Introduction
Dynamic regulatory mechanisms under a variety of
physiological conditions affect the processing and maturation of
proteins in mammalian cells. Glycosylation is an important type of
post-translational modification. More than half of the proteins in
human cells and 50–70% of serum proteins are glycosylated proteins
(1). Mucin-type O-linked
glycosylation (O-glycosylation) and N-linked glycosylation
(N-glycosylation) are the two most important forms of
glycosylation, and changes in either can lead to clinically
significant pathogenesis (2–4). O-glycosylation is considered a
protein modification occurring on proteins that are secreted and
membrane-bound; it serves a key role in protein processing,
secretion, stability, and ligand binding (5). O-glycosylation is associated
with different types of biological processes, such as metabolism,
translation, transcription, cytoskeletal formation, cell cycle
progression and cell signal transduction (6,7).
Abnormal O-glycosylation is associated with a number of
human diseases, including the development of tumors (8). Tumor cells often contain numerous
altered O-glycosylated proteins, which qualitatively and/or
quantitatively change sugar molecule expression (9). Some O-glycosylated proteins are
usually adopted as tumor biomarkers in the circulation, such as
cancer antigen (CA) 19-9 and CA-125 (9).
O-glycosylation of proteins most commonly
occurs in the serine and threonine residues, but it can also occur
in the tyrosine, hydroxylysine and hydroxyproline residues.
Glycosylation is initiated in the endoplasmic reticulum (ER)
(10). However, O-Xyl (proteoglycan)
and N-acetylgalactosamine (GalNAc) type O-glycosylation
(O-GalNAc) are initiated in the Golgi apparatus (10). More than 80% of cell membrane
proteins and extracellular secreted proteins are O-GalNAc
glycosylated proteins (11). This
type of glycosylation is mediated through transferring GalNAc from
UDPGalNAc to threonine or serine residues. The GalNAc
aminotransferase peptide, which contains up to 20 isoenzymes,
catalyzes this reaction (10,12). The
protein encoded by core 1 synthase glycoprotein-GalNAc
3-β-galactosyltransferase 1 (C1GALT1) generates the common
core 1 O-glycan structure, Gal-β-1-3GalNAc-R (T antigen), by the
transfer of galactose (Gal) from UDP-Gal to GalNAc-α-1-R (Tn
antigen) (13). The formation of
complex O-glycan structures requires further modification of the T
antigen (14). To date, at least
eight different O-glycan core structures have been described. The
core 1 type O-glycan forms the basis of O-glycosylation
modification and its synthesis is mainly regulated by C1GALT1
(15). The Tn antigen can form core
3 structure under the catalysis of
β1,3-N-acetylglucosaminyltransferase 6. Core 1 and 3 structures are
catalyzed by β1,6-N-acetylglucosaminyltransferase to form core 2
and 4, respectively (16).
There are three species of
β1,6-N-acetylglucosaminyltrans-ferases in mammals: Two of them
catalyze the core 2 structure constitution, and one catalyzes the
constitution of core 2 or 4 structures (17) (Fig.
1). In addition, there are several other core structures that
are less common than the aforementioned ones (17).
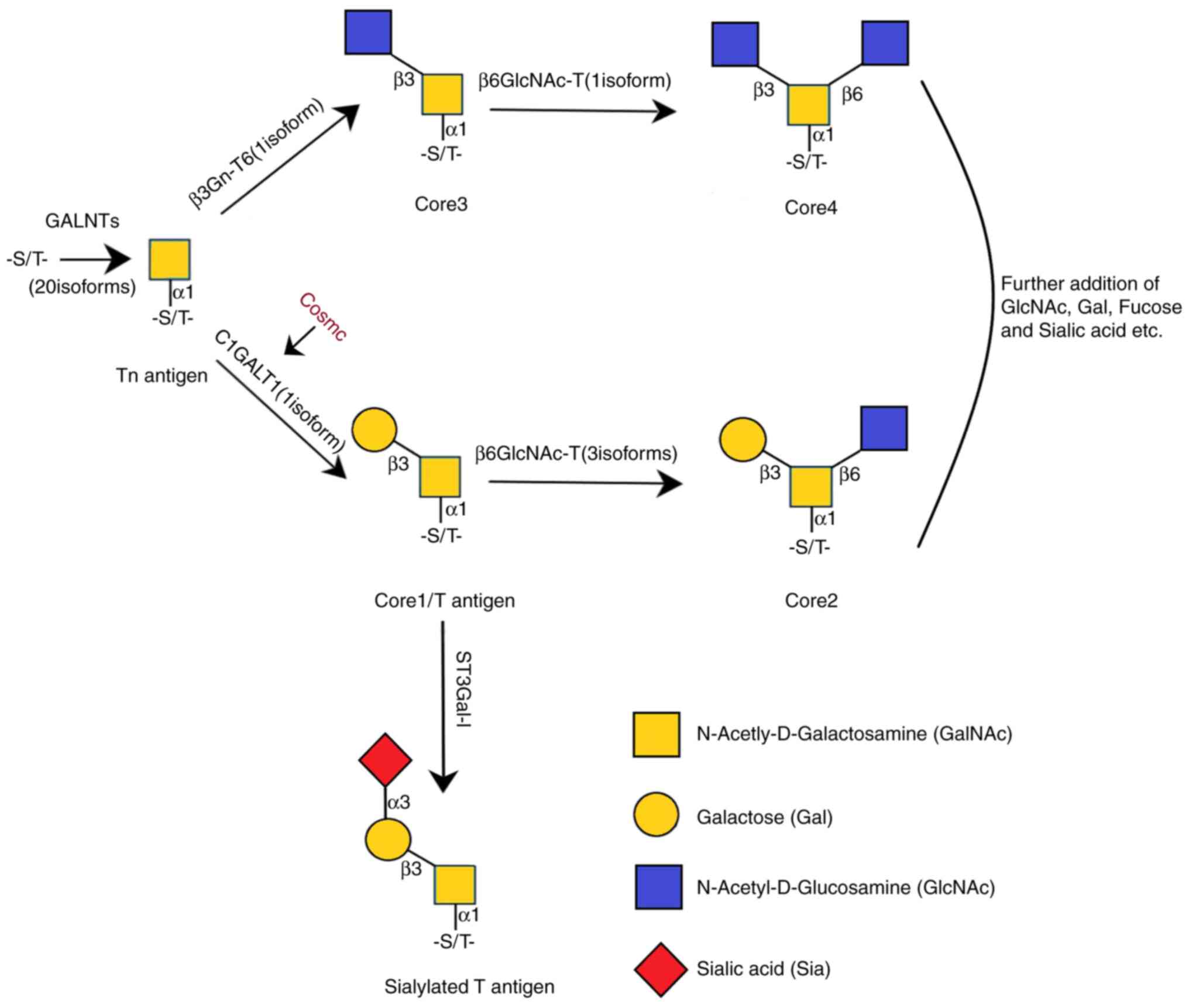 | Figure 1.O-glycosylation model. C1GALT1
transfers Gal from UDP-Gal to Tn antigen to form core 1 O-glycan
structure, T antigen. Core 1 is a precursor for numerous extended
mucin-type O-glycans on the cell surface and secreted
glycoproteins. The structure of core 3 is catalyzed by
β1,3-N-acetylglucosaminyltransferase 6. Core 1 and 3 core
structures can be further modified to form the structure of core 2
and 4, respectively, by the catalysis of
β1,6-N-acetylglucosaminyltransferases. Gal, galactose; GalNAc,
N-acetylgalactosamine; C1GALT1, core 1 synthase glycoprotein-GalNAc
3-β-galactosyltransferase 1; GlcNAc, N-acetylglucosamine; GALNT,
N-acetylgalactosaminyltransferase. |
The C1GALT1 gene is located on chromosome
7p22.1-p21.3, and the protein T-synthase, encoded by the gene, has
been considered to be a core mucin-type
O-glycosyltransferase that resides in the Golgi apparatus
(17), and is synthesized due to its
particular chaperone, Cosmc, in the ER (18). Aryal et al (18) reported that T-synthase could
co-immunoprecipitate with Cosmc. Cosmc can interact with the
deactivated T-synthase, partially restoring the enzyme activity
in vitro, and is a specific partner of T-synthase folding
and maturation (18). This enzyme
adds β1,3-bonded galactose to the existing GalNAc to produce a
common core O-glycan structure. Core 1 is the precursor of many
cell surface mucin O-glycans and secreted glycoproteins, and is the
basis for the formation of complex O-glycans, such as core 2
structure and sialylated T antigens (19). Furthermore, C1GALT1 serves a
vital role in numerous biological functions, including
angiogenesis, platelet production and kidney development (20,21).
The expression of normal O-glycans is associated
with health and homeostasis, whereas abnormal glycosylation is
associated with cancer and other pathologies. Abnormal
glycosylation is involved in cancer cell invasion, migration,
angiogenesis, intercellular contact and epithelial-mesenchymal
transition (EMT) (1,22–24).
Previous studies have revealed that changes in the level of
glycosyltransferase are associated with cancer (25,26). In
addition, C1GALT1 has been associated with the metastasis
and progression of various types of cancer, such as liver and
gastric cancer (27,28).
C1GALT1 can act as an oncogene or a tumor
suppressor gene in various types of conditions. C1GALT1 high
expression in liver cancer tissues is associated with advanced
tumors, poor prognosis and metastasis (29). On the contrary, another study has
presented C1GALT1 as a tumor suppressor gene in various types of
tumors (30).
Chugh et al (30) discovered that C1GALT1
expression is higher in well-differentiated pancreatic cancer
tissues compared with in poorly differentiated pancreatic cancer
tissues. Moreover, in cancer tissues, T antigen expression is lower
compared with that of the Tn antigen (30). C1GALT1 has been shown to be a
tumor suppressor gene in pancreatic cancer since the absence of
C1GALT1 expression promotes the development and metastasis
of pancreatic cancer (30). However,
in another study, C1GALT1 served a different role (31). Liu et al (31) found that in C1GALT1-knockout
mice, spontaneous gastroenteritis and consequently gastric antral
adenocarcinoma were improved in the gastric mucosal epithelial
cells, which indicates that C1GALT1-mediated
O-glycosylation is very important for gastric mucosal and
gastric homeostasis protection (31). However, the use of samples from
different sources or at different tumor stages may have contributed
to the observed dual function of C1GALT1; hence, the true
role of the gene remains elusive. The present review focuses on the
role of C1GALT1 in health and disease.
C1GALT1 in normal development and
non-neoplastic diseases
C1GALT1 in normal development
C1GALT1 and glycosylation are essential for
normal development, especially during angiogenesis, platelet
production and kidney development (32). Impaired T-synthase activity has been
associated with different types of human diseases, including
inflammatory or immune-mediated diseases, and cancer (33).
Xia et al (20) targeted deletion of the C1GALT1
gene, resulting in normal development of heterozygous mice and the
mating of 364 viable offspring. A total of 228 (63%)
T-syn+/- progenies and 136 (37%) T-syn+/+ progenies
were identified by genotyping, but no T-syn-/- progenies
were identified (20). Whether
deletion of two alleles of C1GALT1 led to fetal death of 293
(E9-16) embryos was analyzed (20).
Genotyping revealed the offspring of 142 (48%) T-syn+/-, 78
(27%) T-syn+/+ and 73 (25%) T-syn-/- (20). T-synthase activity was decreased in
T-syn+/- embryos at E12, and there was no activity in
T-syn-/- embryos (20). These
results confirm that all active T-synthase are encoded by
C1GALT1 at this stage of development. T-synthase was found
to be different from the typical glycosyltransferase (34). T-syn-/- embryos in E9
developed normally, but then they gradually developed significant
bleeding in the spinal cord and brain. The T-syn-/- embryos
all died at E13 or E14 (20). In the
T-syn-/- embryos, the only exception detected was poor
angiogenesis (19,20). This phenomenon may be explained by
isolation of endothelial cells from extracellular matrix and
supporting pericytes (20). If mice
lack growth factor B, they cannot recruit peripheral cells to the
developing cerebral vessels, and bleeding will occur in late embryo
or perinatal period (35). By
contrast, the T-syn-/- embryo always died at E14; this means
that in the process of angiogenesis, one or more endothelial
proteins are inseparable from core-1-derived O-glycans (20). This possibility may be further
explored by constructing an endothelial cell model of
C1GALT1-targeted deletion (21).
Although O-glycans are considered to be ubiquitous
in various tissues and types of cells, the expression of
hematopoietic and endothelial cells is high throughout postpartum
and embryonic development (15,36,37). Fu
et al (21) generated mice
lacking T-synthase specifically in endothelial and hematopoietic
cells (named EHC-T-syn-/- mice model). The Tn antigen can be
expressed in hematopoietic, lymphatic and endothelial cells and
arteries, but not in other types of cells (21). The mice developed lymphatic vessel
defects and abnormal lymphatic function. Unlike mice with complete
C1GALT1-knockout, EHC-T-syn-/- mice have no ‘cerebral
hemorrhage’ and ‘partial onset’ embryonic lethality (21). The phenotypic difference may be due
to O-glycans of other types of cells, such as nerve cells and
parietal cells, which contribute to blood vessel development in
nerve tissues (21). However,
EHC-T-syn-/- mice exhibited high neonatal mortality, vascular
system disorder and impaired lymphatic function (21). At the time of autopsy, ~75% of
EHC-T-syn-/- mice exhibited extensive small intestinal bleeding,
which may be one of the reasons for the lethality of EHC-T-syn-/-
after birth (21). Abnormal blood
vessels in the blind end of EHC-T-syn-/- mice exhibited abnormal
function/development of lymphatic vessels, constituting abnormal
links between lymphatic vessels and blood (21). These hyperemic lymphatic vessels are
found in mice that lack fasting-induced adipokines and have defects
in the signaling proteins SLP-76 and SYK (38). These observations suggest that
constant O-glycoprotein expression is required for the maintenance
of lymphatic vessels, angiogenesis and the separation of blood and
lymphatic vessels during development.
In addition, C1GALT1 is very important in the
formation of the follicular basal layer (FBL) and the follicular
environment. The basement membrane provides structural and
selective filters for molecules. The environment is regulated by
the FBL in the follicle at the time of development (39). It has been demonstrated that the
oocyte is important in producing FBL (40). Mice with C1GALT1
oocyte-specific deletion do not synthesize main
1β1,3-galactosyltransferase 1 (named T-synthase as well), and thus
are not able to constitute main 1 derived O-glycan (41). Therefore, the FBL changes the
distribution of laminin and collagen (39) and causes the follicles to combine to
form multiple follicles, with two or more oocytes in a follicle.
Therefore, C1GALT1 expression serves a part in keeping the
normal structure of FBL and the follicular environment.
Additionally, a series of experiments have revealed
that C1GALT1 is very important for platelet production and
renal homeostasis. Kudo et al (42) conditionally knocked out
C1GALT1 and constructed ‘Mx1-C1’ mice, so that the deletion
of the C1GALT1 gene was limited to bone marrow cells. Mx1-C1
mice exhibited severe thrombocytopenia. The hematology parameters
indicated a marked decrease in platelet count. However, white and
red blood cell counts, as well as the levels of hemoglobin, were
normal. Notably, giant platelets were in the peripheral blood
smear, while the morphology of other cells was normal. Compared
with the platelets of wild-type (WT) mice, those of Mx1C1 mice were
larger. Tail bleeding time measurement indicated that bleeding in
WT mice was prevented 6 min after cutting the tail, while bleeding
time in Mx1-C1 mice was markedly prolonged (>10 min), indicating
that C1GALT1 expression is important for hemostasis and
platelet production (42).
In plt1 mice constructed by Alexander et al
(32), T-synthase showed residual
enzyme activity, and through a series of experiments, it was
revealed that C1GALT1 had a very important role in platelet
production and renal homeostasis. Alexander et al (32) treated C57BL/6 mice with N-Ethyl-n
nitrosourea (ENU) and produced generation III (G3). In lineage 76,
multiple mice exhibited lower platelet counts, consistent with the
isolation of ENU-induced mutations that cause thrombocytopenia.
Lineage 76 with recessive mutation was called plt1 (32). plt1/plt1 mice had 40% of the platelet
count of WT mice, and all major organs were histologically normal
except the kidneys, which exhibited structural distortion of the
glomerulus-renal tubules (32). The
levels of creatine, blood urea and urinary protein in plt1/plt1
mice were higher compared with those in WT mice. The kidneys
exhibited inflammatory infiltration, ductal stenosis, glomerular
loss and cortical atrophy. From the 10th week, plt1/plt1 mice began
to get sick, and by day 200, 90% of the mice had died (32). It was demonstrated that the activity
of T-synthase in plt1/plt1 mice was <5% of that in WT mice. Plt1
mutation could lead to severe but incomplete loss of T-synthase
activity (32).
Decrease of T-synthase activity can lead to exposure
to the Tn antigen. The Tn antigen could not be detected in WT mice,
but could be detected in plt1 mice. plt1/plt1 mice died of a severe
kidney disease accompanied by massive proteinuria and
glomerulonephritis (32).
Podocalyxin, which develops from podocytes of the kidney, has been
discovered to be a core TN protein in the kidney (43). Mice with low levels of podocalyxin
shortly died after birth from anuria and renal dysplasia,
consistent with the anti-adhesion effects of podocalyxin on the
podocyte surface for ensuring that the filtration gap is obstructed
(44). Plt1/plt1 mice can produce
urine, which proves that low glycosylated podocalyxin can maintain
part of renal function. However, kidney disease in mice indicates
that podocalyxin glycosylation mediated by T-synthase is crucial
for the maintenance of normal renal function and structure
(32). These results suggest that
some pathological changes in the kidney may be associated with a
decrease of T-synthase activity, which does not depend on the
influence of intrinsic defects and immune factors. In addition to
kidney diseases, further attention should be given to IgA
nephropathy (IgAN).
IgAN
The decreased activity of T-synthase has a close
association with human diseases, the most notable being IgAN (an
ordinary important glomerulonephritis). IgAN has been considered to
be the most common reason of renal failure and glomerulonephritis
globally, and it is an immune-mediated disease characterized by
abnormal glycosylation (45). IgAN
accounts for 37–58% of biopsy-confirmed primary glomerulonephritis
in China (46–48). Within 10 years after diagnosis,
approximately one-third of patients with IgAN will progress to the
final stage of kidney disease (49,50). Two
case-control studies have discovered that Chinese population
susceptibility and C1GALT1 gene polymorphism are associated
with the IgAN variations of the C1GALT1 gene; in particular,
the haplotypes YATIG, YAGDA and YATDG were associated with the
susceptibility to IgAN (51,52). Abnormal O-glycosylation of
IgA1 has been identified in IgAN, which was an important
breakthrough in the study of its pathogenesis (53). IgA1 glycosylation defects result in
elevated galactose-deficient IgA1 (Gd-IgA1) and immunocomplex, and
are associated with IgAN development (53).
There is evidence that the Gd-IgA1 level is
heritable (54,55). Using a genome-wide approach, Gale
et al (56) identified common
genetic factors that influence Gd-IgA1 levels in East Asian and
Caucasian populations. Gale et al (56) studied hundreds of patients with IgAN
from the UK and China, revealing that C1GALT1 is an
important genetic determinant of Gd-IgA1 level, which is an
independent risk factor for progressive IgAN. Compared with that in
ethnicity-matched healthy subjects, the Gd-IgA1 level is increased
in patients with IgAN and is associated with disease severity
(56). Chinese patients with IgAN
have lower levels of Gd-IgA1 than Caucasian patients (56). This suggests that there may be
ethnical differences in the pathogenic importance of IgA1
O-glycosylation changes.
Kiryluk et al (57) used in vitro small interfering
(si)RNA knockdown to demonstrate that C1GALT1 can determine
the secretion rate of Gd-IgA1 in serum IgA1-producing cells. Xing
et al (58) discovered that
C1GALT1 expression in peripheral B lymphocytes of patients
with IgAN has a negative correlation with increased Gd-IgA1 levels
and is markedly downregulated compared with the increase of Gd-IgA1
level. The aforementioned study involved 30 patients with IgAN and
30 healthy volunteers in China (58). Gd-IgA1 level was measured by an
enzyme-linked immunosorbent assay, and the results revealed that
Gd-IgA1 levels ranged between 8.55 and 14.48 U/ml in patients with
IgAN and between 3.97 and 12.15 U/ml in healthy controls (58). In comparison with those in healthy
controls, Gd-IGA1 levels were determined to be significantly higher
in patients with IgAN (P<0.001) (58). By reverse transcription-quantitative
PCR, the expression levels of C1GALT1 were detected in
peripheral B lymphocytes of both patients with IgAN and healthy
controls, revealing that C1GALT1 expression was
significantly downregulated in patients with IgAN compared with
that in healthy controls (P=0.04) (58). It has been suggested that a decrease
in C1GALT1 expression in B lymphocytes may contribute to the
increased production of Gd-IgA1 and eventually lead to IgAN
pathogenesis (59). One difficulty
in exploring the role of C1GALT1 in IgAN is that only a
small proportion of plasma cells secrete IgA1, which is associated
with the disease. The identification and isolation of these plasma
cells are difficult, but it is important for elucidating the role
of C1GALT1 in IgAN. Studying the real cause of the lack of
C1GALT1 expression may illustrate the pathogenesis of IgAN
and contribute to finding new treatments for the disease.
Tn syndrome
In addition to IgAN, another disease that is closely
associated with decreased T-synthase activity is Tn syndrome. Tn
syndrome is an infrequent blood disorder characterized by exposure
of the Tn antigen on the surface of human red blood cells,
granulocytes, platelets and lymphocytes (60). Patients can present as asymptomatic,
or can exhibit mild hemolysis, thrombocytopenia and/or leukopenia,
which are usually considered to be caused by the reaction of Tn
antigens with naturally occurring anti-Tn antibodies (61). These antibodies may be IgM
condensation agglutinin-type and appear to be autoantibodies
against carbohydrate I antigens on adult red blood cells (62). Another possible pathological
mechanism is the abnormal function of glycoproteins on leukocytes
or platelets. Since glycoproteins have an important role in the
function of these cells, changes in glycosylation may impair the
function of glycoproteins (62).
The expression of Tn antigen and T-synthase activity
loss is the result of Cosmc mutation, which has been widely
confirmed (36,63–66). A
study by Vainchenker et al (60) has demonstrated the existence of the
Tn antigen on stem cells of the Tn clone, and Tn syndrome is
derived from acquired somatic changes in Cosmc in the early blood
progenitor cells. Wang et al (67) constructed a mouse model with a
targeted deletion of Cosmc in hematopoietic cells/endothelial cells
(EHC Cosmc−/y), which caused fatal perinatal bleeding in
~90% of mice. The surviving mice developed macrothrombocytopenia
and severely prolonged caudal bleeding time (67). Compared with those in wild-type
(Cosmc+/y) mice, platelets in EHC Cosmc−/y
mice were lacking T-synthase activity. The decrease in T-synthase
activity was associated with the expression of the Tn antigens on
the surfaces of most platelets from EHC Cosmc−/y mice
(67). These experiments
convincingly suggest that thrombocytopenia and hemorrhage in
patients with Tn syndrome are primarily caused by the lack of
functional Cosmc.
High expression of Tn antigen is associated with Tn
syndrome, as well as with cancer (68). According to statistics, >70% of
human cancers may express Tn antigen, including colon (69), breast, ovarian and uterine cervical
epithelial cancer (70–72). The expression of Tn antigen is
closely associated with a poor prognosis, and it is an attractive
target for the development of new diagnostic and therapeutic
methods (70).
Inflammatory bowel disease
Inflammatory bowel disease (IBD), consisting of
ulcerative colitis (UC) and Crohn's disease (CD), is a chronic
inflammatory disease. Although the exact cause of IBD remains
unclear, it is generally believed to be jointly caused by
environmental factors and genetic susceptibility. At the same time,
intestinal microorganisms serve an important role in the occurrence
and development of IBD (73).
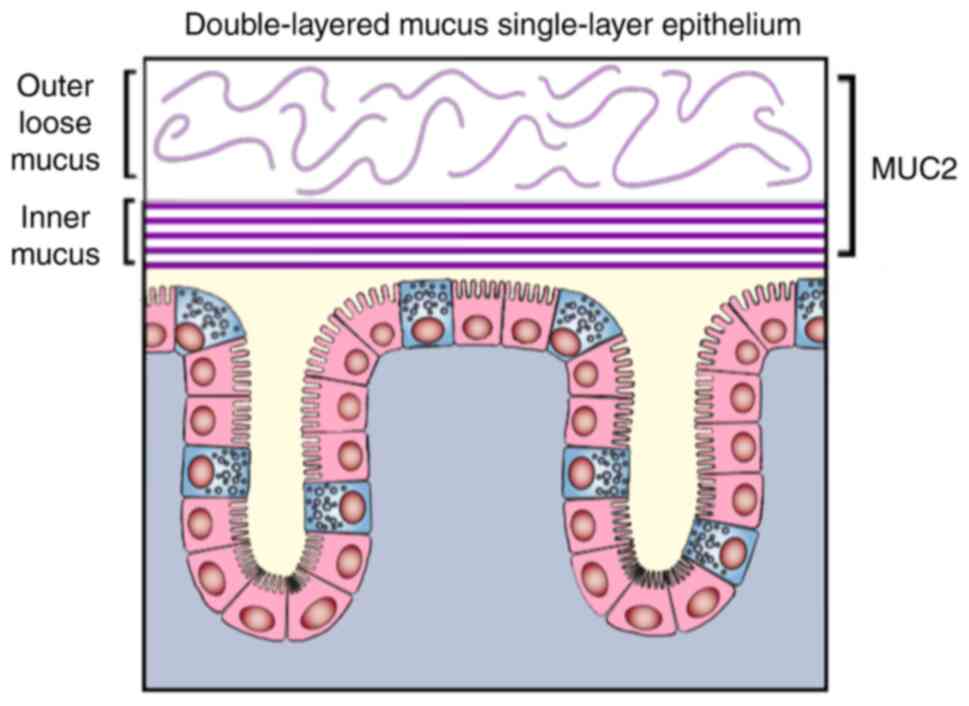
The colonic mucus layer is divided into two layers.
The inner layer adheres to epidermic cells, and in healthy
conditions it is impermeable to bacteria. The primary mucin (MUC)
secreted by colon cells is MUC2, which is generally
O-glycosylated (74)
(Fig. 2). Active human UC is
associated with a mucus layer with structural and functional
defects, such as a thinner mucus layer and increased permeability
to bacteria (75,76). Studies have reported that patients
with active UC have lower levels of carbohydrates in their mucus
layer compared with those in healthy controls and patients with
dormant disease (77–79). Defects in the inner mucus layer can
result in increased bacterial association with epithelial cells,
which may trigger inflammation (80). In serum, reduced galactosylation of
IgG is considered a diagnostic marker for IBD disease (80). The function of suitable mucin
glycosylation is also proven by the fact that mice defective in
core 1derived Oglycans have poor glycosylated MUC2 and develop
spontaneous colitis resembling UC (75).
Fu et al (75)
established a mouse model of colitis evoked by intestinal
epithelial cells lacking C1GALT1. The clinical
manifestations and pathological features are very similar to those
observed in humans (75). The mice
developed transient colitis immediately at 3 weeks of age, which
subsided at 6 weeks, but relapsed at 8 weeks; the severity of the
disease could be reduced by broad-spectrum antibiotic treatment in
mice with metronidazole and vancomycin (75). Additionally, the mice exhibited colon
tumors when they were older. Immunohistochemistry and histology
proved that these tumors were invasive adenocarcinoma, and the
tumor tissue expressed abundant Tn antigen (75). The association between genetic
variations in C1GALT1 and the microbiota in hundreds of
patients with CD and healthy controls has also been studied
(81). Polymorphisms around
C1GALT1 (rs10486157) and COMSC (rs4825729) have been
associated with changes in the composition of the microbiota of the
colonic mucosa (81). These results
support the association between C1GALT1 or
O-glycosylation and host regulation of the microbiome and
suggest a role for the intestinal microbiome in the pathogenesis of
IBD. Improvements in understanding the molecular etiology of IBD,
especially pathways involving glycans, may facilitate the
development of therapeutic drugs.
The high embryonic lethality exhibited by
C1GALT1-knockout mice prevents the development of an
effective C1GALT1 deficiency animal model. Simultaneously,
it also demonstrates that C1GALT1 and O-glycosylation
are vital in normal development. One study has demonstrated that
numerous membrane glycoproteins expressing Tn antigen and/or
truncated O-glycans may be dysfunctional due to degradation and/or
folding errors (82). Therefore, the
expression of normal O-glycans is associated with health and
homeostasis, while the truncation of O-glycans and Tn antigens is
associated with pathologies. The association between the role of
C1GALT1 in angiogenesis, platelet production and kidneys,
and the pathways it may regulate requires further research.
C1GALT1 as an oncogene
C1GALT1 functions as an oncogene in some
cases. The role of the gene in tumor cells and its association with
different types of related signaling pathways and molecules have
been shown in previous research.
Liver cancer
It is known that O-glycosylation can
regulate receptor tyrosine kinases (RTKs), such as fibroblast
growth factor receptor 2, MET and epidermal growth factor receptor
(EGFR) (27,83–86).
Changes in RTK activities are associated with cancer progression
and occurrence (27). The hepatocyte
growth factor (HGF)/c-Met signaling pathway is important in tumor
invasion and metastasis (87). The
HGF/c-Met axis is involved in cell proliferation, movement,
differentiation, invasion, angiogenesis and apoptosis via
activation of multiple downstream signaling pathways (87–89).
C1GALT1 can activate the HGF/c-Met signaling pathway and
increase mucin O-glycan expression in liver cancer cells, which
promotes the proliferation of cells (27). High protein and mRNA expression
levels of C1GALT1 are usually associated with a poor
prognosis and metastasis in hepatocellular carcinoma tumors
(27). Overexpression of
C1GALT1 in hepatocellular carcinoma activates the HGF
signaling pathway through the regulation of dimerization and
O-glycosylation level of the MET protein (27). Additionally, C1GALT1
expression can regulate the proliferation and viability of hepatoma
cells both in vivo and in vitro (27).
Wu et al (27) reported that C1GALT1 enhanced cell
proliferation triggered by HGF through MET. The aforementioned
study revealed that C1GALT1 expression was upregulated in
hepatocellular carcinoma. According to the immunohistochemical
analysis of 32 non-tumor liver tissues and 72 primary
hepatocellular carcinoma tissue specimens, C1GALT1
expression was upregulated in 54% of hepatocellular carcinoma
tissues, but only in 19% of non-neoplastic liver tissues
(Mann-Whitney U test, P=0.002) (27). Compared with non-tumor liver tissues,
C1GALT1 expression was frequently upregulated in
hepatocellular carcinoma tumors. High C1GALT1 expression was
associated with poor prognosis and tumor metastasis (27). Moreover, the study revealed an
important correlation between the expression levels of phospho-MET
and C1GALT1 (R=20.73, P<0.0001) (27). Additionally, MET dimerization and
phosphorylation were decreased by knocking out C1GALT1 in
hepatocellular carcinoma cells, and MET HGF-induced activation was
enhanced by C1GALT1 overexpression (27). The trypan blue rejection test
revealed that C1GALT1-enhanced cell viability was significantly
inhibited by blocked MET activity (27). The proliferation of the cells was
decreased by knocking out C1GALT1 through HGF (27). On the contrary, the HGF-induced cell
proliferation was enhanced by C1GALT1 overexpression (27). Therefore, the aforementioned study
offers new insights into glycosylation in the regulation of RTK
activities.
Gastric cancer
According to preclinical patterns of gastric
cancer, activation of the HGF/c-Met signaling pathway is able to
improve EMT (27,90); nevertheless, further studies are
required to determine whether C1GALT1 can promote tumor
malignancy or activate the HGF/c-Met signaling pathway in gastric
cancer cells. One study has revealed that changes in RTK genome
have been observed in ~37% of patients with gastric cancer
(91). The occurrence and
development of gastric cancer is promoted actively by RTK, which is
considered as a target for cancer treatment (92,93).
The ephrin (EPH) receptor is the largest of the RTK
family and is usually upregulating in tumors, which promotes tumor
development (94–97). These receptors are popular drug
targets (98,99). The human EPH receptor consists of a
neighboring EPHA and five EPHB domains. Ephrin A1 is a ligand of
the EPHA receptor and has been shown to be upregulated in gastric
cancer, promoting EMT (100,101).
Lee et al (28) observed that
C1GALT1 expression increased in gastric adenocarcinoma and
was associated with a poor prognosis. Soluble ephrin A1-mediated
cell migration is promoted by C1GALT1 through the activation
of EPHA2 in gastric cancer. Immunohistochemical staining of 25
cases of gastric adenocarcinoma revealed that C1GALT1
protein expression was higher in 80% of the gastric adenocarcinoma
tissues than in matched non-tumor gastric tissues, and the low
expression levels of C1GALT1 protein were observed in only
4% of the cases (28). In addition
to lymph node metastasis and tumor invasion, high C1GALT1
expression is often associated with higher histological grade and
advanced cancer stage (stage III and IV), and it is an independent
prognostic factor of poor survival (28). C1GALT1 silencing inhibits
gastric cancer cell invasion, migration and viability (MKN45 and
AGS cells), as well as metastasis and tumor growth (28). C1GALT1-knockdown in AGS cells
affects multiple functional pathways. Silencing C1GALT1
decreases phosphorylation and O-glycation levels of HER2 and
EGFR, as well as inhibiting gastric cancer cell migration (28). Although other pathways are also
involved, the viability of cells may be promoted by C1GALT1
at least in part through the activation of HER2 and EGFR.
Prostate cancer
There is increasing evidence that galectins may
interact with abnormal glycosylation and may be associated with
cancer progression. Galectin-4 expression is consistently lower in
patients with primary prostate cancer compared with in patients
with lethal metastatic prostate cancer (102). Galectin-4 activates HER2, EGFR,
IGF1 and HER3 receptors in a carbohydrate-dependent manner
(102). Tsai et al (102) discovered that C1GALT1
expression in primary tumors is lower than that in
castration-resistant prostate cancer. In metastatic prostate cancer
samples, it was demonstrated by immunohistochemical analysis that
C1GALT1 was highly expressed in 70% of the samples, and this
high expression was closely associated with advanced tumor stage
(102). During prostate cancer
progression, C1GALT1 expression is increased and castration
resistance is promoted. Notably, metastatic prostate cancer cell
lines exhibit high C1GALT1 gene and protein expression
levels (102). The aforementioned
findings indicate that there is a close association between tumor
malignant transformation and the change of protein
O-glycosylation and castration resistance. Tumor metastasis
may be promoted through interaction with lectin in prostate cancer.
Therefore, the significance of O-glycosylation in tumor
diagnosis and treatment required to be further explored.
Esophageal cancer
MUC1 is a type I transmembrane mucin, consisting of
two subunits, MUC1-N and MUC1-C. High MUC1 expression is associated
with a poor prognosis and tumor progression in different types of
cancer, making it an oncoprotein (103–106).
MUC1 can regulate the WNT signaling pathway by forming
intracellular complexes with β-catenin, which in turn can
co-activate the expression of cyclin-D1 in the nucleus, ultimately
promoting tumorigenesis by allowing cancer cells to avoid apoptotic
pathways (107). MUC1 is greatly
expressed in esophageal squamous cell carcinoma (ESCC) and ESCC
cell migration and invasion can be inhibited by silencing MUC1.
Wang et al (108) analyzed MUC1 expression through a
large-scale database. MUC1 gene copy number in 102 ESCC tumor
samples among 132 ESCC samples was greatly lower than that in 30
cases of adjacent esophageal squamous epithelium. Wang et al
(108) also analyzed C1GALT1
expression through the large-scale ONCOMINE database. The average
gene copy number of C1GALT1 in 30 ESCC samples was higher
than that in 102 normal esophageal epithelia (108). These data indicate that both MUC1
and C1GALT1 are abundantly expressed in ESCC. In addition, 7
of the 10 pairs of ESCC samples with high MUC1
O-glycosylation had significantly higher expression levels
of C1GALT1 than normal tissues, indicating that
C1GALT1 was positively associated with MUC1
O-glycosylation in ESCC (108). MUC1
O-glycosylation/C1GALT1 expression in ESCC without
lymph node metastasis was greatly lower in ESCC with lymph node
metastasis, and there was a negative association between survival
and MUC1 O-glycosylation/C1GALT1 co-expression
(108). The aforementioned results
suggest that it is possible for MUC1
O-glycosylation/C1GALT1 to be prognostic elements and
have diagnostic significance in ESCC, which proposes new insights
for targeting MUC1 O-glycosylation and C1GALT1 for
inhibiting ESCC metastasis.
Zhang et al (51) demonstrated the role of C1GALT1
expression in the development of radioresistant esophageal cancer.
C1GALT1 protein expression in esophageal cancer tissues was
higher than that in adjacent normal tissues. Poor prognosis, lymph
node metastasis and TNM staging were associated with upregulation
of C1GALT1 expression. In addition, high levels of
C1GALT1 increased the resistance of esophageal cancer cells
to radiation therapy (51).
Similarly, Dong et al (109)
demonstrated that C1GALT1 could enhance radiation resistance
and malignant phenotype of laryngeal cancer cells. Thus,
C1GALT1 is very important in carcinogenic resistance to
radiotherapy.
Cholangiocarcinoma
C1GALT1 serves a role in the development of
cholangiocarcinoma. Cholangiocarcinoma tissues have higher
C1GALT1 expression than normal bile ducts (110). Additionally, elevated
C1GALT1 expression in cancer tissues is associated with
advanced cell grade, larger tumor size and tumor stage (110). The inhibition of C1GALT1 can
significantly inhibit the viability, migration and invasion of
cholangiocarcinoma cells, whereas overexpression of C1GALT1
can promote these abilities (111).
This indicates that C1GALT1 is critical for cancer
progression in cholangiocarcinoma.
Head and neck cancer
Lin et al (13) demonstrated that C1GALT1
expression is upregulated in head and neck squamous cell carcinoma
(HNSCC), and high C1GALT1 expression is associated with poor
clinicopathological characteristics. In addition, C1GALT1
can modify the O-glycans on the EGFR. Previous studies have
revealed that O-glycan modification can influence the behavior of
cancer cells and their signal transduction pathway (27,83,85,112).
Phosphorylation RTK array assay in HNSCC indicated that the
phosphorylation of MET and EGFR is mostly decreased by
C1GALT1 knockout or knockdown (13). The EGFR signaling pathway is
important in the invasion and survival of tumor cells in HNSCC
(113). Lin et al (13) provided evidence via mass spectrometry
that EGFR has GalNAc type O-glycans, indicating that C1GALT1
can modify EGFR. Subsequently, SAS cells overexpressing
C1GALT1 were constructed. The EGF-induced EGFR
phosphorylation at Y1068 was improved by C1GALT1
overexpression, and HNSCC cell invasion, migration and activity was
also improved (13). EGF-EGFR
binding affinity was decreased by the knockout of C1GALT1 in
SAS cells, and the EGFR signaling pathway was inhibited.
Additionally, the invasion, migration and viability of SAS cells
treated with erlotinib, an EGFR tyrosine kinase inhibitor, were
reversed (13). The aforementioned
results indicated that C1GALT1 may change the glycosylation
of EGFR. In HNSCC cells, C1GALT1 enhances the binding
affinity to the EGF ligand, as well as phosphorylation of EGFR,
increasing the malignant phenotype.
Ovarian cancer
Immature truncated O-glycans have usually been
detected in the ovarian cancer cells of human beings, and evidence
indicates that these changes in glycosylation expression can
contribute to various types of cancer, including colon and ovarian
cancer, which usually express short O-glycans (114,115).
Chou et al (116) evaluated the prognostic value of
C1GALT1 expression through analysis of patients with ovarian
cancer in a public database, generating survival curves of each
patient. In all patients with ovarian cancer followed for 20 years,
a low overall survival rate was associated with high C1GALT1
expression (hazard ratio, 1.19; 95% CI, 1.04–1.37; P=0.014)
(116). These results indicate that
targeting C1GALT1 may be a promising strategy for ovarian
cancer (116). Further research on
C1GALT1 is essential for an improved understanding of the
occurrence of ovarian cancer.
Overall, the aforementioned findings indicate that
C1GALT1 promotes tumor development. However, in other cases,
C1GALT1 may also have a tumor-suppressing effect.
C1GALT1 as a tumor suppressor
In the aforementioned types of tumor,
C1GALT1 expression is usually upregulated during
tumorigenesis. However, the expression levels of C1GALT1 in
colorectal and pancreatic cancer are different from the
aforementioned types of tumor.
Pancreatic cancer
The loss of C1GALT1 in mice caused increased
truncated O-glycan expression, which caused the metastasis of
pancreatic ductal adenocarcinoma (PDAC) (30). Genetically engineered KPC and KPCC
mice models were created by breeding KrasG12D/+,
Pdx1-Cre and LSLTrp53R172H/+ with
C1galt1loxP/loxP (30).
The KPC pattern was adopted to create pancreas-specific
C1GALT1 depletion (KPCC mice) and monitor pancreatic tumor
progression and growth in these mice (30). The survival time of KPCC mice
(median, 102 days) was longer compared with that of KPC mice
(median, 200 days), and KPCC mice developed early pancreatic
intraepithelial neoplasia at 3 weeks, PDAC at 5 weeks and
metastases at 10 weeks compared with KPC mice (30). Moreover, metastases to distant organs
in KPC mice were observed after 20 weeks (30). Compared with other PDAC animal
patterns, KPCC is considered to be the predominant PDAC mouse
pattern to present primary metastasis (117,118).
Compared with KPC mice, pancreatic tumors in KPCC mice have been
considered to be more metastatic and aggressive, and Tn production
is increased, while the number of stromata is decreased (30). Pathological analysis of tumor tissues
has shown that most KPCC tumors are poorly differentiated or
undifferentiated, while most KPC animals have moderate to highly
differentiated tumors (30). It is
worth noting that when C1GALT1 is conditionally inactivated
without the background of carcinogenic mutations, the pancreas
appears normal (30). This indicates
that loss of C1GALT1 alone does not lead to the formation of
PDAC. Loss of C1GALT1 is associated with p53 and KRAS
mutations leading to faster progression of PDAC (30).
According to experiments performed in cell lines,
human PDAC cells with C1GALT1 gene knockout have greatly
developed MUC16 abnormal glycosylation, tumorigenicity and
invasion, proliferation and increased expression of Tn carbohydrate
antigen compared with a control group (PDAC cells without
C1GALT1-knockout) (30).
Growth factor receptor activation, such as HER2 and EGFR, as well
as activation of downstream effectors, such as Akt and focal
adhesion kinase (FAK) proteins, is promoted, and MUC16 activates
metastasis signals and interacts with FAK (119). PDAC cell migration is induced by
the activated signals of Akt and FAK, which may possibly explain
the increased migration of C1GALT1-knockout cells (30). It is necessary to conduct further
research on this topic in the future.
Colorectal cancer (CRC)
The expression of Tn antigen is associated with
various types of cancer metastasis and progression (120). For example, immature truncated
O-glycans (like the Tn antigen) can usually be detected in human
CRC (121). Bergstrom et al
(122) argued that there was no
association between cancer progression and Tn antigen by adopting a
CRC murine pattern. Instead, intestinal inflammation has been shown
to lead to eventual tumorigenesis rather than abnormal
O-glycosylation (122). Mice
lacking core 1-derived O-glycans (IECC1galt1-/-) developed
spontaneous colitis. Between 18 and 24 months, ~90% of mice
developed colon tumors, with an average of 3 tumors, of various
sizes (122). In vivo
analysis revealed that Tn exposure itself did not significantly
promote colon inflammation and tumorigenesis. Thus, the incidence
of carcinogenesis in patients who have UC may be decreased by core
inflammatory pathway inhibition. Nevertheless, Dong et al
(123) indicated that forced
knockout of C1GALT1 in HCT116 cells significantly induced Tn
antigen expression and contributed to metastasis and progression of
CRC. It seems that Tn antigen can be adopted as an underlying
target of therapeutic intervention (124,125).
T-synthase deficiency in CRC cells may lead to the activation of
the EMT signaling pathway. EMT is important in cancer progression
(126,127). Knockout of C1GALT1 in HCT116
cells can greatly enhance the adhesion and proliferation of cells
and induce Tn antigen expression (123). Moreover, E-cadherin (a typical
epithelial marker) was markedly decreased in
C1GALT1-knockout HCT 116 cells, accompanied by an enhanced
expression of mesenchymal markers including snail and fibronectin
(123). These observations indicate
that T-synthase deficiency can induce abnormal
O-glycosylation in cells, subsequently promoting
carcinogenesis by activating the EMT process (123).
The aforementioned studies reported the dual role
of C1GALT1 in cancer (carcinogenesis and cancer
suppression), and the association between this gene and several
molecules and signaling pathways has been explored. This may
provide new therapeutic strategies for cancer treatment. Table I shows the role of C1GALT1 in
different types of cancer.
 | Table I.Role of C1GALT1 in different types of
cancer. |
Table I.
Role of C1GALT1 in different types of
cancer.
| First author,
year | Cell lines | Model | Effects | Expression in
cancer | Type of cancer | (Refs.) |
|---|
| Wang et al,
2020 | HA22T, PLC5 | In vitro, in
vivo, human tissue | Regulation of the
O-glycosylation level of the MET protein activates the HGF
signaling pathway | Upregulation | Hepatocellular
carcinoma | (87) |
| Zhang et al,
2018 | ECa109 | In vitro, in
vivo, human tissue | Radiation
resistance is inhibited by glycosylation of the modifier β1
integrin | Upregulation | Esophageal
cancer | (51) |
| Lee et al,
2020 | AGS | In vitro, in
vivo, human tissue | Activation of
EPHA2-promoted cell migration mediated by soluble Ephrin A1 | Upregulation | Gastric cancer | (28) |
| Huang et al,
2015 | HUCCT1 | In vitro,
human tissue | C1GALT1-knockout
inhibits the malignant behavior of bile duct cancer cells | Upregulation |
Cholangiocarcinoma | (111) |
| Lin et al,
2018 | OEC-M1, FaDu | In vitro, in
vivo, human tissue | C1GALT1-knockdown
blocks O-glycan extension on EGFR and inhibits EGFR signal
transduction | Upregulation | Head and neck
squamous cell carcinoma | (13) |
| Chou et al,
2017 | ES-2 | In vitro,
human tissue | Regulates the
expression of multiple genes associated with tumor stem cells in
ovarian cancer cells | Upregulation | Ovarian cancer | (116) |
| Chugh et al,
2018 | T3M4 | In vitro, in
vivo, human tissue | C1GALT1-knockdown
promotes the occurrence and metastasis of pancreatic
adenocarcinoma | Downregulation | Pancreatic ductal
adenocarcinoma | (30) |
Cosmc and integrin β1
Cosmc is the molecular partner of T-synthase,
helping T-synthetase to fold correctly in the ER (128). This chaperone is encoded by Cosmc
in the X chromosome (human Xq24, mouse Is Xc3). Cosmc is located in
the ER. The newly synthesized T-synthase needs Cosmc to avoid
incorrect folding, aggregation and degradation. Human Cosmc is a
type II transmembrane protein with 318 amino acids (36). It has a short N-terminal domain, a
transmembrane domain and a large C-terminal domain in the
cytoplasm, which can independently act as a molecular chaperone for
T-synthase (36). Cosmc protein
itself does not possess galactosyltransferase activity. However,
the expression of functional T-synthase must be accompanied by the
presence of Cosmc (129). There is
26% homology in amino acid sequence between human T-synthase and
human Cosmc, indicating that they are from the same ancestor
(36,129). In humans, Cosmc and T-synthase are
universally expressed and work cooperatively, but their expression
levels vary by tissue or cell type (15,130).
Zeng et al (131) reported
that the promoter structures of Cosmc and T-synthase are similar.
The CpG islands in the 5′ flanking regions of human Cosmc and
T-synthase are gene promoters, and they each contain two SP1/3
binding sites (131). Chromatin
immunoprecipitation analysis and site-directed mutagenesis analysis
of any SP1/3 site confirmed the important role of the SP1/3
sequence in regulating these two genes (131). In patients with Tn syndrome lacking
functional Cosmc, T-synthase activity is completely absent,
indicating that Cosmc is an important partner in the formation of
active T-synthase (131). Upon lack
of functional Cosmc, T-synthase will be reversely transported from
the ER back to the cytoplasm, ubiquitinated and degraded in a 26S
proteasome-dependent manner (131).
Lack of Cosmc is fatal to mice embryos (37,132).
Knockout of Cosmc or T-synthase in mice causes the expression of Tn
antigen and embryonic lethality (20,132).
Wang et al (132) found that
mice with obvious absence of Cosmc have lung and gastrointestinal
bleeding, chylous ascites and growth retardation, and this state is
similar to the conditional T-synthase deletion in hematopoietic
cells and endothelial cells observed in mice. These findings
indicate that the lack of O-glycans in endothelial cells can lead
to misconnection of blood/lymphatic vessels, and that T-synthase
and its molecular chaperone Cosmc are both necessary for the proper
development of blood vessels (21).
Acquired mutations in Cosmc are associated with a
number of diseases, such as IgA nephropathy and Tn syndrome. Some
of the Cosmc gene has genetic deletion in invasive human melanoma
LOX cells, while point mutations exist in other cell lines, causing
Cosmc inactivation and elevating Tn antigen expression (64). For example, human cervical cancer
cells exhibit Cosmc deletion (65).
In pancreatic cancer, epigenetic silencing by Cosmc promoter
methylation leads to inactivation of T-synthase, accompanied by
abnormal O-glycosylation (133). Additionally, Cosmc point mutations
are found in several epithelial samples of patients with UC
(75), but it is unclear if this is
associated with an increased risk of colon cancer.
Cosmc is required for the functional expression of
T-synthase. The expression of Tn antigen and T-synthase activity
loss is a result of human Cosmc loss, which is associated with
several diseases (60,61,63,66,134–136),
such as Tn syndrome (61), IgAN
(137) and human tumor (68). Thus, although these outcomes do not
elucidate the Cosmc chaperone impact, the role of Cosmc in
O-glycosylation seems to specifically rely on T-synthase.
The proper function of T-synthase requires the molecular chaperone
Cosmc, and the integrin β1 subunit may be involved in mediating
these functions.
C1GALT1 can regulate the activity and
glycosylation of integrin β1 (29).
Integrin β1 belongs to the integrin family, which consists of
transmembrane proteins. It can transduce changes in the
extracellular mechanical state and chemical environment of the
cell, which can lead to cytoskeletal changes. It participates in a
wide range of functional activities, such as cell proliferation,
invasion, adhesion and inflammation (138). According to previous studies, there
is a close association between integrin β1 and improvement in
therapeutic drug resistance in different hematopoietic malignancies
and solid tumors, and drug resistance of tumors is mediated by
integrin β1 at the cellular level (139,140).
A study has indicated that blocking integrin β1 inhibits breast
cancer cell proliferation and induces apoptosis (141). Integrin β1 has a close association
with TNM grade and tumor size in liver cancer (142). High expression levels of integrin
β1 are associated with worse survival in patients with liver cancer
(143). Moreover, a previous study
has indicated that C1GALT1 induces hepatocellular carcinoma cell
adhesion to extracellular stroma proteins via integrin β1, as well
as inducing cancer cell migration and invasion (29). C1GALT1 regulates integrin β1 activity
as well as its downstream signaling through the modification of the
O-glycan on integrin β1 (29,144).
The interaction between MET and integrin in the regulation of
development, immunity and invasion of cancer cells has been
previously reported (145–147). Since HGF-triggered cell
proliferation is enhanced by C1GALT1 via MET, it is a reasonable
assumption that the signaling pathways of MET and integrin β1
promote C1GALT1-mediated HCC malignancies synergistically. These
findings further prove that mucin-type O-glycosylation is
important in regulating cancer malignancies, indicating that
C1GALT1 may be a promising therapeutic candidate.
Targeting integrin β1 with inhibitory antibodies
can increase the sensitivity of hepatocellular carcinoma cells to
radiation (148). Moreover, the
inhibition of integrin β1 using antibodies or siRNAs causes
dose-dependent radiation sensitization of head and neck cancer
cells (149). The downregulation of
integrin β1 in laryngeal carcinoma can inhibit
glycosylation-mediated radiation resistance (123). In esophageal cancer, C1GALT1 can
regulate the signaling pathway of the downstream FAK and modify the
O-glycan structure on integrin β1 (51). Moreover, in esophageal cancer cells,
integrin β1 blocking antibodies and FAK inhibitors can enhance
radiation-induced apoptosis (51).
In conclusion, the aforementioned results indicate
that C1GALT1 and integrin β1 signaling pathways can synergistically
promote intrinsic radiation resistance mediated by glycosylation,
although the detailed mechanism of this phenomenon remains
elusive.
Itraconazole, an inhibitor of C1GALT1
Itraconazole is a common antifungal drug with
anticancer effects. Itraconazole has been beneficial in patients
with ovarian cancer, recurrent non-small cell lung cancer, prostate
cancer and other types of cancer, either as a single drug or in
combination therapy in clinical trials (150–153).
Lin et al (13) proposed
itraconazole as a new important C1GALT1 inhibitor in head
and neck cancer. Lin et al (13) screened the ZINC database for
compounds that could bind to the C1GALT1 protein. A total of
seven drugs were found not to be standard anticancer treatments and
had fewer side effects. Only itraconazole significantly increased
the expression of Tn antigens on several cell surfaces (13). At the same time, itraconazole
significantly decreased the protein expression levels of
C1GALT1, while mRNA expression was not significantly
affected, suggesting that itraconazole may affect the protein level
of C1GALT1 through post-translational modification (13). In general, C1GALT1 protein
folding errors are transported to the proteasome and then degraded
(154). The proteasome degradation
pathway involves ubiquitination, and itraconazole increases
ubiquitinated C1GALT1. The results of the cell thermal
displacement analysis revealed that when using itraconazole to
treat SAS and OEC-M1 cells, the melting temperature of
C1GALT1 decreased, and itraconazole decreased the protein
expression levels of C1GALT1 in a dose-dependent manner at a
constant melting temperature (13).
Vicia villosa agglutinin pull-down tests indicated that
itraconazole increased the Tn antigen on EGFR (13). SAS cells that overexpressed
C1GALT1 and OEC-M1 cells with inhibited C1GALT1 were
injected in a mouse xenotransplantation model (13). The tumor growth rate and volume of
SAS cells increased significantly, while the tumor growth of OEC-M1
cells decreased significantly (13).
C1GALT1-mediated tumor growth was partially reversed by
itraconazole in SAS cells (13). The
aforementioned results indicate that C1GALT1 greatly
influences HNSCC, and silencing C1GALT1 may potentially be
an underlying treatment for tumors (13). Although C1GALT1 expression in
mice is partially inhibited by itraconazole, targeting
C1GALT1 via genetic molecular pathways can have great
therapeutic potential for cancer treatment.
Conclusion
Glycosylation is a common, complex and diverse
post-translational modification. This diverse polysaccharide has a
wide scope of biological functions. Mammalian angiogenesis,
platelet production and kidney development are inseparable from
O-glycosylation. The orderly construction of sugar molecules
in normal cells involves substrate-specific glycosyltransferases
(51,155). C1GALT1 and glycosylation are
essential for normal development. Impaired T-synthase activity has
been associated with different types of human diseases, including
inflammatory or immune-mediated diseases, and cancer. The present
review highlighted the relevance of C1GALT1 in the
pathogenesis of IgAN, Tn syndrome, IBD and various types of
cancer.
The change in glycosylation was discovered in a
malignant transformation 60 years ago, and this change is
considered to be one of the hallmarks of human cancer pathogenesis
(156). Abnormal
O-glycosylation, which found in various types of tumor, is
very important in metastasis progression (83,157–159).
The abnormal O-glycosylation of proteins on malignant tumor
cell surface participates in different steps of tumor progression
and regulates intercellular and intracellular signal transduction,
thus inducing angiogenesis, EMT, metastasis and cell proliferation
(157,160). The protein encoded by the
C1GALT1 gene is a key mucin-type O-glycosyltransferase
located in the Golgi apparatus (17). Galactose transfers to Tn antigen with
its molecular chaperone Cosmc, forming Galβ1-3GalNAcαSer/Thr
structure (T antigen, core 1 structure) (83). In cases of hepatocellular carcinoma
and cholangiocarcinoma, C1GALT1 expression is usually
upregulated during tumorigenesis (27,109).
Additionally, C1GALT1 silencing can inhibit cancer cell migration,
invasion and proliferation, which inhibits metastasis and tumor
growth (27,28). The C1GALT1 and integrin β1
signaling pathways can synergistically promote
glycosylation-mediated intrinsic radiation resistance (149). Abnormal O-glycosylation is
involved in the process of EMT (123). In addition, changes in
C1GALT1 expression can cause short O-glycan expression in
different types of cancer, which leads to cancer progression
(120). C1GALT1 expression
in mice is inhibited partially by itraconazole (13). Targeting C1GALT1 via genetic
molecular pathways can have great therapeutic potential for cancer
treatment. On the contrary, C1GALT1 acts as a tumor
suppressor gene in colon and pancreatic cancer (30,123).
Using samples from different sources or at different tumor stages
may contribute to the observed duality in the C1GALT1
function, rendering the true role of this gene still elusive.
In conclusion, it is of great necessity to
implement further studies for exploring the role of C1GALT1
and O-glycosylation, as well as its molecular chaperone
Cosmc, and their interaction with different C1GALT1 targets,
such as integrin β1, in the clinical setting. Future studies will
help improve the understanding of certain pathologies and find new
ways to treat and prevent disease in the future.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
XJS was in charge of the writing and revision of
the manuscript. MZ and XS were involved in articles and data
collection. WL was involved in the making of the table. XM was
involved in figure preparation and supervision. Data authentication
is not applicable. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
O-glycosylation
|
O-linked glycosylation
|
|
N-glycosylation
|
N-linked glycosylation
|
|
ER
|
endoplasmic reticulum
|
|
GalNAc
|
N-acetylgalactosamine
|
|
C1GALT1
|
core 1 synthase glycoprotein-GalNAc
3-β-galactosyltransferase 1
|
|
O-GalNAc
|
GalNAc type
O-glycosylation
|
|
Tn antigen
|
Thomsen-nouveau antigen
(GalNAc-α-1-R)
|
|
T antigen
|
Thomsen-Friedenreich antigen
(Gal-β-1-3GalNAc-R)
|
|
ESCC
|
esophagus squamous cell carcinoma
|
|
HNSCC
|
head and neck squamous cell
carcinoma
|
|
EGFR
|
epidermal growth factor receptor
|
|
CRC
|
colorectal cancer
|
|
EMT
|
epithelial-mesenchymal transition
|
|
FAK
|
focal adhesion kinase
|
|
IgAN
|
IgA nephropathy
|
|
Gd-IgA1
|
galactose-deficient immunoglobulin
A1
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
References
|
1
|
Apweiler R, Hermjakob H and Sharon N: On
the frequency of protein glycosylation, as deduced from analysis of
the SWISS-PROT database. Biochim Biophys Acta. 1473:4–8. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Theodoratou E, Thaçi K, Agakov F,
Timofeeva MN, Štambuk J, Pučić-Baković M, Vučković F, Orchard P,
Agakova A, Din FV, et al: Glycosylation of plasma IgG in colorectal
cancer prognosis. Sci Rep. 6:280982016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vajaria BN and Patel PS: Glycosylation: A
hallmark of cancer. Glycoconj J. 34:147–156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Munkley J and Elliott DJ: Hallmarks of
glycosylation in cancer. Oncotarget. 7:35478–3589. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shan A, Lu J, Xu Z, Li X, Xu Y, Li W, Liu
F, Yang F, Sato T, Narimatsu H and Zhang Y: Polypeptide
N-acetylgalactosaminyltransferase 18 non-catalytically regulates
the ER homeostasis and O-glycosylation. Biochim Biophys Acta Gen
Subj. 1863:870–882. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tian E and Ten Hagen KG: Recent insights
into the biological roles of mucin-type O-glycosylation. Glycoconj
J. 26:325–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kudelka MR, Antonopoulos A, Wang Y, Duong
DM, Song X, Seyfried NT, Dell A, Haslam SM, Cummings RD and Ju T:
Cellular O-Glycome Reporter/Amplification to explore O-glycans of
living cells. Nat Methods. 13:81–16. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gupta R, Leon F, Rauth S, Batra SK and
Ponnusamy MP: A Systematic review on the implications of O-linked
glycan branching and truncating enzymes on cancer progression and
metastasis. Cells. 9:4462020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cervoni GE, Cheng JJ, Stackhouse KA,
Heimburg-Molinaro J and Cummings RD: O-glycan recognition and
function in mice and human cancers. Biochem J. 477:1541–1564. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Joshi HJ, Narimatsu Y, Schjoldager KT,
Tytgat H, Aebi M, Clausen H and Halim A: SnapShot: O-Glycosylation
pathways across kingdoms. Cell. 172:632.e22018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li LX, Ashikov A, Liu H, Griffith CL,
Bakker H and Doering TL: Cryptococcus neoformans UGT1 encodes a
UDP-Galactose/UDP-GalNAc transporter. Glycobiology. 27:87–98. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bennett EP, Mandel U, Clausen H, Gerken
TA, Fritz TA and Tabak LA: Control of mucin-type O-glycosylation: A
classification of the polypeptide GalNAc-transferase gene family.
Glycobiology. 22:736–56. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin MC, Chien PH, Wu HY, Chen ST, Juan HF,
Lou PJ and Huang MC: C1GALT1 predicts poor prognosis and is a
potential therapeutic target in head and neck cancer. Oncogene.
37:5780–5793. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saeland E, Belo AI, Mongera S, van Die I,
Meijer GA and van Kooyk Y: Differential glycosylation of MUC1 and
CEACAM5 between normal mucosa and tumour tissue of colon cancer
patients. Int J Cancer. 131:117–128. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ju T, Brewer K, D'Souza A, Cummings RD and
Canfield WM: Cloning and expression of human core 1
beta1,3-galactosyltransferase. J Biol Chem. 277:178–186. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tran DT and Ten Hagen KG: Mucin-type
O-glycosylation during development. J Biol Chem. 288:6921–6929.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tu L and Banfield DK: Localization of
Golgi-resident glycosyltransferases. Cell Mol Life Sci. 67:29–41.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aryal RP, Ju T and Cummings RD: The
endoplasmic reticulum chaperone Cosmc directly promotes in vitro
folding of T-synthase. J Biol Chem. 285:2456–2462. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guzman-Aranguez A and Argüeso P: Structure
and biological roles of mucin-type O-glycans at the ocular surface.
Ocul Surf. 8:8–17. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia L, Ju T, Westmuckett A, An G, Ivanciu
L, McDaniel JM, Lupu F, Cummings RD and McEver RP: Defective
angiogenesis and fatal embryonic hemorrhage in mice lacking core
1-derived O-glycans. J Cell Biol. 164:451–459. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu J, Gerhardt H, McDaniel JM, Xia B, Liu
X, Ivanciu L, Ny A, Hermans K, Silasi-Mansat R, McGee S, et al:
Endothelial cell O-glycan deficiency causes blood/lymphatic
misconnections and consequent fatty liver disease in mice. J Clin
Invest. 118:3725–3737. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pinho SS and Reis CA: Glycosylation in
cancer: Mechanisms and clinical implications. Nat Rev Cancer.
15:540–555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen CH, Wang SW, Chen CW, Huang MR, Hung
JS, Huang HC, Lin HH, Chen RJ, Shyu MK and Huang MC: MUC20
overexpression predicts poor prognosis and enhances EGF-induced
malignant phenotypes via activation of the EGFR-STAT3 pathway in
endometrial cancer. Gynecol Oncol. 128:560–567. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen CH, Hsiao SM, Chang TC, Wu WY and Lin
HH: Clinical and urodynamic effects of baclofen in women with
functional bladder outlet obstruction: Preliminary report. J Obstet
Gynaecol Res. 42:560–565. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang X, Dong W, Zhou H, Li H, Wang N,
Miao X and Jia L: α-2,8-Sialyltransferase is involved in the
development of multidrug resistance via PI3K/Akt pathway in human
chronic myeloid leukemia. IUBMB Life. 67:77–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ma H, Miao X, Ma Q, Zheng W, Zhou H and
Jia L: Functional roles of glycogene and N-glycan in multidrug
resistance of human breast cancer cells. IUBMB Life. 65:409–422.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu YM, Liu CH, Huang MJ, Lai HS, Lee PH,
Hu RH and Huang MC: C1GALT1 enhances proliferation of
hepatocellular carcinoma cells via modulating MET glycosylation and
dimerization. Cancer Res. 73:5580–5590. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee PC, Chen ST, Kuo TC, Lin TC, Lin MC,
Huang J, Hung JS, Hsu CL, Juan HF, Lee PH and Huang MC: C1GALT1 is
associated with poor survival and promotes soluble Ephrin
A1-mediated cell migration through activation of EPHA2 in gastric
cancer. Oncogene. 39:2724–2740. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu CH, Hu RH, Huang MJ, Lai IR, Chen CH,
Lai HS, Wu YM and Huang MC: C1GALT1 promotes invasive phenotypes of
hepatocellular carcinoma cells by modulating integrin β1
glycosylation and activity. PLoS One. 9:e949952014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chugh S, Barkeer S, Rachagani S,
Nimmakayala RK, Perumal N, Pothuraju R, Atri P, Mahapatra S, Thapa
I, Talmon GA, et al: Disruption of C1galt1 gene promotes
development and metastasis of pancreatic adenocarcinomas in Mice.
Gastroenterology. 155:1608–1624. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu F, Fu J, Bergstrom K, Shan X, McDaniel
JM, McGee S, Bai X, Chen W and Xia L: Core 1-derived mucin-type
O-glycosylation protects against spontaneous gastritis and gastric
cancer. J Exp Med. 217:e201823252020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Alexander WS, Viney EM, Zhang JG, Metcalf
D, Kauppi M, Hyland CD, Carpinelli MR, Stevenson W, Croker BA,
Hilton AA, et al: Thrombocytopenia and kidney disease in mice with
a mutation in the C1galt1 gene. Proc Natl Acad Sci USA.
103:16442–16447. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ju T, Xia B, Aryal RP, Wang W, Wang Y,
Ding X, Mi R, He M and Cummings RD: A novel fluorescent assay for
T-synthase activity. Glycobiology. 21:352–362. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lowe JB and Marth JD: A genetic approach
to Mammalian glycan function. Annu Rev Biochem. 72:643–691. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Soriano P: Abnormal kidney development and
hematological disorders in PDGF beta-receptor mutant mice. Genes
Dev. 8:1888–1896. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ju T and Cummings RD: A unique molecular
chaperone Cosmc required for activity of the mammalian core 1 beta
3-galactosyltransferase. Proc Natl Acad Sci USA. 99:16613–16618.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Xia L and McEver RP: Targeted disruption
of the gene encoding core 1 beta1-3-galactosyltransferase
(T-synthase) causes embryonic lethality and defective angiogenesis
in mice. Methods Enzymol. 416:314–331. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Abtahian F, Guerriero A, Sebzda E, Lu MM,
Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, et al:
Regulation of blood and lymphatic vascular separation by signaling
proteins SLP-76 and Syk. Science. 299:247–251. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Anderson WA and Spielman A: Permeability
of the ovarian follicle of Aedes aegypti mosquitoes. J Cell Biol.
50:201–221. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Batista F, Lu L, Williams SA and Stanley
P: Complex N-glycans are essential, but core 1 and 2 mucin
O-glycans, O-fucose glycans, and NOTCH1 are dispensable, for
mammalian spermatogenesis. Biol Reprod. 86:1792012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Berkholtz CB, Lai BE, Woodruff TK and Shea
LD: Distribution of extracellular matrix proteins type I collagen,
type IV collagen, fibronectin, and laminin in mouse
folliculogenesis. Histochem Cell Biol. 126:583–592. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kudo T, Sato T, Hagiwara K, Kozuma Y,
Yamaguchi T, Ikehara Y, Hamada M, Matsumoto K, Ema M, Murata S,
Ohkohchi N, et al: C1galt1-deficient mice exhibit thrombocytopenia
due to abnormal terminal differentiation of megakaryocytes. Blood.
122:1649–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kerjaschki D, Sharkey DJ and Farquhar MG:
Identification and characterization of podocalyxin-the major
sialoprotein of the renal glomerular epithelial cell. J Cell Biol.
98:1591–1596. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Doyonnas R, Kershaw DB, Duhme C, Merkens
H, Chelliah S, Graf T and McNagny KM: Anuria, omphalocele, and
perinatal lethality in mice lacking the CD34-related protein
podocalyxin. J Exp Med. 194:13–27. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pirulli D, Crovella S, Ulivi S, Zadro C,
Bertok S, Rendine S, Scolari F, Foramitti M, Ravani P, Roccatello
D, et al: Genetic variant of C1GalT1 contributes to the
susceptibility to IgA nephropathy. J Nephrol. 22:152–159.
2009.PubMed/NCBI
|
|
46
|
Zhou FD, Zhao MH, Zou WZ, Liu G and Wang
H: The changing spectrum of primary glomerular diseases within 15
years: A survey of 3331 patients in a single Chinese centre.
Nephrol Dial Transplant. 24:870–876. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Li LS and Liu ZH: Epidemiologic data of
renal diseases from a single unit in China: Analysis based on
13,519 renal biopsies. Kidney Int. 66:920–923. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pan X, Xu J, Ren H, Zhang W, Xu Y, Shen P,
Li X, Wang W, Chen X, Wu P, et al: Changing spectrum of
biopsy-proven primary glomerular diseases over the past 15 years: A
single-center study in China. Contrib Nephrol. 181:22–30. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Barratt J and Feehally J: IgA nephropathy.
J Am Soc Nephrol. 16:2088–2097. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
D'Amico G: Natural history of idiopathic
IgA nephropathy and factors predictive of disease outcome. Semin
Nephrol. 24:179–196. 2004. View Article : Google Scholar
|
|
51
|
Zhang C, Deng X, Qiu L, Peng F, Geng S,
Shen L and Luo Z: Knockdown of C1GalT1 inhibits radioresistance of
human esophageal cancer cells through modifying β1-integrin
glycosylation. J Cancer. 9:2666–2677. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li GS, Zhang H, Lv JC, Shen Y and Wang HY:
Variants of C1GALT1 gene are associated with the genetic
susceptibility to IgA nephropathy. Kidney Int. 71:448–453. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Novak J, Julian BA, Mestecky J and Renfrow
MB: Glycosylation of IgA1 and pathogenesis of IgA nephropathy.
Semin Immunopathol. 34:365–382. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Kiryluk K, Moldoveanu Z, Sanders JT, Eison
TM, Suzuki H, Julian BA, Novak J, Gharavi AG and Wyatt RJ: Aberrant
glycosylation of IgA1 is inherited in both pediatric IgA
nephropathy and Henoch-Schönlein purpura nephritis. Kidney Int.
80:79–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lomax-Browne HJ, Visconti A, Pusey CD,
Cook HT, Spector TD, Pickering MC and Falchi M: IgA1 glycosylation
is heritable in healthy twins. J Am Soc Nephrol. 28:64–68. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gale DP, Molyneux K, Wimbury D, Higgins P,
Levine AP, Caplin B, Ferlin A, Yin P, Nelson CP, Stanescu H, et al:
Galactosylation of IgA1 is associated with common variation in
C1GALT1. Am Soc Nephrol. 28:2158–2166. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kiryluk K, Li Y, Moldoveanu Z, Suzuki H,
Reily C, Hou P, Xie J, Mladkova N, Prakash S, Fischman C, et al:
GWAS for serum galactose-deficient IgA1 implicates critical genes
of the O-glycosylation pathway. PLoS Genet. 13:e10066092017.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Xing Y, Li L, Zhang Y, Wang F, He D, Liu
Y, Jia J, Yan T and Lin S: C1GALT1 expression is associated with
galactosylation of IgA1 in peripheral B lymphocyte in
immunoglobulin a nephropathy. BMC Nephrol. 21:182020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Xie LS, Qin W, Fan JM, Huang J, Xie XS and
Li Z: The role of C1GALT1C1 in lipopolysaccharide-induced IgA1
aberrant O-glycosylation in IgA nephropathy. Clin Invest Med.
33:E5–E13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Vainchenker W, Vinci G, Testa U, Henri A,
Tabilio A, Fache MP, Rochant H and Cartron JP: Presence of the Tn
antigen on hematopoietic progenitors from patients with the Tn
syndrome. J Clin Invest. 75:541–546. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Berger EG: Tn-syndrome. Biochim Biophys
Acta. 1455:255–268. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Ju T, Wang Y, Aryal RP, Lehoux SD, Ding X,
Kudelka MR, Cutler C, Zeng J, Wang J, Sun X, et al: Tn and
sialyl-Tn antigens, aberrant O-glycomics as human disease markers.
Proteomics Clin Appl. 7:618–631. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ju T and Cummings RD: Protein
glycosylation: Chaperone mutation in Tn syndrome. Nature.
437:12522005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Ju T, Lanneau GS, Gautam T, Wang Y, Xia B,
Stowell SR, Willard MT, Wang W, Xia JY, Zuna RE, et al: Human tumor
antigens Tn and sialyl Tn arise from mutations in Cosmc. Cancer
Res. 68:1636–1646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Schietinger A, Philip M, Yoshida BA, Azadi
P, Liu H, Meredith SC and Schreiber H: A mutant chaperone converts
a wild-type protein into a tumor-specific antigen. Science.
314:304–308. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Crew VK, Singleton BK, Green C, Parsons
SF, Daniels G and Anstee DJ: New mutations in C1GALT1C1 in
individuals with Tn positive phenotype. Br J Haematol. 142:657–667.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Wang Y, Jobe SM, Ding X, Choo H, Archer
DR, Mi R, Ju T and Cummings RD: Platelet biogenesis and functions
require correct protein O-glycosylation. Proc Natl Acad Sci USA.
109:16143–16148. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Springer GF: T and Tn, general carcinoma
autoantigens. Science. 224:1198–1206. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Itzkowitz SH, Yuan M, Montgomery CK,
Kjeldsen T, Takahashi HK, Bigbee WL and Kim YS: Expression of Tn,
sialosyl-Tn, and T antigens in human colon cancer. Cancer Res.
49:197–204. 1989.PubMed/NCBI
|
|
70
|
Tsuchiya A, Kanno M, Kawaguchi T, Endo Y,
Zhang GJ, Ohtake T and Kimijima I I: Prognostic relevance of tn
expression in breast cancer. Breast Cancer. 6:175–180. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Inoue M, Ton SM, Ogawa H and Tanizawa O:
Expression of Tn and sialyl-Tn antigens in tumor tissues of the
ovary. Am J Clin Pathol. 96:711–716. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Terasawa K, Furumoto H, Kamada M and Aono
T: Expression of Tn and sialyl-Tn antigens in the neoplastic
transformation of uterine cervical epithelial cells. Cancer Res.
56:2229–2232. 1996.PubMed/NCBI
|
|
73
|
Perez-Muñoz ME, Bergstrom K, Peng V,
Schmaltz R, Jimenez-Cardona R, Marsteller N, McGee S, Clavel T, Ley
R, Fu J, et al: Discordance between changes in the gut microbiota
and pathogenicity in a mouse model of spontaneous colitis. Gut
Microbes. 5:286–295. 2014. View Article : Google Scholar
|
|
74
|
Johansson ME, Sjövall H and Hansson GC:
The gastrointestinal mucus system in health and disease. Nat Rev
Gastroenterol Hepatol. 10:352–361. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Fu J, Wei B, Wen T, Johansson ME, Liu X,
Bradford E, Thomsson KA, McGee S, Mansour L, Tong M, et al: Loss of
intestinal core 1-derived O-glycans causes spontaneous colitis in
mice. J Clin Invest. 121:1657–1666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Johansson ME, Gustafsson JK,
Holmén-Larsson J, Jabbar KS, Xia L, Xu H, Ghishan FK, Carvalho FA,
Gewirtz AT, Sjövall H and Hansson GC: Bacteria penetrate the
normally impenetrable inner colon mucus layer in both murine
colitis models and patients with ulcerative colitis. Gut.
63:281–291. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Campbell BJ, Finnie IA, Hounsell EF and
Rhodes JM: Direct demonstration of increased expression of
Thomsen-Friedenreich (TF) antigen in colonic adenocarcinoma and
ulcerative colitis mucin and its concealment in normal mucin. J
Clin Invest. 95:571–576. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Clamp JR, Fraser G and Read AE: Study of
the carbohydrate content of mucus glycoproteins from normal and
diseased colons. Clin Sci (Lond). 61:229–234. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Larsson JM, Karlsson H, Crespo JG,
Johansson ME, Eklund L, Sjövall H and Hansson GC: Altered
O-glycosylation profile of MUC2 mucin occurs in active ulcerative
colitis and is associated with increased inflammation. Inflamm
Bowel Dis. 17:2299–2307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Theodoratou E, Campbell H, Ventham NT,
Kolarich D, Pučić-Baković M, Zoldoš V, Fernandes D, Pemberton IK,
Rudan I, Kennedy NA, et al: The role of glycosylation in IBD. Nat
Rev Gastroenterol Hepatol. 11:588–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Jacobs JP, Lin L, Goudarzi M, Ruegger P,
McGovern DP, Fornace AJ Jr, Borneman J, Xia L and Braun J:
Microbial, metabolomic, and immunologic dynamics in a relapsing
genetic mouse model of colitis induced by T-synthase deficiency.
Gut Microbes. 8:1–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ju T, Aryal RP, Kudelka MR, Wang Y and
Cummings RD: The Cosmc connection to the Tn antigen in cancer.
Cancer Biomark. 14:63–81. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Hung JS, Huang J, Lin YC, Huang MJ, Lee
PH, Lai HS, Liang JT and Huang MC: C1GALT1 overexpression promotes
the invasive behavior of colon cancer cells through modifying
O-glycosylation of FGFR2. Oncotarget. 5:2096–2106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu SY, Shun CT, Hung KY, Juan HF, Hsu CL,
Huang MC and Lai IR: Mucin glycosylating enzyme GALNT2 suppresses
malignancy in gastric adenocarcinoma by reducing MET
phosphorylation. Oncotarget. 7:11251–11262. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wu YM, Liu CH, Hu RH, Huang MJ, Lee JJ,
Chen CH, Huang J, Lai HS, Lee PH, Hsu WM, et al: Mucin
glycosylating enzyme GALNT2 regulates the malignant character of
hepatocellular carcinoma by modifying the EGF receptor. Cancer Res.
71:7270–7279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Huang MJ, Hu RH, Chou CH, Hsu CL, Liu YW,
Huang J, Hung JS, Lai IR, Juan HF, Yu SL, et al: Knockdown of
GALNT1 suppresses malignant phenotype of hepatocellular carcinoma
by suppressing EGFR signaling. Oncotarget. 6:5650–5665. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Wang H, Rao B, Lou J, Li J, Liu Z, Li A,
Cui G, Ren Z and Yu Z: The Function of the HGF/c-Met Axis in
Hepatocellular Carcinoma. Front Cell Dev Biol. 8:552020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Lee HE, Kim MA, Lee HS, Jung EJ, Yang HK,
Lee BL, Bang YJ and Kim WH: MET in gastric carcinomas: Comparison
between protein expression and gene copy number and impact on
clinical outcome. Br J Cancer. 107:325–333. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Inokuchi M, Otsuki S, Fujimori Y, Sato Y,
Nakagawa M and Kojima K: Clinical significance of MET in gastric
cancer. World J Gastrointest Oncol. 7:317–327. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Toiyama Y, Yasuda H, Saigusa S, Matushita
K, Fujikawa H, Tanaka K, Mohri Y, Inoue Y, Goel A and Kusunoki M:
Co-expression of hepatocyte growth factor and c-Met predicts
peritoneal dissemination established by autocrine hepatocyte growth
factor/c-Met signaling in gastric cancer. Int J Cancer.
130:2912–2921. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Deng N, Goh LK, Wang H, Das K, Tao J, Tan
IB, Zhang S, Lee M, Wu J, Lim KH, et al: A comprehensive survey of
genomic alterations in gastric cancer reveals systematic patterns
of molecular exclusivity and co-occurrence among distinct
therapeutic targets. Gut. 61:673–684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Bradley CA, Salto-Tellez M, Laurent-Puig
P, Bardelli A, Rolfo C, Tabernero J, Khawaja HA, Lawler M, Johnston
PG and Van Schaeybroeck S; MErCuRIC consortium, : Targeting c-MET
in gastrointestinal tumours: Rationale, opportunities and
challenges. Nat Rev Clin Oncol. 15:1502018. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Sierra JC, Asim M, Verriere TG, Piazuelo
MB, Suarez G, Romero-Gallo J, Delgado AG, Wroblewski LE, Barry DP,
Peek RM Jr, et al: Epidermal growth factor receptor inhibition
downregulates Helicobacter pylori-induced epithelial inflammatory
responses, DNA damage and gastric carcinogenesis. Gut.
67:1247–1260. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Xi HQ, Wu XS, Wei B and Chen L: Eph
receptors and ephrins as targets for cancer therapy. J Cell Mol
Med. 16:2894–2909. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Vaught D, Brantley-Sieders DM and Chen J:
Eph receptors in breast cancer: Roles in tumor promotion and tumor
suppression. Breast Cancer Res. 10:2172008. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Herath NI and Boyd AW: The role of Eph
receptors and ephrin ligands in colorectal cancer. Int J Cancer.
126:2003-2011.PubMed/NCBI
|
|
97
|
Lisle JE, Mertens-Walker I, Rutkowski R,
Herington AC and Stephenson SA: Eph receptors and their ligands:
Promising molecular biomarkers and therapeutic targets in prostate
cancer. Biochim Biophys Acta. 1835:243–257. 2013.PubMed/NCBI
|
|
98
|
Pasquale EB: Eph receptors and ephrins in
cancer: Bidirectional signalling and beyond. Nat Rev Cancer.
10:165–180. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Boyd AW, Bartlett PF and Lackmann M:
Therapeutic targeting of EPH receptors and their ligands. Nat Rev
Drug Discov. 13:39–62. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Wen Q, Chen Z, Chen Z, Chen J, Wang R,
Huang C and Yuan W: EphA2 affects the sensitivity of oxaliplatin by
inducing EMT in oxaliplatin-resistant gastric cancer cells.
Oncotarget. 8:47998–48011. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Huang J, He Y, Mcleod HL, Xie Y, Xiao D,
Hu H, Chen P, Shen L, Zeng S, Yin X, et al: miR-302b inhibits
tumorigenesis by targeting EphA2 via Wnt/ β-catenin/EMT signaling
cascade in gastric cancer. BMC Cancer. 17:8862017. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Tsai CH, Tzeng SF, Chao TK, Tsai CY, Yang
YC, Lee MT, Hwang JJ, Chou YC, Tsai MH, Cha TL and Hsiao PW:
Metastatic progression of prostate cancer is mediated by autonomous
binding of Galectin-4-O-glycan to cancer cells. Cancer Res.
76:5756–5767. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Luo H, Guo W, Wang F, You Y, Wang J, Chen
X, Wang J, Wang Y, Du Y, Chen X, et al: miR-1291 targets mucin 1
inhibiting cell proliferation and invasion to promote cell
apoptosis in esophageal squamous cell carcinoma. Oncol Rep.
34:2665–2673. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Shi M, Chen D, Yang D and Liu XY:
CCL21-CCR7 promotes the lymph node metastasis of esophageal
squamous cell carcinoma by up-regulating MUC1. J Exp Clin Cancer
Res. 34:1492015. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Su H, Hu N, Yang HH, Wang C, Takikita M,
Wang QH, Giffen C, Clifford R, Hewitt SM, Shou JZ, et al: Global
gene expression profiling and validation in esophageal squamous
cell carcinoma and its association with clinical phenotypes. Clin
Cancer Res. 17:2955–2966. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Agata N, Ahmad R, Kawano T, Raina D,
Kharbanda S and Kufe D: MUC1 oncoprotein blocks death
receptor-mediated apoptosis by inhibiting recruitment of caspase-8.
Cancer Res. 68:6136–6144. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Kufe DW: MUC1-C oncoprotein as a target in
breast cancer: Activation of signaling pathways and therapeutic
approaches. Oncogene. 32:1073–1081. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Wang Y, Liao X, Ye Q and Huang L: Clinic
implication of MUC1 O-glycosylation and C1GALT1 in esophagus
squamous cell carcinoma. Sci China Life Sci. 61:1389–1395. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Dong X, Luo Z, Wang Y, Meng L, Duan Q, Qiu
L, Peng F and Shen L: Altered O-glycosylation is associated with
inherent radioresistance and malignancy of human laryngeal
carcinoma. Exp Cell Res. 362:302–310. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Chen P, Chang A, Huang M and Wu Y:
Abstract 1400: C1GALT1 regulates malignant phenotypes of
cholangiocarcinoma cells. Cancer Res. 792019.doi:
10.1158/1538-7445.AM2019-1400. PubMed/NCBI
|
|
111
|
Huang MC, Huang MJ and Wu YM: P0247:
C1GAlT1 is overexpressed in cholangiocarcinoma and C1GAlT1
knockdown inhibits malignant behaviors of cholangiocarcinoma cells.
J Hepatol. 62 (Suppl):S3992015. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Lin MC, Huang MJ, Liu CH, Yang TL and
Huang MC: GALNT2 enhances migration and invasion of oral squamous
cell carcinoma by regulating EGFR glycosylation and activity. Oral
Oncol. 50:478–484. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Leemans CR, Snijders P and Brakenhoff RH:
The molecular landscape of head and neck cancer. Nat Rev Cancer.
18:269–282. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Ghazizadeh M, Ogawa H, Sasaki Y, Araki T
and Aihara K: Mucin carbohydrate antigens (T, Tn, and sialyl-Tn) in
human ovarian carcinomas: Relationship with histopathology and
prognosis. Hum Pathol. 28:960–966. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Davidson B, Gotlieb WH, Ben-Baruch G,
Kopolovic J, Goldberg I, Nesland JM, Berner A, Bjåmer A and Bryne
M: Expression of carbohydrate antigens in advanced-stage ovarian
carcinomas and their metastases-A clinicopathologic study. Gynecol
Oncol. 77:35–43. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Chou CH, Huang MJ, Liao YY, Chen CH and
Huang MC: C1GALT1 seems to promote in vitro disease progression in
ovarian cancer. Int J Gynecol Cancer. 27:863–871. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Leach SD: Mouse models of pancreatic
cancer: The fur is finally flying. Cancer Cell. 5:7–11. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Mazur PK and Siveke JT: Genetically
engineered mouse models of pancreatic cancer: Unravelling tumour
biology and progressing translational oncology. Gut. 61:1488–1500.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Muniyan S, Haridas D, Chugh S, Rachagani
S, Lakshmanan I, Gupta S, Seshacharyulu P, Smith LM, Ponnusamy MP
and Batra SK: MUC16 contributes to the metastasis of pancreatic
ductal adenocarcinoma through focal adhesion mediated signaling
mechanism. Genes Cancer. 7:110–124. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Fujita-Yamaguchi Y: Renewed interest in
basic and applied research involving monoclonal antibodies against
an oncofetal Tn-antigen. J Biochem. 154:103–105. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Sun X, Ju T and Cummings RD: Differential
expression of Cosmc, T-synthase and mucins in Tn-positive
colorectal cancers. BMC Cancer. 18:8272018. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Bergstrom K, Fu J, Johansson ME, Liu X,
Gao N, Wu Q, Song J, McDaniel JM, McGee S, Chen W, et al: Core 1-
and 3-derived O-glycans collectively maintain the colonic mucus
barrier and protect against spontaneous colitis in mice. Mucosal
Immunol. 10:91–103. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Dong X, Jiang Y, Liu J, Liu Z, Gao T, An G
and Wen T: T-synthase deficiency enhances oncogenic features in
human colorectal cancer cells via activation of
epithelial-mesenchymal transition. Biomed Res Int.
2018:95323892018. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Scheid E, Major P, Bergeron A, Finn OJ,
Salter RD, Eady R, Yassine-Diab B, Favre D, Peretz Y, Landry C, et
al: Tn-MUC1 DC vaccination of rhesus macaques and a phase I/II
trial in patients with nonmetastatic castrate-resistant prostate
cancer. Cancer Immunol Res. 4:881–892. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Sakai K, Yuasa N, Tsukamoto K,
Takasaki-Matsumoto A, Yajima Y, Sato R, Kawakami H, Mizuno M,
Takayanagi A, Shimizu N, et al: Isolation and characterization of
antibodies against three consecutive Tn-antigen clusters from a
phage library displaying human single-chain variable fragments. J
Biochem. 147:809–817. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Piyush T, Rhodes JM and Yu LG: MUC1
O-glycosylation contributes to anoikis resistance in epithelial
cancer cells. Cell Death Discov. 3:170442017. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Freire-de-Lima L, Gelfenbeyn K, Ding Y,
Mandel U, Clausen H, Handa K and Hakomori SI: Involvement of
O-glycosylation defining oncofetal fibronectin in
epithelial-mesenchymal transition process. Proc Natl Acad Sci USA.
108:17690–17695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
An G, Wei B, Xia B, McDaniel JM, Ju T,
Cummings RD, Braun J and Xia L: Increased susceptibility to colitis
and colorectal tumors in mice lacking core 3-derived O-glycans. J
Exp Med. 204:1417–1429. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Kudo T, Iwai T, Kubota T, Iwasaki H,
Takayma Y, Hiruma T, Inaba N, Zhang Y, Gotoh M, Togayachi A and
Narimatsu H: Molecular cloning and characterization of a novel
UDP-Gal:GalNAc(alpha) peptide beta 1,3-galactosyltransferase
(C1Gal-T2), an enzyme synthesizing a core 1 structure of O-glycan.
J Biol Chem. 277:47724–47731. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Ju T, Aryal RP, Stowell CJ and Cummings
RD: Regulation of protein O-glycosylation by the endoplasmic
reticulum-localized molecular chaperone Cosmc. J Cell Biol.
182:531–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Zeng J, Mi R, Wang Y, Li Y, Lin L, Yao B,
Song L, van Die I, Chapman AB, Cummings RD, et al: Promoters of
Human Cosmc And T-synthase genes are similar in structure, yet
different in epigenetic regulation. J Biol Chem. 290:19018–19033.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Wang Y, Ju T, Ding X, Xia B, Wang W, Xia
L, He M and Cummings RD: Cosmc is an essential chaperone for
correct protein O-glycosylation. Proc Natl Acad Sci USA.
107:9228–9233. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Mi R, Song L, Wang Y, Ding X, Zeng J,
Lehoux S, Aryal RP, Wang J, Crew VK, van Die I, et al: Epigenetic
silencing of the chaperone Cosmc in human leukocytes expressing tn
antigen. J Biol Chem. 287:41523–41533. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Cartron JP and Nurden AT:
Galactosyltransferase and membrane glycoprotein abnormality in
human platelets from Tn-syndrome donors. Nature. 282:621–623. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Cartron JP, Cartron J, Andreu G, Salmon C
and Bird GW: Selective deficiency of 3-beta-d-galactosyltransferase
(T-transferase) in Tn-polyagglutinable erythrocytes. Lancet.
1:856–857. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Ju T, Otto VI and Cummings RD: The Tn
antigen-structural simplicity and biological complexity. Angew Chem
Int Ed Engl. 50:1770–1791. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Hiki Y: O-linked oligosaccharides of the
IgA1 hinge region: Roles of its aberrant structure in the
occurrence and/or progression of IgA nephropathy. Clin Exp Nephrol.
13:415–423. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Yang D, Tang Y, Fu H, Xu J, Hu Z, Zhang Y
and Cai Q: Integrin β1 promotes gemcitabine resistance in
pancreatic cancer through Cdc42 activation of PI3K p110β signaling.
Biochem Biophys Res Commun. 505:215–221. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Desgrosellier JS and Cheresh DA: Integrins
in cancer: Biological implications and therapeutic opportunities.
Nat Rev Cancer. 10:9–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Matsunaga T, Fukai F, Miura S, Nakane Y,
Owaki T, Kodama H, Tanaka M, Nagaya T, Takimoto R, Takayama T and
Niitsu Y: Combination therapy of an anticancer drug with the
FNIII14 peptide of fibronectin effectively overcomes cell
adhesion-mediated drug resistance of acute myelogenous leukemia.
Leukemia. 22:353–360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Park CC, Zhang H, Pallavicini M, Gray JW,
Baehner F, Park CJ and Bissell MJ: Beta1 integrin inhibitory
antibody induces apoptosis of breast cancer cells, inhibits growth,
and distinguishes malignant from normal phenotype in three
dimensional cultures and in vivo. Cancer Res. 66:1526–1535. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Li Y, Ren Z, Wang Y, Dang YZ, Meng BX,
Wang GD, Zhang J, Wu J and Wen N: ADAM17 promotes cell migration
and invasion through the integrin β1 pathway in hepatocellular
carcinoma. Exp Cell Res. 370:373–382. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Winkler J, Roessler S, Sticht C, DiGuilio
AL, Drucker E, Holzer K, Eiteneuer E, Herpel E, Breuhahn K, Gretz
N, et al: Cellular apoptosis susceptibility (CAS) is linked to
integrin β1 and required for tumor cell migration and invasion in
hepatocellular carcinoma (HCC). Oncotarget. 7:22883–22892. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Fransvea E, Mazzocca A, Antonaci S and
Giannelli G: Targeting transforming growth factor (TGF)-betaRI
inhibits activation of beta1 integrin and blocks vascular invasion
in hepatocellular carcinoma. Hepatology. 49:839–850. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Trusolino L, Bertotti A and Comoglio PM: A
signaling adapter function for alpha6beta4 integrin in the control
of HGF-dependent invasive growth. Cell. 107:643–654. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
146
|
McCall-Culbreath KD, Li Z and Zutter MM:
Crosstalk between the alpha2beta1 integrin and c-met/HGF-R
regulates innate immunity. Blood. 111:3562–3570. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Liu Y, Chattopadhyay N, Qin S, Szekeres C,
Vasylyeva T, Mahoney ZX, Taglienti M, Bates CM, Chapman HA, Miner
JH and Kreidberg JA: Coordinate integrin and c-Met signaling
regulate Wnt gene expression during epithelial morphogenesis.
Development. 136:843–853. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Wu J, Li Y, Dang YZ, Gao HX, Jiang JL and
Chen ZN: HAb18G/CD147 promotes radioresistance in hepatocellular
carcinoma cells: A potential role for integrin β1 signaling. Mol
Cancer Ther. 14:553–563. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Eke I, Dickreuter E and Cordes N: Enhanced
radiosensitivity of head and neck squamous cell carcinoma cells by
β1 integrin inhibition. Radiother Oncol. 104:235–242. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Tsubamoto H, Sonoda T, Yamasaki M and
Inoue K: Impact of combination chemotherapy with itraconazole on
survival of patients with refractory ovarian cancer. Anticancer
Res. 34:2481–2487. 2014.PubMed/NCBI
|
|
151
|
Rudin CM, Brahmer JR, Juergens RA, Hann
CL, Ettinger DS, Sebree R, Smith R, Aftab BT, Huang P and Liu JO:
Phase 2 study of pemetrexed and itraconazole as second-line therapy
for metastatic nonsquamous non-small-cell lung cancer. J Thorac
Oncol. 8:619–623. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Antonarakis ES, Heath EI, Smith DC,
Rathkopf D, Blackford AL, Danila DC, King S, Frost A, Ajiboye AS,
Zhao M, et al: Repurposing itraconazole as a treatment for advanced
prostate cancer: A noncomparative randomized phase II trial in men
with metastatic castration-resistant prostate cancer. Oncologist.
18:163–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Nguyen-Tan PF, Zhang Q, Ang KK, Weber RS,
Rosenthal DI, Soulieres D, Kim H, Silverman C, Raben A, Galloway
TJ, et al: Randomized phase III trial to test accelerated versus
standard fractionation in combination with concurrent cisplatin for
head and neck carcinomas in the Radiation Therapy Oncology Group
0129 trial: Long-term report of efficacy and toxicity. J Clin
Oncol. 32:3858–3866. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Aryal RP, Ju T and Cummings RD:
Identification of a novel protein binding motif within the
T-synthase for the molecular chaperone Cosmc. J Biol Chem.
289:11630–11641. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Schachter H: The joys of HexNAc. The
synthesis and function of N- and O-glycan branches. Glycoconj J.
17:465–483. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Hakomori S: Glycosylation defining cancer
malignancy: New wine in an old bottle. Proc Natl Acad Sci USA.
99:10231–10233. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Hua D, Shen L, Xu L, Jiang Z, Zhou Y, Yue
A, Zou S, Cheng Z and Wu S: Polypeptide
N-acetylgalactosaminyltransferase 2 regulates cellular
metastasis-associated behavior in gastric cancer. Int J Mol Med.
30:1267–1274. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Kariya Y, Kanno M, Matsumoto-Morita K,
Konno M, Yamaguchi Y and Hashimoto Y: Osteopontin O-glycosylation
contributes to its phosphorylation and cell-adhesion properties.
Biochem J. 463:93–102. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Wang ZQ, Bachvarova M, Morin C, Plante M,
Gregoire J, Renaud MC, Sebastianelli A and Bachvarov D: Role of the
polypeptide N-acetylgalactosaminyltransferase 3 in ovarian cancer
progression: Possible implications in abnormal mucin
O-glycosylation. Oncotarget. 5:544–560. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Liu B, Pan S, Xiao Y, Liu Q, Xu J and Jia
L: LINC01296/miR-26a/GALNT3 axis contributes to colorectal cancer
progression by regulating O-glycosylated MUC1 via PI3K/AKT pathway.
J Exp Clin Cancer Res. 37:3162018. View Article : Google Scholar : PubMed/NCBI
|