Introduction
Immunotherapy used in treatment of neoplastic
diseases aims to boost the antitumor response, where
antigen-specific T lymphocytes play an important role. However, the
presence of immunosuppressive cells, such as myeloid-derived
suppressor cells (MDSCs), tumor-associated macrophages (TAMs),
regulatory T lymphocytes (Tregs) and soluble factors [e.g.
interleukin-10 (IL-10), transforming growth factor-β (TGF-β),
vascular endothelial growth factor (VEGF), indoleamine
2,3-dioxygenase (IDO), prostaglandin E2 (PGE2)] in the tumor
microenvironment (TME), contributes to the reduction of the
activity of the effector T cells, thus diminishing the
effectiveness of the applied treatment (1,2). Among
the most potent suppressors of antitumor immunity are
myeloid-derived suppressor cells, a heterogenic population that
consists of monocytic (M-MDSCs) and polymorphonuclear (PMN-MDSCs)
cells, whose differentiation from myeloid precursors is altered by
the tumor-derived factors (3,4). During
carcinogenesis, the accumulation of these cells can be observed in
tumor sites and their frequency is often considered as a biomarker
for predicting the response to the applied immunotherapy and
prognosis of the clinical outcome in patients (5–7). MDSCs
suppress the specific immune response by acting on immunocompetent
cells and promoting escape of the tumor from immune surveillance,
which leads to the progression of the disease. Depletion of amino
acids necessary for T-cell activation, production of reactive
oxygen and nitrogen species, and expression of proteins of a
suppressive nature, such as IL-10, TGF-β and programmed
death-ligand 1 (PD-L1), by MDSCs lead to inhibition of the activity
of antitumor immune cells, as well as induction of Tregs with
suppressor activity (8).
Although recent reports indicate that IL-10 may take
part in induction of MDSCs (9,10), the
role of TME-derived IL-10 in differentiation of MDSCs and
activation of their suppression mechanisms is not fully understood.
Based on the literature, it is known that IL-10 affects the
activity of other myeloid cells in the TME. It hinders the
maturation of dendritic cells (DCs) and reduces the expression of
costimulatory molecules, limiting their ability to induce a
specific antitumor response (11,12).
Moreover, it promotes polarization of macrophages towards M2-type
cells, which in consequence produce elevated amounts of IL-10 that
inhibits the secretion of IL-12 in autocrine fashion (13). In addition, IL-10 impairs the
antitumor activity of CD4+ T cells by reducing the
secretion of Th1-type cytokines, as well as by inducing the
differentiation of Tregs (14,15).
Since these processes invariably lead to tumor progression, IL-10
is regarded as a potential target for antitumor therapies (16). In our previous research, local
elimination of IL-10 was applied in antitumor immunotherapy. Trials
with cyclophosphamide (CY), dendritic cell-based vaccines and
lentiviral vectors (LVs) locally silencing the expression of this
cytokine revealed high efficiency of this kind of treatment in MC38
murine colon carcinoma growth inhibition and showed that the
therapeutic effect was accompanied by a reduction of the percentage
of MDSCs in tumors (17). However,
it was also observed that a few days after application,
intratumorally injected LVs carrying shRNA against IL-10 (shIL-10
LVs) induced an increased influx of PMN-MDSCs into tumors (17). Considering our previous observations,
this study aimed to determine the mechanisms of acting of
lentiviral vectors encoding shRNA against IL-10 with particular
emphasis on their influence on the activity of tumor-derived MDSCs.
Third-generation lentiviral vectors encoding shRNA were used to
silence the expression of IL-10 in ex vivo-generated MDSCs,
as well as to reduce the production of IL-10 in the TME of MC38
colon carcinoma. Lentiviral vectors were injected intratumorally
according to two different schedules, which made it possible to
demonstrate the kinetics of immune response induction after
application of shIL-10 LVs, as well as to establish whether shIL-10
LVs were able to efficiently reduce the IL-10 secretion after
development of antiviral immune response. Since cyclophosphamide
was reported as an IL-10 reducing agent and due to its potential to
increase the antitumor activity of some immunotherapeutic
strategies (18), the intratumoral
activity of shIL-10 LVs was also evaluated when they were applied
in the combined treatment with a low dose of CY. The gathered data
indicate that transduction with shIL-10 LVs results in altered
immunosuppressive activity of ex vivo-generated and
tumor-derived MDSCs. Reduction of immunosuppressive activity of
myeloid cells infiltrating tumors depends on the direct influence
of the vectors on PMN-MDSCs, which were observed to lose their
suppressor activity following transduction with shIL-10 LVs in
in vitro conditions. Nevertheless, this effect appears to be
dependent both on specific silencing of IL-10 expression and
changes mediated by lentiviral transduction itself.
Materials and methods
Mice
Female C57BL/6 mice were obtained from the Center
for Experimental Medicine of the Medical University of Bialystok,
Poland. All experiments were performed in accordance with Directive
2010/63/EU of the European Parliament and of the Council on the
protection of animals used for scientific purposes and were
approved by the 1st Local Ethic Committee for Experiments with the
Use of Laboratory Animals, Wroclaw, Poland (authorization number
11/2015, 33/2018). After the experiments, mice were sacrificed by
cervical dislocation.
Cell culture
The in vivo growing MC38 murine colon
carcinoma from the Tumor Bank of the TNA Radiobiology Institute
(Rijswijk, The Netherlands) was adapted to in vitro
conditions as described by Pajtasz-Piasecka et al (19). MC38/0 cells were maintained in
RPMI-1640 (Gibco) supplemented with 100 U/ml penicillin, 10 mg/ml
streptomycin, 1 mM sodium pyruvate, 0.05 mM 2-mercaptoethanol and
5% fetal bovine serum (FBS; all reagents from Sigma-Aldrich).
Lenti-X 293T cell line (Clontech) was maintained in high-glucose
Dulbecco's Modified Eagle Medium (Gibco) supplemented with 100 U/ml
penicillin, 100 mg/ml streptomycin, 1 mM sodium pyruvate and 10% of
FBS. MDSCs were generated from bone marrow of healthy (wild-type)
C57BL/6 mice isolated according to the procedure described by
Rossowska et al (20). The
cells were cultured in a conditioned medium consisting of 25% RPMI
1640 and 75% supernatant from the above MC38/0 cells
(10×106/10 ml) cultured in hypoxia conditions (1%
O2) for 48 h, supplemented with 10% FBS and 80 ng/ml
recombinant murine granulocyte-macrophage colony-stimulating factor
(rm GM-CSF) (ImmunoTools). The medium was replaced every two days
of culture. On the 6th day, MDSCs were transduced with lentiviral
vectors in the presence of polybrene (10 µg/ml, Sigma-Aldrich).
Production of lentiviral vectors
Lentiviral vectors (LVs) were produced using a
third-generation lentiviral system, which consisted of pMDLg/pRRE,
pRSV-Rev, pMD2.G [the plasmids were a gift from Didier Trono
(Addgene plasmid #12251, 12253, 12259)] and pGLV-H1-GFP + Puro
expression plasmids (EzBiolab). The expression plasmids encoded
three different shRNA sequences against IL-10 (shIL-10) or a
non-targeting sequence of shRNA against human GAPDH (shN), which
was used as a negative control (Fig.
1). Lentiviral vectors were produced and concentrated according
to the procedure described in our previous article (21). Briefly, Lenti-X cells were
co-transfected with plasmids and cultured for 48 h. Supernatant
containing lentiviral vectors was collected and concentrated by
precipitation using PEG 6000 (Sigma-Aldrich). The titer of the
lentiviral vectors was determined by the serial dilution method
using MC38/0 cells.
Determining IL-10 silencing efficiency
in transduced MDSCs
MDSCs were transduced with LVs encoding shRNA
silencing the expression of IL-10. For reference, non-transduced
cells, or cells transduced with control LVs encoding non-targeting
shRNA against human GAPDH, were used. After 3 days, the efficacy of
transduction was determined by flow cytometry as a percentage of
enhanced green fluorescent protein-positive (EGFP+)
cells. The efficiency of IL-10 silencing was evaluated at mRNA and
protein levels using real-time PCR and ELISA, respectively. Total
RNA was isolated using a NucleoSpin RNA kit (Macherey-Nagel) and
reverse-transcribed with a RevertAid First Strand cDNA Synthesis
Kit (Thermo Scientific). Real-time PCR was performed using TaqMan
Universal PCR Master Mix and TaqMan Gene Expression Assay primers
for IL-10 (Applied Biosystems) in reference to the HPRT gene.
Concentration of IL-10 in supernatants from the MDSC 24-h culture
established on the 3rd day after transduction (0.25×106
cells/0.5 ml) was measured by ELISA (BD Biosciences).
Estimation of differentiation level of
transduced MDSCs
Three days after transduction, the phenotype
characterization of MDSCs was performed by flow cytometry. The
cells were labeled with a cocktail of monoclonal antibodies
conjugated with fluorochromes: Anti-CD11b (M1/70) PerCP-Cy5.5,
anti-CD11c (N418) Brilliant Violet (BV) 650, anti-Ly6C (HK1.4) BV
510, anti-Ly6G (1A8) BV 605, anti-MHC II (M5/114.15.2) APC-Cy7,
anti-CD86 (GL-1) PE-Cy7, anti-F4/80 (BM8) Alexa Fluor 700,
anti-PD-L1 (10F.9G2) APC (all from BioLegend). Analysis of the cell
phenotype was performed using LSRFortessa flow cytometer with FACS
Diva software (BD Biosciences). In order to exclude dead cells,
DAPI dye (Invitrogen) was added prior to the analysis.
Determination of suppressive activity
of transduced MDSCs
Spleen cells obtained from healthy (wild-type)
C57BL/6 mice were labeled with CellTrace CFSE (carboxyfluorescein
succinimidyl ester) dye (0.5 µM, 15 min, 37°C; Invitrogen) and
co-cultured with transduced M-MDSCs or PMN-MDSCs sorted with FACS
Aria (BD Biosciences). Cells were cultured in a ratio of 1:1
(1×105 splenocytes and 1×105 M-MDSCs or
1×104 splenocytes and 1×104 PMN-MDSCs) or 2:1
(2×105 splenocytes and 1×105 M-MDSCs or
2×104 splenocytes and 1×104 PMN-MDSCs) in the
presence of concanavalin A (2 µg/ml; Sigma-Aldrich) and rh IL-2
(200 U/ml, ImmunoTools). After 3 days, the cells were labeled with
monoclonal antibodies conjugated with fluorochromes: Anti-CD11b
(M1/70) PerCP-Cy5.5, anti-CD4 (RM4-5) APC, anti-CD8 (53–6.7) PE-Cy
(all from BioLegend). CFSE mean fluorescence intensity (MFI) in
CD4+ and CD8+ cells was measured using the
LSRFortessa (BD Biosciences). Concentrations of interferon-γ
(IFN-γ) and IL-10 in supernatants from the above co-culture were
determined using ELISA kits (eBioscience, Invitrogen and BD
Biosciences, respectively).
Intratumoral application of lentiviral
vectors
Eight to ten-week-old female C57BL/6 mice were
subcutaneously inoculated in the right flank with MC38/0 cells
(1.1×106 cells/0.2 ml/mouse). After the tumor volume had
reached ~50 mm3, mice were randomized and treated with
cyclophosphamide (CY) and/or LVs encoding shRNA against IL-10
(shIL-10 LVs) or with LVs encoding non-targeting shRNA against
human GAPDH (shN LVs). Lentiviral vectors were applied
intratumorally (i.t.; 2×106 TU/50 µl/mouse) three times,
accordingly to the schemes presented in the Results section. The
intratumoral injections were performed with extreme caution and any
mechanical disruption of the tumor tissue were not observed.
Cyclophosphamide (Baxter) was applied i.p. (150 mg/kg body weight)
two days prior to the first LV injection. On the 6th (7–10 mice per
group) or 10th (7–10 mice per group) day of the treatment (LVs) and
on the 8th (3 mice per group) and 12th (9–10 mice per group) day of
the treatment (CY + LVs), mice were sacrificed, and tumors and
tumor-draining lymph nodes (tLNs) were dissected, homogenized and
used for further analyses.
Determination of IL-10 silencing
efficiency in tumors
Concentration of IL-10 in supernatants from 24-h
culture of cells isolated from the tumor tissue (10 mg/ml) was
estimated using ELISA (BD Biosciences).
Analysis of myeloid cell populations
in tumors of LV-treated mice
Tumor cells were isolated from mice were labeled
with a cocktail of monoclonal antibodies conjugated with
fluorochromes: Anti-CD45 (30-F11) V500, anti-CD3 (145-2C11)
PE-CF594, anti-CD19 (1D3) PE-CF594, anti-CD49b (DX5) PE-CF594 (all
from BD Biosciences), anti-CD11b (M1/70) PerCP-Cy5.5, anti-CD11c
(N418) BV 650, anti-F4/80 (BM8) Alexa Fluor 700, anti-Ly6C (HK1.4)
PE, anti-Ly6G (1A8) BV 605 (all from BioLegend). In order to
exclude dead cells, DAPI dye was added prior to the analysis.
Analysis of percentage of tumor-infiltrating myeloid cells as well
as EGFP expression was performed using the LSRFortessa (BD
Biosciences).
Analysis of lymphoid cell populations
in tumors and tumor-draining LNs of LV-treated mice
Tumor and lymph node cells isolated from mice were
stained with LIVE/DEAD Fixable Violet Dead Staining Kit
(Invitrogen) and labeled with a cocktail of monoclonal antibodies
conjugated with fluorochromes: Anti-CD45 (30-F11) BV 605, anti-CD3
(17A2) BV 650, anti-CD4 (RM4-5) FITC, anti-CD8 (53–6.7)
APC/Fire750, anti-CD25 (PC61) PE, anti-CD44 (IM7) PE-Cy7,
anti-CD62L (MEL-14) PerCP-Cy5.5 (all from BioLegend), and
anti-CD49b (DX5) PE-CF594 (BD Biosciences) for lymphoid cell
analysis in tumors and anti-CD4 (RM4-5) PerCP-Cy5.5, anti-CD8
(53-6.7) PE-Cy, anti-CD44 (IM7) FITC, and anti-CD62L (MEL-14) BV
605 (all from BioLegend) for lymphocyte analysis in tLNs. In the
following step, the cells were fixed using eBioscience
Foxp3/Transcription Factor Staining Buffer Set (Invitrogen).
Subsequently, cells from tLNs were labeled with anti-Ki67 (16A8)
APC (BioLegend). Analyses of lymphoid cells in tumors and tLNs were
performed using LSRFortessa (BD Biosciences).
Determination of suppressive activity
of tumor-infiltrating myeloid cells
Tumor cells isolated from mice were labeled with
anti-CD11b monoclonal antibody (M1/70) conjugated with magnetic
nanoparticles (BD IMag). Myeloid (CD11b+) cells were
magnetically separated and co-cultured with CFSE-labeled
splenocytes from healthy mice in a ratio of 1:1 (5×104
tumor-infiltrating CD11b+ cells and 5×104
splenocytes). Co-culture as well as the analysis was performed
according to the procedure described above. CFSE mean fluorescence
intensity (MFI) in CD4+ and CD8+ cells was
measured using the LSRFortessa (BD Biosciences). Concentrations of
IFN-γ and IL-10 in supernatants from the co-culture were determined
using ELISA kits (eBioscience, Invitrogen and BD Biosciences,
respectively).
Statistical analysis
All the data were analyzed using GraphPad Prism 8
software. Normality was verified using the Shapiro-Wilk test.
Statistical significance of normally distributed data was
calculated using Welch's ANOVA followed by post hoc Dunnett's T3
multiple comparison test. For the analysis of non-normally
distributed data, the non-parametric Kruskal-Wallis test followed
by post hoc Dunn's multiple comparison test or the Mann-Whitney U
test was used. P<0.05 was considered to indicate a statistically
significant difference.
Results
IL-10 silencing in in vitro-cultured
MDSCs induced changes in their suppressive activity
In the first step of the study, the effectiveness of
three different nucleotide sequences of shRNA in silencing of
murine IL-10 was determined. The research was conducted using
MDSCs, which were ex vivo-generated during six-day culture
of bone marrow cells in the presence of GM-CSF and the supernatant
collected from MC38 colon carcinoma culture maintained in hypoxia
conditions. Phenotype characterization of the obtained cells was
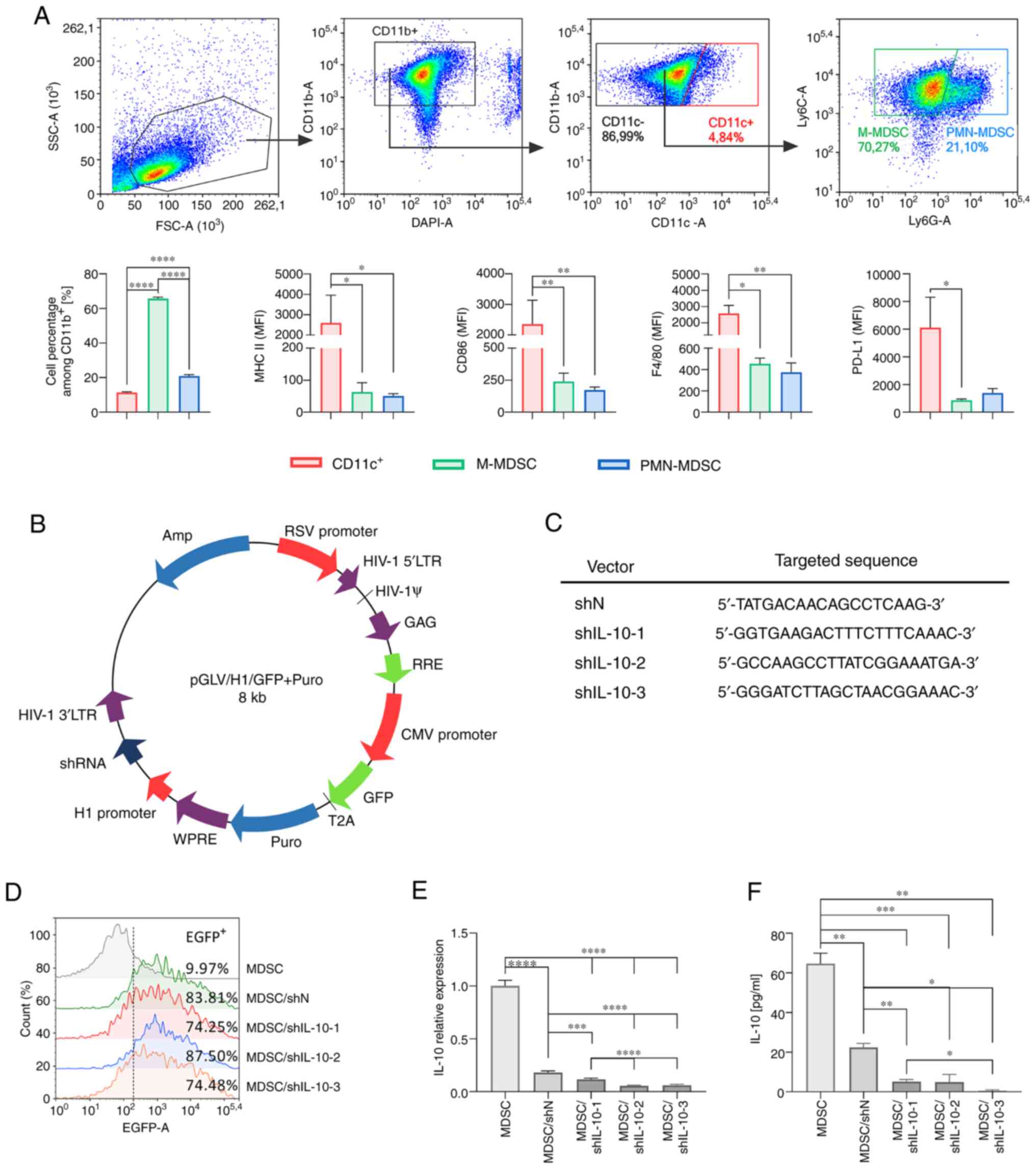
performed using the flow cytometry method according to the scheme
presented in Fig. 1A. On the 6th day
of differentiating cell culture, M-MDSCs
(CD11b+CD11cnegLy6C+Ly6Gneg)
constituted the vast majority in the cell suspension (over 60%),
whereas PMN-MDSCs
(CD11b+CD11cnegLy6CintLy6G+)
accounted for approx. 20% of the cells (Fig. 1A). Apart from MDSCs,
CD11c+ cells corresponding to immature dendritic cells
were also detected (approximately 10% of all cells). As expected,
the obtained MDSCs were characterized by lower expression of
molecules associated with the maturation stage of myeloid cells
such as MHC class II, CD86, F4/80 or PD-L1 compared to
CD11c+ cells. Such phenotype characteristics indicate
typical features of MDSCs (Fig.
1A).
The obtained cell suspension was transduced using
lentiviral vectors encoding EGFP as a reporter gene and shRNAs
against murine IL-10 (Fig. 1B and
C). Transduced cells were characterized by high expression of
EGFP and significantly reduced expression of IL-10 (Fig. 1D-F). Real-time PCR and ELISA
confirmed that the shIL-10-3 sequence was the most effective in
IL-10 silencing, and hence this sequence was chosen for further
experiments where it was referred to as shIL-10.
As a result of transduction with shN or shIL-10 LVs,
decreased percentages of M-MDSCs and PMN-MDSCs in the cell
suspension were detected. However, the efficiency of transduction
in both subpopulations of MDSCs, presented here as a percentage of
EGFP+ cells, was high (Fig.
2A). Although we observed a statistically significant shift in
the expression of selected molecules on the surface of modified
M-MDSCs and PMN-MDSCs in relation to non-transduced cells, these
changes were not dependent on the IL-10 silencing but were related
to lentiviral transduction itself (Figs.
2B and S1). Moreover, compared
to the high expression of these molecules on CD11c+
cells (Fig. S2A and B), it may be
concluded that neither M-nor PMN-MDSCs undergo substantial
phenotypical changes under the influence of lentiviral transduction
and they retain the status of immature cells.
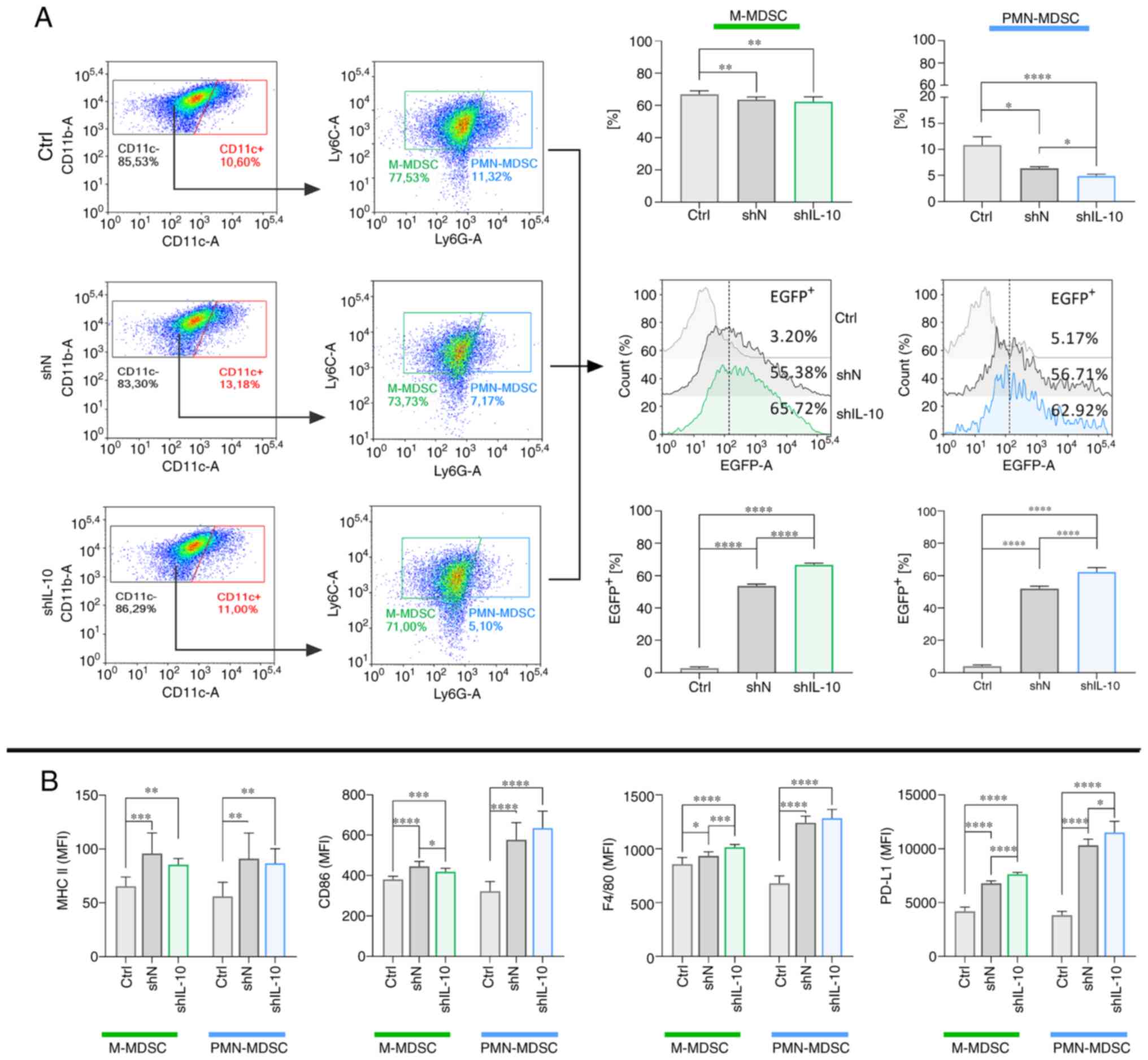 | Figure 2.Characteristics of M-MDSCs and
PMN-MDSCs with the silenced expression of IL-10. (A) Representative
flow cytometric data and bar plots showing the changes in the
proportion of M-MDSCs and PMN-MDSCs in the culture and a percentage
of EGFP+ cells (as a transduction efficacy control)
among identified subpopulations of MDSCs on the 3rd day after IL-10
silencing. (B) Phenotype characteristics of M-MDSCs and PMN-MDSCs
on the 3rd day after IL-10 silencing. The results are given as the
mean ± SD calculated for three independent experiments measured in
triplicates. Statistical significance was calculated using Welch's
ANOVA followed by post hoc Dunnett's T3 multiple comparison test.
Differences with a P<0.05 were regarded as significant
(*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001). M-MDSC,
monocytic myeloid-derived suppressor cells; PMN-MDSC,
polymorphonuclear myeloid-derived suppressor cells; EGFP, enhanced
green fluorescent protein; ctrl, non-transduced cells; shN, cells
transduced with LVs encoding shN sequence; shIL-10, cells
transduced with LVs encoding shIL-10 sequence; MFI, mean
fluorescence intensity. |
The influence of IL-10 silencing on the suppressor
activity of in vitro-cultured M-MDSCs and PMN-MDSCs was
determined using a CFSE-based proliferation assay (Figs. 3, 4
and S3). Since these two
subpopulations employ different mechanisms to suppress T cell
activity, we decided to evaluate their function separately.
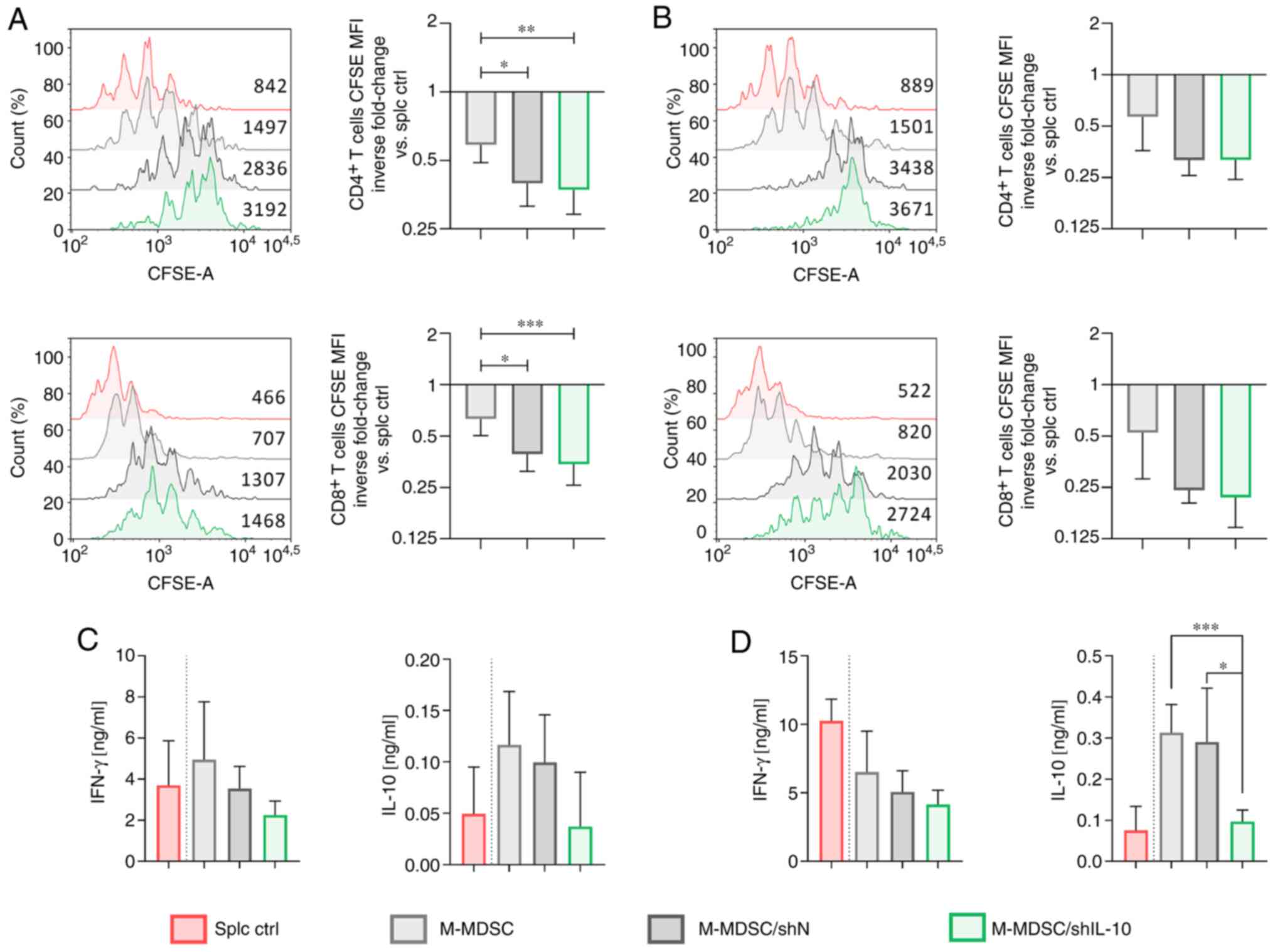 | Figure 3.Influence of IL-10 silencing on the
suppressive activity of M-MDSCs. CFSE-labeled splenocytes obtained
from healthy mice were cultured with M-MDSCs (isolated from the
in vitro culture of MDSCs using the FACS Aria sorter) at the
final ratio of (A and C) 1:1 or (B and D) 2:1. (A and B)
Proliferation of CD4+ and CD8+ spleen-derived
T lymphocytes was measured by CFSE dilution and fluorescence
intensity on representative histograms. Splenocytes cultured with
ConA and IL-2 (splc ctrl) were used as a positive control of
proliferation. The results are presented as the mean inverse
fold-change of CFSE MFI calculated in reference to the splc ctrl
group ± SD [100% splc ctrl MFI value-3089 and 1005 (A,
CD4+, exp. 1 and exp. 2, respectively), 1679 and 475 (A,
CD8+, exp. 1 and exp. 2, respectively), 2882 and 1018
(B, CD4+, exp. 1 and exp. 2, respectively), 1440 and 457
(B, CD8+, exp.1 and exp. 2, respectively)]. (C and D)
Concentrations of IFN-γ and IL-10 in supernatants collected after
72 h co-culture of M-MDSCs with splenocytes measured using ELISA.
The results are expressed as the mean ± SD. Data obtained for two
independent experiments measured in triplicate. Statistical
significance was calculated using Welch's ANOVA followed by post
hoc Dunnett's T3 multiple comparison test. Differences with a
P<0.05 were regarded as significant (*P<0.05, **P<0.01,
***P<0.001). M-MDSC, monocytic myeloid-derived suppressor cells;
MFI, mean fluorescence intensity. |
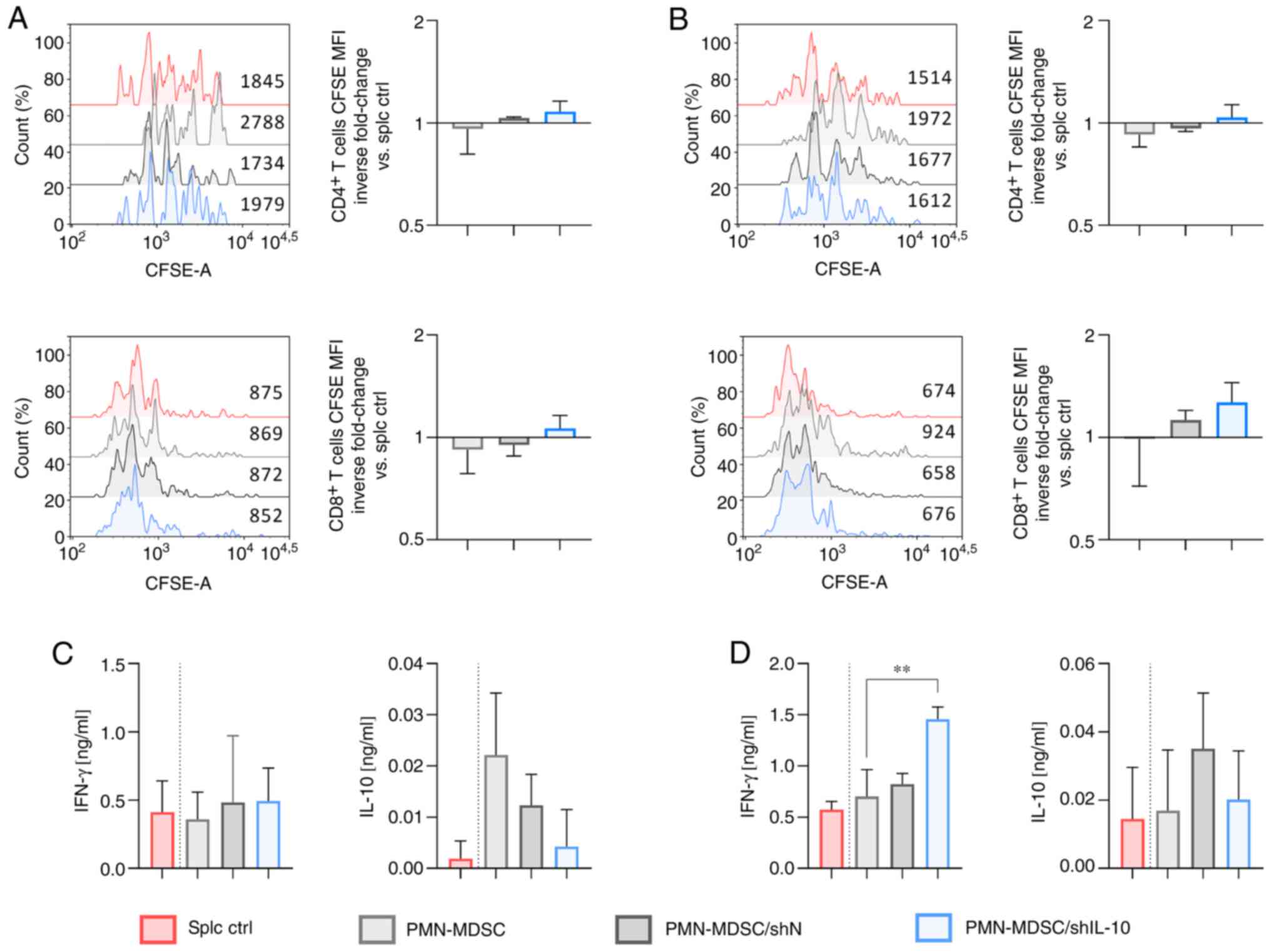 | Figure 4.Influence of IL-10 silencing on the
suppressive activity of PMN-MDSCs. CFSE-labeled splenocytes
obtained from healthy mice were cultured with PMN-MDSCs (isolated
from the in vitro culture of MDSCs using the FACS Aria
sorter) at the final ratio of (A and C) 1:1 or (B and D) 2:1. (A
and B) Proliferation of CD4+ and CD8+
spleen-derived T lymphocytes was measured by CFSE dilution and
presented as fluorescence intensity on representative histograms.
Splenocytes cultured with ConA and IL-2 (splc ctrl) were used as
the positive control of proliferation. The results are presented as
the mean inverse fold change of CFSE MFI calculated by reference to
the splc ctrl group ± SD [100% splc ctrl MFI value-4818 and 2102
(A, CD4+, exp. 1 and exp. 2, respectively), 1662 and 796
(A, CD8+, exp. 1 and exp. 2, respectively), 3813 and
1740 (B, CD4+, exp. 1 and exp. 2, respectively), 1977
and 645 (B, CD8+, exp. 1 and exp. 2, respectively)]. (C
and D) Concentrations of IFN-γ and IL-10 in supernatants collected
after 72 h co-culture of PMN-MDSCs with splenocytes measured using
ELISA. The results are expressed as the mean ± SD. Data obtained
for two independent experiments measured in 1–3 repeats.
Statistical significance was calculated using Welch's ANOVA
followed by post hoc Dunnett's T3 multiple comparison test.
Differences with a P-value <0.05 were regarded as significant
(**P<0.01). PMN-MDSC, polymorphonuclear myeloid-derived
suppressor cells; MFI, mean fluorescence intensity. |
The obtained data showed that the proliferation of
spleen-derived CD4+ and CD8+ T lymphocytes
was the lowest when cells were co-cultured with M-MDSC/shIL-10
(Figs. 3A and B, and S4A). Statistically significant differences
were detected between non-transduced M-MDSCs and M-MDSC/shIL-10
groups (Fig. 3A). It is worth noting
that transduction with shN LVs also resulted in significantly
increased suppressive activity of MDSCs. The highly suppressive
activity of M-MDSC/shIL-10 was also reflected in the production of
cytokines by T lymphocytes. Namely, splenocytes co-cultured with
M-MDSC/shIL-10 secreted decreased amounts of IFN-γ in comparison to
those co-cultured with M-MDSCs or M-MDSC/shN (Fig. 3C and D). On the other hand, the
production of IL-10 in this group was comparable to that obtained
from the positive control of splenocytes cultured without M-MDSCs
(splc ctrl) and considerably lower than in M-MDSCs or M-MDSC/shN
groups (Fig. 3C and D).
The opposite effect was noted when splenocytes were
cultured in the presence of PMN-MDSC/shIL-10. The obtained results
indicate that PMN-MDSC/shIL-10 acts more as an inducer of
CD4+ and CD8+ T lymphocyte proliferation than
as a suppressor (Fig. 4A and B).
Although the differences between groups were not statistically
significant, it should be noted that splenocytes co-cultured in the
presence of PMN-MDSC/shIL-10 (in both a 1:1 and a 2:1 ratio)
proliferated even more intensively compared to control splenocytes,
whereas proliferation in the PMN-MDSC group was lower than in the
splc ctrl group or more or less at the level of the positive
control in the case of the PMN-MDSC/shN group (Fig. 4A and B). Moreover, a statistically
significant difference of CD4+ T lymphocyte
proliferation when cultured in the presence of PMN-MDSCs and
PMN-MDSC/shIL-10 in a ratio of 4:1 was detected (Fig. S4B). Additionally, PMN-MDSC/shIL-10
induced higher production of IFN-γ compared to the PMN/MDSC group,
and the difference was statistically significant when splenocytes
were co-cultured with PMN-MDSCs at a ratio of 2:1, which was not
observed in the co-culture of splc and PMN-MDSC/shN (Fig. 4D). Production of IL-10 in the
PMN-MDSC/shIL-10 group was comparable to the splc ctrl group and
considerably lower than in the PMN-MDSC group (at 1:1 ratio) and
PMN-MDSC/shN group (Fig. 4C and
D).
The obtained data indicate that introduction of
shRNA against IL-10 into M-MDSCs and PMN-MDSCs did not induce the
maturation of the cells towards fully functional myeloid cells such
as macrophages, dendritic cells, or granulocytes. However,
transduction of particular subpopulations of MDSCs with shIL-10 LVs
resulted in changes of their suppressor activity. Namely, it was
observed that M-MDSCs transduced with shIL-10 LVs showed increased
suppressor activity in comparison to untransduced cells. The
opposite effect was observed for the PMN-MDSC subpopulation-their
transduction with shIL-10 LVs resulted in decreased ability to
suppress T cell activity. Since MDSC/shN were also characterized by
lowered expression of IL-10 in comparison to untransduced cells,
the suppressor activity of MDSC/shIL-10 did not differ
significantly from MDSC/shN. However, it was observed that the
effect of transduction was correlated with the efficiency of
reduction of IL-10 expression.
Intratumoral injections of shIL-10 LVs
reduced IL-10 production in TME and influenced the proportions and
activity of myeloid cells infiltrating tumor
In order to determine the influence of lentiviral
vectors carrying shRNA against IL-10 on the activity of immune
cells in tumor tissue, shIL-10 LVs and control shN LVs were
administered to MC38 tumor-bearing mice. Triple, intratumoral
injections of the vectors were conducted according to the schemes
presented in Fig. 5A and B. Tumors
and tumor-draining lymph nodes were harvested on the 6th or 10th
day starting from the first LV injection. It was observed that the
concentration of IL-10 produced by cells isolated from tumors
dissected on both the 6th and 10th day was substantially lower in
the group that received shIL-10 LVs compared to the untreated
control and the shN LV group (Fig. 5C
and D). Detailed analysis of EGFP expression in tumor cells
(characterized here as CD45neg), as well as lymphoid and
myeloid cells infiltrating the TME, revealed that on the 6th day
after LV treatment the CD11b+ myeloid cells were the
only population with enhanced expression of EGFP, whereas the 10th
day myeloid cells in LV groups were characterized by nearly
two-fold higher EGFP expression than in the control group (Figs. S5A and B, and 5E). In the prolonged scheme of treatment,
enhanced expression of EGFP in tumor cells (Fig. S5A and B) was also observed. Further
analyses, carried out on selected subpopulations of myeloid cells,
demonstrated that on the 6th day the percentages of
EGFP+ cells were the highest in the M-MDSC and PMN-MDSC
populations from the shIL-10 LV group and were at the level of 12.4
and 15.2% respectively (Fig. 5F). On
the 10th day, a substantial increase in the percentage of
PMN-MDSC/EGFP+ was observed in both the shN LV group (up
to 35.0%) and the shIL-10 LV group (up to 30.6%) compared to
untreated control (Fig. 5G). The
effectiveness of LV transduction in this subpopulation was
considerably higher than in M-MDSCs (Fig. 5F and G). In the prolonged scheme of
treatment, LVs demonstrated a noticeable increase in the
effectiveness of DC modification. In both the shN and shIL-10
groups, the percentage of DC/EGFP+ was high and varied
within the range of 32–37% (Fig.
5G). It indicates that in the proposed scheme of treatment
myeloid cells infiltrating MC38 tumors seem to be one of the main
recipients of LV transduction in situ.
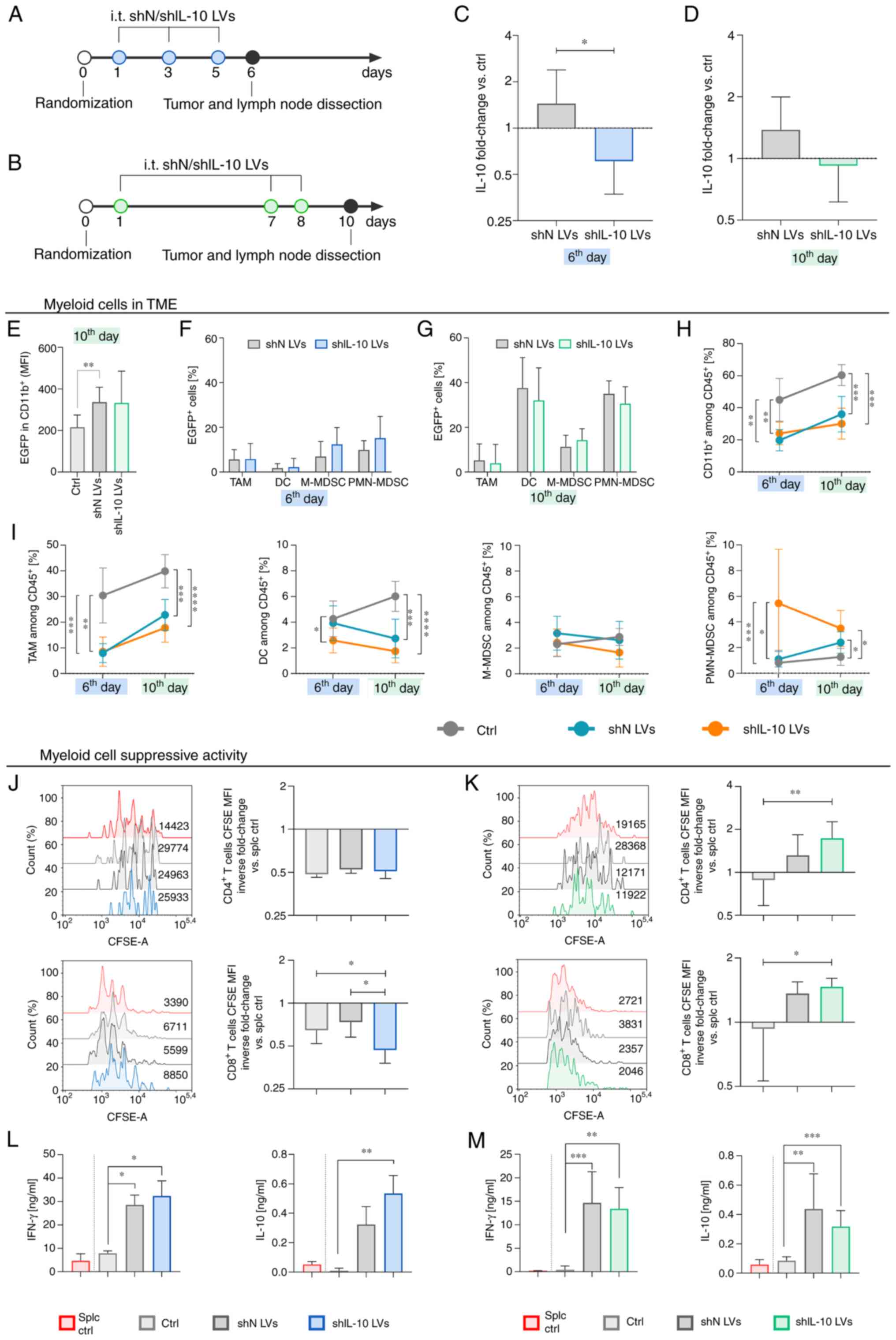 | Figure 5.Influence of the intratumoral
inoculation of IL-10 silencing lentivectors (shIL-10 LVs) on the
myeloid cell activity. Tumors were dissected and analyzed on the
6th or 10th day after triple injection of LVs. (A and B) Schemes
presenting administration schedules of shIL-10 LVs. (C and D) IL-10
production by cells isolated from tumors presented as the mean
fold-change of IL-10 concentration in LV-treated groups in
reference to the untreated group (ctrl) ± SD measured using ELISA
[100% ctrl value-(C) 27.2 pg/ml, (D) 231.6 pg/ml]. (E) EGFP
expression in myeloid cell infiltrating tumors on the 10th day
after the first LV injection. (F and G) Changes in percentages of
EGFP positive cells among selected subpopulations of myeloid cells
on the 6th and 10th day, respectively. (H and I) Changes in
percentages of myeloid cells and myeloid cell subpopulations in
tumors on the 6th and the 10th days after LV inoculation estimated
using multiparameter flow cytometry (TAM:
CD11b+CD11c+F4/80+; DC:
CD11b+CD11c+F4/80negMHCII+;
M-MDSC:
CD11b+CD11cnegF4/80negLy6C+Ly6Gneg;
PMN-MDSC:
CD11b+CD11cnegF4/80negLy6ClowLy6G+).
(J and K) Suppressor activity of myeloid cells estimated using
CFSE-based proliferation assay. The proliferation of
CD4+ and CD8+ spleen-derived T lymphocytes
was measured on the (J) 6th or on the (K) 10th day after LV
injection. Splenocytes cultured with ConA and IL-2 (splc ctrl) were
used as a positive control of proliferation. The results are
presented as the mean inverse fold change of CFSE MFI calculated by
reference to the splc ctrl group ± SD [100% splc ctrl MFI
value-14509 and 4046 (J, CD4+ and CD8+,
respectively), 22382 and 3096 (K, CD4+ and
CD8+, respectively)]. (L and M) Concentrations of IFN-γ
and IL-10 in supernatants collected after 72 h co-culture of
myeloid cells with splenocytes measured using ELISA. The results
are expressed as the mean ± SD. The number of mice per group in
each experiment was 7–10. Statistical significance was calculated
using (H-M) Welch's ANOVA followed by post hoc Dunnett's T3
multiple comparison test, (E) the non-parametric Kruskal-Wallis
test followed by post hoc Dunn's multiple comparison test or (C and
D) the Mann-Whitney U test. Differences with a P<0.05 were
regarded as significant (*P<0.05, **P<0.01, ***P<0.001,
****P<0.0001). shN LVs, lentiviral vectors encoding shN
sequence; shIL-10 LVs, lentiviral vectors encoding shIL-10
sequence; TME, tumor microenvironment; MFI, mean fluorescent
intensity; EGFP, enhanced green fluorescent protein; TAM,
tumor-associated macrophages; DC, dendritic cells; M-MDSC,
monocytic myeloid-derived suppressor cells; PMN-MDSC,
polymorphonuclear myeloid-derived suppressor cells. |
The analysis of changes in myeloid cell influx into
MC38 tumors following the administrations of shIL-10 LVs was
performed using the multiparameter flow cytometry method according
to the scheme presented in Supplemental Fig. S6. The obtained data demonstrated a
significant reduction of the percentage of CD11b+ in the
aftermath of LV injections compared to the untreated control. The
effect was noted on both the 6th and 10th day and there were no
considerable differences between shN and shIL-10 LVs (Fig. 5H). A detailed analysis showed that
LVs induced a significant reduction of TAM and DC populations in
tumors compared to the untreated control group (Fig. 5I). In the case of DCs, the effect was
the most intensive after injection of shIL-10 LVs and the
percentage of the cells decreased over time. In tumors inoculated
with shIL-10 LVs a lower percentage of M-MDSCs than in the
untreated control and in the shN LV group was also detected, which
was particularly evident on the 10th day after the first LV
injection. Interestingly, shIL-10 LVs induced an increased influx
of PMN-MDSCs into tumors. The highest percentage of these cells was
noted on the 6th day and although it decreased over time, on the
10th day the proportions of these cells in the shIL-10 LV group
were still significantly higher than in the control group (Fig. 5I).
The CFSE-based proliferation assay showed that
myeloid cells isolated from shIL-10 LV-treated tumors dissected on
the 6th day were characterized by considerably higher suppressor
activity toward CD8+ T lymphocytes than those isolated
from the untreated control or the shN LV group (Fig. 5J). However, it needs to be
highlighted that the effect was temporary and on the 10th day after
the first injection of shIL-10 LVs myeloid cells lost their
suppressor activity in contrast to those obtained from the
untreated group and the proliferation of CD4+, as well
as CD8+ T lymphocytes was even more intensive than in
the splc ctrl group (Fig. 5K).
Splenocytes co-cultured with myeloid cells isolated from the
LV-treated groups according to two schemes of treatment secreted
significantly higher amounts of IFN-γ and IL-10 compared to those
cultured in the presence of myeloid cells isolated from the
untreated control (Fig. 5L and M).
However, there were no considerable differences in production of
these cytokines between shN LVs and shIL-10 LVs groups.
Analysis of lymphoid cells infiltrating tumors,
performed according to the scheme presented in Fig. S7, showed that there was no
significant influence of shIL-10 LV injections on the percentage of
CD4+ T lymphocytes and NK cells in tumors compared to
the untreated control (Fig. 6A).
Nevertheless, LVs induced a high influx of CD8+ cells
into tumors and there were visible differences in the percentage of
these cells between the shN LV and shIL-10 LV groups. Namely, the
percentage of the cells after injection of shN LVs was the highest
on the 6th day and then slightly decreased, whereas in the group
that received shIL-10 LVs the reaction was delayed and the highest
percentage of CD8+ T lymphocytes was detected on the
10th day and was considerably higher than in the untreated control
and the shN LV group (Fig. 6A). In
order to compare changes in the cytotoxic T lymphocytes (CTLs) and
MDSC influx into tumors, the CTL/MDSCs ratios were calculated. The
analysis demonstrated that the CTL/M-MDSC ratio of the shIL-10 LV
group increased over time and on the 10th day was significantly
higher than in the untreated control and the shN LV group, whereas
the CTL/PMN-MDSC ratio was the lowest for the shIL-10 LV group on
the 6th day and then increased to a similar level as in the other
groups (Fig. 6B and C).
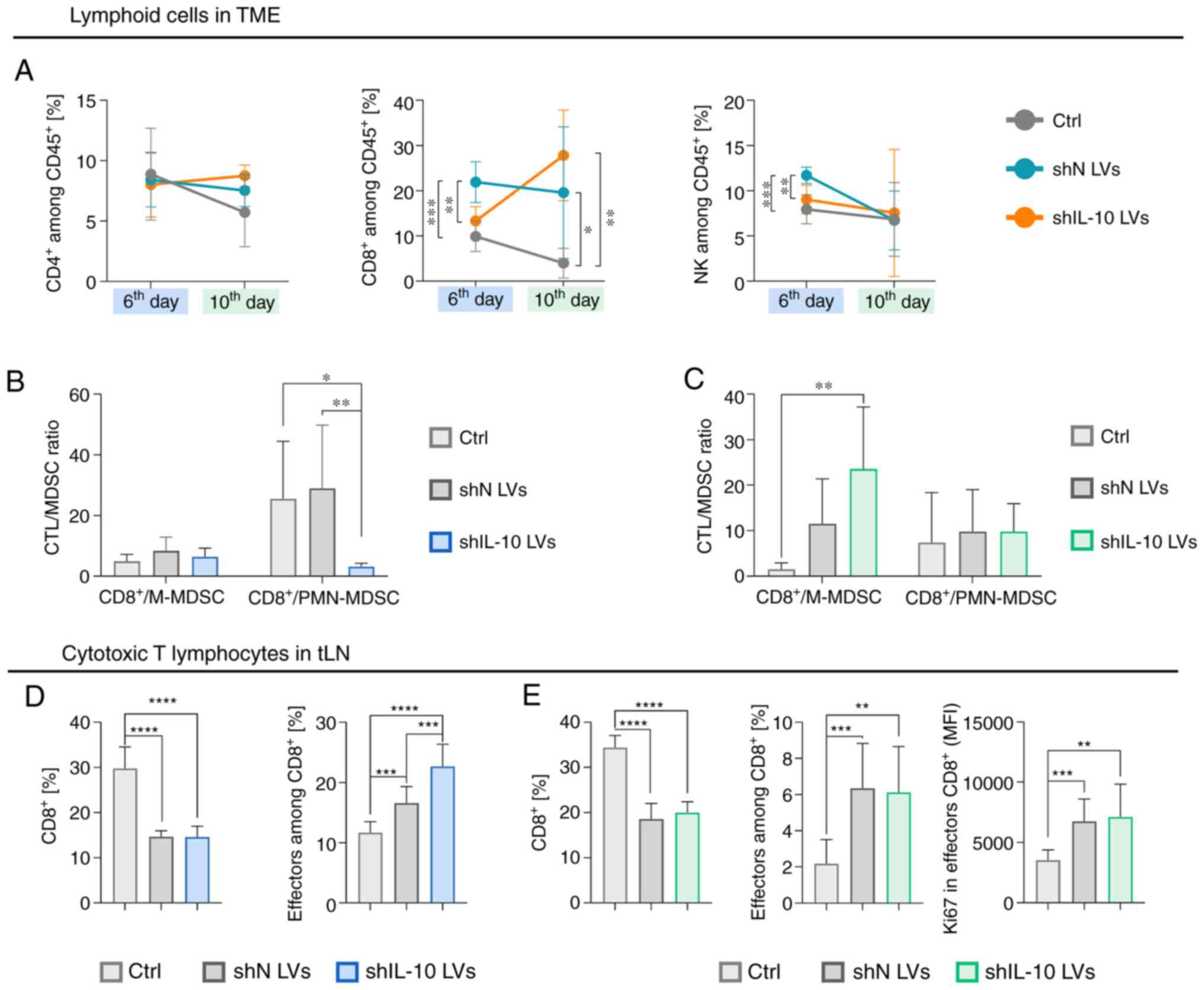 | Figure 6.Influence of the intratumoral
inoculation of IL-10 silencing lentivectors (shIL-10 LVs) on
lymphoid cells in tumors and tumor-draining lymph nodes. Tumors and
lymph nodes were dissected and analyzed using a multiparameter flow
cytometry on the 6th or 10th day after triple injection of LVs. (A)
Changes in percentages of CD4+ T lymphocytes
(CD3+CD49bnegCD4+), and
CD8+ T lymphocytes
(CD3+CD49bnegCD8+), and NK cells
(CD3negCD49b+) in tumors on the 6th and the
10th days after LV inoculation. (B and C) The CTL to MDSC ratio
calculated on the (B) 6th and on the (C) 10th days after LV
injection. (D) Changes in the percentage of CTLs and the percentage
of effectors among them in tLNs on the 6th day after LV injection.
(E) Changes in the percentage of CTLs, the percentage of effectors
among them, and expression of Ki67 by effector CTLs in tLNs on the
10th day after LV injection. The results are expressed as the mean
± SD. The number of mice per group in each experiment was 7–10.
Statistical significance was calculated using (A, D and E) Welch's
ANOVA followed by post hoc Dunnett's T3 multiple comparison test or
(B and C) the non-parametric Kruskal-Wallis test followed by post
hoc Dunn's multiple comparison test. Differences with a P<0.05
were regarded as significant (*P<0.05, **P<0.01,
***P<0.001, ****P<0.0001). shN LVs, lentiviral vectors
encoding shN sequence; shIL-10 LVs, lentiviral vectors encoding
shIL-10 sequence; TME, tumor microenvironment; MFI, mean
fluorescent intensity; M-MDSC, monocytic myeloid-derived suppressor
cells; PMN-MDSC, polymorphonuclear myeloid-derived suppressor
cells; CTL, cytotoxic T lymphocytes; tLN, tumor-draining lymph
nodes. |
The analysis of CD8+ T lymphocytes in
tumor-draining lymph nodes showed a significantly lower percentage
of these cells in shN LV and shIL-10 LV groups than in the
untreated control, which indicated an efflux of the cells from tLNs
in the aftermath of LV injections (Figs.
6D and E, and S8). Although
there were no significant differences in the percentage of CTLs
between the shN and shIL-10 LV groups, on the 6th day the
percentage of effector CTLs in the group receiving shIL-10 LVs was
significantly higher than in the other groups. It showed that
injections of LVs induced intensive mobilization of CD8+
cells in tLNs. However, shIL-10 LVs can be more effective in
activation of effector CTLs.
In sum, the obtained data showed that intratumoral
injections of shIL-10 LVs induced an increased influx of PMN-MDSCs
and delayed infiltration of CD8+ T lymphocytes into the
TME. Considering that shIL-10 LVs induced increased activation of
effector CTLs in tLNs on the 6th day, one can assume that the lower
percentage of CD8+ T lymphocytes in tumors in comparison
to the shN LV-treated group results from the suppressive activity
of PMN-MDSCs which infiltrated into the tumors in the aftermath of
shIL-10 LV injections but have not been transduced yet. Prolonged
observation revealed that on the 10th day, an enhanced percentage
of shIL-10 LV-transduced MDSCs was correlated with a higher T
lymphocyte proliferation rate in the presence of myeloid cells
isolated from the shIL-10 LV group and an increased CTL/M-MDSC
ratio in comparison to the untreated group. It may indicate that
IL-10 downregulation in the tumor contributes to a reduction of
immunosuppression, which depends on PMN-MDSC activity in the
TME.
Pretreatment with cyclophosphamide
reduced the effectiveness of LVs in transduction of myeloid cells
in situ while simultaneously increasing their effect on
mobilization of CTLs and NK cells into MC38 tumor
Due to the well-documented immunomodulatory activity
of low doses of cyclophosphamide and its potential application in
combination with different forms of immunotherapy we decided
determine what the effect of pretreatment with CY on the activity
of shIL-10 LVs in the TME would be. LVs were administered according
to the aforementioned schemes; however, two days before their
application mice received CY in a dose of 150 mg/kg body weight.
Detailed schemes of the treatment are presented in Fig. 7A and B. Tumors, and tumor-draining
lymph nodes were dissected on the 8th and 12th day after CY
injection and since CY was administered two days prior to the first
injection of LVs, the timepoints of analyses correspond to those in
experiments performed without CY (6th and 10th day after LV
injection). The obtained data showed that CY administered alone or
with shN LVs slightly reduced the ability of cells isolated from
tumor tissues to secrete IL-10 in comparison to the untreated
group, which indicated the direct role of CY in reduction of the
concentration of this cytokine in the TME. After application of CY
+ shIL-10 LVs the effect of specific IL-10 silencing was observed,
but the difference between CY and CY + shIL-10 LV groups was
statistically significant only on the 12th day (Fig. 7C and D). Furthermore, the EGFP
analysis demonstrated that the mean intensity of EGFP fluorescence
in CD11b+ myeloid cells from CY + LVs groups was at the
same level as in the CY group (on the 8th day) or the untreated
control (on the 12th day) (Fig. 7E).
On the other hand, an increased intensity of EGFP fluorescence was
observed in tumor cells and lymphoid cells (Fig. S5C and D). It may be concluded that
myeloid cells were less susceptible to LVs in the TME due to the
differentiating activity of CY. Results confirming this hypothesis
are presented in Fig. S9.
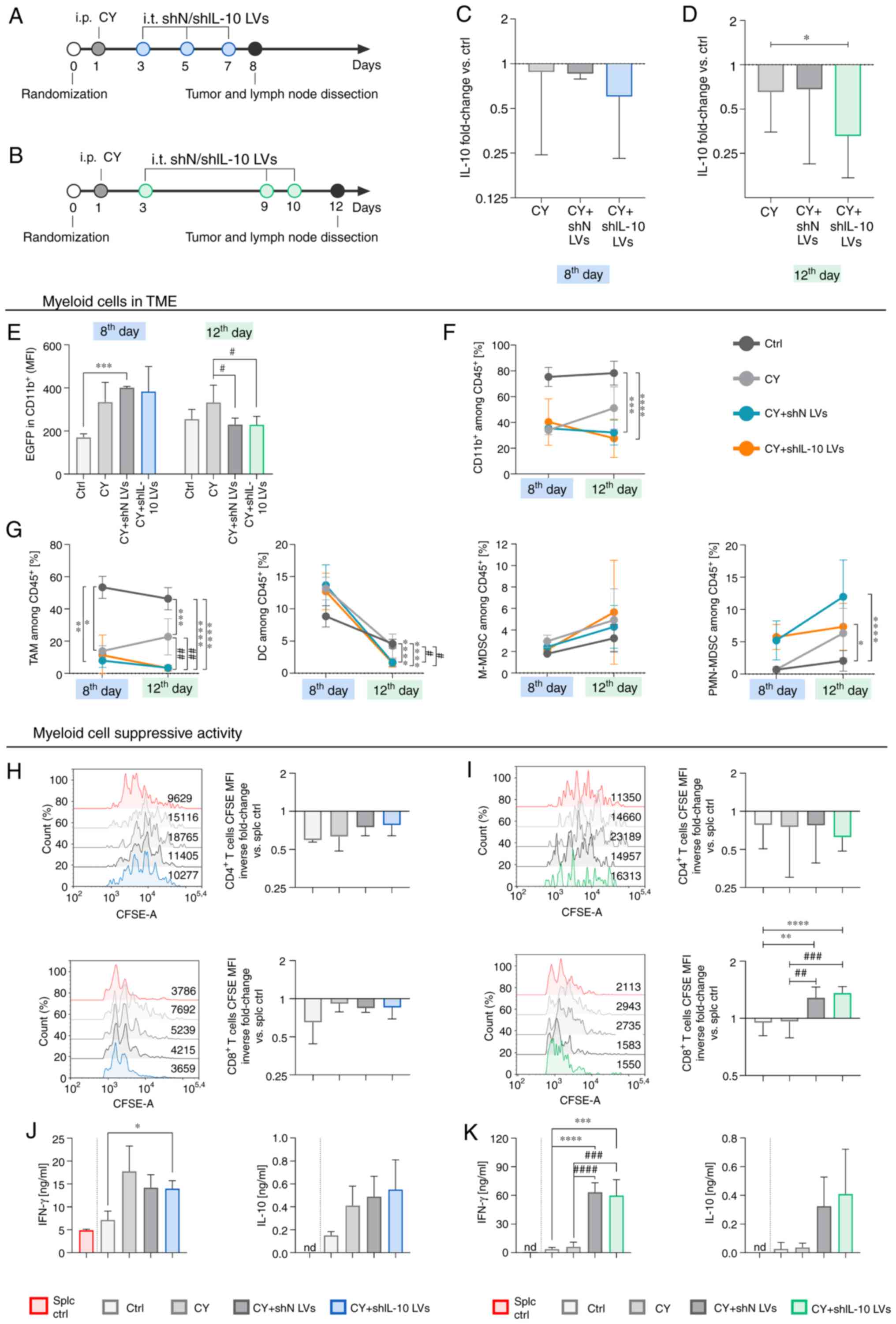 | Figure 7.Myeloid cell activity in MC38 tumor
in aftermath of shIL-10 LV injections preceded by administration of
cyclophosphamide. Tumors were dissected and analyzed on the 8th or
12th day after intraperitoneal administration of CY followed by
triple injection of LVs. (A and B) Schemes presenting the CY and LV
administration schedules. (C and D) IL-10 production by cells
isolated from tumors presented as the mean fold change of IL-10
concentration in CY + LV-treated groups referenced by the untreated
group (ctrl) ± SD measured using ELISA [100% ctrl value-(C) 317
pg/ml and (D) 393 pg/ml]. (E) EGFP expression in myeloid cell
infiltrating tumors. (F and G) Changes in percentages of myeloid
cells and myeloid cell subpopulations in tumors on the 8th and the
12th day after treatment estimated using multiparameter flow
cytometry (TAM:
CD11b+CD11c+F4/80+; DC:
CD11b+CD11c+F4/80negMHCII+;
M-MDSC: CD11b+CD11cnegF4/80negLy6C+Ly6Gneg;
PMN-MDSC:
CD11b+CD11cnegF4/80negLy6ClowLy6G+).
(H and I) Suppressor activity of myeloid cells estimated using
CFSE-based proliferation assay. Proliferation of CD4+
and CD8+ spleen-derived T lymphocytes was measured on
the (H) 8th or on the (I) 12th day after treatment. Splenocytes
cultured with ConA and IL-2 (splc ctrl) were used as a positive
control of proliferation. The results are presented as the mean
inverse fold change of CFSE MFI calculated by reference to the splc
ctrl group ± SD [100% splc ctrl MFI value-9400 and 3767 (H,
CD4+ and CD8+, respectively), 11950 and 2239
(I, CD4+ and CD8+, respectively)]. (J and K)
Concentrations of IFN-γ and IL-10 in supernatants collected after
72 h co-culture of myeloid cells with splenocytes measured using
ELISA (nd, not detected). The results are expressed as the mean ±
SD. The number of mice per group in experiment A was 3 and in
experiment B 9–10. Statistical significance was calculated using
Welch's ANOVA followed by post hoc Dunnett's T3 multiple comparison
test. Differences with a P-value <0.05 were regarded as
significant (*P<0.05, **P<0.01, ***P<0.001,
****P<0.0001, #P<0.05, ##P<0.01,
###P<0.001, ####P<0.0001; differences
related to untreated group were presented as * and those related to
CY-treated group were presented as #). shN LVs, lentiviral vectors
encoding shN sequence; shIL-10 LVs, lentiviral vectors encoding
shIL-10 sequence; CY, cyclophosphamide; TME, tumor
microenvironment; MFI, mean fluorescent intensity; EGFP, enhanced
green fluorescent protein; TAM, tumor-associated macrophages; DC,
dendritic cells; M-MDSC, monocytic myeloid-derived suppressor
cells; PMN-MDSC, polymorphonuclear myeloid-derived suppressor
cells. |
Nevertheless, the combined application of CY and
shIL-10 LVs induced significant changes in the myeloid cell influx
into tumors. Although the application of CY contributed to the
decrease of CD11b+ cells in tumors, which was noticed on
both the 8th and 12th day after treatment starting, LV injections
enhanced the effect, and the percentage of myeloid cells in the
shIL-10 LV group on the 12th day was significantly lower than in
the untreated control and the CY group. The differences between shN
and shIL-10 LV groups were rather small (Fig. 7F). Detailed analysis of myeloid cell
subpopulations revealed that the application of CY with LVs (both
shN and shIL-10) led to almost complete elimination of TAMs and DCs
from the TME (Fig. 7G), while the
percentage of M-MDSCs and PMN-MDSCs increased after application of
CY + LVs (Fig. 7G). In spite of the
increased proportion of MDSCs in tumors after application of CY +
LVs, the CFSE-based proliferation assay showed that although
suppressor activity of myeloid cells isolated from the tumor was
inconsiderably lower than the one detected for the untreated group
on the 8th day, proliferation rates of lymphocytes in these groups
were still lower than in the splc control group (Fig. 7H). Additionally, it was observed that
myeloid cells isolated from tumors of the CY + shIL-10 LV group on
the 12th day showed the highest suppressive activity toward
CD4+ T lymphocytes (Fig.
7I), while the opposite effect was observed in the case of
CD8+ T lymphocytes (Fig.
7I). The influence of the treatment on proliferation of
spleen-derived lymphocytes was reflected in cytokine production by
splenocytes co-cultured with myeloid cells. Data presented in
Fig. 7J indicate a strong influence
of CY on the activity of myeloid cells isolated from tumors on the
8th day. However, on the 12th day, CY was losing its activity and a
large increase in the production of IFN-γ, as well as IL-10 was
detected in the groups receiving CY + LVs (Fig. 7K).
Analysis of lymphoid cells in tumors showed a strong
influence of the combined treatment on the CTL and NK cell influx
into the TME. Cyclophosphamide induced changes in the percentage of
CD8+ T lymphocytes and on the 8th day proportions of
those cells were higher in the CY and CY + LV groups compared to
the untreated control. However, on the 12th day a large influx of
CTLs was observed only after combined treatment (Fig. 8A). On the 12th day, a significant
increase of the percentage of NK cells in tumors was also observed
following the CY + LV treatment (Fig.
8A), while no differences were noted between shN LV and shIL-10
LV groups. It was also demonstrated that the CTL/M-MDSC ratio was
slightly more favorable for the CY + shIL-10 LV group than for the
CY + shN LV group both on the 8th and 12th day, whereas the
CTL/PMN-MDSC ratio was considerably lower for both CY + LV groups
compared to the control groups on the 8th day and increased over
time to the control level (Fig. 8B and
C).
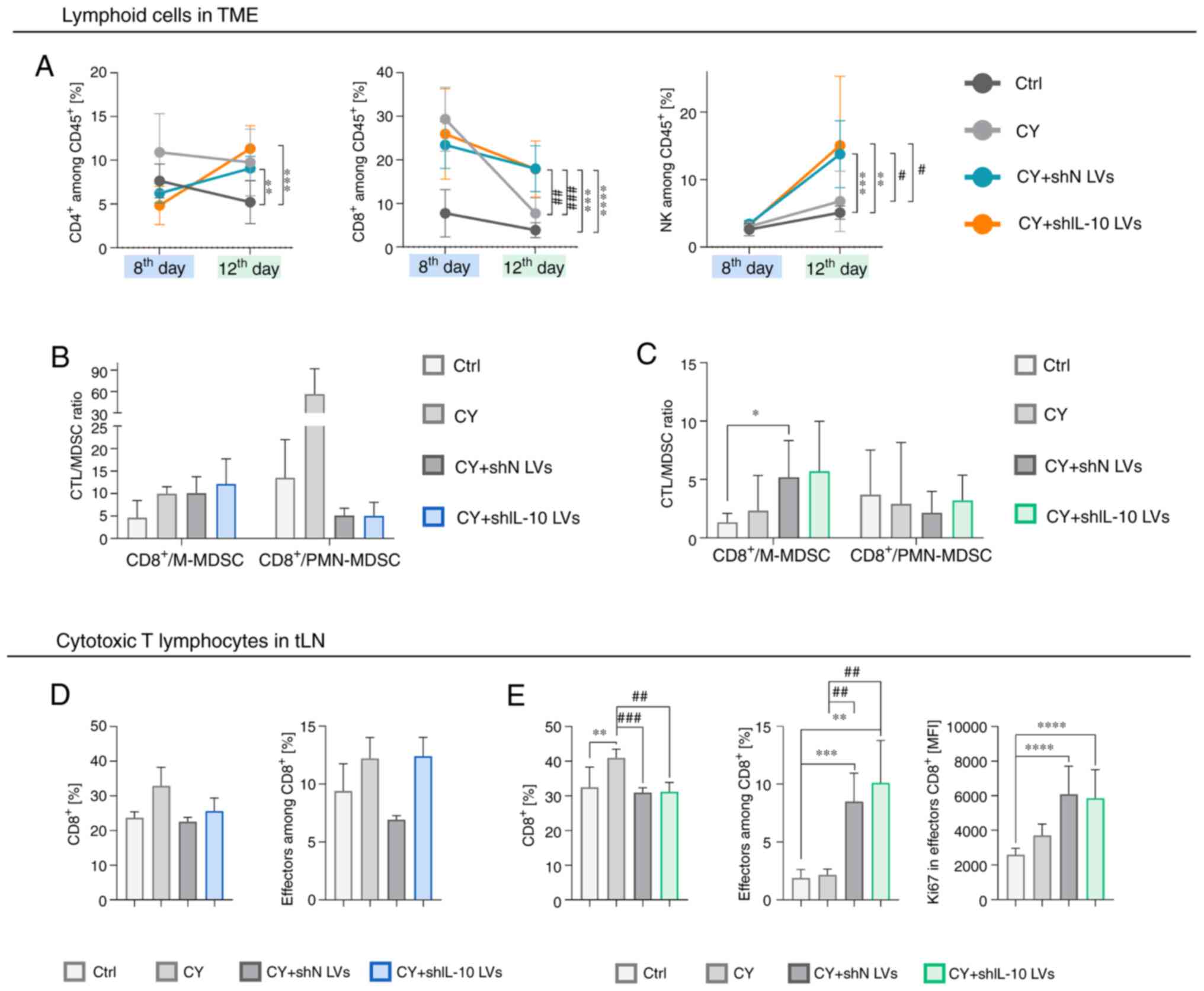 | Figure 8.Lymphoid cell activity in MC38 tumor
and tumor-draining lymph nodes in aftermath of shIL-10 LV
injections preceded by administration of cyclophosphamide. Tumors
and tLNs were dissected and analyzed using flow cytometry on the
8th or 12th day after intraperitoneal administration of CY followed
by triple injection of LVs. (A) Changes in percentages of
CD4+ T lymphocytes
(CD3+CD49bnegCD4+),
CD8+ T lymphocytes
(CD3+CD49bnegCD8+), and NK cells
(CD3negCD49b+) in tumors on the 8th and the
12th days after treatment. (B and C) CTL to MDSC ratio calculated
on the (B) 8th and on the (C) 12th days after treatment. (D)
Changes in the percentage of CTLs and effectors among them in tLNs
on the 8th day after treatment. (E) Changes in the percentage of
CTLs, effectors among them, and expression of Ki67 by effector CTLs
in tLNs on the 12th day after treatment. The results are expressed
as the mean ± SD. The number of mice per group in experiment A was
3 and in experiment B 9–10. Statistical significance was calculated
using (A and D) Welch's ANOVA followed by post hoc Dunnett's T3
multiple comparison test or the (B, C and E) non-parametric
Kruskal-Wallis test followed by post hoc Dunn's multiple comparison
test. Differences with a P<0.05 were regarded as significant
(*P<0.05, **P<0.01, ***P<0.001, ****P<0.0001,
#P<0.05, ##P<0.01,
###P<0.001; differences related to the untreated
group were presented as * and those related to the CY-treated group
were presented as #). shN LVs, lentiviral vectors encoding shN
sequence; shIL-10 LVs, lentiviral vectors encoding shIL-10
sequence; CY, cyclophosphamide; TME, tumor microenvironment; MFI,
mean fluorescent intensity; M-MDSC, monocytic myeloid-derived
suppressor cells; PMN-MDSC, polymorphonuclear myeloid-derived
suppressor cells; CTL, cytotoxic T lymphocytes; tLN, tumor-draining
lymph nodes. |
On the other hand, the analysis of CD8+ T
lymphocytes in tumor-draining lymph nodes indicated that
pretreatment with CY delayed the mobilization of the cells and on
the 8th day there were no significant changes in the percentage and
activity of CTLs in tLNs (Fig. 8D),
while on the 12th day the efflux of CTLs, as well as a significant
increase in the percentage of effector CTLs and a high
proliferation index of these cells, was observed after application
of the combined treatment. A slightly higher percentage of effector
CTLs in the shIL-10 LV group compared to the shN LV group (Fig. 8E) was also observed.
In summary, CY induced the reduction of IL-10 in the
TME by itself, so differences in IL-10 concentration in the TME
could be insufficient to detect changes in proportions and activity
of cells infiltrating tumors treated with CY + LVs. However, we
observed that after the combined treatment, tumor cells and
lymphoid cells were the major targets for intratumorally
administered LVs, and such treatment activated not only
CD8+ T lymphocytes but also NK cells.
Discussion
The main objective of this work was to determine the
mechanisms of the immune response induced by lentiviral vectors
encoding the shRNA sequence that silence the expression of IL-10.
Taking into considerations our previous observations, we focused on
evaluation of suppressor activity of MDSCs following treatment with
shIL-10 LVs. For this purpose, shIL-10 LVs were used for
transduction of ex vivo-generated MDSCs. During the second
stage of this research, lentiviral vectors were injected into MC38
colon carcinoma tumors in order to evaluate the changes in the
local immune response caused by shIL-10 LV-mediated reduction of
IL-10 in the TME.
In the first stage of our research, it was observed
that silencing of IL-10 in MDSCs, which were generated from bone
marrow cells cultured in the presence of MC38 colon carcinoma
cell-derived factors, did not induce their differentiation towards
mature myeloid cell populations. To test whether IL-10 silencing
affected MDSC activity, the effects of MDSC/shIL-10 on T cell
proliferation and their ability to produce IFN-γ and IL-10 were
determined. We decided to examine M-MDSCs and PMN-MDSCs separately,
since these two subpopulations of MDSCs use different suppression
mechanisms. According to the literature, M-MDSCs suppress T cell
activity by secretion of nitrogen oxide, arginase and
immunosuppressive cytokines, whereas PMN-MDSCs do so mostly by the
production of reactive oxygen and nitrogen species, and due to the
low stability of these compounds, they affect lymphocytes during
cell-to-cell contact (3). During our
research, it was observed that T cells proliferated less
intensively in the presence of M-MDSC/shIL-10 than in cultures with
non-transduced cells. Moreover, M-MDSC/shIL-10 decreased the
ability of lymphocytes to produce IFN-γ and IL-10. On the other
hand, it seems that lentiviral vector-mediated silencing of IL-10
in PMN-MDSCs decreased their suppressor activity against T
lymphocytes - these cells divided more often and also secreted more
IFN-γ and less IL-10 than lymphocytes cultured in the presence of
non-transduced PMN-MDSCs. It should be noted that the suppressor
activity of MDSC/shIL-10 did not differ significantly from
MDSC/shN. The changes of the activity of MDSCs transduced with shN
LVs are probably a result of the lentiviral vector influence on
these cells. Since LVs contain viral antigens, as well as viral
RNA, they modulate the activity of myeloid cells by toll-like
receptor stimulation (22).
Consequently, transduction of MDSCs with LVs may result in
increased expression of molecules responsible for the activity of
myeloid cells, e.g. MHC II, CD86, PD-L1, as well as in a changed
cytokine expression profile. Taking into consideration the fact
that MDSCs transduced with shN LVs showed a lower level of IL-10
expression than control cells, but still significantly higher than
MDSC/shIL-10, it can be noted that the effect of MDSC transduction
on their suppressive activity correlated with the efficiency of
reduction of IL-10 expression. Nevertheless, the obtained data
suggest that IL-10 silencing may result in completely opposite
changes in the activity of M-MDSCs and PMN-MDSCs. In the literature
there are few reports on the effect of IL-10 on MDSC activity, and
the recent publications present divergent conclusions. Tanikawa
et al (23) conducted studies
using IL-10 knockout mice. During tumor development in
IL-10−/− mice, they observed higher accumulation of
MDSCs than in wild-type mice. This effect was also correlated with
increased suppressor activity of IL-10−/− MDSCs.
However, research conducted by Noman et al (24) showed that MDSC-mediated suppression
can be partially abolished by IL-10 neutralization, since after
adding anti-IL-10 antibodies to MDSC and T cell cultures, the
percentage of CD8+IFN-γ+ and
CD4+IFN-γ+ cells increased (24). It should be emphasized that in the
mentioned papers, MDSCs were identified as
CD11b+Gr1+, and such phenotypic
characteristics do not allow separation of the two subpopulations
of cells. Considering our results described above, it is possible
that the observations regarding the effect of IL-10 on MDSC
activity depend on the proportion between monocytic and
polymorphonuclear cell subpopulations. However, to confirm this
assumption, further research is needed to elucidate the influence
of IL-10 on the individual mechanisms used by MDSCs.
Interleukin-10, occurring in high concentrations in
the TME, can be produced by MDSCs, but also by other myeloid cell
populations, such as TAMs and tolerogenic DCs, as well as by
tumor-infiltrating lymphocytes, e.g. Tregs and Th2 lymphocytes. In
some cases, IL-10 is also secreted directly by tumor cells
(16). Since MC38 murine colon
carcinoma cells do not produce IL-10 in vitro, it can be
assumed that the main source of this cytokine in MC38 tumors is
immune cells. In order to reduce the concentration of IL-10 in TME,
lentiviral vectors encoding shRNA sequences against IL-10 were
injected into MC38 tumors. The advantage of the lentiviral vectors
is their ability to transduce a wide spectrum of cells, both
proliferating and resting (25).
After intratumoral administration of lentiviral vectors, they can
therefore transduce tumor cells and immune cells present in the
TME. The transduced cells can proliferate and accumulate at the
injection site for up to three weeks after subcutaneous
administration of the vectors (26).
On the other hand, antiviral immune response elicited after LV
injections may lead to the elimination of transduced cells from the
TME by antigen-specific CTLs (22).
There is a constant effort to minimize the limitations of the
lentiviral vector system resulting from low efficiency and
immunogenicity (27,28). Hence, research aimed at determining
the mechanisms triggered by the in vivo application of
lentiviral vectors is of key importance in the development of
LV-based therapies.
Taking this into consideration, in this work,
lentiviral vectors were administered three times according to two
schedules. The first one assumed intratumoral LV injections every
two days and the end of the experiment on the 6th day from the
start of the study. Since adaptive antiviral response takes about
one week to develop (29), at this
timepoint, an immune response after the use of LVs may be observed,
but the elimination of transduced cells should not occur yet. In
the second scheme, the interval between the first and second
administrations of LVs was extended to 6 days, in order to check
whether repeated administration of the vectors after the
development of the adaptive immune response induced by the first
injection would also be effective in reducing the concentration of
IL-10 in the TME, which was measured on the 10th day after the
first LV application. Additionally, performing the experiments in
such a way enabled us to determine the kinetics of changes
occurring in the activity of cells of the immune system due to
lentiviral vector-mediated immunomodulation, as well as due to the
decrease in the concentration of IL-10 in the TME resulting from
transduction of cells with shRNA specific for IL-10.
Analysis of expression of EGFP in the TME, as well
as IL-10 production by cells isolated from tumors confirmed that
intratumorally injected LVs were able to transduce predominantly
myeloid cells inside tumors and deliver sequences encoding shRNA
specific for IL-10, and, as a result, reduce the concentration of
IL-10 in the TME. Due to a low number of leukocytes in the MC38
TME, we were not able to analyze the IL-10 secretion by each
myeloid cell population, but the percentage of EGFP+
cells demonstrates the efficiency of transduction of individual
populations. The main recipients of lentiviral transduction were
PMN-MDSCs, as observed in both schemes of the treatment. However,
on the 10th day after LV inoculations, the percentage of
EGFP+ cells among PMN-MDSCs was almost two-fold higher
than on the 6th day. This effect may be related to the influx of
PMN-MDSCs enhanced by LV injections. A similar response to viral
application was observed by Tan et al (30) during oncolytic virotherapy of
mesothelioma. In this case, recruitment of IL-10-secreting
PMN-MDSCs into the TME resulted in inhibition of DC-mediated
induction of CTLs. Therefore, it can be assumed that elevated
suppressor activity of myeloid cells isolated from tumors on the
6th day of the treatment with shIL-10 LVs is related to the
insufficient efficacy of transduction and, as a result, high
expression of IL-10 in these cells. In contrast, analyses performed
on the 10th day after LV inoculations revealed that myeloid cells
isolated from shIL-10 LV-treated tumors did not suppress the
activity of T cells. We suppose that this effect corresponded to
the higher percentage of transduced PMN-MDSCs, which was presumably
observed as a result of the recruitment of these cells into the TME
after the first injection of LVs and their in-situ
transduction by LVs administered six days later. The loss of
suppressor activity of myeloid cells isolated from tumors injected
with shIL-10 LVs is in line with the results obtained during
analysis of ex vivo-generated PMN-MDSCs transduced with
shIL-10 LVs.
Since the analyses presented in this work were
performed on the 6th and 10th day after LV administration, the
observation was too short to determine the changes in tumor growth
rate induced by LVs. However, our previously published research
demonstrated that triple injection of shIL-10 LVs carried out at
weekly intervals resulted in significant MC38 tumor growth
inhibition, which was 71.5% in relation to the untreated control on
the 23rd day of treatment (17).
Another study of ours aimed to determine the influence of IL-10
elimination, mediated by anti-IL-10 antibodies (Ab), on the
effectiveness of cyclophosphamide and dendritic cell-based therapy
(31). We observed that temporary
systemic reduction of IL-10 did not lead to tumor growth inhibition
but resulted in decreased suppressive activity of splenic-derived
MDSCs. The changes induced by anti-IL-10 Ab led to enhanced
efficiency of dendritic cell-based therapy.
Cyclophosphamide is known for its immunomodulatory
properties and contribution to the enhancement of the anti-tumor
response induced during immunotherapy. Since our previous research
proved that it augments the antitumor response of shIL-10 LVs
(17), schemes of tumor inoculations
with LVs were extended by single i.p. CY administration prior to
the LV injections. It was proved that CY used in low doses causes
the selective elimination of regulatory T lymphocytes, and by
inducing immunogenic death of cancer cells, at the same time
contributes to the increase in the activity of effector T
lymphocytes and supports the formation of immune memory (32). As a result of the changes of the
immune landscape mediated by CY, a decrease of IL-10 expression is
often observed (33–35). In fact, production of IL-10 by cells
isolated from tumors was decreased not only in CY and shIL-10
LV-treated groups, but a slight decrease was also observed in
groups treated with CY as well as CY and shN LVs. Analysis of EGFP
expression in the TME revealed that pretreatment with CY changed
the susceptibility of cells to transduction-in this case only
CD45neg and lymphoid cell transduction was observed.
Lack of transduction of myeloid cells was presumably related to the
effect of cyclophosphamide on the maturation of myeloid cells. TAMs
and DCs identified in the TME of cytostatic-treated mice showed an
increased level of expression of MHC II and CD86 molecules compared
to the untreated control. This could be caused by the accumulation
of DAMPs in the TME, released by cancer cells during the
immunogenic cell death induced by cyclophosphamide (36). The presence of these molecules in the
TME causes an increased influx of dendritic cells, which absorb the
released tumor antigens, and then migrate to the tumor-draining
lymph nodes and activate antigen-specific T cells. Schiavoni et
al (37) found that
cyclophosphamide promotes the expansion of the cross-presentation
dendritic cell population, resulting in stimulation of tumor
antigen-specific cytotoxic T lymphocytes. This is in line with our
observation that on the 8th day after CY administration an increase
of the percentage of CD8+ T cells and, to a lesser
extent, CD4+ T cells among leukocytes infiltrating
tumors was correlated with an increase of the percentage of
effector cells among CD8+ T cells identified in
tumor-draining lymph nodes. The effect of CY on the CTL population
was only temporary and on the 12th day after its administration was
not observed. However, modulation of the TME by CY led to
elicitation of a CTL- and NK-dependent response induced by LVs on
the 12th day of the treatment. There are reports in the literature
indicating that cyclophosphamide may increase the accumulation of
MDSCs, which reduces its therapeutic effect (38,39).
However, the present research did not confirm these
observations-myeloid cells isolated from the CY-treated group did
not show higher T cell suppressor activity than cells from
untreated tumors. Despite the lack of myeloid cell transduction
after treatment with CY and LVs, on the 12th day suppressor
activity of these cells towards CD8+ T cells was
significantly decreased. However, presumably due to the decrease of
IL-10 secretion in the TME mediated by CY, we did not observe any
differences between LV-treated groups. Additionally, since after
pretreatment with CY we did not note that LV transduced myeloid
cells inside tumors, no effect of shIL-10 LV-mediated reduction of
IL-10 production by myeloid cells could be observed.
In conclusion, the obtained data may indicate that
downregulation of IL-10 expression with lentiviral vectors encoding
shRNA against IL-10 in ex-vivo-generated, as well as
tumor-derived MDSCs results in modulation of their suppressor
activity. However, this effect seems to be dependent not only on
the silencing of IL-10 expression but also on the transduction with
lentiviral vectors. Hence, to confirm this assumption, further
studies on the effects of IL-10 depletion on the suppressor
mechanisms used by these cells are required. Such knowledge would
make it possible to establish novel therapeutic regimens, which
could become a foundation for future strategies in antitumor
treatment of patients with a high frequency of MDSCs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research was funded by National Science Centre,
Poland (grant nos. 2014/15/N/NZ4/04817 and
2018/30/E/NZ5/00711).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding authors on reasonable
request.
Authors' contributions
NAG and JR were involved in conceptualization. NAG,
JR and EPP developed the methodology. NAG and JR performed formal
analysis. NAG, KWC, AS, JM, EPP and JR performed the experiments.
NAG and JR prepared the original draft. KWC, AS, JM and EPP
reviewed and edited the manuscript. JR supervised the study. NAG
and JR acquired funding. NAG and JR confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments were performed in accordance with
Directive 2010/63/EU of the European Parliament and of the Council
on the protection of animals used for scientific purposes and were
approved by the 1st Local Ethic Committee for Experiments with the
Use of Laboratory Animals, Wrocław, Poland (authorization no.
11/2015, 33/2018).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Buoncervello M, Gabriele L and Toschi E:
The janus face of tumor microenvironment targeted by immunotherapy.
Int J Mol Sci. 20:43202019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pitt JM, Marabelle A, Eggermont A, Soria
JC, Kroemer G and Zitvogel L: Targeting the tumor microenvironment:
Removing obstruction to anticancer immune responses and
immunotherapy. Ann Oncol. 27:1482–1492. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kumar V, Patel S, Tcyganov E and
Gabrilovich DI: The nature of myeloid-derived suppressor cells in
the tumor microenvironment. Trends Immunol. 37:208–220. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marvel D and Gabrilovich DI:
Myeloid-derived suppressor cells in the tumor microenvironment:
Expect the unexpected. J Clin Invest. 125:3356–3364. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gnjatic S, Bronte V, Brunet LR, Butler MO,
Disis ML, Galon J, Hakansson LG, Hanks BA, Karanikas V, Khleif SN,
et al: Identifying baseline immune-related biomarkers to predict
clinical outcome of immunotherapy. J Immunother Cancer. 5:442017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spencer KR, Wang J, Silk AW, Ganesan S,
Kaufman HL and Mehnert JM: Biomarkers for immunotherapy: Current
developments and challenges. Am Soc Clin Oncol Educ Book.
35:e493–e503. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Masucci GV, Cesano A, Hawtin R, Janetzki
S, Zhang J, Kirsch I, Dobbin KK, Alvarez J, Robbins PB, Selvan SR,
et al: Validation of biomarkers to predict response to
immunotherapy in cancer: Volume I-pre-analytical and analytical
validation. J Immunother Cancer. 4:762016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Anger N and Rossowska J: Myeloid-derived
suppressor cells as a target for anticancer therapy. Postępy
Higieny i Medycyny Doświadczalnej. 72:1179–1198. 2018. View Article : Google Scholar
|
|
9
|
Xiu B, Lin Y, Grote DM, Ziesmer SC,
Gustafson MP, Maas ML, Zhang Z, Dietz AB, Porrata LF, Novak AJ, et
al: IL-10 induces the development of immunosuppressive
CD14(+)HLA-DR(low/-) monocytes in B-cell non-Hodgkin lymphoma.
Blood Cancer J. 5:e3282015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu L, Deng Z, Peng Y, Han L, Liu J, Wang
L, Li B, Zhao J, Jiao S and Wei H: Ascites-derived IL-6 and IL-10
synergistically expand CD14+HLA-DR−/low
myeloid-derived suppressor cells in ovarian cancer patients.
Oncotarget. 8:76843–76856. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Steinbrink K, Wölfl M, Jonuleit H, Knop J
and Enk AH: Induction of tolerance by IL-10-treated dendritic
cells. J Immunol. 159:4772–4780. 1997.PubMed/NCBI
|
|
12
|
Cavani A, Nasorri F, Prezzi C, Sebastiani
S, Albanesi C and Girolomoni G: Human CD4+ T lymphocytes
with remarkable regulatory functions on dendritic cells and
nickel-specific Th1 immune responses. J Invest Dermatol.
114:295–302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sica A, Saccani A, Bottazzi B,
Polentarutti N, Vecchi A, van Damme J and Mantovani A: Autocrine
production of IL-10 mediates defective IL-12 production and
NF-kappa B activation in tumor-associated macrophages. J Immunol.
164:762–767. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dennis KL, Blatner NR, Gounari F and
Khazaie K: Current status of interleukin-10 and regulatory T-cells
in cancer. Curr Opin Oncol. 25:637–645. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fiorentino DF, Bond MW and Mosmann TR: Two
types of mouse T helper cell. IV. Th2 clones secrete a factor that
inhibits cytokine production by Th1 clones. J Exp Med.
170:2081–2095. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sato T, Terai M, Tamura Y, Alexeev V,
Mastrangelo MJ and Selvan SR: Interleukin 10 in the tumor
microenvironment: A target for anticancer immunotherapy. Immunol
Res. 51:170–182. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rossowska J, Anger N, Szczygieł A,
Mierzejewska J and Pajtasz-Piasecka E: Reprogramming the murine
colon cancer microenvironment using lentivectors encoding shRNA
against IL-10 as a component of a potent DC-based
chemoimmunotherapy. J Exp Clin Cancer Res. 37:1262018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sistigu A, Viaud S, Chaput N, Bracci L,
Proietti E and Zitvogel L: Immunomodulatory effects of
cyclophosphamide and implementations for vaccine design. Semin
Immunopathol. 33:369–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pajtasz-Piasecka E, Szyda A, Rossowska J,
Krawczenko A, Indrová M, Grabarczyk P, Wysocki P, Mackiewicz A and
Duś D: Loss of tumorigenicity of murine colon carcinoma MC38/0 cell
line after transduction with a retroviral vector carrying murine
IL-12 genes. Folia Biol (Praha). 50:7–14. 2004.PubMed/NCBI
|
|
20
|
Rossowska J, Pajtasz-Piasecka E, Anger N,
Wojas-Turek J, Kicielińska J, Piasecki E and Duś D:
Cyclophosphamide and IL-12-transduced DCs enhance the antitumor
activity of tumor antigen-stimulated DCs and reduce Tregs and MDSCs
number. J Immunother. 37:427–439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rossowska J, Anger N, Szczygieł A,
Mierzejewska J and Pajtasz-Piasecka E: Intratumoral
lentivector-mediated TGF-β1 gene downregulation as a potent
strategy for enhancing the antitumor effect of therapy composed of
cyclophosphamide and dendritic cells. Front Immunol. 8:7132017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Breckpot K, Escors D, Arce F, Lopes L,
Karwacz K, Van Lint S, Keyaerts M and Collins M: HIV-1 Lentiviral
vector immunogenicity is mediated by toll-like receptor 3 (TLR3)
and TLR7. J Virol. 84:5627–5636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tanikawa T, Wilke CM, Kryczek I, Chen GY,
Kao J, Núñez G and Zou W: Interleukin (IL)-10 ablation promotes
tumor development, growth and metastasis. Cancer Res. 72:420–429.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Noman MZ, Desantis G, Janji B, Hasmim M,
Karray S, Dessen P, Bronte V and Chouaib S: PD-L1 is a novel direct
target of HIF-1α, and its blockade under hypoxia enhanced
MDSC-mediated T cell activation. J Exp Med. 211:781–790. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Breckpot K, Emeagi PU and Thielemans K:
Lentiviral vectors for anti-tumor immunotherapy. Curr Gene Ther.
8:438–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Furmanov K, Elnekave M, Lehmann D, Clausen
BE, Kotton DN and Hovav AH: The role of skin-derived dendritic
cells in CD8+ T cell priming following immunization with
lentivectors. J Immunol. 184:4889–4897. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Olbrich H, Slabik C and Stripecke R:
Reconstructing the immune system with lentiviral vectors. Virus
Genes. 53:723–732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Milone MC and O'Doherty U: Clinical use of
lentiviral vectors. Leukemia. 32:1529–1541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nash AA and Dutia BM: The Immune response
to viral infections. Topley & Wilson's Microbiology and
Microbial Infections. American Cancer Society, . 15–Mar;2010.doi:
10.1002/9780470688618.taw0220. View Article : Google Scholar
|
|
30
|
Tan Z, Liu L, Chiu MS, Cheung KW, Yan CW,
Yu Z, Lee BK, Liu W, Man K and Chen Z: Virotherapy-recruited
PMN-MDSC infiltration of mesothelioma blocks antitumor CTL by
IL-10-mediated dendritic cell suppression. Oncoimmunology.
8:e15186722019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rossowska J, Anger N, Kicielińska J,
Pajtasz-Piasecka E, Bielawska-Pohl A, Wojas-Turek J and Duś D:
Temporary elimination of IL-10 enhanced the effectiveness of
cyclophosphamide and BMDC-based therapy by decrease of the
suppressor activity of MDSCs and activation of antitumour immune
response. Immunobiology. 220:389–398. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abu Eid R, Razavi GSE, Mkrtichyan M, Janik
J and Khleif SN: Old-school chemotherapy in immunotherapeutic
combination in cancer, a low-cost drug repurposed. Cancer Immunol
Res. 4:377–382. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang XM, Zhang NR, Lin XT, Zhu CY, Zou
YF, Wu XJ, He XS, He XW, Wan YL and Lan P: Antitumor immunity of
low-dose cyclophosphamide: Changes in T cells and cytokines
TGF-beta and IL-10 in mice with colon-cancer liver metastasis.
Gastroenterol Rep (Oxf). 8:56–65. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Matar P, Rozados VR, Gervasoni SI and
Scharovsky OG: Down regulation of T-cell-derived IL-10 production
by low-dose cyclophosphamide treatment in tumor-bearing rats
restores in vitro normal lymphoproliferative response. Int
Immunopharmacol. 1:307–319. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xia Q, Geng F, Zhang FF, Liu CL, Xu P, Lu
ZZ, Zhang HH, Kong W and Yu XH: Enhancement of fibroblast
activation protein α-based vaccines and adenovirus boost immunity
by cyclophosphamide through inhibiting IL-10 expression in 4T1
tumor bearing mice. Vaccine. 34:4526–4535. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vanmeerbeek I, Sprooten J, De Ruysscher D,
Tejpar S, Vandenberghe P, Fucikova J, Spisek R, Zitvogel L, Kroemer
G, Galluzzi L and Garg AD: Trial watch: Chemotherapy-induced
immunogenic cell death in immuno-oncology. Oncoimmunology.
9:17034492020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schiavoni G, Sistigu A, Valentini M,
Mattei F, Sestili P, Spadaro F, Sanchez M, Lorenzi S, D'Urso MT,
Belardelli F, et al: Cyclophosphamide synergizes with type I
interferons through systemic dendritic cell reactivation and
induction of immunogenic tumor apoptosis. Cancer Res. 71:768–778.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mikyšková R, Indrová M, Vlková V, Bieblová
J, Šímová J, Paračková Z, Pajtasz-Piasecka E, Rossowska J and
Reiniš M: DNA demethylating agent 5-azacytidine inhibits
myeloid-derived suppressor cells induced by tumor growth and
cyclophosphamide treatment. J Leukoc Biol. 95:743–753. 2014.
View Article : Google Scholar
|
|
39
|
Sevko A, Sade-Feldman M, Kanterman J,
Michels T, Falk CS, Umansky L, Ramacher M, Kato M, Schadendorf D,
Baniyash M and Umansky V: Cyclophosphamide promotes chronic
inflammation-dependent immunosuppression and prevents antitumor
response in melanoma. J Invest Dermatol. 133:1610–1619. 2013.
View Article : Google Scholar : PubMed/NCBI
|