Introduction
Human ovarian cancer (OC) is one of the most lethal
types of cancer in women (1). A
total of 239,000 patients are diagnosed with OC and ~152,000
OC-related deaths are reported worldwide every year (2). OC is a heterogeneous disease comprising
a collection of neoplasms with distinct clinic morphological and
molecular heterogeneity (3). Current
OC therapy includes optimal primary cytoreductive surgery and
systemic chemotherapies comprising taxanes (paclitaxel) and
platinum (cisplatin or carboplatin) compounds (4). Inspite significant advances in surgery
and chemotherapy for OC over the last two decades, this cancer is
associated with poor overall survival of patients (5). In addition, traditional therapy is
characterized by severe side effects and increased drug resistance,
hence novel drugs with higher efficacy should be explored (6). Furthermore, novel personalized
therapies should be developed to improve efficacy in patients with
OC. MicroRNAs are implicated in progression of OC (7). However, a previous study reported
microRNA mediated drug resistance in OC (8). Hence, safety and specificity of
microRNAs based therapeutics needs to be explored further.
Vascular endothelial growth factor, rapamycin
(mTOR), and epidermal growth factor receptor (EGFR) signaling
pathways have been explored extensively for development of OC
therapies (9). Combination of
inhibitors targeting these pathways demonstrates good synergistic
effects in patients with OC (10).
EGFR is an important type of receptor tyrosine kinase and the EGFR
pathway contains epidermal growth factor receptor Her1 (EGFR;
ErbB1), Her2 (ErbB2), Her3 (ErbB3), and Her4 (ErbB4) signaling
molecules. In our previous study, we reported that increased
expression of EGFR is associated with poor prognosis of patients
with OC pathogenesis (11). High
expression levels of ErbB3 and therapeutic regimen targeting ErbB3
were reported in OC cells (12).
High expression levels of ErbB3/Neuregulin 1 in OC cells is
implicated in promotion of omentum metastasis (13). In addition, miR-152 suppresses OC
cell proliferation, migration and invasion, and promotes apoptosis
by inhibiting ErbB3 (14). Recently,
ErbB3 was reported as a potential target in OC treatment (12). A previous study has reported that
35–70% of patients with OC with upregulated EGFR expression have a
poor prognosis (9). Hence, EGFR is a
potential therapeutic target for OC. First-generation EGFR tyrosine
kinase inhibitors (TKIs), such as erlotinib (15) and gefitinib (16) have poor efficacy against OC.
Second-generation EGFR-TKIs, such as dacomitinib (17) are more potent EGFR tyrosine kinase
inhibitors compared with first-generation inhibitors. Currently,
only a few functional assays and combination studies of dacomitinib
with other drugs have been performed against OC (11,18).
Cisplatin is used for OC treatment (19). However, Nuclear factor, erythroid 2
like 2 induced cisplatin resistance in OC by promoting CD99
expression lowers effectiveness of cisplatin (20). To the best of our knowledge, to date
no study has explored the activity of a combination of dacomitinib
and cisplatin on OC cells.
The aim of the present study was to explore
cytotoxicity and the mechanism of action of dacomitinib against OC
cells. This article provides direct evidence that dacomitinib
effectively improved chemosensitivity of cisplatin-resistant human
ovarian cancer cells and expands understanding of dacomitinib
application. These data provide a clue of how to effectively kill
resistant OC cells. However, further studies should be performed to
confirm for the findings of the present study and provide
information on development of effective OC therapy.
Materials and methods
Cell lines and culture conditions
SKOV-3, a human ovarian cancer cell line was
obtained from ATCC. The SKOV3-DDP cell line, which was resistant to
cisplatin was obtained from The Hospital Central Laboratory of
Qingdao University (Qingdao, China). OV4 cells (meaning the OVCAR-4
cell line in the present study) were obtained from the Type Culture
Collection of the Chinese Academy of Sciences. SKOV-3 and SKOV3-DDP
cells were cultured in DMEM (Gibco; Thermo Fisher Scientific Inc.)
containing 10% (v/v) FBS (Gibco; Thermo Fisher Scientific Inc.) at
37°C with 5% CO2, whereas OV4 cells were cultured in
RIPM 1640 (Gibco; Thermo Fisher Scientific Inc.). 100 IU/ml
penicillin and 10 mg/ml streptomycin were added for all sterile
cell culturing.
Wound healing assay
SKOV-3 and OV4 cells (4×105/well) were
plated into 6-well plates and incubated for 48 h in DMEM or RIPM
1640 containing 10% (v/v) FBS at 37°C with 5% CO2. The
cell layer was scratched with a pipette tip to create a fresh wound
in the middle of the wells. Subsequently, FBS-free medium was used
for the wound healing assay. Different concentrations (0, 0.3, 1
and 3 µM) of dacomitinib (cat. no. C9154; Selleck Chemicals) were
added into respective wells. After culturing for 0, 24 and 48 h at
37°C with 5% CO2, photos of each well were taken to
determine cell migration.
Cytotoxicity assay for cells treated
with dacomitinib and cisplatin
SKOV-3 and SKOV3-DDP cells (5×104/well)
were plated in 96-well plates with 100 µl RPMI 1640. Cells were
first treated with 1 µM dacomitinib for 24 h, then 0, 2.5, 5, 10,
20, 40, and 80 µM of cisplatin (DDP) (cat. no. 15663-27-1; Selleck
Chemicals) was added into the wells and cultured at 37°C with 5%
CO2. After 48 h, 20 µl MTT reagent (5 mg/ml; pH=7.4) was
added to the cell culture and left for 4 h, following which 150 µl
DMSO was added for 10 min avoiding light. Optical density (OD)
value of each well was determined at 490 nm using an universal
microplate spectrophotometer.
Cell apoptosis analysis using
fluorescence-activated cell sorting
SKOV3-DDP (1×106) cells treated with or
without dacomitinib and cisplatin were cultured for 24 h at 37°C
with 5% CO2. After washing 3 times with PBS, cells were
stained with a kit containing Annexin V-PE and 7AAD (cat. no.
SY0479; Beijing Biolab Technology Co., Ltd.). Subsequently, cells
were resuspended with 0.2 ml PBS and analyzed using the
fluorescence-activated cell sorting technique using a FACSCantoII
(BD Biosciences) and Flowjo v.10 (Becton, Dickinson & Company).
Both early and late stage apoptosis were assessed.
Western blotting for assessing the
protein expression level of proteins associated with epithelial
mesenchymal transition (EMT) or drug resistance
SKOV-3 and SKOV3-DDP cells treated with dacomitinib
or cisplatin were lysed on ice for 30 min using mammalian cell
lysis RIPA buffer (cat. no. P0013C; Beyotime Insitute of
Biotechnology) containing protease and phosphatase inhibitor
cocktails (1:1,000). Samples were then centrifuged at 13,000 × g
for 20 min at 4°C. Protein concentration was measured by BCA kit
(cat. no. P0012S; Beyotime Institute of Biotechnology).
Supernatants containing 20 µg proteins/lane were mixed with loading
buffer for 10 min at 100°C. Cell lysate proteins were then
separated using 12% SDS-PAGE gels and were transferred to PVDF
membranes. Clipped PVDFs were blocked with 5% non-fat skimmed milk
(dissolved in 1X Tris-buffered saline with 0.1% Tween-20) for 1 h
at room temperature. PVDF membranes were then incubated with
monoclonal primary antibodies against CDH1 (cat. no. Ab76055;
Abcam), SLUG (snail family transcriptional repressor 2) (cat. no.
Ab180714; Abcam), EGFR (cat. no. Ab52894; Abcam), P-EGFR (cat. no.
Ab40815; Abcam), P-GP (cat. no. Ab103477; Abcam), and the internal
reference β-actin (cat. no. 9710; Origene Technologies Inc.)
overnight at 4°C. After washing 3 times with TBST (TBS+0.05%Tween
20), PVDF membranes were incubated with an appropriate horse radish
peroxidase (HRP)-conjugated secondary antibody (cat. no. 15140-122;
Gibco; Thermo Fisher Scientific Inc.), or Goat Anti-Mouse IgG
H&L (HRP) (cat. no. Ab6789; Abcam), or Goat Anti-Rabbit IgG
H&L (HRP) (cat. no. Ab6721; Abcam) respectively. DAB kit (cat.
no. ZLT-9031; Origene Technologies Inc.) was used to determine
protein levels. The dilutions of all the primary antibodies
(1:1,000) and secondary antibodies (1:5,000) were used in the
present study. Image J software (National Institutes of Health) was
used to measure the densitometry of the grey band. CDH1 and SLUG
markers were used for EMT related protein (21,22).
EGFR and P-GP markers were used for drug resistant protein
(23,24).
Determination of differentially
expressed genes (DEGs) and potential proteins associated with low
expression of HER2 (EGFR signaling member) in human OC using
bioinformatics
GSE31432 (Illumina Human HT-12 v.3.0 expression bead
chip) data (25) were retrieved from
the Gene Expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo). The dataset
comprised 4 types of human OC samples: negative group cells treated
with trastuzumab, or pertuzumab, or both. These inhibitors reduce
HER2 expression, hence have similar activity on OC cells as
dacomitinib treatment. DEGs in the negative group and the
drug-treatment group (trastuzumab and pertuzumab) were first
determined (unpaired Student's t-test was used and genes with an
adjusted P-value <0.0004 were defined as DEGs). Functional
proteins were predicted using the String webserver (https://string-db.org/) with Gene Ontology (GO)
enrichment and Kyoto Encyclopedia of Genomics and Genes (KEGG)
pathway analysis.
Statistical analysis
Three or more independent experiments were performed
and data were reported as means ± SD. Statistical analysis were
performed using SPSS Statistical Package v.17 (SPSS, Inc.) or
GraphPad5 software (GraphPad Software Inc.). Unpaired Student's
t-test and one-way ANOVA followed by the post hoc test were
performed to compare means of 2 or more groups, respectively.
P<0.05 was considered to indicate a statistically significant
difference.
Results
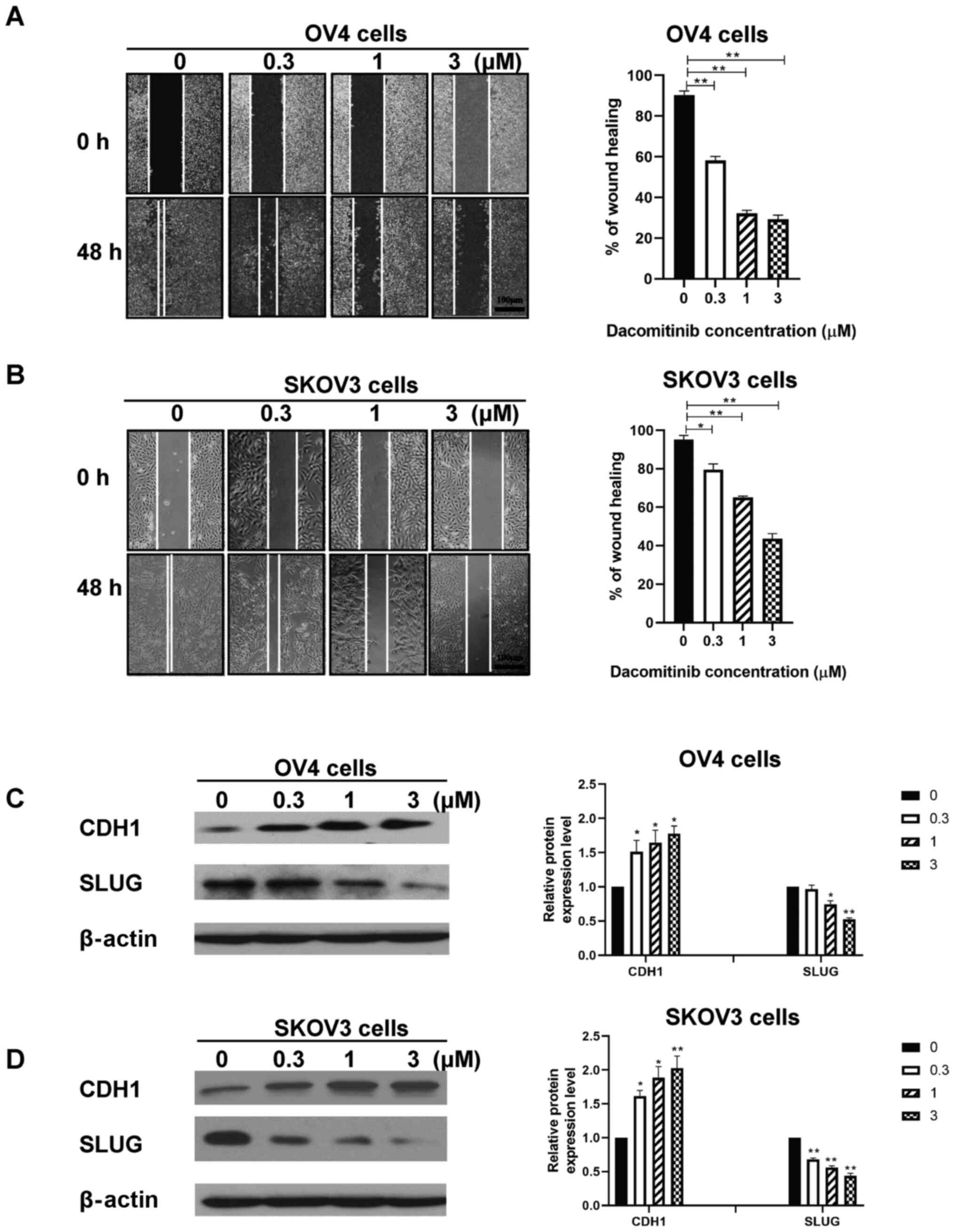
Dacomitinib inhibits migration and
invasive ability of human SKOV3 and OV4 cells in a dose-dependent
manner
Wound healing assay was performed to determine
effect of dacomitinib treatment (0, 0.3, 1, 3 µM) on migration
ability of SKOV3 and OV4 cells. The percentage of wound healing of
OV4 cells treated with 3 µM dacomitinib was 29.30% after 48 h
compared with the control group which had a percentage of wound
healing of 90.33% (Fig. 1A). The
percentage of wound healing of SKOV3 cells treated with 3 µM
dacomitinib was 43.67% after 48 h compared with the control group
which had a percentage of wound healing of 95.33% (Fig. 1B). The aforementioned findings
indicate that dacomitinib inhibits migration ability of human OC
cells. High dosages of dacomitinib demonstrated higher inhibition
of migration ability of OC when compared with control group
(without dacomitinib) (Fig. 1A and
B).
EMT related proteins, CDH1 and SLUG, were chosen to
assess migration and invasive ability of human ovarian cancer cell
following dacomitinib treatment. Western blotting data demonstrated
increased expression of CDH1 and decreased expression of SLUG in
SKOV3 and OV4 cells following treatment with high dacomitinib doses
(3 µM) and vice versa. (Fig. 1C and
D). The aforementioned results indicated that dacomitinib
inhibited migration and invasive ability of OC cells in a
dose-dependent manner.
A combination of dacomitinib and
cisplatin treatment inhibits viability and promotes apoptosis of
human OC cells
To explore the synergistic effects of dacomitinib
with other drugs, cisplatin was chosen and tested on SKOV3 and
SKOV3-DDP cells. Prior to the assay, cisplatin resistant cells were
developed and treated with dacomitinib. Cells were treated with
cisplatin (0, 2.5, 5, 10, 20, 40 and 80 µM) for 24 h. Cells
demonstrated decreased viability following treatment with a higher
dosage of cisplatin (80 µM) (Fig.
2A). Cisplatin had an IC50 of 12.27 µM against SKOV3
cells, and IC50 of 64.34 µM against SKOV3-DDP cells
(drug resistance index=5.24) (data not shown).
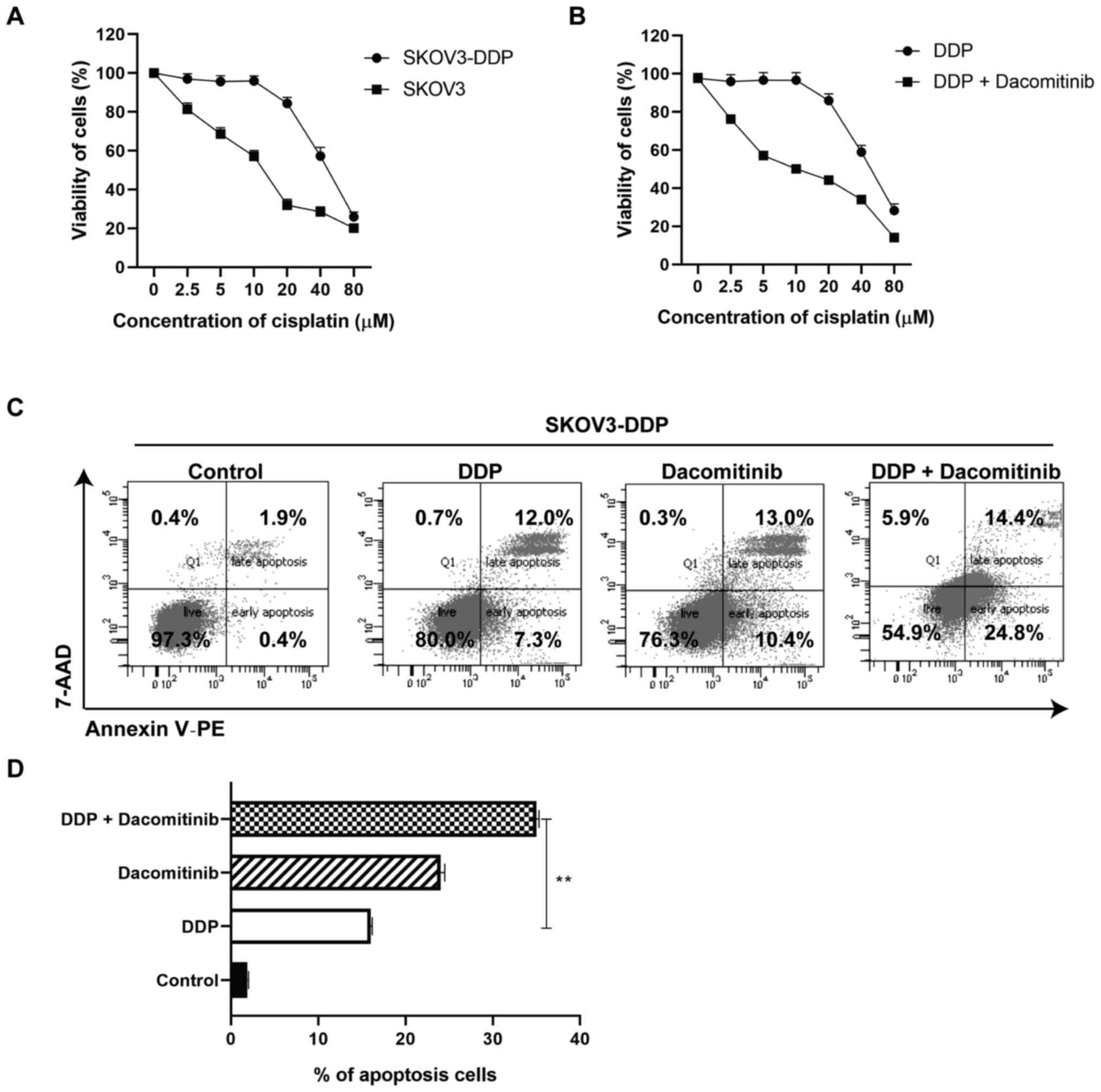 | Figure 2.Viability and cell apoptosis of
cisplatin-resistant ovarian cancer cells treated with dacomitinib.
(A) Viability of SKOV3 and SKOV3-DDP cells after treatment with
cisplatin (0, 2.5, 5, 10, 20, 40, 80 µM) for 24 h. (B) Viability of
SKOV3-DDP cells after treatment with both dacomitinib (1 µM) and
cisplatin (0, 2.5, 5, 10, 20, 40, 80 µM) for 24 h. (C and D) Cell
apoptosis of SKOV3-DDP after treatment with dacomitinib, cisplatin
or both of them for 24 h. **P<0.01. DDP, cisplatin. |
SKOV3-DDP cells were then treated with or without
dacomitinib (1 µM) plus 0, 2.5, 5, 10, 20, 40, 80 µM cisplatin for
24 h. Cells demonstrated decreased viability following treatment
with a higher dosage of cisplatin (80 µM) (Fig. 2B). An IC50 of 11.30 was
observed against SKOV3-DDP cells (a 5.69 times decrease when
compared with IC50 of 64.34 µM against SKOV3-DDP cells)
(data not shown). These findings implied that dacomitinib improved
the antiproliferative effect of cisplatin-resistance in SKOV3-DDP
cells (Fig. 2B). Apoptosis of
SKOV3-DDP cells treated with dacomitinib, cisplatin, or both was
tested using FACS based on Annexin V and 7AAD staining. Apoptotic
assays demonstrated a higher apoptosis rate of cisplatin group
(19.3%), dacomitinib group (23.4%), and dacomitinib plus cisplatin
group (39.2%) compared with the apoptosis rate of the control group
(2.3%). The dacomitinib plus cisplatin group demonstrated a higher
percentage of apoptosis compared with the cisplatin group (DDP)
(Fig. 2C and D). The aforementioned
finding demonstrated that dacomitinib promotes apoptosis of
cisplatin-resistant OC cells.
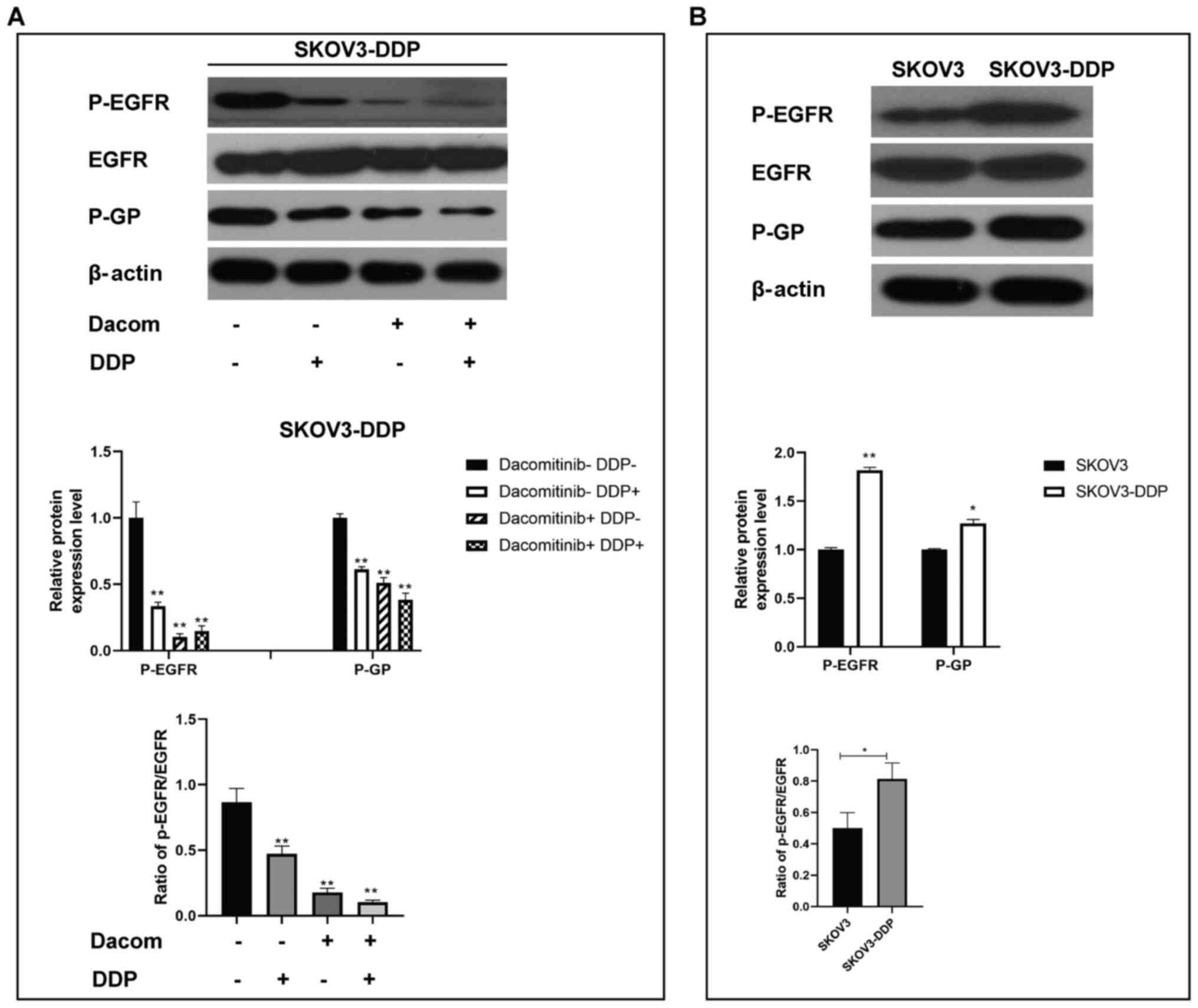
Dacomitinib decreases protein
expression of P-EGFR and P-GP in cisplatin-resistant OC cells
P-EGFR and P-GP protein levels are associated with
EGFR signaling (26,27). Protein levels of p-EGFR and P-GP in
SKOV3-DDP cells treated with dacomitinib and cisplatin were
determined (Fig. 3A and B).
Expression levels of P-EGFR and P-GP in cisplatin, dacomitinib, and
dacomitinib plus cisplatin groups were significantly lower compared
with that in the control group (Fig.
3A). Western blotting analysis demonstrated higher expression
levels of p-EGFR and P-GP in SKOV3-DDP cells (resistant cells)
compared with the levels in SKOV3 cells (Fig. 3B). These findings collectively
implied that dacomitinib, an EGFR inhibitor, regulates p-EGFR and
P-GP directly or indirectly to modulate growth of
cisplatin-resistant OC cells.
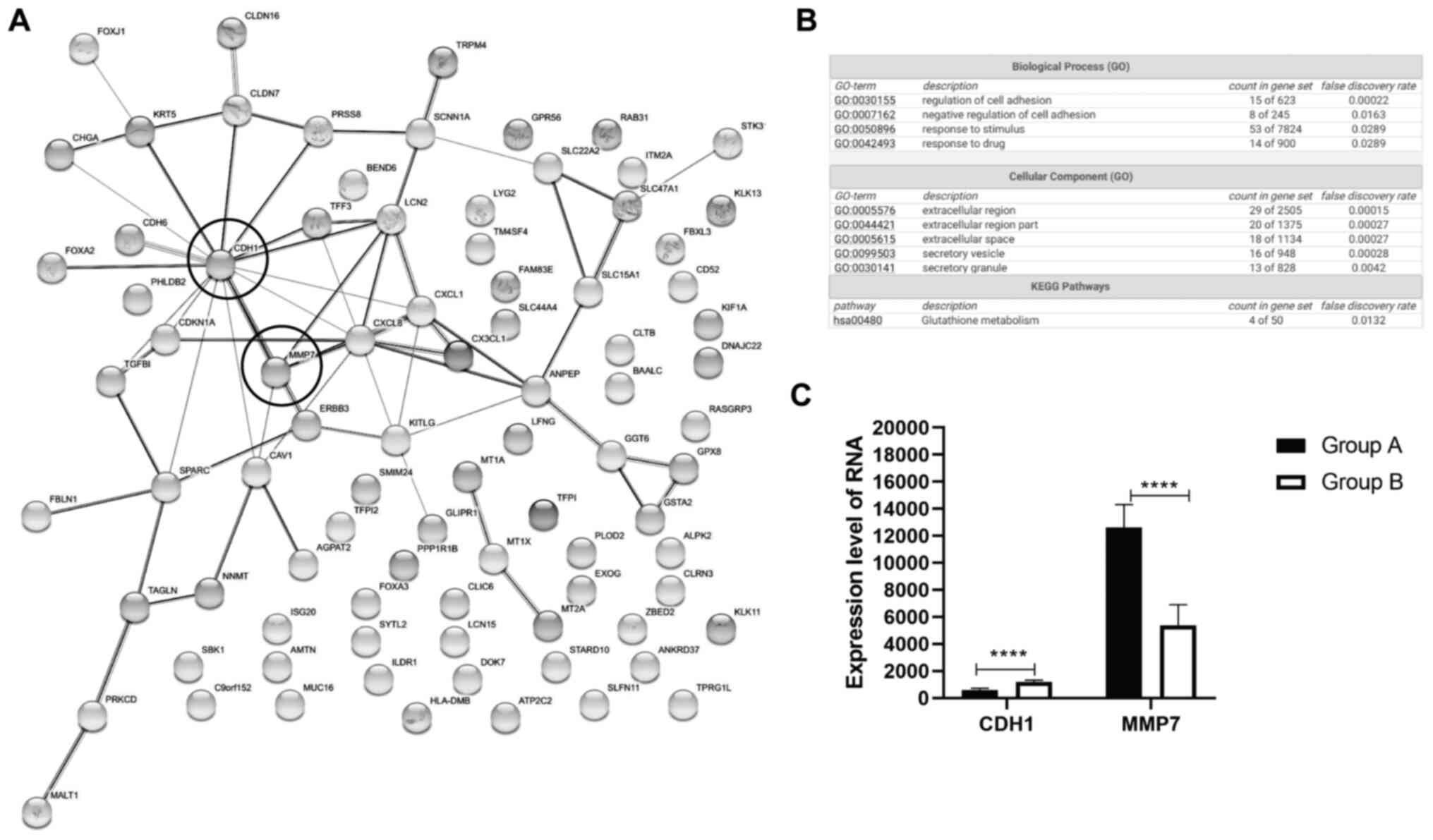
Potential candidate genes CDH1 and
MMP7 are identified in decreased EGFR signaling pathway of OC
To further explore the effect of EGFR inhibitors in
regulation of signaling pathways in human ovarian cancer cells,
SKOV3 samples treated with EGFR inhibitors, such as trastuzumab and
pertuzumab were retrieved from the GEO database (GSE31432).
Dacomitinib, trastuzumab, and pertuzumab are EGFR and HER2
inhibitors (28–30). EGFR inhibitor-treated samples were
used to determine potential genes regulated by dacomitinib. A total
of 6 samples without any treatment were chosen for group A, and 5
samples treated with EGFR inhibitor trastuzumab plus pertuzumab
were chosen for group B. GEO analysis demonstrated that these
samples had good quality of total gene expression (data not shown).
The top 100 genes were identified based on P-value (Table SI).
GO enrichment and KEGG pathway analysis were
performed on these candidate genes using the String webserver. A
total of 66 proteins demonstrated highly related interaction (PPI
enrichment P-value=4.77×10−7) from the top 100 genes
(Fig. 4A). Biological process (GO)
category demonstrated that only 15 proteins were enriched in
regulation of cell adhesion, whereas 8 proteins were enriched in
negative regulation of cell adhesion (Fig. 4B). Cellular component (GO)
demonstrated extracellular region (49 proteins), extracellular
space (18 proteins), and secretory vesicle/granule (29 proteins)
were highly enriched (Fig. 4B). KEGG
pathway analysis showed 4 proteins enriched in glutathione
metabolism signaling (Fig. 4B).
There were several key nodes, such as CDH1
(E-cadherin) and matrix metalloproteinase (MMP7) (Fig. 4A). Expression of CDH1 and MMP7 was
detected in all samples in the GSE31432 dataset. Following EGFR
inhibition, samples of human OC demonstrated increased expression
level of E-cadherin and decreased expression level of MMP7
(Fig. 4C). These findings were
consistent with EGFR inhibition by dacomitinib through reducing
migration and invasive ability of OC by modulation of CDH1.
Discussion
OC drugs are characterized by low efficacy (31). Findings from our previous study
demonstrated that dacomitinib reduces migration of OC cells
(11). In addition, dacomotinib
treatment demonstrated a significant reduction in migration of
cisplatin resistance OC cells in the present study. Further
transwell assays should be performed to confirm the findings of the
wound healing assay. The findings of the current study demonstrated
that dacomitinib decreased expression of EGFR and P-GP in OC
cisplatin-resistant cells. In addition, in the present study
important signaling pathways in OC for the samples with low
expression of EGFR were predicted using GEO analysis and String
webserver. However, the role of these candidate genes in OC should
be confirmed using functional assays after dacomitinib treatment in
future studies.
Dacomitinib is a small-molecule inhibitor of EGFR
(HER1), HER2, and HER4 (32).
Dacomitinib has high cytotoxicity against multiple types of cancer
cells resistant to drugs, such as EGFR inhibitor gefitinib and
erlotinib (33,34). Dacomitinib increases CDH1 and
decreases SLUG expression levels. CDH1 and SLUG proteins are
important EMT related proteins (21,22). EMT
related proteins serve an important role in migration of different
cancer cells including OC cells (35). During EMT, epithelial-type cancer
cells undergo molecular (epigenetic changes), morphological
(biomechanical forces), and functional changes (invasive ability)
(36). High expression levels of
CDH1 is a nonmalignant tumor marker, whereas SLUG binds to the
promoter of CDH1 and inhibits expression of CDH1 (37). The aforementioned studies provide
evidence that dacomitinib inhibits growth of OC cells through
modulation of CDH1 and SLUG expression levels.
In the present study, to determine the effectiveness
of dacomitinib in drug-resistant OC cells, cells were first exposed
to different concentrations of cisplatin. Cisplatin-resistant cells
were then treated with dacomitinib. In the present study,
dacomitinib demonstrated a significant apoptotic effect in cells
exposed to high cisplatin concentrations compared with treatment of
OC cells with cisplatin alone. This implied that a combination of
dacomitinib and cisplatin has a synergistic effect on patients with
OC. The mechanism of action of dacomitinib in killing
drug-resistant OC cells is currently not clear (11).
P-GP (ABCB1) is a 170 kDa transmembrane protein,
expressed from the MDR1 locus (38), and is associated with multiple drug
resistance (39–41). A previous meta-analysis demonstrated
that high expression of EGFR is associated with worse survival
rates of patients with OC, and high expression of P-GP is related
with cisplatin resistance (42).
Hence, in the present study EGFR and P-GP were selected to explore
the function of dacomitinib in drug resistant cells. In the present
study, high expression of EGFR and P-GP were observed in SKOV3-DDP,
but not in SKOV3 cells. The findings of the present study implied
that EGFR and P-GP may participate in progression of resistance. A
recent study reported inhibition of EGFR reverses cisplatin
resistance in OC (43). Cisplatin
exerts its activity by targeting protein kinase G (PKG) (44). Overexpression of PKG2 may inhibit
expression and phosphorylation of EGFR in OC (45). Cisplatin may regulate PKG2 to further
inhibit EGFR in OC, however the exact mechanisms of this need to be
explored. In the present study, dacomitinib treatment reduced
expression of EGFR and P-GP in SKOV3-DDP cells. Hence, dacomitinib
may improve chemosensitivity of cisplatin in OC cells by regulating
expression of EGFR and P-GP.
Dacomitinib treated OC samples were available in the
GEO database (25). In the present
study, western blotting demonstrated that dacomitinib significantly
reduced expression of EGFR. In the present study, samples treated
with other inhibitors had a similar RNA profile as cells treated
with dacomitinib. The findings of the present study revealed
important signaling pathways in OC progression, such as regulation
of cell adhesion, extracellular region part, vesicle,
membrane-bounded vesicle, extracellular space and glutathione
metabolism signaling pathways. Inhibition of EGFR expression
inhibits cell adhesion signaling pathway (46). Dacomitinib may be a potential therapy
for patients with OC.
The present study had several limitations. Firstly,
no transwell invasion assays were used and should be performed by
future studies. Secondly, no propidium iodide was used to evaluate
apoptosis of cell treated with dacomitinib and cisplatin. No in
vivo assays were performed in the present study. Future studies
should perform these to verify the in vitro findings of the
present study.
In conclusion, the present study demonstrated that
dacomitinib inhibits human OC cell viability through modulation of
the protein expression of CDH1 and P-GP. In addition, it decreases
activity of the EGFR signaling pathway improving chemosensitivity
of cisplatin-resistant OC cells. Further studies should be
performed to explore the specific mechanism of dacomitinib effect
on OC development.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This study was partly supported by the Development
Fund of Zibo Maternal and Child Health Hospital and the Key
Research and Development Program of Zibo City (2019gy010009).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LX and YQ conceived, designed, performed all
experiments and wrote the manuscript. LX and YQ confirm the
authenticity of all the raw data. YX and YZ were responsible for
the collection and follow-up of clinical cases. JZ and HW ere
responsible for data statistics. All authors have read and approved
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reid BM, Permuth JB and Sellers TA:
Epidemiology of ovarian cancer: A review. Cancer Biol Med. 14:9–32.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kossaï M, Leary A, Scoazec JY and Genestie
C: Ovarian cancer: A heterogeneous disease. Pathobiology. 85:41–49.
2018. View Article : Google Scholar
|
|
4
|
Angioli R, Palaia I, Zullo MA, Muzii L,
Manci N, Calcagno M and Panici PB: Diagnostic open laparoscopy in
the management of advanced ovarian cancer. Gynecol Oncol.
100:455–461. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ray-Coquard I, Mirza MR, Pignata S,
Walther A, Romero I and du Bois A: Therapeutic options following
second-line platinum-based chemotherapy in patients with recurrent
ovarian cancer: Comparison of active surveillance and maintenance
treatment. Cancer Treat Rev. 90:1021072020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kuroki L and Guntupalli SR: Treatment of
epithelial ovarian cancer. BMJ. 371:m37732020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Deb B, Uddin A and Chakraborty S: miRNAs
and ovarian cancer: An overview. J Cell Physiol. 233:3846–3854.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mihanfar A, Fattahi A and Nejabati HR:
MicroRNA-mediated drug resistance in ovarian cancer. J Cell
Physiol. 234:3180–3191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dinh P, Harnett P, Piccart-Gebhart MJ and
Awada A: New therapies for ovarian cancer: Cytotoxics and
molecularly targeted agents. Crit Rev Oncol Hematol. 67:103–112.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Smolle E, Taucher V, Pichler M, Petru E,
Lax S and Haybaeck J: Targeting signaling pathways in epithelial
ovarian cancer. Int J Mol Sci. 14:9536–9555. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu L, Wu H, Jiang C, Wang H, Gao B, Yan S,
Qi Y and Zhou S: Dacomitinib, a new pan-EGFR inhibitor, is
effective in killing ovarian cancer cells. Discov Med. 22:297–309.
2016.PubMed/NCBI
|
|
12
|
Camblin AJ, Tan G, Curley MD, Yannatos I,
Iadevaia S, Rimkunas V, Mino-Kenudson M, Bloom T, Schoeberl B,
Drummond DC, et al: Dual targeting of IGF-1R and ErbB3 as a
potential therapeutic regimen for ovarian cancer. Sci Rep.
9:168322019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pradeep S, Kim SW, Wu SY, Nishimura M,
Chaluvally-Raghavan P, Miyake T, Pecot CV, Kim SJ, Choi HJ,
Bischoff FZ, et al: Hematogenous metastasis of ovarian cancer:
Rethinking mode of spread. Cancer Cell. 26:77–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li LW, Xiao HQ, Ma R, Yang M, Li W and Lou
G: miR-152 is involved in the proliferation and metastasis of
ovarian cancer through repression of ERBB3. Int J Mol Med.
41:1529–1535. 2018.PubMed/NCBI
|
|
15
|
Gordon AN, Finkler N, Edwards RP, Garcia
AA, Crozier M, Irwin DH and Barrett E: Efficacy and safety of
erlotinib HCl, an epidermal growth factor receptor (HER1/EGFR)
tyrosine kinase inhibitor, in patients with advanced ovarian
carcinoma: Results from a phase II multicenter study. Int J Gynecol
Cancer. 15:785–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schilder RJ, Sill MW, Chen X, Darcy KM,
Decesare SL, Lewandowski G, Lee RB, Arciero CA, Wu H and Godwin AK:
Phase II study of gefitinib in patients with relapsed or persistent
ovarian or primary peritoneal carcinoma and evaluation of epidermal
growth factor receptor mutations and immunohistochemical
expression: A gynecologic oncology group study. Clin Cancer Res.
11:5539–5548. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liao BC, Lin CC and Yang JC: Second and
third-generation epidermal growth factor receptor tyrosine kinase
inhibitors in advanced nonsmall cell lung cancer. Curr Opin Oncol.
27:94–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Momeny M, Zarrinrad G, Moghaddaskho F,
Poursheikhani A, Sankanian G, Zaghal A, Mirshahvaladi S, Esmaeili
F, Eyvani H, Barghi F, et al: Dacomitinib, a pan-inhibitor of ErbB
receptors, suppresses growth and invasive capacity of
chemoresistant ovarian carcinoma cells. Sci Rep. 7:42042017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu J, Zhang L, Li H, Wu S and Liu Z: Nrf2
induced cisplatin resistance in ovarian cancer by promoting CD99
expression. Biochem Biophys Res Commun. 518:698–705. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li R, Ong SL, Tran LM, Jing Z, Liu B, Park
SJ, Huang ZL, Walser TC, Heinrich EL, Lee G, et al: Chronic
IL-1beta-induced inflammation regulates epithelial-to-mesenchymal
transition memory phenotypes via epigenetic modifications in
non-small cell lung cancer. Sci Rep. 10:3772020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miao Y, Liu G and Liu L: Histone
methyltransferase SUV39H2 regulates LSD1-dependent CDH1 expression
and promotes epithelial mesenchymal transition of osteosarcoma.
Cancer Cell Int. 21:22021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao L, Fan T, Shi Z, Ding C, Zhang C,
Yuan Z, Sun Q, Tan C, Chu B and Jiang Y: Design, synthesis and
evaluation of novel ErbB/HDAC multitargeted inhibitors with
selectivity in EGFRT790M mutant cell lines. Eur J Med
Chem. 213:1131732021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hyokai S, Tanaka H, Aihara N and Kamiie J:
Expression of P-glycoprotein and breast cancer resistance protein
in three cases of canine lymphoma showing drug resistance. J Vet
Med Sci. Jan 29–2021.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sims AH, Zweemer AJ, Nagumo Y, Faratian D,
Muir M, Dodds M, Um I, Kay C, Hasmann M, Harrison DJ and Langdon
SP: Defining the molecular response to trastuzumab, pertuzumab and
combination therapy in ovarian cancer. Br J Cancer. 106:1779–1789.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Toolabi M, Moghimi S, Bakhshaiesh TO,
Salarinejad S, Aghcheli A, Hasanvand Z, Nazeri E, Khalaj A,
Esmaeili R and Foroumadi A:
6-Cinnamoyl-4-arylaminothienopyrimidines as highly potent cytotoxic
agents: Design, synthesis and structure-activity relationship
studies. Eur J Med Chem. 185:1117862020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu ZN, Shi ZY, Dang YF, Cheng YN, Guan YH,
Hao ZJ, Tian B, He HW and Guo XL: Pantoprazole pretreatment
elevates sensitivity to vincristine in drug-resistant oral
epidermoid carcinoma in vitro and in vivo. Biomed Pharmacother.
120:1094782019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hao Sun and Wu YL: Dacomitinib in
non-small-cell lung cancer: A comprehensive review for clinical
application. Future Oncol. 15:2769–2777. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hurvitz SA, Caswell-Jin JL, McNamara KL,
Zoeller JJ, Bean GR, Dichmann R, Perez A, Patel R, Zehngebot L,
Allen H, et al: Pathologic and molecular responses to neoadjuvant
trastuzumab and/or lapatinib from a phase II randomized trial in
HER2-positive breast cancer (TRIO-US B07). Nat Commun. 11:58242020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamashita T, Masuda N, Saji S, Araki K,
Ito Y, Takano T, Takahashi M, Tsurutani J, Koizumi K, Kitada M, et
al: Trastuzumab, pertuzumab, and eribulin mesylate versus
trastuzumab, pertuzumab, and a taxane as a first-line or
second-line treatment for HER2-positive, locally advanced or
metastatic breast cancer: Study protocol for a randomized
controlled, non-inferiority, phase III trial in Japan
(JBCRG-M06/EMERALD). Trials. 21:3912020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Huang X and Tang J: Human la protein: An
RNA-binding protein involved in ovarian cancer development and
multidrug resistance. Onco Targets Ther. 13:10721–10727. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Passaro A, Mok T, Peters S, Popat S, Ahn
MJ and de Marinis F: Recent advances on the role of EGFR tyrosine
kinase inhibitors in the management of NSCLC with uncommon, non
exon 20 insertions, EGFR mutations. J Thorac Oncol.
S1556-0864:31102–31103. 2020.(Epub ahead of print).
|
|
33
|
Engelman JA, Zejnullahu K, Gale CM,
Lifshits E, Gonzales AJ, Shimamura T, Zhao F, Vincent PW, Naumov
GN, Bradner JE, et al: PF00299804, an irreversible pan-ERBB
inhibitor, is effective in lung cancer models with EGFR and ERBB2
mutations that are resistant to gefitinib. Cancer Res.
67:11924–11932. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gonzales AJ, Hook KE, Althaus IW, Ellis
PA, Trachet E, Delaney AM, Harvey PJ, Ellis TA, Amato DM, Nelson
JM, et al: Antitumor activity and pharmacokinetic properties of
PF-00299804, a second-generation irreversible pan-erbB receptor
tyrosine kinase inhibitor. Mol Cancer Ther. 7:1880–1889. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kudo-Saito C, Ozaki Y, Imazeki H, Hayashi
H, Masuda J, Ozawa H and Ogiwara Y: Targeting oncoimmune drivers of
cancer metastasis. Cancers (Basel). 13:5542021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Klymenko Y, Kim O and Stack MS: Complex
determinants of epithelial: Mesenchymal phenotypic plasticity in
ovarian cancer. Cancers (Basel). 9:1042017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ahmed AR and Muhammad EM: E-cadherin and
CD10 expression in atypical hyperplastic and malignant endometrial
lesions. J Egypt Natl Canc Inst. 26:211–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gottesman MM and Pastan I: Biochemistry of
multidrug resistance mediated by the multidrug transporter. Annu
Rev Biochem. 62:385–427. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Goldstein LJ, Galski H, Fojo A, Willingham
M, Lai SL, Gazdar A, Pirker R, Green A, Crist W, Brodeur GM, et al:
Expression of a multidrug resistance gene in human cancers. J Natl
Cancer Inst. 81:116–124. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Muthiah D, Henshaw GK, DeBono AJ, Capuano
B, Scammells PJ and Callaghan R: Overcoming P-glycoprotein-mediated
drug resistance with noscapine derivatives. Drug Metab Dispos.
47:164–172. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen Q, Liu X, Luo Z, Wang S, Lin J, Xie
Z, Li M, Li C, Cao H, Huang Q, et al: Chloride channel-3 mediates
multidrug resistance of cancer by upregulating P-glycoprotein
expression. J Cell Physiol. 234:6611–6623. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xu L, Cai J, Yang Q, Ding H, Wu L, Li T
and Wang Z: Prognostic significance of several biomarkers in
epithelial ovarian cancer: A meta-analysis of published studies. J
Cancer Res Clin Oncol. 139:1257–1277. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Poursheikhani A, Yousefi H,
Tavakoli-Bazzaz J and Seyed HG: EGFR blockade reverses cisplatin
resistance in human epithelial ovarian cancer cells. Iran Biomed J.
24:370–378. 2020.PubMed/NCBI
|
|
44
|
Perrotta C, Cervia D, Di Renzo I, Moscheni
C, Bassi MT, Campana L, Martelli C, Catalani E, Giovarelli M,
Zecchini S, et al: Nitric oxide generated by tumor-associated
macrophages is responsible for cancer resistance to cisplatin and
correlated with syntaxin 4 and acid sphingomyelinase inhibition.
Front Immunol. 9:11862018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xu H, Zhang Z, Li P, Lu X, Chen B and Lan
T: Expression of PKG2 in ovarian cancer and its effect on epidermal
growth factor receptor. J BUON. 25:729–735. 2020.PubMed/NCBI
|
|
46
|
Pang J, Jiang P, Wang Y, Jiang L, Qian H,
Tao Y, Shi R, Gao J, Chen Y and Wu Y: Cross-linked hyaluronan gel
inhibits the growth and metastasis of ovarian carcinoma. J Ovarian
Res. 11:222018. View Article : Google Scholar : PubMed/NCBI
|