Introduction
Urinary bladder cancer is the ninth most common malignancy globally and the fourth most common among men in 2017 (1,2). Urothelial carcinoma (UC), also known as transitional cell carcinoma, accounts for ~90% of bladder cancer cases (3). Although non-muscle-invasive UCs are generally not life-threatening, advanced UCs with metastasis are associated with a high risk of mortality (3,4). The 5-year survival rate of patients with stage IV distant metastasis is <5% (5). The standard first-line chemotherapy for advanced UC is platinum-based combination chemotherapy (5).
However, ~40% of the patients with UC are unsuitable candidates for cisplatin-based therapy (6). Furthermore, patients with advanced UC have a poor prognosis owing to the rapid development of resistance to platinum-based drugs. Thus, the treatment options for these patients are particularly limited (5,6). Therefore, there is an urgent need to develop new or efficient chemotherapeutic agents to increase the survival rate of patients with advanced UC.
Temozolomide (TMZ) is an anticancer drug used against various tumors (7,8), particularly glioblastoma (9). TMZ is a non-classical alkylating agent that induces DNA methylation (10). Its cytotoxic mechanism differs from that of conventional therapeutic agents used for treating patients with UC. Its anticancer activity is largely attributed to its DNA methylation ability that induces various outcomes, including cellular senescence, cell cycle arrest and apoptosis (11,12).
Most conventional cancer therapies exert their effects by promoting cell death (13,14). Unfortunately, tumor cells frequently develop resistance to chemotherapy-induced apoptosis. Targeting senescence or autophagy may be an effective alternative to eradicate resistant cancer cells (13,15). In addition, restoring senescence could result in favorable outcomes in patients with cancers, such as hepatocellular carcinoma (14,16,17).
Autophagy is a highly conserved self-degrading process involved in homeostasis maintenance (18). It also serves a vital role in cell survival under environmental perturbations, such as nutrient deprivation and cytotoxic drug treatment (19). Chemotherapy induces autophagy, which may help tumor cells survive by supporting their adaptation to harsh conditions (20). Hence, inhibiting autophagy can augment the therapeutic effects of anticancer drugs (21). Chloroquine (CQ), a traditional anti-malarial drug, has been investigated for its promising role in autophagy inhibition (22,23). As the only clinically available autophagy blocker, CQ may be an adjunctive agent in cancer therapy (24,25). Furthermore, because CQ has been approved by the Food and Drug Administration as an antimalarial drug, it has been commonly used in clinical trials as an autophagy inhibitor (26). The present study was conducted to explore the effects of TMZ and its combinatorial therapeutic regimen with CQ on UC cells.
Materials and methods
Cell lines and culture
Two cell lines (T24 and J82) were used in the present study, which were validated by performing DNA fingerprinting analysis (Korean Cell Line Bank; Korean Cell Line Research Foundation; Fig S1). The cells were cultured in Roswell Park Memorial Institute-1640 medium (Cytiva) at 37°C with 5% CO2. The culture medium weas supplemented with 10% fetal bovine serum (GE Healthcare), 10 mM HEPES (Sigma-Aldrich; Merck KGaA), 2.0 g/l sodium bicarbonate (Sigma-Aldrich; Merck KGaA), 1 mM sodium pyruvate (Thermo Fisher Scientific, Inc.) and 100 U/100 µg/ml penicillin-streptomycin (Thermo Fisher Scientific, Inc.).
Chemicals and antibodies
TMZ (Sigma-Aldrich; Merck KGaA) was dissolved in dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA). CQ (Sigma-Aldrich; Merck KGaA) was dissolved in distilled water, filtered through a 0.2-µM pore membrane, and protected from light. Antibodies against GAPDH (1:1,000; cat. no. 2118S) and p21 (1:1,000; cat. no. 2947S) were purchased from Cell Signaling Technology Inc. Antibodies against LC3 (1:1,000; cat. no. NB100-2220) and p62 (1:2,000; cat. no. NBP1-48320) were purchased from Novus Biologicals LLC. Antibody against p53 (1:500; cat. no. sc-53394) was obtained from Santa Cruz Biotechnology, Inc. Goat anti-rabbit HRP-conjugated secondary antibody (1:2,000; cat. no. ADI-SAB-300-J; Enzo Life Sciences, Inc.) and goat anti-mouse HRP-conjugated secondary antibody (1:2,000; cat. no. P0447; Dako; Agilent Technologies) were used.
Cell viability assay
Cell viability was determined using Cell Counting Kit-8 (Dojindo Molecular Technologies, Inc.) according to the manufacturer's instructions. Cells were treated with TMZ at 10, 20, and 40 µM for 24 h. The inhibitory concentration (IC50) of CQ was determined by treating the cells with CQ concentrations ranging from 1×106 to 1×10−2 µM, with 10-fold dilution. Cell viability was quantified by measuring photometric absorbance at 450 nm using Epoch Microplate Spectrophotometer (Bio Tek Instruments, Inc.) and expressed as a percentage relative to the viability of untreated control cells.
Colony-forming assay
A colony-forming assay was conducted as described previously (27). The number of colonies in each category were recorded from five random fields at ×20 magnification. Colonies with 5–10, 11–50, and >50 cells were classified as small, medium and large, respectively.
Apoptosis assay
Annexin V Apoptosis Detection kit (eBioscience; Thermo Fisher Scientific, Inc.) was used for the apoptosis assay. According to the manufacturer's instructions, cells treated with either 0 or 20 µM TMZ for 24 h at 37°C were prepared, and Annexin V staining was performed. After staining, the cells were placed on ice in dark conditions and analyzed using a BD FACSVerse flow cytometer (BD Bioscience) within 1 h. The relative proportion of apoptotic UC cells was determined. UC cells stained with Annexin V-fluorescein isothiocyanate and propidium iodide (PI) were indicated in a scatter diagram. The cells were divided into necrotic (Q1, Annexin-/PI+), late apoptotic (Q2, Annexin+/PI+), intact (Q3, Annexin-/PI-), and early apoptotic (Q4, Annexin+/PI-) based on the results of Annexin and PI staining.
Scratch wound-healing assay
A scratch wound-healing assay was performed as previously described (28,29). The T24 and J82 cells were treated with either 0 or 20 µM TMZ for 24 h at 37°C. When cells covered 80–90% of the dish, the cells were scratched using 200 µl pipette tips. Cells were washed twice using PBS, and cultured in serum-free medium (Cytiva). At 24 h after the scratch, the number of migrated cells was counted in nine random fields of view at ×40 magnification under light microscope.
Senescence-associated β-galactosidase (SA-β-gal) assay
Senescence was evaluated using an SA-β-gal staining kit (cat. no. CBA-230; Cell Biolabs, Inc.) according to the manufacturer's instructions (30). Color development was detected under a light microscope in 10 random fields at ×40 magnification.
Transwell migration assay
The invasiveness of UC cells was evaluated using a BD BioCoat™ 24-Multiwell Invasion System precoated with BD Matrigel™ Matrix (BD Biosciences) according to the manufacturer's instructions (27). The number of invaded cells in five random fields at ×20 magnification was counted, and the average was recorded.
SP assay
The SP fraction in TMZ-treated UC cells was determined according to a previously published protocol (31). The SP regions were confirmed by treatment with 50 µM verapamil hydrochloride for 30 min at 37°C.
Western blot assay
Cell lysates were prepared by scraping the T24 and J82 cells off the plate after treatment with the EzRIPA lysis kit (ATTO Corporation). The lysates were transferred into microcentrifuge tubes and centrifuged at 15,000 × g for 15 min at 4°C. After transferring the supernatant into a new tube, the protein concentration was measured using a detergent-compatible protein assay reagent (Bio-Rad Laboratories, Inc.). Gel electrophoresis, membrane transfer and blocking, and antibody binding were performed as described previously (28,32). Proteins (25 µg protein/lane) were loaded and separated on 10 or 15% SDS-PAGE gels. The proteins were transferred to polyvinylidene difluoride membranes, and blocked with 5% skim milk for 1 h at room temperature. The membranes were incubated with the aforementioned primary antibodies overnight at 4°C. After washed by PBST (0.05% Tween-20), the membranes were incubated with the aforementioned secondary antibodies at room temperature for 2 h. Chemiluminescence was detected using Luminata Forte Western HRP Substrate (Merck Millipore) by ImageQuant LAS 40000 mini (GE Healthcare). Membranes were reprobed with GAPDH antibody as an internal loading control.
Statistical analysis
Data are expressed as mean ± standard deviation (SD) where indicated. A unpaired two-tailed Student's t-test, and one-way ANOVA were used for statistical comparisons. In addition, Tukey's post hoc test was used. P<0.05 was considered to indicate a statistically significant difference.
Results
TMZ does not induce apoptosis
TMZ did not induce significant cancer cell death in T24 and J82 cells (P>0.05; Fig. 1A). In T24 cells, cell viability of the control group was 100%±1.92%, that of the 10 µM TMZ-treated group was 96.92%±1.18%, that of the 20 µM TMZ-treated group was 95.30%±0.66%, and that of the 40 µM TMZ-treated group was 96.10%±1.24%. In J82 cells, cell viability of the control group was 100%±9.14%, that of the 10 µM TMZ-treated group was 96.47%±9.82%, that of the 20 µM TMZ-treated group was 91.58%±6.84%, and that of the 40 µM TMZ-treated group was 84.70%±5.87%.
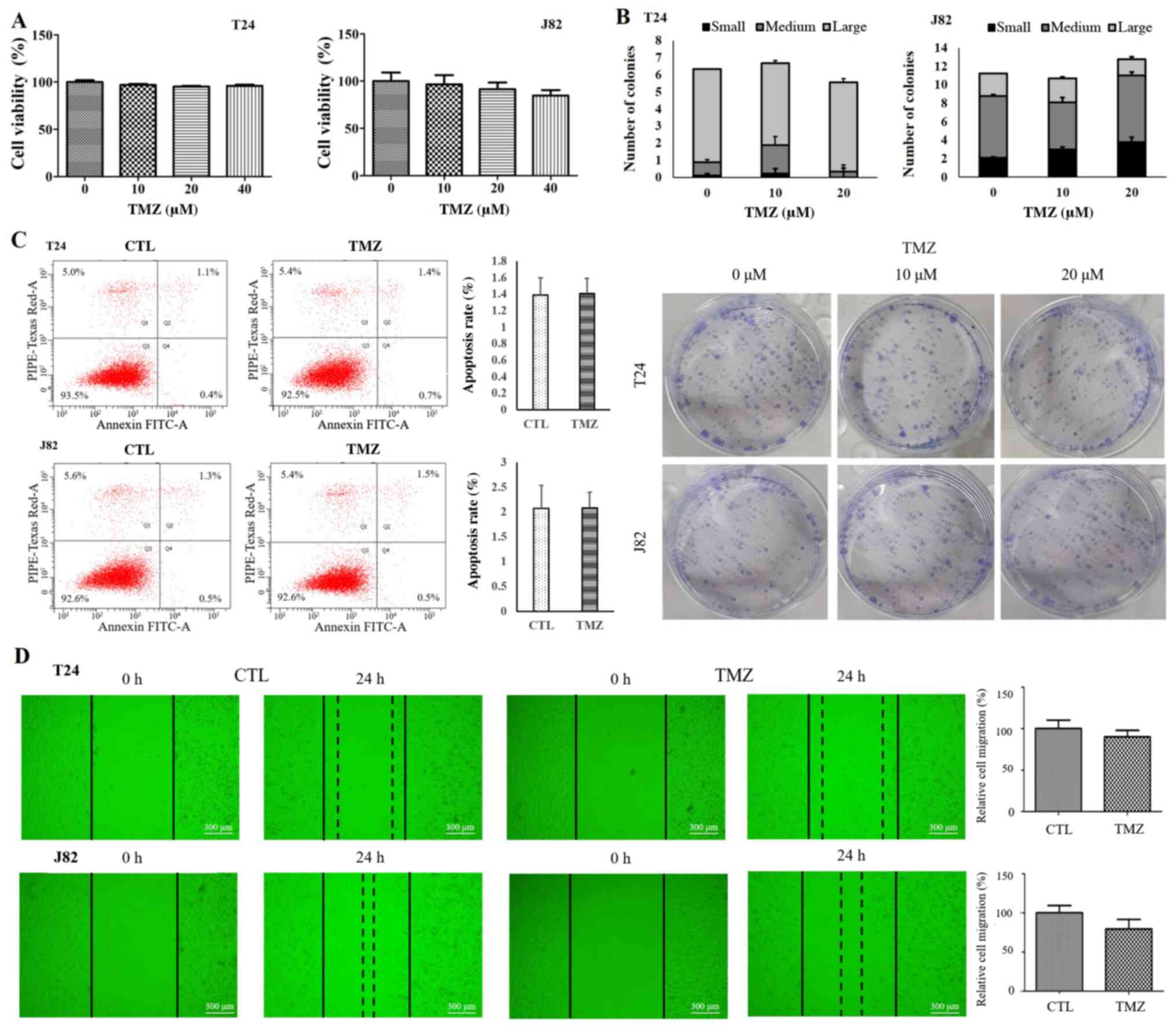 |
Figure 1.
(A) Cell viability assay. TMZ at concentrations of 0, 10, 20 and 40 µM did not significantly alter the cell viability of human urothelial carcinoma cells. Data are presented as mean ± SD. (B) Colony-forming assay. TMZ did not significantly alter the colony-forming ability of human urothelial carcinoma cells. There were no significant changes in the total number or composition of the colonies. Colonies with 5–10, 11–50 and >50 cells were classified as small, medium and large, respectively. Each data point represents the mean ± SD. (C) Apoptosis assay. Results of fluorescence-activated cell sorting of urothelial carcinoma cells stained with Annexin V-FITC and propidium iodide are presented as a scatter diagram. (D) Scratch wound-healing assay. Relative cell migration showed that TMZ did not significantly affect the proliferation and migration capacity of human urothelial carcinoma cells. Black outlines indicate the initial wound area. Dotted lines indicate the edge of the wound after 24 h. CTL, control; TMZ, temozolomide; FITC, fluorescein isothiocyanate.
|
In addition, TMZ did not significantly alter the colony-forming ability in both cell lines (Fig. 1B). Quantification of the cell fractions revealed apoptotic cells (Q2 + Q4) in 1.39%±0.21% of the control group and 1.41%±0.18% of the TMZ-treated T24 cells, and 2.07%±0.47% of the control group and 2.08%±0.32% of the TMZ-treated J82 cells (Fig. 1C). These results indicated that TMZ, at the given concentration, did not affect apoptosis. Moreover, TMZ did not significantly alter the migration capacity (P>0.05) (Fig. 1D).
TMZ induces senescence in UC cells
The SA-β-gal activity was significantly higher in TMZ-treated T24 and J82 cells than in the respective untreated control cells (P<0.001; Fig. 2A). Moreover, some of the TMZ-treated cells exhibited altered morphological features such as a flattened and enlarged shape. Furthermore, the levels of p53 and p21 increased in TMZ-treated T24 cells (P<0.01), and TMZ-treated J82 cells (P<0.05; Fig. 2B).
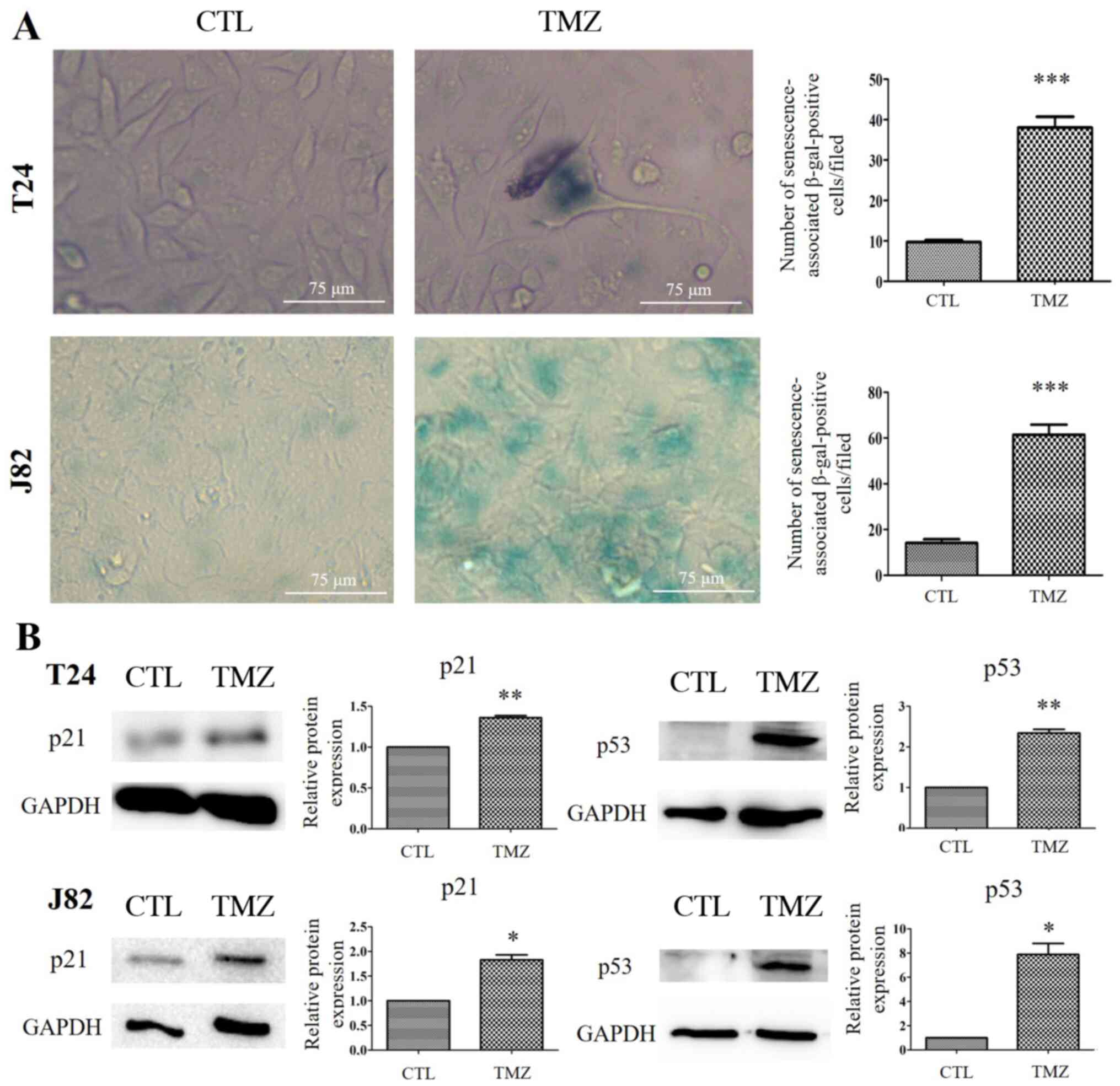 |
Figure 2.
(A) TMZ-treated urothelial carcinoma cells were stained positive for senescence-associated β-gal activity. Some cells had an altered morphology after treatment with TMZ. ***P<0.001. (B) Western blot analysis. TMZ treatment resulted in an increase in the levels of p53 and p21. *P<0.05; **P<0.01. β-gal, β-galactosidase; CTL, control; TMZ, temozolomide.
|
TMZ significantly decreases the invasiveness of UC cells
The transwell migration assay revealed a remarkable decrease in the invasiveness of TMZ-treated UC cells compared with that of the untreated control cells (Fig. 3A). TMZ decreased the invasiveness of T24 and J82 cells to 18.46%±3.92% (P<0.001) and 54.69%±3.40% (P<0.05), respectively, compared with that of their respective untreated control cells.
 |
Figure 3.
(A) Transwell migration assay. In the transwell migration assay, the invasiveness of TMZ-treated urothelial carcinoma cells was remarkably lower than that of untreated CTL cells. TMZ reduced the invasiveness of T24 cells to 18.46%±3.92% compared with the CTL group and that of J82 cells to 54.69%±3.40% compared with CTL group. *P<0.05; ***P<0.001. (B) SP assay. The SP fraction displayed a high dye efflux activity, which appeared as a clearly delineated tail in the lower left portion of the histogram. The SP was validated by verapamil treatment. Representative flow cytometry histograms are shown. TMZ treatment markedly reduced the side population fraction. SP, side population; CTL, control; TMZ, temozolomide.
|
TMZ preferentially depletes the SP fraction of UC cells
Hoechst 33342 staining revealed a delineated SP fraction, identified by flow cytometric analysis (33). Verapamil hydrochloride abrogated the SP region. The SP fraction of T24 cells, accounting for 0.8% of the negative control cells, decreased to 0.1% after TMZ treatment for 24 h (Fig. 3B). Similarly, TMZ treatment decreased the SP fraction of J82 cells from 1.0 to 0.3%.
TMZ enhances the autophagic response in UC cells, and inhibition of autophagy sensitizes UC cells to TMZ
The cytotoxicity of CQ on UC cells was measured using different concentrations of CQ. The IC50 of CQ was 182.72 µM in T24 cells and 441.84 µM in J82 cells. Based on these results, 10 µM CQ was used to inhibit autophagy with limited toxicity (Fig. 4A).
 |
Figure 4.
(A) Measurement of the inhibitory concentration of CQ. Inhibitory concentration of CQ was 182.72 µM for the T24 cells and 441.84 µM for the J82 cells. (B) Cell viability assay and western blot analysis. TMZ and CQ have a synergistic cytotoxic effect. Autophagic flux was induced by TMZ, which was evidenced by p62 degradation and increased LC3-II levels. CQ dramatically increased the levels of both LC3-II and p62. ***P<0.001. (C) Apoptosis assay. The combination of TMZ and CQ markedly induced apoptosis. ***P<0.001. CTL, control; TMZ, temozolomide; CQ, chloroquine.
|
To examine the effect of TMZ on autophagy, the levels of LC3-II were measured (Fig. 4B), which were elevated after TMZ treatment, indicating enhanced autophagy. Treatment with CQ inhibited the autophagic response. Irrespective of the TMZ treatment, CQ dramatically elevated the levels of both LC3-II and p62 in T24 and J82 cells. However, TMZ treatment alone increased the level of LC3-II but decreased the level of p62. These results indicated that CQ treatment inhibited TMZ-induced autophagy in both UC cell lines. Moreover, the cell viability assay results revealed that CQ enhanced the cytotoxicity of TMZ in UC cells. These data demonstrated that the inhibition of autophagy contributed to the synergistic cytotoxic effect of CQ and TMZ on T24 and J82 cells (P<0.001).
The mode of the cell death induced by CQ and TMZ cotreatment was evaluated using an apoptosis assay (Fig. 4C). In both cell lines, apoptosis was significantly increased in the groups cotreated with TMZ and CQ (P<0.001).
Discussion
To identify a potential candidate to overcome the chemoresistance observed in advanced UC, the effect of TMZ on the biological and molecular phenotypes of UC cells was investigated in the present study. TMZ significantly elicited senescence and autophagy in UC cells. In addition, it reduced their malignant potential, including invasiveness. Treatment with TMZ reduced the SP fraction of UC cells. Notably, the results also indicated that CQ (an inhibitor of the autophagic response) combined with TMZ synergistically enhanced the cytotoxicity against UC cells.
Although the effect was statistically insignificant, TMZ caused a slight decrease in the cell viability of both cell lines. Cell viability was reduced by 4.70% in the T24 cells and 8.42% in the J82 cells following treatment with 20 µM TMZ. Meanwhile, apoptosis was minimally induced by the same treatment. Considering that ruling out apoptosis is one of the ways of proving the induction of senescence, these results may indicate senescence (34). Induction of senescence without a statistically significant decrease in cell viability was also observed in another study (35). This is possible because senescence does not invoke immediate cytotoxic effect, and senescent cells are viable and metabolically active (14).
Consistent with these findings, SA-β-gal staining results showed that TMZ treatment was associated with enhanced senescence. Moreover, TMZ increased the expression levels of p53 and p21, which serve a vital role in the onset of senescence (36,37). Several drugs, such as adenosine and metformin, can activate p53-dependent senescence (38,39). In cancers, senescence is associated with a more favorable outcome of cancer therapy (34). It is also considered a novel functional target to induce a growth inhibitory response (34). Previously, it has been reported that cancer cells can be induced into to a senescent state by anticancer treatment; this finding is of particular clinical relevance (40). Furthermore, therapeutic approaches that induce either p21 activation or p53 reactivation have been confirmed to be effective by the induction of a senescence response (14,36).
In the present study, TMZ reduced the invasiveness and SP fraction of UC cells. A decreased SP fraction may contribute to the reduced invasiveness of UC cells to a certain extent, because SP fraction is enriched in those cancer cells that have more malignant features (41). The SP fraction enriched in cancer stem cells may confer therapeutic resistance (33). Therefore, eliminating the SP fraction may represent an effective therapeutic strategy in UC treatment. A reduction in the SP fraction by TMZ treatment implies clinical significance in overcoming resistance to anticancer drugs (41).
In the present study, the protein expression analysis revealed that TMZ elevated LC3-II expression, an indication of autophagy (42). These data suggested that enhanced autophagy may help UC cells to adapt to TMZ treatment. Additionally, the co-treatment of UC cells with TMZ and CQ significantly enhanced the treatment cytotoxicity. Moreover, TMZ and CQ synergistically induced apoptosis, whereas either TMZ or CQ did not induce a significant level of apoptosis. These data suggest that autophagy helped UC cells treated with TMZ to avoid apoptosis. Therefore, the TMZ-treated UC cells underwent apoptosis when autophagy was blocked by CQ. Similarly, other studies have shown that the inhibition of autophagy induces the cytotoxic effect of anticancer agents (20,43,44). CQ suppresses the development of chemoresistance and eliminates resistant cancer cells by blocking autophagy (23,45,46).
It was also observed that CQ inhibited autophagy in UC cells, and that TMZ-induced autophagy was reduced by CQ treatment. Decreased cellular levels of p62 indicate increased autophagic p62 degradation, whereas increased cellular levels of p62 reflect the inhibition of autophagic p62 degradation (47). TMZ can enhance the autophagic flux, as shown by p62 degradation and elevated LC3-II levels (48). In the current study, CQ treatment resulted in a marked increase in the levels of both LC3-II and p62, indicating that CQ inhibited TMZ-induced autophagy. These results suggest that combination therapy with CQ may represent an effective approach to enhance the cytotoxicity of TMZ. Further studies are required to evaluate the effect of the TMZ and CQ combination on normal cells.
In conclusion, TMZ is a promising anticancer agent that can be used to overcome therapeutic resistance. By preferentially reducing SP fraction and inducing senescence, TMZ reduced the malignant features of UC cells. Furthermore, the use of CQ in combination with TMZ is a potential method to enhance the efficacy of TMZ by targeting autophagy. Although further research is warranted to explore the incorporation of TMZ into the therapeutic regimen of patients with UC, the findings of the present study findings indicate the importance of TMZ in treating UC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by BK21 FOUR Future Veterinary Medicine Leading Education and Research Center, and the Basic Science Research Program through the National Research Foundation of Korea, funded by the Ministry of Education, Science and Technology (grant no. 2020R1A2C1010215).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
NYK carried out most of the experiments, analyzed the data and was a major contributor in writing the manuscript. SHH and YY provided critical feedback, contributed to sample preparation and confirmed the authenticity of all the raw data. YK supervised the project, and helped design the research and manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Glossary
Abbreviations
Abbreviations:
|
UC
|
urothelial carcinoma
|
|
TMZ
|
temozolomide
|
|
CQ
|
chloroquine
|
|
SP
|
side population
|
|
PI
|
propidium iodide
|
|
SA-β-gal
|
senescence-associated β-galactosidase
|
|
SD
|
standard deviation
|
References
|
1
|
Sjödahl G, Lauss M, Lövgren K, Chebil G, Gudjonsson S, Veerla S, Patschan O, Aine M, Fernö M, Ringnér M, et al: A molecular taxonomy for urothelial carcinoma. Clin Cancer Res. 18:3377–3386. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A and Bray F: Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 71:96–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gui Y, Guo G, Huang Y, Hu X, Tang A, Gao S, Wu R, Chen C, Li X, Zhou L, et al: Frequent mutations of chromatin remodeling genes in transitional cell carcinoma of the bladder. Nat Genet. 43:875–878. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen M, Zhuang C, Liu Y, Li J, Dai F, Xia M, Zhan Y, Lin J, Chen Z, He A, et al: Tetracycline-inducible shRNA targeting antisense long non-coding RNA HIF1A-AS2 represses the malignant phenotypes of bladder cancer. Cancer Lett. 376:155–164. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koufopoulou M, Miranda PA, Kazmierska P, Deshpande S and Gaitonde P: Clinical evidence for the first line treatment of advanced urothelial carcinoma: Current paradigms and emerging treatment options. Cancer Treat Rev. 89:1020722020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y, Lee J-L, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, et al KEYNOTE-045 Investigators, : Pembrolizumab as second line therapy for advanced urothelial carcinoma. N Engl J Med. 376:1015–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, Ludwin SK, Allgeier A, Fisher B, Belanger K, et al European Organisation for Research and Treatment of Cancer Brain Tumour and Radiation Oncology Groups; National Cancer Institute of Canada Clinical Trials Group, : Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 10:459–466. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Woll PJ, Judson I, Lee SM, Rodenhuis S, Nielsen OS, Buesa JM, Lorigan PC, Leyvraz S, Hermans C, van Glabbeke M, et al: Temozolomide in adult patients with advanced soft tissue sarcoma: A phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 35:410–412. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, et al European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group, : Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang HW, Xu ZK, Song Y and Liu YG: Correlations of MGMT genetic polymorphisms with temozolomide resistance and prognosis of patients with malignant gliomas: A population-based study in China. Cancer Gene Ther. 24:215–220. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roos WP, Batista LF, Naumann SC, Wick W, Weller M, Menck CF and Kaina B: Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 26:186–197. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mhaidat NM, Zhang XD, Allen J, Avery-Kiejda KA, Scott RJ and Hersey P: Temozolomide induces senescence but not apoptosis in human melanoma cells. Br J Cancer. 97:1225–1233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sui X, Chen R, Wang Z, Huang Z, Kong N, Zhang M, Han W, Lou F, Yang J, Zhang Q, et al: Autophagy and chemotherapy resistance: A promising therapeutic target for cancer treatment. Cell Death Dis. 4:e8382013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Acosta JC and Gil J: Senescence: A new weapon for cancer therapy. Trends Cell Biol. 22:211–219. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schmitt CA, Fridman JS, Yang M, Lee S, Baranov E, Hoffman RM and Lowe SW: A senescence program controlled by p53 and p16INK4a contributes to the outcome of cancer therapy. Cell. 109:335–346. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ventura A, Kirsch DG, McLaughlin ME, Tuveson DA, Grimm J, Lintault L, Newman J, Reczek EE, Weissleder R and Jacks T: Restoration of p53 function leads to tumour regression in vivo. Nature. 445:661–665. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang X, Qu K, Tao J, Yin G, Han S, Liu Q and Sun H: Inhibition of CIP2A attenuates tumor progression by inducing cell cycle arrest and promoting cellular senescence in hepatocellular carcinoma. Biochem Biophys Res Commun. 495:1807–1814. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Codogno P and Meijer AJ: Autophagy and signaling: Their role in cell survival and cell death. Cell Death Differ. 12 (Suppl 2):1509–1518. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rosenfeldt MT and Ryan KM: The role of autophagy in tumour development and cancer therapy. Expert Rev Mol Med. 11:e362009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Manic G, Obrist F, Kroemer G, Vitale I and Galluzzi L: Chloroquine and hydroxychloroquine for cancer therapy. Mol Cell Oncol. 1:e299112014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang M, Liao C, Hu Y, Qinwen Pan and Jiang J: Sensitization of breast cancer cells to paclitaxel by dichloroacetate through inhibiting autophagy. Biochem Biophys Res Commun. 489:103–108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mahoney JR and Eaton JW: Chloroquine resistant malaria: Association with enhanced plasmodial protease activity. Biochem Biophys Res Commun. 100:1266–1271. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kimura T, Takabatake Y, Takahashi A and Isaka Y: Chloroquine in cancer therapy: A double-edged sword of autophagy. Cancer Res. 73:3–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Verschooten L, Barrette K, Van Kelst S, Rubio Romero N, Proby C, De Vos R, Agostinis P and Garmyn M: Autophagy inhibitor chloroquine enhanced the cell death inducing effect of the flavonoid luteolin in metastatic squamous cell carcinoma cells. PLoS One. 7:e482642012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Amaravadi RK, Yu D, Lum JJ, Thomas Tikhonenko A and Thompson CB: Inhibition of autophagy with chloroquine enhances therapeutic tumor regression in a mouse model of B cell lymphoma. Cancer Res. 66:14482006.
|
|
26
|
Liu T, Zhang J, Li K, Deng L and Wang H: Combination of an autophagy inducer and an autophagy inhibitor: A smarter strategy emerging in cancer therapy. Front Pharmacol. 11:4082020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim NY, Kim MC and Kim Y: Hypomethylation reduced the aggressive potential of human malignant mesothelioma cells. Cancer Gene Ther. 23:425–432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kim HA, Kim MC, Kim NY and Kim Y: Inhibition of hedgehog signaling reduces the side population in human malignant mesothelioma cell lines. Cancer Gene Ther. 22:387–395. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Z, Niu S, Xu F, Zhao W, Ma R and Chen M: CircAMOTL1 promotes tumorigenesis through miR 526b/SIK2 axis in cervical cancer. Front Cell Dev Biol. 8:5681902020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Leikam C, Hufnagel A, Schartl M and Meierjohann S: Oncogene activation in melanocytes links reactive oxygen to multinucleated phenotype and senescence. Oncogene. 27:7070–7082. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ning ZF, Huang YJ, Lin TX, Zhou YX, Jiang C, Xu KW, Huang H, Yin XB and Huang J: Subpopulations of stem-like cells in side population cells from the human bladder transitional cell cancer cell line T24. J Int Med Res. 37:621–630. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chen M, Wei X, Shi X, Lu L, Zhang G, Huang Y and Hou J: LncRNA HIF1A-AS2 accelerates malignant phenotypes of renal carcinoma by modulating miR-30a-5p/SOX4 axis as a ceRNA. Cancer Biol Med. 18:587–603. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kai K, D'Costa S, Yoon BI, Brody AR, Sills RC and Kim Y: Characterization of side population cells in human malignant mesothelioma cell lines. Lung Cancer. 70:146–151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ewald JA, Desotelle JA, Wilding G and Jarrard DF: Therapy-induced senescence in cancer. J Natl Cancer Inst. 102:1536–1546. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yi G, He Z, Zhou X, Xian L, Yuan T, Jia X, Hong J, He L and Liu J: Low concentration of metformin induces a p53-dependent senescence in hepatoma cells via activation of the AMPK pathway. Int J Oncol. 43:1503–1510. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Olivier M, Petitjean A, Marcel V, Pétré A, Mounawar M, Plymoth A, de Fromentel CC and Hainaut P: Recent advances in p53 research: An interdisciplinary perspective. Cancer Gene Ther. 16:1–12. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chang BD, Xuan Y, Broude EV, Zhu H, Schott B, Fang J and Roninson IB: Role of p53 and p21waf1/cip1 in senescence-like terminal proliferation arrest induced in human tumor cells by chemotherapeutic drugs. Oncogene. 18:4808–4818. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang D, Song J, Wu L, Ma Y, Song C, Dovat S, Nishizaki T and Liu J: Induction of senescence by adenosine suppressing the growth of lung cancer cells. Biochem Biophys Res Commun. 440:62–67. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li P, Zhao M, Parris AB, Feng X and Yang X: p53 is required for metformin-induced growth inhibition, senescence and apoptosis in breast cancer cells. Biochem Biophys Res Commun. 464:1267–1274. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gewirtz DA, Holt SE and Elmore LW: Accelerated senescence: An emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol. 76:947–957. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Moserle L, Ghisi M, Amadori A and Indraccolo S: Side population and cancer stem cells: Therapeutic implications. Cancer Lett. 288:1–9. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tanida I, Ueno T and Kominami E: LC3 and autophagy. Methods Mol Biol. 445:77–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Rotondo R, Oliva MA, Staffieri S, Castaldo S, Giangaspero F and Arcella A: Implication of lactucopicrin in autophagy, cell cycle arrest and oxidative stress to inhibit U87Mg glioblastoma cell growth. Molecules. 25:58432020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Moghadam AR, da Silva Rosa SC, Samiei E, Alizadeh J, Field J, Kawalec P, Thliveris J, Akbari M, Ghavami S and Gordon JW: Autophagy modulates temozolomide-induced cell death in alveolar Rhabdomyosarcoma cells. Cell Death Discov. 4:522018.Erratum in: Cell Death Discov 5: 116, 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ganguli A, Choudhury D, Datta S, Bhattacharya S and Chakrabarti G: Inhibition of autophagy by chloroquine potentiates synergistically anti-cancer property of artemisinin by promoting ROS dependent apoptosis. Biochimie. 107((Pt B)): 338–349. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liang X, Tang J, Liang Y, Jin R and Cai X: Suppression of autophagy by chloroquine sensitizes 5-fluorouracil-mediated cell death in gallbladder carcinoma cells. Cell Biosci. 4:102014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yoon YH, Cho KS, Hwang JJ, Lee SJ, Choi JA and Koh JY: Induction of lysosomal dilatation, arrested autophagy, and cell death by chloroquine in cultured ARPE-19 cells. Invest Ophthalmol Vis Sci. 51:6030–6037. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rao R, Balusu R, Fiskus W, Mudunuru U, Venkannagari S, Chauhan L, Smith JE, Hembruff SL, Ha K, Atadja P, et al: Combination of pan-histone deacetylase inhibitor and autophagy inhibitor exerts superior efficacy against triple-negative human breast cancer cells. Mol Cancer Ther. 11:973–983. 2012. View Article : Google Scholar : PubMed/NCBI
|


















