Introduction
Breast cancer is the most common malignancy in women
and the second leading cause of cancer-associated mortality in
women worldwide (1). Patients with
early-stage breast cancer can be cured (2). Early diagnosis and early treatment of
breast cancer are the key to improving its prognosis (3). Despite marked advances in its
treatment, breast cancer remains a major health problem (4).
Nuclear receptor coactivator 5 (NCOA5) is a nuclear
receptor coregulator, which interacts with α and β estrogen
receptors, as well as an orphan nuclear receptor (nuclear receptor
subfamily 1 group D member 2), independently of the AF2 domain
(5). NCOA5 is considered to have
both coactivator and corepressor functions, and could modulate
estrogen receptor α-mediated transcription (6). In addition, NCOA5 interacts with tumor
suppressor Tat-interacting protein (30 kDa) and regulates c-myc
transcription (7). NCOA5 has been
reported to be involved in the regulation of several malignancies,
such as hepatocellular carcinoma, colorectal cancer and papillary
thyroid cancer (8–14). Ye et al (15) reported that NCOA5 expression was
increased in luminal breast cancer tissues, and high NCOA5
expression was associated with the progression and prognosis of
patients with luminal breast cancer. However, to the best of our
knowledge, the mechanisms underlying the role of NCOA5 in breast
cancer are still unknown.
In the present study, the expression levels of NCOA5
in breast cancer tissues and a series of breast cancer cell lines
were detected. The effects of NCOA5 on the viability, migration,
adhesion and epithelial-mesenchymal transition (EMT) of breast
cancer cells were evaluated using loss-of-function experiments. The
present study aimed to reveal the biological function of NCOA5 in
breast cancer, and provided a potential therapeutic target for
patients with breast cancer.
Materials and methods
Tissue collection
Breast cancer tissues and adjacent normal tissues
(distance from tumor margin, 2 cm) were collected from 25 female
patients with breast cancer who underwent surgical resection at the
People's Hospital of Deyang City (Deyang, China). These patients
were recruited between January 2019 and November 2020. The patients
were aged 35–67 years with a mean age of 51.3 years, and all
patients had not been treated before surgical resection. The
inclusion criteria were: i) Female subjects aged 18–70 years; ii)
histology or cytology confirmed breast cancer; iii) ECOG score was
0–1; iv) the expected survival time of the case was >3 months;
v) left ventricular ejection fraction ≥55%; vi) laboratory
examination data met the following criteria: Hemoglobin ≥90 g/l,
absolute neutrophil count ≥1.5×109/l, platelets
≥100×109/l, total bilirubin ≤1.5 times upper limit of
normal (x ULN), aspartate transaminase/alanine transaminase ≤2.5×
ULN or ≤5× ULN in case of liver metastasis, creatinine ≤1.5× ULN
and international normalized ratio ≤1.5× ULN, partial
thromboplastin time ≤1.5× ULN; and vii) volunteered to participate
in the research and signed the informed consent form. The exclusion
criteria were: i) Patients with heart, liver, kidney and
hematopoietic system diseases; ii) brain metastasis; iii) with
double or multiple cancers; iv) suffering from clinically
significant active, acute, chronic infection or bleeding; v)
hypertension is not under control; vi) pregnant or lactating women,
mental disorders; vii) participate in any other clinical trials
within 1 month before enrollment; viii) received therapy before
surgery; and ix) the researcher judged that the subjects had any
other conditions that were not suitable for the trial. The study
was approved by the Ethics Committee of the People's Hospital of
Deyang City (Deyang, China). All patients signed an informed
consent form prior to enrollment in the present study.
Cell culture and transfection
The human MCF-10A normal breast epithelial cell line
and human breast cancer cell lines (MDA-MB-231, MDA-MB-453 and
SK-BR-3 breast adenocarcinoma cell lines, and MCF-7 invasive ductal
carcinoma cell line) were purchased from The Cell Bank of Type
Culture Collection of The Chinese Academy of Sciences. The cells
were cultured in DMEM (Thermo Fisher Scientific, Inc.) supplemented
with 10% FCS (Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin (Gibco; Thermo Fisher Scientific, Inc.) and 100 µg/ml
streptomycin (Gibco; Thermo Fisher Scientific, Inc.) in a
humidified atmosphere with 5% CO2 at 37°C. The small
interfering RNA (siRNA/si) directly against human NCOA5 (siNCOA5)
and the non-targeting negative control siRNA (siNC) were purchased
from Shanghai GenePharma Co., Ltd. The sequences of siRNAs were as
follows: siNCOA5 sense, 5′-AGGGAUCUUAGAGACUUUCGUTT-3′ and
antisense, 5′-ACGAAAGUCUCUAAGAUCCCUTT-3′; siNC sense,
5′-UUCUCCGAACGUGUCACGUTT-3′ and antisense,
5′-ACGUGACACGUUCGGAGAATT-3′. Cells (5×105 cells/ml) were
plated into the wells and transfected with 50 nM siRNA using
Lipofectamine® 3000 transfection reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C for 6 h, according to the
manufacturer's protocols. At 48 h after transfection, cells were
collected for subsequent experiments.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from tissues or cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.).
The isolated RNA was then reverse transcribed into cDNA using a
Prime-Script® RT reagent kit (Takara Biotechnology Co.,
Ltd.) according to the manufacturer's protocol. The primers used in
the present study were as follows: NCOA5 forward,
5′-CAAGTGCTCCCCTCTGCTAC-3′ and reverse, 5′-CTGTTTGCTGCTGTGGAAAA-3′;
GAPDH forward, 5′-CGACCACTTTGTCAAGCTCA-3′ and reverse,
5′-AGGGGTCTACATGGCAACTG-3′. The specific reaction procedure was:
42°C for 15 min, 85°C for 5 sec, and 4°C until further use. The
cDNA was used as a template for qPCR. The PCR reaction was
performed with the SYBR Green PCR Master Mix (Quantabio) on an
Applied Biosystems 7900HT Fast Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc.). Amplification was
performed using the following conditions: Initial denaturation at
95°C for 1 min, followed by 40 cycles of 95°C for 5 sec and 60°C
for 20 sec. GAPDH was used as a reference gene. Relative gene
expression of NCOA5 was calculated using the 2−ΔΔCq
method (16).
Western blotting
Proteins were extracted from the tissues or cells
using RIPA Lysis Buffer (EMD Millipore). A BCA Protein Assay Kit
(Pierce; Thermo Fisher Scientific, Inc.) was used to determine the
protein concentration of lysates. A total of 50 µg protein per lane
was separated via 10% SDS-PAGE, and the protein was then
transferred to a polyvinylidene difluoride membrane (EMD
Millipore). Next, the membrane was blocked with 10% skim milk at
4°C overnight, followed by incubation at 37°C for 2 h with the
following antibodies: NCOA5 (cat. no. ab70831; dilution, 1:500),
N-cadherin (cat. no. ab18203; dilution, 1:1,000), Vimentin (cat.
no. ab45939; dilution, 1:800), E-cadherin (cat. no. ab212059;
dilution, 1:800) and GAPDH (cat. no. ab9485; dilution, 1:2,000).
All these antibodies were purchased from Abcam. After washing three
times with PBS with 0.05% Tween-20 at room temperature for 5 min
each, the membrane was incubated with the Goat Anti-Rabbit IgG
H&L (HRP) antibody (cat. no. ab6721; dilution, 1:2,000; Abcam)
at 37°C for 1 h. Finally, the membrane was treated with ECL Plus
Western Blotting Substrate (Thermo Fisher Scientific, Inc.) and the
band density was determined by densitometric analysis (Image Lab
v4.0; Bio-Rad Laboratories, Inc.).
MTT
To determine cell viability, a total of
1.5×104 cells were seeded in each well of 96-well
plates. The cells were cultured in DMEM supplemented with 10% FCS
at 37°C in a humidified atmosphere of 5% CO2 for 24, 48,
72 and 96 h. Cell viability was determined using a MTT Kit
(Beyotime Institute of Biotechnology) according to the
manufacturer's protocol. Cells were incubated with MTT solution at
37°C for 4 h, and then dimethyl sulfoxide (Sigma-Aldrich; Merck
KGaA) was added to dissolve the formazan crystals. Absorbance was
detected at a wavelength of 490 nm using a microplate reader
(Bio-Rad Laboratories, Inc.).
Transwell migration assay
Cell migration was analyzed using 6-well transwell
insert chambers with a pore size of 8 µm (Corning, Inc.). The cells
were suspended in DMEM without FCS and then 5×105 cells
were cultured in the upper chamber. DMEM supplemented with 10% FCS
was added to the bottom chamber as a chemoattractant. Following
incubation at 37°C for 24 h, the migrated cells were fixed with 95%
ethanol for 15 min at 37°C. Subsequently, the cells were stained
with 0.3% hematoxylin (Leagene Biotechnology) for 15 min at 37°C.
Cells were observed under a light microscope (Nikon Corporation),
and the number of migrated cells was calculated for five random
fields.
Cell adhesion assay
The 96-well plates were pre-coated with fibronectin
(Sigma-Aldrich; Merck KGaA) and blocked with 1% bovine serum
albumin (Sigma-Aldrich; Merck KGaA) at 37°C for 2 h. Cells were
suspended in serum-free medium and then seeded into the 96-well
plates at a density of 4×103 cells/well. Following
incubation at 37°C for 1 h, the cells were washed with PBS and
fixed in 4% paraformaldehyde at room temperature for 15 min. The
adhesive cells were stained with 0.5% crystal violet (Leagene
Biotechnology) at room temperature for 2 h, and the crystals were
dissolved with sodium dodecyl sulphate (Amresco, LLC) at room
temperature for 30 min. The absorbance at 570 nm was measured using
a microplate reader (Bio-Rad Laboratories, Inc.). Cell adhesion
activity was normalized to the optical density values of the siNC
group (which was set as 100%).
Statistical analysis
All data were obtained from experiments performed in
triplicate and the experiments were repeated at least three times.
The data are presented as the mean ± standard deviation.
Statistical analysis was performed using GraphPad Prism 5 software
(GraphPad Software, Inc.). A paired t-test was used for the
analysis of tumor and adjacent non-tumor samples. An unpaired
t-test was used for comparisons between two groups for cell
experiments. One-way ANOVA followed by Tukey's post hoc test was
used for analysis among three or more groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
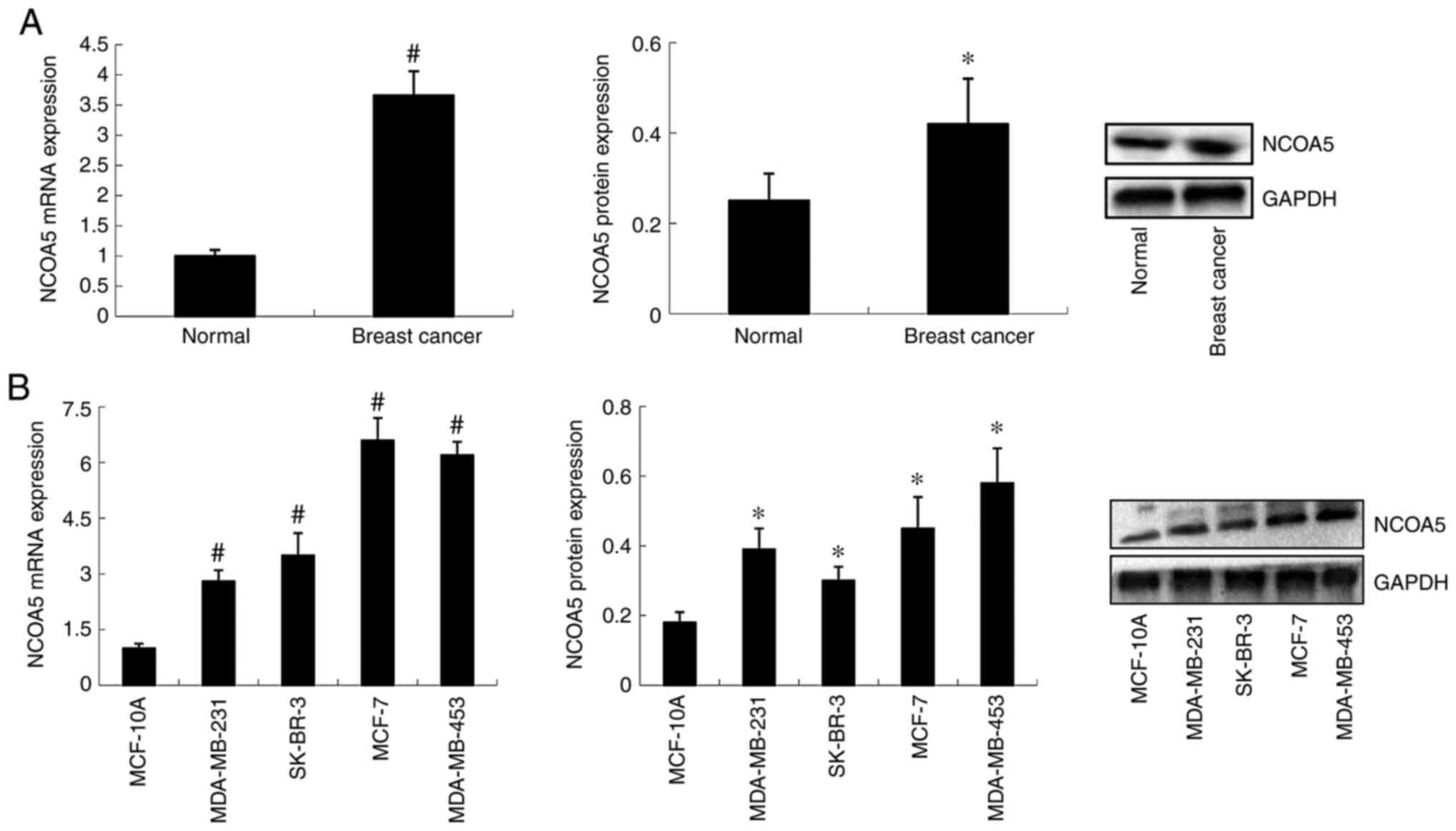
NCOA5 expression in human breast
cancer tissues
Expression levels of NCOA5 in human breast cancer
tissues and adjacent normal tissues were detected by RT-qPCR and
western blotting. As shown in Fig.
1A, compared with that in adjacent normal tissues, NCOA5
expression was increased in breast cancer tissues at both the mRNA
and protein levels.
NCOA5 expression in breast cancer cell
lines
NCOA5 expression in the MCF-10A normal breast
epithelial cell line and a series of breast cancer cell lines
(MDA-MB-231, MDA-MB-453, MCF-7 and SK-BR-3) was detected by RT-qPCR
and western blotting. The results demonstrated that the mRNA and
protein expression levels of NCOA5 were significantly increased in
breast cancer cell lines compared with the normal cell line
(Fig. 1B). Among the breast cancer
cell lines, MDA-MB-453 and MCF-7 cells exhibited higher NCOA5 mRNA
and protein expression than MDA-MB-231 and SK-BR-3 cells.
Subsequently, loss-of-function experiments were performed using the
MDA-MB-453 and MCF-7 cell lines, which exhibited high expression
levels of NCOA5.
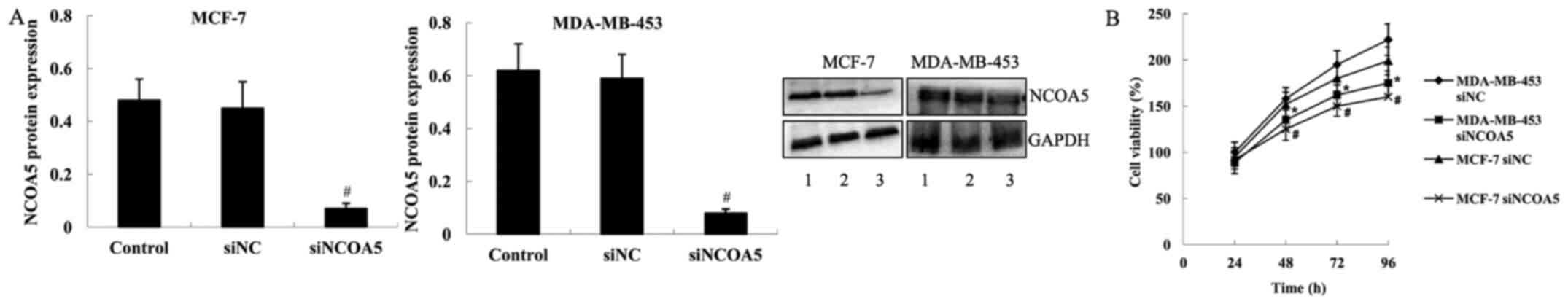
Effect of NCOA5 on the viability of
breast cancer cells
To investigate the biological function of NCOA5 in
breast cancer cells, siNCOA5 was transfected into MDA-MB-453 and
MCF-7 cells to knock down NCOA5 expression. The results of western
blot analysis demonstrated that compared with those in the cells
transfected with siNC, the protein expression levels of NCOA5 were
significantly decreased in MDA-MB-453 and MCF-7 cells transfected
with siNCOA5 (Fig. 2A).
Subsequently, an MTT assay was performed using MDA-MB-453 and MCF-7
cells to examine viability. As shown in Fig. 2B, compared with the cells transfected
with siNC, cell viability was significantly decreased in both
MDA-MB-453 and MCF-7 cell lines transfected with siNCOA5 at 48, 72
and 96 h.
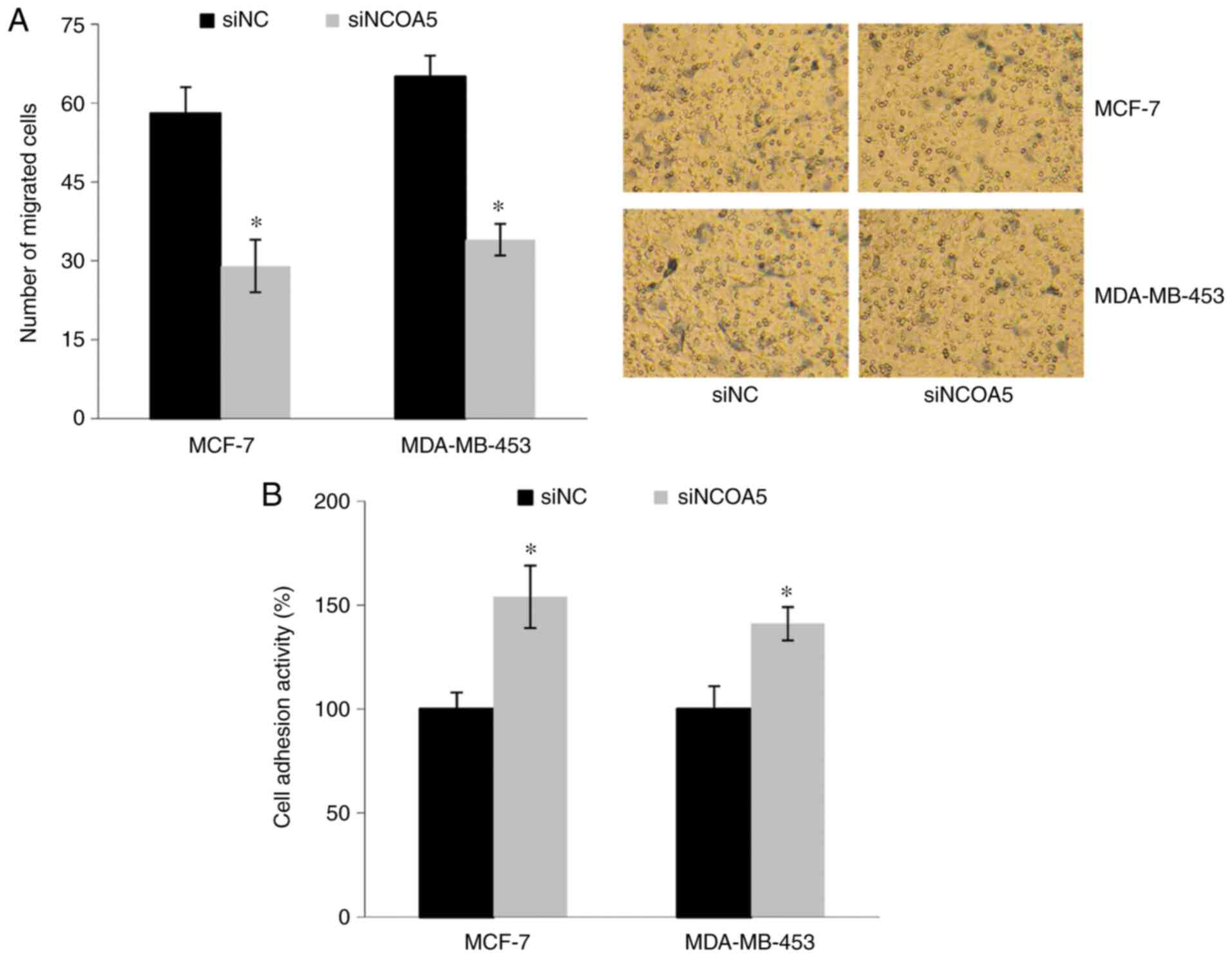
Effect of NCOA5 on the migration and
adhesion abilities of breast cancer cells
Transwell migration assays were performed to
investigate the effect of NCOA5 on cell migration. As shown in
Fig. 3A, the number of migrated
cells was significantly decreased in the siNCOA5 group compared
with the siNC group. Furthermore, a cell adhesion assay revealed
that the adhesion abilities of MDA-MB-453 and MCF-7 cells were
significantly increased following transfection with siNCOA5
(Fig. 3B).
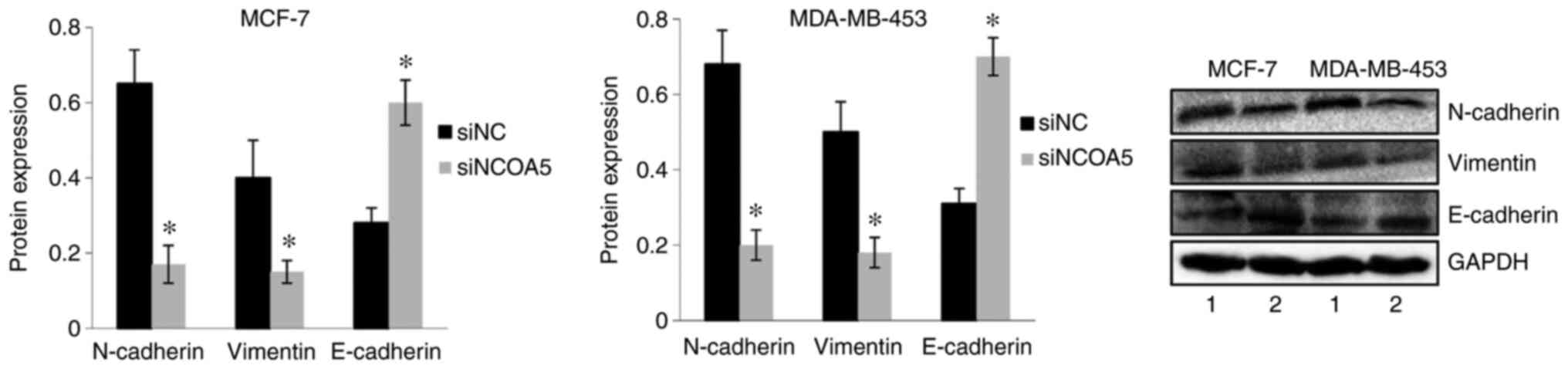
Effect of NCOA5 on EMT of breast
cancer cells
The protein expression levels of N-cadherin,
Vimentin and E-cadherin were examined by western blot analysis to
investigate the effect of NCOA5 on EMT of breast cancer cells.
Compared with those in the cells transfected with siNC, the protein
expression levels of N-cadherin and Vimentin were significantly
decreased, whereas the protein expression levels of E-cadherin were
significantly increased in MDA-MB-453 cells transfected with
siNCOA5. In MCF-7 cells, the expression levels of N-cadherin and
Vimentin were significantly decreased, and E-cadherin expression
was significantly increased in siNCOA5-tranfected cells compared
with siNC-transfected cells (Fig.
4).
Discussion
NCOA5 is a unique nuclear receptor coactivator with
both co-activation and co-repression functions (6). The abnormal expression of estrogen
receptor is associated with various types of cancer (17), and previous studies have demonstrated
that NCOA5 can regulate estrogen receptor-mediated transcription in
human cells (5–7). The role of NCOA5 in human cancer has
attracted increasing attention. Abnormal expression of NCOA5 has
been reported in a variety of tumors, including esophageal squamous
cell carcinoma, hepatocellular carcinoma and cervical cancer
(15,18–21). The
present study demonstrated that NCOA5 expression was significantly
increased in human breast cancer tissues compared with adjacent
normal tissues. Furthermore, NCOA5 expression was significantly
increased in breast cancer cell lines compared with a normal breast
epithelial cell line. The present results were consistent with a
previous report showing that NCOA5 was upregulated in luminal
breast cancer tissues compared with adjacent normal tissues both in
a validated cohort and The Cancer Genome Atlas cohort (15). Additionally, upregulation of NCOA5
has been identified in colorectal cancer (13). Conversely, NCOA5 has been reported to
be downregulated in hepatocellular carcinoma, cervical cancer,
esophageal squamous cell carcinoma, papillary thyroid carcinoma and
osteosarcoma (18–21). These reports suggest that alterations
of NCOA5 are involved in the carcinogenesis and progression of
human cancer.
Accumulating evidence has demonstrated that NCOA5
serves as a tumor suppressor or an oncogene in different tumor
types (12–14,19). For
example, NCOA5 expression is associated with the
clinicopathological features of patients with colorectal cancer
(13). Knockdown of NCOA5 markedly
suppresses the proliferation, migration and invasion of colorectal
cancer cells, induces cell cycle G1 phase arrest, and inhibits
in vivo xenograft growth of colorectal cancer cells
(13). Similarly, knockout of NCOA5
inhibits proliferation and migration in hepatocellular carcinoma
cells (12). By contrast, Zheng
et al (14) reported that
NCOA5 is a tumor suppressor gene in papillary thyroid carcinoma,
and reduced NCOA5 expression is associated with the aggressive
clinicopathological features of patients with papillary thyroid
carcinoma. Additionally, a study revealed that low NCOA5 expression
predicts poor prognosis in human cervical cancer, and
downregulation of NCOA5 results in an increase in proliferation,
migration and invasion of HeLa cells (19). Ye et al (15) demonstrated that high NCOA5 expression
was an independent high risk factor in luminal breast cancer, and
patients with high NCOA5 expression had a lower overall survival
rate. Yuan et al (22)
reported that NCOA5 expression could be stimulated by methionine
and leucine in bovine mammary epithelial cells; however,
phosphatidylinositol 3-kinase inhibition could abolish the
stimulatory effect of methionine and leucine on NCOA5. NCOA5 can
bind to the mTOR promoter, and induce mTOR phosphorylation and
β-casein synthesis (22). However,
the precise role and the cellular mechanism for NCOA5 in breast
cancer are still largely unknown. The present study explored the
biological function of NCOA5 in vitro. NCOA5 expression was
significantly increased in breast cancer cell lines. Therefore,
loss-of-function experiments were performed in MDA-MB-453 and MCF-7
cell lines, which exhibited higher expression levels of NCOA5 than
MDA-MB-231 and SK-BR-3 cell lines. It was demonstrated that
knockdown of NCOA5 suppressed the viability and migration, and
induced adhesion of breast cancer cells, indicating that NCOA5 may
act as a novel oncogene to promote the progression of breast
cancer. The present finding was consistent with previous studies
demonstrating that NCOA5 acts as an oncogene in colorectal cancer
and hepatocellular carcinoma (12,13).
However, in papillary thyroid carcinoma and cervical cancer, NCOA5
acts as a tumor suppressor (14,19). It
was hypothesized that the role of NCOA5 in human cancer may be
dependent on the tissue type. The mechanisms underlying the
biological effects of NCOA5 in different types of human cancer
should be further explored.
The present study further demonstrated that NCOA5
knockdown decreased the expression levels of N-cadherin and
Vimentin, and increased the expression levels of E-cadherin. It has
been demonstrated that N-cadherin, Vimentin and E-cadherin are key
mediators of EMT (23–25). EMT is an important process for
epithelial cancer metastasis (26–28). The
loss of E-cadherin expression or gain of N-cadherin and Vimentin
expression contributes to EMT (25,29,30). The
present results supported the hypothesis that NCOA5 induced EMT in
breast cancer cells, thus promoting tumor cell migration and
invasion. Additional functional experiments are required to
demonstrate the effects of the EMT phenotype of the cells.
Interestingly, the present findings suggested that
NCOA5 could promote proliferation and aggressiveness of breast
cancer cells. However, among all the breast cancer cell lines
examined, the expression levels of NCOA5 were high in the least
aggressive MCF-7 cell line (31). At
present, there are few studies regarding the role of NCOA5 in
breast cancer (15,22), and the association between NCOA5
expression and the aggressiveness of breast cancer cell lines is
still unclear. Whether the expression levels of NCOA5 could be used
to predict the aggressiveness of breast cancer cell lines requires
further investigation.
A limitation of the present study was that
loss-of-function experiments were performed only in breast cancer
cells. More studies on MCF-10A normal breast epithelial cell line
are required to provide a negative control for the experiments
performed using the breast cancer cell lines. Another limitation of
the present study was that it only used an MTT assay to determine
cell viability. Other assays are required to further examine the
effect of NCOA5 on cell proliferation.
In conclusion, the present study demonstrated that
NCOA5 expression was upregulated in human breast cancer tissues and
breast cancer cell lines. Furthermore, the present study revealed
the cellular mechanisms for NCOA5 in breast cancer, and
demonstrated that knockdown of NCOA5 suppressed cell viability and
migration, induced cell adhesion, and inhibited EMT of breast
cancer cells, indicating that NCOA5 serves a tumor-promoting role
in the progression of breast cancer. The present study suggested
NCOA5 as a novel target for the treatment of breast cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YT and FL designed the study, prepared the
manuscript, and confirmed the authenticity of all the raw data. YT,
FL and PX conducted the experiments. All authors were substantially
involved in the research, acquisition of data, analysis and
manuscript preparation. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the People's Hospital of Deyang City (Deyang, China).
All patients signed an informed consent form prior to enrollment in
the present study.
Patient consent for publication
All patients signed an informed consent for
enrollment and publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Januškevičienė I and Petrikaitė V:
Heterogeneity of breast cancer: The importance of interaction
between different tumor cell populations. Life Sci. 239:1170092019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wörmann B: Breast cancer: Basics,
screening, diagnostics and treatment. Med Monatsschr Pharm.
40:55–64. 2017.PubMed/NCBI
|
|
3
|
Zeng Y, Zhang J, Meng J, Numthuam S and
Naruse K: Application of multi-modal imaging mediated by iron
carbon nanoparticles based on reinforcement learning in the
diagnosis of breast nodules. J Nanosci Nanotechnol. 21:1154–1160.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Anastasiadi Z, Lianos GD, Ignatiadou E,
Harissis HV and Mitsis M: Breast cancer in young women: An
overview. Updates Surg. 69:313–317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang Z and Teng CT: Estrogen receptor
alpha and estrogen receptor-related receptor alpha1 compete for
binding and coactivator. Mol Cell Endocrinol. 172:223–233. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sauvé F, McBroom LD, Gallant J, Moraitis
AN, Labrie F and Giguère V: CIA, a novel estrogen receptor
coactivator with a bifunctional nuclear receptor interacting
determinant. Mol Cell Biol. 21:343–353. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang C, Ito M, Piening V, Bruck K, Roeder
RG and Xiao H: TIP30 interacts with an estrogen receptor
alpha-interacting coactivator CIA and regulates c-myc
transcription. J Biol Chem. 279:27781–27789. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu CY and Feng GS: NCOA5, a molecular
link between type 2 diabetes and liver cancer. Hepatobiliary Surg
Nutr. 3:106–108. 2014.PubMed/NCBI
|
|
9
|
Liu X, Liu F, Gao S, Reske J, Li A, Wu CL,
Yang C, Chen F, Luo R and Xiao H: A single non-synonymous NCOA5
variation in type 2 diabetic patients with hepatocellular carcinoma
impairs the function of NCOA5 in cell cycle regulation. Cancer
Lett. 391:152–161. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dhar D, Seki E and Karin M: NCOA5, IL-6,
type 2 diabetes, and HCC: The deadly quartet. Cell Metab. 19:6–7.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Facciorusso A and Barone M: Glucose
intolerance and hepatocellular carcinoma: Recent findings for old
diseases. Hepatobiliary Surg Nutr. 3:91–92. 2014.PubMed/NCBI
|
|
12
|
He J, Zhang W, Li A, Chen F and Luo R:
Knockout of NCOA5 impairs proliferation and migration of
hepatocellular carcinoma cells by suppressing
epithelial-to-mesenchymal transition. Biochem Biophys Res Commun.
500:177–183. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun K, Wang S, He J, Xie Y, He Y, Wang Z
and Qin L: NCOA5 promotes proliferation, migration and invasion of
colorectal cancer cells via activation of PI3K/AKT pathway.
Oncotarget. 8:107932–107946. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zheng ZC, Wang QX, Zhang W, Zhang XH and
Huang DP: A novel tumor suppressor gene NCOA5 is correlated with
progression in papillary thyroid carcinoma. OncoTargets Ther.
11:307–311. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye XH, Huang DP and Luo RC: NCOA5 is
correlated with progression and prognosis in luminal breast cancer.
Biochem Biophys Res Commun. 482:253–256. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ranhotra HS: Estrogen-related receptor
alpha and cancer: Axis of evil. J Recept Signal Transduct Res.
35:505–508. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao S, Li A, Liu F, Chen F, Williams M,
Zhang C, Kelley Z, Wu CL, Luo R and Xiao H: NCOA5
haploinsufficiency results in glucose intolerance and subsequent
hepatocellular carcinoma. Cancer Cell. 24:725–737. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liang Y, Zhang T, Shi M, Zhang S, Guo Y,
Gao J and Yang X: Low expression of NCOA5 predicts poor prognosis
in human cervical cancer and promotes proliferation, migration, and
invasion of cervical cancer cell lines by regulating notch3
signaling pathway. J Cell Biochem. 120:6237–6249. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen GQ, Tian H, Yue WM, Li L, Li SH, Qi
L, Gao C, Si LB and Lu M: NCOA5 low expression correlates with
survival in esophageal squamous cell carcinoma. Med Oncol.
31:3762014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu Y, Wu J, Dong QR and Guo NZ:
Association between expression of nuclear receptor co-activator 5
protein and prognosis in postoperative patients with osteosarcoma.
Oncol Lett. 15:1888–1892. 2018.PubMed/NCBI
|
|
22
|
Yuan X, Zhang L, Cui Y, Yu Y, Gao X and Ao
J: NCOA5 is a master regulator of amino acid-induced mTOR
activation and β-casein synthesis in bovine mammary epithelial
cells. Biochem Biophys Res Commun. 529:569–574. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Zhang T, Qin S, Wang R, Li Y, Zhou
Z, Chen Y, Wu Q and Su F: Effects of UPF1 expression on EMT process
by targeting E cadherin, N cadherin, Vimentin and Twist in a
hepatocellular carcinoma cell line. Mol Med Rep. 19:2137–2143.
2019.PubMed/NCBI
|
|
24
|
Yamashita N, Tokunaga E, Iimori M, Inoue
Y, Tanaka K, Kitao H, Saeki H, Oki E and Maehara Y: Epithelial
Paradox: Clinical significance of coexpression of e-cadherin and
vimentin with regard to invasion and metastasis of breast cancer.
Clin Breast Cancer. 18:e1003–e1009. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Paolillo M and Schinelli S: Extracellular
matrix alterations in metastatic processes. Int J Mol Sci.
20:202019. View Article : Google Scholar
|
|
26
|
Diepenbruck M and Christofori G:
Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no,
maybe? Curr Opin Cell Biol. 43:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chaffer CL, San Juan BP, Lim E and
Weinberg RA: EMT, cell plasticity and metastasis. Cancer Metastasis
Rev. 35:645–654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bill R and Christofori G: The relevance of
EMT in breast cancer metastasis: Correlation or causality? FEBS
Lett. 589:1577–1587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wong SHM, Fang CM, Chuah LH, Leong CO and
Ngai SC: E-cadherin: Its dysregulation in carcinogenesis and
clinical implications. Crit Rev Oncol Hematol. 121:11–22. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang X, Liu G, Kang Y, Dong Z, Qian Q and
Ma X: N-cadherin expression is associated with acquisition of EMT
phenotype and with enhanced invasion in erlotinib-resistant lung
cancer cell lines. PLoS One. 8:e576922013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Guo L, Zhang K and Bing Z: Application of
a co expression network for the analysis of aggressive and non
aggressive breast cancer cell lines to predict the clinical outcome
of patients. Mol Med Rep. 16:7967–7978. 2017. View Article : Google Scholar : PubMed/NCBI
|