Introduction
Tumor suppressors, proto-oncogenes and epigenetic
factors serve a central role in carcinogenesis. Instead of a
treatment that also harms healthy cells, such as chemotherapy,
epigenetic therapy has led to the development of a new therapeutic
option in which side effects are minimized (1). Prostate cancer (PCa) is a disease with
multifactorial etiology, including epigenetic and genomic
alterations (2,3). While the traditional indicator in the
diagnosis of PCa is the PSA level in the blood, this marker may
also increase in other diseases related to the prostate (such as
prostatitis and benign prostatic hyperplasia) (4). Moreover, there is no direct association
between PSA level and the stage of PCa. For the clinical management
of PCa, reliable new diagnostic, predictive, prognostic and
therapeutic biomarkers are needed (5–7).
Micro RNAs (miRNAs/miRs) are non-coding (ncRNA) RNAs
20–25 nucleotides in length that function at the
post-transcriptional stage as regulators of gene expression
(6,8,9).
Depending on whether miRNA is complementary with the target mRNA,
the latter is degraded or translation is ceased (6). The role of miRNAs in the epigenetic
mechanism is among the most interesting subjects of research in
cancer studies (10). Despite
numerous challenges in their clinical application, miRNAs have the
potential to reduce off-target effects and complete traditional,
targeted or immune-based therapies for cancer (11). Multiple studies have been conducted
to elucidate the association between prostate carcinogenesis and
miRNAs (12–20). There are also other studies on the
clinical importance of miRNA gene promoter methylation in PCa
(21–25).
The human genome encodes five DNA methyltransferases
(DNMTs). Namely, DNMT1, DNMT2, DNMT3a, DNMT3b and DNMT3L. DNMT1,
DNMT3A and DNMT3B are canonical C5 DNMTs that catalyze the addition
of methylation markers to genomic DNA (26). Abnormalities in DNMT genes can
trigger malignancy, deteriorate prognosis and complicate treatment.
DNMT inhibition reduces tumor formation by increasing the
expression of tumor suppressor genes and this has resulted in the
inclusion of DNMT inhibitors as anti-cancer targets (27). The effects of DNMT genes on tumors
and therapeutic approaches targeting DNMTs remain a matter of
discussion today (28).
Phosphate and tensin homolog (PTEN) gene is a tumor
suppressor that regulates cell growth and survival via the
phosphoinositol-3 kinase pathway (PI3K) (29). Loss of PTEN expression is observed in
a ~70% of PCa patients (30,31). NK3 Homeobox 1 (NKX3.1) is an
important homeobox gene for prostatic epithelial specification,
proper differentiation and luminal stem cells. Concurrently, this
gene is another tumor suppressor gene that regulates the androgen
receptor (AR)-related signaling pathway. The loss of PTEN in human
prostate cancers may cause a decrease in NKX3.1 expression. Thus,
NKX3.1 is a therapeutic target in the treatment of PTEN-deficient
prostate cancers (32,33). It has been indicated that NKX3.1 is
not expressed in metastatic prostate cancer and may be a valuable
biomarker for precisely determining prostatic origin in poorly
differentiated metastatic carcinomas (34).
Epigenetic interactions are candidates for new
prognostic biomarkers and can be detected by integrating
methylation and miRNA linkages (35,36).
Epigenetic-based miRNAs (epi-miRNAs) are defined as a new class of
miRNAs that suppress enzymes involved in this epigenetic mechanism
(36). These miRNAs can directly
inhibit enzymes, such as DNMTs and histone deacetylases (HDACs),
which are key mediators of epigenetic mechanisms (37). epi-miR29 is the first epi-miRNA
identified in lung cancer (36,38).
However, the number of experimentally validated epi-miRs involved
in these regulatory epigenetic cycles is relatively low (39).
The miR-34 family consists of three members:
miR-34a, miR-34b and miR-34c. While miR-34a is encoded with its own
transcript, miR-34b and miR-34c share a common primary transcript
(40). Since upregulation of the
miR-34 family ceases the cell cycle in the G1 phase and leads to
cell apoptosis, it can serve a tumor suppressive role (41,42).
Vogt et al (43) reported the
CpG methylation levels of miR-34a and miR-34b/c as being between
40–100% in colorectal, pancreatic, breast, ovarian, urothelial and
kidney cancers, and stated that these epi-miRs may have diagnostic
value.
miR-148a indicates different expressions in
different cancers (44). Hamilton
et al (45) emphasized that
miR-148a is subject to stage-specific regulations in PCa. Yu et
al (46) concluded that miR-148a
may be a tumor suppressing miR, indicating that it is downregulated
in gastric cancer. In another study, miR-148a expression was found
to be inversely correlated with p27 expression and was
downregulated in advanced gastric cancer (47). Other studies have also reported the
downregulation of miR-148 in PCa (48,49). By
contrast, miR-148a was found to be a prognostic onco-miR capable of
regulating EGFR and apoptosis in glioblastoma by targeting
mitogen-inducible gene 6 (MIG6) and proapoptotic bim (BCL2L11)
(50). It was also found that
increased miR-148a-3p in serum can be a potential biomarker in PCa
(51). Szczyrba et al
(52) reported the upregulation of
miR-148a (~5-fold increase) in PCa.
miR-152 is a member of the miR-148/152 family.
miR-152 has been indicated to act as a tumor suppressor in human
cancers (53–55). miR-148a and miR-152 have been shown
to target the 3′-UTR region of DNMT1 (56). In another study, miR-152 was reported
to suppress PTEN expression by targeting the 3′-UTR region of PTEN
(57). Contrary to studies reporting
downregulation of miR-152, there are other studies in which miR-152
is upregulated in colorectal, lung and breast cancers (58) and in prostate cancer (6,59).
The miR-200 family has been indicated to facilitate
oncogenic activity via inhibition of the tumor suppressor Ras
Association Domain Family Member 2 (RASSF2) in colon cancer
(60). The oncogenic effects of
miR-200 family were also identified in bladder cancer (61). Moreover, in murine lung
adenocarcinoma cells, the miR-200 family has also been reported to
act as a tumor suppressing miR, inhibiting epithelial-mesenchymal
transition (EMT) and metastasis (62). Furthermore, tumor suppressing miR
negatively regulates the Rho/Rho-associated kinase ROCK signaling
pathway of miR-200 family in hepatacellular carcinoma (63).
In a preliminary study where miR-34b/c, miR-148a and
miR-152 were identified as prognostic onco-miR biomarker candidates
in PCa, it was found that these epi-miRNAs were correlated with the
clinicopathological (PSA, GS and TNM-Staging) characteristics of
the patients (25). It was also
reported that miR-200a/b acted as an onco-miR in the early stage of
PCa and a tumor suppressing miR in the advanced metastatic stage.
To the best of our knowledge, comparative multiple analyzes of
miR-34b/c, 148a, 152 and 200a/b epi-miRNAs with DNMTs, PTEN and
NKX3.1 are not yet available in the literature, which signifies the
novelty of the current study. With regards to the aformentioned
information, the aim of the present study was to determine the
epigenetic interaction between the target genes DNMT1, DNMT3a,
DNMT3b, PTEN and NKX3.1, and miR-34b/c, 148a, 152 and 200a/b
epi-miRNAs in patients with prostate cancer. The aim was also to
determine the miRNA-mRNA regulatory interaction links of each gene
and find their possible roles in diagnostic and therapeutic
prognosis in PCa.
Materials and methods
Collection of clinical samples
The present study was performed with blood samples
taken from 25 healthy patients in the control group, 25 patients
with PCa and 40 patients with metastatic prostate cancer (Met-PCa),
which were collected with the approval of Gazi University Faculty
of Medicine Ethics Committee (Ankara, Turkey; approval no.
G.Ü.E.T-686). Whole blood samples included in the study were
obtained from patients who applied to the Urology Clinics of the
Gazi University Hospital and the Hacettepe University Hospital
(Ankara, Turkey). Informed consent was provided by all patients.
The age ranges of the patients were 54–70 years for PCa, 40–85
years for metastatic cases and 41–59 years for the control group.
Inclusion criteria in the present study were as follows: i) The 65
PCa and Met-PCa patients had not undergone any therapies or
surgeries related to PCa; and ii) the 25 healthy controls have not
had a family history of PCa. The individuals included in the
patient and control groups did not have any known systemic
diseases. The clinicopathological characteristics of the patients
and healthy controls are listed in Table
I. In the metastatic group, a blood sample from one patient had
to be discarded, thus could not be included in the data.
 | Table I.Clinicopathological characteristics
of the patients and controls. |
Table I.
Clinicopathological characteristics
of the patients and controls.
| Characteristic | PCaa (n=25) | Met-PCa (n=39) | Control |
|---|
| Median age, years
(range) | 61 (54–70) | 68 (40–85) | 50 (41–59) |
| PSA levels at
diagnosis, ng/ml |
|
|
|
| <2.5 |
|
| 25 |
|
<10 | 19 | 0 | 0 |
|
10-20 | 5 | 10 | 0 |
|
>20 | 1 | 29 | 0 |
| Gleason score |
|
|
|
| 6 | 10 | 0 | 0 |
| 7 | 11 | 0 | 0 |
| 8 | 3 | 11 | 0 |
| 9 | 0 | 16 | 0 |
| 10 | 1 | 12 | 0 |
| Pathological
stage |
|
|
|
|
T2N0M0 | 15 | 0 | 0 |
|
T3N0M0 | 10 | 0 | 0 |
|
T3N0/1M1 | 0 | 10 | 0 |
|
T3/4N0/1M1 | 0 | 24 | 0 |
|
T4N0/1M1 | 0 | 5 | 0 |
RNA isolation
The blood samples were stored in room temperature
and the steps specified in the RNA isolation kit (miRNeasy
Serum/Plasma Kit; Qiagen GmbH) protocol were applied. After adding
200 µl serum and 1/5 of the QIAzole lysis buffer from the kit,
vortexing was performed to mix the contents. The resulting mixture
was kept at room temperature for 5 min. Subsequently, 200 µl
chloroform was added and vortexing was carried out for 15 sec.
After 2–3 min, the solution was centrifuged at 12,000 x g and 4°C
for 15 min. The top phase containing RNA was transferred to another
tube (~600 µl). In total, 1.5 volumes of 100% ethanol was added and
then the solution was mixed thoroughly by pipetting up and down
several times in the RNeasy MinElute spin column within the 2 ml
collection tube; and centrifuged at 8,000 x g for 15 sec at room
temperature. The flow-through (containing QIAzole lysis reagent or
buffer RWT) was discarded and the spin column was reused in the new
collection tube. Then 700 µl wash buffer RWT was added and the tube
was centrifuged at 8,000 x g for 15 sec at room temperature. The
flow-through was discarded and the spin column was again reused in
the new collection tube. Another washing buffer of about 500 µl RPE
was added and the tube was centrifuged at 8,000 x g for 15 sec at
room temperature. The flow-through was once again discarded and the
spin column was placed in the new collection tube. Subsequently 500
µl of 80% ethanol was added into the RNeasy MinElute spin column
and the tube was centrifuged at 8,000 x g for 2 min at room
temperature. After centrifugation, the RNeasy MinElute spin column
was removed and placed in the new collection tube for the last
time, such that the spin column would not come into contact with
the flow remaining under the collection tube. The spin column with
an open the lid was centrifuged at 14,000 x g 4°C for 5 min to
remove ethanol and was then dried. The dried spin column was
transferred to a new 1.5 ml collection tube; 14 µl RNase-free water
was added to it and was centrifuged at 14,000 x g 4°C for 1 min.
The samples were stored at −20°C until they were studied.
cDNA synthesis and fluorimetric
measurement
The purity and concentrations of the obtained RNAs
were measured by colibri microvolume spectrophotometric method
(Colibri LB915, Titertek-Berthold). The protocol of the cDNA
synthesis kit (QuantiNova reverse transcription kit; Qiagen GmbH)
was followed. The total volume was adjusted to 15 µl by adding 13
µl total RNA and 2 µl qDNA separation mixture. After 2 min at 45°C
in the thermal cycler, the device was stopped and the samples were
placed on ice. Afterwards, 4 µl of reverse transcription master mix
and 1 µl of reverse transcription enzyme were added into each tube
and the final volume was brought to 20 µl. The cDNA was synthesized
by setting the tubes in the Thermal Cycler for 3 min at 25°C, 10
min at 45°C and 5 min at 85°C. The samples were stored at −20°C
until they were studied.
Quantification of mRNA levels using
quantitative (q)PCR
Expression levels of a total of six genes, one being
a reference gene, were measured in the present study. The primers
used are listed in Table II. The
necessary steps were followed according to the manufacturer's
instructions: A total of 15 µl of mixture was prepared; each sample
containing 4.5 µl water, 0.5 µl of the primer pair, 10 µl of the
SYBR master mix (Bioline sensiFAST SYBR 2×; Bioline Reagents Ltd.).
The final volume was made up to 20 µl by adding 5 µl of cDNA. Next,
40 cycles of qPCR was performed using the Qiagen rotor-gene-Q
instrument (Qiagen GmbH) and the thermocycling conditions were as
follows: Denaturation at 95°C for 1 min, annealing at 61°C for 30
sec and extension at 72°C for 1 min. Experiments were conducted in
triplicate. β-actin gene was used as the housekeeping gene.
 | Table II.Targeted primer sequences for reverse
transcription-quantitative PCR. |
Table II.
Targeted primer sequences for reverse
transcription-quantitative PCR.
| Gene | Primer
sequence |
|---|
| β-actin | F:
5′-GAAGATCAAGATCATTGCTCCT-3′ |
|
| R:
5′-ACTCGTCATACTCCTGCTT-3′ |
| DNMT1 | F:
5′-ACCACCATCACATCTCATT-3′ |
|
| R:
5′-GTCTAGCAACTCGTTCTCT-3′ |
| DNMT3a | F:
5′-CGGAACATTGAGGACATCT-3′ |
|
| R:
5′-GTACTGGTACGCACACTC-3′ |
| DNMT3b | F:
5′-GAAGATCAGAGCCGAGAACAA-3′ |
|
| R:
5′-TCAAAGAGAGGGTGGAAGGA-3′ |
| PTEN | F:
5′-TTAGACTTGACCTATATTTATCCA-3′ |
|
| R:
5′-GCGGTGTCATAATGTCTT-3′ |
| NKX3.1 | F:
5′-AGAGACCGAGCCAGAAAGG-3′ |
|
| R:
5′-GCTTCTGCGGCTGCTTAG-3′ |
Statistical analysis
Intergroup mRNA expression levels were analyzed with
the Kruskal Wallis-H test using SPSS 22.0 (IBM Corp.). After the
Kruskal Wallis-H analysis, complementary benchmarking techniques
were used to determine which groups caused the significant
difference. Comparative analysis of three different groups in pairs
(PCa vs. control, Met-PCa vs. control and PCa vs. Met-PCa) was
conducted and confirmed Tamhane's T2 post hoc test. The statistical
data are consistent and there were not any statistical changes in
the present results. In order to examine the correlation between
patient groups and certain clinicopathological parameters (Gleason
Score, PSA level and TNM staging), multinomial logistic regression
analysis was performed. Finally, linear regression analysis was
performed to reveal the association between epi-miRNAs and their
target genes. P<0.05 was considered to indicate a statistically
significant difference.
Results
The expression levels of DNMT1, DNMT3a, DNMT3b and
PTEN were analyzed and statistically significant differences were
found between the PCa and Met-PCa groups, and the control group
(P<0.05; Fig. 1A and B). However,
the expression level of NKX3.1, which is one of the target genes of
the investigated miRNAs, did not exhibit a significant difference
between the PCa and Met-PCa groups, and the control group
(P>0.05; Fig. 1A and B). It is
worth emphasizing that there were statistically significant
differences between the PCa-control and Met-PCa-control groups, but
no significant difference was found between the PCa and Met-PCa
groups (P>0.05). Since NKX3.1 expression level did not differ
between groups, it was not included in the clinicopathological
evaluation of the present study, and hence in complementary
comparative analyzes. When the PCa and Met-PCa groups were compared
with the control group, a significant increase in expression level
of the DNMT1 gene was found to be ~5-fold in the PCa group and
~6-fold in the Met-PCa group (P<0.001; Fig. 1A). Again, when the PCa and Met-PCa
groups were compared with the control, DNMT3a decreased ~5-fold in
the PCa group and ~2-fold in the Met-PCa group. In addition, the
expression level of DNMT3b decreased ~3-fold in the PCa group and
~2-fold in the Met-PCa group (P<0.01; Fig. 1A). When compared to the control,
while decreased expression in DNMT3a and DNMT3b genes was
significant in both PCa and Met-PCa groups, decreased expression in
the PTEN gene was found to be significant only in the Met-PCa group
(P<0.01; Fig. 1A). Moreover, the
expression level of PTEN decreased ~2-fold in the PCa group
compared with the control, and it decreased ~4-fold in the Met-PCa
group (Fig. 1A). In terms of NKX3.1
expression level, no significant difference was found between any
groups (P>0.05; Fig. 1A and B).
The evaluation results of the patients classified according to
their clinicopathological characteristics are provided in Table III (PSA), Table IV (GS) and Table V (TNM). A significant difference was
found only in DNMT1 in both groups (P<0.01).
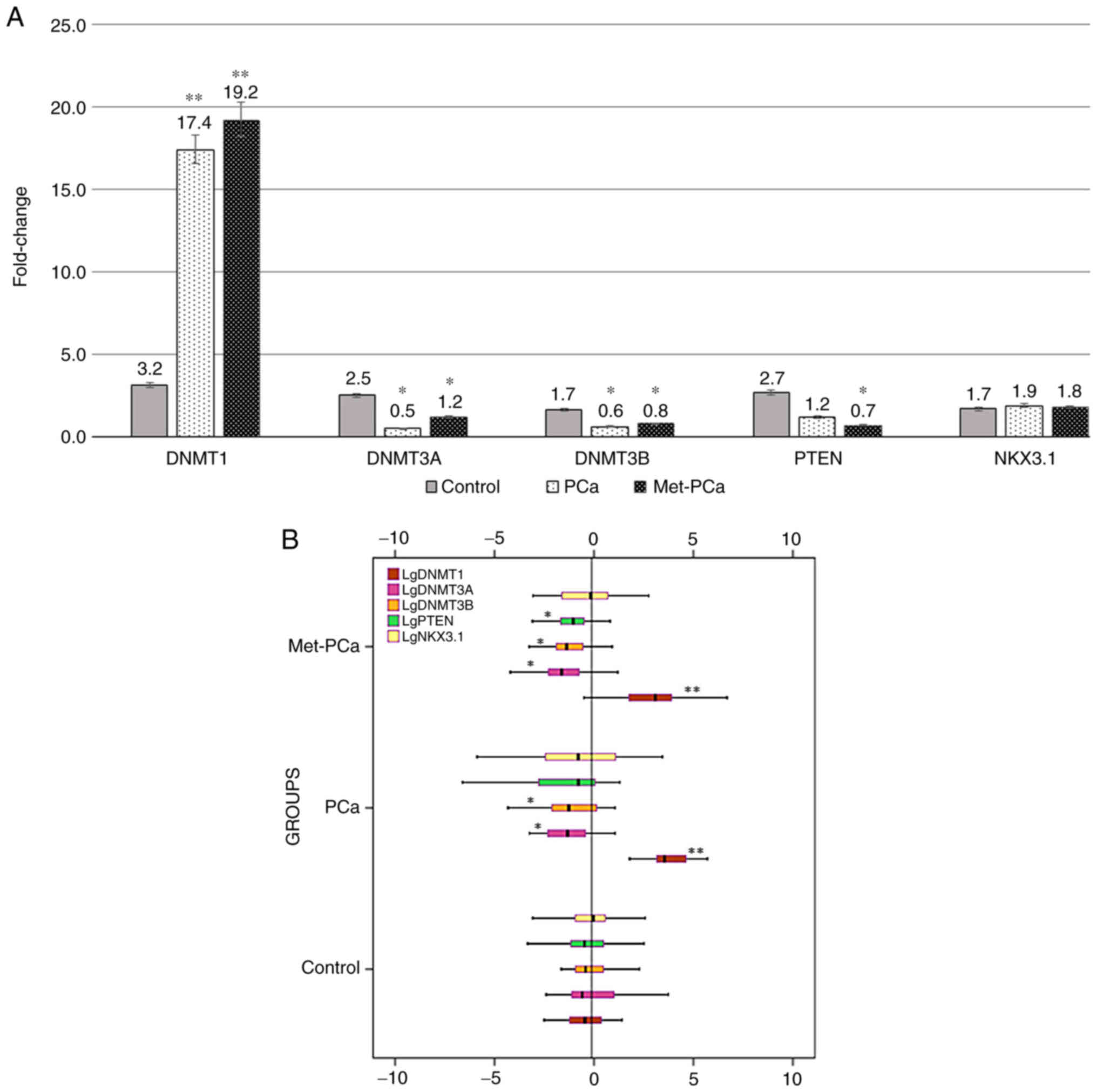 | Figure 1.(A) Fold-changes in mRNA expression
levels of DNMT1, DNMT3a, DNMT3b, PTEN and NKX3.1 in the healthy
group and in patients with PCa and Met-PCa. (B) The box plot of
DNMT1, DNMT3a, DNMT3b, PTEN and NKX3.1 in terms of gene expression
levels. Mean reference line was −0.1. Values are expressed as the
mean ± SEM from triplicate groups. *P<0.01; **P<0.001 vs.
Control. DNMT, DNA methyltransferase; PTEN, phosphate and tensin
homolog; NKX3.1, NK3 Homeobox 1; PCa, prostate cancer; Met-PCa,
metastatic prostate cancer. |
 | Table III.Association between PSA levels and
expression levels of DNMT1, DNMT3a, DNMT3b and PTEN. |
Table III.
Association between PSA levels and
expression levels of DNMT1, DNMT3a, DNMT3b and PTEN.
| A, DNTM1 |
|---|
|
|---|
|
| PCa PSA values
(ng/ml) | Met-PCa PSA values
(ng/ml) |
|---|
|
|
|
|
|---|
| Value | <10 | ≥10 | ≤20 | >20 |
|---|
| FC | 6.08 | 19.56 | 6.69 | 22.95 |
| P-value | 0.014a | 0.005a | 0.001a | 0.0001a |
| Mean ± SD | 19±0.693 | 6±0.492 | 23±0.504 | 7±0.500 |
|
| B,
DNMT3a |
|
|
| PCa PSA
values | Met-PCa PSA
values |
|
|
|
|
| Value | <10 | ≥10 | ≤20 | >20 |
|
| FC | 0.59 | 0.26 | 1.90 | 0.83 |
| P-value | 0.371 | 0.146 | 0.154 | 0.817 |
| Mean ± SD | 0.6±1.099 | 0.3±1.429 | 2±0.746 | 0.8±0.697 |
|
| C,
DNMT3b |
|
|
| PCa PSA
values | Met-PCa PSA
values |
|
|
|
|
| Value | <10 | ≥10 | ≤20 | >20 |
|
| FC | 0.69 | 0.41 | 1.15 | 0.67 |
| P-value | 0.805 | 0.838 | 0.534 | 0.092 |
| Mean ± SD | 0.7±1.587 | 0.4±1.776 | 1.1±1.229 | 0.7±1.199 |
|
| D, PTEN |
|
|
| PCa PSA
values | Met-PCa PSA
values |
|
|
|
|
| Value | <10 | ≥10 | ≤20 | >20 |
|
| FC | 0.75 | 0.47 | 1.04 | 1.58 |
| P-value | 0.955 | 0.874 | 0.636 | 0.806 |
| Mean ± SD | 0.8±0.957 | 0.5±1.096 | 1±0.422 | 1.6±0.423 |
 | Table IV.Associations between the Gleason
Scores and expression levels of DNMT1, DNMT3a, DNMT3b and PTEN. |
Table IV.
Associations between the Gleason
Scores and expression levels of DNMT1, DNMT3a, DNMT3b and PTEN.
| A, DNTM1 |
|---|
|
|---|
|
| PCa Gleason
Scores | Met-PCa Gleason
Scores |
|---|
|
|
|
|
|---|
| Value | 6 | 7 | 8 | 10 | 8 | 9 | 10 |
|---|
| FC | 15.41 | 18.83 | 15.16 | 24.74 | 17.75 | 18.29 | 21.74 |
| P-value | 0.003a | 0.003a | 0.006a | 0.001a | 0.0001a | 0.0001a | 0.0001a |
| Mean ± SD | 15±0.496 | 19±0.578 | 15±0.778 | 25±1.817 | 18±0.566 | 18±0.49 | 22±0.524 |
|
| B,
DNMT3a |
|
|
| PCa Gleason
Scores | Met-PCa Gleason
Scores |
|
|
|
|
| Value | 6 | 7 | 8 | 10 | 8 | 9 | 10 |
|
| FC | 0.53 | 0.52 | 0.53 | 0.33 | 0.30 | 1.89 | 2.58 |
| P-value | 0.36 | 0.241 | 0.215 | 0.433 | 0.22 | 0.65 | 0.43 |
| Mean ± SD | 0.5±1.099 | 0.5±1.176 | 0.5±1.482 | 0.3±5.274 | 0.3±0.774 | 1.9±0.691 | 2.6±0.709 |
|
| C,
DNMT3b |
|
|
| PCa Gleason
Scores | Met-PCa Gleason
Scores |
|
|
|
|
| Value | 6 | 7 | 8 | 10 | 8 | 9 | 10 |
|
| FC | 0.63 | 0.64 | 0.52 | 0.56 | 0.34 | 1.18 | 1.48 |
| P-value | 0.854 | 0.882 | 0.969 | 0.65 | 0.16 | 0.13 | 0.26 |
| Mean ± SD | 0.6±1.491 | 0.6±1.504 | 0.5±1.748 | 0.6±5.56 | 0.3±1.263 | 1.2±1.197 | 1.5±1.206 |
|
| D, PTEN |
|
|
| PCa Gleason
Scores | Met-PCa Gleason
Scores |
|
|
|
|
| Value | 6 | 7 | 8 | 10 | 8 | 9 | 10 |
|
| FC | 0.72 | 0.68 | 0.73 | 0.44 | 0.41 | 2.00 | 0.74 |
| P-value | 0.885 | 0.929 | 0.932 | 0.983 | 0.91 | 0.99 | 0.94 |
| Mean ± SD | 0.7±0.917 | 0.7±0.949 | 0.7±1.211 | 0.4±2.278 | 0.4±0.452 | 2±0.404 | 0.7±0.408 |
 | Table V.Associations between TNM staging and
the expression levels of DNMT1. DNMT3a. DNMT3b and PTEN. |
Table V.
Associations between TNM staging and
the expression levels of DNMT1. DNMT3a. DNMT3b and PTEN.
| A, DNTM1 |
|---|
|
|---|
|
| PCa TNM
classification | Met-PCaTNM
classification |
|---|
|
|
|
|
|---|
| Value | T2N0M0 | T3N0M0 | T3N0/1M1 | T3/4 N0/1M1 | T4 N0/1M1 |
|---|
| FC | 18.31 | 26.04 | 10.41 | 24.66 | 30.55 |
| P-value | 0.002a | 0.001a | 0.0001a | 0.0001a | 0.0001a |
| Mean ± SD | 18±0.472 | 16±0.618 | 10.4±0.581 | 24.66±0.517 | 10.6±0.508 |
|
| B,
DNMT3a |
|
|
| PCa TNM
classification | Met-PCa TNM
classification |
|
|
|
|
| Value | T2N0M0 | T3N0M0 |
T3N0/1M1 | T3/4
N0/1M1 | T4
N0/1M1 |
|
| FC | 0.55 | 0.53 | 0.28 | 2.29 | 1.37 |
| P-value | 0.255 | 0.374 | 0.071 | 0.768 | 0.805 |
| Mean ± SD | 0.5±1.117 | 0.5±1.156 | 0.3±0.812 | 2.3±0.714 | 1.4±0.833 |
|
| C,
DNMT3b |
|
|
| PCa TNM
classification | Met-PCa TNM
classification |
|
|
|
|
| Value | T2N0M0 | T3N0M0 |
T3N0/1M1 | T3/4
N0/1M1 | T4
N0/1M1 |
|
| FC | 0.74 | 0.50 | 0.43 | 1.31 | 0.96 |
| P-value | 0.889 | 0.804 | 0.451 | 0.127 | 0.261 |
| Mean ± SD | 0.7±1.46 | 0.5±1.518 | 0.4±1.274 | 1.3±1.206 | 1±1.32 |
|
| D, PTEN |
|
|
| PCa TNM
classification | Met-PCa TNM
classification |
|
|
|
|
| Value | T2N0M0 | T3N0M0 |
T3N0/1M1 | T3/4
N0/1M1 | T4
N0/1M1 |
|
| FC | 0.76 | 0.62 | 2.83 | 0.61 | 0.49 |
| P-value | 0.953 | 0.678 | 0.389 | 0.762 | 0.792 |
| Mean ± SD | 0.8±0.932 | 0.6±0.97 | 2.8±0.499 | 0.6±0.468 | 0.5±0.527 |
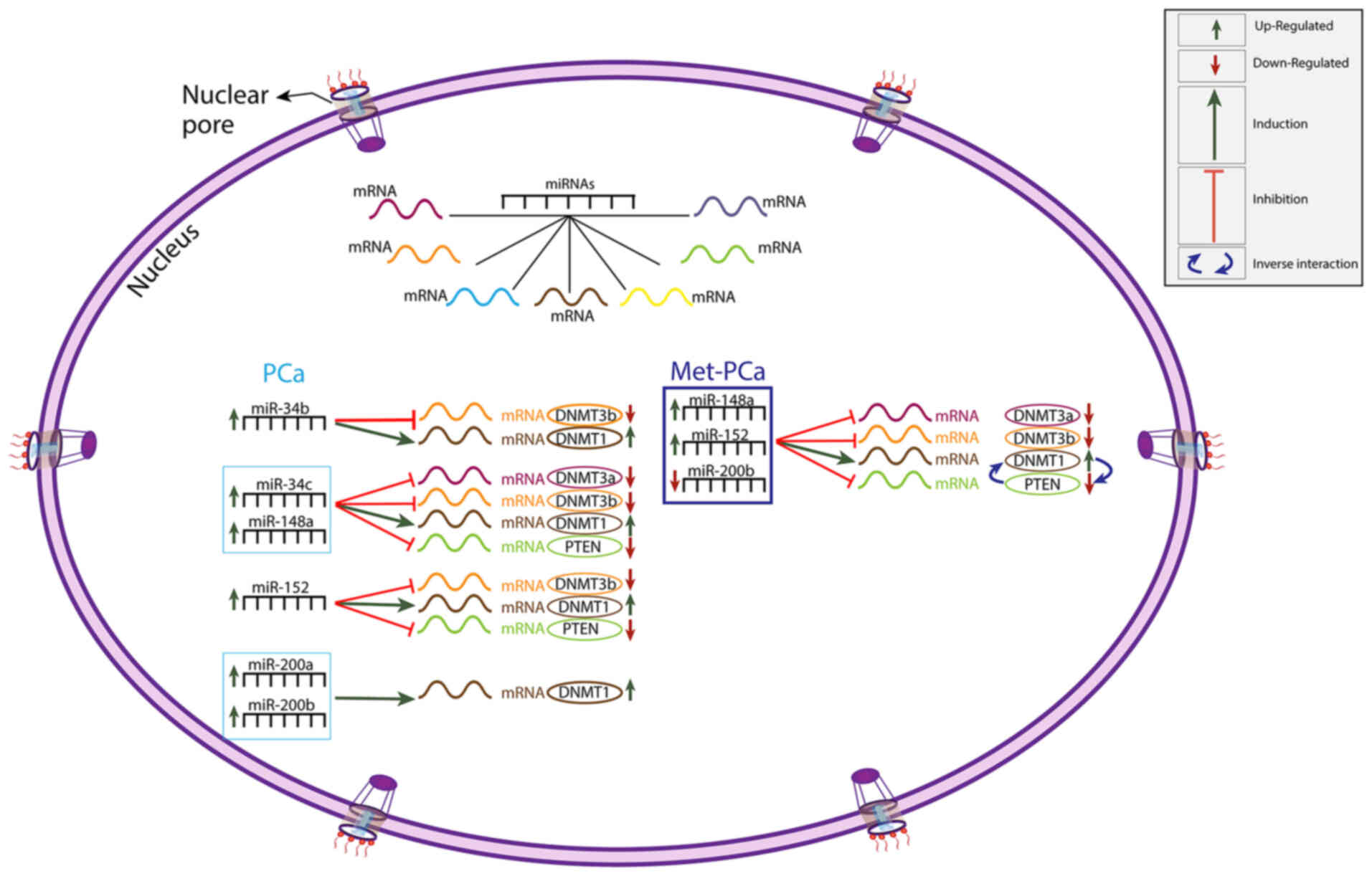
According to the results of linear regression
analysis between epi-miRNAs and their target genes, in the PCa
group, there was a statistically significant association between
miR-34b and DNMT1/DNMT3b; between miR-34c/miR-148a and all target
genes studied; between miR-152 and DNMT1/DNMT3b/PTEN; and between
miR-200a/b and DNMT1 (P<0.05) (Table
VI). In the same patient group, a highly significant
association was observed between all miRNAs investigated and DNMT1
(P<0.01; Table VI). Since an
inversely proportional interaction between DNMT1 and tumor
suppressor genes has been demonstrated, the effects of DNMT1 on
PTEN (a tumor suppressor gene) were investigated. In the present
study, while there was no statistically significant difference
between PTEN and DNMT1 in the PCa and control groups (P>0.05), a
highly significant difference was found between PTEN and DNMT1 in
the Met-PCa group (P<0.0001) (Table
VI). In the Met-PCa group, miR-148a, miR-152 and miR-200b
indicated a statistically significant association with all target
genes (P<0.01; Table VI). In the
same group, miR-34b, miR-34c and miR-200a did not indicate a
statistically significant association with the target genes
(P>0.05; Table VI). In the
control group, no statistically significant association was
identified between epi-miRNAs and target genes (P>0.05; Table VI). The present findings indicate a
significant association between epi-miRNAs and target genes, which
are presented in Fig. 2. It is worth
noting that the miRNAs can regulate and interaction with numerous
mRNAs.
 | Table VI.P-values between the expression
levels of epi-miRNAs and expression levels of DNMT1, DNMT3a, DNMT3b
and PTEN. |
Table VI.
P-values between the expression
levels of epi-miRNAs and expression levels of DNMT1, DNMT3a, DNMT3b
and PTEN.
| A, PCa |
|---|
|
|---|
|
| P-values of
Epi-miRNAs | Genes |
|---|
|
|
|
|
|---|
| Genes | miR-34b | miR-34c | miR-148a | miR-152 | miR-200a | miR-200b | DNMT1 |
|---|
| DNMT1 | 0.004a | 0.034a | 0.001a | 0.009a | 0.011a | 0.002a |
|
| DNMT3a | 0.37 | 0.01 a | 0.042a | 0.156 | 0.67 | 0.951 |
|
| DNMT3b | 0.043a | 0.023a | 0.007a | 0.022a | 0.127 | 0.305 |
|
| PTEN | 0.058 | 0.037a | 0.008a | 0.004a | 0.918 | 0.835 | 0.089 |
|
| B,
Met-PCa |
|
|
| P-values of
Epi-miRNAs | Genes |
|
|
|
|
| Genes | miR-34b | miR-34c |
miR-148a | miR-152 |
miR-200a |
miR-200b | DNMT1 |
|
| DNMT1 | 0.951 | 0.127 | 0.016a | 0.002a | 0.979 | 0.001a |
|
| DNMT3a | 0.856 | 0.283 | 0.018a | 0.004a | 0.404 | 0.015a |
|
| DNMT3b | 0.057 | 0.054 | 0.002a | 0.007a | 0.077 | 0.001a |
|
| PTEN | 0.881 | 0.667 | 0.003a | 0.004a | 0.578 | 0.003a | 0.0001a |
|
| C,
Control |
|
|
| P-values of
Epi-miRNAs |
|
|
|
|
|
| Genes | miR-34b | miR-34c |
miR-148a | miR-152 |
miR-200a |
miR-200b | DNMT1 |
|
| DNMT1 | 0.609 | 0.624 | 0.812 | 0.211 | 0.089 | 0.141 |
|
| DNMT3a | 0.107 | 0.223 | 0.942 | 0.054 | 0.199 | 0.763 |
|
| DNMT3b | 0.06 | 0.051 | 0.578 | 0.261 | 0.05 | 0.86 |
|
| PTEN | 0.907 | 0.097 | 0.951 | 0.78 | 0.167 | 0.272 | 0.479 |
Discussion
miRNA and epigenetic regulators control expression
of protein-coding genes. A specific group of miRNAs, defined as
epi-miRNAs, and epigenetic regulators form a powerful network of
reciprocal expressions and interactions. These genetic circuits are
regulated by a double-negative feedback loop, in which a miRNA
inhibits an epigenetic regulator and is subsequently suppressed by
the same regulator (37,64). The epi-miRNA circuit, which is
sensitive to the cellular environment, serves a ‘transition switch’
role, providing strong stability to random cell passages in
homeostatic situations. Reale et al (39) showed that miRs enhance their
regulatory potential by interacting with epigenetic regulatory
genes. They also found that the same miR behaves as an epi-miR in a
particular cell line, but may not indicate any epigenetic effects
in another cell line. For this reason, the ‘epi-miRNome’ is
significant for the successful personalized treatment of cancer.
Epi-miRs are particularly noteworthy to elucidate the
etiopathogenesis of the disease and the similarity between cells in
the same tissue and/or cells derived from the same type of tumors.
In this respect, the present study has the potential to shed light
on the follow-up and treatment prediction of prostate cancer.
Increased mRNA expression of DNMT1 has been
indicated in prostate cancer and benign prostate epithelial cell
lines (65). Progressively
increasing expression of DNMT1 is associated with urothelial
carcinogenesis in the development and precancerous stages of
bladder nodular invasive carcinomas (66,67). In
the present study, a significant increase in the expression level
of the DNMT1 gene was observed in the PCa and Met-PCa groups, and a
significant decrease in DNMT3a, DNMT3b and PTEN gene expression
levels was also noted. There are many studies reporting that the
expression of the DNMT1 enzyme increases in cancer, as well as
studies with the opposite results. One of the reasons for this
discrepancy is that DNMT1 upregulation is not a secondary effect of
increased cancer cell proliferative activity, but may also be
caused by mutations in this enzyme (28,68). The
decrease in PTEN gene expression and increase in DNMT1 expression
in both patient groups are consistent with the literature. These
results suggest that DNMT1 may cause a decrease in the expression
of PTEN by causing hypermethylation of its promoter. In the present
study, DNMT1, which was found to be highly expressed, downregulated
PTEN expression; and the antagonistic interaction between
DNMT1-PTEN was found to be significant only in the Met-PCa group.
This result suggests that DNMT1 primarily regulates the PTEN gene
at the metastatic stage, and DNMT1/PTEN relative expression may be
associated with a less favorable prognosis. Furthermore, it was
concluded in the present study that high DNMT1 expression combined
with low PTEN expression may represent prognostic markers for
overall survival in patients with Met-PCa. This result suggests
that the PTEN suppressor gene may be the target of other epigenetic
regulatory enzymes in addition to DNMT1 in the metastatic process,
leading to multiple changes, such as loss of heterozygosity and
allelic deletion. In other words, the decrease in PTEN accompanying
the increase in DNMT1 expression level is perhaps rare in
early-stage prostate cancer, but may be encountered frequently with
tumor progression. In parallel to the study by Qi et al
(69) on bladder cancer, the present
study demonstrated that the DNMT1 expression indicated a
significant association with clinicopathological features (PSA, GS
and TNM) in the PCa and Met-PCa groups. Statistical significance of
only the DNMT1 gene in terms of all clinicopathological stages
proves that this gene serves an active role in both the early stage
and the advanced stage of PCa. The present result indicates the
potential of DNMT1 as a therapeutic target and candidate for a
biomarker. Since a literature search did not return any results for
studies that associate DNMTs with prostate cancer according to
clinicopathological features, it wasn't possile to make a
comparison in terms of the latter part of the present results. It
has been indicated that DNMT3b (70)
and DNMT3a (71) can be potential
targets in developing appropriate diagnostics and therapeutics in
breast cancer. Ma et al (72)
noted that DNMT1- and DNMT3a-targeting tumor suppressor genes in
pituitary adenomas were upregulated, and were associated with
aggressive tumor behavior and high methylation. It has also been
demonstrated that DNMT1 is positively correlated with tumor size,
clinical stage, histological grading, lymph node metastasis,
vascular invasion, recurrence and prognosis in renal cell carcinoma
(73). In urological cancers, DNMTs
have also been investigated (74–76). The
vast majority of studies have highlighted the importance of the
methylation pattern in the discovery of new anticancer
therapeutics. Bowen et al (33) showed that loss of NKX3.1, a
gatekeeper suppressor, increased prostate epithelial cell
proliferation in mice, but NKX3.1 mRNA expression was not affected
by PTEN. Therefore, it was suggested that the interaction of tumor
suppressors PTEN and NKX3.1 may be responsible for the loss of
PTEN. The loss of function of NKX3.1 caused inflammation-induced
prostate cancer through abnormal cellular plasticity and impairment
of cellular differentiation in a mouse model (77). In the present study, no significant
difference was found in NKX3.1 gene expression between any groups
and the control. The reason thelatter result differed from those in
the literature may be due to the fact that human serum instead was
used in the present study rather than mouse tissue.
Majid et al (42) investigated prostate cancer in a
xenograft mouse model and found that miR-34b inhibited cell
proliferation, colony formation and migration/invasion by directly
targeting Akt and its downstream genes, and induced apoptosis by
stopping the cell cycle at G0/G1. In the same study, the increased
expression of miR-34b was reported to downregulate DNMTs and HDACs
by inducing partial demethylation and active chromatin
modifications. Shiina et al (78) reported that downregulation of miR-34b
upregulated androgen receptor (AR) in African-Americans with PCa,
pointing to a potential association between race and tumorigenesis.
Conversely, these low expressions levels possibly stemming from
allelic deletions and/or loss of heterozygosity should not be
overlooked. Contrary to the above studies, Chamani et al
(79) found that expressions of
DNMT1, DNMT3a and DNMT3b were down-regulated in hepatocellular
cancer cells treated with dendrosomal nanocurcumin, while miR-34a,
−34b and −34c were upregulated. It was demonstrated that there is
an inverse proportion between the expression level of miR-34 and
DNMTs. In a preliminary study (25),
it was found that the percentages of promoter methylation of
miR-34b and miR-34c decreased in the PCa and Met-PCa groups, and
this hypomethylation resulted in an increased expression level of
miR-34b/c. Furthermore, high miR-34b/c levels were associated
directly with PSA, GS and TNM staging. In the current study,
parallel to the literature, an inversely proportional relationship
was identified between the highly expressed miR-34c and target
genes DNMT3a, 3b and PTEN. In addition, it was found that high
levels of DNMT1 were associated with high levels of miR-34b and
miR-34c in the PCa group. We hypothesized that the high level of
miR-34b/c may stem from the mutant DNMT1. However, the conclusion
emphasizing that high DNMT1 level was associated with high miR-34b
and miR-34c levels was not consistent with the findings of Majid
et al (42). The reason for
this may be the pleiotropic role of miR-34b, as well as the
different tissue sources included in the study protocol,
differences in sample selection, sample size, ethnic composition,
mutations in the DNMT1 enzyme and regulations of DNMT1 enzyme with
different miRNAs. In the Met-PCa group, no statistically
significant difference was found between miR-34b/c and target
genes. By increasing the expression of DNMT1 in the early stages of
PCa and suppressing the PTEN gene, miR-34c may represent a
potential diagnostic and prognostic target of a new regulatory
pathway: miR-34c-DNMT1-PTEN, although this would have to be
confirmed in further in vivo studies.
Sengupta et al (80) demonstrated that in hormone-resistant
prostate cancer cell lines, ectopic expression of miR-148a induces
apoptosis by suppressing DNMT1 expression. They also noted that
there was an antagonistic relationship between miR-148a and DNMT1
expression levels. miR-148a regulated the expression of DNMT genes
by binding to the coding region of DNMT1 and DNMT3b (56,81).
Braconi et al (56)
identified that the precursors of miR-148a and miR-152 increased
the expression of methylation-sensitive tumor suppressor genes
Rassf1a and p16INK4a by decreasing DNMT1 protein expression in
human cholangiocarcinoma cell line. Hamilton et al (45) found that miR-148a targets both
oncogenes and tumor suppressors, such as PTEN in PCa, and its
expression level varies according to the stage of the disease.
Moreover, Li et al (44)
indicated that miR-148a-3p was upregulated in certain cancers and
downregulated in others. miR-148a was upregulated in glioblastoma
(82) and in osteosarcoma (83), which reflects the aggressiveness of
the cancers. In another study, miR-148a induced activation of the
phosphatidylinositol 3-kinase (PI3K) signaling pathway in
osteosarcomas by targeting PTEN (84). Furthermore, miR-148a increased
β-catenin expression by inhibiting PTEN expression (85). Dybos et al (51) investigated miR-148a-3p in PCa and
reported that high miR-148a-3p was much more than a general marker
of imbalance, such as infections in the body. The aforementioned
studies support our hypothesis, drawing attention to the potential
role of miR-148a overexpression in PCa development. Consistent with
the literature, in the PCa and Met-PCa groups, it was revealed that
hypomethylated miR-148a interacted inversely with target genes
DNMT3a, 3b and PTEN; however, unlike in the literature, it
interacted directly with DNMT1. It was revealed that high DNMT1
expression levels were associated with high miR-148a level in both
patient groups. This result suggests that the expression level of
miR-148a may have been increased due to the mutant DNMT1 gene.
A highly significant association between DNMT1 and
PTEN genes was identified in the Met-PCa group. A possible
mechanism for the increased expression of miR-148a is the
hypomethylation of the miR-148a host gene promoter that stimulates
transcription of this miRNA due to its onco-miR nature. It was
revealed that miR-148a downregulated PTEN expression by targeting
DNMT1. PTEN activity, in addition to epigenetic silencing and
mutations, can also be regulated in a variety of ways, such as
transcriptional inhibiting, abnormal protein localization and
post-translational modifications. The present findings revealed
that the DNMT1 gene was in the upstream cascade of PTEN and it
downregulated PTEN via promoter hypermethylation that promoted
metastasis of PCa. The significance of the linkage between DNMT1
and PTEN genes in the metastatic stage of the disease suggests that
tumor suppressor gene deletion may be involved in the metastatic
state.
Another non-coding RNA that serves key roles in PCa
pathogenesis is miR-152. It has been reported that the expression
level of miR-152 was significantly decreased in PCa tissues with
high Gleason Score (53,54). Theodore et al (54) reported that expression of miR-152
decreased due to the high methylation profile in PCa cell lines, in
contrast to increased expression of DNMT1. They also noted that
miR-152/DNMT1 epigenetic regulation may serve a key role in
aggressiveness of PCa tumors, particularly in African-American
populations. In another study, the expression level of miR-152
decreased in PCa tissue due to the high methylation profile
(86). In contrast to the study by
Ramalho-Carvalho et al (86),
high plasma miR-152 levels have been proposed as a diagnostic
biomarker for other cancers such as lung, colorectal and breast
cancer (58). Matin et al
(6) reported that overexpression of
miR-152-3p increased the proliferation and migration of prostate
cancer cells. Moya et al (59) identified one of the four plasma
miRNAs (a highly expressed miR-152) that has the potential to
improve the diagnostic power of the existing PSA biomarker for PCa.
In a preliminary study (25), we
found that the increase in the expression of miR-152 as a result of
promoter hypomethylation was directly proportional to PSA, GS and
TNM staging in both PCa and Met-PCa groups. Our results did not
support the finding of the study by Zhu et al (53), Theodore et al (54) and Ramalho-Carvalho et al
(86). The reasons for this are that
detected levels of miRNA can vary significantly, especially
depending on the extraction methodology applied in serum samples
(pre-extraction steps such as sample collection, sampling,
processing and storage, and internal controls used), sample size,
tissue type studied and racial differences. Moreover, since miRNA
secretion is a selective process, the circulatory levels are not a
true reflection of intracellular levels (87). In addition, due to the unknown
effects of the coagulation process on extracellular miRNAs in the
blood, levels of miRNA concentrations in serum rather than in
plasma were examined. Therefore, comparisons among miRNA
irregularities in different samples became even more difficult. For
example, while miR-152 was upregulated in serum (88), it was downregulated in tissue samples
(89) from patients with bladder
cancer. The discrepancy might be attributed to the metastatic role
of miR-152, known as exosomal-miR or metasta-miR, possibly via
exosomal escape (90,91). Exosomes derived from prostate cancer
cells contribute to the chemo-resistance of cancer (91). In the present study, high levels of
miR-152 were further upregulated due to the resistance in the
metastatic stage, even resulting in loss of PTEN. Also, certain
miRNAs are released by tumor cells as argonaute (AGO)-bound
complexes or from dead cells in apoptotic bodies; thus, one source
of miRNAs is tumor cells (92).
Perhaps the source of high levels of miR-152s in the present study
may have been PCa cells. Chen et al (58) reported similar results. They
investigated the plasma values of onco-miR-21 and the tumor
suppressor miR-152 and found that the expression levels of both
miRs were increased. In the PCa and Met-PCa groups from the current
study, it was demonstrated that upregulation of miR-152 was
significantly associated with upregulation of DNMT1, and with
downregulation of DNMT3b and PTEN. The current findings were
consistent with a nasopharyngeal carcinoma study by Huang et
al (57), who showed miR-152
inhibited PTEN expression by targeting the 3′UTR of the PTEN mRNA.
The present study also revealed an inversely proportional
relationship between miR-152 and PTEN expression levels in both
groups. Moreover, a highly significant association was demonstrated
between miR-152 and DNMT1 expression in the Met-PCa group, as it
was between miR-148a and DNMT1. The present result suggests that
mutant DNMT1 gene might increase the expression level of miR-152
and this causes PTEN gene to be silenced by methylation. To the
best of our knowledge, the miR-152-DNMT1-PTEN pathway has not yet
been investigated in PCa, therefore, it was not possible to
directly compare the current results with other data from the
literature.
Downregulation of the miRNA-200 family is a
potential tumor-suppressor in PCa (93). Low miRNA-200 family expression causes
upregulation of zinc finger E homeobox binding transcription
factors (ZEB)1 and ZEB2, which are best known for their role in
sustaining EMT (94). miR-200a and b
cause the growth of endometrial cancer cells in vitro, by
suppressing PTEN gene expression (95). In another study on endometrial
cancer, miR-200a, miR-200b and miR-429 were found to be onco-miRs
targeting PTEN (96). Suo et
al (97) reported that miR-200a,
which was overexpressed in the ovarian cancer tissue and cell
lines, inhibited PTEN expression and had a carcinogenic effect for
this type of cancer. As is known, target genes of miR-200a/b
include DNMT3a, DNMT3b and DNMT1 (71). The downregulation of miR-200b and
miR-200c-mediated DNMTs increased the chemotherapeutic sensitivity
of ovarian cancer cells in vivo by reversing cisplatin
resistance (98). In a breast cancer
cell line, Zeng et al (99)
identified that flap endonuclease 1, which is an important gene in
DNA replication, excision repair and telomere maintenance, caused
DNMT1 upregulation, which was associated with downregulation of
miR-200a. The miR-200 family generally behaves in a cancer-specific
manner, their expression is increased in certain cancers but
decreased in other cancers. Our precious study demonstrated the
pleitropic effect of miR-200a/b family in local/locally advanced
and metastatic PCa (25). An
increase in miR-200a and miR-200b expression levels due to promoter
hypomethylation was observed in the local/locally advanced PCa
group; and low expression levels due to promoter hypermethylation
were observed in the metastatic group. In other words, it was
determined that miR-200a/b behaves as an onco-miR in the early
stage of PCa, but as a tumor suppressor-miR in the advanced
metastatic stage. In the current study, it was determined in
patients with Met-PCa that there was a highly significant
association between all target genes and downregulated miR-200b, as
well as between all target genes and upregulated miR-148a and
miR-152. In the metastatic group, it was indicated that miR-200b,
which is downregulated as a result of hypermethylation in its
promoter, interacted synergistically with target genes of DNMT3a,
DNMT3b and PTEN, but antagonistically with DNMT1. In the PCa
patient group, there was a significant association between high
miR-200a and high DNMT1 expression. The current findings indicate
that downregulated miR-200b in Met-PCa suppresses the PTEN gene
and, conversely, stimulates the DNMT1 gene. In the control group,
no significant association was found between any epi-miRNAs and
target genes investigated. This result indicates that the altered
expression of gene-regulating epi-miRNAs serves an active role in
the etiopathogenesis of PCa, and that these epi-miRNAs may
represent potential biomarker candidates.
The complex association between miRNAs and
epigenetic signature is an important milestone for monitoring gene
expression profiles in cancer. Notably, epi-miR-epi trilateral
regulatory feedback associations between miRNAs and epigenetic
patterns can target epigenetic modifying enzymes (37). To the best of our knowledge, the
present study was the first analyzing the signaling relationship
between epi-miRs and target genes, which is associated with
prognostic effects of miR-34b/c, miR-148a, mir-152 and miR-200a/b
epi-miRNAs and DNMTs, PTEN and NKX3.1, in the local and metastatic
stages of prostate cancer development. Examing PCa cell lines for
functional role of these miRNAs and target genes in relation to PCa
will help clarify this association.
In summary, miR-148a, miR-152 and miR-200b targeting
DNMT1/PTEN expression levels may represent powerful and distinctive
biomarker candidates, and serve as predictive, prognostic and
therapeutic targets, particularly in patients with advanced stage
PCa. Further studies are needed to explain the precise detailed
interaction of PTEN with DNMT1 in PCa in the pattern of tumor
suppressor-miR, onco-miR and/or metasta-miR. The current findings
are novel and pioneering in that they highlight the relative and
holistic expression profiles of epi-miRNAs and target mRNAs in the
diagnostic and prognostic process of PCa. The present results may
lead the way for other studies with larger cohorts.
Acknowledgements
Not applicable.
Funding
The present study was conducted with financial
support from the Gazi University Research Fund (project code no.
01/2020-19).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
VG and EK conceived, designed and performed the
research. EK interpreted experimental data. Statistical analysis
was performed by VG. VG and EK confirm the authenticity of all the
raw data. Clinical data analysis and sample selection were
performed by EK, CYB and SS. EK wrote the manuscript. All authors
read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Gazi University Faculty of Medicine, Ankara, Turkey
(approval no. G.Ü.E.T-686). All patients provided written informed
consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
Authors' information
The author ORCID IDs are as follows: Dr Venhar
Gurbuz, 0000-0002-9777-5173; Professor Sinan Sozen,
0000-0002-2573-3927; Professor Cenk Y. Bilen 0000-0003-2770-7762;
and Professor Ece Konac 0000-0001-5129-2515.
References
|
1
|
Stahl M, Kohrman N, Gore SD, Kim TK,
Zeidan AM and Prebet T: Epigenetics in cancer: A hematological
perspective. PLoS Genet. 12:e10061932016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malik SS, Batool R, Masood N and Yasmin A:
Risk factors for prostate cancer: A multifactorial case-control
study. Curr Probl Cancer. 42:337–343. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pandareesh MD, Kameshwar VH and Byrappa
KK: Prostate carcinogenesis: Insights in relation to epigenetics
and inflammation. Endocr Metab Immune Disord Drug Targets.
21:253–267. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prcic A, Begic E and Hiros M: Usefulness
of total PSA value in prostate diseases diagnosis. Acta Inform Med.
24:156–161. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bickers B and Aukim-Hastie C: New
molecular biomarkers for the prognosis and management of prostate
cancer-the post PSA era. Anticancer Res. 29:3289–3298.
2009.PubMed/NCBI
|
|
6
|
Matin F, Jeet V, Moya L, Selth LA,
Chambers S; Australian Prostate Cancer BioResource, ; Clements JA
and Batra J: A plasma biomarker panel of four MicroRNAs for the
diagnosis of prostate cancer. Sci Rep. 8:66532018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Filella X, Fernández-Galan E, Bonifacio RF
and Foj L: Emerging biomarkers in the diagnosis of prostate cancer.
Pharmgenomics Pers Med. 11:83–94. 2018.PubMed/NCBI
|
|
8
|
Sharma S, Kelly TK and Jones PA:
Epigenetics in cancer. Carcinogenesis. 31:27–36. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramassone A, Pagotto S, Veronese A and
Visone R: Epigenetics and MicroRNAs in cancer. Int J Mol Sci.
19:4592018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bosutti A, Zanconati F, Grassi G, Dapas B,
Passamonti S and Scaggiante B: Epigenetic and miRNAs dysregulation
in prostate cancer: The role of nutraceuticals. Anticancer Agents
Med Chem. 16:1385–1402. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lai X, Eberhardt M, Schmitz U and Vera J:
Systems biology-based investigation of cooperating microRNAs as
monotherapy or adjuvant therapy in cancer. Nucleic Acids Res.
47:7753–7766. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pesta M, Klecka J, Kulda V, Topolcan O,
Hora M, Eret V, Ludvikova M, Babjuk M, Novak K, Stolz J and Holubec
L: Importance of miR-20a expression in prostate cancer tissue.
Anticancer Res. 30:3579–3583. 2010.PubMed/NCBI
|
|
13
|
Brase JC, Johannes M, Schlomm T, Fälth M,
Haese A, Steuber T, Beissbarth T, Kuner R and Sültmann H:
Circulating miRNAs are correlated with tumor progression in
prostate cancer. Int J Cancer. 128:608–616. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Watahiki A and Wang Y, Morris J, Dennis K,
O'Dwyer HM, Gleave M, Gout PW and Wang Y: MicroRNAs associated with
metastatic prostate cancer. PLoS One. 6:e249502011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Al-Kafaji G, Said HM, Alam MA and Al Naieb
ZT: Blood-based microRNAs as diagnostic biomarkers to discriminate
localized prostate cancer from benign prostatic hyperplasia and
allow cancer-risk stratification. Oncol Lett. 16:1357–1365.
2018.PubMed/NCBI
|
|
16
|
Bhagirath D, Yang TL, Bucay N, Sekhon K,
Majid S, Shahryari V, Dahiya R, Tanaka Y and Saini S: microRNA-1246
is an exosomal biomarker for aggressive prostate cancer. Cancer
Res. 78:1833–1844. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Paziewska A, Mikula M, Dabrowska M,
Kulecka M, Goryca K, Antoniewicz A, Dobruch J, Borowka A, Rutkowski
P and Ostrowski J: Candidate diagnostic miRNAs that can detect
cancer in prostate biopsy. Prostate. 78:178–185. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang M, Wang F and Zhang X: miRNA-627
inhibits cell proliferation and cell migration, promotes cell
apoptosis in prostate cancer cells through upregulating MAP3K1,
PTPRK and SRA1. Int J Clin Exp Pathol. 11:255–261. 2018.PubMed/NCBI
|
|
19
|
Tu J, Peng Q, Shen Y, Hong Y, Zhu J, Feng
Z, Zhou P, Fan S, Zhu Y and Zhang Y: Identification of biomarker
microRNA-mRNA regulatory pairs for predicting the docetaxel
resistance in prostate cancer. J Cancer. 10:5469–5482. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Bi CW, Zhang GY, Bai Y, Zhao B and Yang H:
Increased expression of miR-153 predicts poor prognosis for
patients with prostate cancer. Medicine (Baltimore). 98:e16705.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lynch SM, O'Neill KM, McKenna MM, Walsh CP
and McKenna DJ: Regulation of miR-200c and miR-141 by methylation
in prostate cancer. Prostate. 76:1146–1159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Daniunaite K, Dubikaityte M, Gibas P,
Bakavicius A, Lazutka JM, Ulys A, Jankevicius F and Jarmalaite S:
Clinical significance of miRNA host gene promoter methylation in
prostate cancer. Hum Mol Genet. 26:2451–2461. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Torres-Ferreira J, Ramalho-Carvalho J,
Gomez A, Menezes FD, Freitas R, Oliveira J, Antunes L, Bento MJ,
Esteller M, Henrique R and Jerónimo C: miR-193b promoter
methylation accurately detects prostate cancer in urine sediments
and miR-34b/c or miR-129-2 promoter methylation define subsets of
clinically aggressive tumors. Mol Cancer. 16:262017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Barros-Silva D, Costa-Pinheiro P, Duarte
H, Sousa EJ, Evangelista AF, Graça I, Carneiro I, Martins AT,
Oliveira J, Carvalho AL, et al: MicroRNA-27a-5p regulation by
promoter methylation and MYC signaling in prostate carcinogenesis.
Cell Death Dis. 9:1672018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gurbuz V, Kiliccioglu I, Dikmen AU, Bilen
CY, Sozen S and Konac E: Comparative analysis of epi-miRNA
expression levels in local/locally advanced and metastatic prostate
cancer patients. Gene. 758:1449632020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lyko F: The DNA methyltransferase family:
A versatile toolkit for epigenetic regulation. Nat Rev Genet.
19:81–92. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Subramaniam D, Thombre R, Dhar A and Anant
S: DNA methyltransferases: A novel target for prevention and
therapy. Front Oncol. 4:802014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang J, Yang C, Wu C, Cui W and Wang L:
DNA methyltransferases in cancer: Biology, paradox, aberrations,
and targeted therapy. Cancers (Basel). 12:21232020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Maehama T, Taylor GS and Dixon JE: PTEN
and myotubularin: Novel phosphoinositide phosphatases. Annu Rev
Biochem. 70:247–279. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Robinson D, Van Allen EM, Wu YM, Schultz
N, Lonigro RJ, Mosquera JM, Montgomery B, Taplin ME, Pritchard CC,
Attard G, et al: Integrative clinical genomics of advanced prostate
cancer. Cell. 162:4542015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sun J, Li S, Wang F, Fan C and Wang J:
Identification of key pathways and genes in PTEN mutation prostate
cancer by bioinformatics analysis. BMC Med Genet. 20:1912019.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lei Q, Jiao J, Xin L, Chang CJ, Wang S,
Gao J, Gleave ME, Witte ON, Liu X and Wu H: NKX3.1 stabilizes p53,
inhibits AKT activation, and blocks prostate cancer initiation
caused by PTEN loss. Cancer Cell. 9:367–378. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bowen C, Ostrowski MC, Leone G and Gelmann
EP: Loss of PTEN accelerates NKX3.1 degradation to promote prostate
cancer progression. Cancer Res. 79:4124–4134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gurel B, Ali TZ, Montgomery EA, Begum S,
Hicks J, Goggins M, Eberhart CG, Clark DP, Bieberich CJ, Epstein JI
and Marzo AM: NKX3.1 as a marker of prostatic origin in metastatic
tumors. Am J Surg Pathol. 34:1097–1105. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shivakumar M, Lee Y, Bang L, Garg T, Sohn
KA and Kim D: Identification of epigenetic interactions between
miRNA and DNA methylation associated with gene expression as
potential prognostic markers in bladder cancer. BMC Med Genomics.
10 (Suppl 1):S302017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Memari F, Joneidi Z, Taheri B, Aval SF,
Roointan A and Zarghami N: Epigenetics and Epi-miRNAs: Potential
markers/therapeutics in leukemia. Biomed Pharmacother.
106:1668–1677. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Arif K, Elliott E, Haupt L and Griffiths
L: Regulatory mechanisms of epigenetic miRNA relationships in human
cancer and potential as therapeutic targets. Cancers (Basel).
12:29222020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fabbri M, Garzon R, Cimmino A, Liu Z,
Zanesi N, Callegari E, Liu S, Alder H, Costinean S,
Fernandez-Cymering C, et al: MicroRNA-29 family reverts aberrant
methylation in lung cancer by targeting DNA methyltransferases 3A
and 3B. Proc Natl Acad Sci USA. 104:15805–15810. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reale E, Taverna D, Cantini L, Martignetti
L, Osella M, De Pittà C, Virga F, Orso F and Caselle M:
Investigating the epi-miRNome: Identification of epi-miRNAs using
transfection experiments. Epigenomics. 11:1581–1599. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Misso G, Di Martino MT, De Rosa G, Farooqi
AA, Lombardi A, Campani V, Zarone MR, Gullà A, Tagliaferri P,
Tassone P and Caraglia M: Mir-34: A new weapon against cancer? Mol
Ther Nucleic Acids. 3:e1942014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tarasov V, Jung P, Verdoodt B, Lodygin D,
Epanchintsev A, Menssen A, Meister G and Hermeking H: Differential
regulation of microRNAs by p53 revealed by massively parallel
sequencing: MiR-34a is a p53 target that induces apoptosis and
G1-arrest. Cell Cycle. 6:1586–1593. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Majid S, Dar AA, Saini S, Shahryari V,
Arora S, Zaman MS, Chang I, Yamamura S, Tanaka Y, Chiyomaru T, et
al: miRNA-34b inhibits prostate cancer through demethylation,
active chromatin modifications, and AKT pathways. Clin Cancer Res.
19:73–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vogt M, Munding J, Grüner M, Liffers ST,
Verdoodt B, Hauk J, Steinstraesser L, Tannapfel A and Hermeking H:
Frequent concomitant inactivation of miR-34a and miR-34b/c by CpG
methylation in colorectal, pancreatic, mammary, ovarian,
urothelial, and renal cell carcinomas and soft tissue sarcomas.
Virchows Arch. 458:313–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Li Y, Deng X, Zeng X and Peng X: The role
of Mir-148a in cancer. J Cancer. 7:1233–1241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hamilton MP, Rajapakshe KI, Bader DA,
Cerne JZ, Smith EA, Coarfa C, Hartig SM and McGuire SE: The
landscape of microRNA targeting in prostate cancer defined by
AGO-PAR-CLIP. Neoplasia. 18:356–370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yu B, Lv X, Su L, Li J, Yu Y, Gu Q, Yan M,
Zhu Z and Liu B: MiR-148a functions as a tumor suppressor by
targeting CCK-BR via inactivating STAT3 and akt in human gastric
cancer. PLoS One. 11:e01589612016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Guo SL, Peng Z, Yang X, Fan KJ, Ye H, Li
ZH, Wang Y, Xu XL, Li J, Wang YL, et al: miR-148a promoted cell
proliferation by targeting p27 in gastric cancer cells. Int J Biol
Sci. 7:567–574. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Walter BA, Valera VA, Pinto PA and Merino
MJ: Comprehensive microRNA profiling of prostate cancer. J Cancer.
4:350–357. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Porkka KP, Pfeiffer MJ, Waltering KK,
Vessella RL, Tammela TL and Visakorpi T: MicroRNA expression
profiling in prostate cancer. Cancer Res. 67:6130–6135. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Kim J, Zhang Y, Skalski M, Hayes J, Kefas
B, Schiff D, Purow B, Parsons S, Lawler S and Abounader R:
microRNA-148a is a prognostic oncomiR that targets MIG6 and BIM to
regulate EGFR and apoptosis in glioblastoma. Cancer Res.
74:1541–1553. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Dybos SA, Flatberg A, Halgunset J, Viset
T, Rolfseng T, Kvam S and Skogseth H: Increased levels of serum
miR-148a-3p are associated with prostate cancer. APMIS.
126:722–731. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Szczyrba J, Löprich E, Wach S, Jung V,
Unteregger G, Barth S, Grobholz R, Wieland W, Stöhr R, Hartmann A,
et al: The MicroRNA profile of prostate carcinoma obtained by deep
sequencing. Mol Cancer Res. 8:529–538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhu C, Li J, Ding Q, Cheng G, Zhou H, Tao
L, Cai H, Li P, Cao Q, Ju X, et al: miR-152 controls migration and
invasive potential by targeting TGFα in prostate cancer cell lines.
Prostate. 73:1082–1089. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Theodore SC, Davis M, Zhao F, Wang H, Chen
D, Rhim J, Dean-Colomb W, Turner T, Ji W, Zeng G, et al: MicroRNA
profiling of novel African American and Caucasian prostate cancer
cell lines reveals a reciprocal regulatory relationship of miR-152
and DNA methyltranferase 1. Oncotarget. 5:3512–3525. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Li B, Xie Z and Li B: miR-152 functions as
a tumor suppressor in colorectal cancer by targeting PIK3R3. Tumor
Biol. 37:10075–10084. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Braconi C, Huang N and Patel T:
MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor
suppressor gene expression by interleukin-6 in human malignant
cholangiocytes. Hepatology. 51:881–890. 2010.PubMed/NCBI
|
|
57
|
Huang S, Li X and Zhu H: MicroRNA-152
targets phosphatase and tensin homolog to inhibit apoptosis and
promote cell migration of nasopharyngeal carcinoma cells. Med Sci
Monit. 22:4330–4337. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen H, Liu H, Zou H, Chen R, Dou Y, Sheng
S, Dai S, Ai J, Melson J, Kittles RA, et al: Evaluation of plasma
miR-21 and miR-152 as diagnostic biomarkers for common types of
human cancers. J Cancer. 7:490–499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Moya L, Meijer J, Schubert S, Matin F and
Batra J: Assessment of miR-98-5p, miR-152-3p, miR-326 and miR-4289
expression as biomarker for prostate cancer diagnosis. Int J Mol
Sci. 20:11542019. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Carter JV, O'Brien SJ, Burton JF, Oxford
BG, Stephen V, Hallion J, Bishop C, Galbraith NJ, Eichenberger MR,
Sarojini H, et al: The microRNA-200 family acts as an oncogene in
colorectal cancer by inhibiting the tumor suppressor RASSF2. Oncol
Lett. 18:3994–4007. 2019.PubMed/NCBI
|
|
61
|
Yang R, Xu J, Hua X, Tian Z, Xie Q, Li J,
Jiang G, Cohen M, Sun H and Huang C: Overexpressed miR-200a
promotes bladder cancer invasion through direct regulating
Dicer/miR-16/JNK2/MMP-2 axis. Oncogene. 39:1983–1996. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Schliekelman MJ, Gibbons DL, Faca VM,
Creighton CJ, Rizvi ZH, Zhang Q, Wong CH, Wang H, Ungewiss C, Ahn
YH, et al: Targets of the tumor suppressor miR-200 in regulation of
the epithelial-mesenchymal transition in cancer. Cancer Res.
71:7670–7682. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wong CM, Wei L, Au SL, Fan DN, Zhou Y,
Tsang FH, Law CT, Lee JM, He X, Shi J, et al: MiR-200b/200c/429
subfamily negatively regulates Rho/ROCK signaling pathway to
suppress hepatocellular carcinoma metastasis. Oncotarget.
6:13658–13670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Osella M, Riba A, Testori A, Corà D and
Caselle M: Interplay of microRNA and epigenetic regulation in the
human regulatory network. Front Genet. 5:3452014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Nakagawa T, Kanai Y, Ushijima S, Kitamura
T, Kakizoe T and Hirohashi S: DNA hypermethylation on multiple CpG
islands associated with increased DNA methyltransferase DNMT1
protein expression during multistage urothelial carcinogenesis. J
Urol. 173:1767–1771. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Nakagawa T, Kanai YAE, Saito Y, Kitamura
T, Kakizoe T and Hirohashi S: Increased DNA methyltransferase 1
protein expression in human transitional cell carcinoma of the
bladder. J Urol. 170:2463–2466. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Patra SK, Patra A, Zhao H and Dahiya R:
DNA methyltransferase and demethylase in human prostate cancer. Mol
Carcinog. 33:163–171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang W and Xu J: DNA methyltransferases
and their roles in tumorigenesis. Biomark Res. 5:12017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Qi D, Li J, Que B, Su J, Li M, Zhang C,
Yang M, Zhou G and Ji W: Long non-coding RNA DBCCR1-003 regulate
the expression of DBCCR1 via DNMT1 in bladder cancer. Cancer Cell
Int. 16:812016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Roscigno G, Quintavalle C, Donnarumma E,
Puoti I, Diaz-Lagares A, Iaboni M, Fiore D, Russo V, Todaro M,
Romano G, et al: MiR-221 promotes stemness of breast cancer cells
by targeting DNMT3b. Oncotarget. 7:580–592. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Pang Y, Liu J, Li X, Xiao G, Wang H, Yang
G, Li Y, Tang SC, Qin S, Du N, et al: MYC and DNMT3A-mediated DNA
methylation represses microRNA-200b in triple negative breast
cancer. J Cell Mol Med. 22:6262–6274. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ma HS, Wang EL, Xu WF, Yamada S, Yoshimoto
K, Qian ZR, Shi L, Liu LL and Li XH: Overexpression of DNA
(Cytosine-5)-methyltransferase 1 (DNMT1) And DNA
(Cytosine-5)-methyltransferase 3A (DNMT3A) is associated with
aggressive behavior and hypermethylation of tumor suppressor genes
in human pituitary adenomas. Med Sci Monit. 24:4841–4850. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li M, Wang Y, Song Y, Bu R, Yin B, Fei X,
Guo Q and Wu B: Aberrant DNA methyltransferase 1 expression in
clear cell renal cell carcinoma development and progression. Chin J
Cancer Res. 26:371–381. 2014.PubMed/NCBI
|
|
74
|
Graça I, Sousa EJ, Costa-Pinheiro P,
Vieira FQ, Torres-Ferreira J, Martins MG, Henrique R and Jerónimo
C: Anti-neoplastic properties of hydralazine in prostate cancer.
Oncotarget. 5:5950–5964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Jagadeesh S, Sinha S, Pal BC, Bhattacharya
S and Banerjee PP: Mahanine reverses an epigenetically silenced
tumor suppressor gene RASSF1A in human prostate cancer cells.
Biochem Biophys Res Commun. 362:212–217. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Agarwal S, Amin KS, Jagadeesh S, Baishay
G, Rao PG, Barua NC, Bhattacharya S and Banerjee PP: Mahanine
restores RASSF1A expression by down-regulating DNMT1 and DNMT3B in
prostate cancer cells. Mol Cancer. 12:992013. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Le Magnen C, Virk RK, Dutta A, Kim JY,
Panja S, Lopez-Bujanda ZA, Califano A, Drake CG, Mitrofanova A and
Abate-Shen C: Cooperation of loss of NKX3.1 and inflammation in
prostate cancer initiation. Dis Model Mech. 11:dmm0351392018.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shiina M, Hashimoto Y, Kato T, Yamamura S,
Tanaka Y, Majid S, Saini S, Varahram S, Kulkarni P, Dasgupta P, et
al: Differential expression of miR-34b and androgen receptor
pathway regulate prostate cancer aggressiveness between
African-Americans and caucasians. Oncotarget. 8:8356–8368. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chamani F, Sadeghizadeh M, Masoumi M and
Babashah S: Evaluation of MiR-34 family and DNA methyltransferases
1, 3A, 3B gene expression levels in hepatocellular carcinoma
following treatment with dendrosomal nanocurcumin. Asian Pac J
Cancer Prev. 17:219–224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Sengupta D, Deb M and Patra SK:
Antagonistic activities of miR-148a and DNMT1: Ectopic expression
of miR-148a impairs DNMT1 mRNA and dwindle cell proliferation and
survival. Gene. 660:68–79. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Duursma AM, Kedde M, Schrier M, le Sage C
and Agami R: miR-148 targets human DNMT3b protein coding region.
RNA. 14:872–877. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hua D, Mo F, Ding D, Li L, Han X, Zhao N,
Foltz G, Lin B, Lan Q and Huang Q: A catalogue of glioblastoma and
brain MicroRNAs identified by deep sequencing. OMICS. 16:690–699.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Ma W, Zhang X, Chai J, Chen P, Ren P and
Gong M: Circulating miR-148a is a significant diagnostic and
prognostic biomarker for patients with osteosarcoma. Tumour Biol.
35:12467–12472. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Zhang H, Wang Y, Xu T, Li C, Wu J, He Q,
Wang G, Ding C, Liu K, Tang H and Ji F: Increased expression of
microRNA-148a in osteosarcoma promotes cancer cell growth by
targeting PTEN. Oncol Lett. 12:3208–3214. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yuan K, Lian Z, Sun B, Clayton MM, Ng IOL
and Feitelson MA: Role of miR-148a in hepatitis B associated
hepatocellular carcinoma. PLoS One. 7:e353312012. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Ramalho-Carvalho J, Gonçalves CS, Graça I,
Bidarra D, Pereira-Silva E, Salta S, Godinho MI, Gomez A, Esteller
M, Costa BM, et al: A multiplatform approach identifies miR-152-3p
as a common epigenetically regulated onco-suppressor in prostate
cancer targeting TMEM97. Clin Epigenetics. 10:402018. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Collino F, Deregibus MC, Bruno S, Sterpone
L, Aghemo G, Viltono L, Tetta C and Camussi G: Microvesicles
derived from adult human bone marrow and tissue specific
mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS
One. 5:e118032010. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Jiang X, Du L, Wang L, Li J, Liu Y, Zheng
G, Qu A, Zhang X, Pan H, Yang Y and Wang C: Serum microRNA
expression signatures as novel noninvasive biomarkers for
prediction and prognosis of muscle-invasive bladder cancer.
Oncotarget. 7:36733–36742. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Dudziec E, Miah S, Choudhry HM, Owen HC,
Blizard S, Glover M, Hamdy FC and Catto JW: Hypermethylation of CpG
islands and shores around specific microRNAs and mirtrons is
associated with the phenotype and presence of bladder cancer. Clin
Cancer Res. 17:1287–1296. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Kim J, Yao F, Xiao Z, Sun Y and Ma L:
MicroRNAs and metastasis: Small RNAs play big roles. Cancer
Metastasis Rev. 37:5–15. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Pan J, Ding M, Xu K, Yang C and Mao LJ:
Exosomes in diagnosis and therapy of prostate cancer. Oncotarget.
8:97693–97700. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Turchinovich A, Samatov T, Tonevitsky A
and Burwinkel B: Circulating miRNAs: Cell-cell communication
function? Front Genet. 4:1192013. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Pang Y, Young CY and Yuan H: MicroRNAs and
prostate cancer. Acta Biochim Biophys Sin (Shanghai). 42:363–369.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Guo F, Kerrigan BC, Yang D, Hu L,
Shmulevich I, Sood AK, Xue F and Zhang W: Post-transcriptional
regulatory network of epithelial-to-mesenchymal and
mesenchymal-to-epithelial transitions. J Hematol Oncol. 7:192014.
View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Wu Q, Lu RL, Li JX and Rong LJ: MiR-200a
and miR-200b target PTEN to regulate the endometrial cancer cell
growth in vitro. Asian Pac J Trop Med. 10:498–502. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Yoneyama K, Ishibashi O, Kawase R, Kurose
K and Takeshita T: miR-200a, miR-200b and miR-429 are onco-miRs
that Target the PTEN gene in endometrioid endometrial carcinoma.
Anticancer Res. 35:1401–1410. 2015.PubMed/NCBI
|
|
97
|
Suo HB, Zhang KC and Zhao J: MiR-200a
promotes cell invasion and migration of ovarian carcinoma by
targeting PTEN. Eur Rev Med Pharmacol Sci. 22:4080–4089.
2018.PubMed/NCBI
|
|
98
|
Liu J, Zhang X, Huang Y, Zhang Q, Zhou J,
Zhang X and Wang X: miR-200b and miR-200c co-contribute to the
cisplatin sensitivity of ovarian cancer cells by targeting DNA
methyltransferases. Oncol Lett. 17:1453–1460. 2019.PubMed/NCBI
|
|
99
|
Zeng X, Qu X, Zhao C, Xu L, Hou K, Liu Y,
Zhang N, Feng J, Shi S, Zhang L, et al: FEN1 mediates miR-200a
methylation and promotes breast cancer cell growth via MET and EGFR
signaling. FASEB J. 33:10717–10730. 2019. View Article : Google Scholar : PubMed/NCBI
|