Introduction
Thyroid carcinoma is a tumor of the endocrine system
with increasing incidence and morbidity (1,2). The
most common type of thyroid carcinoma is papillary thyroid
carcinoma (PTC), which accounts for ~80% of all thyroid carcinoma
cases (3). Hormonotherapy and
surgical resection are considered to be the two primary approaches
for treating thyroid carcinoma (4,5).
Increasing evidence has suggested that pseudogenes
consist of functional rather than junk DNA (6,7), and an
estimated 18,000 pseudogenes are present in the human genome
(8). Pseudogenes can regulate gene
expression at multiple levels, and are involved in various
biological processes, including cancer development and progression
(9). The BRAF pseudogene has been
shown to act as a competing endogenous RNA (ceRNA) in lymphoma
(10). Additionally, other studies
have demonstrated that both pituitary tumor-transforming 3
pseudogene and protein disulfide isomerase family A member 3
pseudogene 1 are upregulated in hepatocellular carcinoma (11,12),
while double homeobox A pseudogene (DUXAP) 8 promotes non-small
cell lung cancer (NSCLC) cell proliferation and invasiveness
(13). Liao et al (14) revealed that pseudogene legumain
(LGMN) promoted glioblastoma by sponging microRNA (miR)-495-3p.
However, the effects of pseudogene LGMN on thyroid carcinoma
progression remain elusive.
MicroRNAs (miRNAs/miRs) are small non-coding RNAs,
20–22 nucleotides in length (15,16).
Pathologically, miRNAs can regulate gene expression during cancer
progression (17,18). Ye et al (19) indicated that miR-204 inhibited
thyroid cancer progression, and miR-338 was found to suppress
thyroid carcinoma tumorigenesis by targeting Ras-associated binding
protein 23 (20). Additional studies
have also revealed that miR-495 regulates glioblastoma and
colorectal cancer progression (14,21).
Autophagy is a lysosome-mediated cellular
degradation process (22), exerting
opposing roles in cancer. ATG3 and p62 serve keys roles in cancer
by regulating the autophagy pathway (22). Autophagy has been reported to promote
apoptosis, but also to enhance chemotherapeutic resistance
(23–25). Another study suggested that autophagy
could initiate and contribute to thyroid carcinoma (26). Therefore, in the current study, we
hypothesized that autophagy may be a potential effector for LGMN in
thyroid carcinoma.
The present study aimed to investigate the role of
LGMN in thyroid carcinoma. Determining the molecular mechanism
underlying the effects of LGMN on thyroid carcinoma progression may
provide novel insights into the development of targeted therapies
for this pathology.
Materials and methods
Cell culture
The human thyroid carcinoma cell lines, CAL-62 and
SW579, were obtained from The Cell Bank of Type Culture Collection
of The Chinese Academy of Sciences. The immortalized normal human
thyroid follicular epithelial cell line, Nthy-ori3-1, was purchased
from CoBioer Biosciences Co., Ltd. (cat. no. CBP61205). HUVECs were
purchased from Shanghai Zhong Qiao Xin Zhou Biotechnology Co., Ltd.
HUVECs, CAL-62 and SW579 cells were cultured in DMEM supplemented
with 1% penicillin/streptomycin and 10% FBS, at 37°C (5%
CO2) in a humidified atmosphere. Nthy-ori3-1 cells were
maintained in RPMI-1640 with 10% FBS and 1%
penicillin/streptomycin.
Construction of stable cell lines
293T cells (The Cell Bank of Type Culture Collection
of The Chinese Academy of Sciences) were transfected with 1 µg
pLKO.1-LGMN, 0.5 µg pVSVG (Addgene, Inc.) and 0.5 µg pPAX2
(Addgene, Inc.) plasmids, and the resulting 2nd generation
lentiviruses were collected after 36–48 h of transfection. The
viruses (MOI=4) were used to infect CAL-62 and SW579 cells.
Following incubation for 24 h, cells were cultured in the presence
of 2 µg/ml puromycin for 1 week to obtain resistant cells.
Puromycin (1 µg/ml) were used for maintenance. The short hairpin
RNA (shRNA) sequences used were as follows: Sh-negative control
(NC), 5′-AUUCGGUUCAAGGUCCAUUGGG-3′; sh-LGMN-1,
5′-CCUGCCGGAUAACAUCAAU-3′; and sh-LGMN-2,
5′-CGUGGAAGAUCUGACUAAA-3′.
Transfection
Autophagy-related gene 3 (ATG3) cDNA (Genscript
Biotech) or LGMN (Genscript Biotech) was subcloned into the
pcDNA3.1 vector (Addgene, Inc.), 1 µg pcDNA3.1-ATG3 or
pcDNA3.1-LGMN vector was used to transfect CAL-62 and SW579 cells
with Lipofectamine® 2000 (Thermo Fisher Scientific,
Inc.) at room temperature. Following transfection for 24 h, the
cells were harvested for further analysis. NC mimic (50 µM),
miR-495 mimic (50 µM), NC inhibitor (50 µM), miR-495 inhibitor (50
µM) were transfected into CAL-62 and SW579 cells with
Lipofectamine® RNAiMAX (Thermo Fisher Scientific, Inc.)
at room temperature. The interval transfection and subsequent
experiments was 48 h. NC mimic, 5′-CAUUCAUCCAUCAAUCGGGCAGGCCUU−3′;
miR-495 mimic, 5′-UUACCGAUCCAAUUUCCGGACGGUUAC−3′; NC inhibitor,
5′-UUCAGGCAAUCCAAAUGCAGG−3′; and miR-495 inhibitor,
5′-AAUGGGACUUCCAUCGGAAUCCU-3′.
Colony formation assay
A total of 2×104 cells were seeded into
each well of a 6-well plate, and cultured for ~1 week. Following
colony formation, the cells were stained with 0.5% crystal violet
at room temperature for 1–2 h.
Transwell invasion assay
CAL-62 and SW579 cells were seeded into the upper
chambers of Matrigel-precoated inserts (BD Biosciences), at a
density of 1×105 cells in 250 µl serum-free medium. In
addition, 300 µl medium supplemented with 20% FBS was added to the
bottom chamber as a chemoattractant. Following incubation at 37°C
for 24 h, invasive cells were stained with 0.05% crystal violet
solution at room temperature for 1–2 h. A light microscope (CKX53;
Olympus Corporation) was used to observe migratory cells in lower
chamber (magnification, ×100).
Cell Counting Kit 8 (CCK-8) assay
CAL-62 and SW579 cells were seeded into 96-well
plates at a density of 1×103 cells/well. The cells were
then transfected with the indicated oligonucleotides and incubated
for different lengths of time. Subsequently, the cells were
incubated with 10 µl CCK-8 reagent (Vazyme Biotech Co., Ltd.) for 3
h at 37°C (5% CO2), after which the absorbance of each
well was determined at a wavelength of 450 nm.
Western blot analysis
Total protein were extracted by RIPA buffer (Beijing
Solarbio Science & Technology Co. Ltd.) from CAL-62 and SW579
cells. Protein concentration was determined by bicinchoninic acid
(BCA) method and ~50 µg/lane of protein was separated by 10%
SDS-PAGE, prior to transfer onto PVDF membranes. Following blocking
with 5% non-fat milk at room temperature for 1 h, the membranes
were incubated with primary antibodies overnight at 4°C. The
following primary antibodies were used: Anti-ATG3 (cat. no.
PA5-17018; 1:1,000; Thermo Fisher Scientific, Inc.), anti-p62 (cat.
no. ab155686; 1:1,000; Abcam) and anti-GAPDH (cat. no. 8884;
1:2,000; Cell Signaling Technology, Inc.). The membranes were then
incubated with the following secondary antibodies at room
temperature for 1 h: Anti-mouse HRP-conjugated IgG (cat. no. 7076;
1:3,000) or anti-rabbit HRP-conjugated IgG (cat. no. 7074;
dilution, 1:3,000) (both Cell Signaling Technology, Inc.). Enhanced
chemiluminescent (ECL) reagent (ThermoFisher Scientific Inc.) was
used to probe protein bands, which was visualized by ChemiDoc™ MP
and Image Lab v.4.1 software (Bio-Rad Laboratories, Inc.).
The Cancer Genome Atlas (TCGA)
analysis
The gene expression data from thyroid carcinoma
samples, including 512 tumor and 337 normal tissues, were
downloaded from TCGA (https://tcga.xenahubs.net). Comparisons between the
expression levels of LGMN were conducted using Student's t-test
(unpaired, two-tailed).
Tube formation assay
Growth factor-reduced Matrigel was used to coat
24-well plates at 37°C for 4–5 h. Subsequently, HUVECs at a density
of 3×103 cells/well were seeded into the coated plates
in conditioned medium (Ham's F-12K + 0.1 mg/ml Heparin + 0.03–0.05
mg/ml ECGs + 10% FBS + 1% P/S). Tube formation was observed under a
light microscope (magnification, ×200).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA was extracted from CAL-62 and SW579 cells
using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). A total of 1 µg RNA was reverse transcribed into
cDNA using the PrimeScript RT reagent kit (Takara Bio, Inc.)
according to the manufacturer's instructions. qPCR was carried out
with SYBR Green kit (Roche Applied Science) to determine relative
gene expression, and quantified using the 2−ΔΔCq method
(27). The following thermocycling
conditions were used: Initial denaturation at 95°C for 5 min,
followed by 40 cycles of 95°C for 30 sec, 60°C for 30 sec and 72°C
for 30 sec. The primer sequences used were as follows: LGMN
forward, 5′-GTTGAGAGGCGATGCAGAAG-3′ and reverse,
5′-GCAGGTGGTTCATCATGGAC-3′; ATG3 forward,
5′-CAATGGGCTACAGGGGAAGA-3′ and reverse, 5′-ATCCGCCATCACCATCATCT-3′;
p62 forward, 5′-CACAGAGGAGAAGAGCAGCT-3′ and reverse,
5′-TGGAGTTCACCTGTAGACGG-3′; miR-495 forward,
5′-ACCTGAAAAGAAGTTGCCCA-3′ and reverse,
5′-GCACCATGTTTGTTTCGTCAC−3′; and β-actin forward,
5′-ACTCTTCCAGCCTTCCTTCC-3′ and reverse,
5′-CGTACAGGTCTTTGCGGATG-3′.
Statistical analysis
GraphPad Prism 8.0 software (GraphPad Software,
Inc.) was used to compare the differences between two groups, using
unpaired Student's t-test. The differences among multiple groups
were assessed using one-way ANOVA followed by Tukey's post hoc
test. The data are expressed as the mean ± SD, and P<0.05 was
considered to indicate a statistically significant difference.
Results
LGMN depletion attenuates thyroid
carcinoma progression
To investigate the tumorigenic role of LGMN in
thyroid carcinoma, the shRNA gene knockdown approach was employed.
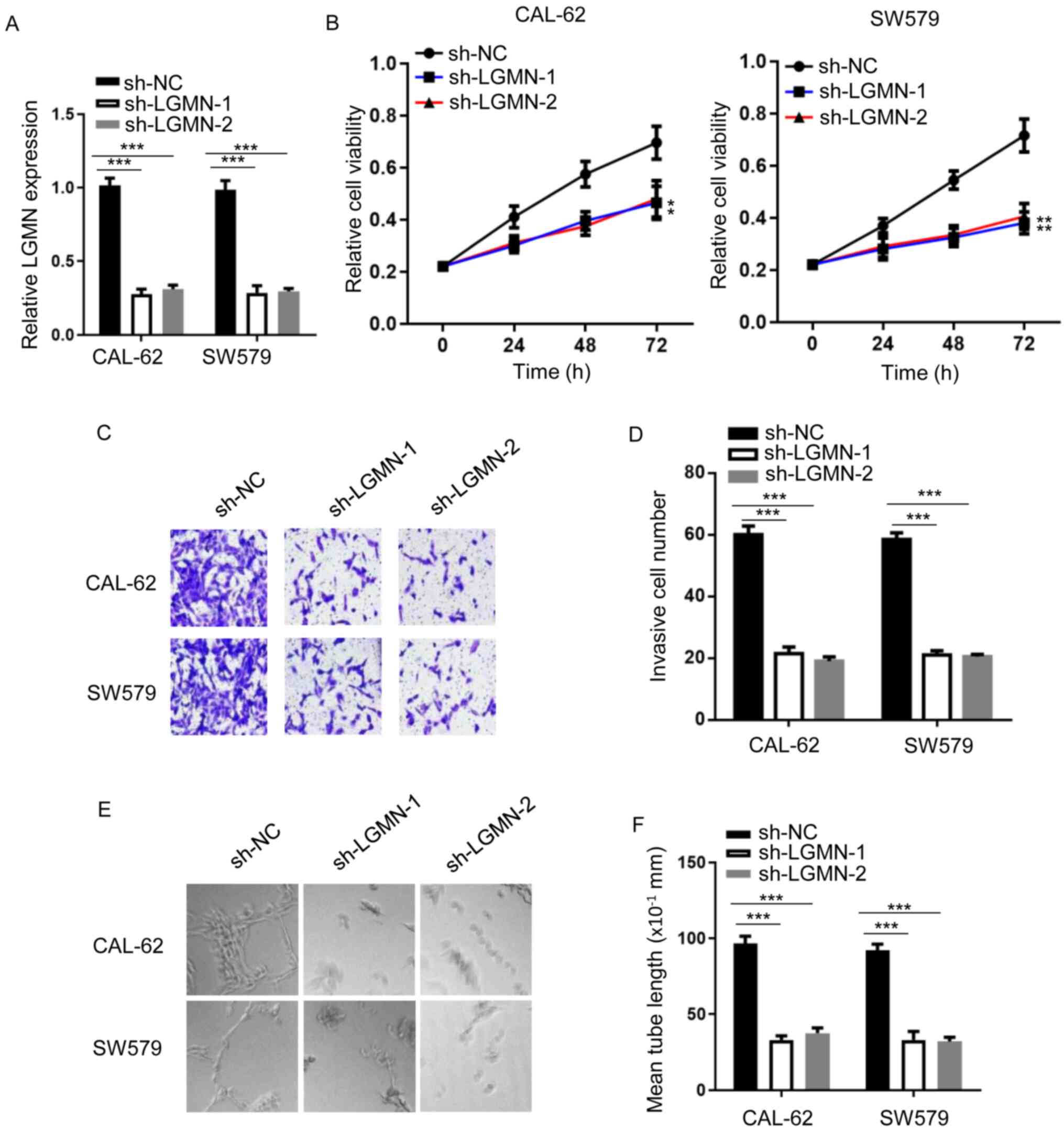
shRNAs against LGMN were synthesized, and RT-qPCR analysis was used
to confirm knockdown efficiency (Fig.
1A). LGMN-knockdown notably impaired CAL-62 and SW579 cell
proliferation (Fig. 1B). Similarly,
LGMN-knockdown decreased the invasive capacity of both cell lines
(Fig. 1C and D). Furthermore, a tube
formation assay was conducted to evaluate the tube formation
ability of HUVECs treated with conditioned medium from thyroid
carcinoma cells. The results showed that LGMN-knockdown attenuated
the tube formation ability of HUVECs compared with the sh-NC group
(Fig. 1E and F). These findings
suggested that LGMN depletion inhibited the tumor phenotype of
thyroid carcinoma cells.
LGMN is associated with thyroid
carcinoma
To investigate the potential effect of pseudogene
LGMN on thyroid carcinoma, clinical TCGA datasets of thyroid
carcinoma samples were obtained and analyzed using bioinformatics
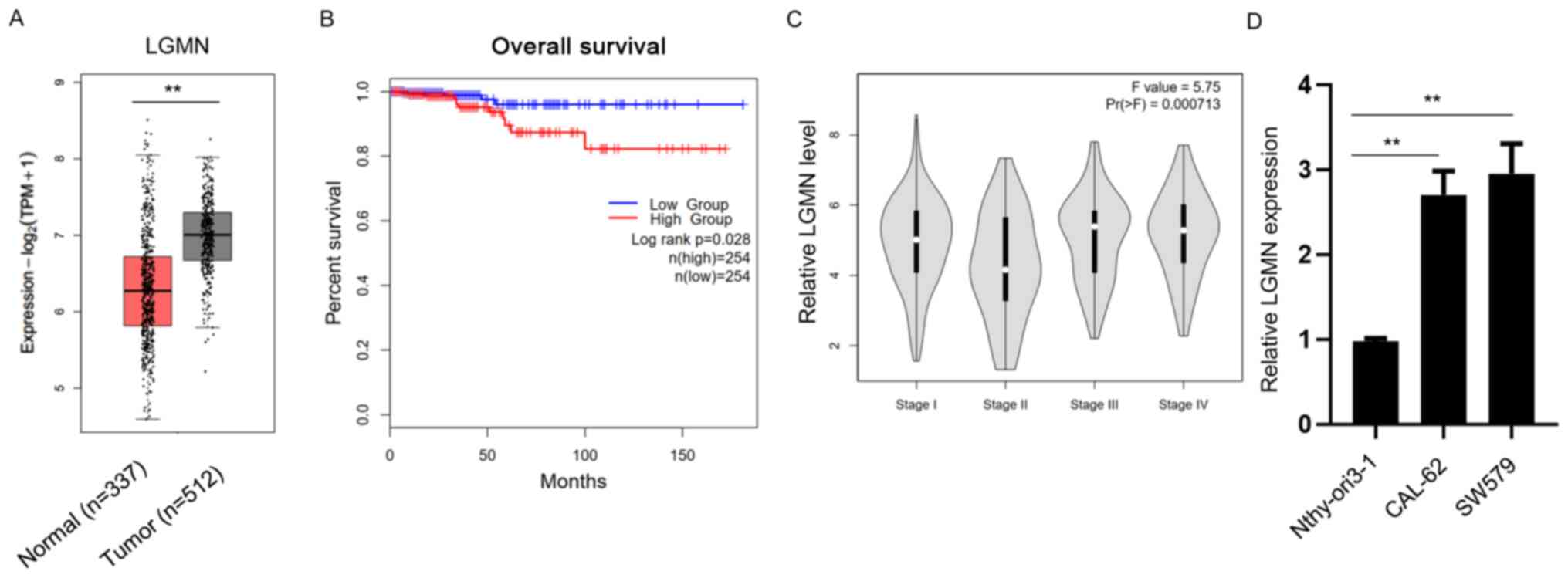
approaches. Bioinformatics analysis revealed that LGMN was
significantly upregulated in thyroid carcinoma tissues compared
with normal tissues (Fig. 2A).
Furthermore, the overall survival (OS) rate was evaluated in the
high- and low-LGMN expression groups. The results showed that the
OS rate was higher in patients in the low-LGMN group compared with
those in the high-LGMN group (Fig.
2B). Additionally, the analysis indicated that higher LGMN
expression levels were associated with higher grades of thyroid
carcinoma (Fig. 2C). Subsequently,
RT-qPCR analysis was performed to detect the expression levels of
LGMN in the normal thyroid epithelial cell line (Nthy-ori3-1) and
thyroid carcinoma CAL-62 and SW579 cells. The results demonstrated
that LGMN was significantly upregulated in thyroid carcinoma cell
lines (Fig. 2D). The aforementioned
findings suggested that LGMN promoted the development of thyroid
carcinoma.
miR-495 modulates autophagy
miRNAs have been reported to act as interacting
factors for pseudogenes in various cancer types (28,29). A
study reported that miR-495 could sponge LGMN in glioblastoma
(14). Herein, RT-qPCR analysis was
performed to determine the expression levels of miR-495 in CAL-62
and SW579 cells transfected with sh-NC, sh-LGMN-1 or sh-LGMN-2. The
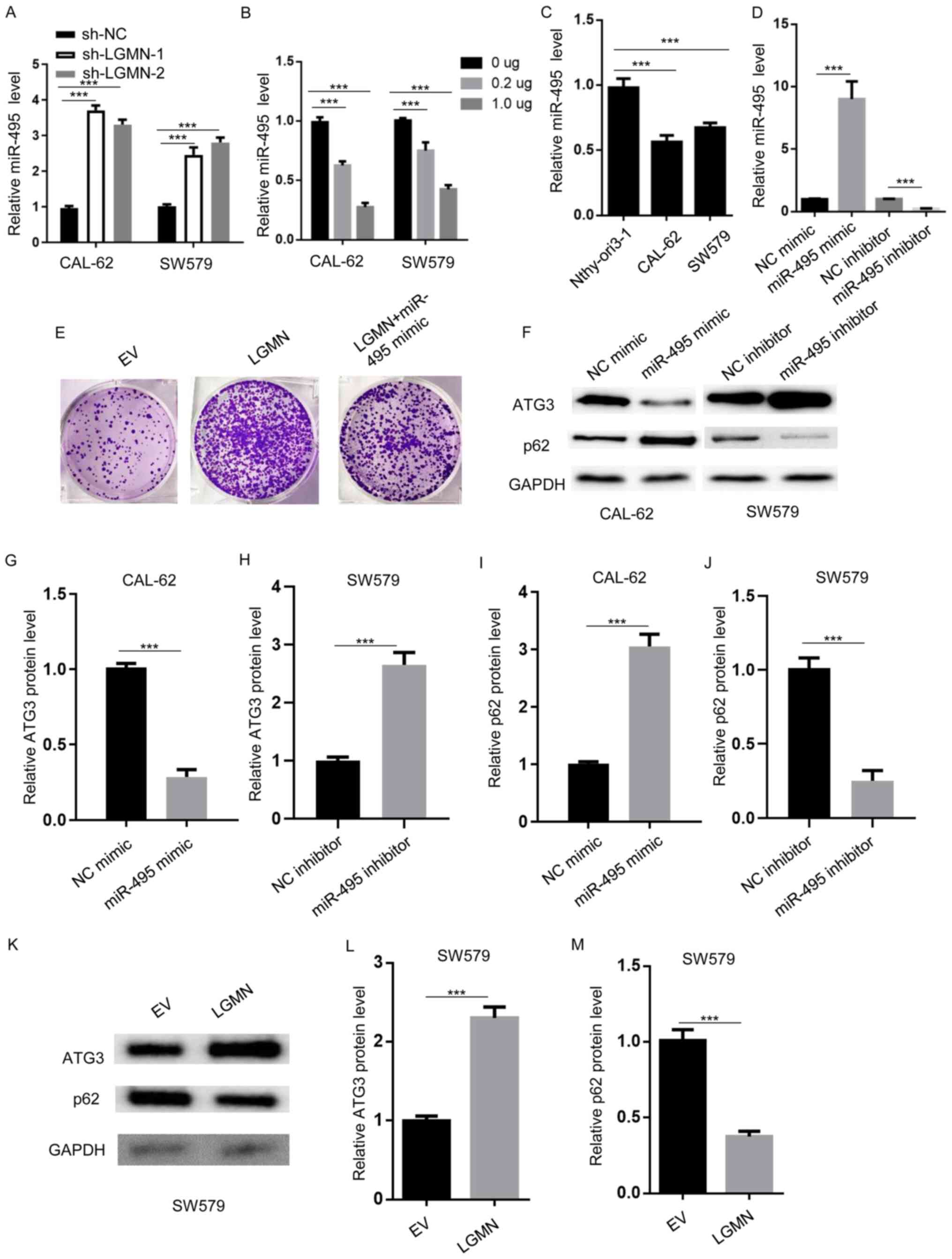
results revealed that miR-495 was significantly upregulated
following LGMN-knockdown (Fig. 3A).
In addition, following LGMN-overexpression in CAL-62 and SW579
cells, RT-qPCR showed that miR-495 levels were downregulated in a
dose-dependent manner (Fig. 3B).
Moreover, miR-495 expression levels were lower in thyroid carcinoma
CAL-62 and SW579 cells compared with normal thyroid epithelial
cells (Nthy-ori3-1) (Fig. 3C).
miR-495 levels were upregulated in miR-495 mimic-transfected, and
downregulated in miR-495 inhibitor-transfected CAL-62 cells
(Fig. 3D). In order to determine
whether miR-495 acts as key mediator for LGMN-regulated
tumorigenesis, a colony formation assay was conducted to
demonstrated that LGMN overexpression increased the number of
CAL-62-cell colonies compared with the control, which was
attenuated by miR-495 mimic transfection (Fig. 3E).
 | Figure 3.miR-495 modulates autophagy. (A)
RT-qPCR analysis was performed to determine the miR-495 expression
levels in CAL-62 and SW579 cells transfected with sh-NC, sh-LGMN-1
or sh-LGMN-2. ***P<0.001. (B) RT-qPCR analysis of miR-495
expression levels in CAL-62 and SW579 cells transfected with the
indicated amounts of pcDNA3.1-LGMN. ***P<0.001. (C) RT-qPCR was
performed to establish the miR-495 expression levels in normal
thyroid epithelial cells (Nthy-ori3-1) and thyroid carcinoma cells
(CAL-62 and SW579). ***P<0.001. (D) RT-qPCR analysis of miR-495
expression levels in CAL-62 cells transfected with NC mimics,
miR-495 mimics, NC inhibitor and miR-495 inhibitor. ***P<0.001.
(E) Colony formation assay to determine the colony number of CAL-62
cells transfected with EV (pcDNA3.1), pcDNA3.1-LGMN, or
pcDNA3.1-LGMN plus miR-495 mimics. (F-J) Western blot analysis was
performed to measure the protein expression levels of ATG3 and p62
in CAL-62 cells transfected with NC or miR-495 mimics, and SW579
cells transfected with NC or miR-495 inhibitors. ***P<0.001.
(K-M) Western blot analysis was carried out to determine the
protein expression levels of ATG3 and p62 in SW579 cells
transfected with EV (pcDNA3.1) or pcDNA3.1-LGMN. ***P<0.001.
Experiments were performed for three biological replicates; western
blotting was performed once (n=1). miR-495, microRNA-495; LGMN,
pseudogene legumain; RT-q, reverse transcription-quantitative; sh-,
short hairpin RNA; NC, negative control; ATG3, autophagy-related
gene 3; EV, empty vector. |
Subsequently, the present study aimed to identify
the potential downstream effectors of miR-495. A previous report
revealed that miR-495 attenuated ATG3 expression and promoted that
of p62 (30). Therefore, western
blot analysis was conducted to determine whether miR-495 could
regulate the protein expression levels of ATG3 and p62 in thyroid
carcinoma. To investigate whether miR-495 had a role in regulating
autophagy in both CAL-62 and SW579 cells, western blotting was
conducted in both cell lines using mimic or inhibitor. The results
demonstrated that transfection with miR-495 mimics markedly reduced
ATG3 and promoted p62 expression in CAL-62 cells, while the miR-495
inhibitor exerted opposing effects in SW579 cells (Fig. 3F-J). Furthermore, LGMN overexpression
increased ATG3, and suppressed p62 expression in SW579 cells
(Fig. 3K-M). Collectively, these
results indicated that miR-495 was modulated by LGMN to regulate
autophagy signaling.
Autophagy serves a crucial role in
miR-495-regulated thyroid carcinoma progression
Since the miR-495/autophagy axis may act as an
effector of LGMN-mediated thyroid carcinoma progression, the
current study aimed to further investigate the role of autophagy in
the LGMN-modulated thyroid carcinoma phenotype. Firstly, the
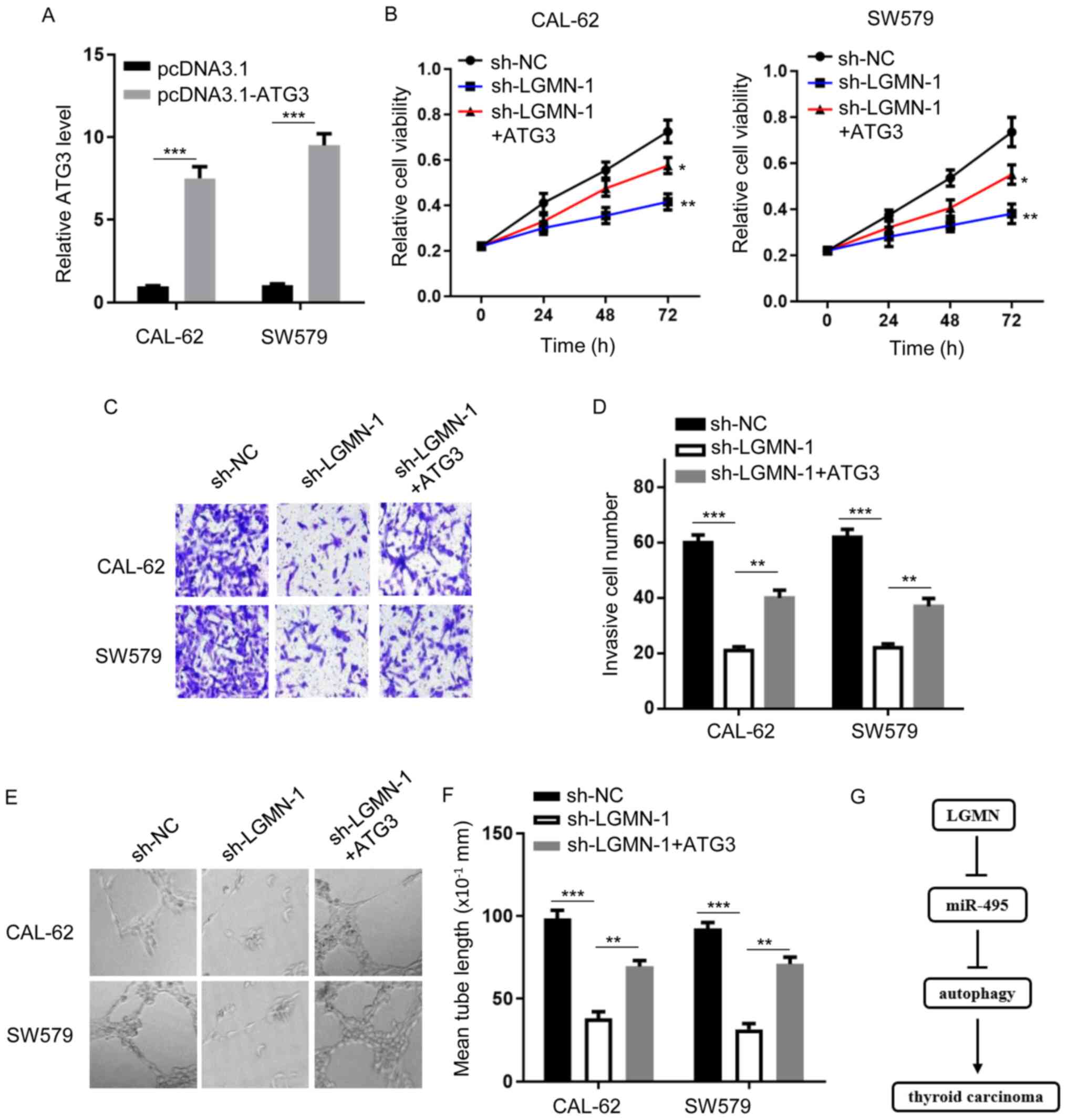
expression level of ATG3 was evaluated in CAL-62 and SW579 cells
transfected with or without an ATG3 overexpression plasmid
(Fig. 4A). CCK-8 and Transwell
assays showed that LGMN-knockdown attenuated cellular proliferation
and invasiveness, respectively, whereas ATG3 overexpression rescued
the effects of LGMN silencing, to a certain degree (Fig. 4B-D). In addition, impaired tube
formation ability was observed in HUVECs transfected with sh-LGMN,
which was reversed by ATG3 overexpression (Fig. 4E and F). Overall, the results
indicate that autophagy serves a critical role in
LGMN/miR-495-regulated thyroid carcinoma progression.
Discussion
The results of the present study suggested that LGMN
plays a promotive role in the development of the thyroid carcinoma
phenotype by modulating the proliferation, invasion and tube
formation abilities of HUVECs. Notably, miR-495 and autophagy were
identified as downstream effectors of LGMN in thyroid carcinoma
cells (Fig. 4G). Therefore, the
current study proposes a potential novel mechanism underlying
thyroid carcinoma progression.
PTC accounts for ~80% of all thyroid carcinoma cases
(10,31). Currently, the available diagnostic
and therapeutic approaches for thyroid carcinoma remain
ineffective, and limited targeted therapy approaches have been
developed. The results of the present study suggested that LGMN may
be used as a biomarker to facilitate the diagnosis of thyroid
carcinoma, and that the RNA levels of LGMN may be used for patient
classification, the patients with high and low levels of LGMN may
be sensitive for different treatment options.
Studies have demonstrated that the pseudogenes BRAF
and DUXAP10 are dysregulated in thyroid carcinoma (10,32).
Consistent with these findings, the current study indicated that
LGMN was upregulated in thyroid carcinoma, and that this increase
in expression was associated with a poorer outcome. LGMN has also
been shown to exert its function by interacting with miR-495
(14), and the present study
confirmed that LGMN could negatively regulate miR-495.
miR-495 plays a crucial role in several types of
cancer, including gastric cancer, NSCLC and lung cancer (33–35).
Herein, miR-495 was found to induce the cellular proliferation,
invasion and tube formation abilities of HUVECs. To the best of our
knowledge, the present study was the first to report the role of
miR-495 in thyroid carcinoma, and the results showed that miR-495
could modulate the expression of autophagy-related genes. However,
it was hypothesized that miR-495 may also affect additional
signaling pathways and downstream effectors to exert its
function.
Emerging evidence has suggested that ATG3 and p62
serve a key role in autophagy. Herein, ATG3 was indicated to
restore the LGMN-regulated tumorigenesis of thyroid carcinoma. In
conclusion, the results of the current study suggest that LGMN may
be considered an important regulator of thyroid carcinoma
progression, while the miR-495/autophagy axis might serve as a
downstream effector of LGMN. However, the study included a number
of limitations that should be addressed in the future; for
instance, the use of animal models to investigate the role of LGMN,
and in addition to autophagy, other potential downstream targets
could also be identified.
In conclusion, the findings of the present study
also provide novel insights to increase the current understanding
of thyroid carcinoma progression. Furthermore, these findings may
aid the clinical diagnosis of thyroid carcinoma, and suggest a
potential treatment by means of targeting the
LGMN/miR-495/autophagy. High levels of LGMN and low expression of
miR-495 may be beneficial for an improved treatment outcome and
prognosis.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
JS, YP and EY designed the present study. JS, YP, JL
and QH performed the experiments. HZ, LS and EY analyzed the data
and prepared the figures. EY drafted the manuscript. JS and EY
confirmed the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Borran S, Ahmadi G, Rezaei S, Anari MM,
Modabberi M, Azarash Z, Razaviyan J, Derakhshan M, Akhbari M and
Mirzaei H: Circular RNAs: New players in thyroid cancer. Pathol Res
Pract. 216:1532172020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rossi ED, Faquin WC and Pantanowitz L:
Cytologic features of aggressive variants of follicular-derived
thyroid carcinoma. Cancer Cytopathol. 127:432–446. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tang KT and Lee CH: BRAF mutation in
papillary thyroid carcinoma: Pathogenic role and clinical
implications. J Chin Med Assoc. 73:113–128. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ito Y, Jikuzono T, Higashiyama T, Asahi S,
Tomoda C, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Kuma K and
Miyauchi A: Clinical significance of lymph node metastasis of
thyroid papillary carcinoma located in one lobe. World J Surg.
30:1821–1828. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhao J, Xu C, Yao J, Yu C, Liao L and Dong
J: Statins and thyroid carcinoma: A meta-analysis. Cell Physiol
Biochem. 47:1422–1431. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Stewart GL, Enfield KSS, Sage AP, Martinez
VD, Minatel BC, Pewarchuk ME, Marshall EA and Lam WL: Aberrant
expression of pseudogene-derived lncRNAs as an alternative
mechanism of cancer gene regulation in lung adenocarcinoma. Front
Genet. 10:1382019. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shih JH, Chen HY, Lin SC, Yeh YC, Shen R,
Lang YD, Wu DC, Chen CY, Chen RH, Chou TY and Jou YS: Integrative
analyses of noncoding RNAs reveal the potential mechanisms
augmenting tumor malignancy in lung adenocarcinoma. Nucleic Acids
Res. 48:1175–1191. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poliseno L: Pseudogenes: Newly discovered
players in human cancer. Sci Signal. 5:re52012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao-Jie L, Ai-Mei G, Li-Juan J and Jiang
X: Pseudogene in cancer: Real functions and promising signature. J
Med Genet. 52:17–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin JD, Fu SS, Chen JY, Lee CH, Chau WK,
Cheng CW, Wang YH, Lin YF, Fang WF and Tang KT: Clinical
manifestations and gene expression in patients with conventional
papillary thyroid carcinoma carrying the BRAF(V600E) mutation and
BRAF pseudogene. Thyroid. 26:691–704. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang JL, Cao SW, Ou QS, Yang B, Zheng SH,
Tang J, Chen J, Hu YW, Zheng L and Wang Q: The long non-coding RNA
PTTG3P promotes cell growth and metastasis via up-regulating PTTG1
and activating PI3K/AKT signaling in hepatocellular carcinoma. Mol
Cancer. 17:932018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kong Y, Zhang L, Huang Y, He T, Zhang L,
Zhao X, Zhou X, Zhou D, Yan Y, Zhou J, et al: Pseudogene PDIA3P1
promotes cell proliferation, migration and invasion, and suppresses
apoptosis in hepatocellular carcinoma by regulating the p53
pathway. Cancer Lett. 407:76–83. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun M, Nie FQ, Zang C, Wang Y, Hou J, Wei
C, Li W, He X and Lu KH: The pseudogene DUXAP8 promotes
non-small-cell lung cancer cell proliferation and invasion by
epigenetically silencing EGR1 and RHOB. Mol Ther. 25:739–751. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao K, Qian Z, Zhang S, Chen B, Li Z,
Huang R, Cheng L, Wang T, Yang R, Lan J, et al: The LGMN pseudogene
promotes tumor progression by acting as a miR-495-3p sponge in
glioblastoma. Cancer Lett. 490:111–123. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim YW, Kim EY, Jeon D, Liu JL, Kim HS,
Choi JW and Ahn WS: Differential microRNA expression signatures and
cell type-specific association with Taxol resistance in ovarian
cancer cells. Drug Des Devel Ther. 8:293–314. 2014.PubMed/NCBI
|
|
16
|
Duan W, Kong X, Li J, Li P, Zhao Y, Liu T,
Binang HB, Wang Y, Du L and Wang C: LncRNA AC010789.1 promotes
colorectal cancer progression by targeting microRNA-432-3p/ZEB1
axis and the Wnt/β-catenin signaling pathway. Front Cell Dev Biol.
8:5653552020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kafshdooz L, Pourfathi H, Akbarzadeh A,
Kafshdooz T, Razban Z, Sheervalilou R, Ebrahimi Sadr N, Khalilov R,
Saghfi S, Kavetskyy T, et al: The role of microRNAs and
nanoparticles in ovarian cancer: A review. Artif Cells Nanomed
Biotechnol. 46:241–247. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han D, Li J, Wang H, Su X, Hou J, Gu Y,
Qian C, Lin Y, Liu X, Huang M, et al: Circular RNA circMTO1 acts as
the sponge of microRNA-9 to suppress hepatocellular carcinoma
progression. Hepatology. 66:1151–1164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ye M, Dong S, Hou H, Zhang T and Shen M:
Oncogenic role of long noncoding RNAMALAT1 in thyroid cancer
progression through regulation of the miR-204/IGF2BP2/m6A-MYC
signaling. Mol Ther Nucleic Acids. 23:1–12. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shu T, Yang L, Sun L, Lu J and Zhan X:
CircHIPK3 promotes thyroid cancer tumorigenesis and invasion
through the Mirna-338-3p/RAB23 axis. Med Princ Pract. Oct
26–2020.(Epub ahead of Print). View Article : Google Scholar
|
|
21
|
Qian J, Garg A, Li F, Shen Q and Xiao K:
LncRNA LUNAR1 accelerates colorectal cancer progression by
targeting the miR4953p/MYCBP axis. Int J Oncol. 57:1157–1168.
2020.PubMed/NCBI
|
|
22
|
Levy JMM, Towers CG and Thorburn A:
Targeting autophagy in cancer. Nat Rev Cancer. 17:528–542. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pagotto A, Pilotto G, Mazzoldi EL,
Nicoletto MO, Frezzini S, Pastò A and Amadori A: Autophagy
inhibition reduces chemoresistance and tumorigenic potential of
human ovarian cancer stem cells. Cell Death Dis. 8:e29432017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chude CI and Amaravadi RK: Targeting
autophagy in cancer: Update on clinical trials and novel
inhibitors. Int J Mol Sci. 18:12792017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amaravadi R, Kimmelman AC and White E:
Recent insights into the function of autophagy in cancer. Genes
Dev. 30:1913–1930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wei W, Hardin H and Luo QY: Targeting
autophagy in thyroid cancers. Endocr Relat Cancer. 26:R181–R194.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Yan L, Yue C, Xu Y, Jiang X, Zhang L and
Wu J: Identification of potential diagnostic and prognostic
pseudogenes in hepatocellular carcinoma based on
pseudogene-miRNA-mRNA competitive network. Med Sci Monit.
26:e9218952020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou Z, Wang Y, Xia N, Lv T, Yuan X and
Song Y: Pseudogene KRT17P3 drives cisplatin resistance of human
NSCLC cells by modulating miR-497-5p/mTOR. Cancer Sci. 112:275–286.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li W, Yang Y, Hou X, Zhuang H, Wu Z, Li Z,
Guo R, Chen H, Lin C, Zhong W, et al: MicroRNA-495 regulates
starvation-induced autophagy by targeting ATG3. FEBS Lett.
590:726–738. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Somuncu E, Karatas A, Ferahman S, Saygili
N, Yilmaz E, Ozturk O and Kapan M: The investigation of foxe1
variations in papillary thyroid carcinoma. Int J Clin Exp Pathol.
8:13458–13464. 2015.PubMed/NCBI
|
|
32
|
Li J, Jiang L, Liu Z, Li Y, Xu Y and Liu
H: Oncogenic pseudogene DUXAP10 knockdown suppresses proliferation
and invasion and induces apoptosis of papillary thyroid carcinoma
cells by inhibition of Akt/mTOR pathway. Clin Exp Pharmacol
Physiol. 47:1473–1483. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Eun JW, Kim HS, Shen Q, Yang HD, Kim SY,
Yoon JH, Park WS, Lee JY and Nam SW: MicroRNA-495-3p functions as a
tumor suppressor by regulating multiple epigenetic modifiers in
gastric carcinogenesis. J Pathol. 244:107–119. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen X, Xu Y, Liao X, Liao R, Zhang L, Niu
K, Li T, Li D, Chen Z, Duan Y and Sun J: Plasma miRNAs in
predicting radiosensitivity in non-small cell lung cancer. Tumour
Biol. 37:11927–11936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Halvorsen AR, Sandhu V, Sprauten M, Flote
VG, Kure EH, Brustugun OT and Helland Å: Circulating microRNAs
associated with prolonged overall survival in lung cancer patients
treated with nivolumab. Acta Oncol. 57:1225–1231. 2018. View Article : Google Scholar : PubMed/NCBI
|