Introduction
Melanoma is one of the leading causes of
cancer-related deaths worldwide (1).
In 2020, approximately 100,350 new melanoma cases were expected in
the United States of America (2),
indicating that melanoma incidence would increase by approximately
35.8% in 2020 compared with that in 2015 (3). Accordingly, there has been a global
increase in the incidence of melanoma. Patients with melanoma
exhibit resistance to cancer therapy. Therefore, there is an urgent
need to develop novel therapeutics and treatment strategies against
this disease (4). Notably, malignant
melanoma accounts for 65% of deaths caused by various types of
melanoma; however, early diagnosis could improve therapeutic
outcomes. Dacarbazine (DTIC) is used for treating melanoma.
However, clinical studies have reported that DTIC administration
can result in various side effects such as vomiting, nausea, and
anorexia (5). In the last few
decades, DTIC has been widely used to treat patients with melanoma,
despite observed side effects. Accordingly, DTIC is frequently
co-administered with other anticancer drugs or immunotherapeutics
to alleviate these side effects (6).
Moreover, a previous study has reported the co-administration of
DTIC and saponin, a substance of natural origin, in an attempt to
reduce the DTIC dose (7). Although
several hypotheses have been proposed, the anticancer mechanism of
DTIC needs to be comprehensively elucidated (6).
Oxyresveratrol (ORT), a naturally occurring
stilbenoid, was first isolated from the heartwood of Artocarpus
lakoocha, a monkey fruit in tropical regions (8). Previous studies have demonstrated the
proliferation-inhibitory activity of ORT against stomach and
ovarian cancers (9). Reportedly, the
enhanced tyrosinase activity associated with melanoma is mitigated
following treatment with ORT (10,11).
Furthermore, the antioxidative capacity of ORT is two-fold higher
than that of resveratrol (12). ORT
consumption may exert beneficial effects on human health, as
circulating ORT has an absorption rate of more than 50% in tissues
(13).
It is well known that malignant melanoma can develop
resistance to chemotherapy. Hence, various reports have recommended
administering additional anticancer drugs in combination with DTIC,
for malignant melanoma (14–17). However, therapeutic efficacy of the
ORT/DTIC combination in melanoma has not been previously reported.
In the present study, we examined synergistic
proliferation-inhibitory effect of the ORT/DTIC combination against
a malignant melanoma cell line.
Materials and methods
Cell culture
The human malignant melanoma cell line WM-266-4 and
the mouse macrophage cell line RAW264.7 were procured from the
Korean Cell Line Bank. The cells were cultured in Dulbecco's
modified Eagle's medium (DMEM) supplemented with 100 U/ml
penicillin, 100 µg/ml streptomycin, and 10% heat-inactivated fetal
bovine serum. The culture reagents used in this study were
purchased from Welgene. The cell lines were subcultured in 100-mm
cell culture plates (Sarstedt), with fresh DMEM replaced every
three days. All cells were cultured at 37°C in a humidified
atmosphere containing 5% CO2.
Cell viability
Cell viability was measured using the WST-1 assay
(Takara Bio). In brief, WM-266-4 cells (5×104
cells/well) were cultured in 96-well cell culture plates (SPL Life
Science) and incubated at 37°C for 3 h in a humidified atmosphere
containing 5% CO2. Next, the cells were treated with ORT
(50-170 µM; Sigma-Aldrich; Merck KGaA; ORT-treated group), DTIC
(0.5–2.5 mM; Tokyo Clinic Industry; DTIC-treated group), or an ORT
(70 µM)/DTIC (0.5–2.5 mM) combination (co-treatment group) for 48
h. After 48 h, each well was washed twice with phosphate-buffered
saline (PBS) and 10 µl WST-1 reagent was added to 90 µl DMEM. After
2 h, the absorbance of the mixture was measured at 450 nm using a
microplate reader (EL800; Bio-Tek).
Radical scavenging activity
The antioxidant potential of ORT was determined
using the 2,2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH;
Sigma-Aldrich; Merck KGaA) assay, following a previously described
method with minor modifications (18). In brief, 150 µl of DPPH solution (100
µM), dissolved in 100% ethanol, was added to each well of a 96-well
plate, followed by the addition of 50 µl of the half-maximal
inhibitory concentration (IC50) of ORT, DTIC, or the
ORT/DTIC combination. For the control group, 50 µl of 14.04 M
dimethyl sulfoxide and/or 1 M HCl was added instead of drugs. The
absorbance of the reaction mixture was measured at 520 nm, using a
microplate reader (BioTek EL800), after incubation for 10 min. The
DPPH radical scavenging activity was calculated as follows: DPPH
radical scavenging activity (%) = [(1-(A/B)] ×100, where A and B
are the absorbance values of the treatment and control groups,
respectively.
Measurement of cytokine
concentrations
In brief, RAW264.7 cells were treated with
lipopolysaccharide (LPS; Sigma-Aldrich; Merck KGaA) to induce
cytokine expression. Next, the cells were immediately treated with
the IC50 of ORT, DTIC, or the ORT/DTIC combination for
48 h. The culture medium was collected to examine interleukin
(IL)-6 (DY406; R&D Systems) and tumor necrosis factor (TNF)-α
(DY410; R&D Systems) levels using enzyme-linked immunosorbent
assay kits (R&D Systems) according to the manufacturer's
instructions.
Wound healing assay
Cell migration was analyzed using the CytoSelect
24-well wound healing assay (Cell Biolabs). First, square blocks
were inserted into each well of a 24-well plate. WM-266-4 cells
(5×105 cells/well) were seeded in 24-well plates, and
the cells were cultured for 24 h. The cells were washed twice with
PBS (pH 7.4) and treated with the IC50 of ORT, DTIC, or
the ORT/DTIC combination. After 24 h, cell migration to the wound
area was comparatively analyzed using a microscope (CK40;
Olympus).
Cell cycle analysis
In brief, WM-266-4 cells seeded onto 100-mm cell
culture plates were treated with the IC50 of ORT, DTIC,
or the ORT/DTIC combination for 24 h. The cells were harvested and
fixed with 70% (w/v) ethanol at 20°C for at least 4 h. The fixed
cells were washed thrice with PBS and treated with a propidium
iodide (PI; Sigma-Aldrich; Merck KGaA) solution (50 µg/ml PI, 0.1
mg/ml RNase [Sigma-Aldrich; Merck KGaA], and 0.1% Triton X-100
[v/v; Sigma-Aldrich; Merck KGaA] in PBS). Then, the treated cells
were incubated at 25°C for 30 min in the dark and analyzed using a
flow cytometer (FACSCalibur Cell Analyzer; BD Biosciences). The
data were analyzed using the ModFit Lt software (version 4.0;
Verity Software House, ME, USA).
Analysis of apoptosis
After seeding onto 100-mm cell culture plates,
WM-266-4 cells were treated with the IC50 of ORT, DTIC,
or the ORT/DTIC combination for 48 h and then transferred into a
conical tube. The cells were then washed thrice with PBS and
suspended in 200 µl of Annexin V binding buffer, a component of the
apoptosis detection kit (BD Biosciences). The cells were incubated
with 5 µl PI and 5 µl fluorescein isothiocyanate-conjugated Annexin
V for 20 min at room temperature in the dark. Apoptosis was
measured using a flow cytometer (FACSCalibur Cell Analyzer). The
fluorescence intensities of PI and Annexin V-FITC were analyzed at
excitation/emission wavelengths of 488/617 nm and 488/530 nm,
respectively.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from untreated WM-266-4
cells and WM 266-4 cells treated with the IC50 of ORT,
DTIC, or the ORT/DTIC combination, using the TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.). Complementary DNA
(cDNA) was synthesized from total RNA using a first-strand
synthesis kit (GeneAll). The cDNA concentration was determined
using a NanoDrop spectrophotometer (Coliri LB 915; JC Bio). qRT-PCR
was performed using CFX Connect Real-Time PCR (Bio-Rad) and
BrightGreen 2X qPCR MasterMix (ABM, Vancouver, Canada), with 50
amplification cycles. In the present study, the expression levels
of NOTCH2, NOTCH3, MYC, CCND1, BAX, CASP3 and CASP9
were comparatively analyzed among different treatment groups. The
primers used in the qRT-PCR analysis are listed in Table I. The expression levels of target
genes were normalized to those of GAPDH (19–24).
Relative gene expression levels were calculated using the
2−ΔΔCt method (25).
 | Table I.Primer sequences used for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences used for reverse
transcription-quantitative PCR.
| Gene | Forward primer
(5–3) | Reverse primer
(5–3) |
|---|
| NOTCH2 |
CGTTTAGTCAGGAATATGCGG |
GGACACATTTATGTACCCAGAG |
| NOTCH3 |
GCTTCTCAGGTCCTCGCTGT |
GGCACAGTGACAGGTGAAGG |
| MYC |
CCAGCAGCGACTCTGAGG |
CCAAGACGTTGTGTGTTC |
| CCND1 |
CGATGCCAACCTCCTCAACGA |
TCGCAGACCTCCAGCATCCA |
| BAX |
GGATGCGTCCACCAAGAAG |
GCCTTGAGCACCAGTTTGC |
| CASP3 |
ATGGTTTGAGCCTGAGCAGA |
GGCAGCATCATCCACACATAC |
| CASP9 |
GCAGGCTCTGGATCTCGGC |
GCTGCTTGCCTGTTAGTTCGC |
| GAPDH |
CGGAGTCAACGGATTTGGTCGTAT |
AGCCTTCTCCATGGTGGTGAAGAC |
Western blotting
Proteins were extracted from WM-266-4 cells treated
with the IC50 of ORT, DTIC, or the ORT/DTIC combination,
using the M-PER mammalian protein extraction reagent (Thermo Fisher
Scientific, Inc.). Protein concentration was determined using a
bicinchoninic acid protein assay kit (Thermo Fisher Scientific,
Inc.). For each group, 25 µg of total protein was subjected to
sodium dodecyl sulfate-polyacrylamide gel electrophoresis using a
10–12% gel. Next, the resolved proteins were transferred onto
polyvinylidene fluoride membranes (0.45 µm; EMD Millipore). The
membranes were blocked with 5% skim milk or 5% bovine serum albumin
and incubated overnight at 4°C with anti-β-actin, anti-BAX,
anti-CASP3, anti-CASP9, anti-MYC, anti-CCND1, anti-NOTCH2 and
anti-NOTCH3 antibodies diluted 1,000-fold (Cell Signaling
Technology). Then, the membranes were probed with secondary
antibodies, diluted 1,000-fold, for 2 h at room temperature. The
antigen-antibody complexes were visualized using enhanced
chemiluminescence western blotting detection reagents (Thermo
Fisher Scientific, Inc.). ChemiDoc XRS (Bio-Rad) and the Image Lab
software (version 4.1; Bio-Rad) were used to analyze the blot
images.
Statistical analysis
All results of the present study were analyzed using
analysis of variance at a 5% significance level. Data were treated
by one-way ANOVA and Tukey's honestly significant difference test
(Tukey's HSD test). ANOVA was performed using SPSS version 20.0
(IBM Corp.). In the present study, all experiments were performed
at least thrice.
Results
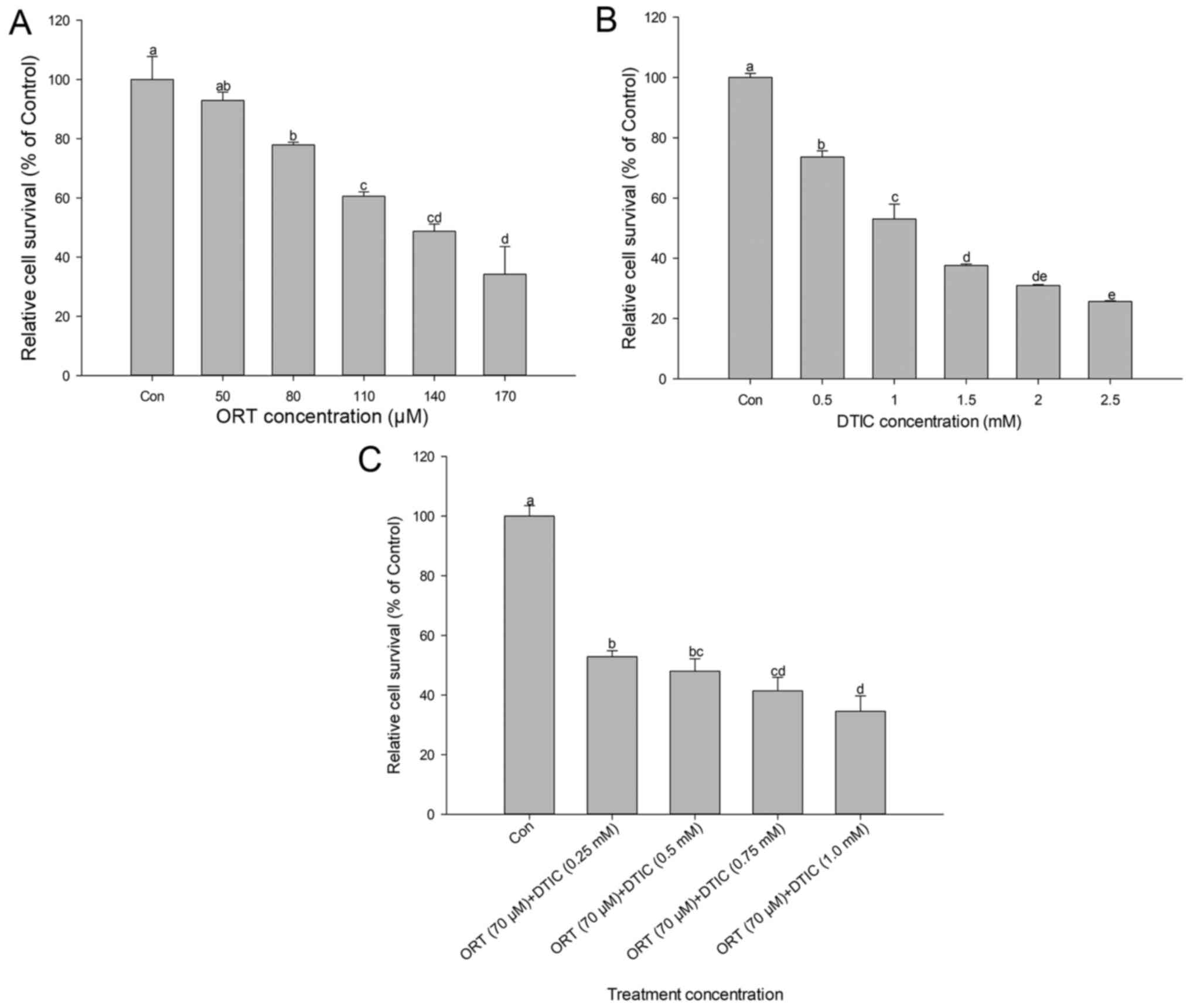
The dose-dependent effects of ORT, DTIC, and the
ORT/DTIC combination on cell viability are shown in Fig. 1. ORT and DTIC decreased cell
viability in a dose-dependent manner. Compared with that of the
control cells, the percentage viability of cells treated with 50,
80, 110, 140 and 170 µM ORT was approximately 92.9, 77.9, 60.6,
48.7 and 34.2%, respectively. Compared with that of the control
cells, the percentage viability of cells treated with 0.5, 1, 1.5,
2 and 2.5 mM DTIC was approximately 73.6, 53.0, 37.6, 30.9 and
25.6%, respectively. The IC50 values of ORT and DTIC
were approximately 140 µM and 1 mM, respectively. The cells were
co-treated with 70 µM ORT and various DTIC concentrations (0.25–1.0
mM) (Fig. 1C). The percentage
viability of cells co-treated with 0.25, 0.50, 0.75 and 1.0 mM DTIC
was 52.9, 48.0, 41.4 and 34.6%, respectively, when compared with
that of the control cells. This indicated that DTIC decreased cell
viability in a dose-dependent manner. In particular, the viability
of cells co-treated with 70 µM ORT and 0.25 mM DTIC was 52.9%.
Thus, the IC50 of the ORT/DTIC combination was
approximately two-fold lower than that of ORT and four-fold lower
than that of DTIC.
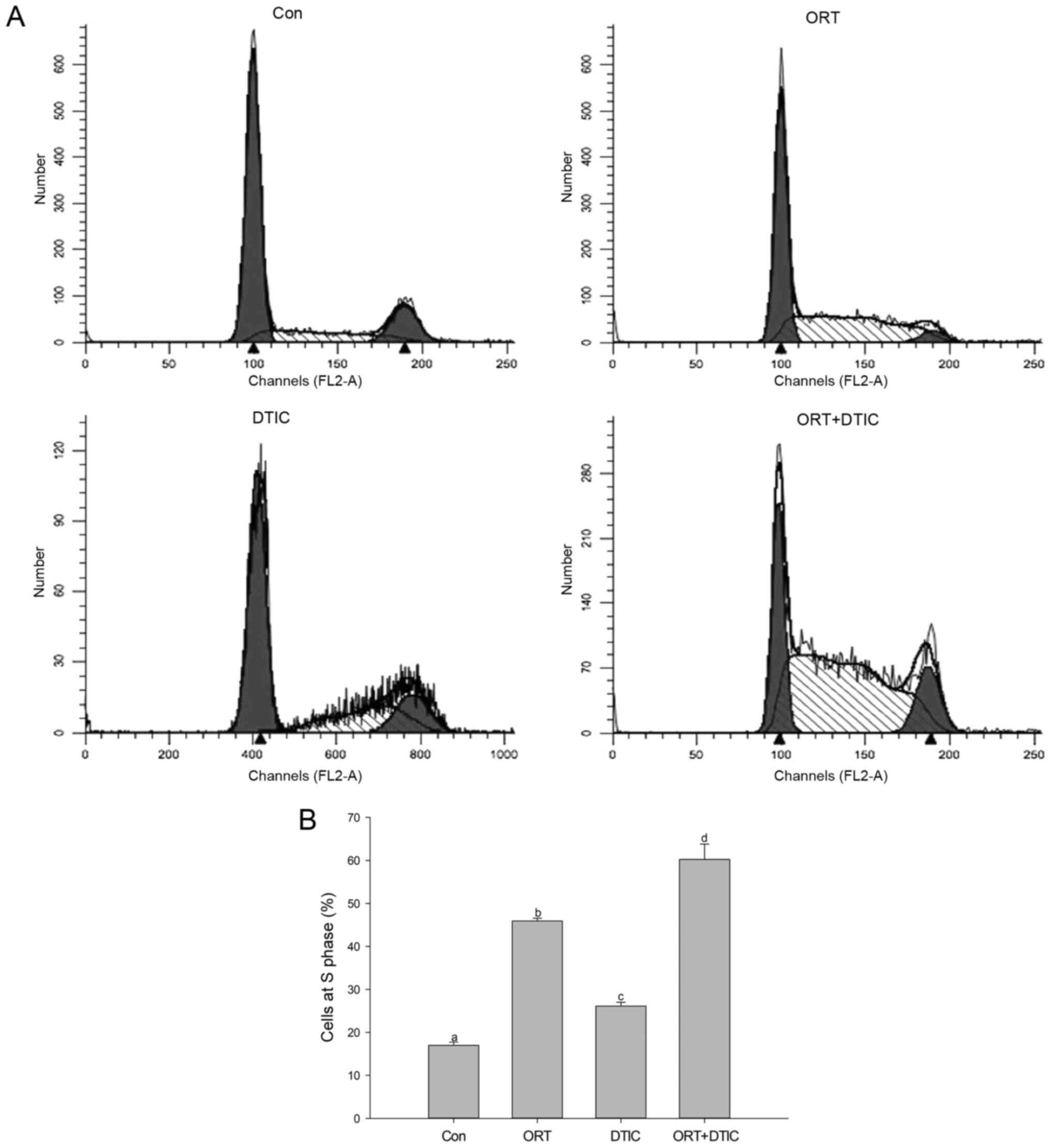
Flow cytometry was performed to examine the effects
of ORT, DTIC, and the ORT/DTIC combination on the cell cycle
(Fig. 2). The proportion of WM-266-4
cells arrested in the S phase was examined in the different
treatment groups (Fig. 2A). The
analysis revealed that the cell proportions arrested at the S phase
in the control, ORT-treated, DTIC-treated, and co-treatment groups
were 17.00±0.74, 45.93±0.65, 26.13±0.87 and 60.22±3.58%,
respectively (Fig. 2B). This
suggested that ORT and DTIC synergistically induced cell cycle
arrest following co-administration.
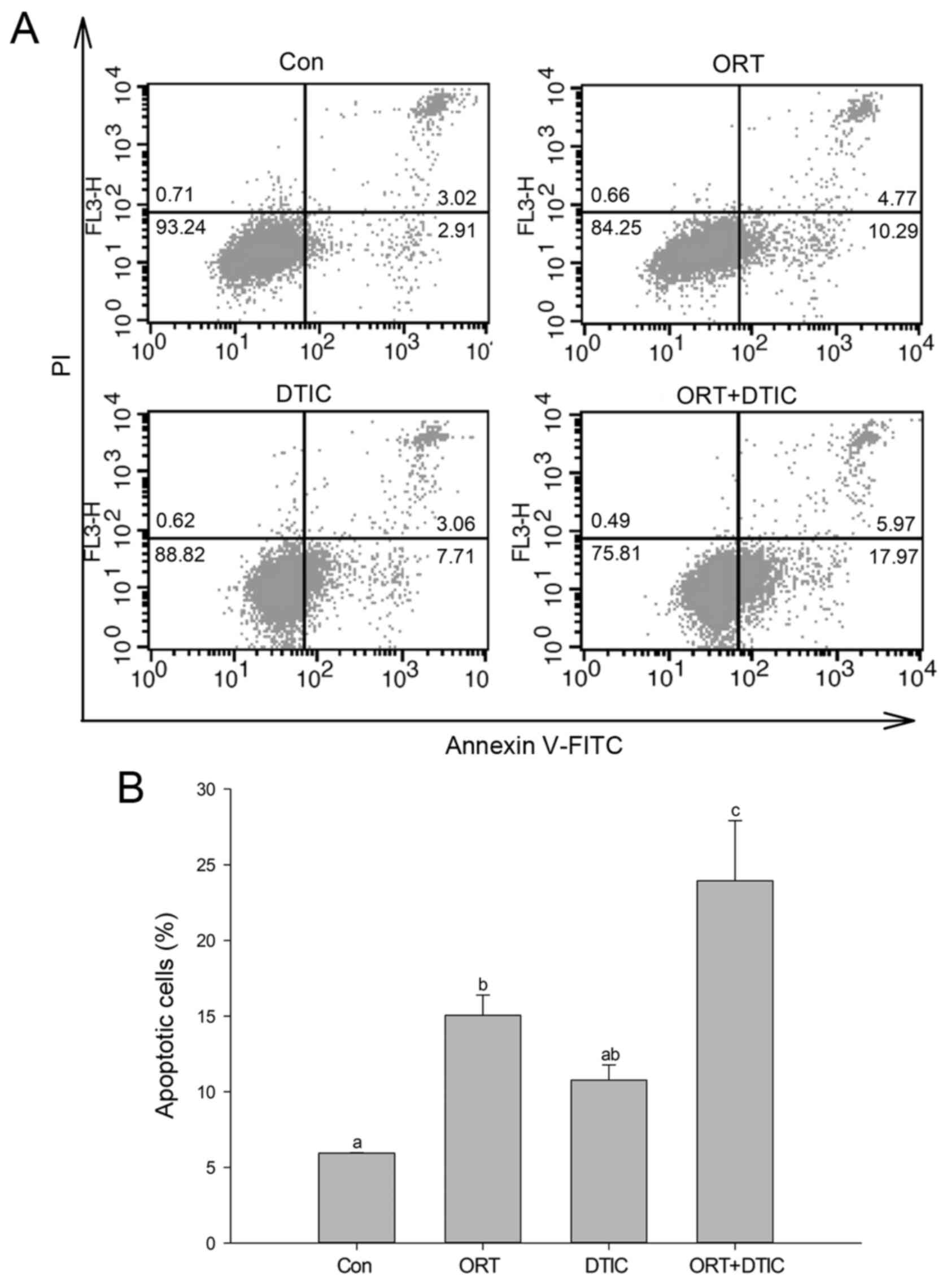
Next, flow cytometry with Annexin V/PI double
staining was employed to assess apoptosis in WM-266-4 cells.
Apoptotic cells were classified as follows: early apoptotic cells
(Annexin V+/PI−) and late apoptotic cells
(Annexin V+/PI+). The pattern of apoptosis
varied between the control and treatment groups (Fig. 3A). The proportion of cells exhibiting
total apoptosis (early apoptosis plus late apoptosis) in the
control, ORT-treated, DTIC-treated, and co-treatment groups was
5.94±0.03, 15.06±1.34, 10.77±1.00 and 23.94±3.98%, respectively
(Fig. 3B). Compared with that in the
control group, the proportion of apoptotic cells was increased in
the ORT- and DTIC-treated groups. Additionally, the proportion of
apoptotic cells was further increased in the co-treatment groups
when compared with that in the ORT- and DTIC-treated groups,
indicating that ORT and DTIC synergistically induced apoptosis in
WM-266-4 cells.
Next, the mRNA expression levels of genes related to
the cell cycle and apoptosis were measured using qRT-PCR (Fig. 4). Among genes associated with the S
phase, the relative mRNA expression levels of NOTCH2,
NOTCH3, and CCND1 varied in the ORT-treated group when
compared with those in the control group. The expression levels of
NOTCH2, NOTCH3, and CCND1 were markedly higher in the
DTIC-treated group than in the control group. However, the
expression levels of these genes in the co-treatment group were
similar to those observed in the control group (Fig. 4). The expression pattern of
MYC markedly differed from that of the three genes. Compared
with that in the control group, the expression of MYC was
increased in the ORT-treated group. However, MYC expression
in the DTIC-treated and co-treatment groups was approximately
seven-fold higher than that in the control group (Fig. 4). On analyzing the expression levels
of CASP3 and CASP9 (apoptosis-related genes), no
specific pattern was observed. Compared with those in the control
group, BAX expression levels were markedly decreased in the
ORT-treated, DTIC-treated, and co-treatment groups, with the lowest
levels observed in the co-treatment group. Compared with those in
the control group, the expression levels of CASP3 were
markedly reduced in the ORT-treated group and considerably elevated
in the DTIC-treated group. The expression levels of CASP3 in
the co-treatment groups were similar to those in the control group;
however, the expression levels of CASP9 were upregulated by
approximately 2.5-fold in the co-treatment group as compared to
those in the control group (Fig.
4).
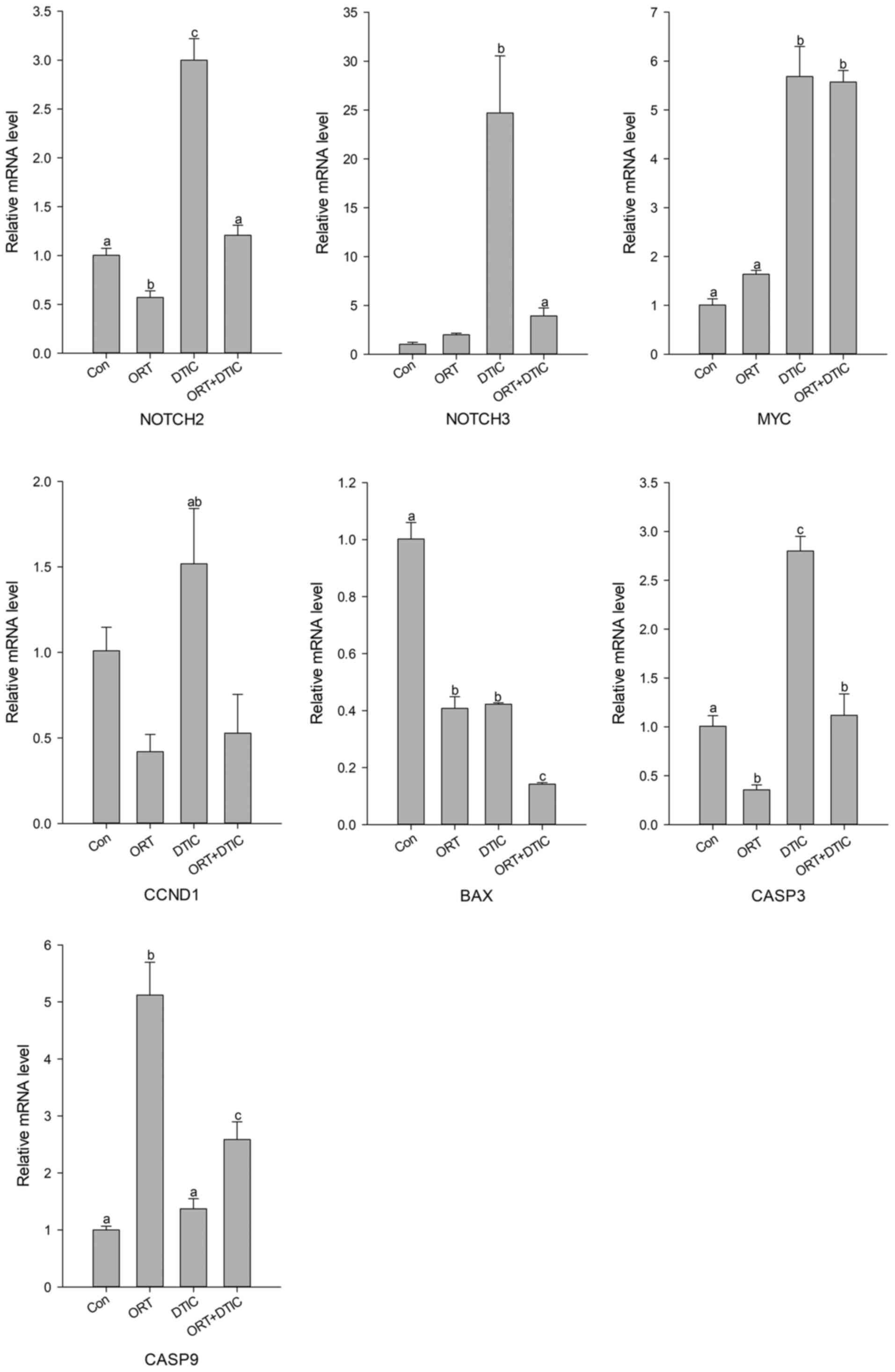 | Figure 4.mRNA expression levels of seven genes
in the Con (untreated), ORT-treated, DTIC-treated and ORT+DTIC
co-treatment groups. The relative mRNA expression levels of NOTCH2,
NOTCH3, MYC, CCND1, BAX, CASP9 and CASP3 were analyzed using
reverse transcription-quantitative PCR. Values followed by the same
small letter are not significantly different (P<0.05). ORT,
oxyresveratrol; DTIC, dacarbazine; Con, control; CCND1, cyclin D1;
CASP, caspase. |
The effects of ORT, DTIC, and the ORT/DTIC
combination on the expression levels of NOTCH2, NOTCH3, and BAX
were examined using western blotting. Compared with those in the
control, ORT-treated, and DTIC-treated groups, the expression
levels of NOTCH2, NOTCH3, and BAX were markedly reduced in the
co-treatment group (Fig. 5A and B).
The expression level of CCND1 was markedly higher in the
co-treatment group than in the ORT- and DTIC-treated groups.
Furthermore, the expression level of MYC in the ORT-treated,
DTIC-treated, and co-treatment groups was higher than that in the
control group. Compared with that in the DTIC-treated group, the
expression level of MYC was increased in the ORT-treated and
co-treatment groups. In the ORT-treated, DTIC-treated, and
co-treatment groups, the expression levels of CASP3 and CASP9 were
higher than those in the control group.
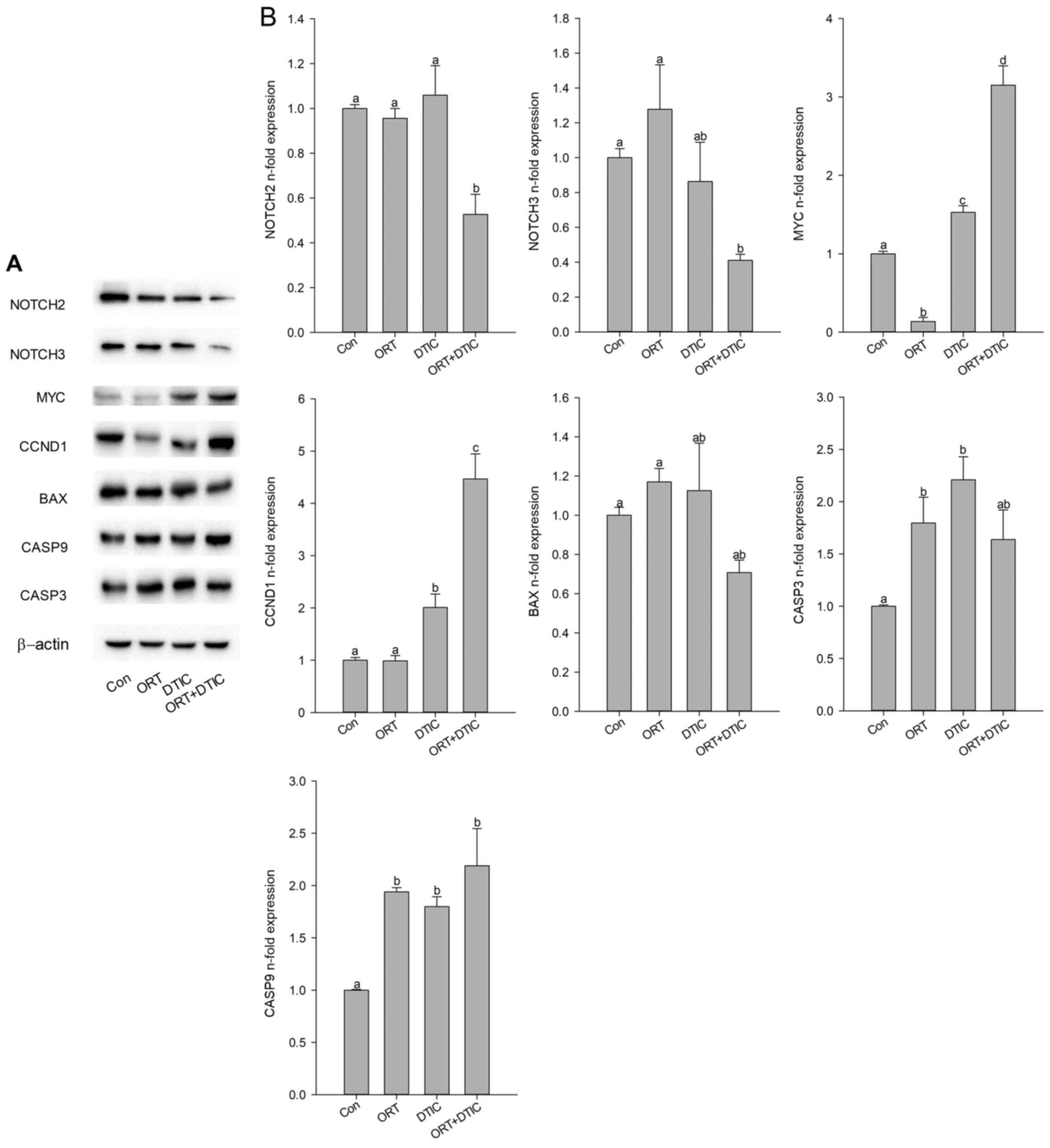 | Figure 5.Protein expression levels of seven
genes in the Con (untreated), ORT-treated, DTIC-treated and
ORT+DTIC co-treatment groups. (A) Protein expression was analyzed
using western blotting. (B) Protein expression levels of NOTCH2,
NOTCH3, MYC, CCND1, BAX, CASP9 and CASP3 were normalized with those
of β-actin. Values followed by the same small letter are not
significantly different (P<0.05). ORT, oxyresveratrol; DTIC,
dacarbazine; Con, control; CCND1, cyclin D1; CASP, caspase. |
Discussion
In the present study, the WST-1 assay was used to
examine the effects of ORT, DTIC, or the ORT/DTIC combination on
WM-266-4 melanoma cell viability. This assay enables the
measurement of intact cell viability. The IC50 of the
ORT, DTIC, or ORT/DTIC combination was estimated using the WST-1
assay. The viability of WM-266-4 cells decreased to 50% following
treatment with 140 µM ORT, 1 mM DTIC, or the 70 µM ORT/0.25 mM DTIC
combination (Fig. 1). This indicated
that the concentration of DTIC, an anticancer drug, required to
suppress the proliferation of WM-266-4 cells by 50% decreased from
1 mM to 0.25 mM in the presence of 70 µM ORT. The cytotoxicity of
ORT, an antioxidant, is reportedly lower than that of DTIC
(26). Moreover, ORT is not
cytotoxic in normal cells, such as human keratinocyte and mouse
melanocyte cells, at relatively high concentrations (27,28).
The DPPH radical scavenging activity assay is widely
utilized to measure the antioxidant potential of chemicals
(29). ORT, a stilbenoid and
aglycone of mulberroside A, has four hydroxyl groups on the benzene
ring. The structural characteristics of ORT indicate that it is a
potent antioxidant. ORT is frequently employed to alleviate
oxidative stress, which is a common cause of cellular dysfunction,
injury, and death (30). As shown in
Fig. S1, the antioxidant capacities
of ORT and DTIC were approximately 12.55±0.003 and 1.26±0.005%,
respectively, thus indicating the potent antioxidant capacity of
ORT. The antioxidant capacity of ORT (12.55±0.003%) was lower than
that of the ORT/DTIC combination (16.18±0.01%). This suggests that
the ORT/DTIC combination exhibited synergistic antioxidant
activities.
IL-6, which is involved in T cell differentiation
and proliferation, mediates various functions, including vital
pro-inflammatory functions in response to infection or injury.
However, excessive production of IL-6 contributes to chronic
inflammation, which leads to various diseases, including chronic
arthritis and osteoporosis (31–33).
TNF-α plays an important role in the innate immune response against
LPS (34). Reportedly, ORT mitigates
the LPS-induced increase in TNF-α levels (35). The effects of ORT and DTIC on the
production of IL-6 and TNF-α were evaluated by treating RAW264.7
cells with the IC50 of ORT, DTIC, or the ORT/DTIC
combination. As shown in Fig. S2A,
the lowest IL-6 concentration was observed following ORT treatment
(0.33±0.08 ng/ml). Treatment with DTIC increased IL-6 concentration
(3.76±0.71 ng/ml). However, co-treatment with ORT and DTIC
decreased the concentration of IL-6 to 0.69±0.06 ng/ml, which was
marginally higher than that in the ORT-treated group (Fig. S2A). These results demonstrate that
ORT suppresses the DTIC-induced increase in IL-6 levels, indicating
that the ORT/DTIC combination exerts a synergistic inhibitory
effect on IL-6 production. As shown in Fig. S2B, the concentration of TNF-α in the
ORT- and DTIC-treated groups was lower than that in the control
group. The concentration of TNF-α was the lowest in the
co-treatment group. These findings indicate that co-treatment with
ORT and DTIC can synergistically mitigate LPS-induced IL-6 and
TNF-α production. Previous studies have revealed that TNF-α and
IL-6 are involved in the mitogen-activated protein kinase (MAPK)
pathway. Thus, co-treatment with ORT and DTIC may modulate the MAPK
pathway (36). The levels of IL-6
and TNF-α were downregulated in RAW264.7 cells, suggesting that the
combination of ORT and DTIC could exert synergistic effects in
vitro and in vivo, which might allow the coexistence of
cancer and immune cells.
The analysis of cell cycle arrest is an important
tool for examining the effects of chemicals on the cell cycle
(37). DTIC has been reported to
induce S phase cell cycle arrest (38). As shown in Fig. 2, the proportion of cells arrested
during the S phase was higher in the co-treatment group
(60.22±3.58%) than in the ORT-treated (45.93±0.65%) and
DTIC-treated groups (26.13 ± 0.87 %). Thus, the proportion of cells
arrested in the S phase in the co-treatment group was two-fold
higher than that in the DTIC-treated group (Fig. 2). S phase arrest correlates with the
expression of various proteins. For example, upregulated expression
of CCND1 is associated with S phase arrest (39). Another study has reported that MYC
overexpression promotes S phase arrest (40). Additionally, the downregulated
expression of NOTCH2 and NOTCH3 and upregulated expression of CCND1
and MYC reportedly reduce cell migration ability (41). As shown in Fig. 5, western blotting revealed that the
expression of NOTCH2 and NOTCH3 was downregulated in the
co-treatment group. Additionally, the expression levels of CCND1
and MYC were higher in the co-treatment group than in the control
group. The expression level of MYC in the ORT-treated group was
similar to that in the co-treatment group, indicating that ORT
promoted cell cycle arrest at the S phase by upregulating MYC
expression. The expression level of BAX was lower in the
co-treatment group than in the ORT- and DTIC-treated groups.
However, the expression levels of CASP3 and CASP9 did not markedly
differ among the three treatment groups. Compared with those in the
control group, the protein expression levels of CASP3 and CASP9
were increased in the three treatment groups. The qRT-PCR results
were consistent with western blotting results in some cases, while
varying in others (Fig. 4).
As shown in Fig. S3,
cell migration ability seemed lower in the co-treatment group than
in the ORT- and DTIC-treated groups. Although the results in
Fig. S3 alone could not say that
the co-treatment has an inhibitory effect on cell migration, since
no quantification was performed, these results were consistent with
the decreased expression of NOTCH2 and NOTCH3 in the co-treatment
group (Fig. 5). These findings
suggest that the combination of ORT and DTIC synergistically
promotes S phase arrest by regulating the expression levels of
NOTCH2, NOTCH3, CCND1, and MYC.
ORT reportedly promotes apoptosis by upregulating
the expression of CASP3 and CASP9 (42). Additionally, ORT induces autophagy by
upregulating the expression levels of LC3 I and LC3 II, which are
involved in the autophagy pathway (42). As shown in Fig. 3, the proportion of cells exhibiting
apoptosis was markedly higher in the co-treatment group
(23.94±3.98%) than in the ORT-treated (15.06±1.34%) and
DTIC-treated groups (10.77±1.00%), thus implying that the
combination of ORT and DTIC synergistically promoted apoptosis in
WM-266-4 cells. Previous studies have revealed that decreased BAX
expression and increased CASP3 and CASP9 expression can be
correlated with apoptosis (26). The
expression levels of BAX, CASP3, and CASP9, especially in the
co-treatment group (Fig. 5), were
consistent with the proportion of cells exhibiting apoptosis
(Fig. 3). Therefore, the results
presented in Fig. 5 may support the
effects of ORT/DTIC co-treatment. The expression levels of CASP3
and CASP9 were higher in the co-treatment group than in the control
group. However, co-treatment with ORT and DTIC did not
synergistically upregulate the expression levels of CASP3 and
CASP9, as observed following individual treatments. Thus, although
treatment with ORT and DTIC enhanced CASP3 and CASP9 expression,
co-treatment did not demonstrate this effect, indicating that
co-treatment failed to demonstrate synergistic effects in terms of
CASP3/9 expression. The cleaved forms of CASP3/9 were often
employed to show the variation in expression of CASP3/9 but we had
a difficulty in the detection of cleaved forms of CASP3/9 due to
the lack of experiences to deal with the expressed proteins. We
planned to study further the detailed signaling pathway including
the cleaved forms of CASP3/9 for co-treatment with ORT and
DTIC.
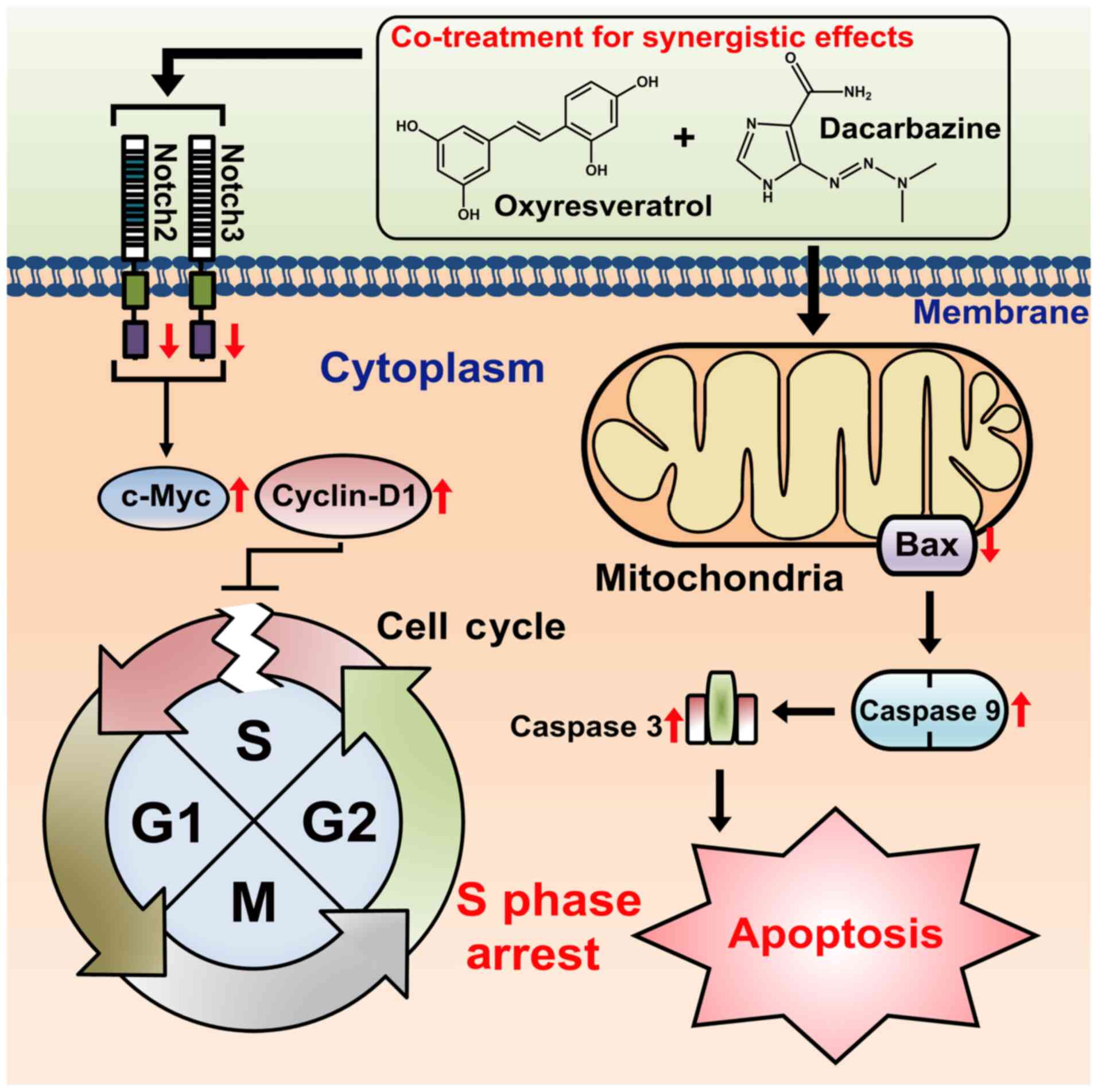
Herein, based on the observed results, we propose a
possible mechanism underlying synergistic proliferation-inhibitory
effects of the ORT/DTIC combination against WM-266-4 cells. The
ORT/DTIC combination induced S phase arrest and apoptosis (Fig. 6). The treatment combination decreased
the levels of NOTCH2 and NOTCH3 and consequently upregulated the
expression levels of MYC and CCND1, which has been previously
reported (41). Thus, the treatment
combination promoted cell cycle arrest at the S phase and
downregulated BAX expression in the mitochondria. The upregulated
expression of CASP3 and CASP9 may promote apoptosis. This proposed
mechanism is supported by results observed in a previous study
demonstrating that the downregulated BAX expression results in the
concomitant upregulation of CASP3 and CASP9 expression (26). The mechanism proposed in this study
suggests that co-treatment with ORT and DTIC promotes apoptosis in
WM-266-4 cells. Additionally, this co-treatment strategy enables
the administration of lower DTIC concentrations in the presence of
ORT, which exhibits low cytotoxicity (43,44).
In conclusion, the findings of present study suggest
that the synergistic proliferation-inhibitory effects of the
ORT/DTIC combination against WM-266-4 cells can be attributed to
the NOTCH signaling pathway and its downstream signaling pathways
(CCND1 and MYC), as well as the BAX signaling pathway (CASP3 and
CASP9). The dose of DTIC, a known cancer drug, can be reduced when
co-administered with antioxidants, such as ORT, an antioxidant with
low cytotoxicity.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by a grant from the
National Research Foundation of Korea (grant no.
NRF-2015R1A2A2A0100650).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SGL conceived the study, performed the experiments
and collected the data. SGL and DGL analyzed the data. NC and YHJ
assessed and confirm the authenticity of the raw data. SGL drafted
the manuscript. SGL and YHJ interpreted the data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
cDNA
|
complementary DNA
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
DPPH
|
2,2-diphenyl-1-picryl-hydrazyl-hydrate
|
|
DTIC
|
dacarbazine
|
|
IC50
|
half-maximal inhibitory
concentration
|
|
IL
|
interleukin
|
|
LPS
|
lipopolysaccharide
|
|
ORT
|
oxyresveratrol
|
|
PI
|
propidium iodide
|
|
RT-qPCR
|
reverse transcription-quantitative
PCR
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1
|
Khoobchandani M, Ganesh N, Gabbanini S,
Valgimigli L and Srivastava MM: Phytochemical potential of Eruca
sativa for inhibition of melanoma tumor growth. Fitoterapia.
82:647–653. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu B, Wang Y, Yu X, Zhang H, Zhu J, Wang
C, Chen F, Liu C, Wang J and Zhu H: Cuprous oxide
nanoparticle-inhibited melanoma progress by targeting melanoma stem
cells. Int J Nanomedicine. 12:2553–2567. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ma C and Armstrong AW: Severe adverse
events from the treatment of advanced melanoma: A systematic review
of severe side effects associated with ipilimumab, vemurafenib,
interferon alfa-2b, dacarbazine and interleukin-2. J Dermatolog
Treat. 25:401–408. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SG and Chae YS: New systemic treatment
for malignant melanoma. Korean J Intern Med (Korean Assoc Intern
Med). 85:357–363. 2013.PubMed/NCBI
|
|
7
|
Baharara J, Amini E, Nikdel N and
Salek-Abdollahi F: The cytotoxicity of dacarbazine potentiated by
sea cucumber saponin in resistant B16F10 melanoma cells through
apoptosis induction. Avicenna J Med Biotechnol. 8:112–119.
2016.PubMed/NCBI
|
|
8
|
Mongolsuk S, Robertson A and Towers R: 2:
4: 3: 5-Tetrahydroxystilbene from Artocarpus lakoocha. J
Chem Soc. 429:2231–2233. 1957. View Article : Google Scholar
|
|
9
|
Tan Y, Liu C and Chen R: Phenolic
constituents from stem bark of Morus wittiorum and their
anti-inflammation and cytotoxicity. Zhongguo Zhongyao Zazhi.
35:2700–2703. 2010.(In Chinese). PubMed/NCBI
|
|
10
|
Choi SM, Kim DH, Chun KS and Choi JS:
Carnosol induces apoptotic cell death through ROS-dependent
inactivation of STAT3 in human melanoma G361 cells. Appl Biol Chem.
62:1–11. 2019. View Article : Google Scholar
|
|
11
|
Kim YM, Yun J, Lee C-K, Lee H, Min KR and
Kim Y: Oxyresveratrol and hydroxystilbene compounds. Inhibitory
effect on tyrosinase and mechanism of action. J Biol Chem.
277:16340–16344. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chung KO, Kim BY, Lee MH, Kim YR, Chung
HY, Park JH and Moon JO: In-vitro and in-vivo anti-inflammatory
effect of oxyresveratrol from Morus alba L. J Pharm Pharmacol.
55:1695–1700. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qiu F, Komatsu K, Saito K, Kawasaki K, Yao
X and Kano Y; Pharmacokinetic study of mulberroside A and its
metabolites in rat, : Pharmacological properties of traditional
medicines. XXII. Pharmacokinetic study of mulberroside A and its
metabolites in rat. Biol Pharm Bull. 19:1463–1467. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim J, Cho N, Kim EM, Park KS, Kang YW,
Nam JH, Nam MS and Kim KK: Cudrania tricuspidata leaf
extracts and its components, chlorogenic acid, kaempferol, and
quercetin, increase claudin 1 expression in human keratinocytes,
enhancing intercellular tight junction capacity. Appl Biol Chem.
63:1–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McDermott DF, Mier JW, Lawrence DP, van
den Brink MR, Clancy MA, Rubin KM and Atkins MB: A phase II pilot
trial of concurrent biochemotherapy with cisplatin, vinblastine,
dacarbazine, interleukin 2, and interferon α-2B in patients with
metastatic melanoma. Clin Cancer Res. 6:2201–2208. 2000.PubMed/NCBI
|
|
16
|
Cocconi G, Bella M, Calabresi F, Tonato M,
Canaletti R, Boni C, Buzzi F, Ceci G, Corgna E, Costa P, et al:
Treatment of metastatic malignant melanoma with dacarbazine plus
tamoxifen. N Engl J Med. 327:516–523. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bedikian AY, Millward M, Pehamberger H,
Conry R, Gore M, Trefzer U, Pavlick AC, DeConti R, Hersh EM, Hersey
P, et al Oblimersen Melanoma Study Group, : Bcl-2 antisense
(oblimersen sodium) plus dacarbazine in patients with advanced
melanoma: The Oblimersen Melanoma Study Group. J Clin Oncol.
24:4738–4745. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen H-M, Muramoto K, Yamauchi F, Fujimoto
K and Nokihara K: Antioxidative properties of histidine-containing
peptides designed from peptide fragments found in the digests of a
soybean protein. J Agric Food Chem. 46:49–53. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Monzani E, Facchetti F, Galmozzi E,
Corsini E, Benetti A, Cavazzin C, Gritti A, Piccinini A, Porro D,
Santinami M, et al: Melanoma contains CD133 and ABCG2 positive
cells with enhanced tumourigenic potential. Eur J Cancer.
43:935–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Utikal J, Leiter U, Udart M, Kaskel P,
Peter RU and Krähn GM: Expression of c-myc and bcl-2 in primary and
advanced cutaneous melanoma. Cancer Invest. 20:914–921. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Choi HJ, Yee S-B, Park SE, Im E, Jung JH,
Chung HY, Choi YH and Kim ND: Petrotetrayndiol A induces cell cycle
arrest and apoptosis in SK-MEL-2 human melanoma cells through
cytochrome c-mediated activation of caspases. Cancer Lett.
232:214–225. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shanehbandi D, Zarredar H, Asadi M, Zafari
V, Esmaeili S, Seyedrezazadeh E, Soleimani Z, Jadid HS, Eyvazi S,
Feyziniya S, et al: Anticancer impacts of Terminalia catappa
extract on SW480 colorectal neoplasm cell line. J Gastrointest
Cancer. 50:1–7. 2019.
|
|
23
|
Orangi M, Pasdaran A, Shanehbandi D,
Kazemi T, Yousefi B, Hosseini BA and Baradaran B: Kazemi T, Yousefi
B, Hosseini BA and Baradran B: Cytotoxic and apoptotic activities
of methanolic subfractions of Scrophularia oxysepala against
human breast cancer cell line. Evid Based Complement Alternat Med.
2016:85406402016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu X, Liu Y, Wang L, He J, Zhang H, Chen
X, Li Y, Yang J and Tao J: Gambogic acid induces apoptosis by
regulating the expression of Bax and Bcl-2 and enhancing caspase-3
activity in human malignant melanoma A375 cells. Int J Dermatol.
48:186–192. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yadav V, Varshney P, Sultana S, Yadav J
and Saini N: Moxifloxacin and ciprofloxacin induces S-phase arrest
and augments apoptotic effects of cisplatin in human pancreatic
cancer cells via ERK activation. BMC Cancer. 15:5812015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu S, Zheng Z, Zhang X, Chen F and Wang M:
Oxyresveratrol and trans-dihydromorin from the twigs of Cudrania
tricuspidata as hypopigmenting agents against melanogenesis. J
Funct Foods. 13:375–383. 2015. View Article : Google Scholar
|
|
28
|
Hu S, Chen F and Wang M: Photoprotective
effects of oxyresveratrol and Kuwanon O on DNA damage induced by
UVA in human epidermal keratinocytes. Chem Res Toxicol. 28:541–548.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bougatef A, Hajji M, Balti R, Lassoued I,
Triki-Ellouz Y and Nasri M: Antioxidant and free radical-scavenging
activities of smooth hound (Mustelus mustelus) muscle
protein hydrolysates obtained by gastrointestinal proteases. Food
Chem. 114:1198–1205. 2009. View Article : Google Scholar
|
|
30
|
Lim YH, Kim KH and Kim JK: Source,
biosynthesis, biological activities and pharmacokinetics of
oxyresveratrol. Korean J Food Sci Technol. 47:545–555. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Strassmann G, Masui Y, Chizzonite R and
Fong M: Mechanisms of experimental cancer cachexia. Local
involvement of IL-1 in colon-26 tumor. J Immunol. 150:2341–2345.
1993.PubMed/NCBI
|
|
32
|
Takagi N, Mihara M, Moriya Y, Nishimoto N,
Yoshizaki K, Kishimoto T, Takeda Y and Ohsugi Y: Blockage of
interleukin-6 receptor ameliorates joint disease in murine
collagen-induced arthritis. Arthritis Rheum. 41:2117–2121. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang XG, Bataille R, Jourdan M, Saeland
S, Banchereau J, Mannoni P and Klein B: Granulocyte-macrophage
colony-stimulating factor synergizes with interleukin-6 in
supporting the proliferation of human myeloma cells. Blood.
76:2599–2605. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee AK, Sung SH, Kim YC and Kim SG:
Inhibition of lipopolysaccharide-inducible nitric oxide synthase,
TNF-α and COX-2 expression by sauchinone effects on I-kappaBalpha
phosphorylation, C/EBP and AP-1 activation. Br J Pharmacol.
139:11–20. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Choi EM and Hwang JK: Effects of Morus
alba leaf extract on the production of nitric oxide, prostaglandin
E2 and cytokines in RAW264.7 macrophages. Fitoterapia. 76:608–613.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Naserian M, Ramazani E, Iranshahi M and
Tayarani-Najaran Z: The role of SAPK/JNK pathway in the synergistic
effects of metformin and dacarbazine on apoptosis in Raji and Ramos
lymphoma cells. Curr Mol Pharmacol. 11:336–342. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pitot HC and Sirica AE: The stages of
initiation and promotion in hepatocarcinogenesis. Biochim Biophys
Acta. 605:191–215. 1980.PubMed/NCBI
|
|
38
|
Koprowska K, Hartman ML, Sztiller-Sikorska
M and Czyz ME: Parthenolide enhances dacarbazine activity against
melanoma cells. Anticancer Drugs. 24:835–845. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Prall OW, Sarcevic B, Musgrove EA, Watts
CK and Sutherland RL: Estrogen-induced activation of Cdk4 and Cdk2
during G1-S phase progression is accompanied by increased cyclin D1
expression and decreased cyclin-dependent kinase inhibitor
association with cyclin E-Cdk2. J Biol Chem. 272:10882–10894. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sheen J-H and Dickson RB: Overexpression
of c-Myc alters G(1)/S arrest following ionizing radiation. Mol
Cell Biol. 22:1819–1833. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao WX, Wu ZM, Liu W and Lin JH: Notch2
and Notch3 suppress the proliferation and mediate invasion of
trophoblast cell lines. Biol Open. 6:1123–1129. 2017.PubMed/NCBI
|
|
42
|
Rahman MA, Bishayee K, Sadra A and Huh SO:
Oxyresveratrol activates parallel apoptotic and autophagic cell
death pathways in neuroblastoma cells. Biochim Biophys Acta, Gen
Subj. 1861:23–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lorenz P, Roychowdhury S, Engelmann M,
Wolf G and Horn TF: Oxyresveratrol and resveratrol are potent
antioxidants and free radical scavengers: Effect on nitrosative and
oxidative stress derived from microglial cells. Nitric Oxide.
9:64–76. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kim SH and Chung SH: Comparison of high
dose interferon-α2b immunotherapy and dacarbazine chemotherapy as
postoperative treatment of malignant melanoma. J Korean Orthop
Assoc. 51:426–431. 2016. View Article : Google Scholar
|