Introduction
Gastric cancer is one of the most common
malignancies worldwide, as the fifth most frequently diagnosed
cancer and the third-leading cause of cancer-associated mortality,
particularly in East Asian countries (1). Its 5-year overall survival rate is
<30% (2). Most patients are
diagnosed at an advanced stage (3).
Some clinical trials have tested new targeted drugs for advanced
gastric cancer (4–6); however, the results are disappointing
due to notable toxic effects and low response rate (7). Thus, it is important to investigate the
molecular pathogenesis and develop novel drugs for patients with
gastric cancer.
The occurrence of gastric cancer is an intricate
process, involving the abnormal expression of several genes
(8,9). Tankyrases (TNKS), as member of the poly
(ADP-ribose) polymerase (PARP) family, has two subtypes, TNKS1 and
TNKS2 (10). Both subtypes have 85%
overlap in the amino acid sequence (10). TNKS participates in various
biological processes. For example, TNKS hyperactivates the
Wnt/β-catenin pathway by destabilizing AXIN (11). Furthermore, TNKS regulates
PARsylation of BLZF1, as well as CASC3, thereby recruiting RNF146
and subsequent ubiquitination (12).
In addition, it participates in centrosome maturation during
prometaphase by regulating PARsylation of HEPACAM2/MIKI (13). It may regulate vesicle trafficking,
as well as subcellular distribution of SLC2A4/GLUT4-vesicles
(14). By mediating PARsylation of
TERF1, TNKS is involved in telomere length (15). It has been reported that TNKS
expression is upregulated in different types of cancer, including
gastric cancer (16). In addition,
TNKS1 expression is significantly associated with stage and
differentiation of gastric cancer (17). Recently, it was demonstrated that
TNKS knockdown can inhibit the proliferation, invasion and
epithelial-to-mesenchymal transition (EMT) process in
hepatocellular carcinoma cells (11). However, the underlying molecular
mechanisms of TNKS in gastric cancer remain unclear.
Several signaling pathways, such as EMT, contribute
to tumor invasion and metastasis (18). EMT has a profound influence on the
early events of metastatic spread of gastric cancer cells (18). In the EMT process, the expression of
adhesion proteins, such as E-cadherin, decrease, followed by loss
of polarity of epithelial tumor cells and loosening of cell
connections (19). The interaction
between epithelial cells gradually disappears (19). Subsequently, the expression of
mesenchymal cell characteristics (N-cadherin and Vimentin) and
matrix metalloproteinases (MMPs) increases, and the expression of
signal transduction proteins, such as Twist, is activated (20). Tumor cells have acquired the ability
to resist apoptosis and degrade the extracellular matrix, and their
migratory ability increases, resulting in invasion and metastasis
(21). Clinically, EMT is associated
with poor prognosis of patients with gastric cancer (22). Activation of the Wnt/β-catenin
pathway accelerates the EMT process, thereby promoting invasion and
metastasis of gastric cancer (23,24).
HLY78 can bind to the DAX domain of Axin, which has been widely
used as the Wnt/β-catenin pathway agonist (25).
Dihydroartemisinin (DHA) is the main component of
artemisinin extracted from the traditional Chinese medicine,
Artemisia annua (26).
Several studies have reported that DHA exerts broad biological
characteristics, including antitumor effects (26–28). As
reported in a recent study, DHA can restrain proliferative,
migrative and invasive abilities of gastric cancer cells (27). Furthermore, DHA prevents
Helicobacter pylori-induced gastric cancer by inhibiting
NF-κB activity (28). However, the
exact molecular mechanism of DHA remains unclear. Thus, the present
study aimed to investigate the effects of DHA on proliferation,
migration, the Wnt/β-catenin pathway, as well as the EMT process in
gastric cancer cells.
Materials and methods
Patients and tissue specimens
A total of 87 pairs of gastric cancer tissues and
adjacent normal tissues were collected from patients (42 men and 45
women; mean age, 60.1 years; age range, 43–72 years) following
surgical resection at The First Hospital of Yulin between February
2018 and February 2020. Normal tissues were at least 5 cm away from
tumor tissues. The inclusion criteria were as follows: i) Patients
did not receive chemotherapy or radiotherapy prior to surgery; ii)
patients were diagnosed as primary gastric cancer; iii) patients
did not have other types of cancer; iv) patients did not have any
history of surgery and v) patients did not have any concomitant
diseases. Patients without complete clinical information were
excluded from the present study. Tissue samples were transferred
into liquid nitrogen following surgery and stored at −80°C. All
specimens were fixed in 10% formalin, followed by gradient alcohol
dehydration, transparent xylene and paraffin embedding within 24 h.
Patients were diagnosed by two pathologists, in line with the
guidelines of the Union for International Cancer Control (29). The present study was approved by the
Ethics Committee of The First Hospital of Yulin (Yulin, China;
approval no. 2018031) and written informed consent was provided by
all patients prior to the study start.
Immunohistochemistry
Gastric cancer tissues were fixed overnight with 10%
formalin solution at 4°C. Paraffin-embedded gastric cancer tissues
were cut into 4-µm-thick sections. Following dewaxing and
rehydrating, the sections were incubated with 3%
H2O2 for 20 min to inhibit endogenous
peroxidase activity at room temperature. Following antigen
retrieval, the sections were blocked with 5% BSA blocking solution
at room temperature for 1 h. The sections were incubated with
primary antibodies against TNKS (1:200; cat. no. 18030-1-AP), AXIN2
(1:150; cat. no. 20540-1-AP), Vimentin (1:200; cat. no.
10366-1-AP), β-catenin (1:100; cat. no. 17565-1-AP), E-cadherin
(1:100; cat. no. 20874-1-AP) and N-cadherin (1:100; cat. no.
22018-1-AP) overnight at 4°C (all purchased from ProteinTech Group,
Inc.). Following the primary incubation, membranes were incubated
with HRP-conjugated secondary antibodies (1:1,000; cat. no. ab6721;
Abcam) for 2 h at room temperature. DAB reagent (Sigma-Aldrich;
Merck KGaA) was used for color development. The sections were
stained with hematoxylin for 3 min at room temperature, and
differentiated with 1% hydrochloric acid in ethanol for 15 sec, and
1% ammonia water for 1 min. Following a series of ethanol
dehydration, the sections were made transparent with xylene and
sealed with neutral resin. Images were observed under a light
microscope (Olympus Corporation) at magnification of ×200. The
optical density values were determined using ImageJ software
(version 1.48; National Institutes of Health). Furthermore, the
semi-quantitative values of TNKS, AXIN2 and β-catenin expression
were assessed via the percentage of positive cells (<5%, 0
point; 5–25%, 1 point; 26–50%, 2 points; 51–75%, 3 points and
76–100%, 4 points) and staining intensity (no staining, 0 point;
light yellow, 1 point; brown, 2 points and tan, 3 points), as
previously described (30). The
product of the two was defined as follows: 0, negative (−) and
>1 positive (+).
Cell culture
The human gastric cancer cell lines, AGS and HGC-27,
were purchased from Shanghai Zhongqiao Xinzhou Biotechnology Co.,
Ltd. Cells were maintained in RPMI-1640 medium supplemented with
10% fetal bovine serum (FBS), at 37°C with 5% CO2 and
95% saturated humidity environment (all purchased from Gibco;
Thermo Fisher Scientific, Inc.). When the cells reached 80%
confluence, they were digested with 0.25% trypsin. After passaging
three times, cells were harvested and seeded into a 6-well plate at
a density of 3×105 cells/well.
Transient transfection
Small interfering RNAs (siRNAs) targeting TNKS1 or
TNKS2 (5 nM, Sangon Biotech, Co., Ltd.) and the corresponding siRNA
negative control (si-NC; Sangon Biotech, Co., Ltd.) were separately
transfected into AGS and HGC-27 cells using
Lipofectamine® 2000 transfection reagent (cat. no.
11668019; Thermo Fisher Scientific, Inc.). The siRNA sequences were
as follows: hTNKS1 forward, 5′-GCAUGGAGCUUGUGUUAAUUU-3′ and
reverse, 5′-AUUAACACAAGCUCCAUGCUU-3′; hTNKS2 forward,
5′-GAGGGUAUCUCAUUAGGUAUU-3′ and reverse,
5′-UACCUAAUGAGAUACCCUCUU-3′; and si-NC forward,
5′-CGUUACUUUUGUGUAGUACAA-3′ and reverse,
5′-UUGUACUACACAAAAGUAACG-3′. After 48 h, transfection efficiency
was verified via western blotting.
Cell Counting Kit-8 (CCK-8)
AGS and HGC-27 cells were respectively seeded into
96-well plates at a density of 5×103 cells/well. After
the cells fully adhered, the original culture medium was discarded.
Subsequently, each group was treated with different concentrations
of HLY78 (cat. no. HY-122816, MCU; 0, 5, 10, 20, 30, 40, 50 and 100
µM) or DHA (cat. no. HY-N0176; MCE; 0, 5, 10, 20, 30, 50 and 100
µM) in RPMI-1640 complete medium (100 µl/well). Following
incubation for 48 h at 37°C, cells were cultured in medium
containing CCK-8 (100 µl, RPMI-1640 complete medium + 10 µl CCK-8;
Beyotime Institute of Biotechnology) for 1 h. Cell viability was
analyzed at a wavelength of 450 nm, using a microplate reader
(Bio-Rad Laboratories, Inc.).
Western blotting
AGS and HGC-27 cells were seeded into 6-well plates
at a density of 5×105 cells/well and lysed on ice using
RIPA lysis buffer (Beyotime Institute of Biotechnology). Following
centrifugation at 12,000 × g for 20 min at 4°C, the supernatant was
harvested. Protein concentration was determined using the BCA
protein concentration determination kit (Thermo Fisher Scientific,
Inc). The protein samples were diluted by an equal volume of 2X
loading buffer and heated for 10 min to denature the protein. A
total of 40 µl protein sample was added to each well, separated via
12% SDS-PAGE, transferred onto PVDF membranes (MilliporeSigma) and
blocked with 5% skimmed milk powder for 1 h at 4°C. The membranes
were incubated with primary antibodies against TNKS (1:1,000; cat.
no. 18030-1-AP), AXIN2 (1:1,000; cat. no. 20540-1-AP), TWIST
(1:1,000; cat. no. 10366-1-AP), MMP2 (1:1,000; cat. no.
10366-1-AP), Vimentin (1:1,000; cat. no. 10366-1-AP), β-catenin
(1:2,000; cat. no. 17565-1-AP), E-cadherin (1:1,000; cat. no.
20874-1-AP), N-cadherin (1:500; cat. no. 22018-1-AP) and β-actin
(1:1,000; cat. no. 20536-1-AP) overnight at 4°C (all purchased from
ProteinTech Group, Inc.). Following the primary incubation,
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (1:5,000; cat. nos. ab205719 and ab6721;
Abcam) for 1 h at room temperature. ECL color luminescent liquid
(GE Healthcare) was evenly added to the PVDF membranes and allowed
to react for 1 min. The gray values of the target proteins were
quantified using ImageJ software (version 1.48; National Institutes
of Health).
Colony formation assay
AGS and HGC-27 cells were seeded into a 6-well plate
at a density of 5×103 cells/well. Following transfection
or treatment for 24 h, cells were digested using trypsin, and 2 ml
cell suspension was added to each well. Cell colony formation was
observed every 2 days and the medium was changed every 3–4 days.
The medium was discarded after 1 week and cells were fixed with 600
µl methanol for 30 min at 4°C. Cells were subsequently stained with
600 µl 0.1% crystal violet for 20 min at room temperature. Stained
cells were observed under a light microscope (Olympus Corporation)
at magnification of ×200.
Wound healing assay
AGS and HGC-27 cells were seeded into a 6-well plate
at a density of 5×103 cells/well. Once the bottom of the
plate was covered by cells and the confluence was up to 95% under
the microscope field of view, a 10 µl pipette tip was used to
scratch the cell monolayers. The original medium was discarded and
the plates were washed three times with PBS to remove cell debris.
The cells were serum-starved during the wound healing assay.
Following treatment with 5 nM si-TNKS1, 5 nM si-TNKS2, 20 µM HLY78
or 30 µM DHA, cells were observed at 0 and 48 h. Images were
observed under a light microscope (Olympus Corporation) at
magnification of ×200.
Migration assay
The Transwell chamber (Corning, Inc.) was placed
into a 24-well plate. Serum-free medium was used to routinely
prepare the cells into a single cell suspension. AGS and HGC-27
cells were plated in the upper chambers of Transwell plates
(5×103 cells/well), while 500 µl medium supplemented
with 10% FBS was plated in the lower chambers. Following incubation
for 12 h at room temperature, the 24-well plate was washed 2–3
times with PBS and the migratory cells were fixed with 4%
paraformaldehyde for 15 min at 4°C. Cells were subsequently stained
with Giemsa for 30 min at room temperature and counted in three
randomly selected fields using a light microscope (Olympus
Corporation).
Immunofluorescence
AGS and HGC-27 cells were seeded into a 6-well plate
at a density of 5×103 cells/well and covered with a
cover glass. Once the cells adhered to the wall, the glass slide
was fixed with 4% paraformaldehyde for 15 min at 4°C. The sections
were incubated with penetrating agent for 20 min at room
temperature and subsequently blocked with blocking solution for 30
min at room temperature. Sections were incubated with primary
antibodies against TNKS (1:200; cat. no. 18030-1-AP), AXIN2 (1:150;
cat. no. 20540-1-AP) and β-catenin (1:100; cat. no. 17565-1-AP)
overnight at 4°C (all purchased from ProteinTech Group, Inc.).
Following the primary incubation, the sections were incubated with
Alexa Fluor® 488-conjugated secondary antibody (1:500;
cat. nos. ab150077 and ab150113; Abcam) for 90 min at room
temperature in the dark. Sections were stained with DAPI solution
for 15 min at room temperature, dried and the anti-quenching
mounter was mounted. Sections were observed under a fluorescence
microscope at magnification of ×200.
Statistical analysis
Statistical analysis was performed using SPSS 22.0
software (IBM Corp.). Unpaired Student's t-test was used to compare
differences between two groups, while one-way ANOVA followed by
Tukey's post hoc test were used to compare differences between
multiple groups. The χ2 test was used to assess the
association between TNKS expression and the clinicopathological
characteristics of patients with gastric cancer. P<0.05 was
considered to indicate a statistically significant difference.
Results
Activation of TNKS, Wnt/β-catenin and
EMT in gastric cancer
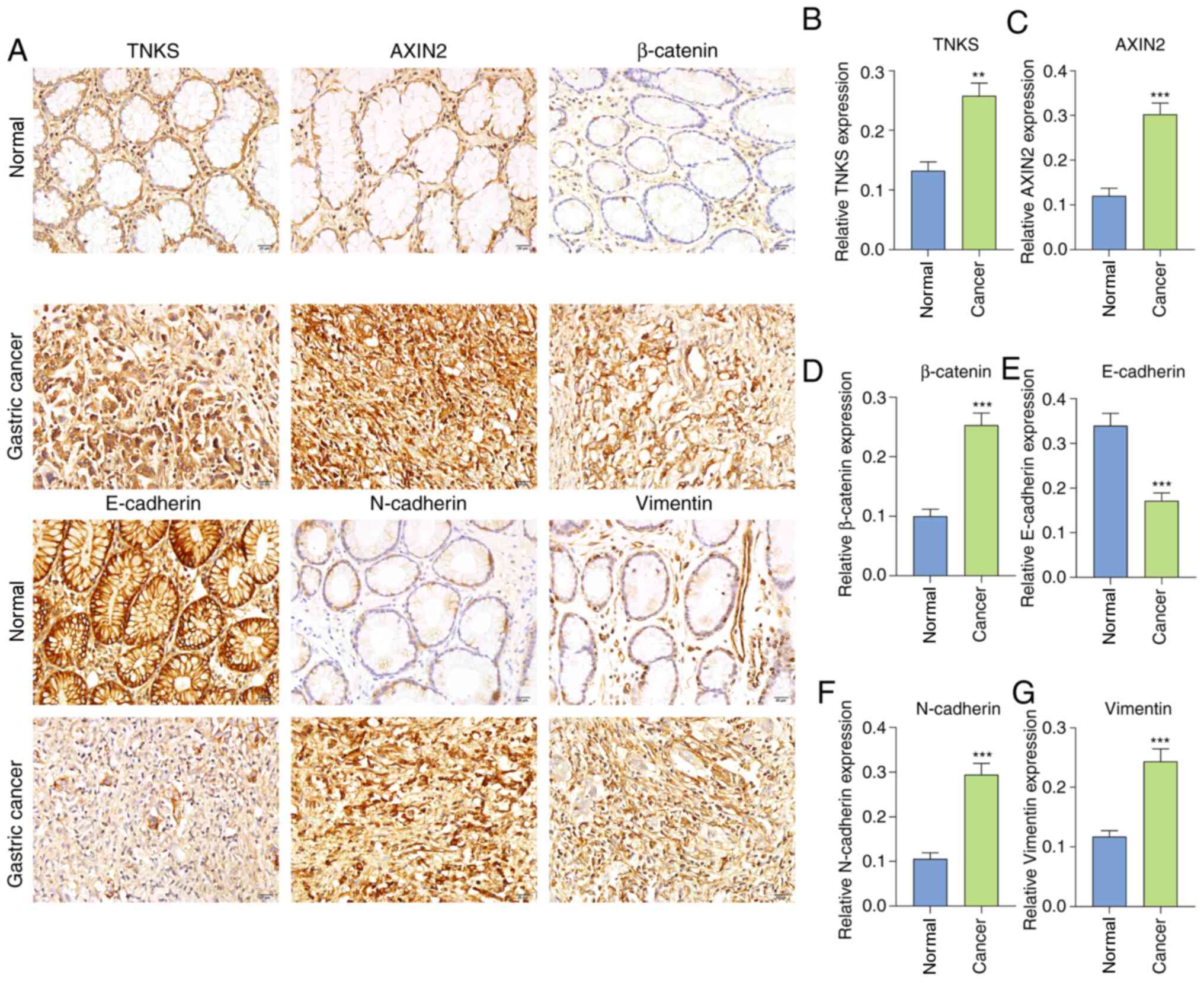
The expression levels of TNKS, Wnt/β-catenin and EMT
proteins were detected in 87 pairs of gastric cancer tissues and
adjacent normal tissues. The results demonstrated that TNKS
expression was 1.95 times higher in gastric cancer tissues compared
with normal tissues (P<0.01; Fig. 1A
and B). In the Wnt/β-catenin pathway, AXIN2 (P<0.001;
Fig. 1A and C) and β-catenin
(P<0.001; Fig. 1A and D) both
exhibited higher expression in gastric cancer tissues compared with
normal tissues. The positive rates of TNKS, AXIN2 and β-catenin in
gastric cancer, as well as adjacent normal tissues were calculated
in a cohort of 87 patients with gastric cancer, as listed in
Table I. Furthermore, the expression
of EMT-related proteins was detected. Among them, E-cadherin
(P<0.001; Fig. 1A and E) was
downregulated, while N-cadherin (P<0.001; Fig. 1A and F) and Vimentin (P<0.001;
Fig. 1A and G) were upregulated in
gastric cancer tissues compared with normal tissues. Consistent
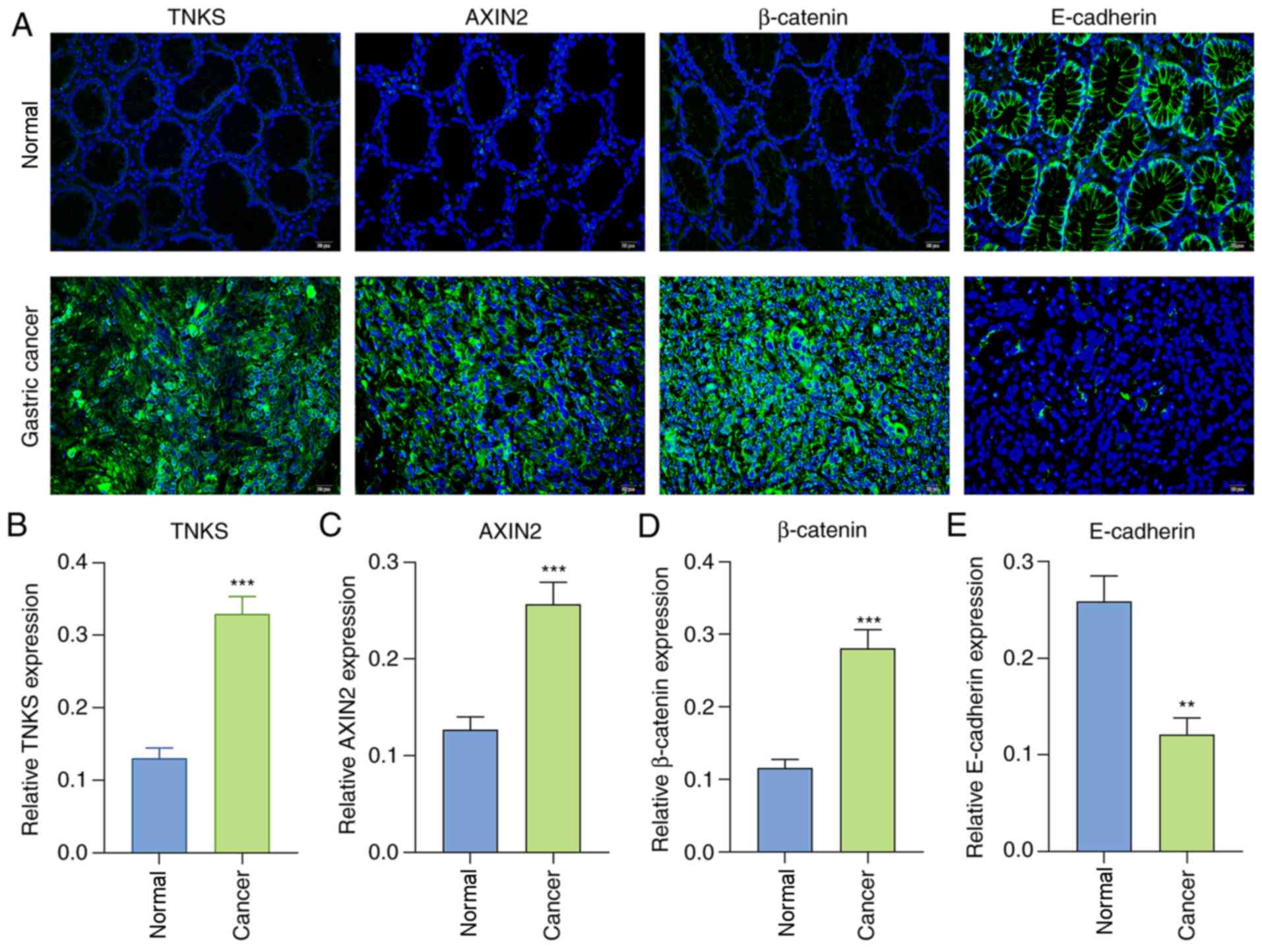
with immunohistochemistry, immunofluorescence analysis demonstrated
that TNKS (P<0.001; Fig. 2A and
B), AXIN2 (P<0.001; Fig. 2A and
C) and β-catenin (P<0.001; Fig.
2A and D) expression levels were distinctly highly in gastric
cancer tissues compared with normal tissues. Furthermore,
E-cadherin expression was downregulated by 0.47-fold in gastric
cancer tissues compared with normal tissues (P<0.01; Fig. 2A and E). Collectively, the TNKS,
Wnt/β-catenin and EMT pathways are activated in gastric cancer
(16,18,23). The
association between TNKS expression and the clinicopathological
characteristics of patients with gastric cancer was also evaluated.
As presented in Table II, TNKS
expression was significantly associated with depth of invasion
(P=0.012), lymph metastasis (P=0.001), TNM stage (P=0.018) and
survival status (P<0.001).
 | Table I.Expression levels of TNKS, AXIN2 and
β-catenin in 87 gastric cancer tissues and 87 adjacent normal
tissues from a cohort of patients with gastric cancer. |
Table I.
Expression levels of TNKS, AXIN2 and
β-catenin in 87 gastric cancer tissues and 87 adjacent normal
tissues from a cohort of patients with gastric cancer.
|
| Gastric cancer
tissues (n=87) | Adjacent normal
tissues (87) |
|
|---|
|
|
|
|
|
|---|
| Protein | Positive rate, n
(%) | Negative rate, n
(%) | Positive rate, n
(%) | Negative rate, n
(%) | P-value |
|---|
| TNKS | 61 (70.1) | 26 (29.9) | 21 (24.1) | 66 (75.9) | <0.0001 |
| AXIN2 | 57 (65.5) | 30 (34.5) | 23 (26.4) | 64 (73.6) | <0.0001 |
| β-catenin | 68 (78.2) | 19 (21.8) | 23 (26.4) | 64 (73.6) | <0.0001 |
 | Table II.Association between TNKS expression
and the clinicopathological characteristics of patients with
gastric cancer (n=87). |
Table II.
Association between TNKS expression
and the clinicopathological characteristics of patients with
gastric cancer (n=87).
|
|
| TNKS
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Characteristic | Total (n=87) | Positive, %
(n=61) | Negative, %
(n=26) | χ2 | P-value |
|---|
| Sex |
|
|
|
|
|
|
Male | 42 | 28 (66.7) | 14 (33.3) | 0.461 | 0.497 |
|
Female | 45 | 33 (73.3) | 12 (26.7) |
|
|
| Age, years |
|
|
|
|
|
|
<60 | 43 | 32 (74.4) | 11 (25.6) | 0.752 | 0.386 |
|
≥60 | 44 | 29 (65.9) | 15 (34.1) |
|
|
| BMI |
|
|
|
|
|
|
<24 | 27 | 17 (63.0) | 10 (37.0) | 0.978 | 0.613 |
|
24-27.9 | 31 | 23 (74.2) | 8 (25.8) |
|
|
|
≥28 | 29 | 21 (72.4) | 8 (27.6) |
|
|
| Smoking |
|
|
|
|
|
| No | 46 | 33 (71.7) | 13 (28.3) | 0.123 | 0.726 |
|
Yes | 41 | 28 (68.3) | 13 (31.7) |
|
|
| Drinking |
|
|
|
|
|
| No | 39 | 24 (61.5) | 15 (38.5) | 2.481 | 0.115 |
|
Yes | 48 | 37 (77.1) | 11 (22.9) |
|
|
| Helicobacter pylori
infection |
|
|
|
|
|
| No | 54 | 37 (68.5) | 17 (31.5) | 0.173 | 0.677 |
|
Yes | 33 | 24 (72.7) | 9 (27.3) |
|
|
| Depth of
invasion |
|
|
|
|
|
|
T1/T2 | 36 | 19 (52.8) | 17 (47.2) | 6.363 | 0.012a |
|
T3/T4 | 51 | 40 (78.4) | 11 (21.6) |
|
|
| Lymph
metastasis |
|
|
|
|
|
| N0 | 27 | 9 (33.3) | 18 (66.7) | 11.379 | 0.001b |
|
N1/N2/N3 | 60 | 43 (71.7) | 17 (28.3) |
|
|
| TNM stage |
|
|
|
|
|
|
I–II | 40 | 22 (55.0) | 18 (45.0) | 5.572 | 0.018a |
|
III–IV | 47 | 37 (78.7) | 10 (21.3) |
|
|
| Survival
status |
|
|
|
|
|
|
Dead | 33 | 24 (72.7) | 9 (27.3) | 23.351 |
<0.001c |
|
Alive | 54 | 11 (20.4) | 43 (79.6) |
|
|
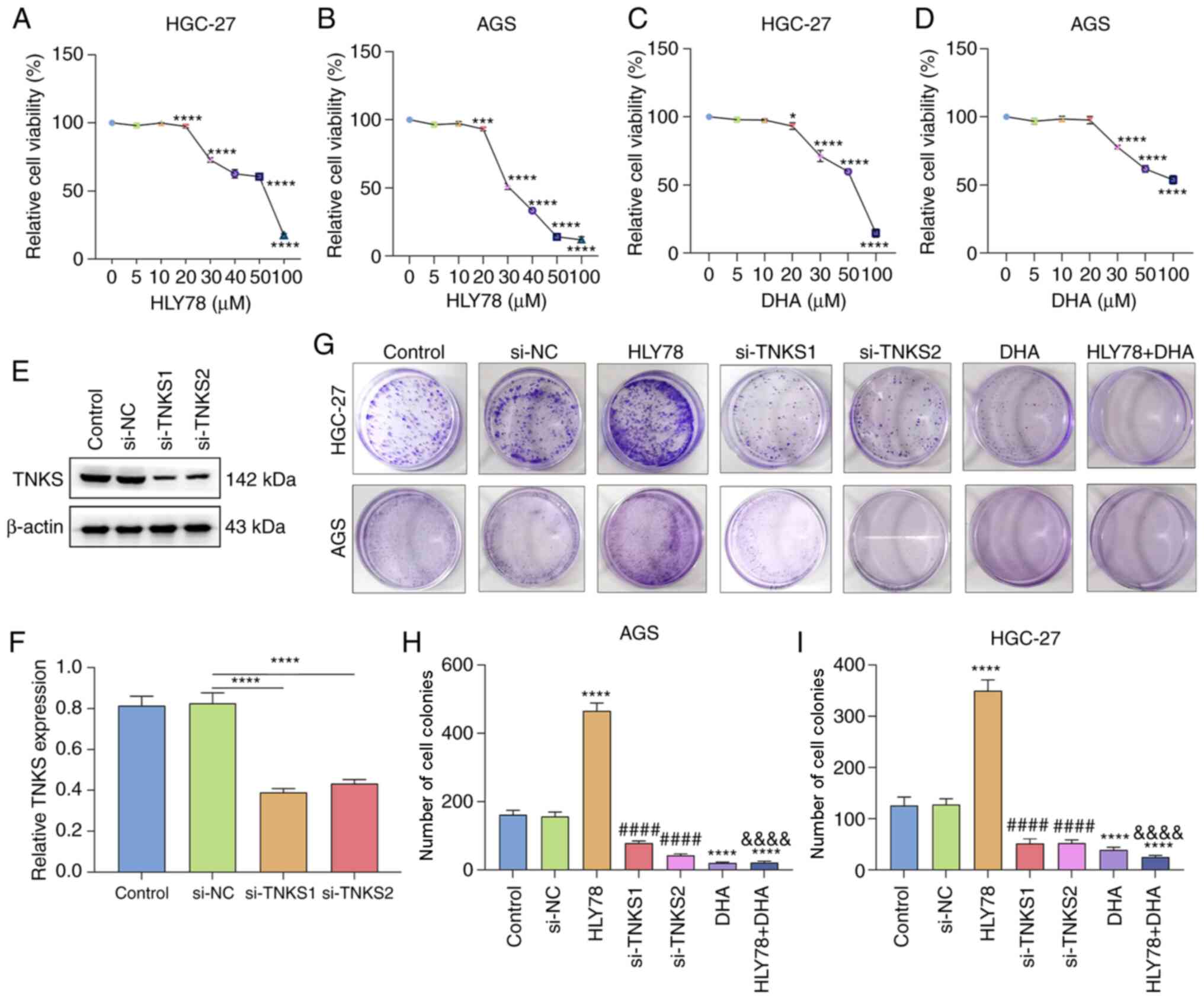
DHA treatment significantly suppresses
proliferation of gastric cancer cells partly by TNKS2
Gastric cancer cells were treated with different
concentrations of HLY78, a Wnt activator. The results of the CCK-8
assay demonstrated that the viability of HGC-27 and AGS cells
decreased as the concentration of HLY78 increased (both
P<0.0001; Fig. 3A and B). HLY78
(20 µM) was selected as the optimal concentration for subsequent
analyses. The viability of HGC-27 and AGS cells treated different
concentrations of DHA was assessed. The results demonstrated that
DHA significantly suppressed viability of the gastric cancer cells
in a concentration-independent manner (both P<0.0001; Fig. 3C and D). DHA (30 µM) was determined
the optimal concentration. Western blot analysis confirmed that
TNKS expression decreased following transfection with si-TNKS1 and
si-TNKS2 (P<0.0001; Fig. 3E and
F). Cell colony formation was assessed for the transfected or
treated gastric cancer cells. The results demonstrated that HLY78
significantly increased the colony formation ability by 2.78-fold
and 2.88-fold for HGC-27 and AGS cells compared with the controls
(both P<0.0001; Fig. 3G-I).
Furthermore, the colony formation ability decreased following
transfection with si-TNKS1 or si-TNKS2. Treatment with DHA notably
decreased the number of cell colonies by 0.31-fold and 0.13-fold in
HGC-27 and AGS cells compared with the controls. In addition,
treatment with DHA significantly attenuated the enhancement of
colony formation induced by HLY78. Taken together, these results
suggest that DHA significantly suppresses proliferation partly by
silencing TNKS2 expression in gastric cancer cells.
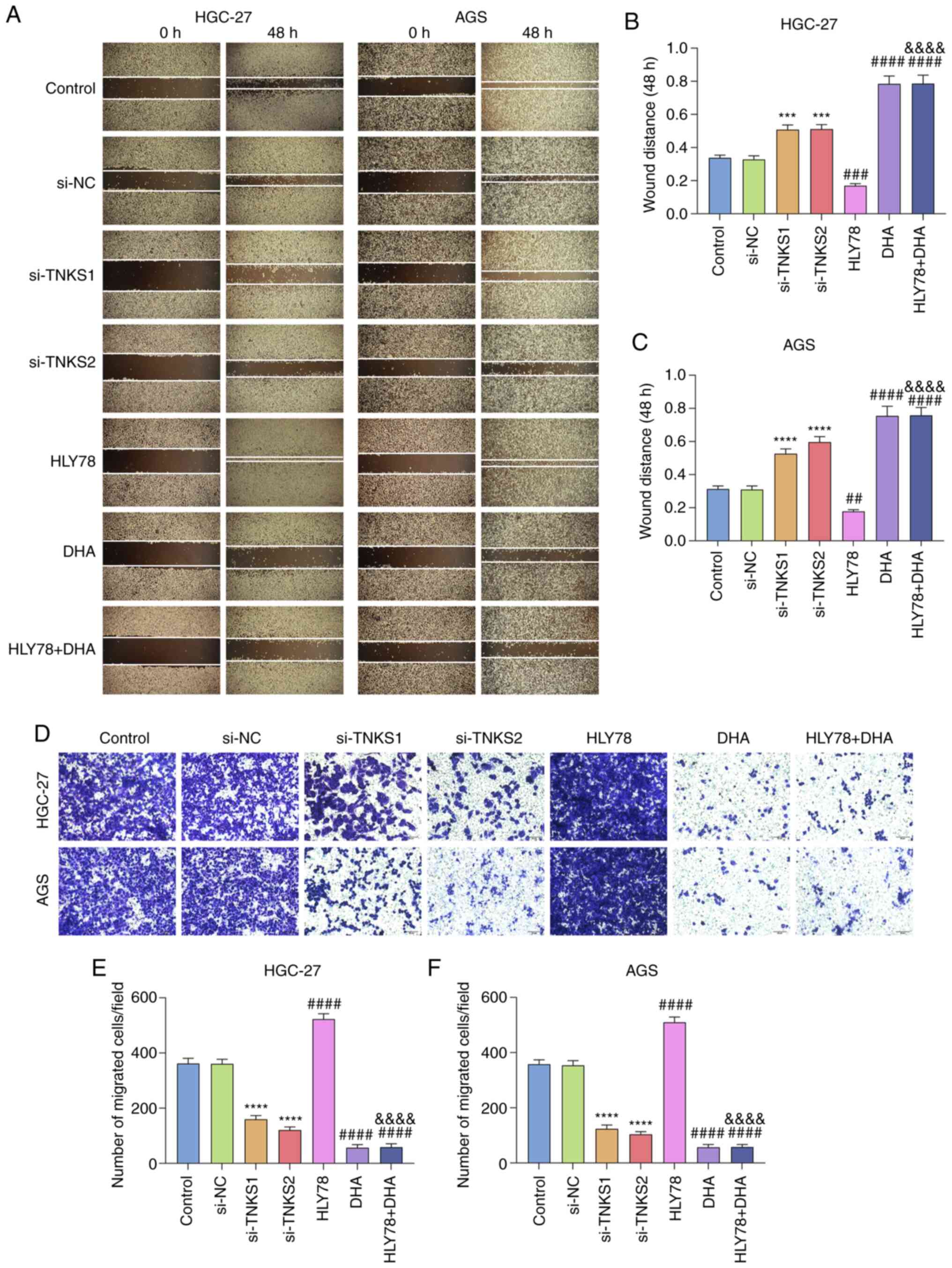
DHA treatment suppresses migration of
gastric cancer cells partly by silencing TNKS
The wound healing and Transwell assays were
performed to assess the migratory ability of treated gastric cancer
cells. The results demonstrated that HLY78 elevated the migration
ability of HGC-27 and AGS cells (P<0.001 and P<0.01; Fig. 4A-C). The wound distance was
significantly shorter following transfection with si-TNKS1 by
1.55-fold and 1.56-fold or transfection with si-TNKS2 by 1.70-fold
and 1.93-fold (P<0.0001 or P<0.001). Furthermore, treatment
with DHA significantly weakened the migratory ability of gastric
cancer cells by 2.33-fold and 2.41-fold. DHA attenuated the effect
of HLY78 on cell migration (all P<0.0001). The results of the
Transwell assay demonstrated that the number of migratory cells
increased by 1.45-fold and 1.42-fold following treatment with HLY78
(all P<0.0001; Fig. 4D-F).
Conversely, TNKS knockdown or treatment with DHA decreased the
number of migratory cells. The increase in the number of migratory
cells induced by HLY78 was attenuated following co-treatment with
DHA. Taken together, these results suggest that DHA inhibits the
migratory ability of gastric cancer cells partly by silencing
TNKS.
DHA suppresses activation of the EMT
process and the Wnt/β-catenin pathway in gastric cancer cells
The present study investigated whether DHA can
affect activation of EMT and the Wnt/β-catenin pathway in gastric
cancer cells via western blot analysis. The results demonstrated
that DHA significantly decreased TNKS expression by 0.42-fold
(P<0.0001; Fig. 5A and B).
Furthermore, HLY78 elevated TNKS expression by 1.84-fold in gastric
cancer cells, which was ameliorated following DHA co-treatment
(both P<0.0001). HLY78 treatment increased AXIN2 expression by
0.55-fold, the effects of which were reversed following treatment
with DHA (both P<0.0001; Fig. 5A and
C). MMP2 expression is used to assess the invasive ability of
tumor cells (20). The results
demonstrated that DHA significantly inhibited MMP2 expression by
0.47-fold and ameliorated the increase in MMP2 expression induced
by HLY78 (both P<0.0001; Fig. 5A and
D). In Fig. 5A and E, TWIST
expression was increased by HLY78 and decreased by DHA in gastric
cancer cells both (P<0.0001). DHA treatment significantly
decreased Vimentin expression by 0.69-fold (P<0.001), and
improved HLY78-induced increase in Vimentin expression
(P<0.0001; Fig. 5A and F).
Furthermore, β-catenin (Fig. 5A and
G) and N-cadherin (Fig. 5A and
H) expression levels significantly decreased following
treatment with DHA (both P<0.01), the effects of which were
ameliorated following treatment with HLY78 (both P<0.0001). In
Fig. 5A and I, DHA significantly
increased E-cadherin expression by 2.25-fold (P<0.0001), while
HLY78 suppressed E-cadherin expression by 0.50-fold in gastric
cancer cells. Collectively (P<0.0001), these results suggest
that DHA inactivates EMT and the Wnt/β-catenin pathway in gastric
cancer cells.
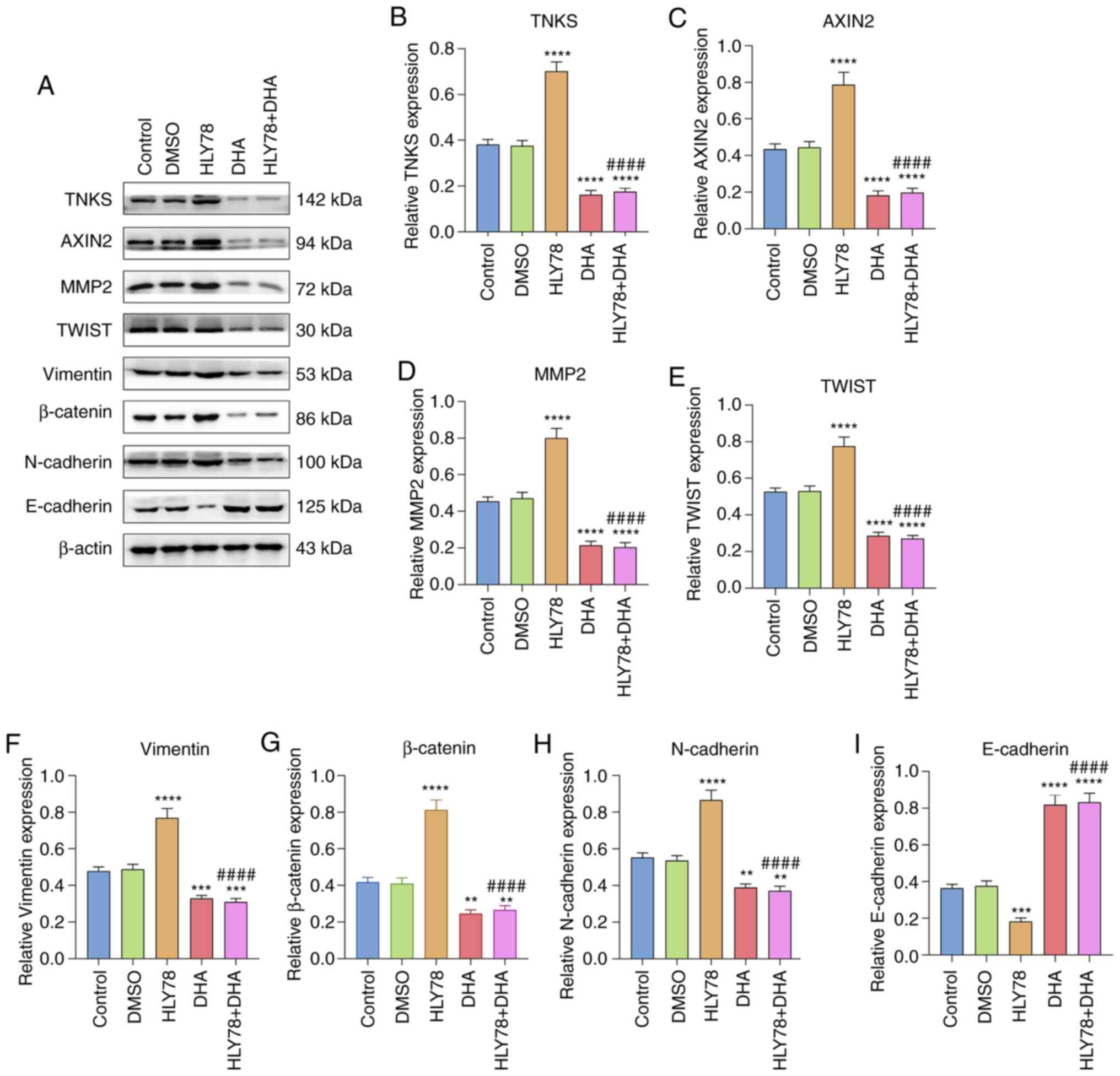 | Figure 5.DHA inactivates EMT and the
Wnt/β-catenin pathway in gastric cancer. (A) Western blot analysis
was performed to detect the expression levels of EMT- and
Wnt/β-catenin pathway-related proteins in gastric cancer cells
treated with HLY78 and/or DHA. (B) TNKS, (C) AXIN2, (D) MMP2, (E)
TWIST, (F) Vimentin, (G) β-catenin, (H) N-cadherin and (I)
E-cadherin expression levels were quantified according to western
blotting. **P<0.01, ***P<0.001 and ****P<0.0001 vs. the
DMSO group; ####P<0.0001 vs. the HLY78 group. DHA,
Dihydroartemisinin; EMT, epithelial-to-mesenchymal transition;
TNKS, Tankyrases; MMP, matrix metalloproteinase. |
DHA suppresses activation of the EMT
process and the Wnt/β-catenin pathway in gastric cancer partly by
silencing TNKS
The effects of TNKS knockdown and DHA on EMT and the
Wnt/β-catenin pathway in gastric cancer cells were investigated. As
expected, TNKS expression significantly decreased by 0.47-fold and
0.22-fold in gastric cancer cells treated with si-TNKS1/si-TNKS2 or
DHA (all P<0.0001; Fig. 6A and
B). Furthermore, TNKS1 or TNKS2 knockdown significantly
decreased AXIN2 expression by 0.39-fold and 0.38-fold (both
P<0.0001; Fig. 6A and C), MMP2 by
0.37-fold and 0.35-fold (both P<0.0001; Fig. 6A and D), TWIST by 0.28-fold and
0.54-fold (both P<0.0001; Fig. 6A and
E), Vimentin by 0.55-fold and 0.51-fold (both P<0.0001;
Fig. 6A and F), β-catenin by
0.32-fold and 0.30-fold (both P<0.0001; Fig. 6A and G) and N-cadherin by 0.28-fold
and 0.53-fold (both P<0.0001; Fig. 6A
and H). In Fig. 6A and I,
E-cadherin expression significantly increased by 1.95-fold and
2.00-fold following transfection with si-TNKS1/si-TNKS2 (all
P<0.0001). Similar results were observed following treatment
with DHA (all P<0.0001). Taken together, these results suggest
that DHA inhibits activation of EMT and the Wnt/β-catenin pathway
in gastric cancer cells partly by silencing TNKS.
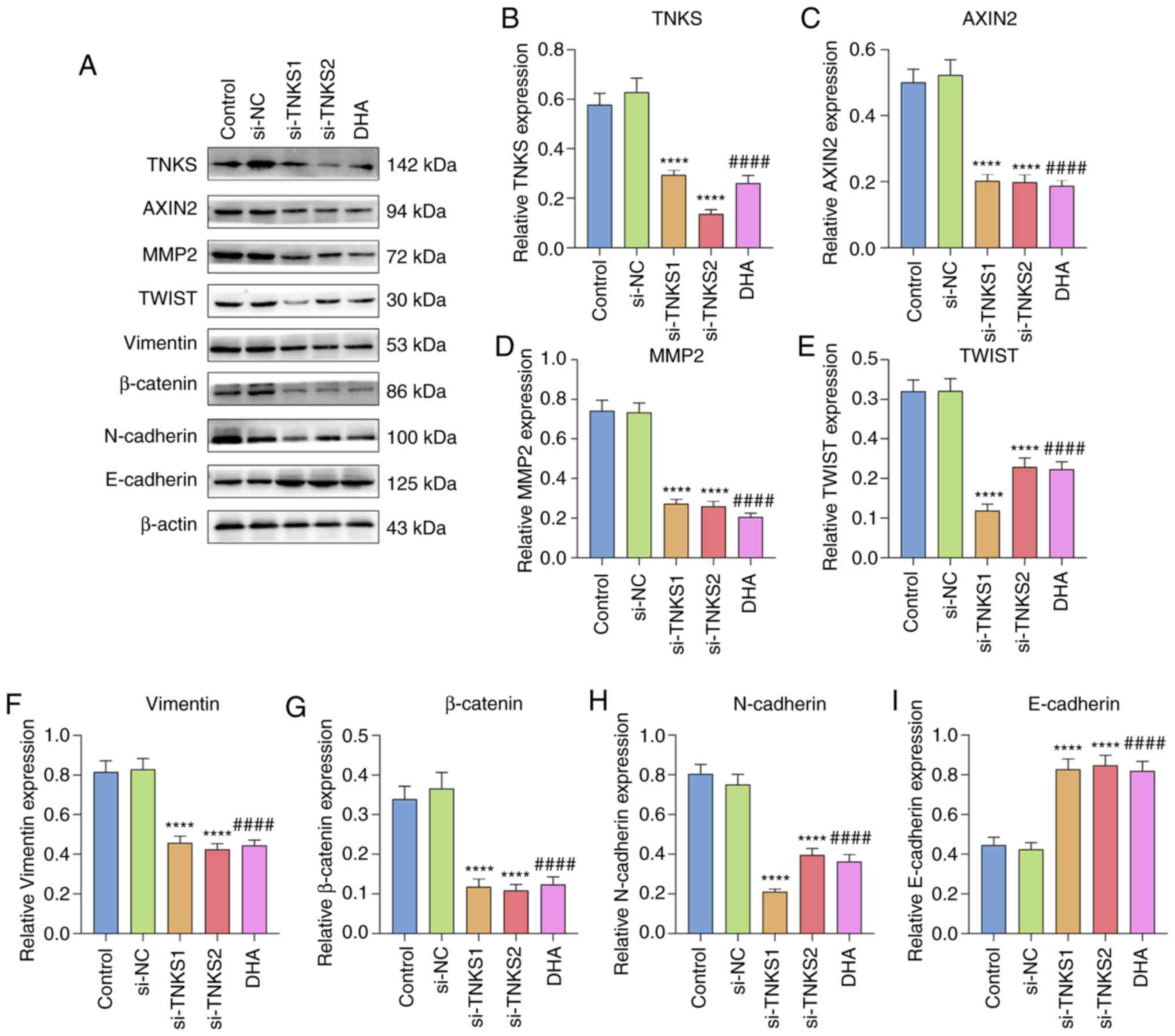 | Figure 6.TNKS knockdown and DHA suppress
activation of EMT and the Wnt/β-catenin pathway in gastric cancer.
(A) Western blot analysis was performed to detect the expression
levels of EMT- and Wnt/β-catenin pathway-related proteins in
gastric cancer cells transfected with si-TNKS1/si-TNKS2 or treated
with DHA. (B) TNKS, (C) AXIN2, (D) MMP2, (E) TWIST, (F) Vimentin,
(G) β-catenin, (H) N-cadherin and (I) E-cadherin expression levels
were quantified according to western blotting. ****P<0.0001 vs.
the si-NC group; ####P<0.0001 vs. the control group.
TNKS, Tankyrases; DHA, Dihydroartemisinin; EMT,
epithelial-to-mesenchymal transition; si, small interfering; MMP,
matrix metalloproteinase; NC, negative control. |
DHA inactivates the Wnt/β-catenin
pathway in gastric cancer partly via silencing TNKS
Immunofluorescence analysis was performed to detect
the expression levels of TNKS, AXIN2 and β-catenin in HGC-27 and
AGS cells. Consistent with western blotting, TNKS expression
significantly decreased following transfection with
si-TNKS1/si-TNKS2 or treatment with DHA in HGC-27 cells (all
P<0.0001; Fig. 7A and B).
Conversely, HLY78 treatment significantly increased TNKS
expression, which was ameliorated by DHA. In Fig. 7A and C, TNKS knockdown or DHA
significantly decreased AXIN2 expression in HGC-27 cells. AXIN2
expression was elevated following treatment with HLY78, which was
reversed following co-treatment with DHA (all P<0.0001).
β-catenin expression significantly decreased in HGC-27 cells
induced by TNKS knockdown or DHA (P<0.0001 or P<0.001;
Fig. 7A and D). Conversely, HLY78
significantly increased β-catenin expression, which was reversed
following DHA co-treatment. Similar results were observed in AGS
cells. Both TNKS knockdown and DHA decreased TNKS (P<0.05,
P<0.01 or P<0.001; Fig. 7A and
E), AXIN2 (all P<0.0; 001 Fig. 7A
and F) and β-catenin (P<0.01, P<0.001 or P<0.0001;
Fig. 7A and G) expression levels in
gastric cancer cells. Collectively, these results suggest that DHA
inactivates the Wnt/β-catenin pathway in gastric cancer partly by
silencing TNKS.
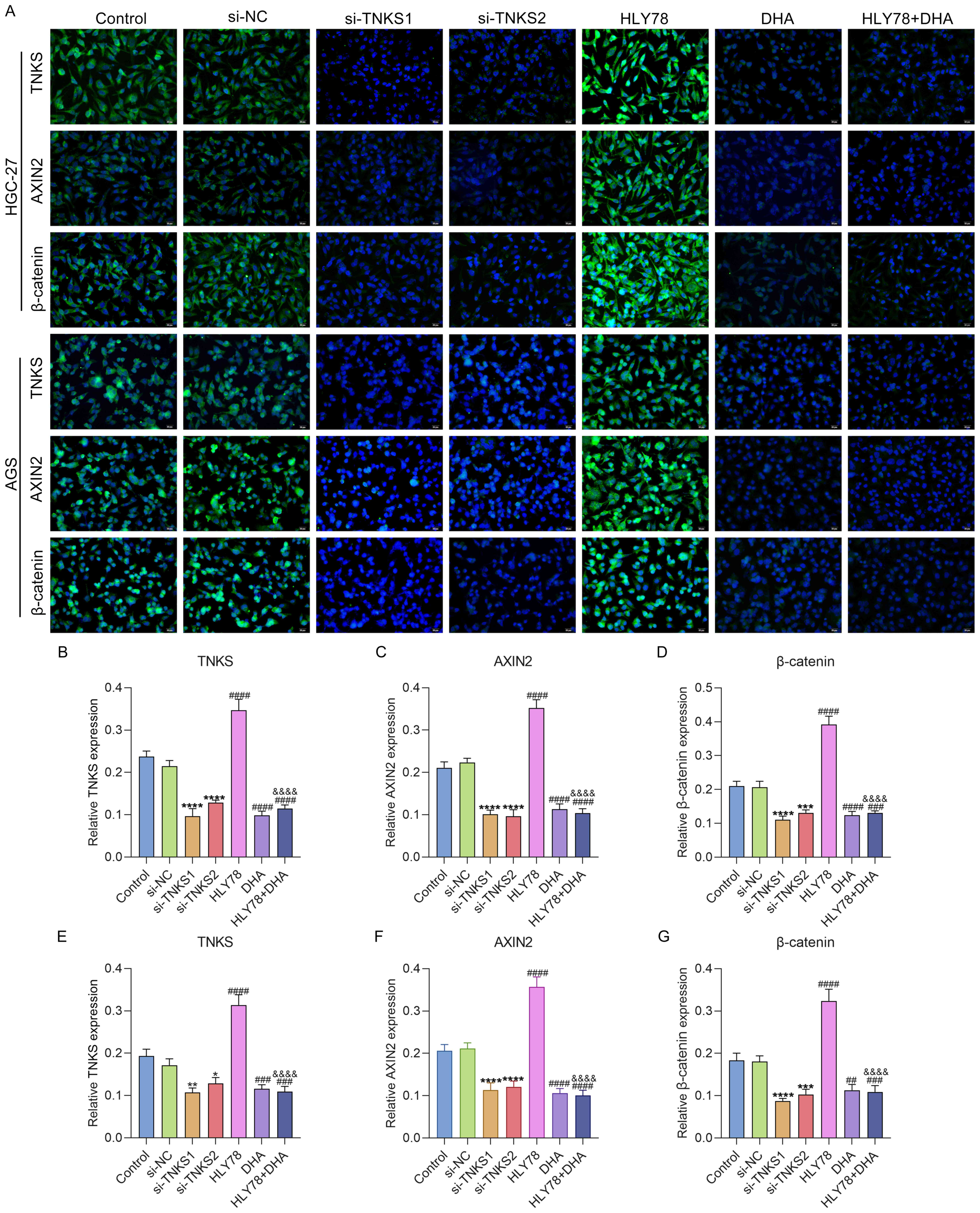 | Figure 7.TNKS knockdown and DHA inactivate the
Wnt/β-catenin pathway in gastric cancer. (A) Immunofluorescence
analysis of TNKS, AXIN2 and β-catenin in HGC-27 and AGS gastric
cancer cells treated with si-TNKS1/si-TNKS2, HLY78, DHA and HLY78 +
HLY78. (B) TNKS, (C) AXIN2 and (D) β-catenin expression levels were
quantified for HGC-27 cells. (E) TNKS, (F) AXIN2 and (G) β-catenin
expression levels were quantified for AGS cells. *P<0.05,
**P<0.01, ***P<0.001 and ****P<0.0001 vs. the si-NC group;
##P<0.01, ###P<0.001 and
####P<0.0001 vs. the control group;
&&&&P<0.0001 vs. the HLY78 group.
TNKS, Tankyrases; DHA, Dihydroartemisinin; si, small interfering;
NS, negative control. |
Discussion
The results of the present study demonstrated that
DHA and inhibition of TKNS suppressed proliferation, migration, the
EMT process, as well as the Wnt/β-catenin pathway in gastric
cancer. Following co-treatment with Wnt activator, HLY78, the
inhibitory effect of DHA was not affected. Inhibition of TNKS is
considered a therapeutic strategy for different types of cancer,
such as hepatocellular carcinoma (11) and gastric cancer (17). The results of the present study
demonstrate that DHA inhibited TNKS expression. Thus, DHA may be a
promising drug for the treatment of gastric cancer.
The EMT process and Wnt/β-catenin pathway contribute
to migration and invasion in gastric cancer (19,24,31). In
the present study, activation of the EMT process and Wnt/β-catenin
pathway were detected in gastric cancer tissues. The results
demonstrated that DHA inhibited activation of the Wnt/β-catenin
pathway and EMT process in gastric cancer. HLY78, a Wnt activator,
activated Wnt/β-catenin, as well as the EMT process, thereby
promoting proliferation and migration of gastric cancer cells
(32). Notably, DHA ameliorated the
HLY78-induced malignant transformation in gastric cancer cells,
suggesting that DHA may be a promising novel drug for the treatment
of gastric cancer. Vimentin, as a mesenchymal marker, promotes
gastric cancer cell migration and adhesion (19). AXIN2 protein functions in the
canonical Wnt pathway (33). TWIST
is associated with EMT process in gastric cancer (34). The expression levels of Vimentin,
AXIN2 and TWIST were suppressed following treatment of gastric
cancer cells with DHA. For gastric cancer, the loss of E-cadherin
expression may stimulate cells to transform into a more invasive
characteristic via the EMT process (19). The results of the present study
suggest that DHA can recover E-cadherin expression in gastric
cancer cells. MMPs are involved in tumor cell invasion and
migration, as well as degradation of the extracellular matrix
(35). MMP-2, an important member of
the MMPs family, is overexpressed in gastric cancer (36–38). Its
high expression can induce migration, as well as invasion in
gastric cancer cells (35). In the
present study, DHA distinctly decreased MMP2 expression in gastric
cancer cells, suggesting that DHA can ameliorate the invasion and
metastasis in gastric cancer. It has been reported that DHA can
suppress EMT formation in breast cancer cells (39) and esophageal cancer cells (40). Furthermore, treatment with DHA can
inhibit the proliferative ability of squamous cancer cells via the
Wnt/β-catenin pathway (41). Thus,
DHA inactivates EMT and the Wnt/β-catenin pathway in gastric cancer
cells.
TNKS inhibitor is considered a candidate drug for
inactivation of the Wnt/β-catenin process in cancers (42). It has been reported that TNKS induces
aerobic glycolysis and proliferation by activating the
Wnt/β-catenin pathway in ovarian cancer (43). Furthermore, its overexpression is
closely associated with poor clinical outcomes of patients with
colorectal cancer (44). However,
the role of TNKS in gastric cancer remains unclear. The results of
the present study demonstrated that TNKS expression was higher in
gastric cancer tissues compared with normal tissues. Furthermore,
TNKS knockdown suppressed the proliferation and migration,
Wnt/β-catenin, as well as the EMT process in gastric cancer cells.
Thus, TNKS inhibition may be used as a potential treatment strategy
for gastric cancer. Collectively, these results suggest that
treatment with DHA significantly inhibits TNKS expression in
gastric cancer, suggesting that DHA may be an inhibitor of TNKS.
However, further studies are required to validate the results
presented here.
In conclusion, the results of the present study
demonstrated that DHA or TNKS inhibition suppressed proliferation
and migration of gastric cancer cells. Furthermore, the
Wnt/β-catenin pathway and EMT process were inactivated following
treatment with DHA or TNKS knockdown. DHA distinctly decreased TNKS
expression, suggesting that TNKS may be a potential target of DHA.
However, the present study is not without limitations. First, the
therapeutic effects of DHA on gastric cancer need to be further
investigated in vivo. Secondly, whether TNKS is a direct
target of DHA requires further investigation. Thus, prospective
studies will aim to investigate the therapeutic effects of DHA on
gastric cancer and determine its underlying molecular
mechanisms.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Key R & D
plan of Shaanxi Province (grant no. 2020SF-236), the Project of
Hebei Medical Science Research Project (grant no. 20191127) and the
Science and Technology Research and Development Project of Yulin
City in 2020 (grant no. YF-2020-039).
Availability of data and materials
The datasets analyzed during the current study are
available from the corresponding author upon reasonable
request.
Authors' contributions
HM conceived and designed the present study. YM, PZ
and QZ performed most of the experiments, analyzed the data and
drafted the initial manuscript. XW, QM, XL and BC performed some
experiments and analyzed the data, and drafted and revised the
manuscript. HM and YM confirmed the authenticity of all the raw
data. All authors have read and approved the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Hospital of Yulin (Yulin, China; approval
no. 2018031) and written informed consent was provided by all
patients prior to the study start.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
DHA
|
Dihydroartemisinin
|
|
TNKS
|
Tankyrases
|
|
EMT
|
epithelial-to-mesenchymal
transition
|
|
MMPs
|
matrix metalloproteinases
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
Arnold M, Abnet CC, Neale RE, Vignat J,
Giovannucci EL, McGlynn KA and Bray F: Global burden of 5 major
types of gastrointestinal cancer. Gastroenterology.
159:335–349.e15. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Venerito M, Link A, Rokkas T and
Malfertheiner P: Review: Gastric cancer-Clinical aspects.
Helicobacter. 24 (Suppl 1):e126432019. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu J, Zhang Y, Jia R, Yue C, Chang L, Liu
R, Zhang G, Zhao C, Zhang Y, Chen C, et al: Anti-PD-1 antibody
SHR-1210 combined with apatinib for advanced hepatocellular
carcinoma, gastric, or esophagogastric junction cancer: An
open-label, dose escalation and expansion study. Clin Cancer Res.
25:515–523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Doi T, Shitara K, Naito Y, Shimomura A,
Fujiwara Y, Yonemori K, Shimizu C, Shimoi T, Kuboki Y, Matsubara N,
et al: Safety, pharmacokinetics, and antitumour activity of
trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug
conjugate, in patients with advanced breast and gastric or
gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet
Oncol. 18:1512–1522. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bando H, Doi T, Muro K, Yasui H, Nishina
T, Yamaguchi K, Takahashi S, Nomura S, Kuno H, Shitara K, et al: A
multicenter phase II study of TAS-102 monotherapy in patients with
pre-treated advanced gastric cancer (EPOC1201). Eur J Cancer.
62:46–53. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Shitara K, Bang YJ, Iwasa S, Sugimoto N,
Ryu MH, Sakai D, Chung HC, Kawakami H, Yabusaki H, Lee J, et al:
Trastuzumab deruxtecan in previously treated HER2-positive gastric
cancer. N Engl J Med. 382:2419–2430. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun X, Yang S, Feng X, Zheng Y, Zhou J,
Wang H, Zhang Y, Sun H and He C: The modification of ferroptosis
and abnormal lipometabolism through overexpression and knockdown of
potential prognostic biomarker perilipin2 in gastric carcinoma.
Gastric Cancer. 23:241–259. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang Q, Lu P, Wang T, Zheng Q, Li Y, Leng
SX, Meng X, Wang B, Xie J and Zhang H: Sitagliptin affects gastric
cancer cells proliferation by suppressing Melanoma-associated
antigen-A3 expression through Yes-associated protein inactivation.
Cancer Med. 9:3816–3828. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guo HL, Zhang C, Liu Q, Li Q, Lian G, Wu
D, Li X, Zhang W, Shen Y, Ye Z, et al: The Axin/TNKS complex
interacts with KIF3A and is required for insulin-stimulated GLUT4
translocation. Cell Res. 22:1246–1257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Huang J, Qu Q, Guo Y, Xiang Y and Feng D:
Tankyrases/β-catenin signaling pathway as an anti-proliferation and
anti-metastatic target in hepatocarcinoma cell lines. J Cancer.
11:432–440. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Liu S, Mickanin C, Feng Y,
Charlat O, Michaud GA, Schirle M, Shi X, Hild M, Bauer A, et al:
RNF146 is a poly(ADP-ribose)-directed E3 ligase that regulates axin
degradation and Wnt signalling. Nat Cell Biol. 13:623–629. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozaki Y, Matsui H, Asou H, Nagamachi A,
Aki D, Honda H, Yasunaga S, Takihara Y, Yamamoto T, Izumi S, et al:
Poly-ADP ribosylation of Miki by tankyrase-1 promotes centrosome
maturation. Mol Cell. 47:694–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chi NW and Lodish HF: Tankyrase is a
golgi-associated mitogen-activated protein kinase substrate that
interacts with IRAP in GLUT4 vesicles. J Biol Chem.
275:38437–38444. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cook BD, Dynek JN, Chang W, Shostak G and
Smith S: Role for the related poly(ADP-Ribose) polymerases
tankyrase 1 and 2 at human telomeres. Mol Cell Biol. 22:332–342.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pai SG, Carneiro BA, Mota JM, Costa R,
Leite CA, Barroso-Sousa R, Kaplan JB, Chae YK and Giles FJ:
Wnt/beta-catenin pathway: Modulating anticancer immune response. J
Hematol Oncol. 10:1012017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gao J, Zhang J, Long Y, Tian Y and Lu X:
Expression of tankyrase 1 in gastric cancer and its correlation
with telomerase activity. Pathol Oncol Res. 17:685–690. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yue B, Song C, Yang L, Cui R, Cheng X,
Zhang Z and Zhao G: METTL3-mediated N6-methyladenosine modification
is critical for epithelial-mesenchymal transition and metastasis of
gastric cancer. Mol Cancer. 18:1422019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bure IV, Nemtsova MV and Zaletaev DV:
Roles of E-cadherin and noncoding RNAs in the
epithelial-mesenchymal transition and progression in gastric
cancer. Int J Mol Sci. 20:2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ji L, Zhang B and Zhao G: Liver X receptor
α (LXRα) promoted invasion and EMT of gastric cancer cells by
regulation of NF-κB activity. Hum Cell. 30:124–132. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu X, Chen L, Liu L and Niu X:
EMT-mediated acquired EGFR-TKI resistance in NSCLC: Mechanisms and
strategies. Front Oncol. 9:10442019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li S, Cong X, Gao H, Lan X, Li Z, Wang W,
Song S, Wang Y, Li C, Zhang H, et al: Tumor-associated neutrophils
induce EMT by IL-17a to promote migration and invasion in gastric
cancer cells. J Exp Clin Cancer Res. 38:62019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wei Y, Zhang F, Zhang T, Zhang Y, Chen H,
Wang F and Li Y: LDLRAD2 overexpression predicts poor prognosis and
promotes metastasis by activating Wnt/β-catenin/EMT signaling
cascade in gastric cancer. Aging (Albany NY). 11:8951–8968. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li G, Su Q, Liu H, Wang D, Zhang W, Lu Z,
Chen Y, Huang X, Li W, Zhang C, et al: Frizzled7 promotes
epithelial-to-mesenchymal transition and stemness via activating
canonical Wnt/β-catenin pathway in gastric cancer. Int J Biol Sci.
14:280–293. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang S, Yin J, Chen D, Nie F, Song X, Fei
C, Miao H, Jing C, Ma W, Wang L, et al: Small-molecule modulation
of Wnt signaling via modulating the Axin-LRP5/6 interaction. Nat
Chem Biol. 9:579–585. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo H, Vong CT, Chen H, Gao Y, Lyu P, Qiu
L, Zhao M, Liu Q, Cheng Z, Zou J, et al: Naturally occurring
anti-cancer compounds: Shining from Chinese herbal medicine. Chin
Med. 14:482019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fan HN, Zhu MY, Peng SQ, Zhu JS, Zhang J
and Qu GQ: Dihydroartemisinin inhibits the growth and invasion of
gastric cancer cells by regulating cyclin D1-CDK4-Rb signaling.
Pathol Res Pract. 216:1527952020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Su T, Li F, Guan J, Liu L, Huang P, Wang
Y, Qi X, Liu Z, Lu L and Wang D: Artemisinin and its derivatives
prevent Helicobacter pylori-induced gastric carcinogenesis
via inhibition of NF-κB signaling. Phytomedicine. 63:1529682019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
In H, Solsky I, Palis B, Langdon-Embry M,
Ajani J and Sano T: Validation of the 8th edition of the AJCC TNM
staging system for gastric cancer using the national cancer
database. Ann Surg Oncol. 24:3683–3691. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao W, Deng C, Han Q, Xu H and Chen Y:
Carvacrol may alleviate vascular inflammation in diabetic db/db
mice. Int J Mol Med. 46:977–988. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu J, Huang C, Peng C, Xu F, Li Y, Yutaka
Y, Xiong B and Yang X: Stromal fibroblast activation protein alpha
promotes gastric cancer progression via epithelial-mesenchymal
transition through Wnt/β-catenin pathway. BMC Cancer. 18:10992018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Guo X, Zhang L, Fan Y, Zhang D, Qin L,
Dong S and Li G: Oxysterol-binding protein-related protein 8
inhibits gastric cancer growth through induction of er stress,
inhibition of wnt signaling, and activation of apoptosis. Oncol
Res. 25:799–808. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mazzoni SM and Fearon ER: AXIN1 and AXIN2
variants in gastrointestinal cancers. Cancer Lett. 355:1–8. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu AN, Zhu ZH, Chang SJ and Hang XS:
Twist expression associated with the epithelial-mesenchymal
transition in gastric cancer. Mol Cell Biochem. 367:195–203. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang T, Hou J, Jian S, Luo Q, Wei J, Li Z,
Wang X, Bai P, Duan B, Xing J and Cai J: miR-29b negatively
regulates MMP2 to impact gastric cancer development by suppress
gastric cancer cell migration and tumor growth. J Cancer.
9:3776–3786. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jiang XJ, Lin J, Cai QH, Zhao JF and Zhang
HJ: CDH17 alters MMP-2 expression via canonical NF-κB signalling in
human gastric cancer. Gene. 682:92–100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ni YJ, Lu J and Zhou HM: Propofol
suppresses proliferation, migration and invasion of gastric cancer
cells via regulating miR-29/MMP-2 axis. Eur Rev Med Pharmacol Sci.
23:8606–8615. 2019.PubMed/NCBI
|
|
38
|
Zhao L, Niu H, Liu Y, Wang L, Zhang N,
Zhang G, Liu R and Han M: LOX inhibition downregulates MMP-2 and
MMP-9 in gastric cancer tissues and cells. J Cancer. 10:6481–6490.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ju RJ, Cheng L, Peng XM, Wang T, Li CQ,
Song XL, Liu S, Chao JP and Li XT: Octreotide-modified liposomes
containing daunorubicin and dihydroartemisinin for treatment of
invasive breast cancer. Artif Cells Nanomed Biotechnol. 46 (Suppl
1):616–628. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen X, He LY, Lai S and He Y:
Dihydroartemisinin inhibits the migration of esophageal cancer
cells by inducing autophagy. Oncol Lett. 20:942020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hui HY, Wu N, Wu M, Liu Y, Xiao SX and
Zhang MF: Dihydroartemisinin suppresses growth of squamous cell
carcinoma A431 cells by targeting the Wnt/β-catenin pathway.
Anticancer Drugs. 27:99–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Solberg NT, Waaler J, Lund K, Mygland L,
Olsen PA and Krauss S: TANKYRASE inhibition enhances the
antiproliferative effect of PI3K and EGFR inhibition, mutually
affecting β-CATENIN and AKT signaling in colorectal cancer. Mol
Cancer Res. 16:543–553. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang HY, Shen JX, Wang Y, Liu Y, Shen DY
and Quan S: Tankyrase promotes aerobic glycolysis and proliferation
of ovarian cancer through activation of Wnt/β-catenin signaling.
Biomed Res Int. 2019:26863402019.PubMed/NCBI
|
|
44
|
Ma Z, Han C, Xia W, Wang S, Li X, Fang P,
Yin R, Xu L and Yang L: circ5615 functions as a ceRNA to promote
colorectal cancer progression by upregulating TNKS. Cell Death Dis.
11:3562020. View Article : Google Scholar : PubMed/NCBI
|