Introduction
Myxoid liposarcoma (MLPS) is the second most common
subtype of LPS, accounting for 30% of all cases of LPS and 10% of
all soft tissue sarcomas (STSs) (1).
MLPS is most frequently localized to the deep tissues of the thigh
(2), and has a distinct morphology,
clinical course, molecular markers and chromosomal translocation.
MLPS can be divided into two subgroups: i) Low grade, also known as
pure MLPS; and ii) high grade, also known as round cell (RC) LPS
(3). Differentiation between these
two subtypes relies on cellular framework with an accepted cut-off
of 5% for the universally accepted RC component (RCC) (4). Previous studies have shown that the
percentage of the hypercellular component is strongly associated
with impaired outcome, higher incidence of distant metastasis and
poorer prognosis, but with no differentiation by management between
the two subtypes (5,6).
Surgical resection with adjuvant radiotherapy (RT)
and possible chemotherapy is the primary management option for MLPS
(5,7). Several studies have investigated the
clinical management outcomes of MLPS, but could not identify a
single, unified strategy (5,7). Lack of a well-defined protocol
increases the risk of local and distant relapses, negatively
impacting patient overall survival (OS) (6). The aim of the present study was to
establish an updated algorithm for the multidisciplinary management
of MLPS, addressing the roles of surgical resection, chemotherapy
and RT in optimizing overall patient prognosis. The study
encompasses an individualized approach incorporating demographic,
prognostic and specific tumor prognostic parameters.
Search methodology
Search strategy
Research engine search was conducted on the second
week of January 2021 using the PubMed (https://pubmed.ncbi.nlm.nih.gov/), Medline (https://ovidsp.dc2.ovid.com/ovid-a/ovidweb.cgi),
Scopus (https://www.scopus.com/search/form.uri?display=basic#basic)
and Google Scholar databases (https://scholar.google.com/). The medical subject
headings (MeSH) and terms used at the start of our search strategy
were as follows: ‘Myxoid liposarcoma’, ‘liposarcoma’, ‘prognostic
factors’, ‘adjuvant chemotherapy’, ‘adjuvant radiotherapy’,
‘postoperative radiotherapy’ and ‘round cell myxoid liposarcoma’.
For further information regarding current trials, the
ClinicalTrials.gov Registry Platform (https://clinicaltrials.gov/ct2/home) was searched
using the following three MeSH terms: ‘Myxoid liposarcoma’,
‘adjuvant radiotherapy’, ‘liposarcoma’ and ‘chemotherapy’.
Randomized and non-randomized trials, observational studies and
case studies were included. Search language was restricted to
English.
Inclusion and exclusion criteria
The retrieved databases were independently screened
starting with the title, then the abstract; finally, full-text
articles were reviewed. Studies about MLPS alone and different
types of LPS (including the subtype MLPS) were included. The
studies mainly tackled pathophysiology, histology, prognostic
parameters and treatment modalities (surgical and non-surgical).
Studies not involving MLPS, review articles, studies performed on
animals, studies on the molecular aspect of MLPS, studies on MLPS
of the trunk only, studies of retroperitoneal MLPS, non-English
written studies and Abstracts were excluded. The data were
extracted and summarized in a uniform excel sheet format. The excel
sheet consisted mainly of the author, years, numbers of cases,
treatment, follow-ups, outcomes and conclusion. The information
from the retrieved studies that was not relevant to the present
study was not added to the excel sheet. The current review aimed to
develop an integrated algorithm that combined the available
extracted literature focusing on the treatment modality outcomes
and the prognostic values to help guide toward favoring a treatment
over another.
Quality assessment
The risks of bias were assessed independently by two
of the authors. A revised tool to assess risk of bias in randomized
clinical trials (RCTs), known as the risk of bias 2 (2019 version)
was used for RCT risk of bias (8).
The updated version of the tool is structured into six domains
accompanied by signaling questions. The six domains were as
follows: i) Bias arising from the randomization process; ii) bias
due to deviations from intended interventions (effect of assignment
to intervention); iii) bias due to deviations from intended
interventions (effect of adhering to intervention); iv) bias due to
missing outcome data; v) bias in measurement of the outcome; and
vi) bias in selection of the reported result. Each item was
recorded as ‘high risk’, ‘low risk’ or ‘some concern risk’.
The ‘Newcastle-Ottawa Quality Assessment Form for
Cohort Studies’ was used for quality assessment of the included
cohort studies (9). The quality of
each study was further graded as ‘good’, ‘fair’ or ‘poor’ according
to the points it received in different components.
The included articles were critically reviewed by
the authors of the present review, who are orthopedic surgeons and
orthopedic oncologists for basic assessment of confidence levels of
each article. Confidence level was not assigned specifically, but
only those assigned to a high quality study relevant to the subject
were considered for recommendation and analysis. Any
inconsistencies were managed through discussions among authors.
Study compilation and algorithm
A total of 3 RCTs, 1 ongoing RCT, 5 prospective
studies (1 randomized), 1 case study and 27 retrospective studies
were selected (Table SI). The main
stratification of the algorithm was based on the size of the tumor
(<5, 5–10 and >10 cm). The second stratification was based on
the depth of the tumor [superficial (histology, type of resection
and location) for further adjuvant or neoadjuvant treatment if
needed, or deep]. Each patient was then stratified according to its
demographical and tumor characteristics.
Prognostic factors of MLPS
Age
Multiple prognostic factors influence the OS and
cancer-specific survival of patients with MLPS (10). Historically, age has been considered
a major attributor to the overall natural progression of MLPS, and
thus stratification of management should be conducted based on age
cut-offs for optimization of care with the most effective modality.
Wu et al (11) demonstrated
that increased age was associated with poorer OS and
cancer-specific survival in patients with MLPS. An age of >30
years was shown to be an independent risk factor for poorer OS, and
patients >60 years of age had a worse prognosis than those
>30 years old (11). Another
study indicated that an age of >60 years was an independent
predictor of worse OS and disease-free survival (DFS) for extremity
and trunk MLPS (12). In addition,
an epidemiological study (13) on
the prognostic factors of MLPS revealed that patients >40 years
of age had a higher probability of developing RC tumors, which was
itself a predictor of worse DFS. Although a specific age was not
identified, elderly patients had a higher tendency to acquire MLPS
with a greater percentage of RC tumors (13). In a study of the effects of age and
histology on operable LPS, Greto et al (14) found that patients >65 years of age
had a higher risk of high grade histology and local recurrence (LR)
over a follow-up period of 8.6 years. In a study of 95 patients
with MLPS with or without RC differentiation, an age of >45
years, tumor size of >10 cm (significant only with univariate
analysis) and tumor necrosis were all found to be associated with a
worse OS (15). The hypothetical
suggestions behind age and poor prognosis are a decrease in patient
immunity and inferior host DNA repair mechanisms, which result in a
higher probability of mismatch errors, and cause oncogene
activation and/or amplification or defects in tumor suppressor
genes (16). A study conducted at
the University of Groningen Medical Center revealed that patients
>45 years old had the worst prognosis (17). Dürr et al (10) found that patients with MLPS aged
between 45 and 60 years had a worse prognosis for OS. This finding
was reinforced by multivariate analysis in a number of other
studies (15,18,19). A
nomogram of 2,163 patients with STS was constructed by the Memorial
Sloan-Kettering Cancer Center to analyze the disease-specific
survival (DSS) of common types of STS (20). The major predictors of survival were
listed, including age, and it was demonstrated that patients >65
years old had a 6% reduction of 8 years DSS compared with those
>45 years old (18). On the other
hand, a study conducted at the Cleveland Clinic revealed no
significant difference in prognosis between patients older or
younger than 45 years of age (19).
Additionally, various studies conducted in the early 1990s have
shown that age at diagnosis has no prognostic value (21,22). The
median age of MLPS presentation is 45 years, and most studies have
selected 45 years as the cut-off value to assess the prognosis of
patients with MLPS, primarily since it provides the strongest
statistical power (23). However,
when 45-year-old patients were compared with those between 60 and
65 years old (as is the case in the majority of reported studies),
it was well established that a higher age was associated with a
poorer prognosis (14,15). Thus, age is as an impactful
influencing factor for patients with MLPS. As such, in the present
study, an organogram or algorithm was constructed using an age of
>65 years (±5 years) as the cut-off for the need for any
additional or more aggressive therapeutic intervention (Figs. 1–3),
in view of its negative prognostic value.
Histology
Multiple subtypes of LPS exist, including
pleomorphic, well-differentiated myxoid and RC. RC is the less
differentiated form of MLPS, and both can be frequently found in
the same tumor (19). Fiore et
al (6) studied the prognostic
factors of multiple subtypes of MLPS, and found that RCLPS (which
is graded as type II or III in contrast to pure MLPS, also known as
grade I) exhibited more aggressive behavior, with a 22% incidence
of distant metastasis, compared with 5% in primary MLPS. In a
retrospective study of 49 patients on the clinicopathological
prognostic factors of MLPS, ten Heuvel et al (17) established that patents with MLPS and
>5% RCC had a higher tendency to develop metastasis, with an
associated poorer survival (P=0.0004 and P=0.03 from univariate and
multivariate analysis, respectively). This finding was further
reinforced by Antonescu et al (18) (P=0.01 and P=0.02 from univariate and
multivariate analysis, respectively). Furthermore, Smith et
al (19) revealed that >5%
RCC is a factor for poorer prognosis in univariate analysis only
(P<0.05). In the Mayo Clinic study, 35% of the 14 patients with
MLPS (5-25% RCC) developed metastasis, and 29% died from the
disease, although this finding was not statistically significant
(15). For patients with >25%
RCC, 58% succumbed to associated metastases and 54% died from
causes associated with the primary tumor, which was statistically
significantly in both univariate and multivariate analyses
(15). Evans (24) observed a similar finding regarding
the effect of MLPS with 25% RC on OS, although the sample size was
small. Patients with pure RCMLPS have a poorer prognosis than those
with pure MLPS. Several cut-offs have been listed to identify which
range of RCC would be the best attributor of overall prognosis (as
stated in the aforementioned studies) (15,17,18).
Nevertheless, other studies have stated that any RC component is
associated with poorer outcome (3,17,19,25,26).
For example, Haniball et al (4) investigated the prognostic factors
associated with MLPS, and demonstrated that a higher RCC predicts a
worse survival outcome. Thus, it was concluded that patients with
>5% RCC should be considered for adjuvant RT and/or chemotherapy
(4,27). Histological prognosis of RCMLPS was
also demonstrated by Bartlett et al (28), regarding its impact on survival and
LR. In addition, the importance of adjuvant RT was further
elucidated by Yang et al (29), Pisters et al (30) and Harrison et al (31), who determined that high-grade LPS
(i.e. RC) was highly affected by adjuvant RT, which prevents LR
post-limb salvage surgery. However, 5% RCC was selected as a
cut-off for more aggressive surgical and non-surgical treatment in
the present study, as shown in Fig.
1. In addition, since grading is the most impactful factor of
prognosis compared with size, age and location (as indicated by the
nomogram constructed by the Memorial Sloan-Kettering Cancer Center)
(20), the safest surgical and
non-surgical treatment was selected where applicable to decrease
the risk of LR where possible (32).
Nevertheless, it is important to mention that this nomogram was
constructed for all histological LPSs, including MLPS.
Size
The average size of MLPS is ~8–12 cm, with a range
between 1.5 and 25 cm (10). Several
studies have discussed the prognostic value of tumor size in
patients with MLPS. Orson et al (33) and Reitan et al (34) both demonstrated that larger tumors
were associated with poorer prognosis. Moreover, four larger
studies (7,15,18,20)
assessed several prognostic factors for MLPS with varying results.
One study showed that tumors >5 cm were associated with worse
DSS compared with those <5 cm (17). Univariate analysis revealed that
tumors >10 cm indicated lower OS rates compared with patients
with smaller tumors (15); however,
this finding was not statistically significant for multivariable
analysis. Zheng et al (7)
showed that the greater the diameter of the tumor, the lower the OS
rate, and the higher the risk of metastasis. A similar result has
been demonstrated in various other studies illustrating the impact
of tumor size on prognosis (28,35–38). For
example, in a study of >3,752 patients with LPS, Callegaro et
al (20) demonstrated that a
tumor size of 11 cm was associated with worse 5-year survival rate
than that of patients with 4-cm tumors, and that patients aged
>69 years had a worse 5-year survival rate than those >42
years old; these findings were both statistically significant.
Notably, the aforementioned studies included various histological
types of LPS, including MLPS. On the other hand, a study conducted
by Smith et al (19)
demonstrated that size did not affect the clinical outcomes of
patients with MLPS and Nishida et al (12) stated that depth and size were not
independent prognostic factors for survival. In a study by Dürr
et al (10), the mean size of
the MLPS was 12 cm. It was demonstrated that tumor size had a
significant prognostic value in terms of OS, and that RT decreased
the size of the tumor, but did not affect LR (10). These findings are in contrast to
those of Guadagnolo et al (39), who showed a significant decrease in
LR. Differentiating size cut-offs has several advantages in MLPS.
Although the literature revealed no real consensus on which tumor
sizes are associated with the worst prognostic outcome, most
studies have used cut-offs of <5, 5–10 and >10 cm. It is
important to note that larger tumors have a greater probability of
being closer to essential structures, which may jeopardize the
extent of resection in limb salvage surgery. Thus, the current
review suggests that when wide surgical resection (R0) is not
possible, the only alternative is either marginal (R1) or
intralesional (R2) resection, which increases the risk of
recurrence with or without affecting survival. Therefore, it is
imperative to consider tumor size when treating patients with MLPS,
as it may guide clinicians to the appropriate timely management for
optimization of cancer-free survival, while minimizing the risk to
vital structures.
Location, depth and sex
Various studies have identified that trunk LPS is
associated with a poorer outcome than extremity LPS (12,19,40).
However, the difference in survival and prognosis between tumors of
the upper and lower extremities is not commonly discussed in
literature. No significant difference was observed by Wu et
al (11) when comparing upper
and lower extremity LPS, though univariate analysis revealed a
lower OS in patients with lower extremity LPS. Lansu et al
(13) demonstrated that upper
extremity LPS was associated with a poorer OS (P=0.00001) in
patients when followed-up over 7.6 years. Muratori et al
(41) found that metastasis-free
survival for lower extremity tumors was higher compared with that
of the upper extremities, although without statistical
significance. Nishida et al (12) studied the role of location and depth
in terms of OS and DFS, with a follow-up period of 12 months. The
OS and DFS for upper and lower extremity MLPS were 80, 69, 93 and
81%, respectively, but did not reach statistical significance
(12). A possible explanation for
this may be the smaller surface area of the upper compared with the
lower extremity, and thus, there is less space for the tumor to
increase in size without affecting vital neurovascular structures.
In addition, due to the crowded anatomy of the upper extremity in
terms of compartments and neurovascular structures, the probability
of complete resection of the tumor is decreased compared with that
of the lower extremity, assuming the same lesion size (12).
In terms of depth, patients with deep tumors
experienced 85 and 70% OS and DFS rates, respectively, and 100 and
78% OS and DFS rates for superficial tumors, respectively, but
without statistical significance (12,20). In
a study of LPS, Gronchi et al (42) revealed that deep tumors were
associated with worse DFS and OS times compared with superficial
tumors. A similar finding was observed by Greto et al
(14) surrounding the prognosis of
deep compared with superficial tumors. Aiba et al (43) did not reveal an association between
depth and histological response in patients with MLPS, and Bartlett
et al (28) demonstrated that
deep tumors were associated with a higher risk of LR (without
statistical significance) compared with superficial tumors, during
a 5.4-year follow-up period.
Male patients have been shown to have significantly
worse survival compared with female patients with MLPS (11). Vos et al (44) demonstrated that male sex was the only
significant factor for the risk of LR in patients with LPS
(P=0.037). The same prognostic impact of male sex was further
reinforced by Toulmonde et al (45); however the study was conducted on
patients with retroperitoneal MLPS. By contrast, Salduz et
al (46) did not reveal any
effect of sex on the prognosis of patients with MLPS. This
evidence-based literature guided the selection of more aggressive
treatment post-surgical resection in male patients, or patients
with upper extremity or deep tumors in the present study, as is
apparent from the resulting organograms (Figs. 1–3).
Surgical treatment in MLPS
Surgical resection remains the first-line treatment
for almost all cases of STS, including primary MLPS of the
extremities, with the exception of some diffusely metastatic
conditions (7,47,48).
Historically, amputation was once considered the standard therapy
to attain local control in patients with extremity STS (49). However, improvements in imaging,
implementation of adjuvant therapy and technical advances in
reconstructive surgical procedures have decreased the requirement
for amputation. In 1982, a randomized control study of 43 patients
conducted by Rosenberg et al (50) showed that limb-sparing surgery with
RT was an effective treatment in patients with high-grade STS of
the extremities, with a LR rate of 15%, and no difference in OS and
DFS rate compared with amputation. Limb salvage surgery in MLPS is
beneficial for the following reasons: i) The low potential for
local invasion and distant metastasis compared with other STSs; and
ii) its high radiosensitivity, insuring adequate preoperative
shrinkage of the tumor, and securing margin-free surgical resection
while minimizing the risk of recurrence (3,51). The
challenge to surgeons resecting soft tissue tumors is removing the
mass with a sufficient margin of surrounding normal tissue, while
maximizing postoperative physical function (52). Detailed surgical approaches and
techniques for resecting STSs are beyond the scope of this article.
However, MLPS resection is broadly divided into R0 and R1 (53). In very rare cases, the surgeon may
fail to completely resect the tumor, primarily due to adherence to
neurovascular bundles. In these cases, it is considered to be R2.
R0 is accomplished through resection of the lesion, its
pseudocapsule and/or reactive zone with a surrounding of completely
normal tissue, but without removing the entire compartment. The
cut-off margin of normal tissue varies between 1 and 2 cm, or if
the fascial plane is intact (53).
On the other hand, R1 is achieved by also removing the lesion as
aforementioned; however, the plane of dissection is through the
pseudocapsule, and thus, may potentially retain microscopic tissue
portions at the margin of the wound (53).
The importance of R0 has been demonstrated by Zheng
et al (7). In their study,
MLPS with R0 had no LR compared with 61% LR in patients with R1
(7). The average follow-up period
for patients with R0 was 43.7 months, and 60.9% of patients who
underwent R1 experienced LR, with an average follow-up period of 75
month. Nevertheless, no significant difference was observed between
the different surgical modalities (7). Notably, patients who underwent R1 and
R0 had a tumor size of 17.2±8.8 and 8.6±4.7 cm, respectively, with
a significant difference in tumor size; of the patients who
underwent R0, 5 received adjuvant chemotherapy or RT, and 3
succumbed to the disease (7).
Furthermore, Dürr et al (10)
observed the same finding with no recurrences following R0 compared
with 33% after R1, despite the use of RT and chemotherapy in the
majority of patients. A retrospective study by Nishida et al
(12) (with an 8-year follow-up
period) revealed that MLPS with R0 could be locally controlled with
surgery alone, without the need for RT. No decrease in LR rate was
observed in the minority of patients treated with RT. Moreover, no
significant advantage was observed with RT to prevent LR in
patients with negative margins (12). Haniball et al (4) did not obtain promising results from
postoperative RT in patients with R1 or R2. The risk of LR in this
study was found to be strictly associated with the margins and the
presence of >5% RCC, irrespective of whether the patients
received RT or not (4).
RT in MLPS
The primary characteristic of MLPS, compared with
other types of STS, is high radiosensitivity (54). RT can be used either preoperatively
or postoperatively (55), and
preoperative RT has gained popularity in STS. The main advantages
of preoperative RT are reduction in the field and dose, as well as
potential tumor shrinkage. In 2004, Pitson et al (56) highlighted a superior preoperative
response to RT in patients with MLPS compared with that for other
subtypes of STS, and there was a significantly greater reduction in
tumor size when treated with RT compared with undifferentiated STS.
In 2007, Engström et al (36)
reported a marked decrease in MLPS volume following preoperative
RT; 23 out of 30 irradiated tumors showed a median reduction in
volume of 52%, and it was postulated that RT induced the
histopathological accumulation of mature lipoma-like areas and
tumor volume reduction that may facilitate resectability. No LR was
observed in patients with MLPS who had received preoperative RT, as
demonstrated by Chung et al (51). Only one instance of LR was reported
by Salduz et al (46) in a
study of 23 patients with MLPS treated with preoperative RT.
Finally, Moreau et al (3)
concluded that RT prevented local relapse (P<0.001) and also
induced a 7-fold decrease in the 5-year LR rate of patients with
positive margins (P<0.05).
Moreover, Chowdhry et al (57) observed differences between patients
who had received preoperative RT (LR 3%) compared with those who
underwent postoperative RT (LR 11%). Since 1979, preoperative RT
combined with conservative surgery has been the standard treatment
for patients with MLPS, with no observable LR (39). However, the aforementioned study also
included patients referred to their center following resection,
thus undergoing preoperative RT was not always an option. In these
patients, postoperative RT was associated with 94% local control
despite the fact that 29% patients received R1 or undetermined
margin resection (39). Fiore et
al (6) reported on mixed groups
of patients where some received perioperative RT and others
underwent surgery alone, and multivariate analysis revealed that
postoperative RT was associated with a lower LR rate. Furthermore,
ten Heuvel et al (17)
identified a significant decrease in LR when surgery was coupled
with postoperative RT (8% vs. 44%; P=0.01). However, the most
convincing argument for RT was demonstrated by Hatano et al
(58), who showed that MLPS treated
by R1 or R2, combined with RT, resulted in 100% local control in 10
patients over a follow-up period of 58 months. The choice between
using RT as a preoperative or postoperative modality in MLPS is
still under debate. However, the aforementioned findings on
preoperative RT in STS, including MLPS, have shown it to be an
extensively promising modality for the management of MLPS. The
reasons for this include a reduction in late toxicity, improved
outcome, maintaining adequate local control and allowing borderline
tumors to become surgically accessible (38,59). Le
Grange et al (38) showed
that preoperative RT resulted in a marked decrease in the volume of
soft tissue tumors, which was most apparent in MLPS. Preoperative
RT was shown to be an effective treatment, primarily for high-risk
borderline tumors, justifying its importance as the modality of
choice in RCMLPS due to its high-grade features, risk of
recurrence, large deep features and its proximity to sensitive
locations (38). Notably, tumor
volume reduction in patients who underwent preoperative RT was
inversely proportional to tumor grade (38). Postoperative RT has been associated
with a higher risk of fibrosis, joint stiffness and marginally
predictive edema compared with preoperative RT (59). In addition, preoperative RT allows a
lower threshold for high-dose postoperative RT, which decreases the
risk of dose-associated radiation toxicity (59). The radiosensitivity of MLPS,
including RCMLPS, was shown by a reduction in tumor size, as well
as an increasing percentage of hyalinization, intra-mural fat and
necrosis, enabling its use for the optimization and facilitation of
surgical resection when used preoperatively (60). In 92% of cases, preoperative RT
decreased MLPS size by an average of 25–30% in tumors of 12 cm at
48 days post treatment initiation, thus shrinking the tumor to ~8
cm with a hyalinization content of almost 100% (60). Notably, 7/13 patients in the study
also received preoperative chemotherapy (60). Roberge et al (61) demonstrated an 82% median decrease in
tumor volume following external beam RT. This radiosensitivity was
primarily associated with damage to the medium-sized arterioles
(62). Although Eilber and Eckardt
(48) demonstrated that necrotic
content post-RT was associated with a decreased risk of recurrence
and improved survival, this finding was not proven to be accurate
according to Wortman et al (60) and Mullen et al (63).
The recent DOREMY trial (NCT02106312) is
investigating the possibility of an RT dose reduction for MLPS
without jeopardizing clinical outcomes (https://clinicaltrials.gov/ct2/show/NCT02106312).
Through their phase II results (13)
it was revealed that a 36-Gy dose delivered in once-daily 2-Gy
fractions resulted in the same clinical outcome with a possible
decrease in toxicity. These findings reinforce the role of
preoperative RT in MLPS where adequate surgical resection is
unachievable or where the tumor is inoperable (13,36).
Postoperative RT was once more commonly used than preoperative RT
for STSs (64). However, since 2004,
there has been a steady increase in the use of preoperative RT.
Postoperative RT showed no superior benefit in terms of survival
and LR compared with preoperative RT (64). In addition, local control is better
achieved using preoperative RT (65). Overall, the timing of use for each
modality is multifactorial, and should be evaluated on a
case-by-case basis. It is safe to conclude that postoperative RT
may be used as an adjunct treatment for use in patients with small
tumors (<10 cm) where the true depth and approximation to vital
structures is underestimated, leading to non-optimal resection.
Nevertheless, due to its high dose, higher late morbidity is
expected in postoperative RT, but a higher rate of infection is
seen in preoperative RT (66).
Currently, the standard treatment for STS management is external
beam intensity modulated RT due to its superior local control
compared with brachytherapy (67).
The majority of studies (25,35,42,43,64)
comparing preoperative and postoperative RT include all STS
subtypes, not just MLPS. However, to the best of our knowledge,
high-quality evidence solely addressing comparative studies of RT
timing in MLPS is lacking.
Chemotherapy in MLPS
The role of chemotherapy in patients with MLPS has
been discussed from several perspectives: i) As a neoadjuvant for
local control of primary MLPS; ii) as an adjuvant in a
postoperative setting; and iii) for the treatment of metastatic
MLPS. Adjuvant chemotherapy in STS (including MLPS) has been
studied in multiple randomized control trials in the 1990s, with a
meta-analysis published in The Lancet, concluding that
doxorubicin was associated with a significant improvement in
recurrence-free survival with no associated improvement in OS
(68). Another study emphasized the
role of ifosfamide to treat primary STS with differing conclusions
(2). In 2004, Eilber and Eckardt
(48) investigated the effects of
both doxorubicin and ifosfomide in patients with high-risk primary
STS (including M/RCLPS), demonstrating that ifosfomide was
associated with improved DSS compared with patients who did not
receive chemotherapy (P=0.0003). This association was the strongest
for tumors >10 cm, high grade (e.g. RC), primary and extremity
LPS. In addition, M/RCLPS was shown to be an independent prognostic
factor for improved DSS (P=0.03) (48). In positive-margin MLPS and post
re-excision, complementary RT combined with adjuvant chemotherapy
should be implemented depending on the histology, type, extension
of the tumor and size (29,69,70).
Previously, neoadjuvant chemotherapy was rarely
discussed for the treatment of MLPS. A phase II clinical trial of
neoadjuvant trabectedin in patients with advanced localized MLPS
demonstrated that 1.5 mg/m2 trabectedin every 3 weeks
had significant efficacy and minimal toxicity (42). Tumors with the complete disappearance
of malignant tissue were in the lower extremities, with a size
between 5 and 10 cm (42). A study
conducted by Guadagnolo et al (39) stated that for patients with tumor
size >10 cm, treatment should be supplemented with neoadjuvant
doxorubicin-based therapy. Axitinib has shown promising results for
the treatment of MLPS, exerting its anti-angiogenic and
anti-tumorigenic effects through the VEGF signaling pathway
(71). In unresectable pulmonary
metastatic and extrapulmonary metastatic STS, including MLPS and
RCLPS, patient prognosis is unfavorable, thus systemic chemotherapy
is required, and surgery should be considered as a palliative
treatment option (41).
Several clinical trials have investigated the role
of combined adjuvant chemotherapy (e.g., trabectidin) and RT in
MLPS (42,51). This combination has the potential to
decrease RT-associated toxicity, and may also act in a synergistic
manner for tumor eradication (42,72).
Furthermore, the TRASTS trial has shown that chemotherapy-RT
combination in patients with MLPS is safe, and a potential step
towards the delivery of combination therapy (42,51,72,73). It
is important to note that although the extent of necrosis
post-neoadjuvant chemo-RT in MLPS was not large, the percentage was
not associated with the outcome (63). Thus, volume reduction should be the
primary focus to reach a tumor size that is appropriately
resectable.
Discussion
MLPS has distinctive features compared with other
types of STS. Specific chromosomal translocations have been
identified in MLPS, which consist of fusions between the fusion
binding protein gene and the C/EBP homologous protein (CHOP) gene
[(t12;16)(q13;p11)] in 90% of tumors, and between the RNA-binding
protein related to Ewing sarcoma and CHOP genes [(t12;22)(q13;q12)]
in >5% of tumors (17). In
difficult cases, PCR detection of these translocations allows for a
specific diagnosis of MLPS (15).
Among the STSs, fine needle aspiration biopsy (FNAB) is of
significant efficacy in adult myxoid STSs, with the majority being
diagnosed with a reasonable level of accuracy, based solely on
their cytological distinctions (74,75).
Nonetheless, FNAB is not very accurate in differentiating subtypes
of MLPS, including RCMLPS (23,74).
Despite the distinct prognostic factors for OS in the presence of
>5% RCC, limited data are available that may influence the
course of treatment. MLPS and RCLPS were considered as a single
entity in the present study, and both have been included in the
resulting organograms.
MLPS has a distinctive pattern of extrapulmonary
metastases, and screening and follow-ups should include history
taking, thorough physical exams and imaging (i.e. whole body MRI
and CT scans of the chest, abdomen, pelvis and thorax) (76).
Surgical resection remains the first-line treatment
for MLPS. The resection margin is one of the most important factors
affecting survival in patients with STS, as observed by Kim et
al (77) over a median follow-up
period of 48 months. However, due to its high radiosensitivity, few
centers advocate more conservative resection in MLPS, and
preoperative RT is the preferred type of adjuvant therapy (50). However, chemotherapy may also serve a
minor role, particularly in tumors >10 cm in size (50).
Nevertheless, the specific prognostic factors and
standardized algorithm required to maximize survival rate with the
highest quality of life remain limited. Thus, a detailed algorithm
with the potential prognostic and therapeutic factors (combining
size, grade, location and follow-up) to detect recurrences is
required to establish a schema for clinical guidance. Several
prognostic factors influence LR rate and OS in MLPS (18), the most important of which are
surgical margins, the presence of a RCC and tumor size (4,12,17). The
latter is of utmost importance, and serves as a considerable
limiting factor in management modification (e.g. the benefit of
incorporating adjuvant chemotherapy in tumors >10 cm in size)
(69). A retrospective study of 34
patients with MLPS with a 65-month mean follow-up period showed
that tumor size was an independent risk factor for MLPS metastasis,
with an average tumor diameter of 21.7 cm (7). Another study of 49 patients with a
median follow-up of 101 months demonstrated that tumor grade, size
and age negatively impacted survival (17). Haniball et al (4) analyzed the prognostic factors and
metastatic patterns in primary M/RCLPS, and discovered that age
>50 years, size >10 cm and >5% RCC on histology were
significant factors for a poor prognosis (P=0.027, P=0.03 and
P=0.0001, respectively). The study was conducted over a 10-year
follow-up period with 130 tumors, including 88 with <5% RCC and
42 with >5% RCC (4). It was also
suggested that adjuvant chemotherapy and RT should be considered
for patients with >5% RCC (4). In
a multicenter retrospective study of 418 cases of MLPS and RCLPS,
Moreau et al (3) demonstrated
that with a 5.2-year follow-up period, a tumor diameter >10 cm
was associated with an increased risk of metastasis and death
(P=0.024), and that RT effectively prevented LR, and should
therefore be administered as a neoadjuvant. By contrast, Nishida
et al (12) concluded that
size and depth did not affect patient prognosis in MLPS of the
extremities. Thus, the majority of studies suggest that size is a
critical factor in the overall prognosis of patients with MLPS.
Hence, in the present study, the management organogram was
stratified based on size cut-offs of <5, 5–10 and >10 cm
(Figs. 1–3).
There is adequate data recommending the use of R0
surgical resection for MLPS, with the aim to achieve negative
margins (55). Nevertheless, the
definition of negative-margin R0 varies considerably, complicating
the comparison between different studies (78–80). In
the present study, a 1-cm negative margin was adopted for the
algorithm, which was based on the National Comprehensive Cancer
Network (NCCN) STS clinical practice guidelines (55). Moreover, intramuscular margins should
not receive the same consideration as extra-compartmental margins.
Deep fascia, periosteum and fatty tissues around the vascular
bundles may act as a barrier, and thus, a few millimeters of
healthy tissue are enough to ensure margin-free resection.
Nevertheless, intracompartmental margins should be ≥1 cm for the
optimization of surgical resection.
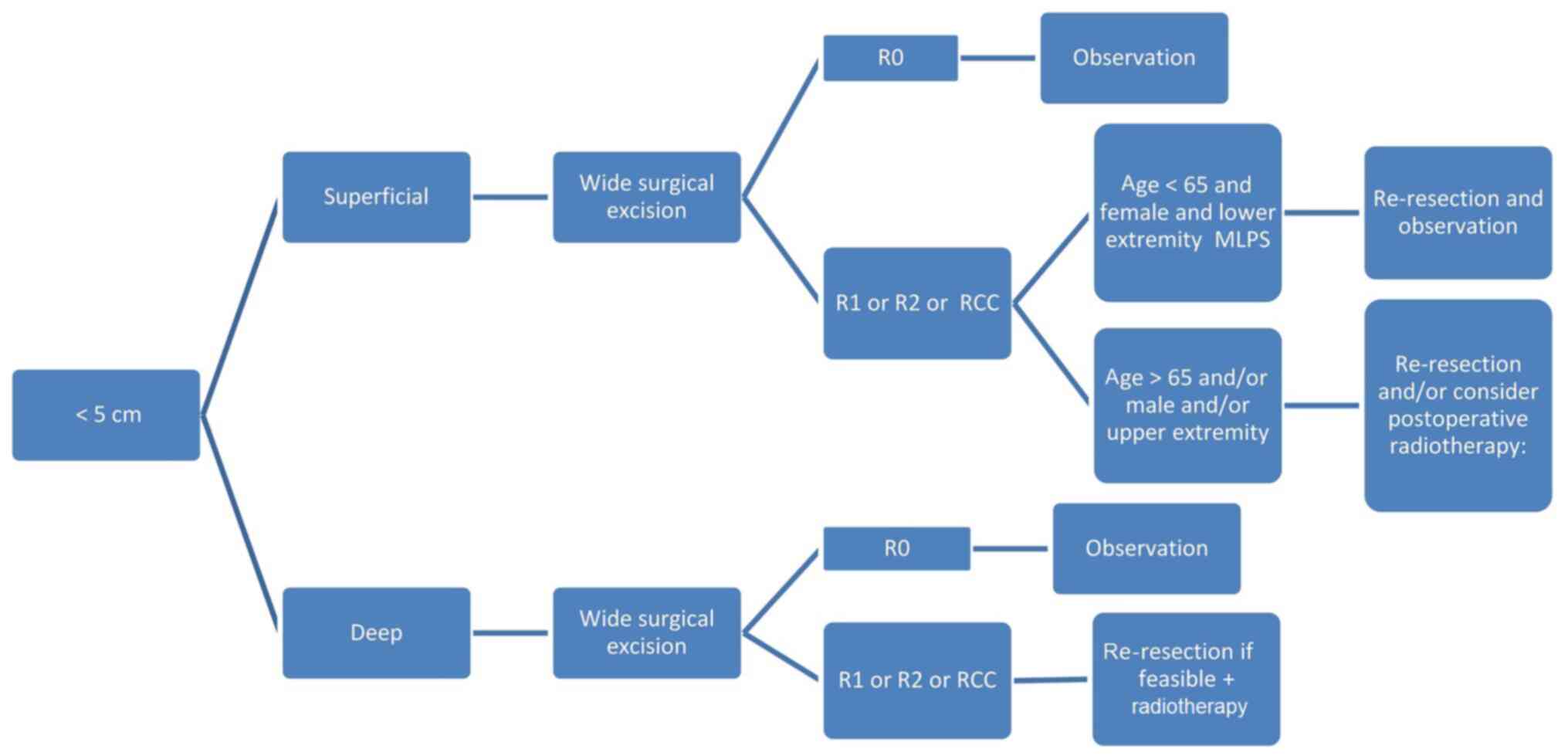
For the management of superficial MLPS <5 cm
(Fig. 1), R0 excision should be
adopted as the surgical procedure of choice. If the surgical
margins are disease-free (margins >1 cm or an intact fascial
plane), observation with frequent follow-ups is required,
regardless of age. This is reinforced by multiple studies
demonstrating that surgery without RT is the favored treatment type
to enable local control, especially if confirmed by negative-margin
resection in tumors <5 cm (6,11,12). If
the surgical margin shows positive microscopic margins (R1),
positive macroscopic margins (R2) or if pathology indicates RCMLPS,
the next actions are stratified as follows: i) Repeated resection
and observation if the patient is <65 years old, female and with
lower extremity MLPS; or ii) consider postoperative RT if the
patient is >65, male or has upper extremity MLPS (Fig. 1).
The analogy behind this management has been
demonstrated by several studies (11–14,18),
showing that age >65 years, female sex and lower extremity MLPS
were associated with lower LR-free survival, distant
metastasis-free survival, OS, DSS and local control, respectively
(6). Age >65 years is an
independent prognostic factor affecting local and distant
recurrence, as well as OS (12,18). In
fact, age is a well-defined negative prognostic factor for clinical
outcome in patients with MLPS, which can be attributed to
associated comorbidities, late tumor presentation and genomic
alterations present in older patients (55). Lower control rates on the upper
extremity are primarily attributed to the anatomical difficulty in
achieving R0 (12), which explains
the use of postoperative RT if positive surgical margins are
identified. The adopted postoperative RT regimen can be used for
high-grade STS alone or low grade tumors with the aforementioned
risk factors (NCCN clinical practice guidelines) (55).
If the MLPS size is <5 cm (Fig. 1), deep and with negative R0 margins
(i.e., wide local resection), the same management as superficial
MLPS is recommended, which is post-operative follow-up. This is
based on studies (7,12,20)
showing that tumor depth is not a prognostic factor, and does not
impact cancer-free survival in MLPS. However, if the tumor is deep
and with post-operative resection or R2 margins (i.e.
intralesional), or if it has an RCC, repeated resection is
recommended if feasible, as well as administration of postoperative
RT (Fig. 1).
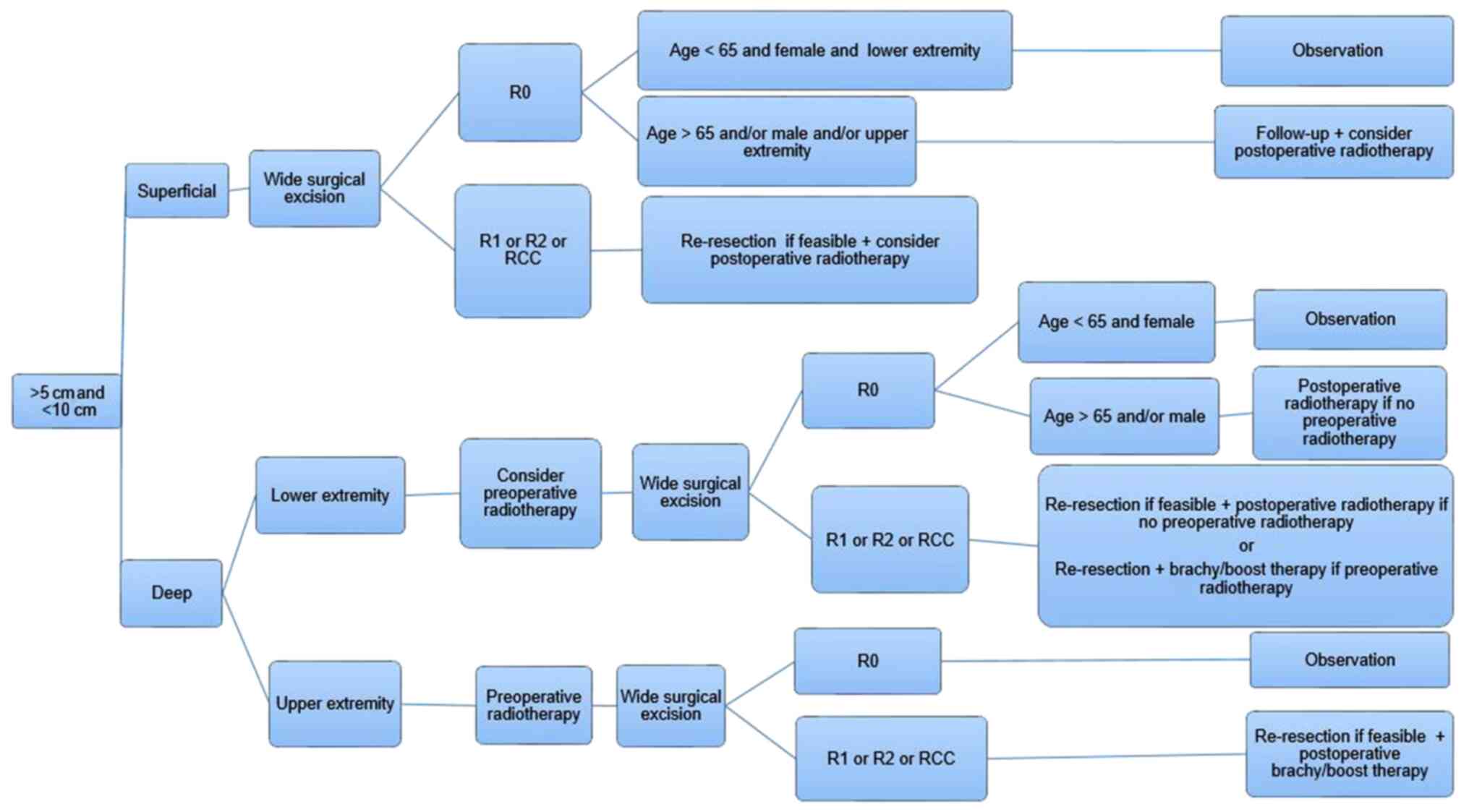
There is currently limited literature surrounding
the management of MLPS with a size between 5 and 10 cm. In the
current algorithm, surgery with negative-margin resection may be
enough to treat superficial tumors, but only in female patients
<65 years old with lower extremity tumors. Thus, postoperative
RT should be considered as an adjuvant treatment in all other
patients. If the surgical margins are positive (R1 or R2), or the
tumor has an RCC, repeat resection and postoperative RT should be
implemented, irrespective of age, sex or tumor location (Fig. 2).
If the tumor is deep and is in the lower
extremities, preoperative RT should be considered before R0
surgical excision. In patients who proceed directly to surgery,
only female patients <65 years old may not require postoperative
radiation if R0 is achieved. When the surgical margins are
positive, or if the tumor has an RCC, re-resection and
postoperative RT should be implemented, irrespective of age, sex
and tumor location. For patients that have received neoadjuvant
radiation, brachy/boost therapy should be administered to minimize
radiation exposure, while targeting residual margins and limiting
the possibility of LR (Fig. 2).
Due to the challenging aspects of resection, deep
tumors located in the upper extremities should first be treated
with preoperative RT, and then R0 surgical resection. If the
surgical margins are positive (R1 or R2) or the tumor has an RCC,
then repeated resection should be implemented if possible, as well
as postoperative brachy/boost therapy (Fig. 2).
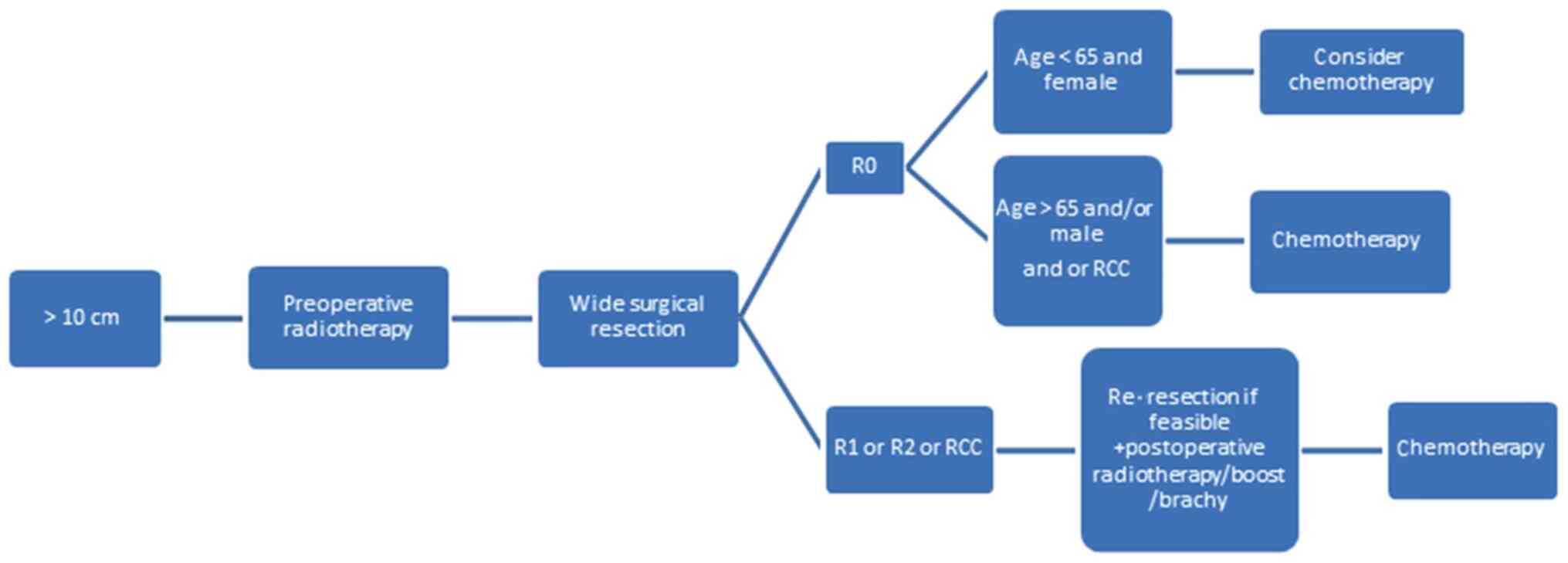
Finally, irrespective of depth and location, MLPSs
>10 cm in size should be managed with preoperative RT, which
minimizes the size of the tumor and permits R0 (Fig. 3). In patients with negative margins,
chemotherapy should be recommended, especially where an RCC is
identified or if the patient is male or aged >65 years. In
positive-margin resection, patients will require repeated resection
with brachy/boost therapy and chemotherapy (Fig. 3).
In the case of metastatic MLPS, all patients should
be treated with neoadjuvant chemotherapy (3). Recurrent MLPS should be addressed with
the same algorithm, but following the suggestions for the high-risk
category.
The present updated algorithm will guide the
surgeon towards a stepwise management plan based on clinical
evidence, risk and prognostic factors for the optimization of
decision making and OS. However, additional clinical trials are
warranted to better fortify therapeutic guidelines for the
treatment of MLPS. Furthermore, a meta-analysis would be a
beneficial addition to the current literature, as it would allow
for improved stratification of the level of evidence of the
available literature. Finally, a multidisciplinary approach,
including the surgeon, oncologist and radiation oncologist, is
critical to optimizing an individualized treatment plan for
patients with MLPS.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YT, AB, ASN and SS all contributed equally to the
study design, data collection and analysis, and writing or
reviewing of the manuscript. Data authentication is not applicable.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MLPS
|
myxoid liposarcoma
|
|
STS
|
soft tissue sarcoma
|
|
RC
|
round cell
|
|
RT
|
radiotherapy
|
|
LR
|
local recurrence
|
|
OS
|
overall survival
|
|
DFS
|
disease-free survival
|
|
DSS
|
disease-specific survival
|
|
R0
|
wide local resection
|
|
R1
|
marginal resection
|
|
R2
|
intralesional resection
|
|
FNAB
|
fine needle aspiration biopsy
|
|
RCC
|
RC component
|
|
NCCN
|
National Comprehensive Cancer
Network
|
References
|
1
|
Conyers R, Young S and Thomas DM:
Liposarcoma: Molecular genetics and therapeutics. Sarcoma.
2011:4831542010.PubMed/NCBI
|
|
2
|
De Vita A, Mercatali L, Recine F, Pieri F,
Riva N, Bongiovanni A, Liverani C, Spadazzi C, Miserocchi G,
Amadori D and Ibrahim T: Current classification, treatment options,
and new perspectives in the management of adipocytic sarcomas. Onco
Targets Ther. 9:6233–6246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moreau LC, Turcotte R, Ferguson P, Wunder
J, Clarkson P, Masri B, Isler M, Dion N, Werier J, Ghert M, et al:
Myxoidround cell liposarcoma (MRCLS) revisited: An analysis of 418
primarily managed cases. Ann Surg Oncol. 19:1081–1088. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Haniball J, Sumathi VP, Kindblom LG, Abudu
A, Carter SR, Tillman RM, Jeys L, Spooner D, Peake D and Grimer RJ:
Prognostic factors and metastatic patterns in primary
myxoid/round-cell liposarcoma. Sarcoma. 2011:5380852011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hoffman A, Ghadimi MP, Demicco EG,
Creighton CJ, Torres K, Colombo C, Peng T, Lusby K, Ingram D,
Hornick JL, et al: Localized and metastatic myxoid/round cell
liposarcoma: Clinical and molecular observations. Cancer.
119:1868–1877. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fiore M, Grosso F, Lo Vullo S,
Pennacchioli E, Stacchiotti S, Ferrari A, Collini P, Lozza L,
Mariani L, Casali PG and Gronchi A: Myxoid/round cell and
pleomorphic liposarcomas: Prognostic factors and survival in a
series of patients treated at a single institution. Cancer.
109:2522–2531. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zheng K, Yu XC, Xu M and Yang Y: Surgical
outcomes and prognostic factors of myxoid liposarcoma in
extremities: A retrospective study. Orthop Surg. 11:1020–1028.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Higgins JP, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343:d59282011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wells GA, Shea B, O'Connell D, Peterson J,
Welch V, Losos M and Tugwell P: The Newcastle-Ottawa scale (NOS)
for assessing the quality of non-randomized studies in
meta-analysis. 2000.
|
|
10
|
Dürr HR, Rauh J, Baur-Melnyk A, Knösel T,
Lindner L, Roeder F, Jansson V and Klein A: Myxoid liposarcoma:
local relapse and metastatic pattern in 43 patients. BMC Cancer.
18:3042018. View Article : Google Scholar
|
|
11
|
Wu J, Qian S and Jin L: Prognostic factors
of patients with extremity myxoid liposarcomas after surgery. J
Orthop Surg Res. 14:902019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nishida Y, Tsukushi S, Nakashima H and
Ishiguro N: Clinicopathologic prognostic factors of pure myxoid
liposarcoma of the extremities and trunk wall. Clin Orthop Relat
Res. 468:3041–3046. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lansu J, Bovée JVMG, Braam P, van Boven H,
Flucke U, Bonenkamp JJ, Miah AB, Zaidi SH, Thway K, Bruland ØS, et
al: Dose reduction of preoperative radiotherapy in myxoid
liposarcoma: A nonrandomized controlled trial. JAMA Oncol.
7:e2058652021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Greto D, Saieva C, Loi M, Terziani F,
Visani L, Garlatti P, Lo Russo M, Muntoni C, Becherini C, Topulli
J, et al: Influence of age and subtype in outcome of operable
liposarcoma. Radiol Med. 124:290–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kilpatrick SE, Doyon J, Choong PF, Sim FH
and Nascimento AG: The clinicopathologic spectrum of myxoid and
round cell liposarcoma. A study of 95 cases. Cancer. 77:1450–1458.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cohen HJ: Biology of aging as related to
cancer. Cancer. 74.(7 Suppl):S2092–S2100. 1994.
|
|
17
|
ten Heuvel SE, Hoekstra HJ, van Ginkel RJ,
Bastiaannet E and Suurmeijer AJ: Clinicopathologic prognostic
factors in myxoid liposarcoma: A retrospective study of 49 patients
with long-term follow-up. Ann Surg Oncol. 14:222–229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Antonescu CR, Tschernyavsky SJ, Decuseara
R, Leung DH, Woodruff JM, Brennan MF, Bridge JA, Neff JR, Goldblum
JR and Ladanyi M: Prognostic impact of P53 status, TLS-CHOP fusion
transcript structure, and histological grade in myxoid liposarcoma:
A molecular and clinicopathologic study of 82 cases. Clin Cancer
Res. 7:3977–3987. 2001.PubMed/NCBI
|
|
19
|
Smith TA, Easley KA and Goldblum JR:
Myxoid/round cell liposarcoma of the extremities. A
clinicopathologic study of 29 cases with particular attention to
extent of round cell liposarcoma. Am J Surg Pathol. 20:171–180.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Callegaro D, Miceli R, Bonvalot S,
Ferguson PC, Strauss DC, van Praag VVM, Levy A, Griffin AM, Hayes
AJ, Stacchiotti S, et al: Development and external validation of a
dynamic prognostic nomogram for primary extremity soft tissue
sarcoma survivors. EClinicalMedicine. 17:1002152019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gustafson P: Soft tissue sarcoma:
Epidemiology and prognosis in 508 patients. Acta Orthop Scand
Suppl. 259:2–31. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang HR, Hajdu SI, Collin C and Brennan
MF: The prognostic value of histologic subtypes in primary
extremity liposarcoma. Cancer. 64:1514–1520. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kilpatrick SE, Ward WG and Bos GD: The
value of fine-needle aspiration biopsy in the differential
diagnosis of adult myxoid sarcoma. Cancer. 90:167–177. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Evans HL: Liposarcoma a study of 55 cases
with a reassessment of its classification. Am J Surg Pathol.
3:507–523. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fiore M, Ford S, Callegaro D, Sangalli C,
Colombo C, Radaelli S, Frezza AM, Renne SL, Casali PG and Gronchi
A: Adequate local control in high-risk soft tissue sarcoma of the
extremity treated with surgery alone at a reference centre: Should
radiotherapy still be a standard? Ann Surg Oncol. 25:1536–1543.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spillane AJ, Fisher C and Thomas JM:
Myxoid liposarcoma-the frequency and the natural history of
nonpulmonary soft tissue metastases. Ann Surg Oncol. 6:389–394.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lemeur M, Mattei JC, Souteyrand P,
Chagnaud C, Curvale G and Rochwerger A: Prognostic factors for the
recurrence of myxoid liposarcoma: 20 cases with up to 8 years
follow-up. Orthop Traumatol Surg Res. 101:103–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bartlett EK, Curtin CE, Seier K, Qin LX,
Hameed M, Yoon SS, Crago AM, Brennan MF and Singer S: Histologic
subtype defines the risk and kinetics of recurrence and death for
primary extremity/truncal liposarcoma. Ann Surg. Jul 5–2019.(Epub
ahead of print). PubMed/NCBI
|
|
29
|
Yang JC, Chang AE, Baker AR, Sindelar WF,
Danforth DN, Topalian SL, DeLaney T, Glatstein E, Steinberg SM,
Merino MJ and Rosenberg SA: Randomized prospective study of the
benefit of adjuvant radiation therapy in the treatment of soft
tissue sarcomas of the extremity. J Clin Oncol. 16:197–203. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pisters P, Harrison LB, Leung D, Woodruff
JM, Casper ES and Brennan MF: Long-term results of a prospective
randomized trial of adjuvant brachytherapy in soft tissue sarcoma.
J Clin Oncol. 14:859–868. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Harrison LB, Franzese F, Gaynor JJ and
Brennan MF: Long-term results of a prospective randomized trial of
adjuvant brachytherapy in the management of completely resected
soft tissue sarcomas of the extremity and superficial trunk. Int J
Radiat Oncol Biol Phys. 27:259–265. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kattan MW, Leung DH and Brennan MF:
Postoperative nomogram for 12-year sarcoma-specific death. J Clin
Oncol. 20:791–796. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Orson GG, Sim FH, Reiman HM and Taylor WF:
Liposarcoma of the musculoskeletal system. Cancer. 60:1362–1370.
1987. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Reitan JB, Kaalhus O, Brennhovd IO, Sager
EM, Stenwig AE and Talle K: Prognostic factors in liposarcoma.
Cancer. 55:2482–2490. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Beane JD, Yang JC, White D, Steinberg SM,
Rosenberg SA and Rudloff U: Efficacy of adjuvant radiation therapy
in the treatment of soft tissue sarcoma of the extremity: 20-year
follow-up of a randomized prospective trial. Ann Surg Oncol.
21:2484–2489. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Engström K, Bergh P, Cederlund CG,
Hultborn R, Willen H, Aman P, Kindblom LG and Meis-Kindblom JM:
Irradiation of myxoid/round cell liposarcoma induces volume
reduction and lipoma-like morphology. Acta Oncol. 46:838–845. 2007.
View Article : Google Scholar
|
|
37
|
Knebel C, Lenze U, Pohlig F, Lenze F,
Harrasser N, Suren C, Breitenbach J, Rechl H, von Eisenhart-Rothe R
and Mühlhofer HML: Prognostic factors and outcome of liposarcoma
patients: A retrospective evaluation over 15 years. BMC Cancer.
17:4102017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
le Grange F, Cassoni A and Seddon B:
Tumour volume changes following pre-operative radiotherapy in
borderline resectable limb and trunk soft tissue sarcoma. Eur J
Surg Oncol. 40:394–401. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Guadagnolo BA, Zagars GK, Ballo MT, Patel
SR, Lewis VO, Benjamin RS and Pollock RE: Excellent local control
rates and distinctive patterns of failure in myxoid liposarcoma
treated with conservation surgery and radiotherapy. Int J Radiat
Oncol Biol Phys. 70:760–765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Oh YJ, Yi SY, Kim KH, Cho YJ, Beum SH, Lee
YH, Suh JS, Hur H, Kim KS, Kim SH, et al: Prognostic model to
predict survival outcome for curatively resected liposarcoma: A
multi-institutional experience. J Cancer. 7:1174–1180. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Muratori F, Bettini L, Frenos F,
Mondanelli N, Greto D, Livi L, Franchi A, Roselli G, Scorianz M,
Capanna R and Campanacci D: Myxoid liposarcoma: Prognostic factors
and metastatic pattern in a series of 148 patients treated at a
single institution. Int J Surg Oncol. 2018:89287062018.PubMed/NCBI
|
|
42
|
Gronchi A, Hindi N, Cruz J, Blay JY,
Lopez-Pousa A, Italiano A, Alvarez R, Gutierrez A, Rincón I,
Sangalli C, et al: Trabectedin and RAdiotherapy in soft tissue
sarcoma (TRASTS): Results of a phase I study in myxoid liposarcoma
from Spanish (GEIS), Italian (ISG), French (FSG) sarcoma groups.
EClinicalMedicine. 9:35–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Aiba H, Yamada S, Mizutani J, Yamamoto N,
Okamoto H, Hayashi K, Kimura H, Takeuchi A, Miwa S, Higuchi T, et
al: Preoperative evaluation of the efficacy of
radio-hyperthermo-chemotherapy for soft tissue sarcoma in a case
series. PLoS One. 13:e01952892018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Vos M, Koseła-Paterczyk H, Rutkowski P,
van Leenders GJLH, Normantowicz M, Lecyk A, Sleijfer S, Verhoef C
and Grünhagen DJ: Differences in recurrence and survival of
extremity liposarcoma subtypes. Eur J Surg Oncol. 44:1391–1397.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Toulmonde M, Bonvalot S, Méeus P, Stoeckle
E, Riou O, Isambert N, Bompas E, Jafari M, Delcambre-Lair C, Saada
E, et al: Retroperitoneal sarcomas: Patterns of care at diagnosis,
prognostic factors and focus on main histological subtypes: A
multicenter analysis of the French sarcoma group. Ann Oncol.
25:735–742. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Salduz A, Alpan B, Valiyev N, Özmen E,
İribaş A, Ağaoğlu F, Bayram A, Bilgiç B and Özger H: Neoadjuvant
radiotherapy for myxoid liposarcomas: Oncologic outcomes and
histopathologic correlations. Acta Orthop Traumatol Turc.
51:355–361. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chao AH, Mayerson JL, Chandawarkar R and
Scharschmidt TJ: Surgical management of soft tissue sarcomas:
Extremity sarcomas. J Surg Oncol. 111:540–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Eilber FR and Eckardt J: Surgical
management of soft tissue sarcomas. Semin Oncol. 24:526–533.
1997.PubMed/NCBI
|
|
49
|
Sahu A, Sagar R, Sarkar S and Sagar S:
Psychological effects of amputation: A review of studies from
India. Ind Psychiatry J. 25:4–10. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rosenberg SA, Tepper J, Glatstein E, Costa
J, Baker A, Brennan M, DeMoss EV, Seipp C, Sindelar WF, Sugarbaker
P and Wesley R: The treatment of soft-tissue sarcomas of the
extremities: Prospective randomized evaluations of (1) limb-sparing
surgery plus radiation therapy compared with amputation and (2) the
role of adjuvant chemotherapy. Ann Surg. 196:305–315. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chung PW, Deheshi BM, Ferguson PC, Wunder
JS, Griffin AM, Catton CN, Bell RS, White LM, Kandel RA and
O'Sullivan B: Radiosensitivity translates into excellent local
control in extremity myxoid liposarcoma: A comparison with other
soft tissue sarcomas. Cancer. 115:3254–3261. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Endo M and Lin PP: Surgical margins in the
management of extremity soft tissue sarcoma. Chin Clin Oncol.
7:372018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Enneking WF, Spanier SS and Goodman MA: A
system for the surgical staging of musculoskeletal sarcoma. Clin
Orthop Relat Res. 106–120. 1980.PubMed/NCBI
|
|
54
|
Skorpil M, Rydén H, Wejde J, Lidbrink E,
Brosjö O and Berglund J: The effect of radiotherapy on fat content
and fatty acids in myxoid liposarcomas quantified by MRI. Magn
Reson Imaging. 43:37–41. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
von Mehren M, Randall RL, Benjamin RS,
Boles S, Bui MM, Ganjoo KN, George S, Gonzalez RJ, Heslin MJ, Kane
JM, et al: Soft tissue sarcoma, version 2.2018, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
16:536–563. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Pitson G, Robinson P, Wilke D, Kandel RA,
White L, Griffin AM, Bell RS, Catton CN, Wunder JS and O'Sullivan
B: Radiation response: An additional unique signature of myxoid
liposarcoma. Int J Radiat Oncol Biol Phys. 60:522–526. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chowdhry V, Goldberg S, DeLaney TF, Cote
GM, Chebib I, Kim J, Lozano-Calderón SA and De Amorim Bernstein K:
Myxoid liposarcoma: Treatment outcomes from chemotherapy and
radiation therapy. Sarcoma. 2018:80291572018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hatano H, Ogose A, Hotta T, Kawashima H,
Sugita T, Sasamoto R and Endo N: Treatment of myxoid liposarcoma by
marginal or intralesional resection combined with radiotherapy.
Anticancer Res. 23:3045–3049. 2003.PubMed/NCBI
|
|
59
|
Davis AM, O'Sullivan B, Turcotte R, Bell
R, Catton C, Chabot P, Wunder J, Hammond A, Benk V, Kandel R, et
al: Late radiation morbidity following randomization to
preoperative versus postoperative radiotherapy in extremity soft
tissue sarcoma. Radiother Oncol. 75:48–53. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wortman JR, Tirumani SH, Tirumani H,
Shinagare AB, Jagannathan JP, Hornick JL and Ramaiya NH:
Neoadjuvant radiation in primary extremity liposarcoma: Correlation
of MRI features with histopathology. Eur Radiol. 26:1226–1234.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Roberge D, Skamene T, Nahal A, Turcotte
RE, Powell T and Freeman C: Radiological and pathological response
following pre-operative radiotherapy for soft-tissue sarcoma.
Radiother Oncol. 97:404–407. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
de Vreeze RS, de Jong D, Haas RL, Stewart
F and van Coevorden F: Effectiveness of radiotherapy in myxoid
sarcomas is associated with a dense vascular pattern. Int J Radiat
Oncol Biol Phys. 72:1480–1487. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mullen JT, Hornicek FJ, Harmon DC, Raskin
KA, Chen YL, Szymonifka J, Yeap BY, Choy E, DeLaney TF and Nielsen
GP: Prognostic significance of treatment-induced pathologic
necrosis in extremity and truncal soft tissue sarcoma after
neoadjuvant chemoradiotherapy. Cancer. 120:3676–3682. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lazarev S, McGee H, Moshier E, Ru M,
Demicco EG and Gupta V: Preoperative vs postoperative radiation
therapy in localized soft tissue sarcoma: Nationwide patterns of
care and trends in utilization. Pract Radiat Oncol. 7:e507–e516.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zagars GK, Ballo MT, Pisters PW, Pollock
RE, Patel SR and Benjamin RS: Preoperative vs. postoperative
radiation therapy for soft tissue sarcoma: A retrospective
comparative evaluation of disease outcome. Int J Radiat Oncol Biol
Phys. 56:482–488. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
O'sullivan B and Davis AM: A randomized
phase III trial of pre-operative compared to post-operative
radiotherapy in extremity soft tissue sarcoma. Int J Radiat Oncol
Biol Phys. 51:151–152. 2001. View Article : Google Scholar
|
|
67
|
Alektiar K, Brennan M and Singer S: Local
control comparison of IMRT vs. brachytherapy in primary high-grade
extremity Sarcoma. Int J Radiat Oncol Biol Phys. 75:S652009.
View Article : Google Scholar
|
|
68
|
Adjuvant chemotherapy for localised
resectable soft-tissue sarcoma of adults, . Meta-analysis of
individual data. Sarcoma meta-analysis collaboration. Lancet.
350:1647–1654. 1997.PubMed/NCBI
|
|
69
|
Katz D, Boonsirikamchai P, Choi H, Lazar
AJ, Wang WL, Xiao L, Park MS, Ravi V, Benjamin RS and Araujo DM:
Efficacy of first-line doxorubicin and ifosfamide in myxoid
liposarcoma. Clin Sarcoma Res. 2:22012. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Prestwich RJ, Taylor RE and Grimer R:
Metastatic myxoid liposarcoma: Aggressive multimodality management.
Clin Oncol (R Coll Radiol). 17:1302005. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Kerr LT, Donoghue JF, Wilding AL and Johns
TG: Axitinib has antiangiogenic and antitumorigenic activity in
myxoid liposarcoma. Sarcoma. 2016:34846732016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Gronchi A, Hindi N, Blay JY, Redondo A,
Sanfilippo R, Morosi C, Jurado JC, Fra PL, Martinez-Trufero J,
Morales CMV, et al: Trabectedin and radiotherapy in soft-tissue
sarcoma (TRASTS) study: An international, prospective, phase II
trial in localized myxoid liposarcoma-A collaborative Spanish
(GEIS), Italian (ISG) and French (FSG) group study. J Clin Oncol.
38 (15 Supppl):S115142020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Chen M and Kirsch DG: Safely combining
trabectedin with radiotherapy to treat myxoid liposarcoma.
EClinicalMedicine. 9:5–6. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Wakely PE Jr, Geisinger KR, Cappellari JO,
Silverman JF and Frable WJ: Fine-needle aspiration cytopathology of
soft tissue: Chondromyxoid and myxoid lesions. Diagn Cytopathol.
12:101–105. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Kilpatrick SE and Ward WG:
Myxofibrosarcoma of soft tissues: Cytomorphologic analysis of a
series. Diagn Cytopathol. 20:6–9. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Asano N, Susa M, Hosaka S, Nakayama R,
Kobayashi E, Takeuchi K, Horiuchi K, Suzuki Y, Anazawa U, Mukai M,
et al: Metastatic patterns of myxoid/round cell liposarcoma: A
review of a 25-year experience. Sarcoma. 2012:3451612012.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Kim HS, Lee J, Yi SY, Jun HJ, Choi YL, Ahn
GH, Seo SW, Lim DH, Ahn YC, Park JO and Kim SJ: Liposarcoma:
Exploration of clinical prognostic factors for risk based
stratification of therapy. BMC Cancer. 9:2052009. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Rydholm A and Rööser B: Surgical margins
for soft-tissue sarcoma. J Bone Joint Surg Am. 69:1074–1078. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Trovik CS: Local recurrence of soft tissue
sarcoma: A Scandinavian sarcoma group project. Acta Orthop Scand.
72:1–27. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Kawaguchi N, Matumoto S and Manabe J: New
method of evaluating the surgical margin and safety margin for
musculoskeletal sarcoma, analysed on the basis of 457 surgical
cases. J Cancer Res Clin Oncol. 121:555–563. 1995. View Article : Google Scholar : PubMed/NCBI
|