Introduction
Cervical cancer (CVC) is currently the second
leading cause of mortality in women worldwide, and according to the
in 2018 World Health Report of the World Health Organization, the
average age-adjusted incidence of CVC cancer was 13.1 per 100,000
(1). Morbidity and mortality rates
are higher in developing countries compared with developed
countries (2). Vaccination against
the human papillomavirus (HPV) can reduce the incidence of CVC,
although CVC cannot be completely prevented (3). Further research is therefore needed
for the development of CVC treatment strategies beyond the methods
used for CVC diagnosis such as smear cytology and colposcopy.
CVC is highly associated with oncogenic mutations
caused by HPV infection, which are responsible for host cell
proliferation (4). Furthermore,
most HPV types, including HPV16, HPV18 and HPV84, encode
oncoproteins such as E6 and E7 that overcome host tumor-suppressor
and cell-cycle checkpoint proteins like p53, cyclin E, p21, and p27
(5–8). Current treatments for CVC include
surgery, radiation therapy and chemotherapy (9,10).
In the case of chemotherapy, certain drugs such as cisplatin and
doxorubicin are preferred for reducing the preoperative tumor size
or preventing postoperative recurrence, as well as for treating
metastases (11). However, some
patients have reported some adverse side effects or presented with
resistance to drug therapy (12,13).
Apoptosis is one of the main mechanisms of action of
chemotherapeutic cancer drugs and is a type of
mitochondria-mediated cell death (14). Apoptotic stress activates
pro-apoptotic agents such as BAX, which are regulated by the
activated Bcl-2 molecule. Activated BAX stimulates the release of
cytochrome c from the mitochondria. In addition, the
released cytochrome c induces apoptosis by proteolysis,
membrane damage and DNA cleavage through activation of Apaf-1,
caspase-9 and caspase-3 (15). In
particular, oxidative stress-induced cell-death pathways promote
mitochondrial fission, which is associated with lipid accumulation
(16,17). In addition, doxorubicin can induce
lipid droplet accumulation in the cytoplasm in various types of
cancer, including colorectal cancer, lymphoma and breast cancer,
which participate in apoptosis (18). One important strategies for
treating CVC may therefore involve the induction of apoptosis by
regulating mitochondrial function.
Recently, aspirin has been used for its anticancer
effects in various types of tumor, including gastric, esophageal
and colon cancers (18). For
example, in gastric cancer cell line SGC7901, the survival of cells
treated with 3.0 and 10.0 mmol/l aspirin for 24 h was decreased by
44.6 and 88.5%, respectively (19). Data from a clinical report
indicated that aspirin may help inhibiting breast cancer metastasis
(20,21). Subsequently, in a previous study,
we designed an aspirin conjugated chalcone derivative that had
anti-inflammation and anti-tumor properties and prepared
nanoparticle (NP) formulations, since some anticancer drugs,
including the chemical used in the present study, have non-polar
properties that make them difficult to be used in an injectable
form (22). This revealed that
aspirin and 2′-hydroxy-2,3,5′-trimethoxychalcone linked polymeric
nanoparticles (AS-DK143-NPs) selectively targeted cancer cells and
significantly reduce tumor sizes in a breast cancer xenograft model
with low toxicity to healthy tissues. In the present study extended
our previous observation by performing additional experiments to
provide a comprehensive understanding of the role of AS-DK143-NPs
against CVC in several representative solid cancer cell lines such
as HepG2, BXPC-3, AGS and HeLa.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), Roswell
Park Memorial Institute (RPMI) medium, fetal bovine serum (FBS) and
penicillin/streptomycin (P/S) were purchased from Welgene Inc.
WST-1 and bovine serum albumin (BSA) were purchased from
Sigma-Aldrich; Merck KGaA. A TUNEL assay kit was obtained from
Promega Corporation. Hoechst dye, Mito-tracker™ Red
CMXRos and the Alexa-Fluor 488-conjugated donkey anti-Rabbit
antibody were purchased from Thermo Fisher Scientific, Inc. The
anti-Ki-67 antibody was purchased from Abcam (cat. no. ab15580).
The aspirin conjugated DK143
[(E)-2-(4-(2,3-dimethoxyphenyl)acryloyl)-4-methoxyphenyl-2-acetoxybenzoate,
AS-DK143] and AS-DK143-loaded methoxy poly(ethylene
glycol)-poly(L-lactide) NPs (AS-DK143-NPs) were prepared and
characterized according to our previous report (22). HeLa cell line, HepG2, which is a
hepatoblastoma cell line, and AGS cell line were purchased from the
Korean Cell Line Bank; Korean Cell Line Research Foundation. BXPC-3
cells were obtained from the Japanese Collection of Research
Bioresources Cell Bank.
Cell line authentication
Cell lines were authenticated using short tandem
repeat (STR) analysis as described in 2012 in the ANSI standard
(ASN-0002) by the American Type Culture Collection Standards
Development Organization and by Reid et al (23) The match criterion is based on an
algorithm that compares the number of shared alleles between the
reference and sample profile.
Preparation of
(E)-2-(4-(2,3-dimethoxyphenyl)acryloyl)-4-methoxyphenyl-2
acetoxybenzo ate (AS-DK143)-loaded polymeric micelles
(E)-3-(2,3-dimethoxyphenyl)-1-(2-hydroxy-5-methoxyphenyl)prop-2-en-1-one
(DK143), one of the chalcone derivatives, was used to synthesize
AS-DK143 using the following procedures.
1-(3-Dimethylaminopropyl)-3-ethylcarbodimide hydrochloride
(EDC·HCl, 0.641 g, 3.3 mmol) and 4-dimethylaminopyridine (DMAP,
0.816 g, 3.3 mmol) were added to chloroform (50 ml) containing
aspirin (0.574 g, 3 mmol) and DK143 (1.0 g, 3 mmol). The solution
was stirred at room temperature for 24 h. At the end of the
reaction, ice water was added to the mixture solution and acidified
with 6 N HCl. After extraction with ethyl acetate (100 ml, × 3),
the organic layer was purified using silica gel flash column
chromatography to yield 33.3% (0.48 g) as a pale-yellow solid.
Preparation of AS-DK143-loaded
mPEG-PLA nanoparticles (NPs)
AS-DK143 polymeric micelles (AS-DK143-NPs) were
prepared using the thin-film hydration method. Briefly, methoxy
poly(ethylene glycol)-b-poly(D,L-lactide) (mPEG-PLA, 60 mg) and
AS-DK143 (10 mg) were dissolved in dichloromethane (50 ml) and then
removed by rotary evaporator under reduced pressure at 40°C. The
film obtained from this process contained AS-DK143 distributed in
an amorphous form. The film was continuously hydrated in distilled
water (50 ml) at room temperature. The solution was filtered
through a syringe filter (0.2 µm) and lyophilized for 48 h.
Cell viability and cell
morphology
HeLa (human cervical cancer cell line) and HepG2
cells (human liver cancer cell line) were cultured in DMEM
supplemented with 10% FBS and 1% P/S and placed at 37°C in a
humidified incubator containing 5% CO2. AGS (human
gastric adenocarcinoma hyperdiploid cell line) and BXPC-3 cells
(human pancreatic cancer cell line) were cultured in RPMI
supplemented with 10% FBS and 1% P/S and placed at 37°C in a
humidified incubator containing 5% CO2. Cells were
seeded at the density of 1×104 cells/well in 96-well
plates, cultured for 24 h and treated with different concentration
of AS-DK143-NPs (1, 3, 10, 30, 100 or 300 µM) for 24 h. To
determine the cell viability, cells were incubated with 10 µl of
WST-1 for 2 h at 37°C. Absorbance was read at 450 nm using an iMARK
spectrophotometer (Bio-Rad Laboratories, Inc.). Cell-morphology
images were captured using an inverted microscope (magnification,
×200; Nikon Corporation).
TUNEL assay
HeLa cells were plated in 8-chamber slides at a
density of 0.5 × 104 cells/well, and the cells were
treated with AS-DK143-NPs for 24 h. Cells were fixed in 4%
paraformaldehyde (PFA) and permeated in 0.1% TritonX-100 solution
for 5 min at room temperature. Cells were incubated with
equilibration buffer for 5 min and treated with recombinant
terminal deoxynucleotidyl transferase (rTdT) reaction solution
(consisting of equilibration buffer, a mixture of biotinylated
nucleotides, and rTdT enzyme) for 1 h at 37°C. The cells were
immersed in 2X SSC buffer (NaCl and sodium citrate) and washed with
phosphate-buffered saline. The cells were incubated with
streptavidin-conjugated horseradish peroxidase solution for 30 min.
The cells were reacted with 3,3′-diaminobenzidine (DAB) solution
(DAB chromogen and DAB substrate). Subsequently, the cells were
mounted and observed under a light microscope.
Immunocytochemistry
HeLa cells (1×105 cells/well) were seeded
in 30-mm dishes and treated with AS-DK143-NPs for 24 h. Cells were
then fixed with 4% PFA for 10 min at room temperature (RT) and
permeabilized using 0.1% Triton-X 100 for 15 min at RT. The cells
were blocked for 1 h in 5% BSA at room temperature and incubated
with a primary antibody against Ki-67 (1:1,000) for 24 h at 4°C.
Subsequently, cells were incubated for 1 h at RT with a
fluorophore-conjugated secondary antibody (1:1,000). Cells were
further incubated with Hoechst solution for 20 min at room
temperature. Fluorescence was observed using a K1-fluo microscope
(magnification, ×200; Nanoscope Systems, Inc.).
Mitochondrial labeling
HeLa cells were seeded in 30-mm confocal dishes at
the density of 1×105 cells/well and were treated with 10
and 30 µM of AS-DK143-NPs for 24 h. Cells were loaded with 500 nM
Mito-tracker™ Red CMXRos (excitation: 579 nm, emission:
599 nm) for 30 min at 37°C. The medium was replaced and
mitochondrial images were obtained using a Zeiss KSM 780 microscope
(magnification, 400; Ziess GmbH).
Reverse transcription quantitative
(RT-q)PCR
Total RNA was isolated from HeLa cells using TRIzol
reagent (Thermo Fisher Scientific, Inc.) and mixed with chloroform.
The resulting supernatant was incubated with isopropanol for 20 h
at 4°C and then centrifuged at 8,000 × g for 15 min at 4°C. The
total RNA was quantified using a NanoDrop device
(NanoDrop™ 2000/2000c spectrophotometer; Thermo Fisher
Scientific Inc.). cDNA was synthesized using 1 µg RNA and the
Superscript III First Strand cDNA Synthesis Kit. qPCR was performed
on an Exicyler™ 96 instrument (Bioneer Corporation)
using SYBR master mix (Bioneer Corporation). The following PCR
thermocycling conditions were used: Initial denaturation at 95°C
for 10 min, 40 cycles of denaturation for 10 sec at 95°C and
annealing at 60°C for 30 sec, followed by a final extension step
for 30 sec at 72°C. The sequences of the primers were as follows:
CD36 forward, 5′-TGGAACAGAGGCTGACAACTT-3′, reverse,
5′-TTGATTTTGATAGATATGGGATGC-3′; BAX forward,
5′-GCGTCCACCAAGAAGCTGAG-3′, reverse, 5′-ACCACCCTGGTCTTGGATCC-3′;
Bcl2 forward, 5′-TGTGGCCTTCTTTGAGTTCG-3′, reverse,
5′-TCACTTGTGGCCCAGATAGG-3′; p53 forward,
5′-TGTGGAGTATTTGGATGACA-3′, reverse, 5′-GAACATGAGTTTTTTATGGC-3′;
and β-actin forward, 5′-GTGATGGTGGGCATGGGTC-3′ and reverse,
5′-ACGGCCAGAGGCGTACAGGG-3′. The relative expression levels were
normalized to endogenous control β-actin and were expressed as
2−ΔΔCq (24).
3-dimension (3D) holotomography
To detect the effects of AS-DK143-NPs on live cells,
HeLa cells (1×105 cells/well) were seeded in 60-mm
Tomodishes and treated with 30 µM AS-DK143-NPs at 36°C for 30, 60
and 120 min. 3D holotomography images of live cells were obtained
using an HT-1 microscope (magnification, ×600; Tomocube, Inc.). The
cell structures were scanned using a digital micromirror device and
reconstructed using a 3D refractive index tomogram. The masses of
the cytoplasm and lipid droplets were analyzed based on densities
of 0.135 g/ml and 0.178 g/ml, respectively.
Statistical analysis
Data are expressed as the means ± standard deviation
of at least three independent experiments. Statistical analyses
were performed using GraphPad Prism software version 4.0 (GraphPad
Software, Inc.). Data were compared using Student's t-test and
one-way ANOVA followed by with Tukey's post hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Effect of AS-DK143 on different human
cancer cell lines
Fig. 1 shows the
chemical structure of AS-DK143 and the formed NPs that were used as
an efficient drug delivery system. Cell viability assay was
performed to evaluate the anti-tumor effects of AS-DK143-NPs on the
four cell lines HepG2, BXPC-3, AGS and HeLa. Cells were treated
with increasing concentration of AS-DK143 for 24 h. The inhibitory
rate was determined using WST-1 assay. As presented in Fig. 2, the IC50 values of
AS-DK143-NPs were 18.54, 35.74, 9.28 and 4.17 µM in HepG2, BXPC-3,
AGS and HeLa cells, respectively. The AS-DK143-NPs revealed a
reducing effect on cell viability in HeLa cells compared with other
cell lines.
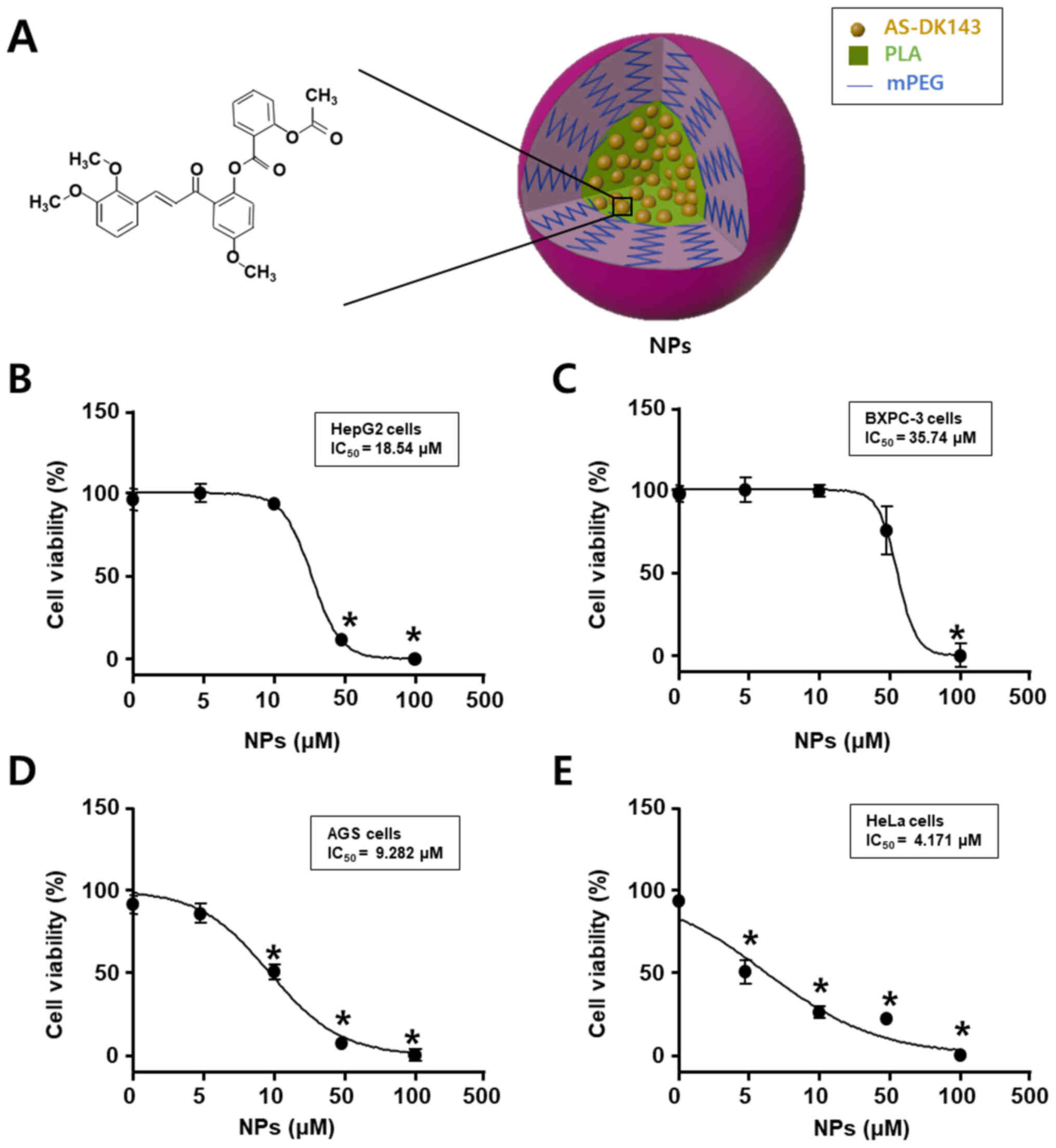 | Figure 1.Anticancer effects of AS-DK143 in
various types of cancer cell. (A) Schematic representation of
AS-DK143-NPs. (B) HepG2, (C) Bxpc-3, (D) AGS and (E) HeLa cells
were treated with various concentrations (1, 3, 10, 30, 100 or 300
µM) of NPs for 24 h. Cell viability was evaluated using WST-1
assay. *P<0.05 vs. untreated group. AS, aspirin; NPs,
nanoparticles; mPEG-PLA, methoxy poly(ethylene
glycol)-b-poly(D,L-lactide). |
Anticancer effects of AS-DK143-NPs on
HeLa cells
To determine the anticancer effects of AS-DK143-NPs,
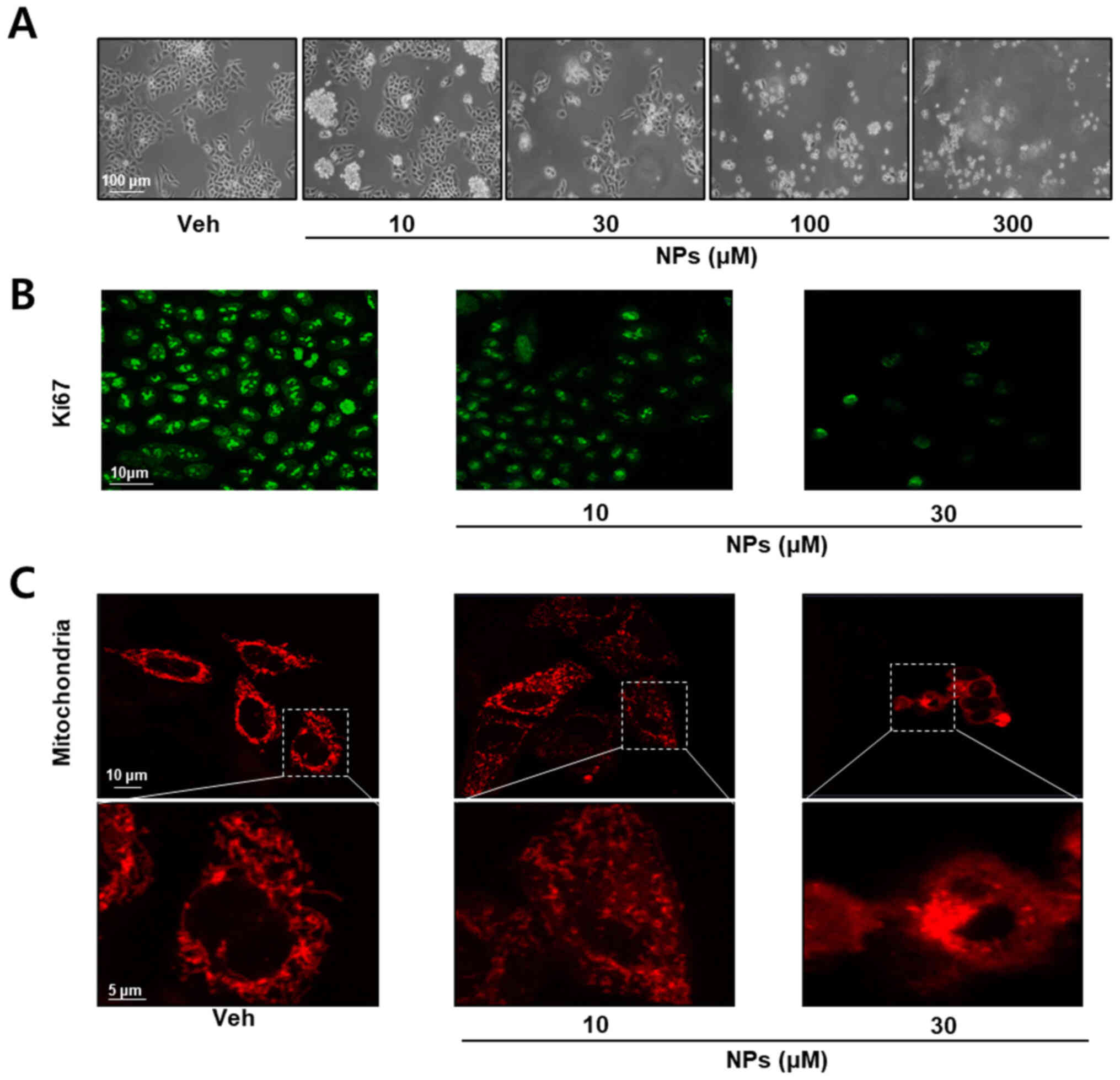
we performed cell morphology and immunocytochemistry assays. As
presented in Fig. 2A, cells were
treated with different concentrations of AS-DK143-NPs (10, 30, 100
and 300 µM) for 24 h. AS-DK143-NPs treatment induced changes in
cell morphology and viability. Subsequently, the anticancer effects
of AS-DK143-NPs were confirmed by studying cell proliferation
arrest using Ki-67 antibody for immunocytochemical staining. As
presented in Fig. 2B, AS-DK143-NPs
treatment decreased Ki-67 expression in HeLa cells. In addition,
AS-DK143-NPs induced mitochondrial fission (Fig. 2C).
AS-DK143-NPs induces apoptosis in HeLa
cells
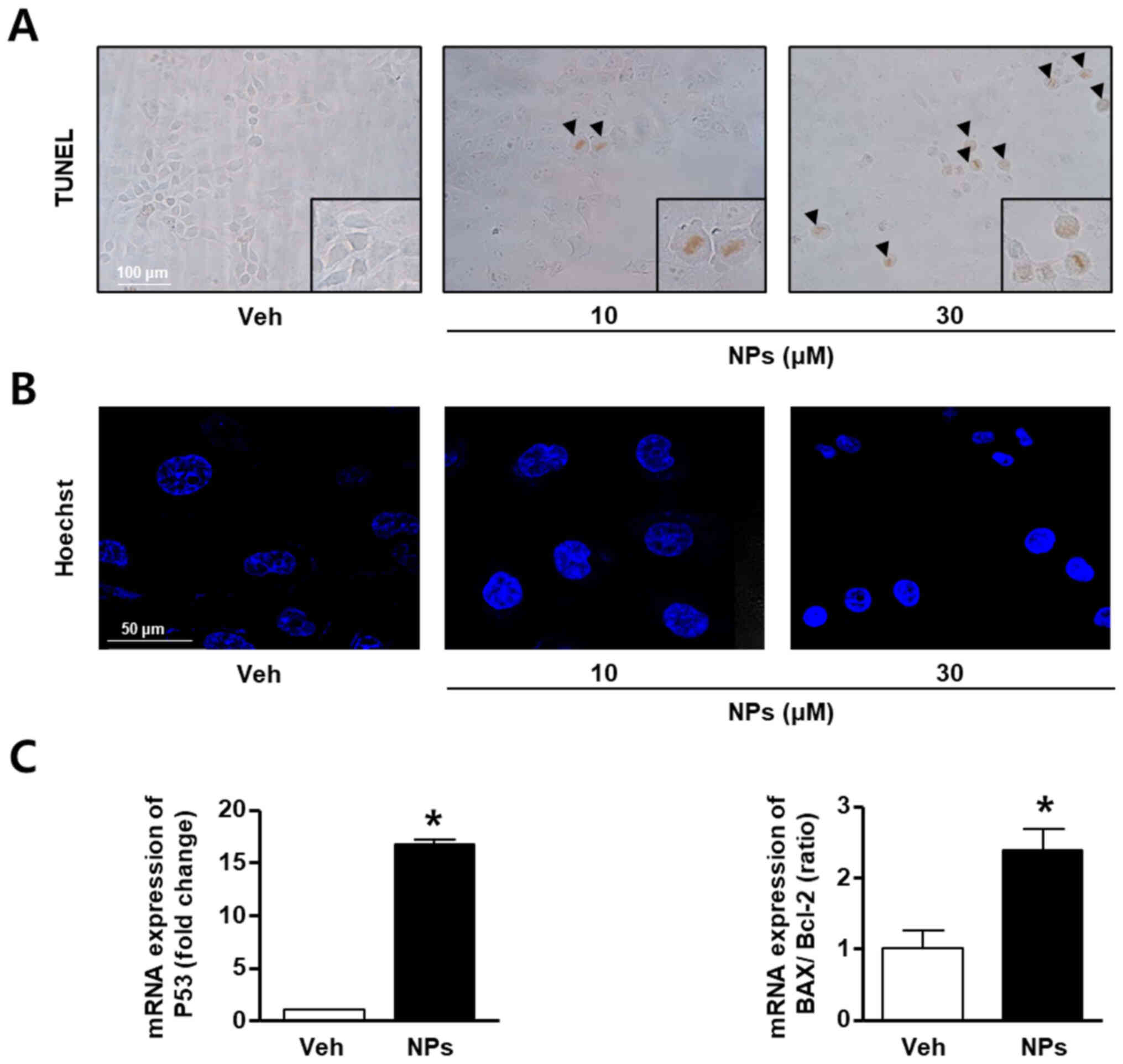
To determine whether AS-DK143-NPs could induce
apoptosis in HeLa cells, TUNEL assays, Hoechst staining and RT-qPCR
were performed. HeLa cells were grown in the absence or presence of
AS-DK143-NPs (10 and 30 µM) for 24 h. The results demonstrated that
AS-DK143-NPs increased TUNEL-positive staining in a concentration
dependent manner, whereas the vehicle control-treated cells were
not stained with the TUNEL solution (Fig. 3A). In addition, AS-DK143-NPs
induced nucleolus aggregation in HeLa cells (Fig. 3B). Furthermore, we confirmed that
AS-DK143-NPs induced HeLa apoptosis by evaluating the mRNA
expression levels of certain apoptosis-related genes. As presented
in Fig. 3C, AS-DK143-NPs
significantly increased the expression level of p53 and the
BAX/Bcl-2 ratio.
AS-DK143-NPs induces lipid
accumulation in HeLa cells
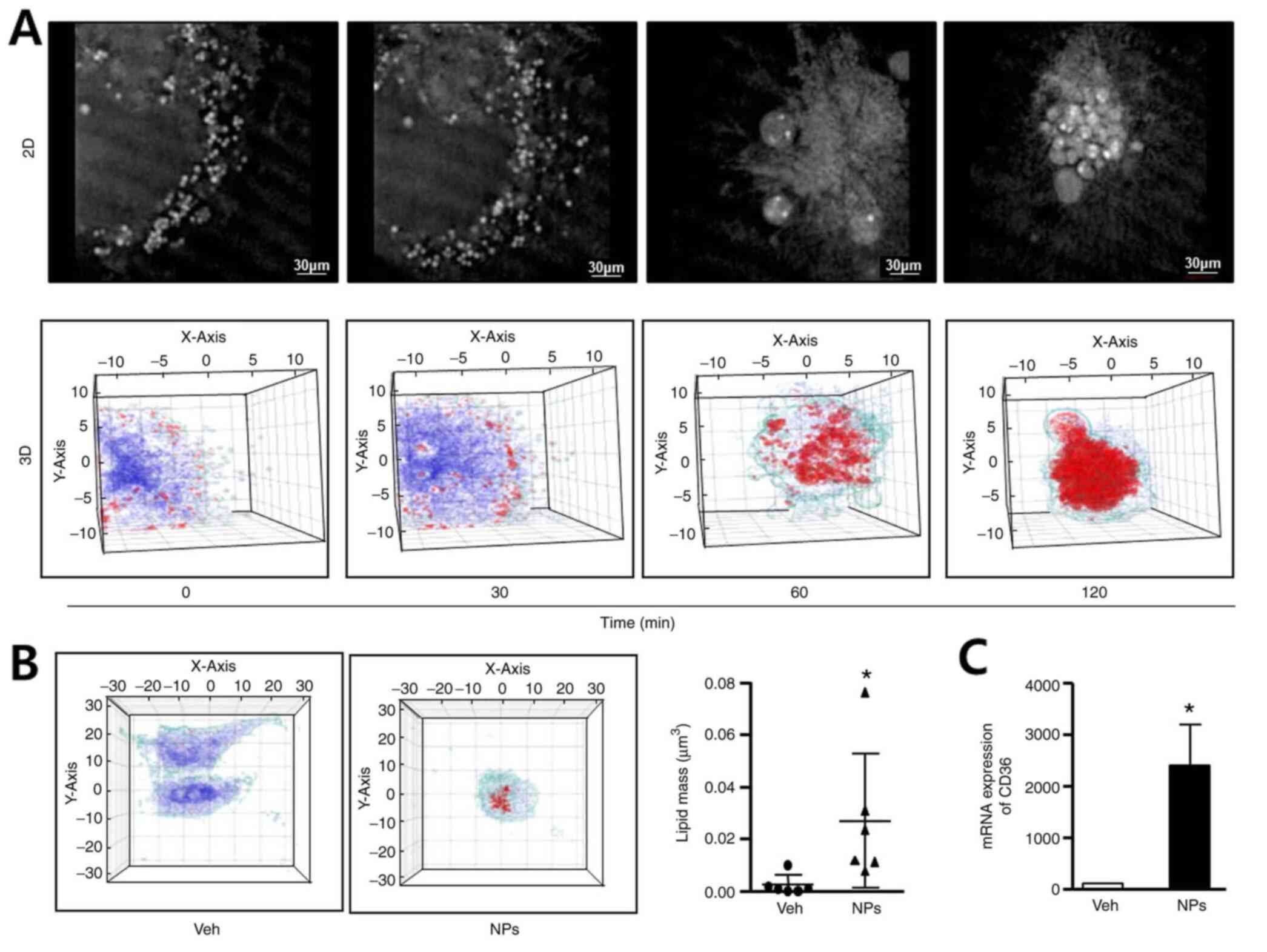
To determine whether AS-DK143-NPs could induce lipid
accumulation, we performed live cell imaging, 3-D holographic
imaging using a holotomographic microscope and determined CD36 mRNA
expression by RT-qPCR. Hela cells were treated with AS-DK143-NPs
(10 µM) for 2 h and 2D images representing their cell morphology
were taken (Fig. 4A). We observed
the formation of apoptotic bodies over time. The 3D images revealed
lipid droplet formation (red color) and lipid accumulation occurred
in a time-dependent manner. The 3D holotomographic-imaging assay
was performed to evaluate lipid masses in live cells. The results
demonstrated that lipid accumulation was not altered in vehicle
treated cells but was significantly increased (~6-fold) in HeLa
cells treated with AS-DK143-NPs (Fig.
4B). As presented in Fig. 4C,
AS-DK143-NPs significantly increased the mRNA level of CD36
compared with the vehicle.
Discussion
CVC therapy may include a surgical method, depending
on the progression of the lesion (25). Doxorubicin can be used to treat CVC
and various types of cancer, such as breast and bladder cancer,
Kaposi's sarcoma, lymphoma and acute lymphocytic leukemia. However,
anthracycline-based anticancer drugs such as doxorubicin can cause
toxicity and permanent damage in cardiac cells (26). Identifying effective anticancer
drugs with reduced side effects is therefore crucial in the
development of anticancer treatment strategy for CVC, which may
improve patient survival and enable surgery. Aspirin was the first
synthetic antipyretic and anti-inflammatory analgesic and was used
for various purposes, including reducing cardiovascular risk,
especially myocardial infarction and ischemic stroke (27,28).
DK143 is synthesized from selected plant-derived anticancer drugs
(29). In the present study, we
attempted to synthesize NPs carrying both compounds, aspirin and
DK143, which have excellent anticancer properties and no adverse
side effects on the heart (22).
The results from the present study demonstrated that
AS-DK143-NPs may have a stronger cytotoxic effect on HeLa cells
compare with other human cancer cell lines, including HepG2, BXPC-3
and AGS cell lines. We also determined whether AS-DK143-NPs could
induce cell cycle regulation in HeLa cells. Ki-67 is closely
related to tumor cell proliferation. Thus, it is not expressed
during the stationary phase (G0) of the cell cycle, but is
expressed during the proliferative phases (G1, S, G2 and M phases)
(30). The present study
demonstrated that AS-DK143-NPs reduced Ki-67 expression. However,
these results raised the question of whether AS-DK143-NPs may exert
an anticancer effect by inducing apoptosis.
Apoptosis is induced by cellular signals that
originate from mitochondria (31).
BAX and Bcl-2 play important regulatory roles in apoptosis
induction (32). An increased
BAX/Bcl-2 expression ratio has been reported to promote apoptotic
signals triggered by anticancer drugs (33). In the present study, we observed
that apoptosis induced by AS-DK143-NPs could inhibit HeLa cell
viability. Furthermore, apoptosis induction by AS-DK143-NPs was
confirmed by performing TUNEL assays and staining cell nuclei with
the Hoechst dye. The results from these analyses suggested that
AS-DK143-NPs may exert anticancer effects by regulating
apoptosis.
Apoptosis may also be induced by various cellular
molecules, including lipids (34).
Lipid accumulation in cancer cells caused by anticancer drugs can
activate apoptosis related signaling cascades, resulting in the
development of apoptotic bodies (35). These bodies promote tumor cell
death via excessive CD36 expression (36). In the present study, we observed
that AS-DK143-NPs induced significant lipid accumulation, CD36
expression and lipid droplet formation in HeLa cells (Fig. 4).
In future studies, in order to investigate how lipid
accumulation by AS-DK143-NPs induces apoptosis in HeLa cell, we
need to confirm that lipid droplet accumulation via high glucose or
TGFb2 exposure in cancer cells induces apoptosis directly. Our
results indicated that AS-DK143-NPs may represent a potential
treatment strategy against CVC. Further investigation on the
anticancer effects of AS-DK143-NPs in tumor-bearing animal models,
the possible adverse effects on normal cells and comparative
studies including negative and positive controls is needed to
determine whether AS-DK143-NPs may be considered as a potential
anticancer drug that could induce cancer cell apoptosis. Future
studies will perform flow cytometry (FACS) analyses for cell cycle
analysis and additional western blot analysis, including
apoptosis-related proteins (BAX, BCL2 and Caspase 3/7), for more
detailed apoptosis evaluation. In addition, we are going to conduct
cytochrome c oxidase assay for additional mitochondrial fate
evaluation.
Acknowledgements
Not applicable.
Funding
This study was supported by the Basic Science Research Program
through the National Research Foundation of Korea funded by the
Ministry of Science and ICT (grant no. 2021R1I1A1A01049147), the
Korea Health Technology R&D Project through the Korea Health
Industry Development Institute funded by the Ministry of Health
& Welfare (grant no. HI18C2383), Republic of Korea, the Korea
Basic Science Institute (KBSI) under the R&D programs (grant
no. D110710) supervised by the Ministry of Science and ICT. This
work was supported by the Technology Development Program (grant no.
S3054194) funded by the Korean Ministry of SMEs and Startup.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KPL, SB, KSP and BSM conceived and designed the
study. MSY, KPL, SB and JSP performed the experiments. BSH, SJL,
SJO, DHL, RL and SHK analyzed the data. KPL and SB drafted the
initial manuscript. KSP and BSM revised the manuscript and
confirmed the authenticity of all the raw data. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arbyn M, Weiderpass E, Bruni L, de Sanjosé
S, Saraiya M, Ferlay J and Bray F: Estimates of incidence and
mortality of cervical cancer in 2018: A worldwide analysis. Lancet
Glob Health. 8:e191–e203. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Maine D, Hurlburt S and Greeson D:
Cervical cancer prevention in the 21st century: Cost is not the
only issue. Am J Public Health. 101:1549–1555. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lowndes CM: Vaccines for cervical cancer.
Epidemiol Infect. 134:1–12. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yim EK and Park JS: The role of HPV E6 and
E7 oncoproteins in HPV-associated cervical carcinogenesis. Cancer
Res Treat. 37:319–324. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thomas M, Pim D and Banks L: The role of
the E6-p53 interaction in the molecular pathogenesis of HPV.
Oncogene. 18:7690–7700. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fischer M, Uxa S, Stanko C, Magin TM and
Engeland K: Human papilloma virus E7 oncoprotein abrogates the
p53-p21-DREAM pathway. Sci Rep. 7:26032017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan X, Liu Y and Chen JJ: Down-regulation
of p21 contributes to apoptosis induced by HPV E6 in human mammary
epithelial cells. Apoptosis. 10:63–73. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Photopulos GJ: Surgery or radiation for
early cervical cancer. Clin Obstet Gynecol. 33:872–882. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kumar L and Gupta S: Integrating
Chemotherapy in the Management of Cervical Cancer: A Critical
Appraisal. Oncology. 91 (Suppl 1):8–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hoffman MS, Roberts WS, Bryson SC,
Kavanagh JJ Jr, Cavanagh D and Lyman GH: Treatment of recurrent and
metastatic cervical cancer with cis-platin, doxorubicin, and
cyclophosphamide. Gynecol Oncol. 29:32–36. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu H, Luo H, Zhang W, Shen Z, Hu X and
Zhu X: Molecular mechanisms of cisplatin resistance in cervical
cancer. Drug Des Devel Ther. 10:1885–1895. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Serkies K and Jassem J: Concurrent weekly
cisplatin and radiotherapy in routine management of cervical
cancer: A report on patient compliance and acute toxicity. Int J
Radiat Oncol Biol Phys. 60:814–821. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sarosiek KA, Ni Chonghaile T and Letai A:
Mitochondria: Gatekeepers of response to chemotherapy. Trends Cell
Biol. 23:612–619. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brunelle JK and Letai A: Control of
mitochondrial apoptosis by the Bcl-2 family. J Cell Sci.
122:437–441. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Willems PH, Rossignol R, Dieteren CE,
Murphy MP and Koopman WJ: Redox Homeostasis and Mitochondrial
Dynamics. Cell Metab. 22:207–218. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jana BA, Chintamaneni PK, Krishnamurthy
PT, Wadhwani A and Mohankumar SK: Cytosolic lipid excess-induced
mitochondrial dysfunction is the cause or effect of high fat
diet-induced skeletal muscle insulin resistance: A molecular
insight. Mol Biol Rep. 46:957–963. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wong A, Chen S, Yang LK, Kanagasundaram Y
and Crasta K: Lipid accumulation facilitates mitotic
slippage-induced adaptation to anti-mitotic drug treatment. Cell
Death Discov. 4:1092018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang L, Zhu H, Liu D, Liang S, Xu H, Chen
J, Wang X and Xu Z: Aspirin suppresses growth of human gastric
carcinoma cell by inhibiting survivin expression. J Biomed Res.
25:246–253. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sostres C, Gargallo CJ and Lanas A:
Aspirin, cyclooxygenase inhibition and colorectal cancer. World J
Gastrointest Pharmacol Ther. 5:40–49. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Johnson KE, Ceglowski JR, Roweth HG,
Forward JA, Tippy MD, El-Husayni S, Kulenthirarajan R, Malloy MW,
Machlus KR, Chen WY, et al: Aspirin inhibits platelets from
reprogramming breast tumor cells and promoting metastasis. Blood
Adv. 3:198–211. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee DY, Lee KP, Baek S, Park JS, Kim YJ,
Kim KN, Kim SR and Yoon MS: Anti-breast cancer activity of
aspirin-conjugated chalcone polymeric micelles. Macromol Res.
29:105–110. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reid Y, Storts D, Riss T and Minor L:
Authentication of Human Cell Lines by STR DNA Profiling Analysis.
In: Assay Guidance Manual. [Internet]. Markossian S, Grossman A,
Brimacombe K, Arkin M, Auld D, Austin CP, Baell J, Chang TDY,
Coussens NP, Dahlin JL, et al: Eli Lilly & Company and the
National Center for Advancing Translational Sciences. (Bethesda,
MD). 2004.
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sarenac T and Mikov M: Cervical Cancer,
Different Treatments and Importance of Bile Acids as Therapeutic
Agents in This Disease. Front Pharmacol. 10:4842019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cai F, Luis MAF, Lin X, Wang M, Cai L, Cen
C and Biskup E: Anthracycline-induced cardiotoxicity in the
chemotherapy treatment of breast cancer: Preventive strategies and
treatment. Mol Clin Oncol. 11:15–23. 2019.PubMed/NCBI
|
|
27
|
Vane JR and Botting RM: The mechanism of
action of aspirin. Thromb Res. 110:255–258. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ittaman SV, VanWormer JJ and Rezkalla SH:
The role of aspirin in the prevention of cardiovascular disease.
Clin Med Res. 12:147–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee DH, Jung Jung Y, Koh D, Lim Y, Lee YH
and Shin SY: A synthetic chalcone,
2′-hydroxy-2,3,5′-trimethoxychalcone triggers unfolded protein
response-mediated apoptosis in breast cancer cells. Cancer Lett.
372:1–9. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li LT, Jiang G, Chen Q and Zheng JN: Ki67
is a promising molecular target in the diagnosis of cancer
(review). Mol Med Rep. 11:1566–1572. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Indran IR, Tufo G, Pervaiz S and Brenner
C: Recent advances in apoptosis, mitochondria and drug resistance
in cancer cells. Biochim Biophys Acta. 1807:735–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Khanzadeh T, Hagh MF, Talebi M, Yousefi B,
Azimi A, Hossein Pour Feizi AA and Baradaran B: Investigation of
BAX and BCL2 expression and apoptosis in a resveratrol- and
prednisolone-treated human T-ALL cell line, CCRF-CEM. Blood Res.
53:53–60. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Naseri MH, Mahdavi M, Davoodi J, Tackallou
SH, Goudarzvand M and Neishabouri SH: Up regulation of Bax and down
regulation of Bcl2 during 3-NC mediated apoptosis in human cancer
cells. Cancer Cell Int. 15:552015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Su LJ, Zhang JH, Gomez H, Murugan R, Hong
X, Xu D, Jiang F and Peng ZY: Reactive Oxygen Species-Induced Lipid
Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid Med
Cell Longev. October 13–2019.(Epub ahead of print). doi:
10.1155/2019/5080843. View Article : Google Scholar
|
|
35
|
Cruz ALS, Barreto EA, Fazolini NPB, Viola
JPB and Bozza PT: Lipid droplets: Platforms with multiple functions
in cancer hallmarks. Cell Death Dis. 11:1052020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Frank AC, Ebersberger S, Fink AF, Lampe S,
Weigert A, Schmid T, Ebersberger I, Syed SN and Brüne B: Apoptotic
tumor cell-derived microRNA-375 uses CD36 to alter the
tumor-associated macrophage phenotype. Nat Commun. 10:11352019.
View Article : Google Scholar : PubMed/NCBI
|