Introduction
Pancreatic ductal adenocarcinoma (PDAC) is one of
the most refractory cancers in humans, with an overall 5-year
survival rate of only 9% (1,2). PDAC has an extremely poor prognosis
that has not improved much in the last few decades (3,4). Most
PDAC cases are diagnosed at an advanced stage, and patients with
unresectable PDAC generally receive systemic chemotherapy. Most of
the operative cases also need adjuvant chemotherapy to prevent
tumor recurrence of cancer. However, the beneficial effects of
standard regimens of chemotherapy are not sufficient, giving rise
to an urgent requirement of a novel treatment strategy to improve
the prognosis of PDAC.
Pancreatic ductal adenocarcinoma is generally
characterized by desmoplasia, which consists of an extracellular
matrix and stromal cells, including pancreatic stellate cells
(PSCs). Desmoplasia is considered one of the main reasons for the
resistance of pancreatic cancer to chemotherapy (5,6).
Furthermore, activated PSCs in the tumor microenvironment enhance
the malignancy of pancreatic cancer cells (PCCs), namely their
ability to invade and metastatize (7,8).
Therefore, modulating the activation of PSCs is a promising
therapeutic strategy for improving PDAC prognosis. We have
previously reported the suppression of PSC activation by inhibition
of PSC autophagy using chloroquine, a lysosomal inhibitor, and
mitigation of tumor progression by chloroquine in a murine PDAC
model (8). There are also other
reports demonstrating the use of chloroquine for treating PDAC;
some indicating promising results (9–12).
Long-term use of chloroquine, however, leads to adverse effects,
such as retinopathy and cardiac complications, in the clinical
setting (12–14).
In the past few decades, various tumor-specific drug
delivery systems (DDSs) using nanotechnology such as polymers and
liposomes, have emerged (15). In
1986, nano-sized DDS was advocated, which selectively accumulated
in tumor tissues due to its enhanced permeability and retention
(EPR) effect (16,17). Research has shown promising effects
of nanoparticles, loaded with therapeutic agents, on several types
of cancers, although this research remains limited overall
(15). Inter-alia, poly
lactic-co-glycolic acid (PLGA) nanoparticle is an outstanding DDS
carrier, which has a high safety profile, approved by the Food and
Drug Administration (FDA) and European Medicines Agency (EMA),
morphological stability, and excellent biocompatibility and
biodegradability (18,19).
In this study, we hypothesized that the treatment of
PSCs with DDS nanoparticles loaded with chloroquine could suppress
PSC activation efficiently and regulate desmoplasia. We developed a
novel nano-drug of chloroquine-loaded PLGA nanoparticles (Nano-CQ),
aiming to enhance the effect of anti-cancer drugs, like
gemcitabine, by anti-stromal pre-treatment with Nano-CQ in an in
vivo murine PDAC model. To the best of our knowledge, the
effect of chloroquine-loaded PLGA nanoparticles targeting PSCs on
PDAC in vivo as a pre-treatment for chemotherapy has not
been previously evaluated.
Materials and methods
Cells
We extracted human PSCs from fresh pancreatic cancer
surgical specimens using the outgrowth method and immortalized them
as described in our previous reports (20,21). The
pancreatic cancer cell line SUIT-2 was purchased from Japan Health
Science Research Resources Bank (Osaka, Japan). Both PSCs and
SUIT-2 cells were maintained in Dulbecco's modified Eagle's medium
(DMEM) supplemented with 10% fetal bovine serum at 37°C and 10%
CO2, as described previously (22). Cells were confirmed to be free of
mycoplasma and used within eight passages for each experiment.
Treatment agents
Indocyanine green was purchased from Tokyo Chemical
Industry (#I0535). Chloroquine phosphate was purchased from
Sigma-Aldrich (#PHR1258). Gemcitabine was purchased from Eli Lilly,
Japan [Gemzar Injection (4224403D1030)]. Medetomidine was purchased
from Kyoritsu Seiyaku. Midazolam was purchased from Sandoz.
Butorphanol was purchased from Meiji Seika Pharma. We consigned the
manufacturing of the PLGA nanoparticles, namely the indocyanine
green-loaded nanoparticles (Nano-ICG) and chloroquine-loaded
nanoparticles (Nano-CQ), to Sentan Iryou Kaihatsu. PLGA was used as
a matrix for the nanoparticles, and polyvinyl alcohol was used as a
dispersing agent. The particle size was analyzed by light
scattering method, using the Nanotrac Wave-EX150 system (Microtrac
BEL Corp.); the median diameters (D50) of Nano-ICG and Nano-CQ were
210 and 205 nm, respectively. These nanoparticles contained 4%
indocyanine green (wt/wt) and 1.5% chloroquine (wt/wt),
respectively. All the agents were dissolved in phosphate-buffered
saline (PBS).
Mice
Seven-week-old BALB/c AJcl nu/nu female mice were
purchased from Clea. After one week of acclimation, the mice
received immortalized PSCs (5×105) with SUIT-2 cells
(5×105) or luciferase-expressing SUIT-2 cells
(5×105) suspended in 50 µl DMEM. Cells were
orthotopically transplanted into the pancreatic tail under general
anesthesia, using combination anesthetic (0.3 mg/kg of
medetomidine, 4.0 mg/kg of midazolam, and 5.0 mg/kg of butorphanol)
administered by intraperitoneal injection (23). The median body weight of mice was
21.63 g (ranges, 19.51–23.79) at the time of implantation. They
were fed ad libitum and were kept under a 12-h light/12-h
dark cycle. The mouse room was kept at a temperature of 20–26°C and
a humidity of 40–70%. All mice were euthanized by dislocating
cervical vertebra under general anesthesia at the end of
experiments or if a humane endpoint was reached; defined as a loss
of >15% of body weight, a tumor volume >1.2 cm3,
severe ascites, vomiting, or inability to ambulate or rise for food
and water. However, no animals reached these humane endpoints.
Tumor accumulation assay
Tumor implantation and dissemination were verified
by measuring luciferin emission using the in vivo imaging
system (IVIS) Spectrum (Caliper Life Sciences), after injecting 150
mg D-luciferin (#LK10000; Oz Biosciences) into the intraperitoneal
cavity of mice. After verifying tumor formation, either Nano-ICG
(0.2 mg indocyanine green per mouse, n=4) or free indocyanine green
(0.2 mg per mouse, n=4) was injected into a tail vein. Indocyanine
green fluorescence was measured continuously using the IVIS
Spectrum (excitation: 745 nm, emission: 840 nm). Luciferin emission
and indocyanine green fluorescence were quantified using Living
Image software, version 4.1 (Summit Pharmaceuticals International
Corporation).
Immunohistochemistry
Formalin-fixed, paraffin embedded tumor tissues were
sliced into 4-µm-thick sections and endogenous peroxidase activity
was blocked with methanol containing 0.3% hydrogen peroxidase for
30 min at room temperature. Antigen retrieval was performed by
boiling in a microwave oven (citrate buffer, pH 6.0). Afterwards,
the tissues were incubated with mouse anti-α-smooth muscle actin
(αSMA) antibody (1:100, #M0851; Dako) overnight at 4°C and stained
with EnVision+System-HRP Labeled Polymer Anti-Mouse (#K4001; Dako).
Activated PSCs were identified based on their spindle-like shape
and αSMA-positive staining. The αSMA-positive area was measured
using analysis application Hybrid cell count (BZ-H3C, Keyence).
Cellular uptake assay
SUIT-2 cells (5×105/well) or immortalized
pancreatic stellate cells (imPSCs) (5×105/well) were
cultured in a 6-well plate. After 24 h of incubation at 37°C and
10% CO2, cells were treated with Nano-ICG (10 µM
indocyanine green) or free indocyanine green (10 µM) for 1 h and
washed with PBS. The intracellular indocyanine green fluorescence
was observed under BZ-X700 (Keyence) fluorescent microscope. Nuclei
were counterstained using Hoechst 33342 (#H342; Dojindo). Each
experiment was conducted in triplicate wells and repeated
twice.
In vivo treatment experiment
We conducted the treatment experiments in seven
different groups using the SUIT-2 cells and imPSCs co-transplanted
model: chloroquine group (n=3), low-dose chloroquine group (n=3),
Nano-CQ group (n=3), gemcitabine group (n=3), gemcitabine +
chloroquine group (n=3), gemcitabine + Nano-CQ group (n=4), and
control (vehicle) group (n=6). The treatment agents were
administered according to the following schedule: cells were
implanted on day 0; chloroquine (50 mg/kg) was administered on days
7, 8, 9, 14, 15, 16, 21, 22 and 23; low-dose chloroquine (30
mg/kg), Nano-CQ (30 mg/kg chloroquine), and vehicle were
administered on days 7, 14 and 21; gemcitabine (40 mg/kg) was
administered on days 10, 17 and 24. All mice were sacrificed on day
28, with all orthotopic tumors were resected and measured. Tumor
volume was calculated using the following formula: π/6 × L × W × H,
where L is the largest tumor diameter, W is the smallest diameter,
and H is the height. The maximum tumor volume was ~0.8
cm3.
Statistical analysis
Results are presented as the mean ± standard error.
The effects of Nano-CQ on tumor volume, tumor weight and αSMA
expression were analyzed using ANOVA followed by the Tukey's test.
Statistical significance was defined as P<0.05. All statistical
analyses were performed using JMP 14 software (SAS Institute).
Results
PLGA nanoparticle acts as a drug
delivery system in pancreatic tumor
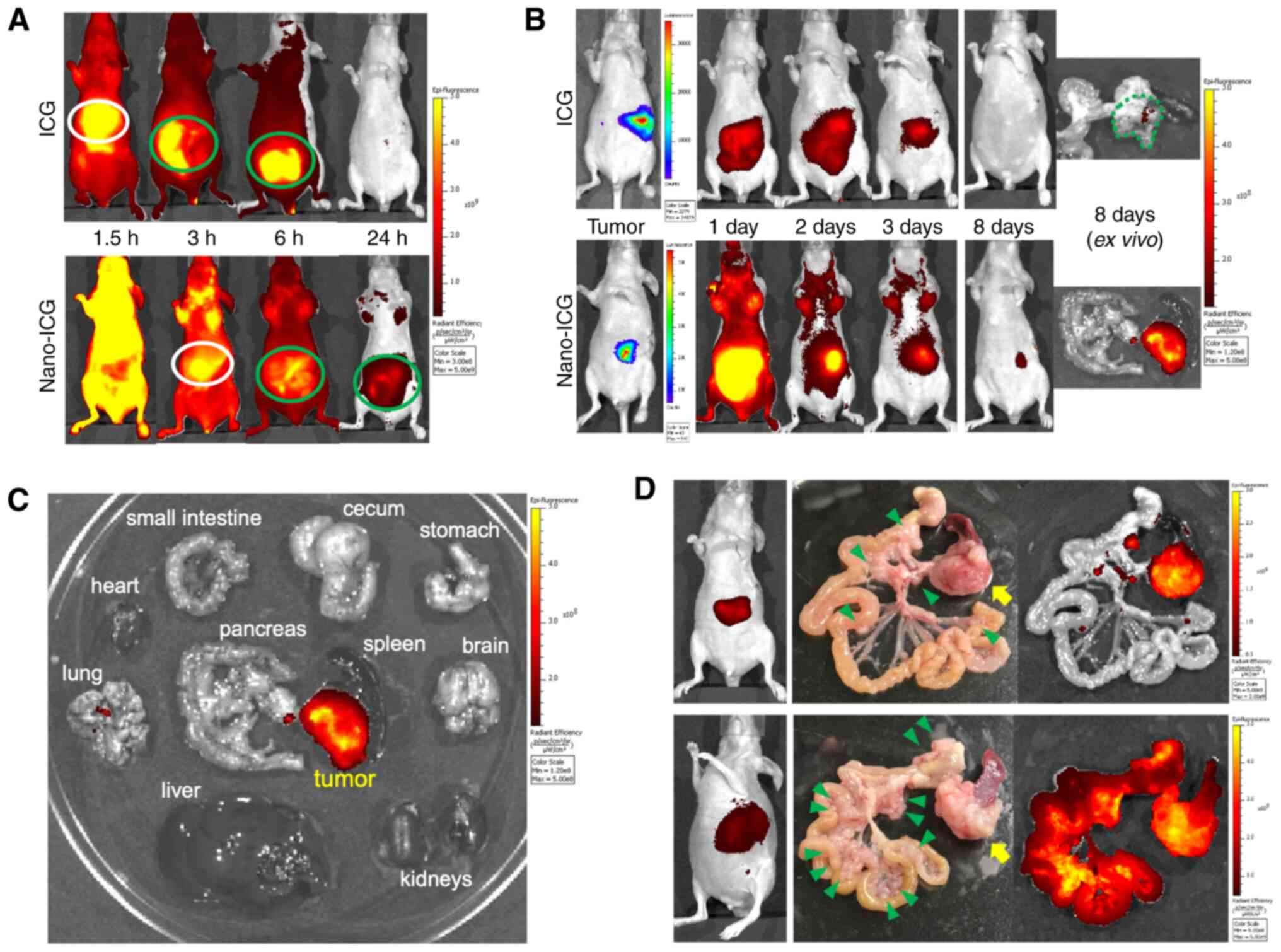
The ability of PLGA nanoparticles to deliver drugs
to the murine pancreatic tumor was evaluated using Nano-ICG.
Nano-ICG or free indocyanine green was injected into a tail vein of
the mouse orthotopically co-implanted with PCCs and PSCs. In
vivo, indocyanine green is metabolized in the liver and
subsequently secreted into the bile (24,25). On
short-term observation, free indocyanine green was taken into the
liver and secreted into the bile; thereafter, it disappeared from
the systemic circulation quickly and was discharged through the
intestine. In contrast, Nano-ICG needed substantially more time for
liver uptake after administration (Fig.
1A). These results indicate the enhanced retentivity of this
PLGA nanocarrier in the blood.
Long-term observations revealed the accumulation and
retention of Nano-ICG particles for 8 days in the pancreatic tumor.
On the other hand, no obvious accumulation of free indocyanine
green was observed in the tumors 8 days after administration
(Fig. 1B). These findings suggest
that the EPR effect of this nanosystem is sufficient in vivo
applications. Furthermore, there was no obvious accumulation of
Nano-ICG in other major organs, including normal pancreatic tissue
(Fig. 1C). Nano-ICG accumulated in
disseminated nodules as well as in the primary pancreatic tumor,
regardless of their number or size (Fig.
1D). Based on these findings, it is clear that this DDS can be
used to treat not only localized PDACs but also metastatic
ones.
Histologically, Nano-ICG accumulated in the
αSMA-positive area (Fig. 2A). In
vitro analysis showed that PSCs, rather than PCCs, had higher
fluorescent intensity for Nano-ICG and Nano-ICG showed much
improved intracellular uptake than free indocyanine green in both
PCCs and PSCs (Fig. 2B). These
findings suggest that the uptake of PLGA nanocarrier is higher in
PSCs than PCCs.
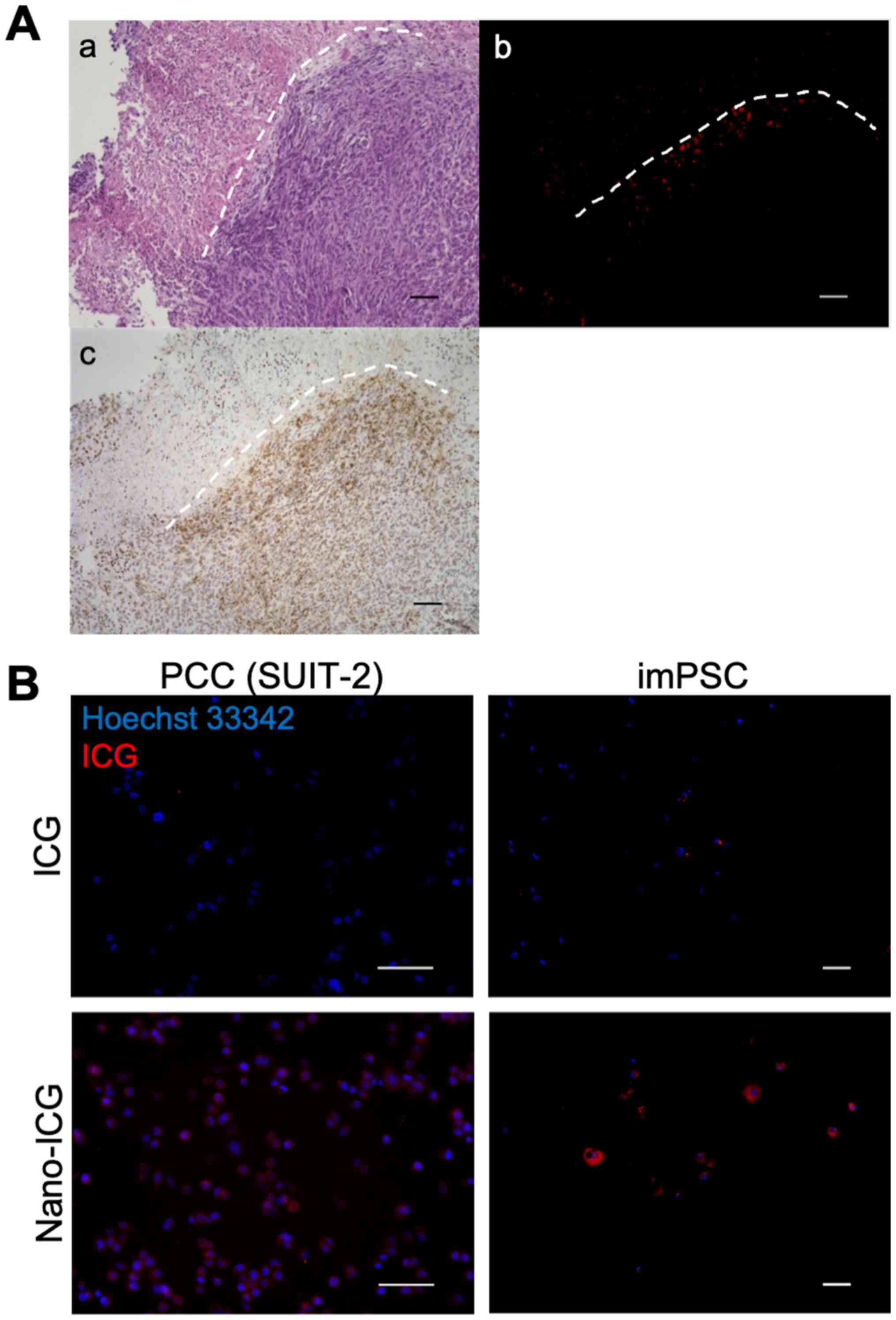 | Figure 2.Nano-ICG preferentially accumulates
in PSCs. (A) Representative photomicrographs of orthotopic tumor
stained with (a) HE, (b) fluorescence for ICG and (c) αSMA
immunostaining. Nano-ICG was observed in the αSMA-positive area.
The white, short, dashed lines show the contour of the viable
tumor. Magnification, ×100; scale bars, 100 µm. (B) Representative
photomicrographs of PCCs (SUIT-2) and imPSCs washed at 1 h after
addition of free ICG or Nano-ICG. Nano-ICG showed more
intracellular uptake compared with free ICG, and PSCs showed
stronger intracellular fluorescence of Nano-ICG compared with PCCs.
Original magnification, ×200 (left panels) or ×100 (right panels);
scale bars, 100 µm. HE, hematoxylin eosin; αSMA, α-smooth muscle
actin; PCC, pancreatic cancer cell; ICG, indocyanine green; PSC,
pancreatic stellate cells; im, immortalized. |
Nano-CQ efficiently reduces the
density of activated PSCs and enhanced the antitumor effect of
gemcitabine
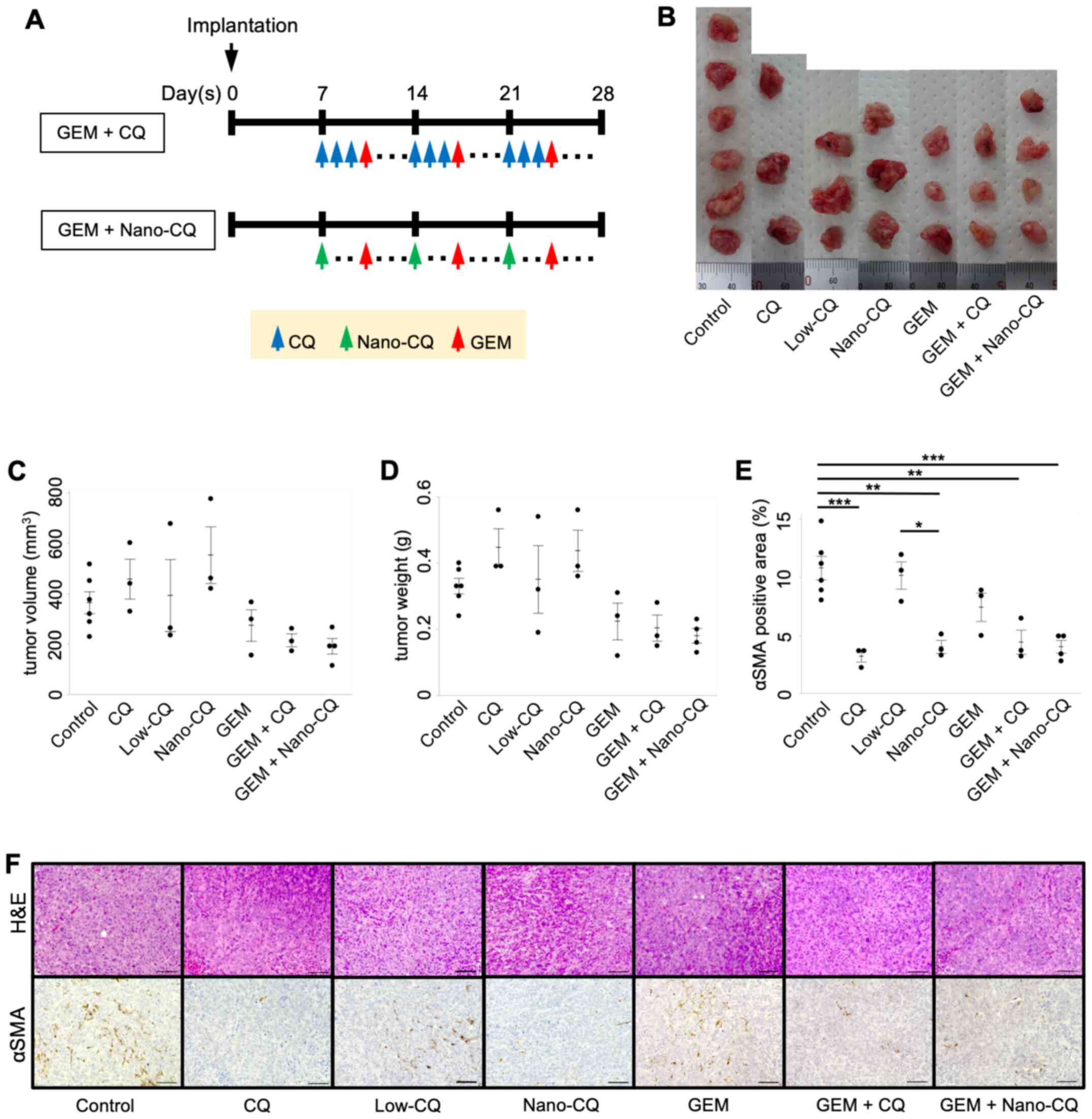
To assess the antitumor effects of
chloroquine-loaded PLGA and its potential effects on PSC
activation, we used a single administration of Nano-CQ (total
chloroquine dose: 90 mg/kg), free chloroquine (total dose: 90 mg/kg
or 450 mg/kg), or combination therapy with the anti-cancer drug
gemcitabine, in the orthotopic murine PDAC model (Fig. 3A). The treatment started 7 days
post-implantation and the antitumor effect was assessed by
measuring the tumor volume and weight on day 28. The gemcitabine,
gemcitabine + chloroquine, and gemcitabine + Nano-CQ groups showed
the antitumor effect compared with the control (vehicle) group and
the latter two groups seemed to show the more effect than the
former, although no statistical significance was detected (Fig. 3B-D); indicating that Nano-CQ
successfully enhanced the effect of gemcitabine at the lower
chloroquine dose.
The effect of Nano-CQ on PSC activation was assessed
using immunohistochemistry of αSMA. The αSMA-positive area in the
tumor was significantly decreased in the Nano-CQ (P=0.001) and
chloroquine (P<0.001) groups, compared to the control group
(Fig. 3E and F). Similar results
were observed in the combination therapy groups (Fig. 3E and F). Of note, no inhibitory
effect on PSC activation was observed in the low-dose chloroquine
group, which had the same chloroquine dosage as the Nano-CQ group
(Fig. 3E and F).
Discussion
In this study, Nano-ICG showed great ability to
selectively accumulate in pancreatic tumor tissues and to remain
there for much longer than free indocyanine green, in a murine PDAC
model. Our findings suggest that this PLGA nanoparticle-DDS could
be useful for the treatment of PDAC. Furthermore, Nano-CQ
successfully enhanced the antitumor effect of gemcitabine by
reducing the density of activated PSCs in the tumor tissue.
Consistent with previous reports, free indocyanine
green showed rapid uptake by the liver and quick disappearance from
the circulation in mice (26,27). In
contrast, Nano-ICG uptake and retention by the liver was much
higher and longer than free indocyanine green. These findings
indicate that this nanocarrier has enhanced retentivity in the
blood and it avoids the reticuloendothelial system and opsonization
due to the presence of a hydrophilic surface layer of polyvinyl
alcohol (18,28). Nano-ICG showed highly selective
accumulation in the tumor of the orthotopic murine PDAC model
without the need for any active targeting, such as tumor-specific
antibodies (29,30); this is due to Nano-ICG's EPR effect,
suggesting this nanosystem is a useful DDS for the treatment of
pancreatic tumor. Furthermore, Nano-ICG showed high accumulation in
the disseminated nodules, indicating that this DDS may be
beneficial in treating PDAC since more than half of the patients
with this cancer have metastasis at the time of diagnosis (2). In addition, although the effect of this
DDS was evaluated in a murine PDAC model in this study, this simple
DDS could be applied to the tumors of other organs or even
nontumorous diseases, such as inflammatory diseases because it
functions by passive targeting (18,19,31).
The distribution of Nano-ICG was mostly observed in
the αSMA-positive area, which was located in the peripheral region
of the tumor tissues, suggesting higher Nano-ICG uptake by PSCs
than PCCs. Indeed, studies have shown that PSCs have a higher
uptake capacity for extracellular components and drugs than PCCs
(32,33). Altogether, this nanosystem is
considered to affect PSCs more strongly than PCCs. Moreover, high
interstitial fluid pressure and low perfusion due to poorly
developed blood vessels are considered to be the main reasons for
inefficient drug delivery to cancer cells in solid tumors (5,6,34–36).
Thus, Nano-ICG was observed in the peripheral region of the tumor
tissues.
Recently, chloroquine has demonstrated the ability
to interfere with various physiological processes, a property that
might be useful in cancer therapy (12,37). We
have previously reported on chloroquine's antitumor property,
inhibiting cancer-stromal interaction via inhibition of PSC
autophagy (8). However, the dose we
used in this study was much lower than the dose used in previous
studies (8,12). Thus, the single use of Nano-CQ or
free chloroquine could not show antitumor effects in the present
study.
We have measured αSMA as it is a known marker of
activated PSCs (38,39), which play a central role in producing
extracellular matrix to form desmoplasia. In this study, we
intended to modulate the desmoplastic stroma of PDAC and to make it
easier for chemotherapeutics to reach the cancer cells by
administrating Nano-CQ as a pre-treatment agent. Thus, we
considered that reducing activated PSCs was the most important
indicator. A similar reduction in the density of activated PSCs was
achieved in the Nano-CQ group, compared to the chloroquine group,
in spite of using one-fifth of the chloroquine dose used in the
former compared to the latter group. In contrast, low-dose free
chloroquine, which had the same chloroquine dosage as in the
Nano-CQ group, did not reduce PSC activation. These results suggest
that this nanosystem could reduce the dose of chloroquine needed
while preserving its effectiveness. Considering the adverse effects
of chloroquine, including retinopathy and cardiac complications,
which have been well-established in clinical practice (12–14),
this DDS could help reduce these risks by its high tumor
selectivity and low chloroquine doses needed.
In this study, we aimed to enhance the antitumor
effect of gemcitabine via modulation of desmoplasia in PDAC using
pre-treatment with Nano-CQ. This combination therapy showed
promising results, as intended. Nano-CQ preferentially accumulated
in PSCs in the pancreatic tumor, reduced the density of activated
PSCs before administration of gemcitabine, and then synergistically
enhanced the antitumor effect of gemcitabine successfully. These
findings indicate that Nano-CQ shows long-lasting effectiveness, at
lower doses, due to its highly selective tumor accumulation,
retention, and sustained drug release property. However, according
to some reports, the EPR effect of nano drugs needs to be augmented
by enhancing tumor blood flow using vasodilators and other drugs
for further effectiveness in the clinical setting (40–43).
Such augmentation of tumor blood flow may further enhance Nano-CQ
accumulation in tumors.
The findings of this study have some limitations.
First, we did not conduct a large-scale treatment experiment that
could serve as a basis for clinical application. Second, we decided
treatment regimens, such as the dose of drugs, based on previous
studies. Further research is needed, however, to determine the
optimal regimen. Third, the optimization of the size and drug
content rate of the PLGA nanoparticles is also needed in the future
research.
In conclusion, the nanosystem described in our
study, which is based on PLGA nanoparticles, was capable of acting
as a DDS for pancreatic tumors, even in those inducing metastasis.
Moreover, Nano-CQ pre-treatment efficiently enhanced the antitumor
effect of gemcitabine by reducing PSC activation in vivo.
Nano-CQ showed an adequate effect at lower doses of chloroquine,
compared to free chloroquine, indicating that this nanosystem could
reduce the dose of chloroquine needed and, consequently, mitigate
its adverse effects. Although further large-scale studies and
investigations are needed to translate our findings to the clinical
practice, including determining the optimal drug concentration in
the nanocarrier, dose, and schedule of treatment, Nano-CQ offers a
promising pre-treatment strategy for PDAC in combination with
gemcitabine.
Acknowledgements
The authors would like to thank Ms. S. Sadatomi and
Ms. E. Manabe (Department of Surgery and Oncology, Kyushu
University Hospital) for their advice.
Funding
The present study was partially supported by The
Japan Society for the Promotion of Science Grants-in-Aid and for
Young Scientists Grant (grant nos. JP18H02880, JP19H03732,
JP19K18153 and JP20H03754), The Takeda Science Foundation, The
Kobayashi Foundation for Cancer Research and The Shinnihon
Foundation of Advanced Medical Treatment Research.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SM, KN, KO and MN conceived and designed the study.
SM performed all experiments and data curation, and drafted the
manuscript. SM, AS, WG and NI analyzed and interpretated the
curated data. KN and MN supervised the project, and confirm the
authenticity of all the raw data. SM, KN, NI and KO revised the
manuscript. MN approved the final version of the manuscript for
publication. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all the
participants before using their pancreatic cancer surgical
specimens for the establishment of PSCs, and the study was approved
by The Ethics Committee of Kyushu University (approval no.
2019-462; Fukuoka, Japan). All animal experiments were approved by
The Ethics Committee of Kyushu University (approval no. A20-248-1;
Fukuoka, Japan) and were conducted in accordance with the Japanese
laws associated with animal experiments, the institutional
regulations of Kyushu University, the Science Council of Japan
guidelines for proper conduct of animal experiments (44) and the National Institutes of Health
guide for the care and use of laboratory animals (45).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
αSMA
|
α-smooth muscle actin
|
|
DDS
|
drug delivery system
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
EPR effect
|
enhanced permeability and retention
effect
|
|
imPSC
|
immortalized pancreatic stellate
cell
|
|
IVIS
|
in vivo imaging system
|
|
Nano-CQ
|
nanoparticles loaded with
chloroquine
|
|
Nano-ICG
|
nanoparticles loaded with indocyanine
green
|
|
PBS
|
phosphate-buffered saline
|
|
PCC
|
pancreatic cancer cell
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
PDAC
|
pancreatic ductal adenocarcinoma
|
|
PLGA
|
poly lactic-co-glycolic acid
|
|
PSC
|
pancreatic stellate cell
|
References
|
1
|
Everett JN and Simeone DM: Pancreatic
cancer. World cancer report: Cancer Research for Cancer Prevention.
Wild CP, Weiderpass E and Stewart BW: International Agency for
Research on Cancer; Lyon: pp. 367–373. 2020
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Neoptolemos JP, Kleeff J, Michl P,
Costello E, Greenhalf W and Palmer DH: Therapeutic developments in
pancreatic cancer: Current and future perspectives. Nat Rev
Gastroenterol Hepatol. 15:333–348. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Aslan M, Shahbazi R, Ulubayram K and
Ozpolat B: Targeted therapies for pancreatic cancer and hurdles
ahead. Anticancer Res. 38:6591–6606. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hosein AN, Brekken RA and Maitra A:
Pancreatic cancer stroma: An update on therapeutic targeting
strategies. Nat Rev Gastroenterol Hepatol. 17:487–505. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tanaka HY and Kano MR: Stromal barriers to
nanomedicine penetration in the pancreatic tumor microenvironment.
Cancer Sci. 109:2085–2092. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bachem MG, Schünemann M, Ramadani M, Siech
M, Beger H, Buck A, Zhou S, Schmid-Kotsas A and Adler G: Pancreatic
carcinoma cells induce fibrosis by stimulating proliferation and
matrix synthesis of stellate cells. Gastroenterology. 128:907–921.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Endo S, Nakata K, Ohuchida K, Takesue S,
Nakayama H, Abe T, Koikawa K, Okumura T, Sada M, Horioka K, et al:
Autophagy is required for activation of pancreatic stellate cells,
associated with pancreatic cancer progression and promotes growth
of pancreatic tumors in mice. Gastroenterology. 152:1492–1506.e24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Monma H, Iida Y, Moritani T, Okimoto T,
Tanino R, Tajima Y and Harada M: Chloroquine augments TRAIL-induced
apoptosis and induces G2/M phase arrest in human pancreatic cancer
cells. PLoS One. 13:e01939902018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Molejon MI, Swayden M, Fanale D, Bintz J,
Gayet O, Soubeyran P and Iovanna J: Chloroquine plays a
cell-dependent role in the response to treatment of pancreatic
adenocarcinoma. Oncotarget. 9:30837–30846. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balic A, Sørensen MD, Trabulo SM, Sainz B
Jr, Cioffi M, Vieira CR, Miranda-Lorenzo I, Hidalgo M, Kleeff J,
Erkan M, et al: Chloroquine targets pancreatic cancer stem cells
via inhibition of CXCR4 and hedgehog signaling. Mol Cancer Ther.
13:1758–1771. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pascolo S: Time to use a dose of
Chloroquine as an adjuvant to anti-cancer chemotherapies. Eur J
Pharmacol. 771:139–144. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Marmor MF, Kellner U, Lai TYY, Melles RB
and Mieler WF; American Academy of Ophthalmology, : Recommendations
on screening for chloroquine and hydroxychloroquine retinopathy
(2016 Revision). Ophthalmology. 123:1386–1394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chatre C, Roubille F, Vernhet H, Jorgensen
C and Pers YM: Cardiac complications attributed to chloroquine and
hydroxychloroquine: A systematic review of the literature. Drug
Saf. 41:919–931. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shi J, Kantoff PW, Wooster R and Farokhzad
OC: Cancer nanomedicine: Progress, challenges and opportunities.
Nat Rev Cancer. 17:20–37. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Matsumura Y and Maeda H: A new concept for
macromolecular therapeutics in cancer chemotherapy: Mechanism of
tumoritropic accumulation of proteins and the antitumor agent
smancs. Cancer Res. 46:6387–6392. 1986.PubMed/NCBI
|
|
17
|
Maeda H, Nakamura H and Fang J: The EPR
effect for macromolecular drug delivery to solid tumors:
Improvement of tumor uptake, lowering of systemic toxicity, and
distinct tumor imaging in vivo. Adv Drug Deliv Rev. 65:71–79. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Danhier F, Ansorena E, Silva JM, Coco R,
Le Breton A and Préat V: PLGA-based nanoparticles: An overview of
biomedical applications. J Control Release. 161:505–522. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ding D and Zhu Q: Recent advances of PLGA
micro/nanoparticles for the delivery of biomacromolecular
therapeutics. Mater Sci Eng C. 92:1041–1060. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ikenaga N, Ohuchida K, Mizumoto K, Cui L,
Kayashima T, Morimatsu K, Moriyama T, Nakata K, Fujita H and Tanaka
M: CD10+ pancreatic stellate cells enhance the
progression of pancreatic cancer. Gastroenterology. 139:1041–1051,
1051.e1-1051.e8. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kozono S, Ohuchida K, Eguchi D, Ikenaga N,
Fujiwara K, Cui L, Mizumoto K and Tanaka M: Pirfenidone inhibits
pancreatic cancer desmoplasia by regulating stellate cells. Cancer
Res. 73:2345–2356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ohuchida K, Mizumoto K, Murakami M, Qian
LW, Sato N, Nagai E, Matsumoto K, Nakamura T and Tanaka M:
Radiation to stromal fibroblasts increases invasiveness of
pancreatic cancer cells through tumor-stromal interactions. Cancer
Res. 64:3215–3222. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawai S, Takagi Y, Kaneko S and Kurosawa
T: Effect of three types of mixed anesthetic agents alternate to
ketamine in mice. Exp Anim. 60:481–487. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cherrick GR, Stein SW, Leevy CM and
Davidson CS: Indocyanine green: Observations on its physical
properties, plasma decay, and hepatic extraction. J Clin Invest.
39:592–600. 1960. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Desmettre T, Devoisselle JM and Mordon S:
Fluorescence properties and metabolic features of indocyanine green
(ICG) as related to angiography. Surv Ophthalmol. 45:15–27. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang G, Liu F, Zhang B, He Y, Luo J and
Bai J: Imaging of pharmacokinetic rates of indocyanine green in
mouse liver with a hybrid fluorescence molecular tomography/x-ray
computed tomography system. J Biomed Opt. 18:0405052013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qi B, Crawford AJ, Wojtynek NE, Holmes MB,
Souchek JJ, Almeida-Porada G, Ly QP, Cohen SM, Hollingsworth MA and
Mohs AM: Indocyanine green loaded hyaluronan-derived nanoparticles
for fluorescence-enhanced surgical imaging of pancreatic cancer.
Nanomedicine. 14:769–780. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Acharya S and Sahoo SK: PLGA nanoparticles
containing various anticancer agents and tumour delivery by EPR
effect. Adv Drug Deliv Rev. 63:170–183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang T, Yang S, Petrenko VA and Torchilin
VP: Cytoplasmic delivery of liposomes into MCF-7 breast cancer
cells mediated by cell-specific phage fusion coat protein. Mol
Pharm. 7:1149–1158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu ST, Fowler AJ, Garmon CB, Fessler AB,
Ogle JD, Grover KR, Allen BC, Williams CD, Zhou R, Yazdanifar M, et
al: Treatment of pancreatic ductal adenocarcinoma with tumor
antigen specific-targeted delivery of paclitaxel loaded PLGA
nanoparticles. BMC Cancer. 18:4572018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Katsuki S, Matoba T, Koga JI, Nakano K and
Egashira K: Anti-inflammatory nanomedicine for cardiovascular
disease. Front Cardiovasc Med. 4:872017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ikenaga N, Ohuchida K, Mizumoto K, Akagawa
S, Fujiwara K, Eguchi D, Kozono S, Ohtsuka T, Takahata S and Tanaka
M: Pancreatic cancer cells enhance the ability of collagen
internalization during epithelial-mesenchymal transition. PLoS One.
7:e404342012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hessmann E, Patzak MS, Klein L, Chen N,
Kari V, Ramu I, Bapiro TE, Frese KK, Gopinathan A, Richards FM, et
al: Fibroblast drug scavenging increases intratumoural gemcitabine
accumulation in murine pancreas cancer. Gut. 67:497–507. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Libutti SK, Tamarkin L and Nilubol N:
Targeting the invincible barrier for drug delivery in solid
cancers: Interstitial fluid pressure. Oncotarget. 9:35723–35725.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chauhan VP, Stylianopoulos T, Boucher Y
and Jain RK: Delivery of molecular and nanoscale medicine to
tumors: Transport barriers and strategies. Annu Rev Chem Biomol
Eng. 2:281–298. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Meng H and Nel AE: Use of nano engineered
approaches to overcome the stromal barrier in pancreatic cancer.
Adv Drug Deliv Rev. 130:50–57. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pelt J, Busatto S, Ferrari M, Thompson EA,
Mody K and Wolfram J: Chloroquine and nanoparticle drug delivery: A
promising combination. Pharmacol Ther. 191:43–49. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bynigeri RR, Jakkampudi A, Jangala R,
Subramanyam C, Sasikala M, Rao GV, Reddy DN and Talukdar R:
Pancreatic stellate cell: Pandora's box for pancreatic disease
biology. World J Gastroenterol. 23:382–405. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Apte MV, Park S, Phillips PA, Santucci N,
Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA,
et al: Desmoplastic reaction in pancreatic cancer: Role of
pancreatic stellate cells. Pancreas. 29:179–187. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Maeda H: Nitroglycerin enhances vascular
blood flow and drug delivery in hypoxic tumor tissues: Analogy
between angina pectoris and solid tumors and enhancement of the EPR
effect. J Control Release. 142:296–298. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Seki T, Fang J and Maeda H: Enhanced
delivery of macromolecular antitumor drugs to tumors by
nitroglycerin application. Cancer Sci. 100:2426–2430. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Suzuki M, Hori K, Abe I, Saito S and Sato
H: A new approach to cancer chemotherapy: Selective enhancement of
tumor blood flow with angiotensin II. J Natl Cancer Inst.
67:663–669. 1981.PubMed/NCBI
|
|
43
|
Li CJ, Miyamoto Y, Kojima Y and Maeda H:
Augmentation of tumour delivery of macromolecular drugs with
reduced bone marrow delivery by elevating blood pressure. Br J
Cancer. 67:975–980. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Science Council of Japan, . No: 2 Expanded
Committee on Establishment of Guidelines for Proper Conduct of
Animal Experiments. Guidelines for Proper Conduct of Animal
Experiments. http://www.scj.go.jp/en/animal/June 1–2006
|
|
45
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals, . Guide for the Care and Use of Laboratory Animals. 8th
edition. National Academies Press; Washington, DC: 2011
|