Introduction
Oral squamous cell carcinoma (OSCC) has the
biological characteristics of uncontrollable invasiveness, high
mortality and extensive hypoxia (1).
Data from the Surveillance, Epidemiology, and End Results in 2000
suggested that 28,900 people in the US are diagnosed with OSCC
every year, of which ~7,400 deaths are reported annually (2). Although the comprehensive sequence
therapy of this cancer has achieved outstanding results, the
quality of life of patients with advanced stage OSCC is still very
poor (3,4). Hence, the in depth study of the
mechanism of OSCC is crucial for the selection of appropriate tumor
markers and the discovery of new therapeutic strategies. Previous
studies have focused on OSCC protein-coding genes (5,6). Recent
studies have shown that lncRNA may serve an important role in the
pathogenesis of OSCC (7–9).
Most lncRNAs were initially considered as
nonfunctional ‘noise’, by-products of RNA polymerase II
transcription and without biological function (10). However, lncRNA had been found to be
involved in X chromosome silencing, genomic imprinting, gene
modification, transcriptional activation, transcriptional
interference, and nuclear transport (11–13).
Hence, the role of lncRNAs has gradually attracted extensive
attention. With extensive studies in recent years, lncRNAs have
been demonstrated to regulate gene expression at the epigenetic,
transcriptional and posttranscriptional levels, which is more
direct and rapid in regulating cell proliferation, migration and
differentiation (14,15). A previous study demonstrated that
lncRNA is involved in diverse pathological processes of
tumorigenesis and development at different levels (16). In addition, lncRNA has been
demonstrated to participate in a number of important physiological
and pathological processes, such as cell proliferation (17), differentiation (18), tissue formation (19) and organ formation (20).
The abnormal expression of lncRNA had been reported
in the first-generation map in 2011, which involved as many as 19
kinds of cancer (21). The lncRNA
can act as a regulator of gene expression through gene
modification, transcription and postprocessing (22). In the past few years, numerous
studies have shown that the upregulation and downregulation of
lncRNA are involved in the development and progress of OSCC
(23,24). High expression of some lncRNAs in
OSCC (such as lncRNA PDIA3P, lncRNA FGD5-AS1 and lncRNA HOXA11-AS)
can promote the proliferation, invasion, and migration of cancer
cells (25–27).
LncRNA MEG3 is located on the human chromosome
14q32.3, with a length of ~1600 nucleotides (28). This transcript consists of 10 exons
and is expressed in maternal alleles (29). In 1998, Schuster Gossler and his
colleagues found MEG3 was highly expressed in the paraxial mesoderm
of mouse embryos, suggesting it may be involved in regulating the
process of myogenesis (30).
Subsequently, Takahashi et al and Zhou et al found
the developmental defect of skeletal muscle and perinatal death in
MEG3 knockout mice, indicating that MEG3 may serve an instrumental
role in the regulation of embryo formation and skeletal muscle
development (31,32). In addition, MEG3 also functions by
regulating the differentiation of mouse embryonic stem cells by
promoting the interaction between the accessory component of
Polycomb repressive complex-2 JARID2 with its' core component EZH2
(33,34). Recent research has revealed that
lncRNA MEG3 is expressed in normal human tissues and lowly
expressed in gastric cancer, breast cancer, etc. (35,36).
However, its expression in OSCC is rarely reported. The present
study aimed to investigate the effects of lncRNA MEG3 on the
proliferation, migration and p53 pathway of SCC25 and CAL27 OSCC
cells. lncRNA MEG3 may be a therapeutic target for patients with
OSCC.
Materials and methods
Participants
The OSCC tissues and corresponding normal tissues
(>2 cm from the edge of the tumor) were collected from 72
patients who underwent surgical resection in the Department of
Stomatology, the Fourth Affiliated Hospital of Hebei Medical
University between January 2015 and December 2016. The patients had
complete data and definite diagnosis by pathological examination
and were all first episode patients. The subjects had no treatment
for OSCC before the operation and signed written informed consent.
The samples were collected from 35 males and 37 females with a mean
age of 53 years (range, 21–75 years). The Ethics Committee of the
Fourth Affiliated Hospital (Shijiazhuang, China) approved the
experiment and supervised and guided the whole experimental process
(approval no. 201411EC040). All patients were followed up
immediately after discharge. By 31st December 2019, 49 cases
survived and 23 cases succumbed to the disease.
Materials and reagents
The following materials were used to investigate the
expression of the p53 gene: primers of MEG3, p53,
pcDNA3.1-MEG3 overexpression plasmids and an empty control plasmid
(Wanleibio Co., Ltd.). The normal oral mucosa cell line (hNOK) and
OSCC cell line SCC25 and CAL27 were purchased from BeNa Culture
Collection; Beijing Beina Chunglian Institute of Biotechnology).
Dulbecco's Modified Eagle's Medium (DMEM) and fetal bovine serum
(FBS) were purchased from Gibco; Thermo Fisher Scientific, Inc.
BSA, DMSO and MTT reagent were purchased from Sigma-Aldrich; The
expression of the p53 gene was detected using
TRIzol® RNA isolation kit, Lipofectamine®
2000 liposome, Matrigel, Invitrogen Superscript Reverse
Transcriptase kit and SYBR Green qPCR Master Mix kit which were all
purchased from Invitrogen (Thermo Fisher Scientific, Inc.). Rabbit
anti-human monoclonal and goat anti-rabbit secondary antibodies
were purchased from Santa Cruz Biotechnology Co., Ltd.
Reverse transcription-quantitative
(RT-q)PCR experiment
The total RNA was extracted from the appropriate
tissues using TRIzol for RNA quality inspection, and cDNA was
prepared via reverse transcription. Fluorescence quantitative
analysis was performed according to the operational instructions of
the SYBR Green qPCR Master Mix kit. According to the target
sequence, the corresponding upstream and downstream primers were
designed and synthesized for PCR amplification. GAPDH was used as
internal reference gene. The primer sequences were as follows:
lncRNA MEG3 forward, 5′-CTTTTCTGGGGGAATGGGG-3′ and reverse,
5′-AGAGGGGTGGGAAGGGACT-3′; p53 forward,
5′-ACCACCATCCACTACAACTACAT-3′ and reverse,
5′-CAGGACAGGCACAAACACG-3′; and GADPH forward
5′-CTTAGTTGCGTTACACCCTTTCTTG-3′ and reverse,
5′-CTGTCACCTTCACCGTTCCAGTTT-3′. The thermocycling conditions were
as follows: pre-denaturation at 95°C for 10 min, denaturation at
95°C for 15 sec, annealing at 60°C for 30 sec, elongation at 72°C
for 30 sec and final extension at 72°C for 2 min 30 sec. The
expression of lncRNA was calculated with GAPDH as internal
reference. After three independent experiments, the data were
obtained by using the following formula: ∆Cq=Cq lncRNA-Cq GAPDH;
and ∆∆Cq=(Cq lncRNA-Cq GAPDH)-(Cq lncRNA-Cq GAPDH) experimental
group-(Cq lncRNA-Cq GAPDH) control group. When the relative
expression of OSCC and normal tissues were counted, the relative
expression of each sample (2−∆∆Cq) and the changes in
the lncRNA in different tissues were compared (37).
Cell culture and plasmid
transfection
The SCC25 and CAL27 cells were seeded in DMEM
containing 10% FBS and cultured in a 95% CO2 incubator
at 37°C. The medium was changed, and subculturing was performed
every 3–4 days. The cells in the logarithmic growth phase were
seeded on a 6-well plate and transfected when the cells grew to
~70% confluency. The PEGFP-N1 plasmid (Clontech; Takara Bio USA)
transfection was performed according to the manufacturer's
instructions of the Lipofectamine 2000 transfection reagent. The
transfection concentration was 40 nmol/l at 37°C for 20 min. After
transfection, the cells were cultured in the incubator for 6 h, and
then the medium was replaced with DMEM containing 10% FBS. After 48
h of transient transfection, the RNA was extracted to test the
transfection efficiency.
Experimental grouping
The experiment was divided into the following: blank
control group (NC), untransfected SCC25 and CAL27 cells; vector
group (empty vector), SCC25 and CAL27 cells transfected with
pcDNA3.1 empty control plasmid; and overexpression group
(pcDNA3.1-MEG3), SCC25 and CAL27 cells transfected with
pcDNA3.1-MEG3.
MTT assay
The SCC25 and CAL27 cells were seeded on 96-well
plates with 1×104 cells in each well. After the cells
adhered to the wall, transfection was performed. Each group was set
with 3 multiple wells. After 6, 24, 48, 72 and 96 h of culture, 10
µl of MTT solution was added to each well, and the incubation was
continued for at 37°C for 4 h. DMSO was used for purple formazan
dissolution. The OD value was measured at 570 nm by a microplate
analyzer, and the cell growth curve was plotted (38).
Transwell chamber assay
For the migration experiment, the experimental
groups were the same as those in section. After 48 h, the
transfected cells were collected, and the cells were suspended in
serum-free medium at a density of 2.5×105 cells/ml.
Then, 200 and 600 µl of the serum-free medium were added to the
upper and lower chambers of 6.5 mm Transwell with 8.0 µm pore
polycarbonate membrane insert (Corning, Inc.), respectively, which
was stored at 37°C for 1 h. The medium in the well was absorbed and
discarded, and the chamber was washed twice with PBS. Then, 200 µl
of the cell suspension was added in the upper chamber, and 800 µl
of the medium containing 30% FBS was added to the lower chamber
cultured at 37°C for 48 h. The Transwell chamber was cleaned with
PBS, fixed with 4% paraformaldehyde at 25°C for 20 min and stained
with 0.5% crystal violet for 5 min. The cells migrated to the lower
layer of the microporous membrane were counted under an inverted
microscope (magnification, ×200). Five visual fields/samples were
counted, and the mean was determined. For the invasion assay,
Matrigel gel was thawed at 4°C and diluted with serum-free medium
at the ratio 1:3 under a clean worktable. The Transwell chambers
were placed in 24-well plates coated with 40 µl of Matrigel and
then incubated in an incubator at 37°C for 2 h. The remaining steps
were the same as those listed for the migration experiment.
Flow cytometric analysis
The cells were inoculated in 6-well plates with
5×105 cells per well. After 48 h of transfection, the
cells in each group were collected and washed twice with PBS, and
500 µl of the binding buffer was added to gently suspend the cells.
Annexin VLIGHT 650/PI apoptosis detection kit was purchased from
Wanleibio Co., Ltd., which was used according to the manufacturer's
protocols. Annexin V-light 650 (5 µl) was added, and the mixture
was evenly mixed. Then, 10 µl of propidium iodide was added, and
the mixture was evenly mixed again. The cells were incubated at
room temperature for 15 min, and apoptosis was detected via flow
cytometry (using Beckman Analysis 2.0) and analyzed using Kaluza
Analysis 2.0 (Beckman Coulter, Inc.).
Western blot analysis
The protein was extracted using total protein
extraction kit (Wanleibio Co., Ltd.). The protein concentrations
were detected using bicinchoninic acid protein quantification kit
(Wanleibio Co., Ltd.). After 48 h of transfection, the p53 protein
was detected using a Bio-Rad vertical electrophoresis system. Then,
5% of concentration gel and 12% of separation gel were selected as
electrophoresis plastic. Next, 5X Loading Buffer and PBS were used
to dilute the protein sample, which was boiled in a water bath for
5 min, and 20 µl solution was prepared with 40 µg protein loaded
per lane. The proteins were transferred to PVDF blotting membrane
at 80V for 1.5 h, and 5% (M/V) skimmed milk powder with TBST (0.05%
Tween20) buffer was used for standby. After the transfer, the PVDF
membrane was removed, immersed in TBST in the shaker at speed of
100 rpm for 5 min at 25°C. Then, TBST buffer was removed and the
PVDF membrane was immersed in skimmed milk powder solution in the
shaker at speed of 100 r/min for 1 h. The transferred proteins were
probed with respective primary antibodies against b-actin (1:1,000;
cat. no. WL01845, Wanleibio Co., Ltd.) and P53 (1:500, cat. no.
WL01333, Wanleibio Co., Ltd.) overnight at 4°C. After the membrane
was washed with TBST buffer, the horseradish peroxidase-labeled
secondary antibody (1:5,000, cat. no. WLA023, Wanleibio Co., Ltd.)
was diluted with 5% skimmed milk powder/TBST solution to the
relevant concentration. Then, the membrane was added into the
second antibody solution, and the signals were developed using an
enhanced chemiluminescent kit (cat. no. WLA003, Wanleibio Co.,
Ltd.) and was prepared for color development. The images were
collected, exposed and the captured using Gel-Pro Analyzer 4.0
(Media Cybernetics, Inc.) system (39).
Statistical analysis
SPSS 22.0 software (IBM Corp.) was used for
statistical analysis. The measurement data was expressed as mean ±
SD. The measurement data was normally distributed, and the square
difference was the same. The significance of the average values was
analyzed using one-way ANOVA with a Tukey's HSD post hoc test.
χ2-test and Fisher's Exact test were performed to
analyze the count data. P<0.05 was considered to indicate a
statistically significant difference.
Results
lncRNA MEG3 expression in OSCC tissues
and cells
RT-qPCR was employed to detect the relative
expression of lncRNA MEG3 in OSCC tissues and corresponding normal
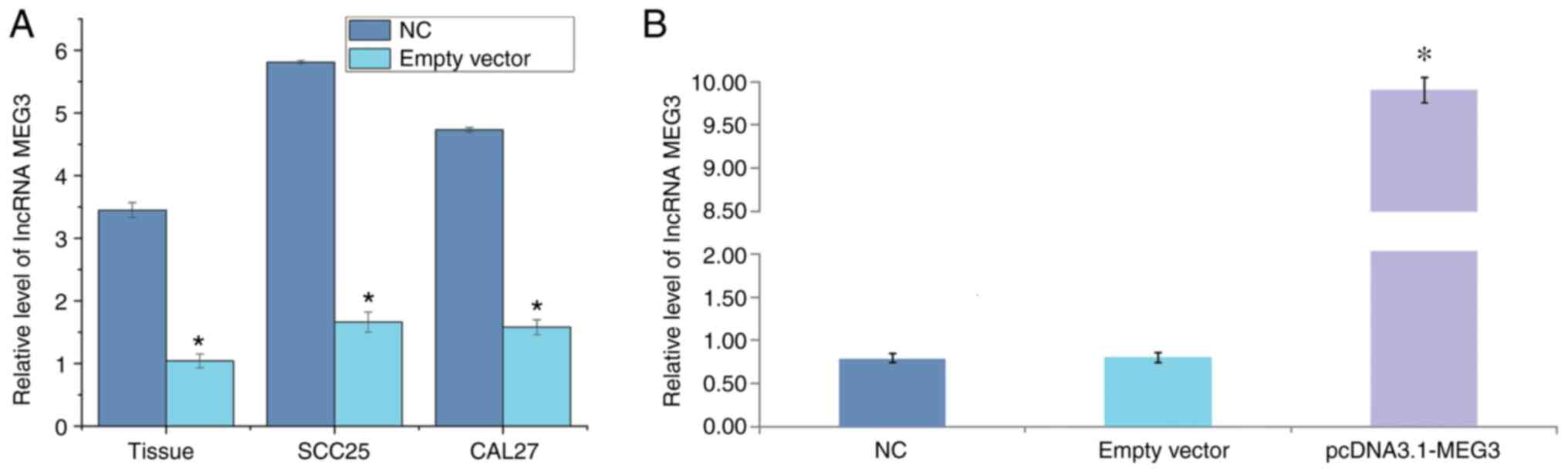
tissues. The results showed that the expression of MEG3 in the OSCC
tissues was significantly lower than that in the normal tissues
(P<0.05). Compared with normal oral mucosa cells, the expression
of lncRNA MEG3 in SCC25 and CAL27 cells was significantly lower
(P<0.05; Table I and Fig. 1A).
 | Table I.Expression of long non-coding RNA
maternally expressed 3 in OSCC tissues and cells. |
Table I.
Expression of long non-coding RNA
maternally expressed 3 in OSCC tissues and cells.
| Group | Tissues (n=5) | SCC25 (n=5) | CAL27 (n=5) |
|---|
| Normal | 3.45±0.12 | 5.81±0.03 | 4.73±0.03 |
| OSCC |
1.66±0.16a |
1.04±0.11a | 1.58±0.12 |
| Q-statistic | 27.853 | 106.259 | 74.5543 |
| P-value | 0.001 | 0.001 | 0.001 |
Construction of lncRNA MEG3
overexpression plasmid vector
Compared with the blank control group (0.75±0.56)
and empty vector group (0.77±0.65), the relative expression level
of lncRNA MEG3 mRNA in the overexpression group (9.87±0.87) was
significantly increased (P<0.05; Fig.
1B).
lncRNA MEG3 expression level is
significantly associated with clinicopathological features in
patients with OSCC
According to the median value of lncRNA MEG3
expression level, patients with OSCC were divided into the
high-(n=30) and low-expression (n=42) groups. The associations
between the expression of lncRNA MEG3 and patient age, sex, smoking
status, tumor location, clinical stage, lymph node metastasis,
distant metastasis and survival status were analyzed. The
expression of lncRNA MEG3 was not associated with age, sex, smoking
or tumor location (P>0.05), but was associated with clinical
stage, lymph node metastasis, distant metastasis and survival
status (P<0.05; Table II).
 | Table II.Association of long non-coding RNA
maternally expressed 3 expression with clinicopathological
characteristics of patients with oral squamous cell carcinoma. |
Table II.
Association of long non-coding RNA
maternally expressed 3 expression with clinicopathological
characteristics of patients with oral squamous cell carcinoma.
|
|
| MEG3 |
|
|
|---|
|
|
|
|
|
|
|---|
| Clinical
characteristics | n-value | High (30) | Low (42) |
c2-value | P-value |
|---|
| Age (years) |
|
|
|
|
|
|
<53 | 32 | 15 | 17 | 0.643 | 0.515 |
|
≥53 | 40 | 15 | 25 |
|
|
| Sex |
|
|
|
|
|
|
Male | 35 | 16 | 19 | 0.459 | 0.853 |
|
Female | 37 | 14 | 23 |
|
|
| Smoking |
|
|
|
|
|
|
Yes | 42 | 20 | 22 | 1.469 | 0.361 |
| No | 30 | 10 | 20 |
|
|
| Tumor location |
|
|
|
|
|
| Tongue
cancer | 30 | 12 | 18 | – | 0.003 |
|
Gingival carcinoma | 22 | 9 | 13 |
| 1.000 |
|
Carcinoma of the buccal
mucosa | 10 | 5 | 5 |
| 0.732 |
|
Others | 10 | 4 | 6 |
| 1.000 |
| Clinical stage |
|
|
|
|
|
| T1 | 28 | 18 | 10 | – | 0.003 |
| T2 | 25 | 6 | 19 |
| 0.044 |
| T3 | 15 | 5 | 10 |
| 0.500 |
| T4 | 4 | 1 | 3 |
| 0.600 |
| Lymphatic
metastasis |
|
|
|
|
|
|
Yes | 36 | 10 | 26 | 4.629 | 0.034 |
| No | 36 | 20 | 16 |
|
|
| Distant
metastasis |
|
|
|
|
|
|
Yes | 18 | 2 | 16 | 9.219 | 0.0028 |
| No | 54 | 28 | 26 |
|
|
| Survival
status |
|
|
|
|
|
|
Living | 49 | 27 | 22 | 11.392 | 0.002 |
|
Dead | 23 | 3 | 20 |
|
|
Effect of lncRNA MEG3 on the
proliferation of OSCC cells
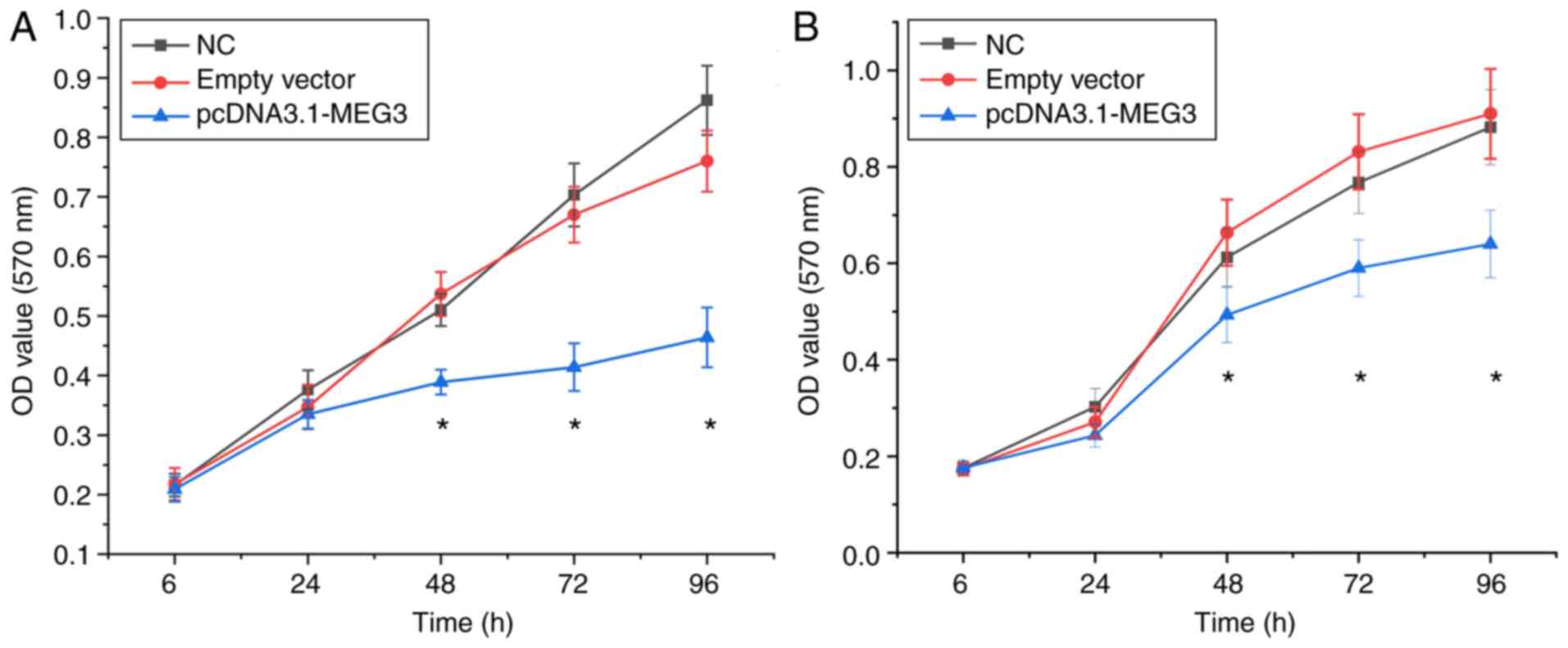
After transfection for 6 and 24 h, no significant
difference was found in the cell proliferation of each group
(P>0.05). After transfection for 48, 72 and 96 h, the
proliferation of the blank control and vector groups were
significantly higher than that of the overexpression group
(P<0.05). These results indicated that the overexpression of
lncRNA MEG3 resulted in decreased proliferation of SCC25 and CAL27
cells (Fig. 2A and B).
Effect of lncRNA MEG3 on migration of
OSCC cells
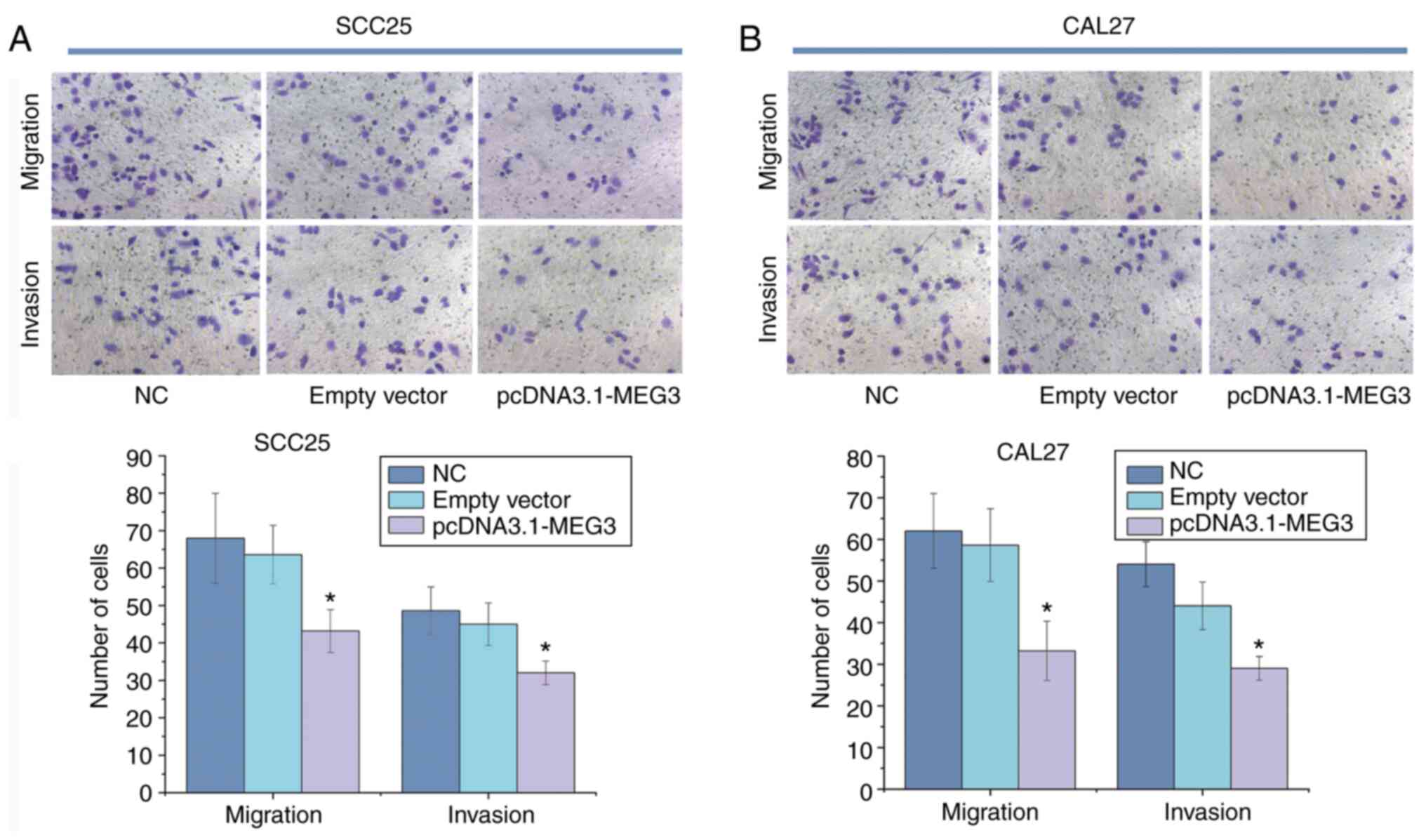
After overexpression of lncRNA MEG3 was established
in the SCC25 and CAL27 cells, the numbers of cells migrating and
invading the membrane in the blank control and vector groups were
significantly higher compared with the overexpression group
(P<0.05).
In SCC25 cells, the numbers of migrated and invaded
cells in the blank control were 68.00±11.98 and 48.60±6.39,
respectively. The numbers of cells migrated and invaded in the
empty vector group were 63.60±7.83 and 45.00±5.70, respectively. In
the lncRNA MEG3 group, the number migrated cells was 43.20±5.72,
and the number of invaded cells was 32.00±3.16.
In CAL27 cells, the number of migrated and invaded
cells in the blank control was 62.21±8.57 and 54.00±5.39,
respectively. The number of migrated and invaded cells in the empty
vector group was 58.60±8.73 and 44.31±5.13, respectively. In the
lncRNA MEG3 group, the number of migrated cells was 33.20±7.21, and
the number of invaded cells was 29.00±2.86. These results indicated
that the overexpression of lncRNA MEG3 resulted in the decreased
migration and invasion of SCC25 and CAL27 cells (Table III and Fig. 3).
 | Table III.Number of migrated and invaded cells
in each group. |
Table III.
Number of migrated and invaded cells
in each group.
| Cell line | Migrated cells,
n | Invaded cells,
n |
|---|
| SCC25 |
|
|
| NC | 68.00±11.98 | 48.60±6.39 |
|
Vehicle | 63.60±7.83 | 45.00±5.70 |
|
pcDNA3.1-MEG3 | 43.20±5.72 | 32.00±3.16 |
|
Q-statistic | 4.7592 |
4.4522 |
|
P-value | 0.0143 | 0.0211 |
| CAL27 |
|
|
| NC | 62.21±8.57 | 54.00±5.39 |
|
Vehicle | 58.60±8.73 | 44.31±5.13 |
|
pcDNA3.1-MEG3 | 33.20±7.21 | 29.00±2.86 |
|
Q-statistic | 6.1877 | 5.8146 |
|
P-value | 0.0024 | 0.0038 |
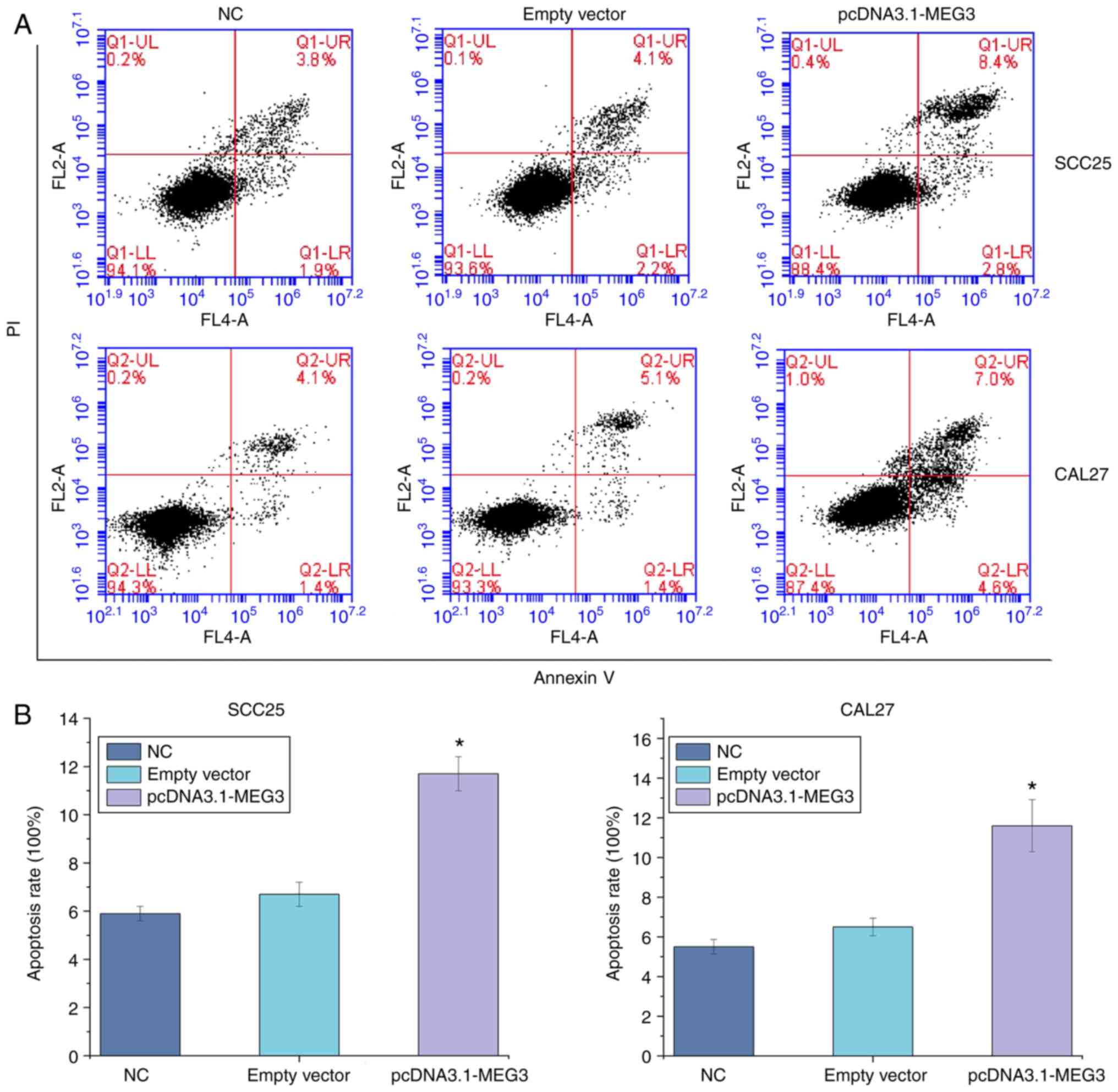
Apoptosis of OSCC cells is induced by
lncRNA MEG3
The apoptosis rates of SCC25 in the blank control,
vector and overexpression groups were 5.93±0.30, 6.70±0.50 and
11.7±0.71%, respectively. Meanwhile, the apoptosis rates of CAL27
in the blank control, vector and overexpression groups were
5.52±0.37, 6.50±0.44 and 11.6±1.31%, respectively. Compared with
the blank control and vector groups, the apoptosis rate of the
overexpression group was significantly increased (P<0.05),
indicating that the overexpression of the lncRNA MEG3 may promote
the apoptosis of SCC25 and CAL27 cells (Fig. 4).
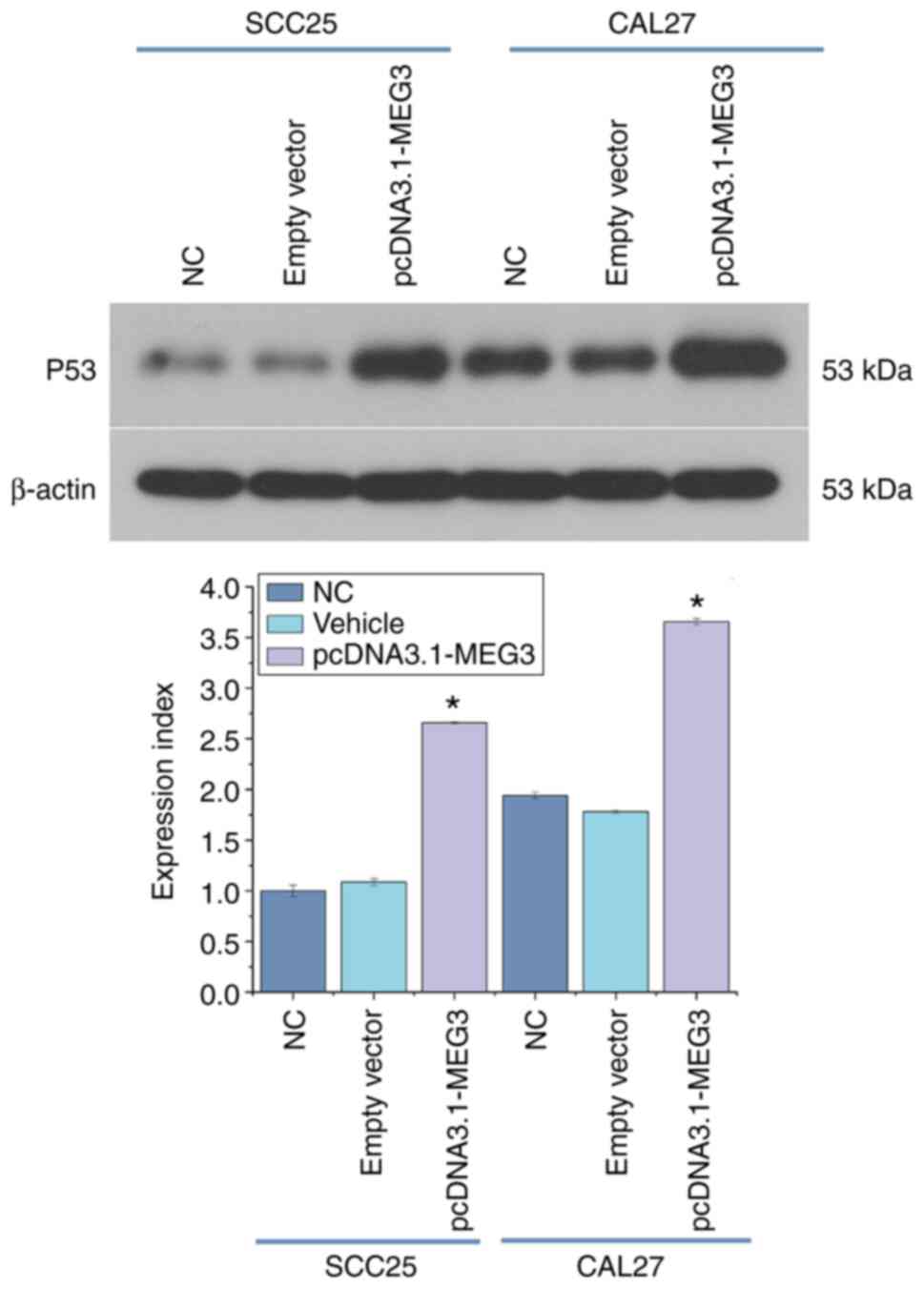
Influence of lncRNA MEG3 on p53
expression
Western blot analysis was used to detect the
expression of the p53 protein in the blank control group
(1.04±0.056), vector group (1.09±0.35) and overexpression group
(2.66±0.012) in SCC25 cells. Meanwhile, the expression of p53
protein in the blank control group (1.94±0.33), vector group
(1.78±0.017) and overexpression group (3.66±0.032) in CAL27 cell
was also detected. The results indicated that the overexpression of
lncRNA MEG3 enhanced the expression of the p53 protein. RT-qPCR was
used to detect the expression of p53 mRNA in each group. Compared
with the blank control group and vector group, the expression level
of the p53 mRNA in the overexpression group was significantly
increased (P<0.05). The overexpression of the lncRNA MEG3
resulted in promotion of the expression of the p53 signaling
pathway (Fig. 5).
Discussion
lncRNA MEG3 serves the role of a tumor suppressor
gene in malignant tumors, such as liver, gastric and bladder
cancers (40). The loss-of-function
or decreased expression of this transcript directly results in
cancer. In the present study, expression levels in 72 OSCC tissue
samples were detected. The results indicated that the expression of
lncRNA MEG3 in the OSCC tissues was significantly lower than that
in the corresponding normal tissues. This expression trend was
consistent with that of other cancer types and was closely
associated with the clinical stage, lymph node metastasis, distant
metastasis and survival status of patients with OSCC. The results
also suggested that the expression of lncRNA MEG3 was closely
related to the clinical stage, lymph node metastasis, distant
metastasis and survival status. To further explore the effects of
lncRNA MEG3 in the OSCC cells, SCC25 and CAL27 cells were
transfected using Lipofectamine 2000 to overexpress lncRNA MEG3 and
observe changes in the biological behavior of SCC25 and CAL27
cells. With the use of MTT, Transwell chamber and flow cytometry
assays, overexpression of lncRNA MEG3 was found to significantly
inhibit the proliferation and invasion capacity of SCC25 and CAL27
cells and promote apoptosis. These results indicated that lncRNA
MEG3 had a significant inhibitory effect on the OSCC cells. This
phenomenon indicates that lncRNA MEG3 also serves an important role
in OSCC.
LncRNA MEG3 can directly bind the p53 DNA domain,
stimulate the activation of p53-mediated association and promote
the apoptosis of cancer cells (36,41,42).
Therefore, lncRNA MEG3 may also inhibit cell activity and induce
apoptosis by promoting p53 gene expression in OSCC. Moreover, the
expression level of the p53 gene was detected. The results showed
that following overexpression of lncRNA MEG3, the expression of p53
increased significantly in SCC25 cells. This result suggested that
lncRNA MEG3 may represent an upstream regulator of p53 activation,
which is consistent with the results obtained by Zhang et al
(43). Therefore, the upregulation
of lncRNA MEG3 expression and activation of p53 pathway may have
therapeutic potential when used to inhibit the proliferation and
invasion of OSCC cells.
The abnormal expression of lncRNA MEG3 (such as
upregulation or downregulation) may result in the development
primary malignant tumors, which may be due to the methylation or
hypermethylation of a gene promoter (44,45).
Epigenetic silencing of tumor suppressor genes is associated with
the hypermethylation of the promoter CpG island (CGI), which can be
considered an early warning of cancer. The MEG3 promoter region
contains numerous CGIs and has two different methylation regions
(DMR), namely, IG-DMR and MEG3-DMR, which are located upstream of
the MEG3 gene, which is the imprinting control center of the
DLK1-MEG3 locus (46,47). A previous study demonstrated that the
methylation of MEG3 promoter in patients with esophageal squamous
cell carcinoma directly led to the inhibition of MEG3 expression,
resulting in the inhibition of tumor cell apoptosis (48). The aforementioned studies
demonstrated that the abnormal methylation of the MEG3 promoter may
promote the progress of malignant tumors, thus inhibiting the
formation and occurrence of methylation and promoting the
expression of tumor suppressor genes. These mechanisms are expected
to represent novel targets for tumor treatment.
In conclusion, the present study confirmed that
lncRNA MEG3 inhibits the proliferation, migration and invasion
capacities of SCC25 and CAL27 cells by targeting p53 and promoting
apoptosis. A theoretical basis and experimental data for potential
prevention and treatment of OSCC with lncRNA MEG3 as a target have
been identifies. However, other targeted pathways besides the
regulatory mechanism of MEG3-p53 have not been fully elucidated and
need to be further studied. In the later stages, in addition to
silencing lncRNA MEG3 to further confirm its effect on the OSCC
cell function, its inhibitory effect on tumor growth in an in
vivo model should also be analyzed. The potential target genes
and signaling pathways of lncRNA MEG3 need to be explored further
to provide additional experimental basis for the early application
of lncRNA MEG3 as a molecular target for clinical tumor
therapy.
Acknowledgements
Not applicable.
Funding
The present study was funded by Hebei Key Project
Plan of Medical Science Research (grant no. 20210213).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WW and TL designed the study, and performed the
experiments alongside HL. WW wrote the manuscript and analyzed the
data alongside YQ. KL helped with the investigation and formal
analysis of data. YL and QD contributed to the methodology and data
analysis. All authors read and approved the final version of the
manuscript. KL, YL and QD confirmed the authenticity of all the raw
data.
Ethics approval and consent to
participate
The Ethics Committee of the Fourth Affiliated
Hospital (Shijiazhuang, China) approved the experiment and
supervised and guided the whole experimental process (approval no.
201411EC040). All patients provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yang B, Dong K, Guo P, Guo P, Jie G, Zhang
G and Li T: Identification of key biomarkers and potential
molecular mechanisms in oral squamous cell carcinoma by
bioinformatics analysis. J Comput Biol. 27:40–54. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kademani D: Oral cancer. Mayo Clin Proc.
82:878–887. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Joseph JP, Harishankar MK, Pillai AA and
Devi A: Hypoxia induced EMT: A review on the mechanism of tumor
progression and metastasis in OSCC. Oral Oncol. 80:23–32. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim SY, Han YK, Song JM, Lee CH, Kang K,
Yi JM and Park HR: Aberrantly hypermethylated tumor suppressor
genes were identified in oral squamous cell carcinoma (OSCC). Clin
Epigenetics. 11:1162019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chong CE, Lim KP, Gan CP, Marsh CA, Zain
RB, Abraham MT, Prime SS, Teo SH, Silvio Gutkind J, Patel V and
Cheong SC: Over-expression of MAGED4B increases cell migration and
growth in oral squamous cell carcinoma and is associated with poor
disease outcome. Cancer Lett. 321:18–26. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sun C and Li J: Expression of MiRNA-137 in
oral squamous cell carcinoma and its clinical significance. J BUON.
23:167–172. 2018.PubMed/NCBI
|
|
7
|
Qiu YL, Liu YH, Ban JD, Wang WJ, Han M,
Kong P and Li BH: Pathway analysis of a genome-wide association
study on a long non-coding RNA expression profile in oral squamous
cell carcinoma. Oncol Rep. 41:895–907. 2019.PubMed/NCBI
|
|
8
|
Jin T, Guo Y and Huang Z, Zhang Q and
Huang Z, Zhang Y and Huang Z: Vitamin D inhibits the proliferation
of oral squamous cell carcinoma by suppressing lncRNA LUCAT1
through the MAPK pathway. J Cancer. 11:5971–5981. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang X, Yang S, Lv X, Wang L and Li C:
Overexpression of LncRNA SNHG1 were suitable for oncolytic
adenoviruse H101 therapy in oral squamous-cell carcinoma.
OncoTargets Ther. 13:13033–13039. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gullerova M: Long non-coding RNA. Genomic
Elements in Health, Disease and Evolution. Felekkis K and
Voskarides K: Springer; New York, NY: pp. 83–108. 2015, View Article : Google Scholar
|
|
11
|
Cui HB, Ge HE, Wang YS and Bai XY:
MiR-208a enhances cell proliferation and invasion of gastric cancer
by targeting SFRP1 and negatively regulating MEG3. Int J Biochem
Cell Biol. 102:31–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bayarmaa B, Wu Z, Peng J, Wang Y, Xu S,
Yan T, Yin W, Lu J and Zhou L: Association of LncRNA MEG3
polymorphisms with efficacy of neoadjuvant chemotherapy in breast
cancer. BMC Cancer. 19:8772019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Yao T, Wang Y, Yu J, Liu Y and
Lin Z: Long noncoding RNA MEG3 is downregulated in cervical cancer
and affects cell proliferation and apoptosis by regulating miR-21.
Cancer Biol Ther. 17:104–113. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dykes IM and Emanueli C: Transcriptional
and post-transcriptional gene regulation by long non-coding RNA.
Genom Proteom Bioinf. 15:177–186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vafadar A, Shabaninejad Z, Movahedpour A,
Mohammadi S, Fathullahzadeh S, Mirzaei HR, Namdar A, Savardashtaki
A and Mirzaei H: Long non-coding RNAs as epigenetic regulators in
cancer. Curr Pharm Design. 25:3563–3577. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huarte M: The emerging role of LncRNAs in
cancer. Nat Med. 21:1253–1261. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu C, Li S, Sun D and Yang S: lncRNA PVT1
accelerates progression of non-small cell lung cancer via targeting
miRNA 526b/EZH2 regulatory loop. Oncol Lett. 19:1267–1272.
2020.PubMed/NCBI
|
|
18
|
Han X, Zheng J, Wang Y and Gao Z: MiRNA
29a inhibits colon cancer growth by regulation of the
PTEN/Akt/GSK3β and Wnt/β catenin signaling pathways. Oncol Lett.
16:2638–2644. 2018.PubMed/NCBI
|
|
19
|
Zheng Z, Li W, Xu J, Xie B, Yang M, Huang
H, Li H and Wang Q: LncMSEN1, a mantle specific LncRNA
participating in nacre formation and response to polyI: C
stimulation in pearl oyster Pinctada fucata martensii. Fish
Shellfish Immunol. 96:330–335. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu S, Wu L, Qi H and Xu M:
LncRNA/circRNA-miRNA-mRNA networks regulate the development of root
and shoot meristems of Populus. Ind Crop Prod. 133:333–347. 2019.
View Article : Google Scholar
|
|
21
|
Gibb EA, Vucic EA, Enfield KS, Stewart GL,
Lonergan KM, Kennett JY, Becker-Santos DD, MacAulay CE, Lam S,
Brown CJ and Lam WL: Human cancer long non-coding RNA
transcriptomes. PLoS One. 6:e259152011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
He Q, Long J, Yin Y, Li Y, Lei X, Li Z and
Zhu W: Emerging roles of lncRNAs in the formation and progression
of colorectal cancer. Front Oncol. 9:15422020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guo QY, Wang H and Wang Y: LncRNA H19
polymorphisms associated with the risk of OSCC in Chinese
population. Eur Rev Med Pharmacol Sci. 21:3770–3774.
2017.PubMed/NCBI
|
|
24
|
Liu L, Zhan Y, Huang Y and Huang L: LncRNA
FGD5-AS1 can be predicted as therapeutic target in oral cancer. J
Oral Pathol Med. 49:243–252. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sun CC, Zhang L, Li G, Li SJ, Chen ZL, Fu
YF, Gong FY, Bai T, Zhang DY, Wu QM and Li DJ: The lncRNA PDIA3P
interacts with miR-185-5p to modulate oral squamous cell carcinoma
progression by targeting cyclin D2. Mol Ther Nucleic Acids.
9:100–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Niu X, Yang B, Liu F and Fang Q: LncRNA
HOXA11-AS promotes OSCC progression by sponging miR-98-5p to
upregulate YBX2 expression. Biomed Pharmacother. 121:1096232020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang X, Li H and Shi J: LncRNA HOXA11-AS
promotes proliferation and cisplatin resistance of oral squamous
cell carcinoma by suppression of miR-214-3p expression. Biomed Res
Int. 2019:86451532019.PubMed/NCBI
|
|
28
|
Zhou Y, Zhang X and Klibanski A: MEG3
noncoding RNA: A tumor suppressor. J Mol Endocrinol. 48:R45–R53.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang J, Liang Y, Huang X, Guo X, Liu Y,
Zhong J and Yuan J: STAT3-induced upregulation of lncRNA MEG3
regulates the growth of cardiac hypertrophy through
miR-361-5p/HDAC9 axis. Sci Rep. 9:4602019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schuster-Gossler K, Bilinski P, Sado T,
Ferguson-Smith A and Gossler A: The mouse GTL2 gene is
differentially expressed during embryonic development, encodes
multiple alternatively spliced transcripts, and may act as an RNA.
Dev Dyn. 212:214–228. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Takahashi N, Okamoto A, Kobayashi R,
Shirai M, Obata Y, Ogawa H, Sotomaru Y and Kono T: Deletion of
GTL2, imprinted non-coding RNA, with its differentially methylated
region induces lethal parent-origin-dependent defects in mice. Hum
Mol Genet. 18:1879–1888. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou Y, Cheunsuchon P, Nakayama Y, Lawlor
MW, Zhong Y, Rice KA, Zhang L, Zhang X, Gordon FE, Lidov HG, et al:
Activation of paternally expressed genes and perinatal death caused
by deletion of the GTL2 gene. Development. 137:2643–2652. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Stadtfeld M, Apostolou E, Akutsu H, Fukuda
A, Follett P, Natesan S, Kono T, Shioda T and Hochedlinger K:
Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse
induced pluripotent stem cells. Nature. 465:175–181. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kaneko S, Bonasio R, Saldaña-Meyer R,
Yoshida T, Son J, Nishino K, Umezawa A and Reinberg D: Interactions
between JARID2 and noncoding RNAs regulate PRC2 recruitment to
chromatin. Mol Cell. 53:290–300. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wei GH and Wang X: lncRNA MEG3 inhibit
proliferation and metastasis of gastric cancer via p53 signaling
pathway. Eur Rev Med Pharmacol Sci. 21:3850–3856. 2017.PubMed/NCBI
|
|
36
|
Hu D, Su C, Jiang M, Shen Y, Shi A, Zhao
F, Chen R, Shen Z, Bao J and Tang W: Fenofibrate inhibited
pancreatic cancer cells proliferation via activation of p53
mediated by upregulation of LncRNA MEG3. Biochem Biophys Res
Commun. 471:290–295. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhao Y and Hong L: lncRNA-PRLB confers
paclitaxel resistance of ovarian cancer cells by regulating
RSF1/NF-κB signaling pathway. Cancer Biother Radiopharm.
36:202–210. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao H, Zheng GH, Li GC, Xin L, Wang YS,
Chen Y and Zheng XM: Long noncoding RNA LINC00958 regulates cell
sensitivity to radiotherapy through RRM2 by binding to
microRNA-5095 in cervical cancer. J Cell Physiol. 234:23349–23359.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Al-Rugeebah A, Alanazi M and Parine NR:
MEG3: An oncogenic long non-coding RNA in different cancers. Pathol
Oncol Res. 25:859–874. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Zhao J, Geng J, Chen F, Wei Z, Liu
C, Zhang X, Li Q, Zhang J, Gao L, et al: Long Non-Coding RNA MEG3
knockdown attenuates endoplasmic reticulum stress-mediated
apoptosis by targeting p53 following myocardial infarction. J Cell
Mol Med. 23:8369–8380. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Uroda T, Anastasakou E, Rossi A, Teulon
JM, Pellequer JL, Annibale P, Pessey O, Inga A, Chillón I and
Marcia M: Conserved pseudoknots in lncRNA MEG3 are essential for
stimulation of the p53 pathway. Mol Cell. 75:982–995.e9. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang LL, Hu D and Zou LH: Low expression
of lncRNA MEG3 promotes the progression of oral squamous cell
carcinoma by targeting miR-21. Eur Rev Med Pharmacol Sci.
22:8315–8323. 2018.PubMed/NCBI
|
|
44
|
Dong Z, Zhang A, Liu S, Lu F, Guo Y, Zhang
G, Xu F, Shi Y, Shen S, Liang J and Guo W: Aberrant
methylation-mediated silencing of lncRNA MEG3 functions as a ceRNA
in esophageal cancer. Mol Cancer Res. 15:800–810. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Molina-Pinelo S, Salinas A, Moreno-Mata N,
Ferrer I, Suarez R, Andrés-León E, Rodríguez-Paredes M, Gutekunst
J, Jantus-Lewintre E, Camps C, et al: Impact of DLK1-DIO3 imprinted
cluster hypomethylation in smoker patients with lung cancer.
Oncotarget. 9:4395–4410. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hill KE, Kelly AD, Kuijjer ML, Barry W,
Rattani A, Garbutt CC, Kissick H, Janeway K, Perez-Atayde A,
Goldsmith J, et al: An imprinted non-coding genomic cluster at
14q32 defines clinically relevant molecular subtypes in
osteosarcoma across multiple independent datasets. J Hematol Oncol.
10:1072017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang J, Lin Z, Gao Y and Yao T:
Downregulation of long noncoding RNA MEG3 is associated with poor
prognosis and promoter hypermethylation in cervical cancer. J Exp
Clin Cancer Res. 36:52017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Gao Y, Huang P and Zhang J:
Hypermethylation of MEG3 promoter correlates with inactivation of
MEG3 and poor prognosis in patients with retinoblastoma. J Transl
Med. 15:2682017. View Article : Google Scholar : PubMed/NCBI
|