Introduction
Colorectal cancer (CRC) is the fourth most fatal
cancer globally, with almost a million annual deaths (1,2). It has
the second and third highest incidence rate among cancers in men
and women, respectively (2). Early
detection offers a survival rate of up to 90% (3); however, the disease remains virtually
asymptomatic until later stages, at which point the survival rate
declines severely to <10% (1,3). This
underscores the great importance of pursuing early biomarkers of
CRC.
The current gold standard in CRC screening is
colonoscopy (1,4–6).
However, despite its proven success, it remains under-utilized
(7), probably due to its invasive
nature. Several non-invasive biomarkers have been suggested with
varying rates of success including fecal immunochemical testing
(1,4)
and expression of circulating RNA (8). Furthermore, several epigenetic
alterations have been implicated in CRC (4). For example, hypermethylation of the
septin 9 promoter has been linked to CRC, and early reports
suggested moderate sensitivity and specificity (9). However, larger-scale investigations
showed that the sensitivity of CRC detection was <50% using this
method (10). A stool DNA analysis
of multiple targets was developed by Cologuard® (Exact
Sciences Corporation), and two independent studies validated its
excellent specificity and sensitivity related to CRC (11,12).
However, both studies found that the sensitivity of the test
dropped <50% for patients with advanced precancerous
lesions.
Circular RNA (circRNA) molecules have emerged as
promising disease biomarkers due to their stability and increased
half-life compared with their linear counterparts (13,14). The
expression of several circRNA targets has been shown to be altered
in CRC tissue compared with normal adjacent tissue (13–15).
Mechanistically, several biological functions have been reported
for circRNA, such as sponging microRNA (16), binding to proteins (17), acting as protein scaffolds (18) and interacting with RNA polymerase II
(19).
However, due to a lack of reproducibility, findings
obtained using basic research are rarely transferred to clinical
practice (20–22). Investigations by Prinz et al
(23) and by Begley and Ellis
(24) revealed that 92 and 89% of
the surveyed reverse-transcription-quantitative PCR (RT-qPCR)
studies could not be reproduced, respectively, even when the
experiments were repeated by the same laboratories in which the
original experiments were conducted. In two separate reports
(20,25), Stephen Bustin, the first author of
the Minimum Information for Publication of Quantitative Real-Time
PCR Experiments (MIQE) guidelines (26), has been extremely critical of the
validity of the results from several studies, citing a number of
factors that could lead to erroneous results. These factors include
a lack of information regarding the PCR conditions and PCR
efficiency, as well as dependence on a single reference gene for
normalization. In the present study, these issues were addressed
using a stringent, more controlled RT-qPCR approach.
The aim of the present study was to investigate
whether the altered expression of circRNA molecules in CRC tissue
can also be detected in the blood, in order to evaluate their
ability to serve as potential non-invasive biomarkers. The
literature was scanned for circRNA candidates reported to be
deregulated in CRC by two or more independent research groups, four
of which were selected for further analysis based on the
involvement of their parent genes in CRC. The expression patterns
of their linear counterparts were also examined to gain further
insight into the regulatory pathways of the genes that encode them.
Using this approach, novel findings on the gene expression patterns
of leukocytes of CRC patients are reported, which may have
potential for use in CRC screening.
Materials and methods
Patient and control enrollment, sample
acquisition and ethical approval
This case-control study was performed on 74
volunteers aged 31–85 years. The patients with CRC (n=42; mean age
± SD=57.2 ±12.5 years) included 29 males and 12 females (one
patient with missing data). The healthy control group (n=32; mean
age ± SD= 49.3 ±10.5 years old) consisted of 19 males and 13
females. Patients with CRC were included if they were Saudis with a
confirmed diagnosis of CRC at any tumor-node-metastasis (TNM) stage
using histopathological and CT scan biopsies. Non-Saudi patients
were excluded from the study. The inclusion criteria for the
healthy controls were as follows: i) they had to be Saudis; ii)
free of any metabolic or chronic diseases, such as hypertension,
diabetes mellitus II and other endocrine disorders); and iii)
without any family history of CRC or any other tumor during the
time of the study. Samples (2 ml whole blood) were collected in
EDTA tubes from all participants. The participants routinely
visited the Day Care Unit of King Abdulaziz University Hospital
(Jeddah, Saudi Arabia) (patients with CRC) or the Blood Bank Unit
of King Fahad General Hospital (Jeddah, Saudi Arabia) (controls) in
the period August 2015-July 2016). The purpose of the research was
explained to the participants, from whom written consent was
obtained. The Unit of Biomedical Ethics at The Faculty of Medicine,
King Abdulaziz University, approved this study (approval no.
261-15).
Selection criteria for candidate
circRNA molecules
A literature search was performed in PubMed
(https://pubmed.ncbi.nlm.nih.gov/) and
PubMed Central (https://www.ncbi.nlm.nih.gov/pmc/) databases for all
articles that reported altered expression of circRNAs in CRC tissue
[search terms used were: (circRNA OR circular RNA) and (CRC OR
colorectal cancer) in the title/abstract fields]. Scanning both the
main manuscripts and the supplementary materials, 13 circRNA
candidates were found to be reported by at least two independent
research groups (Table I) (27–50).
From these, four candidates were selected for the purpose of this
study, based on the functional implications of their host genes in
CRC: ciRS-7 (27–30), circular methyltransferase-like 3
(circMETTL3; 30,31), circular SNF2 histone linker PHD RING helicase
(circSHPRH; 32,33) and circular ubiquitin-specific peptidase 3
(circUSP3; 30,34). The linear isoforms of these circRNA candidates
were also selected for further analysis, except for ciRS-7, which
has no linear counterpart.
 | Table I.circRNAs that were reported by at
least 2 independent groups to be deregulated in CRC. |
Table I.
circRNAs that were reported by at
least 2 independent groups to be deregulated in CRC.
| circRNA ID | Parent Gene | (Refs.) |
|---|
|
hsa_circ_0001946 | CDR1AS
(ciRS-7) | (27–30) |
|
hsa_circ_0000523 | METTL3 | (30,31) |
|
hsa_circ_0001649 | SHPRH | (32,33) |
|
hsa_circ_0002138 | USP3 | (30,34) |
|
hsa_circ_0000284 | HIPK3 | (28,35) |
|
hsa_circ_0006990 | VAPA | (36,37) |
|
hsa_circ_0026344 | ACVRL1 | (38,39) |
|
hsa_circ_0000826 | ANKRD12 | (40,41) |
|
hsa_circ_0001313 | CCDC66 | (42,43) |
|
hsa_circ_0020397 | DOCK1 | (44,45) |
|
hsa_circ_0026782 | ITGA7 | (30,46) |
|
Hsa_circ_0001821 | PVT1 | (36,47) |
|
hsa_circ_0000518 | RPPH1 | (41,48) |
|
hsa_circ_0072088 | ZFR | (49,50) |
RNA extraction and purity
Unless otherwise stated, all centrifugation steps of
the RNA extraction protocol were carried out in room temperature.
RNA was extracted from leukocytes using QIAamp RNA blood mini kit
(Qiagen GmbH; cat. no. 52304) following the manufacturer's
instructions. Briefly, 1 ml of whole blood from each sample was
mixed with 5 ml buffer EL, incubated for 15 min on ice and
centrifuged at 400 × g for 10 min at 4°C. The supernatant was then
discarded, the resulting pellet was resuspended in 2 ml buffer EL,
and centrifugation was repeated. The supernatant was discarded, and
600 µl RLT buffer supplemented with 1% β-mercaptoethanol was added
to the pellet. The lysate was added to a QIAshredder column, then
mixed with 600 µl 70% ethanol. The lysate-ethanol mixture was
transferred to a spin column and centrifuged at 8,000 × g for 15
sec (two successive loads to add the whole lysate-ethanol mixture)
and the flow-through was discarded. Then, 700 µl of buffer RW1 was
added, the mixture was centrifuged at 8,000 × g for 15 sec, and the
flow-through was discarded. This step was repeated with 500 µl
buffer RPE, after which 500 µl buffer RPE was added, and the
mixture was centrifuged at 20,000 × g for 3 min. The spin column
was transferred to a new collection tube and 50 µl RNase-free water
was added. After centrifugation at 8,000 × g for 1 min, the eluate
was stored at −20°C.
cDNA synthesis
A total of 300 ng RNA from each sample was reverse
transcribed using random hexamers in 20-µl reactions using a
High-Capacity cDNA Reverse Transcription kit (Applied Biosystems;
cat. no. 4368814) according to the manufacturer's protocol.
Briefly, all RNA samples were adjusted to a final concentration of
30 ng/µl and 10 µl of each sample was mixed with 2 µl 10X random
hexamers, 0.8 µl 25X dNTP mix, 2 µl 10X RT buffer, 1 µl reverse
transcriptase, 3.2 µl nuclease-free water and 1 µl RNase inhibitor
(Applied Biosystems; cat. no. N8080119). The thermal cycler for the
cDNA synthesis reaction was set for 10 min at 25°C, followed by 120
min at 37°C and finally 5 min at 85°C to inactivate the reverse
transcriptase.
Primer design
Primers were designed using the PrimerBLAST tool
from the National Center for Biotechnology Information-US National
Library of Medicine (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi?LINK_LOC=BlastHome)
and purchased from Macrogen. The primers sequences are listed in
Table II.
 | Table II.Primer sequences for each gene. |
Table II.
Primer sequences for each gene.
| Gene name | Accession no. or
circBase ID | Primer | Sequence,
5′-3′ | Amplicon length,
bp |
|---|
| ciRS-7 |
hsa_circ_0001946 | Forward |
ACCCAGTCTTCCATCAACTGG | 112 |
|
|
| Reverse |
GCCATCGGAAACCCTGGATA |
|
| circMETTL3 |
hsa_circ_0000523 | Forward |
ACAGAGCAAGAAGATCTACGGA | 113 |
|
|
| Reverse |
GAAGCTGTGCTGGGCTTAGG |
|
| circSHPRH |
hsa_circ_0001649 | Forward |
CCGAATTGGACAGACAAAACCT | 136 |
|
|
| Reverse |
TTCTGACCACAGCTTCCACTT |
|
| circUSP3 |
hsa_circ_0002138 | Forward |
CAGGAGCCAAGGGGATAACA | 258 |
|
|
| Reverse |
GGTTGGTTAAAGGTACTTGTGCAT |
|
| linMETTL3 | NM_019852.5 | Forward |
TTTTCCGGTTAGCCTTCGGG | 226 |
|
|
| Reverse |
TTCCGTAGATCCAAGTGCCC |
|
| linSHPRH | NM_001042683.3 | Forward |
TGGCTCTGAGGAATCGTGTG | 280 |
|
|
| Reverse |
GCACAGATTGGGCAAGGTTC |
|
| linUSP3 | NM_006537.4 | Forward |
CCCGGCTAGAAGCGACAC | 229 |
|
|
| Reverse |
AGTCAAACAGACCCAAGGGC |
|
| GAPDH | NM_002046.7 | Forward |
TCACCAGGGCTGCTTTTAAC | 389 |
|
|
| Reverse |
GATGATCTTGAGGCTGTTGTCA |
|
| RPLP1 | NM_001003.3 | Forward |
GTCCTTCCGAGGAAGCTAAGG | 187 |
|
|
| Reverse |
ATTGATCTTATCCTCCGTGACTGT |
|
| RPL13A | NM_012423.4 | Forward |
GCTAAACAGGTACTGCTGGG | 99 |
|
|
| Reverse |
AGCCAGGTACTTCAACTTGTTTC |
|
RT-qPCR
The resulting cDNA was used for qPCR using the
SsoAdvanced™ Universal SYBR®-Green Supermix (Bio-Rad
laboratories, Inc.; cat. no. 1725272) in 11-µl reaction volumes and
final concentrations of 500 nM for the forward and reverse primers.
To ensure equal additions of the cDNA template to all assays, the
master mix was prepared with the cDNA template. To avoid inter-run
variation, all assays for each sample were carried out on the same
run. At least one duplicate of each reaction was set up, and all
replicates had a Cq standard error of <1 Cq. The PCR cycling
protocol included 2 min at 95°C, followed by 40 cycles of 95°C for
15 sec and 60°C for 30 sec (data collection). These cycles were
followed by 95°C for 30 sec, then an incremental rise from 65 to
95°C, during which data were collected every 5 sec at 0.5°C
intervals. Efficiency-corrected Cq values and corrected relative
expression 2−ΔΔCq method (51) were determined using CFX Manager
version 3.1 (Bio-Rad Laboratories, Inc.) and were used for all
subsequent analyses. Initially, three reference genes were used for
normalization: GAPDH, ribosomal protein lateral stalk subunit P1
(RPLP1) and ribosomal protein L13A (RPL13A) (see Table II for accession nos.). GAPDH was not
used in subsequent experiments due to instability in our
conditions, as explained in the Results section.
Reference gene stability
Reference gene stability was determined using the
‘target stability value’ tool in CFX Manager version 3.1 (Bio-Rad
Laboratories, Inc.), following the manufacturer' protocol.
PCR efficiency
The PCR efficiency was calculated using the online
qPCR Efficiency Calculator tool (Thermo Fisher Scientific, Inc.)
(52).
Statistical analysis
Optimal cut-off points for the receiver operating
characteristic (ROC) curves were calculated using the web tool
easyROC v.1.3.1 (53). Welch's
two-tailed t-test, Welch's one-way ANOVA test, ROC curves, area
under the curve (AUC), sensitivity, specificity, positive
predictive value (PPV), negative predictive value (NPV) and
Pearson's correlation coefficient were calculated using the Prism
version 9.0.0 software (GraphPad Software, Inc.). As no significant
differences were found among the groups by Welch's one-way ANOVA
test, post-hoc analysis was not performed.
Results
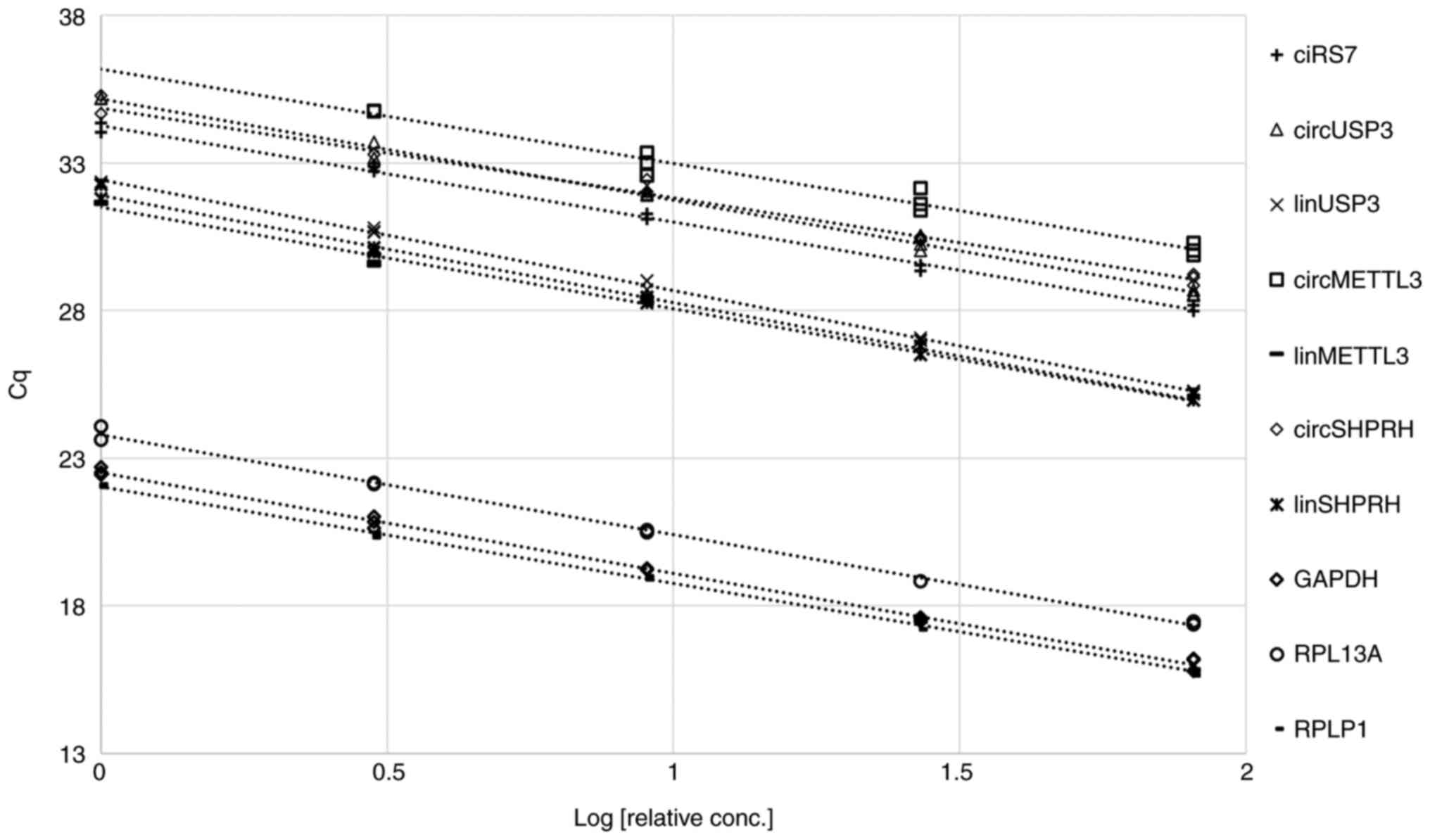
Determination of assay efficiency
To ensure that the results of the present study
would be consistent over a wide range of concentrations in the
aforementioned PCR conditions, with minimal effect of any PCR
inhibitors or unspecific reactions, the efficiency of the qPCR
assays was first determined. Each assay was performed on five
three-fold serial dilutions of cDNA from a representative leukocyte
sample, and Cq values were plotted against the logarithm of the
relative concentration of the cDNA templates (Fig. 1). All assays yielded straight lines
(R2>0.97) with efficiencies of 100±15% (Table III). These efficiencies were
factored into all subsequent Cq calculations to account for any
small variation across concentrations. This approach ensured that
PCR assays were optimal and that reproducible results would be
obtained regardless of the amount of template used in the
reaction.
 | Table III.Assay efficiencies, slopes and
R2 values for the trend line of the Cq vs. logarithm of
relative cDNA template concentration plots. |
Table III.
Assay efficiencies, slopes and
R2 values for the trend line of the Cq vs. logarithm of
relative cDNA template concentration plots.
| Assay | R2 | Slope | Efficiency, % |
|---|
| ciRS-7 | 0.996 | −3.2684 | 102 |
| circMETTL3 | 0.973 | −3.1918 | 106 |
| circSHPRH | 0.985 | −3.0368 | 113 |
| circUSP3 | 0.995 | −3.432 | 96 |
| linMETTL3 | 0.977 | −3.4373 | 95 |
| linSHPRH | 0.995 | −3.6273 | 89 |
| linUSP3 | 0.998 | −3.7538 | 85 |
| GAPDH | 0.997 | −3.4109 | 96 |
| RPL13A | 0.997 | −3.3788 | 98 |
| RPLP1 | 0.998 | −3.2805 | 102 |
Initial analysis of circMETTL3,
circSHPRH, circUSP3, their linear counterparts and ciRS-7
Welch's two-tailed t-test was used to analyze the
expression patterns of circMETTL3, circSHPRH, circUSP3 and their
linear counterparts, as well as ciRS-7 (Table SI) in CRC and normal samples (n=8
each). The data were normalized to RPLP1, RPL13A and GAPDH. Both
linear (P=0.002) and circular (P=0.03) isoforms of METTL3 were
significantly upregulated (2.2- and 1.7-fold, respectively) in
patients with CRC compared with the controls. The linear USP3 was
significantly upregulated 2.1-fold (P<0.0001); however, its
circular isoform only showed a trend towards upregulation (2.2-fold
change; P=0.11). Finally, ciRS-7 and the linear SHPRH were not
differentially regulated (P=0.54 and 0.59, respectively), although
circSHPRH was upregulated 1.6-fold (P=0.03). Based on these
findings, circMETTL3, circUSP3 and their linear counterparts were
selected for further analysis in the remainder of the samples
(n=74).
circMETTL3, circUSP3 and their linear
counterparts are upregulated in CRC
Although GAPDH is commonly used as a reference gene
in RT-qPCR studies of cancer, several reports have documented its
overexpression in CRC (54,55). To address this issue, the CFX Manager
‘target stability value’ tool was used to examine the stability of
all three selected reference genes in 74 samples. The tool's
recommendations for mean CV and mean M-values for homogeneous
samples are <0.25 and <0.5, respectively. The only
combination that met these criteria consisted of RPL13A and RPLP1
(Table IV). The other three
possible combinations, all of which included GAPDH, satisfied
neither criterion. Therefore, GAPDH is unsuitable as a reference
gene in leukocytes from patients with CRC and was consequently
removed from subsequent normalization calculations.
 | Table IV.CV and mean M-value for each
combination of reference genes. Recommendations shown are taken
from the Target Stability Value tool in the CFX Manager
software. |
Table IV.
CV and mean M-value for each
combination of reference genes. Recommendations shown are taken
from the Target Stability Value tool in the CFX Manager
software.
| Reference gene
combination | Mean CV
(recommended <0.25) | Mean M-value
(recommended <0.5) |
|---|
|
RPL13A-RPLP1-GAPDH | 0.3519 | 0.8652 |
| RPL13A-RPLP1 | 0.1597 | 0.4596 |
| RPL13A-GAPDH | 0.3683 | 1.0501 |
| RPLP1-GAPDH | 0.3818 | 1.0858 |
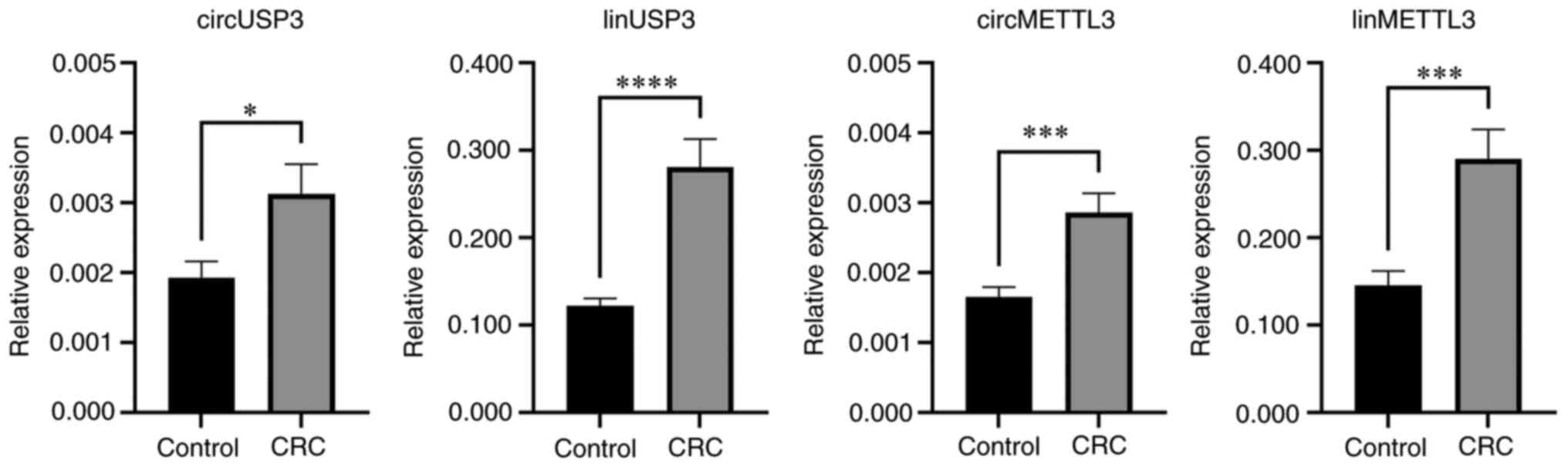
Further analysis of a total of 42 CRC patients and
32 controls revealed that circMETTL3, circUSP3, as well as their
linear counterparts, were significantly upregulated in leukocytes
from patients with CRC (Fig. 2;
Table V). The linear transcript of
USP3 had the highest average upregulation, with a 2.3-fold increase
(P<0.0001), while its circular isoform had the lowest
upregulation of (1.6-fold; P=0.016). The expression of the linear
transcript of METTL3 nearly doubled on average in CRC samples
(P=0.0003), and its circular isoform exhibited a 1.7-fold increase
(P=0.0003).
 | Table V.Parameters of relative expression
between CRC and normal samples. |
Table V.
Parameters of relative expression
between CRC and normal samples.
|
| Mean corrected
relative expression (control) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Assay | Control | CRC | Difference between
means ± SEM | Average fold change
± SEM | Fold change
P-value |
|---|
| circMETTL3 | 0.0017 | 0.0027 | 0.0012±0.0003 | 1.730±0.191 | 0.0003 |
| linMETTL3 | 0.1456 | 0.2902 | 0.1446±0.0378 | 1.993±0.259 | 0.0003 |
| circUSP3 | 0.0019 | 0.0031 | 0.0012±0.0005 | 1.623±0.251 | 0.0158 |
| linUSP3 | 0.1224 | 0.2808 | 0.1584±0.0335 | 2.294±0.274 | <0.0001 |
None of the transcripts were differentially
regulated based on cancer stage (Welch's one-way ANOVA), sex
(Welch's two tailed t-test), or age (Welch's one-way ANOVA,
Figs. S1, S2, and S3,
respectively).
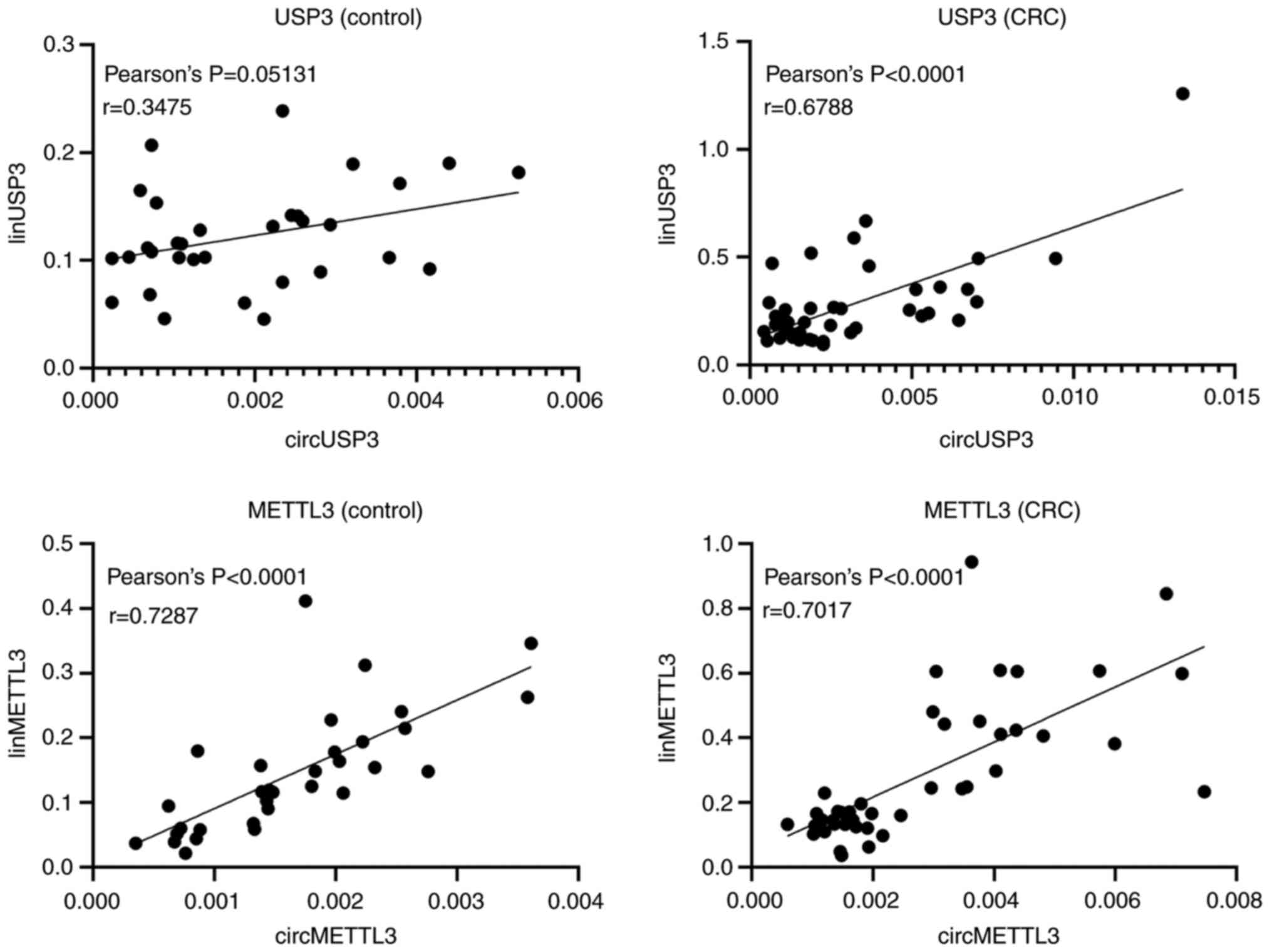
Correlation between the expression
patterns of circular and linear transcripts
To determine whether there was a correlation between
circular and linear transcripts of the genes, Pearson's
coefficients were calculated between circular and linear isoforms
in the CRC and control groups (Fig.
3). There was a strong positive correlation between the
circular and linear isoforms of METTL3, both in patients with CRC
(r=0.7287; P<0.0001) and in healthy controls (r=0.7017;
P<0.0001). Interestingly, while there was no correlation between
circular and linear USP3 transcripts in the leukocytes from healthy
controls (r=0.3475; P=0.0513), a strong positive correlation was
observed in patients with CRC (r=0.6788; P<0.0001).
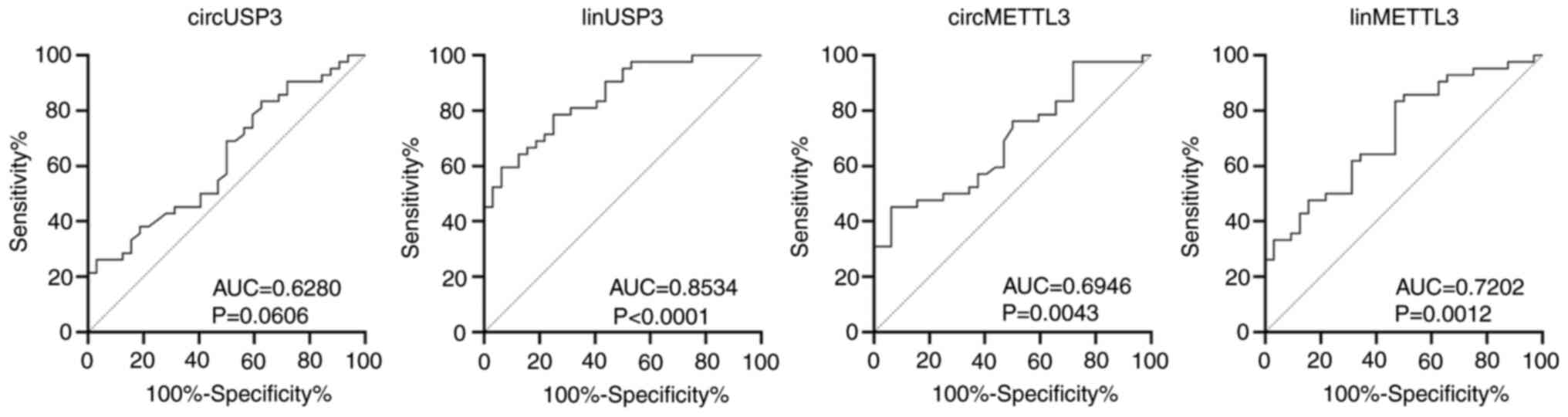
Linear USP3 is a potential candidate
as a non-invasive CRC biomarker
To determine the diagnostic ability of the candidate
transcripts, the AUC was calculated for each assay (Fig. 4, Table
VI). The linear USP3 had an AUC of 0.8534 (P<0.0001) with
sensitivity, specificity, PPV and NPV of 79, 75, 81 and 73%,
respectively. The linear METTL3 assay had excellent sensitivity
(83%) and moderate PPV and NPV (70 and 71%, respectively), albeit
with poor specificity (53.1%). circMETTL3 and circUSP3 exhibited
excellent specificity (94 and 97%, respectively) and PPV (91 and
92%, respectively), but had poor sensitivity and NPV (<50%).
 | Table VI.Receiver operating characteristic
analysis of circUSP3, circMETTL3 and their linear counterparts. |
Table VI.
Receiver operating characteristic
analysis of circUSP3, circMETTL3 and their linear counterparts.
| Assay | AUC | P-value | Sensitivity, % | Specificity, % | PPV, % | NPV, % | Normalized
expression cut-off |
|---|
| circMETTL3 | 0.6946 | 0.0043 | 45.2 | 93.8 | 90.5 | 56.6 | ≥0.00296 |
| linMETTL3 | 0.7202 | 0.0012 | 83.3 | 53.1 | 70.0 | 70.8 | ≥0.12576 |
| circUSP3 | 0.6280 | 0.0606 | 26.2 | 96.9 | 91.7 | 50.0 | ≥0.00493 |
| linUSP3 | 0.8534 | <0.0001 | 78.6 | 75.0 | 80.5 | 72.7 | ≥0.14594 |
Discussion
Suboptimal qPCR assays can lead to erroneous
results. Despite the recommendations of the MIQE guidelines
(26), which are considered the
benchmark for RT-qPCR studies, the majority of published articles
still fail to report the efficiency of their assays, use one
reference gene for normalization and do not clearly report detailed
information about their PCR conditions (20,25). To
make a stronger claim for the diagnostic ability of our assays,
their robustness and reproducibility were ensured by showing
evidence of their optimal efficiency and accounted for these
efficiencies in the relative expression calculations. Moreover,
although three reference genes were initially included, a
combination of two reference genes was ultimately used for
normalization. The observation that GAPDH was an unsuitable
reference gene in the conditions used in this study compounds the
impracticality of dependence on a lone reference gene. Moreover,
each step taken in the process was described in order to provide
complete transparency, which should be an obviously indispensable
practice, but is still widely abandoned in the field (20,25).
Using this stringent RT-qPCR approach, to the best of our
knowledge, the present study reports the first time the
upregulation of both the circular and the linear isoform of USP3
and METTL3 in leukocytes from patients with CRC.
All transcripts showed promising diagnostic ability,
but the linear isoform of USP3 was remarkable. Its upregulation
pattern did not differ based on the available clinicopathological
data of the patients, making it a potentially excellent biomarker
for detecting CRC at the early stages, when the survival rates are
high. Validation of this assay in a larger study cohort is
encouraged to confirm its predictive power in cancer and to apply
it in a wide range of cancer types to examine whether its
upregulation is CRC-specific or common among cancer types.
Despite the observation that circUSP3 is upregulated
in leukocytes from patients with CRC, Ruan et al (34) and Bachmayr-Heyda et al
(30) reported its downregulation in
CRC tissue compared with normal adjacent tissue. The same applies
to circMETTL3, which was found to be upregulated in leukocytes from
patients with CRC in the present study, but which Jin et al
(31) and Bachmayr-Heyda et
al (30) identified as
downregulated in 12 CRC cell lines and in CRC tissue, respectively.
Not much is known about the mechanistic role of circUSP3, although
dual luciferase and knockdown/overexpression experiments by Jin
et al (31) revealed sponging
of miR-31 by circMETTL3, leading to the deactivation of the
Wnt/β-catenin signaling pathway. It may be hypothesized, therefore,
that despite activation of Wnt/β-catenin signaling in CRC tissue,
leukocytes can still deactivate this pathway by overexpressing
circMETTL3.
The observed overexpression of the linear
transcripts of USP3 and METTL3 in leukocytes from patients with CRC
is consistent with their upregulation in CRC tissue (56–63). The
linear isoform of USP3 is involved in the DNA damage response and
its expression is elevated in a number of solid cancers (56). In an interesting multifaceted
investigation, Das et al (56) showed that USP3 promoted cell cycle
progression in a number of cancer cell lines by inhibiting
ubiquitination of the oncogene CDC25A. The linear isoform of METTL3
expresses the only catalytic unit in the methyltransferase complex.
It methylates adenosine residues of RNA at N6 and its levels are
elevated in numerous cancers, leading to global hypermethylation
(57). Nonetheless, whether the
mechanisms of action for USP3 and METTL3 in leukocytes are similar
to those in CRC tissue in diseased subjects still needs to be
verified by further research.
In conclusion, the present study provides the first
evidence of the upregulation of circMETTL3 and circUSP3, along with
their linear isoforms, in the leukocytes from patients with CRC.
This study has the added strength of avoiding some of the critical
errors that can lead to irreproducible RT-qPCR results. These four
transcripts may represent good candidates for more extensive
studies on their potential involvement in CRC progression, and the
linear isoform of USP3 has great prospect as a non-invasive
biomarker for CRC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
This research and the article processing charge were
funded by the Deanship of Scientific Research at King Abdulaziz
University, Jeddah (grant no. G: 284-130-1435).
Availability of data and materials
The datasets used and/or analyzed during this study
are available from the corresponding author on reasonable
request.
Authors' contributions
HC conceptualized the experiments. BA designed the
experiments. AAG acquired the samples, and BA and AAG performed the
experiments. BA, MIK and HC analyzed the data. BA wrote the
manuscript. MIK and HC revised the manuscript for important
intellectual content and managed the project. BA and HC confirmed
the authenticity of all the raw data. All authors have read and
approved the manuscript.
Ethics approval and consent to
participate
The purpose of the study was explained to all
participants and their written consent was obtained before
proceeding. The Unit of Biomedical Ethics at The Faculty of
Medicine, King Abdulaziz University, approved this study (approval.
no. 261-15).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuipers EJ, Grady WM, Lieberman D,
Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ and Watanabe T:
Colorectal cancer. Nat Rev Dis Primers. 1:150652015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yiu AJ and Yiu CY: Biomarkers in
colorectal cancer. Anticancer Res. 36:1093–1102. 2016.PubMed/NCBI
|
|
4
|
Bray C, Bell LN, Liang H, Collins D and
Yale SH: Colorectal cancer screening. WMJ. 116:27–33.
2017.PubMed/NCBI
|
|
5
|
Singh H, Nugent Z, Demers AA, Kliewer EV,
Mahmud SM and Bernstein CN: The reduction in colorectal cancer
mortality after colonoscopy varies by site of the cancer.
Gastroenterology. 139:1128–1137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brenner H, Stock C and Hoffmeister M:
Effect of screening sigmoidoscopy and screening colonoscopy on
colorectal cancer incidence and mortality: Systematic review and
meta-analysis of randomised controlled trials and observational
studies. BMJ. 348:g24672014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
US Preventive Services Task Force, ;
Bibbins-Domingo K, Grossman DC, Curry SJ, Davidson KW, Epling JW
Jr, García FA, Gillman MW, Harper DM, Kemper AR, et al: Screening
for colorectal cancer: US Preventive Services Task Force
recommendation statement. JAMA. 315:2564–2575. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bosch LJ, Carvalho B, Fijneman RJ, Jimenez
CR, Pinedo HM, van Engeland M and Meijer GA: Molecular tests for
colorectal cancer screening. Clin Colorectal Cancer. 10:8–23. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grützmann R, Molnar B, Pilarsky C,
Habermann JK, Schlag PM, Saeger HD, Miehlke S, Stolz T, Model F,
Roblick UJ, et al: Sensitive detection of colorectal cancer in
peripheral blood by septin 9 DNA methylation assay. PLoS One.
3:e37592008. View Article : Google Scholar
|
|
10
|
Church TR, Wandell M, Lofton-Day C, Mongin
SJ, Burger M, Payne SR, Castaños-Vélez E, Blumenstein BA, Rösch T,
Osborn N, et al: Prospective evaluation of methylated SEPT9 in
plasma for detection of asymptomatic colorectal cancer. Gut.
63:317–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imperiale TF, Ransohoff DF, Itzkowitz SH,
Levin TR, Lavin P, Lidgard GP, Ahlquist DA and Berger BM:
Multitarget stool DNA testing for colorectal-cancer screening. N
Engl J Med. 370:1287–1297. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Redwood DG, Asay ED, Blake ID, Sacco PE,
Christensen CM, Sacco FD, Tiesinga JJ, Devens ME, Alberts SR,
Mahoney DW, et al: Stool DNA testing for screening detection of
colorectal neoplasia in Alaska native people. Mayo Clin Proc.
91:61–70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang P and He X: Current research on
circular RNAs associated with colorectal cancer. Scand J
Gastroenterol. 52:1203–1210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lei B, Tian Z, Fan W and Ni B: Circular
RNA: A novel biomarker and therapeutic target for human cancers.
Int J Med Sci. 16:292–301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ng WL, Mohd Mohidin TB and Shukla K:
Functional role of circular RNAs in cancer development and
progression. RNA Biol. 15:995–1005. 2018.PubMed/NCBI
|
|
16
|
Hansen TB, Jensen TI, Clausen BH, Bramsen
JB, Finsen B, Damgaard CK and Kjems J: Natural RNA circles function
as efficient microRNA sponges. Nature. 495:384–388. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ashwal-Fluss R, Meyer M, Pamudurti NR,
Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N and
Kadener S: circRNA biogenesis competes with pre-mRNA splicing. Mol
Cell. 56:55–66. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Du WW, Fang L, Yang W, Wu N, Awan FM, Yang
Z and Yang BB: Induction of tumor apoptosis through a circular RNA
enhancing Foxo3 activity. Cell Death Differ. 24:357–370. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Zhang XO, Chen T, Xiang JF, Yin
QF, Xing YH, Zhu S, Yang L and Chen LL: Circular intronic long
noncoding RNAs. Mol Cell. 51:792–806. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bustin SA: The reproducibility of
biomedical research: Sleepers awake! Biomol Detect Quantif.
2:35–42. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Contopoulos-Ioannidis DG, Ntzani E and
Ioannidis JP: Translation of highly promising basic science
research into clinical applications. Am J Med. 11:477–484. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ioannidis JP: Evolution and translation of
research findings: From bench to where? PLoS Clin Trials.
1:e362006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Prinz F, Schlange T and Asadullah K:
Believe it or not: How much can we rely on published data on
potential drug targets? Nat Rev Drug Discov. 10:7122011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Begley CG and Ellis LM: Drug development:
Raise standards for preclinical cancer research. Nature.
483:531–533. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bustin S and Nolan T: Talking the talk,
but not walking the walk: RT-qPCR as a paradigm for the lack of
reproducibility in molecular research. Eur J Clin Invest.
47:756–774. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Bustin SA, Benes V, Garson JA, Hellemans
J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL,
et al: The MIQE guidelines: Minimum information for publication of
quantitative real-time PCR experiments. Clin Chem. 55:611–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weng W, Wei Q, Toden S, Yoshida K,
Nagasaka T, Fujiwara T, Cai S, Qin H, Ma Y and Goel A: Circular RNA
ciRS-7-A promising prognostic biomarker and a potential therapeutic
target in colorectal cancer. Clin Cancer Res. 23:3918–3928. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Barbagallo C, Brex D, Caponnetto A,
Cirnigliaro M, Scalia M, Magnano A, Caltabiano R, Barbagallo D,
Biondi A, Cappellani A, et al: LncRNA UCA1, upregulated in CRC
biopsies and downregulated in serum exosomes, controls mRNA
expression by RNA-RNA interactions. Mol Ther Nucleic Acids.
7:229–241. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tang W, Ji M, He G, Yang L, Niu Z, Jian M,
Wei Y, Ren L and Xu J: Silencing CDR1as inhibits colorectal cancer
progression through regulating microRNA-7. Onco Targets Ther.
10:2045–2056. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bachmayr-Heyda A, Reiner AT, Auer K,
Sukhbaatar N, Aust S, Bachleitner-Hofmann T, Mesteri I, Grunt TW,
Zeillinger R and Pils D: Correlation of circular RNA abundance with
proliferation-exemplified with colorectal and ovarian cancer,
idiopathic lung fibrosis, and normal human tissues. Sci Rep.
5:80572015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Jin Y, Yu LL, Zhang B, Liu CF and Chen Y:
Circular RNA hsa_circ_0000523 regulates the proliferation and
apoptosis of colorectal cancer cells as miRNA sponge. Braz J Med
Biol Res. 51:e78112018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li F, Huang Q, Gong Z, Wang H and Chen J:
Diagnostic and prognostic roles of circ-SHPRH for solid cancers: A
meta-analysis. Onco Targets Ther. 12:4351–4357. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ji W, Qiu C, Wang M, Mao N, Wu S and Dai
Y: Hsa_circ_0001649: A circular RNA and potential novel biomarker
for colorectal cancer. Biochem Biophys Res Commun. 497:122–126.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ruan H, Deng X, Dong L, Yang D, Xu Y, Peng
H and Guan M: Circular RNA circ_0002138 is down-regulated and
suppresses cell proliferation in colorectal cancer. Biomed
Pharmacother. 111:1022–1028. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zeng K, Chen X, Xu M, Liu X, Hu X, Xu T,
Sun H, Pan Y, He B and Wang S: CircHIPK3 promotes colorectal cancer
growth and metastasis by sponging miR-7. Cell Death Dis. 9:4172018.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li XN, Wang ZJ, Ye CX, Zhao BC, Li ZL and
Yang Y: RNA sequencing reveals the expression profiles of circRNA
and indicates that circDDX17 acts as a tumor suppressor in
colorectal cancer. J Exp Clin Cancer Res. 37:3252018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li XN, Wang ZJ, Ye CX, Zhao BC, Huang XX
and Yang L: Circular RNA circVAPA is up-regulated and exerts
oncogenic properties by sponging miR-101 in colorectal cancer.
Biomed Pharmacother. 112:1086112019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen S, Zhang L, Su Y and Zhang X:
Screening potential biomarkers for colorectal cancer based on
circular RNA chips. Oncol Rep. 39:2499–2512. 2018.PubMed/NCBI
|
|
39
|
Yuan Y, Liu W, Zhang Y, Zhang Y and Sun S:
CircRNA circ_0026344 as a prognostic biomarker suppresses
colorectal cancer progression via microRNA-21 and microRNA-31.
Biochem Biophys Res Commun. 503:870–875. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Z, Song N, Wang Y, Zhong J, Gu T,
Yang L, Shen X, Li Y, Yang X, Liu X, et al: Analysis of
differentially expressed circular RNAs for the identification of a
coexpression RNA network and signature in colorectal cancer. J Cell
Biochem. 120:6409–6419. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Xu H, Wang C, Song H, Xu Y and Ji G:
RNA-Seq profiling of circular RNAs in human colorectal cancer liver
metastasis and the potential biomarkers. Mol Cancer. 18:82019.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang L, Peng X, Lu X, Wei Q, Chen M and
Liu L: Inhibition of hsa_circ_0001313 (circCCDC66) induction
enhances the radio-sensitivity of colon cancer cells via tumor
suppressor miR-338-3p: Effects of cicr_0001313 on colon cancer
radio-sensitivity. Pathol Res Pract. 215:689–696. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hsiao KY, Lin YC, Gupta SK, Chang N, Yen
L, Sun HS and Tsai SJ: Noncoding effects of circular RNA CCDC66
promote colon cancer growth and metastasis. Cancer Res.
77:2339–2350. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang XL, Xu LL and Wang F:
Hsa_circ_0020397 regulates colorectal cancer cell viability,
apoptosis and invasion by promoting the expression of the miR-138
targets TERT and PD-L1. Cell Biol Int. 41:1056–1064. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhang R, Xu J, Zhao J and Wang X:
Silencing of hsa_circ_0007534 suppresses proliferation and induces
apoptosis in colorectal cancer cells. Eur Rev Med Pharmacol Sci.
22:118–126. 2018.PubMed/NCBI
|
|
46
|
Li X, Wang J, Zhang C, Lin C, Zhang J,
Zhang W, Zhang W, Lu Y, Zheng L and Li X: Circular RNA circITGA7
inhibits colorectal cancer growth and metastasis by modulating the
Ras pathway and upregulating transcription of its host gene ITGA7.
J Pathol. 246:166–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang Z, Su M, Xiang B, Zhao K and Qin B:
Circular RNA PVT1 promotes metastasis via miR-145 sponging in CRC.
Biochem Biophys Res Commun. 512:716–722. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zheng X, Chen L, Zhou Y, Wang Q, Zheng Z,
Xu B, Wu C, Zhou Q, Hu W, Wu C and Jiang J: A novel protein encoded
by a circular RNA circPPP1R12A promotes tumor pathogenesis and
metastasis of colon cancer via Hippo-YAP signaling. Mol Cancer.
18:472019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bian L, Zhi X, Ma L, Zhang J, Chen P, Sun
S, Li J, Sun Y and Qin J: Hsa_circRNA_103809 regulated the cell
proliferation and migration in colorectal cancer via
miR-532-3p/FOXO4 axis. Biochem Biophys Res Commun. 505:346–352.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Zhang P, Zuo Z, Shang W, Wu A, Bi R, Wu J,
Li S, Sun X and Jiang L: Identification of differentially expressed
circular RNAs in human colorectal cancer. Tumour Biol.
39:10104283176945462017.PubMed/NCBI
|
|
51
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 4:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
ThermoFisher Scientific: qPCR efficiency
calculator. https://www.thermofisher.com/uk/en/home/brands/thermo-scientific/molecular-biology/molecular-biology-learning-center/molecular-biology-resource-library/thermo-scientific-web-tools/qpcr-efficiency-calculator.htmlDecember
23–2020PubMed/NCBI
|
|
53
|
Goksuluk D, Korkmaz S, Zararsiz G and
Karaağaoğlu AE: easyROC: An interactive web-tool for ROC curve
analysis using R language environment. Contributed Res. 8:213–230.
2016.
|
|
54
|
Zhu Y, Yang C, Weng M, Zhang Y, Yang C,
Jin Y, Yang W, He Y, Wu Y, Zhang Y, et al: Identification of
TMEM208 and PQLC2 as reference genes for normalizing mRNA
expression in colorectal cancer treated with aspirin. Oncotarget.
8:22759–22771. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Guo C, Liu S and Sun MZ: Novel insight
into the role of GAPDH playing in tumor. Clin Transl Oncol.
15:167–172. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Das S, Chandrasekaran AP, Suresh B, Haq S,
Kang JH, Lee SJ, Kim J, Kim J, Lee S, Kim HH, et al: Genome-scale
screening of deubiquitinase subfamily identifies USP3 as a
stabilizer of Cdc25A regulating cell cycle in cancer. Cell Death
Differ. 27:3004–3020. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Li Y, Ge YZ, Xu L, Xu Z, Dou Q and Jia R:
The potential roles of RNA N6-methyladenosine in urological tumors.
Front Cell Dev Biol. 8:5799192020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wang H, Xu B and Shi J: N6-methyladenosine
METTL3 promotes the breast cancer progression via targeting Bcl-2.
Gene. 722:1440762020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Wang Q, Geng W, Guo H, Wang Z, Xu K, Chen
C and Wang S: Emerging role of RNA methyltransferase METTL3 in
gastrointestinal cancer. J Hematol Oncol. 13:572020. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Zeng C, Huang W, Li Y and Weng H: Roles of
METTL3 in cancer: Mechanisms and therapeutic targeting. J Hematol
Oncol. 13:1172020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Fan L, Chen Z, Wu X, Cai X, Feng S, Lu J,
Wang H and Liu N: Ubiquitin-specific protease 3 promotes
glioblastoma cell invasion and epithelial-mesenchymal transition
via stabilizing snail. Mol Cancer Res. 10:1975–1984. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fang CL, Lin CC, Chen HK, Hseu YC, Hung
ST, Sun DP, Uen YH and Lin KY: Ubiquitin-specific protease 3
overexpression promotes gastric carcinogenesis and is predictive of
poor patient prognosis. Cancer Sci. 109:3438–3449. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Liao XH, Wang Y, Zhong B and Zhu SY: USP3
promotes proliferation of non-small cell lung cancer through
regulating RBM4. Eur Rev Med Pharmacol Sci. 6:3143–3151.
2020.PubMed/NCBI
|