Introduction
Glioblastoma multiforme (GBM) is the most common
type of malignant tumour of the brain in adults (1). There are currently no curative
treatments for this tumour entity, thus resulting in a median
survival of 14 months (1,2). This could be due to the heterogeneity
in terms of genetic and epigenetic alterations (3), which triggers GBM tumourigenesis.
Cancer stem cells may be considered as a therapeutic target for the
treatment of GBM (4). Furthermore,
it has been suggested that the tumour environment can maintain GBM
growth, resistance to conventional therapy and immune escape
(4,5). Paez-Gonzalez et al suggested
that neuronal networks could regulate the proliferation and
differentiation of normal neural stem cells in the developed brain
(6).
Although they have been recognized as crucial
components of central and peripheral neurotransmission,
acetylcholine (ACh) and its receptors (AChR) are evolutionarily
conserved and are also broadly expressed in several other
non-neuronal cell types, including bronchial epithelial, glial,
pulmonary vascular, ovarian and cancer cells (7,8).
Νicotinic AChRs (nAChRs), as the main regulators of
complex inhibitory and stimulatory networks, mediate the production
and release of growth, angiogenic and neurogenic factors in cancer
cells (7,9). Furthermore, it has been reported that
nAChRs regulate the activation of intracellular signalling pathways
in a cell type-specific manner (10).
Emerging evidence has suggested that alpha 7 subunit
(α7) nAChRs (11) regulate the
cholinergic anti-inflammatory pathways (12,13).
Among these pathways, a study showed that ACh dose-dependently
inhibited the TNF-α and IL-6 pathways in human macrophages
(14). α7 nAChRs are characterized
by the presence of the genetically duplicated fusion gene
CHRFAM7A (15,16). The chimeric gene product, dup α7,
interacts with the α7 subunits to form a dominant-negative and
dysfunctional receptor. The hybrid, resulting from the fusion of
the partial duplication of CHRNA7 with FAM7,
CHRFAM7A, is expressed in the brain and peripheral tissues
(15–18).
The present study aimed to investigate the role of
ACh, nicotine, α-bungarotoxin (α-BTX) and
3-(2,4-dimethoxybenzylidene) anabaseine (GTS-21) in inhibiting the
proliferation of primary GBM cells and GBM cell lines, namely
A-172, G-28 and U-87.
Materials and methods
Patient characteristics
The data and samples of the patient sample used in
our previous study were reclaimed The samples are collected between
01/2006 and 12/2012 (Departments of Neurosurgery and
Neuropathology, University of Giessen, Germany) (19). At the time of diagnosis, the mean age
of the 44 patients with GBM was 57.4±15.7 years (f:m=13:31), while
the progression-free survival (PFS) time of the patients was 16
months. In the present study, all cases received a gross total
resection. The overall survival (OS) of patients with MGMT
promoter methylation was longer (OS, 23 months; range, 14.8–29.2)
compared with those without MGMT promoter methylation (OS,
11 months; range, 5.5–16.5) (19).
Inclusion criteria was diagnosed GBM, exclusion criteria were
missing consent, missing files and Karnofsky-Index < 80. All
patients provided written informed consent prior to enrolment in
the study (Table I).
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value |
|---|
| Total cases, n | 44 |
| Age at diagnosis,
years | 57.4 |
| Male, n | 31 |
| Female, n | 13 |
| Survival
methylated, months | 22 |
| Survival
unmethylated, months | 11 |
| Resection, n | 41 |
| Missing data,
n | 3 |
Chemicals and cell lines
Nicotinehydrogentatrate (cat. no. N3260) and ACh
were purchased from Sigma-Aldrich (Merck KGaA). GTS-21 (cat. no.
4557) was obtained from Tocris Bioscience, while α-BTX (cat. no.
B1601) was purchased from Thermo Fisher Scientific, Inc. The A172,
G28, U87 cell lines were purchased from ATCC. U87 cell line is a
glioblastoma of unknown origin (HTB-14).
Tissue specimens from patients with histologically
confirmed GBM were immediately collected after surgery. To isolate
cells for the primary cell cultures, the tissues were finely
chopped in DMEM (Dubelcco's modified Eagles medium) and trypsinized
using an 0.05% EDTA/trypsin solution. The trypsin reaction was
stopped by adding 10 ml DMEM and to obtain a single cell
suspension, the minced tissues were passed through a 60-µm cell
strainer (Sigma-Aldrich; Merck KGaA). Subsequently, cells were
washed with PBS (Phosphate buffered saline). Following
centrifugation, the cell pellet was resuspended in DMEM and the
cells were then seeded into 25 cm2 culture flasks
(Greiner Bio-One GmbH) and cultured in an incubator at 37°C and 5%
CO2. Then, following the appropriate number of passages,
the cells were expanded to 175 cm2 flasks (Greiner
Bio-One GmbH). For cell cryopreservation, the cells were first
washed with PBS, centrifugated, resuspended in DMSO (Merck KGaA)
and finally stored in liquid nitrogen. Frozen cells were thawed in
a 37°C-water bath and were then cultured as adherent monolayers in
25-cm2 flasks. The clinicopathological characteristics
of patients are listed in Table
I.
RNA isolation and cDNA synthesis
For total RNA isolation from frozen specimens, the
RNeasy Lipid Tissue Mini Kit® (Qiagen GmbH) was used. To
ensure proper tissue dissociation, the specimens were treated with
a lysis reagent, provided by the kit, for 2 min in a bead mill at
5,000 rpm. The RNA concentration was measured with
NanoDrop® 1000 spectrophotometer (Thermo Fisher
Scientific Inc.). cDNA synthesis was carried out using 0.5 µg total
RNA/sample as a template with the RT2 First Strand Kit
(Qiagen GmbH).
Gene expression analysis
For the simultaneous analysis of 31 genes, including
28 target genes and three housekeeping genes, namely RPL13A,
TBB and GAPDH, a Custom RT2 Profiler PCR
Array was used, containing lyophilized primers for each target gene
(CAPH12576; cat. no. 330231; Qiagen GmbH). The analysis was carried
out on the StepOnePlus quantitative PCR cycler (Thermo Fisher
Scientific, Inc.) using the RT2 SYBR®-Green
Mastermix (Qiagen GmbH) according to the manufacturer's
recommendations. For the analysis, the qPCR results from all
samples were merged and uploaded into the online analysis tool
provided by the manufacturer. The online tool calculates the
ΔΔCq-based fold change in the expression of each target gene
normalized to that of the housekeeping genes.
MTT assay
To evaluate cell survival and proliferation, a MTT
assay was performed following treatment of cells with GTS-21
(Tocris Bioscience), ACh or nicotine (both from Sigma-Aldrich;
Merck KGaA). Briefly, cells were seeded into 96-well plates at a
density of 104 cells/well. All cell lines were treated
with 6.25, 12.5, 25 or 50 µM GTS-21, ACh or nicotine. The negative
control cells for the GTS-21 group were treated with 0.1% DMSO,
while those of the ACh and nicotine groups with 0.1%
H2O. The MTT reagent (Sigma-Aldrich; Merck KGaA) was
diluted in DMEM at a ratio of 1:10. Following treatment of cells
with the corresponding compounds for 24, 48 and 72 h, the culture
medium was removed and the cells were supplemented with 200 µl of
MTT-DMEM solution and incubated at 37°C in an atmosphere of 95% air
and 5% CO2 for an additional 90 min. Subsequently, the
cells were lysed in 150 µl isopropanol/hydrochloric acid solution
(Sigma-Aldrich; Merck KGaA) for 15 min and the absorbance value in
each well was measured using a microplate reader at a wavelength of
562 nm, with a reference wavelength of 630 nm.
Statistical analysis
Statistical analysis was implemented using SPSS 20
(IBM Corp.). The comparison of different concentrations of GTS-21
and α-bungarotoxin were performed using Kruksal-Wallis-test (post
hoc: Bonferroni). P<0.05 was considered to indicate a
statistically significant difference. Data are presented as the
mean ± SEM.
Results
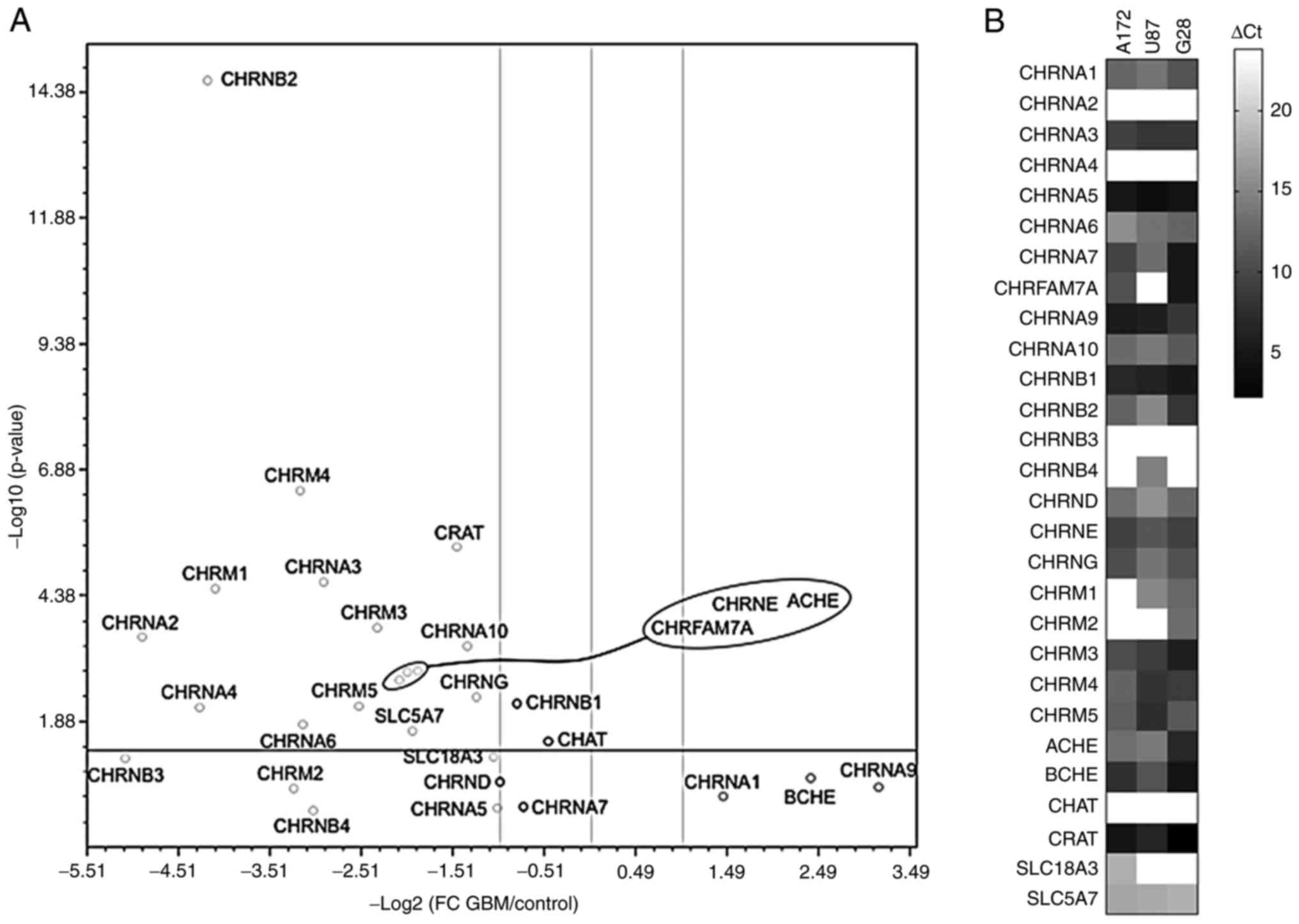
Cholinergic system-related gene
expression is markedly deregulated in GBM
Using a custom-made qPCR array covering the majority
of components of the cholinergic system, the expression levels of
these genes were assessed in 44 tissue samples obtained from
patients with primary GBM and compared with those in five tissue
samples from healthy subjects (Fig.
1A). The results demonstrated that the expression of the
majority of genes encoding the receptor subunits was significantly
downregulated (fold changes, log2 >-1; P<0.01).
Additionally, the gene expression levels of ACHE and those
of the dominant-negative form of CHRNA7 and the duplicate
fusion gene product of CHRFAM7A were also significantly
suppressed. In addition, the expression levels of three genes,
namely CHRNB1, encoding the nicotinic β1 receptor subunit,
CHAT, encoding choline acetyltransferase and CHRNA7,
encoding the nicotinic α7 receptor subunit, remained unchanged.
The expression levels of the cholinergic
system-related genes were also determined in the established GBM
cell lines A172, U87 and G28 (Fig.
1B). CHRNA2 was not expressed in any of the three cell
lines examined. By contrast, CHRNA1, CHRNA9 and BCHE
were expressed in all cell lines. The most significant differences
in the gene expression profile between primary GBM cells and cell
lines were observed in CHAT expression, as CHAT was
not expressed in A172, U87 and G28 cells compared with primary GBM
cells. This finding could be attributed to the loss of ACh
synthesis. Despite the similarities, the cell lines exhibited
quantitative and qualitative heterogeneity in the expression of
several genes, including CHRM2, which was expressed in G28,
but not in A172 and U87 cells and the fusion gene product
CHRFAM7A, which was not expressed in U87 cells.
Overall, the aforementioned findings revealed
significant differences in the expression of cholinergic
system-related genes in GBM. More specifically, the expression of
CHRNA7 remained unchanged in all cell lines examined,
indicating that these cell lines may serve as important tools for
evaluating the role of CHRNA7 in GBM. Furthermore, the
heterogeneity in the expression of CHRFAM7A could contribute
to a better understanding of the participation of the
dominant-negative regulator in the cholinergic system.
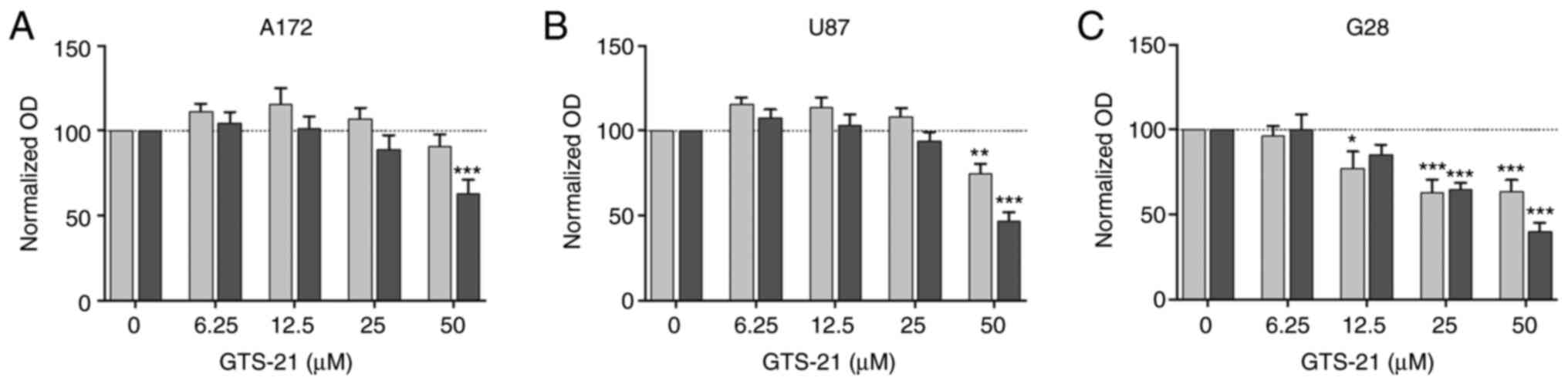
GTS-21, but no other cholinergic
agonists, inhibits GBM cell proliferation
Subsequently, the present study aimed to investigate
the effect of the activation of the α7-composed nAChR on cellular
proliferation. Therefore, the three GBM cell lines A172, U87 and
G28 were stimulated with increasing concentrations of GTS-21, a
well-known partial agonist of α7 and α4β2 subunits, for 24 and 48
h. Since both α4 and β2 subunits were not expressed in the current
cell model, GTS-21 served as a sole α7 agonist. Cell treatment with
50 µM GTS-21 significantly attenuated the proliferation of all cell
lines at 48 h (Fig. 2). However, the
extent of reduction differed among the different GBM cell lines
(A172 cells, 63.3±7.5%; G28 cells, 39.4±5.3%; U87 cells,
47.2±4.8%). Additionally, GTS-21 exerted an antiproliferative
effect on G28 cells even after treatment with 12.5 µM GTS-21 for 24
h (Fig. 2C). However, treatment of
cells with equimolar concentrations of cholinergic agonists, namely
nicotine and ACh, had no effect on cell viability (Fig. S1). In summary, pharmacological
stimulation of the α7 receptor subunits with GTS-21 reduced GBM
cell proliferation.
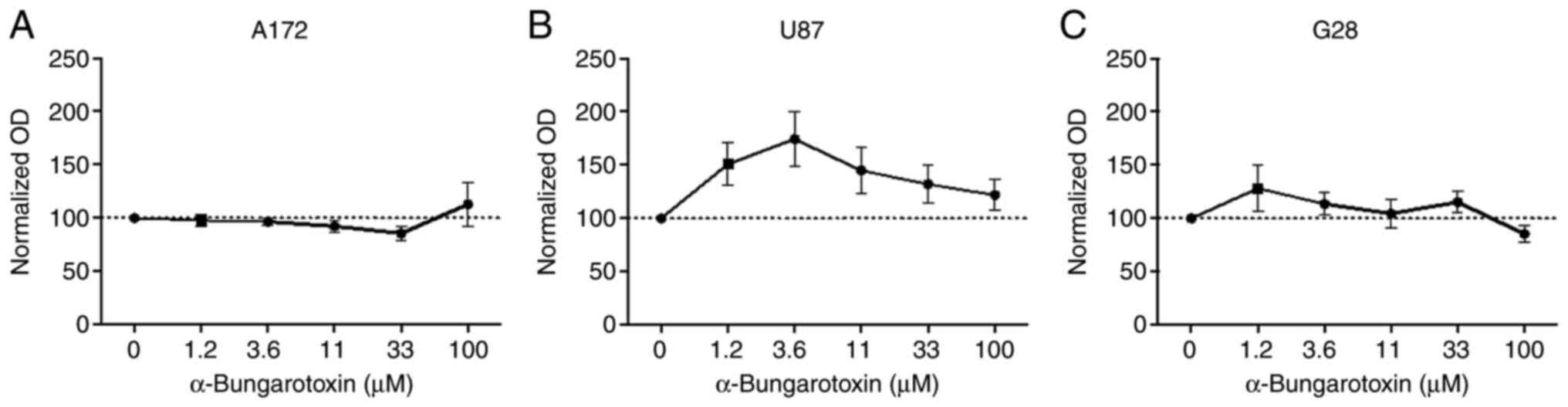
α7 subunit affects the
antiproliferative capacity of GTS-21
To investigate whether the antiproliferative effect
of GTS-21 was associated with the α7 subunit, GBM cells were
co-treated with 50 µM GTS-21 and increasing concentrations of α-BTX
(1.2–100 µM), an antagonist of the homo-pentameric α7 receptor, for
48 h. The results showed that treatment of A172 and G28 cells with
α-BTX had no effect on their proliferation capacity (Fig. 3A and C). However, the proliferation
rate of U87 cells treated with 3.6 µM α-BTX was notably increased
(174.6±21.71%; Fig. 3B). This
finding indicated that the proliferative effects of GTS-21 could be
abolished by α-BTX only in U87 cells.
GTS-21 inhibits the proliferation of
primary GBM cells
A total of eight primary GBM cell cultures were
treated with 6.25, 12.5, 25 or 50 µM GTS-21 for 48 h. The
proliferation rate of primary tumour cells treated with different
doses of GTS-21 was significantly reduced compared with A172, G28
and U87 cells (Fig. 4), suggesting
that all primary cells responded to treatment. The median
inhibition rate (loss of proliferation) in primary GBM cells was
51%, ranging between 36 and 84%.
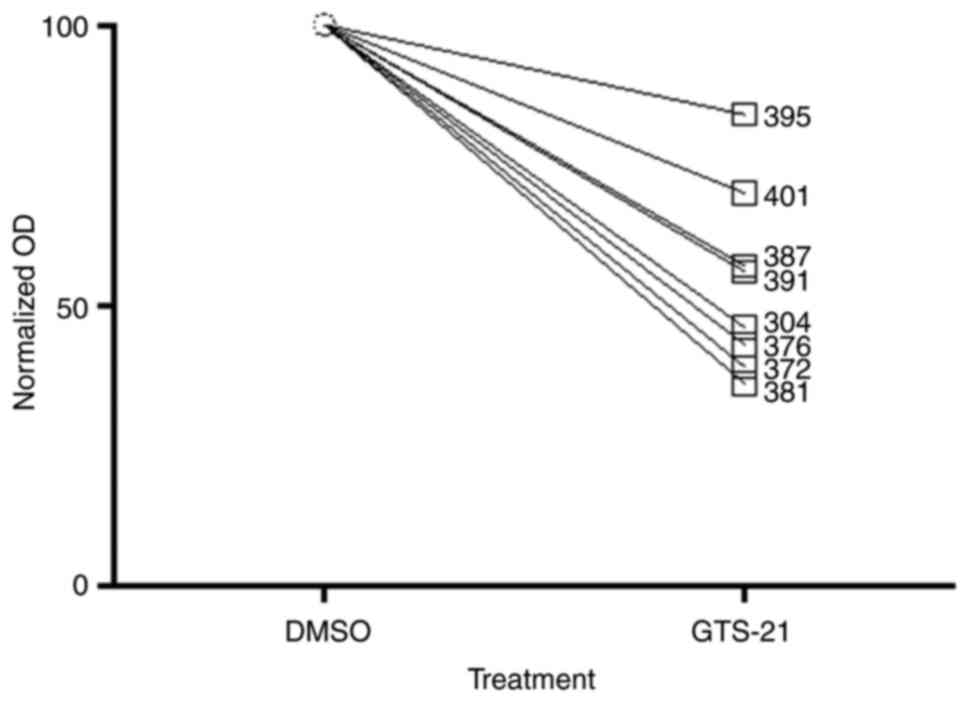 | Figure 4.GTS-21 treatment inhibits
proliferation of cultured primary GBM cells. A total of 8 primary
GBM cell lines (395, 401, 387, 391, 304, 376, 372 and 381) were
treated with GTS-21 (50 µM) for 48 h, after which cell
proliferation was assessed using an MTT assay. The OD of each
sample was normalized to the corresponding DMSO-treated control
sample. Data are presented as the mean ± SEM (n=4). GBM,
glioblastoma multiforme; GTS-21, 3-(2,4-dimethoxybenzylidene). |
Discussion
Normal brain function depends on the presence and
interaction between diverse neurotransmitters and neuropeptides.
Emerging evidence has demonstrated that neuropeptides and
neurotransmitters, as well as their cognate receptors, are
expressed by astrocytes (20).
Therefore, the ligand-receptor-mediated cellular responsiveness
could also be considered as an adjunctive therapy approach to GBM.
However, persistent invasiveness and resistance to standard therapy
remain the major challenges in the development of more effective
treatment options for GBM.
Nevertheless, novel therapies are urgently needed to
improve PFS and OS of patients with GBM. Bavo et al
(21) showed that the activation of
nAChR, composed of α7 and α9α10 subunits, could promote GBM cell
proliferation. Another study demonstrated that the decreased
expression levels of CHRFAM7A were associated with the onset
of Alzheimer's disease, supporting its role in pathological
conditions. By contrast, CHRFAM7A was upregulated in
schizophrenia and bipolar disorders (11). Furthermore, previous study (17,22)
demonstrated that CHRFAM7A was a stoichiometric
dominant-negative regulator of a7 nAChR. Instead, no mutations have
been identified in GBM occurring at the CHRNA7 loci, supporting its
normal expression in these cancer cells (23). To the best of our knowledge, the
current study was the first to investigate the effect of
CHRNA7, encoding α7 nAChR, and CHRFAM7A on three GBM
cell lines, GBM tissues and normal brain tissues (control).
Pucci et al (24) reported the effect of α7 nAChR on the
proliferation of GBM cells. In the current study, the potential
role of GTS-21 as a partial agonist of α7 nAChR was investigated in
GBM cell lines. The results revealed that the proliferation ability
of all cell lines was significantly attenuated following treatment
with GTS-21. However, the optimal concentration for inhibiting the
cell proliferation differed among the three cell lines tested, with
G28 cells being the most sensitive, since G28 cells showed the
highest expression of CHRFAM7A. Interestingly, antagonizing
the aforementioned strong effect by means of cell treatment with
α-BTX, showed that the cell proliferation was only restored in U87
cells, possibly due to a lack of CHRFAM7A expression.
Furthermore, the expression of CHRNA1 and
CHRNA9, encoding the a1 and a9 subunits of nAChR, and that
of BCHE, encoding the ACh-degrading enzyme,
butyrylcholinesterase, was notably upregulated. This finding
confirmed the previously reported findings, showing that GBM cells
could express the α7 and α9α10 subunits of nAChRs (21). A previous study also demonstrated
that CHRNA1 and CHRNA9 upregulation was associated
with shorter OS in patients with GBM (10).
The results suggested that the upregulation of α7
nAChR in heterologous cell lines could suppress the cell
proliferation. It has been reported that α7 nAChR is upregulated in
the GBM vessel endothelium, GBM cells and tumour-associated
macrophages, thus suggesting that α7 nAChR could be a potential
target for the treatment of GBM (25). However, the effect of GTS-21 was only
abrogated in U87 cells, which did not express CHRFAM7A,
following treatment with α-BTX, suggesting that GTS-21 could act in
an α7 nAChR-independent manner.
The limitation of our study is absence of data
related to TERT and IDH mutation and very small population.
In conclusion, the present study showed that GBM
cell treatment with GTS-21, an agonist of the α7 receptor,
attenuated their proliferation. However, treatment with α-BTX
restored the proliferation of U87 cells pre-treated with GTS-21.
These findings suggested that GTS-21 may be considered as a novel
treatment approach to GBM, due to its α7 nAChR-independent
mechanism of action. However, further in vivo studies are
required to elucidate the underlying mechanism of action and
potential therapeutic application of GTS-21.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors contributions
MAK designed the current study, wrote and reviewed
the data and literature of the manuscript, and analyzed the data.
BK collected and analyzed the data. FU performed statistical
analysis and reviewed the data and literature of the manuscript.
MAW and MKFB analyzed the data. PDF analyzed the data, performed
statistical analysis and reviewed the data and literature of the
manuscript. FPS and HG designed the current study and reviewed the
data and literature of the manuscript. All authors read and
approved the final manuscript.MAK and FU confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The current study was approved by the local Ethics
Committee Justus-Liebig-University Giessen (application no. AZ
07/09). All research followed the international and national
regulations in accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GBM
|
glioblastoma multiforme
|
|
CSC
|
cancer stem cell
|
|
NSC
|
neural stem cell
|
|
Ach
|
acetylcholine
|
|
AChR
|
acetylcholine receptor
|
|
nAChR
|
nicotine acetylcholine receptor
|
|
MGMT
|
O6-Methylguanine-DNA-methyltransferase
|
|
CHAT
|
choline acetyltransferase
|
|
CHRM
|
cholinergic receptors muscarinic
|
|
GTS-21
|
(3E)-3-[(2,4-dimethoxyphenyl)methylene]-3,4,5,6-tetrahydro-2,3′-bipyridine
|
|
AD
|
Alzheimer's disease
|
|
BChE
|
butyrylcholinesterase
|
References
|
1
|
Krex D, Klink B, Hartmann C, von Deimling
A, Pietsch T, Simon M, Sabel M, Steinbach JP, Heese O, Reifenberger
G, et al: Long-term survival with glioblastoma multiforme. Brain.
130:2596–2606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus Concomitant and Adjuvant Temozolomide
for Glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maher EA: Malignant glioma: Genetics and
biology of a grave matter. Genes Dev. 15:1311–1333. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bonavia R, Inda MM, Cavenee WK and Furnari
FB: Heterogeneity maintenance in glioblastoma: A social network.
Cancer Res. 71:4055–4060. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hadjipanayis CG and Van Meir EG: Brain
cancer propagating cells: Biology, genetics and targeted therapies.
Trends Mol Med. 15:519–530. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Paez-Gonzalez P, Asrican B, Rodriguez E
and Kuo CT: Identification of distinct ChAT+ neurons and
activity-dependent control of postnatal SVZ neurogenesis. Nat
Neurosci. 17:934–942. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kuol N, Stojanovska L, Apostolopoulos V
and Nurgali K: Role of the nervous system in tumor angiogenesis.
Cancer Microenviron. 11:1–11. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li ZZ, Wang YL, Yu YH, Xing YL and Ji XF:
Aclidinium bromide inhibits proliferation of osteosarcoma cells
through regulation of PI3K/Akt pathway. Eur Rev Med Pharmacol Sci.
23:105–112. 2019.PubMed/NCBI
|
|
9
|
Wessler I and Kirkpatrick CJ:
Acetylcholine beyond neurons: The non-neuronal cholinergic system
in humans. Br J Pharmacol. 154:1558–1571. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Spina R, Voss DM, Asnaghi L, Sloan A and
Bar EE: Atracurium Besylate and other neuromuscular blocking agents
promote astroglial differentiation and deplete glioblastoma stem
cells. Oncotarget. 7:459–472. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pohanka M: Alpha7 nicotinic acetylcholine
receptor is a target in pharmacology and toxicology. Int J Mol Sci.
13:2219–2238. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shytle RD, Mori T, Townsend K, Vendrame M,
Sun N, Zeng J, Ehrhart J, Silver AA, Sanberg PR and Tan J:
Cholinergic modulation of microglial activation by alpha 7
nicotinic receptors. J Neurochem. 89:337–343. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pavlov VA and Tracey KJ: The cholinergic
anti-inflammatory pathway. Brain Behav Immun. 19:493–499. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rosas-Ballina M and Tracey KJ: Cholinergic
control of inflammation. J Intern Med. 265:663–679. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gault J, Robinson M, Berger R, Drebing C,
Logel J, Hopkins J, Moore T, Jacobs S, Meriwether J, Choi MJ, et
al: Genomic organization and partial duplication of the human
alpha7 neuronal nicotinic acetylcholine receptor gene (CHRNA7).
Genomics. 52:173–185. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sinkus ML, Graw S, Freedman R, Ross RG,
Lester HA and Leonard S: The human CHRNA7 and CHRFAM7A genes: A
review of the genetics, regulation, and function.
Neuropharmacology. 96:274–288. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Lucas-Cerrillo AM, Maldifassi MC,
Arnalich F, Renart J, Atienza G, Serantes R, Cruces J,
Sánchez-Pacheco A, Andrés-Mateos E and Montiel C: Function of
partially duplicated human α77 nicotinic receptor subunit CHRFAM7A
gene: Potential implications for the cholinergic anti-inflammatory
response. J Biol Chem. 286:594–606. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Locke DP, Archidiacono N, Misceo D,
Cardone MF, Deschamps S, Roe B, Rocchi M and Eichler EE: Refinement
of a chimpanzee pericentric inversion breakpoint to a segmental
duplication cluster. Genome Biol. 4:R502003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kolodziej MA, Weischer C, Reinges MHT, Uhl
E, Weigand MA, Schwarm FP, Schänzer A, Acker T, Quint K, Uhle F and
Stein M: NDRG2 and NDRG4 Expression Is Altered in Glioblastoma and
Influences Survival in Patients with MGMT-methylated Tumors.
Anticancer Res. 36:887–897. 2016.PubMed/NCBI
|
|
20
|
Murphy S and Pearce B: Functional
receptors for neurotransmitters on astroglial cells. Neuroscience.
22:381–394. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bavo F, Pucci S, Fasoli F, Lammi C,
Moretti M, Mucchietto V, Lattuada D, Viani P, De Palma C, Budriesi
R, et al: Potent antiglioblastoma agents by hybridizing the
onium-alkyloxy-stilbene based structures of an α7-nAChR, α9-nAChR
antagonist and of a pro-oxidant mitocan. J Med Chem.
61:10531–10544. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Araud T, Graw S, Berger R, Lee M, Neveu E,
Bertrand D and Leonard S: The chimeric gene CHRFAM7A, a partial
duplication of the CHRNA7 gene, is a dominant negative regulator of
α7*nAChR function. Biochem Pharmacol. 82:904–914. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brennan CW, Verhaak RGW, McKenna A, Campos
B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ,
Berman SH, et al: The somatic genomic landscape of glioblastoma.
Cell. 155:462–477. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Pucci S, Fasoli F, Moretti M, Benfante R,
Di Lascio S, Viani P, Daga A, Gordon TJ, McIntosh M, Zoli M, et al:
Choline and nicotine increase glioblastoma cell proliferation by
binding and activating α7- and α9- containing nicotinic receptors.
Pharmacol Res. 163:1053362021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zheng Z, Zhang J, Jiang J, He Y, Zhang W,
Mo X, Kang X, Xu Q, Wang B and Huang Y: Remodeling tumor immune
microenvironment (TIME) for glioma therapy using multi-targeting
liposomal codelivery. J Immunother Cancer. 8:e0002072020.
View Article : Google Scholar : PubMed/NCBI
|