Introduction
Ewing sarcoma is the second most common osseous
disease in children and adolescents, and predominantly occurs in
the pelvic bones, femur and chest wall (1,2).
Pathologically, it stems from primitive neuroepithelial cells,
which are small round cells with high expression levels of CD99
with the potential to differentiate into various types of
mesenchymal cell (1,3,4). Ewing
sarcoma is characterized by the fusion of the EWS gene on
chromosome 22q12 (1).
Ewing sarcoma is treated with adjuvant chemotherapy
following wide excision or amputation with or without radiotherapy,
as determined by the National Comprehensive Cancer Network (NCCN)
guidelines (5,6). Despite various treatment strategies,
the 5-year-overall survival (OS) rate is reported to have been only
65% in Turkey between 2001 and 2010, which may be associated with
the high metastatic rate noted at an early stage (7). In addition, metastases are often
regarded as the most significant predictor of OS in patients with
Ewing sarcoma (8,9). Other potential factors include primary
tumor location, tumor volume and lactate dehydrogenase levels
(10,11). Non-metastatic Ewing sarcoma exhibits
relatively mild symptoms and favorable prognosis compared with the
metastatic type of the disease (8).
Therefore, the description of its clinical characteristics and the
investigation into its prognostic factors can assist clinical
decision-making.
With regard to non-metastatic Ewing sarcoma, surgery
is more useful than chemotherapy due to the high probability of en
bloc tumor resection (8,10). Chemotherapy is the main adjuvant
treatment method for Ewing sarcoma and was used in ~92% of
non-metastatic Ewing sarcoma in previous studies (11,12).
Furthermore, it has been demonstrated to be a favorable predictor
for OS (7–12). Chemotherapy decreases the
proliferation rate of the remaining tumor cells and improves
patient survival time (1). With
regard to radiotherapy, it has also been reported that chemotherapy
is an appropriate regimen for local control in patients with Ewing
sarcoma (13).
A nomogram is a visual, statistical predictive tool
that has been designed to identify prognostic factors and predict
disease-specific prognosis for each patient. Its predictive
accuracy is superior to that of transitional prognostic scoring
systems, such as the American Joint Committee on Cancer (AJCC)
Tumor-Node-Metastasis staging system (6). However, to the best of our knowledge,
the application of nomograms in the prediction of the prognosis for
patients with non-metastatic Ewing sarcoma has not been previously
investigated. In the present study, a machine learning model
(random forest) and survival analysis methods, including the
Kaplan-Meier (K-M) curve and Cox proportional hazards regression
model, were used to identify independent prognostic variables for
non-metastatic Ewing sarcoma from large sample data derived from
publicly available sources [Surveillance, Epidemiology and End
Results (SEER) database]. Subsequently, nomograms were constructed
to estimate OS and cause-specific survival (CSS). Furthermore,
external validation was performed to evaluate the accuracy and
reproducibility of the nomograms in clinical applications.
Materials and methods
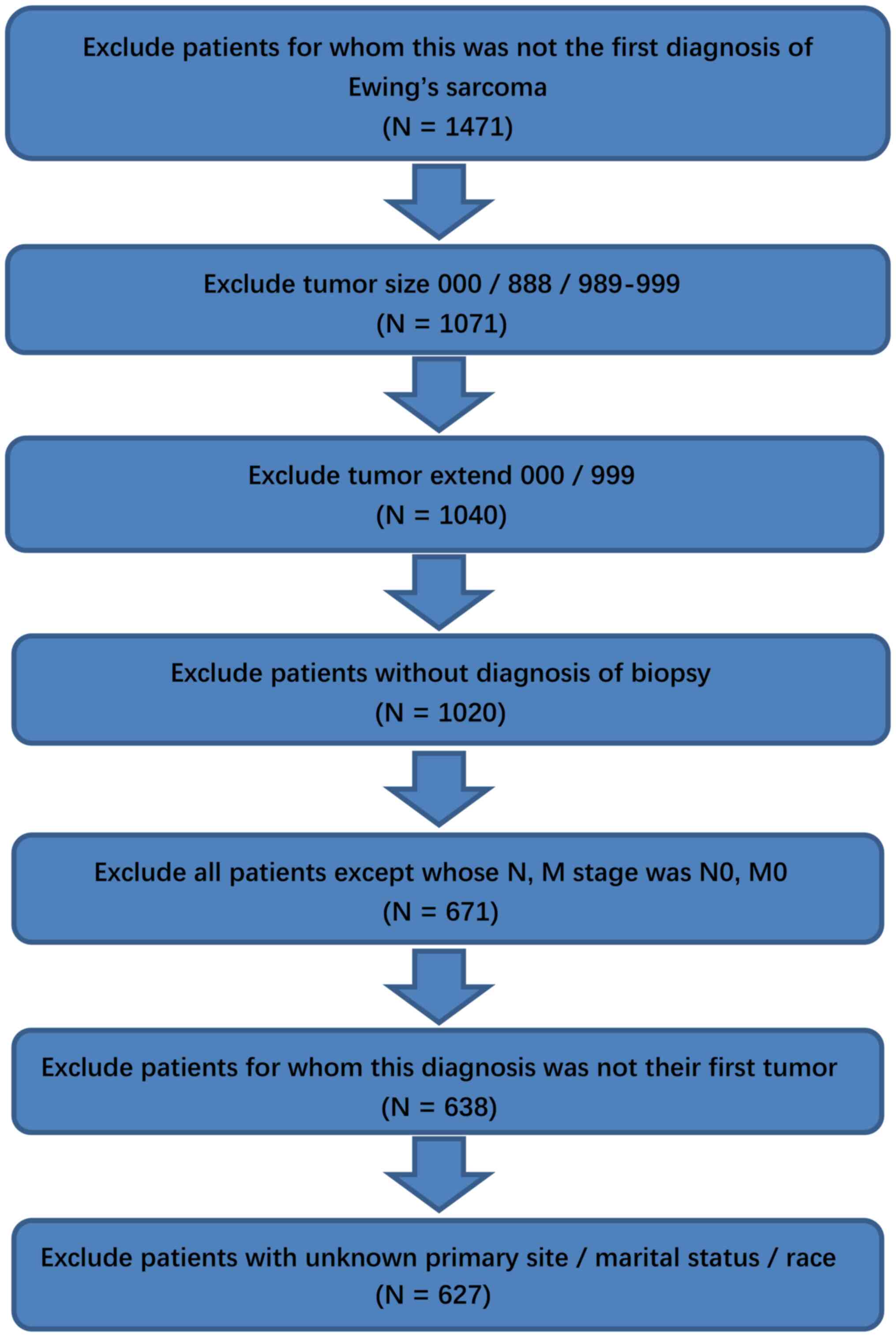
Patient selection
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Zhengzhou University
(no. KEYAN-2018-LW-023; Zhengzhou, China). The data analyzed in the
program were identified between January 2005 and December 2014 from
the SEER database (https://seer.cancer.gov/). Only patients diagnosed
with Ewing sarcoma as the primary malignancy were included.
Patients with tumors that were not first tumors (patients with no
prior history of tumors or patients with tumors that were not
primary tumors) were excluded from the present study. The tumor
size data were collected with the exception of the codes
000/888/999/989-998 (‘000’ indicated no mass or no tumor found;
‘888’ and ‘999’ indicated not applicable or unknown; ‘989-998’
indicated 989 mm or larger but not accurately recorded). The tumor
extension data were also collected with the exception of the codes
000/888/999/989-998. The exclusion criteria were as follows:
Patients without biopsy diagnosis, patients with the N0M0 stage and
patients who did not have Ewing sarcoma as their primary
malignancy, patients with unknown surgical information, and
patients with unknown marital status and ethnicity. Several studies
have reported that marital status is an important prognostic factor
for various tumor types (14,15).
Therefore, in order to include marital status in the subsequent
analysis, subjects with unknown marital status were removed. The
patient selection is shown in the flow chart in Fig. 1. Patient characteristics and the
original dataset are available in Tables SI and SII, respectively.
A cohort was formed for validation according to the
AJCC 6 and 7th edition guidelines using the same exclusion criteria
from the SEER database [this was a cohort with only AJCC stage as
the independent variables and survival data as the dependent
variable from the same SEER database using SEER*STAT (version
8.3.6; seer.cancer.gov/seerstat), and all patients with
unknown AJCC stage were excluded from this cohort] (6). In addition, non-metastatic Ewing
sarcoma cases between January 2013 and December 2018 at The First
Affiliated Hospital of Zhengzhou University (Zhengzhou, China) were
collected for external validation using the Electronic Medical
Record System using the same inclusion and exclusion criteria.
Despite the rarity of the disease, an external dataset comprising
26 cases was finally collected. Patient characteristics are shown
in Table SIII.
Data extraction
The variables analyzed in the present study included
the baseline demographic features of the patients (age at
diagnosis, sex and ethnicity), the tumor characteristics [size,
extension, primary site, International Classification of Diseases
for Oncology, 3rd Edition (ICD.O.3) histological subtype and AJCC T
stage] (6,16), the medical treatment (surgery,
radiation recode and chemotherapy recode) and the socioeconomic
variables (marital status, degree of education, family income and
employment status). Age was divided into three subgroups, namely
children (<15 years), adolescents and young adults (15–39
years), and old adults (>39 years) according to the NCCN
Guidelines for Adolescent and Young Adult Oncology (17). The endpoint of the present study was
patient death, which was presented as OS and CSS. The follow-up
endpoint of all patients was December 31, 2014.
Statistical analysis
The categorical variables were expressed as
percentages and the continuous variables were presented as the
median (range). Subsequently, the continuous variables were
transformed into categorical variables, such as age, tumor size and
tumor extension. According to the NCCN guidelines (version 2017)
(6), age (<15, 15–39 and >39
years), tumor size (<80 and ≥80 mm) and tumor extension (<300
and ≥300 mm) were divided into different subgroups. In addition,
patients were divided into subgroups according to the median values
for socioeconomic variables. The χ2 test was adopted to
assess the distinction between the sub-variables of each
categorical variable. The same processes were performed based on OS
and CSS. To compare the OS and CSS between subtypes, a K-M curve
was used. For the K-M survival analysis, a log-rank test was used
to compare the survival curves of each variable.
A random forest plot was established for entire
variables. The Mean Decrease Gini (MDG) was used to estimate how
each variable contributed to the OS and CSS. Additionally, the
out-of-bag error was used to assess the accuracy of the
classification in the random forest. The Cox proportional hazards
model was constructed based on the results of the univariate
analysis. The significant variables of the univariate analysis were
screened to structure the subsets used as the data of the full Cox
model. The non-significant chemotherapy effects were also retained
for subsequent analysis. As a result, a reduced Cox model was
constructed by sorting out the significant predictors from the full
Cox model. Although the surgical information was not significant in
the full Cox model, it was reserved for subsequent analysis in the
reduced Cox model. Following double selections, the Cox model with
the optimum predictors was eventually selected.
Based on the reduced Cox model, the nomograms were
constructed for the evaluation of OS and CSS. The discriminations
between predicted and observed values were evaluated via the
concordance index (C-index) of internal validation. Their
calibration was subsequently estimated using the corresponding
calibration curve. In addition, to assess the accuracy of the final
models, the external validation and concordance of the two AJCC
editions was performed as the last step.
In the present study, R version 3.5.0 software
(https://www.r-project.org/) was used to
identify the statistically significant variables with a two-sided
P<0.05 (α=0.05). The R packages ggplot2 (version 3.3.2;
http://cloud.r-project.org/web/packages/ggplot2/index.html),
survminer (version 0.4.8; http://cloud.r-project.org/web/packages/survminer/index.html),
survival (version 3.2–7; http://cloud.r-project.org/web/packages/survival/index.html),
rms (version 6.0-1; http://cloud.r-project.org/web/packages/rms/index.html)
and randomForest (version 4.6–14; http://cloud.r-project.org/web/packages/randomForest/index.html)
were used to establish the model and draw the curves, as well as
the nomograms.
Results
Baseline characteristics
A total of 1,471 cases from the SEER database were
selected between 2005 and 2014 as primary malignant cases. These
cases were filtered, and an entire cohort containing 627 patients
was included based on the inclusion and exclusion criteria. The
patient characteristics are listed in Table SI, and the raw dataset of the entire
primary cohort comprising 1,471 patients with non-metastatic Ewing
sarcoma is shown in Table SII. At
the end of the follow-up period, 502 patients were alive and 125
patients did not survive. The median age was 17 years (range, 0–85
years) and the highest proportion was noted in the 15–39-year-old
subgroup. There were 252 women and 375 men. The median tumor size
was 70 mm (range, 5–950 mm) and the median tumor extension was 400
mm (range, 100–820 mm). With regard to the primary tumor site, the
distribution was as follows: Head/face/neck (8.0%), limbs (48.8%),
thorax/abdomen (19.9%) and trunk (23.3%). The ICD.O.3 histology
groups were adamantinoma of long bones (4.5%) and Ewing sarcoma
(95.5%). In view of the limited number of AJCC T3 (0.8%) cases, the
main comparison χ2 test was only performed in the AJCC
T1 and AJCC T2 cases (53.1 vs. 46.1%). AJCC T was the only variable
to do this filtration in the χ2 test, and T3 cases were
not deleted in all subsequent analysis processes. Surgery was
performed in 76.6% of patients, with chemotherapy recorded in 92.0%
of the cases, whereas radiation was noted in 40.7% of the cases.
The median OS was 40.00 months (range, 0.00–119.00 months).
Univariate analysis
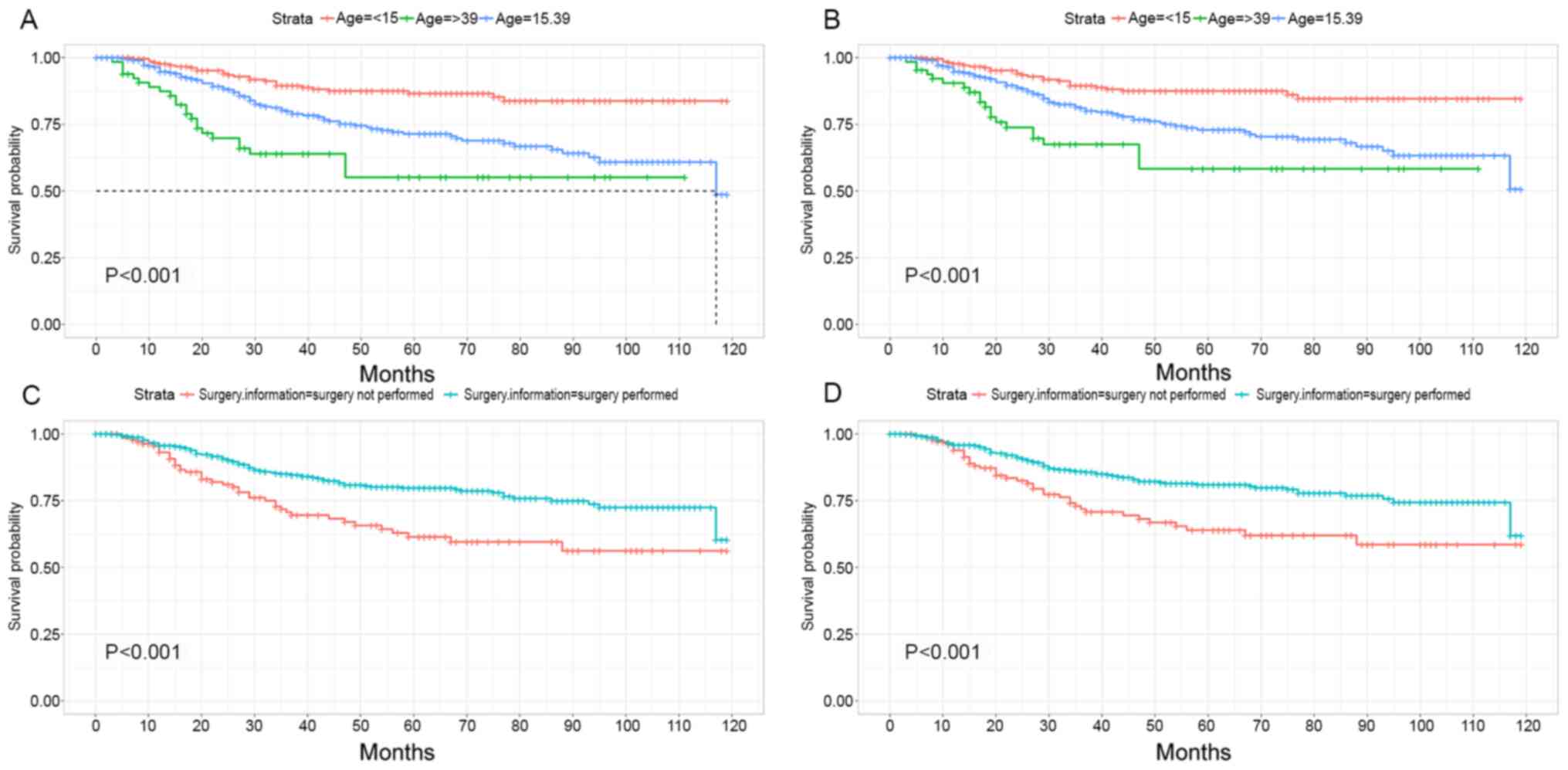
The K-M survival analysis for OS and CSS is
summarized in Table I. Patients who
were >39 years exhibited the poorest OS and CSS, whereas
patients whose age was <15 years exhibited the best prognosis
(P<0.001, Fig. 2A; P<0.001,
Fig. 2B; P-values apply to the
log-rank tests among all categories, not the comparison between any
two categories). Improved OS/CSS was achieved in patients who
underwent surgery compared with patients who did not undergo
surgery (P<0.001, Fig. 2C;
P<0.001, Fig. 2D). In addition,
the MDG values of the random forests were estimated for OS [out of
bag (OOB)=21.37%] and CSS (OOB=19.62%), along with the outcomes of
the parametric or the non-parametric tests (Table I). Variance homogeneous and normal
distributed continuous variables could be compared by Student's
t-test, otherwise, the Mann-Whitney U-test or Kruskal-Wallis H-test
should be used. The Student's t-test had conditions of use. The
c2 test was used for discontinuous variables. In
Table II, tumor features and
medical treatments were significantly associated with OS and CSS,
with the exception of the chemotherapy recode. Chemotherapy recode
was also associated with the survival ending (the endpoint of the
present study was patient death, which was presented as OS and CSS,
and the follow-up endpoint of all patients was December 31, 2014).
The parameters age, sex and marital status (data not shown)
significantly influenced the prognosis of the patients. Finally, 11
elements (age, sex, tumor size, tumor extension, primary site,
ICD.O.3 histology, AJCC T, surgery information, radiation recode,
chemotherapy recode and marital status) were further analyzed using
the Cox regression model.
 | Table I.Results of single factor analysis and
random forest analysis for OS (OOB=21.37%) and CSS
(OOB=19.62%). |
Table I.
Results of single factor analysis and
random forest analysis for OS (OOB=21.37%) and CSS
(OOB=19.62%).
|
| Overall
survival | Cause-specific
survival |
|---|
|
|
|
|
|---|
| Variables | P-value of
parametricor non-parametric test | P-value of
Kaplan-Meier survival analysis | MDG
(OOB=21.37%) | P-value of
parametric or non-parametric test | P-value of
Kaplan-Meier survival analysis | MDG
(OOB=19.62%) |
|---|
| Age (Kruskal-Wallis
H-test) (<15 vs. 15–39 vs. >39) |
<0.001a |
<0.001a | 14.654 |
<0.001a |
<0.001a | 13.376 |
| Tumor size (<80
vs. ≥80 mm) |
<0.001a |
<0.001a | 7.346 |
<0.001a |
<0.001a | 6.730 |
| Tumor extension
(<300 vs. ≥300 mm) |
<0.001a |
<0.001a | 6.686 |
<0.001a |
<0.001a | 6.141 |
| Ethnicity (Black
vs. White vs. Other) | 0.134 | 0.130 | 8.877 | 0.129 | 0.170 | 8.443 |
| Sex (female vs.
male) | 0.013a | 0.031a | 8.383 | 0.008a | 0.020a | 7.580 |
| Primary site (head,
face, neck vs. limbs vs. thorax, abdomen vs. trunk) |
<0.001a |
<0.001a | 19.793 |
<0.001a | 0.001a | 18.782 |
| Histological
subtype (adamantinoma of long bones vs. Ewing sarcoma) | 0.027a | 0.025a | 1.221 | 0.010a | 0.011a | 1.127 |
| AJCC T stage (T1
vs. T2 vs. T3) |
<0.001a |
<0.001a | 7.447 |
<0.001a |
<0.001a | 7.015 |
| Surgery information
(surgery not performed vs. surgery performed) | 0.003a |
<0.001a | 7.517 | 0.004a |
<0.001a | 7.085 |
| Radiation recode
(no/unknown vs. yes) |
<0.001a |
<0.001a | 8.049 | 0.001a |
<0.001a | 8.092 |
| Chemotherapy recode
(no/unknown vs. yes) | 0.263 | 0.280 | 5.285 | 0.507 | 0.500 | 4.954 |
| Marital status at
diagnosis (married vs. single/separated/divorced/widowed) | 0.001a |
<0.001a | 7.912 | 0.006a | 0.002a | 6.787 |
| 9th grade education
(lower 50% vs. upper 50%) | 0.497 | 0.550 | 7.005 | 0.313 | 0.370 | 7.062 |
| High school
education (lower 50% vs. upper 50%) | 0.424 | 0.240 | 6.623 | 0.179 | 0.097 | 6.529 |
| At least bachelor's
degree (lower 50% vs. upper 50%) | 0.690 | 0.510 | 6.017 | 0.562 | 0.420 | 5.823 |
| Median family
income (lower 50% vs. upper 50%) | 0.873 | 0.650 | 5.247 | 0.793 | 0.600 | 4.747 |
| Income status
(lower 50% vs. upper 50%) | 0.905 | 0.980 | 5.635 | 0.852 | 0.810 | 5.361 |
| Unemployed (lower
50% vs. upper 50%) | 0.379 | 0.260 | 6.068 | 0.313 | 0.220 | 5.487 |
| Non-manual worker
(lower 50% vs. upper 50%) | 0.711 | 0.900 | 5.608 | 0.831 | >0.999 | 5.403 |
 | Table II.Cox proportional hazards regression
model for overall survival and cause-specific survival in patients
with non-metastatic Ewing sarcoma. |
Table II.
Cox proportional hazards regression
model for overall survival and cause-specific survival in patients
with non-metastatic Ewing sarcoma.
|
| Overall
survival | Cause-specific
survival |
|---|
|
|
|
|
|---|
| Variable | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Categorical age,
years |
|
|
|
|
| <15 | 1.00
(reference) |
| 1.00
(reference) |
|
|
15–39 | 2.154
(1.375–3.375) |
<0.001a | 2.084
(1.316–3.300) | 0.002a |
|
>39 | 4.334
(2.417–7.770) |
<0.001a | 4.121
(2.248–7.552) |
<0.001a |
| Categorical tumor
extension, mm |
|
|
|
|
|
<300 | 1.00
(reference) |
| 1.00
(reference) |
|
|
≥300 | 2.404
(1.366–4.230) | 0.002a | 2.652
(1.465–4.803) | 0.001a |
| Sex |
|
|
|
|
|
Female | 1.00
(reference) |
| 1.00
(reference) |
|
|
Male | 1.654
(1.124–2.434) | 0.011a | 1.675
(1.120–2.506) | 0.012a |
| Histological
subtype |
|
|
|
|
|
Adamantinoma of long
bones | 1.00
(reference) |
|
|
|
| Ewing
sarcoma | 19.137
(2.466–148.492) | 0.005a |
|
|
| Surgery
information |
|
|
|
|
| Surgery
not performed | 1.00
(reference) |
| 1.00
(reference) |
|
| Surgery
performed | 0.593
(0.407–0.865) | 0.007a | 0.561
(0.378–0.831) | 0.004a |
| Chemotherapy
recode |
|
|
|
|
|
No/Unknown | 1.00
(reference) |
| 1.00
(reference) |
|
|
Yes | 0.308
(0.164–0.581) |
<0.001a | 0.780
(0.400–1.521) | 0.466 |
Multivariate Cox regression and
nomogram
In the Cox proportional hazards model, a higher
hazard ratio (HR) was noted for the parameter of older age for OS
[15–39 years: HR, 2.154; 95% confidence interval (CI), 1.375–3.375;
P<0.001; >39 years: HR, 4.334; 95% CI, 2.417–7.770;
P<0.001] and CSS (15–39 years: HR, 2.084; 95% CI, 1.316–3.300;
P=0.002; >39 years: HR, 4.121; 95% CI, 2.248–7.552; P<0.001)
compared with younger age (<15 years). Larger tumor extension
was also a risk factor for OS (≥300.00 mm: HR, 2.404; 95% CI,
1.366–4.230; P=0.002) and CSS (≥300.00 mm: HR, 2.652; 95% CI,
1.465–4.803; P=0.001). In addition, male subjects appeared to have
poorer OS (HR, 1.654; 95% CI, 1.124–2.434; P=0.011) and CSS (HR,
1.675; 95% CI, 1.120–2.506; P=0.012) than female subjects. The
ICD.O.3 histological results for the higher risk subtype (Ewing
sarcoma) indicated associations with OS (HR, 19.137; 95% CI,
2.466–148.492; P=0.005), but not with CSS (data not shown). The
patients who had undergone surgery exhibited favorable OS (HR,
0.593; 95% CI, 0.407–0.865; P=0.007) and CSS (HR, 0.561; 95% CI,
0.378–0.831; P=0.004). The patients who were treated with
chemotherapy exhibited improved OS (HR, 0.308; 95% CI, 0.164–0.581;
P<0.001), whereas the effects of chemotherapy on CSS (HR, 0.780;
95% CI, 0.400–1.521; P=0.466) were not identified to be
statistically significant.
Based on the reduced Cox models, nomograms were
built to predict the probability of 3- and 5-year OS (Fig. 3A and C; C-index=0.791) and CSS
(Fig. 3B and D; C-index=0.813). The
calibration plots demonstrated the agreement between predictions
and observed values due to the 45-degree-line nearby points
(Fig. 3C and D). Information of the
26 cases for external validation is shown in Table SIII and the median age was 18 years
(range, 1–51 years). The median survival time was 22 months (range,
3–54 months; Table SIII). The
concordances of external validation based on the cohort of The
First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China) were 0.834 for OS and 0.825 for CSS. Although the results of
the K-M survival analysis indicated significant prognostic values
of AJCC stage, the C-indices were 0.531 (OS) and 0.534 (CSS) for
AJCC 6th edition guidelines, and 0.547 (OS) and 0.561 (CSS) for
AJCC 7th edition guidelines, respectively, which were lower than
the nomograms (Fig. S1).
Additionally, the results of the K-M survival analysis indicated
that chemotherapy could not significantly prolong the survival of
the patients (Fig. S2). In the
subgroup analysis, older patients were more likely to undergo
chemotherapy rather than surgery, which might explain the poor
prognosis in older patients (Table
SIV).
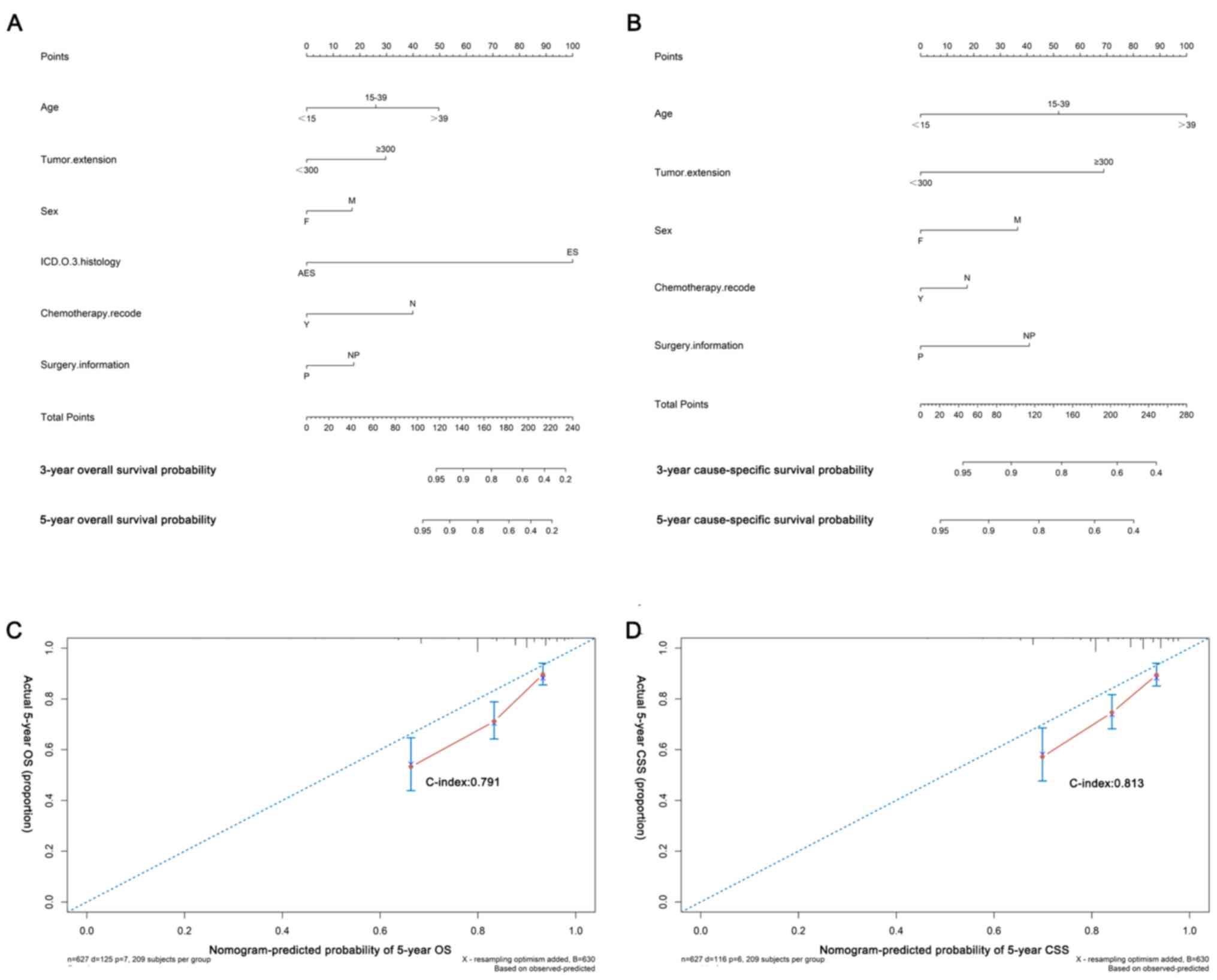 | Figure 3.Nomograms and calibration curves of
(A and C) OS and (B and D) CSS. The C-indices for internal
validation of OS and CSS prediction were 0.791 and 0.813. F,
female; M, male; AES, adamantinoma of long bones; ICD.O.3,
International Classification of Diseases for Oncology, 3rd Edition;
ES, Ewing sarcoma; Y, yes; N, no/unknown; P, surgery performed; NP,
surgery not performed; C-index, concordance index; OS, overall
survival; CSS, cause-specific survival. |
Discussion
Ewing sarcoma is the second most common malignant
osseous tumor occurring in children and adolescents after
osteosarcoma (2). Non-metastatic
Ewing sarcoma has a favorable prognosis compared with the
metastatic type and the identification of its predictors is helpful
to prolong OS and CSS (6). A
nomogram is a widely used prediction model that can incorporate
meaningful factors, and predict OS and CSS of patients. In the
present study, a prognostic nomogram was constructed for patients
with non-metastatic Ewing sarcoma based on the SEER database.
Furthermore, external non-metastatic Ewing sarcoma cases from The
First Affiliated Hospital of Zhengzhou University (Zhengzhou,
China) were used to validate the nomograms in order to provide
reliable prediction models for clinical treatment.
The machine learning model (random forest) and
classical survival analysis (K-M survival curve and Cox
proportional hazards regression model) demonstrated that age, sex,
tumor extension and ICD.O.3 histology were independent prognostic
factors for both OS and CSS. Surgery was identified as a favorable
predictor for both OS and CSS, while chemotherapy was significantly
associated with OS and could potentially influence CSS in a number
of previous studies, but this was not a significant indicator for
CSS in the present study (6,8,17,18).
Other variables, namely ethnicity, tumor size, primary site,
radiotherapy and socioeconomic status (marital status), were not
observed to be independent risk factors associated with OS and
CSS.
In the present series of patients, the mean age was
20.14±13.62 years, with 375 male and 252 female subjects. Old age
and male sex were prognostic factors for OS and CSS as determined
by univariate and multivariate analyses, which was in accordance
with previously reported findings (2,18–24). In
the subgroup analysis, older patients were more likely to undergo
chemotherapy rather than surgery, which might explain the poor
prognosis in older patients (Table
SIV). Therefore, it was suspected that the poor prognosis of
older patients may be due to their low tolerance of chemotherapy
(25,26). Furthermore, the results indicated
that male patients tended to develop large tumors, which may
increase the treatment complexity (Table SIV).
Ewing sarcoma was divided into adamantinoma-like
Ewing sarcoma of long bones and Ewing sarcoma based on its
histological evaluation provided by the SEER database. The
percentages of the incidence of these tumors were 4.5 and 95.5%,
respectively. Due to the lower-grade malignancy of
adamantinoma-like Ewing sarcoma, ICD.O.3 histology was a
significant variable in the current analysis, and Ewing sarcoma
exhibited a poorer prognosis than the adamantinoma of long bones
(27,28).
The tumor extension was an independent prognostic
factor for both OS and CSS in the present analysis. Based on the
results of the subgroup analysis, larger extension limited the
application of surgery, thereby leading to negative prognosis,
which was similar to the findings reported by Arshi et al
(2).
Current therapeutic strategies for Ewing sarcoma
include surgery, chemotherapy and radiotherapy, and their combined
application. Surgery is the standard treatment strategy for Ewing
sarcoma (7,12,23,24). In
the present series, 76.6% of patients underwent surgery and this
treatment method was a favorable prognostic factor for both OS and
CSS. With regard to non-metastatic Ewing sarcoma, surgery is more
useful due to the high probability of en bloc tumor resection
(12,17,18).
Chemotherapy is the main treatment method for Ewing sarcoma, which
was present in ~92% of the cases comprising non-metastatic Ewing
sarcoma in the present study (7,12).
Additionally, chemotherapy has been demonstrated to be a favorable
predictor for OS (7,12). In Europe, the standard
chemotherapeutic strategy for Ewing sarcoma is vincristine,
ifosfamide, doxorubicin and etoposide (VIDE). This regimen is
safely used at a high dose and is administered bi-weekly (5,29). It
destroys any remaining tumor cells that are proliferating slowly,
and further improves patient survival time (1). In addition, in the external validation
cohort of patients in the present study, the chemotherapeutic
regimen used was a VIDE regimen in all cases. The results of the
K-M survival analysis indicated that chemotherapy could
significantly prolong the survival of the patients. Furthermore,
radiotherapy has been reported to be an appropriate regimen for
local control in patients with Ewing sarcoma (13). In the present study, non-significant
results were noted in the Cox model, which was consistent with
Gaspar et al (5), who
demonstrated that radiotherapy did not exhibit a long-term tumor
control and may result in severe side effects.
Based on the six independent variables identified
using univariate and multivariate analyses, nomograms with high AUC
and collaboration were constructed for OS and CSS in order to
predict patient survival probability at certain time points. By
external validation, they were appropriately tested and achieved
satisfying fitting degrees. The C-index was estimated to be 0.931
and 0.724 for OS and CSS, respectively, which was superior to those
for the AJCC 6th and 7th editions. Therefore, this variable could
assist clinicians in making accurate survival evaluations and
generating appropriate therapeutic strategies.
To the best of our knowledge, the present study was
the first to address the use of nomograms in the field of
non-metastatic Ewing sarcoma. The results implied the potential for
clinical application. Despite these promising results, several
limitations were present in the current study. Inaccurate variables
appeared due to the criteria discrepancy in diagnosis and treatment
methods that originated from different registries. Secondly, the
retrospective nature of the present study was a limitation compared
with a prospective study, such as a prospective cohort study and
randomized controlled trial. Thirdly, the study lacked the detailed
information for the chemotherapy and radiotherapy protocols used in
the SEER database, including the treatment time, dose cycle, drug
type and dosage (30). Finally,
although applicable nomograms for OS and CSS have been tested by
internal and external validation protocols alongside the
calibration curves, a limited number of cases in the external
series had a relatively short follow-up time. Additional clinical
data are required to validate and revise the data presented in the
current study. In order to further analyze and improve the
application of nomograms, more strict and accurate models that can
combine the variables examined with genetic factors are required.
Subsequent studies should focus on the complex molecular mechanisms
and expression of genomic biomarkers in order to construct more
accurate nomograms.
In conclusion, age, sex, tumor extension and surgery
were independent prognostic factors for both OS and CSS. In
addition, the Ewing sarcoma subtype was a poor factor, whereas
chemotherapy was a favorable factor with regard to OS. Nomograms
based on reduced Cox models attained a satisfying accuracy in
predicting the survival of patients with non-metastatic Ewing
sarcoma and could assist clinicians in more accurately evaluating
patient survival.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported in part by the
National Natural Science Foundation of China (grant nos. 81702659,
81772856 and 81501203), Youth Fund of Shanghai Municipal Health
Planning Commission (grant no. 2017YQ054) and Henan Medical Science
and Technology Research Project (grant no. 201602031).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
RH, DH, CS, PY, PH, XZ, HY, TM and ZH conceived and
designed the study, collected and/or assembled the data. analyzed
and interpreted the data and wrote the manuscript. RH, DH, CS, PY,
PH, XZ, HY, TM and ZH confirmed the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of The First Affiliated Hospital of Zhengzhou University
(approval no. KEYAN-2018-LW-023; Zhengzhou, China). All patients
enrolled in the present study provided written informed consent for
participation.
Patient consent for publication
Written informed consent was obtained from all 26
patients for publication of the present study. Consent was obtained
from the parents/guardians of the patients under 18 years of age at
the time of data collection.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OS
|
overall survival
|
|
CSS
|
cause-specific survival
|
|
SEER
|
Surveillance, Epidemiology, and End
Results
|
|
AJCC
|
American Joint Committee on Cancer
|
|
ICD.O.3
|
International Classification of
Diseases for Oncology, 3rd Edition
|
|
MDG
|
Mean Decrease Gini
|
|
HR
|
hazard ratio
|
|
CI
|
confidence interval
|
References
|
1
|
Grunewald TGP, Cidre-Aranaz F, Surdez D,
Tomazou EM, de Álava E, Kovar H, Sorensen PH, Delattre O and
Dirksen U: Ewing sarcoma. Nat Rev Dis Primers. 4:52018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arshi A, Sharim J, Park DY, Park HY,
Yazdanshenas H, Bernthal NM and Shamie AN: Prognostic determinants
and treatment outcomes analysis of osteosarcoma and Ewing sarcoma
of the spine. Spine J. 17:645–655. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Scotlandi K, Baldini N, Cerisano V, Manara
MC, Benini S, Serra M, Lollini PL, Nanni P, Nicoletti G, Bernard G,
et al: CD99 engagement: An effective therapeutic strategy for Ewing
tumors. Cancer Res. 60:5134–5142. 2000.PubMed/NCBI
|
|
4
|
Riggi N, Cironi L, Provero P, Suvà ML,
Kaloulis K, Garcia-Echeverria C, Hoffmann F, Trumpp A and
Stamenkovic I: Development of Ewing's sarcoma from primary bone
marrow-derived mesenchymal progenitor cells. Cancer Res.
65:11459–11468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gaspar N, Hawkins DS, Dirksen U, Lewis IJ,
Ferrari S, Le Deley MC, Kovar H, Grimer R, Whelan J, Claude L, et
al: Ewing sarcoma: Current management and future approaches through
collaboration. J Clin Oncol. 33:3036–3046. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Biermann JS, Chow W, Reed DR, Lucas D,
Adkins DR, Agulnik M, Benjamin RS, Brigman B, Budd GT, Curry WT, et
al: NCCN Guidelines Insights: Bone cancer, version 2.2017. J Natl
Compr Canc Netw. 15:155–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Arpaci E, Yetisyigit T, Seker M, Uncu D,
Uyeturk U, Oksuzoglu B, Demirci U, Coskun U, Kucukoner M, Isıkdogan
A, et al: Prognostic factors and clinical outcome of patients with
Ewing's sarcoma family of tumors in adults: Multicentric study of
the Anatolian Society of Medical Oncology. Med Oncol. 30:4692013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Paulussen M, Ahrens S, Burdach S, Craft A,
Dockhorn-Dworniczak B, Dunst J, Fröhlich B, Winkelmann W, Zoubek A
and Jürgens H: Primary metastatic (stage IV) Ewing tumor: Survival
analysis of 171 patients from the EICESS studies. European
Intergroup Cooperative Ewing Sarcoma Studies. Ann Oncol. 9:275–281.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ferreira AJ, Boldrini E, López RVM,
Scapulatempo Neto C, Santos JF and Lopes LF: Characterization,
survival analysis, and expression of IGFR in tumor samples from
patients diagnosed with Ewing family tumors treated at the Barretos
Cancer Hospital. Rev Bras Ortop. 52:705–713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rodríguez-Galindo C, Liu T, Krasin MJ, Wu
J, Billups CA, Daw NC, Spunt SL, Rao BN, Santana VM and Navid F:
Analysis of prognostic factors in ewing sarcoma family of tumors:
Review of St. Jude Children's Research Hospital studies. Cancer.
110:375–384. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Grevener K, Haveman LM, Ranft A, van den
Berg H, Jung S, Ladenstein R, Klco-Brosius S, Juergens H, Merks JH
and Dirksen U: Management and outcome of Ewing sarcoma of the head
and neck. Pediatr Blood Cancer. 63:604–610. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wan W, Lou Y, Hu Z, Wang T, Li J, Tang Y,
Wu Z, Xu L, Yang X, Song D and Xiao J: Factors affecting survival
outcomes of patients with non-metastatic Ewing's sarcoma family
tumors in the spine: A retrospective analysis of 63 patients in a
single center. J Neurooncol. 131:313–320. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ning MS, Perkins SM, Borinstein SC, Holt
GE, Stavas MJ and Shinohara ET: Role of radiation in the treatment
of non-metastatic osseous Ewing sarcoma. J Med Imaging Radiat
Oncol. 60:119–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Buja A, Lago L, Lago S, Vinelli A, Zanardo
C and Baldo V: Marital status and stage of cancer at diagnosis: A
systematic review. Eur J Cancer Care (Engl). 27:2018. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gomez SL, Hurley S, Canchola AJ, Keegan
TH, Cheng I, Murphy JD, Clarke CA, Glaser SL and Martínez ME:
Effects of marital status and economic resources on survival after
cancer: A population-based study. Cancer. 122:1618–1625. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Niu XH: Interpretation of 2020 NCCN
Clinical Practice Guidelines in Oncology-Bone Cancer. Zhonghua Wai
Ke Za Zhi. 58:430–434. 2020.(In Chinese). PubMed/NCBI
|
|
17
|
Coccia PF, Pappo AS, Beaupin L, Borges VF,
Borinstein SC, Chugh R, Dinner S, Folbrecht J, Frazier AL, Goldsby
R, et al: Adolescent and young adult oncology, version 2.2018. J
Natl Compr Cancer Netw. 16:66–97. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bacci G, Longhi A, Ferrari S, Mercuri M,
Versari M and Bertoni F: Prognostic factors in non-metastatic
Ewing's sarcoma tumor of bone: An analysis of 579 patients treated
at a single institution with adjuvant or neoadjuvant chemotherapy
between 1972 and 1998. Acta Oncol. 45:469–475. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jawad MU, Cheung MC, Min ES,
Schneiderbauer MM, Koniaris LG and Scully SP: Ewing sarcoma
demonstrates racial disparities in incidence-related and
sex-related differences in outcome: An analysis of 1631 cases from
the SEER database, 1973–2005. Cancer. 115:3526–3536. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duchman KR, Gao Y and Miller BJ:
Prognostic factors for survival in patients with Ewing's sarcoma
using the surveillance, epidemiology, and end results (SEER)
program database. Cancer Epidemiol. 39:189–195. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Karski EE, Matthay KK, Neuhaus JM, Goldsby
RE and Dubois SG: Characteristics and outcomes of patients with
Ewing sarcoma over 40 years of age at diagnosis. Cancer Epidemiol.
37:29–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
López Guerra JL, Márquez-Vega C,
Ramírez-Villar GL, Cabrera P, Ordóñez R, Praena-Fernández JM and
Ortiz MJ: Prognostic factors for overall survival in paediatric
patients with Ewing sarcoma of bone treated according to
multidisciplinary protocol. Clin Transl Oncol. 14:294–301. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oksüz DC, Tural D, Dincbas FÖ, Dervisoglu
S, Turna H, Hiz M, Kantarci F, Ceylaner B, Koca S and Mandel NM:
Non-metastatic Ewing's sarcoma family of tumors of bone in
adolescents and adults: Prognostic factors and clinical
outcome-single institution results. Tumori. 100:452–458. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wan ZH, Huang ZH and Chen LB: Survival
outcome among patients with Ewing's sarcoma of bones and joints: A
population-based cohort study. Sao Paulo Med J. 136:116–122. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta AA, Pappo A, Saunders N, Hopyan S,
Ferguson P, Wunder J, O'Sullivan B, Catton C, Greenberg M and
Blackstein M: Clinical outcome of children and adults with
localized Ewing sarcoma: Impact of chemotherapy dose and timing of
local therapy. Cancer. 116:3189–3194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lee J, Hoang BH, Ziogas A and Zell JA:
Analysis of prognostic factors in Ewing sarcoma using a
population-based cancer registry. Cancer. 116:1964–1973. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kreicbergs A, Silvferswärd C and Tribukait
B: Flow DNA analysis of primary bone tumors. Relationship between
cellular DNA content and histopathologic classification. Cancer.
53:129–136. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fujii H, Honoki K, Enomoto Y, Kasai T,
Kido A, Amano I, Kumamoto M, Morishita T, Mii Y, Nonomura A and
Takakura Y: Adamantinoma-like Ewing's sarcoma with EWS-FLI1 fusion
gene: A case report. Virchows Arch. 449:579–584. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Womer RB, West DC, Krailo MD, Dickman PS,
Pawel BR, Grier HE, Marcus K, Sailer S, Healey JH, Dormans JP and
Weiss AR: Randomized controlled trial of interval-compressed
chemotherapy for the treatment of localized Ewing sarcoma: A report
from the Children's Oncology Group. J Clin Oncol. 30:4148–4154.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Noone AM, Lund JL, Mariotto A, Cronin K,
McNeel T, Deapen D and Warren JL: Comparison of SEER treatment data
with medicare claims. Med Care. 54:E55–E64. 2016. View Article : Google Scholar : PubMed/NCBI
|