Introduction
Photodynamic therapy (PDT) is a form of treatment
for tumors and pre-cancerous lesions that works by inducing cell
death. The treatment involves a photosensitizing agent which is
topically applied or injected into the blood stream (1,2). It is
absorbed by cells and after a pre-determined amount of time, the
site is exposed to a light source such as a laser or light-emitting
diode (1). When light is applied at
a specific wavelength to the targeted area, the photosensitizing
agent undergoes a reaction that forms reactive oxygen species (ROS)
and kills the targeted cells (1,2) and/or
induces vascular damage (3). To the
host, PDT is usually minimally invasive and minimally toxic as the
photosensitizers do not tend to accumulate in cell nuclei (2,4).
Photosensitizers are divided into families based on
chemical structure including porphyrins, chlorins, and dyes
(5). They are also grouped into
first, second, and third generation photosensitizers (6). The first generation photosensitizer
most commonly used is Photofrin, a mixture of porphyrin dimers and
oligomers (6,7). First generation photosensitizers are
used less frequently today due to side effects such as skin
sensitivity and their weak absorption at 630 nm (8,9). Two
examples of second generation photosensitizers commonly employed in
dermatology practice are aminolevulinic acid (ALA) and the methyl
ester form, methyl aminolevulinate (MAL) (10). Both can be topically applied and have
some selectivity for precancerous and cancerous cells, although MAL
is more lipophilic and absorbed by deeper skin tissues than ALA
(10). These agents are converted
into the highly reactive protoporphyrin IX (PpIX) (11). Notably, rapidly proliferating cells,
such as cancer cells, convert more ALA to PpIX in their
mitochondria than their non-transformed counterparts in the
epidermis. When neoplastic cells are exposed to light at various
wavelengths (classically between 570 and 670 nm) (10), ROS are created which then damage and
destroy target cells. In clinical practice, both red (most commonly
630 nm) and blue (410-420 nm) wavelength light sources are
utilized. Moreover, ‘daylight PDT’ is also employed, using natural
sunlight to activate these photosensitizing agents (10–12).
Finally, third generation photosensitizers are antibody-directed
and were developed to have a strong affinity for tumor cells,
causing less damage to the surrounding tissues (5,6)
The clinical uses of PDT span from multiple
neoplastic indications, skin disorders and ocular conditions.
Considering the two-dose nature of the treatment, topical PDT is
frequently favored over other field therapy options, even if
reportedly less effective than other field therapy options such as
5-fluorouracil (5-FU) (13). It has
been utilized for other dermatologic conditions, including
psoriasis, basal cell carcinoma, verruca, and extramammary Paget's
disease (14). Systemic PDT is also
used to prevent severe vision loss in wet macular degeneration by
targeting the vasculature that gives rise to the condition
(15). Additionally, PDT has been
used in patients with Barrett's esophagus, as well as cancers of
the mouth and lungs (16).
However, there are multiple reports of side effects
of PDT, including redness, pain and photosensitivity. Relevant to
this review, PDT exerts immunomodulatory effects that could limit
its effectiveness (17–23). This review paper highlights our
current understanding of the immunosuppressive effects of PDT with
ALA and will specifically focus on pharmacologic strategies to
mitigate this unwanted effect, which has not specifically been
reviewed in the current literature. The aim is to increase
understanding of this process, which will be especially helpful in
improving the effectiveness of topical PDT in treating tumors or
pre-cancerous lesions.
Overview of PDT-induced
immunosuppression
Despite the numerous indications that PDT is
effectively used, there is strong evidence to suggest that PDT
exerts local immunosuppressive effects (17–23).
Those effects impact the overall success of the modality. Efforts
to mitigate the resulting immunosuppressive effects could help
improve efficacy or expand indications for PDT. For example,
topical PDT has been associated with reactivation of orolabial
herpes simplex virus (HSV) infections (24). Moreover, there is evidence from the
literature, albeit anecdotal, that more aggressive melanomas and
non-melanoma skin cancers can arise in PDT-treated skin (25,26).
However, this is a controversial point due to confounding variables
such as the fact that skin treated with PDT was more likely to
develop skin cancer. Notably, more solid evidence has been provided
by preclinical studies. PDT was initially discovered to be
immunosuppressive in mice using dinitrofluorobenzene (DNFB), a
sensitizing agent that induces a contact hypersensitivity response.
In this study, mice treated with PDT showed a significant decrease
in their cell-mediated immune response to DNFB applied to the
PDT-treated area (20).
When PDT is applied to an area, there is a rapid
invasion of neutrophils, mast cells, monocytes, and macrophages to
destroy abnormal tumor cells (27),
demonstrating a robust anti-tumor immune response in the host.
However, in addition to its immuno-stimulatory effects, PDT causes
an increase in regulatory T cells (Tregs) and myeloid-derived
suppressor cells (MDSCs) (28),
which serve as counterregulatory mechanisms to impede the effector
responses of the immune system (29). MDSCs secrete the immunosuppressive
cytokines such as transforming growth factor-beta (TGF-β) and
interleukin 10 (IL-10), which allows macrophages that migrate into
a tumor to express inhibitor molecules (30) and converts Tregs to an active form,
inhibiting effector T-cell proliferation (31). Tregs also suppress effector T cells
directly, produce IL-10, and enhance MDSCs and regulate their
differentiation through TGF-β signaling (31). Hence, MDSCs and Tregs effectively
‘cross-talk’ through the B7-H1 pathway which directly suppresses
T-cell proliferation to form an immunosuppressive microenvironment
within tumors (29,32) and thus favors tumor growth.
The exact mechanisms by which PDT exerts
immunomodulatory effects is at present unclear. However, through
its ability to generate ROS, PDT has been shown in cell lines and
pre-clinical studies to be a potent generator of the lipid
mediator, Platelet-activating factor
(1-alkyl-2-acetyl-glycerophosphocholine, PAF) (33). Of note, exogenous PAF is known to
induce systemic immunosuppression via its ability to generate Tregs
(33). PAF upregulates IL-10, which
inhibits the host immune response through the mechanism discussed
earlier, but also leads to an increase in Tregs through a
COX-2-mediated process (34,35). COX-2 generates prostaglandins such as
PGE2 which promote blood vessel formation, allowing increased
angiogenesis resulting in enhanced growth/proliferation of
experimental tumor types, including melanoma (36,37). The
activation of COX-2 is also connected to the induction and
expansion of MDSCs (38) and
increasing the levels of immunosuppressive Tregs (35), this mechanism was shown to exhibit
its effects downstream of PAF in experimental models (34), with the overall pathway being
PAFàCOX-2àTregs. Though demonstrated in mice, it has not yet been
verified whether or not this pathway exists in humans.
Although PDT is an effective option in treating
cancerous and precancerous cells, relapses can occur following
treatment (39), likely due to the
immunosuppressive side effects. This review is focused on studies,
summarized in Table I, that have
shown how blocking the immunosuppression can result in enhanced
efficacy of PDT in decreasing tumors, pre-cancerous cells, and
morbidity for patients. The relationship between MDSCs, Tregs, and
COX-2 offers three targets for inhibiting PDT-induced
immunosuppression, and illustrated in Fig. 1. These are the potentially targetable
methods that will be explored in this review.
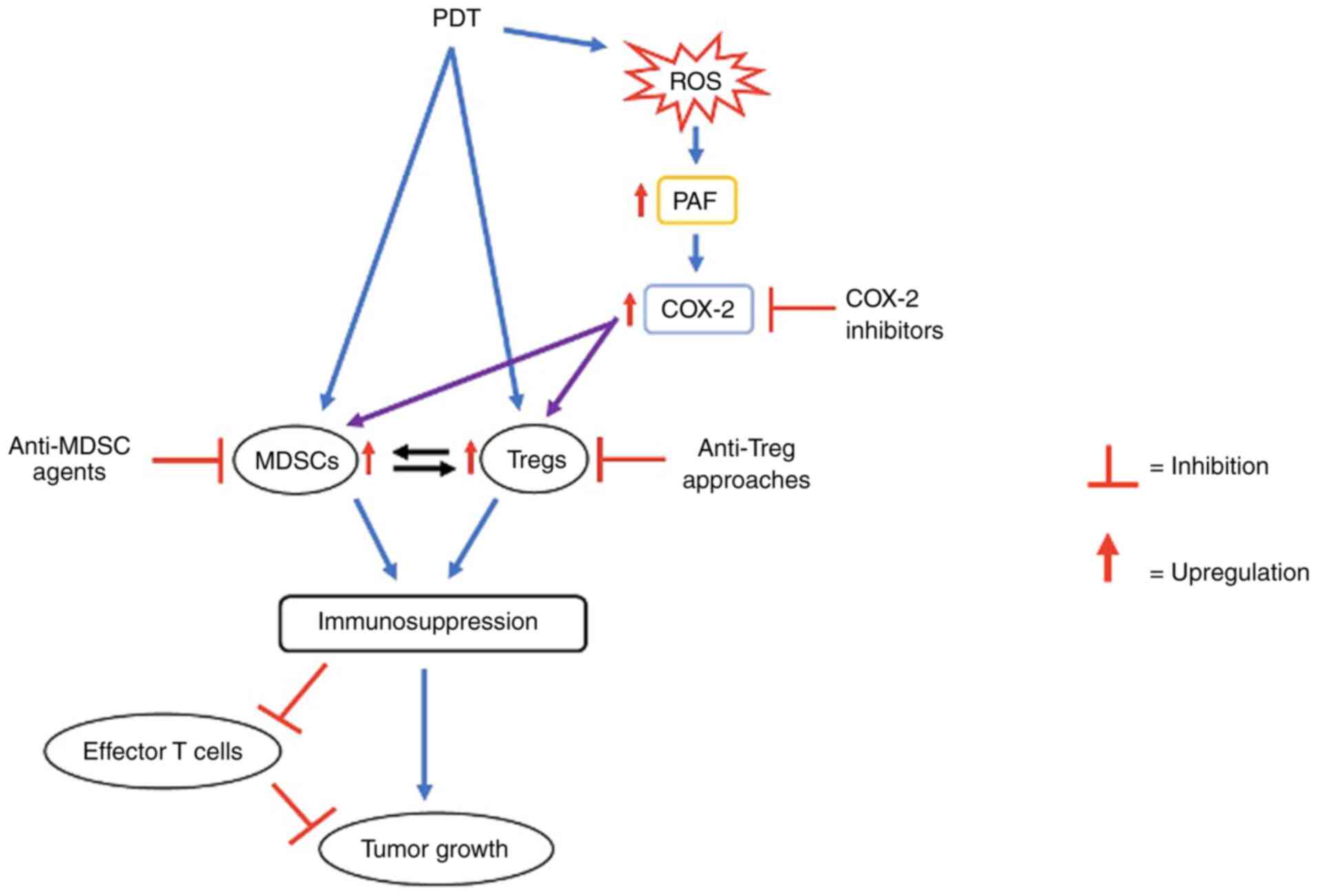 | Figure 1.Schematic representation of the
mechanisms involved in reducing the therapeutic efficacy of PDT. In
this model, PDT, due to its prooxidant effects, generates ROS. As a
consequence of this, PAF lipids are produced. The immunosuppressive
cell types, Tregs and MDSCs, are upregulated via the downstream
COX-2 pathway. This results in the immunosuppression and inhibition
of effector T cells, leading to the reduced antitumor effect of
PDT. Possible approaches to circumvent this PDT-induced
immunosuppression and increase its efficacy include COX-2
inhibitors, anti-Treg approaches and anti-MDSC agents. PDT,
photodynamic therapy; ROS, reactive oxygen species; PAF,
platelet-activating factor; COX-2, cyclooxygenase-2; Tregs,
regulatory T cells; MDSCs, myeloid-derived suppressor cells. |
 | Table I.Summary of studies detailing enhanced
results by inhibiting PDT-induced immunosuppression. |
Table I.
Summary of studies detailing enhanced
results by inhibiting PDT-induced immunosuppression.
| First author,
year | Category | Model | Medication | Results | (Refs.) |
|---|
| Castano et
al, 2008 | Inhibition of
Tregs | Mouse |
Cyclophosphamide | Low-dose
cyclophosphamide + PDT led to permanent antitumor effects against a
highly-metastatic reticulum cell sarcoma by decreasing the number
of Tregs | (27) |
| Reginato et
al, 2013 | Inhibition of
Tregs | Mouse |
Cyclophosphamide | Levels of Tregs and
TGF-β increased with PDT, but were attenuated to baseline levels
with cyclophosphamide + PDT, which increased the effectiveness of
PDT treatment | (21) |
| Oh et al,
2017 | Inhibition of
Tregs | Mouse | Anti-CD25
antibodies | Anti-CD25 therapy
specifically decreased Treg populations in melanoma tumors,
rendering PDT more effective. The systemic immune response was
unaffected | (44) |
| Wachowska et
al, 2020 | Inhibition of
Tregs | Mouse | Epacadostat | Tregs were
decreased to control levels and neutrophil infiltration of tumors
increased, but toxic systemic inflammation was also induced | (18) |
| Korbelik et
al, 2015 | Inhibition of
MDSCs | Mouse | ATRA | Levels of MDSCs
were reduced following injection of PDT-treated cells and ATRA This
extended the time that PDT slowed the growth of the tumor | (17) |
| Mai et al,
2020 | Inhibition of
MDSCs | Mouse | PM-IR780-Met | PM-IR780-Met
decreased oxygen consumption by inhibiting the mitochondrial
respiratory chain. It reduced infiltration of MDSCs into tumors and
increased the levels of effector T cells | (56) |
| Sun et al,
2020 | Inhibition of
MDSCs | Mouse | Sorafenib | When combined with
PDT, sorafenib increased T cell infiltration and inhibited tumor
growth more effectively than PDT alone, and in some cases decreased
tumor size | (28) |
| Ferrario et
al, 2002 | Inhibition of
COX-2 | Mouse | NS-398 | NS-398, a COX-2
inhibitor, was given in combination with PDT, and enhanced PDT
response by resulting in an increased antitumor effect without
causing an increased response in non-tumor cells | (60) |
| Makowski et
al, 2003 | Inhibition of
COX-2 | Mouse | COX-2
inhibitor | Tumor growth was
significantly inhibited, mouse survival rates were increased and a
higher complete cure rate was observed compared with PDT alone when
the COX-2 inhibitor was given chronically after treatment with
PDT | (39) |
| Van der Geer and
Krekels, 2009 | Inhibition of
COX-2 | Humans | Diclofenac | Improved efficacy
of PDT + diclofenac in reducing actinic keratoses, but with
increased side effects. | (64) |
| Rosenberg et
al, 2019 | Increasing
CD4+ T cells | Humans | Calcipotriol plus
5-FU | The combination had
a pro-inflammatory effect, which may have potential as an adjunct
to PDT | (68) |
| Thanos et
al, 2012 | Replenishment of
intracellular ATP | Humans | Nicotinamide | Nicotinamide may
have potential as a low-cost method of reducing PDT-induced
immunosuppression and increasing its effectiveness | (72) |
| Frost et al,
2011 | Decreasing oxygen
consumption | Humans | Reducing radiation
rate | Reducing the rate
of radiation decreased oxygen consumption and was as effective as
high-rate PDT at clearing tumors | (76) |
Inhibition of Tregs
Tregs are among the suppressive immunophenotypes
which have been implicated in mediating immunosuppression or immune
escape mechanisms (40). Multiple
studies have demonstrated that Tregs proliferate at a larger extent
in the tumor microenvironment, implicated in tumor progression and
metastasis, as well as counterbalancing the anti-tumor immune
responses of cancer therapies against malignancies including skin
cancer (41,42). To that end, Treg reprogramming has
been explored as a critical approach to circumvent
immunosuppressive mechanisms to control tumor growth and enhance
the efficacy of therapeutic regimens (42,43). As
stated earlier, PDT activates the host's innate and adaptive immune
systems, leading to a migration of inflammatory cells such as
neutrophils and dendritic cells into the target area. However, a
simultaneous immunosuppressive effect takes place in the tumor
microenvironment, allowing it to evade the immune response. The
first method of blocking this is to target the immunosuppressive
Tregs.
One method is the administration of cyclophosphamide
with PDT, the efficacy of which has been shown in multiple
preclinical studies (17,21,27). In
a mouse model of a highly-metastatic reticulum cell sarcoma, PDT
plus cyclophosphamide administered at a low dose caused a decrease
in Tregs and increased the immune system's response to tumor growth
(21,27). PDT alone caused an increase in
survival and tumor regression among mice, but no permanent cures.
Cyclophosphamide alone also provided a survival advantage and
reduced Tregs but led to no permanent cures. However, when PDT and
cyclophosphamide were given together, the permanent cure rate was
70% (27). This synergistic effect
was attributed to cyclophosphamide's ability to decrease the Treg
population and prevent the immunosuppression induced by PDT
(27).
In another study testing the effects of
cyclophosphamide plus PDT, mice with colon carcinoma CT26 tumors
were treated with either PDT alone or in combination with
cyclophosphamide. The levels of Tregs were measured throughout
treatment. Moreover, because TGF-β is an immunosuppressive cytokine
that both promotes the development of Tregs and allows Tregs to
regulate MDSCs (31), levels of TGF-
β were also measured. The study showed that PDT alone leads to an
increase in the Treg population, but that this effect is negated by
the administration of cyclophosphamide before PDT, which brings
down the Tregs to a level comparable to that of control mice (mice
that were not inoculated with cancer) (21). Additionally, mice treated with PDT
alone had an elevation in TGF-β, while mice treated with PDT and
cyclophosphamide showed a significant decrease in TGF- β levels
that was similar to control mice (21). Furthermore, untreated mice survived a
median of 25 days after tumor inoculation, while the median
survival for mice treated with PDT alone was 29 days (21). However, 9 out of 10 mice treated with
both cyclophosphamide and PDT displayed tumor regression, and all 9
of those mice survived over 90 days (21).
Tregs were also selectively depleted in the tumor
microenvironment of mice in a study by Oh et al (44). This was achieved by injecting
anti-CD25 antibodies that were conjugated to a photosensitizer,
which induces apoptosis in Tregs (44). The purpose of this study was to find
a method that decreased tumor-associated Treg populations without
inducing severe autoimmune or hyper-immune systemic responses.
Overall, tumor growth was inhibited by PDT plus the CD25-targeted
therapy (44). The local
tumor-associated Treg population was depleted without systemic side
effects and the combination caused significant anti-tumor immunity
at the site of the melanoma (44).
The mice did not exhibit significant hyper-immune responses and
continued to have an adaptive immune response against the influenza
virus, demonstrating that the systemic immune response was not
significantly affected (44).
Another method to reduce the number of Tregs is to
inhibit indoleamine 2,3-dioxygenase 1 (IDO). IDO is a
heme-containing enzyme located in multiple tissues of the body that
is expressed during inflammatory diseases and tumorigenesis
(45). IDO is elevated after PDT and
activates Tregs, preventing their conversion to effector T cells
(46). Therefore, inhibiting IDO
decreases Tregs and activates IL-6, which induces an acute
inflammatory response (18). One
study inoculated carcinoma tumor cells in murine models and showed
that targeting IDO with inhibitors such epacadostat decreases Treg
numbers to control levels and causes neutrophil infiltration of
tumors, but also induces severe systemic inflammation at high doses
of epacadostat through an IL-6 mechanism (18). The toxic reaction can be prevented
with anti-IL-6 antibodies, but this negates the anti-tumor effect
of the PDT/epacadostat combination, making its efficacy comparable
to PDT alone (18). While this side
effect is concerning, other studies have inhibited IDO through
other methods without exhibiting the same toxicity. In one study,
IDO was inhibited with a protoporphyrin IX and NLG919 conjugate in
mouse models inoculated with breast cancer cells (47). This amplified PDT's immune response
in tumor cells without significant toxicity of major organs
(47). This study did not measure
changes in Treg levels, but did report increased CT8+ T lymphocyte
levels (47), implying that
decreased Tregs likely played a role in augmented anti-tumor
immunity. The role that IDO plays in the regulation of
inflammation, both within the tumor and systemically, is poorly
understood, and this method requires further investigation in
preclinical and clinical trials to prevent toxic systemic side
effects.
Inhibition of MDSCs
MDSCs are a heterogeneous population of immature
myeloid cells which have been implicated to play important roles
not only in pathological conditions, including cancer progression,
but also in impacting the efficacy of anti-cancer agents (48–50).
Importantly, these MDSC-induced effects are largely governed by
their ability to induce immunosuppression, mediated via the
orchestration of multiple signaling pathways as well as
interactions with several immune cells and mediators (51–53).
Therefore, strategies to target MDSCs have been hypothesized as one
of the promising approaches to overcome immunosuppressive effects,
restore anti-tumoral immunity response and/or enhance the efficacy
of therapeutic agents. However, this is closely tied to the
depletion of other immunophenotypes such as Tregs, thus, it is
difficult, if not impossible, to affect one without affecting the
other cell type(s).
One study by Korbelik et al revealed an
improved cure rate in squamous cell carcinomas when all trans
retinoic acid (ATRA) was administered with a vaccine made from
tumor cells treated with PDT (17).
In this study, squamous cell carcinoma cells were treated with PDT
and injected into mice bearing the same squamous cell carcinoma
tumors. Mice were also injected with ATRA, whose purpose was to
facilitate the conversion of immunosuppressive MDSCs to a
non-suppressive phenotype (54). In
this study, ATRA reduced the number of MDSCs by causing their
differentiation into mature myeloid cells, and overall made the PDT
vaccine more effective by extending the time that PDT slowed the
growth of the tumor (17). Thus,
decreasing the MDSC population allows PDT to be effective against
tumor cells for a longer period of time and reduce its overall
size.
Reversing a hypoxic state in a tumor was also shown
to impede the MDSC-regulated pathway (55,56).
Hypoxia is produced by consuming oxygen after making ATP (adenosine
triphosphate), thus, reversing it can be achieved by interfering
with oxidative phosphorylation (57,58).
This study used platelet membranes as nano-carriers called
PM-IR780-Met, which included encapsulated metformin whose role was
to decrease oxygen consumption by inhibiting the mitochondrial
respiratory chain. This ultimately reduced the levels of MDSCs and
their infiltration into tumor tissues. In turn, this reduced the
number of Tregs being recruited by MDSCs and increased the
infiltration of effector T cells into the tumor, lymph nodes, and
spleen (56). Thus, it can be
inferred that the nano-carrier increased both the anti-tumor and
systemic immune responses. Furthermore, treatment with PDT without
the nanocarrier decreased tumor growth from 7.5-fold to 4-fold, but
the addition of metformin caused growth to decrease to 1.1-fold,
showing a superior anti-tumor response (56). In addition to preventing the
immunosuppressive effects of PDT, a constant supply of oxygen was
supplied by the nano-carrier, and this allowed more ROS to be
generated during PDT, rendering PDT more effective against tumors
(56).
Sorafenib administered with low-dose PDT has been
exploited as another method that enhances the T cell-mediated
antitumor effects. Sorafenib has been shown to reduce MDSC and Treg
populations (28,59), while recruiting more
antigen-processing cells and cytotoxic T cells to the tumor
(28). When combined with PDT,
sorafenib increases T-cell infiltration and inhibits tumor growth
more effectively than PDT alone, and in some cases decreases tumor
size (28). This was attributed to
Sorafenib's ability to limit the interaction between cytotoxic CD8+
T cells and immunosuppressive cells, inducing a stronger anti-tumor
immune response.
Inhibition of COX-2
As discussed earlier, PDT has been shown in mice to
generate systemic immunosuppression through the lipid mediator PAF
in a pathway leading to increased COX-2 expression and levels of
Tregs (33,60). However, COX-1, which is
constitutively expressed in most cells, is not increased by PDT
(60). Eicosanoids and
COX-2-generated prostaglandins such as PGE2 have also been linked
to local immunosuppression (61,62).
This pathway has not yet been demonstrated in humans. Given the
availability of COX inhibitors, this strategy could serve as an
easy target to combat the PDT-induced immunosuppression and relapse
of pre-cancerous cells. In particular, selective COX-2 inhibitors
such as celecoxib are safe for short-term use and may also decrease
the painful side effects of PDT, so this method merits additional
investigation.
Although not yet tested in humans, combination
therapies involving selective COX-2 inhibitors have been shown to
improve the therapeutic effectiveness of PDT in treating solid
tumors in mice (35,39,60,63). In
one example, NS-398, a COX-2 inhibitor, was given in combination
with PDT, and caused decreased levels of PGE2 and VEGF, which
enhanced PDT's response in tumor cells of mouse carcinomas and
sarcomas and resulted in a significant increase in tumor cures
(compared to PDT alone) (60).
Furthermore, the combination did not cause an increased response in
non-tumor cells; specifically, it did not affect skin sensitization
nor did it cause increased skin damage in sites without tumor cells
(60).
In a second example by Makowski et al, there
was no increased PDT efficacy in vitro when tumor cells were
incubated with COX-2 inhibitors (39). This result was unexpected, especially
following the results of Ferrario et al that showed PDT's
effect potentiated by the addition of a COX-2 inhibitor (60). This prompted the group to conduct two
different in vivo experiments: For one set of mice the COX-2
inhibitor before PDT, and the other received it chronically after
illumination with PDT. The former showed no increased anti-tumor
response, but with the latter, there was a statistically
significant retardation of a poorly differentiated colon
adenocarcinoma C-26 tumor growth, increased mouse survival, and
higher complete cure rate compared to PDT alone (39). The proposed mechanism by Makowski
et al is that the COX-2 inhibitor decreases angiogenic
factors-which is synergistic with PDT's ability to cause vascular
damage-and triggers apoptosis in tumor cells (39). This would explain why the anti-tumor
effects were only increased when COX-2 was administered after PDT,
since the tumor would have more difficulty repairing blood vessels
following the vascular damage caused by PDT. This study
demonstrated that COX-2 inhibitors may improve the efficacy of PDT
through methods other than inhibiting immunosuppression.
Diclofenac is a nonsteroidal anti-inflammatory drug
(NSAID) that functions as a cyclooxygenase-1 (COX-1) and COX-2
inhibitor. It has been shown in small clinical trials to improve
the efficacy of PDT in reducing actinic keratoses when used as an
adjuvant therapy, likely by targeting COX-2 receptors on actinic
keratosis (64). Although not a
COX-2 selective inhibitor, diclofenac shows promise because it is
already used as a treatment on its own for actinic keratosis
(33). Adjuvant therapy would not
only make the treatment more effective, but also inhibit the
immunosuppression caused by PDT. However, more patients reported
pain, sometimes unbearable, and side effects such as pruritus,
scaling, and crusting during PDT when used in conjunction with
diclofenac (64). This might result
in the diclofenac and PDT combination being reserved for small
areas of skin.
Conclusions
With a better understanding of the mechanisms of
immunosuppression of PDT with ALA, we can inhibit them and offer
patients more effective treatments with potentially fewer side
effects. This is the first review to specifically address methods
of inhibiting immunosuppression for PDT with ALA. However, multiple
options may not be considered practical due to the risk of side
effects. For example, ATRA is only regularly used by oncologists
and cyclophosphamide is only commonly used by rheumatologists,
nephrologists, and dermatologists. Although diclofenac might not be
a popular option due to side effects, the fact that it potentiated
the effects of PDT merits exploration of other non-selective
COX-inhibitors that could be used in conjunction with PDT. The
approaches that use anti-CD25, sorafenib, abatacept, and COX-2
inhibitors are more realistic in most settings. There are likely
other options, yet to be determined, that involve different
pathways for blocking PDT-induced immunosuppression.
It should be noted that other promising strategies
are being developed to augment tumor-specific production of PpIX to
include the use of topical vitamin D analogues (65). One effect of vitamin D receptor
activation involves its ability to increase Tregs (66,67),
which could result in an ‘immunosuppressive phenotype’. However,
combinations of topical vitamin D agonists (calcipotriol) with 5-FU
chemotherapy appear to result in a more pro-inflammatory effect
which has been reported to result in long-term remissions (68). Hence, the exact effects of vitamin D
as an adjunct to PDT on the skin immune system is an area of future
investigation. Additionally, topical and oral nicotinamide (vitamin
B3) replenish cellular ATP after irradiation with UV light
(69). Through this mechanism, it
has been shown to reduce the immunosuppression associated with both
high and low-dose PDT, making it more effective against actinic
keratoses and nonmelanoma skin cancers (70–73).
This is a useful discovery, as nicotinamide is low-cost, readily
available, and has few, if any side effects (74). Finally, immunosuppression has been
shown to decrease by simply reducing the rate of irradiation,
perhaps because of the decreased oxygen consumption at lower rates
(75,76). In pre-clinical trials, lower rates of
PDT (15 or 45 mWcm−2) were as effective as high-rate PDT
(75 mWcm−2) in clearing tumors (77).
Though the immunosuppressive pathways appear
complex, there is considerable rationale for pharmacologic
strategies to target this unwanted effect. Further investigations
documenting the exact level and mechanisms of PDT-induced
immunosuppression demonstrated in mice need to be pursued in
humans. In addition, there is a need for studies testing both the
ability of various strategies such as COX inhibitors to inhibit
PDT-induced immunosuppression with a clinical benefit such as
improved clearance of actinic keratosis.
Acknowledgements
Not applicable.
Funding
This research was supported in part by grants from
the National Institutes of Health (grant nos. R01 HL062996 and
ES031087) and Veteran's Administration Merit Award (grant no.
5I01BX000853).
Availability of data and materials
Not applicable.
Authors' contributions
SB and CAR developed the concept and contributed to
the majority of the manuscript writing. RPS and JBT made final
revisions to the manuscript. Data authentication is not applicable.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
PDT
|
photodynamic therapy
|
|
ROS
|
reactive oxygen species
|
|
ALA
|
5-aminolevulinic acid
|
|
MAL
|
methyl aminolevulinate
|
|
PpIX
|
protoporphyrin IX
|
|
5-FU
|
5-fluorouracil
|
|
DNFB
|
dinitrofluorobenzene
|
|
MDSCs
|
myeloid-derived suppressor cells
|
|
Tregs
|
regulatory T cells
|
|
HSV
|
herpes simplex virus
|
|
TGF-β
|
transforming growth factor β
|
|
IL-10
|
interleukin-10
|
|
PAF
|
platelet-activating factor
|
|
COX-2
|
cyclooxygenase-2
|
|
PGE2
|
prostaglandin E2
|
|
IFNγ
|
interferon γ
|
|
IDO
|
indoleamine 2,3-dioxygenase
|
|
ATRA
|
all trans retinoic acid
|
|
ATP
|
adenosine triphosphate
|
|
PM-IR780-Met
|
platelet membranes as nano-carriers to
co-encapsulate metformin and IR780
|
|
NSAID
|
nonsteroidal anti-inflammatory
drugs
|
|
COX-1
|
cyclooxygenase-1
|
References
|
1
|
Dougherty TJ and Marcus SL: Photodynamic
therapy. Eur Cancer. 28:1734–1742. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sharman WM, Allen CM and van Lier JE:
Photodynamic therapeutics: Basic principles and clinical
applications. Drug Disc Today. 4:507–517. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ochsner M: Photophysical and
photobiological processes in the photodynamic therapy of tumours. J
Photochem Photobiol B. 39:1–18. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moan J: Porphyrin photosensitization and
phototherapy. Photochem Photobiol. 43:681–690. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Allison RR, Downie GH, Cuenca R, Hu XH,
Childs CJ and Sibata CH: Photosensitizers in clinical PDT.
Photodiagnosis Photodyn Ther. 1:27–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kwiatkowski S, Knap B, Przystupski D,
Saczko J, Kędzierska E, Knap-Czop K, Kotlińska J, Michel O,
Kotowski K and Kulbacka J: Photodynamic therapy-mechanisms,
photosensitizers and combinations. Biomed Pharmacother.
106:1098–1107. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Abrahamse H and Hamblin MR: New
photosensitizers for photodynamic therapy. Biochem J. 473:347–364.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang J, Jiang C, Figueiró Longo JP,
Azevedo RB, Zhang H and Muehlmann LA: An updated overview on the
development of new photosensitizers for anticancer photodynamic
therapy. Acta Pharm Sin B. 8:137–146. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chatterjee DK, Fong LS and Zhang Y:
Nanoparticles in photodynamic therapy: An emerging paradigm. Adv
Drug Deliv Rev. 60:1627–1637. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gold M: ALA-PDT and MAL-PDT: What makes
them different. J Clin Aesthet Dermatol. 2:44–47. 2009.
|
|
11
|
Cantisani C, Paolino G, Faina V, Frascani
F, Cantoresi F, Bianchini D, Gilda Fazia and Stefano Calvieri:
Overview on topical 5-ALA photodynamic therapy use for non melanoma
skin cancers. Int J Photoen. 2014:3048622014. View Article : Google Scholar
|
|
12
|
Morton CA and Braathen LR: Daylight
photodynamic therapy for actinic keratoses. Am J Clin Dermatol.
19:647–656. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wehner MR: Comparing the efficacy of field
treatments for actinic keratosis: A critical appraisal of a
randomized trial in the New England Journal of Medicine. Br J
Dermatol. 182:1343–1344. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taub AF: Photodynamic therapy in
dermatology: History and horizons. J Drugs Dermatol. 3 (Suppl
1):S8–S25. 2004.PubMed/NCBI
|
|
15
|
Armbrecht AM: A prospective study of
visual function and quality of life following PDT in patients with
wet age related macular degeneration. Br J Ophthalmol.
88:1270–1273. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
McBride G: Studies expand potential uses
of photodynamic therapy. J Natl Cancer Inst. 94:1740–1742. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Korbelik M, Banáth J and Saw K:
Immunoregulatory cell depletion improves the efficacy of
photodynamic therapy-generated cancer vaccines. Int J Mol Sci.
16:27005–27014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wachowska M, Stachura J, Tonecka K, Fidyt
K, Braniewska A, Sas Z, Kotula I, Rygiel TP, Boon L, Golab J and
Muchowicz A: Inhibition of IDO leads to IL-6-dependent systemic
inflammation in mice when combined with photodynamic therapy.
Cancer Immunol Immunother. 69:1101–1112. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Reddan JC, Anderson CY, Xu H, Hrabovsky S,
Freye K, Fairchild R, Tubesing KA and Elmets CA: Immunosuppressive
effects of silicon phthalocyanine photodynamic therapy. Photochem
Photobiol. 70:72–77. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lynch DH, Haddad S, King VJ, Ott MK,
Straight R and Jolles CJ: Systemic immunosuppression induced by
photodynamic therapy (PDT) is adoptively transferred by
macrophages. Photochem Photobiol. 49:453–458. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Reginato E, Mroz P, Chung H, Kawakubo M,
Wolf P and Hamblin MR: Photodynamic therapy plus regulatory T-cell
depletion produces immunity against a mouse tumour that expresses a
self-antigen. Br J Cancer. 109:2167–2174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mroz P and Hamblin MR: The
immunosuppressive side of PDT. Photochem Photobiol Sci. 10:751–758.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Falk-Mahapatra R and Gollnick SO:
Photodynamic therapy and immunity: An update. Photochem Photobiol.
96:550–559. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nobbe S, Trüeb RM, French LE and Hofbauer
GFL: Herpes simplex virus reactivation as a complication of
photodynamic therapy. Photodermatol Photoimmunol Photomed.
27:51–52. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wolf P, Fink-Puches R, Reimann-Weber A and
Kerl H: Development of malignant melanoma after repeated topical
photodynamic therapy with 5-Aminolevulinic acid at the exposed
site. Dermatology. 194:53–54. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fiechter S, Skaria A, Nievergelt H, Anex
R, Borradori L and Parmentier L: Facial basal cell carcinomas
recurring after photodynamic therapy: A retrospective analysis of
histological subtypes. Dermatology. 224:346–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Castano AP, Mroz P, Wu MX and Hamblin MR:
Photodynamic therapy plus low-dose cyclophosphamide generates
antitumor immunity in a mouse model. Proc Natl Acad Sci USA.
105:5495–5500. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sun X, Cao Z, Mao K, Wu C, Chen H, Wang J,
Wang X, Cong X, Li Y, Meng X, et al: Photodynamic therapy produces
enhanced efficacy of antitumor immunotherapy by simultaneously
inducing intratumoral release of sorafenib. Biomaterials.
240:1198452020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lindau D, Gielen P, Kroesen M, Wesseling P
and Adema GJ: The immunosuppressive tumour network: Myeloid-derived
suppressor cells, regulatory T cells and natural killer T cells.
Immunology. 138:105–115. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang Y, Ma Y, Fang Y, Wu S, Liu L, Fu D
and Shen X: Regulatory T cell: A protection for tumour cells. J
Cell Mol Med. 16:425–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee CR, Kwak Y, Yang T, Han JH, Park SH,
Ye MB, Lee W, Sim KY, Kang JA, Kim YC, et al: Myeloid-derived
suppressor cells are controlled by regulatory T cells via TGF-β
during murine colitis. Cell Rep. 17:3219–3232. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fujimura T, Kambayashi Y and Aiba S:
Crosstalk between regulatory T cells (Tregs) and myeloid derived
suppressor cells (MDSCs) during melanoma growth. Onco Immunol.
1:1433–1434. 2012.PubMed/NCBI
|
|
33
|
Ferracini M, Sahu RP, Harrison KA, Waeiss
RA, Murphy RC, Jancar S, Konger RL and Travers JB: Topical
photodynamic therapy induces systemic immunosuppression via
generation of platelet-activating factor receptor ligands. J Invest
Dermatol. 135:321–323. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sahu RP, Petrache I, van Demark MJ, Rashid
BM, Ocana JA, Tang Y, Yi Q, Turner MJ, Konger RL and Travers JB:
Cigarette smoke exposure inhibits contact hypersensitivity via the
generation of platelet-activating factor agonists. J Immunol.
190:2447–2454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Q, Yao Y, Konger RL, Sinn AL, Cai S,
Pollok KE and Travers JB: UVB radiation-mediated inhibition of
contact hypersensitivity reactions is dependent on the
platelet-activating factor system. J Invest Dermatol.
128:1780–1787. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zelenay S, van der Veen AG, Böttcher JP,
Snelgrove KJ, Rogers N, Acton SE, Chakravarty P, Girotti MR, Marais
R, Quezada SA, et al: Cyclooxygenase-dependent tumor growth through
evasion of immunity. Cell. 162:1257–1270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ke J, Yang Y, Che Q, Jiang F, Wang H, Chen
Z, Zhu M, Tong H, Zhang H, Yan X, et al: Prostaglandin E2 (PGE2)
promotes proliferation and invasion by enhancing SUMO-1 activity
via EP4 receptor in endometrial cancer. Tumor Biol. 37:12203–12211.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fujita M, Kohanbash G, Fellows-Mayle W,
Hamilton RL, Komohara Y, Decker SA, Ohlfest JR and Okada H: COX-2
blockade suppresses gliomagenesis by inhibiting myeloid-derived
suppressor cells. Cancer Res. 71:2664–2674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Makowski M, Grzela T, Niderla J, Łazarczyk
M, Mróz P, Kopeé M, Legat M, Strusińska K, Koziak K, Nowis D, et
al: Inhibition of cyclooxygenase-2 indirectly potentiates antitumor
effects of photodynamic therapy in mice. Clin Cancer Res.
9:5417–5422. 2003.PubMed/NCBI
|
|
40
|
Togashi Y and Nishikawa H: Regulatory T
cells: Molecular and cellular basis for immunoregulation. Curr Top
Microbiol Immunol. 410:3–27. 2017.PubMed/NCBI
|
|
41
|
Najafi M, Farhood B and Mortezaee K:
Contribution of regulatory T cells to cancer: A review. J Cell
Physiol. 234:7983–7993. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Moreno Ayala MA, Li Z and DuPage M: Treg
programming and therapeutic reprogramming in cancer. Immunology.
157:198–209. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yan S, Zhang Y and Sun B: The function and
potential drug targets of tumour-associated Tregs for cancer
immunotherapy. Sci China Life Sci. 62:179–186. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oh DS, Kim H, Oh JE, Jung HE, Lee YS, Park
JH and Lee HK: Intratumoral depletion of regulatory T cells using
CD25-targeted photodynamic therapy in a mouse melanoma model
induces antitumoral immune responses. Oncotarget. 8:47440–47453.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mellor AL, Lemos H and Huang L:
Indoleamine 2,3-Dioxygenase and tolerance: Where are we now? Front
Immunol. 8:13602017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Baban B, Chandler PR, Sharma MD, Pihkala
J, Koni PA, Munn DH and Mellor AL: IDO activates regulatory T cells
and blocks their conversion into Th17-Like T cells. J Immuno.
183:2475–2483. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Huang Z, Wei G, Zeng Z, Huang Y, Huang L,
Shen Y, Sun X, Xu C and Zhao C: Enhanced cancer therapy through
synergetic photodynamic/immune checkpoint blockade mediated by a
liposomal conjugate comprised of porphyrin and IDO inhibitor.
Theranostics. 9:5542–5557. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Consonni FM, Porta C, Marino A, Pandolfo
C, Mola S, Bleve A and Sica A: Myeloid-derived suppressor cells:
Ductile targets in disease. Front Immunol. 10:9492019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pawelec G, Verschoor CP and
Ostrand-Rosenberg S: Myeloid-derived suppressor cells: Not only in
tumor immunity. Front Immunol. 10:10992019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zuo H, Hou Y, Yu Y, Li Z, Liu H, Liu C, He
J and Miao L: Circumventing myeloid-derived suppressor
cell-mediated immunosuppression using an oxygen-generated and
-economized nanoplatform. ACS ACS Appl Mater Interfaces.
12:55723–55736. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Groth C, Hu X, Weber R, Fleming V,
Altevogt P, Utikal J and Umansky V: Immunosuppression mediated by
myeloid-derived suppressor cells (MDSCs) during tumour progression.
Br J Cancer. 120:16–25. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ostrand-Rosenberg S, Sinha P, Beury DW and
Clements VK: Cross-talk between myeloid-derived suppressor cells
(MDSC), macrophages, and dendritic cells enhances tumor-induced
immune suppression. Semin Cancer Biol. 22:275–281. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bruno A, Mortara L, Baci D, Noonan DM and
Albini A: Myeloid derived suppressor cells interactions with
natural killer cells and pro-angiogenic activities: Roles in tumor
progression. Fronti Immunol. 10:7712019. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Iclozan C, Antonia S, Chiappori A, Chen DT
and Gabrilovich D: Therapeutic regulation of myeloid-derived
suppressor cells and immune response to cancer vaccine in patients
with extensive stage small cell lung cancer. Cancer Immunol
Immunother. 62:909–918. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Larue L, Myrzakhmetov B, Ben-Mihoub A,
Moussaron A, Thomas N, Arnoux P, Baros F, Vanderesse R, Acherar S
and Frochot C: Fighting hypoxia to improve PDT. Pharmaceuticals
(Basel). 12:1632019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Mai X, Zhang Y, Fan H, Song W, Chang Y,
Chen B, Shi J, Xin X, Teng Z, Sun J and Teng G: Integration of
immunogenic activation and immunosuppressive reversion using
mitochondrial-respiration-inhibited platelet-mimicking
nanoparticles. Biomaterials. 232:1196992020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou Z, Zhang B, Wang H, Yuan A, Hu Y and
Wu J: Two-stage oxygen delivery for enhanced radiotherapy by
perfluorocarbon nanoparticles. Theranostics. 8:4898–4911. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ji C, Lu Z, Xu Y, Shen B, Yu S and Shi D:
Self-production of oxygen system CaO2/MnO2
@PDA-MB for the photodynamic therapy research and switch-control
tumor cell imaging. J Biomed Mater Res B Appl Biomater.
106:2544–2552. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cao M, Xu Y, Youn J, Cabrera R, Zhang X,
Gabrilovich D, Nelson DR and Liu C: Kinase inhibitor Sorafenib
modulates immunosuppressive cell populations in a murine liver
cancer model. Lab Invest. 91:598–608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ferrario A, von Tiehl K, Wong S, Luna M
and Gomer CJ: Cyclooxygenase-2 inhibitor treatment enhances
photodynamic therapy-mediated tumor response. Cancer Res.
62:3956–3961. 2002.PubMed/NCBI
|
|
61
|
Zhang Z, Huang S, Wu S, Qi J, Li W, Liu S,
Cong Y, Chen H, Lu L, Shi S, et al: Clearance of apoptotic cells by
mesenchymal stem cells contributes to immunosuppression via PGE2.
EBioMedicine. 45:341–350. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim SH, Roszik J, Cho S-N, Ogata D, Milton
DR, Peng W, Menter DG, Ekmekcioglu S and Grimm EA: The COX2
effector microsomal PGE2 synthase 1 is a regulator of
immunosuppression in cutaneous melanoma. Clin Cancer Res.
25:1650–1663. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ferrario A and Gomer CJ: Targeting the
tumor microenvironment using photodynamic therapy combined with
inhibitors of cyclooxygenase-2 or vascular endothelial growth
factor. Methods Mol Biol. 635:121–132. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Van der Geer S and Krekels GAM: Treatment
of actinic keratoses on the dorsum of the hands: ALA-PDT versus
diclofenac 3% gel followed by ALA-PDT. A placebo-controlled,
double-blind, pilot study. J Dermatolog Treat. 20:259–265. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Rollakanti K, Anand S and Maytin EV:
Topical calcitriol prior to photodynamic therapy enhances treatment
efficacy in non-melanoma skin cancer mouse models. Proc SPIE Int
Soc Opt Eng. 9308:93080Q2015.PubMed/NCBI
|
|
66
|
Ni C, Gan X, Li X, Sun H, Chen Z and Lu H:
Vitamin D alleviates acute graft-versus-host disease through
promoting the generation of Foxp3+ T cells. Ann Transl Med.
7:7482019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
van der Aar AM, Sibiryak DS, Bakdash G,
van Capel TM, van der Kleij HP, Opstelten DJ, Teunissen MB,
Kapsenberg ML and de Jong EC: Vitamin D3 targets epidermal and
dermal dendritic cells for induction of distinct regulatory T
cells. J Allergy Clin Immunol. 127:1532–1540.e7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Rosenberg AR, Tabacchi M, Ngo KH,
Wallendorf M, Rosman IS, Cornelius LA and Demehri S: Skin cancer
precursor immunotherapy for squamous cell carcinoma prevention. JCI
Insight. 4:e1254762019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Park J, Halliday GM, Surjana D and Damian
DL: Nicotinamide prevents ultraviolet radiation-induced cellular
energy loss. Photochem Photobiol. 86:942–948. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Gensler HL: Prevention of
photoimmunosuppression and photocarcinogenesis by topical
nicotinamide. Nutr Cancer. 29:157–162. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Gensler HL, Williams T, Huang AC and
Jacobson EL: Oral niacin prevents photocarcinogenesis and
photoimmunosuppression in mice. Nutr Cancer. 34:36–41. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Thanos SM, Halliday GM and Damian DL:
Nicotinamide reduces photodynamic therapy-induced immunosuppression
in humans. Br J Dermatol. 167:631–636. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Surjana D, Halliday GM, Martin AJ, Moloney
FJ and Damian DL: Oral nicotinamide reduces actinic keratoses in
phase II double-blinded randomized controlled trials. J Invest
Dermatol. 132:1497–1500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Knip M, Douek IF, Moore WPT, Gillmor HA,
McLean AEM, Bingley PJ and Gale EA; European Nicotinamide Diabetes
Intervention Trial Group, : Safety of high-dose nicotinamide: A
review. Diabetologia. 43:1337–1345. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Foster TH, Murant RS, Bryant RG, Knox RS,
Gibson SL and Hilf R: Oxygen consumption and diffusion effects in
photodynamic therapy. Radiat Res. 126:296–303. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Frost GA, Halliday GM and Damian DL:
Photodynamic therapy-induced immunosuppression in humans is
prevented by reducing the rate of light delivery. J Invest
Dermatol. 131:962–968. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ericson MB, Sandberg C, Stenquist B,
Gudmundson F, Karlsson M, Ros A-M, Rosén A, Larkö O, Wennberg AM
and Rosdahl I: Photodynamic therapy of actinic keratosis at varying
fluence rates: Assessment of photobleaching, pain and primary
clinical outcome. Br J Dermatol. 151:1204–1212. 2004. View Article : Google Scholar : PubMed/NCBI
|















