Introduction
Colorectal cancer (CRC), including colon and rectal
cancer, is the third most common cancer in both males and females,
with ~1.36 million new cases per year and the fourth leading cause
of cancer-related deaths worldwide (1). With the development of the economy and
changes in the dietary structure of individuals, the morbidity and
mortality rates of CRC have increased in recent years (2–4). The
5-year survival rate of CRC is only 50–60% (5). At present, the survival time of
patients has increased with the development of science and
technology, and the improvement of medical standards; however,
patients still have poor prognosis and distant metastasis. The
quality of life is also affected in patients with CRC (6). Multidisciplinary comprehensive
treatment is an important treatment principle for CRC, including
surgery, radiation therapy, chemotherapy, immunotherapy and Chinese
traditional treatment (7). Surgical
treatment to remove the lesion is the preferred treatment for CRC,
which also reduces the symptoms, prolongs the life span of the
patient and improves the quality of life (8). As the early symptoms of CRC are not
obvious, once clinical symptoms appear, the patient is already in
late stage cancer, therefore surgery is not suitable and medical
treatment is administered instead (9). Traditional anticancer drugs have low
selectivity and high toxicity, whereas developing new drugs is time
consuming and is associated with high costs. Thus, in recent years,
the idea of ‘new use of old drugs’ provided a new research
direction for the treatment of CRC (10).
Penicillin is an old and widely used antibiotic,
with low toxicity and high efficiency (11). It can be used for pharyngitis,
scarlet fever, cellulitis caused by hemolytic streptococcus,
pneumonia caused by pneumococcus, otitis media, meningitis, tetanus
and gas gangrene caused by clostridium (12). The mechanism of action of penicillin
is to hinder the formation of the bacterial wall and destroy the
structure of the bacterial wall. As human cells have no cell wall,
penicillin has the least side effects among various types of
antibiotics (13). In addition,
penicillin has almost no toxic effects on the human body, and
previous studies have used penicillin in human experimental
studies, indicating that penicillin is harmless to human cells
(14,15). The degradation product of penicillin,
as a hapten, which can be combined with protein to produce IgE,
which is the basis of immediate allergic reactions (16). There are few studies investigating
the use of antibiotics in antitumor treatment alone (17,18), and
no reports of the anti-cancer effect of penicillin. In the present
study, penicillin was used to treat CRC cells to observe their
effects on the growth, migration and invasion.
The mitochondrion is a double-membraned organelle
(19). Its main function is to
produce ATP via oxidative phosphorylation, to provide energy for
eukaryotic cells (20). The second
function of mitochondria is associated with apoptosis following
changes in mitochondrial membrane permeability (21). Under the stimulation of certain cell
death signals, such as reactive oxygen species (ROS. and DNA
damage, the outer membrane permeability of mitochondria increases,
and a series of changes occur, including cytochrome c
release, reduction of the mitochondrial transmembrane potential and
a change in the redox state in the cell (22). As a result, the mitochondrial
respiratory chain and electron transfer are blocked, and the energy
metabolism of the cell is impaired (23). Finally, cytochrome c and other
pro-apoptotic proteins (Bax and Bid) are released into the
cytoplasm, promoting apoptosis (24).
Autophagy refers to a phenomenon in which cells use
lysosomes to degrade misfolded proteins or damaged organelles to
maintain a normal intracellular environment, which is common in
eukaryotic cells (25). Studies have
found that autophagy has a two-way role in the survival and death
of tumor cells (26,27). Moderate autophagy can clear damaged
organelles, so that cells can respond correctly to external stimuli
and damage, and survive. However, excessive autophagy can promote
cell apoptosis and cause cell damage (28). During autophagy,
microtubule-associated protein 1 light chain 3 (LC3) enzymatically
decomposes a small piece of polypeptide and becomes LC3-I, after
which LC3-I binds with phosphatidylethanolamine to become LC3-II.
Therefore, the ratio of LC3-II/LC3-I is often used in experimental
studies to assess the level of autophagy (29). In another study, the mRFP-GFP-LC3
dual-fluorescence autophagy system was used to track changes in LC3
and the autophagic flux. The level of autophagy activity and
autophagic flux was evaluated by observing the bright spots of
green fluorescent protein (FP) and monomeric red FP under
fluorescence and confocal microscopy (30). Observation of autophagosomes by
transmission electron microscopy is the gold standard for
determining changes in autophagy. Under electron microscopy,
autophagosomes appear as double-layered or multi-layered vacuoles
containing cytoplasmic components, including mitochondria,
endoplasmic reticulum, and ribosomes (31).
In the present study the effect of penicillin on the
growth and metastasis of CRC cells was investigated first, then the
underlying mechanism. The results provide a new experimental basis
and research direction for the clinical treatment of CRC.
Materials and methods
Cell culture and treatment
The human CRC cell lines, HCT116, HT29 and SW620
were purchased from the Cell Bank of Shanghai Chinese Academy of
Sciences. Long-acting penicillin (powder was purchased from North
China Pharmaceutical Group Corp and dissolved in PBS. The cells
were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.),
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.
and 1% (Penicillin/Streptomycin) at 37°C in a humidified incubator
with 5% CO2.
Western blot analysis
The HCT-116 cell line was washed twice with PBS and
lysed in RIPA buffer (Beijing Solarbio Science and Technology Co.,
Ltd.) containing 2 mM phenylmethylsulfonyl fluoride (PMSF). Protein
concentration was determined using the bicinchoninic acid method
(CoWin Biosciences). The proteins were separated using 12% SDS-PAGE
and transferred to 0.45 µm PVDF membranes, blocked with 10% skimmed
milk at room temperature for 2 h. The membrane was then incubated
with the following primary antibodies overnight at 4°C: β-actin
(1:1,000; cat. no. 4ab020185; 4A Biotech Co., Ltd.), LC3 (1:1,000;
cat. no. NB100-2220; Novus Biologicals), cytochrome c
(1:1,000; cat. no. NB100-56503; Novus Biologicals), cleaved
caspase-3 (1:1,000; cat. no. 9661; Cell Signaling Technology,
Inc.), caspase-3 (1:1,000; cat. no. 14220; Cell Signaling
Technology, Inc.), COX4 (1:1,000; cat. no. 4844; Cell Signaling
Technology, Inc.). Then, the membrane was incubated with
HRP-labeled secondary antibodies [(goat anti-rat, cat. no. A0192;
goat anti-rabbit, (cat. no. A0208) (both 1:10,000) (both from
Beyotime Institute of Biotechnology)] at room temperature for 1 h.
The proteins bands were detected with a gel imaging and analysis
system (Tanon Scuence and Technology Co., Ltd.). Densitometry was
performed using ImageJ software (National Institutes of Health).
The HCT-116 cells were seeded into 6-well plates, at a density of
5.0×105, cells/well in 2 ml medium, then divided into
four groups: Control, 10 mM 3-methyladenine (3′MA; MedChemExpress),
500 U/ml penicillin and penicillin+3-MA groups. After the cells
were incubated for 24 h, the protein was extracted for western blot
analysis.
Mitochondrial isolation
The HCT116 cell line (1×107) was
collected and washed with PBS, and cytochrome c release was
determined using a mitochondrial isolation kit (Beyotime Institute
of Biotechnology). First, Mitochondria Isolation Solution
containing PMSF (Beijing Soleibao Technology Co., Ltd., China) was
added to the cells and they were incubated in an ice bath for 15
min. A glass homogenizer was used to grind the cells followed by
centrifugation at 1,000 × g for 10 min at 4°C. The liquid
supernatant was moved to a fresh tube and centrifuged again at
11,000 × g for 10 min at 4°C. The sediment was mixed with
Mitochondrial Lysate Solution to obtain the mitochondrial proteins.
The supernatant was centrifuged at 12,000 × g for 20 min at 4°C to
obtain cytosolic proteins, then analyzed using western blot
analysis.
Cell viability assay
Viability of the HCT-116, HT-29 and SW620 cell lines
was determined using a MTT assay at the indicated times. The
HCT-116, HT29 and SW620 cell lines (2×103 cells/well)
were seeded in 96-well plates (Corning Inc.) and cultured for 24 h
in RPMI-1640 supplemented with 10% FBS. The cells were then treated
with penicillin (0, 50, 100, 200, 500 and 1,000 U/ml) for 1–4 days,
then 10 µl MTT (Sigma-Aldrich, Merck KGaA) solution (5 mg/ml in
PBS) was added to each well. The plates were incubated for another
3–4 h at 37°C. Intracellular formazan crystals were dissolved by
adding 100 µl dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) to
each well. Cell proliferation was determined by measuring the
absorbance at 490 nm with a spectrophotometer (Multiskan MK3;
Thermo Fisher Scientific, Inc.). The experiments were performed 3
times.
Wound healing assay
For the wound healing assay, the HCT-116, HT-29 and
SW620 cell lines (3–5×105 cells/well) were seeded in
6-well plates and cultured until 100% confluence. The monolayer was
carefully scratched with a sterile 200 µl pipette tip. Floating
cells were removed by a gentle wash with cold PBS, then cells were
cultured with RPMI-1640 containing 2% FBS and incubated with or
without long-acting penicillin (500 U/ml) for 72 h. Representative
images were captured under an inversion fluorescence microscope and
the gap closures were quantitated using the ImageJ software
(v1.8.0.112; National Institutes of Health). All the experiments
were performed at least 3 times.
Transwell invasion assay
Transwell invasion assays were performed using
Transwell chambers with 8-µm pore size filter membranes (EMD
Millipore). The polycarbonate filter was coated with Matrigel (30
µg/well; BD Matrigel Matrix) at 37°C for 1 h. Then, the chambers
were inserted into 24-well culture plates. The HCT-116, HT-29 and
SW620 cell lines were starved overnight in assay media (RPMI-1640
without FBS), then single-cell suspensions were seeded into the
upper chamber (5×104 cells/well in RPMI-1640 without
FBS). The lower chamber was filled with 600 µl RPMI-1640 containing
10% FBS. Following incubation at 37°C for 24 h, the non-invaded
cells on the upper side of the filter were removed with a cotton
swab. The invaded cells were fixed in methanol for 30 min at room
temperature, stained with 0.1% crystal violet at room temperature
for 15 min, and observed under a fluorescent microscope (Olympus
corporation at ×100 magnification. All the experiments were
performed at least 3 times.
Immunofluorescence
The HCT-116 cells were seeded at 1×104
cells/ml in an 8-well plate and transfected with the GFP-RFP-LC3
lentivirus after cell adherence. Tandem fluorescent-tagged LC3
(GFP-RFP-LC3) lentiviral vector was purchased from Shanghai
GeneChem Co., Ltd. The cells were treated with long-acting
penicillin (500 U/ml) for 1 day following transfection for 72 h,
then observed under a confocal microscope (Olympus Corp.). After
the cells were transfected with LC3-GFP-RFP lentivirus, LC3 had
both green and red colors in the cytoplasm, but only showed green
puncta when in the autophagolysosomes. All the experiments were
performed at least 3 times.
Flow cytometry
Apoptosis of the HCT-116 cell line was determined
using the Annexin V-PE Apoptosis Detection kit (BD Biosciences).
Briefly, the cells were seeded into 6-well plates, then treated
with long-acting penicillin (0, 50, 100, 200 and 500 U/ml) at 37°C
for 24 h. Following which, the cells were washed twice with cold
PBS, then resuspended in 1X binding buffer. A total of 100 µl cell
suspension was transferred to a 1.5 ml culture tube, to which 1 µl
Annexin V-PE and 1 µl 7-AAD were added. The mixture was incubated
for 15 min at room temperature in the dark. The results were
immediately analyzed using a FACS flow cytometer (FACSARIA II; BD
Biosciences) and the FlowJo software (v10.0.7r2; FlowJo LLC). All
experiments were performed at least 3 times.
The cell cycle staining solution (MultiSciences
Biotech Co., Ltd.) was used to analyze the cell cycle of the cells.
The HCT-116 cell line was seeded (5×105 cells/well) into
6-well plates, then treated with long-acting penicillin (0, 50,
100, 200 and 500 U/ml) at 37°C for 24 h. The treated and untreated
cells were washed twice in cold PBS. After centrifugation at 2,000
× g for 3 min at 4°C, the washed pellets were resuspended in 300 µl
DNA staining solution, containing 3 µl permeabilization solution
and incubated at room temperature for 30 min in the dark. DNA
content was analyzed using a flow cytometer (FACSARIA II; BD
Biosciences). The results were analyzed using the cell cycle
analysis software ModFit LT v4.1 (Verity Software House, Inc.,).
All the experiments were performed at least 3 times.
Electron microscopy
The HCT-116 cell line was fixed in 4%
paraformaldehyde in 0.1 M phosphate buffer for 4 h at 4°C. The
cells were washed with 0.1 M phosphate buffer and post-fixed in 1%
osmium tetroxide for 2 h at 4°C. Then, the cells were dehydrated
with ethanol and embedded in Epon-812 resin, and, finally,
polymerized for 2 days at 65°C. Ultrathin sections (70 to 90 nm)
were stained with uranyl acetate and lead citrate at room
temperature for 30 min. The ultrathin sections were observed under
a Hitachi-7000 electron microscope (Hitachi Ltd.).
Energy metabolism analysis of living
cell mitochondria
The HCT-116 cell line was transferred at a density
of 1×104 cells/well in unbuffered DMEM (Gibco; Thermo
Fisher Scientific, Inc.) at pH 7.4 to an XF24 Seahorse assay plate
to measure the mitochondrial oxygen consumption rate (OCR) with an
XF analyzer (XF24; Seahorse Bioscience, Inc.) at different time
points (0, 20, 40, 60 and 80 min). The cells were incubated at 37°C
without CO2 for 1 h for equilibration before initiating
the assay. To measure bioenergetics, the basic OCR was measured
first. The cells were treated with oligomycin (1 µmol/l) to inhibit
ATP synthase, then carbonyl cyanide was added to
trifluoromethoxyphenyl hydr (1 µmol/l) to produce maximum uncoupled
respiration. Non-mitochondrial respiration was determined by adding
rotenone (0.5 µM) and antimycin A (0.5 µM). Under these
well-defined conditions, the effects of penicillin on the
mitochondrial basal respiration, ATP-linked respiration, maximum
respiration capacity and reserve respiration capacity of the CRC
cells was analyzed.
Statistical analysis
SPSS v19.0 (IBM Corp.) was used for data analysis
and GraphPad Primer v8 (GraphPad Software, Inc.) was used for graph
generation. Data conforming to the normal distribution are
presented as the mean ± SD. An unpaired t-test was used to compare
two groups and one-way ANOVA followed by Bonferroni's post hoc test
was used to compare >2 groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
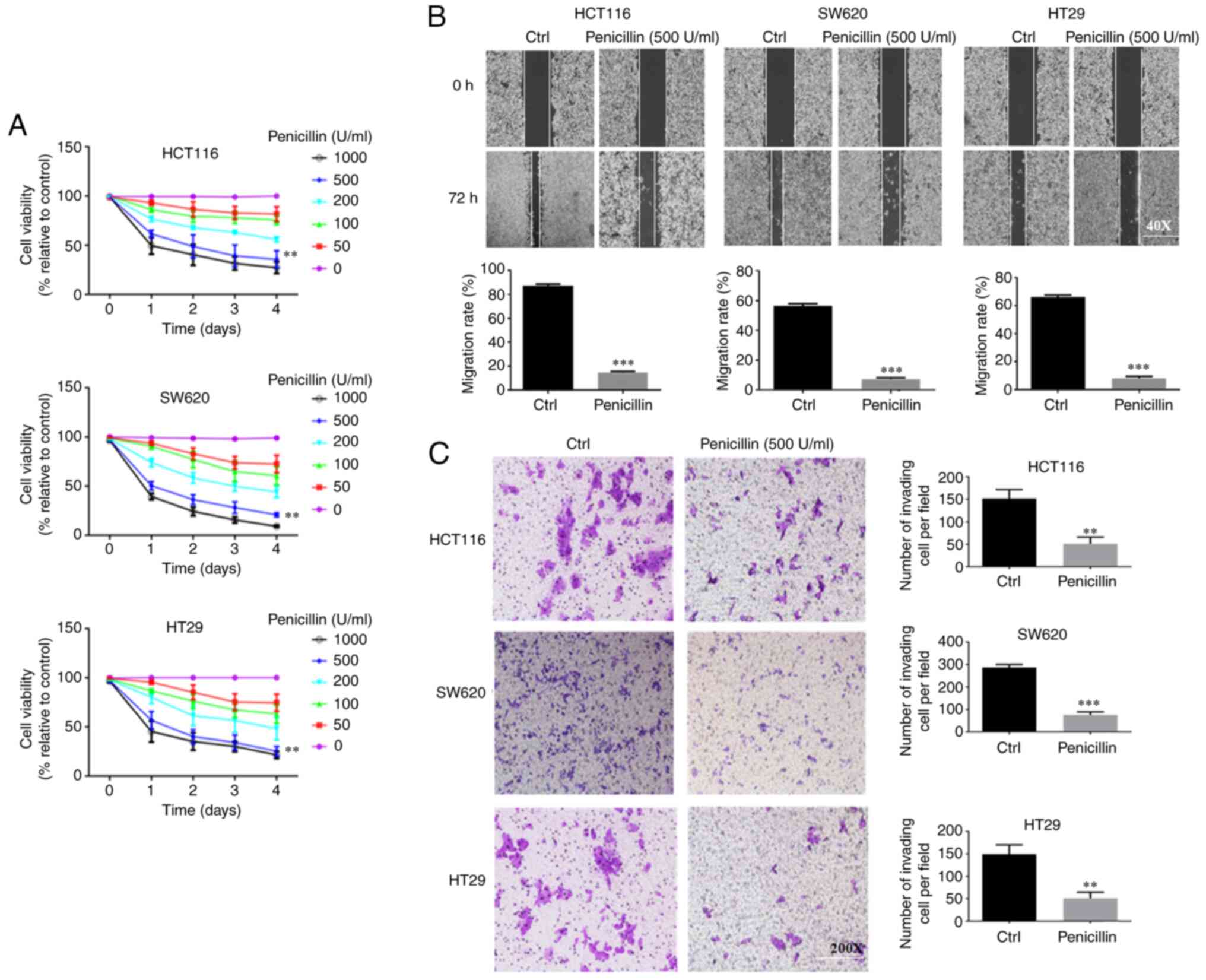
Penicillin has an inhibitory effect on
the growth, migration and invasion of the CRC cell lines
First, 0–1,000 U/ml long-acting penicillin was used
to treat the HCT-116, HT29 and SW620 cell lines to determine its
effect on cell growth. As shown in Fig.
1A, as the concentration of long-acting penicillin increased,
the activity of the CRC cells gradually weakened. In addition, cell
viability decreased after the first day, and 500 U/ml was the most
effective inhibition concentration. At 1,000 U/ml, the inhibitory
effect on cells began to stabilize. The results of the three CRC
cell lines showed the same trend.
Next, the most effective inhibitory concentration of
long-acting penicillin (500 U/ml) was used to treat the CRC cell
lines to determine its effect on the migration and invasion
abilities of the CRC cell lines. As shown in Fig. 1B, at 72 h, the control group of the
three different CRC cell lines had migrated substantially toward
the middle of the scratch, while the penicillin-treated cells
migrated significantly less compared with that in the control
group. As shown in Fig. 1C, for all
three cell lines, the control group had more invading cells
compared with that in the penicillin group. Thus, penicillin could
inhibit the migration and invasion abilities of the CRC cell
lines.
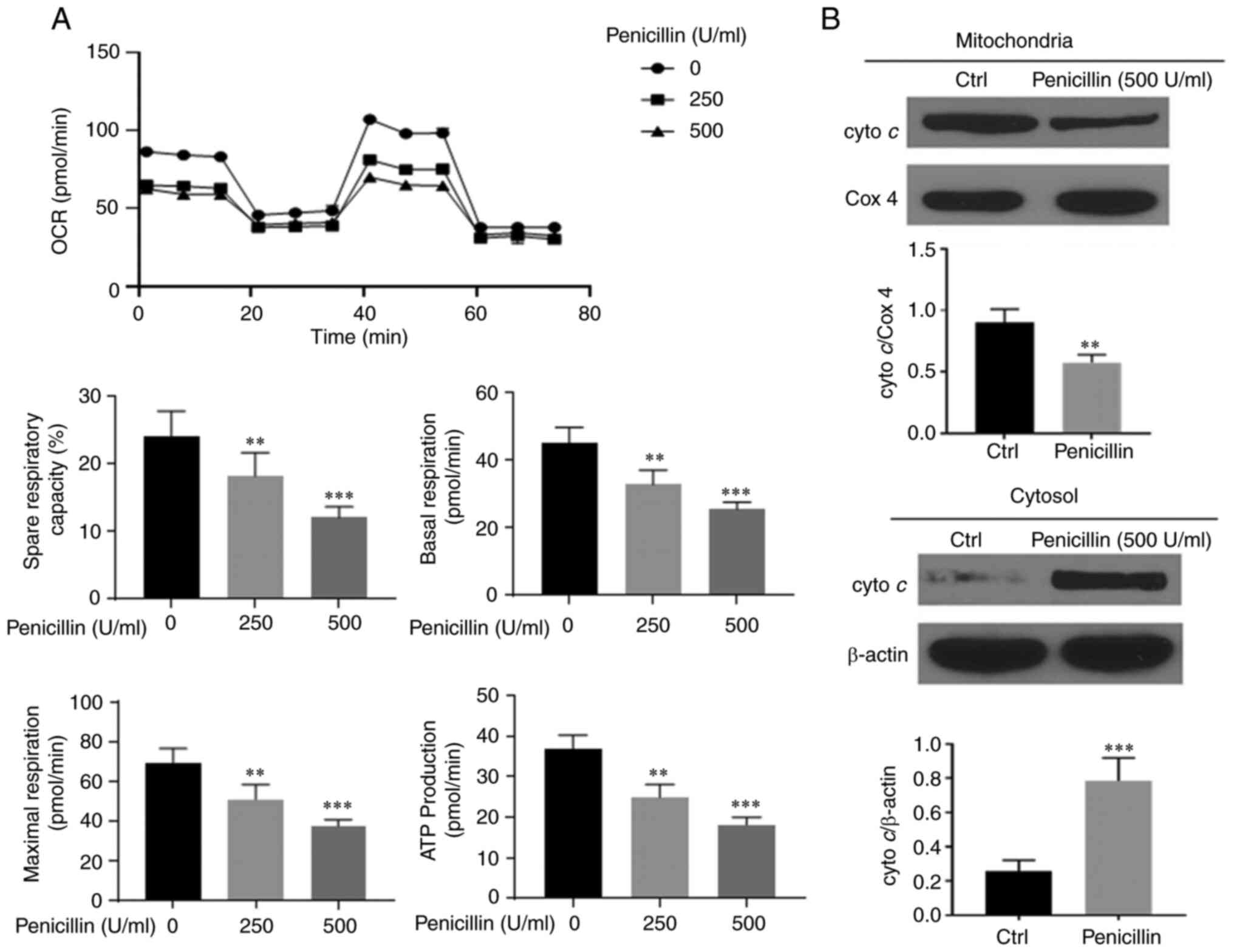
Penicillin disrupts mitochondrial
function of the HCT-116 cell line
To investigate whether penicillin caused
dysregulation of mitochondrial energy metabolism in the HCT-116
cell line, a mitochondrial energy metabolism experiment was
performed and the results are shown in Fig. 2A. After treatment of the HCT-116
cells with long-acting penicillin (250 and 500 U/ml) for 24 h, the
entire OCR curve of the HCT-116 cell line mitochondrial aerobic
respiration shifted downward. A significant change was observed
even at a 250 U/ml. In addition, inhibition was observed for the
main parameters of mitochondrial aerobic respiration, including
basal respiration capacity, ATP production, maximum respiration
capacity and respiration potential. To further determine whether
dysfunction of the mitochondria in the HCT-116 cell line occurred,
western blot analysis was used to measure cytochrome c
protein expression and the results are shown in Fig. 2B. After the HCT116 cell line was
treated with 500 U/ml penicillin, the cytochrome c protein
expression level in the mitochondria and the cytoplasm was
measured. The results showed that the protein expression level of
cytochrome c in the mitochondria and cytoplasm decreased and
increased, respectively indicating that cytochrome c moved
from the mitochondria into the cytoplasm. Thus, penicillin could
cause energy metabolism disorders in the mitochondria of CRC cell
lines.
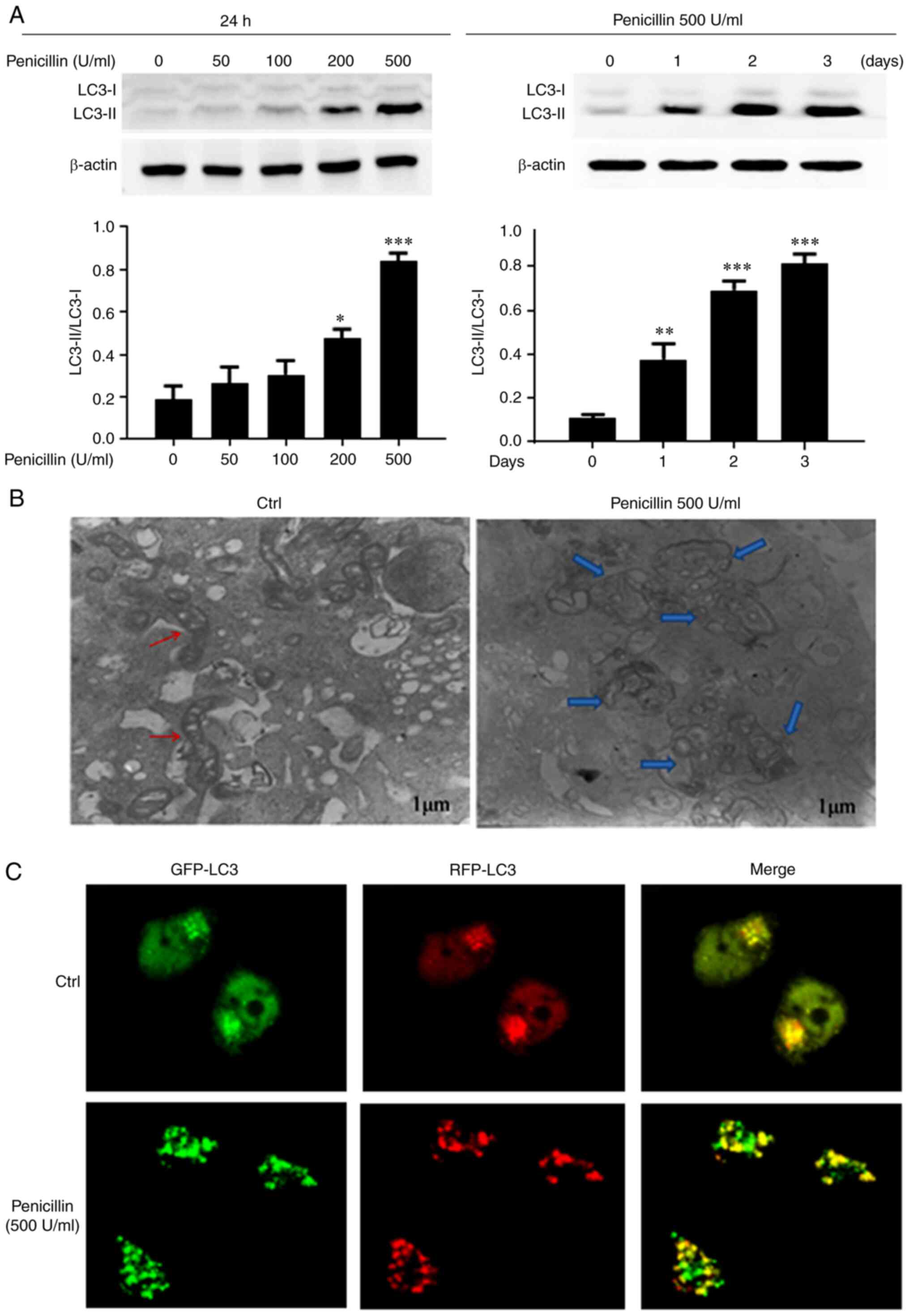
Penicillin induces autophagy in the
HCT-116 cell line
To investigate the effect of penicillin on autophagy
in the HCT-116 cell line, western blot analysis of
autophagy-related proteins, transmission electron microscope and
LC3 autophagy lentivirus were used to examine the level of
autophagy. First, different concentrations of long-acting
penicillin (0, 50, 100, 200, and 500 U/ml) were used to treat the
HCT-116 cell line for 24 h, then long-acting penicillin at 500 U/ml
was also added to the cells for different periods of time (0, 1, 2
and 3 days) to observe the protein expression level of
autophagy-related protein, LC3. As shown in Fig. 3A, penicillin increased the conversion
of cytoplasmic LC3-I to membrane-type LC3-II and the expression
level of LC3-II/LC3-I increased with the increase in penicillin
concentration, showing dose dependence.
Transmission electron microscopy was also used to
further observe changes in autophagosomes after penicillin
treatment of the HCT-116 cell line. As shown in Fig. 3B, after the HCT-116 cell line was
treated with 500 U/ml long-acting penicillin for 24 h,
autophagosomes with double membrane structure (blue arrows) were
observed under the electron microscope, mainly mitochondrial
swelling and irregular mitochondrial crest. The control group
showed normal mitochondria (red arrows), and no autophagosomes.
Therefore, penicillin could induce autophagy in the HCT-116 cell
line and could damage the mitochondrial structure of CRC cells.
As shown in Fig. 3C,
after treating the HCT-116 cell line with 500 U/ml long-acting
penicillin for 24 h, GFP-LC3 green fluorescent spots, RFP-LC3 red
fluorescent spots, and RFP-LC3 and GFP-LC3 double-positive
fluorescent spots all increased, further demonstrating that
following penicillin treatment of the HCT-116 cells, intracellular
autophagosomes and autophagy increased.
Effects of penicillin on the cell
cycle and apoptosis in the HCT116 cell line
Next, flow cytometry was used to examine the effect
of penicillin on the cell cycle and cell apoptosis in the HCT-116
cell line. Different concentrations of long-acting penicillin (0,
50, 100, 200, and 500 U/ml) were used to treat the HCT-116 cell
line for 24 h and the results are shown in Fig. 4. Penicillin significantly increased
the number of HCT-116 cells in the G1 phase and
decreased the number of cells in the S phase, indicating that
penicillin could block cell cycle progression in the HCT-116 cell
line at the G1 phase. A concentration dependence was
also observed. In addition, after treatment with long-acting
penicillin, the percentage of early apoptotic cells increased
significantly. Furthermore, different concentrations of penicillin
(0, 100, 200, 500 U/ml) were added to the HCT-116 cell line for 24
h, then western blot analysis was used to detect the expression of
apoptosis-related protein, caspase-3. The results showed that
penicillin could promote the apoptosis of the HCT-116 cell line.
After the autophagy inhibitor, 3-MA inhibited autophagy, the
protein expression level of caspase-3 was downregulated.
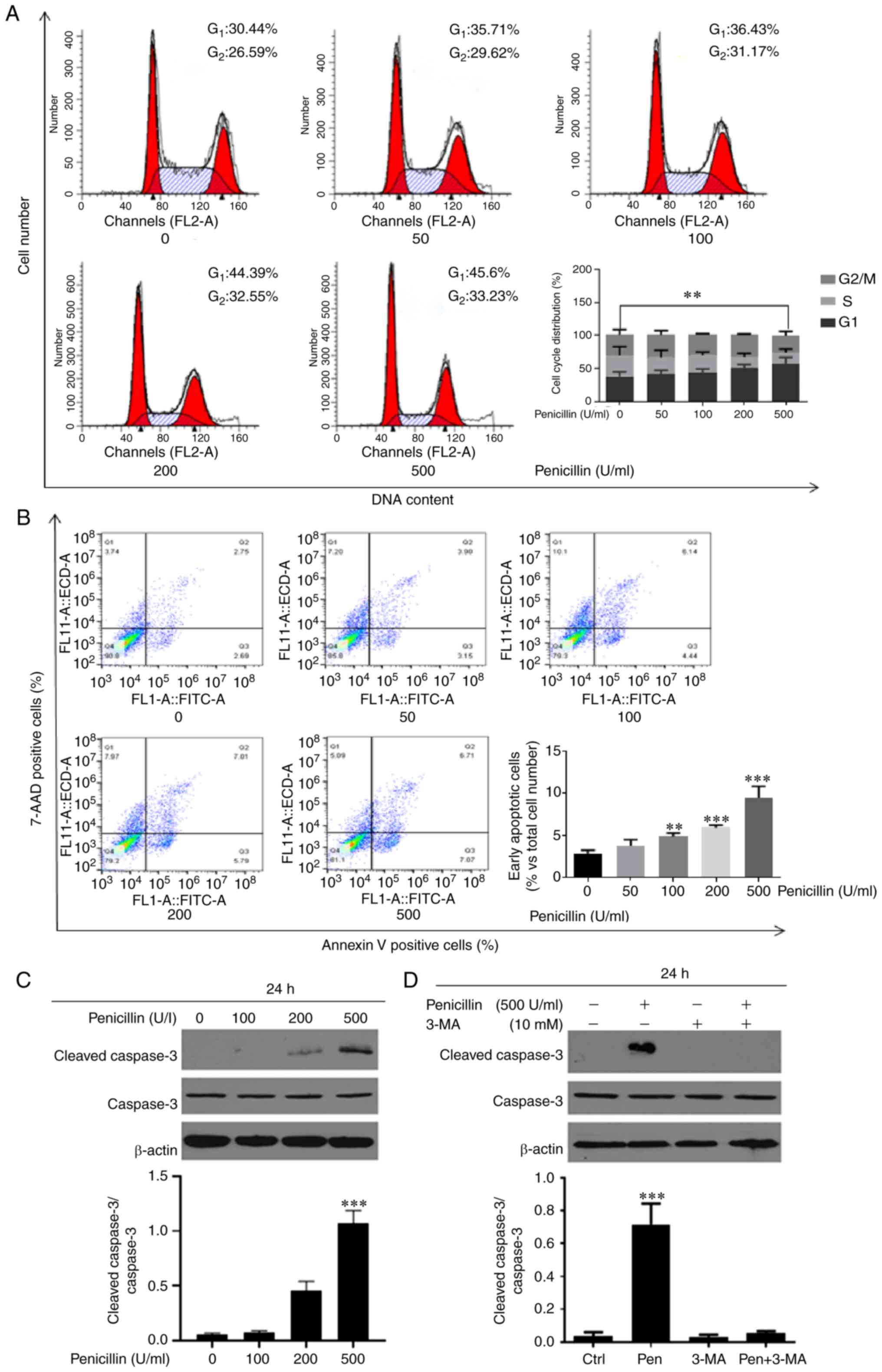 | Figure 4.Penicillin blocks the CRC cell cycle
in the G1 phase and increases the number of early apoptotic cells
in the HCT116 cell line. The HCT116 cell line was treated with
long-acting penicillin (0, 50, 100, 200, and 500 U/ml. for 24 h,
then (A) cell cycle and (B) apoptosis was analyzed. (C) Different
concentrations of penicillin (0, 100, 200, 500 U/ml) were added to
the HCT116 cell line for 24 h and the protein expression level of
cleaved caspase-3 was increased and was dose-dependent. (D) After
the autophagy inhibitor, 3-MA inhibited autophagy, the protein
expression level of caspase-3 was decreased. **P<0.01,
***P<0.001 vs. control. Ctrl, control; pen, penicillin. |
Discussion
As a common malignant tumor in the world, CRC is one
of the leading causes of cancer-associated death. In recent years,
the number of new patients with CRC has exceeded 1 million,
resulting in 700,000 deaths worldwide each year (32). Therefore, identifying a safe and
effective drug is important in the current research on CRC.
Previously, it has been reported that antibiotics could enhance the
therapeutic effect of chemotherapy drugs on CRC (33); however, to the best of our knowledge,
there has been no report on whether antibiotics alone can treat
tumors. Penicillin is an antibacterial drug commonly used in
clinical practice; it has few toxic side effects and is cheaper
compared with other antibiotics (streptomycin, cephalosporins and
vancomycin) (34). In view of its
clinical safety, we hypothesized that it could be used in antitumor
therapy.
In the present study, long-acting penicillin was
used to treat the CRC cell lines and it was found that penicillin
inhibited the growth of the CRC cell lines, and 500 U/ml was the
optimal inhibitory concentration. In addition, the wound healing
and Transwell invasion assays showed that penicillin significantly
inhibited the migration and invasion of the CRC cell lines. These
findings suggested that penicillin had antitumor properties, and
could inhibit the growth, migration and invasion of the CRC cell
lines. During normal cell growth, abnormal apoptosis and cell cycle
progression can cause malignant cells to proliferate. Inhibiting
tumor cell proliferation can be achieved by regulating abnormal
cell apoptosis and abnormal cell cycle (35). In the present study, it was found
that penicillin blocked the cell cycle of the HCT116 cell line at
the G1 phase and increased the number of early apoptotic
cells, suggesting that penicillin could increase the apoptosis by
blocking the cell cycle.
Studies have shown that mitochondria not only
produce and provide energy, but also participate in a variety of
physiological and pathological reactions (21,36,37).
Mitochondrial dysfunction can lead to various pathological changes,
such as the inflammatory response, oxidative stress response and
apoptosis. Changes caused by mitochondrial structure and function
injury, such as abnormal function of cellular respiratory chain
enzymes, membrane potential decline and permeability change,
calcium ion overload and pro-apoptotic protein overexpression,
ultimately lead to apoptosis and are an important way to inhibit
cell growth (38). The release of
cytochrome c is associated with the increase in
mitochondrial outer membrane permeability and membrane potential
(39). Existing studies show that
CRC cell growth inhibition and mitochondrial function is associated
(40–42). To identify the mechanism by which
penicillin inhibited CRC cell growth migration and invasion,
mitochondrial energy metabolism was analyzed to examine the cell
mitochondrial energy metabolism, and it was found that penicillin
caused mitochondria energy metabolic disorders, where basic
respiration capacity, maximal respiration capacity, respiration
potential, and ATP generation were significantly inhibited.
Mitochondrial energy metabolism disorder leads to increased
mitochondrial membrane permeability, which releases cytochrome
c into the cytoplasm, further promoting the release of
apoptotic factors and finally leading to cell apoptosis (43). In the present study, the protein
expression level of cytochrome c was significantly
upregulated by penicillin treatment in a dose-dependent manner.
This suggested that penicillin caused mitochondrial function damage
in the HCT116 cell line, led to energy metabolism disorder and
promoted the release of cytochrome c.
There are numerous ways to induce tumor cell death,
including several non-apoptotic forms of death in addition to
apoptosis, in which autophagic cell death is an important one
(44). Autophagy is a basic
physiological process widely present in eukaryotic cells. It
removes harmful protein aggregates, damaged organelles and some
pathogens through lysosomal degradation, to maintain the
homeostasis of the cells (45,46).
Appropriate autophagy is crucial to cell homeostasis, but if
autophagy is dysregulated, it causes a several diseases, including
neurodegeneration and tumors, and excessive autophagy can lead to
cell death (47). Therefore,
autophagy plays an important role in antitumor drug therapy. In the
present study, penicillin induced autophagy in the HCT116 cell
line, which in turn caused apoptosis. LC3 is known to be in two
forms, LC3-I and LC3-II. When autophagy begins, inactive LC3-I
binds with phosphatidylethanolamine and is transformed into the
active LC3-II, and activated LC3-II aggregates on the autophagic
membrane; therefore, the ratio of LC3-II/LC3-I reflects the degree
of autophagy to a certain extent (48,49). In
the present study, the ratio of LC3-II/LC3-I was significantly
upregulated by penicillin in a time- and dose-dependent manner. The
number of autophagosomes increased following penicillin treatment
as observed using confocal microscopy. Furthermore, mitochondrial
structure damage was observed with transmission electron
microscopy.
Some studies have shown that the use of antibiotics
increases the risk of CRC or colorectal adenoma, particularly in
the proximal colon (50,51), and other studies have shown that
long-term antibiotics, such as tetracycline, can reduce the risk of
rectal cancer (52,53). A previous study also showed that when
the ampicillin/amoxicillin treatment strategy is switched from
anti-anaerobic to anti-aerobic, the incidence of CRC is reversed
(54). The size and pattern of the
effect of oral antibiotics on the risk of CRC differs depending on
the anatomical location of the cancer. The proximal colon is the
first location to be exposed to antibiotics and different
colorectal anatomical locations determine the distribution of
different advocacy flora (55). Oral
anti-anaerobic drugs can destroy the microbiota organization and
structure in the colon and significantly impact the risk of CRC
(56). As the intestinal flora is
mainly composed of anaerobic bacteria, it is of note that the CRC
tissue is rich in a multi-microbial invasive biofilm, particularly
the right tumor (57). The greater
impact of antibiotics on the proximal colon may reflect the
destruction of biofilm formation related to carcinogenesis
(51). Antibiotics can reduce the
risk of CRC; however, the intestinal flora is also affected. In the
current study, it was found that penicillin had an antitumor
effect, which is a new finding; however, further research should be
performed in a clinical setting.
In conclusion, the role of penicillin in
antimicrobial therapy has been widely known; however, reports of
penicillin use in antineoplastic therapy are rare. In the present
study, using cultured CRC cell lines it was demonstrated that
penicillin could inhibit the growth and promote CRC cell apoptosis.
The results provide the basis for evaluating the antitumor effects
of penicillin. To further understand the antitumor properties of
penicillin, the inhibitory effects of penicillin on CRC cells
should be investigated in animal experiments, which is the aim of
our future experiments, to establish a cell derived xenograft tumor
model to further verify the inhibitory effect of penicillin on CRC
cell lines. Such experiments would further reveal the effects of
penicillin on the growth and metastasis of CRC cells, and provide a
basis for the clinical treatment of CRC.
Acknowledgements
Not applicable.
Funding
This study was supported by Ningbo Science and
Technology Innovation Team Program (grant no. 2014B82002), the
National Natural Science Foundation of China (grant no. 81370165),
the Fang Runhua Fund of Hong Kong and the K. C. Wong Magna Fund in
Ningbo University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SB and WC conceived and designed the study. FH, YW
and CL performed the experiments, interpreted the results and wrote
the manuscript. SY, YZ and YX analyzed and checked all the data.
All authors read and approved the final manuscript and confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kitahara CM, Berndt SI, de González AB,
Coleman HG, Schoen RE, Hayes RB and Huang WY: Prospective
investigation of body mass index, colorectal adenoma, and
colorectal cancer in the prostate, lung, colorectal, and ovarian
cancer screening trial. J Clin Oncol. 31:2450–2459. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ji Q, Zhang L, Liu X, Zhou L, Wang W, Han
Z, Sui H, Tang Y, Wang Y, Liu N, et al: Long non-coding RNA MALAT1
promotes tumour growth and metastasis in colorectal cancer through
binding to SFPQ and releasing oncogene PTBP2 from SFPQ/PTBP2
complex. Br J Cancer. 111:736–748. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pellat A, Deyra J, Coriat R and Chaussade
S: Results of the national organised colorectal cancer screening
program with FIT in Paris. Sci Rep. 8:41622018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bhandari A, Woodhouse M and Gupta S:
Colorectal cancer is a leading cause of cancer incidence and
mortality among adults younger than 50 years in the USA: A
SEER-based analysis with comparison to other young-onset cancers. J
Investig Med. 65:311–315. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siegel RL, Ward EM and Jemal A: Trends in
colorectal cancer incidence rates in the United States by tumor
location and stage, 1992–2008. Cancer Epidemiol Biomarkers Prev.
21:411–416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Souwer ETD, Bastiaannet E, Steyerberg EW,
Dekker JT, van den Bos F and Portielje JEA: Risk prediction models
for postoperative outcomes of colorectal cancer surgery in the
older population-a systematic review. J Geriatr Oncol.
11:1217–1228. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chakedis J and Schmidt CR: Surgical
treatment of metastatic colorectal cancer. Surg Oncol Clin N Am.
27:377–399. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ueno H, Hase K, Hashiguchi Y, Shinto E,
Shimazaki H, Yamamoto J, Nakamura T and Sugihara K: Potential
causes of stage migration and their prognostic implications in
colon cancer: A nationwide survey of specialist institutions in
Japan. Jpn J Clin Oncol. 44:547–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Das S, Ciombor KK, Haraldsdottir S and
Goldberg RM: Promising new agents for colorectal cancer. Curr Treat
Options Oncol. 19:292018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benzathine Penicillin G: Drugs and
Lactation Database (LactMed). National Library of Medicine;
Bethesda, MD: 2006
|
|
12
|
Dalal A, Eskin-Schwartz M, Mimouni D, Ray
S, Days W, Hodak E, Leibovici L and Paul M: Interventions for the
prevention of recurrent erysipelas and cellulitis. Cochrane
Database Syst Rev. Jun 20–2017.(Epub ahead of print). doi:
10.1002/14651858.CD009758.pub2. View Article : Google Scholar
|
|
13
|
Shenoy ES, Macy E, Rowe T and Blumenthal
KG: Evaluation and management of penicillin allergy: A review.
JAMA. 321:188–199. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Padari H, Metsvaht T, Germovsek E, Barker
CI, Kipper K, Herodes K, Standing F, Oselin K, Tasa T, Soeorg H and
Lutsar I: Pharmacokinetics of penicillin G in preterm and term
neonates. Antimicrob Agents Chemother. 62:e02238–17. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Llor C, Perez A, Carandell E,
Garcia-Sangenis A, Rezola J, Llorente M, Gestoso S, Bobé F,
Román-Rodríguez M, Cots JM, et al: Efficacy of high doses of
penicillin versus amoxicillin in the treatment of uncomplicated
community acquired pneumonia in adults. A non-inferiority
controlled clinical trial. Aten Primaria. 51:32–39. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ahlstedt S: Penicillin allergy-can the
incidence be reduced? Allergy. 39:151–164. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suehiro Y, Takemoto Y, Nishimoto A, Ueno
K, Shirasawa B, Tanaka T, Kugimiya N, Suga A, Harada E and Hamano
K: Dclk1 inhibition cancels 5-FU-induced cell-cycle arrest and
decreases cell survival in colorectal cancer. Anticancer Res.
38:6225–6230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kee JY, Han YH, Mun JG, Um JY and Hong SH:
Pharmacological effect of prohibited combination pair Panax ginseng
and Veratrum nigrum on colorectal metastasis in vitro and in vivo.
J Ethnopharmacol. 220:177–187. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jayashankar V, Mueller IA and Rafelski SM:
Shaping the multi-scale architecture of mitochondria. Curr Opin
Cell Biol. 38:45–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Roger AJ, Munoz-Gomez SA and Kamikawa R:
The origin and diversification of mitochondria. Curr Biol.
27:R1177–R1192. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vidali S, Aminzadeh S, Lambert B,
Rutherford T, Sperl W, Kofler B and Feichtinger RG: Mitochondria:
The ketogenic diet-A metabolism-based therapy. Int J Biochem Cell
Biol. 63:55–59. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kalpage HA, Bazylianska V, Recanati MA,
Fite A, Liu J, Wan J, Mantena N, Malek MH, Podgorski I, Heath EI,
et al: Tissue-specific regulation of cytochrome c by
post-translational modifications: Respiration, the mitochondrial
membrane potential, ROS, and apoptosis. FASEB J. 33:1540–1553.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Giorgi C, Marchi S, Simoes ICM, Ren Z,
Morciano G, Perrone M, Patalas-Krawczyk P, Borchard S, Jędrak P,
Pierzynowska K, et al: Mitochondria and reactive oxygen species in
aging and age-related diseases. Int Rev Cell Mol Biol. 340:209–344.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ryter SW, Ma KC and Choi AMK: Carbon
monoxide in lung cell physiology and disease. Am J Physiol Cell
Physiol. 314:C211–C27. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Levine B and Klionsky DJ: Development by
self-digestion: Molecular mechanisms and biological functions of
autophagy. Dev Cell. 6:463–477. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yun CW and Lee SH: The roles of autophagy
in cancer. Int J Mol Sci. 19:34662018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu HM and Hu F: The role of autophagy and
mitophagy in cancers. Arch Physiol Biochem. 9:1–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Amaravadi R, Kimmelman AC and White E:
Recent insights into the function of autophagy in cancer. Genes
Dev. 30:1913–1930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schaaf MB, Keulers TG, Vooijs MA and
Rouschop KM: LC3/GABARAP family proteins: Autophagy-(un)related
functions. FASEB J. 30:3961–3978. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhai Y, Lin P, Feng Z, Lu H, Han Q, Chen
J, Zhang Y, He Q, Nan G, Luo X, et al: TNFAIP3-DEPTOR complex
regulates inflammasome secretion through autophagy in ankylosing
spondylitis monocytes. Autophagy. 14:1629–1643. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reggiori F and Ungermann C: Autophagosome
maturation and fusion. J Mol Biol. 429:486–496. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dekker E, Tanis PJ, Vleugels JLA, Kasi PM
and Wallace MB: Colorectal cancer. Lancet. 394:1467–1480. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klose J, Trefz S, Wagner T, Steffen L,
Preissendörfer Charrier A, Radhakrishnan P, Volz C, Schmidt T,
Ulrich A, Dieter SM, et al: Salinomycin: Anti-tumor activity in a
pre-clinical colorectal cancer model. PLoS One. 14:e02119162019.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Benzathine Penicillin G: Drugs and
Lactation Database [LactMed (Internet)]. National Library of
Medicine; Bethesda, MD: 2006. Nov 16–2020
|
|
35
|
Alimbetov D, Askarova S, Umbayev B, Davis
T and Kipling D: Pharmacological targeting of cell cycle, apoptotic
and cell adhesion signaling pathways implicated in chemoresistance
of cancer cells. Int J Mol Sci. 19:16902018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bock FJ and Tait SW: Mitochondria as
multifaceted regulators of cell death. Nat Rev Mol Cell Biol.
21:85–100. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abate M, Festa A, Falco M, Lombardi A,
Luce A, Grimaldi A, Zappavigna S, Sperlongano P, Irace C, Caraglia
M and Misso G: Mitochondria as playmakers of apoptosis, autophagy
and senescence. Semin Cell Dev Biol. 98:139–153. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pistritto G, Trisciuoglio D, Ceci C,
Garufi A and D'Orazi G: Apoptosis as anticancer mechanism: Function
and dysfunction of its modulators and targeted therapeutic
strategies. Aging. 8:603–619. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gogvadze V, Orrenius S and Zhivotovsky B:
Analysis of mitochondrial dysfunction during cell death. Methods
Mol Biol. 1264:385–393. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lawrie TA, Green JT, Beresford M, Wedlake
L, Burden S, Davidson SE, Lal S, Henson CC and Andreyev HJ:
Interventions to reduce acute and late adverse gastrointestinal
effects of pelvic radiotherapy for primary pelvic cancers. Cochrane
Database Syst Rev. Jun 23–2018.(Epub ahead of print). doi:
10.1002/14651858.CD012529.pub2.
|
|
41
|
Burlaka AP, Ganusevich II, Vovk AV,
Burlaka AA, Gafurov MR and Lukin SN: Colorectal cancer and
mitochondrial dysfunctions of the adjunct adipose tissues: A case
study. Biomed Res Int. 2018:21690362018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Akagi J and Baba H: Hydrogen gas restores
exhausted CD8+ T cells in patients with advanced
colorectal cancer to improve prognosis. Oncol Rep. 41:301–311.
2019.PubMed/NCBI
|
|
43
|
Chimenti MS, Sunzini F, Fiorucci L, Botti
E, Fonti GL, Conigliaro P, Triggianese P, Costa L, Caso F, Giunta
A, et al: Potential role of cytochrome c and tryptase in psoriasis
and psoriatic arthritis pathogenesis: Focus on resistance to
apoptosis and oxidative stress. Front Immunol. 9:23632018.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ai L, Xu A and Xu J: Roles of PD-1/PD-L1
pathway: Signaling, cancer, and beyond. Adv Exp Med Biol.
1248:33–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhou ZX, Zhao LY, Lin T, Liu H, Deng HJ,
Zhu HL, Yan J and Li GX: Long-term oncologic outcomes of
laparoscopic vs open surgery for stages II and III rectal cancer: A
retrospective cohort study. World J Gastroenterol. 21:5505–5512.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Stolz A, Ernst A and Dikic I: Cargo
recognition and trafficking in selective autophagy. Nat Cell Biol.
16:495–501. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ktistakis NT and Tooze SA: Digesting the
expanding mechanisms of autophagy. Trends Cell Biol. 26:624–635.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Heintze J, Costa JR, Weber M and Ketteler
R: Ribose 5-phosphate isomerase inhibits LC3 processing and basal
autophagy. Cell Signal. 28:1380–1388. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schlafli AM, Adams O, Galvan JA, Gugger M,
Savic S, Bubendorf L, Schmid RA, Becker KF, Tschan MP, Langer R and
Berezowska S: Prognostic value of the autophagy markers LC3 and
p62/SQSTM1 in early-stage non-small cell lung cancer. Oncotarget.
7:39544–39555. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dejea CM, Wick EC, Hechenbleikner EM,
White JR, Mark Welch JL, Rossetti BJ, Peterson SN, Snesrud EC,
Borisy GG, Lazarev M, et al: Microbiota organization is a distinct
feature of proximal colorectal cancers. Proc Natl Acad Sci USA.
111:18321–18326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tomkovich S, Dejea CM, Winglee K, Drewes
JL, Chung L, Housseau F, Pope JL, Gauthier J, Sun X, Mühlbauer M,
et al: Human colon mucosal biofilms from healthy or colon cancer
hosts are carcinogenic. J Clin Invest. 129:1699–1712. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tang X, Wang X, Zhao YY, Curtis JM and
Brindley DN: Doxycycline attenuates breast cancer-related
inflammation by decreasing plasma lysophosphatidate concentrations
and inhibiting NF-kappaB activation. Mol Cancer. 16:362017.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sapadin AN and Fleischmajer R:
Tetracyclines: Nonantibiotic properties and their clinical
implications. J Am Acad Dermatol. 54:258–265. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang J, Haines C, Watson AJM, Hart AR,
Platt MJ, Pardoll DM, Cosgrove SE, Gebo KA and Sears CL: Oral
antibiotic use and risk of colorectal cancer in the United Kingdom,
1989–2012: A matched case-control study. Gut. 68:1971–1978. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dejea CM, Fathi P, Craig JM, Boleij A,
Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM,
Huso DL, et al: Patients with familial adenomatous polyposis harbor
colonic biofilms containing tumorigenic bacteria. Science.
359:5922018. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Drewes JL, White JR, Dejea CM, Fathi P,
Iyadorai T, Vadivelu J, Roslani AC, Wick EC, Mongodin EF, Loke MF,
et al: High-resolution bacterial 16S rRNA gene profile
meta-analysis and biofilm status reveal common colorectal cancer
consortia. NPJ Biofilms Microbiomes. 3:342017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dejea CM, Wick EC, Hechenbleikner EM,
White JR, Mark Welch JL, Rossetti BJ, Peterson SN, Snesrud EC,
Borisy GG, Lazarev M, et al: Microbiota organization is a distinct
feature of proximal colorectal cancers. Proc Natl Acad Sci USA.
111:18321–18326. 2014. View Article : Google Scholar : PubMed/NCBI
|