Introduction
Prostate cancer (PCa) is the most frequently
diagnosed type of cancer, and the second leading cause of
cancer-related mortality in American men (1). Castration-resistant PCa (CRPCa) is the
final stage of PCa, for which there is currently a relative lack of
treatment options. Therefore, the development of effective and
innovative therapies against PCa is necessary.
Adenosine deaminases acting on RNA (ADARs) are
enzymes that catalyze the adenosine-to-inosine (A-to-I) editing of
double-stranded RNA (dsRNA) substrates (2,3). At
present, two different enzymes with ADAR activity, double-stranded
RNA-specific adenosine deaminase (ADAR1) and double-stranded
RNA-specific editase 1 (ADAR2), have been identified in mammals
(3,4). Previous studies have highlighted the
dependence of certain types of cancer on ADAR1 expression (5,6). For
example, high expression levels of ADAR1 are closely associated
with metastasis and poor prognosis in carcinoma (7,8). ADAR1
also functions as a checkpoint that limits antitumor immunity by
preventing the sensing of endogenous dsRNA (9). However, a limited number of studies
have aimed to characterize the expression patterns of ADAR1 in PCa
development.
Therefore, the aim of the present study was to
investigate the effect of ADAR1 on PCa. The expression levels of
ADAR1 were detected in PCa and CRPCa tissues, as well as CRPCa cell
lines. ADAR1 was then knocked down in two stable cell lines, DU145
and PC3. The effects of ADAR1-knockdown on cellular proliferation
and apoptosis were subsequently determined, and the interaction
between ADAR1 and the phosphorylation of H2A.X variant histone was
evaluated. These results suggested ADAR1 as a promising target for
anti-cancer therapy in CRPCa.
Materials and methods
Cells lines and, tissue samples
The cell lines, RWPE-1 (cat. no. CRL-11609), LnCap
(cat. no. CRL-1740), 22RV1 (cat. no. CRL-2505), DU145 (cat. no.
HTB-81) and PC3 (cat. no. CRL-1435) were purchased from the
American Type Culture Collection. RWPE-1 cells were maintained in
keratinocyte serum-free medium (Thermo Fisher Scientific, Inc.),
DU145 cells were maintained in DMEM (Thermo Fisher Scientific,
Inc.), and LnCap, 22RV1 and PC3 cells were maintained in RPMI 1640
(Sigma-Aldrich; Merck KGaA). The media were supplemented with 10%
FBS (Thermo Fisher Scientific, Inc.), and the cells were cultured
at 37°C in a humidified atmosphere containing 5% CO2.
DU145 and PC3 cells were transfected with negative control (NC)
small interfering (si)RNA and ADAR1-specific siRNAs (Shanghai Gene
Pharma Co., Ltd.), and the suppressed expression of ADAR1 was
confirmed using western blot analysis.
Preliminary screening of ADAR1 protein expression
via immunohistochemistry (IHC) and western blot analysis with an
antibody against ADAR1, was conducted on tissues from patients
diagnosed with benign prostatic hyperplasia (BPH), PCa and CRPCa at
The Fifth Affiliated Hospital of Guangzhou Medical University.
All procedures carried out in studies involving
patients were conducted in accordance with the ethical standards of
the institutional and/or national research committee, as well as
with the 1964 Declaration of Helsinki and its later amendments, or
comparable ethical standards. The present study was a retrospective
study reviewed and approved by the Ethics Committee, The Fifth
Affiliated Hospital, Guangzhou Medical University (Guangzhou,
China). Written informed consent was obtained from all
patients.
Sample collection
Samples were obtained from patients (28 male
patients, including 10 BPH, 10 PCa and 8 CRPCa cases; age, 63–74
years) who underwent surgery at the Department of Urology, the
Fifth Affiliated Hospital of Guangzhou Medical University
(Guangzhou, China) between March 2019 and September 2020. BPH
tissue samples were obtained from transurethral resection of the
prostate; PCa and CRPCa tissue samples were obtained from radical
prostatectomy. Pathological evaluation of these tissues was
confirmed. Patients with CRPCa were selected according to the
European Association of Urology guidelines (10). The inclusion criteria were as
follows: i) Histologically confirmed diagnosis of BPH, PCa or
CRPCa; ii) aged between 60 and 75 years; and iii) willing to donate
tissue samples. Patients who had incomplete information or a
history of other malignant tumors were excluded. In the present
study, patient characteristics (age, prostate volume, preoperative
prostate-specific antigen level, Gleason score and clinical stage)
were collected retrospectively. The characteristics of the patients
are displayed in Table I.
 | Table I.Characteristics of patients with BPH,
PCa and CRPCa (n=28). |
Table I.
Characteristics of patients with BPH,
PCa and CRPCa (n=28).
| Characteristic | BPH (n=10) | PCa (n=10) | CRPCa (n=8) |
|---|
| Age (years), median
(range) | 68.4 (64–73) | 67.2 (63–70) | 69.5 (67–74) |
| Prostate volume
(cm3), median (range) | 78.2 (65–91) | 46.2 (37–53) | 46.3 (38–55) |
| Preoperative
prostate-specific antigen (ng/ml), median (range) | 3.6 (0.8–10.5) | 22.1 (12.6–32.1) | 57.6 (42.5–81.4) |
| Source of tissue | Transurethral
resection of the prostate | Radical
prostatectomy | Radical
prostatectomy |
| Gleason score, median
(range) |
| 7.8 (6–10) | 8.8 (7–10) |
| Clinical stage |
|
|
|
| T1 |
| 3 | 1 |
| T2 |
| 4 | 3 |
| T3 |
| 3 | 2 |
| T4 |
| 0 | 2 |
IHC
A standard IHC protocol was used to stain the tissue
samples, using a rabbit monoclonal antibody against ADAR1 [Cell
Signaling Technology, Inc.; cat. no. 81284] (11). Briefly, paraffin-embedded tissue
sections were deparaffinized, rehydrated and subjected to antigen
retrieval. Samples were incubated with anti-ADAR1 diluted with TBS
(1:800). All samples were observed under a light microscope (Carl
Zeiss AG) and automated image quantification was conducted using
ImageJ2× software (National Institutes of Health).
Western blotting
The tissue and cell samples were homogenized in
lysis buffer containing: 0.1 mol/l NaCl, 0.01 M Tris-HCl (pH 7.5),
1 mM EDTA and 1 µg/ml aprotinin. After centrifugation at 16,000 × g
for 5 min (4°C), total protein content in the supernatants was
determined using a Bradford Protein Assay kit (Bio-Rad
Laboratories, Inc,). In total, 40–80 µg protein was separated via
8% SDS-PAGE and then transferred to nitrocellulose membranes
(12,13). The membranes were blocked for 60 min
with blocking buffer [composed of 5% skimmed milk in TBS-Tween-20
(0.25 M Tris-HCl pH 7.5, 0.1% Tween-20 and 0.15 M NaCl)], at 37°C,
and subsequently incubated with anti-GAPDH (1:1,000; cat. no.
5174), anti-β-actin (1:1,000; cat. no. 4970), anti-β-tubulin
(1:1,000; cat. no. 2128), anti-phosphorylated (p)-H2A.X (1:2,000;
cat. no. 2577), anti-H2A.X (1:2,000; cat. no. 7631), anti-cleaved
caspase-3 (1:1,000; cat. no. 9661), anti-caspase-3 (1:1,000; CST;
cat. no. 9662), anti-cleaved PARP (1:500; cat. no. 5625) and
anti-PARP (1:500; cat. no. 9542) antibodies (all CST Biological
Reagents Co., Ltd.) overnight at 4°C. The primary antibodies were
detected using an HRP-conjugated secondary anti-rabbit IgG antibody
(1:2,000; cat. no. 7076; CST Biological Reagents Co., Ltd.) for 1 h
at 37°C. Bands were visualized using 3,3′-diaminobenzidine reagent
at room temperature for 2 min, and were semi-quantified via
scanning with a Molecular Imager (Bio-Rad Laboratories, Inc.). The
results were analyzed with ImageJ2× and Origin 8.0 software
(National Institutes of Health).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from cell lines using the
RNeasy mini kit (Qiagen, Inc.) per the manufacturer's protocol, and
was quantified through spectrophotometric measurement using a
NanoDrop system (Thermo Fisher Scientific, Inc.). qPCR analysis was
conducted using the Verso One-Step SYBR qRT-PCR kit (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Primers were custom-synthesized and obtained from
Guangzhou HYY Co. (http://www.hyymed.com/) (Table II). The amplification reaction was
performed as follows: Initial denaturation for 5 min at 95°C,
followed by 30 cycles at 95°C for 10 sec, 60°C for 15 sec and 72°C
for 20 sec. β-actin was used as a control for relative
quantification. Rotor-Gene Software (Qiagen, Inc.) was used for
comparative concentration analysis (14,15). All
reactions were carried out in triplicate, and the 2−ΔΔCq
method was used to determine the relative quantification of mRNA
expression (16).
 | Table II.Primers used for reverse
transcription-quantitative PCR analysis. |
Table II.
Primers used for reverse
transcription-quantitative PCR analysis.
| Gene | Primer | Sequence
(5′-3′) |
|---|
| Double-stranded
RNA-specific adenosine deaminase | Forward |
CGCCCTCTTTGACAAGTCCT |
|
| Reverse |
GGGATTGTGCCTTCTCCGTTC |
| β-actin | Forward |
TGGCACCAGCACAATGAA |
|
| Reverse |
CTAAGTCATAGTCCGCCTAGAAGCA |
Transfection
DU145 and PC3 cells were seeded into 6-well plates
at a density of 1×104 cells/well, and transfected with
negative control siRNA (NC-siRNA sense, 5′-UUCUCCGAACGUGUCACGUTT-3′
and antisense, 5′-ACGUGACACGUUCGGAGAATT-3′) or ADAR1-specific siRNA
(ADAR1-siRNA-a sense, 5′-GCAUCUGACCCGUGCUAUUTT-3′ and antisense,
5′-AAUAGCACGGGUCAGAUGCTT-3′; ADAR1-siRNA-b sense,
5′-GCAGCCCAUUUAUCUCAAATT-3′ and antisense,
5′-UUUGAGAUAAAUGGGCUGCTT-3′; and ADAR1-siRNA-c sense,
5′-GCUUCAACACUCUGACUAATT-3′ and antisense,
5′-UUAGUCAGAGUGUUGAAGCTT-3′) (final concentration, 20 nmol/l; all
Shanghai GenePharma Co., Ltd.) using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. Following culture for 48 h at 37°C,
transfection efficiency was assessed by western blotting. After
transfection, cells were cultured for 24 h and then used for
subsequent experimentation.
Cell Counting Kit 8 (CCK-8) assay
Cell viability was measured using the CCK-8 kit
(Dojindo Molecular Laboratories, Inc.) following the manufacturer's
protocol. Briefly, cells were seeded into a 96-well plate at a
density of 8×103 cells/well, and incubated with CCK-8
reagent at 37°C for 4, 24, 48 and 72 h. The optical density was
measured at 450 nm to estimate the number of viable cells.
Apoptosis analysis
Apoptosis was analyzed using an Annexin V-FITC/PI
kit (eBioscience; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Cells in each group were seeded into a
6-well plate at a density of 1×105 cells/well, and
transfected with NC siRNA and ADAR1-specific siRNAs. After
incubation for a further 2 days, the cells were harvested, washed
with PBS and resuspended in staining buffer (Annexin V-FITC/PI kit)
at a final density of 3×105/ml. Then, apoptosis was
analyzed using a FACScan flow cytometer (FACSCalibur; BD
Biosciences).
Statistical analysis
Statistical analysis was performed using SPSS
software ver. 28.0 (SPSS, Inc.) and graphs were generated using
GraphPad Prism 8 (GraphPad Software, Inc.). Statistical results are
presented as the mean ± SD of ≥3 independent experiments. Unpaired
Student's t-test was used to compare differences between two
groups. One-way or two-way ANOVA were used for multiple
comparisons, followed by Dunnett's or Tukey's post hoc tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
Patient characteristics including age, prostate
volume, preoperative prostate-specific antigen (PSA), source of
tissue, Gleason score and clinical stage are summarized in Table I. In the present study, 28 male
patients aged 63–74 were enrolled, including 10 BPH, 10 PCa and 8
CRPCa cases. The mean prostate volume in each group was 78.2
(65–91), 46.2 (37–53) and 46.3 (38–55) cm3,
respectively. The mean preoperative PSA was 3.6 (0.8–10.5), 22.1
(12.6–32.1) and 57.6 (42.5–81.4) ng/ml, respectively.
Histopathological Gleason score was determined based on the
criteria of the World Health Organization. The mean Gleason score
of patients with PCa was 7.8 (6–10), and
that of patients with CRPCa was 8.8 (7–10).
Tumors were staged according to the eighth edition American Joint
Committee on Cancer Staging Manual (17). The number of PCa patients at stages
T1, T2 and T3 was 3, 4 and 3, respectively. The number of CRPCa
patients at stages T1, T2, T3 and T4 was 1, 3, 2 and 2,
respectively.
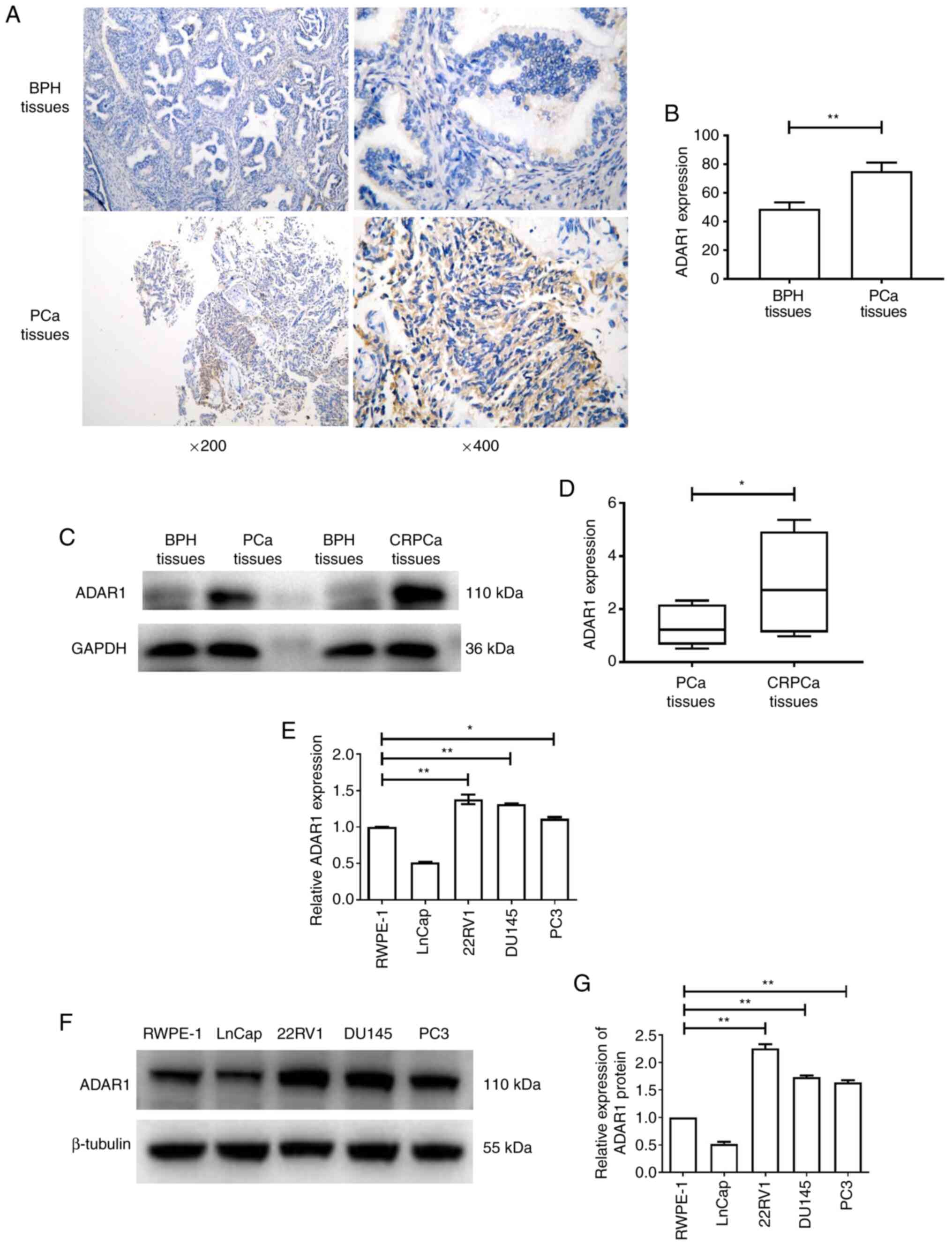
ADAR1 is highly expressed in
CRPCa
The expression level of ADAR1 was determined using
IHC images of PCa tissues. Via IHC staining and automated image
quantification, ADAR1 expression was found to be significantly
increased in PCa, compared with BPH tissues (Fig. 1A and B). Furthermore, PCa and CRPCa
tissues were extracted and probed with an ADAR1 monoclonal
antibody. Notably, higher protein expression levels of ADAR1 were
detected in CRPCa tissues compared with PCa tissues (Fig. 1C and D). Furthermore, RT-qPCR and
western blot analysis demonstrated that the mRNA and protein
expression levels of ADAR1 were decreased in LNCaP cells and
significantly increased in the CRPCa cell lines (22RV1, DU145 and
PC3) compared with RWPE-1 cells (Fig.
1E-G). These results suggest that ADAR1 is upregulated in CRPCa
tissues and cell lines.
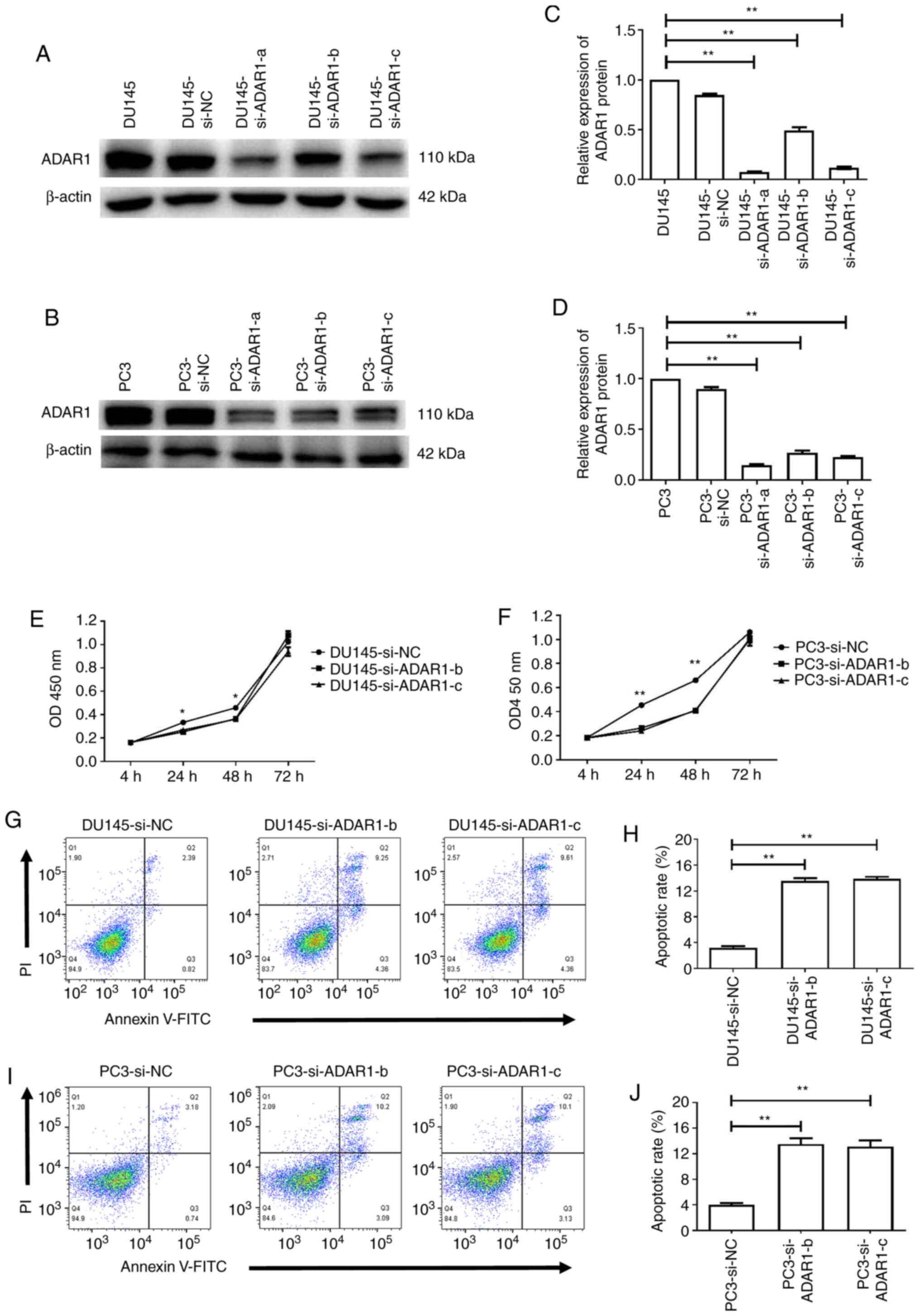
ADAR1-knockdown inhibits the
proliferation and induces the apoptosis of DU145 and PC3 cells
DU145 and PC3 cells that are considered to be
androgen receptor negative (with high expression levels of ADAR1)
were transiently transfected with ADAR1-specific siRNAs. Total
protein was isolated and analyzed via western blotting 48 h
post-transfection. Compared with the blank (no siRNA) and
mock-transfected cells (DU145 si-NC and PC3 si-NC), the expression
level of ADAR1 was markedly suppressed in cells transfected with
ADAR1 siRNAs (Fig. 2A-D).
Next, the potential effects of ADAR1-knockdown on
the proliferation of DU145 and PC3 cells were assessed. As shown in
Fig. 2E and F, in both cell lines,
ADAR1-knockdown significantly inhibited cellular proliferation
compared with the untransfected cells after 24 and 48 h
(P<0.05). However, there was no statistically significant
difference in cellular proliferation 72 h post-transfection.
Flow cytometry was used to assess whether
ADAR1-knockdown was associated with DU145 and PC3 cell apoptosis.
The results demonstrated that the apoptotic rate of ADAR1
siRNA-transfected cells was increased compared with that of the
untransfected cells (Fig. 2G-J).
Thus, these results indicated that knockdown of ADAR1 expression
inhibited the proliferation and induces the apoptosis of DU145 and
PC3 cells.
ADAR1-Knockdown induces DU145 and PC3
cell apoptosis by promoting the phosphorylation of H2AX
Next, it was determined whether the phosphorylation
of H2AX during ADAR1-knockdown induced the apoptosis of DU145 and
PC3 cells. The expression levels of p-H2A.X, H2A.X, cleaved
caspase-3, caspase-3 cleaved PARP and PARP were detected using
western blotting (Fig. 3A and B).
Results showed that DU145 si-ADAR1 cells and PC3 si-ADAR1-c cells
exhibited markedly increased activation of caspase-3 and cleavage
of PARP, which was accompanied by the phosphorylation of H2AX
(Fig. 3C and D). Collectively, these
data confirm that ADAR1-knockdown inhibits the proliferation and
induces the apoptosis of DU145 and PC3 cells by promoting the
phosphorylation of H2A.X.
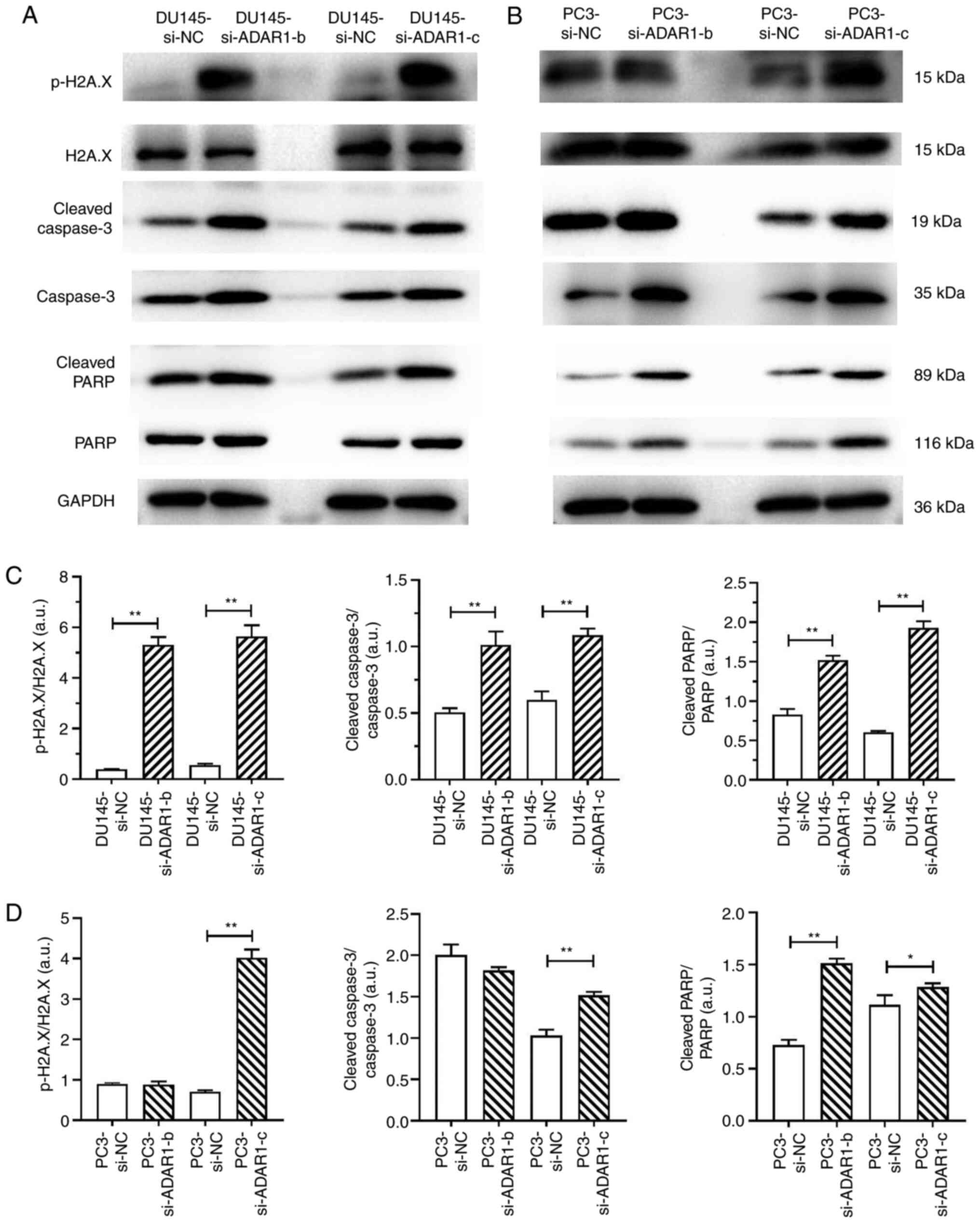 | Figure 3.ADAR1-knockdown induces the apoptosis
of DU145 and PC3 cells by promoting the phosphorylation of H2AX.
Lysates from transfected (A) DU145 and (B) PC3 cells were analyzed
via western blotting to detect the expression levels of apoptotic
markers (cleaved caspase-3, caspase-3, cleaved PARP and PARP), and
the DNA double-strand-break markers, p-H2AX and H2AX. Data are
representative of three independently performed experiments.
Protein expression levels of p-H2AX, H2AX, cleaved caspase-3,
caspase-3, cleaved PARP and PARP in (C) DU145 and (D) PC3 cells.
Statistical results are presented as the mean ± SD of five
independent experiments. *P<0.05 and **P<0.01. ADAR1,
double-stranded RNA-specific adenosine deaminase; si (RNA), small
interfering; NC, negative control; p-, phosphorylated; H2AX, H2A.X
variant histone. |
Discussion
RNA editing, also known as RNA modification, was
first discovered by Benne et al (18) in mitochondrion-encoded mRNA of the
trypanosome. Dysregulated RNA editing has been associated with
multiple cancer types (19). A-to-I
RNA editing is the posttranscriptional deamination of A-to-I in
dsRNA, which is catalyzed by enzymes of the ADAR family (20). Currently, three members of the ADAR
family have been identified, namely ADAR1 (encoded by ADAR), ADAR2
(encoded by ADARB1) and adenosine deaminase RNA-specific B2
(inactive; encoded by ADARB2) (3,21). ADAR1
was the first identified active A-to-I RNA enzyme that was more
ubiquitously expressed in different tissues. While both ADAR1 and
ADAR2 serve roles in tumorigenesis, additional editing events
regulated by ADAR1 have been observed in some cancer types, along
with its abundant expression (22).
Thus, ADAR1 activation in cancer is an interesting topic that
requires continued in-depth investigation.
The present study revealed that ADAR1 expression was
significantly increased in PCa tissues, and that higher ADAR1
protein expression was apparent in CRPCa tissues and cell lines
(22RV1, DU145 and PC3). Therefore, these results suggest an
important role for ADAR1 in PCa progression. In most tumor types,
ADAR1 expression is elevated compared with that in matched normal
tissues, indicating that RNA editing may supplement genomic DNA
alterations and drive tumorigenesis (6,23,24).
These data suggest that the loss of ADAR1 may be associated with
antitumor activities.
In the current study, ADAR1 expression was knocked
down using siRNA transfection, which was shown to exert antitumor
effects on DU145 and PC3 cells 24 and 48 h post-transfection.
However, after 72 h in culture, there was no statistically
significant difference in cellular proliferation between NC siRNA
and ADAR1-specific siRNAs. These data suggest that ADAR1-mediated
RNA editing is a complicated process regulated by various factors
and signaling pathways, which requires further exploration in the
future. It has been revealed that ADAR1 regulates the apoptosis of
stressed cells, and that numerous anti-apoptotic genes contain 3′
untranslated regions in the dsRNA that are protected by ADAR1
(25,26). In the present study, flow cytometric
analysis demonstrated that ADAR1-knockdown significantly induced
apoptosis, which indicated that knocking down ADAR1 may act as a
potential inhibitor of CRPCa expansion by promoting apoptosis.
The critical role of caspases in regulating
apoptosis has been well documented in PCa (27,28).
H2AX is a ubiquitous member of the H2A histone family, and the
generation of its phosphorylated form has been suggested to affect
dsRNA stability and the repair of DNA double-strand breaks
(29–31). The present results demonstrated that
the expression levels of cleaved caspase-3 and cleaved PARP, and
the phosphorylation of H2AX were significantly increased in
ADAR1-knockdown cells.
In conclusion, the present results indicated that
ADAR1-knockdown inhibited the proliferation and induced the
apoptosis of CRPCa cells by promoting the phosphorylation of H2AX.
These findings provide a novel insight into the direct anticancer
effect of ADAR1 silencing, and support its application as a
promising anticancer treatment method in CRPCa.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Science and
Technology Program of Guangzhou City of China (grant no.
20201A011107 and 20201A011106), the Guangzhou Municipal Science and
Technology Project (grant no. 202102020531), the Natural Science
Foundation of Guangdong Province (grant no. 2021A1515010065) and
the National Natural Science Foundation of China (grant no.
81974392).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, RZ, GX and JB conceived and designed the study.
JB, SL, ZC and YY conducted the experiments and performed data
analysis. XL and GX confirm the authenticity of all the raw data.
RZ and XL drafted the initial manuscript. XL guided the writing.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was a retrospective study reviewed
and approved by the Ethics Committee of The Fifth Affiliated
Hospital, Guangzhou Medical University in Guangzhou, China
(approval no. GZMU-2019-021), and all patients provided written
informed consent for the use of their tissue samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bass BL: RNA editing by adenosine
deaminases that act on RNA. Annu Rev Biochem. 71:817–846. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishikura K: Functions and regulation of
RNA editing by ADAR deaminases. Annu Rev Biochem. 79:321–349. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hundley HA and Bass BL: ADAR editing in
double-stranded UTRs and other noncoding RNA sequences. Trends
Biochem Sci. 35:377–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu H, Golji J, Brodeur LK, Chung FS, Chen
JT, deBeaumont RS, Bullock CP, Jones MD, Kerr G, Li L, et al:
Tumor-derived IFN triggers chronic pathway agonism and sensitivity
to ADAR loss. Nat Med. 25:95–102. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gannon HS, Zou T, Kiessling MK, Gao GF,
Cai D, Choi PS, Ivan AP, Buchumenski I, Berger AC, Goldstein JT, et
al: Identification of ADAR1 adenosine deaminase dependency in a
subset of cancer cells. Nat Commun. 9:54502018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun Y, Fan J, Wang B, Meng Z, Ren D, Zhao
J, Liu Z, Li D, Jin X and Wu H: The aberrant expression of ADAR1
promotes resistance to BET inhibitors in pancreatic cancer by
stabilizing c-Myc. Am J Cancer Res. 10:148–163. 2020.PubMed/NCBI
|
|
8
|
Liu X, Fu Y, Huang J, Wu M, Zhang Z, Xu R,
Zhang P, Zhao S, Liu L and Jiang H: ADAR1 promotes the
epithelial-to-mesenchymal transition and stem-like cell phenotype
of oral cancer by facilitating oncogenic microRNA maturation. J Exp
Clin Cancer Res. 38:3152019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ishizuka JJ, Manguso RT, Cheruiyot CK, Bi
K, Panda A, Iracheta-Vellve A, Miller BC, Du PP, Yates KB, Dubrot
J, et al: Loss of ADAR1 in tumours overcomes resistance to immune
checkpoint blockade. Nature. 565:43–48. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cornford P, van den Bergh RCN, Briers E,
Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N,
Gandaglia G, Gillessen S, et al: EAU-EANM-ESTRO-ESUR-SIOG
Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of
Relapsing and Metastatic Prostate Cancer. Eur Urol. 79:263–282.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan Y, Li X, Xu Y, Zhao H, Su Z, Lai D,
Yang W, Chen S, He Y, Li X, et al: Mitochondrial E3 ubiquitin
ligase 1 promotes autophagy flux to suppress the development of
clear cell renal cell carcinomas. Cancer Sci. 110:3533–3542. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo Y, Balasubramanian B, Zhao ZH and Liu
WC: Marine algal polysaccharides alleviate aflatoxin B1-induced
bursa of Fabricius injury by regulating redox and apoptotic
signaling pathway in broilers. Poult Sci. 100:844–857. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu WC, Ou BH, Liang ZL, Zhang R and Zhao
ZH: Algae-derived polysaccharides supplementation ameliorates heat
stress-induced impairment of bursa of Fabricius via modulating
NF-κB signaling pathway in broilers. Poult Sci. 100:1011392021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Balasubramanian B, Guo Y, Qiu SJ,
Jha R and Liu WC: Dietary Enteromorpha polysaccharides
supplementation improves breast muscle yield and is associated with
modification of mRNA transcriptome in broiler chickens. Front Vet
Sci. 8:6639882021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu WC, Guo Y, Zhihui Z, Jha R and
Balasubramanian B: Algae-derived polysaccharides promote growth
performance by improving antioxidant capacity and intestinal
barrier function in broiler chickens. Front Vet Sci. 7:6013362020.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Amin MB, Greene FL, Edge SB, Compton CC,
Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR and
Winchester DP: The eighth edition AJCC cancer staging manual:
Continuing to build a bridge from a population-based to a more
‘personalized’ approach to cancer staging. CA Cancer J Clin.
67:93–99. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benne R, Van Den Burg J, Brakenhoff JP,
Sloof P, Van Boom JH and Tromp MC: Major transcript of the
frameshifted coxll gene from trypanosome mitochondria contains four
nucleotides that are not encoded in the DNA. Cell. 46:819–826.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kung CP, Maggi LB Jr and Weber JD: The
role of RNA editing in cancer development and metabolic disorders.
Front Endocrinol (Lausanne). 9:7622018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dominissini D, Moshitch-Moshkovitz S,
Amariglio N and Rechavi G: Adenosine-to-inosine RNA editing meets
cancer. Carcinogenesis. 32:1569–1577. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nishikura K: A-to-I editing of coding and
non-coding RNAs by ADARs. Nat Rev Mol Cell Biol. 17:83–96. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu LD and Öhman M: ADAR1 editing and its
role in cancer. Genes (Basel). 10:122018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Paz-Yaacov N, Bazak L, Buchumenski I,
Porath HT, Danan-Gotthold M, Knisbacher BA, Eisenberg E and Levanon
EY: Elevated RNA editing activity is a major contributor to
transcriptomic diversity in tumors. Cell Rep. 13:267–276. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kung CP, Cottrell KA, Ryu S, Bramel ER,
Kladney RD, Bao EA, Freeman EC, Sabloak T, Maggi L Jr and Weber JD:
Evaluating the therapeutic potential of ADAR1 inhibition for
triple-negative breast cancer. Oncogene. 40:189–202. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sakurai M, Shiromoto Y, Ota H, Song C,
Kossenkov AV, Wickramasinghe J, Showe LC, Skordalakes E, Tang HY,
Speicher DW and Nishikura K: ADAR1 controls apoptosis of stressed
cells by inhibiting Staufen1-mediated mRNA decay. Nat Struct Mol
Biol. 24:534–543. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Walkley CR and Kile BT: Cell death
following the loss of ADAR1 mediated A-to-I RNA editing is not
effected by the intrinsic apoptosis pathway. Cell Death Dis.
10:9132019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J and Yuan J: Caspases in apoptosis and
beyond. Oncogene. 27:6194–6206. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang H, Salinas RA, Leal BZ,
Kosakowska-Cholody T, Michejda CJ, Waters SJ, Herman TS,
Woynarowski JM and Woynarowska BA: Caspase-mediated apoptosis and
caspase-independent cell death induced by irofulven in prostate
cancer cells. Mol Cancer Ther. 3:1385–1396. 2004.PubMed/NCBI
|
|
29
|
Collins PL, Purman C, Porter SI, Nganga V,
Saini A, Hayer KE, Gurewitz GL, Sleckman BP, Bednarski JJ, Bassing
CH and Oltz EM: DNA double-strand breaks induce H2Ax
phosphorylation domains in a contact-dependent manner. Nat Commun.
11:31582020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Talasz H, Helliger W, Sarg B, Debbage PL,
Puschendorf B and Lindner H: Hyperphosphorylation of histone H2A. X
and dephosphorylation of histone H1 subtypes in the course of
apoptosis. Cell Death Differ. 9:27–39. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Firsanov DV, Solovjeva LV and Svetlova MP:
H2AX phosphorylation at the sites of DNA double-strand breaks in
cultivated mammalian cells and tissues. Clin Epigenetics.
2:283–297. 2011. View Article : Google Scholar : PubMed/NCBI
|