Introduction
Worldwide, in 2018 there were an estimated 570,000
cases of cervical cancer and 311,000 women died due to this
malignancy (1). In Sweden, since the
1960s, an organized, population-based cervical screening program
has substantially reduced cervical cancer incidence and mortality
(2,3). However, more recent data suggest that
cervical cancer is on the rise once again in Sweden (4). A key aim of cervical screening
programs, such as that in Sweden, is to identify and treat women
with precancerous lesions, cervical intraepithelial neoplasia
(CIN), before these lesions develop into invasive cancer (5). Once high-grade CIN has been identified
and treated, more intense, prolonged follow-up is needed compared
to the general population of women (6). Follow-up is of critical importance,
because these patients are at long-term risk for developing
recurrent disease (7,8). Yet, evidence-based recommendations for
the most appropriate post-therapeutic screening protocols are
lacking (9).
It is now clearly-established that the high-risk
human papilloma virus (HPV) is the key contributor to the
development of cervical cancer. Consequently, testing for HPV is a
vital component of all aspects of cervical cancer screening. This
includes the use of HPV tests to evaluate the risk of recurrent
disease in patients who have been treated for high-grade CIN
(10). Numerous studies have shown
that HPV testing provides essential information concerning the
chances of disease recurrence in this vulnerable population of
women (11–15).
A particular concern vis-à-vis cervical cancer risk
is among women treated for high-grade CIN at a more advanced age.
In the Swedish cancer registry study of all 132,493 women treated
for CIN3 between 1958–2002, the relative risk of subsequent
cervical cancer rose steadily with each decade of life. An
accelerated risk was noted for women above 60 years of age
(8). On the one hand, among women in
the more senior age group, the overall prevalence of HPV positivity
appears to be quite low (16). On
the other hand, however, once infected with HPV, the infection may
be more persistent than among younger women, and is associated with
a high prevalence of cervical dysplasia (16–18). The
potential contribution of persistent HPV infection to cervical
cancer risk has, thus, been particularly emphasized for this age
group. Further, these findings underscore the need to find
appropriate algorithms for the more senior women to optimize
cervical cancer surveillance (16).
Incomplete excision of high-grade CIN has also been
implicated as a risk factor for recurrence of high-grade cervical
dysplasia (19–23). In the literature, reports about the
percentage of unclear or uncertain margins range widely, from as
low as 3% up to as high as 50–60%, with the overall percentage
being approximately 23%, according to a fairly-recent meta-analysis
(20). Compared to when both the
endocervical and ectocervical margins were considered clear, the
risk was an estimated four to six-fold higher for recurrent
high-grade cervical dysplasia with one or both unclear margins
(20,24).
Given the recognized etiologic role of HPV, however,
questions have been raised about the actual importance of margin
status in predicting recurrence after treatment of high-grade
cervical dysplasia. In our 2020 long-term follow-up registry study
of 991 women with histopathologically-confirmed high-grade CIN who
underwent conization from 2000 to 2007, a single post-conization
HPV result was available for a small subgroup of the patients
(19). Among the 84 patients with
positive HPV findings, those with positive/uncertain margins showed
a significantly increased risk of recurrent/residual CIN2+ (over
two-fold) compared to women with HPV positive findings but with
clear margins. In contrast, however, among the 105 women with
negative post-conization HPV findings, margin status was not found
to be significantly associated with recurrence. We noted the need
for further investigation of this question with more complete
post-conization HPV data for a cohort of patients followed after
treatment. This is one of the aims of the present study.
In that light, most recently, within the framework
of an investigation comparing clinician-sampled and self-sampled
specimens at early post-conization follow-up, complete HPV and
cytologic results, as well as practically complete colposcopic
results were obtained for a large cohort of patients (25). We now incorporate these early
post-conization data into a longer-term follow-up study extending
for over six years. Full data on margin excision status and other
aspects of initial treatment are included, as well as consideration
of age and comorbid conditions that may impact on HPV acquisition
and/or CIN progression (19,26). Herein, our main focus is upon
recurrence of high-grade cervical dysplasia, seeking to identify
the factors that independently contribute to treatment failure.
Materials and methods
Design of the study, population and
location
Between October 2014 and January 2017, all patients
who had been treated for the first time by conization for
histologically-confirmed high-grade CIN (CIN2+) or adenocarcinoma
in-situ (AIS) were eligible to participate in this study. The
hospitals in which the patients had been treated were: Karolinska
University Hospital, Danderyd Hospital or South General Hospital,
all within Stockholm County, Sweden.
The patients were contacted soon after treatment by
Ellinor Östensson, EÖ, who scheduled the 1st follow-up visit at
Karolinska University Hospital. Approximately six months
post-conization was the aimed time interval for this 1st follow-up
visit. Often after repeated attempts to find a suitable time, all
532 patients were scheduled and then attended this 1st
follow-up.
After coming to the Karolinska University Hospital
for the 1st follow-up visit, EÖ met with each woman to present the
study procedures. These included self-collection of vaginal and
urine samples for HPV testing, as reported in a previous study
(25); completion of a
questionnaire, as reported in previous studies (27,28);
gynecologic examination with colposcopy and cervical sampling as
clinical follow-up. The overall aim of the study was stated to be
prevention of cervical cancer. The participants were assured of
complete confidentiality and full freedom to withdraw from the
study at any time with no consequences whatsoever. Permission to
review the patient's medical records was included in the informed
consent. The options for the informed consent were: Agreement to
participate and decline to participate. Karolinska Ethics Committee
approved the study protocol (approval nos. 2006/1273-31,
2014/2034-3). One patient declined to participate. Two patients
were found to have microinvasive squamous cell carcinoma upon
histopathological re-examination, when already enrolled in the
study. These two patients were excluded from further follow-up
analyses herein. Thus, the total number of patients in the present
study is 529.
1st follow-up visit: Gynecologic
examination with colposcopy, clinician-collected cervical samples,
other procedures
One of the two gynecologists (Dr. Andersson or Dr.
Mints), who performed colposcopy and cervical sampling, first met
with each patient. Punch biopsies were directed by colposcopy, and
were obtained from visible lesions. The biopsies were
histologically graded with the analysis done at Karolinska
University Hospital. Standard procedures were followed, using the
CIN classification (29). Patients
in whom a recurrent lesion was found were sent for follow-up
treatment. This was re-excision or simple total hysterectomy, based
upon clinical evaluation and other considerations.
Enrolled women were followed according to national
guidelines using cytology co-testing. The liquid-based method
(ThinPrep®, Hologic, Marlbororgh, MA, USA) was used for
cytology and the Cobas 4800 assay (Roche Molecular Diagnostics,
Pleasanton, CA, USA) for standard HPV testing. Samples were taken
from the endocervix using cervical brushes and from the ectocervix
with plastic spatulas. The samples were transferred into PreservCyt
liquid-based cytology (LBC) vials according to European guidelines
(30). The LBC was carried out at
the Cytology Department of the Karolinska University Hospital,
following the Bethesda system (31).
The HPV DNA testing performed on-site was with the hospital's
standard: Cobas 4800 HPV (Roche Diagnostics).
In addition, as part of the participation in the
study, but not used for clinical decision-making,
clinician-collected cervical samples (Abbott), self-collected
vaginal samples (VSS) and urine samples were analyzed for
comparative HPV testing at 1st follow-up. The procedure employed a
multiplex real-time polymerase chain reaction (PCR) test which
detects HPV16, HPV18, as well as other high-risk HPV: 31, 33, 35,
39, 45, 51, 52, 56, 58, 59, 66, 68. The results of this comparative
testing are described in detail in a previous study (25). Herein, the results of the Abbott
clinician-collected cervical samples and VSS are mainly presented
for the patients in whom recurrent disease was detected.
Subsequent follow-up
Subsequent follow-up was based upon the results from
LBC and Cobas HPV from the first follow-up. Insofar as cytological
abnormalities were found, and/or the Cobas HPV result was positive,
the patient was referred for a second follow-up. This second
follow-up employed the same standard protocol as the first
follow-up, and was based upon Swedish National Guidelines. The
second follow-up was usually scheduled approximately one year after
the first follow-up. Insofar as the cytology was negative for
intraepithelial lesions or malignancy (NILM) and the HPV Cobas
findings were also negative, Swedish National Guidelines were that
the patient should return to routine triennial screening. This
routine screening was envisioned to include HPV testing using Cobas
from a clinician-taken sample and cytologic examination, with
colposcopy performed according to clinician discretion.
Review of medical records
The entire medical record of each patient was
thoroughly reviewed through December 2020. Information was obtained
on age at the time of conization, the modality of conization, grade
of dysplasia in the excised cone, number of cone pieces and margin
status in the cone biopsy. Excisions were considered incomplete
when dysplasia was found along the specimen margin, termed
‘unclear’ or when the margin status was uncertain. Assessment of
the resection margins was further subdivided into: i) Ectocervical
only, ii) endocervical only or iii) both margins unclear or
uncertain. All comorbid diagnoses were noted. These were also
categorized as conditions assumed to interact with HPV acquisition
or CIN progression: autoimmune disorders, malignancy, infection
with hepatitis or human immunodeficiency virus, diabetes mellitus,
genetic disorders or organ transplantation (19,26).
Diagnosed recurrent/residual disease was defined as
histologically-confirmed high-grade CIN on biopsy taken at
colposcopy during any of the follow-up examinations.
Statistical analysis
A power analysis was performed prior to the study.
Therein, it was estimated that 500 patients were needed for
statistical significance at an alpha level of P<0.05. Extensive
univariate and bivariate analyses were first undertaken. The latter
was performed using the Pearson χ2 test or Fisher's
exact test if any expected cell was less than five, with one degree
of freedom. All comparisons were two-sided. Salient
dichotomizations were thereby made, as described in the Results
section. Statistica (13.5.0.17/TIBCO-2018) and SPSS
(IBM-version-25.0; IBM, Armonk, NY, USA) were used for statistical
analysis. Multiple logistic regression was used to compute odds
ratios (OR) and 95% confidence intervals (CI) with the outcome
being detected recurrence of high-grade CIN.
Results
Study overview and detection of
recurrence
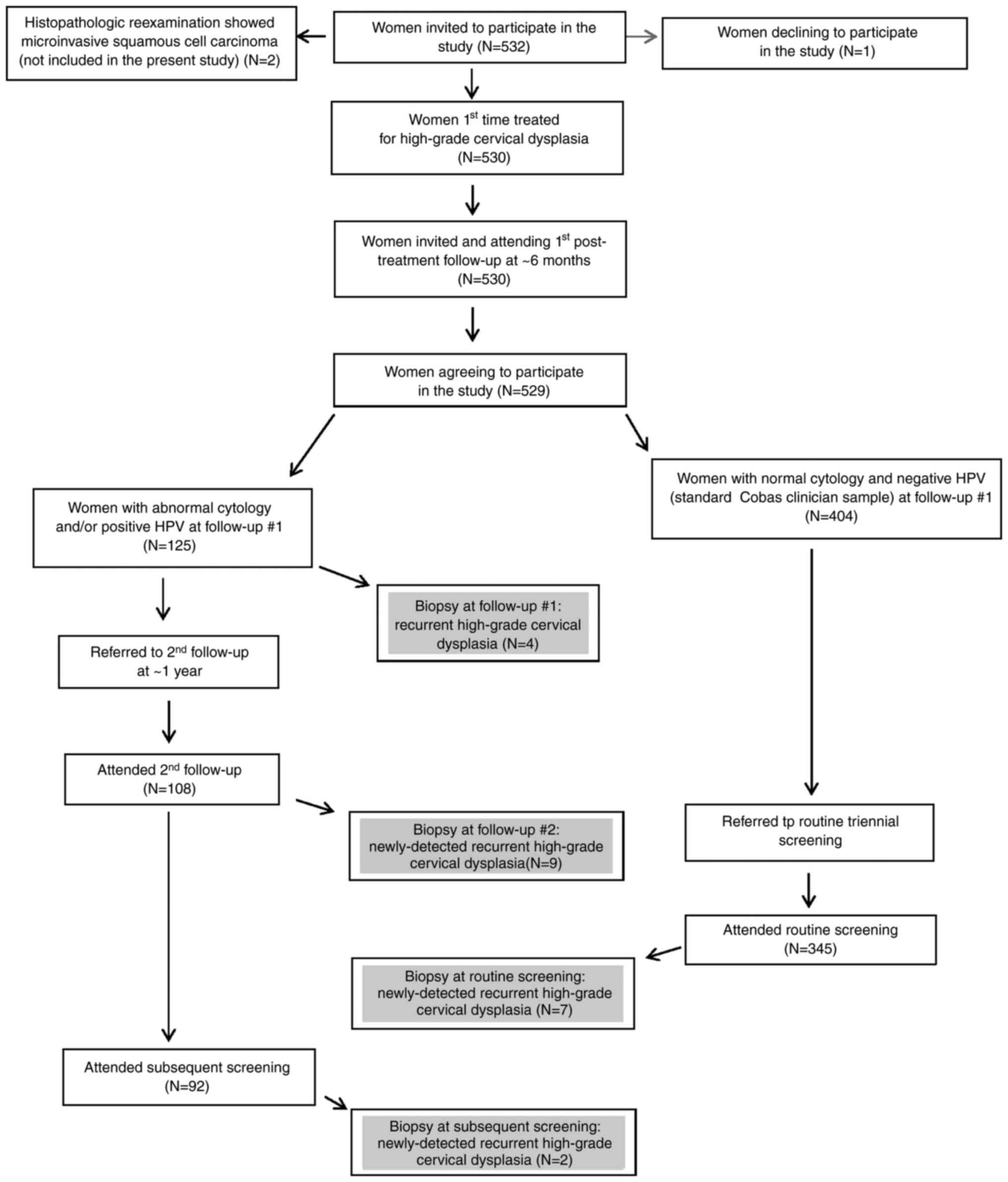
A summary of the number of women included in each
step of the study is provided in Fig.
1. The detected recurrent cases are highlighted therein.
Demographic and other baseline
univariate findings
Among the 529 patients included in the present
study, the mean age was 34.3 years at the time of treatment.
Altogether, 188 (35.5%) of the patients were below age 30 at the
time of treatment, and seventy-six patients (14.4%) were age 45 or
above at that time. Over two-thirds of the patients in this cohort
were employed and well over half were university educated (25,27,28).
Most of the patients (85%) were treated using the
contoured-loop excision of the transformation zone (C-LETZ)
surgical method. Seventy-seven patients (14.6%) were treated by
laser conization, while three patients were treated with ablation.
In the vast majority of the patients, a single cone piece was
excised. The margin excision status revealed that the endocervical
and ectocervical margins were both clear in 73% of the patients.
Both these margins were either unclear or uncertain in 56 (10.6%)
patients, while in the remaining 87 patients only one of the
margins was clear. The histology of the excised cone was CIN2 in
133 of the patients (25%), CIN2/3 in 368 patients (70%), CIN3/AIS
in 15 patients and AIS in 13 patients.
Univariate findings from 1st
follow-up
Over half the 529 patients came to 1st follow-up
within six months after treatment; all but four of the patients
came to 1st follow-up within one year after treatment. The latter
four patients came within 15 months after treatment. At first
follow-up the cytology results were available for all 529 patients;
these were normal, NILM, in 453 (86%) of the patients. There were
also complete data for HPV results at 1st follow-up; these were
positive in 86 (16.3%) of the patients. Altogether, 37 patients
(7%) had both abnormal cytology and HPV positive findings at 1st
follow-up. Four cases of recurrent high-grade CIN were detected at
1st follow-up.
Univariate findings from 2nd
follow-up
Excluding the four patients in whom recurrence was
detected at 1st follow-up, 121 patients of the 529 patients were
referred to 2nd follow-up to which 108 of these patients attended.
The majority of these patients attended 2nd follow-up within one
year after the 1st follow-up; over 90% came within two years after
the 1st follow-up. The longest time interval between the 1st and
2nd follow-up was 3.5 years. At 2nd follow-up, 74 women had NILM,
31 had abnormal cytology and cytology was missing for three
patients. Forty-three women had a positive HPV result and 51 had a
negative HPV result at that time, while HPV data were not available
for fourteen patients at 2nd follow-up. Nine cases of recurrent
high-grade CIN were detected at 2nd follow-up.
Univariate findings from routine
follow-up
There were 404 women with normal cytology and
negative HPV at 1st follow-up; these women were referred to routine
triennial screening. Altogether 345 attended (85.4%). Among the
women who had attended 2nd follow-up without detected recurrence,
92 attended the subsequent routine screening. At the latter
screening occasion, abnormal cytology was recorded in 34 women and
NILM in 345, whereas cytology results were missing for 58 women who
attended routine screening. The HPV results at routine follow-up
were positive in 36 women, negative in 299 patients and were
missing in 102 patients. Nine new cases of recurrent high-grade CIN
were detected at routine follow-up.
Comorbidity
Altogether 136 patients (25.7%) had one or more
comorbid diagnosis reported in their medical records. Overall, the
most frequent were psychiatric disorders in 51 patients, among whom
44 patients were noted to have clinical depression. Fifty-two
patients (9.8%) had a comorbid diagnosis assumed to interact with
HPV acquisition or CIN progression, the most common being
autoimmune conditions in 37 patients. The autoimmune conditions
were varied and included rheumatoid arthritis, systemic lupus
erythematosus, immune thrombocytopenia, hypothyroidism,
inflammatory bowel disease and multiple sclerosis.
A comorbid malignancy was noted in ten patients
(1.9%). Two patients had had comorbid infectious disease associated
with HPV acquisition/progression and two patients had type 2
diabetes mellitus. Genetic disorders were reported in four
patients. Thirteen patients had two or more comorbid diagnoses
reported in their medical records.
Detailed examination of the detected
recurrent cases
Tables I, II and III
provide an in-depth profile of each patient in whom recurrent
high-grade CIN was detected at the 1st, 2nd or routine follow-up,
respectively. Table I shows that all
four recurrent cases detected at 1st follow-up were of squamous
pathology on biopsy: high-grade squamous intraepithelial lesions
(HSIL). A wide age distribution is observed among these patients.
Only one of these four patients did not have clear ecto- and
endocervical margins. All had HPV positive findings by the standard
Cobas and Abbott clinician-taken samples, as well as from VSS. The
patient with uncertain margins and one of the two patients in the
age group between 56 and 60 also had HPV16 positive findings from
clinician-taken samples and VSS, whereas none showed HPV18
positivity. The other patient within the 56 to 60 age group was the
only one to have transformation zone 3 (TZ3) on colposcopy. She was
also the only one in whom any comorbidity was reported, namely an
autoimmune disorder and chronic obstructive pulmonary disease.
 | Table I.Patients with recurrent high-grade
cervical intraepithelial neoplasia detected at the 1st follow-up
visit. |
Table I.
Patients with recurrent high-grade
cervical intraepithelial neoplasia detected at the 1st follow-up
visit.
| Age, years | Surgical
method | Ectocervical
margin | Endocervical
margin | Cone piece # | Excised cone
histology | Cytology 1st
FU | TZ3 on
colposcopy | HPVa 1st FU | HPV 16b | HPV 18b | Diagnosed
comorbidity |
|---|
| 26–30 | Laser | Clear | Clear | 1 | CIN2 | HSIL | No | (+) | (−) | (−) | No |
| 31–35 | C-LETZ | Uncertain | Uncertain | 1 | CIN3 | AGC | No | (+) | (+) | (−) | No |
| 56–60 | C-LETZ | Clear | Clear | 1 | CIN2 | HSIL | Yes | (+) | (−) | (−) | Autoimmune disorder
& COPD |
|
| C-LETZ | Clear | Clear | 1 | CIN3 | HSIL | No | (+) | (+) | (−) | No |
 | Table II.Patients with recurrent high-grade
cervical intraepithelial neoplasia detected at the 2nd
follow-up. |
Table II.
Patients with recurrent high-grade
cervical intraepithelial neoplasia detected at the 2nd
follow-up.
| A, Recurrent
adenocarcinoma in situ detected at the 2nd follow-up
visit |
|---|
|
|---|
| Age, years | Surgical
method | Ectocervical
margin | Endocervical
margin | Cone piece # | Excised cone
histology | TZ3 1st FU
colposcopy | Cytology 1st
FU | HPV 1st
FUe | HPV 16 | HPV 18 | HPV 2nd
FUa | Cytology 2nd
FU | Diagnosed
comorbidity |
|---|
| 31–35 | Laser | Clear | Clear | 1 | AIS | Yes | AGC | (+)b | (−) | (+)d | (+) | AIS | No |
|
| C-LETZ | Clear | Unclear | 1 | AIS | Yes | AGC | (+)c | (−) | (+)d | (+) | AGC | No |
|
| B, Recurrent
high-grade squamous intraepithelial lesions detected at the 2nd
follow-up visit |
|
| Age,
years | Surgical
method | Ectocervical
margin | Endocervical
margin | Cone piece
# | Excised cone
histology | TZ3 1st FU
colposcopy | Cytology 1st
FU | HPV 1st
FUe | HPV 16 | HPV 18 | HPV 2nd
FUa | Cytology 2nd
FU | Diagnosed
comorbidity |
|
| 26–30 | C-LETZ | Clear | Clear | 1 | CIN3 | No | LSIL | (+) | (−) | (−) | (+) | HSIL | No |
|
| C-LETZ | Clear | Unclear | 1 | CIN3 | No | LSIL | (+) | (−) | (+)f | (+) | LSIL | No |
| 31–35 | C-LETZ | Clear | Clear | 1 | CIN2 | No | NILM | (+) | (−) | (−) | (+) | HSIL | No |
| 41–45 | C-LETZ | Uncertain | Uncertain | 2 | CIN2 | No | HSIL | (+) | (+)g | (−) | (+) | HSIL | No |
|
| Laser | Clear | Unclear | 1 | CIN3 | No | HSIL | (+) | (−) | (−) | Missing | HSIL | No |
| 46–50 | C-LETZ | Clear | Unclear | 1 | CIN3 | No | NILM | (+) | (+)g | (−) | (+) | HSIL | Depression |
|
| C-LETZ | Clear | Unclear | 1 | CIN3 | Yes | LSIL | (+) | (−) | (+)g | (+) | HSIL | No |
 | Table III.Patients with recurrent high-grade
cervical intraepithelial neoplasia detected at subsequent routine
screening. |
Table III.
Patients with recurrent high-grade
cervical intraepithelial neoplasia detected at subsequent routine
screening.
| A, Recurrent
adenocarcinoma in situ detected at subsequent routine
screening |
|---|
|
|---|
| Age, years | Surgical
method | Ecto-cervical
margin | Endo-cervical
margin | Cone piece # | Excised cone
histology | TZ3 1st FU
colpo-scopy | Cytology 1st
FU | HPV 1st
FUa | HPV 16a | HPV 18a | HPV 2nd
FUb | Cytology 2nd
FU | HPV routine
screenb | Cytology routine
screen | Diagnosed
comorbidity |
|---|
| 36–40 | C-LETZ | Uncertain | Uncertain | 3 | AIS | No | NILM |
(+)c | (−) | (+)c | No 2nd FU | No 2nd FU | (+) | AIS | Autoimmune
disorder |
| 41–45 | C-LETZ | Uncertain | Uncertain | 1 | CIN3/AIS | Yes | NILM |
(−) | (−) | (−) | No 2nd FU | No 2nd FU | (+) | AIS | No |
|
| B, Recurrent
high-grade squamous intraepithelial lesions detected at subsequent
routine screening |
|
| Age,
years | Surgical
method | Ecto-cervical
margin | Endo-cervical
margin | Cone piece
# | Excised cone
histology | TZ3 1st FU
colpo-scopy | Cytology 1st
FU | HPV 1st
FUa | HPV
16a | HPV
18a | HPV 2nd
FUb | Cytology 2nd
FU | HPV routine
screenb | Cytology routine
screen | Diagnosed
comorbidity |
|
| 26–30 | Laser | Unclear | Clear | 1 | CIN3 | No | NILM | (−) | (−) | (−) | No 2nd FU | No 2nd FU | (+) | ASC-US | No |
|
| Laser | Clear | Clear | 1 | CIN2 | No | NILM | (−) | (−) | (−) | No 2nd FU | No 2nd FU | (+) | LSIL | Other infectious
diseasef |
| 31–35 | C-LETZ | Clear | Clear | 1 | CIN3/AIS | No | NILM | (−) | (−) | (−) | No 2nd FU | No 2nd FU | (−) | NILM | No |
| 46–50 | C-LETZ | Uncertain | Unclear | 3 | CIN3 | Yes | NILM | (−) | (−) | (−) | No 2nd FU | No 2nd FU | Missing | NILM | Hypertension |
|
| C-LETZ | Uncertain | Uncertain | 1 | CIN3 | Yes | HSIL | (+)d | (+)d | (−) | Missing | HSIL | Missing | NILM | Neurologic
disorder |
|
| C-LETZ | Clear | Unclear | 3 | CIN3 | Yes | ASC-US | (+)e | (+)e | (−) | (+) | NILM | (+) | NILM | No |
| 51–55 | C-LETZ | Clear | Unclear | 1 | CIN3 | No | NILM | (−) | (−) | (−) | No 2nd FU | No 2nd FU | (+) | NILM | Interacting
infectious diseasef
& autoimmune disorder |
Table II is divided
into two sub-parts: Table IIA
presents the two patients in whom adenocarcinoma in situ,
AIS, was found on biopsy at 2nd follow-up, while the seven patients
with recurrent HSIL are presented in Table IIB. Both patients with recurrent AIS
had TZ3 on colposcopy. The patient treated by laser conization had
clear margins. By standard Cobas and VSS, the overall HPV results
were negative at 1st follow-up. It was only by the
clinician-sampling using the Abbott method that overall HPV
positivity at 1st follow-up was revealed for this patient. The
other patient with recurrent AIS had been treated by C-LETZ
conization with an unclear endocervical margin. Whereas VSS had
been negative, both clinician-sampling methods revealed HPV
positivity. With the Abbott method, HPV18 was positive. At 2nd
follow-up, HPV positivity was detected in both patients using the
standard Cobas method.
The seven patients with recurrent HSIL detected at
2nd follow-up also show a wide age distribution. All four patients
above age 40 at the time of treatment had unclear or uncertain
endocervical margins. One of them was treated by laser conization;
another patient treated by C-LETZ had two cone pieces. All seven
patients had HPV positive findings at 1st follow-up by all three
methods. Positive HPV16 or HPV18 was found in four of these seven
patients via VSS, while the clinician-taken sample did not reveal
one case of HPV18 positivity. Among the six patients in whom this
was assessed at 2nd follow-up, HPV was positive. Comorbidity
(depression) was noted in only one of the nine patients with
detected recurrence at 2nd follow-up.
Table III is also
divided into two sub-parts. In Table
IIIA are the two patients with recurrent adenocarcinoma in
situ, AIS, found at subsequent routine screening. All the
margins had been uncertain in both cases. At 1st follow-up only
Abbott clinician-samples were positive in one patient (HPV18); in
the other patient all HPV testing was negative. Since cytology was
NILM at that time, neither patient was referred to 2nd follow-up.
It was only thereafter at routine screening that HPV on Cobas was
positive and cytology revealed AIS. An autoimmune disorder was a
diagnosed comorbidity in one of these patients.
Among the seven patients with recurrent HSIL
(Table IIIB) detected at routine
screening, five had NILM cytology and negative HPV via standard
Cobas (as well as by Abbott clinician sample and VSS) at 1st
follow-up and, therefore, were not referred to 2nd follow-up. In
three of these patients, at least one margin had been unclear or
uncertain. At subsequent routine screening, Cobas HPV was positive
in three of these cases, but negative in one patient and missing in
the other. The other two patients attended 2nd follow-up having had
abnormal cytology at 1st follow-up as well as positive HPV at 1st
follow-up. Neither of these patients had entirely clear margins at
conization. Four of these seven patients had diagnosed comorbidity,
including autoimmune and infectious diseases which are assumed to
interact with HPV acquisition and/or CIN progression.
Bivariate analysis vis-à-vis detected
recurrence of high-grade cervical dysplasia
In Table IV
comparisons are made between the patients in whom recurrent
high-grade cervical dysplasia was detected and those in whom
recurrence was not detected. There were significantly more patients
aged 45 or above with detected recurrence. Surgical method did not
differ significantly between these two groups. However, 64% of the
patients with detected recurrence had at least one unclear or
uncertain margin, whereas this was the case for 25% of the patients
without detected recurrence. Neither the number of cone pieces nor
the histology of the excised cone differed significantly for the
patients with vs. without diagnosed recurrence. Comorbidity did not
significantly distinguish these two groups, nor did the finding of
TZ3 on colposcopy at 1st follow-up.
 | Table IV.Comparison of the patients with and
without detected recurrence of high-grade cervical intraepithelial
neoplasia. |
Table IV.
Comparison of the patients with and
without detected recurrence of high-grade cervical intraepithelial
neoplasia.
| Variable | No recurrence of
high-grade CIN detected | Recurrent
high-grade CIN detected | P-value |
|---|
| Age at conization,
years |
|
| <0.01 |
|
<45 | 440 | 13 |
|
|
≥45 | 67 | 9 |
|
| Surgical
method |
|
| NS |
|
C-LETZ | 432 | 17 |
|
|
Laser | 72 | 5 |
|
|
Ablationa |
3 |
|
|
| Margin excision
statusb |
|
| <0.001 |
| Both
margins clear | 378 | 8 |
|
| Only
ectocervical margin unclear/uncertain | 41 | 1 |
|
| Only
endocervical margin unclear/uncertain | 38 | 7 |
|
| Both
margins unclear/uncertain | 50 | 6 |
|
| Number of cone
piecesa |
|
| NS |
|
One | 416 | 18 |
|
| Two or
more | 88 | 4 |
|
| Histology of
excised cone |
|
| NS |
|
CIN2 | 128 | 5 |
|
| CIN2/3
or worse | 379 | 17 |
|
| Any diagnosed
comorbidity |
|
| NS |
| No | 378 | 15 |
|
|
Yes | 129 | 7 |
|
| Any diagnosed
comorbidity linked to HPV or CIN progressionc |
|
| NS |
| No | 458 | 19 |
|
|
Yes | 49 | 3 |
|
| Diagnosed comorbid
malignancy |
|
| NS |
| No | 497 | 22 |
|
|
Yes | 10 | 0 |
|
| Colposcopy at 1st
follow-up: TZ3d |
|
| NS |
| No | 298 | 14 |
|
|
Yes | 205 | 8 |
|
| Cytology at 1st
follow-up |
|
| <0.001 |
|
NILM | 444 | 9 |
|
|
Abnormal | 63 | 13 |
|
| Cytology at 2nd
(referred) follow-upe,f |
|
| <0.001 |
|
NILM | 73 | 1 |
|
|
Abnormal | 21 | 10 |
|
| Cytology at routine
follow-upe,g |
|
| <0.01 |
|
NILM | 340 | 5 |
|
|
Abnormal | 30 | 4 |
|
| HPV at 1st
follow-up (Standard Cobas) |
|
| <0.001 |
|
Negative | 435 | 8 |
|
|
Positive | 72 | 14 |
|
| HPV at 2nd
(referred) follow-up (Standard Cobas)e,h |
|
| <0.001 |
|
Negative | 51 | 0 |
|
|
Positive | 34 | 9 |
|
| HPV at routine
follow-up (Standard Cobas)e,i |
|
| <0.001 |
|
Negative | 298 | 1 |
|
|
Positive | 30 | 6 |
|
| Persistent HPV
positivity (Standard Cobas)e,j |
|
| <0.01 |
| No (HPV
positive at 1st follow-up, HPV negative at 2nd follow-up) | 27 | 0 |
|
| Yes
(HPV positive at 1st & 2nd follow-up) | 28 | 8 |
|
| Persistent HPV
positivity (Standard Cobas)e,k |
|
| NS |
| No (HPV
positive at 1st follow-up, HPV negative at routine follow-up) | 36 | 0 |
|
| Yes
(HPV positive at 1st follow-up & routine follow-up) | 15 | 1 |
|
Cytology at 1st follow-up was significantly more
often abnormal among the patients with detected recurrence at any
of the follow-up times (59%) compared to those in whom no
recurrence was detected (12%). Excluding the four patients in whom
recurrence was detected at 1st follow-up, abnormal cytology at 2nd
follow-up was significantly more frequent among patients with
detected recurrence (91%) vs. those in whom no recurrence was
detected (22%). Among the nine patients in whom recurrence was
detected at subsequent follow-up, four (44.4%) had abnormal
cytology at that follow-up occasion, whereas 30 (8.1%) of the 370
patients without detected recurrence in whom cytology was reported
at subsequent follow-up had an abnormal result.
Likewise, standard Cobas HPV was significantly more
often positive at each follow-up among patients with detected
recurrence compared to those in whom no recurrence was detected. At
1st follow-up, 64% of the 22 patients in whom recurrence was
detected showed HPV positivity, vs. 14% among the 507 patients
without detected recurrence. Excluding the four patients in whom
recurrence was detected at 1st follow-up, at 2nd follow-up, all
nine patients with detected recurrence in whom these results were
available showed HPV positivity. In contrast, 34 of the 85 patients
without detected recurrence in whom HPV results were available at
2nd follow-up showed HPV positivity at that occasion. HPV results
were available for seven of the nine patients in whom recurrence
was detected at subsequent routine follow-up, and were positive in
six cases (85.7%). In contrast, among the 328 patients without
detected occurrence in whom these HPV was assessed at routine
follow-up, only 30 patients showed HPV positivity.
The limited available data on persistent HPV
positivity indicates that all eight patients with detected
recurrence and HPV positivity with standard Cobas at 1st follow-up
also showed positivity at 2nd follow-up. In contrast, nearly 50% of
the patients without detected recurrence who had HPV positive
results with standard Cobas at 1st follow-up had negative HPV at
2nd follow-up. Of the nine patients in whom recurrence was detected
at subsequent follow-up, one had HPV positive findings with
standard Cobas at 1st follow-up and at subsequent follow-up. Among
those without detected recurrence, there were 15 patients with HPV
positivity both at 1st follow-up and at routine screening, while 36
patients had HPV positive findings at 1st follow-up but not at
routine screening.
Age-related bivariate findings
The age-related findings are presented in Table V, with stratification of the patients
below age 30, those aged 30 to 44 and patients aged 45 or above.
The statistical analysis compares all patients below age 45 with
those aged 45 or above. Firstly, it is seen that significantly more
of the patients aged 45 or above were treated by the laser method
(24%) compared to 13% among those below age 45. There were no
significant age-related differences regarding margin excision
status.
 | Table V.Age-related comparisons. |
Table V.
Age-related comparisons.
| Variable | <30 y.o. at
treatment | 30–44 y.o. at
treatment | P-value | ≥45 y.o. at
treatment |
|---|
| Surgical
method |
|
| <0.05 |
|
|
C-LETZ | 155 | 236 |
| 58 |
|
Laser | 31 | 28 |
| 18 |
|
Ablationa | 2 | 1 |
| 0 |
| Margin excision
statusb |
|
| NS |
|
| Both
ectocervical & endocervical clear | 140 | 190 |
| 56 |
|
Ectocervical margin only
unclear/uncertain | 16 | 21 |
| 5 |
|
Endocervical margin only
unclear/uncertain | 13 | 22 |
| 10 |
| Both
margins unclear/uncertain | 19 | 32 |
| 5 |
| Transformation zone
3 on colposcopy at 1st follow-upc |
|
| <0.001 |
|
| No | 130 | 157 |
| 25 |
|
Yes | 56 | 107 |
| 50 |
| Cytology at 1st
follow-up |
|
| <0.05 |
|
|
NILM | 161 | 234 |
| 58 |
|
Abnormal | 27 | 31 |
| 18 |
| HPV at 1st
follow-up (Standard Cobas) |
|
| <0.05 |
|
|
Negative | 158 | 228 |
| 57 |
|
Positive | 30 | 37 |
| 19 |
| Any further
follow-up after 1st follow-upd |
|
| NS |
|
| No | 24 | 28 |
| 9 |
|
Yes | 163 | 236 |
| 65 |
| Cytology at 2nd
(referred) follow-upd,e |
|
| NS |
|
|
NILM | 33 | 29 |
| 12 |
|
Abnormal | 6 | 18 |
| 7 |
| HPV at 2nd
(referred) follow-up (Standard Cobas)d,f |
|
| NS |
|
|
Negative | 25 | 20 |
| 6 |
|
Positive | 14 | 18 |
| 11 |
| Cytology at routine
follow-upd,g |
|
| NS |
|
|
NILM | 128 | 173 |
| 44 |
|
Abnormal | 11 | 17 |
| 6 |
| HPV at routine
follow-up (Standard Cobas)d,h |
|
| NS |
|
|
Negative | 90 | 170 |
| 39 |
|
Positive | 13 | 17 |
| 6 |
| Any diagnosed
comorbidity |
|
| 0.07 |
|
| No | 133 | 210 |
| 50 |
|
Yes | 55 | 55 |
| 26 |
| Any comorbidity
linked to HPV or CIN progressioni |
|
| NS |
|
| No | 166 | 245 |
| 66 |
|
Yes | 22 | 20 |
| 10 |
| Diagnosed comorbid
malignancy |
|
| <0.01 |
|
| No | 188 | 260 |
| 71 |
|
Yes | 0 | 5 |
| 5 |
| Recurrence of
high-grade CIN detected at follow-up |
|
| <0.01 |
|
| No | 184 | 256 |
| 67 |
|
Yes | 4 | 9 |
| 9 |
A highly significant difference was noted regarding
the prevalence of TZ3 on colposcopy (67%) at 1st follow-up among
patients aged 45 or above vs. 36% for patients below age 45.
Patients aged 45 or above significantly more often had abnormal
cytology at 1st follow-up (24%) vs. 13% seen in patients younger
than 45. Further analysis among the eighteen patients age ≥45 with
abnormal cytology at 1st follow-up reveals that six patients had
HSIL, four had low-grade squamous intraepithelial lesions (LSIL)
and eight had atypical squamous cells of undetermined-significance
(ASC-US). In contrast, ten patients below age 45 had HSIL, one
patient had ASC-H (atypical squamous cells cannot exclude HSIL) and
23 had LSIL. Atypical glandular cells (AGC) were found in five
patients below age 45. Twenty-five percent of the patients aged 45
or above were found to have HPV positivity using the standard Cobas
method at 1st follow-up. This was significantly more than among
those below age 45 (15%).
Approximately 12% of the patients had no further
follow-up after the 1st follow-up, with a fairly similar percentage
in each of the age groups. At 2nd and routine follow-up, there were
no significant age-related differences in cytology or HPV.
Borderline significantly more patients aged 45 or
above had any diagnosed comorbidity. Five of the 76 patients aged
45 or above had a diagnosed comorbid cancer (6.6%) vs. 1.1% in the
younger patients.
The previously noted significant age-related
difference in detected recurrence rate is presented in more detail
here in Table V. Recurrence was
detected in 2.1% of the patients below age 30, 3.4% in those aged
30 to 44 and 11.8% for patients aged 45 or above.
Bivariate findings vis-à-vis margin
status
The focus of Table
VI is on margin status. For statistical analysis, this is
dichotomized as both margins clear vs. any or both margins unclear
or uncertain. The margin status did not differ significantly in
relation to the surgical method employed. However, with more than
one cone piece there was a significantly greater likelihood that at
least one margin was unclear or uncertain compared to when only one
cone piece was taken. Further analysis reveals that although the
surgical method was not significantly associated with margin
status, there was a greater likelihood of more than one cone piece
with C-LETZ compared to laser surgical method (Pearson
χ2=13.9, P=0.0002). There were only two cases in which
more than one cone piece was reported with laser, whereas in 90
cases using C-LETZ more than one cone piece was reported. In 85% of
cases with one or more margins uncertain or unclear, the histology
of the excised cone was CIN2/3 or worse. More severe cone histology
was significantly less frequent (71%) when both margins were
clear.
 | Table VI.Comparisons related to margin
status. |
Table VI.
Comparisons related to margin
status.
| Variable | Both margins
clear | P-value | Only ectocervical
margin unclear/uncertain | Only endocervical
margin unclear/uncertain | Both margins
unclear/uncertain |
|---|
| Surgical
method |
| NS |
|
|
|
|
C-LETZ | 329 |
| 33 | 36 | 51 |
|
Laser | 54 |
| 9 | 9 | 5 |
|
Ablationa | 3 |
| 0 | 0 | 0 |
| Cone
piecesa |
| <0.01 |
|
|
|
| Only
one | 326 |
| 34 | 40 | 34 |
| More
than one | 57 |
| 8 | 5 | 22 |
| Histology of
excised cone |
| <0.01 |
|
|
|
|
CIN2 | 112 |
| 8 | 5 | 8 |
| CIN2/3
or worse | 274 |
| 34 | 40 | 48 |
| Cytology at 1st
follow-up |
| <0.05 |
|
|
|
|
NILM | 338 |
| 39 | 32 | 44 |
|
Abnormal | 48 |
| 3 | 13 | 12 |
| HPV at 1st
follow-up (Standard Cobas) |
| NS |
|
|
|
|
Negative | 326 |
| 40 | 34 | 43 |
|
Positive | 60 |
| 2 | 11 | 13 |
| Cytology & HPV
(Standard Cobas) at 1st follow-up |
| NS |
|
|
|
| NILM
and negative HPV | 301 |
| 37 | 29 | 37 |
|
Abnormal cytology &/or
positive HPV | 85 |
| 5 | 16 | 19 |
Abnormal cytology at 1st follow-up was significantly
more common when one or more margin was unclear or uncertain.
Although there was a greater percentage of HPV positivity with one
or both unclear margins, HPV positivity at 1st follow-up did not
significantly differ according to margin status. As noted, at least
one of these abnormal findings was needed to refer the patient to
2nd follow-up. At the bottom of Table
VI, it is seen that there was no significant association
between abnormal cytology and/or positive HPV at 1st follow-up and
unclear or uncertain margins. Altogether 103 patients with at least
one unclear or uncertain margin had negative HPV and normal
cytology at 1st follow-up. According to the current protocol, these
103 patients were returned to routine screening.
Multiple logistic regression findings
with detected recurrence as the outcome
Table VII displays
three multiple logistic regression models with detected recurrence
of high-grade CIN as the outcome. All the models are highly
statistically significant and the data are complete, i.e. all 529
patients are included in the analysis. The first model includes
four independent variables that each significantly predicts
detected recurrence. These predictors are age 45 or above at the
time of conization, incomplete lesion excision, i.e. one or both
unclear or uncertain margins at conization, positive HPV at 1st
follow-up using the standard Cobas clinician-taken sample and
abnormal cytology at 1st follow-up. The second model includes one
more independent variable, any diagnosed comorbidity, which did not
significantly predict detected recurrence. This second model had
only a slightly higher overall χ2 than the first model
with one less independent variable. In the third model, comorbidity
with conditions that have been linked to HPV acquisition and/or CIN
progression was included, rather than any diagnosed comorbidity.
The latter independent variable yielded a slightly higher odds
ratio, but still non-significant prediction of detected
recurrence.
 | Table VII.Prediction of detected recurrent
high-grade cervical intraepithelial neoplasia in 529 treated
patients assessed via multiple logistic regression. |
Table VII.
Prediction of detected recurrent
high-grade cervical intraepithelial neoplasia in 529 treated
patients assessed via multiple logistic regression.
| Model
χ2 |
Variable | OR | −95% CI | +95% CI | P-value |
|---|
| 53.2a | Age 45 or
above | 3.5 | 1.3 | 9.9 | <0.05 |
|
| One or more unclear
or uncertain margins | 5.3 | 2 | 14.2 | <0.001 |
|
| HPV positive at 1st
follow-up (Standard Cobas) | 5.8 | 2 | 16.8 | <0.01 |
|
| Abnormal cytology
at 1st follow-up | 3.9 | 1.4 | 11 | <0.05 |
| 53.3a | Age 45 or
above | 3.4 | 1.2 | 9.6 | <0.05 |
|
| One or more unclear
or uncertain margins | 5.4 | 2 | 14.5 | <0.001 |
|
| HPV positive at 1st
follow-up (Standard Cobas) | 5.8 | 2 | 16.7 | <0.01 |
|
| Abnormal cytology
at 1st follow-up | 3.9 | 1.4 | 11.2 | <0.05 |
|
| Any diagnosed
comorbidity | 1.3 | 0.4 | 3.6 | NS |
| 53.4a | Age 45 or
above | 3.4 | 1.2 | 9.6 |
<0.05 |
|
| One or more unclear
or uncertain margins | 5.4 | 2 | 14.6 |
<0.001 |
|
| HPV positive at 1st
follow-up (Standard Cobas) | 5.8 | 2 | 16.8 |
<0.01 |
|
| Abnormal cytology
at 1st follow-up | 3.8 | 1.3 | 11 |
<0.05 |
|
| Any diagnosed
comorbidity linked to HPV or to CIN progressionb | 1.5 | 0.4 | 5.8 | NS |
Adjusting for all the other significant independent
variables, i.e. age, HPV status at 1st follow-up and abnormal
cytology, we performed further multiple logistic regression. It was
found that only unclear or uncertain ectocervical margins were
non-significant for predicting detected recurrence. That analysis
included 428 patients and there was only one patient with detected
recurrence who had only unclear or uncertain ectocervical margins.
In contrast, however, only unclear or uncertain endocervical
margins did significantly predict detected recurrence [OR=6.2 (95%
CI: 1.8–21.4) P=0.004]. Altogether 431 patients were included in
that analysis, with seven detected recurrences among those with
only unclear or uncertain endocervical margins. When both margins
were unclear or uncertain, the OR was 5.5 (95% CI: 1.6–18.9,
P=0.006) for detected recurrence. There were six detected
recurrences among those with both margins unclear or uncertain, and
the overall analysis included 442 patients.
Discussion
A unique feature of the present study is practically
complete attendance to early follow-up after treatment of
high-grade CIN for a sizable cohort of patients, coupled with
complete data vis-à-vis HPV status and cytology plus nearly
complete colposcopy data at that early follow-up occasion. In
addition, complete information was obtained about diagnosed
comorbidity. These unique features are combined with full data on
surgical method, margin excision status and histology of the
excised cone. After first follow-up, the patients were triaged as
per national guidelines, with subsequent data available on a very
large percentage, 88.4%, of this cohort who continued to attend
follow-up as per recommendation. In other words, this study
combines the ideal situation of complete early follow-up with a
clearly-described situation thereafter, for at least four years, up
to a maximum of more than six years. During that period, recurrent
high-grade CIN was detected in altogether twenty-two patients.
Given the completeness of the early follow-up data,
powerful multivariate models could be generated to identify four
significant independent risk factors for detected recurrence of
high-grade CIN. These were: age 45 or above at the time of
conization, one or more unclear or uncertain excision margins,
positive HPV and abnormal cytology at 1st follow-up. In
contradistinction to previous long-term findings (19), diagnosed comorbidity which is
potentially linked to HPV or to CIN progression was not found to
significantly predict detected recurrence in the present study.
Surgical method and histology of the excised cone, likewise, were
not found to be directly associated with increased detected
recurrence risk, similarly to other investigations (19,32).
The strong association between margin status and
recurrence risk found herein is consistent with a number of other
studies (19–23). Moreover, the findings that incomplete
excision from the endocervical or both margins, but not from
ectocervical margins alone show significant multivariate
association with detected recurrence, are consistent with other
investigations (19,20,33). Of
particular note is that in the present study, this increased risk
is not significantly associated with HPV positivity at early
follow-up. Moreover, patients with negative HPV and NILM cytology
at 1st follow-up were thereafter referred to routine triennial
screening, at which time five of the seven patients in whom
recurrence was detected had at least one unclear or uncertain
margin. Incomplete resection was significantly more likely with
more severe cone histology. The latter, however, in itself, did not
show a direct relation with recurrence.
Although, overall, there was no significant
association found between age and margin status, most of the
patients aged 45 or above in whom recurrence was detected also had
at least one unclear or uncertain margin. Specifically, the margins
were reportedly clear only in the two patients above age 45 with
recurrence detected at 1st follow-up. The seven other patients aged
45 or above in whom recurrence was subsequently detected all had at
least one unclear or uncertain margin. Thus, the present findings
would seem to support the recommendation (20,21) that
larger and thereby more complete excisions should be made when
treating women in the post-menopausal age group with high-grade
CIN.
Significantly more women aged 45 or above were
treated with laser rather than with contoured loop excision of the
transformation zone, C-LETZ. Although surgical method did not show
a significant association with detected recurrence, with the laser
technique, there was a significantly greater likelihood that only
one cone piece would be generated. In turn, with a single cone
piece, the chances of having clear margins was significantly
greater. It should also be noted that none of the women aged 45 or
above with detected recurrence had been treated with laser.
Although the laser technique is a higher-cost procedure requiring
particular colposcopic expertise (34,35), it
might be inferred from the results of the present study that the
laser technique would be a good option for women in this age
group.
Concordant with other reports (36), in the present study, the patients
near or in the post-menopausal age group, i.e. 45 or above,
significantly more often had the finding of transition zone 3, TZ3,
on colposcopy compared to the women below age 45. This finding
indicates that the squamo-columnar junction is located in the
endocervix, thereby resulting in an unsatisfactory colposcopic
examination (37). Although
colposcopy was performed at 1st follow-up in all but four of the
529 patients, biopsies were only taken from visible lesions, which
were very few, thirteen altogether. High-grade cervical dysplasia
may also be found on biopsies taken from colposcopically negative
sites (38). It can therefore be
surmised that the actual number of recurrences may have been
underestimated (25). This
underestimation could well have been even more pronounced among
patients aged 45 or above due to the higher percentage of TZ3 on
colposcopy.
Patients aged 45 or above significantly more often
had abnormal cytology and positive HPV at 1st follow-up, compared
to those younger than 45. Nevertheless, fifty-one patients (67%) of
the seventy-six patients aged 45 or above had NILM on cytology and
negative HPV at 1st follow-up, and were therefore referred directly
to routine triennial screening, according to national guidelines.
At that screening occasion, recurrence was detected in two more
patients above age 45, both with unclear endocervical margins but
with NILM and negative HPV at 1st follow-up. Notably, one of these
two patients also had two comorbid diagnoses (infectious disease
and autoimmune disorder) that have been linked to HPV and CIN
progression (19,26). This patient also had a positive HPV
finding at the subsequent routine screening.
With complete data on HPV as well as cytology at 1st
follow-up for all 529 patients, a fuller picture emerges of the
major contributors to recurrent high-grade CIN. Abnormal cytology
slightly surpassed age in its power to predict recurrence. A
positive HPV result is seen as a very powerful predictor, with a
higher odds ratio, although lower statistical significance than
margin status.
Positive HPV as well as abnormal cytology at 2nd
follow-up and at routine follow-up each also showed significant
association with detected recurrence in bivariate analysis. The
importance of persistent HPV positivity after treatment of
high-grade CIN has been emphasized (39). Patterns of persistent HPV infection
after treatment of high-grade CIN were the focus of a previous
study (9) since persistence is
considered to be the key contributor of progression to invasive
cervical cancer. The difficulties in finding the optimal protocol
for assessing post-therapeutic HPV persistence were underscored in
a previous study (9). In the present
study, the patients with positive HPV at 1st follow-up were
referred to 2nd follow-up. As seen from Table IV, 57% of the patients at 2nd
follow-up had persistent HPV findings, with significantly more
persistent HPV among the patients in whom recurrence was detected.
However, there was some loss to follow-up among the 125 patients
who were referred to 2nd follow-up. Moreover, at 2nd follow-up and
particularly at subsequent routine screening, there were
substantial missing HPV data. As shown in Table IIIB, regarding the two patients
with detected recurrence at routine screening who had also attended
2nd follow-up, complete HPV data indicating persistent HPV
positivity at all three occasions was seen in only one patient.
These subsequent HPV data were missing in the other patient.
Heretofore in the present paper, the main focus
vis-à-vis the HPV results has been on the clinician-taken cervical
samples via the standard Cobas method. Albeit missing in a few of
the patients who attended 2nd follow-up and in a substantial number
of those who attended routine follow-up, these results could be
assessed and compared across the three screening occasions. As
described in detail in a previous study (25), at 1st follow-up complete data were
also available for vaginal self-taken samples, VSS, and
clinician-taken cervical samples via the Abbott method. Based on
the results available at that time for 1st and 2nd follow-up,
overall HPV positivity was found by each of these three methods in
all eleven patients in whom recurrence was detected and who had
squamous pathology. However, in the remaining two patients in whom
the recurrent pathology was glandular, HPV positivity was not
detected by VSS, but only by clinician-taken cervical samples
(These findings are shown in Tables
I and II). In both patients
with glandular pathology, HPV18, an especially potent risk
indicator (40,41), was found to be positive with
clinician sampling. Those limited data seemed to suggest that
self-sampling should not be recommended for patients with glandular
pathology, but only for those in whom the pathology was squamous
(25).
We can herein ask whether more insights are gleaned
from the additional follow-up data for subsequent routine
screening. With respect to the two patients in whom recurrent
glandular pathology was detected at that later time (Table IIIA), concordantly, vaginal
self-sampling did not reveal a positive HPV result. In one patient
only the Abbott clinician-taken sample (not Cobas) was positive
and, moreover, indicated HPV18 positivity. In the other patient
with glandular pathology, however, HPV positivity was not detected
in either of the clinician-sampled cervical specimens at 1st
follow-up. We herein describe the additional analysis of the 27
patients without detected recurrence in whom there was glandular
histology in the excised cone and/or AGC on cytology at any of the
follow-up periods. This reveals that three patients showed
positivity on both Abbott clinician-taken samples and VSS, in 22
patients both were HPV negative, one patient showed HPV positivity
only on VSS and in one patient only the clinician sample was
positive. Comparing VSS and Cobas clinician-taken samples shows
nearly identical findings, except that there were 23 patients with
HPV negative on both, and in no case was Cobas positive when VSS
was negative. Taken together, these results seem to suggest that
VSS may not be inferior to clinician-sampling for follow-up of
patients with glandular pathology. Further study of this question
is needed.
Scrutiny is warranted, as well, of the patients with
squamous pathology in whom recurrence was detected at subsequent
routine follow-up. The two patients who also attended 2nd follow-up
had positive HPV in both clinician-taken samples, while VSS was
positive in only one of these cases. Among the five patients with
recurrent squamous pathology and who only attended subsequent
routine screening, HPV at 1st follow-up was negative not only by
the standard Cobas, but also from the Abbott clinician-taken
samples and VSS. The overriding conclusion from analyzing all nine
recurrent cases detected at subsequent routine follow-up is that
repeated HPV testing is indispensable for adequate follow-up of
patients treated for high-grade cervical intraepithelial dysplasia.
Assessment of high-risk subtypes HPV16 and 18 is particularly
advisable.
Besides the missing HPV data for 102 of the 437
patients who came to routine follow-up (23%), 61 patients did not
attend any subsequent follow-up after the 1st one. This is of major
concern, given the elevated risk of recurrent high-grade cervical
intraepithelial dysplasia and invasive cervical cancer. Home
self-sampling for HPV could well be a realistic solution. Our
earlier study among 479 women from the present cohort treated for
high-grade CIN indicated that the vast majority were amenable to
performing self-sampling at home and considered self-sampling to
have been implemented without difficulty (27). Notably, self-sampling in our study
was performed in the clinic restroom, which was less comfortable
than the home environment (25).
In the most recent period through the end of the
year 2020 for which we have follow-up data on the present cohort, a
new situation arose due to the global COVID-19 pandemic. In Sweden,
during the 1st wave of the COVID epidemic, cervical screening was
largely cancelled, including the cancellation of 192 000 cervical
screening appointments in Stockholm (42). Concordantly, a number of reports
worldwide indicate a precipitous decline in cervical screening
(43–47). The actual and potential consequences
of the COVID-19 pandemic vis-à-vis increased cervical cancer
incidence, treatment delay and mortality have been underscored
(45,48,49).
Home self-sampling for HPV is widely endorsed as the key strategy
to avoid these pandemic-related, adverse consequences (42,44,47,50–53). As
succinctly stated in a previous study (50): ‘The new imperatives of the COVID-19
pandemic support self-sampled HPV testing as the primary cervical
screening method’. That conclusion is justified, given the
reliability of self-sampling (25,54–57),
together with its acceptability and cost-effectiveness (27,58–63).
Considering the elevated risk of recurrence
associated with increased age and the importance of persistent HPV
infection, home self-sampling for HPV could be particularly helpful
for women in the post-menopausal age group. In a recent
population-based Swedish study 893 women aged 60 to 75 performed
VSS at home, with 4.4% positivity and 2.5% persistent positivity at
2nd sampling (64). The authors
concluded that vaginal self-sampling at home was well-accepted
among women in this age group.
Although the present results did not show
significantly increased comorbid conditions among the patients with
detected recurrence, further inspection of Table I,Table
II,Table III suggests that in
individual cases these disorders, especially autoimmune conditions,
may have been contributory. Notably, in a long-term large-scale
Australian study (65), autoimmune
conditions were found to be associated with increased cervical
dysplasia, supporting the need for ‘expansion of cervical cancer
preventative programs to include these at-risk females’.
A recent meta-analysis indicates that, compared to
surgical excision alone, prophylactic HPV vaccination is associated
with a significantly decreased risk of recurrence among patients
treated for high-grade CIN (66).
Unfortunately, data concerning HPV vaccination are not available
for the present cohort, and would be an important consideration in
future studies. Co-infection with Epstein-Barr virus, EBV, might
also be considered in future studies, given some reports of its
possible contribution to progression of cervical lesions (67).
In conclusion, the results of the present study
indicate that four factors significantly and independently
contribute to the risk of recurrent high-grade cervical
intraepithelial neoplasia, CIN. Among these, incomplete excision of
the CIN lesion was the most significant predictor of recurrence,
and, therefore, warrants more intensive subsequent screening,
regardless of the early post-conization HPV findings. An early
post-conization positive HPV finding was also a powerful,
independent predictor recurrent high-grade CIN. Nevertheless, over
one-third of the patients with detected recurrence had a negative
early post-conization HPV finding. These patients were returned to
routine screening, at which time in most (but not all the cases),
the HPV finding was positive. This underscores the vital need for
repeated evaluation of HPV. Especially during this on-going
pandemic crisis, vaginal self-sampling, VSS, for HPV is strongly
recommended. However, caution and further investigation regarding
VSS are still needed insofar as the cervical pathology is glandular
and not squamous. Abnormal early post-conization cytology was also
a significant independent predictor of recurrent high-grade CIN.
This finding supports the current national guidelines of more
intensive follow-up for patients with abnormal cytology
post-conization.
Women aged 45 or above were also at higher risk of
recurrence. Besides implementing all the above-recommended measures
vis-à-vis the three other independent risk factors, special
attention is warranted to women in this age group. Among the
pertinent issues are choice of surgical modality (laser may be
preferable), performance of wider, more complete excision, as well
as the increased likelihood of an inadequate colposcopic
examination associated with transition zone 3, TZ3, which is often
found among women in the more senior age group.
Although not statistically significant, relevant
comorbidity, warrants consideration in clinical decision-making
about follow-up. This appears to be especially relevant for
autoimmune conditions.
Overall, women who have been treated for high-grade
cervical intraepithelial neoplasia are at increased risk for
recurrent disease and progression to cervical cancer, and therefore
require careful, individualized follow-up to avoid these potential
adverse consequences. The results of the present study provide
insights that can substantially contribute to the practical
realization of this aim.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Swedish
Cancer Foundation (grant no. 110544, CAN2011/471), the Karolinska
Institute (Cancer Strategic Grants; grant no. 5888/05-722), the
Swedish Research Council (grant no. 521-2008-2899), the Stockholm
County Council (grant nos. 20130097 and 20160155) and the Gustaf V
Jubilee Fund (grant nos. 154022 and 151202).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SAn, DM, SAl and EÖ confirm the authenticity of the
raw data. SAn conceived and designed the study, performed
colposcopy, cervical sampling and punch biopsies of visible
lesions, assessed the authenticity of the raw data, reviewed the
data, collected the related literature, and revised the manuscript.
DM participated in the design and conception of the study, assessed
the authenticity of the raw data, prepared the data set for
analysis, collected related literature, participated in the
statistical analysis, drafting and revision of the manuscript. KB
performed the statistical analysis, collected the related
literature, wrote and revised the manuscript. SAl participated in
the design and conception of the study, assessed the authenticity
of the raw data, prepared the data set for analysis, collected the
related literature and revised the manuscript. EÖ participated in
the design and conception of the study, was responsible for
identifying and recruiting all the participants, met with all the
participants, gave the instructions for self-sampling, assessed the
authenticity of the raw data, prepared the data set for analysis,
collected the related literature and revised the manuscript. MM
participated in the design and conception of the study, performed
colposcopy, cervical sampling and punch biopsies of visible
lesions, and revised the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Karolinska Ethics Committee approved the study
protocol (approval nos. 2006/1273-31, 2014/2034-3). All
participants provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AGC
|
atypical glandular cells
|
|
AIS
|
adenocarcinoma in-situ
|
|
ASC-H
|
atypical squamous cells cannot exclude
HSIL
|
|
ASC-US
|
atypical squamous cells of
undetermined significance
|
|
CI
|
confidence intervals
|
|
CIN
|
cervical intraepithelial neoplasia
|
|
C-LETZ
|
contoured-loop excision of the
transformation zone
|
|
HPV
|
high-risk human papillomavirus
|
|
HSIL
|
high-grade squamous intraepithelial
lesions
|
|
LBC
|
liquid-based cytology
|
|
LSIL
|
low-grade squamous intraepithelial
lesions
|
|
NILM
|
negative for intraepithelial lesions
or malignancy
|
|
OR
|
odds ratio
|
|
PCR
|
polymerase chain reaction
|
|
TZ3
|
transformation zone 3
|
|
VSS
|
vaginal self-sampling
|
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
R, Torre L and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwise for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hemminki K, Li X and Vaittinen P: Time
trends in the incidence of cervical and other genital squamous cell
carcinomas and adenocarcinomas in Sweden, 1958–1996. Eur J Obstet
Gynecol Reprod Biol. 101:64–69. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gunnell AS, Ylitalo N, Sandin S, Sparén P,
Adami HO and Ripatti S: A longitudinal Swedish study on screening
for squamous cell carcinoma and adenocarcinoma: Evidence of
effectiveness and overtreatment. Cancer Epidemiol Biomarkers Prev.
16:2641–2648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Screening SNC, Registry. Förebyggande av
livmoderhalscancer i Sverige 2017, . Available from. http://www.nkcx.se/templates/_rsrapport_2017.pdf
|
|
5
|
Andrae B, Kemetli L, Sparén L, Strander B,
Ryder W, Dillner J and Törnberg S: Screening-preventable cervical
cancer risks: Evidence from a nationwide audit in Sweden. J Natl
Cancer Inst. 100:622–629. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ebisch R, Rutten D, IntHout J, Melchers W,
Massuger L, Bulten J, Bekkers R and Siebers A: Long-lasting
increased risk of human papillomavirus-related carcinomas and
premalignancies after cervical intraepithelial neoplasia grade 3: A
population-based cohort study. J Clin Oncol. 35:2542–2550. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pettersson BF, Hellman K, Vaziri R,
Andersson S and Hellström AC: Cervical cancer in the screening era:
Who Fell victim in spite of successful screening programs? J
Gynecol Oncol. 22:76–82. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strander B, Andersson-Ellström A, Milsom I
and Sparén P: Long term risk of invasive cancer after treatment for
cervical intraepithelial neoplasia grade 3: Population based cohort
study. BMJ. 335:10772007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoffman S, Le T, Lockhart A, Sanusi A, Dal
Santo L, Davis M, McKinney D, Brown M, Poole C, Willame C and Smith
JS: Patterns of persistent HPV infection after treatment for
cervical intraepithelial neoplasia (CIN): A systematic review. Int
J Cancer. 141:8–23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Arbyn M, Ronco G, Anttila A, Meijer CJ,
Poljak M, Ogilvie G, Koliopoulos G, Nauclen P, Sankaranarayanan R
and Peto J: Evidence regarding human papillomavirus testing in
secondary prevention of cervical cancer. Vaccine. 30 (Suppl
5):F88–F99. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brismar S, Johansson B, Borjesson M, Arbyn
M and Andersson S: Follow-up after treatment of cervical
intraepithelial neoplasia by human papillomavirus genotyping. Am J
Obstet Gynecol. 201:17.e1–e8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kocken M, Uijterwaal M, de Vries A,
Berkhof J, Ket J, Helmerhorst J and Meijer C: High-risk
papillomavirus testing versus cytology in predicting post-treatment
disease in women treated for high-grade cervical disease: A
systematic review and meta-analysis. Gynecol Oncol. 125:500–507.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Persson M, Brismar Wendel S, Ljungblad L,
Johansson B, Weiderpass E and Andersson S: High-risk human
papillomavirus E6/E7 mRNA and L1 DNA as markers of
residual/recurrent cervical intraepithelial neoplasia. Oncol Rep.
28:346–352. 2012.PubMed/NCBI
|
|
14
|
Garutti P, Borghi C, Bedoni C, Bonaccorsi
G, Greco P, Tognon M and Martini F: HPV-based strategy in follow-up
of patients treated for high-grade cervical intra-epithelial
neoplasia: 5-year results in a public health surveillance setting.
Eur J Obstet Gynecol Reprod Biol. 210:236–241. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bruhn L, Andersen S and Hariri J:
HPV-testing versus HPV-cytology co-testing to predict the outcome
after conization. Acta Obstet Gynecol Scand. 97:758–765. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hermansson RS, Olovsson M, Hoxell E and
Lindström AK: HPV prevalence and HPV-related dysplasia in elderly
women. PLoS One. 13:e01893002018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rodriguez A, Schiffman M, Herrero R,
Hildesheim A, Bratti C, Sherman M, Solomon D, Guillén D, Alfaro M,
Morales J, et al: Longitudinal study of human papillomavirus
persistence and cervical intraepithelial neoplasia grade 2/3:
Critical role of duration of infection. J Natl Cancer Inst.
102:315–324. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Plummer M, Schiffman M, Castle P,
Maucort-Boulch D and Wheeler C: A 2-year prospective study of human
papillomavirus persistence among women with a cytological diagnosis
of atypical squamous cells of undetermined significance or
low-grade squamous intraepithelial lesion. J Infect Dis.
195:1582–1589. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Alder S, Megyessi D, Sundström K,
Östensson E, Mints M, Belkić K, Arbyn M and Andersson S: Incomplete
excision of cervical intraepithelial neoplasia as a predictor of
the risk of recurrent disease-a 16 year follow-up study. Am J
Obstet Gynecol. 222:172e1–172e12. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arbyn M, Redman C, Verdoodt F, Kyrgiou M,
Tzafetas M, Ghaem-Maghami S, Petry KU, Leeson S, Bergeron C,
Nieminen P, et al: Incomplete excision of cervical precancer as a
predictor of treatment failure: A systematic review and
meta-analysis. Lancet Oncol. 18:1665–1679. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fernández-Montolí ME, Tous S, Medina G,
Castellarnau M, García-Tejedor A and de Sanjosé S: Long-term
predictors of residual or recurrent cervical intraepithelial
neoplasia 2–3 after treatment with a large loop excision of the
transformation zone: A retrospective study. BJOG. 127:377–387.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reich O, Pickel H, Lahousen M, Tamussino K
and Winter R: Cervical intraepithelial neoplasia III: long-term
outcome after cold-knife conization with clear margins. Obstet
Gynecol. 97:428–430. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reich O, Lahousen M, Pickel H, Tamussino K
and Winter R: Cervical intraepithelial neoplasia III: Long-term
follow-up after cold-knife conization with involved margins. Obstet
Gynecol. 99:193–196. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ghaem-Maghami S, Sagi S, Majeed G and
Soutter WP: Incomplete excision of cervical intraepithelial
neoplasia and risk of treatment failure: A meta-analysis. Lancet
Oncol. 8:985–993. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Östensson E, Belkić K, Ramqvist T, Mints M
and Andersson S: Self-sampling for high-risk human papillomavirus
as a follow-up alternative after treatment of high-grade cervical
intraepithelial neoplasia. Oncol Lett. 21:2402021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alder S: Prevention of Cervical Cancer in
Countries with High and Low Incidence of the Disease. Doctoral
Dissertation Stockholm Sweden: Karolinska Institute; 2018
|
|
27
|
Andersson S, Belkić K, Mints M and
Östensson E: Is self-sampling to test for HPV an acceptable option
among women who have been treated for high-grade cervical
intraepithelial neoplasia? PLoS One. 13:e01990382018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Andersson S, Belkić K, Safer Demirbüker S,
Mints M and Östensson E: Perceived cervical cancer risk among women
treated for high-grade cervical intraepithelial neoplasia: The
importance of specific knowledge. PLoS One. 12:e1901562017.
View Article : Google Scholar
|
|
29
|
Richart RM: Cervical intraepithelial
neoplasia. Pathol Annu. 8:301–328. 1973.PubMed/NCBI
|
|
30
|
Jordan J, Martin-Hirsch P, Arbyn M,
Schenck U, Baldauf JJ, Da Silva D, Anttila A, Nieminen P and
Prendiville W: European guidelines for clinical management of
abnormal cervical cytology-part-2. Cytopathology. 20:5–16. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Solomon D, Davey D, Kurman R, Moriarty A,
O'Connor D, Prey M, Raab S, Sherman M, Wilbur D, Wright T, et al:
The 2001 Bethesda System: Terminology for reporting results of
cervical cytology. JAMA. 287:2114–2119. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Martin-Hirsch PL, Paraskevaidis E, Bryant
A, Dickinson HO and Keep SL: Surgery for cervical intraepithelial
neoplasia. Cochrane Database Syst Rev. CD0013182010.doi:
10.1002/14651858.CD001318.pub2. PubMed/NCBI
|
|
33
|
Ghaem-Maghami S, De-Silva D, Tipples M,
Lam S, Perryman K and Soutter W: Determinants of success in
treating cervical intraepithelial neoplasia. BJOG. 118:679–684.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Fallani MG, Penna C, Fambrini M and
Marchionni M: Laser CO2 vaporization for high-grade
cervical intraepithelial neoplasia: A long-term follow-up series.
Gynecol Oncol. 91:130–133. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wongtiraporn W and Laiwejpithaya S,
Sangkarat S, Benjapibal M, Rattanachaiyanont M, Ruengkhachorn I,
Chaopotong P and Laiwejpithaya S: Long term outcomes of laser
conization for high grade cervical intraepithelial neoplasia in
Thai women. Asian Pac J Cancer Prev. 15:7757–7761. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Frank J: The colposcopic examination. J
Midwifery Womens Health. 53:447–452. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Manley K, Simms R, Platt S, Patel A and
Bahl R: Unsatisfactory colposcopy: Clinical decision making in
conditions of uncertainty. BMC Med Inform Decis Mak. 17:1252017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Baasland I, Hagen B, Vogt C, Valla M and
Romundstad PR: Colposcopy and additive diagnostic value of biopsies
from colposcopy-negative areas to detect cervical dysplasia. Acta
Obstet Gynecol Scand. 95:1258–1263. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bogani G, Pinelli C, Chiappa V, Martinelli
F, Lopez S, Ditto A and Rapagliesi F: Age-specific predictors of
cervical dysplasia recurrence after primary conization: Analysis of
3,212 women. J Gynecol Oncol. 31:e602020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Andersson S, Larson B, Hjerpe A,
Silfverswärd C, Sällström J, Wilander E and Rylander E:
Adenocarcinoma of the uterine cervix: The presence of human
papillomavirus and the method of detection. Acta Obstet Gynecol
Scand. 82:960–965. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dahlström L, Ylitalo N, Sundström K,
Palmgren J, Ploner A, Eloranta S, Sanjeevi C, Andersson S, Rohan T,
Dillner J, et al: Prospective study of human papillomavirus and
risk of cervical adenocarcinoma. Int J Cancer. 127:1923–1930. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Dillner J: Covid-19: Challenges and
opportunities for cancer screening: An example from cervical cancer
in Sweden. International Agency for Research on Cancer COVID-19 and
cancer screening. May;2021.
|
|
43
|
de Pelsemaeker MC, Guiot Y, Vanderveken J,
Galant C and Van Bockstal M: The impact of the COVID-19 pandemic
and the associated Belgian governmental measures on cancer
screening, surgical pathology and cytopathology. Pathobiology.
88:46–55. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Feletto E, Grogan P, Nickson C, Smith M
and Canfell K: How has COVID-19 impacted cancer screening?
Adaptation of services and the future outlook in Australia. Public
Health Res Pract. 30:e30420262020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kregting LM, Kaljouw S, de Jonge L, Jansen
EEL, Peterse EFP, Heijnsdijk EAM, van Ravesteyn NT,
Lansdorp-Vogelaar I and de Kok IMCM: Effects of cancer screening
restart strategies after COVID-19 disruption. Br J Cancer.
12:1516–1523. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Miller M, Xu L, Qin J, Hahn E, Ngo-Metzger
Q, Mittman B, Tewari D, Hodeib M, Wride P, Saraiya M and Chao CR:
Impact of COVID-19 on cervical cancer screening rates among women
aged 21–65 in a large integrated health care system-Southern
California January 1-September 30, 2019 and January 1-September 30,
2020. MMWR Morb Mortal Wkly Rep. 70:109–113. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Gorin SNS, Jimbo M, Heizelman R, Harmes K
and Harper D: The future of cancer screening after COVID-19 may be
at home. Cancer. 127:498–503. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Burger E, Jansen E, Killen J, de Kok U,
Smith M, Sy S, Dunnewind N, Campos N, Haas J, Kobrin S, et al:
Impact of COVID-19-related care disruptions on cervical cancer
screening in the United States. J Med Screen. 28:213–216. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Nogami Y, Kobayashi Y, Tsuji K, Yokota M,
Nishio H, Nakamura M, Yamagami W, Morisada T, Tominaga E, Banno K
and Aoki D: Impact of the COVID-19 epidemic at a high-volume
facility in gynecological oncology in Tokyo, Japan: A single-center
experience. J Ovarian Res. 13:1052020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ajenifuja K, Belinson J, Goldstein A,
Desai K, de Sanjose S and Schiffman M: Designing low-cost, accurate
cervical screening strategies that take into account COVID-19: A
role for self-sampled HPV typing. Infect Agent Cancer. 15:612020.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ciavattini A, Carpini G, Giannella L,
Arbyn M, Kyrgiou M, Joura E, Sehouli J, Carcopino X, Redman C,
Nieminen P, et al: European Federation for Colposcopy (EFC) and
European Society of Gynaecological Oncology (ESGO) joint
considerations about human papillomavirus (HPV) vaccination,
screening programs, colposcopy, and surgery during and after the
COVID-19 pandemic. Int J Gynecol Cancer. 30:1097–1100. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cohen M, Powell A, Coleman J, Keller J,
Livingston A and Anderson J: Special ambulatory gynecologic
considerations in the era of coronavirus disease 2019 (COVID-19)
and implications for future practice. Am J Obstet Gynecol.
223:372–378. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Feldman S and Haas J: How the corona
disease-2019 may improve care: Rethinking cervical cancer
prevention. J Natl Cancer Inst. 113:djaa0892020.
|
|
54
|
Arbyn M, Verdoodt F, Snijders P, Verhoef
V, Suonio E, Dillner L, Minozzi S, Bellisario C, Banzi R, Zhao FH,
et al: Accuracy of human papillomavirus testing on self-collected
versus clinician-collected samples: A meta-analysis. Lancet Oncol.
15:172–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Arbyn M, Snijders PJ, Meijer CJ, Berkhof
J, Cuschieri K, Kocjan BJ and Poljak M: Which high-risk HPV assays
fulfill criteria for use in primary cervical cancer screening? Clin
Microbiol Infect. 21:817–826. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jentschke M, Chen K, Arbyn M, Hertel B,
Noskowicz M, Soergel P and Hillemans P: Direct comparison of two
vaginal self-sampling devices for the detection of human
papillomavirus infections. J Clin Virol. 82:46–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Bergengren L, Kaliff M, Larsson G,
Karlsson M and Helenius G: Comparison between professional sampling
and self-sampling for HPV-based cervical cancer screening among
postmenopausal women. Int J Gynecol Obstet. 142:359–364. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Östensson E, Hellström AC, Hellman K,
Gustavsson I, Gyllensten U, Wilander E, Zethraeus N and Andersson
S: Projected cost-effectiveness of repeat high-risk human
papillomavirus testing using self-collected vaginal samples in the
Swedish cervical cancer screening program. Acta Obstet Gynecol
Scand. 92:830–840. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Galbraith KV, Gilkey MB, Smith JS, Richman
AR, Barclay L and Brewer NT: Perceptions of mailed HPV self-testing
among women at higher risk for cervical cancer. J Community Health.
39:849–856. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Arrossi S, Ramos S, Straw C, Thouyaret L
and Orellana L: HPV testing: A mixed-method approach to understand
why women prefer self-collection in a middle-income country. BMC
Public Health. 16:8322016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Racey C and Gesink D: Barriers and
facilitators to cervical cancer screening among women in rural
Ontario, Canada: The role of self-collected HPV testing. J Rural
Health. 32:136–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Mao C, Kulasingam S, Whitham H, Hawes S,
Lin J and Kiviat N: Clinician and patient acceptability of
self-collected human papillomavirus testing for cervical cancer
screening. J Womens Health. 26:609–615. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Andersson S, Belkić K, Mints M and
Östensson E: Acceptance of self-sampling among long-term cervical
screening non-attenders with HPV positive results: Promising
opportunity for specific cancer education. J Cancer Educ.
36:126–133. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lindström AK, Hermansson RS, Gustavsson I,
Hedlund Lindberg J, Gyllensten U and Olovsson M: Cervical dysplasia
in elderly women performing repeated self-sampling for HPV testing.
PLoS One. 13:e02077142018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Foster E, Malloy M, Jokubaitis V, Wrede D,
Butzkueven H, Sasadeusz J, Van Doornum S, Macrae F, Unglik G,
Brotherton J and van der Walt A: Increased risk of cervical
dysplasia in females with autoimmune conditions-results from an
Australia database linkage study. PLoS One. 15:e02348132020.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Jentschke M, Kampers J, Becker J,
Sibbertsen P and Hillemanns P: Prophylactic HPV vaccination after
conization: A systematic review and meta-analysis. Vaccine.
38:6402–6409. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Joharinia N, Faghihinezhad S, Seyedi K,
Farhadi A, Hosseini SY, Safaei A, Baharampour H and Sarvari J:
Co-existing of HSV1/2 or EBV infection with the presence of
high-risk HPV DNA in cervical lesions in the southwest of Iran.
Asian Pac J Cancer Prev. 21:1459–1464. 2020. View Article : Google Scholar : PubMed/NCBI
|