Introduction
Thymic cancer is a thymic epithelial tumor with
strong metastatic characteristics, and 50–65% of patients exhibit
distant metastases when diagnosed (1). At present, surgical resection is the
primary method for treating thymic cancer (2), but the application of surgical
resection has limitations. Therefore, the identification of novel
diagnostic markers and therapeutic targets is of great importance
for the clinical treatment of thymic cancer.
Metastasis-associated-lung-adenocarcinoma-transcript-1 (MALAT1) is
a long non-coding RNA (lncRNA) that cannot be translated into
protein in vitro (3).
Previous studies have shown that MALAT1 is closely associated with
various diseases, including lung cancer, renal cell carcinoma and
esophageal squamous cell carcinoma (4–6). MALAT1
can promote cervical cancer cell proliferation and migration, and
can regulate the expression of genes associated with apoptosis
(7). Han et al (8) reported that MALAT1 could promote cancer
cell proliferation and migration by activating the ERK/MAPK
pathway. High expression levels of MALAT1 can be a marker of poor
prognosis in patients with hepatocellular carcinoma (9). Therefore, MALAT1 is considered a
potential tumor marker.
High-mobility group AT-hook 2 (HMGA2) belongs to the
non-histone chromosome high-mobility group family, and is located
on chromosome 12q 13–15 (10). The
human HMGA2 gene consists of five exons and four introns (10). HMGA2 functions as a transcription
factor by altering the structure of chromatin, and by interacting
with DNA and target proteins (10).
HMGA2 is usually highly expressed in numerous malignant tumors and
is closely associated with increased invasiveness (11–15). Tan
et al (unpublished data) showed that small interfering (si)
RNA targeting HMGA2 attenuated epithelial-mesenchymal transition
(EMT) in thymic cancer cells via the Wnt/β-catenin pathway. The
present study investigated the effects of inhibiting the expression
of MALAT1 on the proliferation and apoptosis of thymic cancer
cells. Further molecular experiments were performed to verify
whether si-MALAT1 exerted its effect by regulating the expression
of HMGA2. The present study may provide some insights into the
treatment of thymic cancer.
Materials and methods
Cell culture and transfection
The thymic cancer cell line IU-TAB-1 was obtained
from Applied Biological Materials, Inc. (cat. no. T8001), while
293T, A549 and HCT-116 cells (used as controls) were provided by
the Stem Cell Bank of the Chinese Academy of Sciences. Cells were
cultured in RPMI-1640 medium (293T cells) (cat. no. SH30022.01B;
HyClone; Cytiva), Prigrow II medium (IU-TAB-1 cells) (cat. no.
TM002; Abmgood), F-12K medium (A549 cells) (cat. no. 21127-022;
Gibco; Thermo Fisher Scientific, Inc.) or McCoy's 5A medium
(HCT-116 cells) (cat. no. 16600-082; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (cat.
no. 10270-106; Gibco; Thermo Fisher Scientific, Inc.) in an
atmosphere containing 5% CO2 and 95% atmospheric air at
37°C for 24 h. The medium was replaced every 24 h and the cells
were subcultured or cryopreserved when the cell density reached
70–80%.
The sequence of the full-length cDNA of MALAT1
(accession no. NR_002819.4) was obtained from the NCBI database.
IU-TAB-1 cells were transfected with MALAT1 siRNA using
Lipofectamine® 2000 reagent (cat. no. 13778030;
Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Briefly, 100 pmol siRNA and 5 ml
Lipofectamine® RNAiMAX were added to 250 µl Opti-MEM at
4°C for 20 min, and then the mixture was added to the cell culture
plates and incubated at 37°C in a 5% CO2 incubator for
48 h before subsequent experiments. The cells were divided into
five groups: Control (untransfected cells), MALAT1-siRNA1,
MALAT1-siRNA2, MALAT1-siRNA3 and non-targeting negative control
(NC). The transfection efficiency was determined by RT-qPCR.
Cell Counting Kit-8 (CCK-8) assay
IU-TAB-1 cells were seeded in a 96-well plate at
5×103 cells/ml using Prigrow II medium containing 10%
fetal bovine serum. Cells from each group were treated for 48 h. To
evaluate cell proliferation, 10 µl CCK-8 solution (cat. no. C1706;
Bioswamp Wuhan Bienle Biotechnology Co., Ltd.) was added to each
well, and the cells were cultured at 37°C for 4 h. The optical
density at 450 nm was measured using a plate reader (Multiskan FC;
Thermo Fisher Scientific, Inc.).
Flow cytometry
IU-TAB-1 cells were cultured for 24 h and then
harvested. Next, 1 ml pre-cooled PBS was added before centrifuging
the cells at 1,000 × g at 4°C for 5 min. Next, 10 µl Annexin V-FITC
and 10 µl propidium iodide were added. The apoptotic rate and cell
cycle were detected using flow cytometry (NovoCyte; Agilent
Technologies, Inc.), and the data were analyzed with CytExpert
software (Beckman Coulter, Inc.; version 2.0). One-step
fluorescence compensation strategy was used to eliminate any
interference with the fluorescein isothiocyanate channel.
RT-qPCR
The expression levels of HMGA2, MALAT1 and
miR-145-5p in each cell line were detected by RT-qPCR. Total RNA
was extracted from 1×106 cells using TRIzol®
reagent according to the manufacturer's protocol (Invitrogen;
Thermo Fisher Scientific, Inc.). cDNA was synthesized using a
reverse transcriptase kit (cat. no. 639505; Takara Bio, Inc.). qPCR
was performed with a CFX Connect 96 Real-Time PCR Detection System
(Bio-Rad Laboratories, Inc.) using SYBR Green PCR kit (cat. no.
KM4101; Kapa Biosystems; Roche Diagnostics). Each reaction was
conducted in duplicate using the following thermocycling
conditions: Denaturation at 95°C for 3 min, followed by 39 cycles
of 95°C for 5 sec, 56°C for 10 sec and 72°C for 25 sec; 65°C for 5
sec and 95°C for 50 sec. The results were analyzed by the
2−ΔΔCq method (16).
GAPDH and U6 were used as the reference genes for mRNA and miRNA,
respectively. The primers were designed and provided by Wuhan
Tianyihuiyuan Biotechnology Co., Ltd., and their sequences are
listed in Table I.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Primer | Sequence (5′-3′) |
|---|
| HMGA2-F | TTCAGCCCAGGGACAA |
| HMGA2-R | CCAGGCAAGGCAACAT |
| MALAT1-F | TAACCAGGCATAACAC |
| MALAT1-R | CGAAGACACAGAGACC |
| miR-145-5p-F |
GGGGTCCAGTTTTCCCAG |
| miR-145-5p-R |
AACTGGTGTCGTGGAGTCGGC |
| U6-F |
CTCGCTTCGGCAGCACA |
| U6-R |
AACGCTTCACGAATTTGCGT |
| GAPDH-F |
CCACTCCTCCACCTTTG |
| GAPDH-R |
CACCACCCTGTTGCTGT |
Western blotting
After treatment for 48 h, cells were washed with
cold PBS and subjected to a lysis buffer (cat. no. 180006; Bioswamp
Wuhan Bienle Biotechnology Co., Ltd.), and the proteins were
quantified using the bicinchoninic acid assay kit (cat. no. 180007;
Bioswamp Wuhan Bienle Biotechnology Co., Ltd.). Proteins (20
µg/lane) were separated via 12% SDS-PAGE and were then transferred
onto PVDF membranes. The membranes were blocked with a buffer
containing 5% non-fat milk in PBS with 0.05% Tween-20 for 2 h at
room temperature and then incubated with the following primary
antibodies (all from Bioswamp Wuhan Bienle Biotechnology Co.,
Ltd.): Anti-HMGA2 (1:1,000; cat. no. PAB40807), anti-cyclin D1
(1:1,000; cat. no. MAB37160), anti-cyclin E (1:1,000; cat. no.
PAB36461), anti-Bax (1:1,000; cat. no. MAB30681), anti-Bcl-2
(1:1,000; cat. no. PAB30041) and anti-GAPDH (1:2,000; cat. no.
PAB36264) overnight at 4°C. After three washes with PBS with 10%
Tween-20, the membranes were incubated with horseradish
peroxidase-conjugated secondary goat anti-rabbit IgG (1:20,000;
cat. no. PAB160011; Bioswamp Wuhan Bienle Biotechnology Co., Ltd.)
for 2 h at 4°C. Protein bands were visualized by enhanced
chemiluminescence detection (Tanon-5200; Tanon Science and
Technology Co., Ltd.) and analyzed using Tanon GIS software
(version 4.2; Tanon Science and Technology Co., Ltd.). GAPDH was
used as a loading control for normalization.
Luciferase reporter assay
IU-TAB-1 cells were co-transfected with 50 nM
miR-145-5p mimics or miR-145-5p inhibitor plus 200 ng MALAT1-3′-UTR
or HMGA2-3′-UTR using Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) following the manufacturer's
instructions. After 48 h, the cells were lysed using the
Dual-Luciferase Reporter Assay System (Promega Corporation), and
luciferase activity was measured using a GloMax20/20 Luminometer
(Promega Corporation). Luciferase activity was normalized to the
Renilla luciferase signal in IU-TAB-1 cells. The constructs
were prepared as follows: MALAT1-3′-UTR sense strand,
5′-CTAGCTTGGAGAAGATAGAAGTTTGAAGTGGAAAACTGGAAGACAGAAGTACGGGAAGGCGAAGAAAAG-3′
and antisense strand,
5′-CTAGACTTTTCTTCGCCTTCCCGTACTTCTGTCTTCCAGTTTTCCACTTCAAACTTCTATCTTCTCCAA-3′;
HMGA2-3′-UTR sense strand,
5′-CTAGCAAGACCCAAAGGCAGCAAAAACAAGAGTCCCTCTAAAGCAGCTCAAAAGAAAGCAGAAGCCACTG-3′
and antisense strand,
5′-CTAGACAGTGGCTTCTGCTTTCTTTTGAGCTGCTTTAGAGGGACTCTTGTATTTTGCTGCCTTTGGGTCTT-3′.
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance followed by Tukey's test for multiple
comparisons using SPSS 19.0 software (IBM Corp.). All experiments
were performed independently three times. All figures were prepared
using GraphPad Prism 5.0 software (GraphPad Software, Inc.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
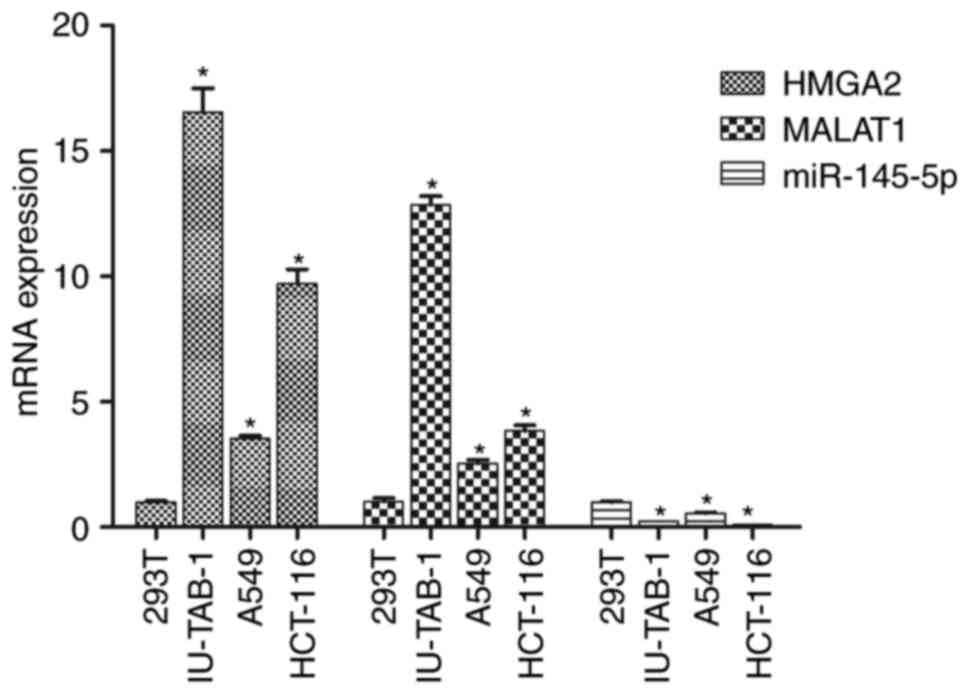
HMGA2, MALAT1 and miR-145-5p
expression in IU-TAB-1, A549, HCT-116 and 293T cells
293T cells are a normal human renal epithelial cell
line, which is often used as a control group for detecting tumor
cytokine expression. As shown in Fig.
1, compared with 293T cells, mRNA expression of HMGA2 and
MALAT1 was significantly increased in IU-TAB-1, A549 and HCT-116
cells (P<0.05), while the expression of miR-145-5p was
significantly decreased (P<0.05). Among all the cell lines
evaluated, IU-TAB-1 cells showed the largest fold change in HMGA2
and MALAT1 expression, as well as a significant change in
miR-145-5p expression. Therefore, IU-TAB-1 cells were selected for
subsequent experiments.
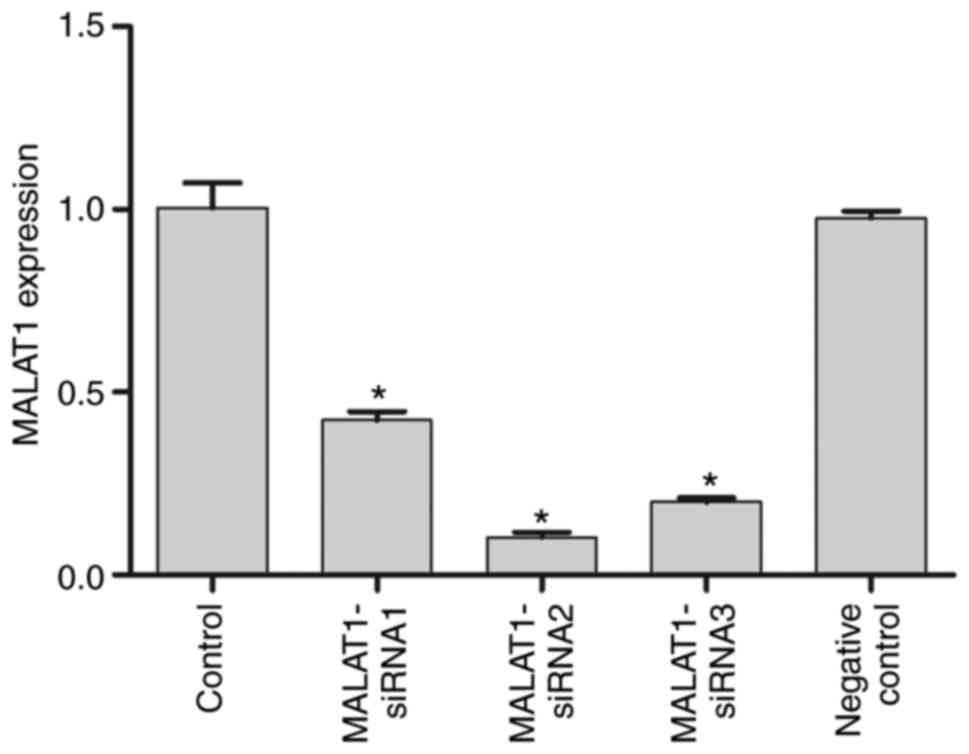
Inhibition of MALAT1 expression
The mRNA expression levels of MALAT1 in the control,
MALAT1-siRNA1, MALAT1-siRNA2, MALAT1-siRNA3 and NC groups confirmed
MALAT1 silencing following siRNA transfection (Fig. 2). The results revealed that all three
MALAT1 siRNA molecules significantly downregulated the expression
of MALAT1 compared with that of control and NC IU-TAB-1 cells
(P<0.05). MALAT1-siRNA2 resulted in the lowest MALAT1 expression
levels among the three siRNA reagents used and was thus used to
silence MALAT1 in subsequent experiments.
si-MALAT1 attenuates cell
proliferation and promotes apoptosis in IU-TAB-1 cells
To examine the effect of MALAT1 on cell
proliferation, a CCK-8 assay was performed (Fig. 3A). Transfection with si-MALAT1
significantly suppressed cell proliferation of IU-TAB-1 cells
compared with the control and NC (P<0.05). Flow cytometry
revealed that si-HMGA2 significantly increased the apoptotic rate
of IU-TAB-1 cells compared with that of control and NC cells
(P<0.05; Fig. 3B), and
significantly decreased the number of cells in G1 phase
while increasing the number of cells in G2 phase
(P<0.05; Fig. 3C). Next, the
expression of cell cycle and apoptosis-related proteins was
examined (Fig. 3D). The protein
expression of cyclin D1, cyclin E, Bcl-2 and HMGA2 significantly
decreased, whereas that of Bax significantly increased (P<0.05
in all cases) compared with the control and NC groups, indicating
that MALAT1 silencing promoted apoptosis and inhibited cell cycle
progression in IU-TAB-1 cells.
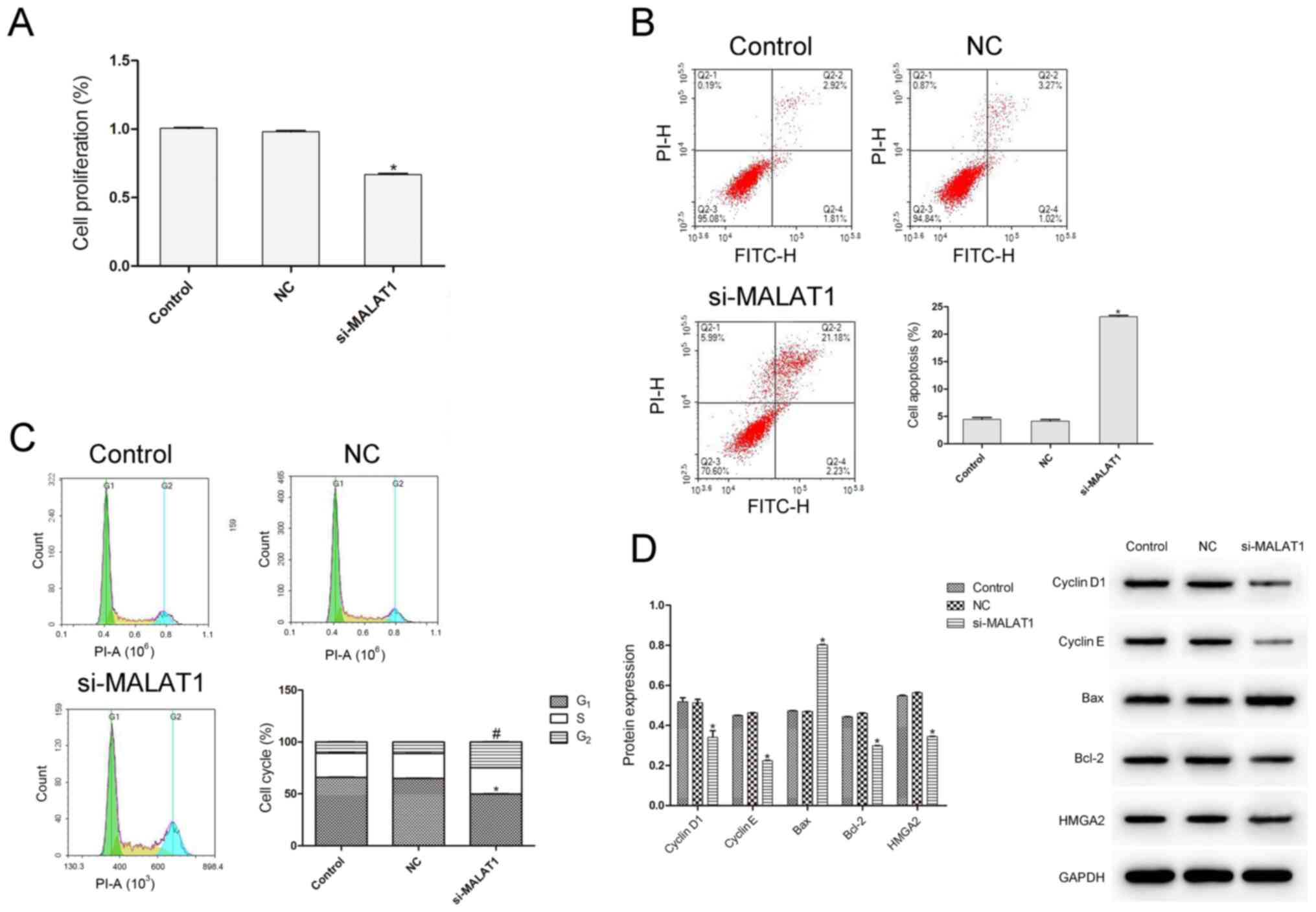 | Figure 3.Effect of si-MALAT1 on cell
proliferation and apoptosis in IU-TAB-1 cells. (A) si-MALAT1
inhibited the cell proliferation of IU-TAB-1 cells. (B) Cell
apoptosis and (C) cell cycle progression were analysed by flow
cytometry. *P<0.05 cells in G1 phase vs. Control
(n=3); #P<0.05 cells in G2 phase vs.
Control (n=3). (D) Cell cycle and apoptosis-related proteins were
detected by western blotting. Compared with the control and NC
groups, si-MALAT1 significantly suppressed cell proliferation, the
number of cells in G1 phase and the expression of cyclin
D1, cyclin E, Bcl-2 and HMGA2, while it increased the apoptotic
rate, the number of cells in G2 phase and the expression
of Bax. *P<0.05 vs. control (n=3). MALAT1,
metastasis-associated-lung-adenocarcinoma-transcript-1; HMGA2, high
mobility group AT-hook 2; NC, negative control; si, small
interfering; FITC, flurorescein isothiocyanate; PE,
phycoerythrin. |
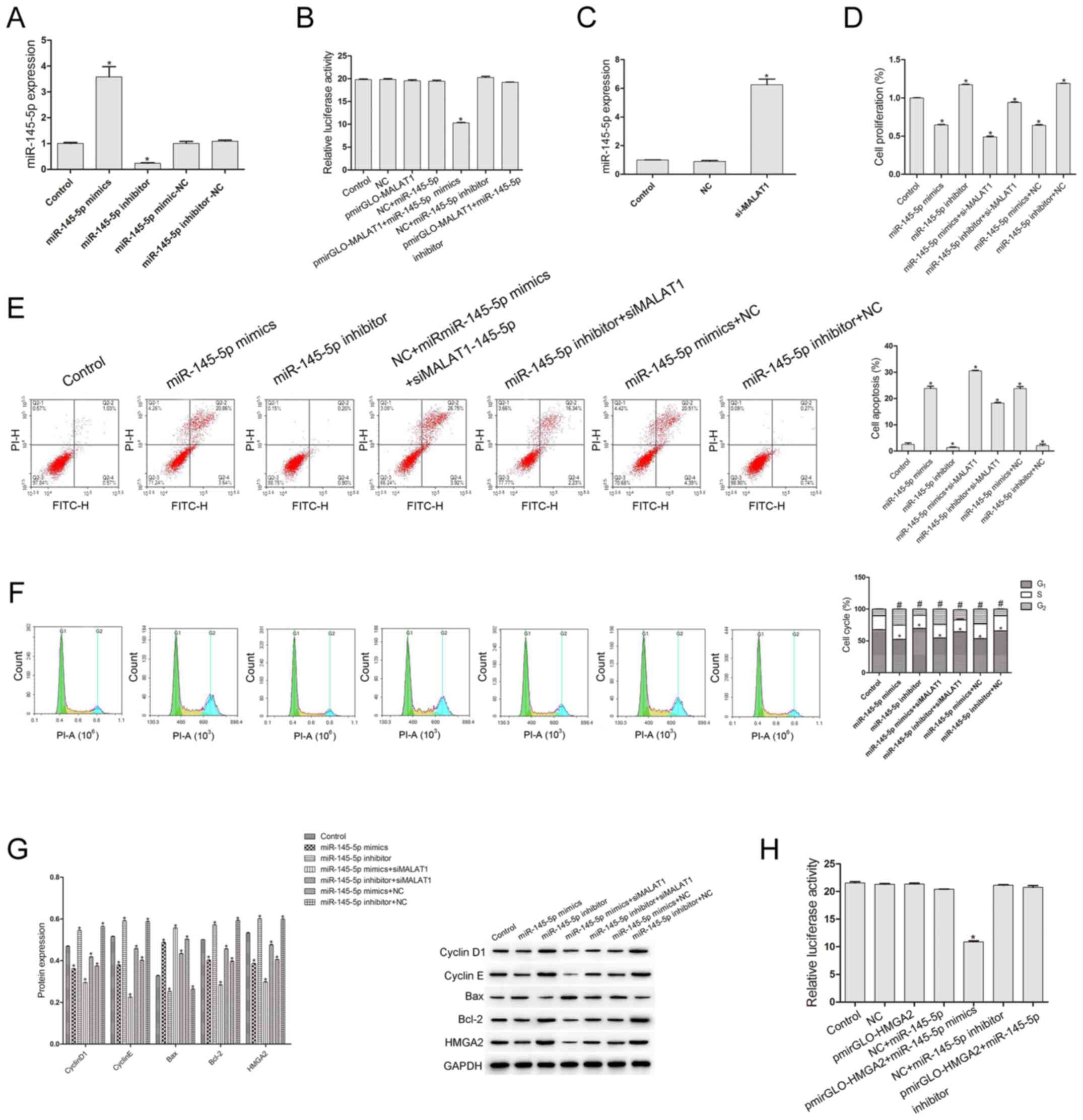
Association between MALAT1 and
miR-145-5p, and between HMGA2 and miR-145-5p
The transfection efficiency of miR-145-5p mimics and
inhibitor was first examined (Fig.
4A). Compared with that of the control and NC groups, the
expression of miR-145-5p in the miR-145-5p mimics group increased
significantly (P<0.05), while it decreased significantly in the
miR-145-5p inhibitor group (P<0.05), suggesting that the
transfections of miR-145-5p mimics and inhibitor were successful.
To study the association between miR-145-5p and MALAT1, a
dual-luciferase reporter assay was conducted. The luciferase
reporter vector pmirGLO-MALAT1 3′-UTR was co-transfected with
miR-145-5p mimics or inhibitor. The results shown in Fig. 4B indicate that, compared with the
control group, the relative luciferase activity of other groups did
not change significantly (P>0.05), except for the
pmirGO-MALAT1+miR-145-5p mimics group (P<0.05), suggesting that
miR-145-5p specifically binds to MALAT1. Next, the expression of
miR-145-5p was evaluated, and the results showed that si-MALAT1
could significantly increase the expression of miR-145-5p
(P<0.05) (Fig. 4C). To study the
effect of miR-145-5p on the biological characteristics of IU-TAB-1
cells, miR-145-5p inhibitor and miR-145-5p mimics and si-MALAT1
were used. As shown in Fig. 4D and
E, compared with that of the control group, the cell
proliferation rate of the miR-145-5p inhibitor group was
significantly increased and the apoptosis rate was decreased
(P<0.05), while the proliferation rate of the miR-145-5p mimics
group was significantly decreased and the apoptosis rate was
significantly increased (P<0.05). Compared with the control
group, the proliferation rate in miR-145-5p mimics + si-MALAT1
group was significantly decreased (P<0.05), while this group
exhibited the highest apoptosis rate (Fig. 4D and E), indicating that there may be
a synergistic effect between si-MALAT1 and miR-145-5p mimics.
Compared with the control, the number of cells in G1
phase in the miR-145-5p inhibitor group was significantly increased
(P<0.05) and in the miR-145-5p inhibitor + si-MALAT1 group it
was significantly decreased (P<0.05), the number of cells in
G2 phase in the miR-145-5p inhibitor group was
significantly decreased (P<0.05) and in the miR-145-5p inhibitor
+ si-MALAT1 group it was significantly increased (P<0.05), while
the miR-145-5p mimics and miR-145-5p mimics + si-MALAT1 group had
the opposite effects (Fig. 4F). As
shown in Fig. 4G, compared with the
control, the expression levels of Cyclin D1, Cyclin E, Bcl-2 and
HMGA2 in the miR-145-5p inhibitor group were significantly
increased (P<0.05), and in the miR-145-5p inhibitor + si-MALAT1
group they were significantly decreased (P<0.05), while Bax
expression in the miR-145-5p inhibitor group was significantly
decreased (P<0.05) and in the miR-145-5p inhibitor + si-MALAT1
group they were significantly increased (P<0.05). On the other
hand, Cyclin D1, Cyclin E, Bcl-2 and HMGA2 expression in the
miR-145-5p mimics group and miR-145-5p mimics + si-MALAT1 group was
significantly decreased compared with the control, while Bax
expression was significantly increased (P<0.05; Fig. 4G). The association between HMGA2 and
miR-145-5p was examined. As shown in Fig. 4H, compared with the control group,
the relative luciferase activity of other groups did not change
significantly (P>0.05), except for the pmirGLO-HMGA2+miR-145-5p
mimics group (P<0.05), suggesting that there was specific
binding between HMGA2 and miR-145-5p.
Discussion
The occurrence and development of malignant tumors
are associated with cell proliferation, apoptosis, invasion and
metastasis. Abnormal lncRNA expression can change the above
biological processes, and plays a key role in tumorigenesis and
development (17). MALAT1 is an
8.7-kb gene located on human chromosome 11q13, which is controlled
by multiple promoters. It is widely distributed in mammalian normal
tissues and abnormally expressed in multiple malignant tumors,
affecting tumor proliferation, apoptosis, invasion, metastasis and
drug resistance (3). Whether MALAT1
is overexpressed in thymic cancer has not yet been determined.
IU-TAB-1, A549 and HCT-116 are common types of tumor cells. Studies
(11–15) have shown that MALAT1 is highly
expressed in a variety of malignant tumors, such as lung, colon and
ovarian cancer, and its enhanced expression is closely associated
with enhanced tumor aggressiveness and disease prognosis.
Therefore, the present study hypothesized that the occurrence and
development of thymic cancer may also be associated with abnormally
high MALAT1 expression. The present study detected the expression
of MALAT1 in the thymic cancer cell line IU-TAB-1, and it used
non-small cell lung cancer A549, human colon cancer HCT-116 and
293T cells as controls. Our results showed that the expression of
MALAT1 was the highest in IU-TAB-1 cells, suggesting that MALAT1 is
highly expressed in thymic cancer.
MALAT1 acts as a competitive endogenous messenger
RNA (18). Liu et al
(19) found that MALAT1 can inhibit
the expression of the target gene growth factor receptor-bound
protein 2 in cervical cancer cells by competitively binding to
miR-124, thereby inhibiting the growth and invasion of cervical
cancer cells, and increasing their apoptosis. MALAT1 knockout
inhibits the migration and invasion of gallbladder cancer cells,
and increases their apoptosis (20).
Yang et al (21) reported
that MALAT1 also has a role in regulating the cell cycle. MALAT1 is
expressed at high levels in cell nuclei. At the G2/M
phase, MALAT1 is localized in the cytoplasm, where interacts with a
large number of nuclear factors and heterogeneous nuclear
ribonucleoprotein (hnRNP) C protein (22). Downregulating the expression of
MALAT1 causes cytoplasmic ectopic hnRNP C expression in
G2/M phase, which, in turn, causes G2/M phase
arrest (23). To investigate whether
MALAT1 has a similar effect on the proliferation and apoptosis of
thymic cancer cells, CCK-8 assay and flow cytometry were used to
detect cell proliferation and apoptosis rates in the present study.
The results showed that inhibiting the expression of MALAT1 can
significantly reduce the proliferation of thymic cancer cells and
promote their apoptosis. The cell cycle results showed that, after
the inhibition of MALAT1, the cell cycle of thymic cancer cells was
mainly blocked in the G2 phase, which is consistent with
the results observed in other types of tumor, such as pancreatic
cancer, liver cancer and squamous cell carcinoma (23–25). To
verify this finding, the expression levels of cell cycle- and
apoptosis-related proteins were detected, and the results supported
the above conclusions, suggesting that si-MALAT1 can inhibit the
proliferation of thymic cancer cells and promote their
apoptosis.
miRNA is a type of non-coding RNA that is widely
involved in cell growth, differentiation, proliferation and
apoptosis (26). miR-145-5p, as a
tumor suppressor miRNA, is downregulated in colorectal cancer, and
inhibits the proliferation, invasion and migration of colorectal
cancer cells. miR-145-5p also plays a suppressive role in lung
(27), breast (28), cervical (29) and prostate cancer (30). In the present study, the expression
of miR-145-5p in thymic cancer cells was significantly lower than
that in 293T cells. After promoting the expression of miR-145-5p,
the proliferation rate of thymic cancer cells was significantly
decreased, and the apoptosis rate was significantly increased.
Luciferase reporter assay revealed that MALAT1 specifically binds
to miR-145-5p, and the miR-145-5p mimics + si-MALAT1 group had the
highest apoptosis rate and lowest cell proliferation rate,
indicating that there may be a synergistic effect between si-MALAT1
and miR-145-5p mimics. si-MALAT1 plays a role in inhibiting
proliferation and promoting apoptosis by enhancing the expression
of miR-145-5p. The present study also confirmed that HMGA2 could
specifically bind to miR-145-5p, that HMGA2 was highly expressed in
IU-TAB-1 cells and that the expression of HMGA2 was significantly
reduced after promoting the expression of miR-145-5p. The apoptosis
rate in thymic cancer cells was increased, while the cell
proliferation rate was decreased, suggesting that miR-145-5p can
inhibit the expression of HMGA2. Sun et al (31) determined via microarray analysis that
MALAT1 can regulate EMT by regulating Snail, and knocking down
MALAT1 can upregulate the expression of E-cadherin and zona
occludens-1, and reduce the activity of β-catenin and vimentin. Xu
et al (32) found that the
EMT of breast cancer cells was reduced after inhibiting MALAT1
activity. Tan et al (unpublished data) confirmed that
inhibition of HMGA2 activity can inhibit the EMT of thymic cancer
cells. Therefore, it was hypothesized that si-MALAT1 can inhibit
the activity of HMGA2 by promoting the expression of miR-145-5p,
thus, inhibiting the EMT of thymic cancer cells. To confirm this
hypothesis, further studies are required. The present study did not
use clinical tissues, which limits the clinical significance of the
study and is one of its limitations. However, the main aim of the
present study was to evaluate the effect of MALAT1 on the
proliferation and apoptosis of thymic cancer cells. In future
studies, the effect of MALAT1 should be further examined using
in vivo experiments. Rong et al (33) observed that MALAT1-knockdown
repressed NSCLC tumorigenicity by inhibiting cell proliferation and
invasion, and promoting apoptosis via regulating miR-515-5p/EEF2.
Although the types of tumor cells investigated in the present study
were not consistent with the aforementioned study, the current
results confirmed those of the study by Rong et al (33). The present study focused on thymic
cancer, which is rarer in clinical practice, and the current
results may therefore have a high clinical value and may provide a
possible molecular-targeted therapy for patients with thymic
cancer.
In conclusion, si-MALAT1 inhibits the proliferation
and apoptosis of IU-TAB-1 cells, and its mechanism may involve
promoting the expression of miR-145-5p and inhibiting that of
HMGA2.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The data used to support the findings of the present
study are available from the corresponding author upon request.
Authors' contributions
ST and JC collected and analyzed the data, and
confirm the authenticity of all the raw data. ST drafted the
manuscript. JC revised the manuscript. Both authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Fink C, Henzler T, Shirinova A, Apfaltrer
P and Wasser K: Thoracic magnetic resonance imaging: Pulmonary
thromboembolism. J Thorac Imaging. 28:171–177. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Qureshi S, West M and Kirk AJB: Surgical
resection for thymic malignancy. Lung Cancer. 63 (Suppl):S382009.
View Article : Google Scholar
|
|
3
|
Gutschner T, Monika H and Diederichs S:
MALAT1-a paradigm for long noncoding RNA function in cancer. J Mol
Med (Berl). 91:791–801. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gutschner T, Hämmerle M, Eissmann M, Hsu
J, Kim Y, Hung G, Revenko A, Arun G, Stentrup M, Gross M, et al:
The Noncoding RNA MALAT1 is a critical regulator of the metastasis
phenotype of lung cancer cells. Cancer Res. 73:1180–1189. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hirata H, Hinoda Y, Shahryari V, Deng G,
Nakajima K, Tabatabai ZL, Ishii N and Dahiya R: Long noncoding RNA
MALAT1 promotes aggressive renal cell carcinoma through Ezh2 and
interacts with miR-205. Cancer Res. 75:1322–1331. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hu L, Wu Y, Tan D, Meng H, Wang K, Bai Y
and Yang K: Up-regulation of long noncoding RNA MALAT1 contributes
to proliferation and metastasis in esophageal squamous cell
carcinoma. J Exp Clin Cancer Res. 34:72015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Wang T, Huang HQ, Li W, Cheng XL
and Yang J: Human MALAT-1 long non-coding RNA is overexpressed in
cervical cancer metastasis and promotes cell proliferation,
invasion and migration. J BUON. 20:1497–1503. 2016.
|
|
8
|
Han Y, Wu Z, Wu T, Huang Y, Cheng Z, Li X,
Sun T, Xie X, Zhou Y and Du Z: Tumor-suppressive function of long
noncoding RNA MALAT1 in glioma cells by downregulation of MMP2 and
inactivation of ERK/MAPK signaling. Cell Death Dis. 7:e21232016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Konishi H, Ichikawa D, Yamamoto Y, Arita
T, Shoda K, Hiramoto H, Hamada J, Itoh H, Fujita Y, Komatsu S, et
al: Plasma level of metastasis-associated lung adenocarcinoma
transcript 1 is associated with liver damage and predicts
development of hepatocellular carcinoma. Cancer Sci. 107:149–154.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Narita M, Narita M, Krizhanovsky V, Nuñez
S, Chicas A, Hearn SA, Myers MP and Lowe SW: A novel role for
high-mobility group a proteins in cellular senescence and
heterochromatin formation. Cell. 126:503–514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Di Cello F, Hillion J, Hristov A, Wood LJ,
Mukherjee M, Schuldenfrei A, Kowalski J, Bhattacharya R, Ashfaq R
and Resar LM: HMGA2 participates in transformation in human lung
cancer. Mol Cancer Res. 6:743–750. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Malek A, Bakhidze E, Noske A, Sers C,
Aigner A, Schäfer R and Tchernitsa O: HMGA2 gene is a promising
target for ovarian cancer silencing therapy. Int J Cancer.
123:348–356. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang ML, Chen CC and Chang LC: Gene
expressions of HMGI-C and HMGI(Y) are associated with stage and
metastasis in colorectal cancer. Int J Colorectal Dis.
24:1281–1286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hristov AC, Cope L, Reyes MD, Singh M,
Iacobuzio-Donahue C, Maitra A and Resar LM: HMGA2 protein
expression correlates with lymph node metastasis and increased
tumor grade in pancreatic ductal adenocarcinoma. Mod Pathol.
22:43–49. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bartuma H, Panagopoulos I, Collin A,
Trombetta D, Domanski HA, Mandahl N and Mertens F: Expression
levels of HMGA2 in adipocytic tumors correlate with morphologic and
cytogenetic subgroups. Mol Cancer. 8:362009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TDL: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi D and Zhang Y, Lu R and Zhang Y: The
long non-coding RNA MALAT1 interacted with miR-218 modulates
choriocarcinoma growth by targeting Fbxw8. Biomed Pharmacother.
97:543–550. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bai RM and Wang CX: Progress of long
non-coding RNA MALAT1 in tumor research. J Clin Oncol.
21:1139–1145. 2016.
|
|
19
|
Liu S, Song L, Zeng S and Zhang L:
MALAT1-miR-124-RBG2 axis is involved in growth and invasion of
HR-HPV-positive cervical cancer cells. Tumour Biol. 37:633–640.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang SH, Zhang WJ, Wu XC, Zhang MD, Weng
MZ, Zhou D, Wang JD and Quan ZW: Long non-coding RNA Malat1
promotes gallbladder cancer development by acting as a molecular
sponge to regulate miR-206. Oncotarget. 7:37857–37867. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang F, Yi F, Han X, Du Q and Liang Z:
MALAT-1 interacts with hnRNP C in cell cycle regulation. FEBS Lett.
587:3175–3181. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miyagawa R, Tano K, Mizuno R, Nakamura Y,
Ijiri K, Rakwal R, Shibato J, Masuo Y, Mayeda A, Hirose T and
Akimitsu N: Identification of cis- and trans-acting
factors involved in the localization of MALAT-1 noncoding RNA to
nuclear speckles. RNA. 18:738–751. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang YJ, Tang XM, Shi MM, Wen CL and Shen
BY: MiR-216a decreases MALAT1 expression, induces G2/M arrest and
apoptosis in pancreatic cancer cells. Biochem Biophys Res Commun.
483:816–822. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yao K, Zhang GJ, Huang ZM and Yao GX:
Expression and function of long non-coding RNA-MALAT1 in liver
cancer. Chin J Gen Surg. 1:90–96. 2016.
|
|
25
|
Kangboonruang K, Wongtrakoongate P,
Lertsuwan K, Khachonkham S, Changkaew P, Tangboonduangjit P,
Siripoon T, Ngamphaiboon N and Chairoungdua A: MALAT1 decreases the
sensitivity of head and neck squamous cell carcinoma cells to
radiation and cisplatin. Anticancer Res. 40:2645–2655. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Esquela-Kerscher A and Slack FJ:
Oncomirs-microRNAs with a role in cancer. Nat Rev Cancer.
6:259–269. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Li Y, Liu J, Fan Y, Li X, Dong M,
Liu H and Chen J: Expression levels of microRNA-145 and
microRNA-10b are associated with metastasis in non-small cell lung
cancer. Cancer Biol Ther. 17:272–279. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding Y, Zhang C, Zhang J, Zhang N, Li T,
Fang J, Zhang Y, Zuo F, Tao Z, Tang S, et al: miR-145 inhibits
proliferation and migration of breast cancer cells by directly or
indirectly regulating TGF-β1 expression. Int J Oncol. 50:1701–1710.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou X, Yue Y, Wang R, Gong B and Duan Z:
MicroRNA-145 inhibits tumorigenesis and invasion of cervical cancer
stem cells. Int J Oncol. 50:853–862. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xie SG, Xie YY, Zhang LL and Huang Q:
Effect of miR-145 targeting DAB2 on the migration and invasion of
prostate cancer PC3 cells. Heredity. 36:50–57. 2014.PubMed/NCBI
|
|
31
|
Sun R, Qin C, Jiang B, Fang S, Pan X, Peng
L, Liu Z, Li W, Li Y and Li G: Down-regulation of MALAT1 inhibits
cervical cancer cell invasion and metastasis by inhibition of
epithelial-mesenchymal transition. Mol Biosyst. 12:952–962. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xu S, Sui S, Zhang J, Bai N, Shi Q, Zhang
G, Gao S, You Z, Zhan C, Liu F and Pang D: Downregulation of long
noncoding RNA MALAT1 induces epithelial-to-mesenchymal transition
via the PI3K-AKT pathway in breast cancer. Int J Clin Exp Pathol.
8:4881–4891. 2015.PubMed/NCBI
|
|
33
|
Rong F, Liu L, Zou C, Zeng J and Xu Y:
MALAT1 promotes cell tumorigenicity through regulating
miR-515-5p/EEF2 axis in non-small cell lung cancer. Cancer Manag
Res. 12:7691–7701. 2020. View Article : Google Scholar : PubMed/NCBI
|