Introduction
Cervical cancer, one of the most common types of
malignancy worldwide, is the fourth leading cause of
cancer-associated mortality among females (1). Despite significant advances in
prevention, screening and diagnostic methods, certain cases are
diagnosed at locally advanced stages, such as Federation of
Gynecology and Obstetrics (FIGO) stages IIIA, IIIB and IVA.
Platinum-based concurrent chemoradiotherapy (CCRT) is currently
used as the standard treatment strategy for patients with advanced
disease (2–4). However, compared with patients with
early-stage disease, the prognosis of these patients is
unfavorable, with a 5-year survival rate of <60% (5,6).
Reducing the tumor size by administering neoadjuvant chemotherapy
(NAC) was indicated to facilitate the success of hysterectomy,
resulting in improved prognosis (7,8);
therefore, the use of NAC for the treatment of cervical cancer has
attracted significant attention in recent years (8). However, if NAC fails to sufficiently
reduce the tumor size to perform hysterectomy, the treatment
strategy is altered to radiation therapy, which delays the
initiation of core treatment and results in unfavorable prognosis
(9,10). Thus, if it were possible to predict
the response to NAC, this would have the potential to become one of
the major strategies to treat patients with locally advanced
cervical squamous cell carcinoma, providing a greater variety of
treatment options for patients. Therefore, to identify eligible
patients to receive NAC, there is an urgent requirement to discover
biomarkers indicating the response to NAC in patients with locally
advanced cervical squamous cell carcinoma.
The T-box (TBX) gene family consists of five
subfamilies, including T, Tbx1, Tbx2, Tbx6 and Tbr1. TBX genes have
a crucial role in organogenesis and pattern formation in vertebrate
and invertebrate species (11).
Transcription factors of the T-box families serve essential roles
throughout development (12).
Increased expression levels of TBX2 and TBX3, which are included in
the Tbx2 subfamily, are thought to be associated with the oncogenic
process. TBX2 is a transcription factor involved in embryonic
development, cell cycle regulation and cancer (13,14).
Cancer cells widely express TBX2 and it has been indicated to allow
cancer cells to bypass senescence by suppressing the cell cycle
regulators p21 and p14 (15–17). Suppressing p21 reportedly resulted in
chemoresistance by modulating the G1/S cell cycle
transition and inhibiting chemotherapy-induced apoptosis in lung
cancer (18). In addition, TBX2
expression levels were indicated to be upregulated in melanoma
(16), breast cancer (17,19),
prostate cancer (20), non-small
cell lung cancer (21), gastric
cancer (22), laryngeal squamous
cell carcinoma (23) and pancreatic
cancer (24). Furthermore, ectopic
TBX2 expression was associated with resistance to DNA-damaging
chemotherapeutic agents, such as doxorubicin and cisplatin
(25–27). However, to the best of our knowledge,
the relationship between TBX2 expression and platinum-based
chemotherapy in cervical squamous cell carcinoma has remained
largely elusive.
The present study investigated the value of TBX2
expression as an indicator of the effectiveness of NAC by
determining the relationship between TBX2 expression in tumors and
the response to NAC in patients with locally advanced uterine
cervical squamous carcinoma. Furthermore, the effect of small
interfering RNA (siRNA/si)-mediated knockdown of TBX2 expression on
the sensitivity of cervical cancer cells to cisplatin was evaluated
in vitro.
Materials and methods
Patient study
A total of 46 patients with FIGO stage IIIA and IIIB
uterine cervical squamous cell carcinoma who underwent NAC at Osaka
City University Hospital (Osaka, Japan) between April 1995 and
March 2010, were retrospectively evaluated. The inclusion criteria
were as follows: Patients were diagnosed as uterine cervical
squamous cell carcinoma histologically, stages IIIA and IIIB,
patients who underwent NAC and patients with available medical
records to analyze. Patients for whom sufficient medical records
were unavailable were excluded. A punch biopsy of tumor tissue was
performed before the administration of chemotherapy (cisplatin).
Clinical variables including age, FIGO stage, tumor size and the
effect of NAC treatment were obtained for each patient. Patients
were divided into two groups based on their response to NAC: i) NAC
effective group (n=25), which included patients who were
successfully treated with NAC, underwent a hysterectomy and
received radiotherapy; and ii) NAC ineffective group (n=21), which
comprised patients who experienced NAC treatment failure and only
received radiotherapy. The effect of NAC was evaluated by pelvic
examination and computed tomography or magnetic resonance imaging.
Successful NAC was defined as the stage being reduced to stage I or
II, making a hysterectomy possible, while in cases with
unsuccessful NAC, the tumor size was not sufficiently reduced to
perform a hysterectomy. All patients were administered 50, 75 or
100 mg/m2 cisplatin (Bristol Myers Squibb), which was
based on the renal function and age of the patients (28), intra-arterially via balloon-occluded
arterial infusion three times over 30 min. Written informed consent
was obtained from all patients prior to the tumor biopsy and the
experimental protocol was approved by the Institutional Review
Board of Osaka City University Hospital (Osaka, Japan; approval no.
4276).
Immunohistochemical (IHC)
staining
The sections (4 µm thick) were prepared from the
paraffin-embedded tissue blocks. The sections were deparaffinized
and endogenous peroxidase activity was blocked by immersing in 3%
hydrogen peroxidase in methanol. Antigen retrieval was performed by
immersing the samples in 10 mm citrate buffer (pH 6.0) and heating
the samples in an autoclave at 110°C for 20 min. IHC staining was
performed using the Dako LSAB2 Peroxidase kit (cat. no. K0675;
Agilent Technologies, Inc.). In brief, after blocking endogenous
peroxidase activity and performing antigen retrieval, tissue
sections were incubated with a rabbit polyclonal anti-TBX2 antibody
(1:50 dilution; cat. no. LS-C402301; LifeSpan BioSciences, Inc.) in
a humidity chamber at 4°C overnight. Following incubation with the
primary antibody, the sections were incubated with biotinylated
goat anti-mouse and anti-rabbit immunoglobulin G secondary
antibodies included in the Dako LSAB2 Peroxidase kit (cat. no.
K0675; Agilent Technologies, Inc.) at room temperature for 10 min.
The sections were subsequently incubated with a
streptavidin-peroxidase complex and 3,3′-diaminobenzidine was used
as the chromogen. The specificity control was prepared in the same
way with omission of the primary antibodies.
TBX2 expression levels were quantitatively scored
according to the weighted scoring method established by Sinicrope
et al (29). The IHC score
was determined using a light microscope (magnification, ×400) based
on the average percentage of stained tumor cells, which was scored
using a scale of 0–4 as follows: 0 (<5%), 1 (5–25%), 2 (25–50%),
3 (50–75%) and 4 (>75%). The intensity of cell staining was
scored as 1+ (weak), 2+ (moderate) and 3+ (intense). Weighted
scores were calculated by multiplying the score of the stained
tumor cell percentage and the score of the staining intensity.
Cell lines and culture
The CaSki cervical cancer cell line (cat. no.
IFO50007) was purchased from the Japanese Collection of Research
Bioresources Cell Bank. Cells were cultured in RPMI-1640 medium
(Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin, and
maintained in an incubator with 5% CO2 at 37°C.
Small interfering (si)RNA transfection
and cell survival assay
CaSki cells were seeded into 96-well plates
(2×103 cells/well) and divided into two groups: i)
Treated group, in which cells were transfected with TBX2-specific
siRNA (customized siTBX2; Sigma-Aldrich; Merck KGaA); and ii)
control group, in which cells were transfected with nontargeting
siRNA (Mission® Universal Negative Control #1; cat. no.
SIC001-10NMOL; Sigma-Aldrich; Merck KGaA). The siTBX2 sequence was
as follows: Sense, 5′-CCAAUGAACUGCAGAGCAU[dT][dT]-3′ and antisense,
5′-AUGCUCUGCAGUUCAUUGG[dT][dT]-3′. siTBX2 transfections were
performed using Lipofectamine® RNAiMax (Invitrogen;
Thermo Fisher Scientific, Inc.) strictly following the
manufacturer's protocol. After confirming cell adhesion in both
groups, the control group was provided with fresh medium containing
nontargeting siRNA transfection complexes, whereas the transfection
group was treated with fresh medium containing siTBX2 transfection
complexes. Following 24 h of incubation at 37°C, the cells in each
group were incubated for an additional 48 h at 37°C in fresh medium
containing 0, 2.5, 5.0, 7.5, 10 or 50 µM cisplatin. Cell viability
was subsequently measured using a Cell Counting Kit (CCK)-8 assay
(Dojindo Molecular Technologies, Inc.). In brief, 10 µl CCK-8 and
100 µl RPMI-1640 were added to each well, followed by incubation at
37°C for 2 h. The absorbance of each well was measured at a
wavelength of 450 nm using a microplate reader (Corona Electric
Co., Ltd.). The percentage of viable cells in comparison with the
control cells was evaluated using dose-response curves.
Reverse transcription-quantitative PCR
(RT-qPCR)
RT-qPCR was performed using TaqMan chemistry. The
procedure was performed according to the manufacturer's protocol.
TaqMan primer and probes for TBX2 (cat. no. Hs00911929_m1) and
hypoxanthine phosphoribosyltransferase 1 (cat. no. Hs02800695_m1)
were obtained from Thermo Fisher Scientific, Inc. Total RNA was
extracted from CaSki cells using the RNeasy Mini kit (Qiagen GmbH).
Total RNA (1 µg) was reverse transcribed into cDNA using a
High-Capacity cDNA Reverse Transcription kit (Thermo Fisher
Scientific, Inc.). qPCR was subsequently performed on an ABI 7500
Fast Real-Time PCR detection system (Applied Biosystems; Thermo
Fisher Scientific, Inc.) using TaqMan Fast Universal PCR Master Mix
(Thermo Fisher Scientific, Inc.). The following thermocycling
conditions were used for qPCR: Initial denaturation at 95°C for 20
sec, followed by 40 cycles of 95°C for 3 sec and 60°C for 30 sec.
The 2−∆∆Cq method was used to calculate the relative
changes in gene expression for the RT-qPCR experiments (30).
Statistical analysis
Statistical analysis was performed using EZR version
1.3 software (Saitama Medical Center, Jichi Medical University)
(31). Values are expressed as the
mean ± standard deviation. Statistically significant differences
between groups were determined using unpaired Student's t-tests.
Fisher's exact test was used to determine the association between
categorical variables in the different patient groups. The
Kaplan-Meier method and log-rank tests were used for survival
analysis. Mann-Whitney U-tests were used to compare the IHC
weighted scores. A receiver operating characteristic (ROC) curve
was used to determine the cutoff value of TBX2 score to predict the
effect of NAC treatment. P<0.05 was considered to indicate a
statistically significant difference.
Results
Patient characteristics
The 46 patients in the present study were divided
into two groups based on treatment efficacy: NAC effective group
(n=25) and NAC ineffective group (n=21). Differences in age, body
height, body weight, FIGO stage and tumor size were analyzed and no
statistically significant differences in these variables were
identified between the two groups (Table
I).
 | Table I.Clinicopathological features of
patients in the NAC effective and NAC ineffective groups. |
Table I.
Clinicopathological features of
patients in the NAC effective and NAC ineffective groups.
| Variable | NAC effective group
(n=25) | NAC ineffective
group (n=21) | P-value |
|---|
| Age, years |
|
|
|
| Mean ±
SD | 49.3±13.2 | 53.7±11.2 | 0.241a |
|
Range | 24–69 | 37–68 |
|
| Body height,
cm |
|
|
|
| Mean ±
SD | 154.5±6.67 | 152.1±5.09 | 0.182a |
|
Range | 138–166 | 143–162 |
|
| Body weight,
kg |
|
|
|
| Mean ±
SD | 53.01±8.76 | 48.73±8.19 | 0.096a |
|
Range | 37–78 | 38–67 |
|
| International
Federation of Gynecology and Obstetrics stage |
|
| 1b |
|
IIIA | 1 | 0 |
|
|
IIIB | 24 | 21 |
|
| Tumor size, mm |
|
| 0.464a |
| Mean ±
SD | 46.6±16.8 | 50.2±12.5 |
|
TBX2 protein expression levels in
cervical squamous cell carcinoma tissues
IHC was used to analyze TBX2 protein expression
levels in cervical squamous cell carcinoma tissues and the weighted
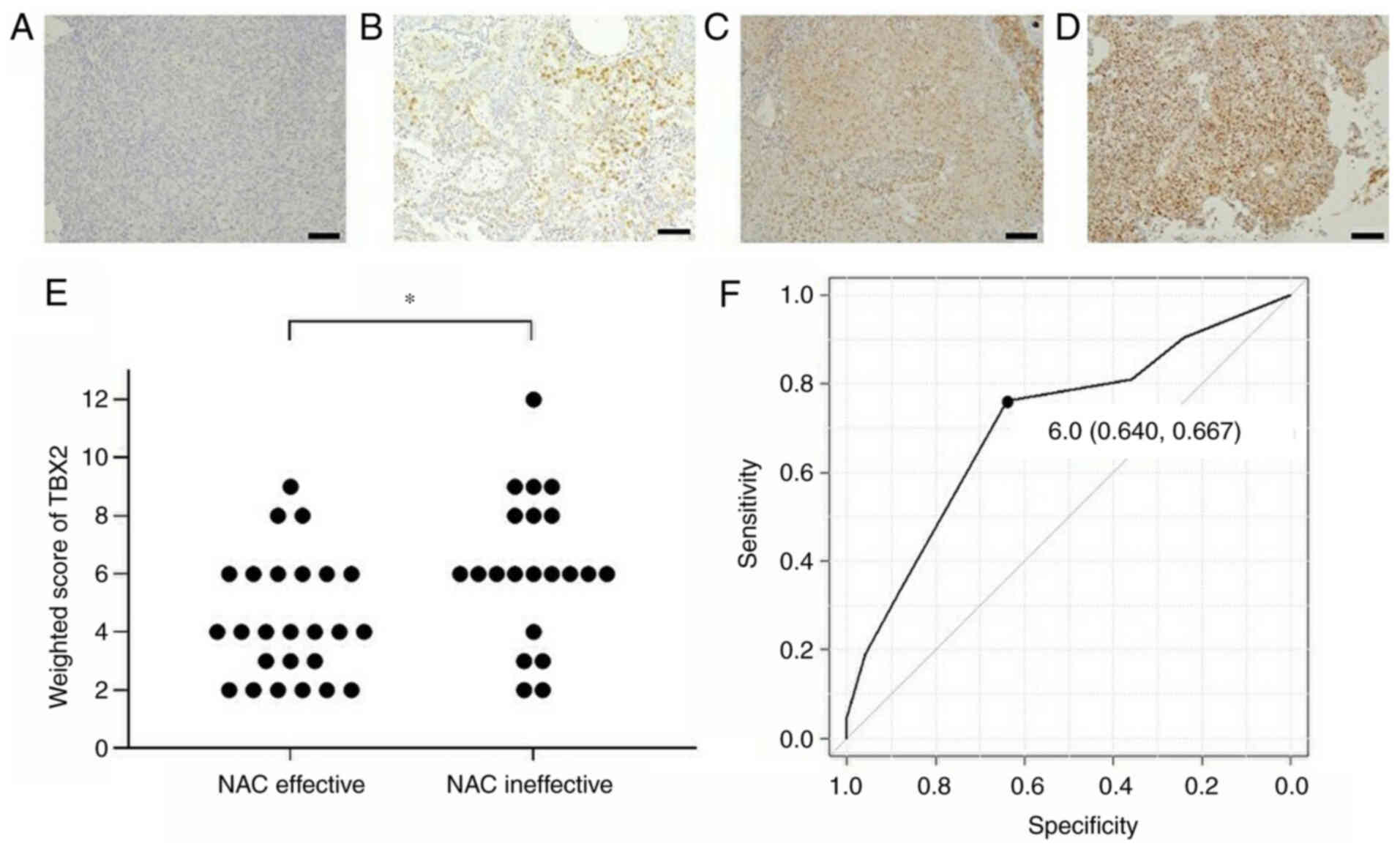
scores of both groups were calculated (Fig. 1A-E). TBX2 was indicated to be
predominantly located in the cell nuclei. The weighted score of the
NAC ineffective group was significantly increased compared with
that in the NAC effective group (P=0.0138; Fig. 1E). A ROC curve was used to determine
the cutoff value of TBX2 scores to predict the effectiveness of NAC
treatment (Fig. 1F). The ROC curve
indicated that with a cutoff value of 6, TBX2 scores were able to
predict the effectiveness of NAC, with a specificity of 64% and
sensitivity of 67%. The area under the curve was 0.662 with a 95%
confidence interval of 0.505–0.819. The patients were subsequently
divided into the following two groups based on their weighted
scores: Low TBX2 expression (weighted score, ≤4; n=21) and high
TBX2 expression (weighted score, ≥6; n=25). No statistically
significant differences were observed in the patient
characteristics between these two groups (Table II). These results suggested that
TBX2 expression may be associated with the efficacy of NAC.
 | Table II.Clinicopathological features of
patients in the TBX2 low and high groups. |
Table II.
Clinicopathological features of
patients in the TBX2 low and high groups.
| Variable | Low TBX2 expression
(score ≤4) (n=21) | High TBX2
expression (score ≥6) (n=25) | P-value |
|---|
| Age, years |
|
|
|
| Mean ±
SD | 50.4±12.7 | 52.0±12.6 | 0.659a |
|
Range | 24–69 | 29–68 |
|
| Body height,
cm |
|
|
|
| Mean ±
SD | 154.0±7.22 | 153.0±5.00 | 0.573a |
|
Range | 138–166 | 143–162 |
|
| Body weight,
kg |
|
|
|
| Mean ±
SD | 53.10±9.61 | 49.34±7.60 | 0.146a |
|
Range | 37–78 | 38–67 |
|
| International
Federation of Gynecology and Obstetrics stage |
|
| 1b |
|
IIIA | 1 | 0 |
|
|
IIIB | 20 | 25 |
|
| Tumor size, mm |
|
| 0.036a |
| Mean ±
SD | 42.8±14.1 | 52.8±14.4 |
|
Association between NAC sensitivity
and TBX2 expression
Whether there was a difference in NAC effectiveness
between the low TBX2 expression and high TBX2 expression groups was
subsequently evaluated. In the low TBX2 expression group, NAC was
effective in 76.2% of cases. By contrast, in the high TBX2
expression group, NAC was effective in 36% of patients. Of note,
this difference in effectiveness of NAC between the two groups was
statistically significant (P=0.009; Table III). These results indicated that
the low TBX2 expression group may be more sensitive to NAC compared
with the high TBX2 expression group.
 | Table III.Number of patients with low (score of
≤4) and high (score of ≥6) TBX2 expression in the NAC effective and
NAC ineffective groups. |
Table III.
Number of patients with low (score of
≤4) and high (score of ≥6) TBX2 expression in the NAC effective and
NAC ineffective groups.
| TBX2
expression | NAC effective
group | NAC ineffective
group | P-value |
|---|
| Low | 16 (76.2) | 5
(23.8) | 0.009a |
| High | 9
(36.0) | 16 (64.0) |
|
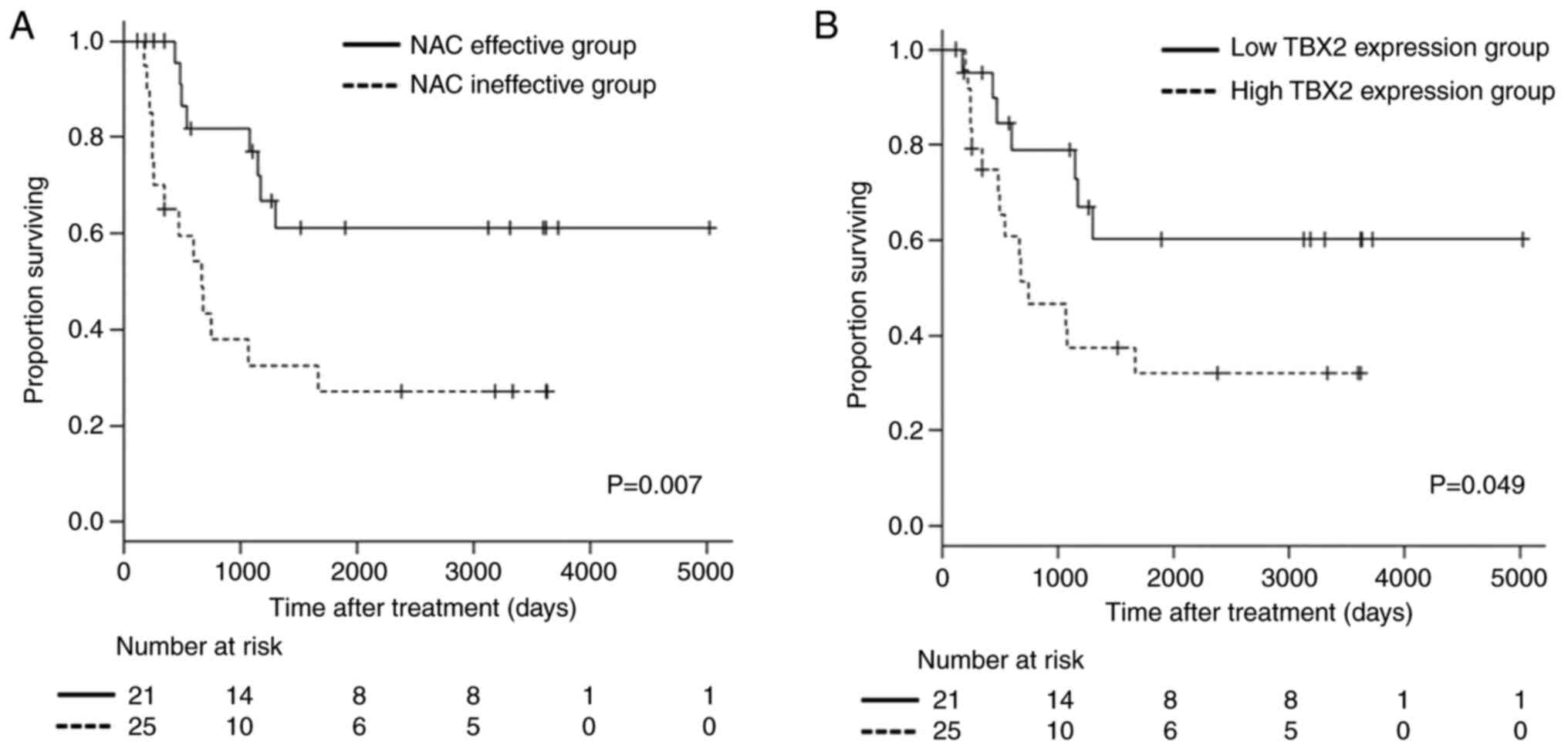
Survival analysis
The patients' follow-up period varied from 124 to
5,015 days. The overall survival of patients in the NAC effective
group was significantly improved compared with that in the NAC
ineffective group (P=0.007; Fig.
2A). In addition, the low TBX2 expression group had a more
favorable overall survival compared with the high TBX2 expression
group (P=0.049; Fig. 2B). These
results suggested that TBX2 expression may help predict the
prognosis of patients with locally advanced uterine cervical
squamous cell carcinoma who received NAC treatment.
Effect of TBX2 knockdown on the
cisplatin sensitivity of cervical cancer cells
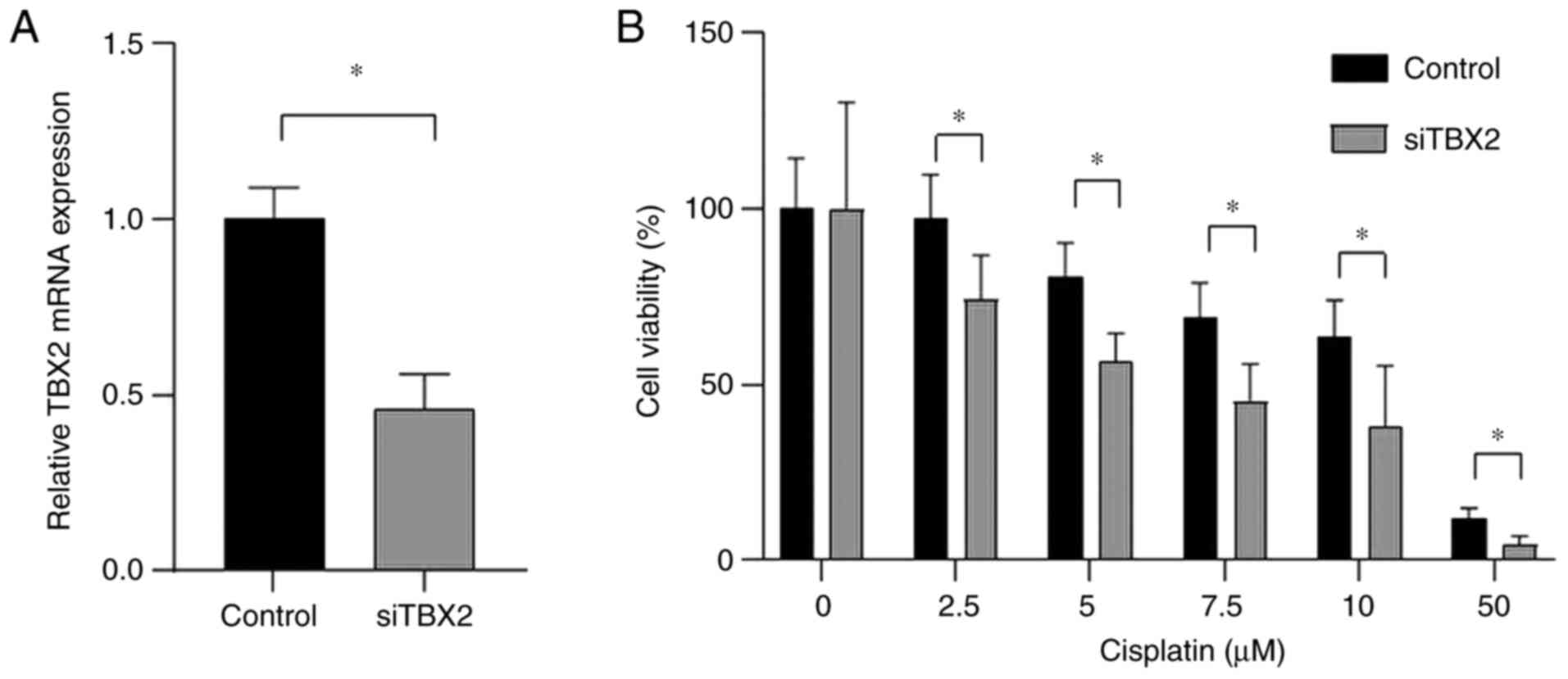
RT-qPCR analysis revealed that TBX2 mRNA expression
levels were significantly downregulated following transfection with
siTBX2 compared with control cells (Fig.
3A). Of note, CaSki cells transfected with siTBX2 had a
significantly enhanced sensitivity to cisplatin compared with
control cells (P<0.01; Fig. 3B).
These results suggested that TBX2 may be involved in the mechanism
through which cisplatin exerts cytotoxic effects on cancer
cells.
Discussion
NAC is a useful treatment option for patients with
cervical cancer; therefore, a significant amount of research has
focused on the use of NAC for patients with cervical cancer in
recent years. For instance, Sala et al (8) reported that NAC improved the survival
outcome for patients with stage IB2-IVA uterine cervical cancer in
a multicenter retrospective analysis. Chen et al (32) demonstrated that NAC reduced the
probability of lymph node metastasis for patients with stage
IB1-IIB uterine cervical cancer. In addition, de Vincenzo et
al (33) reported that the
administration of NAC followed by conization in stage IB2-IIA1
cervical cancer resulted in improved fertility. Sun et al
(34) also reported that treatment
with NAC provided an improved quality of life for patients with
stage IB2-IIA cervical cancer.
Platinum-based CCRT is currently used as a standard
treatment strategy for patients with locally advanced uterine
cervical squamous cell carcinoma (2–4).
However, the prognosis of these patients remains unfavorable,
suggesting that improvements in the available treatments are
required. Effective NAC treatment has been reported to reduce the
tumor size, allow patients to undergo hysterectomy and potentially
improve patient prognosis (7,8).
However, if NAC is ineffective, there are currently no alternatives
to surgical resection other than radiotherapy, which may lead to
unfavorable prognosis due to the delay in the initiation of core
treatment (35). Therefore,
identifying biomarkers that are able to predict the efficacy of NAC
remains important for selecting patients that are likely to benefit
from NAC treatment.
Cisplatin and other platinum-containing drugs exert
antitumor effects by covalently binding to DNA in cancer cells
(36), which facilitates apoptosis
by inhibiting DNA replication (37).
Usually, platinum-based chemotherapy is initially effective;
however, cancer cells may later develop resistance to these agents
(38). Potential mechanisms of
platinum resistance include decreased cellular uptake of cisplatin
(39,40), increased cisplatin detoxification
capacity (41), enhanced DNA damage
repair capacity (42,43), deactivation of apoptotic signaling
pathways (44) and other epigenetic
modifications that occur at both the cellular and molecular levels
(45,46).
TBX2 is a transcription factor that was discovered
to be involved in the regulation of cell cycle progression during
cancer and embryonic development (13,14).
TBX2 was indicated to be widely expressed in cancer cells and
permits them to bypass senescence by suppressing the cell cycle
regulators p21 and p14ARF (15–17). In
addition to its role in cell cycle regulation, p21 also mediates
apoptotic signaling pathways (47).
Several studies have demonstrated that p21 has a proapoptotic role
in cancer (48). For instance, p21
induction by RNA activation enhanced antitumor activity by
promoting cell cycle arrest and apoptotic cell death in bladder
cancer cells (48). Transcriptional
activation of p21 inhibited the viability of hepatocellular
carcinoma cells and significantly increased cell apoptosis by
downregulating the expression levels of anti-apoptotic proteins,
including survivin and Bcl-xL, and upregulating the expression of
executioner caspase-3 and −9 (49).
In addition, TBX2 has been reported to contribute to cancer cell
resistance to therapeutic agents, such as cisplatin (25,26), by
modulating the G1/S cell cycle transition and inhibiting
chemotherapy-induced apoptosis via suppression of the cell cycle
regulator p21 (18). Those reports
regarding cancer cell resistance to cisplatin are consistent with
the present results and they support the present results.
The results of the present study predicted NAC
efficacy in patients with locally advanced uterine cervical
squamous cell carcinoma by determining TBX2 expression levels in
histological samples obtained prior to the initiation of treatment.
Patients with downregulated TBX2 protein expression levels were
more susceptible to NAC treatment and more likely to undergo
successful surgery following NAC treatment, resulting in improved
prognosis.
To the best of our knowledge, the present study was
the first to suggest the potential of determining TBX2 expression
in tumors to predict the efficacy of NAC treatment in patients with
locally advanced uterine cervical squamous cell carcinoma. In
addition, these results may improve the response to NAC treatment
in patients with any stage of cervical squamous cell carcinoma by
making it possible to select eligible patients that are likely to
benefit from NAC treatment.
However, only 46 patients were included in the
present retrospective study; therefore, the major limitations of
the present study are the small number of patients used and the
retrospective design. And even though human papillomavirus (HPV)
infection status is a crucial factor when investigating uterine
cervical cancer, those data of the patients included in the present
study were not available. Further studies involving a larger number
of cases and data including the HPV infection status are required
to address this issue and validate the present findings.
Furthermore, the study was performed at a single institution
without any external validation. In addition, the present study
remains a hypothesis-exploratory study. Therefore, future studies
are required to be performed at multiple centers to validate the
present results.
In conclusion, the results of the present study
indicated that TBX2 expression may represent a useful predictor of
the response to NAC for patients with locally advanced uterine
cervical squamous cell carcinoma. These results may be applied to
patients with any stage of cervical squamous cell carcinoma to
predict whether they may benefit from NAC treatment.
Acknowledgements
The authors would like to thank Dr Yukimi Kira
(Research Support Platform of Osaka City University Graduate School
of Medicine; Osaka, Japan) for their technical support.
Funding
The present study was funded by The Osaka Medical
Research Foundation for Intractable Diseases (grant no.
26-2-47).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author upon reasonable
request.
Authors' contributions
YI, TF and TS designed the study. YI, HM, SN, YA, MS
and MY performed the experiments and collected the data. YI, TF, TY
and TS analyzed the data. YI and TF wrote the manuscript. YI and TF
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study protocol was approved by the
Institutional Review Board of Osaka City University Hospital
(approval no. 4276; Osaka, Japan). Written informed consent was
obtained from all patients prior to participation.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Japan Society of Gynecologic Oncology
(eds), . Formulation Committee of the Treatment Guidelines for
Cervical Cancer, 2011. Kanehara & Co.; Tokyo: 2011, (In
Japanese).
|
|
4
|
National Comprehensive Cancer Network.
NCCN Clinical Practice Guidelines in Oncology, . Cervical Cancer
Version II. 2013, https://www2.tri-kobe.org/nccn/guideline/gynecological/english/cervical.pdf
|
|
5
|
Morris M, Eifel PJ, Lu J, Grigsby PW,
Levenback C, Stevens RE, Rotman M, Gershenson DM and Mutch DG:
Pelvic radiation with concurrent chemotherapy compared with pelvic
and para-aortic radiation for high-risk cervical cancer. N Engl J
Med. 340:1137–1143. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Eifel PJ, Winter K, Morris M, Levenback C,
Grigsby PW, Cooper J, Rotman M, Gershenson D and Mutch DG: Pelvic
irradiation with concurrent chemotherapy versus pelvic and
para-aortic irradiation for high-risk cervical cancer: An update of
radiation therapy oncology group trial (RTOG) 90-01. J Clin Oncol.
22:872–880. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ishiko O, Sumi T, Yasui T, Matsumoto Y,
Kawamura N, Ogita S, Kamino T, Nakamura K and Yamada R:
Balloon-occluded arterial infusion chemotherapy, simple total
hysterectomy and radiotherapy as a useful combination-therapy for
advanced cancer of the uterine cervix. Oncol Rep. 7:141–144.
2000.PubMed/NCBI
|
|
8
|
Sala P, Bogliolo S, Barra F, Fazio A,
Maramai M, Cassani C, Gardella B, Babilonti L, Giannelli F,
Mammoliti S, et al: Neoadjuvant chemotherapy followed by radical
surgery versus concurrent chemo-radiotherapy in the treatment of
locally advanced cervical cancer: A multicenter retrospective
analysis. J Invest Surg. 8:1–7. 2020. View Article : Google Scholar
|
|
9
|
Souhami L, Gil RA, Allan SE, Canary PC,
Araújo CM, Pinto LH and Silveira TR: A randomized trial of
chemotherapy followed by pelvic radiation therapy in stage IIIB
carcinoma of the cervix. J Clin Oncol. 9:970–977. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tattersall MH, Lorvidhaya V, Vootiprux V,
Cheirsilpa A, Wong F, Azhar T, Lee HP, Kang SB, Manalo A and Yen
MS: Randomized trial of epirubicin and cisplatin chemotherapy
followed by pelvic radiation in locally advanced cervical cancer.
Cervical cancer study group of the Asian oceanian clinical oncology
association. J Clin Oncol. 13:444–451. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang F, Xing P, Song F, Du X, Wang G,
Chen K and Yang J: The role of T-box genes in the tumorigenesis and
progression of cancer. Oncol Lett. 12:4305–4311. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Papaioannou VE: The T-box gene family:
Emerging roles in development, stem cells and cancer. Development.
141:3819–3833. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bilican B and Goding CR: Cell cycle
regulation of the T-box transcription factor tbx2. Exp Cell Res.
312:2358–2366. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Abrahams A, Parker MI and Prince S: The
T-box transcription factor Tbx2: Its role in development and
possible implication in cancer. IUBMB Life. 62:92–102.
2010.PubMed/NCBI
|
|
15
|
Peres J, Davis E, Mowla S, Bennett DC, Li
JA, Wansleben S and Prince S: The highly homologous T-Box
transcription factors, TBX2 and TBX3, have distinct roles in the
oncogenic process. Genes Cancer. 1:272–282. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vance KW, Carreira S, Brosch G and Goding
CR: Tbx2 is overexpressed and plays an important role in
maintaining proliferation and suppression of senescence in
melanomas. Cancer Res. 65:2260–2268. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jacobs JJ, Keblusek P, Robanus-Maandag E,
Kristel P, Lingbeek M, Nederlof PM, van Welsem T, van de Vijver MJ,
Koh EY, Daley GQ and van Lohuizen M: Senescence bypass screen
identifies TBX2, which represses Cdkn2a (p19(ARF) and is amplified
in a subset of human breast cancers. Nat Genet. 26:291–299. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Zhu LJ, Yang YC, Wang ZX and Wang
R: miR-224 promotes the chemoresistance of human lung
adenocarcinoma cells to cisplatin via regulating G1/S
transition and apoptosis by targeting p21(WAF1/CIP1). Br J Cancer.
15:339–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Taneja P, Maglic D, Kai F, Zhu S, Kendig
RD, Fry EA and Inoue K: Classical and novel prognostic markers for
breast cancer and their clinical significance. Clin Med Insights
Oncol. 4:15–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nandana S, Tripathi M, Duan P, Chu CY,
Mishra R, Liu C, Jin R, Yamashita H, Zayzafoon M, Bhowmick NA, et
al: Bone metastasis of prostate cancer can be therapeutically
targeted at the TBX2-WNT signaling axis. Cancer Res. 77:1331–1344.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang Z and Guo Y: High TBX2 expression
predicts poor prognosis in non-small cell lung cancer. Neoplasma.
61:476–480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yu H, Liu BO, Liu A, Li K and Zhao H:
T-box 2 expression predicts poor prognosis in gastric cancer. Oncol
Lett. 10:1689–1693. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang Y, Li Z, Zhong Q, Li G, Zhang Y and
Huang Z: Association of TBX2 and P21 expression with
clinicopathological features and survival of laryngeal squamous
cell carcinoma. Int J Clin Exp Med. 7:5394–5402. 2014.PubMed/NCBI
|
|
24
|
Mahlamäki EH, Bärlund M, Tanner M,
Gorunova L, Höglund M, Karhu R and Kallioniemi A: Frequent
amplification of 8q24, 11q, 17q and 20q-specific genes in
pancreatic cancer. Genes Chromosomes Cancer. 35:353–358. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Davis E, Teng H, Bilican B, Parker MI, Liu
B, Carriera S, Goding CR and Prince S: Ectopic Tbx2 expression
results in polyploidy and cisplatin resistance. Oncogene.
27:976–984. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ismail A and Bateman A: Expression of TBX2
promotes anchorage-independent growth and survival in the
p53-negative SW13 adrenocortical carcinoma. Cancer Lett.
278:230–240. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wansleben S, Davis E, Peres J and Prince
S: A novel role for the anti-senescence factor TBX2 in DNA repair
and cisplatin resistance. Cell Death Dis. 4:e8462013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuji K, Yamada R, Kawabata M, Mitsuzane
K, Sato M, Iwahashi M, Kitayama S and Nakano R: Effect of balloon
occluded arterial infusion of anticancer drugs on the prognosis of
cervical cancer treated with radiation therapy. Int J Radiat Oncol
Biol Phys. 32:1337–1345. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: Bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kanda Y: Investigation of the freely
available easy-to-use software ‘EZR’ for medical statistics. Bone
Marrow Transplant. 48:452–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen B, Wang L, Ren C, Shen H, Ding W, Zhu
D, Mao L and Wang H: The effect of neoadjuvant chemotherapy on
lymph node metastasis of FIGO stage IB1-IIB cervical cancer: A
systematic review and meta-analysis. Front Oncol. 10:5702582020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Vincenzo R, Ricci C, Fanfani F, Gui B,
Gallotta V, Fagotti A, Ferrandina G and Scambia G: Neoadjuvant
chemotherapy followed by conization in stage IB2-IIA1 cervical
cancer larger than 2 cm: A pilot study. Fertil Steril. 115:148–156.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun Z, Huang B, Liu C, Yang Y, Rao Y, Du Y
and Ma Y: Comparison of neoadjuvant treatments followed by radical
surgery or chemoradiation on quality of life in patients with stage
IB2-IIA cervical cancer. Gynecol Oncol. 157:536–541. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Panici PB, Bellati F, Manci N, Pernice M,
Plotti F, Di Donato V, Calcagno M, Zullo MA, Muzii L and Angioli R:
Neoadjuvant chemotherapy followed by radical surgery in patients
affected by FIGO stage IVA cervical cancer. Ann Surg Oncol.
14:2643–2648. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bose RN: Biomolecular targets for platinum
antitumor drugs. Mini Rev Med Chem. 2:103–111. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang D and Lippard SJ: Cellular processing
of platinum anticancer drugs. Nat Rev Drug Discov. 4:307–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Brabec V and Kasparkova J: Molecular
aspects of resistance to antitumor platinum drugs. Drug Resist
Updat. 5:147–161. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Morimoto A, Serada S, Enomoto T, Kim A,
Matsuzaki S, Takahashi T, Ueda Y, Yoshino K, Fujita M, Fujimoto M,
et al: Annexin A4 induces platinum resistance in a chloride-and
calcium-dependent manner. Oncotarget. 5:7776–7787. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Surowiak P, Materna V, Kaplenko I,
Spaczyński M, Dietel M, Lage H and Zabel M: Augmented expression of
metallothionein and glutathione S-transferase pi as unfavourable
prognostic factors in cisplatin-treated ovarian cancer patients.
Virchows Arch. 447:626–633. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Martin LP, Hamilton TC and Schilder RJ:
Platinum resistance: The role of DNA repair pathways. Clin Cancer
Res. 14:1291–1295. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu RY, Dong Z, Liu J, Yin JY, Zhou L, Wu
X, Yang Y, Mo W, Huang W, Khoo SK, et al: Role of eIF3a in
regulating cisplatin sensitivity and in translational control of
nucleotide excision repair of nasopharyngeal carcinoma. Oncogene.
30:4814–4823. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Q, Shi S, He W, Padilla MT, Zhang L,
Wang X, Zhang B and Lin Y: Retaining MKP1 expression and
attenuating JNK-mediated apoptosis by RIP1 for cisplatin resistance
through miR-940 inhibition. Oncotarget. 5:1304–1314. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liu RY, Dong Z, Liu J, Zhou L, Huang W,
Khoo SK, Zhang Z, Petillo D, Teh BT, Qian CN and Zhang JT:
Overexpression of asparagine synthetase and matrix
metalloproteinase 19 confers cisplatin sensitivity in
nasopharyngeal carcinoma cells. Mol Cancer Ther. 12:2157–2166.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: A cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fujiwara K, Daido S, Yamamoto A, Kobayashi
R, Yokoyama T, Aoki H, Iwado E, Shinojima N, Kondo Y and Kondo S:
Pivotal role of the cyclin-dependent kinase inhibitor p21WAF1/CIP1
in apoptosis and autophagy. J Biol Chem. 283:388–397. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen Z, Place RF, Jia ZJ, Pookot D, Dahiya
R and Li LC: Antitumor effect of dsRNA-induced p21(WAF1/CIP1) gene
activation in human bladder cancer cells. Mol Cancer Ther.
7:698–703. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Wu ZM, Dai C, Huang Y, Zheng CF, Dong QZ,
Wang G, Li XW, Zhang XF, Li B and Chen G: Anti-cancer effects of
p21WAF1/CIP1 transcriptional activation induced by dsRNAs in human
hepatocellular carcinoma cell lines. Acta Pharmacol Sin.
32:939–946. 2011. View Article : Google Scholar : PubMed/NCBI
|