Introduction
Mucinous breast carcinoma (MBC) is a rare variant of
breast cancer accounting for 1–6% of all primary breast carcinomas,
and is characterized by small clusters of tumor cells floating in
lakes of partitioned mucin (1,2). MBC has
a more favorable prognosis compared with non-specific invasive
ductal carcinoma (IDC), as most cases are associated with a high
expression of estrogen and/or progesterone receptors
(ER/PR+) and a low expression of HER2 (3,4). In
addition, most studies have reported that MBCs have a lower
frequency of axillary lymph node metastases compared with IDCs
(5), which also suggests that the
treatment of MBC should be different from IDC, and additional
detection methods should be used to guide the treatment of MBC.
According to the tumor components, MBCs are divided into two
subtypes: Pure MBC (PMBC), which is defined as a tumor with a
mucinous component of >90%, and mixed MBC (MMBC), which is
defined as a tumor with a 51–90% mucinous component and admixing,
usually with an infiltrating ductal epithelial component (6,7). A
previous study reported a difference in prognosis for PMBCs and
MMBCs, with a lower frequency of axillary lymph node metastases and
a more favorable outcome in the former subtype (8). However, whether the treatment of these
two types of breast cancer should be differentiated remains
unknown.
The Oncotype DX 21-gene recurrence score (RS) assay
is calculated based on the results of a reverse transcription
(RT)-PCR assay of 21 prospectively selected genes in tumor tissues
(9). Over the past decade, the
21-gene RS has been widely used by clinicians to assist with
predicting the outcomes and guides therapeutic decisions in
patients with ER-positive/HER2-negative breast cancer, and it has
become the only genomic test recommended by National Comprehensive
Cancer Network guidelines (10,11).
Further validation studies also confirmed its ability to predict
the benefit from chemotherapy (CT) both in node-negative and
node-positive cases (12,13). Notably, the majority of MBCs have
favorable features, including being ER-positive and HER2-negative
and having a lower incidence rate of nodal metastasis, which
matches the criteria of the 21-gene genomic test (14), thereby suggesting that a considerable
number of patients with MBC may avoid unnecessary CT after the
Oncotype DX 21-gene test. However, at present, data on the RS of
MBC remains limited, due to its relative rarity, and it remains
unknown whether the accuracy, practicability and effectiveness of
the 21-gene RS test in guiding the treatment of IDC is also
suitable for MBC due to tumor heterogeneity (15).
The present study retrospectively investigated the
clinicopathological features and treatment patterns of 49 cases of
MBC, and the Oncotype DX 21-gene RS test was performed in 29 cases
of MBC. We hypothesized that the results of the 21-gene test could
be used to guide the treatment in patients with MBC. Furthermore,
the clinicopathological features and the 21-gene RSs were compared
between patients with PMBC and those with MMBC. In addition, the
individual gene expression from the 21-gene test was also analyzed
between the PMBC and MMBC groups.
Materials and methods
Patients and follow-up
In total, 50 women who were diagnosed with MBC and
treated at the Department of Thyroid and Breast Surgery, Affiliated
Hospital of Zunyi Medical University (Guizhou, China) between
February 2010 and February 2021, were retrospectively included.
During this period, a total of 3,081 patients were diagnosed with
breast cancer, and MBC accounted for 1.59%. The main inclusion
criteria were as follows: Female, without distant metastasis at
first diagnosis and confirmed to be MBC by the Pathology Department
of The Affiliated Hospital of Zunyi Medical University. The main
exclusion criteria were as follows: Male, bilateral cancer,
presence of distant metastasis and unavailability of tissue
samples. A total of 49 patients were included in this study
according to the aforementioned criteria, and 1 patient was
excluded due to the inability to obtain tissue samples. All
available clinicopathological data, including age, menstrual
status, tumor size, lymph node status, TNM stage,
immunohistochemistry (IHC) results and treatment were collected
from the medical records. The patients received all therapeutic
procedures, such as surgery, adjuvant CT, irradiation and
hormonotherapy at the same institution (Table I).
 | Table I.Detailed IHC and adjuvant treatment
information of the enrolled patients. |
Table I.
Detailed IHC and adjuvant treatment
information of the enrolled patients.
| Case ID | ER status | PR status | HER2
statusa | Ki67, % | Molecular
subtype | Chemotherapy | Endocrine
therapy | Irradiation |
|---|
| MMBC1 | Positive | Negative | Negative | 30 | B | Yes | Yes | Yes |
| MMBC2 | Positive | Negative | Negative | 2 | B | Yes | Yes | No |
| MMBC3 | Positive | Negative | Negative | 30 | B | Yes | Yes | Yes |
| MMBC4 | Positive | Positive | Negative | 10 | A | No | Yes | No |
| MMBC5 | Positive | Positive | Negative | 10 | A | Yes | Yes | No |
| MMBC6 | Positive | Positive | Negative | 10 | A | Yes | Yes | No |
| MMBC7 | Positive | Positive | Negative | 5 | A | Yes | Yes | No |
| MMBC8 | Positive | Positive | Positive | 20 | B/HER2 | Yes | Yes | No |
| MMBC9 | Positive | Negative | Negative | 20 | B | No | Yes | No |
| MMBC10 | Positive | Positive | Positive | 10 | B/HER2 | Yes | Yes | No |
| MMBC11 | Positive | Positive | Positive | 20 | B/HER2 | Yes | Yes | Yes |
| PMBC1 | Positive | Positive | Negative | 5 | A | No | Yes | No |
| PMBC2 | Positive | Positive | Negative | 10 | A | Yes | Yes | No |
| PMBC3 | Positive | Positive | Negative | 15 | B | Yes | Yes | No |
| PMBC4 | Positive | Positive | Negative | 20 | B | Yes | Yes | No |
| PMBC5 | Positive | Positive | Negative | 10 | A | No | Yes | No |
| PMBC6 | Positive | Positive | Negative | 20 | B | Yes | Yes | Yes |
| PMBC7 | Positive | Positive | Negative | 10 | A | No | Yes | No |
| PMBC8 | Positive | Positive | Negative | 15 | A | No | Yes | No |
| PMBC9 | Positive | Positive | Negative | 3 | B | Yes | Yes | No |
| PMBC10 | Positive | Positive | Negative | 20 | B | No | No | No |
| PMBC11 | Positive | Positive | Negative | 10 | B | No | Yes | No |
| PMBC12 | Positive | Positive | Negative | 10 | A | No | Yes | No |
| PMBC13 | Negative | Negative | Negative | 80 | TNBC | No | No | No |
| PMBC14 | Positive | Positive | Negative | 20 | B | Yes | Yes | No |
| PMBC15 | Positive | Positive | Negative | 10 | A | Yes | Yes | No |
| PMBC16 | Positive | Positive | Negative | 5 | A | No | Yes | No |
| PMBC17 | Positive | Negative | Negative | 5 | B | Yes | Yes | No |
| PMBC18 | Positive | Positive | Negative | 60 | B | Yes | Yes | Yes |
| PMBC19 | Negative | Negative | Positive | 20 | HER2 | Yes | No | No |
| PMBC20 | Negative | Negative | Positive | 40 | HER2 | Yes | No | Yes |
| PMBC21 | Positive | Positive | Negative | 15 | A | Yes | Yes | No |
| PMBC22 | Positive | Negative | Negative | 10 | B | No | Yes | No |
| PMBC23 | Positive | Positive | Negative | 5 | A | No | Yes | Yes |
| PMBC24 | Positive | Positive | Negative | 10 | A | Yes | Yes | No |
| PMBC25 | Positive | Positive | Negative | 10 | A | No | Yes | No |
| PMBC26 | Positive | Positive | Negative | 10 | A | Yes | Yes | Yes |
| PMBC27 | Positive | Positive | Negative | 5 | A | No | Yes | No |
| PMBC28 | Positive | Positive | Negative | 10 | A | No | Yes | No |
| PMBC29 | Positive | Positive | Negative | 20 | B | No | Yes | No |
| PMBC30 | Positive | Positive | Negative | 10 | A | No | Yes | Yes |
| PMBC31 | Positive | Positive | Negative | 50 | B | Yes | Yes | No |
| PMBC32 | Positive | Negative | Negative | 20 | B | Yes | Yes | Yes |
| PMBC33 | Positive | Positive | Negative | 40 | B | Yes | Yes | Yes |
| PMBC34 | Positive | Positive | Negative | 10 | A | Yes | Yes | No |
| PMBC35 | Positive | Positive | Negative | 1 | A | No | Yes | No |
| PMBC36 | Positive | Positive | Negative | 10 | A | Yes | Yes | No |
| PMBC37 | Positive | Positive | Negative | 10 | A | No | Yes | No |
| PMBC38 | Negative | Negative | Negative | 5 | TNBC | No | No | No |
The time to follow-up was from the date of surgery
to the date of recent follow-up. Patient follow-up was accomplished
by specialized staff at the Department of Thyroid and Breast
Surgery, Affiliated Hospital of Zunyi Medical University, and
routine correspondence and telephone calls were used for follow-up.
The follow-ups were performed every 3 months during the first 2
years, every 6 months during the next 3 years, then once a year
thereafter. Overall survival (OS) time was calculated from the date
of surgery to the occurrence death of any cause. Disease-free
survival (DFS) time was estimated from the date of surgery until
the date of first proven recurrence, including local/regional
recurrence and distant metastasis at any site. The last follow-up
was conducted in April 2021. The current study was approved by the
Ethical Committee of The Affiliated Hospital of Zunyi Medical
University. All procedures were in accordance with the 1964
Declaration of Helsinki and its later amendments. Written informed
consent was provided by all the patients, and all tissue samples
used were from paraffin embedded tissues following surgery. The
tumor tissue was fixed with 10% neutral buffered formalin at room
temperature overnight, and the 4-µm thick tissue sections were used
for pathological evaluation.
Hematoxylin & eosin (H&E)
staining
H&E-stained slides of the MBCs were reviewed
according to the 2012 World Health Organization classification
criteria (16). The histological
sections were stained with hematoxylin for 8–10 min and eosin for
4–5 sec at room temperature, then the stained sections were
observed under a light microscope (magnifications ×40 and ×100).
PMBCs were defined as having a mucinous component of >90% and
MMBC was defined with a 51–90% mucinous component. In addition,
hypocellular MBC (type A) and hypercellular MBC (type B) were also
determined based on cell cluster density (17).
IHC analyses
ER, PR, HER-2 status and the Ki-67 index were
evaluated using IHC. Briefly, the 4-µm thick tissue sections were
incubated with the immunohistochemical antigen repair buffer (cat.
no. MVS-0099; Beijing Strong Biotechnologies, Inc.) for 20 min
after dewaxing in xylene for 60 min and rehydrated in a descending
alcohol series (100, 95 and 75%) at room temperature. Subsequently,
the tissue sections were blocked using an endogenous biotin
blocking kit (cat. no. BLK-0002; Beijing Strong Biotechnologies,
Inc.) for 10 min at room temperature. After washing with PBS, the
tissue sections were incubated for 32 min at 42°C with primary
antibodies targeted against ER (cat. no. kit-0012; clone SP1;
1:100; rabbit monoclonal), PR (cat. no. kit-0013; clone SP2; 1:100;
rabbit monoclonal), HER2 (cat. no. Kit-0043; clone MXR001; 1:100;
rabbit monoclonal) and Ki-67 (cat. no. RMA-0731; clone MXR002;
1:100; rabbit monoclonal) (all from Beijing Strong Biotechnologies,
Inc.). After washing with PBS, the tissue sections were processed
with a MaxvisionTM HRP kit (cat. no. kit-5004; Beijing Strong
Biotechnologies, Inc.). The IHC results were judged by experienced
pathologists using a light microscope (magnification, ×40 and
×100), and the ER and PR were regarded as positive if >1% of
nuclei were stained (18). With
respect to Ki-67, a range of 500–1,000 cells were counted to
calculate the percentage of positive tumor cell nuclei, including
hot spot areas (19). The molecular
subtype was classified according to the 2013 St. Gallen expert
panel consensus (20). All
histological and IHC tumor slides were evaluated independently by
two pathologists.
Fluorescence in situ hybridization
(FISH)
HER2 status was considered to be positive if >10%
of the tumor cells showed a score of 3+ from IHC or showed a
>2.2-fold increase in FISH using a HER2 DNA Probe kit (cat. no.
2J01-30; Abbott Molecular Inc.) (21). Briefly, after the samples were
deparaffinized, dehydrated and air-dried, the tissue sections were
handled with pre-treatment solution at 80°C for 30 min. Then, the
sections were immersed in protease solution at 37°C for 34 min,
followed by immersion in wash buffer (70, 80 and 100% ethanol).
Subsequently, the tissue sections were incubated with the probe
mixture [10 µl HER2 probe (226 kb; 10 ng/µl) and 10 µl CEP17 probe
(9 kb; 20 ng/µl)] at 74°C for 5 min, then the cover slip was sealed
with Fixogum rubber cement (cat. no. 12101ES62; Marabu GmbH and Co.
KG) for 10 min at room temperature, and the samples were
subsequently incubated overnight at 37°C. Next, the samples were
washed with post-hybridization wash buffer at room temperature for
15 min. After air-drying, 10 µl DAPI (cat. no. 30-804840; Abbott
Molecular Inc.) was added to the target area and a cover glass was
added and the samples were incubated at −20°C for 10 min. After the
slides were stored in the dark and left at room temperature, the
FISH results were judged by experienced pathologists using a
fluorescence microscope (magnification, ×40 and ×100).
Testing using the 21-gene RS
assay
The Oncotype DX 21-gene test was performed by AmoyDx
Diagnostics Co., Ltd. Briefly, the H&E-stained slides were
reviewed by pathologists to ensure that the paraffin section
contained sufficient tumor tissue. RNA was then extracted from the
unstained breast tumor formalin fixed paraffin-embedded (FFPE)
sections using a RNeasy FFPE RNA kit (cat. no. 172348; AmoyDx
Diagnostics Co., Ltd), and the concentration was measured after
verifying the absence of DNA contamination. Gene-specific RT was
performed at 65°C for 5 min and 37°C for 60 min using the
PrimeScript RT Master Mix kit (Takara Biotechnology, Co., Ltd.).
Subsequently, standardized quantitative PCR was performed using
Premix Ex Taq™ (Takara Bio, Inc.) in 384-well plates and an Applied
Biosystems Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and the following thermocycling conditions were
used: Initial denaturation at 95°C for 10 min, 95°C for 20 sec and
60°C for 45 sec (for 40 cycles). The 16 genes examined comprised of
five proliferation-related genes [Ki-67, aurora kinase A (AURKA),
baculoviral IAP repeat containing 5 (BIRC5), cyclin B1 (CCNB1) and
MYB proto-oncogene like 2 (MYBL2)], two metastasis-related genes
[MMP11 and cathepsin V (CTSV)], two HER2-related genes [growth
factor receptor bound protein 7 (GRB7) and HER2], four
hormone-related genes [ER, PR, BCL2 and signal peptide CUB domain
and EGF like domain containing 2 (SCUBE2)] and three independent
genes [glutathione S-transferase mu 1 (GSTM1), BAG cochaperone 1
(BAG1) and CD68], which were normalized according to five reference
genes (ACTB, GAPDH, RPLP0, GUSB and TFRC). Therefore, 16
cancer-related genes in 21 genes can be used to predict the outcome
of patients. The expression of the genes was confirmed in
triplicate, and the relative gene expression was calculated using
the 2−∆∆Cq method (22),
and the RS was calculated based on the Oncotype DX formula
(10). According to the RS results,
patients were categorized into low-risk (RS <18),
intermediate-risk (RS ≥18-30) and high-risk (RS ≥30) groups
(23). For further analysis, the
individual gene expression of the 16-cancer genes was measured, and
the distribution of the 16-cancer gene expression in PMBC and MMBC
cases was analyzed.
Statistical analysis
The clinicopathological characteristics were
presented as patient number and percentage and the other data was
expressed as the mean ± standard deviation and range. The
χ2 test or Fisher's exact test were used to evaluate
associations between PMBC and MMBC, while the Kruskal-Wallis test
was used to compare quantitative characteristics. Logistic
regression was used in multivariate analyses to identify risk
factors impacting lymph node metastasis. The Kaplan-Meier
estimation (log-rank test) was used to assess DFS and OS rate, and
the Cox proportional hazard model was used to analyze the
prognostic factors of patients with MBC. The Mann-Whitney test was
used to assess the distribution of the 21-gene RS in the different
subgroups, and to compare the expression levels of the 16 cancer
genes between subgroups. P<0.05 was considered to indicate a
statistically significant difference. SPSS version 22.0 software
(IBM Corp.) was used for all the statistical analyses.
Results
Patients and baseline
clinicopathological features
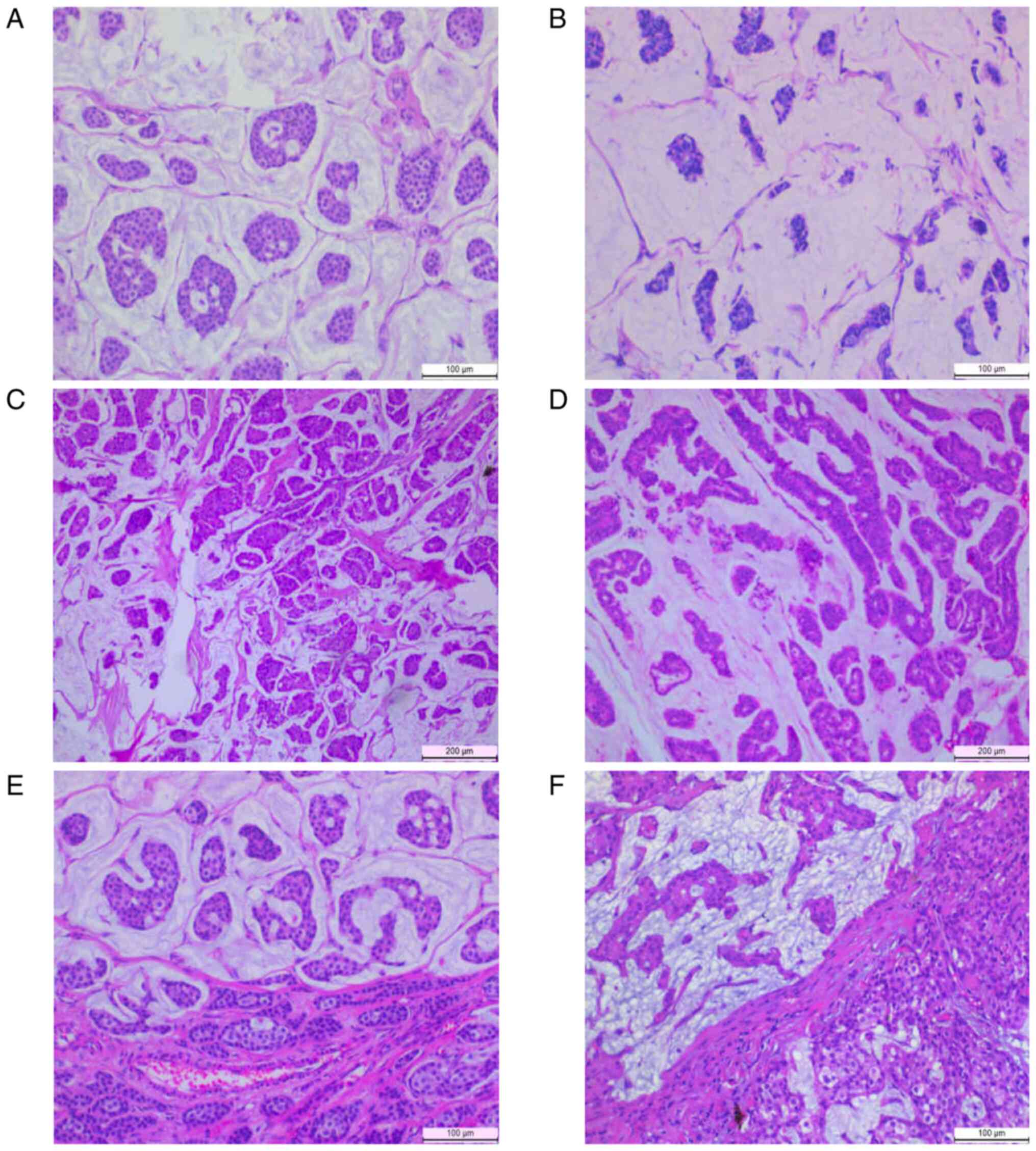
In total, 49 cases diagnosed as MBC (38 PMBCs and 11
MMBCs) were included in this analysis and the pathological changes
of various typical MBCs are shown in Fig. 1. The median age at diagnosis was 52.3
± 12.8 years (range, 33–87 years), and 44.9% of these patients were
postmenopausal. The median tumor size was 3.2 ± 1.8 cm (range,
1.0–8.5 cm) at diagnosis, and 29.2% of cases had axillary lymph
node involvement. According to IHC and FISH results, 45 (91.8%) and
38 (77.6%) patients with MBC were ER and PR positive, respectively.
In 5 (10.2%) of the patients with MBC, HER2 positivity was
detected, while 34.7% of all patients had ≥20% Ki-67 expression.
For the molecular subtype, 49.0% (n=24) were classified as luminal
A, 42.8% (n=21) as luminal B, 4.1% (n=2) as HER2-rich and 4.1%
(n=2) as triple negative. The detailed clinicopathological
characteristics of the patients are shown in Table II.
 | Table II.Clinicopathological features of
patients with mucinous breast carcinoma (n=49). |
Table II.
Clinicopathological features of
patients with mucinous breast carcinoma (n=49).
| Parameters | Number (%) |
|---|
| Age, years |
|
|
≤50 | 26 (53.1) |
|
≥50 | 23 (46.9) |
| Menstruation |
|
|
Premenopausal | 27 (55.1) |
|
Postmenopausal | 22 (44.9) |
| Tumor size,
pTa |
|
| T1 | 18 (37.5) |
| T2 | 18 (37.5) |
| T3 | 12 (25.0) |
| Nodal status,
pNa |
|
| N0 | 34 (70.8) |
| N1 | 7 (14.6) |
| N2 | 6 (12.5) |
| N3 | 1 (2.1) |
| TNM
stagea |
|
| I | 13 (27.1) |
| II | 26 (54.2) |
|
III | 9 (18.7) |
| Subtype |
|
|
PMBC | 38 (77.6) |
|
MMBC | 11 (22.4) |
| ER status |
|
|
Positive | 45 (91.8) |
|
Negative | 4 (8.2) |
| PR status |
|
|
Positive | 38 (77.6) |
|
Negative | 11 (22.4) |
| HER2 status |
|
|
Positive | 5 (10.2) |
|
Negative | 44 (89.8) |
| Ki67, % |
|
|
<20 | 32 (65.3) |
|
≥20 | 17 (34.7) |
| Molecular
subtypeb |
|
| Luminal
A | 24 (49.0) |
| Luminal
Bc | 21 (42.8) |
|
HER2 | 2 (4.1) |
| Triple
negative | 2 (4.1) |
The mean age at diagnosis in patients with PMBC and
MMBC was 51.5 ± 13.4 years (range, 33–87 years) and 54.9 ± 10.8
years (range, 33–78 years), respectively (P=0.25), and the mean
tumor size in PMBCs and MMBCs was 3.19 ± 1.8 cm (range, 1.2–8.5 cm)
and 3.17 ± 1.6 cm (range, 1.0–5.0 cm), respectively (P=0.914). The
data showed no significant differences between PMBCs and MMBCs with
respect to TNM stage (P=0.261), molecular subtype (P=0.17), status
of ER (P=0.562), status of PR (P=0.398) and Ki-67 expression
(P=0.395). However, a significantly higher incidence rate of
axillary lymph node involvement was observed in MMBCs comparison
with that in PMBCs (72.7 vs. 16.2%, respectively; P=0.001). The
clinicopathological characteristics of the PMBCs and MMBCs are
detailed in Table III. Similarly,
the results of multivariate analysis demonstrated that the only
high-risk factor of lymph node metastasis in patients with MBC was
the pathological subtype (P=0.018; Table IV). Furthermore, the status of HER2
had a marginal P-value (P=0.068) in the two groups, and a higher
incidence rate was observed in MMBCs compared with that in PMBCs
(27.3 vs. 5.3%).
 | Table III.Comparison of clinicopathological
characteristics in patients with PMBC and MMBC. |
Table III.
Comparison of clinicopathological
characteristics in patients with PMBC and MMBC.
| Characteristic | PMBC | MMBC | P-value |
|---|
| Mean age ± SD
(range), years | 51.5±13.4
(33–87) | 54.9±10.8
(39–78) | 0.25 |
| Mean tumor size ±
SD (range), cm | 3.19±1.8
(1.2–8.5) | 3.17±1.6
(1.0–5.0) | 0.914 |
| Nodal status,
pNa |
|
| 0.001 |
| N0 | 31 | 3 |
|
|
N1-3 | 6 | 8 |
|
| TNM
stagea |
|
| 0.261 |
| I | 11 | 2 |
|
| II | 21 | 5 |
|
|
III | 5 | 4 |
|
| ER status |
|
| 0.562 |
|
Positive | 34 | 11 |
|
|
Negative | 4 | 0 |
|
| PR status |
|
| 0.398 |
|
Positive | 31 | 7 |
|
|
Negative | 7 | 4 |
|
| HER2 status |
|
| 0.068 |
|
Positive | 2 | 3 |
|
|
Negative | 36 | 8 |
|
| Ki67, % |
|
| 0.395 |
|
<20 | 26 | 6 |
|
|
≥20 | 12 | 5 |
|
| Molecular
subtypeb |
|
| 0.17 |
| Luminal
A | 21 | 4 |
|
| Luminal
B | 14 | 7 |
|
|
HER2 | 2 | 0 |
|
| Triple
negative | 2 | 0 |
|
 | Table IV.Logistic regression analysis of
factors predicting lymph node metastasis. |
Table IV.
Logistic regression analysis of
factors predicting lymph node metastasis.
|
|
|
|
|
| 95% CI |
|---|
|
|
|
|
|
|
|
|---|
| Parameters | B | S.E | Wald | P-value | Lower | Upper |
|---|
| Age (<50 vs.
>50 years) | −1.274 | 0.928 | 1.885 | 0.170 | 0.045 | 1.724 |
| Tumor size (<2
vs. >2 cm) | 0.791 | 0.858 | 0.849 | 0.375 | 0.410 | 11.853 |
| ER (positive vs.
negative) | 19.138 | 17425.283 | 0.000 | 0.999 | N/A | N/A |
| PR (positive vs.
negative) | 0.056 | 1.116 | 0.030 | 0.960 | 0.119 | 9.419 |
| HER2 (positive vs.
negative) | 20.274 | 17425.283 | 0.000 | 0.999 | N/A | N/A |
| Ki67 (<20 vs.
>20%) | 0.809 | 0.938 | 0.743 | 0.389 | 0.357 | 14.116 |
| Subgroup (PMBC vs.
MMBC) | 2.629 | 1.116 | 2.527 | 0.018 | 1.556 | 123.560 |
| Constant | −21.074 | 17425.283 | 0.000 | 0.999 |
|
|
Treatment and prognosis in patients
with MBC
A total of 98.0% of the patients with MBC in the
present study underwent radical mastectomies (1 patient refused
surgery), and the first-line treatment selections following surgery
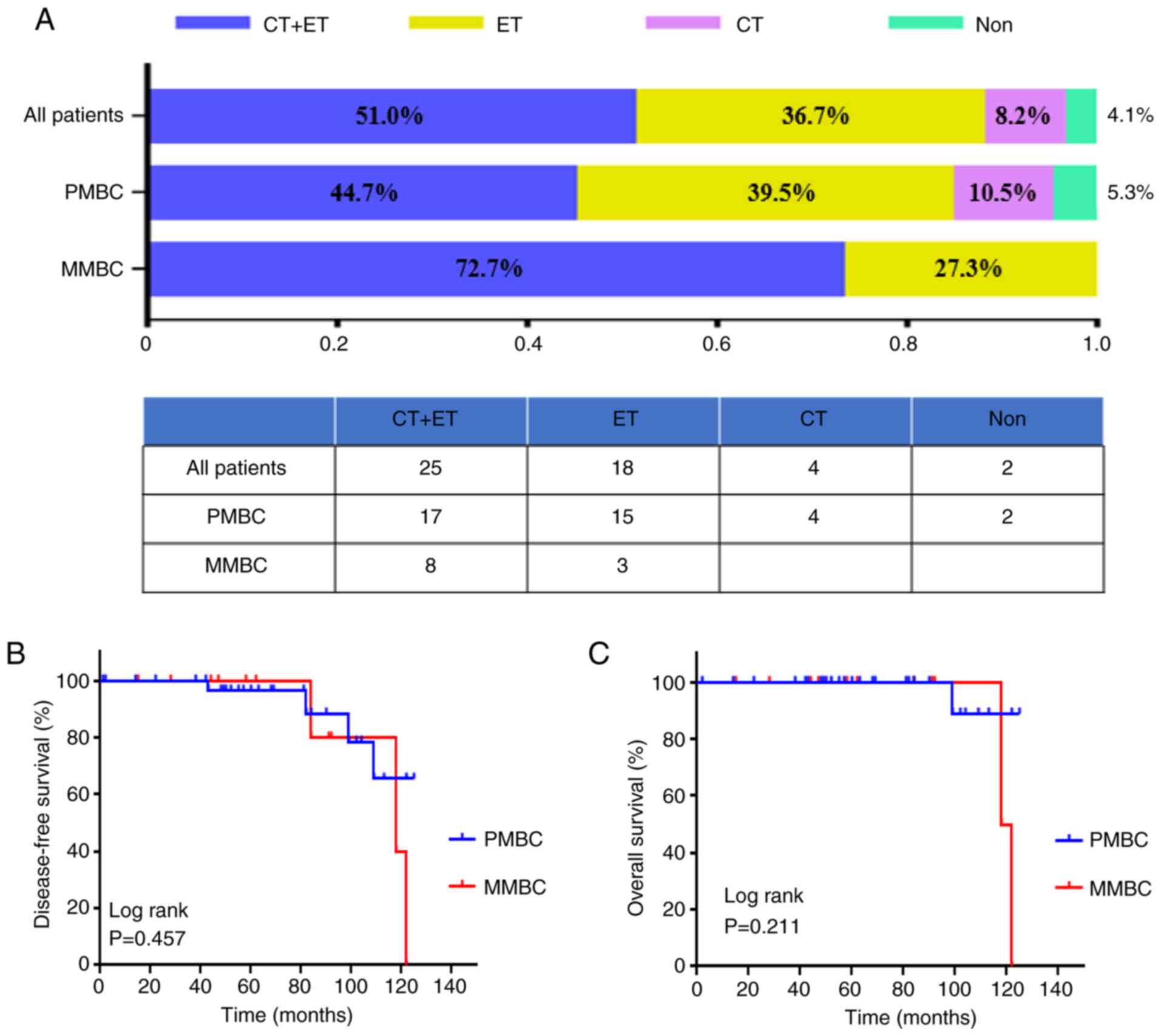
in the MBC cases with different subtypes are presented in Fig. 2A. Overall, 8.2% (n=4), 36.7% (n=18)
and 51% (n=25) of enrolled patients received CT, endocrine therapy
(ET) and CT followed by ET as first-line treatment according to the
molecular subtypes, respectively. The detailed adjuvant treatment
of the MBC cases with various molecular subtypes are shown in
Table I. Of all the patients with
HER2 expression amplification, only 1 patient (20%) received
trastuzumab therapy. In the PMBC and MMBC cases, the proportion of
those receiving CT was 55.3 and 72.7%, respectively, and there was
no statistical significance (P=0.102; data not shown).
The mean follow-up time for patients with MBC was
65.6 months (range, 2–125 months), and 2 patients were lost during
this time. As shown in Fig. 2B and
C, the 5-year DFS and 5-year OS rates for MBC was 97 and 100%,
respectively, and this result was not statistically significant
between PMBCs and MMBCs (log-rank test; P=0.457).
During the study period, distant metastases were
found in 5 patients with high TNM stage (3 cases with stage III and
2 cases with stage II), and 2 of these patients died from lung
metastases (both HER2 expression positive). In addition, 1 patient
with PMBC with no recurrence died of a cardiovascular accident. The
causes of treatment failure in MBCs cases are presented in Table V. In addition, Cox multivariate
analysis did not identify any statistically significant factors
associated with the prognosis of patients with MBC (Table VI).
 | Table V.Disease recurrence and survival
profile of the enrolled patients. |
Table V.
Disease recurrence and survival
profile of the enrolled patients.
|
Recurrence/metastasis sites | Pathological
subtype | Molecular
subtype | Stage | TTRa, month | Outcome |
|---|
| Chest wall and
lung | MMBC | B/HER2 | T3N2M0 | 50 | Death |
| Lung | PMBC | B | T2N0M0 | 76 | Survival |
| Bone and lung | MMBC | B/HER2 | T3N1M0 | 79 | Death |
| Bone | PMBC | A | T3N2M0 | 72 | Survival |
| Bone | PMBC | A | T2N0M0 | 58 | Survival |
 | Table VI.Prognostic significance of the
clinicopathological factors on DFS and OS in patients with MBC. |
Table VI.
Prognostic significance of the
clinicopathological factors on DFS and OS in patients with MBC.
|
| DFS | OS |
|---|
|
|
|
|
|---|
|
|
| 95% CI |
| 95% CI |
|---|
|
|
|
|
|
|
|---|
| Parameters | P-value | Lower | Upper | P-value | Lower | Upper |
|---|
| Age (<50 vs.
>50 years) | 0.239 | 0.220 | 2.548 | 0.975 | 0.280 | 32.050 |
| Tumor size (<2
vs. >2 cm) | 0.939 | N/A | N/A | 0.945 | 0.410 | 11.853 |
| Nodes (positive vs.
negative) | 0.986 | 0.940 | 10.256 | 0.953 | N/A | N/A |
| Subgroup (PMBC vs.
MMBC) | 0.952 | N/A | N/A | 0.999 | N/A | N/A |
| ER (positive vs.
negative) | 0.980 | N/A | N/A | 0.990 | N/A | N/A |
| PR (positive vs.
negative) | 0.960 | N/A | N/A | 0.969 | 0.119 | 9.419 |
| HER2 (positive vs.
negative) | 0.944 | N/A | N/A | 0.966 | N/A | N/A |
| Ki67 (<20 vs.
>20%) | 0.498 | 0.830 | 3.361 | 0.830 | 0.051 | 10.836 |
Comparison of Oncotype DX 21-gene RS
and individual gene expression between the PMBC and MMBC
groups
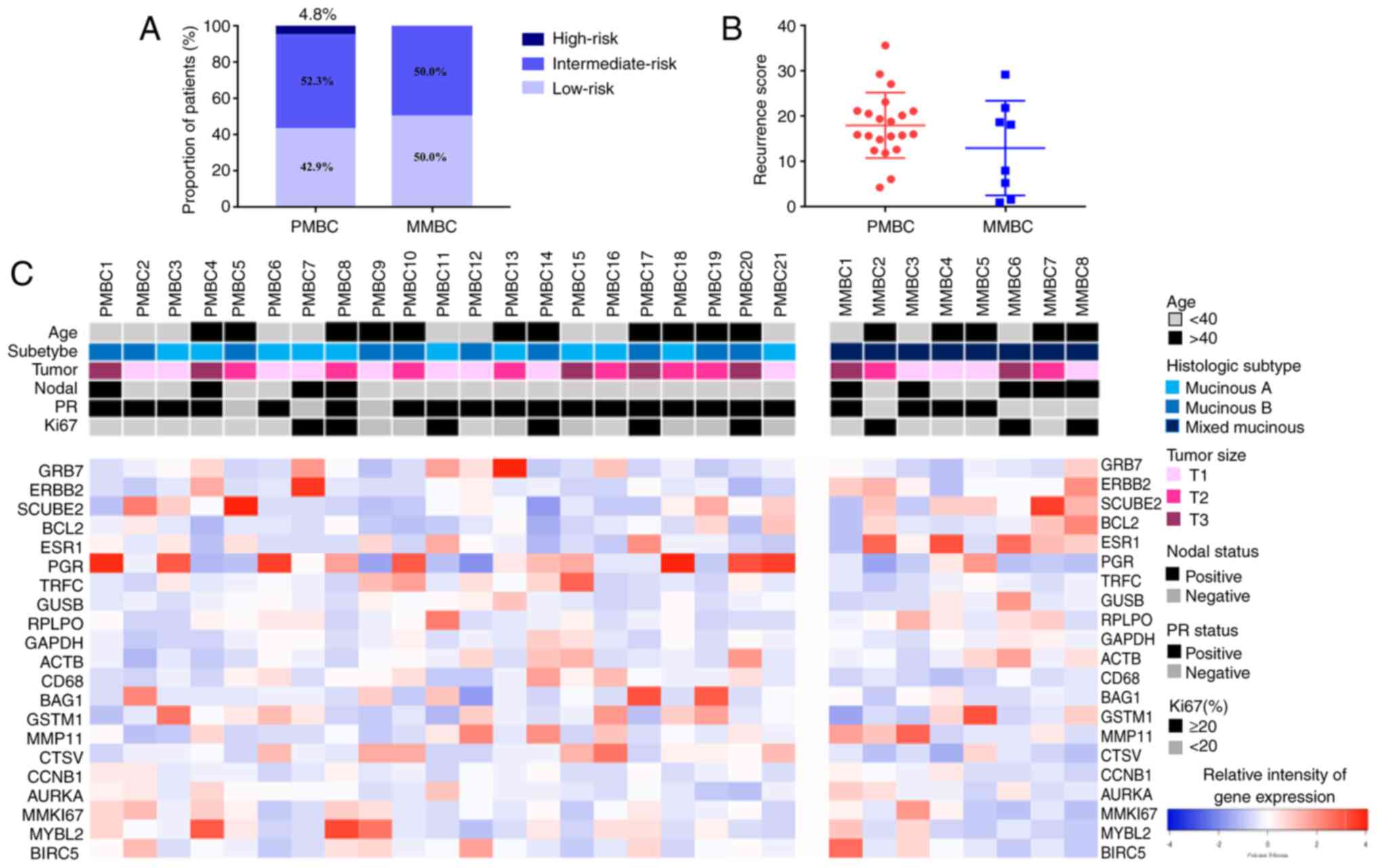
In the present study, 29 of the 42 enrolled
ER-positive and HER2-negative MBC cases underwent Oncotype DX
21-gene testing (the sample quality of 13 cases did not meet the
test) and the results were evaluable, which included 21 PMBCs and 8
MMBCs. According to the criteria of 21-gene test RS stratification,
51.7% patients (15/29) were in the low-risk group (RS <18) with
a mean RS of 10.5 ± 5.6, 44.8% patients (13/29) were in the
intermediate-risk group (RS ≥18-30) with a mean RS of 22.3±5.2, and
3.5% patients (1/29) were in the high-risk group (RS ≥30) (RS,
35.7). The proportions of low-, intermediate- and high-risk RS were
42.9, 52.3 and 4.8%, respectively, among PMBCs, and 50.0, 50.0 and
0% in the MMBCs (P=0.91; Fig. 3A).
The mean 21-gene RS in the PMBC and MMBC groups was 18.0 and 13.0,
respectively (P=0.151; Fig. 3B).
Notably, based on the traditional RS treatment recommendation,
37.9% of patients with MBC in the present study could avoid CT
(Fig. S1).
The individual gene expression of the 16 cancer
genes from the 21-gene test between the PMBC and MMBC groups was
analyzed. The histograms of the distribution of cancer gene
expression in the different histological-type subgroups are
presented in Fig. 3C. In general,
the expression levels of the genes from the proliferation and HER2
groups did not differ significantly between the PMBC and MMBC
cases. In the metastasis group, the expression level of CTSV
(P=0.005) was significantly higher in the PMBC group compared with
that in the MMBC group. In the ER group, the expression level of PR
(P=0.018) was significantly higher in the PMBC group compared with
that in the MMBC group, and the expression level of ESR1 had a
marginal P-value (P=0.053). Furthermore, in the independent group,
the expression level of CD68 was higher in the PMBC group
(P=0.003), while the expression levels of GSTM1 and BAG1 did not
differ significantly between groups. The detailed expression of the
16 cancer genes between the PMBC and MMBC groups are shown in
Table VII.
 | Table VII.Comparison of individual gene
expression levels of the 16 cancer genes from the 21-gene RS in
patients with PMBC and MMBC. |
Table VII.
Comparison of individual gene
expression levels of the 16 cancer genes from the 21-gene RS in
patients with PMBC and MMBC.
| A. Proliferation
group |
|---|
|
|---|
| Gene name | AEI in PMBC ± SD
(range) | AEI in MMBC ± SD
(range) | P-value |
|---|
| CCNB1 | 0.93±1.17
(0.5–5.58) | 1.26±0.41
(0.41–1.53) | 0.793 |
| AURKA | 1.23±0.76
(0.44–3.9) | 1.0±0.59
(0.41–1.98) | 0.649 |
| MKI67 | 1.56±1.75
(0.31–8.45) | 1.15±1.29
(0.21–4.16) | 0.324 |
| MYBL2 | 2.27±4.45
(0.3–21.27) | 0.94±0.93
(0.21–2.67) | 0.168 |
| BIRC5 | 1.48±1.49
(0.17–7.06) | 1.22±1.14
(0.24–3.46) | 0.401 |
|
| B. Invasion
group |
|
| Gene
name | AEI in PMBC ± SD
(range) | AEI in MMBC ± SD
(range) | P-value |
|
| MMP11 | 1.2±0.98
(0.28–4.07) | 1.82±1.66
(0.47–5.38) | 0.324 |
| CTSV | 1.59±1.37
(0.42–6.62) | 0.61±0.48
(0.16–1.6) | 0.005 |
|
| C. ER
group |
|
| Gene
name | AEI in PMBC ± SD
(range) | AEI in MMBC ± SD
(range) | P-value |
|
| SCUBE2 | 1.53±1.66
(0.14–6.24) | 1.92±1.84
(0.43–6.06) | 0.401 |
| BCL2 | 1.29±1.26
(0.31–5) | 1.37±1.00
(0.5–2.94) | 0.457 |
| ESR1 | 1.22±1.07
(0.31–4.98) | 2±1.12
(0.48–3.65) | 0.053 |
| PGR | 2.83±3.19
(0.05–12.9) | 0.7±0.86
(0.04–2.53) | 0.018 |
|
| D. HER2
group |
|
| Gene
name | AEI in PMBC ± SD
(range) | AEI in MMBC ± SD
(range) | P-value |
|
| GRB7 | 2.04±3.12
(0.35–14.5) | 0.91±0.51
(0.43–1.86) | 0.457 |
| ERBB2 | 1.26±1.62
(0.32–7.96) | 1.57±0.90
(0.4–2.84) | 0.103 |
|
| E. Independent
group |
|
| Gene
name | AEI in PMBC ± SD
(range) | AEI in MMBC ± SD
(range) | P-value |
|
| CD68 | 1.38±10.8
(0.32–4.66) | 0.63±0.22
(0.42–1.04) | 0.003 |
| BAG1 | 1.6±1.83
(0.36–6.7) | 0.96±0.44
(0.43–1.74) | 0.684 |
| GSTM1 | 1.62±1.66
(0.14–5.9) | 1.13±1.17
(0.09–3.63) | 0.457 |
Discussion
MBC is a rare histological type of primary breast
cancer and a previous epidemiological survey reported that the
incidence rate of MBC in Caucasians was lower compared with that in
Africans (24). Prior studies
indicated that the majority of MBC cases were ER-positive,
HER2-negative tumors without node metastasis, which suggested that
the treatment of MBC should be different from IDC (14). Therefore, it is necessary to divide
patients into different subgroups according to the recurrence risk
and it can be used to choose more reasonable adjuvant therapy. The
21-gene RS has been proved to assist clinicians with therapeutic
decisions; however, data on the RS of MBC remains limited and to
the best of our knowledge, this topic has not been addressed in
large studies. The present study assessed the clinicopathological
features, treatment and prognosis of patients with MBC. More
importantly, it evaluated the distribution pattern and clinical
value of the 21-gene RS in patients with MBC. To the best of our
knowledge, the current study represents the first study focused on
comparing the 21-gene RS and individual gene expression for
patients with PMBC and those with MMBC.
In the current study, from the 3,081 patients with
invasive breast cancer, 49 (1.59%) had MBC and the incidence rate
was similar to that of other studies (1,2,25). The present results demonstrated that
postmenopausal women accounted for 44.9% of all MBCs, and 91.8%
(45/49) and 77.6% (38/49) MBC cases were ER- and PR-positive,
respectively, which were consistent with previous findings
(3,4). In addition, 29.2% of MBCs had axillary
lymph node metastases, which was higher than the incidence rate of
axillary metastases, ranging from 3–26%, reported in the literature
(1,2,4,5). This may be because 22.4% of the cases
(11/49) in the current study were MMBCs. Next, the present study
compared the clinicopathological characteristics of patients with
PMBC and those with MMBC. There were no significant differences
between PMBCs and MMBCs with respect to age, tumor size, TNM stage,
ER status, PR status and Ki-67 expression. However, patients with
MMBC showed a significantly higher incidence rate of axillary nodal
metastases compared with those with PMBC (72.7 vs. 16.2%), which
was consistent with previous studies (8,26).
Notably, a higher incidence rate of HER2 positivity was observed in
MMBCs in comparison with PMBCs (27.3 vs. 5.3%), and this phenomenon
has been confirmed by other study (27).
The present study also assessed the treatment and
prognosis in PMBC and MMBC cases. According to the clinical stage
and molecular subtype, 55.3% of PMBCs and 72.7% of PMBCs received
CT, while the proportion of PMBC and MMBC cases receiving ET was
84.2 and 100.0%, respectively. With a mean follow-up of 65.6 months
(range, 2–125 months), it was demonstrated that patients with MBC
had excellent 5-year DFS (97.0%) and OS (100.0%) rates, which was
similar to findings of other studies (3,28,29).
However, the difference in the 5-year DFS and OS rates between
PMBCs and MMBCs were statistically insignificant, which was not
consistent with previous studies (8,17). This
phenomenon could be explained by the relatively short follow-up
time and small number of patients with metastasis and those that
died.
In the present study, Oncotype DX 21-gene testing
was performed in 29 ER-positive/HER2-negative patients with MBC,
including 21 PMBCs and 8 MMBCs. The results indicated that 51.7% of
MBC cases were in the low-risk group, with a mean RS of 10.5 ± 5.6,
although 4 patients (26.7%) had lymph node metastases. The
intermediate-risk group included 13 patients with MBC, which had a
mean RS of 22.3 ± 5.2, and 5 patients (28.5%) in this group had
lymph node metastases. In addition, only 1 node-negative cases was
classified into the high risk group, with a mean RS of 35.7. These
results showed a lower proportion of patients with low-risk and a
higher proportion of patients with intermediate-risk compared with
that in the study by Turashvili et al (29), which may be due to the fact that the
patients included in the current study have more high-risk clinical
factors. Based on the traditional RS treatment recommendation,
37.9% of patients with MBC in the present study could avoid CT, and
27.6% of them could choose CT or not. Notably, the NSABP B-20 study
reported that only patients which had a RS ≥31 benefited the most
from adjuvant CT (30), and the
TAILORx study only recommended that patients with a RS ≥26 receive
adjuvant CT (31). Furthermore, the
Southwest Oncology Group-8814 and Eastern Cooperative Oncology
Group E2197 studies extended the application of the 21-gene RS
assay to the lymph node positive population, as well as advocated
RS use in patients with 1–3 positive lymph nodes and considered
omitting adjuvant CT in those with a RS <18 (12,13).
However, this requires further research for confirmation. In
addition, previous research has analyzed the association between RS
and the prognosis of MBC and found that it is no significant
differences in DFS and OS rates among MBC patients in different RS
risk groups (15). However, it is
difficult to analyze the association between RS and prognosis in
the study as only 2 patients had metastasis (PMBC14, RS, 18.87 and
PMBC2, RS, 15.63).
Next, the current study performed a comparison of
the 21-gene RS between PMBCs and MMBCs and the data revealed there
was no statistically significant differences between the two
groups. This result suggests that PMBCs and MMBCs may have similar
21-gene RS with the same molecular subtypes
(ER+/HER2−), but larger sample studies are
required to confirm this conclusion. Analysis of the individual
cancer gene expression differences from the 21-gene RS between
PMBCs and MMBCs was performed, and three of these genes were
differently expressed in PMBC compared with MMBC. As a key element
in tumor growth and metastasis, a high expression level of CTSV was
previously shown to be associated with poor prognosis in breast
cancer (32). In the current study,
the expression of CTSV was significantly higher in PMBCs compared
with that in MMBCs, which suggested that the cell invasive ability
of the former may be higher compared with that of the latter.
However, this phenomenon is not consistent with the fact that the
lymph node metastasis rate of patients with MMBC was higher
compared with that in patients with PMBC and further studies are
required to verify the association between CTSV and MBC. PR is the
main downstream signal molecule in the ER signaling pathway
(33), and PR status was defined as
a predictor for RS according to previous analyses in the Plan B and
NASBP B20 studies (30,34). The present data revealed that the
expression level of PR in the PMBC group was significantly higher
compared with that in the MMBC group, which suggested that PMBC had
a more favorable response to ET. CD68 is a marker of macrophages
and its expression can indicate the infiltration of tumor
lymphocytes (35). A previous study
confirmed that a high level of CD68 protein expression was
associated with poor prognosis in patients with breast cancer
(36). In the current study, the
expression level of CD68 was higher in the PMBC group compared with
the MMBC group, which indicated that the immune status was
different between the two groups, which warrants further
investigation.
The current study has some limitations. First, the
number of MBC cases was limited due to its relatively low
incidence. Second, the study was single-centered and retrospective,
which could cause selection bias. Finally, the follow-up time was
relatively short and ongoing, and a longer follow-up would be of
benefit for further conclusions for MBC.
In conclusion, the main purpose of the present study
was to evaluate the distribution pattern and clinical value of
21-gene RS in patients with MBC. The clinicopathological data and
prognosis of 38 patients with PTMC and 11 patients with MMBC were
analyzed and a total of 29 ER-positive and HER2-negative patients
with MBC underwent the Oncotype DX 21-gene test. The results showed
patients with MBC had favorable prognosis, and patients with PMBC
and MMBC had low- and intermediate-risk RS, which suggested that a
considerable proportion of patients may be able to avoid CT;
however, further research and clinical trials should be conducted
to confirm the observations. There were no statistically
significant differences between PMBCs and MMBCs in the 21-gene RS,
but the high expression level of PR-related genes in PMBCs
indicated that they may have an improved response to ET compared
with MMBCs. In addition, CTSV and CD68 expression showed a
significant difference between the PMBC and MMBC groups, which may
indicate that they have different tumor characteristics and further
studies are required to verify the association of these gene
expression patterns on MBC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Zunyi Science and
Technology Project [Zunshi Kehe Hz Zi (2020) grant no. 258],
Guizhou Province Science Plan Program (Qian Ke He Foundation-ZK
[2021] General 461), National Natural Science Foundation of China
(grant no. 81860715) and Doctor Foundation of Affiliated Hospital
of Zunyi Medical University (grant no. 201712).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RC, YW, JW and XC conceived the study. RC, YW, TL,
JL, NT and GF collected and interpreted the data. JW and XC confirm
the authenticity of the raw data. RC, YW, NT and JW performed the
data analysis. RC and YW wrote the manuscript. XC, TL and JL
reviewed and edited the manuscript. RC, TL and JL acquired the
funding. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures were performed in accordance with the
ethical standards of the Ethical Committees of Affiliated Hospital
of Zunyi Medical University and the Declaration of Helsinki of
1964. The present study was reviewed and approved by the Ethical
Committee of Affiliated Hospital of Zunyi Medical University. All
patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kim HS, Yoo TK, Park WC and Chae BJ: The
prognostic value of HER2 status and efficacy of anti-HER2 therapy
in patients with HR-positive mucinous breast cancer: A nationwide
study from the Korean Breast Cancer Society. Breast Cancer Res
Treat. 180:461–470. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Di Saverio S, Gutierrez J and Avisar E: A
retrospective review with long term follow up of 11,400 cases of
pure mucinous breast carcinoma. Breast Cancer Res Treat.
111:541–547. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bae SY, Choi MY, Cho DH, Lee JE, Nam SJ
and Yang JH: Mucinous carcinoma of the breast in comparison with
invasive ductal carcinoma: Clinicopathologic characteristics and
prognosis. J Breast Cancer. 14:308–313. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Barkley CR, Ligibel JA, Wong JS, Lipsitz
S, Smith BL and Golshan M: Mucinous breast carcinoma: A large
contemporary series. Am J Surg. 196:549–551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cao AY, He M, Liu ZB, Di GH, Wu J, Lu JS,
Liu GY, Shen ZZ and Shao ZM: Outcome of pure mucinous breast
carcinoma compared to infiltrating ductal carcinoma: A
population-based study from China. Ann Surg Oncol. 19:3019–3027.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanagiri T, Ono K, Baba T, So T, Yamasaki
M, Nagata Y, Uramoto H, Takenoyama M and Yasumoto K:
Clinicopathologic characteristics of mucinous carcinoma of the
breast. Int Surg. 95:126–129. 2010.PubMed/NCBI
|
|
7
|
Lei L, Yu X, Chen B, Chen Z and Wang X:
Clinicopathological characteristics of mucinous breast cancer: A
retrospective analysis of a 10-year study. PLoS One.
11:e01551322016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Skotnicki P, Sas-Korczynska B, Strzepek L,
Jakubowicz J, Blecharz P, Reinfuss M and Walasek T: Pure and mixed
mucinous carcinoma of the breast: A comparison of clinical outcomes
and treatment results. Breast J. 22:529–534. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Paik S, Shak S, Tang G, Kim C, Baker J,
Cronin M, Baehner FL, Walker MG, Watson D, Park T, et al: A
multigene assay to predict recurrence of tamoxifen-treated,
node-negative breast cancer. N Engl J Med. 351:2817–2826. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Green N, Al-Allak A and Fowler C: Benefits
of introduction of oncotype DX® testing. Ann R Coll Surg
Engl. 101:55–59. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sparano JA, Gray RJ, Makower DF, Pritchard
KI, Albain KS, Hayes DF, Geyer CE, Dees EC, Goetz MP, Olson JA, et
al: Adjuvant chemotherapy guided by a 21-gene expression assay in
breast cancer. N Engl J Med. 379:111–121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goldstein LJ, Gray R, Badve S, Childs BH,
Yoshizawa C, Rowley S, Shak S, Baehner FL, Ravdin PM, Davidson NE,
et al: Prognostic utility of the 21-gene assay in hormone
receptor-positive operable breast cancer compared with classical
clinicopathologic features. J Clin Oncol. 26:4063–4071. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Albain KS, Barlow WE, Shak S, Hortobagyi
GN, Livingston RB, Yeh IT, Ravdin P, Bugarini R, Baehner FL,
Davidson NE, et al: Prognostic and predictive value of the 21-gene
recurrence score assay in postmenopausal women with node-positive,
oestrogen-receptor-positive breast cancer on chemotherapy: A
retrospective analysis of a randomised trial. Lancet Oncol.
11:55–65. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang W, Chen X, Lin L, Fei X, Garfield DH,
Hong J, Gao W, Zhu S, Wu J, Huang O, et al: Distribution and
clinical utility of the 21-gene recurrence score in pure mucinous
breast cancer patients: A case-control study. J Cancer.
9:3216–3224. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu J, Ding S, Lin L, Fei X, Lin C,
Andriani L, Goh C, Huang J, Hong J, Gao W, et al: Comparison of the
distribution pattern of 21-gene recurrence score between mucinous
breast cancer and infiltrating ductal carcinoma in Chinese
population: A retrospective single-center study. Cancer Res Treat.
52:671–679. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lebeau A and Denkert C: Updated WHO
classification of tumors of the breast: The most important changes.
Pathologe. 42:270–280. 2021.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kashiwagi S, Onoda N, Asano Y, Noda S,
Kawajiri H, Takashima T, Ohsawa M, Kitagawa S and Hirakawa K:
Clinical significance of the sub-classification of 71 cases
mucinous breast carcinoma. Springerplus. 2:4812013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hammond ME, Hayes DF, Dowsett M, Allred
DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS,
Hayes M, et al: American Society of Clinical Oncology/College of
American Pathologists guideline recommendations for
immunohistochemical testing of estrogen and progesterone receptors
in breast cancer. J Clin Oncol. 28:2784–2795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dowsett M, Nielsen TO, A'Hern R, Bartlett
J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, et
al: Assessment of Ki67 in breast cancer: Recommendations from the
international Ki67 in Breast Cancer working group. J Natl Cancer
Inst. 103:1656–1664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ; Panel members, :
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen International Expert Consensus on the
Primary Therapy of Early Breast Cancer 2013. Ann Oncol.
24:2206–2223. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wolff AC, Hammond MEH, Allison KH, Harvey
BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P,
Hanna W, et al: Human epidermal growth factor receptor 2 testing in
breast cancer: American society of clinical Oncology/College of
American pathologists clinical practice guideline focused update. J
Clin Oncol. 36:2105–2122. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sparano JA, Gray RJ, Makower DF, Pritchard
KI, Albain KS, Hayes DF, Geyer CE, Dees EC, Perez EA, Olson JA, et
al: Prospective validation of a 21-gene expression assay in breast
cancer. N Engl J Med. 373:2005–2014. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Abdulrahman G, Opeyemi R and Ganiyu A:
Epidemiology of breast cancer in Europe and Africa. J Cancer
Epidemiol. 2012:9156102012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yim HE, Kim JH, Ahn MS, Jung Y, Roh J,
Park SH, Kim TG, Choi JK and Kang SY: Clinicopathological and
molecular analysis of 45 cases of pure mucinous breast cancer.
Front Oncol. 10:5587602020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ranade A, Batra R, Sandhu G, Chitale RA
and Balderacchi J: Clinicopathological evaluation of 100 cases of
mucinous carcinoma of breast with emphasis on axillary staging and
special reference to a micropapillary pattern. J Clin Pathol.
63:1043–1047. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Erhan Y, Ciris M, Zekioglu O, Erhan Y,
Kapkac M, Makay O and Ozdemir N: Do clinical and
immunohistochemical findings of pure mucinous breast carcinoma
differ from mixed mucinous breast carcinoma. Acta Chir Belg.
109:204–208. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang J, He ZY, Dong Y, Sun JY, Zhang WW
and Wu SG: The Distribution and Outcomes of the 21-gene recurrence
score in T1-T2N0 Estrogen receptor-positive breast cancer with
different histologic subtypes. Front Genet. 9:6382018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Turashvili G, Brogi E, Morrow M, Hudis C,
Dickler M, Norton L and Wen HY: The 21-gene recurrence score in
special histologic subtypes of breast cancer with favorable
prognosis. Breast Cancer Res Treat. 165:65–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paik S, Tang G, Shak S, Kim C, Baker J,
Kim W, Cronin M, Baehner FL, Watson D, Bryant J, et al: Gene
expression and benefit of chemotherapy in women with node-negative,
estrogen receptor-positive breast cancer. J Clin Oncol.
24:3726–3734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sparano JA, Gray RJ, Ravdin PM, Makower
DF, Pritchard KI, Albain KS, Hayes DF, Geyer CE Jr, Dees EC, Goetz
MP, et al: Clinical and genomic risk to guide the use of adjuvant
therapy for breast cancer. N Engl J Med. 380:2395–2405. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Toss M, Miligy I, Gorringe K, Mittal K,
Aneja R, Ellis I, Green A and Rakha E: Prognostic significance of
Cathepsin V (CTSV/CTSL2) in breast ductal carcinoma in situ. J Clin
Pathol. 73:76–82. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mohammed H, Russell IA, Stark R, Rueda OM,
Hickey TE, Tarulli GA, Serandour AA, Serandour AA, Birrell SN,
Bruna A, et al: Progesterone receptor modulates ERα action in
breast cancer. Nature. 523:313–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gluz O, Nitz UA, Christgen M, Kates RE,
Shak S, Clemens M, Kraemer S, Aktas B, Kuemmel S, Reimer T, et al:
West German study group phase III planB trial: First prospective
outcome data for the 21-gene recurrence score assay and concordance
of prognostic markers by central and local pathology assessment. J
Clin Oncol. 34:2341–2349. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
de Groot AF, Blok EJ, Charehbili A, Engels
CC, Smit VTHBM, Dekker-Ensink NG, Putter H, Meershoek-Klein
Kranenbarg E, van de Velde CJH, Liefers GJ, et al: Strong CD8+
lymphocyte infiltration in combination with expression of HLA class
I is associated with better tumor control in breast cancer patients
treated with neoadjuvant chemotherapy. Breast Cancer Res Treat.
175:605–615. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pelekanou V, Villarroel-Espindola F,
Schalper KA, Pusztai L and Rimm DL: CD68, CD163, and matrix
metalloproteinase 9 (MMP-9) co-localization in breast tumor
microenvironment predicts survival differently in ER-positive and
-negative cancers. Breast Cancer Res. 20:1542018. View Article : Google Scholar : PubMed/NCBI
|