Introduction
Medullary thyroid carcinoma (MTC) is a malignant
tumor derived from thyroid parafollicular cells (C cells) (1,2). Hazard
et al (3) first reported the
disease in 1959. MTC is more common among women and middle-aged
individuals. MTC is characterized by local infiltration and growth,
and early lymphatic metastasis to the blood channel (4). Clinically, MTC can be classified into
hereditary (20–30% of cases) and sporadic (70–80% of cases) types
(5). MTC is a relatively rare type
of thyroid cancer, accounting for 3–5% of all thyroid tumors, with
a mortality rate of 13.4% (6). The
clinical incidence of MTC is relatively low; however, its
malignancy is higher than that of differentiated thyroid carcinoma,
between the severity of papillary carcinoma and undifferentiated
carcinoma. MTC is an intermediate-grade malignant tumor with rapid
progression, high recurrence rate, high metastasis rate and a poor
prognosis. Up to 70% of patients with MTC have already suffered
from cervical lymph node metastasis when the disease invades the
thyroid nodule. Among these patients, about 10% have also already
suffered from distant metastasis (7,8).
Therefore, early detection and timely treatment are crucial. Color
Doppler ultrasound is the clinically preferred imaging examination
tool for MTC. As a convenient and non-invasive diagnostic method,
Color Doppler ultrasound plays an irreplaceable role in the early
diagnosis and late follow-up of patients with MTC (9). However, ultrasound, as the most widely
used method in the diagnosis of MTC, has several limitations.
Sometimes it is not easy to differentiate papillary thyroid
carcinoma (PTC) from thyroid adenoma, as the sensitivity is not
favorable. Fine-needle aspiration (FNA) is used as the gold
standard for the preoperative diagnosis of MTC; however, the
sensitivity of FNA cytology alone for the diagnosis of MTC may be
relatively low due to different sampling methods and angles
(10). The rate of misdiagnosis and
missed diagnosis is high (11). MTC
originates from C cells that can secrete a variety of
neuroendocrine polypeptides, such as calcitonin (CT) and
carcinoembryonic antigen (CEA), therefore CT and CEA can be adopted
as tumor markers. In fact, CT and CEA have been found to have
crucial value in the clinical auxiliary diagnosis, assessment and
monitoring of MTC (12,13). In the present study, color Doppler
ultrasound was used in combination with the detection of serum CT
and CEA levels for the diagnosis of MTC, and the clinical value of
the combined detection was determined.
Patients and methods
Clinical subjects
A total of 39 patients with MTC were enrolled into
the MTC group. The patients were hospitalized at the Affiliated
Hospital of Qingdao University (Qingdao, Shandong, China) from
January 2012 to December 2018. They were diagnosed and verified
through surgical procedures and pathology laboratory tests. The
inclusion criteria were as follows: i) Patients diagnosed with
sporadic MTC; ii) patients with complete color Doppler ultrasound
and serum CT and CEA examination data; iii) patients without a
history of malignant tumors in other organs other than the thyroid
gland; iv) patients who had signed informed consent forms; v)
patients who cooperated with researchers. The exclusion criteria
were as follows: i) Patients with hereditary MTC, pheochromocytoma,
or comorbid malignant tumors in other organs besides the thyroid
gland; ii) patients without complete clinical data, and those who
did not wish to be included in the study; iii) patients with other
diseases, such as liver and kidney dysfunction, cardiovascular
disease and diabetes; and iv) patients who were smokers or
pregnant. In addition, 50 patients with PTC were enrolled into the
PTC group, and 30 patients with thyroid adenoma were enrolled into
the benign control group. Clinicopathological data of the study
subjects in the three groups are presented in Table I. There were no significant
differences in the clinicopathological variables between the three
groups (P>0.05), and subjects in the three groups were
comparable. The present study was approved by the Medical Ethics
Committee of The Affiliated Hospital of Qingdao University
(LIhao:201219) and all participants signed informed consent
forms.
 | Table I.Comparison of the general information
of subjects in the three groups. |
Table I.
Comparison of the general information
of subjects in the three groups.
| Features | MTC group (n=39) | PTC group (n=50) | Benign control
group | χ2 | P-value |
|---|
| Sex |
| Male | 21 | 24 | 14 | 0.436 | 0.804 |
|
Female | 18 | 26 | 16 |
|
|
| Age (years) |
| ≤40 | 15 | 20 | 9 | 0.860 | 0.651 |
|
>40 | 24 | 30 | 21 |
|
|
| Marriage status |
|
Unmarried | 7 | 11 | 4 | 0.946 | 0.623 |
|
Married | 32 | 39 | 26 |
|
|
| Education |
| High
school degree and below | 23 | 32 | 17 | 0.479 | 0.787 |
| College
degree and above | 16 | 18 | 13 |
|
|
| Income (Yuan) |
|
≤3000 | 24 | 32 | 16 | 0.919 | 0.632 |
|
>3000 | 15 | 18 | 14 |
|
|
| Place of
residence |
| Coastal
region | 12 | 13 | 8 | 0.271 | 0.873 |
|
Inland | 27 | 37 | 22 |
|
|
| Occupation |
|
Farmer | 11 | 13 | 8 | 0.288 | 0.991 |
|
Worker | 19 | 27 | 16 |
|
|
|
Cadres | 9 | 10 | 6 |
|
|
Examination methods
For 1.2.1 Color Doppler ultrasound examination, a
PHILIPS iU22, color Doppler ultrasonic diagnostic apparatus, was
used in the present study, with a linear array probe at a frequency
of 7.0–15.0 MHz. During the examination, patients were asked to lie
in the supine position, with their shoulders raised with pads,
their heads tilted backwards and their necks extended. First,
two-dimensional ultrasound was used to examine the thyroid glands.
The size, morphology and capsule of the thyroid gland were
observed. The location, shape, boundary, size, echo type and
calcification of the lesion were analyzed. In addition, the lymph
nodes around the thyroid gland were examined, particularly the
bilateral cervical lymph nodes, to observe the number, size, shape
and internal echo of the lymph nodes. Lymph node metastasis was
analyzed and recorded. Subsequently, the blood flow signals in and
around the lesion were examined with color Doppler flow imaging
(CDFI).
Determination of serum CT and CEA
levels
For specimen collection and pre-treatment, fasting
elbow venous blood (4 ml) was collected from each patient in the
MTC group at 7:00 a.m. prior to surgery and 3 months after surgery.
Blood samples were also collected from patients in the PTC group
and the benign control group at 7:00 a.m. Samples were coagulated
at room temperature (25°C), followed by centrifugation at 2,264 × g
for 20 min to separate the upper serum. Lipemia and hemolysis were
excluded.
Serum CT and CEA levels were measured using the
electrochemiluminescence method based on the double-antibody
sandwich principle. A Cobas e601 full-automatic chemiluminescence
immunoassay system from Roche Diagnostics was used. The reagents
were provided by Roche Diagnostics (CEA, cat. no. 11731629322; CT,
cat. no. 06445853190). All detection procedures were strictly
performed in accordance with the kit instructions provided by the
manufacturer, and the quality control met the requirements.
Categorization of findings
Pathological diagnosis was the gold standard.
Consistencies between ultrasound examination and pathological
diagnosis were defined as true positives, while inconsistencies
between misdiagnosis, missed diagnosis and uncertainty were defined
as false negatives. In the control group, consistencies between
ultrasound examination and pathological diagnosis were defined as
true negatives, while inconsistencies between them (misdiagnosis
and uncertainty) were defined as false positives. Serum CT and CEA
levels exceeding the cut-off levels were positive, and serum CT and
CEA levels lower than or equal to the cut-off levels were negative.
The joint examinations had a positive outcome if one or more
detection results were positive. If all detection results were
negative, the joint examinations could be regarded as negative.
Statistical analysis
For statistical analysis, SPSS 19.0 (IBM Corp.) was
used. Measurement data exhibited a skewed distribution, and were
expressed as the median and quartile range [M (25–75%)]. The
non-parametric Kruskal-Wallis H test was used for comparisons among
multiple groups, while the Mann-Whitney U test or Bonferroni test
was used for pairwise comparisons between two groups. Enumeration
data were analyzed using the χ2 test. With the thyroid
adenoma group as a control, receiver operating characteristic (ROC)
curves of the CT and CEA of the participants were drawn, and the
areas under the curves (AUC) were calculated. The optimal cut-off
values for CT and CEA for the diagnosis of MTC were determined
according to the maximum Youden indexes. Taking pathological
diagnosis as the gold standard, the examination results were true
positive (a), false positive (b), false negative (c) and true
negative (d). The formula used for calculation was as follows:
Sensitivity=a/(a + c); specificity=d/(d + b); accuracy=(a + d)/(a +
b + c + d); positive predictive value=a/(a + b); negative
predictive value=d/(d + c); positive likelihood ratio=(a/a +
c)/(b/b + d); and negative likelihood ratio=(c/a + c)/(d/b + d).
P<0.05 indicated a statistically significant difference.
Results
Comparison of color Doppler
ultrasonographic features among MTC, PTC and thyroid adenoma
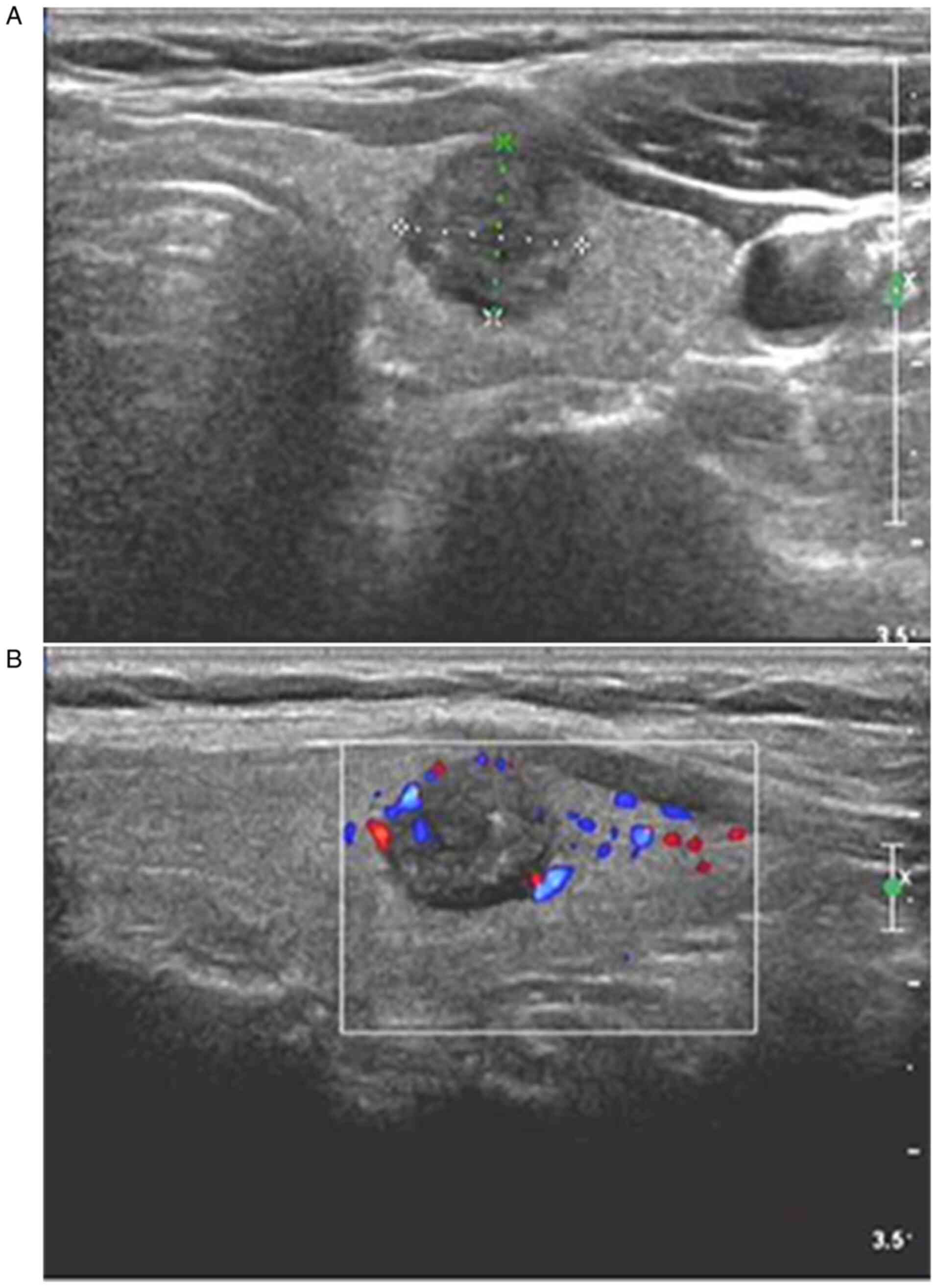
CDFI of MTC samples revealed that MTC samples had a
clear boundary, regular shape (oval or round), an aspect ratio of
≤1, hypoecho or extreme hypoecho, mostly coarse calcification, a
rich and convoluted internal blood flow, and discontinuous
peripheral blood flow signals (Fig.
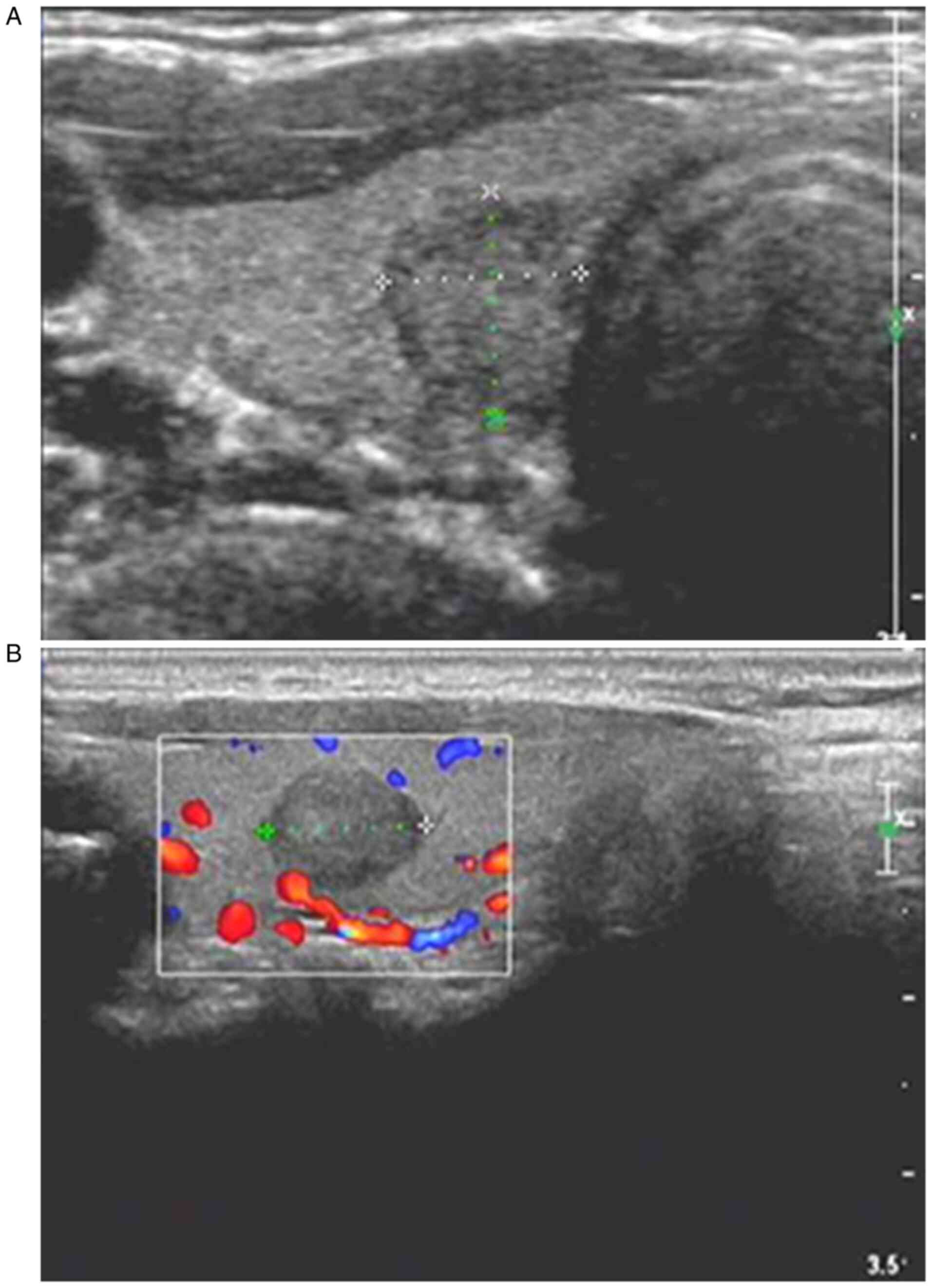
1). CDFI of PTC samples demonstrated that PTC samples had an
unclear boundary, irregular shape, an aspect ratio of >1,
hypoecho or extreme hypoecho, mostly microcalcification, and rich
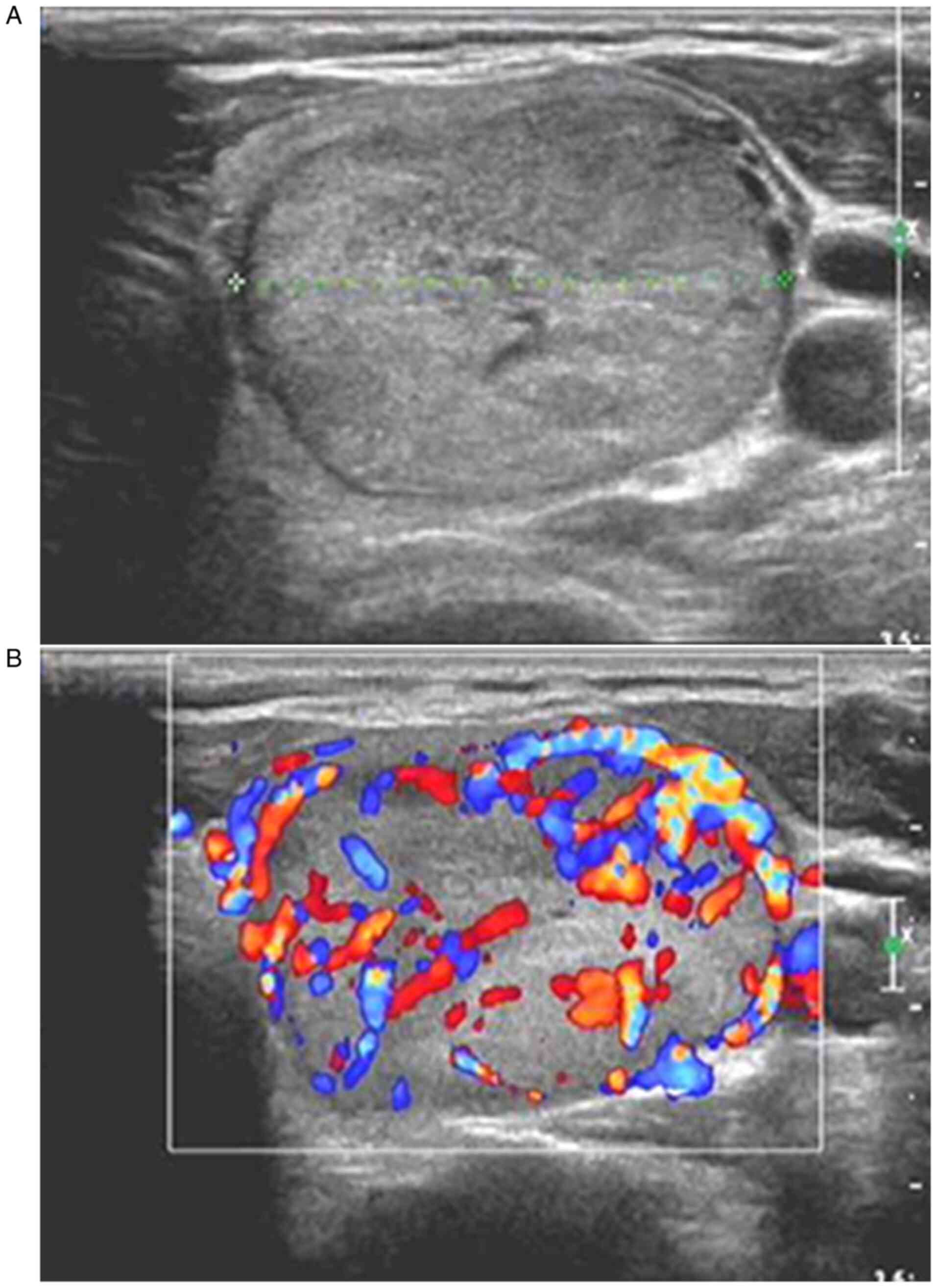
or unrich internal blood flow signals (Fig. 2). CDFI of thyroid adenoma samples
revealed that the thyroid adenoma samples had a clear boundary,
regular shape (oval or round), an aspect ratio of ≤1, hypoecho or
extreme hypoecho, no calcification in the interior, neither rich
nor unrich internal blood flow signals, and a regular course of
peripheral blood flow signals (Fig.
3). The comparison of color Doppler ultrasonographic features
between MTC and PTC samples is shown in Table II, and the comparison of the
ultrasonic features between MTC and thyroid adenoma samples is
presented in Table III.
 | Table II.Comparison of color Doppler
ultrasonographic features between MTC and PTC. |
Table II.
Comparison of color Doppler
ultrasonographic features between MTC and PTC.
| Ultrasonic
imaging | MTC (n=39) | PTC (n=50) | χ2 | P-value |
|---|
| Position |
| Middle
upper pole | 23 | 28 | 0.030 | >0.05 |
| Lower
pole | 16 | 21 |
|
|
| Echo |
|
Hypoecho or extreme
hypoecho | 37 | 44 | 1.265 | >0.05 |
| Isoecho
or hyperecho | 2 | 6 |
|
|
| Boundary |
|
Clear | 24 | 13 | 11.392 | <0.01 |
|
Unclear | 15 | 37 |
|
|
| Shape |
| Round
or oval | 25 | 12 | 14.507 | <0.01 |
|
Irregular | 14 | 38 |
|
|
| Aspect ratio |
|
>1 | 6 | 32 | 21.165 | <0.01 |
| ≤1 | 33 | 18 |
|
|
| Calcification |
| No | 2 | 10 | 24.128 | <0.01 |
|
Microcalcification | 11 | 32 |
|
|
| Course
calcification | 26 | 8 |
|
|
| Internal blood
flow |
| No | 3 | 5 | 13.390 | <0.01 |
| Not
rich | 11 | 32 |
|
|
|
Rich | 25 | 13 |
|
|
| Lymph node
metastasis |
| No | 21 | 43 | 11.214 | <0.01 |
|
Yes | 18 | 7 |
|
|
 | Table III.Comparison of the ultrasonic features
between MTC and thyroid adenoma. |
Table III.
Comparison of the ultrasonic features
between MTC and thyroid adenoma.
| Ultrasonic
imaging | MTC (n=39) | Thyroid adenoma
(n=30) | χ2 | P-value |
|---|
| Position |
| Middle
upper pole | 23 | 18 | 0.007 | >0.05 |
| Lower
pole | 16 | 12 |
|
|
| Echo |
|
Hypoecho or extreme
hypoecho | 37 | 19 | 11.030 | <0.01 |
| Isoecho
or hyperecho | 2 | 11 |
|
|
| Boundary |
|
Clear | 24 | 20 | 0.193 | >0.05 |
|
Unclear | 15 | 10 |
|
|
| Shape |
| Round
or oval | 25 | 23 | 0.361 | >0.05 |
|
Irregular | 14 | 17 |
|
|
| Aspect ratio |
|
>1 | 6 | 3 | 0.433 | >0.05 |
| ≤1 | 33 | 27 |
|
|
| Calcification |
| No | 2 | 25 | 45.609 | <0.01 |
|
Microcalcification | 11 | 4 |
|
|
| Course
calcification | 26 | 1 |
|
|
| Internal blood
flow |
| No | 3 | 8 | 15.99 | <0.01 |
| Not
rich | 11 | 17 |
|
|
|
Rich | 25 | 5 |
|
|
| Lymph node
metastasis |
| No | 21 | 30 | 18.733 | <0.01 |
|
Yes | 18 | 0 |
|
|
Comparison of serum CT and CEA levels
between the MTC, PTC and thyroid adenoma groups
The levels of serum CT and CEA in patients with MTC
were significantly higher compared with those of patients with PTC
and thyroid adenoma (P<0.01). However, there was no significant
difference in the levels of serum CT and CEA between patients with
PTC and those with thyroid adenoma (P>0.05; Table IV).
 | Table IV.Comparison of the levels of serum CT
and CEA among the three groups. |
Table IV.
Comparison of the levels of serum CT
and CEA among the three groups.
| Groups | N | CT (pg/ml) | U | P-value | CEA (ng/ml) | U | P-value |
|---|
| MTC group | 39 | 345.35 (69.34,
1123.44) |
|
| 42.75 (10.67,
102.45) |
|
|
| PTC group | 50 | 3.12 (1.79,
4.12)a | 256.500 | <0.01 | 2.79 (1.38,
3.76)a | 288.000 | <0.01 |
| Benign control
group | 30 | 2.98 (1.15,
3.98)b | 178.000 | <0.01 | 2.64 (1.07,
3.57)b | 139.000 | <0.01 |
Association between serum CT and CEA
levels, and various clinicopathological features of patients with
MTC
The serum levels of CT and CEA in patients with MTC
were not significantly associated with sex and age (both
P>0.05); however, they were significantly associated with the
maximum tumor diameter, lymph node metastasis and the state of the
patients after treatment (all P<0.01; Table V).
 | Table V.Relationship between serum CT and CEA
levels and different clinicopathological features of the patients
with MTC. |
Table V.
Relationship between serum CT and CEA
levels and different clinicopathological features of the patients
with MTC.
| Groups | N | CT (pg/ml) | U | P-value | CEA (ng/ml) | U | P-value |
|---|
| Sex |
|
Male | 21 | 362.19 (58.86,
1210.45) | 425.300 | >0.05 | 46.67 (8.12,
114.61) | 466.900 | >0.05 |
|
Female | 28 | 329.24 (75.67,
1015.36) |
|
| 39.82 (11.23,
98.67) |
|
|
| Age (years) |
|
≤50 | 23 | 327.16 (81.43,
986.75) | 619.200 | >0.05 | 37.54 (12.36,
96.34) | 568.300 | >0.05 |
|
>50 | 16 | 359.78 (61.43,
1225.34) |
|
| 47.61 (9.13,
116.78) |
|
|
| Tumor diameter
(cm) |
| ≤1 | 12 | 102.33 (31.42,
186.31) | 89.000 | <0.01 | 26.54 (6.79,
35.27) | 93.000 | <0.01 |
|
>1 | 27 | 489.43 (125.42,
1361.27) |
|
| 68.79 (37.68,
120.67) |
|
|
| Lymph node
metastasis |
|
Yes | 18 | 782.34 (213.56,
1364.21) | 102.000 | <0.01 | 86.79 (43.22,
33.24) | 113.000 | <0.01 |
| No | 21 | 95.13 (21.56,
159.45) |
|
| 18.67 (7.64,
32.77) |
|
|
| Before and after
treatment |
| Before
treatment | 39 | 345.35 (69.34,
1123.44) | 236.000 | <0.01 | 42.75 (10.67,
102.45) | 201.000 | <0.01 |
| After
treatment | 39 | 5.26 (2.63,
7.34) |
|
| 4.32 (2.17,
5.25) |
|
|
Comparison of the diagnostic value of
color Doppler ultrasound, and serum CT and CEA levels alone, and
color Doppler ultrasound combined with serum CT and CEA levels for
the diagnosis of MTC
With postoperative pathological diagnosis as the
gold standard, among the 39 patients with MTC, consistencies
between ultrasound examination and pathological diagnosis were
found in 30 patients (true positive), while inconsistencies between
the two diagnostic methods were detected in 9 patients (false
negative). Among the 30 patients with thyroid adenoma,
consistencies between ultrasound examination and pathological
diagnosis were found in 28 patients (true negative), while
inconsistencies was detected in 2 patients (false positive).
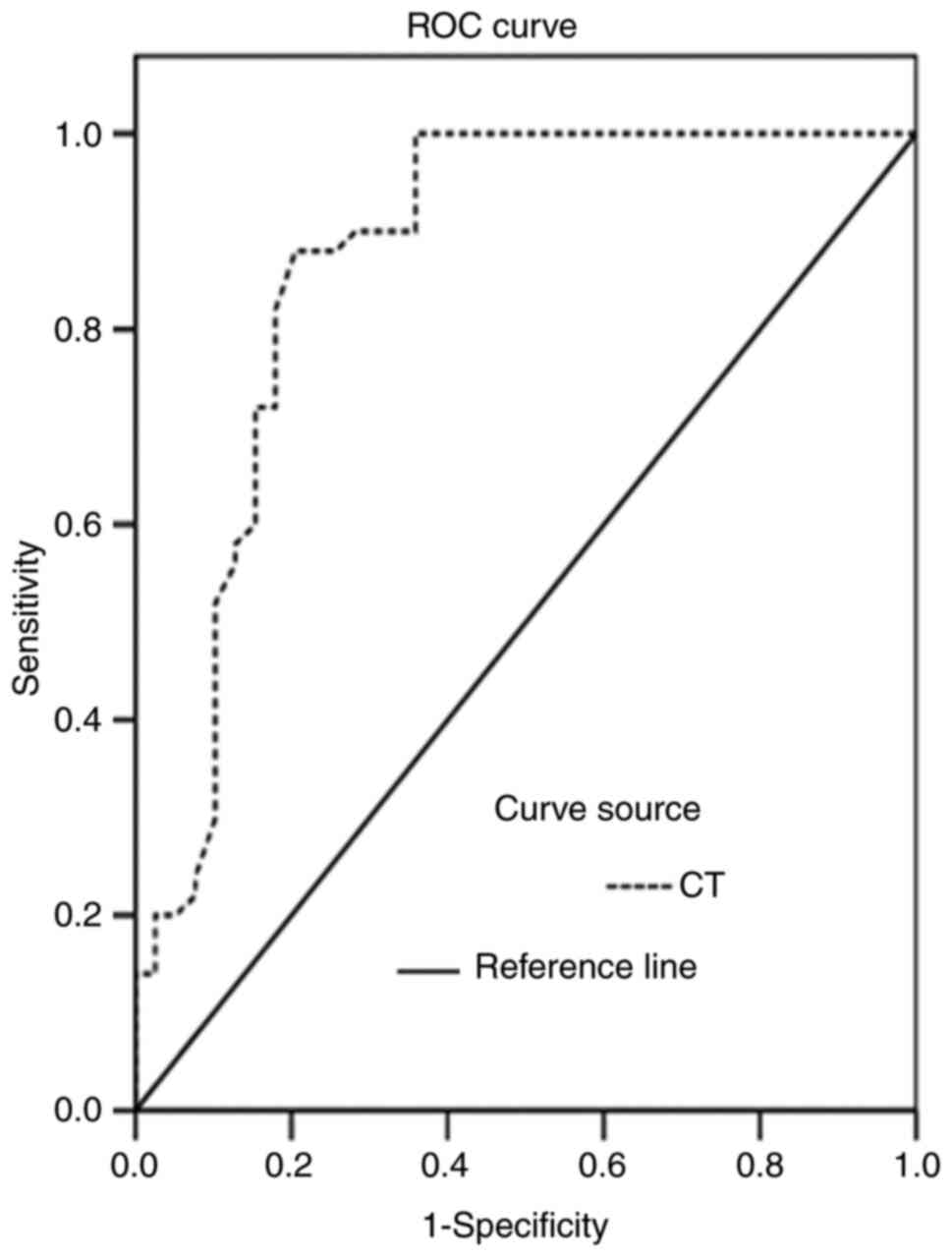
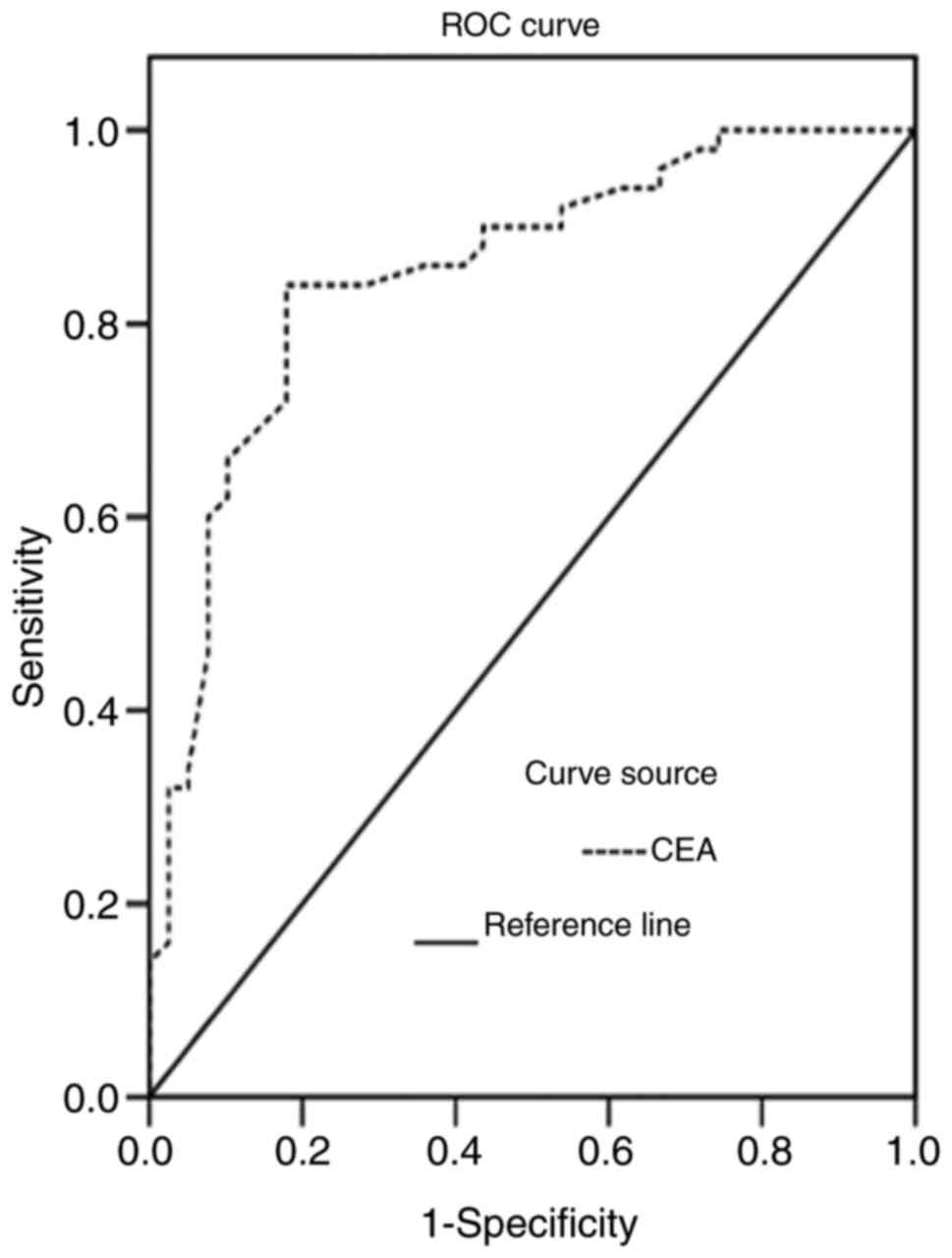
The areas under the ROC curves for CT and CEA levels
in the diagnosis of MTC were 0.838 and 0.789, respectively,
(Figs. 4 and 5). The CT and CEA values corresponding to
the maximum Youden indices of CT and CEA (0.675 and 0.641) were
selected as the optimal critical values for the diagnosis of MTC.
The critical values of CT and CEA were 6.70 pg/ml and 4.60 ng/ml,
respectively. The diagnostic values of color Doppler ultrasound,
serum CT or serum CEA alone, and color Doppler ultrasound combined
with serum CT and CEA for the diagnosis of MTC were analyzed
(Table VI). Color Doppler
ultrasound combined with serum CT and CEA levels exhibited a
significantly higher sensitivity compared with color Doppler
ultrasound, serum CT or serum CEA alone (all P<0.05). The
positive likelihood ratios of ultrasound, serum CT, serum CEA and
the combined detection of MTC were 22.62, 22.54, 10.29 and 14.16,
respectively, and the negative likelihood ratios were 0.24, 0.36,
0.33 and 0.06, respectively.
 | Table VI.Comparison of the diagnostic value of
color Doppler ultrasound, serum CT, serum CEA, and color Doppler
ultrasound combined with serum CT and CEA in diagnosing MTC [%
(n)]. |
Table VI.
Comparison of the diagnostic value of
color Doppler ultrasound, serum CT, serum CEA, and color Doppler
ultrasound combined with serum CT and CEA in diagnosing MTC [%
(n)].
| Detection
indices | Sensitivity | Specificity | Accuracy | Predictive positive
value | Predictive negative
value |
|---|
| Ultrasound | 76.92 (30/39) | 96.67 (29/30) | 84.06 (58/69) | 93.75 (30/32) | 75.68 (28/37) |
| CT | 74.36 (29/39) | 96.67 (29/30) | 84.06 (58/69) | 96.67 (29/30) | 72.50 (29/40) |
| CEA | 68.23 (27/39) | 93.33 (28/30) | 78.26 (54/69) | 90.00 (27/30) | 69.23 (27/39) |
| Ultrasound combined
with CT and CEA | 94.87
(37/39)a | 93.33 (28/30) | 91.30 (63/69) | 90.24 (37/41) | 92.86 (26/28) |
| χ2 | 8.724 | 0.702 | 4.490 | 1.391 | 5.482 |
| P-value | 0.033 | 0.873 | 0.213 | 0.708 | 0.140 |
Discussion
Medullary thyroid carcinoma (MTC) is a highly
malignant tumor and prone to local spread or distant metastasis. It
is crucial to find a correct early diagnosis prior to surgical
intervention in order to make an appropriate surgical scheme to
avoid a second surgery and improve the survival rate (14). It has been shown that the
proto-oncogene RET mutation is the main pathogenic factor in MTC
and can be used to detect MTC (15).
However, this method has several limitations, such as a high cost,
poor diagnostic yield in patients with sporadic MTC. And it is not
able to differentiate the various subtypes of MTC pathogenic genes.
Thus, the detection of the gene cannot be readily applied to
clinical practice on a large scale. In addition, pre-operative
fine-needle aspiration (FNA) has limitations due to its poor
diagnostic positive rate and inability to evaluate lymph node
metastasis. Therefore, researchers have been searching for
economical, efficient and specific indicators and methods for the
pre-operative evaluation of MTC.
At present, neck ultrasonography is the preferred
diagnostic method for MTC. Under ultrasound, MTC can be
preliminarily diagnosed by comprehensively analyzing features,
including morphology, boundary, aspect ratio, internal components,
blood flow signals and the cervical lymph nodes of thyroid nodules.
Ultrasonic signs of MTC have a few common features with those of
papillary thyroid carcinoma (PTC) and thyroid adenoma. MTC is
generally round or oval in shape, similar to thyroid tumors, with
an aspect ratio of ≤1. Lee et al (16) discovered that about 67.40% of MTC
nodules were round or oval, and only 13% of nodules had an aspect
ratio of > in MTC. Kim et al (17) came to a similar conclusion based on
the following clinical observation: The boundary of MTC nodules was
mostly clear, similar to benign lesions (18), while the boundary of PTC nodules was
irregular, unclear or burr-like. Calcification is considered an
important sign of a thyroid malignant tumor (19). Coarse calcification is common in MTC,
which is related to amyloid deposition in the intercellular
substances of C cells. Microcalcification is common in PTC, whereas
thyroid adenoma usually exhibits no calcification. Intranodular
hypoecho and extreme hypoecho are common manifestations of MTC and
PTC ultrasound images (20). In the
present study, it was demonstrated that among patients with MTC,
64.10% of tumors had a round or oval nodule, 61.54% had a clear
boundary, and 84.62% had an aspect ratio ≤1, which is consistent
with the findings of previous studies. In addition, 66.67% of
patients in the MTC group had tumors with course calcification,
which was relatively higher than the results reported by Choi et
al (19), which may be related
to the method used for the patient selection process. There were 32
patients (64.00%) with microcalcification in the PTC group and 25
patients (83.33%) without calcification in the benign control
group. The blood flow signal characteristics of nodules are of
great value in judging their benign and malignant properties.
Compared with PTC, the blood flow signal shown in MTC ultrasound is
richer (21). In addition, under
color Doppler ultrasound, the characteristics of blood flow signals
between MTC and thyroid adenoma are often similar; that is, both
are surrounded by the peripheral blood flow signals of nodules.
However, differences between them can be found through careful
observation. The peripheral blood flow around MTC is discontinuous,
while adenoma can surround the tumor by 1/2 or more, and the course
of blood vessels is regular (22).
The correct diagnosis of MTC under ultrasound requires a
comprehensive judgment based on nodule morphology, edge, aspect
ratio, calcification and blood flow. In the present study, the
sensitivity of ultrasonic diagnosis of MTC was unsatisfactory
(76.92%); consequently, other examinations had to be used along
with ultrasonic diagnosis to improve the diagnostic value.
The application value of calcitonin (CT) has been
evaluated by a series of prospective non-randomized studies.
Routine serum CT screening can be adopted to detect the early
proliferation of C cells and MTC, thus improving the detection rate
of MTC (23,24). CT scans have a very high accuracy to
rule out the possibility of MTC if the serum CT levels are lower
than the reference upper limit (25). The carcinoembryonic antigen (CEA)
level also has an important reference value for the diagnosis of
thyroid cancer (26), and it is more
valuable when serum CT and CEA levels increase simultaneously. As
characteristic secretion products of MTC, serum CT and CEA levels
are significantly increased in the majority of cases with MTC
(27), which was also verified in
the present study. In the present study, it was also demonstrated
that the levels of serum CT and CEA in the MTC group were
significantly higher compared with those in the PTC and benign
control groups. Among the 39 patients with MTC, 29 had increased
levels of CT, and 27 had elevated levels of CEA. Of course, this
does not mean that all patients with elevated serum CT necessarily
suffer from MTC. Equally, normal levels of serum CT and CEA cannot
rule out the possibility of MTC. The accurate diagnosis of MTC can
be achieved by combining the CT scan and CEA level results with
other imaging techniques, such as ultrasonic characteristics. Serum
CT and CEA levels can be helpful tools that may be used to evaluate
the severity of the disease, and high levels of CT and CEA are
indicative of a large tumor with lymph node metastasis (28). In the present study, CT and CEA
levels in patients with tumor diameters of >1 cm and those with
lymph node metastasis were significantly higher compared with those
in patients with tumor diameters ≤1 cm and those without lymph node
metastasis. Therefore, serum CT and CEA levels were also prognostic
predictors of MTC. Serum CT and CEA levels are also helpful for
monitoring the recurrence and metastasis of MTC following surgery.
There is at least one study demonstrating that the aforementioned
indices can be used to reveal the probability of recurrence or
metastasis prior to imaging tests (29). The dynamic observation of serum CT
and CEA levels following surgery can play a crucial role in judging
the effectiveness of the surgical intervention and risk of tumor
recurrence (30). According to the
ROC curves of CT and CEA serum levels, when CT and CEA values
corresponding to the maximum Youden indices of CT (0.675) and CEA
(0.641) were selected as the optimal cut-off values (6.70 pg/ml and
4.60 ng/ml, respectively) for the diagnosis of MTC, the sensitivity
of CT and CEA for the diagnosis of MTC was 74.36 and 68.23%,
respectively. However, the sensitivity of spate examination was
low. The occurrence of false positives and false negatives cannot
be ruled out by in vitro diagnostic tests, and other methods
need to be combined to improve the diagnostic accuracy.
The results of the present study demonstrated that
the sensitivity of ultrasound combined with measuring serum CT and
CEA levels for the diagnosis of MTC was 94.87%, which was
significantly higher than that of single tests (P<0.05). The
positive and negative likelihood rations of combined detection were
14.16 and 0.06, respectively. When the positive likelihood ratio
was >10 or the negative likelihood ratio was <0.1, the
possibility of diagnosing or excluding a certain disease was
significantly increased.
It should be acknowledged that there are a few
limitations to the present study. For example, the present study
did not carry out a systematic follow-up on the long-term
therapeutic effect. In the future, the authors aim to focus on the
association between the changes in serum CT and CEA levels and the
survival rate of patients.
In conclusion, ultrasound combined with measuring
serum CT and CEA levels may be a helpful technique for the early
detection of MTC and was shown to be effective in reducing the
missed diagnosis rate. Nonetheless, the specificity of the combined
examination was reduced. Therefore, it remains necessary to rely on
pathological morphology, immunohistochemistry and specific staining
technique to confirm the diagnosis. The detection of RET
gene mutation can provide an early genetic diagnosis of MTC, which
is the future developmental direction.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XY designed the study and drafted the manuscript. JX
and JS were responsible for the collection and analysis of the
experimental data. LY, RG and ZY performed Color Doppler ultrasound
examination and revised the manuscript critically for important
intellectual content. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
The Affiliated Hospital of Qingdao University (LIhao:201219),
China. Patients who participated in this research, signed the
informed consent and had complete clinical data. Signed written
informed consents were obtained from the patients and/or
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mohammadi M and Hedayati M: A brief review
on the molecular basis of medullary thyroid carcinoma. Cell J.
18:485–492. 2017.PubMed/NCBI
|
|
2
|
Bae YJ, Schaab M and Kratzsch J:
Calcitonin as biomarker for the medullary thyroid carcinoma. Recent
Results Cancer Res. 204:117–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hazard JB, Hawk WA and Crile G Jr:
Medullary (solid) carcinoma of the thyroid; a clinicopathologic
entity. J Clin Endocrinol Metab. 19:152–161. 1959. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Elisei R, Alevizaki M, Conte-Devolx B,
Frank-Raue K, Leite V and Williams GR: 2012 European thyroid
association guidelines for genetic testing and its clinical
consequences in medullary thyroid cancer. Eur Thyroid J. 1:216–231.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roman S, Lin R and Sosa JA: Prognosis of
medullary thyroid carcinoma: Demographic, clinical, and pathologic
predictors ofsurvival in 1252 cases. Cancer. 107:2134–2142. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferlay J, Colombet M, Soerjomataram I,
Dyba T, Randi G, Bettio M, Gavin A, Visser O and Bray F: Cancer
incidence and mortality patterns in Europe: Estimates for 40
countries and 25 major cancers in 2018. Eur J Cancer. 103:356–387.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kebebew E, Ituarte PH, Siperstein AE, Duh
QY and Clark OH: Medullary thyroid carcinoma: Clinical
characteristics, treatment, prognostic factors, and a comparison of
staging systems. Cancer. 88:1139–1148. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Grande E, Santamaría Sandi J, Capdevila J,
Navarro González E, Zafón Llopis C, Ramón Y Cajal Asensio T, Gómez
Sáez JM, Jiménez-Fonseca P, Riesco-Eizaguirre G and Galofré JC:
Consensus on management of advanced medullary thyroid carcinoma on
behalf of the working group of thyroid cancer of the Spanish
society of endocrinology (SEEN) and the Spanish task force group
for orphan and infrequent tumors (GETHI). Clin Transl Oncol.
18:769–775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang GC, Fried K and Levine PH: Detection
of medullary thyroid microcarcinoma using ultrasound-guided fine
needle aspiration cytology. Cytopathology. 24:92–98. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Essig GF Jr, Porter K, Schneider D, Debora
A, Lindsey SC, Busonero G, Fineberg D, Fruci B, Boelaert K, Smit
JW, et al: Fine needle aspiration and medullary thyroid carcinoma:
The risk of inadequate preoperative evaluation and initial surgery
when relying upon FNAB cytology alone. Endocr Pract. 19:920–927.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Trimboli P, Treglia G, Guidobaldi L,
Romanelli F, Nigri G, Valabrega S, Sadeghi R, Crescenzi A, Faquin
WC, Bongiovanni M and Giovanella L: Detection rate of FNA cytology
in medullary thyroid carcinoma: A meta-analysis. Clin Endocrinol
(Oxf). 82:280–285. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jung KY, Kim SM, Yoo WS, Kim BW, Lee YS,
Kim KW, Lee KE, Jeong JJ, Nam KH, Lee SH, et al: Postoperative
biochemical remission of serum calcitonin is the best predictive
factor for recurrence-free survival of medullary thyroid cancer: A
large-scale retrospective analysis over 30 years. Clin Endocrinol
(Oxf). 84:587–597. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Raue F and Frank-Raue K: Long-term
follow-up in medullary thyroid carcinoma. Recent Results Cancer
Res. 204:207–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wells SA Jr, Asa SL, Dralle H, Elisei R,
Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, et al:
Revised American Thyroid Association guidelines for the management
of medullary thyroid carcinoma. Thyroid. 25:567–610. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Romei C, Ciampi R and Elisei R: A
comprehensive overview of the role of the RET proto-oncogene in
thyroid carcinoma. Nat Rev Endocrinol. 12:192–202. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee S, Shin JH, Han BK and Ko EY:
Medullary thyroid carcinoma: Comparison with papillary thyroid
carcinoma and application of current sonographic criteria. AJR Am J
Roentgenol. 194:1090–1094. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kim SH, Kim BS, Jung SL, Lee JW, Yang PS,
Kang BJ, Lim HW, Kim JY, Whang IY, Kwon HS and Jung CK:
Ultrasonographic findings of medullary thyroid carcinoma: A
comparison with papillary thyroid carcinoma. Korean J Radiol.
10:101–105. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Trimboli P, Giovanella L, Valabrega S,
Andrioli M, Baldelli R, Cremonini N, Rossi F, Guidobaldi L,
Barnabei A, Rota F, et al: Ultrasound features of medullary thyroid
carcinoma correlate with cancer aggressiveness: A retrospective
multicenter study. J Exp Clin Cancer Res. 33:872014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Choi N, Moon WJ, Lee JH, Baek JH, Kim DW
and Park SW: Ultrasonographic findings of medullary thyroid cancer:
Differences according to tumor size andcorrelation with fine needle
aspiration results. Acta Radiol. 52:312–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ganeshan D, Paulson E, Duran C, Cabanillas
ME, Busaidy NL and Charnsangavej C: Current update on medullary
thyroid carcinoma. AJR Am J Roentgenol. 201:W867–W876. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cho KE, Gweon HM, Park AY, Yoo MR, Kim J,
Youk JH, Park YM and Son EJ: Ultrasonographic features of medullary
thyroid carcinoma: Do they correlate with Pre and PostOperative
calcitonin levels? Asian Pac J Cancer Prev. 17:3357–3362.
2016.PubMed/NCBI
|
|
22
|
Woliński K, Rewaj-Łosyk M and Ruchała M:
Sonographic features of medullary thyroid carcinomas-a systematic
review and meta-analysis. Endokrynol Pol. 65:314–318. 2014.
View Article : Google Scholar
|
|
23
|
Costante G, Meringolo D, Durante C,
Bianchi D, Nocera M, Tumino S, Crocetti U, Attard M, Maranghi M,
Torlontano M and Filetti S: Predictive value of serum calcitonin
levels for preoperative diagnosis of medullary thyroid carcinoma in
a cohort of 5817 consecutive patients with thyroid nodules. J Clin
Endocrinol Metab. 92:450–455. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chambon G, Alovisetti C, Idoux-Louche C,
Reynaud C, Rodier M, Guedj AM, Chapuis H, Lallemant JG and
Lallemant B: The use of preoperative routine measurement of basal
serum thyrocalcitonin in candidates for thyroidectomy due to
nodular thyroid disorders: Results from 2733 consecutive patients.
J Clin Endocrinol Metab. 96:75–81. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elisei R, Bottici V, Luchetti F, Di Coscio
G, Romei C, Grasso L, Miccoli P, Iacconi P, Basolo F, Pinchera A
and Pacini F: Impact of routine measurement of serum calcitonin on
the diagnosis and outcome of medullary thyroid cancer: Experience
in 10,864 patients with nodular thyroid disorders. J Clin
Endocrinol Metab. 89:163–168. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Papapetrou PD and Polymeris A: Medullary
thyroid carcinoma surgical cytoreduction induces an increase in
serum calcitonin and carcinoembryonic antigen doubling times. Exp
Clin Endocrinol Diabetes. 120:164–168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boschin IM, Torresan F, Toniato A, Zane M,
Ide EC, Pennelli G, Rampin L, Colletti PM, Rubello D and Pelizzo
MR: Incidental medullary thyroid microcarcinoma revealed by mild
increase of preoperative serum calcitonin levels: Therapeutic
implications. Endocrine. 45:448–453. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Machens A, Hauptmann S and Dralle H:
Prediction of lateral lymph node metastases in medullary thyroid
cancer. Br J Surg. 95:586–591. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rowland KJ, Jin LX and Moley JF:
Biochemical cure after reoperations for medullary thyroid
carcinoma: A meta-analysis. Ann Surg Oncol. 22:96–102. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Rosario PW and Calsolari MR: Usefulness of
serum calcitonin in patients without a suspicious history of
medullary thyroid carcinoma and with thyroid nodules without an
indication for fine-needle aspiration or with benign cytology. Horm
Metab Res. 48:372–276. 2016. View Article : Google Scholar : PubMed/NCBI
|