Introduction
Breast cancer (BC) is the second most common
malignancy worldwide and the most frequent cancer in women
(1), contributing to an estimated
25% of all new cancer cases and ~0.5 million cancer-related deaths
each year (2). Despite progress in
the current BC therapies, including surgery, radiotherapy,
chemotherapy and endocrinotherapy, almost 30% patients with BC,
diagnosed at early-stages, may develop distant metastasis, leading
to death (3,4). So far, a number of
clinicopathological features, including tumor size, histological
grade, lymph node status, hormone receptor (HR) status and human
epidermal growth factor receptor type 2 (HER2) status, have been
used for the diagnosis and prognostic prediction in patients with
BC (5); however, the value of
these traditional markers in predicting the prognosis of BC is
limited (6). Therefore, additional
diagnostic or prognostic biomarkers for early surveillance in
patients with BC is required.
MicroRNAs (miRNAs/miR) are a family of endogenous
small non-coding RNAs, which are 18–23 nucleotides in length, and
are considered to regulate numerous biological processes, including
cell differentiation, proliferation, apoptosis and metastasis
(7–9). To date, miRNA expression signatures
have been found to play a tumor suppressive or oncogenic role in
cancer using translational repression or target degradation and
gene silencing (10). In addition,
miRNAs have been shown to be promising biomarkers for BC as they
can be readily detected in both tumor tissues and body fluids (as
circulating miRNAs), including in plasma, serum or saliva (11–13).
Numerous studies have demonstrated that miR-103a-3p
is an oncomiR in various types of cancer, including thyroid cancer
(14), colorectal cancer (15), gastric cancer (16), oral squamous cell carcinoma
(17), malignant mesothelioma
(18) and salivary adenoid cystic
carcinoma (19). In contrast, Ge
et al (20) reported that
downregulation of miR-103a-3p could inhibit the proliferation and
invasion of prostate cancer. However, the role of miR-103a-3p in BC
has not been elucidated.
The aim of the present study was to detect the
expression level of serum miR-103a-3p in patients with BC, analyze
the association between miR-103a-3p expression and
clinicopathological features, and evaluate the ability of
circulating miR-103a-3p to predict and diagnose BC.
Materials and methods
Clinical samples
A total of 112 women with BC, who were admitted and
received treatment at the Cangzhou Central Hospital (Hebei, China)
between January 2009 and December 2014 were recruited into the
present study. All the patients with BC underwent modified radical
mastectomy or breast-conserving surgery. The serum samples were
collected one day prior to and following surgery. Patients were
included if they were i) histologically confirmed as having
invasive ductal breast carcinoma (IDC) type; ii) had no other
associated malignancies; iii) had complete follow-up
clinicopathological information; and iv) who were disease-free and
followed up for at least 5 years. Patients with any neoadjuvant
treatment prior to surgery or with bilateral or inflammatory BC
were excluded. In addition, a group of 59 age- and sex-matched
healthy volunteers were enrolled as a control group, at the same
institution between January 2013 and December 2014, and serum
samples were also obtained during routine physical examinations.
The mean age was 54.1±9.8 years for patients with BC and 53.9±9.3
years for healthy controls. The peripheral blood samples were
collected from all participants in serum gel separator tubes. Each
sample was centrifuged at 3,000 × g for 10 min at 4°C to separate
serum, then stored at −80°C until further use.
Clinical data, including age, tumor size,
pathological type, lymph-node status, histological grading,
metastasis and TNM stage, were also collected. The tumors were
staged according to the 8th edition of the American Joint Committee
on Cancer (21). Postoperative
routine pathological examination, hormonal estrogen receptors (ER),
progesterone receptors (PR), and HER2 were tested using
immunohistochemistry by two pathologists in a blinded manner at
Department of Pathology, Cangzhou Central Hospital (Cangzhou,
China) independently. All enrolled patients provided written
informed consent for the use of their tissue samples and clinical
information in the present study. The Ethics Committee of Cangzhou
Central Hospital (Hebei, China; approval no. 20210013) approved the
study and was conducted in accordance with the Declaration of
Helsinki.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from the prepared serum
samples using the RNA Isolation kit (Qiagen, Inc.), and the cDNA
was synthesized using the RevertAid First Strand cDNA Synthesis kit
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. RT-qPCR was subsequently performed using the TaqMan miR
assay system (cat. no. A25576; Applied Biosystems; Thermo Fisher
Scientific, Inc.) on an FTC-3000™ System (Funglyn Biotech Inc.).
The thermocycling conditions for RT-qPCR were: Initial denaturation
at 95°C for 1 min, followed by 40 cycles of 95°C for 10 sec and
60°C for 35 sec. Relative expression of miR-103a-3p was normalized
to that of U6 using the 2−∆∆Cq method (22). The following primers (Shanghai
GenePharma Co., Ltd) were used: miR-103a-3p forward,
5′-ATCCAGTGCGTGTCGTG-3′ and reverse, 5′-TGCTAGCAGCATTGTACAGG-3′; U6
forward, 5′-CGCTTCGGCAGCACATATAC-3′ and reverse,
5′-TTCACGAATTTGCGTGTCAT-3′.
Statistical analysis
The data are expressed as the mean ± SD from at
least three independent experiments. Statistical evaluations were
performed using SPSS v20.0 (IBM Corp.). Differences between two
groups were analyzed using an unpaired Student's t-test, while the
expression of miR-103a-3p in BC serum tissues before and after
surgery was compared using a paired Student's t-test. Comparisons
of multiple groups were performed using ANOVA followed by Tukey's
post hoc test. Categorical data were compared using either a
χ2 test or a Fisher's exact test. Based on the median
values of miR-103a-3p expression, patients with BC were classified
into either miR-103a-3p low (n=56) or high expression (n=56)
groups. Receiver operating characteristic (ROC) curves were
utilized to calculate diagnostic accuracy. The survival outcomes,
including overall survival (OS) and recurrence-free survival (RFS)
times, were evaluated using Kaplan-Meier curves and compared using
a log-rank test. OS time was calculated from the date of surgery to
the date of the patient's death or to the date of last follow-up.
RFS time was defined between the date of surgery to the date of BC
recurrence. Prognostic factors were analyzed using Cox regression
proportional hazards analysis. P<0.05 was considered to indicate
a statistically significant difference.
Results
Study population
As shown in Table
I, 112 patients with BC were recruited into the study, and a
total of 59 age-matched healthy female volunteers were used as the
control group. There were 81 (72.3%) patients with lymph-node
involvement and 28 patients (25.0%) with distant metastasis. With
respect to TNM stage, 54 patients with BC (48.2%) were at stage II,
30 patients (26.8%) at stage III, and 28 patients (25.0%) at stage
IV. All other clinicopathological data are shown in Table I.
 | Table I.Association between miR-103a-3p
expression level and clinicopathological features in patients with
breast cancer. |
Table I.
Association between miR-103a-3p
expression level and clinicopathological features in patients with
breast cancer.
|
|
| miR-103a-3p
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
feature | Number (n=112) | Low (n=56) | High (n=56) | P-value |
|---|
| Mean age ± SD,
years |
| 54.7±10.3 | 53.1±9.6 | 0.195a |
| Tumor size, cm, n
(%) |
|
|
| 0.357b |
| ≤2 | 24 (21.4) | 14 (25.0) | 10 (17.9) |
|
|
>2 | 88 (78.6) | 42 (75.0) | 46 (82.1) |
|
| Pathological type, n
(%) |
|
|
| 0.844c |
| IDC
I | 9 (8.0) | 5 (8.9) | 4 (7.1) |
|
| IDC
II | 54 (48.2) | 28 (50.0) | 26 (46.4) |
|
| IDC
III | 49 (43.8) | 23 (41.1) | 26 (46.4) |
|
| Lymph-node status, n
(%) |
|
|
| 0.291b |
|
Negative | 31 (27.7) | 18 (32.1) | 13 (23.2) |
|
|
Positive | 81 (72.3) | 38 (67.9) | 43 (76.8) |
|
| Histological
grading, n (%) |
|
|
| 0.638c |
| I | 10 (8.9) | 6 (10.7) | 4 (7.1) |
|
| II | 56 (50.0) | 29 (51.8) | 27 (48.2) |
|
|
III | 46 (41.1) | 21 (37.5) | 25 (44.6) |
|
| ER, n (%) |
|
|
| 0.686b |
|
Negative | 36 (32.1) | 19 (33.9) | 17 (30.4) |
|
|
Positive | 76 (67.9) | 37 (66.1) | 39 (69.6) |
|
| PR, n (%) |
|
|
| 0.566b |
|
Negative | 47 (42.0) | 25 (44.6) | 22 (39.3) |
|
|
Positive | 65 (58.0) | 31 (55.4) | 34 (60.7) |
|
| HER2, n (%) |
|
|
| 0.018b |
|
Negative | 40 (35.7) | 26 (46.4) | 14 (25.0) |
|
|
Positive | 72 (64.3) | 30 (53.6) | 42 (75.0) |
|
| Molecular
subtyped, n (%) |
|
|
| 0.968c |
| Luminal
A | 36 (32.1) | 19 (33.9) | 17 (30.4) |
|
| Luminal
B | 27 (24.1) | 14 (25.0) | 13 (23.2) |
|
| HER2
enriched | 10 (8.9) | 5 (8.9) | 5 (8.9) |
|
| Triple
negative | 39 (34.8) | 18 (32.1) | 21 (37.5) |
|
| Metastasis, n
(%) |
|
|
| 0.002b |
|
Absent | 84 (75.0) | 49 (87.5) | 35 (62.5) |
|
|
Present | 28 (25.0) | 7 (12.5) | 21 (37.5) |
|
| TNM stage, n
(%) |
|
|
| 0.028b |
| II | 54 (48.2) | 32 (57.1) | 22 (48.2) |
|
|
III | 30 (26.8) | 16 (28.6) | 14 (26.8) |
|
| IV | 28 (25.0) | 8 (14.3) | 20 (25.0) |
|
miR-103a-3p is upregulated in patients
with BC
To primarily investigate the expression level of
miR-103a-3p in BC tissues, sera from 112 patients with BC and 59
healthy controls were collected for RT-qPCR analysis. The results
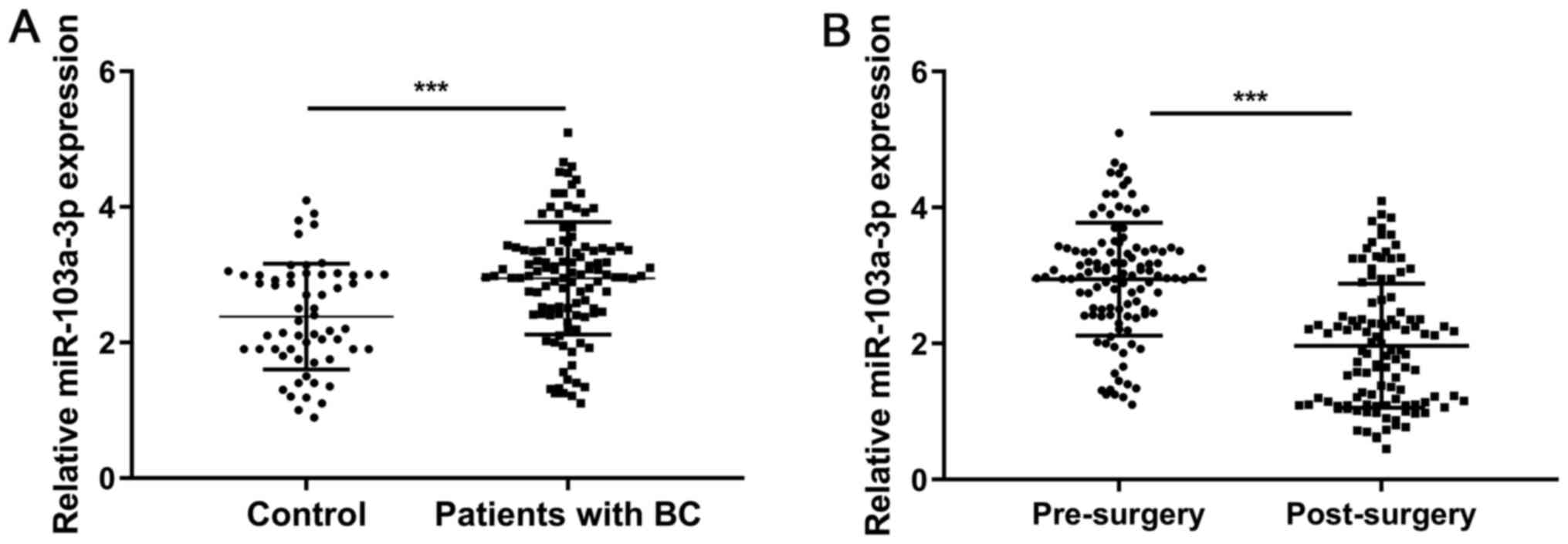
showed that miR-103a-3p expression was significantly upregulated in
patients with BC compared with that in the controls (P<0.001;
Fig. 1A). In addition, serum
miR-103a-3p expression was significantly reduced in patients with
BC following surgery (P<0.001; Fig.
1B). The miR-103a-3p expression level in patients with positive
HER2 status was significantly higher compared with those who are
HER2 negative (P<0.001; Fig.
2A). In addition, miR-103a-3p expression level in patients with
BC and metastasis was significantly higher compared with that in
those without metastasis (P<0.001; Fig. 2B). Comparison of miR-103a-3p
expression level between patients with BC and different TNM stages
showed statistically significant differences between stages II, III
and IV (III vs. II, P<0.001; IV vs. II, P<0.001; IV vs. III,
P<0.001; Fig. 2C). Furthermore,
there was no significant difference between miR-103a-3p expression
levels with respect to the molecular subtypes of BC (Luminal B vs.
Luminal A, P=0.732; HER2 enriched vs. Luminal A, P=0.840; Triple
negative vs. Luminal A, P=0.501; HER2 enriched vs. Luminal B,
P=0.667; Triple negative vs. Luminal B, P=0.341; Triple negative
vs. HER2 enriched, P=0.829; Fig.
2D).
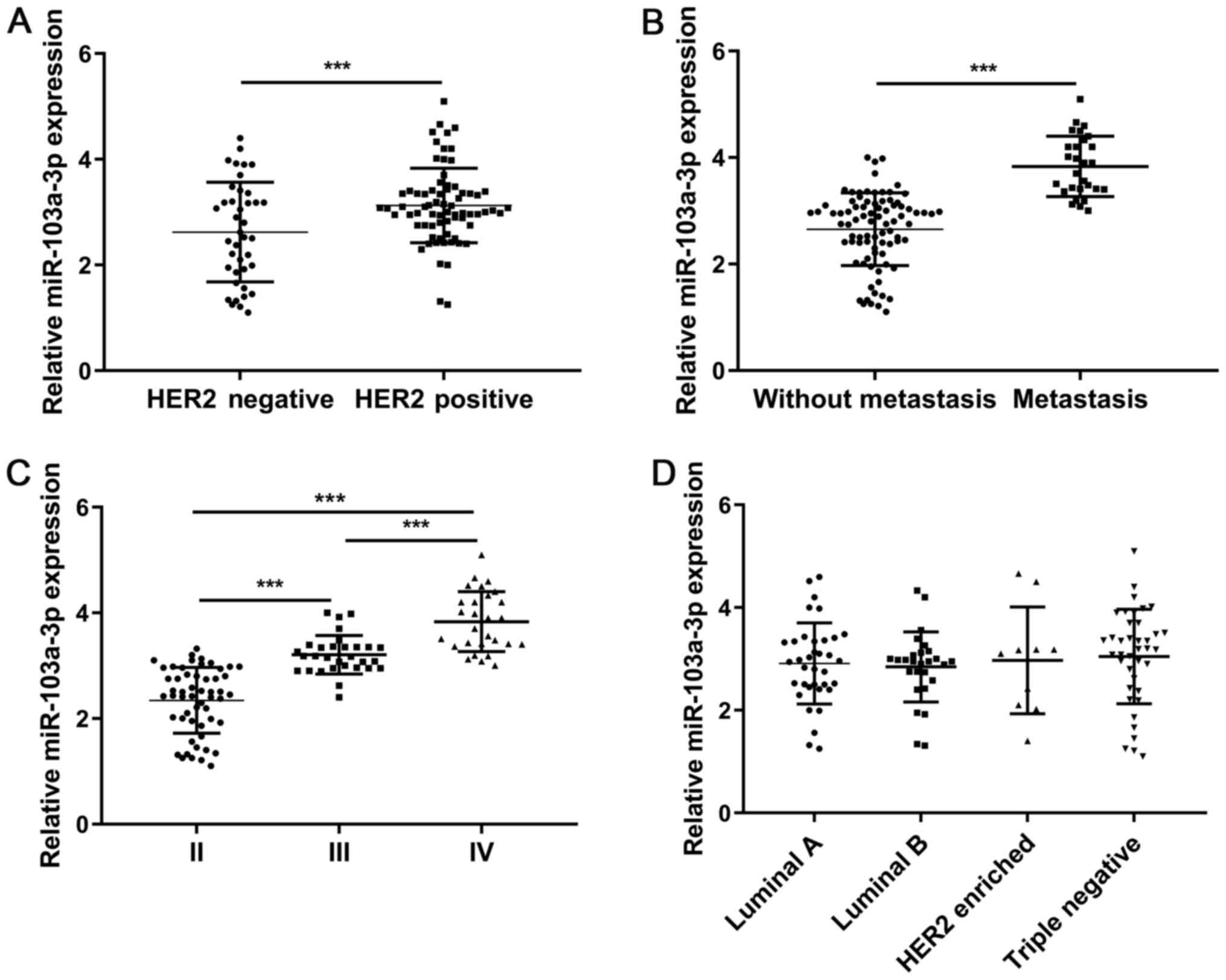 | Figure 2.Reverse transcription-quantitative PCR
analysis of miR-103a-3p expression in serum from patients with BC
stratified by (A) HER2 status, (B) metastasis, (C) TNM stage and
(D) molecular subtypes. Luminal B vs. Luminal A, P=0.732; HER2
enriched vs. Luminal A, P=0.840; Triple negative vs. Luminal A,
P=0.501; HER2 enriched vs. Luminal B, P=0.667; Triple negative vs.
Luminal B, P=0.341; Triple negative vs. HER2 enriched, P=0.829.
***P<0.001. BC, breast cancer; miR, microRNA. |
Association between serum miR-103a-3p
expression level and clinicopathological features of BC
The association between the miR-103a-3p expression
level and the clinicopathological features in patients with BC was
further analyzed. It was found that miR-103a-3p expression was
significantly associated with HER2 status (P=0.018), metastasis
(P=0.002) and TNM stage (P=0.028) (Table I).
Diagnostic value of miR-103a-3p in
patients with BC
To evaluate the diagnostic value of miR-103a-3p in
patients with BC, the performance of serum miR-103a-3p level in
distinguishing between patients with BC and the controls was
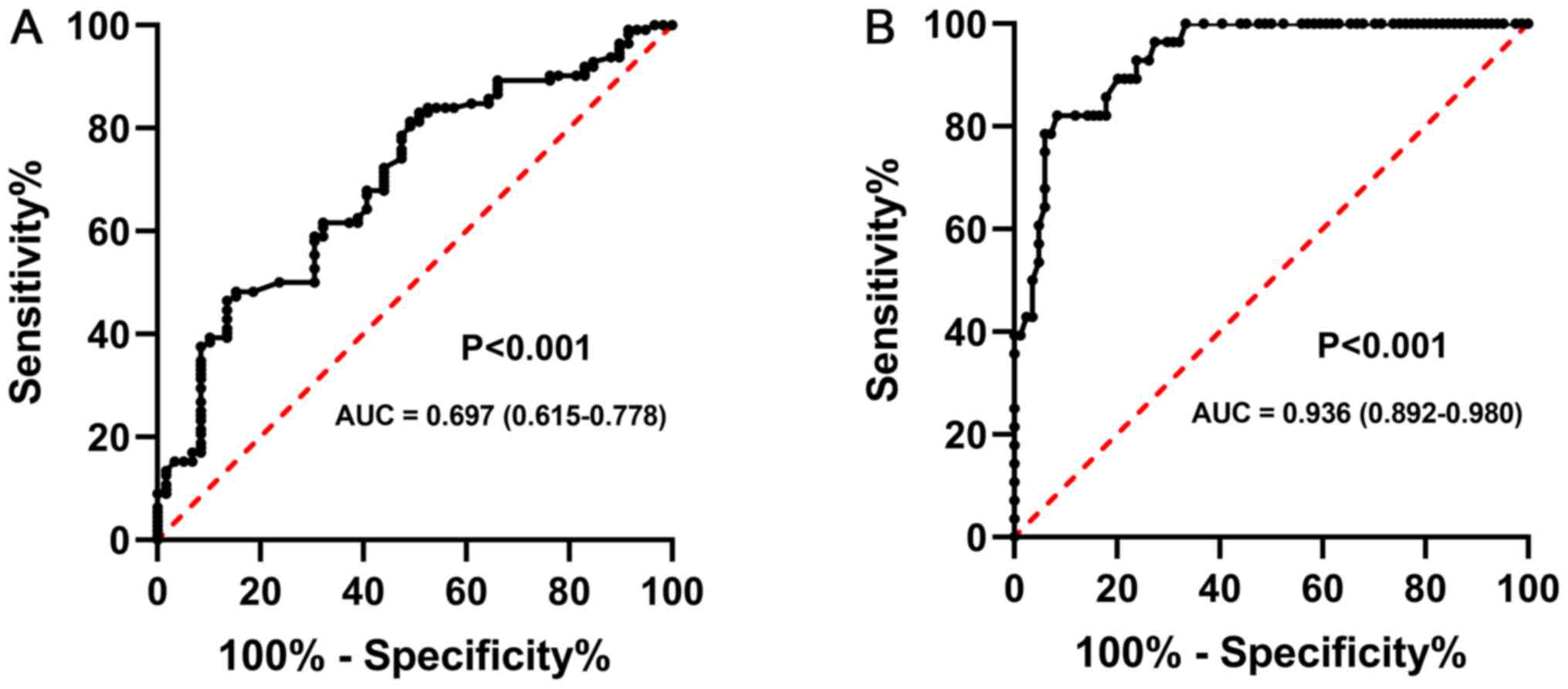
performed using ROC analysis. As shown in Fig. 3A, the optimal diagnostic cut-off
value for miR-103a-3p was 3.01, and the AUC value for miR-103a-3p
was 0.697 [95% confidence interval (CI), 0.615-0.778], with a
sensitivity and specificity of 78.2 and 74.7%, respectively.
Furthermore, ROC analysis also demonstrated that the optimal
diagnostic cut-off value for miR-103a-3p was 3.4, and the AUC value
for miR-103a-3p was 0.936 (95% CI, 0.892–0.980), with a sensitivity
and specificity of 88.6 and 84.0%, respectively, in distinguishing
patients with BC and metastasis from those without metastasis
(Fig. 3B).
Prognostic value of miR-103a-3p in
patients with BC
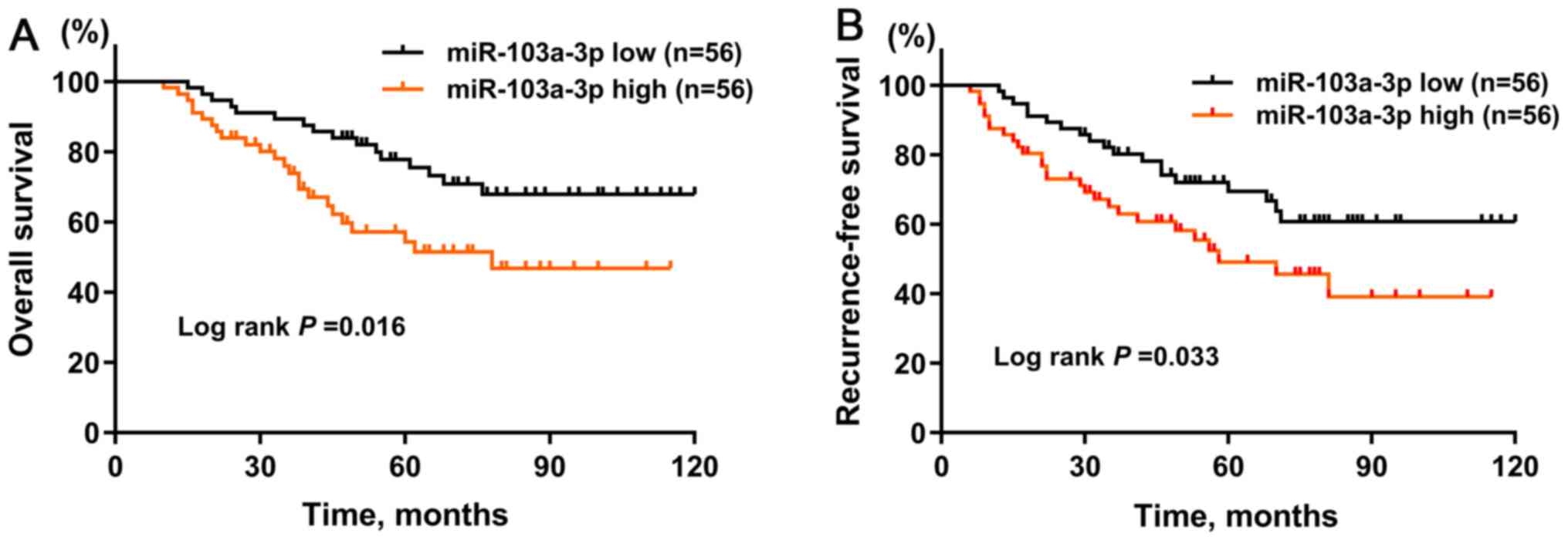
Next, using Kaplan-Meier curves to analyze OS time,
the results showed that patients with BC and a high expression
level of miR-103a-3p was associated with worse OS (P=0.016)
(Fig. 4A) and RFS times (P=0.033)
(Fig. 4B) compared with that in
patients with a low expression level of miR-103a-3p. Univariate Cox
regression analyses demonstrated that HER2 status [hazard ratio
(HR), 2.141; 95% CI, 1.254-3.274; P=0.002), metastasis (HR, 2.841;
95% CI, 1.542-3.984; P=0.001), TNM stage (III vs. II, HR, 2.547;
95% CI, 1.564-3.854; P<0.001; and IV vs. II, HR, 2.951; 95% CI,
1.785-3.641; P<0.001) and high miR-103a-3p expression (HR,
1.774; 95% CI, 1.452-2.051; P=0.005) were independent indicators
for poor OS time in patients with BC (Table II). Multivariate Cox regression
analyses showed that HER2 status [HR, 1.952; 95% CI, 1.112-2.874;
P=0.012), metastasis (HR, 2.412; 95% CI, 1.214-3.174; P=0.005), TNM
stage (III vs. II, HR, 2.471; 95% CI, 1.384-3.641; P=0.001; and IV
vs. II, HR, 2.814; 95% CI, 1.541-3.285; P<0.001) and high
miR-103a-3p expression (HR, 1.612; 95% CI, 1.314-1.854; P=0.023)
were independent indicators for poor OS time in patients with BC
(Table II). In addition,
univariate Cox regression analyses showed that HER2 status (HR,
2.325; 95% CI, 1.653-3.018; P=0.001), metastasis (HR, 3.145; 95%
CI, 2.521-3.954; P<0.001), TNM stage (III vs. II, HR, 2.154; 95%
CI, 1.621-2.963; P<0.001; and IV vs. II, HR, 2.335; 95% CI,
1.841-3.115; P<0.001) and high miR-103a-3p expression (HR,
1.684; 95% CI, 1.351-1.997; P=0.002) were independent indicators
for poor RFS (Table III).
Multivariate Cox regression analyses revealed that HER2 status (HR,
2.010; 95% CI; 1.532-2.991; P=0.009), metastasis (HR, 2.888; 95%
CI, 2.113-3.208; P=0.001), TNM stage (III vs. II, HR, 1.997; 95%
CI, 1.554-2.563; P=0.003; and IV vs. II, HR, 2.117; 95% CI;
1.609-3.695; P<0.001) and high miR-103a-3p expression (HR,
1.333; 95% CI, 1.241-1.763; P=0.029) were independent indicators of
poor RFS time (Table III). Taken
together, the results indicated that miR-103a-3p was an independent
unfavorable prognostic factor in patients with BC.
 | Table II.Univariate and multivariate analyses
of prognostic factors associated with overall survival. |
Table II.
Univariate and multivariate analyses
of prognostic factors associated with overall survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
| 0.952 |
|
|
|
≤50 | Ref |
|
|
|
|
>50 | 1.125
(0.895-1.425) |
|
|
|
| Tumor size, cm |
| 0.184 |
|
|
| ≤2 | Ref |
|
|
|
|
>2 | 1.235
(0.821-1.841) |
|
|
|
| Pathological
type |
| 0.115 |
|
|
| IDC
I | Ref |
|
|
|
| IDC
II | 1.851
(0.912-2.541) | 0.252 |
|
|
| IDC
III | 1.998
(0.925-2.845) | 0.184 |
|
|
| Lymph-node
status |
| 0.098 |
|
|
|
Negative | Ref |
|
|
|
|
Positive | 1.965
(0.841-2.862) |
|
|
|
| Histological
grading |
| 0.118 |
|
|
| I | Ref |
|
|
|
| II | 1.541
(0.852-2.415) | 0.102 |
|
|
|
III | 1.815
(0.841-2.984) | 0.215 |
|
|
| ER |
| 0.521 |
|
|
|
Negative | Ref |
|
|
|
|
Positive | 1.276
(0.862-1.961) |
|
|
|
| PR |
| 0.181 |
|
|
|
Negative | Ref |
|
|
|
|
Positive | 1.452
(0.951-1.864) |
|
|
|
| HER2 |
| 0.002b |
| 0.012b |
|
Negative | Ref |
| Ref |
|
|
Positive | 2.141
(1.254-3.274) |
| 1.952
(1.112-2.874) |
|
| Molecular
subtypea |
| 0.841 |
|
|
| Luminal
A | Ref |
|
|
|
| Luminal
B | 1.241
(0.865-1.874) | 0.546 |
|
|
| HER2
enriched | 0.985
(0.741-1.324) | 0.214 |
|
|
| Triple
negative | 1.141
(0.741-1.685) | 0.623 |
|
|
| Metastasis |
| 0.001b |
| 0.005b |
|
Absent | Ref |
| Ref |
|
|
Present | 2.841
(1.542-3.984) |
| 2.412
(1.214-3.174) |
|
| TNM stage |
|
<0.001b |
|
<0.001b |
| II | Ref |
| Ref |
|
|
III | 2.547
(1.564-3.854) |
<0.001b | 2.471
(1.384-3.641) | 0.001b |
| IV | 2.951
(1.785-3.641) |
<0.001b | 2.814
(1.541-3.285) |
<0.001b |
| miR-103a-3p
expression |
| 0.005b |
| 0.023b |
|
Low | Ref |
| Ref |
|
|
High | 1.774
(1.452-2.051) |
| 1.612
(1.314-1.854) |
|
 | Table III.Univariate and multivariate analyses
of prognostic factors associated with recurrence-free survival. |
Table III.
Univariate and multivariate analyses
of prognostic factors associated with recurrence-free survival.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
| 0.558 |
|
|
|
≤50 | Ref |
|
|
|
|
>50 | 1.010
(0.874-1.351) |
|
|
|
| Tumor size, cm |
| 0.009 |
|
|
| ≤2 | Ref |
|
|
|
|
>2 | 1.351
(0.885-1.652) |
|
|
|
| Pathological
type |
| 0.652 |
|
|
| IDC
I | Ref |
|
|
|
| IDC
II | 1.415
(0.886-2.412) | 0.141 |
|
|
| IDC
III | 1.652
(0.904-2.214) | 0.384 |
|
|
| Lymph-node
status |
| 0.196 |
|
|
|
Negative | Ref |
|
|
|
|
Positive | 1.741
(0.652-2.819) |
|
|
|
| Histological
grading |
| 0.225 |
|
|
| I | Ref |
|
|
|
| II | 1.521
(0.854-1.912) | 0.852 |
|
|
|
III | 1.662
(0.910-2.041) | 0.102 |
|
|
| ER |
| 0.274 |
|
|
|
Negative | Ref |
|
|
|
|
Positive | 1.112
(0.991-1.421) |
|
|
|
| PR |
| 0.206 |
|
|
|
Negative | Ref |
|
|
|
|
Positive | 1.352
(0.928-1.657) |
|
|
|
| HER2 |
| 0.001b |
| 0.009b |
|
Negative | Ref |
| Ref |
|
|
Positive | 2.325
(1.653-3.018) |
| 2.010
(1.532-2.991) |
|
| Molecular
subtypea |
| 0.524 |
|
|
| Luminal
A | Ref |
|
|
|
| Luminal
B | 1.041
(0.925-1.354) | 0.256 |
|
|
| HER2
enriched | 0.912
(0.825-1.319) | 0.741 |
|
|
| Triple
negative | 1.085
(0.952-1.351) | 0.230 |
|
|
| Metastasis |
|
<0.001b |
| 0.001b |
|
Absent | Ref |
| Ref |
|
|
Present | 3.145
(2.521-3.954) |
| 2.888
(2.113-3.208) |
|
| TNM stage |
|
<0.001b |
|
<0.001b |
| II | Ref |
| Ref |
|
|
III | 2.154
(1.621-2.963) |
<0.001b | 1.997
(1.554-2.563) | 0.003b |
| IV | 2.335
(1.841-3.115) |
<0.001b | 2.117
(1.609-3.695) |
<0.001b |
| miR-103a-3p
expression |
| 0.002b |
| 0.029b |
|
Low | Ref |
| Ref |
|
|
High | 1.684
(1.351-1.997) |
| 1.333
(1.241-1.763) |
|
Discussion
BC is an aggressive cancer and commonly diagnosed at
a late stage, with a risk of developing metastasis (23). Recently, a spectrum of miRNAs has
been determined to be of great importance during the progression of
BC, which may benefit BC diagnosis and prognosis (24). For example, Li et al
(25) identified a panel of five
plasma miRNAs (let-7b-5p, miR-122-5p, miR-146b-5p, miR-210-3p and
miR-215-5p) to detect BC with high sensitivity and specificity.
Zhang et al (26) screened
a panel of 3 miRNAs (miR-199a, miR-29c and miR-424) for
differentiating patients with BC from controls, with the highest
diagnostic accuracy.
Various studies have associated miR-103a-3p in tumor
progression. As reported, miR-103a-3p expression levels were
increased in gastric cancer tissues and enhanced overexpression of
miR-103a-3p promoted gastric cancer cell proliferation (16). Zhang et al (14) reported that miR-103a-3p was
overexpressed in thyroid cancer tissues and knocking down its
expression could inhibit cell proliferation, migration and
invasion, and promote thyroid cancer cell apoptosis. In addition,
knocking down miR-103a-3p expression in oral squamous cell
carcinoma could repress cell proliferation and induce apoptosis
(17). Analysis of the clinical
samples in the present study revealed that miR-103a-3p was
upregulated in patients with BC and was also significantly
associated with advanced features of BC, including positive HER2
status, metastasis and a more advanced TNM stage. High expression
of miR-103a-3p was also associated with poor survival outcomes.
miR-103a-3p may represent a potential diagnostic biomarker and
therapeutic target in patients with BC at different TNM stages.
Notably, tumor metastasis is the main obstacle to prognosis in
patients with BC. It was found that serum miR-103a-3p expression
was markedly elevated in patients with BC and tumor metastasis,
which furthers the understanding into the potential role of
miR-103a-3p during BC metastasis.
Numerous studies have discussed the critical role of
circulating miRNA expression as a non-invasive biomarker for early
detection of numerous types of cancer (27–29),
as serum samples are stable, and easily accessible for testing
using RT-qPCR. However, some studies have recently reported that
hemolysis during blood collection or sample processing can alter
the levels of certain proposed miRNAs, such as miR-106a, miR-16 and
miR-17 (30). To avoid having
misleading results, it is vital to investigate whether hemolysis
could affect the expression level of each miRNA in future studies.
Notably, neither miR-103a-3p or U6 have been reported to be
affected by hemolysis (30,31).
Recently, circulating miR-103a-3p has become an important area as a
potential non-invasive biomarker for the diagnosis and prognosis of
multiple types of cancer. For example, Zhang et al (15) established a panel of seven miRNAs
in plasma, including miR-103a-3p, to predict the occurrence of
colorectal cancer. In addition, Weber et al (18) demonstrated that the combination of
mesothelin and miR-103a-3p in plasma could mutually enhance the
diagnostic performance in the detection of malignant mesothelioma.
However, the diagnostic function of miR-103a-3p has not been
elucidated in BC. In the present study, the results indicated that
circulating serum miR-103a-3p expression could discriminate
patients with BC from control subjects prior to surgery. In
addition, miR-103a-3p expression had a high ability to distinguish
patients with BC and metastasis from those without metastasis.
Traditionally, some important predictive or prognostic biomarkers,
including tumor size, tumor grade, lymph node involvement, ER
status and HER2 status have been used for patients with BC
(32). However, tumor tissue is
required for the evaluation of all the aforementioned biomarkers,
which limits their clinical applications. In the present study,
miR-103a-3p was detected easily and stably in peripheral blood, and
could be a new prognostic and predictive marker in patients with
BC. In addition, miR-103a-3p may be a potential therapeutic target
in patients with BC due to its association with tumor metastasis
and stage. For patients with BC and a high expression level of
miR-103a-3p, more precision treatment should be utilized to reduce
the rate of tumor metastasis and improve patient prognosis as
well.
There are some limitations in the present study.
First, there is a lack of an external cohort to validate the
diagnostic and prognostic ability of miR-103a-3p. In addition, the
biological role of miR-103a-3p and its underlying mechanism during
BC initiation and progression remain to be clarified.
In conclusion, the results from the present study
demonstrate that miR-103a-3p was upregulated in patients with BC,
and miR-103a-3p could act as a promising diagnostic and prognostic
biomarker in patients with BC. Further studies are warranted to
validate the diagnostic and prognostic value of miR-103a-3p with
larger sample sizes, and to investigate the biological roles of
miR-103a-3p in BC growth and metastasis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated and/or analyzed during the study
are available from the corresponding author upon reasonable
request.
Author's contributions
HL and HBC conceived and designed the present study.
QZB and WZ collected clinical samples and analyzed the data. HL and
HBC wrote the manuscript. HL and HBC confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All enrolled patients provided written informed
consent for the use if their tissue samples and clinical
information in the study. The Ethics Committee of Cangzhou Central
Hospital (Hebei, China; approval no. 20210013) and was conducted in
accordance with the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
O'Shaughnessy J: Extending survival with
chemotherapy in metastatic breast cancer. Oncologist. 10 (Suppl
3):20–29. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cardoso F, Spence D, Mertz S,
Corneliussen-James D, Sabelko K, Gralow J, Cardoso MJ, Peccatori F,
Paonessa D, Benares A, et al: Global analysis of
advanced/metastatic breast cancer: Decade report (2005–2015).
Breast. 39:131–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Viale G: The current state of breast
cancer classification. Ann Oncol. 23 (Suppl 10):x207–x210. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs: New biomarkers for diagnosis, prognosis, therapy
prediction and therapeutic tools for breast cancer. Theranostics.
5:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tutar Y: miRNA and cancer; computational
and experimental approaches. Curr Pharm Biotechnol. 15:4292014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mishra S, Yadav T and Rani V: Exploring
miRNA based approaches in cancer diagnostics and therapeutics. Crit
Rev Oncol Hematol. 98:12–23. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li B, Zhang F and Li H: miR-1225-5p
inhibits non-small cell lung cancer cell proliferation, migration
and invasion, and may be a prognostic biomarker. Exp Ther Med.
20:1722020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Acunzo M, Romano G, Wernicke D and Croce
CM: MicroRNA and cancer - a brief overview. Adv Biol Regul. 57:1–9.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hamam R, Hamam D, Alsaleh KA, Kassem M,
Zaher W, Alfayez M, Aldahmash A and Alajez NM: Circulating
microRNAs in breast cancer: Novel diagnostic and prognostic
biomarkers. Cell Death Dis. 8:e30452017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chong ZX, Yeap SK and Ho WY: Roles of
circulating microRNA(s) in human breast cancer. Arch Biochem
Biophys. 695:1085832020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ozawa PM, Jucoski TS, Vieira E, Carvalho
TM, Malheiros D and Ribeiro EM: Liquid biopsy for breast cancer
using extracellular vesicles and cell-free microRNAs as biomarkers.
Transl Res. 223:40–60. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang ML, Sun WH, Wu HQ, Liu ZD and Wang
P: Knockdown of microRNA-103a-3p inhibits the malignancy of thyroid
cancer cells through Hippo signaling pathway by upregulating LATS1.
Neoplasma. 67:1266–1278. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang H, Zhu M, Shan X, Zhou X, Wang T,
Zhang J, Tao J, Cheng W, Chen G, Li J, et al: A panel of
seven-miRNA signature in plasma as potential biomarker for
colorectal cancer diagnosis. Gene. 687:246–254. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu X, Miao J, Zhang M, Wang X, Wang Z, Han
J, Tong D and Huang C: miRNA-103a-3p promotes human gastric cancer
cell proliferation by targeting and suppressing ATF7 in vitro. Mol
Cells. 41:390–400. 2018.PubMed/NCBI
|
|
17
|
Zhang G, Chen Z, Zhang Y, Li T, Bao Y and
Zhang S: Inhibition of miR-103a-3p suppresses the proliferation in
oral squamous cell carcinoma cells via targeting RCAN1. Neoplasma.
67:461–472. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Weber DG, Casjens S, Johnen G, Bryk O,
Raiko I, Pesch B, Kollmeier J, Bauer TT and Brüning T: Combination
of miR-103a-3p and mesothelin improves the biomarker performance of
malignant mesothelioma diagnosis. PLoS One. 9:e1144832014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fu M, Chen CW, Yang LQ, Yang WW, Du ZH, Li
YR, Li SL and Ge XY: MicroRNA 103a 3p promotes metastasis by
targeting TPD52 in salivary adenoid cystic carcinoma. Int J Oncol.
57:574–586. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ge J, Mao L, Xu W, Fang W, Wang N, Ye D,
Dong Z, Guan H and Guan C: miR-103a-3p suppresses cell
proliferation and invasion by targeting tumor protein D52 in
prostate cancer. J Invest Surg. 34:984–992. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou J, Lei J, Wang J, Lian CL, Hua L,
Yang LC and Wu SG: Validation of the 8th edition of the American
Joint Committee on Cancer Pathological Prognostic Staging for young
breast cancer patients. Aging (Albany NY). 12:7549–7560. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scully OJ, Bay BH, Yip G and Yu Y: Breast
cancer metastasis. Cancer Genomics Proteomics. 9:311–320.
2012.PubMed/NCBI
|
|
24
|
Koi Y, Tsutani Y, Nishiyama Y, Ueda D,
Ibuki Y, Sasada S, Akita T, Masumoto N, Kadoya T, Yamamoto Y, et
al: Predicting the presence of breast cancer using circulating
small RNAs, including those in the extracellular vesicles. Cancer
Sci. 111:2104–2115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li M, Zou X, Xia T, Wang T, Liu P, Zhou X,
Wang S and Zhu W: A five-miRNA panel in plasma was identified for
breast cancer diagnosis. Cancer Med. 8:7006–7017. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang L, Xu Y, Jin X, Wang Z, Wu Y, Zhao
D, Chen G, Li D, Wang X, Cao H, et al: A circulating miRNA
signature as a diagnostic biomarker for non-invasive early
detection of breast cancer. Breast Cancer Res Treat. 154:423–434.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Valihrach L, Androvic P and Kubista M:
Circulating miRNA analysis for cancer diagnostics and therapy. Mol
Aspects Med. 72:1008252020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Usuba W, Urabe F, Yamamoto Y, Matsuzaki J,
Sasaki H, Ichikawa M, Takizawa S, Aoki Y, Niida S, Kato K, et al:
Circulating miRNA panels for specific and early detection in
bladder cancer. Cancer Sci. 110:408–419. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu XL, Ren LF, Wang HP, Bai ZT, Zhang L,
Meng WB, Zhu KX, Ding FH, Miao L, Yan J, et al: Plasma microRNAs as
potential new biomarkers for early detection of early gastric
cancer. World J Gastroenterol. 25:1580–1591. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kirschner MB, Edelman JJ, Kao SC, Vallely
MP, van Zandwijk N and Reid G: The impact of hemolysis on cell-free
microRNA biomarkers. Front Genet. 4:942013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Iempridee T, Wiwithaphon S, Piboonprai K,
Pratedrat P, Khumkhrong P, Japrung D, Temisak S, Laiwejpithaya S,
Chaopotong P and Dharakul T: Identification of reference genes for
circulating long noncoding RNA analysis in serum of cervical cancer
patients. FEBS Open Bio. 8:1844–1854. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Barzaman K, Karami J, Zarei Z,
Hosseinzadeh A, Kazemi MH, Moradi-Kalbolandi S, Safari E and
Farahmand L: Breast cancer: Biology, biomarkers, and treatments.
Int Immunopharmacol. 84:1065352020. View Article : Google Scholar : PubMed/NCBI
|