Introduction
Gastric cancer (GC) is one of the most common types
of malignancies worldwide (1).
Although the incidence of GC has slowly begun to decline, GC
remains the third leading cause of cancer-associated mortality
worldwide (2). Despite significant
improvements being made in the diagnostic and therapeutic methods
available for GC, the rate of GC recurrence remains high, with a
5-year survival rate of <20% (3).
Metastasis, which is a multi-step process encompassing the
proliferation, invasion, detachment, vascular intravasation and
adhesion of cancer cells, is the main cause of GC recurrence
(4,5). Previous studies have reported a role
for numerous cellular molecular markers in the process of
metastasis, which will help to guide future research on metastasis
(5,6). These should help to identify biomarkers
that can be used to predict the prognosis of patients with GC and
enable the development of effectvie treatment regimens to improve
the survival of patients with GC.
C-X-C motif chemokine receptor 4 (CXCR4), which is a
highly conserved member of the G protein-coupled receptor
subfamily, is a transmembrane receptor of 352 amino acids in
length. CXCR4 serves as the only receptor for stromal cell-derived
factor-1 and has been reported to play an important role in
regulating the differentiation, development and directional
migration of immune cells (7).
Increasing evidence suggest that CXCR4 expression is upregulated in
different types of tumors, where it serves an important role in the
occurrence, growth and metastasis of the tumor (8). In addition, CXCR4 was identified as a
potential unique molecular target for the diagnosis and treatment
of breast (8), lung (9), cervical (10), bladder (11) and colorectal cancer (12). CXCR4 is suggested to serve as a
prognostic indicator in patients with GC, which promotes the
metastasis of GC (13,14).
Vascular endothelial growth factor (VEGF) is
produced by most tumor cells, keratinocytes and macrophages in
wound sites (15). VEGF receptor (R)
is only expressed on the surface of vascular endothelial cells, and
upon binding to its receptor, VEGF can increase vascular
permeability, promote the proliferation of vascular endothelial
cells, and regulate vasculogenesis and postnatal vascular
remodeling (16). VEGF is also known
to act as a lymphangiogenic growth factor, serving an important
role in tumor lymphangiogenesis via activation of the VEGFRs
(17). In addition, signaling
pathways associated with VEGF play important roles in the
occurrence and development of malignant tumor types (18), including breast cancer (19), hepatocellular carcinoma (20) and lung cancer (21). A VEGF genotype was associated with GC
risk in a previous study (22). VEGF
protein expression levels in GC tissues were also reported to be
positively associated with TNM staging and lymph node metastasis in
patients (23).
The present study aimed to determine the expression
levels of CXCR4 and VEGF in a cohort of patients with GC and to
investigate the potential prognostic and predictive values of these
markers in GC. Furthermore, whether detecting the expression levels
of CXCR4 and VEGF can be combined as a novel predictor of GC
survival with more accuracy than the predictive value of either
alone was determined.
Materials and methods
Patient studies
A total of 589 GC surgical cases were recruited from
The Yixing Hospital Affiliated to Medical College of Yangzhou
University (Yixing, China) between January 2000 and December 2006.
The present study protocol was approved by the Institutional Review
Board of Yixing Hospital Affiliated to Medical College of Yangzhou
University (approval no. YXYLL-2021-42). All patients provided
written informed consent prior to participation and all acquired
data were assured of anonymity and confidentiality.
The 589 GC surgical cases were followed up for ≥5
years. Overall survival (OS) was the primary endpoint of the
present analysis, and survival time was calculated from the date of
surgery to the date of death or to the last follow-up. Detailed
clinicopathological characteristics of each patient was obtained
from medical records by the ethics committee of the hospital. The
clinicopathological characteristics, including age, sex,
differentiation stage, depth of invasion, lymph node metastasis,
TNM stage (24), distant metastasis
and tumor diameter were recorded. All tissue sections were fixed in
formalin and embedded in paraffin to construct the tissue
microarray (TMA).
A total of 10 paired fresh tissues were immediately
frozen in liquid nitrogen following surgical resection and stored
at −80°C until subsequent experimentation. The adjacent tissues
were all more than 10 cm away from the cancer tissue. The present
study recruited 5 men and 5 women (age range, 35–68 years; mean
age, 44 years).
Western blotting
Cells or tissues proteins were extracted using RIPA
strong lysis buffer (50 mM pH 7.4 Tris, 150 mM NaCl, 1% Triton
X-100, 1% sodium deoxycholate, 0.1% SDS) and 5 µl PMSF. The
concentration of protein was measured according to the instructions
of the BCA kit (Thermo Fisher Scientific, Inc.). Protein samples
(80 µg/lane) were separated via 10% SDS PAGE, subsequently
transferred onto polyvinylidene fluoride membranes (Beyotime
Institute of Biotechnology) and blocked with 5% skim milk at room
temperature for 2 h. The membranes were washed with Tris-buffered
saline with Tween-20 and incubated with primary antibodies. Western
blotting was performed as previously described (25). The following primary antibodies were
used: Monoclonal rabbit anti-CXCR4 (1:2,000; cat. no. ab181020;
Epitomics; Abcam), monoclonal rabbit anti-VEGF (1:1,000; cat. no.
ab32152; Epitomics; Abcam) and monoclonal mouse anti-β-actin
(1:2,000; cat. no. AF5001; Beyotime Institute of Biotechnology).
Densitometric analysis was performed using ImageJ software (version
1.44; National Institutes of Health), following normalization to
β-actin expression levels.
Construction of the TMA and
immunohistochemistry analysis
CXCR4 and VEGF protein expression levels were
analyzed via immunohistochemistry analysis using a formalin-fixed,
paraffin-embedded TMA containing samples from patients with GC. The
GC TMA included 1,178 cores and each paraffin-embedded tissue
sample punched was 1.5 mm in diameter. These TMAs were heated at
55°C for 20 min and subsequently washed three times with xylene to
remove the paraffin. Subsequently, these chips were washed with
absolute ethyl alcohol. Antigen retrieval step was performed using
sodium citrate and the samples were incubated at 95°C for 30 min.
Serum blocking was subsequently performed for 30 min at room
temperature. Immunostaining was performed as previously described
(25). Briefly, every tissue core
was incubated with monoclonal rabbit anti-VEGF (1:200; cat. no.
ab32152; Epitomics; Abcam) and monoclonal rabbit anti-CXCR4 (1:200;
cat. no. ab181020; Epitomics; Abcam) overnight at 4°C. The staining
scores of the control tissue in each TMA were pre-evaluated as a
quality control of the immunostaining.
Evaluation of immunostaining
The staining of CXCR4 or VEGF was evaluated by two
independent pathologists who were blinded to the clinical data. The
staining results were assessed using a semi-quantitative scoring
system, in which the final score was calculated as the product of
the proportion and intensity scores. The scoring criteria used for
the immunoreactivity score (IRS) was as previously described
(26,27). The scoring system used for CXCR4 and
VEGF expression involved scoring each sample with a score of
between 0 and 12. The intensity of the immunohistochemistry
staining is presented in Fig. 1B and
C. The optimum IRS cut-off value was obtained by receiver
operator characteristic (ROC) analysis, in which the area under the
curve (AUC) at different IRS cut-off values for CXCR4 or VEGF
expression was calculated for an OS of 1, 3 or 5 years. The optimum
cut-off value for the CXCR4 or VEGF IRS was demonstrated to be 5,
as it had the best predictive value for survival (Fig. 2A and B). Thus, samples with an IRS of
0–4 were classified as having low CXCR4 or VEGF expression, while
samples with an IRS of 6–12 were classified as having high CXCR4 or
VEGF expression.
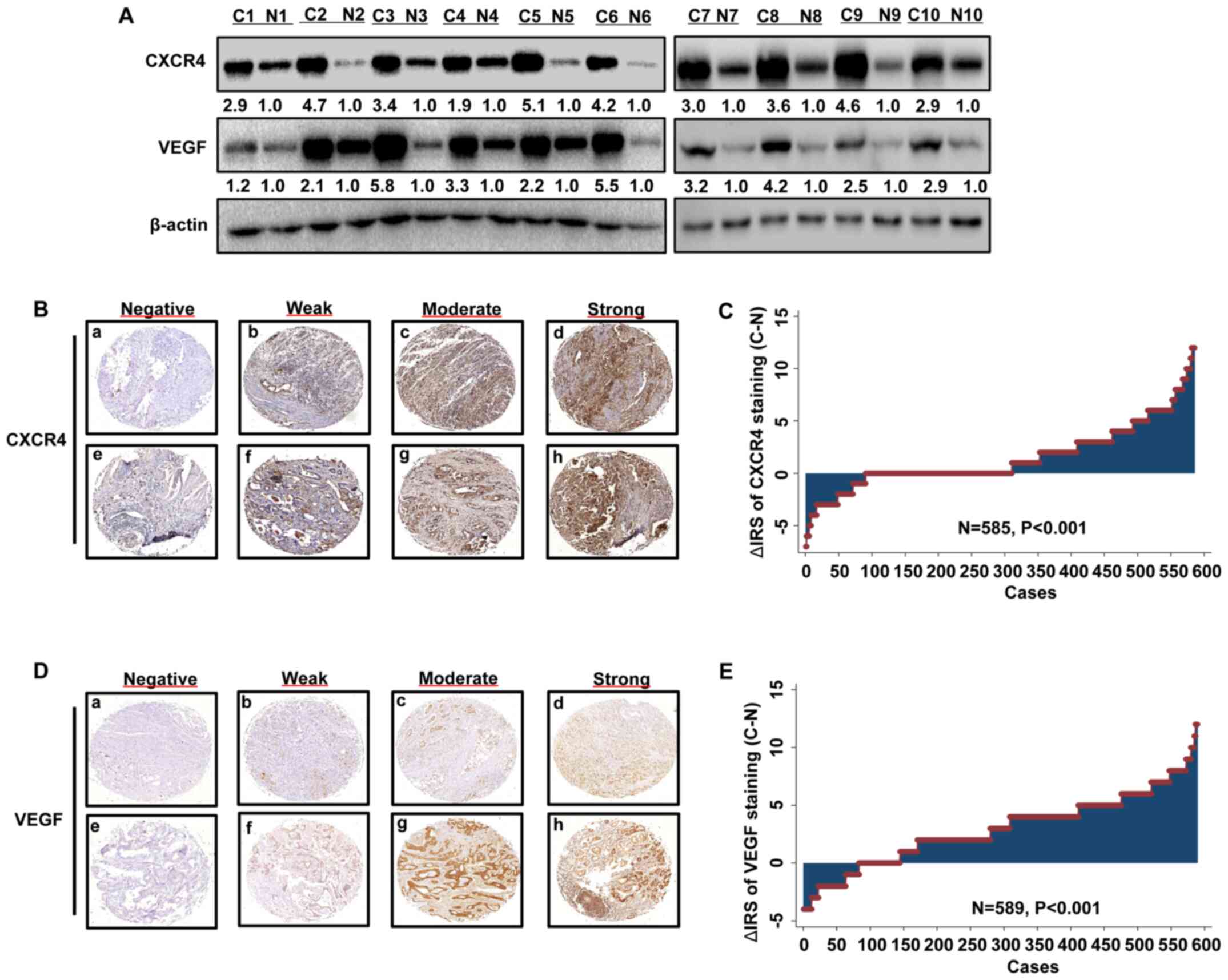 | Figure 1.CXCR4 and VEGF expression levels are
upregulated in GC. (A) Western blot analysis was performed to
detect the protein expression levels of CXCR4 and VEGF, which were
upregulated in GC tissues compared with paired adjacent normal
tissues. Representative images of (B) CXCR4 or (C) VEGF
immunohistochemical staining of the tissue microarray. Panels
(B/C-a, B/C-b, B/C-c and B/C-d) exhibit adjacent normal tissues;
panels (B/C-e, B/C-d, B/C-g and B/C-h) exhibit GC tissues. Negative
staining is presented in panels (B/C-a and B/C-e); weak staining is
presented in panels (B/C-b and B/C-f); moderate staining is
presented in panels (B/C-c and B/C-g) and strong staining is
presented in panels (B/C-d and B/C-h). Original magnification, ×40.
Distribution of (D) CXCR4 and (E) VEGF staining in GC tissues and
paired adjacent normal tissues. CXCR4, C-X-C motif chemokine
receptor 4; VEGF, vascular endothelial growth factor; GC, gastric
cancer; C, cancer; N, normal; IRS, immunoreactivity score. |
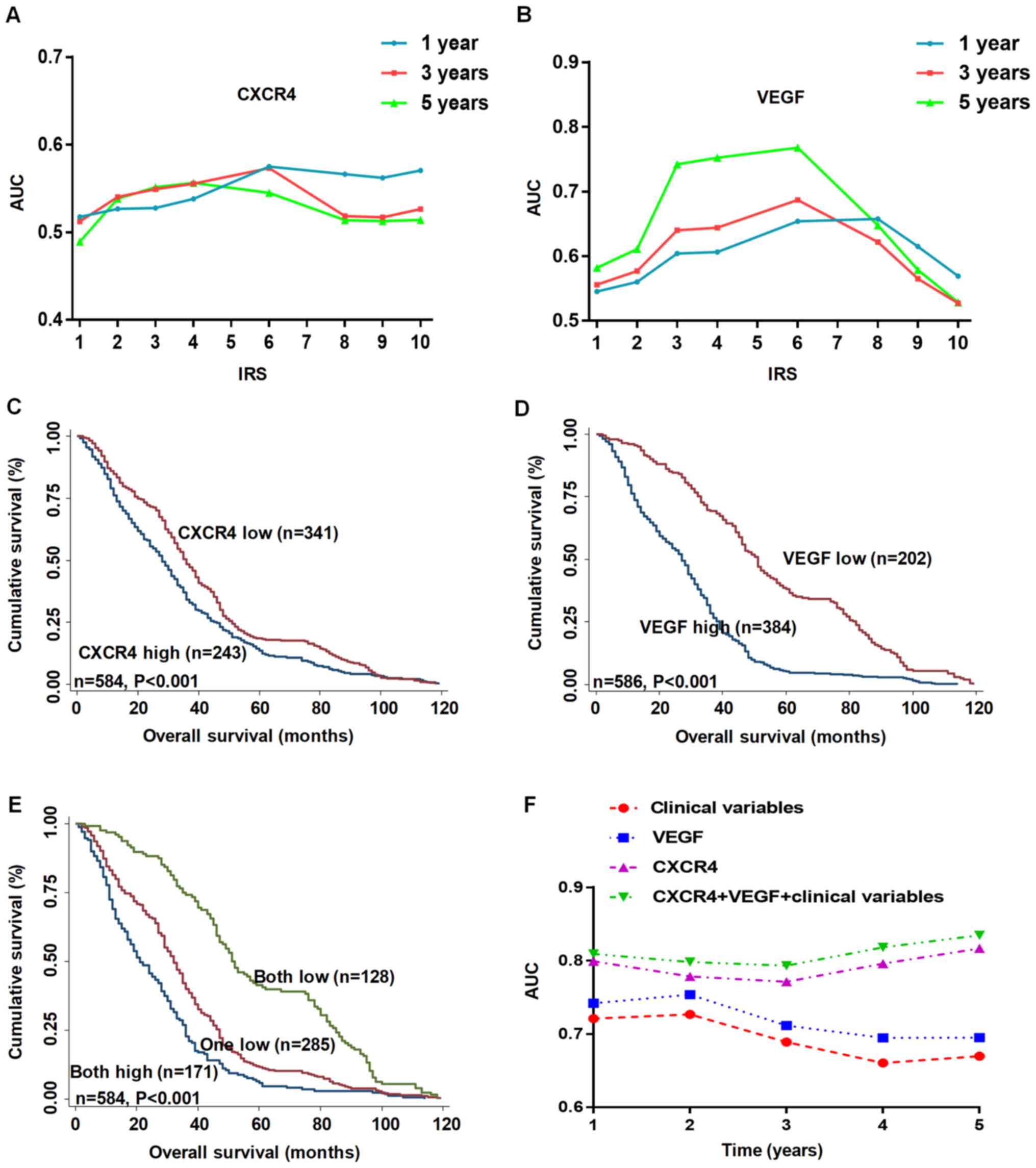 | Figure 2.CXCR4 and VEGF expression levels are
associated with OS of patients with gastric cancer. (A and B) AUC
analysis of different immunoreactivity score cut-off values at 1, 3
and 5 years of OS. Kaplan-Meier survival curves depicting OS
according to the expression levels of (C) CXCR4, (D) VEGF and (E)
combined CXCR4/VEGF in the training cohort. (F) Time-dependent
receiver operator characteristic analyses for clinical risk score
(tumor-node-metastasis stage, histological type and tumor
diameter), or CXCR4, VEGF or CXCR4 and VEGF risk score. CXCR4,
C-X-C motif chemokine receptor 4; VEGF, vascular endothelial growth
factor; OS, overall survival; AUC, area under the curve; IRS,
immunoreactivity score. |
GC cell lines and lentivirus (LV)
production
Human AGS GC cells were purchased from The Cell Bank
of Type Culture Collection of The Chinese Academy of Sciences.
Cells were maintained in RPMI-1640 medium (Hyclone; Cytiva)
supplemented with 10% fetal bovine serum (FBS, Hyclone; Cytiva), at
37°C with 5% CO2.
LV (Shanghai GenePharma Co., Ltd.) was used to
interfere with CXCR4 or VEGF expression. LV-CXCR4, LV-CXCR4-RNA
interference (RNAi, small interfering RNA), LV–VEGF, LV–VEGF-RNAi
and respective controls were transfected into AGS cells.
Transwell migration and invasion
assays
Transwell assays were performed to assess the cell
migratory and invasive abilities. Briefly, the membranes of
Transwell chambers (8-µm pore size; MilliporeSigma) were pre-coated
with Matrigel (MilliporeSigma) for 30 min for the invasion assay at
37°C, but not for the migration assay. A total of 100 µl
4×105/ml cells were subsequently plated in the upper
chambers of the Transwell plates and RPMI-1640 medium supplemented
with 10% FBS was plated in the lower chambers. Following incubation
for 24 h at 37°C, cells in the lower chambers were fixed with
methanol for 10 min and stained with 0.1% crystal violet for 5 min.
Stained cells were counted in five randomly selected fields using
an inverted microscope at (×20 magnification).
Wound healing assay
For the wound healing assay, 5×104/ml
cells were seeded into 6-well plates at 37°C. Following incubation,
the cell monolayers were scratched using a 10 µl pipette tip and
cells were cultured in serum-free medium for 0, 24 or 48 h. The
closure of the wound was visualized in the same field under as
inverted ordinary microscope at 20 multiples. The experiments were
performed in triplicate.
Statistical analysis
Statistical analyses were performed using SPSS 12.0
software (SPSS, Inc.) and STATA statistical software (version 10.1;
StataCorp LP). The Fisher's exact test was used to assess the
association between CXCR4 or VEGF expression and the
clinicopathological characteristics of patients with GC.
Statistical differences in the IRS for CXCR4 or VEGF staining
between tumor tissues and paired adjacent normal tissues (>5 cm
from the tumor tissue) were determined using the paired Wilcoxon
test (raw scores). The correlation between the expression levels of
CXCR4 or VEGF was analyzed using the Spearman's rank-order
correlation test (raw scores). Univariate and multivariate Cox
proportional hazards regression analyses were performed to estimate
the crude hazard ratios (HRs), adjusted HRs and 95% confidence
interval (CI) of HRs. The predictive value of CXCR4 and VEGF on OS
was evaluated using the Kaplan-Meier method, following by a
Weighted Estimation in Cox Regression to determine statistical
significance. P<0.05 was considered to indicate a statistically
significant difference.
Results
CXCR4 and VEGF expression levels are
upregulated in GC tissues compared with adjacent normal
tissues
A total of 10 paired primary GC tissues and matched
adjacent normal tissues were used to detect CXCR4 and VEGF protein
expression levels via western blotting. The expression levels of
CXCR4 and VEGF were upregulated in all tumor tissues compared with
the matched adjacent normal tissues (Fig. 1A). The GC TMA slide comprised 589 GC
tissues and paired adjacent normal tissues. Analysis of the TMA
revealed that CXCR4 and VEGF expression levels were upregulated in
tumor tissues compared with paired adjacent normal tissues
(Fig. 1B-E).
CXCR4 and VEGF expression levels are
associated with clinicopathological characteristics
In the TMA containing samples from patients with GC,
significant associations were observed between high VEGF expression
levels and depth of invasion (P<0.001), lymph node metastasis
(P<0.001), TNM stage (P<0.001) and tumor diameter
(P<0.001), using Fisher's exact analysis. However, no
significant associations were observed between VEGF expression
levels and age, sex, differentiation stage and distant metastasis
(Table I).
 | Table I.Association between the expression
levels of CXCR4 and VEGF and the clinicopathological
characteristics of patients with gastric cancer (n=589). |
Table I.
Association between the expression
levels of CXCR4 and VEGF and the clinicopathological
characteristics of patients with gastric cancer (n=589).
|
| CXCR4
expression | VEGF
expression |
|---|
|
|
|
|
|---|
| Characteristic | Low, n (%) | High, n (%) | P-value | Low, n (%) | High, n (%) | P-value |
|---|
| All patients | 341 (58.1) | 246 (41.9) |
| 204 (34.6) | 385 (65.4) |
| Age, years |
|
| 0.125 |
|
| 0.366 |
|
≤65 | 164 (55.6) | 131 (44.4) |
| 105 (35.5) | 191 (64.5) |
|
>65 | 177 (60.6) | 115 (39.4) |
| 99 (33.8) | 194 (66.2) |
| Sex |
|
| 0.309 |
|
| 0.150 |
|
Male | 265 (58.8) | 186 (41.2) |
| 151 (33.4) | 301 (66.6) |
|
Female | 76 (55.9) | 60 (44.1) |
| 53 (38.7) | 84 (61.3) |
| Differentiation
stage |
|
| 0.043a |
|
| 0.179 |
|
I/II | 302 (56.9) | 229 (43.1) |
| 181 (34.0) | 352 (66.0) |
|
III | 39 (69.6) | 17 (30.4) |
| 23 (41.1) | 33 (58.9) |
| Depth of
invasion |
|
| 0.010a |
|
|
<0.001c |
|
T1/T2 | 122 (65.2) | 65 (34.8) |
| 101 (53.7) | 87 (46.3) |
|
T3/T4 | 219 (54.8) | 181 (45.2) |
| 103 (25.7) | 298 (74.3) |
| Lymph node
metastasis |
|
|
<0.001c |
|
|
<0.001c |
| N0 | 162 (72.0) | 63 (28.0) |
| 114 (50.4) | 112 (49.6) |
|
N1/N2/N3 | 179 (49.4) | 183 (50.6) |
| 90 (24.8) | 273 (75.2) |
| TNM stage |
|
|
<0.001c |
|
|
<0.001c |
|
I/II | 186 (68.1) | 87 (31.9) |
| 132 (47.9) | 142 (52.1) |
|
III/IV | 155 (49.4) | 159 (50.6) |
| 72 (22.9) | 243 (77.1) |
| Tumor diameter,
cm |
|
| 0.021 |
|
|
<0.001c |
| ≤5 | 218 (61.6) | 136 (38.4) |
| 148 (41.7) | 207 (58.3) |
|
>5 | 123 (52.8) | 110 (47.2) |
| 56 (23.9) | 178 (76.1) |
| Distant
metastasis |
|
| 0.002b |
|
| 0.466 |
| M0 | 330 (59.7) | 223 (40.3) |
| 193 (34.8) | 362 (65.2) |
| M1 | 11 (32.4) | 23 (67.6) |
| 11 (32.4) | 23 (67.6) |
The two cancer tissues on these chips had fallen
off, so only the data of 587 patients were obtained. The Fisher's
exact test was also used to assess the association between CXCR4
expression levels and the clinicopathological characteristics of
patients with GC. High CXCR4 expression in the GC tissues was
significantly associated with differentiation stage (P=0.043),
depth of invasion (P=0.010), lymph node metastasis (P<0.001),
TNM stage (P<0.001), tumor diameter (P=0.021) and distant
metastasis (P=0.002). However, no significant associations were
observed between CXCR4 expression and age or sex (Table I).
Upregulated CXCR4 and VEGF expression
levels are associated with poor survival in patients with GC
To determine whether CXCR4 or VEGF expression levels
are associated with OS in patients with GC, Kaplan-Meier survival
curves were used to compare the 5-year overall cumulative survival
between patients with high CXCR4 or VEGF staining and patients with
low CXCR4 or VEGF staining, respectively. As presented in Fig. 2A and B, low CXCR4 or VEGF expression
was 0–4, while high CXCR4 or VEGF expression was 6–12, according to
the IRS. The results demonstrated that high expression levels of
CXCR4 and VEGF were associated with poor OS in patients with GC
(both P<0.05; Fig. 2C and D). In
addition, univariate and multivariate Cox regression analyses were
performed to determine whether CXCR4 or VEGF expression levels and
the clinicopathological characteristics were associated with the OS
of patients with GC. As presented in Table II, depth of invasion, lymph node
metastasis, TNM stage, distant metastasis, tumor diameter, and
CXCR4 and VEGF expression levels were all statistically
significant. Multivariate Cox regression analysis was subsequently
performed to assess the effect of CXCR4 or VEGF expression,
together with the clinical parameters (age, sex, differentiation
stage, depth of invasion and distant metastasis). The results
demonstrated that VEGF expression is an independent and unfavorable
prognostic factor for patients with GC (HR, 0.422; 95% CI,
0.350–0.508; P<0.001; Table
III). Using the same statistical methods, CXCR4 expression was
also identified as an independent and unfavorable prognostic factor
for patients with GC (HR, 0.836; 95% CI, 0.708–0.988; P=0.036;
Table III).
 | Table II.Univariate Cox regression analysis of
VEGF or CXCR4 expression and clinicopathological characteristics
predicting survival in patients with gastric cancer (n=589). |
Table II.
Univariate Cox regression analysis of
VEGF or CXCR4 expression and clinicopathological characteristics
predicting survival in patients with gastric cancer (n=589).
| Characteristic | HR (95% CI) | P-value |
|---|
| Age, years (≤65 vs.
>65) | 1.026
(0.872–1.207) | 0.761 |
| Sex (male vs.
female) | 0.988
(0.815–1.199) | 0.906 |
| Differentiation
stage (I/II vs. III) | 0.817
(0.620–1.077) | 0.152 |
| Depth of invasion
(T1/T2 vs. T3/T4) | 1.858
(1.558–2.215) |
<0.001c |
| Lymph node
metastasis (N0 vs. N1/N2) | 1.917
(1.618–2.269) |
<0.001c |
| TNM stage (I/II vs.
III/IV) | 2.211
(1.871–2.612) |
<0.001c |
| Distant metastasis
(M0 vs. M1) | 1.561
(1.102–2.209) | 0.012a |
| Tumor diameter, cm
(≤5 vs. >5) | 1.560
(1.321–1.843) |
<0.001c |
| CXCR4 expression
(low vs. high) | 0.789
(0.669–0.931) | 0.005b |
| VEGF expression
(low vs. high) | 0.391
(0.326–0.468) |
<0.001c |
 | Table III.Multivariate Cox regression analysis
of CXCR4, VEGF or CXCR4/VEGF expression and clinicopathological
characteristics predicting survival in patients with gastric cancer
(n=589). |
Table III.
Multivariate Cox regression analysis
of CXCR4, VEGF or CXCR4/VEGF expression and clinicopathological
characteristics predicting survival in patients with gastric cancer
(n=589).
| A, CXCR4 |
|---|
|
|---|
| Characteristic | HR (95% CI) | P-value |
|---|
| Age, years (≤65 vs.
>65) | 1.016
(0.863–1.197) | 0.847 |
| Sex (male vs.
female) | 1.081
(0.889–1.314) | 0.437 |
| Differentiation
stage (I/II vs. III) | 0.852
(0.645–1.126) | 0.260 |
| Depth of invasion
(T1/T2 vs. T3/T4) | 1.444
(1.298–1.606) |
<0.001c |
| Distant metastasis
(M0 vs. M1) | 1.404
(0.989–1.994) | 0.058 |
| CXCR4 expression
(low vs. high) | 0.836
(0.708–0.988) | 0.036a |
|
| B, VEGF |
|
|
Characteristic | HR (95%
CI) | P-value |
|
| Age, years (≤65 vs.
>65) | 0.990
(0.841–1.165) | 0.899 |
| Sex (male vs.
female) | 1.125
(0.926–1.368) | 0.235 |
| Differentiation
stage (I/II vs. III) | 0.942
(0.713–1.244) | 0.675 |
| Depth of invasion
(T1/T2 vs. T3/T4) | 1.562
(1.302–1.874) |
<0.001c |
| Distant metastasis
(M0 vs. M1) | 1.736
(1.223–2.463) | 0.002b |
| VEGF expression
(low vs. high) | 0.422
(0.350–0.508) |
<0.001c |
|
| C,
CXCR4/VEGF |
|
|
Characteristic | HR (95%
CI) | P-value |
|
| Age, years (≤65 vs.
>65) | 1.036
(0.880–1.220) | 0.671 |
| Sex (male vs.
female) | 1.084
(0.891–1.318) | 0.420 |
| Differentiation
stage (I/II vs. III) | 1.014
(0.979–1.050) | 0.444 |
| Depth of invasion
(T1/T2 vs. T3/T4) | 1.635
(1.365–1.960) | <0.001 |
| Distant metastasis
(M0 vs. M1) | 1.513
(1.068–2.144) | 0.020a |
| CXCR4/VEGF
expression |
|
|
| Both
low vs. one low | 0.546
(0.437–0.681) |
<0.001c |
| Both
low vs. both high | 0.627
(0.552–0.712) |
<0.001c |
Synergistic effect of detecting CXCR4
and VEGF expression levels on the OS of patients with GC
To determine whether detecting CXCR4 and VEGF
expression levels exerts a synergistic effect on predicting the
prognosis of patients with GC, Kaplan-Meier survival curves were
generated to assess the association between either low CXCR4 and
VEGF expression, high CXCR4 and low VEGF expression, low CXCR4 and
high VEGF expression or high CXCR4 and VEGF expression, and OS. The
results demonstrated that patients with low expression levels of
both CXCR4 and VEGF had the most favorable OS amongst the groups
(P<0.05; Fig. 2E). Multivariate
Cox regression analysis indicated that low expression levels of
CXCR4 or VEGF were independent positive prognostic factors for
patients with GC (both P<0.001; Table III).
Time-dependent ROC analysis was subsequently
performed for the censored data, which indicated that the
combination of the clinical risk score (TNM stage, histological
type and tumor diameter) and CXCR4 and/or VEGF risk scores was
notably higher than either risk score alone in GC TMA cohorts
(Fig. 2F). The AUC at 5 years was
0.670 (95% CI, 0.432–0.671) for the clinical risk score, which
significantly increased to 0.852 (95% CI, 0.527–0.849) when the
clinical risk score was combined with both CXCR4 and VEGF risk
scores.
CXCR4 promotes AGS cell invasion and
migration by regulating VEGF expression
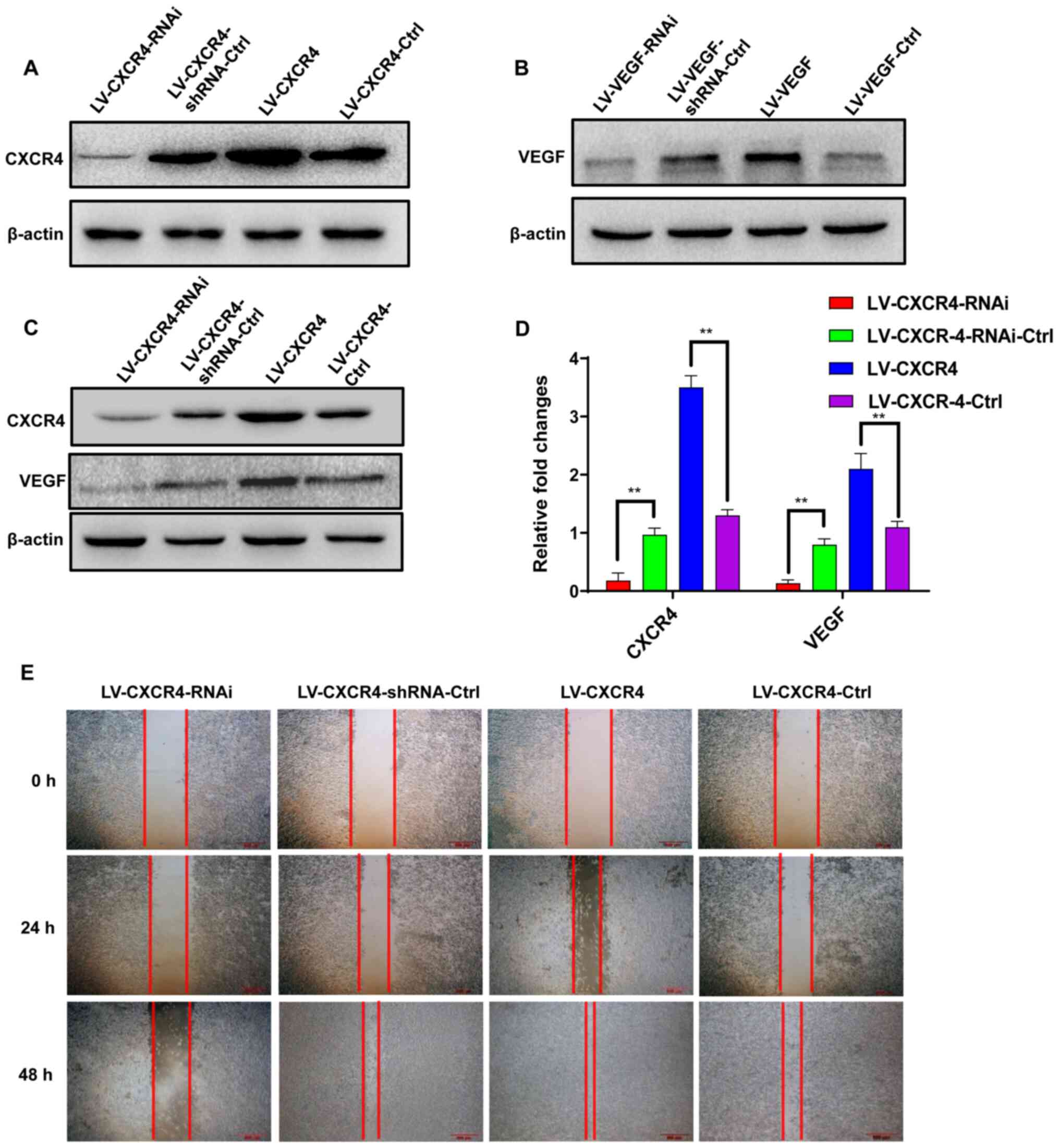
AGS GC cells were infected with LV, and the
LV-mediated overexpression or knockdown of CXCR4 or VEGF was
analyzed via western blotting (Fig. 3A
and B). To determine whether CXCR4 can inhibit AGS cell
invasion and migration by regulating VEGF expression, the
expression levels of VEGF were altered in AGS cells via lentivirus.
As presented in Fig. 3C and D, CXCR4
positively regulated VEGF expression.
As presented in Fig.
3E, the migratory ability of LV-CXCR4-transfected AGS cells
increased compared with the control group, while the migratory
ability of LV-CXCR4-RNAi-transfected AGS cells decreased.
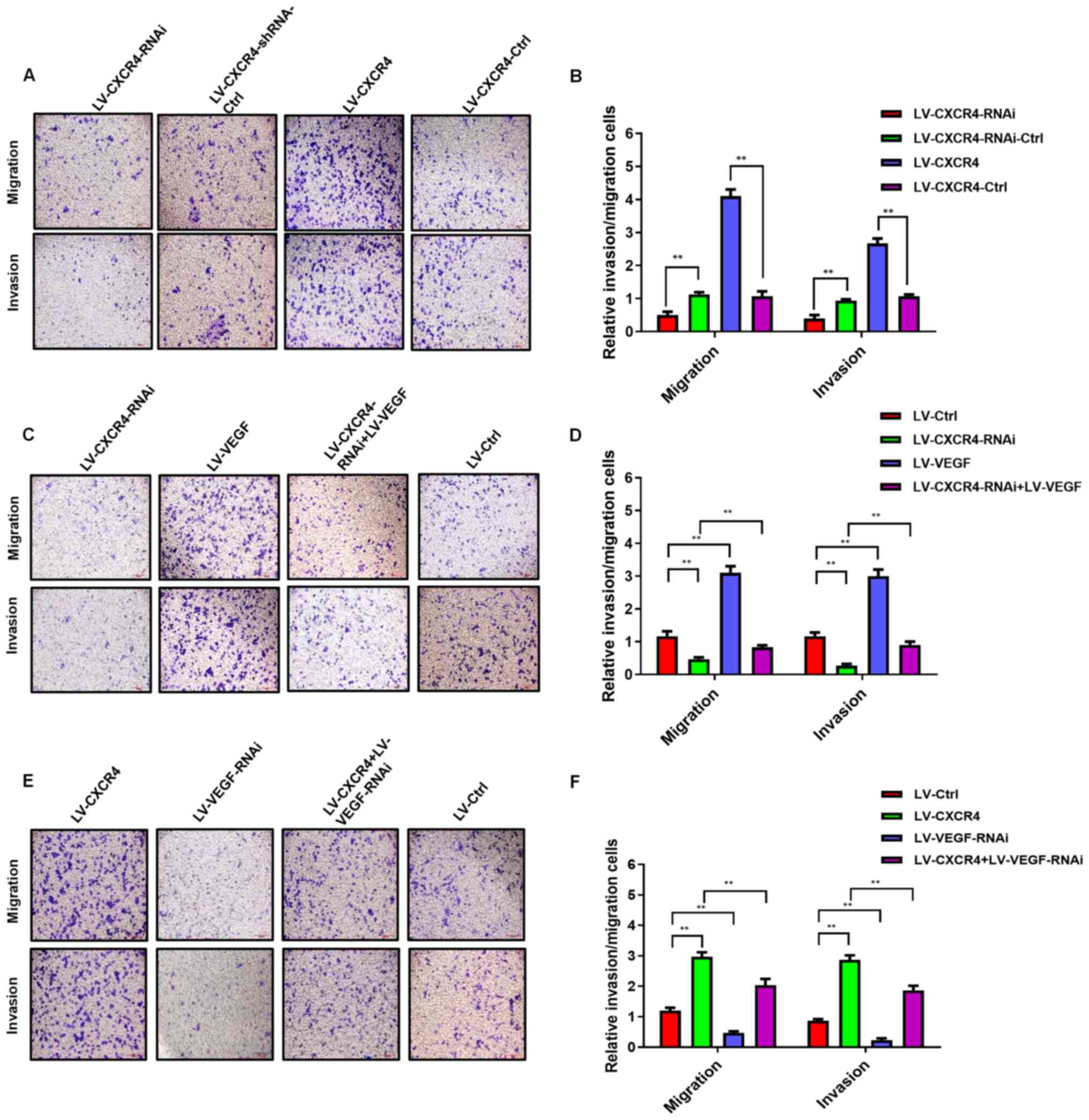
Similarly, the invasive and migratory abilities of
LV-CXCR4-RNAi-transfected AGS cells significantly decreased,
whereas the migratory and invasive abilities in
LV-CXCR4-transfected AGS cells significantly increased compared
with the corresponding control groups (P<0.01; Fig. 4A and B).
The results of the presents study demonstrated that
LV-CXCR4-RNAi-transfected AGS cells had a weaker migratory ability.
Following transfection of AGS cells with LV to increase VEGF
expression, the migratory ability of LV-CXCR4-RNAi-transfected AGS
cells significantly increased (P<0.01; Fig. 4C and D). Conversely, the migratory
ability of LV-CXCR4-transfected AGS cells decreased following
transfection with LV–VEGF-RNAi (P<0.01; Fig. 4E and F). Taken together, these
results suggest that CXCR4 may promote GC cell migration and
invasion by regulating VEGF expression.
Discussion
During the occurrence and development of GC,
cytokines play an important role in the tumor microenvironment by
influencing the survival and proliferation of neoplastic and
vascular cells (18,28). Previous studies have demonstrated
that angiogenic factors are emerging as powerful prognostic tools
(10,29). VEGF and matrix metalloproteinase-9
are two of the most important factors involved in the process of
angiogenesis (30).
CXCR4 is a crucial member of the chemokine receptor
superfamily, and is mainly expressed on granulocytes, T cells, B
cells and dendritic cells (7,31).
Previous studies have demonstrated that CXCR4 is an important
factor associated with human immunodeficiency virus-1 (14,31,32). In
addition, some studies have reported that CXCR4 plays an important
role in the process of cancer growth and metastasis (7,31). CXCR4
was suggested to be a potential target for overcoming therapeutic
resistance to immune checkpoint blockade in patients with
metastatic breast cancer (32).
Overexpression of CXCR4 has been demonstrated to be associated with
a more advanced tumor stage and poorer survival in patients with GC
(14). VEGF expression is widely
distributed in various organs and tissues of the body (16). Oncogenes and tumor suppressor genes
regulate VEGF by increasing or decreasing its expression (33). Several studies have reported that
VEGF expression is closely associated with tumor angiogenesis and
plays a key role in tumor growth and metastasis (18,34).
Furthermore, previous studies have reported that VEGF expression is
upregulated in tumor tissues and the survival rate of patients
decreases as VEGF expression increases (22,35). It
has also been suggested that VEGF may be used as a biomarker to
predict tumor prognosis in different types of cancer, including
lung cancer (36) and breast cancer
(19). The present study aimed to
determine the association between CXCR4, VEGF and the prognosis of
GC.
It is well-known that CXCR4 and VEGF are associated
with cancer growth, invasion and metastasis (37). CXCR4 has been reported to promote
glioma tumor progression and metastasis via VEGF-mediated
angiogenesis (38). In addition,
CXCR4 and VEGF-C expression levels are significantly associated
with lymph node metastasis in non-small cell lung cancer, and CXCR4
and VEGF-C can synergistically promote metastasis in lung cancer
(39). The expression of both CXCR4
and VEGF was also hypothesized to be an effective indicator for
predicting the metastatic potential of nasopharyngeal carcinoma
(40). In addition, upregulated
CXCR4 and VEGF expression levels are associated with increased
rates of colon cancer metastasis (41). The concomitant expression of CXCR4
and VEGF is used as a biomarker for disease-free survival in all
patients (41). These findings
provide clinical evidence that CXCR4 and VEGF play key roles in
GC.
The results of the present study demonstrated that
high VEGF expression in GC tissues was significantly associated
with the depth of invasion, lymph node metastasis, TNM stage and
tumor diameter. In addition, high CXCR4 expression was
significantly associated with differentiation stage, depth of
invasion, lymph node metastasis, TNM stage, tumor diameter and
distant metastasis. Notably, upregulated expression levels of CXCR4
or VEGF were associated with poor OS in patients with GC, as
determined by Kaplan-Meier survival analysis. Furthermore,
univariate and multivariate Cox proportional hazards regression
analyses revealed that both CXCR4 and VEGF expression were
independent negative prognostic factors of GC. In vitro, the
expression levels of CXCR4 and VEGF were either overexpressed or
knocked down using LV transfection, and the results demonstrated
that CXCR4 promoted GC cell invasion and migration by regulating
VEGF expression.
The present study also investigated whether the two
interacting indicators can be integrated to predict the prognosis
of GC more effectively. Through a time-dependent ROC analysis, the
results demonstrated that CXCR4 and VEGF expression together had a
synergetic effect on predicting the prognosis of patients with GC.
Notably, patients with low expression levels of CXCR4 and VEGF had
a more favorable survival outcome as demonstrated by Kaplan-Meier
survival analysis.
Taken together, these results suggest that CXCR4 or
VEGF are both unfavorable prognostic factors for patients with GC.
To the best of our knowledge, the present study was the first to
investigate the potential of the combined value of CXCR4 and VEGF
as efficient prognostic factors for GC. However, further studies
are required to verify the functions of CXCR4 and VEGF in other GC
cell lines, and determine the molecular mechanisms of these two
proteins. Prospective studies will aim to use multicenter samples
to validate the results presented here.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant no. 81773944, awarded to
YQL), the Young Medicine Focus Talent Foundation of Jiangsu
Province (grant no. QNRC2016206, awarded to WMW), the Postgraduate
Research by Practice Innovation Program of Jiangsu Province (grant.
no. KYCX18_2382, awarded to WMW), the Top Talent Support Program
for young and middle-aged people of Wuxi Health Committee (grant
no. 2017, awarded to WMW), the Wuxi City Health Planning Commission
project (grant no. MS201815, awarded to WMW; grant no. Z201907,
awarded to YZ) and the Natural Science Foundation of Jiangsu
Province (grant no. BK20191149, awarded to YZ).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
GC, ZZ and JJ contributed to the conception and
design of the present study, YZ and JJ retrieved data and drafted
the initial manuscript. YL and WW analyzed the data and performed
the statistical analysis. GC and ZZ performed the experiments. YL
and WW confirmed the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Review Board of Yixing Hospital Affiliated to Medical College of
Yangzhou University (approval no. YXYLL-2021-42) and written
informed consent was provided by all participants prior to the
study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gu ML, Zhou XX, Ren MT, Shi KD, Yu MS,
Jiao WR, Wang YM, Zhong WX and Ji F: Blockage of ETS homologous
factor inhibits the proliferation and invasion of gastric cancer
cells through the c-Met pathway. World J Gastroenterol.
26:7497–7512. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Polkowska-Pruszyńska B, Rawicz-Pruszyński
K, Ciseł B, Sitarz R, Polkowska G, Krupski W and Polkowski WP:
Liver metastases from gastric carcinoma: A Case report and review
of the literature. Curr Probl Cancer. 41:222–230. 2017. View Article : Google Scholar
|
|
3
|
Zhao J, Zhao J, Du F, Zhang Y, Shen G, Zhu
H, Ji F, Ma F, Dong L, Kan J, et al: Cardia and non-cardia gastric
cancer have similar stage-for-stage prognoses after R0 resection: A
large-scale, multicenter study in China. J Gastrointest Surg.
20:700–707. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peinado H, Olmeda D and Cano A: Snail, Zeb
and bHLH factors in tumour progression: An alliance against the
epithelial phenotype? Nat Rev Cancer. 7:415–428. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Valastyan S and Weinberg RA: Tumor
metastasis: Molecular insights and evolving paradigms. Cell.
147:275–292. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Altorki NK, Markowitz GJ, Gao D, Port JL,
Saxena A, Stiles B, McGraw T and Mittal V: The lung
microenvironment: An important regulator of tumour growth and
metastasis. Nat Rev Cancer. 19:9–31. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang H, Yuan M, Wu SL, Ba J, Yu X, Mao X
and Jin F: Clinical significance of C-X-C motif chemokine receptor
4 and integrin αvβ6 expression in breast cancer. J Breast Cancer.
23:171–181. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu C, Zhao H, Chen H and Yao Q: CXCR4 in
breast cancer: Oncogenic role and therapeutic targeting. Drug Des
Devel Ther. 9:4953–4964. 2015.PubMed/NCBI
|
|
9
|
Stumpf C, Kaemmerer D, Neubauer E, Sänger
J, Schulz S and Lupp A: Somatostatin and CXCR4 expression patterns
in adenocarcinoma and squamous cell carcinoma of the lung relative
to small cell lung cancer. J Cancer Res Clin Oncol. 144:1921–1932.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lecavalier-Barsoum M, Chaudary N, Han K,
Koritzinsky M, Hill R and Milosevic M: Targeting the CXCL12/CXCR4
pathway and myeloid cells to improve radiation treatment of locally
advanced cervical cancer. Int J Cancer. 143:1017–1028. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nazari A, Khorramdelazad H and Hassanshahi
G: Biological/pathological functions of the CXCL12/CXCR4/CXCR7 axes
in the pathogenesis of bladder cancer. Int J Clin Oncol.
22:991–1000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li LN, Jiang KT, Tan P, Wang AH, Kong QY,
Wang CY, Lu HR and Wang J: Prognosis and clinicopathology of CXCR4
in colorectal cancer patients: A meta-analysis. Asian Pac J Cancer
Prev. 16:4077–4080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jiang Q, Sun Y and Liu X: CXCR4 as a
prognostic biomarker in gastrointestinal cancer: A meta-analysis.
Biomarkers. 24:510–516. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xiang Z, Zhou ZJ, Xia GK, Zhang XH, Wei
ZW, Zhu JT, Yu J, Chen W, He Y, Schwarz RE, et al: A positive
crosstalk between CXCR4 and CXCR2 promotes gastric cancer
metastasis. Oncogene. 36:5122–5133. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Albalawi IA, Mir R and Abu Duhier FM:
Genetic effects of vascular endothelial growth factor A (VEGF-A)
and its association with disease progression in breast cancer
population of Saudi Arabia. Asian Pac J Cancer Prev. 21:139–145.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alagappan VK, Willems-Widyastuti A,
Seynhaeve AL, Garrelds IM, ten Hagen TL, Saxena PR and Sharma HS:
Vasoactive peptides upregulate mRNA expression and secretion of
vascular endothelial growth factor in human airway smooth muscle
cells. Cell Biochem Biophys. 47:109–118. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Eroğlu A, Ersöz C, Karasoy D and Sak S:
Vascular endothelial growth factor (VEGF)-C, VEGF-D, VEGFR-3 and
D2-40 expressions in primary breast cancer: Association with lymph
node metastasis. Adv Clin Exp Med. 26:245–249. 2017. View Article : Google Scholar
|
|
18
|
Chen C, Chi H, Min L and Junhua Z:
Downregulation of guanine nucleotide-binding protein beta 1 (GNB1)
is associated with worsened prognosis of clearcell renal cell
carcinoma and is related to VEGF signaling pathway. J BUON.
22:1441–1446. 2017.PubMed/NCBI
|
|
19
|
Jain S, Ward MM, O'Loughlin J, Boeck M,
Wiener N, Chuang E, Cigler T, Moore A, Donovan D, Lam C, et al:
Incremental increase in VEGFR1* hematopoietic progenitor cells and
VEGFR2* endothelial progenitor cells predicts relapse and lack of
tumor response in breast cancer patients. Breast Cancer Res Treat.
132:235–242. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan Z, Zhuang J, Ji C, Cai Z, Liao W and
Huang Z: Curcumin inhibits hepatocellular carcinoma growth by
targeting VEGF expression. Oncol Lett. 15:4821–4826.
2018.PubMed/NCBI
|
|
21
|
Naykoo NA, Dil-Afroze, Rasool R, Shah S,
Ahangar AG, Bhat IA, Qasim I, Siddiqi MA and Shah ZA: Single
nucleotide polymorphisms, haplotype association and tumour
expression of the vascular endothelial growth factor (VEGF) gene
with lung carcinoma. Gene. 608:95–102. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu W, Dong Z, Hu R and Wang C:
Association of vascular endothelial growth factor (VEGF) gene
polymorphisms with gastric cancer and its development, prognosis,
and survival. Technol Cancer Res Treat. Jan 1–2018.(Epub ahead of
print). doi: 10.1177/1533034617753810.
|
|
23
|
Pang L, Wang J, Fan Y, Xu R, Bai Y and Bai
L: Correlations of TNM staging and lymph node metastasis of gastric
cancer with MRI features and VEGF expression. Cancer Biomark.
23:53–59. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edge SB and Compton CC: The American Joint
Committee on Cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S, Wu X, Chen Y, Zhang J, Ding J,
Zhou Y, He S, Tan Y, Qiang F, Bai J, et al: Prognostic and
predictive role of JWA and XRCC1 expressions in gastric cancer.
Clin Cancer Res. 18:2987–2996. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bai J, Zhou Y, Chen G, Zeng J, Ding J, Tan
Y, Zhou J and Li G: Overexpression of Cullin1 is associated with
poor prognosis of patients with gastric cancer. Hum Pathol.
42:375–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang W, Chen Y, Deng J, Zhou J, Gu X, Tang
Y, Zhang G, Tan Y, Ge Z, Huang Y, et al: Cullin1 is a novel
prognostic marker and regulates the cell proliferation and
metastasis in colorectal cancer. J Cancer Res Clin Oncol.
141:1603–1612. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pedersen LM, Klausen TW, Davidsen UH and
Johnsen HE: Early changes in serum IL-6 and VEGF levels predict
clinical outcome following first-line therapy in aggressive
non-Hodgkin's lymphoma. Ann Hematol. 84:510–516. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Capone F, Guerriero E, Sorice A, Colonna
G, Ciliberto G and Costantini S: Serum cytokinome profile
evaluation: A tool to define new diagnostic and prognostic markers
of cancer using multiplexed bead-based immunoassays. Mediators
Inflamm. 2016:30646432016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeng XH, Ou ZL, Yu KD, Feng LY, Yin WJ, Li
J, Shen ZZ and Shao ZM: Absence of multiple atypical chemokine
binders (ACBs) and the presence of VEGF and MMP-9 predict axillary
lymph node metastasis in early breast carcinomas. Med Oncol.
31:1452014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sojane K, Kangethe RT, Chang CC, Moosa MS,
Lewin SR, French MA and Ndung'u T: Individuals with HIV-1 subtype C
infection and cryptococcal meningitis exhibit viral genetic
intermixing of HIV-1 between plasma and cerebrospinal fluid and a
high prevalence of CXCR4-using variants. AIDS Res Hum Retroviruses.
34:607–620. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen IX, Chauhan VP, Posada J, Ng MR, Wu
MW, Adstamongkonkul P, Huang P, Lindeman N, Langer R and Jain RK:
Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte
infiltration, and improves immunotherapy in metastatic breast
cancer. Proc Natl Acad Sci USA. 116:4558–4566. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Rastegar M, Marjani HA, Yazdani Y,
Shahbazi M, Golalipour M and Farazmandfar T: Investigating effect
of rapamycin and metformin on angiogenesis in hepatocellular
carcinoma cell line. Adv Pharm Bull. 8:63–68. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Honguero Martínez AF, Arnau Obrer A,
Figueroa Almazán S, Martínez Hernández N and Guijarro Jorge R:
Prognostic value of the expression of vascular endothelial growth
factor A and hypoxia-inducible factor 1alpha in patients undergoing
surgery for non-small cell lung cancer. Med Clin (Barc).
142:432–437. 2014. View Article : Google Scholar
|
|
35
|
Chou JC, Lieu FK, Ho DM, Shen HY, Lin PH,
Hu S, Wang SW, Lin H and Wang PS: Regulation of extracellular and
intracellular prolactin on cell proliferation and survival rate
through GHR/JAK2/STAT3 pathway in NSCLC. Chemosphere.
264:1286042021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li H, Takayama K, Wang S, Shiraishi Y,
Gotanda K, Harada T, Furuyama K, Iwama E, Ieiri I, Okamoto I, et
al: Addition of bevacizumab enhances antitumor activity of
erlotinib against non-small cell lung cancer xenografts depending
on VEGF expression. Cancer Chemother Pharmacol. 74:1297–1305. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baci D, Bruno A, Cascini C, Gallazzi M,
Mortara L, Sessa F, Pelosi G, Albini A and Noonan DM:
Acetyl-L-Carnitine downregulates invasion (CXCR4/CXCL12, MMP-9) and
angiogenesis (VEGF, CXCL8) pathways in prostate cancer cells:
Rationale for prevention and interception strategies. J Exp Clin
Cancer Res. 38:4642019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hong X, Jiang F, Kalkanis SN, Zhang ZG,
Zhang XP, DeCarvalho AC, Katakowski M, Bobbitt K, Mikkelsen T and
Chopp M: SDF-1 and CXCR4 are up-regulated by VEGF and contribute to
glioma cell invasion. Cancer Lett. 236:39–45. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bi MM, Shang B, Wang Z and Chen G:
Expression of CXCR4 and VEGF-C is correlated with lymph node
metastasis in non-small cell lung cancer. Thorac Cancer. 8:634–641.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Segawa Y, Oda Y, Yamamoto H, Shiratsuchi
H, Hirakawa N, Komune S and Tsuneyoshi M: Close correlation between
CXCR4 and VEGF expression and their prognostic implications in
nasopharyngeal carcinoma. Oncol Rep. 21:1197–1202. 2009.PubMed/NCBI
|
|
41
|
Wu Y, Jin M, Xu H, Shimin Z, He S, Wang L
and Zhang Y: Clinicopathologic significance of HIF-1α, CXCR4, and
VEGF expression in colon cancer. Clin Dev Immunol. 2010:5375312010.
View Article : Google Scholar : PubMed/NCBI
|