Introduction
Approximately 10 million patients succumb to various
types of cancer annually, despite a wide variety of available
cancer therapies. Malignant cancers demonstrate a poor prognosis,
which is evidenced by a reduced expected lifespan and a greater
difficulty in treatment. The advent of target therapies that
suppress tumor growth, invasion and metastasis, has revolutionized
cancer treatment and given optimism to numerous patients with
cancer. In this regard, target therapy elicits both a specific and
precise action on cancer cells, thereby reducing unpleasant side
effects by contrast to traditional cancer treatment. Target therapy
directed against lung cancer with the EGFR mutation has already
demonstrated encouraging results (1,2).
Consequently, the enthusiasm for target therapy remains high since
almost all types of cancer possess a key cellular factor that
promotes its pathological biochemical metabolism.
The chemokine, monocyte chemoattractant protein-1
(MCP-1), also known as C-C motif chemokine ligand 2 (CCL2), belongs
to the C-C chemokine superfamily, which is comprised of at least 4
members (MCP-1, −2, −3 and −4). MCP-1 binds to a G-protein coupled
receptor and plays a major role in the promotion of inflammation by
modulating monocyte and basophil activity, but not neutrophil or
eosinophil activity (3).
Regardless of the affinities, MCP-1 has an ability to interact with
a number of receptors [e.g., ACKR1, C-C motif chemokine receptor
(CCR)-2, CCR5, CCR10 and CCR11] (4–8); however, previous findings
suggested that CCR2 is the primary MCP-1 receptor. MCP-1 was
initially identified in 1989 and termed glioma-derived chemotactic
factor-2 (GDCF-2) (9). Later,
GDCF-2 was found in the tissue culture media of
phytohemagglutinin-stimulated human mononuclear leukocytes. With
amino acid sequencing and cloning, GDCF-2 was finally renamed MCP-1
(10,11). MCP-1 is also known as tumor-derived
chemotactic factor, as a wide variety of tumor cells can produce it
(12). In addition, MCP-1 is
secreted by a range of cell types in the tumor microenvironment
(TME), such as fibroblasts, tumor-infiltrating monocytes,
endothelial cells and tumor-associated adipocytes (13,14).
The MCP-1 gene (SCYA2) is located on human
chromosome 17q11.2-q21.1 (15).
The precursor MCP-1 comprises 99 amino acids, with 23 amino acids
at the N-terminal, as the hydrophobic signal peptide, whereas the
mature protein is comprised of 76 amino acids, after cleavage of
the signal peptide (Fig. 1A).
There are two forms of the MCP-1 structure, known as I and P
(Fig. 1B and C) (16). For all the MCPs, the N-terminal
residues, 1–6, are essential for chemoattractant activity, and the
first amino acid is necessary for direct receptor binding (17). Handel and Domaille (18) reported that the secondary structure
of MCP-1 consists of one α-helix and four β-sheets (the grey
label), including residues 9–11 (β0), 27–31 (β1), 40–45 (β2) and
51–54 (β3), which are different from the data in the Protein Data
Bank (18). The latter shows that
MCP-1 has three α-helices. Residue 14 can be glycosylated, which
can slightly decrease the potency of its chemotactic activity
(19).
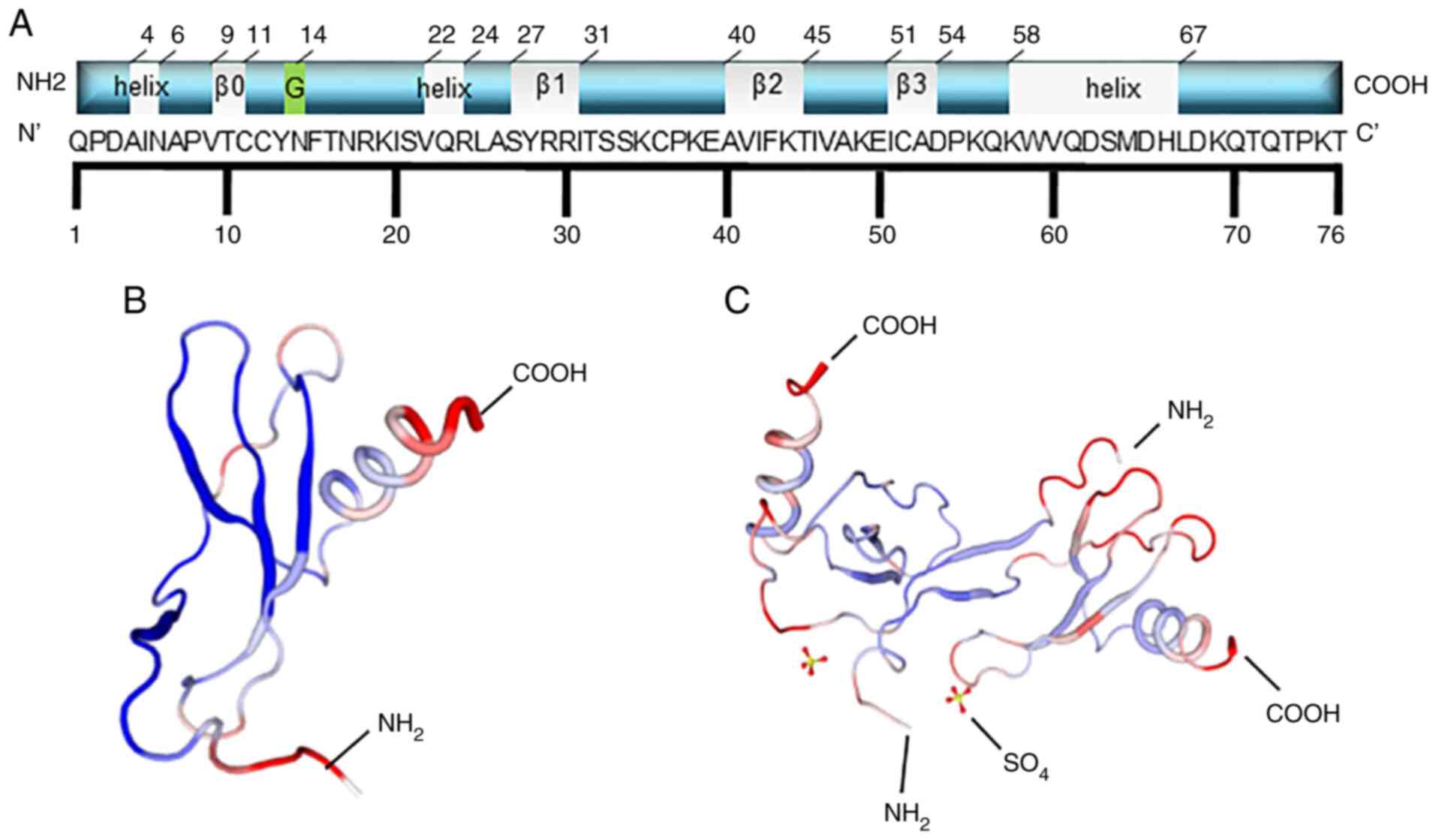 | Figure 1.Schematic structural illustration of
MCP1. (A) The schematic structural illustration. For all MCPs,
N-terminal residues 1–6 are essential for chemoattractant activity,
and the first amino acid is necessary for direct receptor binding.
MCP-1 is composed of 76 amino acids, and the secondary structure of
MCP-1 consisted of one α-helix and four regions of β-sheet (the
grey label), including residues 9–11 (β0), residues 27–31 (β1),
residues 40–45 (β2), residues 51–54 (β3), which is little different
from the data in PDB protein bank. The last one shows that MCP-1
has three α-helix (the grey sections). Residue 14 can be
glycosylated (the green section), which can slightly decrease the
potency of the chemotactic activity of MCP-1. (B and C) The two
forms of secondary structures of MCP-1: (B) is form I and (C) is
form P. The former is the single MCP-1 molecule, while the latter
is the dimer. |
MCP-1 has been associated with several diseases,
such as HIV-1 pathogenesis, cardiovascular disease and cancer. In
the present review, the role and mechanism of MCP-1 in cancer are
to be discussed.
MCP-1 is a key protein in tumor
development
Cancer cell heterogeneity within a tumor is
well-established due to the acquired mutations, as a result of the
selective pressure caused by cell proliferation. Some of these
acquired mutations result in the synthesis of cytokines that either
activate or deactivate signaling pathways, allowing the cancer cell
to escape leukocyte attack or to proliferate faster, leading to a
higher survival probability for cancer cells. Consequently, MCP-1,
secreted by the cancer cells, results in an advantage for the tumor
but a disadvantage for the host, despite the specific signaling
pathway involved. For example, MCP-1 expression is induced by IL-1β
and regulated by NF-κB and activator protein-1 (AP-1) in renal cell
carcinoma and glioblastoma (20,21).
MCP-1 is also a downstream molecule of IL-33, which increases tumor
metastasis and invasion in esophageal carcinoma cells (22). MCP-1 can also be mediated by the
mTOR complex 1 signaling pathway or sushi domain containing 2 in
tumor cells (23). A long
non-coding RNA LINC01296, termed lymph node metastasis associated
transcript 1, activates MCP-1 expression by interacting with hnRNPL
and mediating H3K4 trimethylation (24). MCP-1 expression is also mediated by
PA28γ, which promotes tumor migration, invasion and angiogenesis
(25). In addition, MCP-1
expression is mediated by angiotensin II binding to the angiotensin
type 2 receptor and IL-4 in endothelial cells (26,27).
Furthermore, TGF-β signaling has been associated with MCP-1
expression in fibroblast cells (28). The aforementioned findings indicate
that MCP-1 could be activated by different signaling pathways and
contribute to tumor progression (Fig.
2).
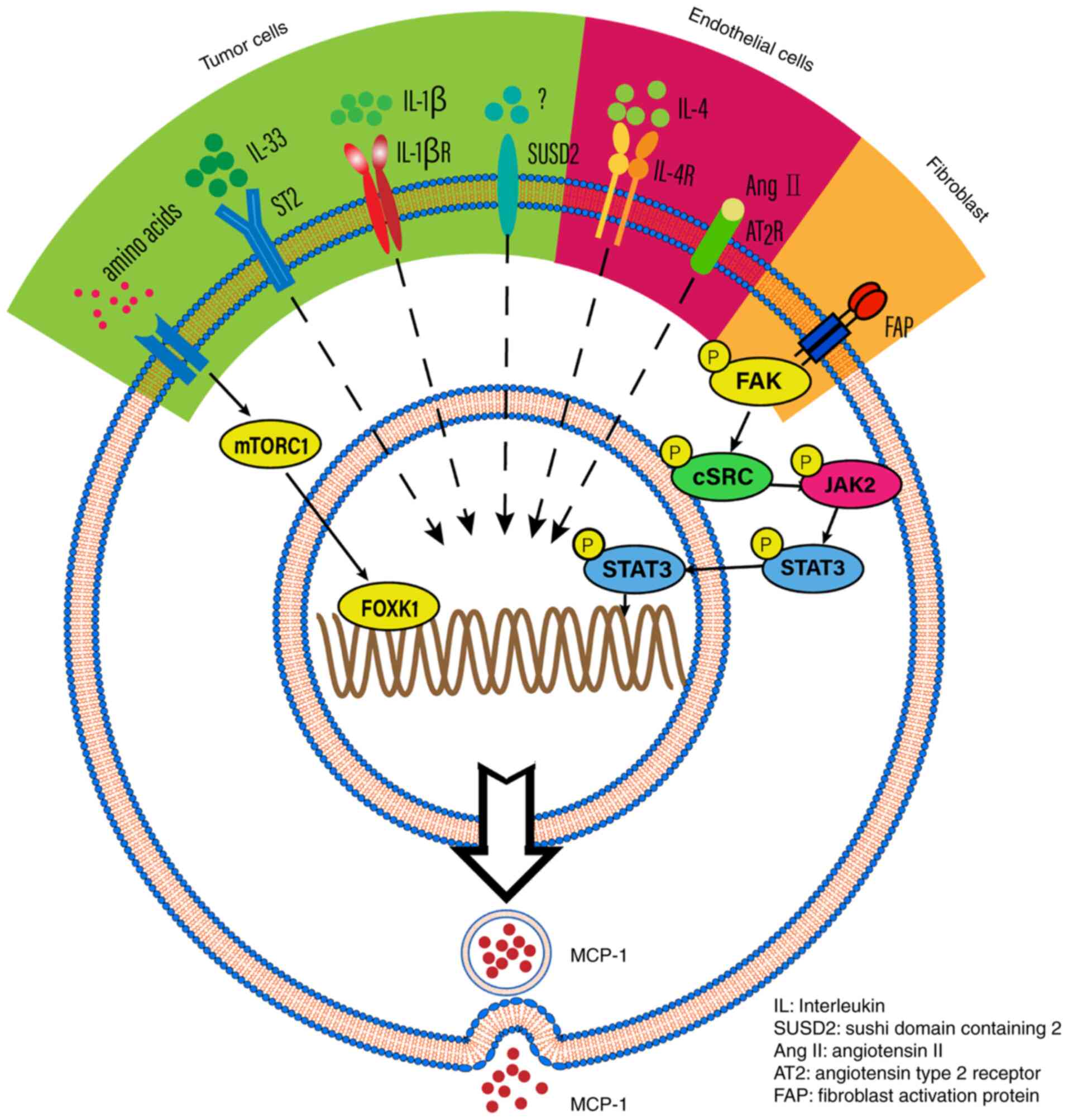 | Figure 2.The potential mechanisms that
regulate MCP-1 expression. MCP-1 expression is induced by IL-1β in
specific tumor cells. MCP-1 is downstream of IL-33, thus IL-33
which binds to its receptor (ST2) may impact on MCP-1 secretion.
MCP-1 is also mediated by mTORC1, which is stimulated by amino
acids. IN addition, MCP-1 expression is also promoted by SUSD2 in
tumor cells. Concerning endothelial cells, angiotensin II and IL-4
can mediate the expression of MCP-1. Fibroblast activation protein
can promote MCP-1 expression in cancer-associated fibroblasts. FAP,
fibroblast activation protein; IL, interleukin; SUSD2, sushi domain
containing 2; AngII, angiotensin II; AT2, angiotensin type 2
receptor; FAP, fibroblast activation protein. |
MCP-1 and CCR2 are expressed in numerous types of
cancer cells (29,30). However, MCP-1 expression may vary
in different cancer cell lines that originate from the same organ.
For example, MCP-1 has a higher expression level in invasive breast
cancer cell lines (e.g., BT594, Hs578T and MDA-MB-231) compared to
non-invasive breast cancer cell lines (e.g., MCF7 and T47D)
(31).
MCP-1 secretion by cancer cells portends to a poor
clinical outcome, due to the induction of both tumor-associated
macrophage infiltration and tumor metastasis in several solid
tumors [e.g., non-small cell lung cancer (NSCLC), prostate cancer,
breast cancer, ovarian cancer, and hepatocellular carcinoma]
(32–36). MCP-1 secretion by Schwann cells also portends to a poor
clinical outcome, due to the induction of perineural invasion
(i.e., the local extension of cancer along nerves) (37). The abnormal stimulation of MCP-1
can be treated with drugs (e.g., minocycline, telmisartan and
zoledronic acid), which have been reported to affect glioblastoma
stromal cells (38).
Previous findings have demonstrated that the TME has
been associated with tumorigenesis due to direct or indirect
interaction between surrounding cells (i.e., stromal cells,
fibroblasts, endothelial cells, and innate and adaptive immune
cells) and tumor cells. The indirect interaction could build a
two-way bridge via various cytokines, chemokines, and other
factors, including MCP-1 (13,14).
MCP-1 has been associated with tumor development in
various manners. For example, i) MCP-1 recognizes and binds
directly to CCR2-expressing cancer cells, which encourages tumor
growth and invasiveness; ii) MCP-1 recruits monocytes into the
tumor, which then differentiate into tumor-associated macrophages
(TAMs) encouraging tumor development and angiogenesis; iii) MCP-1
acts directly on endothelial cells to produce endothelial growth
factors, which encourages angiogenesis (39); and iv) MCP-1 recruits fibrocytes
into the TME and enhances the formation of stroma (40) (Fig.
3). In summary, MCP-1 stimulates activation of various
signaling pathways, which promotes tumor growth on the one hand. On
the other hand, MCP-1 causes immune-suppression, which encourages
tumor growth indirectly. Furthermore, MCP-1 enhances resistance to
tumor drugs. MCP-1 expression increases resistance to an
antiangiogenic agent, while the MCP-1 inhibitor (mNOX-E36), a L-RAN
oligonucleotide chain, restores the sensitivity to the
antiangiogenic agent (41).
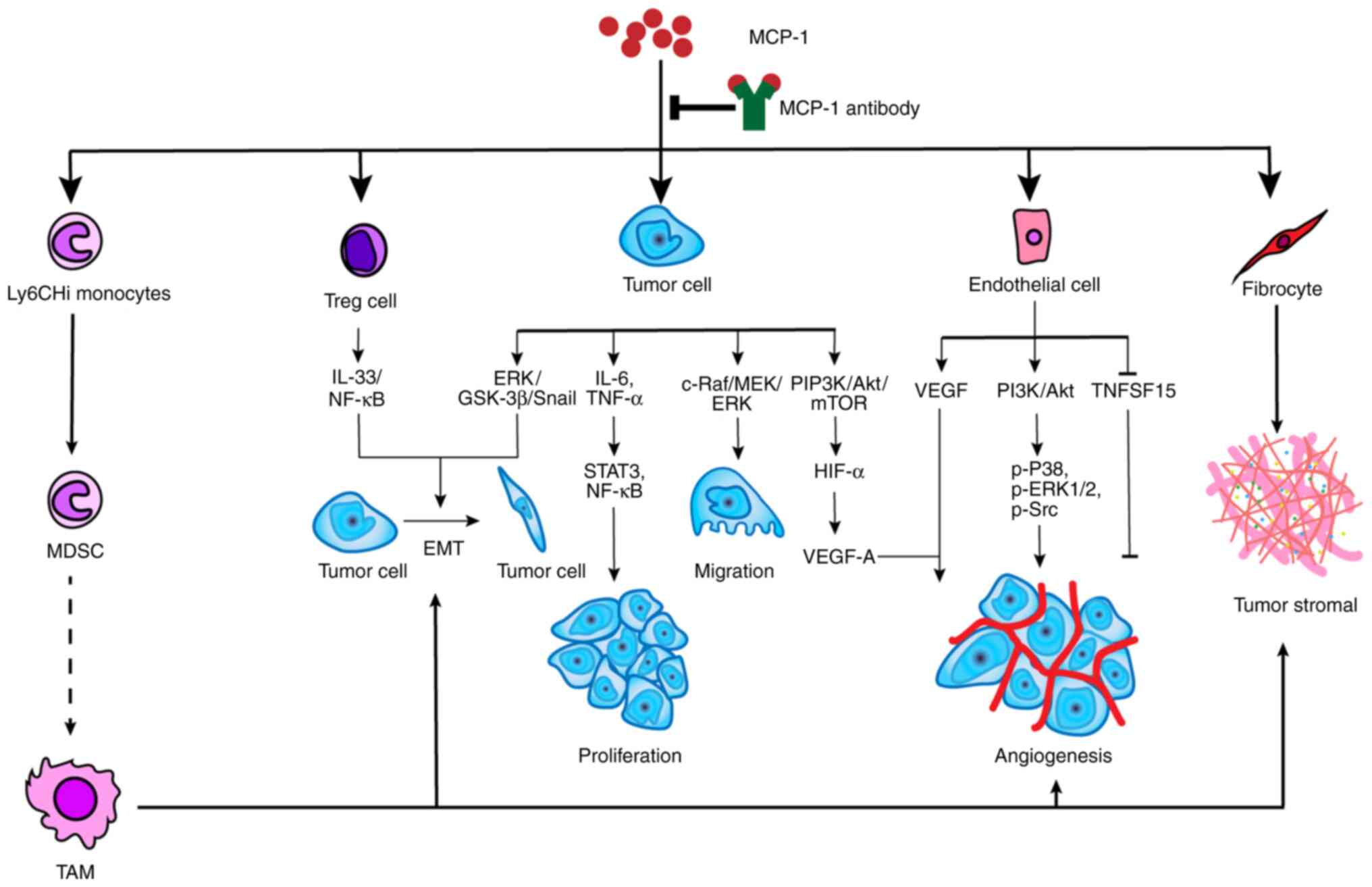 | Figure 3.The potential mechanism of
MCP-1-promoted tumor development. MCP-1 can not only utilize
monocytes, Treg cells, endothelial cells and fibrocytes but also
act on tumor directly to affect tumor development. The precise
mechanism of MDSC transforming into TAM is not clear. TAM advances
tumor development in many respects, including EMT, angiogenesis and
tumor stromal formation. Treg cells and tumor cells can induce the
process of EMT through IL-33/NF-κB and ERK/GSK-3α/Snail,
respectively. MCP-1 facilitates tumor proliferation, migration and
angiogenesis through specific pathways, such as ERK/GSK-3β/Snail
for migration, and PI3K/Akt/mTOR for angiogenesis. In addition,
MCP-1 also promotes tumor angiogenesis by acting on endothelial
cells via the PI3K/Akt signaling pathway and suppressing TNFSF15.
MCP-1 is also a promoter for fibrocytes that synthesize tumor
stromal. Notably, MCP-1 is the engine for tumor development and
shutting off the engine will inhibit this process. TNFS15, tumor
necrosis factor superfamily-15; TAM, tumor-associated macrophage;
MDSC, myeloid-derived suppressor cell; MCP-1, monocyte chemotactic
protein-1; EMT, epithelial-mesenchymal transition; Treg, regulatory
T lymphocyte. |
MCP-1 has been studied mainly in cancer with a high
incidence rate (e.g., breast, prostate and lung cancers). In this
regard, 24.5% of all new cancer cases in women are due to breast
cancer, while 14.1% of all new cancer cases in men are due to
prostate cancer, and 11.4% of all new cancer cases in both men and
women are due to lung cancer according to the International Agency
for Research on Cancer (42).
MCP-1 is associated with tumor development in a multi-faceted
process and we hypothesized that this multi-faceted process may be
analogous in various types of cancer with a high incidence rate.
The multi-faceted process of MCP-1 association with tumor
development will be subsequently discussed.
MCP-1 acts on cancer cells
directly
The MCP-1/CCR2 signaling pathway may operate in an
autocrine manner to promote tumor development, as some cancer cells
secrete MCP-1 and express CCR2 simultaneously. Previous studies
(39–41) have addressed specific mechanisms by which MCP-1 has been
associated with tumor development.
MCP-1 elevates IL-6 and TNF-α, whose downstream
oncogenic signaling pathways involve STAT3 and NF-κB in a
hepatocellular carcinoma mouse model. Furthermore, treatment with
an MCP-1 specific antibody (Ab) blocks the oncogene, c-MYC, which
is downstream of STAT3 and thereby reduces tumor proliferation in a
hepatocellular carcinoma mouse model (36). The aforementioned findings suggest
that MCP-1 plays a key role in the activation of oncogenes and
promotion of tumorigenesis.
In addition, adipocyte secretion of MCP-1, in the
TME, binds to CCR2 on cancer cells and activates the PI3K/Akt/mTOR
signaling pathway. The activation of the PI3K/Akt/mTOR signaling
pathway induces hypoxia inducible factor-1α, which mediates
vascular endothelial growth factor (VEGF)-A expression and thereby
stimulates tumor angiogenesis (35,43).
The activation of the PI3K/Akt/mTOR signaling pathway by MCP-1 also
inhibits autophagy and stimulates tumor proliferation in prostate
cancer cells and osteosarcoma cells (30,44,45).
MCP-1 also induces Akt activation in a dose-dependent manner
(46). In addition to the
activation of the PI3K/Akt/mTOR signaling pathway, the
c-Raf/MEK/ERK and MAPK signaling pathways play a role in
MCP-1-induced tumor migration (47). Furthermore, the IP3-dependent
Akt/PKB signaling pathway is associated with MCP-1-induced tumor
proliferation and migration (48).
MCP-1 can also induce MMP production in cancer cells
and thereby promotes tumor progression (49). MCP-1 enhances the aggressiveness of
NSCLC cells by increasing the level of MMP-9 expression in
vitro (50). MCP-1 induces MMP
production by activating the ERK1/2 and p38 MAPK signaling
pathways, and upregulates MMP by activating c-Raf/Raf-1, MEK, ERK,
MAPK, c-Jun, NF-κB and AP-1 (47,51,52).
MMP cleaves cell-to-cell and cell-extracellular matrix adhesion
components, which promotes cell detachment and leads to
epithelial-mesenchymal transition (EMT) and enhances metastasis
(53). In addition, MCP-1 can
directly induce EMT by activating the ERK/GSK-3β/Snail signaling
pathway (54). Overall, MCP-1
stimulates tumor proliferation and metastasis by activating the
MAPK/ERK and ERK1/2-MMP2/9 signaling pathways, respectively
(48,55).
In addition, previous studies (30,35,44–46)
have found that MCP-1 specifically recognizes the CCR2 receptor and
induces a series of signaling pathways that alter cancer cell
metabolism. The mechanism involved shows consistency among various
tumor types in which MCP1 activates classic signaling pathways,
even though the exact mechanism involved remains unclear. Thus, the
aforementioned findings suggest that MCP-1 may be a novel target
for cancer therapy.
MCP-1 facilitates endothelial cell
angiogenesis
Excessive angiogenesis is a salient feature of
various tumors, which produces a highly unorganized and permeable
tumor vasculature compared with that in normal cells. The leaky
neo-capillaries within the tumor, not only provide less
oxygen/nutrients to the tumor, but also form an abnormal TME
promoting tumor development. The endothelial cells are the
protagonist during the multi-step process of angiogenesis, and
previous studies indicate that MCP-1 interacts with endothelial
cells and may therefore be associated with angiogenesis in tumor
development (56,57).
MCP-1 downregulates the expression level of TNF
superfamily-15 (TNFSF15), which is an inhibitor of
neovascularization (58). In
addition, elevated MCP-1 expression levels were positively
correlated with VEGF expression levels, a potent angiogenic factor
(39). MCP-1 also regulates the
interaction between cancer cells and endothelial cells in
vitro, and promotes endothelial cell migration, thereby
promoting angiogenesis (57). The
binding of MCP-1 to CCR2 activates the PI3K/Akt signaling pathway
and induces phosphorylation of p38, ERK1/2, Src in endothelial
cells in vitro (59).
MCP-1 promotes monocyte/macrophage
recruitment in the TME
In addition to endothelial cells, MCP-1 is also
associated with regulating the immune microenvironment in the
tumor. Myeloid-derived suppressor cells (MDSCs), as a major
regulator of immune responses in cancer, bearing the markers CD11b
(CR3A or integrin αM) and Gr-1 [anti-Gr-1 monoclonal (m)Abs
recognize epitopes common to Ly6C and Ly6G] (60), infiltrate the tumor tissue under
hypoxia, oxidative agents, pro-inflammatory cytokines or nutrient
scarcity (39,61). Monocytic MDSCs are derived from
circulating Ly6Chi monocytes, originate from either a
myeloid or splenic reservoir in a CCR2-dependent manner, and
acquire a pro-inflammatory signature that affects lymphocyte
activity, proliferation and survival (62,63).
In addition, fibroblast activation protein-induced
cancer-associated fibroblasts promote the recruitment of MDSCs via
the production of MCP-1 (64).
Furthermore, MDSC differentiation into TAMs represents one of the
major immune cells in the TME in most types of cancer. Blocking the
MCP-1/CCR2 axis leads to a notable decrease in TAM abundance
(65). TAMs remodel the TME, which
promotes EMT and angiogenesis (65), and are divided into two categories,
the antitumor M1-like and pro-tumor M2-like TAMs. MCP-1 increases
the number of M2 TAMs but decreases the number of M1 TAMs. This
also promotes TAM-dependent lymphangiogenesis in bladder cancer
(24), which results in the immune
escape of tumor cells, initiation of blood vessel growth, and
finally the metastasis of tumor cells. A CCR2 antagonist reduces
the number of M2 TAMs and production of cytokines (i.e., IL-6,
CCL2, KC, G-CSF, MIP-1 and MIP-2), which enhances the efficacy of
tumor therapies (66).
Notably, MCP-1 assists in the recruitment of
monocytes and their differentiation into macrophages, which
suggests that MCP-1 is a key target molecule in tumor development.
Furthermore, MCP-1 modulates the progression of mammary
tumorigenesis, primarily due to its ability to recruit macrophages
to the TME. The loss of MCP-1 expression results in a decline of
macrophage markers, and reduces primary tumor volume and delays
tumor progression in a triple negative breast cancer model
(67).
MCP-1 expression and TAM recruitment demonstrate a
positive correlation, while inhibition of MCP-1 activity reduces
monocyte infiltration, TAM accumulation and tumor incidence
(61). Activation of the
MCP-1/CCR2 axis promotes the recruitment of monocytes and TAMs into
the TME in several tumor types, including sarcoma and breast cancer
(68). Monocyte recruitment into
the tumor metastatic site occurs in an MCP-1-dependent manner, and
their transformation into macrophages promotes tumor proliferation,
metastatic tumor survival/growth, and a poor prognosis in various
types of cancer [e.g., breast, prostate, bladder, kidney and NSCLC)
(34,41,69).
Knockdown of 5′-nucleotidase domain containing 2 notably reduces
TAM recruitment via suppression of the MCP-1/CCR2 signaling pathway
in colorectal carcinoma (70).
Furthermore, as aforementioned, Tgfbr2FspKO improves the
level of MCP-1 secretion and enhances tumor progression associated
with TAM recruitment, which indicates the MCP-1-dependent
attraction of macrophages into the TME depends upon the effects of
the surrounding cytokines. By contrast, recent findings showed that
MCP-1 recruits and activates macrophages to kill cancer cells in
various types of cancer (e.g., gastric and colorectal cancer, and
melanoma), and MCP-1 expression was decreased in small cell lung
cancer (69).
MCP-1 regulates monocyte attraction and infiltration
by the induction of adhesive molecules and cytokines, along with
binding to the CCR2 receptor on monocytes. The signaling pathway of
MCP-1-induced monocyte/TAM recruitment is not clear, but it has
been reported that the JAK/STAT and p42/44 MAPK/c-Jun pathways may
be involved in the activation of macrophages (71,72).
Furthermore, macrophage infiltration promotes angiogenesis with
MCP-1 expression. Elevated MCP-1 expression levels promote both
macrophage infiltration and angiogenesis (73). The number of newly formed vascular
tubes significantly increases when MCP-1-expressing cancer cells
interact with macrophages compared with MCP-1-expressing cancer
cells only (41), indicating that
MCP-1 promotes angiogenesis via macrophage attraction.
MCP-1 recruits regulatory T lymphocytes
(Tregs) into the TME
In addition to monocytes/macrophages in the TME,
MCP-1 also affects the activity of Tregs. MCP-1 recruits Treg
lymphocytes into the TME via the IL-33/NF-κB signaling pathway,
which promotes tumor development, whereas, Treg lymphocyte
recruitment into the TME fails to occur in the absence of MCP-1
(22,74). MCP-1 also recruits Treg lymphocytes
into the TME via the downregulation of TNFSF15, whereby TNFSF15
levels are inversely correlated with the degree of
CD4+CD25+FOXP3+ Treg lymphocyte
infiltration (58). In this
regard, there is a reduction of CD4+ FOXP3+
Treg lymphocytes and induction of CD8+ T-lymphocyte
cytotoxicity, which restricts tumor growth in a CCR2 knockout mouse
lung adenocarcinoma model (75).
Similarly, blocking of the MCP-1/CCR4 signaling pathway using a
CCR4 antagonist inhibits tumor growth and prolongs survival time in
patients with head and neck squamous cell carcinoma (HNSCC)
(76). The aforementioned findings
indicate that MCP-1 recruits Treg lymphocytes into the TME and
reduces the antitumor responses of effector T lymphocytes by
binding to MCP-1 receptors (Fig.
3).
MCP-1 is a potential target for tumor
therapy
MCP-1 directly or indirectly mediates changes in the
tumor, which promotes tumor progression and metastasis. This
suggests that blocking the effects of MCP-1 may serve as a novel
anticancer therapeutic strategy. MCP-1 target therapy is divided
into two categories, MCP-1 inhibitor and MCP-1 neutralizing Ab. The
latter is described, as it has a prospective clinical application
(Table I).
 | Table I.MCP-1 neutralizing antibodies
currently available. |
Table I.
MCP-1 neutralizing antibodies
currently available.
| Name | Type | Company name | Time | Application
(Refs.) | Remarks
(Refs.) |
|---|
| 2H5 | Mouse MCP-1
antibody | eBioscience/BD
Biosciences | 1994 | Umbilical cord
Mesenchymal stem cells (84) | Cross-reacted with
human MCP-1 (85) |
| 5D3-F7 | Recombinant human
MCP-1 antibody | BD Biosciences | 1994 | Human sarcoma
(86) |
|
| AF-479-NA | Mouse MCP-1
antibody | R&D
systems | 2000 | Human gastric
cancer (87); breast cancer
(88) | Cross-reacted with
human MCP-1 (87) |
| MAB479 | Mouse MCP-1
antibody | R&D
systems | 2003 | Mouse lung cancer
(89) |
|
| MAB679 | Human MCP-1
antibody | R&D
systems | 2004 | Human clear cell
renal cell carcinoma (90) |
|
| MAB279 | Human MCP-1
biotinylated antibody | R&D
systems | 2004 | Human glioblastoma
multiforme (91); human breast
cancer cell line MCF10CA1d (CA1d) (78) |
|
| AF-279-NA | Human MCP-1
antibody | R&D
systems | 2004 | Human lung cancer
(92) |
|
|
CNTO888/carlumab | Human MCP-1
antibody | Centocor Inc. | 2007 | Human prostate
cancer (33) |
|
| C1142 | Mouse MCP-1
antibody | Centocor Inc. | 2007 | Prostate cancer
(93) |
|
Previous studies have indicated that MCP-1
overexpression occurs in various cancer cells (34,66).
The blocking of the MCP-1/CCR2 signaling pathway inhibits tumor
progression (29,46) and weakens recruitment of M2
macrophages and Tregs, which activates antitumor CD8+ T
lymphocytes (32,66). MCP-1 neutralizing Ab (CNTO888)
treatment delays tumor growth in an in vivo xenograft mouse
model of prostate cancer (33) and
inhibits the dissemination of estrogen-dependent breast cancer
induced by macrophages in a zebrafish model (77). MCP-1 target therapy inhibits the
development of hepatocellular carcinoma by blocking the oncogenic
IL-6 and TNF-α signaling pathways and activating NK cells in the
TME (36). Treatment with
anti-MCP-1 mAb does not change the total leukocyte recruitment, but
does change neutrophil and M2 macrophage recruitment (32). However, MCP-1 neutralizing Ab
treatment inhibits cancer cell proliferation in vitro, but
not in vivo (78). The
reason for this difference may be due to the fact that the MCP-1
neutralizing mAbs cannot be delivered to the TME in vivo at
an effective concentration for drug efficacy.
In addition, MCP-1 target therapy and other
antitumor therapy can play a synergistic effect. Anti-MCP-1
administration enhances the effect of cisplatin by suppressing
colony formation in HNSCC in vitro (79), and MCP-1 inhibitor (mNOX-E36)
therapy enhances bevacizumab inhibition in tumor progression
(41).
Furthermore, since the N-terminal but not C-terminal
is essential for all MCP signaling and chemotactic activity, this
domain may be a potential target. As early as 1999, Van Coillie
identified the N-terminal truncated MCP-2 (the 6th amino acid
serine to 76th amino acid proline) can block the activity of intact
MCP-1 (80), as the homology
sequences between MCP-1 and MCP-2 is 62% (17,80).
Conclusion
MCP-1 acts as an engine that drives tumor
progression and therefore may serve as an effective therapeutic
target. In this regard, the CCR2 blockade (i.e., the MCP-1
receptor) shows promise due to the antitumor effects it exerts
(29,36,66,75).
However, MCP-1 is secreted not only by tumor cells, but also by
stromal cells surrounding the tumor parenchyma (81). MCP-1 neutralizing Ab treatment may
be more advantageous as a clinical strategy than CCR2 blockade for
three main reasons as indicated below.
First, MCP-1 binds to other CC-chemokine receptors
(besides CCR2), which promote tumorigenesis. Therefore, MCP-1
neutralizing Ab treatment may block not only the tumorigenic
effects of CCR2 binding, but also the tumorigenic effects of the
other CC-chemokine receptor binding. Second, MCP-1 neutralizing Ab
treatment reduces MCP-1 serum levels, which decreases systemic
inflammation and contributes to a favorable prognosis (82). Third, drug development based on
MCP-1 neutralizing Ab treatment appears to have more promise in
delivering a highly efficient therapeutic treatment.
However, the use of MCP-1 neutralizing Ab requires
further research. Traditionally, it has been argued that cessation
of anti-MCP-1 treatment led to a rebound of MCP-1 and a notable
increase in metastases (83). It
points out the issue of utilizing the MCP-1 neutralizing Ab
correctly, which will improve the side effects. Antitumor drugs may
be selected according to tumor types, and observe the principle of
concomitant drugs and full course of treatment. Certainly, it is on
the premise of the development of an ideal antibody of MCP-1.
In conclusion, further research is required to
reveal the role of MCP-1 in cancer progression in order to identify
the most beneficial target and design the most effective
therapeutic strategy.
Acknowledgements
Not applicable.
Funding
Funding was obtained from the General Project of Applied Basic
and Cutting-edge Technology, Tianjin Science and Technology
Commission (grant no. 20JCYBJC01500), and the National Natural
Science Foundation of China (grant nos. 81872325 and 82172640).
Availability of data and materials
Not applicable.
Authors' contributions
LW was the major person in charge of the organizing
and writing of the revised manuscript during the peer review
process. JL searched the associated papers, interpreted the
information and wrote the draft. JT searched the associated papers,
interpreted the information and wrote the outline of the draft. NL
was in charge of writing, interpreting and overseeing the
manuscript. All authors read and approved the final manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
da Cunha Santos G, Shepherd FA and Tsao
MS: EGFR mutations and lung cancer. Annu Rev Pathol. 6:49–69. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paez JG, Jänne PA, Lee JC, Tracy S,
Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et
al: EGFR mutations in lung cancer: Correlation with clinical
response to gefitinib therapy. Science. 304:1497–1500. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deshmane SL, Kremlev S, Amini S and Sawaya
BE: Monocyte chemoattractant protein-1 (MCP-1): An overview. J
Interferon Cytokine Res. 29:313–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Blanpain CD, Migeotte I, Lee B, Vakili J,
Doranz BJ, Govaerts C, Vassart G, Doms RW and Parmentier M: CCR5
binds multiple CC-chemokines: MCP-3 acts as a natural antagonist.
Blood. 94:1899–1905. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bonini JA, Martin SK, Dralyuk F, Roe MW,
Philipson LH and Steiner DF: Cloning, expression, and chromosomal
mapping of a novel human CC-chemokine receptor (CCR10) that
displays high-affinity binding for MCP-1 and MCP-3. DNA Cell Biol.
16:1249–1256. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hemmerich S, Paavola C, Bloom A, Bhakta S,
Freedman R, Grunberger D, Krstenansky J, Lee S, McCarley D, Mulkins
M, et al: Identification of residues in the monocyte chemotactic
protein-1 that contact the MCP-1 receptor, CCR2. Biochemistry.
38:13013–13025. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kashiwazaki M, Tanaka T, Kanda H, Ebisuno
Y, Izawa D, Fukuma N, Akimitsu N, Sekimizu K, Monden M and Miyasaka
M: A high endothelial venule-expressing promiscuous chemokine
receptor DARC can bind inflammatory, but not lymphoid, chemokines
and is dispensable for lymphocyte homing under physiological
conditions. Int Immunol. 15:1219–1227. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schweickart VL, Epp A, Raport CJ and Gray
PW: CCR11 is a functional receptor for the monocyte chemoattractant
protein family of chemokines. J Biol Chem. 275:9550–9556. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Robinson EA, Yoshimura T, Leonard EJ,
Tanaka S, Griffin PR, Shabanowitz J, Hunt DF and Appella E:
Complete amino acid sequence of a human monocyte chemoattractant, a
putative mediator of cellular immune reactions. Proc Natl Acad Sci
USA. 86:1850–1854. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoshimura T, Robinson EA, Tanaka S,
Appella E and Leonard EJ: Purification and amino acid analysis of
two human monocyte chemoattractants produced by
phytohemagglutinin-stimulated human blood mononuclear leukocytes. J
Immunol. 142:1956–1962. 1989.PubMed/NCBI
|
|
11
|
Yoshimura T, Yuhki N, Moore SK, Appella E,
Lerman MI and Leonard EJ: Human monocyte chemoattractant protein-1
(MCP-1). Full-length cDNA cloning, expression in mitogen-stimulated
blood mononuclear leukocytes, and sequence similarity to mouse
competence gene JE. FEBS Lett. 244:487–493. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bottazzi B, Colotta F, Sica A, Nobili N
and Mantovani A: A chemoattractant expressed in human sarcoma cells
(tumor-derived chemotactic factor, TDCF) is identical to monocyte
chemoattractant protein-1/monocyte chemotactic and activating
factor (MCP-1/MCAF). Int J Cancer. 45:795–797. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujisaki K, Fujimoto H, Sangai T,
Nagashima T, Sakakibara M, Shiina N, Kuroda M, Aoyagi Y and
Miyazaki M: Cancer-mediated adipose reversion promotes cancer cell
migration via IL-6 and MCP-1. Breast Cancer Res Treat. 150:255–263.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu Q, Li B, Li Z, Li J and Sun S and Sun
S: Cancer-associated adipocytes: Key players in breast cancer
progression. J Hematol Oncol. 12:952019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mehrabian M, Sparkes RS, Mohandas T,
Fogelman AM and Lusis AJ: Localization of monocyte chemotactic
protein-1 gene (SCYA2) to human chromosome 17q11.2-q21.1. Genomics.
9:200–203. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lubkowski J, Bujacz G, Boqué L, Domaille
PJ, Handel TM and Wlodawer A: The structure of MCP-1 in two crystal
forms provides a rare example of variable quaternary interactions.
Nat Struct Biol. 4:64–69. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Ernst CA and Rollins BJ: MCP-1:
Structure/activity analysis. Methods. 10:93–103. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Handel TM and Domaille PJ: Heteronuclear
(1H, 13C, 15N) NMR assignments and solution structure of the
monocyte chemoattractant protein-1 (MCP-1) dimer. Biochemistry.
35:6569–6584. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Proost P, Struyf S, Couvreur M, Lenaerts
JP, Conings R, Menten P, Verhaert P, Wuyts A and Damme JV:
Posttranslational modifications affect the activity of the human
monocyte chemotactic proteins MCP-1 and MCP-2: Identification of
MCP-2(6–76) as a natural chemokine inhibitor. J Immunol.
160:4034–4041. 1998.PubMed/NCBI
|
|
20
|
Jung Y, Ahn SH, Park H, Park SH, Choi K,
Choi C, Kang JL and Choi YH: MCP-1 and MIP-3α secreted from
necrotic cell-treated glioblastoma cells promote
migration/infiltration of microglia. Cell Physiol Biochem.
48:1332–1346. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee CH, Hung PF, Lu SC, Chung HL, Chiang
SL, Wu CT, Chou WC and Sun CY: MCP-1/MCPIP-1 signaling modulates
the effects of IL-1β in renal cell carcinoma through ER
stress-mediated apoptosis. Int J Mol Sci. 20:61012019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yue Y, Lian J, Wang T, Luo C, Yuan Y, Qin
G, Zhang B and Zhang Y: Interleukin-33-nuclear factor-κB-CCL2
signaling pathway promotes progression of esophageal squamous cell
carcinoma by directing regulatory T cells. Cancer Sci. 111:795–806.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nakatsumi H, Matsumoto M and Nakayama KI:
Noncanonical pathway for regulation of CCL2 expression by an
mTORC1-FOXK1 axis promotes recruitment of tumor-associated
macrophages. Cell Rep. 21:2471–2486. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen C, He W, Huang J, Wang B, Li H, Cai
Q, Su F, Bi J, Liu H, Zhang B, et al: LNMAT1 promotes lymphatic
metastasis of bladder cancer via CCL2 dependent macrophage
recruitment. Nat Commun. 9:38262018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu S, Liu D, Zeng X, Wang J, Liu J, Cheng
J, Lei K, Bai H, Ji N, Zhou M, et al: PA28γ acts as a dual
regulator of IL-6 and CCL2 and contributes to tumor angiogenesis in
oral squamous cell carcinoma. Cancer Lett. 428:192–200. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Castiñeiras-Landeira MI, Rodiño-Janeiro
BK, Paradela-Dobarro B, Batista-Oliveira AL, Raposeiras-Roubín S,
González-Peteiro M, González-Juanatey JR and Álvarez E: Change of
concept about the regulation of angiotensin II-induced monocyte
chemoattractant protein-1 production in human endothelial cells.
Vascul Pharmacol. 80:20–34. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rollins BJ and Pober JS: Interleukin-4
induces the synthesis and secretion of MCP-1/JE by human
endothelial cells. Am J Pathol. 138:1315–1319. 1991.PubMed/NCBI
|
|
28
|
Hembruff SL, Jokar I, Yang L and Cheng N:
Loss of transforming growth factor-beta signaling in mammary
fibroblasts enhances CCL2 secretion to promote mammary tumor
progression through macrophage-dependent and -independent
mechanisms. Neoplasia. 12:425–433. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kuper C, Beck FX and Neuhofer W: Autocrine
MCP-1/CCR2 signaling stimulates proliferation and migration of
renal carcinoma cells. Oncol Lett. 12:2201–2209. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu Y, Cai Z, Galson DL, Xiao G, Liu Y,
George DE, Melhem MF, Yao Z and Zhang J: Monocyte chemotactic
protein-1 (MCP-1) acts as a paracrine and autocrine factor for
prostate cancer growth and invasion. Prostate. 66:1311–1318. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mohamed HT, El-Ghonaimy EA, El-Shinawi M,
Hosney M, Götte M, Woodward WA, El-Mamlouk T and Mohamed MM: IL-8
and MCP-1/CCL2 regulate proteolytic activity in triple negative
inflammatory breast cancer a mechanism that might be modulated by
Src and Erk1/2. Toxicol Appl Pharmacol. 401:1150922020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fridlender ZG, Kapoor V, Buchlis G, Cheng
G, Sun J, Wang LC, Singhal S, Snyder LA and Albelda SM: Monocyte
chemoattractant protein-1 blockade inhibits lung cancer tumor
growth by altering macrophage phenotype and activating CD8+ cells.
Am J Respir Cell Mol Biol. 44:230–237. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Loberg RD, Ying C, Craig M, Yan L, Snyder
LA and Pienta KJ: CCL2 as an important mediator of prostate cancer
growth in vivo through the regulation of macrophage infiltration.
Neoplasia. 9:556–562. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Qian BZ, Li J, Zhang H, Kitamura T, Zhang
J, Campion LR, Kaiser EA, Snyder LA and Pollard JW: CCL2 recruits
inflammatory monocytes to facilitate breast-tumour metastasis.
Nature. 475:222–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun C, Li X, Guo E, Li N, Zhou B, Lu H,
Huang J, Xia M, Shan W, Wang B, et al: MCP-1/CCR-2 axis in
adipocytes and cancer cell respectively facilitates ovarian cancer
peritoneal metastasis. Oncogene. 39:1681–1695. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Teng KY, Han J, Zhang X, Hsu SH, He S,
Wani NA, Barajas JM, Snyder LA, Frankel WL, Caligiuri MA, et al:
Blocking the CCL2-CCR2 axis using CCL2-neutralizing antibody is an
effective therapy for hepatocellular cancer in a mouse model. Mol
Cancer Ther. 16:312–322. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bakst RL, Xiong H, Chen CH, Deborde S,
Lyubchik A, Zhou Y, He S, McNamara W, Lee SY, Olson OC, et al:
Inflammatory monocytes promote perineural invasion via
CCL2-mediated recruitment and cathepsin B expression. Cancer Res.
77:6400–6414. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Salacz M, Kast RE, Saki N, Brüning A,
Karpel-Massler G and Halatsch ME: Toward a noncytotoxic
glioblastoma therapy: Blocking MCP-1 with the MTZ regimen. Onco
Targets Ther. 27:2535–2545. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ueno T, Toi M, Saji H, Muta M, Bando H,
Kuroi K, Koike M, Inadera H and Matsushima K: Significance of
macrophage chemoattractant protein-1 in macrophage recruitment,
angiogenesis, and survival in human breast cancer. Clin Cancer Res.
6:3282–3289. 2000.PubMed/NCBI
|
|
40
|
Kuziel G, Thompson V, D'Amato JV and
Arendt LM: Stromal CCL2 signaling promotes mammary tumor fibrosis
through recruitment of myeloid-lineage cells. Cancers (Basel).
12:20832020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cho HR, Kumari N, Vu HT, Kim H, Park CK
and Choi SH: Increased antiangiogenic effect by blocking
CCL2-dependent macrophages in a rodent glioblastoma model:
Correlation study with dynamic susceptibility contrast perfusion
MRI. Sci Rep. 9:110852019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guru SK, Pathania AS, Kumar S, Ramesh D,
Kumar M, Rana S, Kumar A, Malik F, Sharma PR, Chandan BK, et al:
Secalonic acid-D represses HIF1alpha/VEGF-mediated angiogenesis by
regulating the Akt/mTOR/p70S6K signaling cascade. Cancer Res.
75:2886–2896. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang J, Lu Y and Pienta KJ: Multiple
roles of chemokine (C-C motif) ligand 2 in promoting prostate
cancer growth. J Natl Cancer Inst. 102:522–528. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen Q, Sun W, Liao Y, Zeng H, Shan L, Yin
F, Wang Z, Zhou Z, Hua Y and Cai Z: Monocyte chemotactic protein-1
promotes the proliferation and invasion of osteosarcoma cells and
upregulates the expression of AKT. Mol Med Rep. 12:219–225. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Loberg RD, Day LL, Harwood J, Ying C, John
LN, Giles R, Neeley CK and Pienta KJ: CCL2 is a potent regulator of
prostate cancer cell migration and proliferation. Neoplasia.
8:578–586. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu JF, Chen PC, Chang TM and Hou CH:
Monocyte chemoattractant protein-1 promotes cancer cell migration
via c-Raf/MAPK/AP-1 pathway and MMP-9 production in osteosarcoma. J
Exp Clin Cancer Res. 39:2542020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
He S and Zhang X: The rs1024611 in the
CCL2 gene and risk of gynecological cancer in Asians: A
meta-analysis. World J Surg Oncol. 16:342018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Ito Y, Ishiguro H, Kobayashi N, Hasumi H,
Watanabe M, Yao M and Uemura H: Adipocyte-derived monocyte
chemotactic protein-1 (MCP-1) promotes prostate cancer progression
through the induction of MMP-2 activity. Prostate. 75:1009–1019.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
An J, Xue Y, Long M, Zhang G, Zhang J and
Su H: Targeting CCR2 with its antagonist suppresses viability,
motility and invasion by downregulating MMP-9 expression in
non-small cell lung cancer cells. Oncotarget. 8:39230–39240. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tang CH and Tsai CC: CCL2 increases MMP-9
expression and cell motility in human chondrosarcoma cells via the
Ras/Raf/MEK/ERK/NF-κB signaling pathway. Biochem Pharmacol.
83:335–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Yang CQ, Li W, Li SQ, Li J, Li YW, Kong
SX, Liu RM, Wang SM and Lv WM: MCP-1 stimulates MMP-9 expression
via ERK 1/2 and p38 MAPK signaling pathways in human aortic smooth
muscle cells. Cell Physiol Biochem. 34:266–276. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Orlichenko LS and Radisky DC: Matrix
metalloproteinases stimulate epithelial-mesenchymal transition
during tumor development. Clin Exp Metastasis. 25:593–600. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Li S, Lu J, Chen Y, Xiong N, Li L, Zhang
J, Yang H, Wu C, Zeng H and Liu Y: MCP-1-induced ERK/GSK-3β/snail
signaling facilitates the epithelial-mesenchymal transition and
promotes the migration of MCF-7 human breast carcinoma cells. Cell
Mol Immunol. 14:621–630. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu W, Wang L, Zhang J, Qiao L, Liu Y,
Yang X, Zhang J, Zheng W and Ma Z: Purification of recombinant
human chemokine CCL2 in E. coli and its function in ovarian
cancer. 3 Biotech. 11:82021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Salcedo R, Ponce ML, Young HA, Wasserman
K, Ward JM, Kleinman HK, Oppenheim JJ and Murphy WJ: Human
endothelial cells express CCR2 and respond to MCP-1: Direct role of
MCP-1 in angiogenesis and tumor progression. Blood. 96:34–40. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang S, Xu M, Li F, Wang X, Bower KA,
Frank JA, Lu Y, Chen G, Zhang Z, Ke Z, et al: Ethanol promotes
mammary tumor growth and angiogenesis: The involvement of
chemoattractant factor MCP-1. Breast Cancer Res Treat.
133:1037–1048. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Deng W, Gu X, Lu Y, Gu C, Zheng Y, Zhang
Z, Chen L, Yao Z and Li LY: Down-modulation of TNFSF15 in ovarian
cancer by VEGF and MCP-1 is a pre-requisite for tumor
neovascularization. Angiogenesis. 15:71–85. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Arefieva TI, Kukhtina NB, Antonova OA and
Krasnikova TL: MCP-1-stimulated chemotaxis of monocytic and
endothelial cells is dependent on activation of different signaling
cascades. Cytokine. 31:439–446. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Bronte V, Brandau S, Chen SH, Colombo MP,
Frey AB, Greten TF, Mandruzzato S, Murray PJ, Ochoa A,
Ostrand-Rosenberg S, et al: Recommendations for myeloid-derived
suppressor cell nomenclature and characterization standards. Nat
Commun. 7:121502016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Yang H, Zhang Q, Xu M, Wang L, Chen X,
Feng Y, Li Y, Zhang X, Cui W and Jia X: CCL2-CCR2 axis recruits
tumor associated macrophages to induce immune evasion through PD-1
signaling in esophageal carcinogenesis. Mol Cancer. 19:412020.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Guilliams M, Mildner A and Yona S:
Developmental and functional heterogeneity of monocytes. Immunity.
49:595–613. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Shand FH, Ueha S, Otsuji M, Koid SS,
Shichino S, Tsukui T, Kosugi-Kanaya M, Abe J, Tomura M, Ziogas J
and Matsushima K: Tracking of intertissue migration reveals the
origins of tumor-infiltrating monocytes. Proc Natl Acad Sci USA.
111:7771–7776. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Yang X, Lin Y, Shi Y, Li B, Liu W, Yin W,
Dang Y, Chu Y, Fan J and He R: FAP promotes immunosuppression by
cancer-associated fibroblasts in the tumor microenvironment via
STAT3-CCL2 signaling. Cancer Res. 76:4124–4135. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Laviron M and Boissonnas A: Ontogeny of
tumor-associated macrophages. Front Immunol. 10:17992019.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li X, Yao W, Yuan Y, Chen P, Li B, Li J,
Chu R, Song H, Xie D, Jiang X, et al: Targeting of
tumour-infiltrating macrophages via CCL2/CCR2 signalling as a
therapeutic strategy against hepatocellular carcinoma. Gut.
66:157–167. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Cranford TL, Velázquez KT, Enos RT, Bader
JE, Carson MS, Chatzistamou L, Nagarkatti M and Murphy EA: Loss of
monocyte chemoattractant protein-1 expression delays mammary
tumorigenesis and reduces localized inflammation in the
C3(1)/SV40Tag triple negative breast cancer model. Cancer Biol
Ther. 18:85–93. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Li F, Kitajima S, Kohno S, Yoshida A,
Tange S, Sasaki S, Okada N, Nishimoto Y, Muranaka H, Nagatani N, et
al: Retinoblastoma inactivation induces a protumoral
microenvironment via enhanced CCL2 secretion. Cancer Res.
79:3903–3915. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zheng Y, Wang Z, Wei S, Liu Z and Chen G:
Epigenetic silencing of chemokine CCL2 represses macrophage
infiltration to potentiate tumor development in small cell lung
cancer. Cancer Lett. 499:148–163. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Zhu Z, Hou Q and Guo H: NT5DC2 knockdown
inhibits colorectal carcinoma progression by repressing metastasis,
angiogenesis and tumor-associated macrophage recruitment: A
mechanism involving VEGF signaling. Exp Cell Res. 397:1123112020.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sodhi A and Biswas SK: Monocyte
chemoattractant protein-1-induced activation of p42/44 MAPK and
c-Jun in murine peritoneal macrophages: A potential pathway for
macrophage activation. J Interferon Cytokine Res. 22:517–526. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Biswas SK and Sodhi A: Tyrosine
phosphorylation-mediated signal transduction in MCP-1-induced
macrophage activation: Role for receptor dimerization, focal
adhesion protein complex and JAK/STAT pathway. Int Immunopharmacol.
2:1095–1107. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Kuroda T, Kitadai Y, Tanaka S, Yang X,
Mukaida N, Yoshihara M and Chayama K: Monocyte chemoattractant
protein-1 transfection induces angiogenesis and tumorigenesis of
gastric carcinoma in nude mice via macrophage recruitment. Clin
Cancer Res. 11:7629–7636. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chang AL, Miska J, Wainwright DA, Dey M,
Rivetta CV, Yu D, Kanojia D, Pituch KC, Qiao J, Pytel P, et al:
CCL2 produced by the glioma microenvironment is essential for the
recruitment of regulatory T cells and myeloid-derived suppressor
cells. Cancer Res. 76:5671–5682. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Mittal P, Wang L, Akimova T, Leach CA,
Clemente JC, Sender MR, Chen Y, Turunen BJ and Hancock WW: The
CCR2/MCP-1 chemokine pathway and lung adenocarcinoma. Cancers
(Basel). 12:37232020. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Sun W, Li WJ, Wei FQ, Wong TS, Lei WB, Zhu
XL, Li J and Wen WP: Blockade of MCP-1/CCR4 signaling-induced
recruitment of activated regulatory cells evokes an antitumor
immune response in head and neck squamous cell carcinoma.
Oncotarget. 7:37714–37727. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Svensson S, Abrahamsson A, Rodriguez GV,
Olsson AK, Jensen L, Cao Y and Dabrosin C: CCL2 and CCL5 are novel
therapeutic targets for estrogen-dependent breast cancer. Clin
Cancer Res. 21:3794–3805. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Yao M, Smart C, Hu Q and Cheng N:
Continuous delivery of neutralizing antibodies elevate CCL2 levels
in mice bearing MCF10CA1d breast tumor xenografts. Transl Oncol.
10:734–743. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Wichmann G, Körner C, Boehm A, Mozet C and
Dietz A: Stimulation by monocyte chemoattractant protein-1
modulates the ex-vivo colony formation by head and neck squamous
cell carcinoma cells. Anticancer Res. 35:3917–3924. 2015.PubMed/NCBI
|
|
80
|
Van Coillie E, Van Damme J and Opdenakker
G: The MCP/eotaxin subfamily of CC chemokines. Cytokine Growth
Factor Rev. 10:61–86. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yoshimura T: The production of monocyte
chemoattractant protein-1 (MCP-1)/CCL2 in tumor microenvironments.
Cytokine. 98:71–78. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Laird BJA, Fallon M, Hjermstad MJ, Tuck S,
Kaasa S, Klepstad P and McMillan DC: Quality of life in patients
with advanced cancer: Differential association with performance
status and systemic inflammatory response. J Clin Oncol.
34:2769–2775. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Bonapace L, Coissieux MM, Wyckoff J, Mertz
KD, Varga Z, Junt T and Bentires-Alj M: Cessation of CCL2
inhibition accelerates breast cancer metastasis by promoting
angiogenesis. Nature. 515:130–133. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Shen C, Lie P, Miao T, Yu M, Lu Q, Feng T,
Li J, Zu T, Liu X and Li H: Conditioned medium from umbilical cord
mesenchymal stem cells induces migration and angiogenesis. Mol Med
Rep. 12:20–30. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Luo Y, Laning J, Hayashi M, Hancock PR,
Rollins B and Dorf ME: Serologic analysis of the mouse beta
chemokine JE/monocyte chemoattractant protein-1. J Immunol.
153:3708–3716. 1994.PubMed/NCBI
|
|
86
|
Peri G, Milanese C, Matteucci C, Ruco L,
Zhou D, Sozzani S, Coletta I and Mantovani A: A new monoclonal
antibody (5D3-F7) which recognizes human monocyte-chemotactic
protein-1 but not related chemokines. Development of a sandwich
ELISA and in situ detection of producing cells. J Immunol Methods.
174:249–257. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Zhao C, Bu X, Wang W, Ma T and Ma H:
GEC-derived SFRP5 inhibits Wnt5a-induced macrophage chemotaxis and
activation. PLoS One. 9:e850582014. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Fujimoto H, Sangai T, Ishii G, Ikehara A,
Nagashima T, Miyazaki M and Ochiai A: Stromal MCP-1 in mammary
tumors induces tumor-associated macrophage infiltration and
contributes to tumor progression. Int J Cancer. 125:1276–1284.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Roy RM, Wuthrich M and Klein BS: Chitin
elicits CCL2 from airway epithelial cells and induces
CCR2-dependent innate allergic inflammation in the lung. J Immunol.
189:2545–2552. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Arakaki R, Yamasaki T, Kanno T, Shibasaki
N, Sakamoto H, Utsunomiya N, Sumiyoshi T, Shibuya S, Tsuruyama T,
Nakamura E, et al: CCL2 as a potential therapeutic target for clear
cell renal cell carcinoma. Cancer Med. 5:2920–2933. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lai SW, Liu YS, Lu DY and Tsai CF:
Melatonin modulates the microenvironment of glioblastoma multiforme
by targeting sirtuin 1. Nutrients. 11:13432019. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Zhan Z, Xie X, Cao H, Zhou X, Zhang XD,
Fan H and Liu Z: Autophagy facilitates TLR4- and TLR3-triggered
migration and invasion of lung cancer cells through the promotion
of TRAF6 ubiquitination. Autophagy. 10:257–268. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Loberg RD, Ying C, Craig M, Day LL,
Sargent E, Neeley C, Wojno K, Snyder LA, Yan L and Pienta KJ:
Targeting CCL2 with systemic delivery of neutralizing antibodies
induces prostate cancer tumor regression in vivo. Cancer Res.
67:9417–9424. 2007. View Article : Google Scholar : PubMed/NCBI
|

















