Introduction
Gastric cancer originates from the gastric mucosa
and is one of the most common malignancies worldwide (1). Gastric cancer is associated with high
mortality rates, and as per 2018 GLOBOCAN data, gastric cancer
constitutes 8.2% of all cancer-related mortalities, which continues
to increase the burden on the global public health system (2). The main risk factors of gastric cancer
include a poor diet, a sedentary lifestyle and Helicobacter
pylori infection (3). Despite
recent advancements in treatment strategies, the 5-year survival
rate of patients with gastric cancer remains <10%, and
metastasis of gastric cancer cells is the main cause of mortality
(4,5).
Cisplatin (DDP) is a common anticancer drug used to
treat gastric cancer (6). Most
gastric cancer cells are highly resistant to DDP and the further
development of drug resistance limits the cytotoxic effect of DDP
on gastric cancer cells (7). Thus,
it is important to investigate the molecular mechanisms underlying
drug resistance in gastric cancer cells to provide novel strategies
for the clinical treatment of this disease. C-terminal-binding
protein 1 (CTBP1) is expressed in different types of cancer, such
as lung adenocarcinoma, endometrial carcinoma and glioma (8–10).
Previous studies have reported that CTBP1 is the transcriptional
corepressor of several tumor suppressor genes (11,12), and
various physiological processes, such as proliferation, apoptosis
and epithelial-to-mesenchymal transition (EMT), in different types
of cells, such as cancer cells and cancer stem cells, are regulated
by CTBP1 (13). CTBP1 expression
affects cell proliferation, migration and invasion of different
types of cancer cells. For example, CTBP1 knockdown suppresses the
proliferation and invasion of breast cancer cells by repressing the
EMT process (14). Furthermore,
during the development of gastric cancer, microRNA (miR)-539-3p
inhibits the migration and invasion of gastric tumor cells by
suppressing CTBP1 expression (15).
Notably, high levels of CTBP1 can also promote the proliferation,
migration and invasion of gastric tumor cells (16). Importantly, CTBP1 is reported to
confer DDP resistance to breast cancer cells (17). However, whether CTBP1 could regulate
the DDP resistance of gastric cancer remains unknown.
DNA repair protein RAD51 homolog 1 (RAD51) is a
protein associated with DNA damage repair (18). However, RAD51 expression is also
associated with the development of different types of cancer, such
as ovarian, colorectal and breast cancer (19–21).
STAT5A/B inhibition increases the sensitivity of prostate cancer
cells to radiotherapy through inhibition of RAD51 and DNA repair
(22). In addition, CTBP1 can
enhance the resistance of breast cancer cells to DDP by activating
RAD51 expression (17). However,
whether RAD51 expression affects the resistance of gastric cancer
cells to DDP remains unclear. Thus, the present study aimed to
investigate the effects of CTBP1 and RAD51 on the DDP resistance of
gastric cancer cells.
Materials and methods
Cell culture and treatment
Human gastric cancer cell lines (AGS and HGC-27),
the corresponding DDP-resistant AGS and HGC-27 cell lines (AGS/DDP
and HGC-27/DDP) and the gastric mucosal epithelial cell line
(GES-1) were purchased from The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences. Cells were
maintained in Dulbecco's modified Eagle's medium (Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.). AGS/DDP and HGC-27/DDP cells were maintained in
the same culture medium supplemented with various concentrations of
DDP (2, 4, 6, 8 and 10 µg/ml; Thermo Fisher Scientific, Inc.). All
cells were cultured at 37°C in a humidified atmosphere with 5%
CO2. The usage of these cells was in accordance with the
previous study (23).
Cell transfection
A total of two CTBP1 small interfering (si)RNAs
(siCTBP1-1 and siCTBP1-2; 50 nM) and the negative control (siNC; 50
nM), CTBP1 overexpressing pcDNA3.1 plasmid (pc-CTBP1; 50 nM), RAD51
overexpressing pcDNA3.1 plasmid (pc-RAD51; 50 nM) and empty control
plasmid (pcDNA3.1; 50 nM) were purchased from Shanghai GeneChem
Co., Ltd. Cells were plated into 6-well plates (1×106
cells per well), and transfection was performed when cells in the
logarithmic growth phase reached 80% confluence. Transfection was
performed using Lipofectamine® 2000 reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) at 37°C for 48 h, according to the
manufacturer's instructions. Transfection efficiency was assessed
by western blotting after 48 h.
Cell viability assay
The Cell Counting Kit-8 (CCK-8; Shanghai Yi Sheng
Biotechnology Co., Ltd.) assay was performed to detect cell
viability. Cell suspensions (2×103 cells) were plated
into 96-well plates. Following adhesion of cells (90% confluency),
CCK-8 reagent was diluted with the culture medium and added into
each well for 4 h. The absorbance at 450 nm was measured using a
microplate reader (Molecular Devices, LLC).
Colony formation assay
Cell suspensions were plated into 60-mm culture
dishes at a density of 300 cells/dish. Cells were cultured in the
incubator for 2 weeks, and subsequently fixed with 70% ethanol for
40 min at room temperature and stained with crystal violet (Thermo
Fisher Scientific, Inc.) for 5 min at room temperature. Groups of
>50 cells were considered a clone and were assessed using ImageJ
software (version 1.52r; National Institutes of Health). Stained
cells were observed under a light microscope (Olympus
Corporation).
Apoptosis analysis
Apoptosis was detected by flow cytometric analysis.
Cell suspensions (1×106) were washed three times with
PBS to clear residual FBS from the culture medium. Cells were
subsequently incubated with Annexin V-FITC and propidium iodide
(PI) using an Annexin V-FACS apoptosis detection kit (Beyotime
Institute of Biotechnology) at 37°C in humidified atmosphere for 1
h. Cells were washed three times with PBS, and apoptotic cells were
subsequently analyzed using a FACSAria flow cytometer (Thermo
Fisher Scientific, Inc.) with FlowJo software (version 10.0; FlowJo
LLC; BD Bioscience).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from 1×106 cells
using TRIzol® reagent (Thermo Fisher Scientific, Inc.),
and reverse transcribed into cDNA using a PrimeScript™ RT Reagent
kit (Takara Bio, Inc.) according to the manufacturer's protocol.
cDNA was subsequently amplified using an SYBR Green RT-PCR kit on
an Applied Biosystems 7500 Real-Time PCR system (Thermo Fisher
Scientific, Inc.) and relative expression levels were calculated
using the 2−ΔΔCq method (24). The following thermocycling conditions
were used for qPCR: Initial denaturation for 10 min at 95°C,
followed by 40 cycles of denaturation at 95°C for 15 sec and
annealing/extension at 55°C for 45 sec. GAPDH expression level was
used as a reference control. The following primer sequences were
used for qPCR: CTBP1 forward, 5′-CGACCCTTACTTGTCGGATGG−3′ and
reverse, 5′-TTGACGGTGAAGTCGTTGATG−3′; RAD51 forward,
5′-CTATGTAGCAAAGGGAATGGG−3′ and reverse,
5′-AAGCAGGTAGATGGTGAAGG-3′; and GAPDH forward,
5′-GGAGCGAGATCCCTCCAAAAT−3′ and reverse,
5′-GGCTGTTGTCATACTTCTCATGG-3′.
Western blotting
Total protein was extracted from 1×106
cells using RIPA lysis buffer (Beyotime Institute of
Biotechnology). Total protein was quantified using a bicinchoninic
acid assay (Beyotime Institute of Biotechnology) and proteins (40
µg protein/lane) were separated on 10% gels using SDS-PAGE. The
separated proteins were subsequently transferred onto PVDF
membranes and blocked with 5% skimmed milk powder in Tris-buffered
saline overnight at 4°C. The membranes were incubated with primary
antibodies against CTBP1 (cat. no. ab129181; 1:1,000; Abcam), RAD51
(cat. no. ab133534; 1:1,000; Abcam), Bax (cat. no. ab32503;
1:1,000; Abcam), Bcl-2 (cat. no. ab182858; 1:1,000; Abcam), cleaved
(c)-caspase-3 (cat. no. 9664T; 1:1,000; Cell Signaling Technology,
Inc.), caspase-3 (cat. no. 14220T; 1:1,000; Cell Signaling
Technology, Inc.), c-caspase-9 (cat. no. 20750S; 1:1,000; Cell
Signaling Technology, Inc.), caspase-9 (cat. no. 9508T; 1:1,000;
Cell Signaling Technology, Inc.) and GAPDH (cat. no. ab8245;
1:1,000; Abcam) overnight at 4°C. Following the primary incubation,
membranes were incubated with horseradish peroxidase-conjugated
goat anti-rabbit secondary antibody (cat. no. ab205718; 1:2,000;
Abcam) or anti-mouse horseradish peroxidase-conjugated secondary
antibody (cat. no. ab205719; 1:5,000; Abcam) at room temperature
for 2 h. Protein bands were visualized using Pierce ECL Western
Blotting Substrate (Thermo Fisher Scientific, Inc.). GAPDH was used
as the reference protein. Protein expression was quantified using
ImageJ software (version 1.41; National Institutes of Health).
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8.0 software (GraphPad Software, Inc.). All experiments were
performed in triplicate and data are presented as the mean ± SD.
One-way ANOVA followed by Tukey's post hoc test was used to compare
differences between multiple groups. Pearson's correlation
coefficient was used to assess the correlation between CTBP1 and
RAD51 mRNA expression. P<0.05 was considered to indicate a
statistically significant difference.
Results
CTBP1 expression is upregulated in
DDP-resistant gastric cancer cells
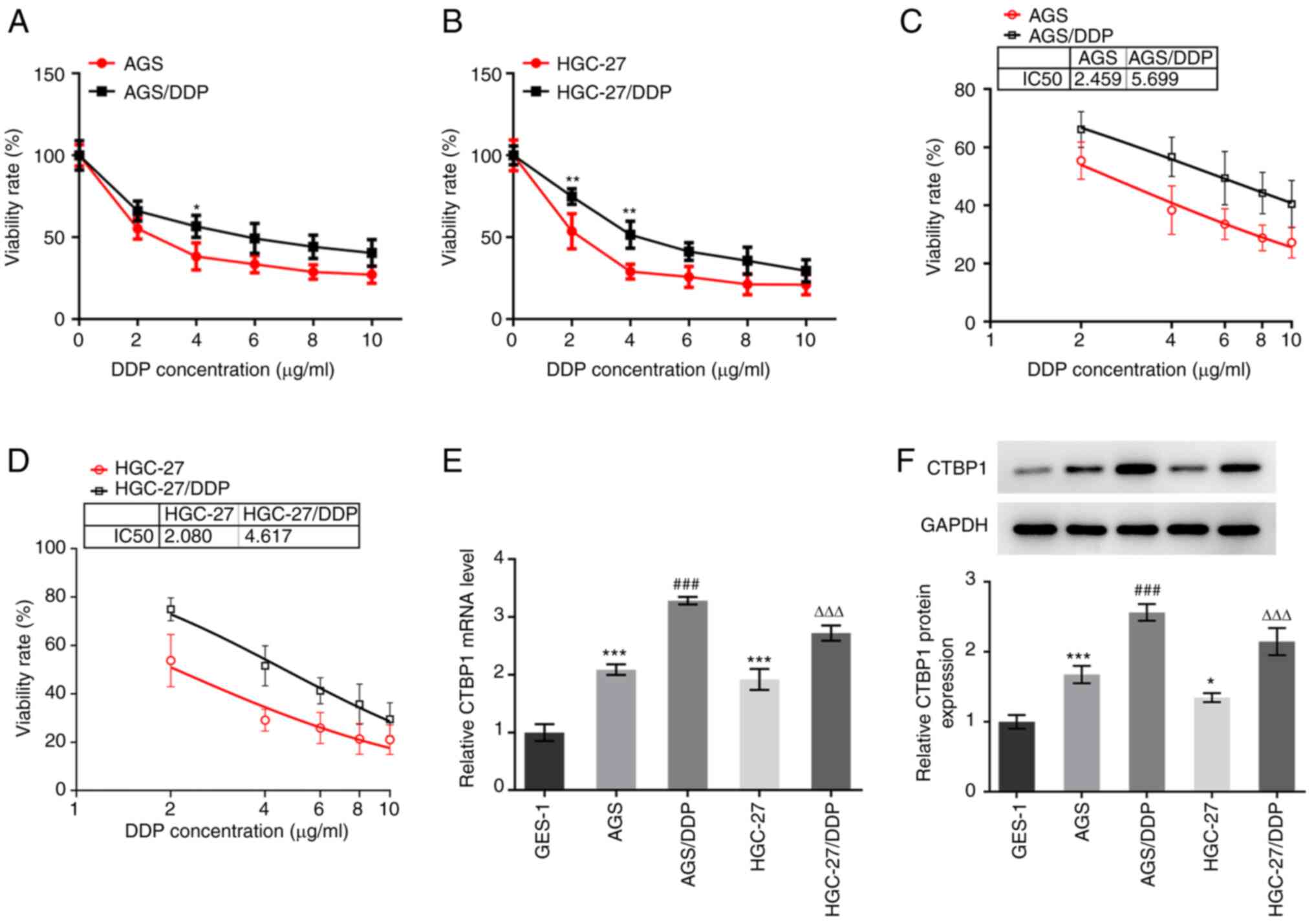
Drug resistance of gastric cancer cells was verified
using the CCK-8 viability assay. As demonstrated in Fig. 1A and B, the viability of
DDP-resistant gastric cancer cells, AGS/DDP (4 µg/ml) and
HGC-27/DDP (2 and 4 µg/ml), was higher compared with that of
gastric cancer cells, AGS and HGC-27. The IC50 values of
DDP in AGS/DDP and HGC-27/DDP cells were determined, which showed
that the IC50 of DDP was higher in AGS/DDP and
HGC-27/DDP cells compared with that in AGS and HGC-27 cells
(Fig. 1C and D). Subsequently, CTBP1
expression was detected in gastric cancer cells and normal gastric
mucosal epithelial cells. CTBP1 mRNA and protein expression levels
were significantly higher in AGS and HGC-27 cells compared with
GES-1 cells (Fig. 1E and F).
Notably, CTBP1 expression was higher in AGS/DDP and HGC-27/DDP
cells compared with expression in AGS and HGC-27 cells. Taken
together, these results suggested that CTBP1 expression is
significantly upregulated in DDP-resistant gastric cancer
cells.
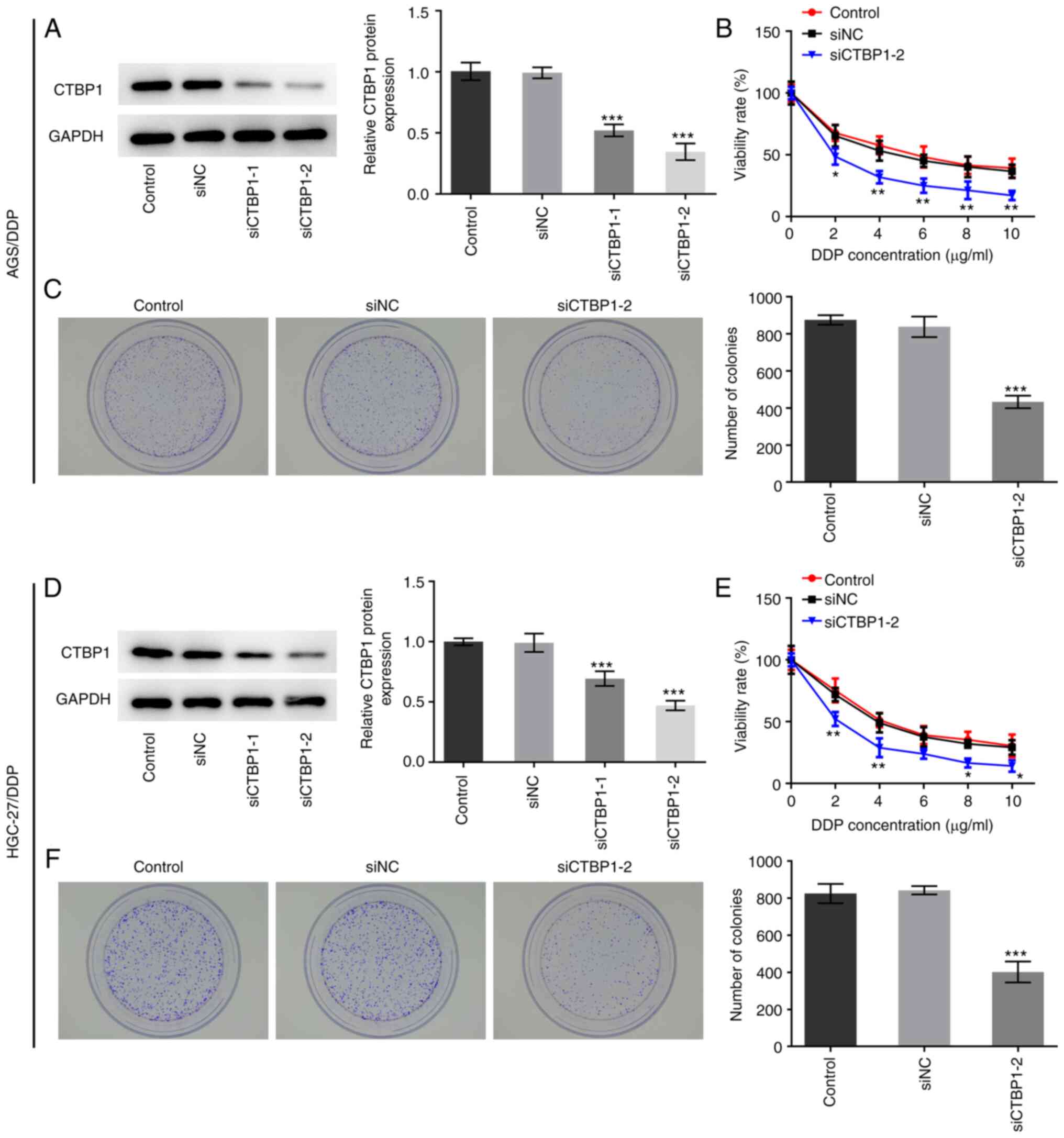
CTBP1 knockdown inhibits the
proliferation of DDP-resistant gastric cancer cells
To detect the effect of CTBP1 on the proliferation
of DDP-resistant gastric cancer cells, CTBP1 expression was
silenced by transfecting cells with siCTBP1-1 or siCTBP1-2. As
presented in Fig. 2A and D, CTBP1
protein expression levels decreased in AGS/DDP and HGC-27/DDP cells
following transfection. Transfection with siCTBP1-2 decreased CTBP1
expression to the greatest extent; therefore, siCTBP1-2 was
selected for subsequent experimentation. Results from the CCK-8
assay demonstrated that CTBP1 knockdown significantly decreased the
viability of AGS/DDP and HGC-27/DPP cells compared with that of the
respective siNC-transfected group (Fig.
2B and E). The inhibitory effects of CTBP1 knockdown on AGS and
HGC-27 cell viability are presented in Fig. S1. Similarly, the results of the
colony formation assay demonstrated that the number of cell
colonies in DDP-resistant AGS and HGC-27 cells significantly
decreased following CTBP1 knockdown (Fig. 2C and F). Collectively, these results
suggested that CTBP1 knockdown suppressed the proliferation of
cisplatin-resistant gastric cancer cells.
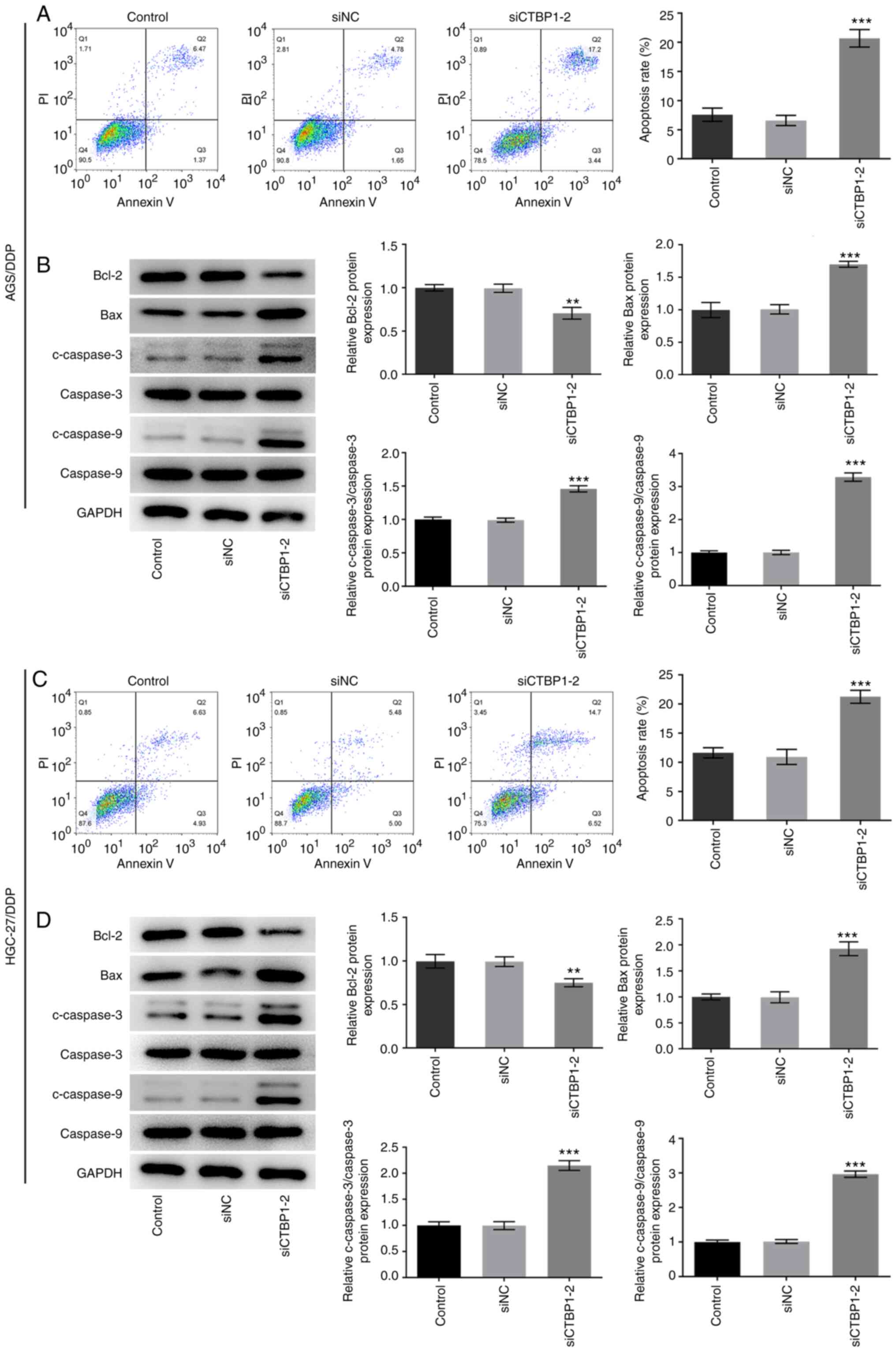
CTBP1 knockdown promotes the apoptosis
of DDP-resistant gastric cancer cells
The effect of CTBP1 on the apoptosis of
DDP-resistant gastric cancer cells was examined. The apoptotic
rates of AGS/DDP and HGC-27/DDP cells were significantly increased
following CTBP1 knockdown compared with the respective siNC group
(Fig. 3A and C, respectively). The
expression levels of the apoptosis-related proteins were detected
by western blotting. As presented in Fig. 3B and D, CTBP1 knockdown in AGS/DDP
and HGC-27/DDP cells significantly decreased Bcl-2 expression but
increased Bax, c-caspase-3 and c-caspase-9 expression compared with
the respective siNC group. Taken together, these results suggested
that CTBP1 knockdown accelerated the apoptosis of DDP-resistant
gastric cancer cells.
RAD51 overexpression abolishes the
anticancer effect of CTBP1 knockdown on DDP-resistant gastric
cancer cells
A previous study reported that CTBP1 expression
influences DDP resistance of breast cancer cells by activating
RAD51 expression (19). In the
present study, RT-qPCR and western blot analysis were used to
evaluate the expression of RAD51 in the aforementioned five cell
lines. RAD51 mRNA and protein expression levels were significantly
upregulated in the AGS and HGC-27 cells compared with expression
levels in the GES-1 cells (Fig. 4A and
B); in addition, RAD51 expression was higher in the AGS/DDP and
HGC-27/DDP cells compared with that in the AGS and HGC-27 cells.
Subsequently, the correlation between CTBP1 and RAD51 mRNA
expression levels was assessed, and a positive correlation was
revealed between CTBP1 and RAD51 mRNA expression (Fig. 4C). AGS/DPP cells exhibited higher
CTBP1 and RAD51 expression levels; therefore, this cell line was
selected to perform the subsequent experiments. RAD51 expression
was notably decreased in AGS/DDP cells following CTBP1 knockdown
(Fig. 4D). Following overexpression
of CTBP1, western blot analysis demonstrated that CTBP1 expression
was markedly increased in the overexpression group (Fig. 4E). As presented in Fig. 4F, elevated CTBP1 expression
significantly increased RAD51 expression.
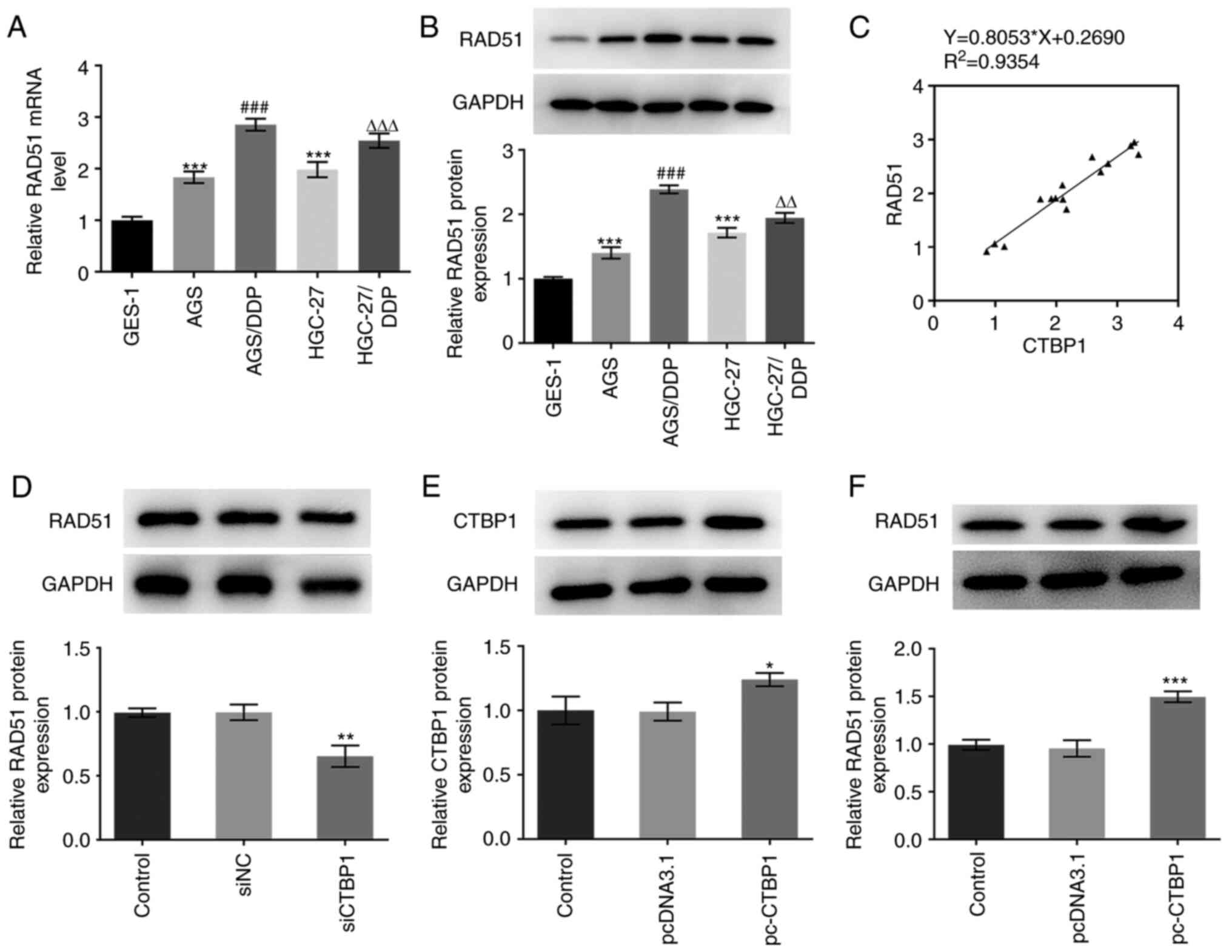 | Figure 4.CTBP1 upregulated RAD51 expression in
DDP-resistant gastric cancer cells. (A) RT-qPCR and (B) western
blot analysis were used to determine the expression levels of RAD51
mRNA and protein, respectively. ***P<0.001 vs. GES-1;
###P<0.001 vs. AGS; ∆∆P<0.01,
∆∆∆P<0.001 vs. HGC-27. (C) Pearson's correlation
analysis between CTBP1 and RAD51 mRNA levels in GES-1, AGS,
AGS/DDP, HGC-27 and HGC-27/DDP cells. (D) Western blot analysis was
performed to detect RAD51 protein expression in AGS/DDP cells
following CTBP1 knockdown. **P<0.01 vs. siNC. (E) Western blot
analysis was performed to detect CTBP1 protein expression in
AGS/DDP cells following transfection with pc-CTBP1. (F) Western
blot analysis was performed to detect RAD51 protein expression in
AGS/DDP cells following transfection with CTBP1 plasmid.
*P<0.05, ***P<0.001 vs. pcDNA3.1. CTBP1, C-terminal-binding
protein 1; DDP, cisplatin; NC, negative control; pc, pcDNA3.1
overexpression vector; RAD51, DNA repair protein RAD51 homolog 1;
si, small interfering RNA. |
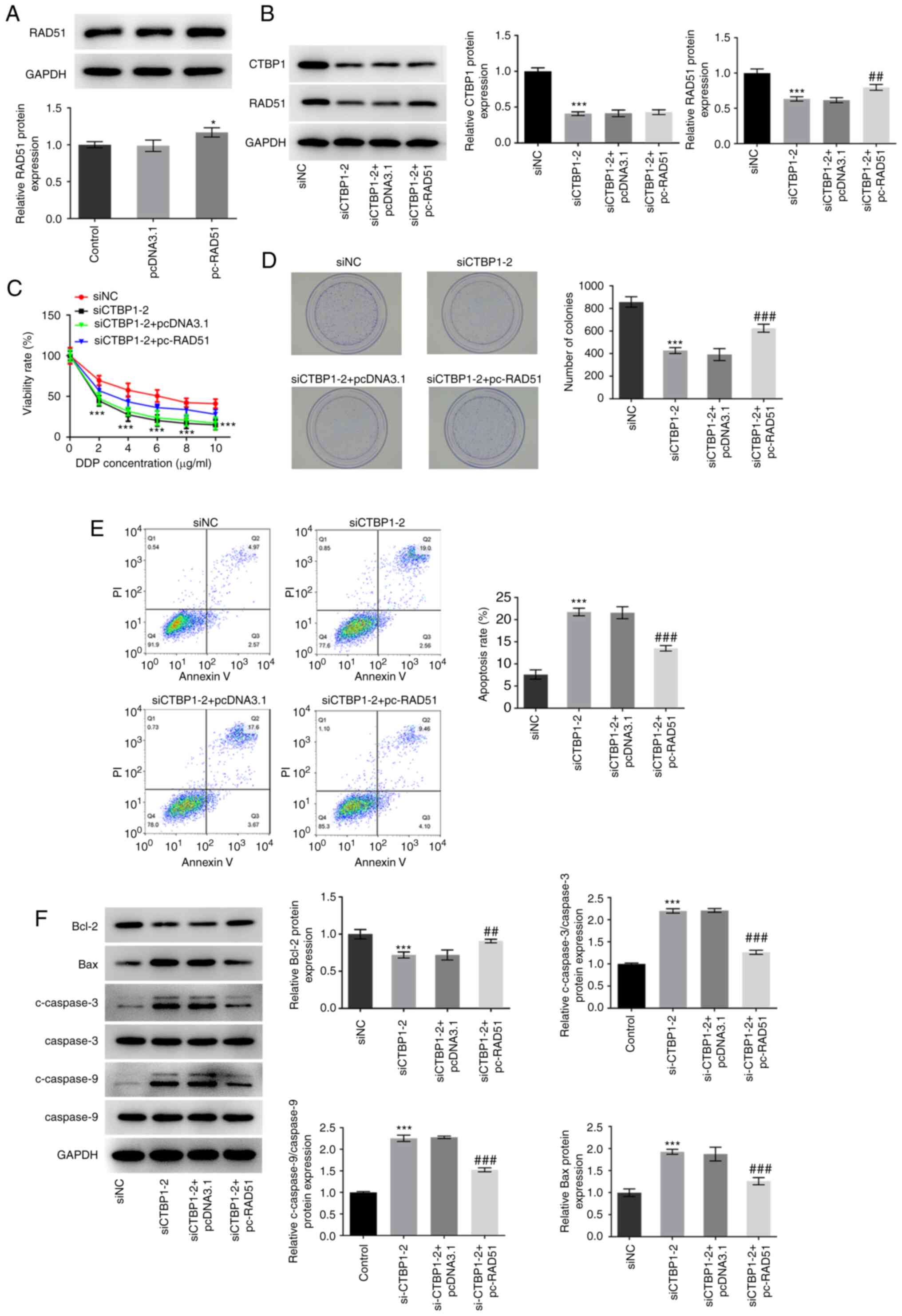
To further determine the association between CTBP1
and RAD51 in gastric cancer, RAD51 overexpressing plasmid was
transfected into AGS/DDP cells (Fig.
5A). A notably reduced CTBP1 protein expression level was
observed in the siCTBP1-2 group compared with the siNC group, and
there was no significant difference in CTBP1 expression between the
siCTBP1-2 + pcDNA3.1 and the siCTBP1-2 + pc-RAD51 groups (Fig. 5B). However, cells transfected with
siCTBP1-2 exhibited a significant downregulation in RAD51 protein
expression in comparison to the siNC group, which was partially
restored by co-transfection with pc-RAD51 (Fig. 5B). In addition, the results
demonstrated that overexpression of RAD51 alleviated the inhibitory
effects of siCTBP1 transfection on the viability and proliferation
of AGS/DDP cells (Fig. 5C and D,
respectively). Similarly, overexpression of RAD51 relieved CTBP1
knockdown-induced apoptosis of DDP-resistant AGS cells (Fig. 5E). Notably, Bcl-2 protein expression
significantly increased, whereas Bax, c-caspase-3 and c-caspase-9
expression levels decreased following overexpression of RAD51 in
CTBP1 knockdown AGS/DDP cells when compared with the siCTBP1-2 +
pcDNA3.1 group (Fig. 5F).
Collectively, these results suggested that CTBP1 may regulate DDP
resistance in gastric cancer cells by modulating RAD51
expression.
Discussion
Gastric cancer is a common malignancy of the
digestive system (2). Despite
advancements in treatment strategies, gastric cancer is associated
with high mortality rates (25),
which is predominantly due to the biological characteristics of
gastric cancer, such as the delayed clinical manifestations, and
the proliferation and metastasis of gastric cancer cells (26). Several anticancer drugs, such as DDP,
exert strong antitumoral effects on gastric cancer cells (27,28).
However, the enhanced resistance of gastric cancer cells to DDP and
other drugs gradually weaken the corresponding anticancer drug
treatment effects (29). Thus, it is
important to determine the molecular mechanisms underlying DDP
resistance of gastric cancer cells.
As a transcription factor, CTBP1 serves a crucial
role in regulating cell proliferation and apoptosis in different
types of cells, such as breast cancer, osteosarcoma and ovarian
cancer (14,30–32).
Furthermore, CTBP1 enhances the occurrence and development of
different types of cancer (26). A
previous study reported that CTBP1 promotes the invasion and
development of tumor-associated macrophages during the development
of non-small cell lung cancer (27).
Furthermore, overexpression of CTBP1 can promote the development of
breast cancer by inducing occurrence and the EMT process (13). During the development of gastric
cancer, miR-644a inhibits the proliferation and invasion of gastric
cancer cells by suppressing CTBP1 expression (28). Furthermore, high levels of CTBP1
induce the migration and invasion of gastric mucosal epithelial
cells (15). The results of the
present study suggested that CTBP1 has the potential to enhance the
development of gastric cancer; CTBP1 knockdown suppressed the
proliferation and induced the apoptosis of DDP-resistant gastric
cancer cells.
RAD51 is a protein observed in different types of
cells, such as those of ovarian, colorectal and breast cancer, and
its expression is key in the process of homologous recombination,
which is one of the major mechanisms of the DNA damage response
(29). When DNA damage occurs, RAD51
forms nucleofilaments with other repair proteins, such as BRCA2 and
partner and localizer of BRCA2, on single-stranded DNA to
facilitate homologous strand pairing and strand exchange during the
DNA damage repair process (30,31).
RAD51 has been reported to enhance the occurrence and development
of colon cancer (32). High RAD51
expression levels can induce the resistance of triple-negative
breast cancer stem cells to chemotherapy drugs (33). In addition, it has been suggested
that inhibition of RAD51 enhances the therapeutic effect of DDP on
gastric cancer (34). Furthermore,
CTBP1 influences the DDP resistance of breast cancer cells by
activating RAD51 expression (19).
The results of the present study demonstrated that CTBP1 expression
was upregulated in DDP-resistant AGS/DDP and HGC-27/DDP gastric
cancer cells. However, the DDP resistance of these cells was
weakened following CTBP1 knockdown. Taken together, these results
suggested that CTBP1 expression may influence the DDP resistance of
gastric cancer cells. Notably, high levels of CTBP1 activated RAD51
expression in AGS/DDP cells, and overexpression of RAD51 abolished
the CTBP1 knockdown-induced decrease in DDP resistance and
apoptosis of DDP-resistant gastric cancer cells. These findings
suggested that CTBP1 expression may be important for RAD51 foci
formation and confer resistance to DDP to gastric cancer cells,
which, in turn, may elevate the cancer cell DNA damage response.
The effect of CTBP1 overexpression on the sensitivity to DDP in AGS
and HCG-27 cells will be explored in future experiments and is a
potential limitation to the present study.
The results of the present study demonstrated the
effect of CTBP1 on the DDP resistance of gastric cancer cells.
Taken together, the results suggested that CTBP1 strengthens the
DDP resistance of gastric cancer cells by activating RAD51
expression, which may provide a novel therapy for the clinical
treatment of patients with gastric cancer. However, the possibility
that CTBP1 works in another way serving as a transcript corepressor
of many types of protein and whether knockdown of CTBP1 changes
other cell death pathways, such as necrosis and autophagy, will be
investigated in future experiments and are limitations of the
present study.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YLW and HYZ conceived the study, interpreted the
data, designed the study, performed the experiments and analyzed
the data. YLW wrote the manuscript and HYZ revised it. All authors
read and approved the final manuscript. YLW and HYZ confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Van Cutsem E, Sagaert X, Topal B,
Haustermans K and Prenen H: Gastric cancer. Lancet. 388:2654–2664.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clevers H: The cancer stem cell: Premises,
promises and challenges. Nat Med. 17:313–319. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang C, Chen R, Zheng F, Tang Y, Wang X,
Chen Z and Lai X: Inhibitory role of ATF3 in gastric cancer
progression through regulating cell EMT and stemness. Cancer Cell
Int. 21:1272021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cats A, Jansen EPM, van Grieken NCT,
Sikorska K, Lind P, Nordsmark M, Meershoek-Klein Kranenbarg E, Boot
H, Trip AK, Swellengrebel HAM, et al: Chemotherapy versus
chemoradiotherapy after surgery and preoperative chemotherapy for
resectable gastric cancer (CRITICS): An international, open-label,
randomised phase 3 trial. Lancet Oncol. 19:616–628. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koizumi W, Tanabe S, Azuma M, Ishido K,
Nishimura K, Sasaki T, Nakatani K, Higuchi K, Nakayama N and Katada
C: Impacts of fluorouracil-metabolizing enzymes on the outcomes of
patients treated with S-1 alone or S-1 plus cisplatin for
first-line treatment of advanced gastric cancer. Int J Cancer.
126:162–170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu Y, Bian T, Feng J, Qian L, Li X, Zhang
Q, Zhang J, Jiang D, Liu J and Shi J: CtBP1 interacts with SOX2 to
promote the growth, migration and invasion of lung adenocarcinoma.
Oncol Rep. 42:67–78. 2019.PubMed/NCBI
|
|
9
|
Wang Y, Dai C, Zhou C, Li W, Qian Y, Wen
J, Wang Y, Han B, Ma J, Xu J, et al: Benzotriazole enhances cell
invasive potency in endometrial carcinoma through CTBP1-mediated
epithelial-mesenchymal transition. Cell Physiol Biochem.
44:2357–2367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao C, Shen Y, Tao X, Xu J, Lu J, Liu C,
Xu Z, Tang Q, Tao T and Zhang X: Silencing of CtBP1 suppresses the
migration in human glioma cells. J Mol Histol. 47:297–304. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moiola CP, De Luca P, Zalazar F, Cotignola
J, Rodríguez-Seguí SA, Gardner K, Meiss R, Vallecorsa P, Pignataro
O, Mazza O, et al: Prostate tumor growth is impaired by CtBP1
depletion in high-fat diet-fed mice. Clin Cancer Res. 20:4086–4095.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Porretti J, Dalton GN, Massillo C, Scalise
GD, Farré PL, Elble R, Gerez EN, Accialini P, Cabanillas AM,
Gardner K, et al: CLCA2 epigenetic regulation by CTBP1, HDACs,
ZEB1, EP300 and miR-196b-5p impacts prostate cancer cell adhesion
and EMT in metabolic syndrome disease. Int J Cancer. 143:897–906.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dcona MM, Morris BL, Ellis KC and Grossman
SR: CtBP-an emerging oncogene and novel small molecule drug target:
Advances in the understanding of its oncogenic action and
identification of therapeutic inhibitors. Cancer Biol Ther.
18:379–391. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han Y, Bi Y, Bi H, Diao C, Zhang G, Cheng
K and Yang Z: miR-137 suppresses the invasion and procedure of EMT
of human breast cancer cell line MCF-7 through targeting CtBP1. Hum
Cell. 29:30–36. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou J, Su M, Zhang H, Wang J and Chen Y:
miR-539-3P inhibits proliferation and invasion of gastric cancer
cells by targeting CTBP1. Int J Clin Exp Pathol. 12:1618–1625.
2019.PubMed/NCBI
|
|
16
|
Wang C, Wang M, Xing B, Chi Z, Wang H, Lie
C and Dong H: C-terminal of E1A binding protein 1 enhances the
migration of gastric epithelial cells and has a clinicopathologic
significance in human gastric carcinoma. Onco Targets Ther.
12:5189–5200. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Deng Y, Guo W, Xu N, Li F and Li J: CtBP1
transactivates RAD51 and confers cisplatin resistance to breast
cancer cells. Mol Carcinog. 59:512–519. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bhattacharyya A, Ear US, Koller BH,
Weichselbaum RR and Bishop DK: The breast cancer susceptibility
gene BRCA1 is required for subnuclear assembly of Rad51 and
survival following treatment with the DNA cross-linking agent
cisplatin. J Biol Chem. 275:23899–23903. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feng Y, Wang D, Xiong L, Zhen G and Tan J:
Predictive value of RAD51 on the survival and drug responsiveness
of ovarian cancer. Cancer Cell Int. 21:2492021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mattiello L, Soliman Abdel Rehim S,
Musella M, Sistigu A, Guarracino A, Vitale S, Corradi F, Galassi C,
Sperati F, Manic G, et al: The targeting of MRE11 or RAD51
sensitizes colorectal cancer stem cells to CHK1 inhibition. Cancers
(Basel). 13:19572021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Malka MM, Eberle J, Niedermayer K, Zlotos
DP and Wiesmuller L: Dual PARP and RAD51 inhibitory drug conjugates
show synergistic and selective effects on breast cancer cells.
Biomolecules. 11:9812021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Maranto C, Udhane V, Hoang DT, Gu L,
Alexeev V, Malas K, Cardenas K, Brody JR, Rodeck U, Bergom C, et
al: STAT5A/B blockade sensitizes prostate cancer to radiation
through inhibition of RAD51 and DNA repair. Clin Cancer Res.
24:1917–1931. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang X, Zhang Q and Guan B: Circ_0110805
knockdown enhances cisplatin sensitivity and inhibits gastric
cancer progression by miR-299-3p/ENDOPDI axis. OncoTargets Ther.
13:11445–11457. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun TT, He J, Liang Q, Ren LL, Yan TT, Yu
TC, Tang JY, Bao YJ, Hu Y, Lin Y, et al: lncRNA GClnc1 promotes
gastric carcinogenesis and may act as a modular scaffold of WDR5
and KAT2A complexes to specify the histone modification pattern.
Cancer Discov. 6:784–801. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Y, Ma J, Lin J, Sun D, Song P, Shi L,
Li H, Wang R, Wang Z and Liu S: Circular RNA circ_ASAP2 regulates
drug sensitivity and functional behaviors of cisplatin-resistant
gastric cancer cells by the miR-330-3p/NT5E axis. Anti-Cancer
Drugs. May 19–2021.(Epub ahead of print). doi:
10.1097/CAD.0000000000001087. View Article : Google Scholar
|
|
28
|
Zhang S, Feng R, Yuan F, Luo Q, Chen X, Li
N and Yang S: The therapeutic effects of dihydroartemisinin on
cisplatin-resistant gastric cancer cells. Curr Pharm Biotechnol.
Feb 16–2021.(Epub ahead of print). doi:
10.2174/1389201022666210217114825. View Article : Google Scholar
|
|
29
|
Zhuo W, Liu Y, Li S, Guo D, Sun Q, Jin J,
Rao X, Li M, Sun M, Jiang M, et al: Long noncoding RNA GMAN,
Up-regulated in gastric cancer tissues, is associated with
metastasis in patients and promotes translation of ephrin A1 by
competitively binding GMAN-AS. Gastroenterology. 156:676–691.e11.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chinnadurai G: The transcriptional
corepressor CtBP: A foe of multiple tumor suppressors. Cancer Res.
69:731–734. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chen X, Zhang Q, Dang X, Song T, Wang Y,
Yu Z, Zhang S, Fan J, Cong F, Zhang W and Duan N: Targeting the
CtBP1-FOXM1 transcriptional complex with small molecules to
overcome MDR1-mediated chemoresistance in osteosarcoma cancer stem
cells. J Cancer. 12:482–497. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
He Y, He Z, Lin J, Chen C, Chen Y and Liu
S: CtBP1/2 differentially regulate genomic stability and DNA repair
pathway in high-grade serous ovarian cancer cell. Oncogenesis.
10:492021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Y, Burness ML, Martin-Trevino R, Guy
J, Bai S, Harouaka R, Brooks MD, Shang L, Fox A, Luther TK, et al:
RAD51 mediates resistance of cancer stem cells to PARP inhibition
in triple-negative breast cancer. Clin Cancer Res. 23:514–522.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
He WL, Li YH, Hou WJ, Ke ZF, Chen XL, Lu
LY, Cai SR, Song W, Zhang CH and He YL: RAD51 potentiates
synergistic effects of chemotherapy with PCI-24781 and
cis-diamminedichloroplatinum on gastric cancer. World J
Gastroenterol. 20:10094–10107. 2014. View Article : Google Scholar : PubMed/NCBI
|