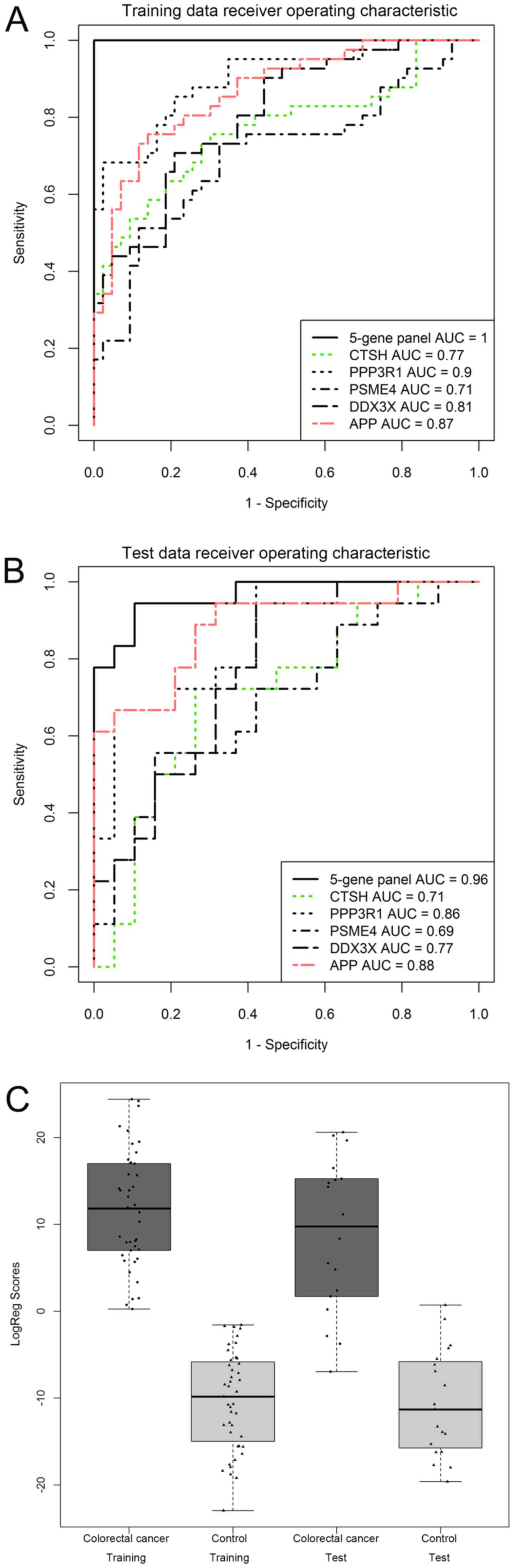
In terms of specificity and accuracy, both the
training and test sets performed well. Sensitivity, specificity and
accuracy in the training set were all 100%, and were 83.3, 94.7 and
89.2% in the test set, respectively. Furthermore, three of the 18
CRC samples in the test set were predicted as negative results, and
one of the 19 healthy control samples in the test set was predicted
as a positive result. These false-negative and false-positive
results require further study in a larger cohort.
The five-gene panel also exhibited a higher ROC AUC
when compared with any single gene in both the training set and
test set, as presented in Fig. 2A and
B. Based on the five gene panel and logistic regression
algorithm, the predictive model performed well in separating CRC
and healthy controls in both the training set and the test set, as
the box-whisker plot illustrates in Fig.
2C. These results suggested this five-gene panel exhibited good
performance for CRC discrimination and might be a potential
biomarker for CRC diagnosis.
Protein networks and immunofunctional
enrichment analysis
To enrich the signaling pathways to reveal the
biological processes underlying these five genes and their
involvement in CRC pathogenesis, we assessed the five individual
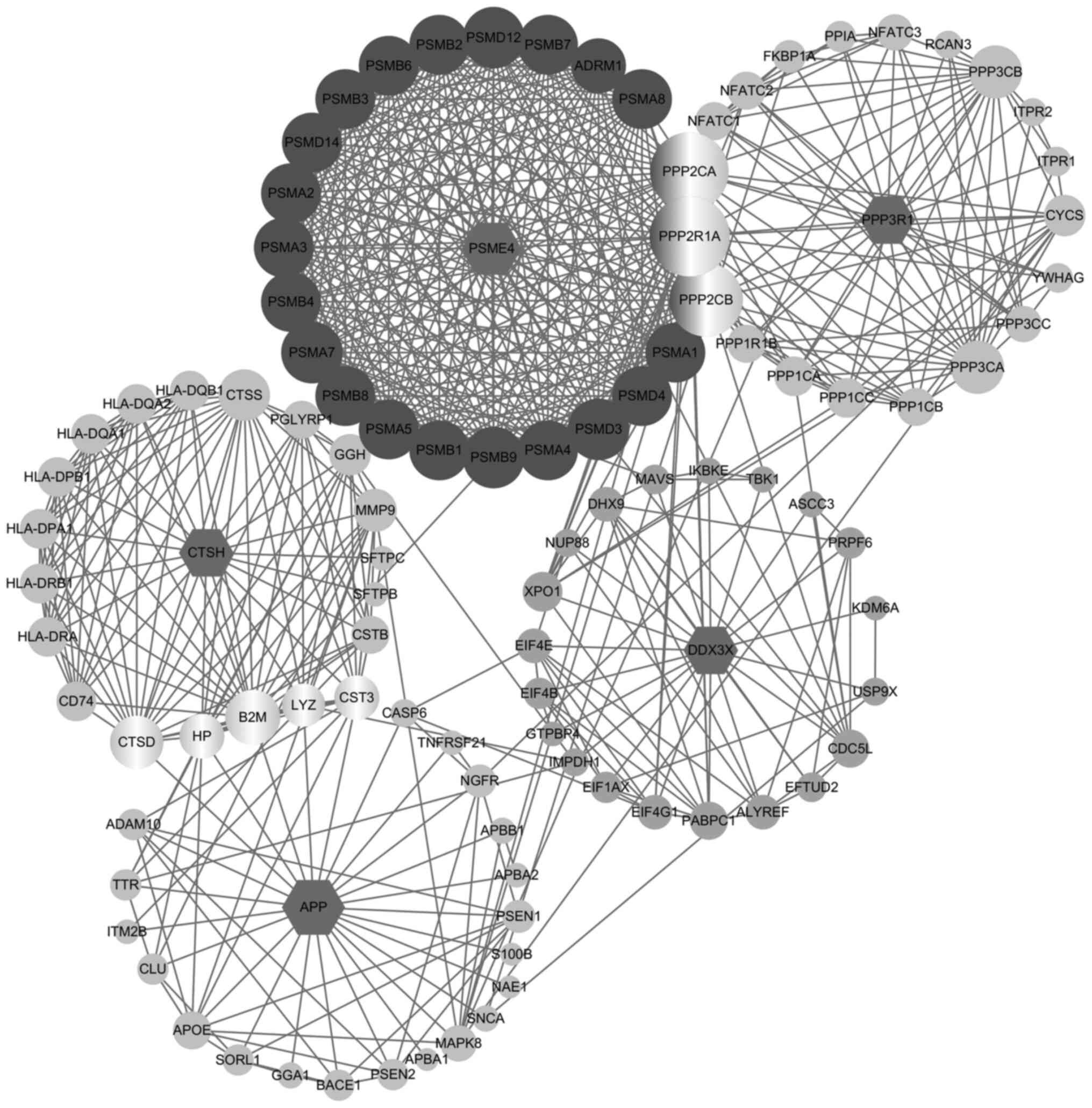
genes (proteins) through protein-protein interactions. This
analysis produced a network including 142 proteins that link these
five genes (proteins) together, as shown in Fig. 3. Subsequently, canonical pathway
analysis found that these genes (proteins) associate to multiple
immune-functions and thus might play important roles in the
interaction between the immune system and colorectal cancer
pathogenesis.
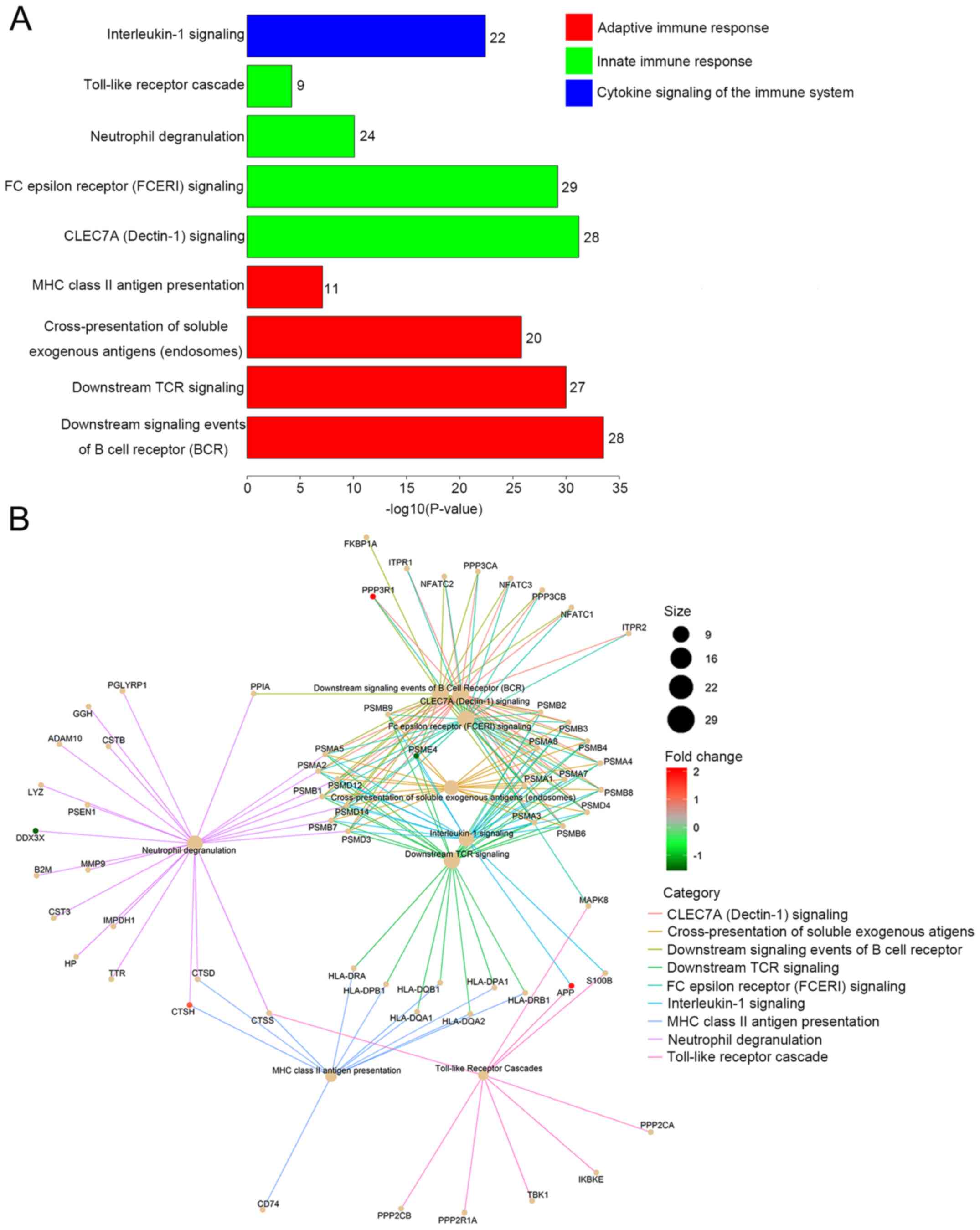
The five genes selected were functionally
categorized based on Reactome annotation terms and pathways
identified with a strict cut-off of adjusted P<0.05, corrected
with the Benjamini-Hochberg method. A total of 152 pathways
consisting of these five CRC-specific genes were identified, and
the top nine immune-related pathways with the highest P-adjusted
values were selected for further analysis. As presented in Fig. 4A, these immune-related pathways were
categorized into three groups: Adaptive immune response, innate
immune response and cytokine signaling of the immune system. The
adaptive immune response group included ‘downstream signaling
events of B cell receptor (BCR)’, ‘downstream T cell receptor (TCR)
signaling’, ‘cross-presentation of soluble exogenous antigens
(endosomes)’ and ‘major histocompatibility complex (MHC) class II
antigen presentation’. The innate immune response group consisted
of ‘CLEC7A (Dectin-1) signaling’, ‘Fc epsilon receptor (FCERI)
signaling’, ‘Toll-like receptor cascade’ and ‘neutrophil
degranulation’. ‘Interleukin-1 signaling’ was the only pathway
identified from the group of cytokine signaling genes in the immune
system. The interactions between the enriched immune-related
pathways and the related candidate genes of each pathway are
indicated in Fig. 4B. These results
indicated that these five CRC-specific genes are mainly associated
with ‘immune responses’, suggesting a close relationship between
immune system variations and the pathogenesis of colorectal
carcinoma.
An immune analysis of the five CRC-specific immune
genes was also summarized as per their effect on immune response,
as presented in Table VI. It was
determined that three of the five candidate genes (PSME4,
PPP3R1 and CTSH) were involved in both the adaptive and
innate immune response; whilst APP was associated with the
innate immune response and cytokine signaling in the immune system,
and DDX3X participated only in the innate immune response.
The gene involved in the highest number of immune response
categories was PSME4, which was involved in five types of
immune responses, including: ‘Signaling by the BCR’, ‘TCR
signaling’, ‘class I MHC mediated antigen processing and
presentation’, ‘C-type lectin receptors (CLRs)’ and ‘FCERI
signaling’.
 | Table VI.Immune analysis of candidate
genes. |
Table VI.
Immune analysis of candidate
genes.
|
|
|
| Immune response
category |
|
|---|
|
|
|
|
|
|
|---|
| Common name | NS Probe ID | Gene class | Adaptive immune
response | Innate immune
response | Cytokine signaling
in the immune system | Synonyms/previous
symbols |
|---|
| PSME4 | NM_014614 | Immune
response | Signaling by the
BCR, TCR signaling, Class I MHC mediated antigen processing and
presentation | CLRs, FCERI | – | PA200,
KIAA0077 |
| PPP3R1 | NM_000945 | Immune
response | Signaling by the
BCR | CLRs, FCERI | – | CALNB1, CNB,
CNB1 |
| APP | NM_000484 | Immune
response/cytokines | – | Toll-like receptor
cascades | Present | AD1 |
| CTSH | NM_004390 | Immune
response | MHC class II
antigen presentation | Neutrophil
degranulation | – | CPSB |
| DDX3X | NM_024005 | Immune
response | – | Neutrophil
degranulation | – | DDX3 |
Survival analysis of candidate
genes
The KM survival curve is able to assess the effect
of any gene or gene combination on survival for various types of
cancer, using >30,000 samples measured using gene chips or
RNA-sequencing (49). As there are
no well-defined gene expression profiles of CRC in the KM plot
database, a KM survival analysis was performed for the five
CRC-specific immune genes to visualize the association between gene
expression and clinical outcome based on the mRNA datasets of 165
rectum adenocarcinoma that collected in the KM plot database
(http://www.kmplot.com/). The CRC OS rates
associated with the five genes are presented in Fig. 5. Of the five genes, only
PPP3R1 demonstrated prognostic power for rectum
adenocarcinoma (P=0.019). High expression of PPP3R1
indicated poorer survival rate (Fig.
5A), consistent with our finding that PPP3R1 was
expressed at higher levels in CRC when compared with controls. The
other four candidate genes showed no prognostic power for rectum
adenocarcinoma.
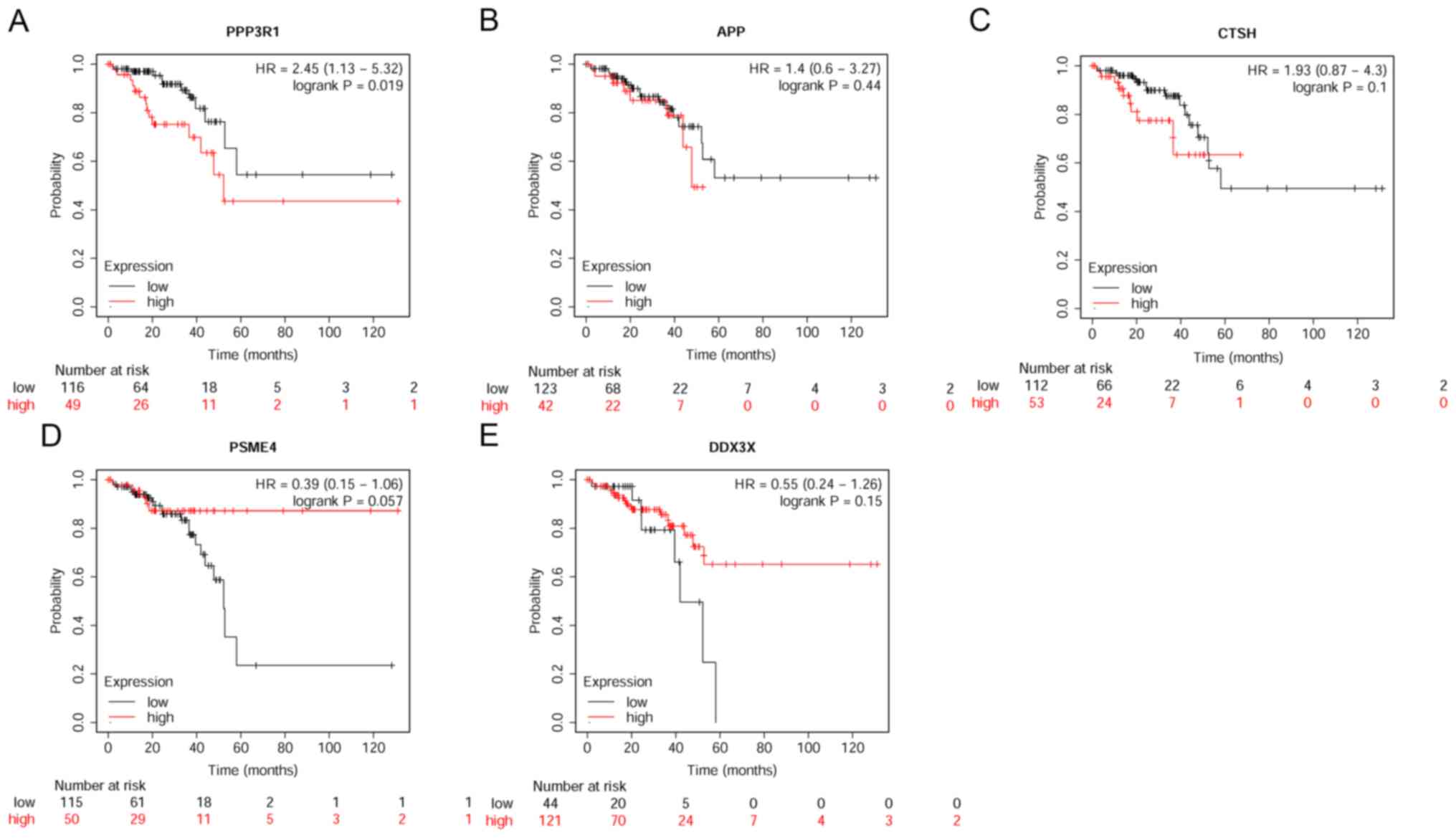 | Figure 5.Survival analysis based on the mRNA
dataset of 165 patients with rectum adenocarcinoma collected by
Kaplan-Meier database. Patients were subdivided into high and low
expression groups by using the percentiles of each mRNA expression
level between the lower and upper quartiles of expression as
cut-off point for five candidate immune-related signatures
associated with survival. (A) PPP3R1, (B) APP, (C)
CSTH, (D PSME or (E) DDX3X. Red and black
lines indicate high- and low-expression level groups, respectively.
HR, hazard ratio; PPP3R1, protein phosphatase 3 regulatory
subunit Bα; APP, amyloid β precursor protein; CTSH,
cathepsin H; PSME4, proteasome activator subunit 4;
DDX3X, DEAD-Box Helicase 3 X-Linked. |
Discussion
The present study compared the peripheral blood
transcriptomes of patients with CRC with those of healthy control
patients to identify CRC-specific immune genes. In doing so, five
candidate genes were selected and used to construct a predictive
model for CRC through a process of feature selection using logistic
regression. The predictive model exhibited strong statistical power
for distinguishing CRC from controls, with an accuracy of 100.0% in
the training set and 89.2% in the independent test set. The
immunofunctional enrichment analysis revealed that the genes were
associated with the adaptive immune response, the innate immune
response and cytokine signaling. From the KM datasets, one of the
five candidate genes (PPP3R1) demonstrated good prognostic
performance in the OS analysis of 165 patients with rectum
adenocarcinoma using the KM plot database (http://www.kmplot.com/). Considering the similarities
in pathogenesis between CRC and rectum adenocarcinoma, it was
reasonable to hypothesize that PPP3R1 may exhibit a similar
prognosis to CRC. These preliminary results are promising; however,
further research with larger cohorts and long-term follow-up is
required to validate the results.
In current clinical practice, CRC screening and
diagnosis relies mainly on the fecal occult blood test (FOBT),
colonoscopy and carcinoembryonic antigen (CEA) detection (50). However, each method has
disadvantages: The sensitivity of FOBT and CEA is limited, and
whilst colonoscopy is the gold standard for CRC diagnosis, the
bowel preparation required and occasional severe complications that
occur limit its application (51).
Furthermore, clinical stratification, treatment and prognosis of
CRC depends on tumor location and TNM staging; however, treatment
outcomes vary, and remain unsatisfactory, suggesting that these
indicators do not provide optimal prognostic information (52).
There is increasing evidence that the pathogenesis,
progression, treatment response and prognosis of CRC are all
significantly influenced by a complex interplay between cancer
cells and the immune system, particularly by the tumor
microenvironment (10,53,54). The
immune cells in peripheral blood constitute a third line of immune
defense; thus, the peripheral blood transcriptome could reflect the
overall immune characteristics of all types of cancer, including
CRC (22). Investigating novel
immune-related gene expression signatures using peripheral blood
transcriptome profiling will provide new strategies for the
diagnosis, treatment and prognosis of CRC in the future.
The present study identified five CRC-specific
immune genes (PPP3R1, APP, CTSH, PSME4 and DDX3X). Of
these, PPP3R1, APP and CTSH were upregulated in the
blood samples from patients with CRC compared with healthy control
samples, whereas PSME4 and DDX3X were downregulated.
The KM survival analysis showed that of the five genes, only
PPP3R1 was closely associated with the clinical prognosis of
CRC, with PPP3R1-upregulation indicating poor survival. This
finding suggests that the survival of CRC patients is associated
with the patients' immune system status and that PPP3R1
might serve as a biomarker for predicting CRC patient
prognosis.
PPP3R1, also named calcineurin B, is one of
the regulatory subunits of calcineurin (CaN). CaN is a
calcium-dependent, calmodulin stimulated serine/threonine protein
phosphatase under the control of Ca2+/calmodulin
(55). CaN is a heterodimer, which
includes a catalytic α subunit and a Ca2+ binding
regulatory β subunit (56,57). The CaN catalytic subunit gene family
consists of three members (serine/threonine-protein phosphatase 2B
catalytic subunits α, β and γ) (58). Lakshmikuttyamma et al
(59) revealed that CaN expression
is closely associated with the development of colon carcinoma, as
indicated by an increased level of CaN phosphatase activity and
higher levels of protein expression in colorectal adenocarcinomas.
The main CaN signaling pathways in CRC are regulated via nuclear
factor of activated T cells 5 (NFAT) and CaN-NFAT, which serve
critical roles in mediating cellular activation of T cell immune
responses (60). Adaptive immune
responses are an essential aspect of tumor-host interactions in
CRC, and the progression from pre-cancerous (adenomatous) colon
lesions to malignant CRC involves a complex pathway associated with
activated T lymphocytes (61). A
previous study demonstrated that CaN and NFAT are constitutively
expressed by intestinal epithelial cells and that these genes
promote CRC development (62). In
early CRC, CaN is activated by microbiota derived toll-like
receptor ligands, and CaN and NFAT promote oncogenesis via
modulation of tumor stem cells in an NFAT-dependent manner
(63,64).
To summarize, upregulated CaN (through
PPP3R1) mediates cellular activation and the immune response
in T cells, reflecting tumor-host interactions and playing an
essential role in the oncogenic processes involved in CRC
development. Therefore, this gene could be considered a
characteristic feature of CRC and of potential importance for CRC
detection. Consistent with the aforementioned previous studies, the
present study demonstrated that PPP3R1 was significantly
increased in the CRC group, and survival analysis also indicated
that high levels of PPP3R1 were associated with a poor
prognosis.
APP is a membrane-bound protein ubiquitously
expressed in a variety of cell types and is also found in neurite
plaques of Alzheimer's disease (AD) as a precursor protein of
β-amyloid (65). To the best of our
knowledge, the majority of research has focused on the role of APP
in AD; however, its biological functions in non-neural cells and
tumors remain unknown (66). Meng
et al (67) demonstrated,
both in vitro and in vivo, that APP is involved in
the proliferation of human colon carcinoma cells. They also
postulated that APP plays a crucial role in the cellular
proliferation and survival of non-neural cells, including colon
carcinoma cells. Another study reported that both CRC and
pancreatic adenocarcinoma upregulated APP, and that patients with
these diseases whose tumors exhibit upregulated APP, present with a
poor prognosis and a short survival time (68). Consistent with these reports, in the
present study, APP was also upregulated in the CRC group,
and it was suggested that APP-upregulation may serve as a
potent CRC diagnostic marker. In addition,
APP-downregulation may prove to be a novel molecular target
for adjuvant and neoadjuvant pharmacological treatment options.
There is notable evidence of an inverse link between
AD and cancer. For example, a previous study suggested that AD was
longitudinally associated with a decreased risk of cancer, and a
history of cancer was associated with a decreased risk of AD in the
group aged ≥65 years Caucasian adults (69). Whether CRC specifically is associated
with AD risk or with other neurodegenerative disorders requires
further investigation.
CTSH is a lysosomal glycoprotein and a member
of the cysteine proteinase family. Together with cathepsins B and
L, CTSH belongs to the peptidase C1 protein family, and can
act both as an aminopeptidase and as an endopeptidase (70). Various types of cysteine proteinase
have critical roles in MHC class II immune responses, apoptosis and
activation of growth factors and hormones (71–74). In
1985, a study observed that pre-operative serum levels of
C-reactive protein (CRP) were inversely correlated with the
activity of cathepsin H and collagenase, and that levels of these
peptidases were raised in rectal and sigmoid tumors (75). This study found that CTSH
activity and protein patterns reflect both cancer stage and site.
CTSH-specific activity is significantly increased in CRC,
and there is a distinct pattern of gene expression during CRC
progression. These findings suggest that CTSH may be particularly
useful in defining Dukes' B and C stage (76) cancer and in distinguishing subsets of
cancer types at a given site (77).
Another study indicated that CTSH levels were significantly
increased in the serum of patients with CRC, and that higher levels
are associated with a poor prognosis (78). A different study reported that
intestinal epithelial cells contain abundant constitutive levels of
the cathepsin proteases. These function in human leukocyte antigen
class II mediated antigen presentation to CD4(+) T lymphocytes in
the presence of the proinflammatory cytokine γ-IFN (79). Consistent with these reports,
CTSH was increased in the serum of patients with CRC in the
present study, and was also shown to be involved in MHC class II
antigen presentation.
DDX3X is a subfamily of the DEAD-box helicase
(DDX), which is the largest RNA helicase family and which
regulates RNA biogenesis by unwinding short RNA duplexes (60). The DDX3X subfamily performs
numerous nuclear functions and plays a role in the regulation of
translation (80,81). DDX3X and DDX3 have dual
roles in different types of cancer; acting either as oncogenes or
as tumor suppressor genes (82).
In CRC, the function of DDX3 remains
controversial. Some studies hypothesize that DDX3 acts as a
tumor suppressive gene with significant prognostic predictive power
in CRC, and have found that a low level of DDX3 indicates
poor prognosis and that downregulation promotes metastasis
(83). In other research,
DDX3 was found to have the opposite effect, acting as an
oncogenic gene in CRC, with upregulation of DDX3 correlated
with the β-catenin/Wnt signaling pathway (84). Inhibition of DDX3 with the
small molecule inhibitor RK-33, which binds to the ATP-binding site
of DDX3, could inhibit Wnt signaling, and this strategy may
indicate a promising therapy in a subset of patients with CRC
(85). In the present study,
DDX3X was a tumor suppressor gene that was downregulated in
the CRC group when compared with the healthy controls.
Understanding the definite role of DDX3X in CRC requires
further investigation.
The most downregulated gene in the present study,
PSME4, also named Proteasome Activator PA200, is a
heat/armadillo repeat protein. It binds to the ends of core or 20S
proteasomes, specifically recognizes acetylated histones and
promotes ATP and ubiquitin-independent degradation of core histones
during spermatogenesis and the DNA damage response (86,87). To
the best of our knowledge, there are currently only a few reports
on the role of PSME4 in cancer, and only one report on the
regulation of proteasome activator PA200 on tumor cell (HeLa
cervical carcinoma and B16.F10 murine melanoma cell) responsiveness
to glutamine and resistance to ionizing radiation (88). The present study is, to the best of
our knowledge, the first to suggest that PSME4 has a role as
a tumor suppressor gene in CRC.
In the present study, peripheral blood transcriptome
profiling analysis identified five immune-system related genes that
could discriminate CRC samples from healthy controls. There were
more patients with left-sided CRC than with right-sided CRC;
however, we previously identified that there are no differences in
blood RNA biomarkers between left- and right-side CRC (31).
Using these five CRC-specific immune genes, the
performance of a predictive model was constructed and evaluated.
Functional enrichment analysis indicated that the five biomarkers
were mainly involved in the following pathways: ‘Signaling by the
BCR’, ‘TCR signaling’, ‘class I MHC mediated antigen processing and
presentation’, ‘CLRs’, ‘FCERI signaling’, ‘toll-like receptor
cascades’, ‘signaling by interleukins’, ‘MHC class II antigen
presentation’ and ‘neutrophil degranulation’. These nine immune
signaling pathways are associated with the adaptive immune
response, the innate immune response and with cytokine signaling.
Survival analysis of the five candidate genes indicated that
upregulation of PPP3R1 predicted a poor survival rate in patients
with CRC, and that the other four candidate genes showed no
significant prognostic power for CRC.
As a case control report, there are some limitations
to the present study. Firstly, the sample size was relatively small
and different genes or more genes with better discriminatory power
may be identified among a larger independent cohort of patients;
second, a peripheral blood transcriptome analysis could reflect
some aspects of immune status rather than global alterations; and
third, the nature of the mechanisms driving these immune-related
transcriptomic biomarkers in peripheral blood is not yet clear, and
the biological functions of some biomarkers require further study.
For example, an in vitro investigation of the biological
functions of genes would be helpful in elucidating mechanisms of
carcinogenesis in CRC, and such a study will be carefully
considered for future work.
Furthermore, it has been reported that
microsatellite instability (MSI) is detected in 15% of all CRC
cases (89). MSI is a hypermutable
phenotype caused by the loss of DNA mismatch repair activity, which
has been observed in different types of cancer cells and thus could
be a potential biomarker for cancer detection (90). This could be a further potential
limitation. However, this present study of blood-based biomarkers
focused on gene expression profiles of blood cells instead of CRC
cells. Investigating the consistency between CRC cell MSI levels
and blood cell gene expression profiles in CRC is required in the
future.
The relationship between bowel microbiota and immune
cells also requires further investigation. Microbiota populations
in the human large bowel usually exist in a symbiotic relationship
with the host, and there is an increasing amount of evidence to
suggest that the intestinal microbiota plays an important role in
the development of CRC (91). For
example, microbiotic imbalances may expose the colon to different
metabolic and inflammatory stimuli (92).
In conclusion, the present study established a
peripheral blood analytic methodology as a promising technology for
the diagnosis of CRC. The results also provide more information
about the immune system-related pathogenic mechanisms involved in
CRC. In addition, these findings may provide clues to potential
novel immunotherapy targets for CRC.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Miss Qian Shi
(Huaxia Bangfu Technology, Inc.), who performed the microarray
experiments.
Funding
The present study was supported by Huaxia Bangfu
Technology, Inc., and funded by The Special Scientific Research
Fund of the Health and Family Planning Commission of Shanghai
Pudong New Area (grant no. PW2018E-02).
Availability of data and materials
The datasets used and/or analyzed during the
present study are available from the corresponding author upon
reasonable request. The microarray datasets generated and/or
analyzed during the current study are available in the Gene
Expression Omnibus (accession no. GSE164191) repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE164191).
Authors' contributions
ZS contributed to the conception of the study,
acquisition of data, performed the histological examination and was
a major contributor in writing the manuscript. WX contributed to
the conception of the study, acquisition of data, performed the
imaging and histological examination, and was a major contributor
in writing the manuscript. YL contributed to the conception and
design of the study, and was a major contributor in writing the
manuscript. YS contributed to the acquisition and analysis of data
and performed the imaging and histological examination. MW
contributed to the bioinformatics analysis and interpretation of
data. RZ contributed to the statistical analysis and interpretation
of data. GS, ZL, LS and CW contributed to the acquisition of data,
and performed the colonoscopy examination. CCL contributed to the
conception and design of the study, and reviewed and edited the
manuscript. LY contributed to the conception of the study, analysis
and interpretation of data, and reviewed and edited the manuscript.
GC contributed to the conception and design of the study, and
performed the imaging and histological examination. CC contributed
to the conception and design of the study, analysis and
interpretation of data, and reviewed and edited the manuscript. GC,
WX and CC confirm the authenticity of all the raw data. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of The Affiliated Hospital of Qingdao University
(Qingdao, China; IRB no. QYFYWZLL25569) and The Ethics Committee of
The Seventh People's Hospital of Shanghai University of Traditional
Chinese Medicine (Shanghai, China; IRB no. 2018-IRBQYYS-029). All
121 participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
CC, YL, MW, RZ and LY are employees of Huaxia
Bangfu Technology, Inc., who partially sponsored this research. The
other authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
TIL
|
tumor infiltrating lymphocytes
|
|
KM
|
Kaplan-Meier
|
References
|
1
|
Basanta D and Anderson ARA: Homeostasis
back and forth: An ecoevolutionary perspective of cancer. Cold
Spring Harb Perspect Med. 7:1–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shiao SL, Ganesan AP, Rugo HS and Coussens
LM: Immune microenvironments in solid tumors: New targets for
therapy. Genes Dev. 25:2559–2572. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Balkwill F and Mantovani A: Inflammation
and cancer: Back to Virchow? Lancet. 357:539–545. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Karin M: Nuclear factor-kappaB in cancer
development and progression. Nature. 441:431–436. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shinko D, Diakos CI, Clarke SJ and Charles
KA: Cancer-related systemic inflammation: The challenges and
therapeutic opportunities for personalized medicine. Clin Pharmacol
Ther. 102:599–610. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Janssen LME, Ramsay EE, Logsdon CD and
Overwijk WW: The immune system in cancer metastasis: Friend or foe?
J Immunother Cancer. 5:792017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Visser KE, Eichten A and Coussens LM:
Paradoxical roles of the immune system during cancer development.
Nat Rev Cancer. 6:24–37. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pernot S, Terme M, Voron T, Colussi O,
Marcheteau E, Tartour E and Taieb J: Colorectal cancer and
immunity: What we know and perspectives. World J Gastroenterol.
20:3738–3750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Markman JL and Shiao SL: Impact of the
immune system and immunotherapy in colorectal cancer. J
Gastrointest Oncol. 6:208–223. 2015.PubMed/NCBI
|
|
10
|
Fletcher R, Wang YJ, Schoen RE, Finn OJ,
Yu J and Zhang L: Colorectal cancer prevention: Immune modulation
taking the stage. Biochim Biophys Acta Rev Cancer. 1869:138–148.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grivennikov SI, Greten FR and Karin M:
Immunity, inflammation, and cancer. Cell. 140:883–899. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Laghi L, Bianchi P, Miranda E, Balladore
E, Pacetti V, Grizzi F, Allavena P, Torri V, Repici A, Santoro A,
et al: CD3+ cells at the invasive margin of deeply
invading (pT3-T4) colorectal cancer and risk of post-surgical
metastasis: A longitudinal study. Lancet Oncol. 10:877–884. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang B, Wang Z, Wu L, Zhang M, Li W, Ding
J, Zhu J, Wei H and Zhao K: Circulating and tumor-infiltrating
myeloid-derived suppressor cells in patients with colorectal
carcinoma. PLoS One. 8:e571142013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kryczek I, Wu K, Zhao E, Wei S, Vatan L,
Szeliga W, Huang E, Greenson J, Chang A, Roliński J, et al:
IL-17+ regulatory T cells in the microenvironments of
chronic inflammation and cancer. J Immunol. 186:4388–4395. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Műzes G, Molnár B and Sipos F: Regulatory
T cells in inflammatory bowel diseases and colorectal cancer. World
J Gastroenterol. 18:5688–5694. 2012. View Article : Google Scholar
|
|
16
|
Märkl B, Wieberneit J, Kretsinger H, Mayr
P, Anthuber M, Arnholdt HM and Schenkirsch G: Number of
intratumoral T lymphocytes is associated with lymph node size,
lymph node harvest, and outcome in node-negative colon cancer. Am J
Clin Pathol. 145:826–836. 2016. View Article : Google Scholar
|
|
17
|
Zeng ZS, Huang Y, Cohen AM and Guillem JG:
Prediction of colorectal cancer relapse and survival via tissue RNA
levels of matrix metalloproteinase-9. J Clin Oncol. 14:3133–3140.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Camus M, Tosolini M, Mlecnik B, Pagès F,
Kirilovsky A, Berger A, Costes A, Bindea G, Charoentong P, Bruneval
P, et al: Coordination of intratumoral immune reaction and human
colorectal cancer recurrence. Cancer Res. 69:2685–2693. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Galon J, Pagès F, Marincola FM, Thurin M,
Trinchieri G, Fox BA, Gajewski TF and Ascierto PA: The immune score
as a new possible approach for the classification of cancer. J
Transl Med. 10:12012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang RF and Wang HY: Immune targets and
neoantigens for cancer immunotherapy and precision medicine. Cell
Res. 27:11–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mileo AM, Nisticò P and Miccadei S:
Polyphenols: Immunomodulatory and therapeutic implication in
colorectal cancer. Front Immunol. 10:7292019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nixon AB, Schalper KA, Jacobs I, Potluri
S, Wang IM and Fleener C: Peripheral immune-based biomarkers in
cancer immunotherapy: Can we realize their predictive potential? J
Immunother Cancer. 7:3252019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ciardiello D, Vitiello PP, Cardone C,
Martini G, Troiani T, Martinelli E and Ciardiello F: Immunotherapy
of colorectal cancer: Challenges for therapeutic efficacy. Cancer
Treat Rev. 76:22–32. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chao S, Cheng C and Liew CC: Mining the
dynamic genome: A method for identifying multiple disease
signatures using quantitative RNA expression analysis of a single
blood sample. Microarrays (Basel). 4:671–689. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liew CC, Ma J, Tang HC, Zheng R and
Dempsey AA: The peripheral blood transcriptome dynamically reflects
system wide biology: A potential diagnostic tool. J Lab Clin Med.
147:126–132. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mohr S and Liew CC: The peripheral-blood
transcriptome: New insights into disease and risk assessment.
Trends Mol Med. 13:422–432. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liew CC: Method for detection of gene
transcripts in blood and uses thereof. Patent US7598031B2. Filed
October 9, 2002; issued January 22, 2004.
|
|
28
|
Omar H, Lim CR, Chao S, Lee MM, Bong CW,
Ooi EJ, Yu CG, Tan SS, Abu Hassan MR, Menon J, et al: Blood gene
signature for early hepatocellular carcinoma detection in patients
with chronic hepatitis B. J Clin Gastroenterol. 49:150–157. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zaatar AM, Lim CR, Bong CW, Lee MM, Ooi
JJ, Suria D, Raman R, Chao S, Yang H, Neoh SB, et al: Whole blood
transcriptome correlates with treatment response in nasopharyngeal
carcinoma. J Exp Clin Cancer Res. 31:762012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Marshall KW, Mohr S, Khettabi FE, Nossova
N, Chao S, Bao W, Ma J, Li XJ and Liew CC: A blood-based biomarker
panel for stratifying current risk for colorectal cancer. Int J
Cancer. 126:1177–1186. 2010.PubMed/NCBI
|
|
31
|
Chao S, Ying J, Liew G, Marshall W, Liew
CC and Burakoff R: Blood RNA biomarker panel detects both left- and
right-sided colorectal neoplasms: A case-control study. J Exp Clin
Cancer Res. 32:442013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liong ML, Lim CR, Yang H, Chao S, Bong CW,
Leong WS, Das PK, Loh CS, Lau BE, Yu CG, et al: Blood-based
biomarkers of aggressive prostate cancer. PLoS One. 7:e458022012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Osman I, Bajorin DF, Sun TT, Zhong H,
Douglas D, Scattergood J, Zheng R, Han M, Marshall KW and Liew CC:
Novel blood biomarkers of human urinary bladder cancer. Clin Cancer
Res. 12:3374–3380. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mok SC, Kim JH, Skates SJ, Schorge JO,
Cramer DW, Lu KH and Liew CC: Use of blood-based mRNA profiling to
identify biomarkers for ovarian cancer screening. Gynecol Obstet
(Sunnyvale). 7:62017. View Article : Google Scholar
|
|
35
|
Hou H, Lyu Y, Jiang J, Wang M, Zhang R,
Liew CC, Wang B and Cheng C: Peripheral blood transcriptome
identifies high-risk benign and malignant breast lesions. PLoS One.
15:e02337132020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zou C, Lyu Y, Jiang J, Cao Y, Wang M, Sang
C, Zhang R, Li H, Liew CC, Cheng C, et al: Use of peripheral blood
transcriptomic biomarkers to distinguish high-grade cervical
squamous intraepithelial lesions from low-grade lesions. Oncol
Lett. 20:2280–2290. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Liu Q, Luo D, Cai S, Li Q and Li X: P-TNM
staging system for colon cancer: Combination of P-stage and AJCC
TNM staging system for improving prognostic prediction and clinical
management. Cancer Manag Res. 10:2303–2314. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Irizarry RA, Bolstad BM, Collin F, Cope
LM, Hobbs B and Speed TP: Summaries of Affymetrix GeneChip probe
level data. Nucleic Acids Res. 31:e152003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi L, Reid LH, Jones WD, Shippy R,
Warrington JA, Baker SC, Collins PJ, de Longueville F, Kawasaki ES,
Lee KY, et al MAQC Consortium, : The MicroArray Quality Control
(MAQC) project shows inter- and intraplatform reproducibility of
gene expression measurements. Nat Biotechnol. 24:1151–1161. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sidiropoulos K, Viteri G, Sevilla C, Jupe
S, Webber M, Orlic-Milacic M, Jassal B, May B, Shamovsky V, Duenas
C, et al: Reactome enhanced pathway visualization. Bioinformatics.
33:3461–3467. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Y, Zhang T, Xiao R, Hao X, Zhang H,
Qu H, Xie B, Wang T and Fang X: Platform-independent approach for
cancer detection from gene expression profiles of peripheral blood
cells. Brief Bioinform. 21:1006–1015. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Algamal ZY and Lee MH: Regularized
logistic regression with adjusted adaptive elastic net for gene
selection in high dimensional cancer classification. Comput Biol
Med. 67:136–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Szklarczyk D, Morris JH, Cook H, Kuhn M,
Wyder S, Simonovic M, Santos A, Doncheva NT, Roth A, Bork P, et al:
The STRING database in 2017: Quality-controlled protein-protein
association networks, made broadly accessible. Nucleic Acids Res.
45:D362–D368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu G, Wang LG, Han Y and He QY:
clusterProfiler: An R package for comparing biological themes among
gene clusters. OMICS. 16:284–287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ferreira JA: The Benjamini-Hochberg method
in the case of discrete test statistics. Int J Biostat. 3:112007.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shannon P, Markiel A, Ozier O, Baliga NS,
Wang JT, Ramage D, Amin N, Schwikowski B and Ideker T: Cytoscape: A
software environment for integrated models of biomolecular
interaction networks. Genome Res. 13:2498–2504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Nagy Á, Lánczky A, Menyhárt O and Győrffy
B: Validation of miRNA prognostic power in hepatocellular carcinoma
using expression data of independent datasets. Sci Rep. 8:92272018.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mihály Z, Kormos M, Lánczky A, Dank M,
Budczies J, Szász MA and Győrffy B: A meta-analysis of gene
expression-based biomarkers predicting outcome after tamoxifen
treatment in breast cancer. Breast Cancer Res Treat. 140:219–232.
2013. View Article : Google Scholar
|
|
49
|
Zhao F and Yu YQ: The prognostic roles of
mRNAs of the exosomes derived from bone marrow stromal cells in
common malignancies: A bioinformatic study. OncoTargets Ther.
11:7979–7986. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ladabaum U, Dominitz JA, Kahi C and Schoen
RE: Strategies for colorectal cancer screening. Gastroenterology.
158:418–432. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Issa IA and Noureddine M: Colorectal
cancer screening: An updated review of the available options. World
J Gastroenterol. 23:5086–5096. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Puppa G, Sonzogni A, Colombari R and
Pelosi G: TNM staging system of colorectal carcinoma: A critical
appraisal of challenging issues. Arch Pathol Lab Med. 134:837–852.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Roelands J, Kuppen PJK, Vermeulen L,
Maccalli C, Decock J, Wang E, Marincola FM, Bedognetti D and
Hendrickx W: Immunogenomic classification of colorectal cancer and
therapeutic implications. Int J Mol Sci. 18:1–20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ge P, Wang W, Li L, Zhang G, Gao Z, Tang
Z, Dang X and Wu Y: Profiles of immune cell infiltration and
immune-related genes in the tumor microenvironment of colorectal
cancer. Biomed Pharmacother. 118:1092282019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Klee CB and Krinks MH: Purification of
cyclic 3,5-nucleotide phosphodiesterase inhibitory protein by
affinity chromatography on activator protein coupled to Sepharose.
Biochemistry. 17:120–126. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Klee CB, Ren H and Wang X: Regulation of
the calmodulin-stimulated protein phosphatase, calcineurin. J Biol
Chem. 273:13367–13370. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Sharma RK, Desai R, Waisman DM and Wang
JH: Purification and subunit structure of bovine brain modulator
binding protein. J Biol Chem. 254:4276–4282. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rusnak F and Mertz P: Calcineurin: Form
and function. Physiol Rev. 80:1483–1521. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lakshmikuttyamma A, Selvakumar P, Kanthan
R, Kanthan SC and Sharma RK: Increased expression of calcineurin in
human colorectal adenocarcinomas. J Cell Biochem. 95:731–739. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sugiura R, Sio SO, Shuntoh H and Kuno T:
Molecular genetic analysis of the calcineurin signaling pathways.
Cell Mol Life Sci. 58:278–288. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Song E, Chen J, Antus B, Wang M, Xie Y,
Yao H and Exton MS: Interleukin-2 enhances susceptibility of colon
cancer cells to FasR mediated apoptosis by up-regulating Fas
receptor level and down-regulating FAP-1 expression. Int J
Immunopathol Pharmacol. 13:113–122. 2000.PubMed/NCBI
|
|
62
|
Gang W, Yu-Zhu W, Yang Y, Feng S, Xing-Li
F and Heng Z: The critical role of calcineurin/NFAT (C/N) pathways
and effective antitumor prospect for colorectal cancers. J Cell
Biochem. 120:19254–19273. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Peuker K, Muff S, Wang J, Künzel S, Bosse
E, Zeissig Y, Luzzi G, Basi M, Strigli A, Ulbricht A, et al:
Epithelial calcineurin controls microbiota-dependent intestinal
tumor development. Nat Med. 22:506–515. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Thomas H: Colorectal cancer: Calcineurin
drives CRC tumorigenesis. Nat Rev Gastroenterol Hepatol.
13:2492016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
O'Brien RJ and Wong PC: Amyloid precursor
protein processing and Alzheimer's disease. Annu Rev Neurosci.
34:185–204. 2011. View Article : Google Scholar
|
|
66
|
Mattson MP: Cellular actions of
beta-amyloid precursor protein and its soluble and fibrillogenic
derivatives. Physiol Rev. 77:1081–1132. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Meng JY, Kataoka H, Itoh H and Koono M:
Amyloid beta protein precursor is involved in the growth of human
colon carcinoma cell in vitro and in vivo. Int J Cancer. 92:31–39.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Venkataramani V, Rossner C, Iffland L,
Schweyer S, Tamboli IY, Walter J, Wirths O and Bayer TA: Histone
deacetylase inhibitor valproic acid inhibits cancer cell
proliferation via down-regulation of the alzheimer amyloid
precursor protein. J Biol Chem. 285:10678–10689. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Roe CM, Fitzpatrick AL, Xiong C, Sieh W,
Kuller L, Miller JP, Williams MM, Kopan R, Behrens MI and Morris
JC: Cancer linked to Alzheimer disease but not vascular dementia.
Neurology. 74:106–112. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Kirschke H, Langner J, Riemann S,
Wiederanders B, Ansorge S and Bohley P: Lysosomal cysteine
proteinases. Ciba Found Symp. 75:15–35. 1979.PubMed/NCBI
|
|
71
|
Lafuse WP, Brown D, Castle L and Zwilling
BS: IFN-gamma increases cathepsin H mRNA levels in mouse
macrophages. J Leukoc Biol. 57:663–669. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Nitatori T, Sato N, Kominami E and
Uchiyama Y: Participation of cathepsins B, H, and L in perikaryal
condensation of CA1 pyramidal neurons undergoing apoptosis after
brief ischemia. Adv Exp Med Biol. 389:177–185. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Mithöfer K, Fernández-del Castillo C,
Rattner D and Warshaw AL: Subcellular kinetics of early trypsinogen
activation in acute rodent pancreatitis. Am J Physiol. 274:G71–G79.
1998.
|
|
74
|
Williams ST and Beart RW Jr: Staging of
colorectal cancer. Semin Surg Oncol. 8:89–93. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Durdey P, Cooper JC, Switala S, King RF
and Williams NS: The role of peptidases in cancer of the rectum and
sigmoid colon. Br J Surg. 72:378–381. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
del Re EC, Shuja S, Cai J and Murnane MJ:
Alterations in cathepsin H activity and protein patterns in human
colorectal carcinomas. Br J Cancer. 82:1317–1326. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Schweiger A, Christensen IJ, Nielsen HJ,
Sørensen S, Brünner N and Kos J: Serum cathepsin H as a potential
prognostic marker in patients with colorectal cancer. Int J Biol
Markers. 19:289–294. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Hershberg RM, Framson PE, Cho DH, Lee LY,
Kovats S, Beitz J, Blum JS and Nepom GT: Intestinal epithelial
cells use two distinct pathways for HLA class II antigen
processing. J Clin Invest. 100:204–215. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Song H and Ji X: The mechanism of RNA
duplex recognition and unwinding by DEAD-box helicase DDX3X. Nat
Commun. 10:30852019. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sharma D and Jankowsky E: The Ded1/DDX3
subfamily of DEAD-box RNA helicases. Crit Rev Biochem Mol Biol.
49:343–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Guenther UP, Weinberg DE, Zubradt MM,
Tedeschi FA, Stawicki BN, Zagore LL, Brar GA, Licatalosi DD, Bartel
DP, Weissman JS, et al: The helicase Ded1p controls use of
near-cognate translation initiation codons in 5 UTRs. Nature.
559:130–134. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Zhao L, Mao Y, Zhou J, Zhao Y, Cao Y and
Chen X: Multifunctional DDX3: Dual roles in various cancer
development and its related signaling pathways. Am J Cancer Res.
6:387–402. 2016.PubMed/NCBI
|
|
83
|
Su CY, Lin TC, Lin YF, Chen MH, Lee CH,
Wang HY, Lee YC, Liu YP, Chen CL and Hsiao M: DDX3 as a strongest
prognosis marker and its downregulation promotes metastasis in
colorectal cancer. Oncotarget. 6:18602–18612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Heerma van Voss MR, Vesuna F, Trumpi K,
Brilliant J, Berlinicke C, de Leng W, Kranenburg O, Offerhaus GJ,
Bürger H, van der Wall E, et al: Identification of the DEAD box RNA
helicase DDX3 as a therapeutic target in colorectal cancer.
Oncotarget. 6:28312–28326. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Cao J, Wang Y, Dong R, Lin G, Zhang N,
Wang J, Lin N, Gu Y, Ding L, Ying M, et al: Hypoxia-induced WSB1
promotes the metastatic potential of osteosarcoma cells. Cancer
Res. 75:4839–4851. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Schmidt M, Haas W, Crosas B, Santamaria
PG, Gygi SP, Walz T and Finley D: The HEAT repeat protein Blm10
regulates the yeast proteasome by capping the core particle. Nat
Struct Mol Biol. 12:294–303. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Qian MX, Pang Y, Liu CH, Haratake K, Du
BY, Ji DY, Wang GF, Zhu QQ, Song W, Yu Y, et al:
Acetylation-mediated proteasomal degradation of core histones
during DNA repair and spermatogenesis. Cell. 153:1012–1024. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Blickwedehl J, Olejniczak S, Cummings R,
Sarvaiya N, Mantilla A, Chanan-Khan A, Pandita TK, Schmidt M,
Thompson CB and Bangia N: The proteasome activator PA200 regulates
tumor cell responsiveness to glutamine and resistance to ionizing
radiation. Mol Cancer Res. 10:937–944. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Boland CR and Goel A: Microsatellite
instability in colorectal cancer. Gastroenterology.
138:2073–2087.e3. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Chang L, Chang M, Chang HM and Chang F:
Microsatellite instability: A predictive biomarker for cancer
immunotherapy. Appl Immunohistochem Mol Morphol. 26:e15–e21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lucas C, Barnich N and Nguyen HTT:
Microbiota, inflammation and colorectal cancer. Int J Mol Sci.
18:13102017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Halfvarson J, Brislawn CJ, Lamendella R,
Vázquez-Baeza Y, Walters WA, Bramer LM, D'Amato M, Bonfiglio F,
McDonald D, Gonzalez A, et al: Dynamics of the human gut microbiome
in inflammatory bowel disease. Nat Microbiol. 2:170042017.
View Article : Google Scholar : PubMed/NCBI
|